Invasive molluscs in Umbrian inland waters: ecological and
Transcription
Invasive molluscs in Umbrian inland waters: ecological and
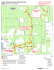
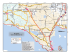
Studi Trent. Sci. Nat., 86 (2009): 99-104 ISSN 2035-7699 99 © Museo Tridentino di Scienze Naturali, Trento 2009 Atti XVIII Convegno Gadio 2008: Un mondo che cambia: successioni ecologiche, invasioni biologiche ed alterazioni antropiche Sessione 5 - Comunicazione orale: Invasioni biologiche Invasive molluscs in Umbrian inland waters: ecological and reproductive aspects Elda Gaino*, Francesca Scoccia, Tisza Lancioni & Alessandro Ludovisi Department of Cellular and and Enviromental Biology, Università degli Studi di Perugia, Via Elce di Sotto, 06123 Perugia, Italy * Corresponding author e-mail: [email protected] SUMMARY - Invasive molluscs in Umbrian inland waters: ecological and reproductive aspects - The increasing colonisation by aquatic invasive molluscs has only recently received scientific attention in Umbria, notwithstanding the possible great impact on the functionality and biodiversity of the freshwater ecosystems. The present contribution highlights the results of investigations carried out on Dreissena polymorpha (Bivalvia) in Lake Trasimeno and on Potamopyrgus antipodarum (Gastropoda) in water courses of the Tiber River basin. This in view to evaluate the impact of habitat type on colonisation and reproduction. Dreissena polymorpha showed a marked selectivity for natural and artificial hard substrates. This preference gives inception to competition with the sponge Ephydatia fluviatilis (Porifera), which overgrow the mollusc, thereby encapsulating its valves. The study of the life cycle showed a tight link between gonadal development and water temperature, the high values of which limit larval development and species dispersal. Analyses on parthenogenetic populations belonging to P. antipodarum showed a different fecundity in relation with habitat type, and the marked selectivity towards various biological substrates (macrophytes, decaying organic materials, periphyton, etc.). The scarce availability of these components together with high current speed, limit the colonisation ability of this species. RIASSUNTO - Molluschi invasivi nelle acque interne dell’Umbria - La crescente colonizzazione da parte di molluschi acquatici invasivi ha ricevuto solo recentemente attenzione scientifica in ambito umbro, nonostante il loro elevato impatto potenziale sul funzionamento e la biodiversità degli ecosistemi d’acqua dolce. Il presente contributo illustra i risultati delle ricerche effettuate su Dreissena polymorpha (Bivalvia) nel Lago Trasimeno e su Potamopyrgus antipodarum (Gastropoda) in corsi d’acqua del bacino del Fiume Tevere. Ciò al fine di valutare l’incidenza delle caratteristiche dell’habitat sulla colonizzazione e riproduzione di queste specie. Dreissena polymorpha mostra una elevata selettività per substrati duri naturali e artificiali. Questa preferenza innesca la competizione con la spugna Ephydatia fluviatilis (Porifera), la quale, potendosi sviluppare sul mollusco, ne determina l’occlusione delle valve. Dall’analisi del ciclo biologico è emersa la stretta dipendenza dello sviluppo delle gonadi dalla temperatura dell’acqua, i cui valori elevati limitano lo sviluppo larvale e la dispersione della specie. L’indagine su popolazioni partenogenetiche di P. antipodarum ha messo in luce la diversa fecondità in relazione al tipo di habitat e la spiccata selettività verso substrati biologici di varia natura (macrofite, residui vegetali deiscenti, periphyton, etc.). La scarsa disponibilità di queste componenti, unitamente ad elevate velocità di corrente, limitano le capacità di colonizzazione della specie. Key words: Dreissena polymorpha, Potamopyrgus antipodarum, habitat selection, reproduction, Lake Trasimeno, Tiber River basin Parole chiave: Dreissena polymorpha, Potamopyrgus antipodarum, selezione di habitat, riproduzione, Lago Trasimeno, bacino Fiume Tevere 1. IntroduCTION There is compelling evidence that, in the past few decades, the human-mediated introduction of non-indigenous species (NIS) is the main driver of biodiversity change in freshwater and terrestrial ecosystems (Sala et al. 2000). The effects on global biodiversity are expected to increase quickly over time in both extent and intensity, in concert with anthropogenic alterations to the environment, such as climate and land use changes. Biological invasions are favoured in disturbed ecosystems and communities, because disturbance opens new niches for the most adaptable invaders (Ross et al. 2001), whose success depends on a close match between their physiological requirements and the environmental characteristics of the system being invaded (Gherardi 2007). A recent list of NIS occurring in Italian inland waters and their spread during the past century is reported by Gherardi et al. (2008). Among the 112 animal species identified, molluscs are represented by 11 species (7 Gastropoda and 4 Bivalvia), two of which - Corbicula fluminea (Müller, 1774) and Dreissena polymorpha (Pallas, 1771) - are also included among the 100 worst invasive species of Europe (DAISIE Project: http://www.europe-aliens.org/ speciesTheWorst.do). Most non-indigenous mollusc species were introduced into Italy in the second half of the 20th century, as a result of the development of commercial traffic (Cianfanelli et al. 2007). Nevertheless, in spite of the wide literature produced, a limited number of researches have focused on the ecology of molluscs in the “alien” environment being invaded. The success of a freshwater invader or the prediction of the in- 100 Gaino et al. Invasive aquatic molluscs in Umbria Dreissena polymorpha 1. lower Mussino Stream 2. upper Tiber River 3. upper Topino River Lake Trasimeno soft sediments hard substrata Potamopyrgus antipodarum Fig. 1 - Map showing the location of sampling sites of invasive molluscs in Lake Trasimeno and in the Tiber River basin in Umbria. Fig. 1 - Siti di campionamento dei molluschi invasivi nel Lago Trasimeno e nel bacino del Fiume Tevere. vasibility of an aquatic system depend on the knowledge of the characteristics of the invader and the specific system being invaded (Moyle & Light 1996). In agreement with Simberloff (2004), in order to acquire the necessary confidence with the invasion process, we need a large catalogue of case studies at local scale. In particular, the knowledge of habitat selectivity, diffusion strategies and specific demographic parameters is essential for making prediction on the spreading and establishment of an invasive species. In this regard, we investigated the reproduction and ecology in populations of the Ponto-Caspian Dreissena polymorpha in a lentic environment (Lake Trasimeno, Italy) and of the New Zealand mudsnail Potamopyrgus antipodarum (Gray, 1843) in running-water systems, belonging to the Tiber river basin. 2. STUDY SITES AND SAMPLING The first record of D. polymorpha in Lake Trasimeno (Spilinga et al. 2000) reports sporadic findings along northern and eastern shores and also around the main is- lands of the lake. This record was followed by an extensive investigation performed by Lancioni and Gaino (2006) on twelve sampling sites located along the shore of the lake (Fig. 1). In each sampling site, a 5m-long transect along the shore was inspected monthly from June 2003 to May 2004 and the abundance of D. polymorpha was recorded together with the type of substrate. On soft sediments, sampling of the mollusc was carried out by means of a Surber net. In addition, presence/absence data of the freshwater sponge Ephydatia fluviatilis (Linnaeus, 1758), often found to coexist with the bivalve, was annotated. In each sampling, 10 bivalves > 8-mm-long (underwater measurement) were randomly collected, measured and then transferred in aerated tanks to the laboratory. The substrate selectivity was evaluated by Pearson’s Chi-squared test of independence by comparing the aggregated data of presence on the various substrates, with the overall frequency of substrates encountered during sampling (Lancioni & Gaino 2007). The similarity of substrate selection between D. polymorpha and E. fluviatilis was also statistically tested by a Chi-squared test. Although the presence of P. antipodarum is known in Italy since 1961 (Berner 1963) and it is fairly widespread Studi Trent. Sci. Nat., 86 (2009): 99-104 in Italian inland waters, only one case was reported for the Umbrian region up to 2007 (Bodon et al. 2005), when it was repeatedly observed within the Tiber River basin, during water quality monitoring surveys. An investigation performed in the sites of recording, evaluated the state of the populations and their potentiality of spreading in the basin in relation with the type of habitat (Gaino et al. 2008). Observations were carried out in three sites showing different environmental features (Fig. 1): 1) lower Mussino Stream (depth: 15 cm; width: 2 m; current speed: ~ 0.1 m s-1; October 2006); 2) upper Tiber River (depth: 25 cm; width: 14 m; current speed: ~ 0.3 m s-1; May 2007); 3) upper Topino River (depth: 20 cm; width: 9 m; current speed: ~ 0.6 m s-1; June 2007). At each sampling site, a 25m-long section of the water course was inspected and the abundance of P. antipodarum was recorded, together with the type of substrate. In order to estimate the density, sampling was carried out by a Surber net (surface area: 100 cm2) and the counting was performed in situ. The collected specimens were then transferred to laboratory in aerated containers for further investigations. The selectivity of the species with respect to the type of substrate was evaluated by Pearson’s Chi-squared test of independence, by comparing the total abundance observed on the various substrates, with the total area of the different substrates encountered during sampling. 3. LABORATORY ANALYSES The gonadal maturation in D. polymorpha was assessed on the basis of the shape of the gonadal cells according to the method suggested by Gist et al. (1997). Morphological observations and measurements were carried out by means of an optical microscope (Leica DMLB), and a photographic archive was created by means of a Leica DC 300F digital camera. Sex attribution in P. antipodarum was performed under Leica MZ6 stereomicroscope (Leica LMS Holdings GmbH, Wetzlar, Germany), equipped with optic fibres. Specimens were anesthetised by adding some drops of chloral hydrate to prevent animal suffering, and then measured from the apex to the inferior rim of the shell by dividing the whole size range into 6 (1mm-distanced) classes. Females were dissected and the total embryos brooded inside each female were counted. 4. RESULTS In Lake Trasimeno, D. polymorpha’s shells are highly polymorphic for their colour, pattern and shape. Shell length varies from less than 1 mm to a maximum of 38 mm. While in the southern areas of the lake D. polymorpha was only sporadic, huge numbers were found in the western sites 9 and 10, in the eastern sites 1 and 2 and in the northern site 5 (Fig. 1). In Lake Trasimeno, D. polymorpha was found to be attached to various hard substrates, varying from concrete landing stages (maximum density ~ 200000 ind. m-2) to rocks (from 114 to 140000 ind. m-2) and artificial substrata (tires, tiles, plastic buoys, pumps and boat keels). The submerged portion of Phragmites australis reed stalks are also colonised (maximum density 2036 ind. 101 m-2), whereas no individuals were observed on silt and only gathered in clumps on sandy substrates. Clumps consist of mixed mussel populations, where the individuals adhere to one another or attach to sand-grain sediments and filamentous algae by means of their byssal threads. The preference of D. polymorpha for hard substrates, with particular reference to concrete, pebbles and rocks, has been confirmed by Chi-squared test to be significant (p= 0.002). Gonadal development of females and males coincide temporally, and is linked to a rapid increase in water temperature (from 4.3°C to 13.4°C in spring). In the population, 82% of the zebra mussels are sexually mature with a female:males ratio of 1:1.16 and there is only one period of successful reproduction per year (from April to June). In the areas where sponges and molluscs coexist, the former tend to overgrow the latter up to the shell encapsulation. The growth and size of sponges vary according to the season, reaching the maximum (300 x 240 mm in length; 60 mm in thickness) in warmer months, while during autumn and winter sponges survive as thin encrusting specimens. The comparison between the mollusc and the sponge distribution showed that, although different in presence, they have statistically equivalent preferences (p= 0.66) with respect to the type of substrate. In most cases (93%), D. polymorpha was found in the same portions of the transects investigated where E. fluviatilis was present (sites 2, 5, 10 and 11). The time progression of E. fluviatilis/D. polymorpha interaction was followed in two sampling sites: here, it was observed that the sponge outcompetes the mussels and gradually envelops the shell, thus leading to the mollusc death. In the three investigated populations of P. antipodarum, no males were found so far. The analysis of abundance data showed that the highest global average density is found in Mussino Stream (1600 ind. m-2), the most diversified environment in terms of substrate offer. By contrast, in Topino River, very low densities were found (33 ind. m-2). The results of the Chi-squared statistics showed that the snail is not evenly distributed on the different substrates, but it selects positively biological substrates, although bare rocks and silt are not avoided. In Mussino Stream, beds of the macrophytes Chara sp. and Vaucheria sp. are positively selected, whereas encrusting periphyton (mainly represented by diatoms) is almost disregarded. It is worth stressing that the highest snail density was found on floating leaves and wood. The comparative analysis of the populations showed that they slightly differ in size composition and fecundity, whereas the number of brooded embryos/female remarkably varies among sites (Fig. 2). The size distribution of the investigated populations showed that all the size classes (1-6) are represented, with the classes 4 and 5 being the most abundant. Females become fecund at a size > 2 mm (Mussino Stream and Topino River) or > 3 mm (Tiber River) and the fecundity comes close to 100% for specimens > 4 mm (Fig. 2). Accordingly, the number of carried embryos increases with size, although it is largely variable within and between populations (Fig. 2). In particular, a markedly reduced number of embryos/female is observed in Topino River, where the population density is also very low. It is worth stressing that Topino River is characterised by the absence of aquatic vegetation and by a water speed that slightly overcomes the upper limit reported in literature for the snail (Doby et al. 1966; Roth 1987). 102 Gaino et al. Invasive aquatic molluscs in Umbria $) b) �� �� �� �� � � � �� �� � � � � � � � � � � � � � � � ��&����"�� �� �� �� �� �� �� � �� �� �� � � � � � � � � � ��&����"�� �� ��� # � $ �! � %� � �! Tiber river �� �� ��&����"�� �� �� �� �� � �� �� �� �� �� � � � � � � � � � � ��&����"�� � � � � � � �� �� �� �� �� �� � �� �� �� � � � ��&����"�� ��&����"�� �� ��� # �� � ��� %� � �! Topino river �� � ��� �)c) �� ��� ��� � � ��� � ! �"� Mussino Stream a) ") �� �� �� �� �� �� � �� �� �� � � �� �� �� �� � �� �� �� �� �� � � � � � � � � � � � � ��&����"�� � � ��&����"�� � � � � � � � � ��&����"�� Fig. 2 - Population structure and size-class fecundity in Potamopyrgus antipodarum from the three sites investigated in the Tiber River basin. a, size distribution; b, percentage of embryos-carrying females; c, number of brooded embryos/female (±SD). Star indicates that the size class 6 includes 1 (Topino River) or 2 (Mussino Stream) individuals. Fig. 2 - Struttura per taglia e fecondità per classe di taglia in Potamopyrgus antipodarum nei tre siti investigati nel bacino del fiume Tevere: a, distribuzione degli individui per taglia; b, percentuale di femmine con embrione; c, numero di embrioni per femmina (±SD). L’asterisco indica che la classe di taglia 6 è occupata da 1 (Torrente Topino) o 2 (Torrente Mussino) individui. 5. DISCUSSION Species spreading, substitution and extinction are commonplace in nature, and are among the forces which can trigger species and ecosystem evolution. They occur over the course of geological times, even in association with climate change (Graumlich & Davis 1993). Therefore, changes in the natural distribution of species should not be viewed as an abnormal event (Lodge 1993). However, human actions have greatly increased the temporal rate of species dispersal, producing, in few decades, a cascade of events that could never be accomplished by means of natural events alone (Lodge 1993). This is particularly true for inland freshwater environments, which are circumscribed systems. Based on the number of documented and potential extinctions, it has been evaluated that freshwater fauna can experience an extinction rate that is five times that of the terrestrial environment (Ricciardi & Rasmussen 1998). Humans have contributed to the spread of organ- isms by the increasing mobility of people and good trade among nations and continents. However, it seems acceptable that the increasing traffic by itself could not have caused a so large effect if NIS were introduced in unaltered environments. In fact, ecosystem modification is often coupled with an increased susceptibility to invasion (Ross et al. 2001). As for our study sites, Lake Trasimeno is a fragile shallow-water system, whose ecological state is strictly linked to the hydrological regime. Water regulation practices, together with the meteorological modifications recorded in Central Italy during the last century (Dragoni 1998; Burzigotti et al. 2003), caused a lowering of the water level of about 2 m, corresponding to a reduction of about 30% in volume. Such a change, which is still in progress, has been accompanied by a modification in the water quality, with particular reference to the salt content and water transparency (unpublished data). Also, significant depletion of the Phragmitetum can be envisaged during the last two decades (M.A.R.U., 1994, Cecchetti et Studi Trent. Sci. Nat., 86 (2009): 99-104 al., 2005). Such environmental changes have certainly affected the riparian zoocoenosis of the lake, with particular reference to the sponge population of E. fluviatilis, which is now found just as small patches on rocks and pebbles (Lancioni & Gaino 2007), whereas museum specimens dated 1963 were collected on reed stalks in the riparian belt of the lake. In such a perturbed environment, spatial and trophic niches are subjected to marked changes, thereby allowing adaptable species, such as D. polymorpha, to invade and spread. Ephydatia fluviatilis, whose spatial and trophic niche is similar to that of the bivalve, may represent a “biological barrier” limiting the diffusion of the species in the lake, provided that the sponge population is not occasionally present. Paradoxically, in this changing scenario, the main constraint to the spreading of the bivalve seems to be the water temperature. In fact, it is well known that the larvae of D. polymorpha cannot survive, settle and metamorphose above 29°C, a temperature which is usually reached along the shore of the lake during the summer period. As for P. antipodarum, it has been often detected in disturbed biotopes in Australia and Europe (Léger & Léger 1974; Ponder 1988; Schreiber et al. 2003), even though high polluted areas are generally avoided (Ghetti 1986). The snail tolerates wide ranges of temperature (Berner 1971; Quinn et al. 1994), salinity and trophic conditions (Proctor et al. 2007), and the current speed seems to represent the main limiting factor for its diffusion (Doby et al. 1966; Roth 1987). Though the first record of this species in Italy dates back to 1961, no investigation have been carried out on the ecology and on the effect of ovoviviparous parthenogenesis on the dispersal of the snail in the Italian peninsula. The comparative analysis performed by Gaino et al. (2008) suggested that fecundity, expressed as number of brooded embryos/female, increases, in parallel with the mean density of the population, in habitats rich in aquatic vegetation, where the current speed is low. These results support the hypothesis that run habitats limit the colonisation by P. antipodarum (Richards et al. 2001), and suggest a direct effect of the habitat on the reproduction rate of the snail. In consideration of the fact that Tiber River collects the most part of the Umbrian water courses, the whole region represents a potential area of expansion for P. antipodarum. However, owing to the lack of punctual information about species distribution and ecology at a regional scale, it is difficult to make prediction about the snail’s spreading in Umbria. 6. CONCLUSIONS The spreading of species out from their native region has an impact on autoctonous biocoenosis. However, before taking a stand against non-indigenous species, we need to be aware about the extent and the effects of the invasion at a regional scale, which is the basic level at which the phenomenon should be faced, from both scientific and political perspectives. In this regard, a significant effort has been devoted during the last decades in drawing maps of distribution and pathways of spreading in Italy of the most invasive spe- 103 cies. However, in order to acquire a proper confidence with the invasion phenomenon, an additional effort is needed to gather data on the biology and ecology of the species in the areas where they are recorded. This step is also necessary in order to make predictions on the spreading of nonindigenous species, and to plan conservation policies for safeguarding indigenous biocoenosis. ACKNOWLEDGEMENTS The research was partially financed by FISR M.I.C.E.N.A. References Berner L., 1963 - Sur l’invasion de la France par Potamopyrgus jenkinsi (Smith). Arch. Molluskenkd., 92: 19-29. Berner L., 1971 - La régulation des naissances chez Potamopyrgus jenkinsi (Smith) en function des resources alimentaires. Proc. 93° Congrés National des Sociétés Savantes 1968: 391-397. Bodon M., Cianfanelli F., Manganelli G., Pezzoli E. & Giusti F., 2005 - Checklist e distribuzione della fauna italiana: 79-81. In: Ruffo S. & F. Stoch (eds), Mollusca Gastropoda Prosobranchia ed Heterobranchia. Mem. Verona, 16: 79-81. Burzigotti R., Dragoni W., Evangelisti C. & Gervasi L., 2003 The Role of Lake Trasimeno (central Italy) in the History of Hydrology and Water Management. Proc. IWHA 3rd International Conference, Alexandria (Egypt): 1-18. Cecchetti A., Ficola M., Lazzerini G., Pedini A. & Segantini F., 2005 - Vegetazione, habitat di interesse comunitario, uso del suolo del Parco del Lago Trasimeno. Parco del Lago Trasimeno, 132 pp. Cianfanelli S., Lori E. & Bodon M., 2007 - Non-indigenous freshwater molluscs and their distribution in Italy. In: Gherardi F. (ed.), Biological invaders in inland waters: profiles, distribution and threads. Springer, Dordrecht, The Netherlands: 103-121. Doby J.M., Chobaud A., Mandahal-Barth G., Rault B. & Chevalier H., 1966 - Extension en Corse du mollusque gasteropode Potamopyrgus jenkinsi (Smith, 1889) (Hydrobiidae). B. Mus. Natl. Hist. Nat., 37: 833-843. Dragoni W., 1998 - Some considerations on climatic changes, water resources and water needs in the Italian region south of the 43°N. In: Brown N. & Issar A. (eds.) Water, Environment and Society in Times of Climatic Change, Kluwer: 241-271. Gaino E., Scoccia F., Lancioni T. & Ludovisi A., 2008 - The invader Potamopyrgus antipodarum in the Tiber River basin (Central Italy). It. J. Zool., 75: 253-261. Gherardi F., 2007 - Biological invasions in inland waters: an overview. In: F. Gherardi (ed.), Biological invaders in inland waters: profiles, distribution and threads. Springer, Dordrecht, The Netherlands: 2-25. Gherardi F., Bertolini S., Bodon M., Cesellato S., Ferraguti M., Lori E., Mura G., Nocita A., Riccardi N., Rossetti G., Rota E., Scalera R., Zerunian S. & Tricarico E., 2008 - Animal xenodiversity in Italian inland waters: distribution, modes of arrival and pathways. Biol. Invasions, 10: 435-454. Ghetti P.F., 1986 - Manuale di applicazione. I macroinvertebrati nell’analisi di qualità dei corsi d’acqua. Indice biotico: E.B.I. modif. Ghetti, 1986. Provincia autonoma di Trento, 104 Gaino et al. Stazione Sperimentale Agraria Forestale, Servizio Protezione Ambiente, 111 pp. Gist D. H., Miller M. C. & Brence W. A., 1997 - Annual reproductive cycle of the zebra mussel in the Ohio River: a comparison with Lake Erie. Arch. Hydrobiol., 138: 335-379. Graumlich L.J. & M.B. Davis, 1993 - Holocene variation in spatial scales of vegetation pattern in the upper Great Lakes. Ecology, 74: 826-839. Lancioni T. & Gaino E., 2006 - The invasive zebra mussel Dreissena polymorpha in Lake Trasimeno (Central Italy): Distribution and reproduction. It. J. Zool., 73: 335-346. Lancioni T. & Gaino E., 2007 - The zebra mussel Dreissena polymorpha: reproduction and competition with the sponge Ephydatia fluviatilis. In: Gherardi F. (ed.), Biological invaders in inland waters: profiles, distribution and threats. Springer, Dordrecht, The Netherlands: 597-611. Léger N. & Léger L., 1974 - L’extension de Potamopyrgus jenkinsi (Smith, 1889) en Corse (julliet 1973). Ann. Parasit. Hum. Comp., 49: 343-347. Lodge D.M., 1993 - Biological invasions: lessons for ecology. Trends. Ecol. Evol., 8: 133-137. M.A.R.U., 1994 - Piano di gestione per il Lago Trasimeno finalizzato al controllo dell’eutrofizzazione. Parte IV. Ministero dell’Ambiente e Regine dell’Umbria, 255 pp. Moyle P.B. & Light T., 1996 - Biological Invasions of fresh water: empirical rules and assembly theory. Biol. Conserv., 78: 149-161. Ponder W.F., 1988 - Potamopyrgus antipodarum - A molluscan coloniser of Europe and Australia. J. Mollus. Stud., 54: 271-285. Proctor T., Kerans B., Ryce E., Dybdahl M., Gustafson D., Hall R., Pickett F., Richards D., Waldeck R.D., Chapman J.,. Wiltshire R. H., Becker D., Anderson M., Pitman B., Lassuy D., Heimowitz P., Dwyer P. & Levri E., 2007 - National Mana- Invasive aquatic molluscs in Umbria gement and the Control Plan for the New Zealand Mudsnail (Potamopyrgus antipodarum). Aquatic Nuisance Species Task Force. Website: http://www.anstaskforce.gov/Documents/NZMS_M&C_Draft_8-06.pdf. Quinn J.M., Steele G.L., Hickey C.W. & Vickers M.L., 1994 - Upper thermal tolerance of twelve New Zealand stream invertebrate species. New Zeal. J. Mar. Fresh., 28: 391-397. Ricciardi A.& Rasmussen J.B., 1998 - Extinction rates of North American Freshwater Fauna. Conserv. Biol., 13: 1220-1222. Richards D.C., Cazier L.D. & Lester G.T., 2001 - Spatial distribution of three snail species, including the invader Potamopyrgus antipodarum, in a freshwater spring. West. N. Am. Naturalist, 61: 375-380. Ross R.M., W.A. Lellis, Bennett R.M. & Johnson C.S., 2001 Landscape determinance of nonindigenous fish invasions. Biol. Invasions., 3: 347-361. Roth G., 1987. Zur Verbreitung und Biologie von Potamopyrgus jenkinsi (E.A. Smith, 1889) im Rhein-Einzugsgebiet (Prosobranchia: Hyidrobiidae). Arch. Hydrobiol., Suppl 79: 49-68. Sala O.E., Chapin III F.S., Armesto J.J., Berlow R., Bloomfield J., . Dirzo R, Huber-Sanwald E., Huenneke L.F., Jackson R.B., Kinzig A., Leemans R., Lodge D., Mooney H.A., Oesterheld M., Poff N.L., Sykes M.T., Walker B.H., Walker M. & Wall D.H., 2000 - Global diversity for the year 2100. Science, 287: 1770-1774. Schreiber E.S.C., Quinn G.P. & Lake P.S., 2003 - Distribution of an alien aquatic snail in relation to flow variability, human activities and water quality. Freshwater Biol., 48: 951-961. Simberloff D., 2004 - Community ecology: is it time to move on? Am. Nat.,163: 787-799. Spilinga C., Chiappafreddo U. & Pirisinu Q., 2000 - Dreissena polymorpha (Pallas) al lago Trasimeno. Riv. Idrobiol., 39: 145-152.