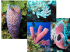The Association of Ophiothrix lineata and Callyspongia vaginalis: A
Transcription
The Association of Ophiothrix lineata and Callyspongia vaginalis: A
P. S. Z. N. I: Marine Ecology, 5 (1): 9-27 (1984) 0 1984 Paul Parey Scientific Publishers, Berlin and Hamburg ISSN 0173-9565/InterCode: MAECDR Accepted: 12.5.1983 The Association of Ophiothrix lineata and Callyspongia vaginalis; A BrittlestarSponge Cleaning Symbiosis? GORDON HENDLER Smithsonian Oceanographic Sorting Center, Smithsonian Institution, Washington, D . C., 20560, U.S.A. c With 7 figures Key words: Ophiothrix lineata, Caflyspongia vaginafis, brittlestar, echinoderm, sponge, symbiosis, predation, periodicity, feeding. Table of contents 10 Problem 10 Material and Methods 10 1. Location 12 2. Elapsed-time cinematography 12 3. Behavioral analyses 13 4 . Size and distribution of brittlestars 13 5. Predation 13 Results 13 1. Distribution of brittlestars 14 2. Circadian rhythm 14 3. Feeding activity 18 4. Fish predation 19 5. Regeneration 19 Discussion 19 1. Obligate commensalism 19 2. Sponges as a refuge from predation 22 3. Sponges as a source of food 24 4. Speculation regarding a cleaning symbiosis 25 Summary 25 Acknowledgements 25 References Abstract. The relationship between a sponge, Callyspongia vaginafis, and an associated brittlestar, Ophiorhriv Zineafa, was examined for mutualistic symbiotic interaction. Cinematography, feeding experiments, and analyses of stomach contents reveal that 0. lineata (unlike other Ophiothrix species) is a non-selective deposit feeder. Its diet consists of detrital particles adhering to the sponge, which are too large to be utilized by the sponge as food. Thus, the brittlestar cleans the inhalent surface of the sponge as it feeds. Since siltation interferes with sponge pumping-activity, it is suggested that the cleaning behavior of 0. lineara may enhance the filtration capability of C. vaginafis. in situ elapsed-time films show that brittlestars expose their arms when they feed, suggesting that they feed only at night because of a need to avoid diurnal predators. Manipulative experiments show that residence in C. vaginalis protects 0. lineafa from predatory fish. A U.S. Copyright Clearance Center Code Statement: 0173-956518410.501-0009$02.50/0 10 HENDLER comparison of the incidence of arm regeneration for brittlestars residing on C. vuginalis, and on a toxic sponge, Neofibularia nolitungere, indicates that factors other than sponge toxicity are involved in protecting sponge-dwellingbrittlestars. Problem According to WESTINGA & HOETJES (1981, p. 149) the fauna inhabiting sponges is “an ecological community, in which, however, interrelationships are not yet clear.” Brittlestars are a common component of that community. Their a:jsociation with sponges has been documented in the scientific literature (WCGINMTE & MACGINITIE, 1949; PEARSE, 1950; HYMAN,1955; BACESCU, 1971; R i h z L E R , 1975) and by the popular press (MURPHY, 1980; SEFTON, 1982). In some cases the association is facultative or fortuitous and the brittlestars involved may be found on or off the sponge. The association of Ophiothrix quinquemaculata (DELLE CHIAJE) with sponges, for example, has been attributed simply to the climbing behavior of the brittlestar or its reaction to water currents (FEDRAet al., 1976; FEDRA, 1977; STACHOWITSCH, 1980). Unfortunately, the ecology of brittlestars which are facultatively associated with sponges has not been investigated in sufficient detail to accurately characterize the interactions between brittlestar and sponge (PEARSE, 1950; WURZIAN, 1977; WESTINGA & HOETJES, 1981). In cases where the two taxa are invariably associated there may be a strict symbiosis, with at least the brittlestar benefiting from the association. The brittlestar Ophiothrix lineata LYMAN,for example, has been reported as an associate of various sponge species. This study examines the interaction of 0. Zineatu with a sponge, Callyspongia vuginulis (LAMARCK), to clarify the nature of the symbiosis between the two organisms. It is possible that sponges offer the brittlestar a refuge from predation. To better understand this potential interaction an additional sponge species was investigated. Neofibularia nolitangere (DUCHASSAING &MICHELO was ~ ) chosen since it is considerably more toxic than Callyspongia vuginulis, and it lharbors several of the same brittlestar species as C. vaginafis. Toxicity cciuld be evaluated as a factor in protecting sponge-dwelling brittlestars, by comparing the physical condition of brittlestars associated with the two species of sponges. The diet and mode of feeding of Ophiothrix lineata were studied to determine whether it uses the sponge as a source of food. Additional complexities of the association were considered. For example, I indicate below that Cullyspongiu vaginalis may benefit because 0. lineata clears debris from the incurrent surface of the sponge host and might thereby enhance the filtering efficiency of the host. Material and Methods 1. Location ’ The study site was a cut in the Belize Barrier Reef to the south of Came Bow Cay ( P ~ ~ Z L&E R MACINTYRE, 1982, p. 11, for location). The area is about 5 m deep, predominantly a sandy bottom with scattered coral heads, sponges, gorgonian colonies, and patches of seagrass. It is subjected to strong tidal and wind-driven currents that can dramatically alter the turbidity of the water in the cut. Fig. 1 (right). At night, the arms of the brittlestar, Ophiothri* lineata, are extended along the outer walls of the sponge, Callyspongia vaginalis. Polyps of the epizoic zoanthid, Pnrazoanthus parasiticus (DURCHASSAING & Mrcm~orrr)are evident on the largest sponge tube. Fig. 2 (bottom). At night, the distal portion of the arms of 0phiorhri.x lineata protrude from the osculum of Callyspongia vaginalis. As the brittlestar’s arms slowly sweep across the inhalent surface of the sponge its attenuate tube-feet and long, slender arm-spines collect the detrital particles on which it feeds. Fig. 3 (right hand bottom). A tube of Callyspongia vaginnlis tom open during the day to reveal a specimen of Ophiothrix lineata. The brittlestar’s disc is covered by fields of low, rough granules alternating with large, bare radial shields. The lines from which the species name derives are visible along the dorsal surface of the arms. 12 HENDLER 2. Elapsed-time cinematography A tube sponge, Callyspongia vaginalis, with a resident population of the brittlestar, Ophiothrix lineata, was filmed in situ for three days using an underwater strobe and a 16mm elapsed-time movie er al., camera set for exposures at 20sec intervals (equipment described in detail by EDGERTON 1968). A buoyant plastic float, used as a current meter, was tethered near the b3se of the colony inside the picture area. The angle of the float line indicated approximate current :<peed(HENDLER, 1982 a), however, only relative fluctuations in current speed are considered in this paper. During filming, the intensity of ambient illumination was monitored continuously with a photometer (United Detector Technology, Inc. 40 X Opto-meter) and a potentiometric strip chart recorder. The photometer sensor was shielded with an uncalibrated diffuser and mounted on an unshaded sector of the laboratory roof on Carrie Bow Cay. 3. Behavioral analyses Using the time-lapse Nm, the movements of the brittlestars and the rates of sediment clearance from the surface of the sponge were analysed with a stop-motion 16-mm projecror (L-W Internahonal224-A MK VI Photo-Optical Data Analyser). Brittlestar activity was meascred by enumerating the brittlestar arms visible-on the outer (inhalent) surface of the sponge at appropriate intervals (Figs. . - 1, 2, 3). To evaluate the rate at which adhering material was removed from the surface sf the sponge, the sponge was dusted with fine sediment before the nocturnal cycle of brittlestar activity and then filmed. Frames of the film were projected and traced on graph paper, and the area covered by sediment in each frame was estimated by counting graph paper squares. The clearance rates wer2 calculated separately for portions of the sponge that were reached by the brittlestar arms and for portions unaffected by the movements of the brittlestars. These results contrast the rate of clearance effected by the brittlestar with the rate of clearance atttributable to gravity and water movement. To clanfy whether brittlestars merely dislodged particles from the surface of the sponge or actually could ingest them, the following experiment was performed. A mixture of brine shrimp eggs, and chopped fish scales and mucus was applied to the surface of a sponge. The eggs measured 0.47 k 0.03 rnm (X k s. d.) diameter. After 15 min, the sponge tubes were quickly ':om open (Fig. 3), the brittlestars were removed, and while still underwater they were put into a plastic bag that was injected with concentrated formaldehyde solution. This treatment ensured that the stomach contents were quickly preserved and that the brittlestars did not regurgitate. Afti:r transferring the preserved samples to alcohol, the gut contents were removed from the brittlestar discs and examined for the presence of brine shrimp eggs. The constituents of the natural diet of Ophiothrix lineata were determined for !)O specimens from 14 Callyspongia vaginah sampled during the afternoon and for 71 specimens from 11 sponges sampled at night. The specimens were preserved in the same manner as those in the brine shrimp ingestion experiment. Stomach contents from each specimen were rinsed into a vial. When particles in the vials settled, all the samples were scrutinized. The quantity of material in each sample was rated on a scale from 1 to 3, based on the relative volume of material in the sample. After gently agitating the sample vial, a subsample of the gut contents from e , x h individual was removed with a wide bore pipette and placed on a well slide beneath a coverslip. A single, randomly chosen microscope field at 100 x magnification was traced using a camera lucida, and the longest axis of each particle in the field was measured from the drawing. The frequen'zy distributions of particles in the stomachs of brittlestars sampled during the day and of those sampled at night were calculated (for 0.05 mm size categories) as a percentage of the total number of particles measured. The naturally occurring accumulation of particles on the outer surface of Caliyspongia vaginalis was sampled for comparison with the brittlestar stomach contents. An underwater suction device (CLARK, 1971), fitted with a 54pm mesh screen over the exhaust valve, was used to capture material clinging to the incurrent wall of the sponge. Collections were made in the afternoon from 20 sponge colonies, and the settled particles in the sample were analysed microscopically and statistically using the same techruque applied to the stomach contents. Brittlestar-sponge symbiosis 13 4. Size and distribution of brittlestars The populations of brittlestars on 12 specimens of CalZyspongia vaginafis and 6 specimens of Neofibufuria nofitungere were removed underwater. For C. vaginalis, every brittlestar visible on the outer wall of the sponge was collected first. Then, the tubes of the sponge colony were opened and the brittlestars within were placed in a separate container. Because of the complex structure of N . nolitangere, the brittlestars collected from the outside and inside of each colony were combined. The specimens collected in the field were anaesthetized and preserved in alcohol in the laboratory. After identifying each brittlestar to species, the disc diameters and maximum arm lengths were measured with calipers, ruler, or ocular micrometer. In addition, the arms of each specimen were examined for signs of autotomy or breakage and regeneration. A regeneration index (RI) was calculated as the fraction of regenerating arms divided by the number of arms examined, multiplied by 100. Arms broken during sampling were disregarded since they could not be critically examined for signs of regeneration. 5. Predation The degree of protection afforded the brittlestars by Callyspongia vaginalis was evaluated in 10 experiments, as follows. A sponge was selected for the experiment with a resident population of Ophiothrix lineuta. Eleven specimens of 0. linearu were removed from another sponge. Five of those brittlestars were placed on the sand at the base of the experimental sponge, and five were placed on the outer wall of the experimental sponge. Finally, one specimen was crushed to attract predators. Attacks by fish that damaged brittlestars beneath, on, and inside the experimental sponge were tallied separately. To determine the percentage of survival after 5 min, the number of 0. lineata remaining on and beneath the sponge were counted. The experimental sponges were not examined to determine the number of resident brittlestars in the sponge tubes, because specimens inside the sponge were never attacked. A related series of 10 experiments was carried out, by placing crushed specimens of 0. liiieafu inside 3 to 5 tubes of a sponge and then an additional crushed specimen on the sand at the base of the sponge. Predatory fish attracted to the area were observed to determine whether they would pursue and attack injured brittlestars inside the sponge. 1. Distribution of brittlestars Different suites of brittlestar species are found on Caffyspongiavaginafis and Neofibularia nofitangere. Fig. 4 shows that 6 species of brittlestars are associated with the two sponges. However, Ophiothrix fineata and 0. oerstedi LUTKENare is restricted restricted to C. vaginafis, while Ophiactis quinqueradia LJUNGMAN to N. nofitangere. Populations of certain brittlestars show different characteristics on the two sponges studied. There are differences in the mean size of the Ophiothrix angulata (SAY)and 0. suensoni L ~ K EonNthe two species of sponges (Fig. 4). For both brittlestar species the mean disc diameter of specimens on Neofibufaria is significantly greater than for specimens on Callyspongia (0. angulata, t = 72.52, n = 130, P < 0.001; 0. suensoni, t = 19.45, n = 142, P < 0.001). The differences in the mean sizes of 0. fineatu inside and outside Caffyspongia vaginafis is more striking, with larger specimens inside, and is also statistically significant (t = 16.34, n = 154, P < 0.001) (Fig. 4). 0. angulata shows the 14 HENDLER same trend (t = 2.40, n = 103, P < O.OZ), as does 0. suensoni (t = 5.46, n = 7 5 , P < 0.001) (Fig. 4). The data for 0. oerstedi are inadequate to test for such a difference in mean disc diameter. 2. Circadian rhythm A specimen of Callyspongia vaginalis was filmed for three days and the patterns shown in the three films were quite similar. The results of the film taken on 19 April, 1982 are shown in Fig. 5. The activity of the brittlestar arms outside the sponge (Figs. 1, 2), begins at dusk and diminishes in the morning. The peak intensity of activity occurs between about 1900 and 2400 h, during darkness. Because there was moonlight during the filming period, photometer values for illumination were recorded at night. There is no evident correlation between the intensity of nocturnal illumination and the activity of brittlestars (Fig. 5 ) . Neither is there an obvious correlation between the strength of the current (generally travelling in surges moving less than 5 cm s-I) and the activity of the brittlestars (Fig. 5 ) . 3. Feeding activity The nocturnal behavior of Ophiothrix lineafu consists of moving its arms like a pendulum, very slowly sweeping across the surface of the sponge (Figs. 1, 2). 5 0.LINEATA 0.SUENSONI 0.ANGULATA 0.OERSTEDI 0.SAVlGNYl 0.QUINQUERADIA SIZE [mm DISC DIAMETER] Fig. 4. Size-frequency distributions of brittlestars (Ophiofhrixlineafa, 0. suensoni, 0. angulafa, 0. oersfedi, Ophiactis savignyi (M. & T.), and 0. quinqueradia) collected from the outside and inside surfaces of Callyspongia vaginalis, and from Neofibularia nolifangere. The disc diameter (mean and s. d.), number of specimens, and regeneration indexare indicated for each sample of brittlestars. 15 Brittlestar-sponge symbiosis The tip of the arm moves at a mean rate of 3.14 k 1.96 cm/min. Only 1.6 % of the 2,566 arms counted during film analysis were lifted off the incurrent wall of Callyspongiu; most arms remain in contact with the sponge (Fig. 5). When the outside of a sponge was dusted with sediment, the moving arms of the brittlestars cleared a swath through the film of sediment clinging to the sponge. Fig.6 illustrates the fraction of the surface of the sponge cleared at intervals after the brittlestars began feeding in the evening. The sediment on the sponge disappears in step with the emergence of brittlestar arms and increasing brittlestar activity (Fig. 6). Comparing the portion of the sponge brushed by the moving brittlestar arms with that area outside the reach of the brittlestars, clearly the portion of the sponge affected by the brittlestars loses silt more rapidly than the remainder of the sponge (Fig. 6). 80 I I I I I I I I I I I I i c 2 40 . . '..... .. ............,.-........ . . . ...... . . .... .' ..... .. - .. .... .. . ..... .. .. .. . . . .......... . .. ..-. . . . . . . . . . . . . . . . . .................... 1.. ................................. TIME [h] Fig. 5. Fluctuations of the intensity of activity of Ophiothrix lineata on Callyspongia vaginalis, expressed as the number of brittlestar arms visible on the outside surface of a sponge (large solid circles) in an elapsed-time film. Also shown are: the numbers of arms lifted off the surface of the sponge (open circles), the relative light intensity (squares), and the angle of the current-meter float (small circles). 16 HENDLER * 0 30 . * * 0 8 * * * * . * LO * * . * . :E * -20 :: LL C> nz U4 a> 0 -10 * * * I-- 3 . 0 .. . . , ,* : f ' . . ' , 40 20 2 60 . . . . . . ao 100 120 * , 140 . , 160 u a K 0 0 404 1 0 . C1 . . . - L 0 t . . . . . * - 1 20 40 60 00 100 120 140 160 T I M E [min] Fig. 6. The rate of clearance of sediment from the surface of a specimen of Callyspongin vaginalis dusted with silt, measured from elapsed-time films for two nights. The rate of clearance csn portions of the sponge reached by Ophiofhrix fineafa (circles) is contrasted with clearance rate for the area not swept by brittlestar arms (squares). The number of 0. lineata arms visible on the sponge (asterisks) is directly proportional to the intensity of brittlestar activity. An experiment utilizing brine-shrimp eggs was performed to determine whether the particles removed from the surface of Callyspongia vaginalis are merely brushed off the sponge or could be ingested by the brittlestars. Of 14 Ophiothrix lineata exposed to the brine-shrimp egg mixture during two night dives, 7 individuals ingested brine-shrimp eggs. Four specimens of 0. lineata were exposed to the mixture during the day, and no brine-shrimp eggs were found in their stomachs. These data are too few to warrant formal seatistical comparison of night versus day behavior. In the stomach contents of 12 specimens collected at night, there was naturally occurring food material, the remaining 2 specimens had empty stomachs. In contrast, 1 specimen from the day sample contained naturally occurring food and 3 lacked food. These data are consistent 'with the cinematographic record of greater feeding activity at night and the results, discussed below, of an analysis of naturally occurring stomach contents. 17 Brittlestar-sponge symbiosis It was rarely possible to discern identifiable particles amongst the material sampled in stomach contents and material sampled from sponges. The samples consisted largely of unidentifiable debris. Some chitinous parts, diatoms, bits of plant tissue, and sponge spicules regularly appeared in small numbers. Foraminifera, fragments of filamentous algae and strands of blue-green algae, copepods, ostracod fragments, gorgonian spicules, and tiny gastropods were occasionally or rarely detected in the stomach contents and from the sponge wall. In addition, fragments of thecate hydroids were collected from the surface of the sponge. T o determine whether the brittlestars selectively removed material from the surface of the sponge, the size frequency distributions of particles from the sponge surface and from the stomach content samples were compared (Fig. 7). The same range of particle sizes occurs on the sponge and in the brittlestar stomachs from night and day collections. Pairwise in the three samples, the proportions of particles in different size categories are not significantly different (x’ = 1.0067 with d.f. = 10, P = 0.9995). This result suggests that the brittlestars do not remove material selectively, at least according to particle size. One has to consider that the size spectrum in the stomach may have been influenced by agglutination or breakdown of particles by the brittlestar and the technique of removing particles from the sponge. However, the similarity of the size-frequency distributions from the three groups is striking. w II 5z ., SURFACE OF SPONGE 20 ’ NIGHT GUT CONTENTS DAY GUT CONTENTS W 0 a w a 10 rnLbb---m cO.05 ~0.10~0.15 cO.20 ~ 0 . 2 540.30 ~ 0 . 3 5~ 0 . 4 0~ 0 . 4 5~ 0 . 5 020.50 Z0.05 20.10 20.15 Z0.20 E0.25 20.30 Z0.35 ’0.40 20.45 S I Z E OF PARTICLE [mm] Fig. 7. Comparison of the size-distributions of particles: siphoned off the surface of Callyspongia vaginalis (number of particles = 1,369), removed from the stomachs of Ophiothrix lineata sampled during the day (n = 499), and removed from the stomachs of 0. tineara sampled at night (n = 3,109). 18 HENDLER It is noteworthy that although the food-particle size distribution is similar for the stomach contents of brittlestars during the day and at night, only 21 % (n = 91) of the brittlestars were feeding during the day compared to 86 % (n := 71) at night. This difference in the incidence of full and empty stomachs in day and night samples is significant (FISHER'SExact Test: n = 162, P < 0.001). The volume of material in the stomachs of animals sampled at night was compared with that in the stomachs of animals collected during the day, by ranking the volume of material from 1 (low volume) to 3 (high volume). For night samples the number of spccimens showing values 1 , 2 , and 3 is, respectively, 28,21, and 12. For the day samples there is a lower volume of stomach contents per specimen, with 18, 2, and 0 individuals in the same categories. Moreover, the hypothesis that the occurrence of the three ranks was the same in the night and day samples is rejected (x' = 12.2568 with d. f. = 2, P = 0.0027). Thus, more brittlestars feed at night and their volume of stomach contents is greater than that of day-specimens. 4. Fish predation Attacks on brittlestars in the lumen of Caflyspongia'vaginalis, and those placed experimentally on t h e outside of the sponge, and on the sand beneath the sponge were analysed. At night, potential predators on brittlestars, such as hermit crabs, squirrelfish, and grunts were present. However, these predators did not approach the experimental sponges or attack Ophiothrix lineata. During the day, fish reached the experimental sponges within 3 to 112 SIX. The predominant species were Hafichoeres bivirtarus (BLOCH),Halichoeres garnotfi (CUVIER & VALENCIENNES), and Scarus croicencis BLOCH.Other fish attracted, with much lower frequency, were Sparisonza chrysopterum (BLOCH& SCHNEIDER), Cafamus bajonado (BLOCH& SCHNEIDER), 2nd Thalassoma bifusciatum (B LOCH). Marauding fish more frequently attacked brittlestars that were on the sand than those that were on the exterior of sponges (48 versus 18 attacks), and the survival of brittlestars on the sponges was higher than for those on the ground (82% versus 64%). In the experiments, Ophiothrix lineata living within the sponges were never attacked by fish. The brittlestars on the sponge and on the ground exhibited frenzied movements that probably intensified attacks by fish and increased the mortality of brittlestars in the experiments. In another series of experiments, comparing fish attacks on injured GI.lineafa beside Caflyspongia vaginafis, versus attacks on injured brittlestars within the sponge tubes, confirmed the effectiveness of the protection provided by the sponge. Fish attacked the brittlestars on the bottom within 4 to 102 sec, but fish did not molest the individuals in the sponge, even when the arms of injured brittlestars protruded from the osculum. The film record shows that most of the fish in the study area were present during the day. Between 0530 and 0800 h, when brittlestar activity virtually ceased, 61 fish were counted by observing a film frame for every 6 min interval. Using the same sampling interval only 9 fish were counted over the much longer period from 1830 to 0530 h. Thus, fish were recorded in greater numbers during Brittlestar-sponge symbiosis 19 the day. The diurnal and nocturnal fish populations were composed of different species. At night, mostly squirrelfish and grunts were filmed, but during the daytime predominantly wrasses, parrotfish, and damselfish were recorded. The latter 3 taxa attacked brittlestars in the predation experiment. 5. Regeneration Regeneration indices (RI) are tabulated in Fig. 4; higher values indicate higher frequency of autotomy or breakage and regrowth. Ophiothrix lineata specimens from the lumen of Callyspongia vaginalis have a higher RI value than specimens collected from the outer surface of the sponge. The same trend is shown by Ophiothrix angulata. Notably, Fig. 4 also shows that brittlestars on the outside of the sponge are considerably smaller than brittlestars on the inside. The RI values of brittlestars were compared between populations from Callyspongia vaginalis and Neofibularia nolitangere to gauge the relative degree of protection provided by the two species of sponges. Ophiothrix suensoni has a higher RI value on Neofibularia than on Callyspongia, but 0. anguluta has a lower R I value on Neofibularia than on Callyspongia. Interestingly, Ophiothrix suensoni and Ophiothrix anguluta both are generally of larger size on Neofibularia than on Callyspongia (see Fig. 4 and “Distribution” - Results section). Discussion 1. Obligate commensalism HYMAN (1955, p. 687) noted that for brittlestars “Their small size and flexible arms fit them for clinging to other animals, especially branching types, and hence it is not surprising that they often occur in association with sponges and coelenterates”. Despite their obvious morphological specializations for occupying large, sessile organisms, the adaptive value of such associations for the brittlestars and sponges is not well understood. Fortunately, it is possible to gauge the significance of the dependence by observation and experiment on brittlestars requiring sponge hosts. It appears that Ophiothrix lineata invariably is associated with sponges. (1977) suggested that the presence of 0. lineata was KISSLING& TAYLOR governed by the availability of suitable sponges, an observation supported by other investigations (CLARK,1933; DEVANEY, 1974). In contrast, the other brittlestar species that live on the same sponges as 0. lineata, live on substrata besides sponges as well (CLARK,1933; DEVANEY, 1974; KISSLING& TAYLOR, 1977). These findings support the hypothesis that 0. Zineara is an obligate commensal of sponges. 2. Sponges as a refuge from predation Abundant evidence has been published showing that brittlestars suffer heavily from predation. RANDALL (1967) reported on 33 species of Caribbean fish that 20 HENDLER feed on brittlestars. They occurred in more than 10 % of the stomach content samples for 10 fish species. He found that Ophiothrix was the most common brittlestar genus identified from fish stomach contents, at least in part because the spines of Ophiothrix species are so easily recognized. Of the 10 fish species referred to for which brittlestars are an important dietary component, 6 fed on Ophiothrix. Those include 3 diurnal wrasses, Bodianus rufus (L.) , Halichoeres 2 porgies, Calamus calamus garnotti, Halichoeres poeyi (STEINDACHNER); (CUVIER& VALENCIENNES), Calamus pennatufa GUICHENOT, and the nocturnal grunt, Anisotremus virginicus (L.). Another wrasse, Thalassorna bifa.i.ciatum, which also ingests Ophiothrix may consume a diet of 30.8 % brittlestars (FEDDERN,1965; RANDALL,1967). There is no evidence of a guild of fish that feed primarily on sponge-dwelling brittlestars, though there is a group of fish which prey largely on sponges (RANDALL & HARTMANN, 1968). Only one of the fish that feeds on sponges, Cantherhines pullus (RANZANI),has been found to ingest brittlestars, and Ophiothrix spp. were found in its stomach contents (RANDALL, 1967; RANDALL & HARTMANN, 1968). Certain fish may occasionally feed on both sponges and brittlestars. For example, BEEBE& TEE-VAN(1928) reported some sponge material in the guts of Halichoeres radiatus (L.), a fish that feeds on brittlestars (RANDALL, 1967). Both brittlestars and sponges are a minor component in the diets of the fishes Anisotremus surinarnensis (BLOCH)and Haemulori album CUVIER& VALENCIENNES (RANDALL, 1967). In European waters crustacean predators that attack Ophiothrix quinquemaculata associated with sponges include the crab Pilumnus hirteffus (L.), the hermit crab Paguristes oculatus (FABR.),and the spiny lobster, Palinurus vulgaris LATR. (VASSEROT, 1965; WURZIAN,1977; STACHOWITSCH, 1979, 1980). There is ambiguous evidence in the literature regarding whether brittlestars are protected from predation by their association with sponges. The potential of the sponge as a physical barrier to predation is obvious. In addition, fish might avoid the toxins or noxins (noxious compounds) synthesized by sponges. BAKUS & THUN(1979) reported that 57 % of the Caribbean sponge species tested were toxic to fish. Neofibularia nolitangere causes intense pain and dermatitis when handled by humans, and it is strongly toxic to fish (BAKUS& THUN,1979). Callyspongia vaginalis was reported by HALSTEAD (1965) to be toxic to fish, but BAKUS& THUN(1979) found that the species liberated noxins that were not lethal to the fish they tested. Not surprisingly, sponge-eating fish feed more & HARTMANN, 1968). often on C. vaginalis than on N . nolitangere (RANDALL However, perhaps because of their chemistry and the presence of spicules in many species, sponges are generally not an attractive food source for most fish. RANDALL & HARTMANN (1968) concluded that, overall, sponge-eating fish are of little importance in controlling populations of Caribbean sponges. Considering the contrasting effects on fish of toxins from the two sponges, Calfyspongia vaginalis and Neofibularia nolitangere, it would be expected that brittlestars associated with the latter, distasteful species would show less damage from predation. However, the results of the regeneration index measurements of brittlestars on C. vaginalis and N . nofitangere were equivocal on this point, perhaps because of the inadequacies of the RI as an index of predation. While the incidence of arm regeneration may parallel the intensity of predation, it is Brittlestar-sponge symbiosis 21 not necessarily directly related to fatal encounters. Brittlestar species that are not ingested by fish can still exhibit a considerable amount of arm regeneration (SHIRLEY,1982). Moreover, regeneration index values are probably biased by the differing regenerative abilities of particular brittlestar size classes and species. The trends in R I values for particular brittlestar species on Callyspongia and Neofibularia were inconsistent. This implies that factors other than sponge toxicity may be involved in the intensity of predation directed at brittlestars on various sponge species. It is possible that the presence or absence of brittlestar species on certain sponge species (Fig. 4) is related to brittlestar requirements for such factors as sponge morphology or microhabitat. The more straightforward data of the fish predation experiments show that Cullyspongia vaginalis offers Ophiothrix lineata protection from fish. Mortality from fish attacks increases for brittlestars inside sponges, over those placed on the outside surface of sponges, to those placed on the substratum near sponges. These results, and evidence of regeneration of 0. lineata on C. vaginalis, indicate that the brittlestar normally suffers a degree of predation while on the sponges. It appears (somewhat unexpectedly) to be the larger individuals, which extend their arms outside the sponge only a t night, that are most seriously affected by predators. The small specimens (both 0. lineata and 0. angulutu), primarily found on the outer wall of the sponge, show less evidence of regeneration. If this observation is not a technical artifact (a product of the difficulty of identifying damage and regeneration in small specimens), it is likely that the tiny specimens on the outside of the sponge simply are not detected by visual predators. Certainly, they are not easily seen by the human observer, because of their small size and their pale coloration. In addition, the occurrence of small individuals outside the sponge and large individuals inside the sponge suggests that there may be settlement and recruitment of Ophiothrix lineata, 0. angulata, and possibly even 0. suensoni on the outside of Callyspongia vaginalis. Subsequently, larger specimens may take residence inside the sponge. However, it is also possible that competition or other negative interactions between size classes (or species), or the activities of predators results in the observed distributions. Since Ophiothrix lineata is a detritus feeder its circadian cycle is likely not a function of the periodical availability of its food, as detritus is generally in constant supply. Rather, the circadian feeding pattern detected with elapsedtime cinematography is probably related to the damage inflicted by predators on the larger specimens of 0. lineatu. The results of several studies on echinoderms suggest that nocturnal activity is a predator avoidance tactic (references in NELSON& VANCE,1979). NELSON& VANCE(1979) demonstrated experimentally that the sea urchin, Centrostephanus coronatus (VERRILL) , which is normally active only at night, is preyed upon by fish when exposed in daytime experiments. The results of the predation experiments using Ophiothrix lineata were analogous. The greater abundance of diurnal than nocturnal predators shown in the elapsed-time film suggest that the brittlestar’s circadian rhythm is an adaptation for predator avoidance. Interestingly, the Mediterranean species, Ophiothrix p i n quernaculata, sometimes a facultative associate on sponges and sometimes 22 HENDLER found in dense monospecific aggregations, maintains its feeding posture continuously rather than hiding during the day. Individuals of 0. quinquemaculata only shift position in response to changing current or to direct disturbance by & MACHAN, 1979). The sort of circadian predatory benthic invertebrates (FEDRA behavior shown by 0. lineata on Callyspongia vaginalis would be nonadaptive for 0. quinquemaculata because 0. quinquemaculafa does not associatto with a permanent host which provides a refuge from predation. A problem with this interpretation of the nocturnal activity pattern of Ophiotlzi-ix lineata (as an escape from predation) is that certain fish wh:,ch prey on Caribbean Ophiothrh spp. are active at night as well as during the day. For example, the nocturnal porkfish (Anisofrernus virginicus) preys on brittlestars (RANDALL, 1967). STACHOWITSCH (1979) found that in the North Adriatic Sea, the brittlestar-eating hermit crab, Paguristes oculatus, is more active during daylight hours than after dark. However, brittlestar-eating pagurids in the Caribbean, such as Petrochirus diogenes L., feed nocturnally (unpubl. obs.). These inconsistencies must be weighed against the observation that the diurnal wrasses were the most important group of brittlestar-predators reported by RANDALL (1967) and were the dominant predators in the predation experiments discussed in the present paper. Evidently, the diurnal crypsis of Ophiothrix lineata enhances the survival of the species. 3. Sponges as a source of food WARNER (1982, p. 168) wrote that “All members of the genus Ophiothrix so far studied - 0. quinquemaculata, 0. spiculata, 0. angulata, and 0. fragiiis - are specialized suspension feeders”. For example, Ophiothrix fragilis (ABILDGAARD) raises its arms in moderate currents and unselectively ingests particles of suspended material that adhere to the tube feet and spines of its arms (WARNER & WOODLEY, 1975). In fact, the elevation on sponges of the closely related Ophiothrix quinquemaculata has been estimated to provide the filter-feeding brittlestar a threefold increase in availability of suspended food particles (FEDRA, 1977). The feeding mode of Ophiothrix lineata differs fundamentally from that of its congeners. The combined observation of arm movements of Ophiothrix lineata on the surface of Callyspongia vaginalis, and their ingestion of particles (i. e., brine-shrimp eggs) on the surface of the sponge, indicate that 0. lineata is a deposit feeder. This conclusion is also affirmed by the similar nature and the size distribution of particles on the surface of the sponge and in the stomachs of the brittlestars. In addition, 0. lineata maintains its arms in contact with the sponge, whereas filter-feeding brittlestars characteristically project their arms into the & TAYLOR (1977) were water above their substratum. Apparently, KISSLING mistaken in supposing that 0. lineata (because of its arm morphology) filterfeeds. In the association between Callyspongia vaginalis and Ophiothrix finesrta, the sponge provides the brittlestar with detrital material and a suitable surface from which to feed. The brittlestar ingests, for the most part, unidentifiable particles of debris, although several taxa of animals and plants are observed in the Brittlestar-sponge symbiosis 23 brittlestar stomach contents. The observations of the brittlestar’s pattern of activity, the contrasting proportion of animals feeding during day and night, and the fluctuations in the volume of stomach contents, all indicate that feeding is primarily a nocturnal behavior. T h e dependence of Ophiothrix lineata on Callyspongia vaginalis for food is clear evidence for a commensal relationship. Ophiothrix lineata could be regarded as a parasite if its food were of value to the sponge, but several lines of evidence indicate that brittlestars do not collect material which can be utilized by sponges. For example, Ophiothrix fragifis responded to both phytoplankton and 60-90 p m particles of radioactively labelled detritus, but under the same experimental regime, three species of sponges showed relatively low uptake of labelled detritus (BEVISS-CHALLINOR & FIELD,1982). Brittlerstars are clearly capable of feeding on particles larger than those readily accessible to digestion by sponges (HENDLER, 1982 b). Brittlestar digestion is extracellular, mediated by enzymes secreted into the lumen of the stomach. Particles, reduced to suitable size by extracellular digestion, are absorbed through the stomach lining by pinocytosis (PENTREATH, 1969; & JANGOUX, 1978). On the other hand, detrital particles DESCHUYTENEER accounted for only about 3 % of the microscopically resolvable, particulate, organic biomass available as food to sponges (REISWIG,1971a). Seemingly. much of the material ingested by brittlestars is detritus, unavailable to sponges as food. The incurrent flow of dernosponges traverses several filter systems including pores in the dermal membrane, inhalent canals, prosopyles, and choanocyte collars (references in REISWIG, 1971 a). The size of the opening in these filters restricts the upper size limit for particles taken by the sponge. In Calfyspongia diffusa (RIDLEY), incurrent pores in the dermal membrane are 50pm, and major incurrent canals from subdermal spaces open into lacunae around choanocyte chambers via 25 p m apertures. The prosopyles are 1p m triangular spaces between choanocyte cell bodies. The size of the pores in the dermal membrane dictates an upper limit of 5 0 p m for particles entering the sponge and particles between 1 and 5 0 p m in size must be phagocytized outside the choanocyte & HILDEMANN, 1982). The parameters for C. vnginnfis are chambers (JOHNSTON probably similar to those for C. diffusa (RUTZLER,pers. comm). REISWIG(1971 a) found that most of the food of sponges was small enough to be phagocytized by the choanocytes. He characterized 95 % of the total plankton collected by sponges as bacteria, unarmored cells, armored cells (fungi, diatoms, etc.), and detritus. However, such plankters (2.5 to 13.5 p m diameter) were an insignificant portion of the sponge diet. Almost all of the sponge’s dietary, particulate organic carbon was composed of particles less than 1.5 pm. The microscopically unresolveable, particulate organic carbon component “provides by far the major carbon source” (REISWIG, 1971 a, p. 582; REISWIC,1974). Although Ophiothrix lineata ingested particles of a size theoretically suitable as food particles for Callyspongia vaginalis, the majority of the particles utilized by the sponge for food could not even be measured with the techniques employed to examine the brittlestar diet. Therefore, the question of brittlestars as parasites of sponges is, at present, unresolved. Although sponge spicules were found in the brittlestar stomach contents, they also were found in the 24 IHENDLER siphon samples from the surface of the sponge and can be assumed to be part of the sediment. Identifiable pieces of sponge tissue were not found in the brittlestar stomach contents, indicating that 0. lineata does not prey on C. vaginalis. 4. Speculation regarding a cleaning symbiosis The feeding activities of Ophiothrix lineatn may benefit Callyspongia vnginnlis. As the brittlestars feed, the incurrent filtering surface of the sponge is cleaned, and this may promote greater pumping efficiency. There is no question that pumping efficiency is of primary importance to sponges. Vital activities of sponges such as feeding, respiration, and reproduction all depend on unob& FLECHSIG, 1979). Sponges survive in structed flow of water (GERRODETTE plankton-poor tropical waters by virtue of their remarkably efficient filterfeeding activities. Thus, they cannot afford to reduce their pumping rates for extended periods of time (REISWIG,1971a, b; 1974). The amount of sediment in suspension can limit distribution of :,ponges (BAKUS,1968). Some demosponges are excluded from low-turbulence environments because they cannot maintain the sediment-free incurrent surfaces necessary for adequate pumping rates (REISWIG,1971a, 1974). For instance, the is more sensitive to suspended sediments sponge Verongia lacunosa (LAMARCK) & FLECHthan any other filter-feeding organism previously tested (GERRODETTE SIG, 1979). Sponges are not without means of cleansing themselves. REISWIG( 1971a) indicated that amoebocytes in the inhalent aquiferous system, capable of capturing 2-5 pm particles, must operate continuously to prevent occlusion of the aquiferous system by suspended particles. Sponges also employ reorganization of subdermal canals and sloughing of particles trapped in sponge mucus for self-cleansing (REISWIG,1971a). Callyspongia vaginalis commonly occurs in sandy lagoons (RAmALL & HARTMANN, 1968). In such habitats it would likely be exposed to relatively heavy sedimentation. Not all specimens of C. vuginalis at the study site had associated brittlestars. Thus, a continuous association with Ophiothrix lineata is not necessary for the survival of the sponge. However, the effectiveness of the brittlestar in removing sediment coating the incurrent surface of the sponge has been amply demonstrated. This is consistent with the hypothesis that the sponge benefits from the activities of the brittlestar. Whether the interaction between Ophiothrix lineata and Callysporigia vaginalis (or other brittlestars and sponges) is truly mutualistic remains to be determined. It may be a facultative relationship (of mutual benefit) rather than obligate mutualism (where both partners are dependent on each other). It would be valuable to evaluate directly the pumping rate of Callyspongia vaginalis with and without associated Ophiothrix lineata. However, LEWIS (1982) has shown that the pumping behavior of C. vaginalis is difficult to record. Certainly, reliable information on the pumping behavior and growth rates of sponges with and without associated brittlestars will be of the utmost inlerest in determining the true nature of sponge-brittlestar associations. Brittlestar-sponge symbiosis 25 Summary 1. Large specimens of Ophiothrix Zineata are concealed in the tubes of CaZlyspongiu vaginafis during the day. At night the brittlestars sweep their arms across the outer surface of the sponge as they feed, simultaneously cleaning the sponge’s inhalent surface. 2. Ophiothrix lineata unselectively ingests detritus (and occassionally, microscopic organisms). Most of these particles are larger than the microscopically unresolvable material utilized as food by sponges. 3. Diurnal fishes such as wrasses, parrotfish and porgies attack 0. Zineata placed outside C. vaginalis, but not those dwelling inside C. vaginalis. Predation on brittlestars was not detected at night. Apparently, the nocturnal activity of the brittlestars is a predator avoidance strategy. 4. It is hypothesized that the brittlestar-sponge relationship is mutualistic. Callyspongia vaginafis provides food and a refuge from predation for 0. lineata, and it is suggested that the pumping efficiency of the sponge may be enhanced by the feedingkleaning activity of the brittlestar. Acknowledgements The analysis of behavior using elapsed-time cinematography was possible with the generous loan of camera and strobe equipment by its inventor, Dr. HAROLD EDGERTON. Mrs. BARBARA LIITMANwas invaluable as a dive buddy, collector, keen observer, and skilled technical assistant. Mrs. LirrMAN graciously provided Fig. 2 and Dr. GEORGE MILLER contributed Fig. 3 for the color plate in this paper. Mr. KJELLSANDVED advised and assisted with cinematography. Smithsonian voluntcers, Mr. ROBERT ADAMS,Mrs. RUTHBLEYLER, and Mr. GEORGE GO patiently analysed clapsed-time films for brittlestar activity patterns and sponge clearance rates. Mrs. BLEYLER and another volunteer, Mrs. F ~ HELLER, Y measured ophiuroids for size and collected data on regeneration. Mrs. SANDY TRAVIS volunteered to measure camera-lucida tracings of microscopic particles. Dr. LEE-ANNHAYEKand Mr. JIM CRAIG provided advice on, and assistance with, statistical analyses. I availed myself of Dr. KLAUSRUTZLER’S knowledge of sponge biology, and he and Drs. DAVIDPAWSON and PORTER KIER, and Mr. JOHNMILLERprovided helpful criticism which improved the manuscript. To all these colleagues, and many helpful friends in Belize, I am indebted and most grateful. Funding for this study was provided through the E. R. FENNIMORE JOHNSON Fund of the Smithsonian Institution, by the Secretary’s Fluid Research Fund (Smithsonian Institution), and from an Exxon grant to Dr. KLAUSR ~ ~ Z L EContribution R. No. 130, Investigations of Marine Shallow-Water Ecosystems Program Reef and Mangrove Study-Belize. References BACESCU, M., 1971: Les spongiaires; un des plus intbressants biotopes benthiques marins. Rapp. Comm. Int. Mer Mtdit., 20: 239-241. BAKUS,G. J., 1968: Sedimentation and benthic invertebrates of Fanning Island, Central Pacific. Mar. Geol., 6: 45-51. - - & M. A . THUN,1979: Bioassays on the toxicity of Caribbean sponges. In: C. LEVI& N. BOURYESNAULT (Eds.), Biologie des Spongiaires. Colloques internationaux du C. N.R. S., 291: 417422. BEEBE,W. & J. TEE-VAN,1928: The fishes of Port-au-Prince Bay, Haiti with a summary of the known species of marine fish of the island of Haiti and Santo Domingo. Zoologica, 10: 1-279. BEVISS-CHALLINOR, M. H. & J. G . FIELD, 1982: Analysis of a benthic community food web using isotopically labelled potential food. Mar. EcoI. Prog. Ser., 9: 223-230. 26 HENDLER CLARK,H. L., 1933: A handbook of the littoral echinoderms of Porto Rico and the other West Indian islands. Scient. Surv. Porto Rico and Virgin Is. N. Y. Acad. Sci., 16: 1-147. CLARK,K.B., 1971: The construction of a collecting device for small aquatic organi:;ms and a method for rapid weighing of small invertebrates. Veliger, 13: 364-367. DESCHUYTENEER, M. & M. JANGOUX, 1978: Comportement alimentaire et structures digestives d’ophioderma longicauda (RETZIUS)(Echinodermafa, Ophiuroidea). Ann. Inst. Octanogr ., Paris, 5 4 127-138. DEVANEY, D. M., 1974: Shallow-water echinoderms from British Honduras, with a description of a new species of Ophiocoma (Ophiuroidea). Bull. Mar. Sci., 24: 122-164. EDGERTON, H. E., V. E. MACROBERTS & K. R. H. READ,1968: An elapsed-time photographic (Eds.), Proceedings of the 8th system fut underwater use. In: N. R. NIUSON& L. HOGBERG International Congress on High-speed Photography, Stockholm. Wiley, New York: 488-491. FEDDERN, H . A . , 1965: The spawning, growth, and general behavior of the bluehead wrasse, Thalassoma bifasciafum (Pisces: Labridae). Bull. Mar. Sci., 15: 896941. FEDRA,K.,1977: Structural features of a North Adriatic benthic community. In: B. F. KEEGAN, & P. J. S. BOADEN (Eds.), Biology of benthic organisms. 11th European marine P. 0. CEIDIGH biology symposium, Galway, October 1976. Pergamon Press, Oxford: 233-246. - - & R. MACHAN, 1979: A self-contained underwater time-lapse cnmera for in siiu long-term observations. Mar. Biol., 55: 239-246. - -, E. M. OLSCHER, C. SCHERUBEL, M. STACHOWITSCH & R. S. WURZIAN, 1976: On the ecology of a North Adriatic benthic community: Distribution, standing crop and composition of the macrobenthos. Mar. Biol., 38: 129-145. GERRODETTE, T. & A. 0. FLECHSIG, 1979: Sediment-induced reduction in the pumping rate of the tropical sponge Verongia lacunosa. Mar. Biol., 55: 103-1 10. HALSTEAD. B. W., 1965: Poisonous and Venomous Marine Animals of the World. Vol. [. Invertebrates. U. S. Govt. Printing Off., Washington, (D. C.). HENDLER, G., 1982 a: Slow flicks show star tricks: Elapsed-time analysis of basketstar (A:;frophyfon muricatum) feeding behavior. Bull. Mar. Sci., 32: 909-918. - -, 1982 b: The feeding biology of Ophioderma hrevispinum (Ophiuroidea: Echinodermata). In: J . M. LAWRENCE (Ed.), Echinoderms. Proceedings of the International Echinoderms Conference, Tampa Bay. Balkema, Rotterdam: 21-27. HYMAN, L., 1955: The Invertebrates. Vol. 4. Echinodermutu. McGraw-Hill, New York. JOHNSTON,I. S. & W. H. HILDEMANN, 1982: Cellular organization in the marine demosponge Callyspongia dqfusa. Mar. Biol., 67: 1-7. KISSLING, D . L . & G.T.TAYLOR, 1977: Habitat factors for reef-dwelling ophiuroids in the Florida Keys. Proc. Third Int. Coral Reef Symp., 1: 225-231. LEWIS,S. M., 1982: Sponge-zoanthid associations: Functional interactions. In: K. R ~ ~ ~ Z&L I.E GR. MACINYYRE (Eds.), The Atlantic Barrier Reef Ecosystem at Carrie Bow Cay, Belize, I: Structure and Communities. Smithson. Contrib. Mar. Sci., 12: 465-474. 1949: Natural History of Marine Animals. McGraw-Hill, New MACGINlnE, G. E. & N. MACGINITIE, York. MURPHY, G., 1980: The omnipresent brittle star. Skin Diver, 29: 54-55. NELSON,B.V. & R. R. VANCE,1979: Die1 foraging patterns of the sea urchin Cenfrostephanus coronatw as a predator avoidance strategy. Mar. Biol., 51: 251-258. PEARSE,A. S., 19.50: Notes on the inhabitants of certain sponges at Bimini. Ecology, 31: 149-151. PENTWEATH, R. J., 1969: The morphology of the gut and a qualitative review of digestive enzymes in some New Zealand ophiuroids. J. Zool., Lond., 159: 413-423. RANDALL, J . E . , 1967: Food habits of reef fishes of the West Indies. Stud. Trop. Oceanogr., 5: 665-847. - - & W. D . HARTMANN, 1968: Sponge-feeding fishes of the West Indies. Mar. Biol., 1: 216225. REISWIG, H. M., 1971 a: Particle feeding in natural populations of three marine demosponges. Biol. Bull., 141: 568-591. - -, 1971 b: In situ pumping activities of tropical Demospongiae. Mar. Biol., 9: 38-50. - -, 1974: Water transport, respiration and energetics of three tropical marine sponges. J. Exp. Mar. Biol. Ecol., 14: 231-249. RUTZLER,K., 1975: Ecology of Tunisian commercial sponges. Tethys, 7: 249-264. - - & I. G. MACINIYRE,1982: The habitat distribution and community structure of the Barrier Reef complex at Carrie Bow Cay, Belize. In: K. RUTZLER& I . G . MACINNRE(Eds.), The Brittlestar-sponge symbiosis 27 Atlantic Barrier Reef Ecosystem at Carrie Bow Cay, Belize, I: Structure and Communities. Smithson. Contrib. Mar. Sci., 12: 9-45, SEFTON, N., 1982: The stars at night. Sea Frontiers, 28: 87-92. SHIRLEY, T. C., 1982: The importance of echinoderms in the diet of fishes of a sublittoral rock reef. In: B. R. CHAPMANN & J. W. TUNNELL (Eds.), South Texas Fauna. Caesar Kleberg Wildlife Research Institute: 49-55. STACHOWITSCH, M., 1979: Movement, activity pattern, and role of a hermit crab population in a sublittoral epifauna community. J. Exp. Mar. Biol. Ecol., 3% 135-150. - -, 1980: The epibiotic and endolithic species associated with gastropod shells inhabited by the hermit crabs Paguristes oculafus and Pagurus cuanensis. P. S. Z . N. I: ‘Marine Ecology, 1: 73101. VASSEROT, J., 1965: Un predateur d’echinodermes s’attaquant particulitrement aux ophiures: La langouste Palinurus vulgaris. Bull. SOC.Zool. Fr., 90: 365-384. WARNER, G., 1982: Food and feeding mechanisms: Ophiuroidea. In: M. JANCOUX & J. M. LAWRENCE (Eds.), Echinoderm Nutrition. Balkema, Rotterdam: 161-181. - - & J . D. WOODLEY, 1975: Suspension-feeding in the brittle-star Ophiofhrixfragilis.J . Mar. Biol. Assoc. U. K., 55: 199-210. WESTINGA, E. s( P. C. HOETIES,1981: The intrasponge fauna of Spheciospongio vesparia (Porifrra, Demospongiae) at Curacao and Bonaire. Mar. Biol., 6 2 139-150. WURZIAN, R. S . , 1977: Predator-prey interaction between the crab Pilronnus hirtellrls (LEACH) and the brittlestar Ophiorhrix yriinquemucnlata (D. CHINE)on a mutual sponge substrate. In: B. F. KEEGAN, P. O’CEIDIGH & P. J . S. BOADEN (Eds.), Biology of Benthic Organisms. Proc. 11th Europ. Mar. Biol. Symp., Galway, October 1976. Pergamon Press, Oxford: 613-620.