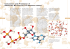Electron Transport Chain and OxPhos
Transcription
Electron Transport Chain and OxPhos
The Electron Transport Chain and Oxidative Phosphorylation The Electron Transport Chain: reductio ad energum- So far, we have not gotten much ATP out of all this cycle turning and oxidizing and old-and-new acetating. Where is the payoff? Earlier, when we discussed the complete oxidation of sugars and related molecules, we denoted that process by starting with the molecule to be oxidized and O2, and ending up with nothing but CO2 and water. In contrast, you may have noticed in the above description that the oxidation of the Krebs cycle molecules does not go “all the way” to CO2 and H20. For each acetate added, 2 CO2 molecules are produced, but no O2 is converted into H2O. In fact, although the Krebs cycle causes oxidation of its substrates as they go around the cycle, no oxygen is involved or required for the Krebs cycle. (This is a popular “trick question” on tests. Be tricked no more!) Instead, the reducing equivalents that are produced are held in the molecules NADH, and FADH2. These will then be used to reduce oxygen to water, getting oxidized back to NAD+ and FAD in the process, restoring the electron carriers. So one way to look at it is that the carriers are being reduced during the Krebs cycle, allowing the production of CO2, and then being oxidized later, to produce water by reducing O2. This “separation” of the complete oxidation of acetate groups into two phases (production of CO2 and reduced carriers, followed by production of H2O and oxidized carriers) serves a very important purpose. The oxidation of the FADH2 and NADH carriers back to their “unloaded” (oxidized) forms is part of a process that is responsible for most of the ATP energy that we derive from glucose and other fuels we consume and break down. The set of reactions that carry the electrons from carriers like NADH and finally to O2 is called the electron transport chain (ETC), or the respiratory chain. The function of this process is to use the chemical energy released during the reoxidation of the carriers and the reduction of O2, to make ATP from ADP and Pi. The production of ATP driven by these redox reactions is called oxidative phosphorylation (sometimes OxPhos for short). The way this happens is a little cell biological miracle. So much so that it took a paradigm-breaking change in thought to get to the correct answer. So first, let’s look at the wrong answer. It is not a fruitless exercise, and the “coming around” of the community from the commonly-held incorrect answer to the unexpected, and at one time unvoiced, correct answer is a beautiful example of the power of consensus-driven hypothesis testing as the most reliable way to arrive at physical truth, even when that truth is unexpected, surprising, or even revolutionary. Energetic Expectations Dashed!- The history of the endeavor to figure out how ATP is produced in the late oxidation steps of glucose metabolism (that is, the conversion of pyruvate into CO2 and H2O with the concomitant generation of ATP) is worth talking about for a minute, because it shows an important, general and beautiful aspect of experimental science as we practice it. The basic principle is that your hypothesis can be wrong, so long as you do the right things to test it. The reactions of glycolysis were worked out years before the oxidative production of ATP in mitochondria was figured out. So the detailed and accurate knowledge gleaned from glycolysis reasonably led to mechanistic expectations about how ATP would be generated after glycolysis. In simplest terms, glycolysis produces ATP through what is called substrate-level phosphorylation. Although this sounds fancy, it is a pretty straightforward idea. A phosphorylated compound with a greater energy of hydrolysis is used to run the reverse reaction of ATP hydrolysis, that is, ATP synthesis from ADP and Pi. (You should convince yourself that the transfer of phosphate from such a high energy compound to ADP can be represented as the chemically balanced combination of two reactions: the phosphate donor being hydrolyzed, and the hydrolysis of ATP being run in reverse. ATP generated in this manner is called substrate-level phosphorylation because the phosphorylated compound that drives the reaction with its free energy of hydrolysis will be a substrate of the enzyme that allows transfer of the donor phosphate onto ADP to produce the desire ATP. It is the intrinsic chemistry of the phosphate donating substrate that allows the production of ATP from ADP to occur spontaneously. If you look at the reactions of glycolysis through the “lens” of substrate level phosphorylation, you will see that the whole show is about the clever generation of two such high energy phosphorylated compounds, 1,3bPG, and PEP, each of which is used to directly phosphorylate ADP to make ATP. Voila, substrate level phosphorylation! See? below is the 1,3bPG reaction, just for review. 1 So when people were trying to figure out how the oxidation of pyruvate to CO2 caused the production of ATP by running the ATP hydrolysis reaction in reverse, it was totally reasonable to expect that nature would use the same trick as it had in glycolysis, that is, by substrate level phosphorylation. Specifically, the field was reasonably and completely dominated by the thought that somewhere in the chemical reactions that took acetate to CO2, and oxidized O2 to H2O, the resulting free energy would be used to make a cranked up phosphorylated compound (or several of them) that would, like 1,3,bPG in the example, react with ADP to make ATP in an energetically favorable manner. The hypothesis went something like: “in the course of the oxidation-reduction reactions that convert pyruvate into CO2 and O2 into H20, high energy phosphorylated molecules are produced that drive the production of ATP from ADP by substrate level phoshporylation”. If you look at the old papers or even the textbooks on this subject in the mid sixties, they all would draw graphical models that included an unknown intermediate X~P that would serve as the high energy phosphate donor in the production of ATP caused during these late oxidative steps. The term oxidative phosphorylation is a common shorthand to describe this coupling. And the simplest model, which was understandably (mis)informed by the successful unraveling of glycolysis, included substrate level phosphorylation as the final step in ATP production. And not surprisingly, both the Beatles and the Rolling Stones (both first big in the sixties) also espoused the substrate level phosphorylation model in their early work. A cell biological solution to a biochemical problem- The actual answer to how the oxidation of acetate (or pyruvate) causes ATP production was totally different from the substrate level phosphorylation model proposed and tested for many years during work on OxPhos (which is how many people refer to oxidative phosphorylation). The solution to the problem dragged researchers who were hard core biochemists into the worlds of cell biology and biophysics. What do I mean by that? Biochemists, especially back in those days but even now, work on biochemical reactions; how molecules are made, broken down, and interact with larger systems in the cell to make life happen. They are often interested in the enzymes that make such things happen at an acceptable rate, and because there are many, many sorts of enzymes, there are many, many scientific endeavors where understanding the biochemical aspects or a process is important and fruitful. Cell biologists focus on how cellular processes occur and many times their work involves or includes thinking about the structure and dynamics of the membrane compartments that define the cell as we know it. This division is a bit arbitrary, since all enzymes are contained in cells, and the dynamics, construction and function of membranes involves many enzymatic processes. Nevertheless, there are definitely people who are more “biochemical” in their research, and others who are more “cell biological” in their approaches. This is because successful science requires lots of focus and lots of expertise in particular approaches. The degree to which this is the case can be seen in the gatherings that scientists go to: Each year the American Society of Biochemisty and Molecular Biology (ASBMB) draws thousands of people who are doing things by more biochemical approaches; in the same year, the annual American Society of Cell Biology (ASCB) meeting attracts thousands of cell biologist. Not surprisingly, many people go to both meetings. Rules of thumb, and not absolute rules, govern this view of science. Anyway, it turns out that the way ATP is made from the oxidation of acetate into CO2 and H2O is a process that intimately involves cell biology, a process that depends on the the properties and existence of membrane compartments. It is through the use of membrane compartments that the energy for ATP production is collected and harnessed. Instead of the free energy for ATP production being stored in some X~P high energy molecule, it turns out that an electrochemical gradient, that is an imbalance of H+ ions and charge across a membrane is created and used to drive the production of ATP. So there is no X~P, and there never was one involved in the the redox reactions that produce H2O by reoxidizing the electron carriers (NADH, FADH2) generated by the Krebs cycle. You can imagine that if a large group of people who are very good at what they do, who are, in fact, the 2 world’s experts in the biochemistry of ATP production all strongly believed that some kind of substrate-level intermediate X~P was being generated in oxidative phosphorylation, then it would have been very hard to change that thinking without some serious counter evidence. It is important that you the student realize that when we say “believe” here, we are talking about the most informed guess or hypothesis derived from experimentally derived information. The fact that substrate level phosphorylation is the way that glycolysis converts ADP back into ATP created the totally reasonable and very heartily embraced idea, or belief, that this same approach was happening in OxPhos. Don’t make a paradigm you can’t break- The entrenched, communal thinking that a phosphorylated intermediate (X~P), that functions like 1,3bPG or PEP, was being produced to drive the mitochondrial synthesis of ATP during oxidation of acetate and production of H2O drove a lot of excellent biochemistry. Gradually, the results of these beautiful and still-valid studies caused the growing concern that the substrate level phosphorlyation model was not describing how oxidative production of ATP was occurring. The previous substrate-level phosphorylation model that came from understanding glycolysis, and was reasonably expected to also be true in mitochondrial oxidative phosphorylation, was what some philosophers of science would call a paradigm, that is, a view or model of a part of the world (in this case the mechanism of oxidative ATP production) *. Before any more progress could be made on this key problem, someone had to propose a new paradigm, that is, a new view or model to explain all the observations that didn’t sync with the old substrate-level phosphorylation view. In other words, the old paradigm had to be broken, or shifted to the new view. These terms, paradigm shift and paradigm break, are used in many cultural contexts, and there are whole books written about the important role of paradigm shifts and paradigm breaking in scientific progress. The most commonly mentioned one is Thomas Kuhn’s famous “The Structure of Scientific Revolutions”. *A set of assumptions, concepts, values, and practices that constitutes a way of viewing reality for the community that shares them, especially in an intellectual discipline. Mitochondria: giving redox reactions space- Both the Krebs cycle and the ETC (and a lot of other processes) occur deep inside the mitochondrion. Many of you have seen pictures of mitochondria, and they are usually draw to look sort of like a kidney bean (or just a kidney come to think of it) or a fancy SoCal swiming pool; sort of an ovoid shape. This is a useful way to introduce the mitochondrion here, and we will use the same graphical convention. But in fact mitochondria are highly dynamic structures that fuse with each other, break into smaller units, and interact with all kinds of cellular parts in the course of their biology. But for now, we will join the legions of kidney shape depicters that have gone before us. The basic feature of the mitochondrion is that it is a closed membranous compartment, with contents that must be imported and exported. The biochemistry going on within the mitochondrion is separated from that going on in the cytoplasm, with lots of communication caused by regulation of the import and export of molecules. This is our first introduction to cellular compartmentalization of metabolism, but it will keep coming up again and again. Mitochondria are membrane-bound organelles, with two separate membranes, an inner one and an outer one. In this sense they are like the gram-negative bacteria from which mitochondria are thought to have evolved, after a probably very rare entry event of one of these bacteria into the cytosol of some pre-mitochondrial eukaryotes. Somehow the unwanted guest gradually became an essential part of the eukaryotic cell, allowing so much of what we are today (). The outer membrane is called… wait for it… the outer membrane (OM) and the inner membrane is called the inner membrane (IM). The space between the inner and outer membrane is called the intermembrane space (IMS), and the interior region enclosed by the outer membrane is called the matrix. The inner membrane is highly folded to promote surface area, and the folds are called cristae. The diagram depicts these sections in our kidney- or swimming poolshaped version of the organelle. 3 Similar to the Gram negative bacteria from whence mitos came, the two membranes are very different in function and composition. The outer membrane has fairly large protein pores, such that metabolites (like ATP, pyruvate, ions, fatty acids, etc.) can get right through. Proteins are restricted from exit or entry, so the IMS has a defined and controlled protein composition, but communicates with the cytoplasm in terms of metabolites and other small molecules. The inner membrane (IM) is a membrane of a different color. This membrane is highly impermeable to even small things like ions, H+, ATP, etc. All movement of things occurs via membrane bound transporters or fancy molecular turnstiles that are embedded in the inner membrane. As you will see, it is this highly impermeable nature that allows mitochondria to extract the majority of energy during the reduction of O2 to H2O. We will also see later that the highly impermeable nature of the mitochondrial inner membrane requires some pretty fancy molecular tricks to get molecules into and out of the matrix where a lot of biochemistry occurs. Whole lotta oxidation goin’ on- The inner membrane of the mitochondrion is the site of the enzymes that catalyse oxidation metabolism and ATP production in oxidative phosphorylation. Our primary focus for now will be the movement of electrons captured during the Krebs cycle (in the form of FADH2 and NADH) along the electron transport chain to their final resting spot in H2O. But this set of enzymes is employed to extract energy from a variety of oxidation reactions, funneling the resulting electrons into a common and useful fate. So learning the details of the electron transport chain will open many metabolic doors, and allow you to understand many of the varied biochemical functions of this now-essential part of the eukaryotic cell. Dr. Mitchell, we presume…To get the field of bioenergetics out of the substrate level phosphorylation rut, it took someone to come forward and break the old paradigm, someone to look at the evidence and put together a radical new idea for how ATP production was being driven. The person most directly responsible for that change was named Peter Mitchell. In truth, these things often involve lots of people all talking, combating, interacting, drinking coffee in the morning, drinking beer after hours and at scientific meetings, writing letters (emails and tweets now (#substratelevelbs; #oxphosisboss) and generally trying to figure out what the heck is going on (there are exceptions, like some of the things Albert Einstein cooked up). And Dr. Mitchell definitely considered a large number of studies to formulate his new model. But it is safe to say he went way out on a limb with his new idea, in the context of the dominant thinking, the dominant paradigm, of that time. He published a seminal paper in 1961 suggesting an entirely new, and it turns out correct, model of oxidative phosphorylation that proposed that during the course of production of H2O from the electrons carried by NADH and FADH2, an electrochemical gradient was produced across the inner membrane of the mitochondrion, and THIS was the way that energy was collected from these reactions to be used for regeneration of ATP from ADP and Pi. Radical! No, wait, Ion! Not surprisingly, at first there were numerous people who were quite skeptical and critical. This is reasonable, because science if full of great yet wrong ideas. But the Mitchell model made many predictions that were all testable, and soon tested, and it re-energized and correctly oriented the field. For this Peter Mitchell was eventually awarded the 1978 Nobel prize. So here is another principle that we can add to our one above. So our new, expanded science adage is: Your hypothesis can be wrong, so long as you do the right things to test…but if your new and radical hypothesis is right, you can get very famous, and it is a lot more fun. Don’t worry, there are still enzymes involved- Although the quotes above have a silly aspect, there is real truth to the idea that correct testing of the wrong hypothesis can and will produce much good science. Correctly done science frees us from the dangers of our unbridled imaginations or our false hopes leading us astray. But it can take a while. The study of the electron transport chain (ETC) is a good example of this “hope-proofing” in action. What I mean is that although the many excellent scientists working on the enzymes of the ETC never found the desired X~P high energy substrate, their unsuccessful search still revealed a huge amount of critical and key information about how oxidative phosphorylation worked. In fact, one could argue that not believing a 4 membrane-oriented, cell biological process was operating helped the field. I say this because the incorrect thought that highly purified enzymes would make it easier to observe the production of the hypothesized X~P intermediate led to a deep and very important understanding of the enzymes that actually do catalyze electron transport and do cause the production of the unanticipated and key transmembrane electrochemical gradient that supplies the energy for ATP production. So lets explore these enzymatic reactions: The enzymes of the ETC- The basic reactions of oxidative phosphorylation start with… ready.. oxidation. At the end of the Krebs cycle, we have NADH, and FADH2, holding the electrons that have been pulled off of Krebs cycle molecules during their oxidation. The net chemical reaction for the Krebs cycle (pay most attention to the reducing equivalents NADH and FADH2 that are produced) is: + 3NAD + FAD + GDP + Pi + AcSCoA + 2H2O + 3NADH + FADH2 + GTP + CoSH + 3H + 2CO2 And as you would expect, during the ETC, the NAD and FAD are regenerated and head back to the Krebs cycle for more action. In addition, O2 is reduced to H2O. But this is done in gradual steps, little by little. In this way, small units of chemical energy are used to create the H+ gradient we talked about above. This strategy allows the maximum “bang for the thermodynamic buck” and is the reason that oxidative metabolism is much more efficient in terms of captured and usable energy than an internal combustion engine. This gradual movement of electrons from carriers such as NADH to oxygen is accomplished by the action of 4 separate protein complexes that catalyze individual redox reactions, in which intermediate molecules sequentially pick up and lose electrons. The complexes were purified in the heyday of studying the enzyme biochemistry of oxidative phosphorylation, and are named Complex I, II, III, and IV. The first two complexes have one biochemical job: to take electrons from the reduced Krebs cycle carriers and place those electrons on a single membrane-bound electron carrier called ubiquinone, or simple Q in its oxidized form. Complex I oxidizes NADH and reduces Q to its two electron reduced form QH2. Complex II converts FADH2 into FAD and reduces Q to QH2. So complex I and complex II are sort of like an “electron funnel” that channels NADH and FADH2 electrons onto the Q carrier to produce QH2. This is a wonderfully simple electron carrier. It has a “business end” that has a structure called a quinone. This structure can be converted with one or two electrons to form a single or doubly reduced molecule. The three forms are shown below. This simple benzoquinone business end is linked to a long lipid molecule (shown in the first form and then called R in the other two in the picture) that keeps it anchored to the surface of the inner mitrochondrial membrane, where all the action happens. This lipid molecule is called “coenzyme Q” (and it is the self-same material that is sold as the supplement/vitamin CoQ10). Complexes I and II: electrons to Q- As mentioned above, both complexes I and II catalyze reactions that convert Q into QH2. The formal name of complex I is NADH:ubiquinone oxidoreductase, and like this long but accurate name suggests, the reaction involves transfer of electrons from NADH to ubiquinone, to make NAD+ (ready for more service in the name of energy metabolism) and the reduced from of coenzyme Q known as QH2. The net chemical reaction looks like this. 5 Complex II is actually an enzyme that we have already encountered, in the Krebs cycle. It is none other than succinate dehydrogenase, (it is at about 7 o’clock on the Krebs cycle). The Krebs version of the succinate dehydrogenase reaction, as you remember from earlier studies, looks like this: succinate + FAD fumarate + FADH2 While the Complex II version looks like this: So you might reasonably ask, in the manner of scholarly discourse, “WFT! How can these be describing the same enzyme when the two reactions are different!?”. And that is a reasonable question. It’s really due to the fact that these enzyme complexes are… complex and usually catalyze a set of internal transfers that start with a small, discrete metabolite that is the electron carrier (or donor) and end with a product that is similarly small, discrete electron acceptor and now carrying the electrons. In both cases, the succinate is the donor of the electrons. But in the version we learned in the Krebs cycle, the succinate dehydrogenase reaction, the acceptor of the succinate electrons is FAD, which ends up being reduced to FADH2. In the version that is described in Complex II, the acceptor or recipient is ubiquinone, or Q which is converted to QH2. What gives? The answer lies in the fact that these complexes usually involve a number of internal transfers of electrons onto and off of various carrier, that we never see. It is true that the first place the succinate electrons go in the succinate dehydrogenase/Complex II) reaction is FAD to become FADH2, and so that aspect of the Krebs cycle is true because we are focused on the flow of carbon metabolites. Also, in the days of discovering the Krebs cycle, spectrophotometric methods were employed that focused on the FAD/FADH2 pair. But when the whole ETC is operating, the FADH2 that is produced in this reaction is tightly bound to Complex II and those electrons are then passed to other electron accepting sites within the complex and ultimately delivered to Q to reduce it to QH2. So when people are talking about the Krebs cycle, they usually leave the electrons on FADH2, but they wend their way through the Complex II carriers and end up on QH2. It, like a lot of metabolism, is a matter of what one is emphasizing and what one is ignoring. Often when people are studying the ETC in isolated mitochondria they will add succinate to isolated mitochondria to start the electron transport chain going. Keep that in mind for later when we talk about this approach to studying mitochondrial respiration. The electrons from NADH or FADH2 (really succinate) end up on the carrier QH2 after the reactions of complexes I and II, but we know they finally end up on H2O (as a result of reducing O2) it is not hard to guess that the next two complexes (III and IV) are involved in getting the electrons from QH2 to O2. Ready…? Cytochrome c: mind your Qs and Qs- We have the electrons from the Krebs cycle now held in the reduced mitochondrial carrier QH2, and the Krebs cycle acceptors NAD and FAD have been restored to continue their good work in oxidation of acetate groups that enter the Krebs cycle. Now what? The electrons from reduced QH2 6 are next placed onto a new carrier called cytochrome c, or cyt c. Complex III (NADH: cytochrome c oxidoreductase) catalyzes this transfer of electrons. cyt c has simple chemistry (and fancy biochemistry). It a protein with a heme cofactor that holds a Fe ion, , just like the iron that we encountered in hemoglobin that uses iron ions to reversibly bind O2. The iron ion in cyt c can either be in the Fe3+ state (oxidized) or the Fe2+ state (reduced). That is it. Complex III has the job of taking the electrons from reduced QH2 and transferring them to cyt c to make the reduced (Fe2+) form. This simple transfer has the net reaction as shown. So the products of the reaction are reduced cyt c (with Fe2+) and re-oxidized Q. One thing to notice about this chemistry is that the transition from QH2 to Q involves the removal of two electrons while the reduction of cyt c involves the transfer of one electron to the Fe3+ in the oxidized cyt c to make Fe2+ in the reduced form. The cool thing about Q as an electron carrier is that due to its quinone chemistry, it can exist in three states: fully reduced (with 2 electrons) partially reduced (with one electron) or fully oxidized, with no electrons. The transfer of electrons from QH2 to cyt c is a fairly complex affair, called the “Q cycle”. The actual chemistry involves simultaneous oxidation of one QH2 to Q, with its two electrons going separate ways. One is actually used to reduce cyt c, and the other is used to reduce a separate Q to the intermediate form QH (I know, it sounds counterproductive). There are actually two distinct sites in Complex III, one for “donor” QH2 (that being oxidized) and one for the distinct “acceptor” Q. This “parting of ways” type reaction is then repeated with a new QH2 and the half-reduced QH, to produce a second reduced cyt c, a second Q and a QH2 from the acceptor QH. So actually a case of “two steps forward and one step back”. There are a number of complex ways to draw this, none of them are very succinct or revealing. Here is mine. The two donor QH2 that provide the electrons are each a separate color (green and magenta) to indicate they are two distinct molecule. The acceptor Q in its various forms is indicated with the brown color Q (so Q, QH. and QH2) to indicate it is the same molecule. In each of the two reactions, the two donated QH2 electrons go to two separate places. One goes to reduce a cytochrome c, and the other goes to reduce the blue acceptor Q. So we are oxidizing two QH2 to produce two Q, reducing two cyt c to make two reduced cyt c, and reducing one Q to make one QH2. That is, as you can check above, the net reaction for complex III written above, but it has a fancier and even inefficient-looking underlying set of occurrences. Why do things run in this crazy way? It turns out that the special separation of the two Q sites has a lot to do with the part of the biochemistry that pertains to proton transport, discussed below. Cytochrome c is the final resting place of the electrons after the Complex III reactions, and now we are finally ready to pass them on to O2 to make water. That is the job of Complex IV Complex IV: the last stop on an electron’s long trip home… The final net reaction in the action of the four complexes involves the regeneration of oxidized cytochrome c and the reduction of oxygen to H20. It is called cytochrome oxidase, and the net reaction is just what you’d predict, four reduced cyt c (with Fe2+) are oxidized, and so donate their electrons to one O2 molecule (because that is how nature packages oxygen for us) to produce two H2Os. Now if you think about it, that is a pretty elaborate reaction, since each cyt c can only donate one electron at a time (since each has one iron that goes from the +2 to the +3 state), and but the oxygen is provided in its molecular form and thus needs a total of 4 electrons to be converted into two H2O. Accordingly, the biochemistry of complex IV is indeed complex. The catalytic cycle involves four separate cyt c molecules giving 7 up their electrons, and a copper center complexing the oxygen atoms during their reduction, and a tyrosine forming a free radical during the festivities, to name a few events during reduction of one oxygen molecule to two H20. First the Q cycle and then these molecular shenanigans. Why is this all so complex? Read on! But first, here is the net reaction of the Complex IV, or cytochrome oxidase enzyme: Redox… is that it?Complexes I, II, III and IV are correctly called complexes because although they have names that sound like a single enzyme, and were originally purified as distinct enzyme activities that are described by those names, they are in fact composed of many proteins, and they are firmly embedded in the membrane. They are, in fact, integral membrane protein complexes that actually span the highly impermeable mitochondrial inner membrane (IM). And it is this transmembrane orientation that is the key to how the electron transport chain leads to production of energy that can be used to convert ADP back into ATP. The simple redox chemistry of the complexes is accurately depicted by the indicated reactions, all grouped together here. But along with this, complexes I, III and IV during their catalytic cycles, push a set number of H+ ions across the membrane to the side that faces the intermembrane space. That is, there is a vectorial or directional aspect to the reactions that involves moving protons, that is, H+ ions, across the membrane. So this process does not affect the chemistry in terms of bond making and bond breaking, but it is an integral part of the function, action and energetics of these reactions. It is important to realize that when the reactions are run in the soluble form that was so brilliantly devised in the enzymology labs of the 1950 and 60s, the “H+ pushing” aspect of the intact complexes would be missed due to the lack of a membrane: when a membrane-embedded complex is solubilized into detergent micelles, there is no membrane defining “inside” and “outside” to allow the generation of a gradient by pushing H+ ions to the outside. Once Peter Mitchell devised his model that explained a lot of hard-to-fit data by including intact membranes and gradient production, experimentalists turned to testing and examining the notion that an H+ gradient was being created as a product of the individual ETC reactions. So the complex I action really requires the membrane with complex I correctly situated in it. The reaction would be depicted like this: Those 4 H+ are being moved across the inner membrane of the mitochondrion in an orientation so that the inside (matrix side) is a bit more negative and the outer side (facing the IMS) is more positive. The energy for this uphill battle is provided by the redox reactions that move the electrons from NADH eventually to Q (magenta arrows). The increased H+ gradient can actually be thought of as a product of the reaction. Hold that idea because it will be a very useful way to think about the mechanism of coupling between the H+ gradient and the synthesis of ATP. In the ETC, three of the four complexes have this added ability to “push” protons across the IM, and in this way they each contribute to the electrochemical potential across the inner membrane of the mitochondrion. Those are complexes I, III and IV. The full electron transport chain, including complexes I-IV, oriented in the mitochondrial inner membrane is shown to the right. The two sides of the membrane are sometimes referred to as “P” and “N”. 8 This stands for “positive” and “negative”, like a battery! And it is! You can see that complex I, III and IV each have a red arrow signifying protons being pumped out across the membrane as part of their action. Complex II is the only complex that does not pump protons, but it is providing electrons to the QH2 pool. Also notice that the process of reducing Q to QH2 is abbreviated here just by showing Q as a “waystation” for electrons flowing. We know better, that both complex I and II reduce Q to QH2 as part of their enzymatic activity. Same with Cyt c. The arrow comes to it and out of it because Q first picks up electrons, and then dumps them into complex III. Mitochondria living up to their potential- The movement of protons across the inner membrane produces an electrochemical potential that is a powerful source of energy. Why? Two reasons: 1) there is a true charge imbalance, literally a voltage, across the membrane that that is positive outside and negative inside, and 2) there is a concentration gradient of protons, higher on the outer side of the membrane than on the inner side. These two separate components will both provide energy for protons to move back down the gradient. That is, both components contribute to free energy of the protons on the “p” or IMS side of the membrane by the following equation, which looks fancy but really says there are two independent features of the proton gradient that contribute to free energy (ΔG), them being the voltage across the membrane (Ψ) and the difference in concentration across the membrane (C2/C1) or ΔpH, depending on how one specifically write the equation. THIS is the source of the free energy that is used to make ATP. The energy stored in the H+ gradient acroos the mitochondrial inner membrane. Directly. No phosphorylated intermediate. No X~P. A voltage and concentration gradient across the membrane, that provides an energy-yielding path back across the membrane. The combination of these two ways of energizing the protons (charge, concentration) is usually referred to as the “proton motive force” or sometime PH+. Importantly, the protons are not consumed, but their movement across the membrane provides energy that can do work. Just like a ball rolling down a hill is giving off energy that can be used to do work even though the ball is not used up or consumed. Or just like water moving over a waterfall is giving off energy that can be used to do work even though the water is not being used up or consumed, protons moving across the energy gradient similarly can do work, and in the case of mitochondria that work is running the “uphill” or endergonic reaction of converting ADP and Pi into ATP. Let’s consider that waterfall analogy a little more. Your yacht wrecks on an uninhabited island in the south pacific, and you need to get your generator going to radio for help, light lights to keep the unknown carnivores away from your campsite, and charge up your iPods. You find a waterfall and hooray, there is plenty of energy in that natural feature for your needs. But to harvest that energy, you need a coupling mechanism to convert the energy of falling water into mechanical energy that turns your generator. So you build a water wheel, that turns the generator crank, and viola, energy from falling water to turn the generator. In the same way, the energy rich proton gradient must be harnessed by a protein complex to couple the energy of protons moving back down the gradient to the formation of ATP. That coupling is accomplished by a miraculous enzyme that is strangely similar to a sort of nano-scale water wheel. It is called ATP synthase. But first, let’s explore the transmembrane proton motive force PH+ a bit more… 9 An experimental interlude: experimental test of proton gradient-driven ATP synthesis- Before we get into the nitty gritty of the ATP synthase (nature’s water wheel), I want to describe some experiments that indicate that the proton gradient across the intact mitochondrial IM is the driving force for ATP synthesis. The first observations that led to this idea were frustratingly negative ones. That is, the inability of really excellent biochemical laboratories to ever see the reactions of the purified ETC complexes cause conversion of ADP and Pi back into ATP. These biochemical experiments always had the complexes removed from the mitochondrial structure, purified in a variety of detergents that allow proteins that normally sit in lipid bilayers to maintain their structure in solution, but without a membrane with distinct sides like we see in the actual mitochondrion. This absence of “coupling” between electron transport reactions (such as NADH transfer of electrons to Q) and ATP synthesis in the test tube is in stark contrast to other biochemical systems. The glycolytic pathway can be observed to make ATP from ADP and Pi when the reactions are run with soluble highly purified enzymes. And this is because the reactions responsible for ADP conversion into ATP are catalyzed by enzymes that directly use ADP and a phosphate donor such as 1,3 bPG (see glycolysis) to bring about the conversion. In contrast, the inability to see direct production of ATP from ADP and Pi when the purified complexes of the ETC were functioning in solution gradually indicated that something was missing or different from the expected substrate level phosphorylation. This “absence of effect” didn’t prove that the effect was absent. But when some of the best laboratories in the world, doing some of the hardest biochemistry in the world were unable to see ATP production when the complexes were operating in solution, it allowed the possibility that ATP production was connected or coupled to the ETC in a fundamentally different way. New answers require asking questions in new ways… Keeping it together to study mitochondria- Another way to study mitochondrial function is…to study mitochondrial function. No, this is not a Zen koan. It simply means that a different approach to studying mitochondrial biochemistry is to look at the processes in the intact, purified organelle. This may seem like a step back from the elegant study of highly purified, functional, detergent-solubilized ETC enzyme complexes; it is certainly a lot easier. To look at functioning mitochondria one has to purify them from some cellular or tissue source, typically from abundant tissues or those that that have high mitochondrial densities (liver, heart, etc.). Then one needs to measure the biochemical processes going on upon adding things like NADH or succinate. To do this, mitochondriists (probably not a real word) or mitochondriacs (actually used in Nick Lane’s great book about mitos) place the purified mitochondria (made by classic cell biological techniques; not super difficult with the correct centrifuges) in a chamber with an electrode that can sense oxygen. This set up is sometimes called an oxygraph, because it records current oxygen concentration in the mitochondrial mix. Since the electron transport chain consumes oxygen, the rate of this process is indicated by the oxygen consumption curve. A typical oxygraph experiment of isololated mitochondria is shown to the right. If everything needed to do oxidative phosphorylation (ATP synthesis) is present except an electron source, the addition of a molecule that can be oxidized by complex I or II will get the ball rolling. Normally people add succinate to the preparation, because NADH can not permeate the intact mitochondrial IM (and because succinate is both cheap and permeant). The graph shows time along the horizontal x-axis and the things being consumed or made on the vertical y axes. What happens is that the addition of the electron donor succinate (“add succinate”) causes a sudden increase in O2 consumption, due to production of H2O. This means the ETC is running in the intact mitochondria when an electron source, (and oxygen) is available. The graph green line shows this process, and represent values on the left axis. Now suppose you measure ATP production, which can also be done by the correct instruments. It is pleasing to see that if ADP (and Pi) are present, when the succinate is added, production of ATP also goes up. This is shown in the red line in the picture, on the right axis. 10 This is a very beautiful system to study how ATP formation is driving by the redox reactions that carry electrons from succinate (or NADH) to 02. But first, lets remember something. This does not work with purified ETC complexes, that is, when complexes I, II, III and IV are combined in solubilized form in the test tube, no matter how much ADP and Pi you add. Those complexes will allow the ETC redox reactions to occur, but ADP and Pi never combine to form ATP in the soluble preparations. So there was something about having the mitochondria intact, with the complexes embedded and correctly oriented in the IM, that allows the electron transport chain to drive ATP synthesis, and we now know that is the proton gradient generated across the IM. But how does one test this notion with an experiment? ATP production and ETC: a nice couple- The observation of ATP production being connected to and requiring the ETC is called “coupling”, for obvious reason. This sort of coupling can also be seen with the glycolytic pathway, but there the consumption of glucose that is coupled to ATP production can be seen with the soluble enzymes, or more typically, in a cytoplasmic preparation of only soluble molecules. Same idea. As I said above, the soluble ETC complexes work fine in doing their individual redox reactions, but together they do not drive the production of ATP. So now we will show you a classic experiment that strongly implicated the proton gradient across the IM as being that entity that couples electron transport to ATP production. First lets do a few control experiments. We have shown that succinate (an electrons source) needs to be added to cause ATP production, just like glucose needs to be added to cause ATP production in glycolysis. What happens if ADP is not present? The answer is shown in an “order of addition” experiment to the right. We show the same kind of experiment, with O2 consumption on the left axis (green), and ATP production on the right axis (red), but instead of adding succinate to a preparation of mitochondria where everything else is present, we first add succinate without ADP or Pi. The arrow indicates where along the time axis the succinate is added. Nothing happens, meaning the slopes for both O2 consumption and ATP production are unchanged. While the lack of ATP production is only expected (since there is no ADP added yet), we also fail to see O2 consumption, despite having succinate present (as indicated by lack of). Furthermore, when ADP and Pi are added after the succinate, Voila!, the whole process starts working: both ETC (left axis; O2 consumption) and ATP production (right axis) go way up. Coupling! Each process requires the other! It is again useful to think about glycolysis, where we would see the same sort of thing. If you had the glycolytic pathway working in a test tube and the system was deprived of ADP, the whole pathway would stop working very quickly because the ADP-requiring reactions would stop, their substrates would build up and all the reactions would quickly cease. What about inhibitors of the mitochondrial enzymes? An inhibitor of complex II/SDH would be expected to stop both O2 consumption and ATP production. There is an inhibitor of the enzyme that produces ATP from ADP and Pi called ATP synthase. This inhibitor is called oligomycin, and that drug also stops both ATP production (not surprisingly) and O2 consumption. In the same vein, inhibitors of the intermediate steps (complex III and IV) also effectively cease both processes. And this is exactly what you would see if similarly studying glycolysis: inhibitors of any intermediate enzyme would stop both glucose consumption and ATP production. So far, the mitochondria looks exactly like a classic ATP producing pathway. But not for long. It is worth mentioning that this “symmetrical” need for both electron donor (succinate) and ADP for either process to run was precisely why people thought that a high energy X~P type molecule was being generated and then consumed in OxPhos. 11 Mitochondria on acid- So with this method to observe coupling between electron transport and ATP production, the nature of coupling could be explored. One of the key observations that indicated a totally different mechanism from substrate level phosphorylation is based on an incredibly simple type of molecule, weak organic acids. Like all acids, these molecules are proton-releasing compounds (acids) that are charged when they are deprotonated, and neutral when they are protonated. However, because they are organic molecules, they are sufficiently hydrophobic that when in the uncharged protonated form, they are soluble in organic solvents, like you may have learned about in O-chem lab. This turns out to be the perfect molecule to examine the importance of a transmembrane proton gradient. That is because the presence of a weak organic acid will create a route for protons to move across an otherwise impermeable membrane. The weak organic acid will exist as both the protonated and deprotonated forms in each aqueous space, with the neutral protonated form moving freely across the normally impermeable inner membrane. This way, a weak organic acid will tend to ferry protons across a lipid bilayer. Thus, if there is a higher concentration of protons on one side of the membrane, this new route of movement will rapidly break down the gradient until it is equal on either side, by simple mass action. The picture below (for some reason, ever darn picture description starts just at the bottom of a page, requiring “page lag”.. I am sorry!) shows an illustration of this process, and a “real” weak organic acid used in mitochondrial experiments called dinitrophenol, or DNP. DNP So what happens when we treat isolated mitochondria in an oxygraph with a weak organic acid like dinitrophenol (DNP). The picture illustrates an experiment in which we observed the usual “coupled” processes of ETC (as indicated by green O2 consumption) and ATP production (in red), and then the effect of addition of DNP at the indicated time. BOOM! Notice two things: 1) that the O2 consumption continues, but the ATP production drops drastically. That is, the flow of electrons and ATP production have been uncoupled. This is why people frequently refer to weak organic acids as “mitochondrial uncouplers”. It turns out that many weak organic acids do this, because the effect of these types of molecules is due to the general solubility properties of weak organic acids rather than some special feature of DNP. This and similar experiments strongly indicated that the proton gradient across the IM was responsible for powering the production of ATP. Put in other words, this experiment indicated that the proton gradient was the “product” of the ETC that is used to drive synthesis of ATP. In glycolysis, the products are actual molecules, that is 1,3 bPG or PEP. These two molecules 12 are made and used to create ATP from ADP. In mitochondrial respiration, complexes I, III, and IV, move protons across the membrane against a gradient, and this mode of energy storage is used to make ATP. That is why you need intact mitochondria to see ATP production driven by the ETC. It is also why you can not see the purified, soluble complexes make ATP in the test tube… there is no way to build up a proton gradient. I alluded to the idea that the proton gradient is a “product” of some of the enzymes of the ETC. So instead of a molecule that is produced in a typical enzymatic reaction, let’s consider the proton gradient across the IM as a reaction product. In a sense it is, and behaves in many ways like a more typical product. As it builds up, the spontaneity of the upstream reactions diminishes. ATP production expends the gradient in a useful manner (as we shall see) and blocking ATP production causes the gradient to build up, just like a product building up in a more traditional pathway like glycolysis. In the picture shown, the gradient is indicated by “PH+”, for proton motive force. The entire (and very complex) ETC is indicated by the net processes of reduced carriers (like succinate or NADH) getting oxidized and oxygen getting reduced to water. This all produces the “product” PH+. This quantity consists of both the chemical gradient and the charge gradient, each caused by the pumping of protons across the IM during the ERC. PH+ is a source of free energy, because the protons will spontaneously move down the gradient. And PH+ is the used up, just like a substrate, to drive ATP synthesis by ATP synthase. Keep this in mind, and we will get back to it a little later. Necessity and sufficiency: light driven ATP synthesis- The experiments with intact mitochondria clearly show that the proton gradient is a necessary condition for ATP production. The proton gradient is where the free energy of the various reactions of complex I-IV ends up, stored in a chemical and charge gradient, a lot like a battery. By removing or lowering this gradient with things like uncouplers, and through a variety of other like-minded experiments, it became clear that the proton gradient was a necessary condition for ATP synthesis by mitochondria. But mitochondria have a lot of things, and parts and stuff, so a related by distinct question was, “is a proton gradient all that you need?”, or put another way, is the proton gradient sufficient to drive ATP synthesis by the enzyme ATP synthase? This question was addressed in a most lovely and elegant experiment. It is one of those cases where it is easy to describe, but very hard to do, because it requires some super challenging biochemistry. But the basic idea is sort of the “Frankenstein” approach, building a biological system to test an idea. Normally, the mitochondrial proton gradient is made though the complex actions of the ETC. But there are a number of much simpler ways that nature can make an identical proton gradient. One of the simplest known is the protein known as bacteriorhodopsin, which is a monomeric protein that spans the purple bacteria membrane seven times. When this protein is properly functioning, it absorbs light and uses that light energy to pump protons across the membrane, making a proton gradient. If the proton gradient is all that is needed, then a gradient produced by this completely distinct way should still do the job just fine, and allow a mammalian ATP synthase to make ATP, even though the relatives of these two proteins (ATP synthase and bacteriorhodopsin) parted ways like about 2 billion years ago! The laboratory of Effram Racker (who was instrumental in many of the key studies of the individual complexes and mitochondrial energetics) combined bacteriorhodopson from the purple sulfer bacteria with ATP synthase purified from beef heart into artificial membranes called “phospholipid vesicles” which are very simple closed membranes made from purified lipid molecules. They set it up so the vesicles would get protons pumped into them when the bacteriorhodopsin was activated, and the ATP synthase would then use the 13 gradient to form ATP, by allowing protons to escape in a productive way. The cool thing is that because the bacteriorhodopsin is light-activated, the experiment literally worked by turning a bright light on the vesicle prep and watching ATP production start. The off and on states of this brilliant experiment are depicted in cartoon form with the yellow squiggles being the activating light. This first version of the experiment was done in 1974, and really put the nail in the coffin of about the chemiosmotic theory. In subsequent versions of the experiment, super pure components were used, to the point that the system works with highly pure bacteriorhodopsin, ATP synthase, and phospholipids. If you can build it, you understand it… light on! light-powered ATP synthesis ATP synthase: what goes around comes around- ATP is produced during OxPhos by the catalytic action of the enzyme ATP synthase. Long after the proton gradient was clearly shown to be necessary and sufficient condition for ATP synthesis, the way by which this protein converts the proton gradient into ATP production was not clear. Gradually the structure of the protein was learned in great detail, and this along with classic enzymology has led to a deep understanding of the action of ATP synthase. The one totally weird thing is why it is called “ATP synthase” when every other ATP-using enzyme almost without exception is called a “synthetase”. I don’t have an answer for this. Oh well, you can’t know everything. The biochemistry text are full of extremely detailed descriptions of this beautiful enzyme, but the basic “forest” view will suffice to gain you admiration and understanding. There are two parts of the enzyme, called Fo and F1. Fo is embedded in the membrane, and F1 is soluble, and can even be purified as a soluble multi subunit enzyme. The soluble F1 subunit has 3 sites (each made up of two proteins, alpha and beta, so ababab is the total count of individual proteins in F1). The sites defined by each ab dimer can be empty, bind ADP + Pi, or bind ATP. Not surprisingly these binding sites are the active sites where the enzyme action is occurring, running the endergonic reaction of converting ADP and Pi into ATP. The Fo subunit resides in the membrane and spans the membrane multiple times; exactly what you would need to harness a proton gradient across a membrane. It turns out that the ATP synthase works by allowing H+ ions to flow back into the matrix by moving through the Fo subunit, and this movement is converted into mechanical energy that pushes the F1 subunit into different conformations that promote ATP synthesis and expulsion from the enzyme. Better still, the motion is a rotary motion, such that there is a shaft that sits in the middle of the Fo protein which actually spins, and this spinning motion drives the changes in the F1 subunits that promote the uphill battle of ATP synthesis. So the analogy of protons in the gradient being like water flowing down a waterfall and turning a water wheel is spookily accurate. There have even been incredible experiments showing that the ATP synthase actually turns like a piston, and this turning motion can be seen with the right microscope and the correct clever tricks! Acceptance is the key- One of the features of the ATP synthase is that the flow of protons through the Fo channel and the movement of the subunits as they cycle through their different conformations are highly interdependent, and this can be shown in two ways: If an inhibitor of the ATP synthase is added to a mitochondrial preparation the ATPase will stop functioning, and the entire ECT will grind to a halt. This is again best viewed by remembering 14 that the proton motive force PH+ can be viewed as a product of the ETC and substrate of the ATP synthase. If the enzyme is inhibited, the proton gradient remains high, and the reactions that produce it eventually (and rapidly) stop. In a similar vein, if there is no ADP, the ATP synthase can not function, and again, the flow of protons through the Fo channel stops, and the whole ETC rapidly slows down. It is important to realize that this connectivity, or coupling between the action of the ATP synthase and the redox complexes of the ETC is due to the fact that the protons have only one way to go down their gradient: by passage through the ATP synthase Fo channel. This tight coupling between the flow of electrons and the production of ATP has a very important functional aspect, and it is this: when ADP is available, the electron transport chain will continue to function. When ADP runs out, the flow of electrons will stop, due to a “pile up” of the gradient, and a slowing of all the upstream reactions that make the gradient. If ADP increases, flow resumes as the protons start flowing through the Fo channel. This is called “acceptor control” and it refers to the fact that the availability of ADP determines the rate of the ETC. The term “acceptor” is used because ADP is a phosphate acceptor. A little byzantine but we have to accept it (ha ha). So this provides a very natural control system for “deciding” how much ETC activity, and hence how much consumption of NADH and succinate, will be occurring. The more ADP, the more consumption of these key input substrates and the more oxidation of the fuels that produce them. 15