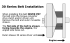A little below the belt
Transcription
A little below the belt
A little below the belt 1 A LITTLE BELOW THE BELT AN ANZUP CANCER TRIALS GROUP PUBLICATION Conducting clinical trial research to improve treatment of bladder, kidney, testicular & prostate cancer ISSUE 2, DECEMBER 2014 Welcome The Australian and New Zealand Urogenital and Prostate (ANZUP) Cancer Trials Group’s Consumer magazine is written for patients, families, carers, and supporters of those with testicular, prostate, kidney and bladder cancers. Below the Belt Consumer Magazine of ANZUP Cancer Trials group is proudly published twice a year. Copyright ANZUP Cancer Trials Group December 2014 2 A LITTLE BELOW THE BELT What’s inside 04 05 06 07 09 10 11 12 15 17 18 19 23 32 33 34 40 41 43 44 About ANZUP Message from the Chair Message from the Executive Officer Message from the CAP Chair Peter Stanford - bladder cancer Chris Reason - testicular cancer Paul Lovelace - kidney cancer Simon Clarke - testicular cancer Where to find safe information on the web ANZUP clinical trials Everyday Heroes The Concept Development process – Dr Ben Tran Inaugural Below the Belt Pedalthon What does a donation look like? Questions to ask your doctor Current ANZUP clinical trials Definitions and phases Definitions and phases Corporate sponsors Annual Scientific Meeting 2015 ANZUP Cancer Trials Group Level 6, Lifehouse Building 119-143 Missenden Road CAMPERDOWN NSW 2050 Locked Bag 77 CAMPERDOWN NSW 1450 Phone +61 2 9562 5033 Email [email protected] Twitter https://twitter.com/ANZUPtrials Welcome to A Little Below the Belt, our second issue of the ANZUP Cancer Trials Group Consumer magazine. In this issue, we meet two men who confronted testicular and bladder cancer head on. We also meet the inspirational Simon Clarke. Simon introduced himself to ANZUP a little more than 12 months ago as a 25-yearold who had recently undergone treatment for testicular cancer. He wanted to create a vehicle that would raise much-needed funds for ANZUP as well as raise awareness of “below the belt” cancers. “Below the Belt Pedalthon” was extraordinary. So too were the funds and awareness he raised to support ANZUP. In this issue, we also explore the journey taken by patients and their families as they navigate the health system after a diagnosis of cancer. We hear from social media expert and ANZUP employee Jenni Beattie who provides insight into the best places to find credible information online. Every new treatment emerging from ANZUP begins as a concept – an idea developed when specialists identify a gap or possible improvement in treatment. In this issue, Dr Ben Tran, medical oncologist, answers some questions and provides a step-by-step overview of how an idea becomes a trial. This also ties in with the need for independent funding that will allow ANZUP to take these trials to concept stage far more quickly than we can now. We hope you enjoy this issue of A Little Below the Belt. A LITTLE BELOW THE BELT 3 About ANZUP The Australian and New Zealand Urogenital and Prostate Cancer Trials Group was founded in 2008 and is a registered, not for profit charity. ANZUP’s mission is to conduct clinical trial research to improve the treatment of bladder, kidney, testicular and prostate cancers. ANZUP is a cooperative and multidisciplinary organisation including collaborations within Australia, New Zealand and international collaborations. ANZUP was formed as a national cooperative clinical trials group encompassing all urologic cancers (prostate, kidney, bladder/urothelial, testis and other related tumours). ANZUP currently has members from Australia and New Zealand from all relevant professional medical, nursing, allied health, basic science and other disciplines. The ANZUP Consumer Advisory Panel (CAP) provides a mechanism for advice to be offered across all ANZUP research activities as well as ensuring community engagement. Cancer Australia primarily funds ANZUP through its Support for Clinical Trials program. ANZUP is about the patient and their families. It is about evidence-based research that changes the way these cancers are treated in Australia and around the world. Ian Davis, ANZUP Chair Research trials outcomes Welcome to this edition of “A Little Below The Belt,” the ANZUP Consumer magazine! ANZUP is the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. ANZUP exists to improve outcomes for people and their families affected by cancers of the urinary system (prostate, kidney, bladder and testicles). We represent all groups involved in the care of people with these cancers, as well as the researchers who are trying to understand why these cancers behave as they do and how we can improve our treatments. A belt won’t hold your trousers up if the loop is not closed. In the same way, we cannot improve outcomes for these “below the belt” cancers unless we close the loop of research. This means taking the discoveries that have been made and working out what they mean in terms of how best to treat these cancers. ANZUP is the “belt buckle” in that process and in another sense we are “braces” as well – we provide support and systems to bring people together to do this research that is so desperately needed. How do we do this? Improvements in health outcomes only come about when the right questions are asked and answered. Here are some examples. If a scientist finds that a particular gene is important in a certain type of cancer, how do we take that information and turn it into a treatment? How do we know which particular people might benefit from those treatments? How do we know if this treatment is better than what we had before? How do we know what effects the treatment might have on that person? How do we help people cope with the effects of that cancer or its treatment? How do we take all that information and get the health system to change to a better way of doing things? How do we know that this is cost effective, both economically and in terms of the unseen costs and problems that our patients and their families experience? The only way to do this is through clinical trials. All the 4 A LITTLE BELOW THE BELT Message from the Chair IAN DAVIS ANZUP provides the opportunities that health carers and researchers need to come together and share their ideas. Ian Davis, ANZUP Chair wonderful laboratory research done in Australia and overseas is very important to provide us with the ideas we need, but unless we prove that we are making a positive difference to people then all that previous research counts for very little. Doctors and other health practitioners need to be able to rely on evidence in order to recommend treatments and give them safely and appropriately. ANZUP performs the clinical trials that generate that evidence. What else do we do? ANZUP provides the opportunities that health carers and researchers need to come together and share their ideas. We need to be constantly on top of all new (and old!) developments. We need to share our clinical insights so that we can understand where the gaps in our knowledge are. We need to train the next generation of researchers, to continue the work and ensure that this momentum is not lost. A key part of this is how we communicate with the broader community. ANZUP takes this very seriously. We have connections with a wide range of stakeholders including various advocacy groups. We are privileged to have a wonderful Consumer Advisory Panel, comprised of people who have been affected by the range of cancers we study and who have strong links back into the community. This means that ANZUP is constantly listening to what the community sees as being important. Our Consumer Advisory Panel members also participate directly in the research we do, by helping us generate information that is understandable, by being included on various grant applications, and by helping us raise the funds we need to do this important work. We are very grateful to all of them for these fantastic contributions. It also works in the other direction. ANZUP listens, but we also have a responsibility to speak to the community to inform them about these cancers and about the outcomes of this research. This magazine is one way of doing that and you will read about these things in the following pages, but we also use our web site (www.anzup.org.au), Community Forum meetings, and other special events. We must never lose sight of why we are doing the work we do: it is for our patients and their families. This edition of the magazine is packed with information. You will read about personal experiences with cancer. You will get an idea of how ANZUP takes a concept and develops it into a full grown research project. You will see that ANZUP is deeply involved in supportive care, including support groups and also looking at the role of carers. You will get a snapshot of the research we are currently doing or planning, and you will find links to more information if you want it. You will see some amazing work done by our supporters to raise funds for ANZUP’s work, including our recent Pedalthon that exceeded all our hopes in terms of raising awareness and additional resources for our work. I’m proud to be part of an organisation like this. It is hard work though, and it takes a long time to do it properly (much longer than any of us would like), and it is usually very expensive (much more than most people realise), and most of us have jobs and other responsibilities in the nonANZUP world too (hello to my wonderful wife – remember me?) ANZUP is a not-for-profit charity and we rely on various sources for our funding, including government and other grants, but those only go so far. They certainly do not extend to covering the costs we need actually to do the trials we need to do. ANZUP is now moving into the fundraising field, and you all know very well that there seems to be constant requests to dip into your pockets. We recognise and support the work that other fundraising groups do but we do have an important point of distinction: we are the only group actually doing the clinical trials that make all the underlying research actually mean something. Please do not hesitate to contact us if you want further information about all this. We are grateful for all your support and we know that this support goes way beyond the financial. Every bit of it goes directly to help us reach our goals of improving outcomes for our patients. Thanks for your interest in ANZUP. I’m sure you will find much of interest in this magazine. A LITTLE BELOW THE BELT 5 Message from the Executive Officer MARG MCJANNETT What a year we have had! As the festive season is upon us, I think it’s worth taking a moment to reflect on the some of the highlights of the past 12 months. In March, we launched our inaugural Community magazine “A Little Below the Belt”. It is our intention that this publication will provide you with important, accessible and accurate information on the work of ANZUP. We encourage you to share it with friends and family. The Annual Scientific Meeting (ASM), held over three days in July, was once again a resounding success. Officially opened by Victorian Parliamentary Secretary for Health, Ms Georgie Crozier MLC, delegates were treated to a scientific program of exceptional quality. Throughout the meeting, our international faculty were extremely generous with both their expertise and engagement. They were ably supported by a superb line up of local speakers and a record number of poster presentations. The broad range of topics covered provided delegates with detail of the most up-to-date management of urological cancers, as well as the psychosocial aspects of treatments and the translation of cutting-edge scientific discoveries. There were also updates on current ANZUP trials, and the very interesting trial concepts presented generated a large amount of discussion. Hopefully they will lead to important trials in the future. This regular event ensures we are on top of world class information that ultimately translates into better outcomes for patients We are extremely grateful to our generous Consumer Advisory Panel, chaired by Belinda Jago: Ray Allen, Matthew Carr, Joe Esposito, Colin O’Brien, Tony Sonneveld OAM, David Swallow, Max Shub, John Stubbs and Peter Stanford and Bill McIlrath for their continued support and collaboration during 2104. Their involvement in diseasespecific teleconferences and ASM planning is vital to ensuring we continue to have a consumer voice at the table. Our Community Engagement Forum at the ASM is another opportunity to provide the community with information about ANZUP, the importance of clinical trials, and the impact a urogenital cancer diagnosis may have on a person and family. We were joined by an experienced team of ANZUP members who engaged and encouraged audience participation. You will be able to read more about this in Belinda Jago’s article on Page 7. Connecting with the community can be quite a challenge and this year, largely due to the extraordinary efforts of Liz Thorp, we saw a significant increase on last year’s numbers with more than 65 community attending. Please consider promoting these events to friends or families if you feel they would find them beneficial. We also launched our new ClinTrial Refer App (see p39) which provides a current list of ANZUP clinical trials underway in cancer centres in Australia and New Zealand. Designed for patients, oncologists, general practitioners and research unit staff, it offers searchable clinical research trial details, hospital locations, inclusion and exclusion criteria, and lay summaries. We hope this will help the broader community identify trials that they may wish to discuss with their doctor. A little more than a year ago, I was approached by a remarkable young man - Simon Clarke, a testicular cancer survivor - who had decided during his treatment that he wanted to create an event to build awareness and raise funds for urogenital cancers. As a consequence he established the Below the Belt Pedalthon. The inaugural event was held at Eastern Creek on 16 September 2014. The rest, as they say, is history. Through Simon’s extraordinary determination and commitment, ably and tirelessly supported by Liz Thorp, the Pedalthon was, by all measures, a HUGE success. It not only raised significant funds to support ANZUP’s research activities but also opened the door for us to better engage with the corporate sector. Our sincere thanks go to Simon and his wonderful family, Dad Andrew, Mum Sally, sister Rose and brothers Cam and Will, and extended family and friends, for their support and generosity. The funds raised will go towards clinical trials research, and concept development meetings to progress our research have already taken place. You can read more about the Pedalthon throughout this magazine. Thank you for your ongoing support. ANZUP would not be able to achieve its high levels of activity without the support, input and commitment of the community. Don’t forget to SAVE THE DATE for the Community Engagement forum where you can meet the experts, 12 July 2015 at the Sofitel Sydney Wentworth. Admission is free, registration on the ANZUP website. Look forward to seeing you there. I wish you and your family best wishes for the festive season. 6 A LITTLE BELOW THE BELT A message from the ANZUP CAP Chair BELINDA JAGO The ANZUP Consumer Advisory Panel (CAP) plays a vital role in the provision of advice and feedback from a consumer perspective on issues as diverse as the direction of general research to community engagement and support. The CAP chair is Belinda Jago who brings a particular knowledge base to the voluntary role through her own experiences as carer to her daughter Bec, who succumbed to kidney cancer at the age of 19. Here she speaks of the CAP’s involvement in the Annual Scientific Meeting, and panel plans for the year ahead. The ANZUP Annual Scientific Meeting (ASM), which takes place in mid-July, provides a key opportunity for CAP members to take part in education sessions which help them provide the most constructive input into ANZUP clinical trial research from a consumer perspective. It also gives CAP members, who through the rest of the year work in small groups or via teleconference, the chance to meet face to face as well as meet with clinicians and researchers. Each year, a Sunday session of the ASM is dedicated to pre-meetings - mostly education sessions for specific interest groups. These include: l A master class for trainee medical clinicians and allied health professionals, focusing on case studies of different urology cancers; l A community engagement forum offering up-to-date information on ANZUP clinical trials and the benefits of being a clinical trial participant; l Importantly, from my perspective as Chair of the CAP, our half-day education session for all CAP members. To be a member of the CAP, you will have been a cancer patient or carer of a family member or close friend, and will have a keen interest in clinical trial research and a passion for advocating to the community the importance of clinical trials. The education sessions inform our personal experience with knowledge about the clinical trial processes: how they start as an idea and, hopefully, end up being the newest and best standard treatment for cancer patients. All the research at ANZUP is about improving outcomes for cancer patients and their families. We are fortunate that many expert researchers and clinicians offer their time to the CAP at these meetings through sessions on subjects such as funding for clinical trials and the grant submission process, the role of ethics in approving a clinical trial, the use of statistics, and the different types of trials developed around proving different theories. These learning opportunities ensure that we have the best possible understanding of the clinical trials process including timing issues, and complexities around ethics and funding approvals, to ensure we can actively engage and contribute to ANZUPs research activities. We also get the chance to discuss our own processes for reviewing clinical trial concepts, what works best, and what we can do to improve our feedback to clinicians and researchers. We also consider the technical A LITTLE BELOW THE BELT 7 language used by clinicians and researchers, and encourage “lay” language summaries for all clinical trial proposals to make sure they are understood. Meeting face to face also means we meet with some of the clinicians and researchers who sit via teleconference with CAP members on the various disease subcommittees. Even just a short meet and greet helps to make these meetings a more successful experience. These disease specific subcommittees are the engine room for considering new ideas and for building concepts for future clinical trials. It is essential for consumers to have input into the design of these trials from a patient point of view. This ensures it is more than likely there will be some benefit to the patient. Quality of life is always a very important consideration. It is essential for consumers to have input into the design of these trials from a patient point of view. This ensures it is more than likely there will be some benefit to the patient. Quality of life is always a very important consideration. The CAP is then given the opportunity to attend the next two days of the scientific program. While this is more technical in nature, the guest speakers, presenting a broad range of information, are always of high calibre. There are probably only ever one or two sessions that “fly” over our heads! Support for, and inclusion of, the CAP by ANZUP ensures that we are a committed and enthusiastic group, very interested in assisting with input into ANZUP clinical trials. As we head into December 2014, planning for the year ahead and next year’s ASM has already begun. Our aim for the coming year is to appoint a Deputy Chair of the CAP to assist the Chair and provide cover for meetings if I am not available. We also hope to have at least two CAP members for each of the disease specific sub-committees and nominations for these are normally sought through ANZUP clinicians. That is how I ended up being connected to ANZUP. We are now a more experienced CAP with most members having been on board for two or more years. More clinical trial research and more consumer involvement will be needed as ANZUP continues to grow. As a CAP member, it is exciting to be part of this successful and growing organisation. The ANZUP Consumer Advisory Panel at the 2014 ASM: L-R Back row: John Stubbs, Ray Allen, Joe Esposito, Leonie Young, Max Shub, Colin O'Brien, ANZUP EO Margaret McJannett, David Swallow, ANZUP Chair Ian Davis. Front Row: CAP Chair Belinda Jago and Tony Sonneveld OAM 8 A LITTLE BELOW THE BELT The journey A great story evokes emotion, persuades, even compels. More importantly, people who have felt something while being lost in a good story want to share it. (Adobe) We hope you are touched by the following stories. Open Communication the key for Ostomy Support Group Meet Peter Stanford, an Ostomy Support Group facilitator at the Cancer Support Centre, Jacaranda Lodge, Sydney Adventist Hospital. Peter joined the group in 2009 after he had bladder cancer surgery and had a stoma fitted. He had been a group member for 12 months when Mike the facilitator asked him to be his 2-i-c. Peter facilitates the Stoma peer support group run on the first Wednesday of each month (details below). The 15 members have a range of stoma (colostomy, ileostomy and urostomy) resulting from either bowel or bladder cancer. The support group session involves a mix of peer group chat and professional presentation. In the first hour, members talk about any issues from the previous month while the second hour is given over to a speaker. Speakers come from a wide range of expertise, such as stoma appliance specialists, stoma nurses and dieticians. The hour dedicated to sharing thoughts and emotions in an environment of trust is important and all members know that what is said in the room stays in the room. It is a chance to open up, share information and feelings. Confidentiality is key and people know they can share sometimes very personal issues and not be judged. Some of the issues that come up regularly include leaking appliances, wind, skin irritations and relationships. New members often hear about the support group from GPs, stoma nurses, or from reading material. They contact the Cancer Support Centre at Jacaranda Lodge and are then introduced into the group. Members are welcome to come alone or with a carer or family member. Some members stay part of the group for years while others may only attend a few sessions. Peter listed the key benefits of the support group as: Camaraderie: All members have stomas. Being part of the group makes them feel less isolated and helps improve coping skills and sense of adjustment. Openness: The peer group is a very open and supportive environment. Information Sharing: From ostomy appliance suppliers to dieticians and nurses – the ostomy area develops rapidly so it is important members hear the latest information. This gives them a sense of empowerment. Peter’s final word about the support group is ‘communication’. In an age of online technology, good, old-fashioned, face to face communication helps break down barriers, forge friendships and strengthen trust and support. Peter said “It is very satisfying to see a member come to the group unsettled and stressed and leave with a smile on their face”. Cancer Support Centre, Jacaranda Lodge Ostomy Support Group Meet: 1st Wed. 10.00am – 11.30am monthly Jacaranda Lodge, Sydney Adventist Hospital, 185 Fox Valley Road, Wahroonga. NSW Contact: Nerolie. Phone: 02 9487 9061 Peter is a member of the ANZUP CAP and sits on the bladder subcommittee. Relevant Links lAust Council of Stoma Associations http://www.australianstoma.com.au lCancer Council NSW http://www.cancercouncil.com.au/ lJacaranda Lodge http://www.sah.org.au/jacaranda-lodge l Stoma Frequently asked questions and answers http://www.australianstoma.com.au/index.php/facingostomy-surgery/2-uncategorised/25-frequently-askedquestions lOther sources of information www.cancerresearchuk.org A LITTLE BELOW THE BELT 9 The journey Chris Reason Just last year, I passed an important milestone. Ten years in remission. It was a day I honestly thought I’d never see, but every day I thank the heavens I did – because so much has come into my life in this disease-free decade: A wonderful wife, two extraordinary children, great friends, and some amazing life moments. And I like to think that all of those moments feel so much better – when you know how close you came to never having any of them. Cancer is evil on every level but it does have one benefit it teaches you the value of life. Not that I was feeling any of that wisdom when I was first diagnosed. I’ll never forget that moment. I was sitting in my urologist’s surgery, he was flicking through his desk diary, and asking about the best time for an operation. His head was down and I honestly thought he was talking into a hands-free phone. But then he looked up at me: “We should operate tomorrow morning – are you available?” he asked. Ten minutes later, I was outside in the corridor, alone and crying. I couldn’t believe that the breakneck-speed of my life had suddenly come to this crashing halt. Initially it was fairly simple and straightforward - I had one testicle removed and some follow-up radiotherapy. But the cancer returned – it spread up into my abdomen and required intensive chemo and finally a marathon operation (a RPLND - a Retroperitoneal Lymph Node Dissection). That procedure not only took 9 hours to perform and 57 metal stitches to repair - it also took the tumours. We’d beaten it! What a feeling. I say “we” of course because it was a team effort – my urologist Dr Peter Nash, the oncology nurses at North Shore Private Hospital, the surgery team at St George’s Hospital, my family and my friends. But one thing I noticed that was missing from the team was outside support. I was surprised that through all the months of the ordeal there was little I could find that offered cancer fighting advice, experience or wisdom. There was no ‘beenthere, done-that’ bloke that I could talk to. And as accommodating as the nurses were, I didn’t think it fair to ask them intimate questions about testicles, sperm counts, sexual function! At times it felt pretty lonely. There were no other blokes in my chemo ward with testicular cancer. And outside the hospital, the subject was one that people had difficulty talking about, understandably. It was even awkward with family. 10 A LITTLE BELOW THE BELT Chris Reason and Kathryn Robinson with twins Lucy and Sam. Photo: Paul Lovelace At times, the ordeal became a pretty isolating experience. I remember at the very low point of the battle, when the outlook wasn’t looking great, I was starting to spiral into depression. The nurses booked me into a psychiatrist. It was the first time in my life I’d had to see one. So when I emerged from my ordeal, I decided I wanted to try and help other blokes who might be about to go through it all. The NSW Cancer Council had launched a program called “Cancer Connect” – it was a phone counselling service for cancer patients. They needed men who had experienced testicular cancer – so I jumped in to help. After a very basic introduction course on counselling, I became part of the program and for the next five years I took random calls from blokes old and young battling testicular cancer in its various forms. I’m not sure how much value I was to them, but I can certainly say the experience was of enormous value to me. I’ve left the program now – but I’ll never forget the guys I met through the many, many phone calls. They are among the many ways I will always remember my own cancer survival - along with the 30cm scar on my abdomen, my chemo-damaged lungs, and the threetimes a year testosterone shot in my backside! That last one is literally a pain in the arse – but it’s better than the alternative! Chris Reason Senior Reporter and Presenter, News. Channel 7 Paul Lovelace, a Sydney-based professional photographer, shares his family’s traumatic cancer story in support of the need for ongoing clinical research into better treatment – and the ultimate goal of finding a cure. Kathleen Lovelace Photo: Paul Lovelace Kathleen Lovelace No one other than those who have experienced it can understand the emotional pain, bafflement, even resentment, of losing someone close to a relentless cancer. Doctors now moved on to a new mode of treatment. Paul said that at the time clinical trials on cancers were underway with the drug Interferon and it was recommended for Kathleen. The trials had shown potential and Kathleen decided to go ahead. Nurses taught her how to self-inject a dose of the drug which she did at home each day for six months. The impact can be even more poignant if there have been times of hope sparked by apparently successful treatment regimens and remissions. It is this period that leaves Paul with the most questions about his mother’s treatment. For Paul Lovelace, the agony of losing his mother Kathleen to cancer in England more than two decades ago is still raw, not only because of the way she was affected by the disease but also because he believes there are unanswered questions about her treatment. “There was no chemo or radiation therapy. I remember my father saying later that the doctors had made a mistake – that they should have followed the surgery with chemo,” he said. “When you reflect on the treatment process you have to ask why they didn’t follow up.” But rather than focus on the negative, he has turned that frustration into a trigger to strongly advocate for any clinical research that may lead to a breakthrough in the cancer battle. Despite great positivity from Kathleen and her family towards Interferon it became obvious that the drug was failing. Sadly, from that point on, deterioration in her condition was rapid. His is the type of story that helps inspire anyone who works with ANZUP to strive to develop new ways to tackle “below the belt” cancers. It is also a stark reminder, should any be needed, of the toll on those left behind and the importance of clarity of treatment message and emotional support. “After about two more months, she just got sicker,” Paul said. “She went into hospital for a week or two to manage the sickness symptoms and then a hospice nurse was allocated to us at home for a week where she died.” Kathleen was in her early 60s when a shock diagnosis confirmed a kidney tumour. The onset of the symptoms had been sudden. The first signs appeared one afternoon, just before lunch with friends: pain, nausea and a general feeling that something wasn’t quite right. Over lunch the symptoms disappeared only to reappear later. It was enough to prompt Kathleen to visit her GP who referred her on for x-rays and scans. The tests revealed the tumour, but there was some encouraging news: her doctors said they believed that its removal through removal of the kidney would eliminate the problem and allow Kathleen to go on to lead a healthy life. “You just feel so helpless. It hurts you so much.” Some years after the death of his mother, Paul was again touched by the cancer scourge. His father, who had been a smoker, was diagnosed with lung cancer and succumbed to the disease. Losing both parents to cancer has added to Paul’s strength of advocacy for any research that might lead to better treatment. “The fact that both parents died of it is always at the back of your mind,” he said. “I am all for any drug or clinical research that can help people or find a cure.” Despite the failure of the drug regimen to help his mother, Paul also believes that the potential of the Interferon trial did have a positive effect, albeit for a relatively short time. After the surgery, the period of respite was short. “She had the surgery, but three months later the tumour came back – in the area where the kidney had been removed,” Paul said. Thanks to Paul Lovelace for providing the picture he took of Chris Reason and family. http://www.paullovelacephotography.com.au A LITTLE BELOW THE BELT 11 The journey Below the Belt Pedalthon founder Simon Clarke tells of his cancer journey and just what set the wheels in motion on a hugely successful fund-raising venture. As a young adult cancer was always an unknown and ominous concept but one I felt I shouldn’t have to consider “until I was older”. Yet in 2012, at the age of 24 - and on the cusp of completing my university degree - I was diagnosed with testicular cancer. With what felt like fairly innocuous symptoms, the diagnosis was a huge shock - so much so that I didn’t know what else to do but pretend nothing had happened and to go back to work. I had never really heard of young men having to deal with anything like this. But why should I have? I’ve since come to realise that is half the problem. Despite its limited public profile, testicular cancer is the most common among young men yet so rarely spoken of. For men, any discussion of what is going on below the belt is often perceived as taboo or met with sniggers. As I began my adventure through the hospital system and waiting rooms I was amazed to find more and more stories with this mentality. Throughout treatment (and now a couple years into remission), my initial shock turned into an overwhelming desire to give back and to find a way to help others in a situation like mine. Most of all I was touched by the impact I had in telling my friends and family that it’s ok to talk about these things. It is not embarrassing, or a stigma, but something that I can actually be proud of - or perhaps worth a good laugh (if only someone had told me that the iPhone app Snapchat would be so popular before I made the username cyclops-sim). With this backdrop, I founded Pedalthon in 2013 - a vehicle to promote awareness and raise critical funds to enable ANZUP to improve the treatments and outcomes of below the belt cancers. Pedalthon’s flagship event, I founded Pedalthon in 2013 a vehicle to promote awareness and raise critical funds to enable ANZUP to improve the treatments and outcomes of below the belt cancers. Simon Clarke 12 A LITTLE BELOW THE BELT where teams competed for four hours to help defeat four cancers, was held in September 2014. Through months of hard work, and an incredible team, our inaugural event attracted about 250 riders across 35 leading corporate and community enterprises. Our combined efforts were able to raise funds net $222,000 and also received recognition from Prime Minister Tony Abbott. It is incredibly difficult to sum up the level of overwhelming support Pedalthon received perhaps something that, initially, we could only dream of. I have been incredibly touched by the way major corporate firms responded to the call to arms. For mine, this target market holds a critical key to unlocking public perception of below the belt cancers and the camaraderie, esprit de corps and sincerity to the cause that each and every one showed was truly special. While I’ll never forget the streams of beaming smiles, perhaps the most moving success out of this year’s Pedalthon was in two separate messages I received from people I had never met before - one from the USA and the other closer to home. Both had stumbled across our message and reached out to say they had been inspired to seek medical advice for themselves. Fundamentally this was the achievement of one of our main goals: to change how we view, act and respond to these cancers. It is for reasons such as these that I believe Pedalthon was able to carve a niche in what is a quite saturated market for charitable effort, and to touch everyone it came across in a way that can only continue to make a difference. Our aim is to grow the event in the coming years – both in terms of participants and funds and, in doing so, to continue to broaden the cycle of awareness. I’m incredibly fortunate to have come across ANZUP and its fantastic staff. I am constantly in awe of the work that ANZUP’s member base is able to achieve and am excited at the prospect of what is possible. I would like to take this opportunity to thank the community for getting behind this event and thank the staff of ANZUP for their support, patience and most of all for their efforts in helping the lives of so many. If you have any comments, questions or would like to participate in next year’s event I would love to hear from you at [email protected] Far left: Simon Clarke with his mother Sally Jones on race day. Photos: Getty he was in the final period of exams for his commerce and science degree at Sydney University and was also working as an intern. The ache was constant. “There was nothing to see and initially the doctor told me not to worry about it. But it still didn’t go away and so two weeks later I went back and said ‘I just don’t feel right’.” The pain was coming from his lower abdomen, towards his right groin, and the doctor ran tests which showed nothing. But Clarke persisted and his doctor upscaled the tests. Within another two weeks he had a CT scan and an MRI. My life had changed in 20 minutes When testicular cancer turns a young man’s head Jill Margo Health editor, The Australian Financial Review As soon as he left the doctor’s consulting rooms, Simon Clarke got into his car and opened Google. The shock hadn’t quite hit him as he scrolled down and began looking for information about testicular cancer. At 23, he was entirely unprepared for the diagnosis he had just received. Not only had he never met anyone with this type of cancer, but he had only vaguely heard of it and had e rroneously assumed it was something that affected older men. Now, sitting outside the family doctor’s rooms on Sydney’s north shore, he couldn’t absorb what he was reading and he noticed his lunch break was almost over. “I didn’t know what else to do so I drove back to work,“ says Simon, then an intern at corporate advisory firm Gresham Partners. By evening, the news had sunk in and he told his p arents. Then he told others. A few weeks earlier, in early November 2012, he’d felt a dull ache in his stomach. It was a stressful time because Then he got the phone call and slipped out to the appointment during lunch. “I remember he looked down and told me I have testicular cancer. I didn’t even know [that possibility] was on the table and there we were, discussing an operation. “In the space of 20 minutes my life had changed.” And the change would be significant. It would shift Clarke’s focus off himself. Rather than taking privilege for granted, he would realise what he had and what he could potentially do for others. Born to Australian parents in New York, he had spent part of his childhood there before the family returned to Sydney and he went to Knox Grammar, where he excelled academically and at sport. Apart from several sport-related broken bones, he had been in excellent health. The world lay open before him. He’d travelled widely and there had never been cause for him to pause to consider where he was going. The diagnosis, however, stopped him in his tracks. “Despite solid family support, it was a solitary time and This story is reproduced with permission. Published 25 June 2014 A LITTLE BELOW THE BELT 13 Now an associate at Gresham, Simon has two personal goals. He wants younger men to know about male cancers so they don’t stumble into it the way he did. And he wants to raise funds for research to understand more about these “below-the-belt” male cancers. The Below the Belt Pedalthon Such research is conducted by ANZUP, Australian and New Zealand Urogenital and Prostate Cancer Trials Group. It is the kind of research that can’t be done by drug companies. Using his own funds, and with the support of ANZUP and the help of friends, Simon has organised a cycling challenge at The Sydney Motor Sport Park (Eastern Creek) this September. Called the Below the Belt Pedalthon, it challenges teams to complete the most laps possible within four hours on the closed track. Simon Clarke with NSWIS Development Cyclist Nathan Bradshaw. Photo: Getty Throughout the day there are also a series of mini challenges. In true race style, garages in the main pit lane will accommodate teams of up to eight cyclists each. it gave me an opportunity for introspection I might not otherwise have had.” The teams – of men and women and of both avid and recreational cyclists – will be drawn from the business community. He spent Christmas in hospital having surgery to remove his right testicle and during his recuperation he began reordering his priorities. Testicular cancer is a younger man's disease He’d noticed the impact his diagnosis had on friends. While they joked about it and suggested he get an upsized prosthesis and a specially tailored suit to show it off, they were deeply shocked. They too didn’t know that testicular cancer is a younger man’s disease and that in Australia, the average age of diagnosis is the mid-30s. It was so confronting, Simon wished he’d been forewarned. To safeguard his fertility he had banked sperm before the operation and had then declined a prosthesis, preferring to live with himself the way he now was. But what to do next? The lack of consensus among his specialists was worrying. “I was constantly trying to learn more about my options, but what scared me was that in the space of a couple of weeks, I saw multiple specialists and they all advised me to do slightly different things. One said I should do chemo, one said I should have radiation and another said I should do nothing, just be in remission and have regular tests – if it spread I could take further action then.” Simon took comfort from the fact that testicular cancer is highly treatable, with relative survival higher than 97 per cent. While he prefers not to use the word “cure”, he opted to watch and wait. “It was a tough decision and now every quarter I have scans and a series of tests to make sure there are no signs of cancer.” 14 A LITTLE BELOW THE BELT For his target market Simon is following a model set by the JPMorgan Corporate Challenge, which began as a modest fun run and is now a major international event. Simon – who is not to be confused with the professional Australian cyclist of the same name – devised, wrote and published a sponsorship prospectus and has a target profit of $100,000 in the first year. All funds raised will go directly to ANZUP, apart from a minor fee to the fund-raising platform. While the group’s infrastructure is funded by Cancer Australia, each clinical trial needs its own funding stream, so applying for grants, which it does, can take several years. A pilot study to test the feasibility of a promising drug, surgery or other supportive care might be able to be done for smaller amounts of money depending on the trial, around $50,000-$250,000. A meaningful clinical trial usually requires more than $1 million to begin. What to look for Common symptoms of testicular cancer l l l l l Painless lump or swelling in either testicle Change in how the testicle feels Ache in the lower abdomen or groin area Sudden build-up of fluid in the scrotum Pain or discomfort in a testicle or in the scrotum Several conditions may cause these symptoms, but if you experience one of them see a doctor. Source: Cancer Australia The journey Searching for Credible Health Information Social media expert Jenni Beattie provides an insight into how to find credible health information online. The power of the internet means that today, more than ever, there is a wealth of information available to help inform our health decisions. While this offers big advantages, it can also leave us feeling overwhelmed. In this article, we hope to provide some tips to help you navigate your way around this increasingly complex information environment. ‘Social media’, ‘online media’, ‘websites’ .… the range of online health information is dizzying! If you have ever searched for health information online you will appreciate that, at times, making the best choices can be both time consuming and stressful. Jenni is the Internal Communications and Projects Manager at ANZUP Over the past five years, there has been a dramatic shift in patients playing a more active role in decision making and searching for more health information. Patients and carers are also sharing their own experiences via online blogs, Twitter or Facebook, and online patient communities. People are now equipped, engaged and empowered, but education is key to ensuring they get the most reputable information. So let’s start! A LITTLE BELOW THE BELT 15 BELT 15 The journey Top 10 Tips for Finding Credible Information 1. The URL (web address) is a good signpost to a site’s credibility. Sites that have an address containing these elements will be reputable: .gov Government sites .edu University/medical school/educational institutions .org Not-for-profit groups with a focus on research 2. Identify, follow and source respected institutions in the relevant field (we have some cancer sites listed for you at the end of this article). 3. Seek out and check references and citations (basis of the research and sources). Make sure material is ‘evidence-based’. 4. If possible, look for any ‘declarations of interest’ in relation to the source of any financial assistance towards the production of the information. 5. Always remember to discuss the information you have found with your GP and medical specialist. Websites and Hashtags of Note l l l l l l l l l http://www.anzup.org.au http://www.cancer.org.au http://www.prostate.org.au/ http://canceraustralia.gov.au http://www.cancercouncil.com.au http://www.cancervic.org.au/ http://www.kidney.org.au/ http://www.cancerinstitute.org.au/ http://www.mskcc.org If you are an active Twitter user, there are also Twitter chats that you can follow. Please view this website to see what is available http://www.symplur.com/healthcare-hashtags/ tweet-chats/ The following hashtags can also be followed on Twitter and are a useful way to aggregate particular interest areas: #blcsm 6. Beware of websites selling cures or health Bladder cancer remedies. 7. Is there a privacy policy on the website? Ensure there is a privacy policy in place if a site requires you to register otherwise provide personal information such as your name or e-mail address, Also ensure your personal information and anonymity are protected and are not being provided or sold to other companies. #tscsm Testicular cancer 8. Check the ‘currency’ of the material: what is the date of publication; is it the most recent version? #PCSM Prostate cancer 9. Never divulge personal information online such as phone numbers, date of birth and addresses. 10. Be careful when and where you comment on information. Google archives everything! Be sure you are happy with what is said before you “publish”. Always re-read what you write and ask yourself if you will be happy with your comments in the future. 16 A LITTLE BELOW THE BELT #kcsm Kidney cancer ANZUP clinical trials The technical explanation of a clinical trial is: to work out the effect of some form of intervention on a group of people with a defined condition. That is, each study is designed to answer scientific questions and find new or better ways to help patients with testicular, kidney, bladder or prostate cancer. The following examples provide an overview of a clinical trial. Perhaps you have a new drug that you think is going to work in cancer. You want to know how safe it is, how often it works and how often it shrinks the cancer. Does it make people live longer? Is it safe? Does it make them feel better? And how does it stack up against the treatments that are already used? ANZUP has a strong record of successful clinical trials. Many of the trials we do don’t involve big blockbuster drugs. As an example, in testicular cancer, we did a clinical trial that showed giving the same drugs one way was better than giving them another way. It doesn’t sound very interesting but think about it like this; - this is a common cancer, these drugs don’t bring in large amounts of revenue for the drug company, so they won’t want to investigate the question. ANZUP did a clinical trial comparing the two types of treatments and we proved that one way was better than the other. This is now the standard way this treatment is given around the world If you are a patient involved in a clinical trial, you are going to receive new treatments not yet available elsewhere. All patients who sign up to a clinical trial are carefully monitored throughout the trial and followed up after the trial. You would more than likely be in a trial that is being carried out in major cancer centres throughout Australia and New Zealand. Throughout your trial, the ANZUP members (doctors, nurses, researchers, radiologists and others specialising in urogenital and prostate cancers) will pull their knowledge and expertise together to design and monitor the trial. How can I find out more about a trial? Before you and your doctor make a decision about your treatment (whether it is in a clinical trial or not), your type of cancer will be diagnosed and ‘staged.’ Staging tells how far the disease has spread. Deciding on treatment depends on many things, including the stage of the disease and your general health. You would most likely be referred to a trial by your own doctor or by a doctor who knows your case. Some patients find out about trials from other sources. In any case, you must have a reasonable understanding of your role in a research study and be freely willing to take part in it. Every clinical trial is designed to answer a set of research questions. There are guidelines for each trial that determine whether it is safe and suitable for you. Each study enrols patients with certain types and stages of cancer, and a certain health status. A study that involves two or more treatments can only yield reliable answers if all the patient cases are the same, so they can be compared with each other. When you see your doctor ask them “Is there a clinical trial that might be suitable for me?” There may not be one available, but if there is, it might be useful for your treatment. Your doctor will then go through all the information related to that clinical trial; what it involves in terms of your time, the tests that are done, how many visits to hospital, the possible side effects of treatment and any other relevant information you may require. If you agree, you will be asked to sign a consent form. The consent form means you have given permission for a drug to be used in a way that it is not ordinarily used, or to use an experimental drug. You would then take part in the clinical trial. If, at any time, you want to come off the trial, you can. You are not locked in. There are many types of clinical trials. Some involve you having to go to hospital and others can be taken at home and you may have to visit the hospital just to make sure everything is going well, and there have been no side effects. Other clinical trials don’t involve a treatment. Some of the trials we are doing involve supportive care questions. In other words, how well are people managing with their diagnosis of cancer and how can we support people better through the process of treatment and after treatment? In this case, there may be very few visits to the hospital. Some of these trials are done online or over the phone. A LITTLE BELOW THE BELT 17 Everyday Heroes Community Fundraising for ANZUP Fundraisers take many shapes and forms and they do it for so many reasons; loved ones, friends, personal goals. Two of our most recent Everyday Heroes raising more than $10,000 between them are featured below. Belinda Jago (ANZUP CAP Chair) started Bec’s Troops in memory of her daughter who died from kidney cancer at the age of 19. Belinda said recently, "Fundraising for ANZUP is all about clinical trial research to improve cancer patient outcomes in bladder, testicular, kidney and prostate cancer. Bec's Troops supports and advocates for this cancer research in memory of Bec who fought her kidney cancer for 5 years and was involved in a clinical trial during that time” Belinda together with Bec’s family and friends, more than 80 runners, took part in The Sunday Age City2Sea to raise over $9,000 for ANZUP in 2014 ($12,000 in 2013). This type of community fundraising raises critical funds but more importantly, provides a positive way to raise awareness about clinical trial research. Charise Alcock participated in perhaps the most picturesque of charity runs, the Knight Frank Pinnacle to Point in Tasmania. The Knight Frank Point to Pinnacle is a 21.4km run or walk from Hobart's iconic Wrest Point Casino to the Pinnacle of the city's majestic Mount Wellington. The course includes 1,270m of climbing and provides an achievable challenge for all levels of athlete from elite runners to recreational joggers and walkers. Charise’s husband Nick recently participated in a clinical trial in Tasmania, and this is what motivated her to raise close to $1,000 for ANZUP. Thank you Love a good fun run, a serious run, swim, walk or ride? Did you know ANZUP is now a registered charity with the Sydney Half Marathon? If you prefer a run to a ride or love them both, did you know you can select ANZUP Cancer Trials Group as the recipient of your fundraising efforts in the Sydney Half Marathon. We would love to see as many ANZUP members, donors; friends and family challenge themselves and their friends to raise funds during the Sydney Half Marathon on Sunday 17 May around beautiful Sydney Harbour. Register here http://www.smhhalfmarathon.com.au/ and select ANZUP as your chosen charity. Thank you and have a great time. Don’t forget to tweet @ANZUPtrials or send us your photos! 18 A LITTLE BELOW THE BELT Dr Ben Tran explains why initial concept development is so important to developing new treatments in testicular, bladder, kidney and prostate cancer. ANZUP Q&A with Dr Ben Tran, Medical Oncologist, Royal Melbourne Hospital ANZUP: Ben, we recently held a very successful fundraising event, the Below the Belt Pedalthon. From this, our riders, supporters and donors raised $222,000. These funds are going to ANZUP to create new concepts. What does a concept development mean? Ben Tran: Concept development involves taking an idea and brainstorming in order to develop it into something strong enough for a grant application. At the present time we don’t have enough money to independently fund our concepts or trials and rely on grants. There is a process to refine the idea/concept, detail the objectives and study population. Essentially, concept development involves considering if an idea is good enough to take forward, and - if it is provide all the input necessary to take it forward. Q: How might an ANZUP member come to think of a new concept? A: ANZUP members are intelligent and inquisitive clinicians and health practitioners who encounter problems in their clinical practice every day. A new concept might simply arise from a clinician identifying a problem in his/her practice that could be solved by a clinical trial. Q: What does it look like when ANZUP members bring a concept to the table? A: A short document, usually a one-pager, is generated by ANZUP members. This would detail a title, objective of the study and rationale behind the idea and possible treatment outcomes. Q: Who can bring an idea to the table? A: Anyone with a concept can bring an idea to the table, and this is one of the greatest values of being an ANZUP member. Q: Who says yes or no to the concept? A: Concepts are generally discussed among a group of experts, led by the chair of the particular subcommittee. After brainstorming the concept, it is up to the committee to decide whether the concept has enough merit to go ahead. By merit, I mean scientifically interesting enough, potentially practice changing and, most importantly, feasible. A LITTLE BELOW THE BELT 19 Q: How does it progress from an idea to a pilot then to a trial? 6. A: 1. Presentation: A concept is presented by an ANZUP member to the relevant ANZUP subcommittee and other ANZUP members 7.Ethics: Ethics approves the protocol and trial 2. Discussion: Group discusses the concept and makes suggestions 3. Endorsement: Group discusses the concept and makes suggestions 4. Letter of intent: A more detailed concept is written as a letter of intent (this includes input from our Consumer Advisory Panel) 5. Trial: A trial management committee is formed and a coordinating centre is appointed (either Clinical Trials Centre [CTC] or a Commercial Research Organisation Research plan: A what, why and how research plan is submitted 8. Protocol: The protocol is written 9. Ethics approval: Ethics approves the protocol and trial 1O. Governance: Governance is established 11. Trial opens: The trial is opened to patients for recruitment 1 PRESENTATION 2 Input from our Consumer Advisory Panel 4 LETTER OF INTENT 7 ETHICS 20 A LITTLE BELOW THE BELT 3 ENDORSEMENT DISCUSSION 8 PROTOCOL 5 TRIAL 9 ETHICS APPROVAL 6 RESEARCH PLAN 10 GOVERNANCE 11 TRIAL OPENS Funding wasn’t available for the concept development before the Pedalthon. These meetings largely happened via teleconference and each participant volunteers their time to be involved. Q: What sources of funding would you look at in each stage? A: Funding wasn’t available for the concept development before the Pedalthon. These meetings largely happened via teleconference and each participant volunteers their time to be involved. Funding is required for the trial to go ahead, to pay for drug trial management, per patient payments etc… Generally, grants are written at the time of concept is fully developed as a Letter of Intent (LOI). focused on developing new and expensive drugs. It has been the role of co-operative groups such as ANZUP to conduct clinical trials to answer clinically relevant questions that don’t involve new drugs. Government money is now scarce and conducting these studies is becoming more difficult. Independent funding is crucial to ensure we continue to ask clinical questions that will help us better care for our patients. Q: What happens in a face to face meeting? A: Concepts are discussed. The person bringing the concept forward will give a short presentation on the concept, including some background and rationale. Committee members will ask questions and diagrams/charts are drawn as the concept is brainstormed. It is also easier to understand each person’s perspective at a face to face meeting. Q: What do you see as three possible treatment changes that ANZUP could bring to fruition in the next three years? A: 1. Improve the treatment and prevention of side effects in chemotherapy treated testicular cancer. 2. Answer the question once and for all: what is the best chemo regimen for muscle invasive bladder cancer? 3. Determine if enzalutamide results in better outcomes when given to castrate-sensitive patients. Q: Where might ANZUP members be coming from for a face to face? For example, all over Australia, or a particular centre, or anywhere around the world? A: Most ANZUP members are located within Australia and come from both capital cities and regional centres. Some ANZUP members also come from New Zealand and other places around the world such as the United States. Q: Why does independent funding make a difference to the progression of a concept from concept to pilot? A: Independent funding is crucial. In the current financial climate, the pharmaceutical industry is Ben is based in Melbourne at The Royal Melbourne Hospital. He is a specialist in medical oncology, the DW Keir Research Fellow and the ANZUP/Tolmar Research Fellow He is also a clinician & translational researcher in l Urological cancers l Drug Development l Cancer Genomics/Molecular Profiling A LITTLE BELOW THE BELT 21 Associate Professor Guy Toner, ANZUP Deputy Chair provides an overview of the recent Concept Development Workshops. The first Concept Development Workshops were held following the Pedalthon fundraiser. re-defining the concept with the help of newly identified collaborators. A new initiative for ANZUP in 2014 was the successful introduction of two Concept Development Workshops (CDW), recently held in Sydney. CDWs have been used successfully by the ANZ Germ Cell (testicular and ovarian) Trials Group (one of ANZUP’s forerunners) and other cooperative trials groups to initiate and develop trial concepts that subsequently resulted in successful and important clinical trials. ANZUP has wanted to introduce CDWs but has lacked the financial resources to do so until this year. 3) An opportunity for the assembled group to brainstorm – considering gaps in current knowledge and new opportunities for studies. There were three major components to these CDWs: 1) All members were invited to submit a brief concept to be considered for discussion at the CDW. The aim is to encourage members to consider new proposals and submit an idea. This can be helpful for concepts that require broad feedback or further refinement. Younger members (trainees for example) can also find this process rewarding. 2) Presentation of submitted concepts to a multidisciplinary group at the CDW including senior investigators, statisticians and before Clinical Trials Centre (CTC) staff. The resulting discussion is often wide-ranging but aims to define clear “next steps”, which might include forming a working party to create a protocol or At the start of the day we had a couple of ideas. By the end of the day we had 4-5 working groups working up concepts to take through the next steps then through to funding Professor Ian Davis 22 A LITTLE BELOW THE BELT The CDWs are not intended to replace existing opportunities to submit a concept to any of the sub-committees nor the brain-storming sessions held at the Annual Scientific Meeting (ASM). Rather, we hope that the CDWs will offer an additional opportunity for concept development and encourage participation from the breadth of our membership and also foster multidisciplinary collaboration. The first workshop was held on 5 November, with a prostate cancer session in the morning and the afternoon devoted to testicular cancer. The second workshop was held on 21 November and included sessions on bladder cancer and kidney cancer. ANZUP plans to hold future CDWs, hopefully as an annual event. There are opportunities to expand the workshops, for example by allowing a full day rather than half-day for each type of cancer. Options to improve the workshop format will be based on feedback from the membership after this year’s inaugural events. ANZUP wishes to acknowledge and thank the very generous contribution of Mrs Ann Waterford. Mrs Waterford has made an annual donation to ANZUP in memory of her husband, Dr Waterford. This donation has supported the inaugural bladder cancer CDW as well as bringing ANZUP members to a bladder cancer meeting immediately prior to this year’s Annual Scientific Meeting in Melbourne. It is only though the generosity of donors like Mrs Waterford that ANZUP can support our members coming together to work on concepts and ideas that will see us improve treatments and outcomes for those affected by bladder and other urogenital cancers. Photo: Getty The Inaugural RIDING FOR UROGENIT AL CANCERS In 2014, ANZUP bit the major event bullet and we are so glad we did! The Below the Belt Pedalthon, a four-hour ride to defeat our four cancers, was held on Tuesday, 16 September at Sydney Motor Sport Park, Eastern Creek. It was a great example of sheer determination and absolute conviction overriding inexperience and fear – not unlike the journey Pedalthon Founder Simon Clarke went through when first diagnosed with testicular cancer. We were both excited and terrified when Simon first approached ANZUP with his Pedalthon idea. We knew this could be one way to speak directly to our key age groups affected by testicular, prostate, kidney and bladder cancer. We wanted them to engage with Simon’s story and hoped they would support the ride and spread the word. In the first few months, we hoped for a couple of sponsors and at best 20 teams providing 100 riders to raise an ambitious target of $50,000. What we didn’t anticipate was the power of Simon’s story, the deep desire to help that it inspired and quite frankly - the level of commitment of the middle aged men and women in Lycra! So how did we go? 35 teams registered to ride, including three community teams. 248 riders took to the track More than $152,000 was raised by individual fundraisers $100,000 197 completed the entire three hours, plus the challenge races through sponsorship, matched giving and donations. This money will go directly towards ANZUP research. A LITTLE BELOW THE BELT 23 Simon was featured in the Australian Financial Review, regional newspapers and on Studio 10 on Network 10. He was interviewed on radio and our coverage on social media rose rapidly. Many of you reading this magazine will have felt the full pain of those three hours on the track. We want to thank you once again for your support and encourage you to keep talking about clinical trials with your family and friends. We met so many riders who shared their stories about family members affected by these cancers. They shared stories of heartbreak and inspiration, and their overwhelming desire to raise funds for research that will change treatments and outcomes. Simon and his family (mother Sally, sister Rose, and brothers Will and Cam, along with his dad Andrew, and his partner Louise) were the powerhouses behind the Pedalthon. They called out to friends and businesses, they donated and shared stories, and they were there on the day to help Simon enjoy the magnificent event he had created. Thanks to the Simon and the support of the teams and corporations who gave so generously we have been able bring the new ideas to the table and start the process of developing new treatment concepts. We will continue with Pedalthon in 2015. We will continue to tell our story, and raise awareness, and hope that by doing so we will help more people understand the importance of Australian clinical trial research. The success of an event such as Pedalthon belongs to many. In particular, we wish to thank the many volunteers who worked tirelessly for days and weeks to help us. To everyone who gave up a day of work using annual leave, or those who gave their free time to volunteer on the day, we thank you! From the fabulous parking marshalls, to the amazing supporters on the registration desk and the general helpers and those who had the venue sparkling clean before we left – thank you most sincerely. Congratulations to the winning team, Custom Creative, who rode the most number of laps on the day. To David Cowling (Clayton Utz) and Jaidan Stevens, who were the highest fundraisers. While Jaidan rode for the three hours, he also raised more money for Pedalthon by having his 10year dreadlocks cut off. To Clayton Utz who had the highest team fundraising effort and to all those who worked the fundraising pages to achieve the magnificent total. 24 A LITTLE BELOW THE BELT Above: Highest fundraising team Clayton Utz. Rolf Behrens, David Cowling, Sonia Goumenis, Joshua Knuckey, Richard Mills, Peter Staciwa, Matthew Wilson. Below: Simon Clarke, Jaidan Stevens and Jenny Sulicich as the first dread is cut off RIDING FOR UROGENIT AL CANCERS The Results Fundraising Clayton Utz blew the fundraising off the track. Congratulations to David Cowling who raised $6,832 and the Clayton Utz team who raised a phenomenal $19,730. Online individual fundraising stands at $153,168 (and growing). With donations, matched funding and sponsorship Pedalthon will have raised net $222,000. Overall team results No. of laps Team Name Team Time Trials 219 210 205 201 179 175 175 165 159 157 153 153 150 147 146 143 141 139 133 13 128 127 121 111 108 101 95 91 89 87 79 78 36 35 1 2 3 4 SUBC Custom Creative.com.au The Origin Power Racers Norton Rose Fulbright Racers Johnson Winter & Slattery Fire & Rescue New South Wales Herbert Smith Freehills Adrian Amer International Towers Sydney Allens Pfizer Pedal Pushers NSW POLICE Baker & McKenzie Gilbert and Tobin Lawyers Cyclops #nochance Origin Spokespeople Tour De Rews Clayton Utz HasBeans The Origin Power House Sparke Helmore Lawyers Herbert Smith Freehills Mark Currell Kemp Strang Skid Marks Pedal-Files Beer Coasters Custom Creative Norton Rose Fulbright Racers SUBC Johnson Winter & Slattery Origin The Origin Power Racers RBS Baker & McKenzie Herbert Smith Freehills Mark Currell Allens Fire and Rescue New South Wales Origin The Origin Power House Sydney Markets Foundation Clayton Utz International Towers Sydney Designcycle Pfizer Pedal Pushers Gilbert and Tobin Lawyers Cyclops #nochance Sparke Helmore Lawyers Herbert Smith Freehills 2 Bank of America Merrill Lynch Origin Spokespeople Kemp Strang Team Gresham HasBeans Beer Coasters NSW POLICE HPC Global Congratulations to K&L Gates our inaugural Champions and winners of the ANZUP BTB Pedalthon Pedal-Files Perpetual Trophy, Specshots & Termimesh Custom Creative. Tour De Rews Skid Marks 00:04:56.000 00:05:11.000 00:05:21.000 00:05:23.000 5 00:05:25.000 6 00:05:33.000 7 00:05:34.000 8 00:05:45.000 9 00:05:47.000 10 00:05:59.000 11 00:06:04.000 12 00:06:22.000 13 00:06:34.000 14 00:06:49.000 15 00:07:07.000 16 00:07:15.000 17 00:07:23.000 18 00:07:23.000 19 00:07:24.000 20 00:07:34.000 21 00:07:48.000 22 00:08:12.000 23 00:08:23.000 24 00:08:30.000 25 00:08:31.000 A LITTLE BELOW THE BELT 25 Thank you to our sponsors Gold sponsors Silver sponsor As an ANZUP project manager and regular commuter cyclist I was very excited to ride in the inaugural Below the Belt Pedalthon. In the weeks and months leading up to the event I registered our team, talked 4 work colleagues into riding with me and started fundraising. However, as the big day drew closer I started to feel less sure of my riding ability. Would the rest of the teams be made up of ‘proper’ cyclists with clip in shoes and scarily-defined calf muscles? The atmosphere at the event briefing was electric and we couldn’t wait to get out on the track. On the start line, the sound of hundreds of shoes being clicked into cleats filled me with excitement and adrenalin. We were off! After one slow lap (it wasn’t really that slow!) it was time to put the pedal to the metal and race. By then the main peloton was far in front of me but in the middle and back of the pack the atmosphere was fun and friendly. We chatted to the other teams and complained together that the ‘flat’ course was not nearly as flat as we had been expecting. The hours flew by and our laps mounted quickly. It was only in the last 30 minutes of the 3 hour event that I started to feel really tired but by then I was determined to finish the 16 laps (64km) I’d been aiming for. I finished my 16th lap with just a minute or two to spare, coincidentally crossing the line with the main peloton. Cruising into the pit lane I felt sweaty and sore but also elated. The event had been an amazing opportunity to challenge myself and to raise much-needed funds for a cause close to my heart. Now I am ‘in training’ for next year’s event. I can’t wait and I am already window-shopping for some drop bars and clip in shoes of my own! Karen Bracken, Trial Project Manager, Clinical Trial Centre Kit sponsor Colla sponteral sor The Herbert Smith Freehills team enjoyed an awesome training ride through the eastern suburbs beaches with Brad McGee in monsoon conditions on 29 August. Brad’s climbing class was clear for all to see through the famous cols of Vaucluse (Col de Water Tower) and Dover Heights (Col de Military Rd). Flooding on the Coogee Malabar Road (and general softness in the HSF team) saw the ride shortened in exchange for an extended breakfast. Over bacon and eggs rolls and confit duck and hash browns (it was in the eastern suburbs after all), Brad held the HSF team transfixed with tales from his time as a pro in Europe. It was a fantastic morning enjoyed by all. Brad gave generously of his time and was a gentleman - a true credit to Australian cycling. Thank you BTB for organising - the experience was worth the sponsorship money alone! Mark Currell, Freehills from Ball-e-tin We also received unbelievable support from the NSWIS Road Cycling Team – Brad McGee OAM and Ben Kersten. They rode with our sponsors and all cyclists on the day. Photo: Getty 26 A LITTLE BELOW THE BELT Photo: Getty Far left: Brad McGee OAM, Margaret McJannett ANZUP EO, Simon Clarke Left: Ben Kersten and Simon Clarke RIDING FOR UROGENIT AL CANCERS Ben rode two super laps with very generous donations from Sydney Markets Foundation ($4,000) and Kemp Strang Lawyers ($500). We are also truly grateful to Origin Energy who quietly provided donations, teams and support staff on the day. We look forward to welcoming everyone to the Below the Belt Pedalthon 2015 on Tuesday 1 September. We have tweaked the format a little to offer plenty more opportunities for our keen cyclists to race with patrons Brad and Ben. We hope you enjoy a few photos from race day. A LITTLE BELOW THE BELT 27 28 A LITTLE BELOW THE BELT A LITTLE BELOW THE BELT 29 Simon Clarke with his specialist Dr Peter Grimison 30 A LITTLE BELOW THE BELT Ms Fiona Scott MP representing the PM See you all at BTB Pedalthon Tuesday 1 September 2015 A LITTLE BELOW THE BELT 31 WHAT DOES A DONATION LOOK LIKE? We are so grateful to those who have already put their hands up for ANZUP. We would also like to thank the many people who have made personal donations directly to ANZUP. Your contributions are making a difference. $50k - $250k Kick off a pilot study Invest in a pilot study to test the feasibility of promising drug therapies, surgical methods, postoperative care and palliative care options. $10k - $500k Give a grant or fund a scholarship Inspire our culture of research by providing a grant or scholarship to clinicians involved in the care of patients with urogenital and prostate cancer. $1m - $5m Support a clinical trial Invest in a clinical trial to test the effectiveness, side effects and best dose of potential treatments for urogenital cancers. WOULD YOU LIKE TO HELP US? Any donation to ANZUP over $2 is fully tax deductible. If you would like to donate to ANZUP, you can donate through our website www.anzup.org.au or by calling ANZUP on +61 2 9562 5033. If you are interested in holding an event to support ANZUP or are considering joining an event such as the City 2 Surf, City 2 Sea, Sydney Marathon, Walk to Work Day or any other community event, please let us know and we will help you find the fundraising pages on GoFundraise and Everyday Hero. 100% of every donation made to ANZUP goes towards producing a clinical trial to improve the treatment of bladder, kidney, testicular and prostate cancers. DONATE NOW 32 A LITTLE BELOW THE BELT Be kind in kind Why in kind makes a difference? Investment and support can come in all shapes and sizes. In kind donations include providing the budget for a specific staff member, meeting room use, auctionable goods for fundraising, advertising support and creative support, and can help us deliver more interesting & educational information. Current ANZUP trials If you would like to know more about any of these trials please discuss it with your GP or specialist Ask questions Questions you may consider if you participate in a clinical trial If you are thinking about taking part in a clinical trial, here are some important questions to ask: l What is the purpose of the study? l What does the study involve? What kinds of tests and treatments? (Find out what is done and how it is done) l What is likely to happen to me with or without this new research treatment? What could the cancer do and what could this treatment do? l What are my options and what are their advantages and disadvantages? l Are there standard treatments for my case and how does the study compare with them? l How could the study affect my daily life? l What side effects could I expect from the study? (There can also be side effects from standard treatments and from the disease itself) l How long will the study last? Will it require an extra time commitment on my part? l Will I have to be hospitalised? If so, how often and for how long? l Will I have any costs? Will any of the treatment be free? l If I am harmed as a result of the research, what treatment would I receive? l What type of long-term follow-up care is part of the study? A LITTLE BELOW THE BELT 33 Testicular Cancer/Germ Cell* Tumours PHASE III Accelerated BEP Trial The current standard practice for the treatment of germ cell tumours is the use of the chemotherapy combination called BEP, which consists of three chemotherapy agents – Blemycin, Etoposide and Cisplatin – administered on a 3-weekly cycle. BEP is given with a drug called pegylated G-CSF (or pegfligrastim) that stimulates white blood cell production. The purpose of this study is to determine whether giving the same dose of BEP on a 2 weekly schedule will be more effective than a 3 weekly schedule and will be well tolerated. The 2 weekly schedule is called “accelerated BEP” and the 3 weekly schedule is called “standard BEP”. Up to 500 patients will be enrolled in the study in Australia, New Zealand and other countries. To date 23 sites have been activated with another 6 ANZ sites in the process of being activated. This study is currently active and recruiting. If this is something you or someone you know is interested in please speak with your doctor. For more information please go to the trials page on the ANZUP website http://www.anzup.org.au/content. aspx?page=trials-p3bep All of the research, all of the hard work, all the money that has been spent up until that point means nothing unless we can close that loop. Unless we can say we know this now, what does that actually mean for improving treatment for our patients because it is only by closing that loop and taking that final step that we actually work out, “Are we doing things better? Can we improve on what we are doing? Professor Ian Davis, ANZUP Chair 34 A LITTLE BELOW THE BELT ANZUP collaborates with the University of Sydney through the National Health and Medical Research Council Clinical Trials Centre (NHMRC CTC). Current site locations for the BCG+MMC ANZUP Clinical Trial ACT • Canberra Hospital NSW • Calvary Mater Newcastle • Concord Repatriation General Hospital • Macquarie Cancer Clinical Trials • Nepean Hospital • Prince of Wales Hospital • Royal North Shore Hospital • Sydney Adventist Hospital • The Tweed Hospital • Westmead Hospital QLD • Icon Cancer Centre (HOCA Wesley) • Princess Alexandra Hospital • Royal Brisbane & Women’s Hospital SA • Royal Adelaide Hospital TAS • Royal Hobart Hospital VIC • Austin Health • Box Hill (Eastern Health) • Peter MacCallum Cancer Centre • Royal Melbourne Hospital *Germ Cells are the cells in the body that develop into sperm or eggs WA • Royal Perth Hospital NZ • Christchurch Hospital • Palmerston North Hospital ANUP have been successfully awarded funding from the Sydney Catalyst Translational Cancer Research Centre for the Phase III Accelerated BEP translational sub-study. This will involve the collection of blood and tissue from participants for future correlative studies. Current ANZUP trials Bladder Cancer Bladder Cancer BCG + MMC Trial BL12 Non-muscle invasive bladder cancer is common and causes substantial suffering. It requires removal or irradiation of the bladder within 5 years in over 30% of people with high risk tumours despite best current treatment. Urothelial cancer is a type of cancer which typically occurs in the urinary system: the kidney, the bladder and associated structures such as the ureters which connect the kidneys to the bladder. A new treatment drug for urothelial cancer is being studied called nab-paclitaxel which will be compared with paclitaxel, which is a chemotherapy drug that is most often used. Recent preliminary studies show promising results from adding MMC, a chemotherapy drug, to current treatment with BCG. Medicines have to be approved for use by Australia’s Therapeutic Goods Administration. BCG is approved in Australia to treat bladder cancer. MMC is approved in Australia as a treatment of early bladder cancer. Before it is approved as a treatment for early bladder cancer, it needs to be tested to see if it is effective. This randomised trial will determine the effects of adding MMC on cure rates, survival, side effects and quality of life. It is anticipated that 500 patients will be enrolled in this study in Australia and New Zealand. To date, 22 patients have been recruited from 6 sites with an addition 6 other sites in the process of being activated. This study is currently active and recruiting. If this is something you or someone you know is interested in please speak with your doctor. For more information please go to the trials page on the ANZUP website http://www.anzup.org.au/content. aspx?page=trials-bcgmmc ANZUP collaborates with the University of Sydney through the National Health and Medical Research Council Clinical Trials Centre (NHMRC CTC). Current site locations for the BCG+MMC ANZUP Clinical Trial This research is being done because we need to identify more effective treatments for urothelial cancer that has progressed after prior chemotherapy. Nab-paclitaxel is a formulation of the chemotherapeutic drug paclitaxel that is combined with a human protein called albumin. Nab-paclitaxel has been tested in other cancers and has shown promising activity in lung cancer, melanoma and pancreatic cancer. Information from research suggests that nab-paclitaxel may be a useful treatment for urothelial cancer. Nab-paclitaxel and paclitaxel are approved in Australia to treat breast cancer and non-small cell lung cancer and paclitaxel is also approved to treat ovarian cancer. Neither of these two drugs is approved in Australia for use in urothelial cancer. The aim of the study is to see if nab-paclitaxel can improve outcomes for patients with urothelial cancer that has now progressed. This study will be conducted in collaboration with the NCIC Clinical Trials Group (NCIC CTG) in Canada. The NCIC CTG is a non-profit research group. About 200 people from Canada, Australia and New Zealand will take part in this study. Approximately 100 participants from 27 Australian sites will take part. NSW • Concord Repatriation General Hospital • Northern Cancer Institute At present, this study is in the startup phase, with the study submitted for ethics approval and trials staff working closely with the sites in preparation for study recruitment. VIC • Footscray Hospital • Royal Melbourne Hospital • The Alfred Hospital This trial is in development WA • Fremantle Hospital and Health Service ANZUP collaborates with the University of Sydney through the National Health and Medical Research Council Clinical Trials Centre (NHMRC CTC). For more information please go to the trials page on the ANZUP website www.anzup.org.au A LITTLE BELOW THE BELT 35 Current ANZUP trials Prostate Cancer ENZAMET The treatment of metastatic prostate cancer (prostate cancer that has spread beyond the prostate gland to other parts of the body), starts with medications that manipulate the hormone levels in the body. Hormonal manipulation occurs in the form of injections called LHRHA (luteinizing hormone releasing hormone analogues) and are often combined with tablets called anti-androgens. These treatments are generally used initially. However, men with metastatic prostate cancer do develop resistance to hormonal manipulation (i.e. so-called “castrate resistant prostate cancer”) and are subsequently treated with chemotherapy. This study has been designed to assess a new antiandrogen tablet called Enzalutamide. Enzalutamide is much stronger than older anti-androgens and has been shown to improve outcomes for men with metastatic prostate cancer. Recent clinical trials of the use of Enzalutamide in men with castrate resistant prostate cancer and those who have previously been treated with chemotherapy have shown that Enzalutamide can decrease PSA levels and shrink or stabilise cancer that has spread to other parts of the body such as bones or lymph nodes. Further, quality of life for men on Enzalutamide was significantly better. The purpose of the ENZAMET trial is to find out if the use of Enzalutamide earlier in the course of treatment for metastatic prostate cancer, may be of more benefit in terms of life expectancy and Quality of life compared to older anti-androgens? We do not yet know if Enzalutamide used in this way will be helpful. The comparison arm is the current standard way of using treatments. We have no reason at this stage to think people will be disadvantaged if they receive that treatment, as it is similar to what they would get if they were not on the trial. Treatment will continue until the cancer starts to grow or there is some other reason to stop treatment. All men will continue to be followed up even after trial treatment finishes. ENZAMET will be an international trial run by ANZUP in multiple centres in Australia, New Zealand, Canada, Ireland and the UK. The aim is to have 1100 participants from these countries. Participants will stay on the study drug until there is evidence of progression and will be followed for a minimum of 3 ½ years from entering the trial. This study is currently active and recruiting. If this is something you or someone you know is interested in please speak with your doctor. For more information please go to the trials page on the ANZUP website http://www.anzup.org.au/content. aspx?page=trials-enzamet 36 A LITTLE BELOW THE BELT ANZUP collaborates with the University of Sydney through the National Health and Medical Research Council Clinical Trials Centre (NHMRC CTC). To date, 29 sites have been activated with another 9 ANZ sites in the process of being activated. 72 patients recruited to date. We have started submission to ethics and regulatory bodies in Europe lead by the collaborative group, Ireland Cooperative Oncology Research Group (ICORG). NSW • Central West Cancer Services • Chris O'Brien Lifehouse • Concord Cancer Centre • Nepean Cancer Care Centre • Northern Cancer Institute • Port Macquarie Base Hospital - NCCI • Prince of Wales Hospital • Riverina Cancer Centre • St George Hospital • St Vincent's Hospital Sydney • Sydney Adventist Hospital • Wollongong Hospital QLD • Gold Coast Hospital • Nambour General Hospital • Princess Alexandra Hospital Brisbane SA • Ashford Cancer Centre • Flinders Medical Centre • Royal Adelaide Hospital TAS • Royal Hobart Hospital VIC • Austin Hospital • Australian Urology Associates • Box Hill (Eastern Health) • Goulburn Valley Health • Monash Cancer Centre - Moorabbin • Peninsula South Eastern Haematology & Oncology Group (PSEHOG) • Peter MacCallum Cancer Centre - East Melbourne • St Vincent's Hospital Melbourne WA • Royal Perth Hospital NT • Royal Darwin Hospital Prostate Cancer ENZARAD Prostate cancer is often treated with powerful X-rays (radiotherapy) instead of surgery. The reasons for choosing radiotherapy or surgery are complex and this is a discussion that men should have with their treating doctors. We will specifically look at men whose cancers have higher risks of coming back after treatment but have not shown any evidence yet of spread outside the prostate. In this situation we are aiming for a cure if possible and the evidence shows that this is more likely when radiotherapy is combined with hormone treatment. This treatment is called Androgen Deprivation Therapy (ADT). ADT is often in the form of injections called LHRHA (luteinizing hormone releasing hormone analogues) and often combined with tablets called anti-androgens. Enzalutamide is a new and stronger anti-androgen that has also been shown to work against prostate cancers that are resistant to other antiandrogens. This is not an alternative treatment to radical prostatectomy. If you are offered a radical prostatectomy you will not be eligible for this trial. ENZARAD is a clinical trial for men with this type of prostate cancer where a decision has been made that radiotherapy is the best treatment. That is this trial is for those men who cannot or are not suitable for radical prostatectomy due to their pathology or core morbidities. ENZARAD will answer this question: will men with prostate cancer apparently confined to the prostate but at high risk of coming back elsewhere benefit, in terms of living longer, from adding Enzalutamide to radiotherapy plus ADT? Clinical trials are the evidence that drive clinical practice. Our trials are run by investigators to answer important clinical questions in terms of improving outcomes for our patients with urological cancers. Associate Professor Guy Toner, ANZUP Deputy Chair ENZARAD will be an international trial run by ANZUP in multiple centres in Australia, New Zealand, Canada, Ireland and the UK. The aim is to have 800 participants from these countries. Participants will stay on study drug until there is evidence of progression and will be followed for a minimum of 3 ½ years from entering the trial. This study is currently active and recruiting. If this is something you or someone you know is interested in please speak with your doctor. For more information please go to the trials page on the ANZUP website http://www.anzup.org.au/ content.aspx?page=trials-enzarad This trial is conducted in collaboration with All Ireland Cooperative Cancer Research Group (ICORG) and the Trans Tasman Radiation Oncology Group (TROG). To date, 14 sites have been activated with 27 patients have been recruited. An additional 11 ANZ sites in the process of being activated. We have started submission to ethics and regulatory bodies in Europe lead by the collaborative group, Ireland Cooperative Oncology Research Group (ICORG). NSW • Calvary Mater Newcastle • Campbelltown Hospital • Central West Cancer Services • Chris O'Brien Lifehouse • Liverpool Hospital • Westmead Hospital QLD • Mater Adult Hospital Radiation Oncology Services • Princess Alexandra Hospital Brisbane • Royal Brisbane & Womens hospital TAS • Royal Hobart Hospital VIC • Box Hill (Eastern Health) • Peter MacCallum Cancer Centre (East Melbourne) • Peter MacCallum Cancer Centre (Moorabbin Campus) A LITTLE BELOW THE BELT 37 Prostate Cancer RAVES Trial Radical prostatectomy is the most common curative approach offered to men with newly-diagnosed prostate cancer. Unfortunately, up to half of these patients will have factors placing them at high risk of cancer reoccurring. Having radiotherapy after an operation is known to improve care rates but what is not known is whether it should be given immediately after the operation or only if PSA rises after surgery – indicating active cancer. Immediate radiotherapy may not benefit all men and can cause serious side effects such as bladder problems and impotence. International lack of consensus on the optimal timing of radiotherapy has resulted in varied clinical practice. This Trans Tasman Radiation Oncology Group (TROG) led Phase III trial will compare the two approaches. If radiotherapy at recurrence results in equivalent outcomes and improved quality of life, it would become the standard treatment. A total of 470 men from Australia and New Zealand will participate. • Peter MacCallum Cancer Centre QLD • Mater Centre • Premion • Princess Alexandra Hospital • Toowoomba Cancer Research Centre • Townsville Hospital • Royal Brisbane & Women’s Hospital WA • Perth Radiation Oncology Centre • Royal Perth Hospital • Sir Charles Gairdner Hospital NZ • Auckland Hospital • Auckland Radiation Oncology • Christchurch Hospital • Dunedin Hospital • Palmerston North Hospital • Waikato Hospital • Wellington Hospital This study is currently active and recruiting. If this is something you or someone you know is interested in please speak with your doctor. For more information please go to the trials page on the ANZUP website Recruiting sites NSW • Calvary Mater Newcastle • Campbelltown Hospital • Central West Cancer Services • Nepean Hospital • Port Macquarie Base Hospital • Riverina Cancer Care Centre • Royal North Shore Hospital • Royal Prince Alfred Hospital • St George Hospital • St Vincent’s Hospital Sydney • Westmead Hospital VIC • The William Buckland Radiotherapy Centre The Alfred • Austin Hospital 38 A LITTLE BELOW THE BELT Many people when confronted with a clinical trial for the first time, may think they are being treated as a guinea pig, but all the evidence shows that people who are participating in clinical trials are receiving the best level of clinical care and have in general better outcomes than those who are not participating. Associate Professor Guy Toner, ANZUP Deputy Chair Current ANZUP trials Prostate Cancer ClinTrial App Living Well with Prostate Cancer In July 2014 ANZUP released its first trial based App. This application was designed for the specialists but will also be a very useful tool for consumers. If you are looking for a trial for your particular cancer you can refer to either the ANZUP website or the new ClinTrial Refer. Prostate cancer is the most common male cancer in developed countries. There are approximately 22,000 men living with advanced prostate cancer today. Research into the impact of advance prostate cancers shows that men report higher levels of psychological distress, poorer quality of life and have an increased risk of suicide compared to men with localised disease. Additionally, many men report they do not receive enough support after their diagnosis. This project is trialling a professionallyled telephone session delivering mindfulness-based cognitive therapy group intervention for men with advance prostate cancer. This program is designed to assist men with stress management and improve their psychological wellbeing. Its effectiveness will be compared to an educational program consisting of the best available resources for men with advanced prostate cancer. This trial is active and recruiting until the end of 2014. If this is something you or someone you know is interested in please speak with your doctor. ANZUP collaborates with Griffith University and Cancer Council Queensland. The ClinTrial Refer ANZUP app provides a current list of all ANZUP and ANZUP co-badged clinical research trials conducted in cancer centres in Australia and New Zealand. Designed for oncologists, general practitioners, research unit staff and patients, ClinTrial Refer ANZUP has searchable clinical research trial details, hospital locations and contacts, and inclusion and exclusion criteria. We hope this will help the community to identify trials that might be suitable. To download the free app, please visit: ANZUP really values the involvement of consumers in clinical trials. The reason for that is that while we may think it is important to do a clinical trial, really it is the consumer who has the best judgement about the importance of the clinical trial. • Apple iTunes: https://itunes.apple.com/au/app/ clintrial-refer-anzup/id894317413?mt=8 • Google Play: https://play.google.com/store/apps/ details?id=com.lps.anzup Or go to the App/Android store and type in ANZUP Associate Professor Guy Toner, ANZUP Deputy Chair A LITTLE BELOW THE BELT 39 BY WAY OF DEFINITION Each magazine, we will be exploring a few terms that you may have come across regarding clinical trials. What is a clinical trial? The information below is provided on the National Health and Medical Research Council website www.australianclinicaltrials.gov.au. Information was accessed 23 January, 2014. Clinical trials are research investigations in which people volunteer to test new treatments, interventions or tests as a means to prevent, detect, treat or manage various diseases or medical conditions. Some investigations look at how people respond to a new intervention and what side effects might occur. This helps determine if a new intervention works, if it is safe, and if it is better than the interventions that are already available. Clinical trials might also compare existing interventions, test new ways to use or combine existing interventions or observe how people respond to other factors that might affect their health (such as dietary changes). The World Health Organization (WHO) definition for a clinical trial is: Clinical trial interventions include but are not restricted to: • experimental drugs • cells and other biological products Any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes. 40 A LITTLE BELOW THE BELT • vaccines • medical devices • surgical and other medical treatments and procedures • psychotherapeutic and behavioural therapies • health service changes • preventive care strategies • educational interventions Researchers may also conduct clinical trials to evaluate diagnostic or screening tests and new ways to detect and treat disease. What does translational mean? Dr Peter Grimison explains “This activity aims to facilitate a “personalised medicine” approach to the treatment of individuals with advanced testicular cancer. We want to determine whether a person’s genetic make-up can tell us reasons why 100% of individuals with advanced testicular cancer are not curable with best standard chemotherapy. We will try and identify genetic variants within an individual that alter how a person’s body processes chemotherapy, and genetic variants within testicular cancer that make it inherently resistant to standard chemotherapy. Results could lead to the design of tailored treatments for such individuals, for example treatments that incorporate a higher dose of or an alternative form of chemotherapy. We will also establish the first national biobank for advanced testicular cancer, which can be used in future collaborative international research. Our ultimate goal is to try and cure 100% of patients with testicular cancer.” DEFINITIONS Blinded Trial Off-Label Use A blinded clinical trial is one where the participants are not told which treatment they are receiving. Off-label use occurs when a drug is prescribed for conditions not approved by the Therapeutics Goods Administration (TGA). Clinical Trial A clinical trial is a scientific and medical study using human subjects to test the safety and benefit of new drugs or medical treatments. Phase I Clinical Trials Phase I clinical trials are initial studies using a small test group to see if a drug is safe for use in humans. Control Group Phase II Clinical Trials In clinical trials, the control group is given a standard treatment (or placebo), which is a different treatment given to the test group, in order to compare the results of the two. Phase II clinical trials are secondary studies that evaluate the efficacy of drugs and their side effects on humans. Double Blind A double blind clinical trial is one where both the participants and the investigators of the trial do not know which participants are receiving which treatment. Dose Finding Study Clinical trial where different doses of drugs are given to different groups of participants to determine which dose is most beneficial. Efficacy Efficacy is the ability to produce results. In cancer clinical trials, it means the ability of the treatment being tested to control or eradicate a cancer, or to help someone feel better or liver longer. Phase III Clinical Trials Phase III clinical trials are expanded clinical trials usually involving a large number of participants often across many facilities or locations. Phase III testing looks at the benefit/risk relationship of new medications. They often study whether the new drug is better than current treatments. Phase IV Clinical Trials Phase IV clinical trials are when drugs that have been approved for sale to the general public, either over the counter or by prescription, are continually studied in a larger population for rare or unexpected side effects. Placebo A placebo is an ineffective treatment given in clinical trials to compare to a new medication. Ethics Committee Placebo Effect An institutional review board is made up of physicians, medical personnel, community advocates, and others. It is their job to ensure that participants in clinical trials are protected and being treated ethically. The placebo effect describes physical or emotional changes a clinical trial participant experiences based on their expectations, and not because they have been given a new medication. Exclusion criteria Preclinical Testing Criteria that will make a potential participant ineligible to participate in a clinical trial. This might include age, overall health, and abnormal kidney or liver function. Preclinical testing is testing done prior to clinical trials and prior to any testing on humans. It usually involves research and testing on animals or cells. Inclusion criteria Randomised Inclusion criteria are the criteria that participants must meet in order to qualify for participation in a clinical trial. This might include type of cancer, extent of the disease, and former treatments received. Randomised clinical trials are when participants are chosen at random to receive different treatments so that the effects of each can be compared. Informed consent Informed consent is the process for clinical trials participants to be made aware of all aspects of what they can expect during the clinical study before deciding whether to participate. This includes treatments, time and travel commitments and potential risks. Participants must sign a document confirming their consent. Recruiting Recruiting refers to the phase of a clinical trial where participants are screened for eligibility and invited to participate. Side effects Side effects are unwanted, often negative results of a treatment. A LITTLE BELOW THE BELT 41 The National Health and Medical Research Council defines the phases of trials as: PHASE I clinical trials are done to test a new biomedical intervention for the first time in a small group of people (20-80) to evaluate safety (e.g. to determine a safe dosage range and identify side effects). 42 A LITTLE BELOW THE BELT PHASE II clinical trials are done to study an intervention in a larger group of people (several hundred) to determine efficacy (whether it works as intended) and to further evaluate its safety. PHASE III studies are done to study the efficacy of an intervention in large groups of trial participants (from several hundred to several thousand) by comparing the intervention to other standard or experimental interventions (or to non-interventional standard care) as well as to monitor adverse effects and to collect information that will allow the intervention to be used safely. PHASE ll lll lV PHASE l PHASE PHASE INCREASING SIZE AND COST PHASE IV studies are done after an intervention has been marketed. These studies are designed to monitor the effectiveness of the approved intervention in the general population and to collect information about any adverse effects associated with widespread use over longer periods of time. ANZUP welcomes as the official travel Partner Members of ANZUP in New Zealand will be pleased to hear their travel costs will be supported by a new relationship with Air New Zealand. In an agreement recently announced, Air New Zealand will provide a number (10) of return economy airfares between New Zealand and Australia. This partnership will provide ANZUP with a significant cost saving and will enable members to travel for face-to-face meetings in Australia. ANZUP Board Member and Chair of the Fundraising and Promotions sub-committee Joe Esposito said recently “this type of in-kind support helps us meet our goals while reducing the costs otherwise associated with these meetings”. Executive Officer Margaret McJannett said, “This is the start of what we hope will be a longterm partnership with Air New Zealand. Travel costs for the ASM and face-to-face meetings are significant and having access to this kind of travel support allows us to bring our NZ members across so they can participate in our research activities helps our bottom line while also helping us progress trial concepts. ”. The partnership is in place for twelve months and we look forward to continued discussions with Air New Zealand regarding subsequent years. Medical Oncologist, Dr Nicola Lawrence from Auckland was able to attend the recent concept development workshop for bladder and renal cancer through the generous sponsorship provided by Air New Zealand. If you are travelling for business or pleasure to NZ or the US we ask you to keep Air New Zealand’s support in mind when booking your fares. ANZUP wishes to thank our corporate supporters for 2014 A LITTLE BELOW THE BELT 43 IN THIS FIGHT THERE ARE NO RULES. Hear from ANZUP's experts about your below the belt cancer and the latest clinical trials available to you. Sunday 12 July 2015. Sofitel Sydney Wentworth. Free entry but bookings essential. www.anzup.org.au FIGHT CANCER BELOW THE BELT • TESTICULAR • PROSTATE • BLADDER • KIDNEY • 44 A LITTLE BELOW THE BELT