medpor contain
Transcription
medpor contain
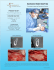
MEDPOR CONTAIN Space Maintenance for Bone Graft Material MEDPOR CONTAIN Illustrative Guide MEDPOR CONTAIN Sheet MEDPOR CONTAIN Implants may be used in conjunction with bone grafting and bone filling materials to assist in “containing” the materials by providing space maintenance necessary for regenerative healing. The implant may be shaped by cutting or trimming to fit a patient's specific needs. Bone Graft Bone The MEDPOR CONTAIN Implant is subperiosteally draped over the area of missing bone and tacked or sutured into place with the bone graft material incased within it. Because of the white color of the biomaterial it typically does not show through overlying tissue. Tacks Graft showing tissue integration of the implant. The MEDPOR CONTAIN Implant should be completely covered with healthy, well vascularized tissue. MEDPOR Biomaterial allows for tissue ingrowth because of its interconnecting open pore structure. MEDPOR CONTAIN Implants are radiolucent and will not interfere with radiographic assessment of the bone graft material. When the reconstructed alveolar ridge bone achieves maturation, remove the MEDPOR CONTAIN Implant approximately 2-3mm beyond the margins of the dental implant placement area, both lingual and labial sides (or on the facial and palatal sides). The remaining margins of the MEDPOR CONTAIN Implant may be left in place in the subperiosteal space, so as not to disturb the fibrovascular integration and periosteal blood supply to the underlying bone. MEDPOR CONTAIN The MEDPOR CONTAIN Implant is designed to create and maintain space in the alveolar ridge when bone graft material is used to re-establish new bone for support of dental implants. The MEDPOR CONTAIN Implant is made of porous polyethylene and is intended to stabilize, support and provide space maintenance for bone graft materials, in the maxilla, mandible and zygoma. The MEDPOR CONTAIN Implant is designed to create and maintain space in the alveolar ridge when bone graft material is used to re-establish new bone for support of dental implants. MEDPOR is a biocompatible, porous polyethylene material. The interconnecting, omnidirectional pore structure allows for fibrovascular in-growth and integration of the patient's tissue.1. More than 250,000 procedures have been performed with porous polyethylene and more than 350 clinical reports in cranial, reconstructive, oculoplastic and cosmetic applications have been published. MEDPOR CONTAIN is an ultra-thin MEDPOR Sheet designed specifically for use in dental procedures requiring bone graft containment. 2. Features • Biocompatible MEDPOR has 20 years of proven use in cranial-maxillofacial procedures • MEDPOR Biomaterial may be bent and cut to fit to individual patient contours • MEDPOR CONTAIN is fixated with tacks, screws or sutures • Semi-rigid material may provide space maintenance necessary for regenerative healing • Because of the white color of the biomaterial, it typically does not show through overlying tissue • During dental implant placement, noncrestal portions of the CONTAIN Implant can remain in place • Permits soft tissue integration Tissue Ingrowth MEDPOR Biomaterial may allow for tissue ingrowth because of its interconnecting open pore structure.1. Animal data has demonstrated that MEDPOR Surgical Implant material permits tissue ingrowth. Histologic analyses of biopsies from human implants have also demonstrated tissue ingrowth.1. M M M M M A cross-section of the porous CONTAIN Implant Sheet shows the MEDPOR CONTAIN material (M) visible within the spaces, pulled away from tissues due to histologic artifact/processing. Hypercellular fibro-collagenous tissue is adjacent to the CONTAIN Implant Sheet surface and within its pores. A closer view shows tissue integrated within a pore of the CONTAIN Implant Sheet. MEDPOR CONTAIN CAT# DESCRIPTION A B C 81060 81061 81062 81063 81064 81065 81066 81067 81068 CONTAIN CONTAIN CONTAIN CONTAIN CONTAIN CONTAIN CONTAIN CONTAIN CONTAIN 15 25 35 15 25 35 15 25 35 30 30 45 30 30 45 30 30 45 0.25 0.25 0.25 0.35 0.35 0.35 0.45 0.45 0.45 C A B MEDPOR CONTAIN (0.25mm Sheet) Thickness shown at 400% 15mm x 30mm Catalog # 81060 25mm x 30mm Catalog # 81061 35mm x 45mm Catalog # 81062 MEDPOR CONTAIN (0.35mm Sheet) Thickness shown at 400% 15mm x 30mm Catalog # 81063 25mm x 30mm Catalog # 81064 35mm x 45mm Catalog # 81065 MEDPOR CONTAIN (0.45mm Sheet) Thickness shown at 400% 15mm x 30mm Catalog # 81066 Illustrations are not actual size. Please consult dimensional descriptions. 25mm x 30mm Catalog # 81067 35mm x 45mm Catalog # 81068 A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery. The information presented is intended to demonstrate the breadth of Stryker product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area. Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Medpor, Medpor Contain, Stryker. All other trademarks are trademarks of their respective owners or holders. Literature Number: 9410-400-197 Rev. None UnDe/P.S. Copyright © 2010 Stryker Printed in USA 1. Liu JK, Gotfried ON, Cole CD, Dougherty, WR, Couldwell WT, "MEDPOR Porous Polyethylene Implant for Cranioplasty and Skull Base Reconstruction" Neurosurgery [April 2004] 2. Spagnoli D, Misiek D, "A New Option for Stabilizing and Supporting Bone Graft Materials" [2010] Stryker Craniomaxillofacial Kalamazoo, MI 49002 USA t: 269 324 5346 toll free: 800 962 6558 f: 877 648 7114 www.stryker.com