Collier, Catherine. J., Sven Uthicke, and Michelle Waycott
Transcription
Collier, Catherine. J., Sven Uthicke, and Michelle Waycott
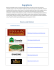
Limnol. Oceanogr., 56(6), 2011, 2200–2210 2011, by the Association for the Sciences of Limnology and Oceanography, Inc. doi:10.4319/lo.2011.56.6.2200 E Thermal tolerance of two seagrass species at contrasting light levels: Implications for future distribution in the Great Barrier Reef Catherine J. Collier,a,* Sven Uthicke,b and Michelle Waycotta a School of Marine and Tropical Biology, James Cook University, Townsville, Queensland, Australia Institute of Marine Science, Townsville, Queensland, Australia b Australian Abstract This study assessed metabolism, growth, and survival of two seagrass species at three different seawater temperatures (27uC, 30uC, and 33uC) under saturating (400 mmol photons m22 s21) and limiting (40 mmol photons m22 s21) light over 1 month. Halodule uninervis grown at 33uC was within its physiological optimum temperature range, exhibiting 2.33 higher photosynthetic rates than at 27uC, and increased net shoot carbon (C) production (up to 103 higher) at saturating light levels. In contrast, 33uC exceeded the optimum temperature threshold for Zostera muelleri, resulting in critical metabolic imbalances with large reductions in photosynthesis and increases in leaf respiration. This led to substantially lower growth rates (0–2% of those at 27uC) and lower final biomass (only 10% of that at 27uC) in the 33uC treatment after 1 month. This decline at higher temperatures occurred at both light levels, but it was more severe in limiting light, where the C balance went into deficit. H. uninervis in the Great Barrier Reef (GBR) exists well within its optimal temperature range and should continue to thrive at projected future temperatures, at least under saturating light levels. In contrast, Z. muelleri currently exists near its upper thermal threshold, and future temperature increases of the magnitude investigated here would likely lead to the contraction of the range of this species from the northern GBR—potentially by more than 1000 km. This could have ecologically significant ramifications, because Z. muelleri is often the only GBR species that currently inhabits muddy estuarine areas, which are critical fisheries habitats. Seagrasses are marine flowering plants, and they are globally distributed and ecologically valued for their high rates of productivity, coastal nutrient cycling, and as a habitat that supports fisheries species and as a direct food source for obligate seagrass feeders such as dugongs (Orth et al. 2006; Heck et al. 2008; Unsworth and Cullen 2010). In tropical regions, including the Indo-Pacific, where coastal marine resources provide up to 100% of daily protein needs for the communities living along the coast, seagrasses are a vital fisheries habitat (Unsworth and Cullen 2010). Seagrass-dominated ecosystems also support productivity and biodiversity of adjacent habitats, particularly mangroves and coral reefs (Heck et al. 2008). Global seagrass loss and the factors contributing to it are therefore of critical concern for the sustainability of these coastal ecosystems and the communities and industries supported by them (Orth et al. 2006; Waycott et al. 2009). Plant metabolism is responsive to temperature in a largely predictable manner, whereby the balance between carbon uptake (photosynthesis) and consumption (respiration) is affected. The photosynthetic processes of higher plants are highly sensitive to temperature, with increases in photosynthesis occurring up to a physiological optimum, followed by sharp reductions in photosynthetic efficiency after temperatures exceed specific thresholds (Berry and Bjorkman 1980; Bulthius 1987; McDonald 2003). Respiration rates can continue to rise with increasing temperatures, even after photosynthesis starts to decline. When this happens, the availability of fixed carbon through photosynthesis no longer balances C requirements as temperatures rise, and the plants go into deficit (i.e., the ratio of * Corresponding author: [email protected] photosynthesis to respiration, or P : R, , 1). Such metabolic imbalances in plants are generally associated with the remobilization of storage reserves, as well as adjustment of morphology and productivity as a means to reduce respiratory requirements (Chapin et al. 1987; Ralph et al. 2007; Collier et al. 2009), but the consequences of temperature-induced metabolic imbalances for seagrasses are largely untested. The optimum temperatures for photosynthesis and for maximizing P : R ratios are also not known for many—particularly tropical—seagrass species (Bulthius 1987; Campbell et al. 2006; Rasheed and Unsworth 2011). Many environmental factors influence plant growth and physiology, but there is typically one factor that principally limits growth (Schulze et al. 2002). Seagrasses have relatively high minimum light requirements, yet their predominance in coastal habitats exposes them to conditions of low and highly variable light (Dennison 1987; Waycott et al. 2009). Natural variability in the light environment occurs through daily and lunar cycles as incident light and water depth change, and runoff and resuspension of bottom sediments can cause chronic and acute reductions in light (Ralph et al. 2007). This variable light environment is a particularly strong feature of the local environment in northern Australia, including the Great Barrier Reef (Carruthers et al. 2002; De’ath and Fabricius 2010). Declining water quality has led to conditions in which light levels have become the primary limiting factor in many seagrass meadows, and although this tends to have local effects, it is a globally significant problem (Waycott et al. 2009). Seagrasses have adapted to their highly variable light environment primarily by meeting respiratory and growth requirements with a 2200 Seagrass thermal tolerance 2201 Fig. 1. (A) Annual maximum monthly sea-surface temperature in the Indo-Pacific region (2002–2009, http://www.bio-oracle.ugent. be) and the distribution of Halodule uninervis and Zostera muelleri (Waycott et al. 2004). (B) Sea-surface temperature in northeast Australia showing the Great Barrier Reef and the study site. combination of photosynthetic C fixation and reallocation of reserves (Ralph et al. 2007). There are a number of different processes and scales at which this balance can be achieved. For example, the efficiency of light capture and photosynthesis can be increased to boost photosynthetic rates (Enrı́quez 2005), while at the same time, the demands for fixed C can be lowered by reducing growth rates and the amount of biomass retained by the plant (Fourqurean and Zieman 1991; Collier et al. 2009). Plant-scale responses that affect meadow structure, density, and productivity are significant at a broader ecological level because they are critical to the habitat and food value of seagrass meadows. Halodule uninervis is a tropical seagrass species common throughout the Indo-Pacific and east African coast, whereas Zostera muelleri (syn. Zostera capricornii) is a tropical to temperate species occurring in Australia and New Zealand only (Fig. 1) (Lee Long et al. 2000; Waycott et al. 2004). The two species only overlap in distribution in northeastern Australia and the Great Barrier Reef (GBR). In the GBR, the mean annual temperature is 25.8uC (Lough 2007), and in the hottest month (usually around February), mean monthly temperatures range from 27.5uC in the southern GBR to 31.5uC in the north (Fig. 1). Seasurface temperature in the GBR is projected to increase between 1uC and 3uC by 2100 under the Intergovernmental Panel on Climate Change (IPCC) A2 scenario (Lough 2007). At the higher end of this range, summer water temperatures would exceed 33uC. Although temperatures can reach 33uC in seagrass meadows under extreme conditions in the GBR (e.g., low tide), they do not persist, and therefore long-term summer temperatures of 33uC will be well above temperatures that occur within the distributional range of Z. muelleri. The objectives of this work were to: (1) test the thermal tolerance of two seagrass species with different geographical distributions to water temperatures at the lower and higher ends of the range projected to occur in the GBR within the current century, (2) identify the effects of light limitation on plant responses to these temperature increases, and (3) infer, using the observed thermal tolerances, likely changes to the future distributions of these species due to climate change. We hypothesized that H. uninervis would have a higher temperature threshold than Z. muelleri and that exceedance of the optimum metabolic temperature thresholds would be expressed at a plant scale. Methods Two seagrass species, Halodule uninervis Ascherson (Cymodoceaceae) and Zostera muelleri Irmisch ex Ascher- 2202 Collier et al. son (Zosteraceae), were tested for the combined effects of increased water temperature and reduced light intensity. The two species were collected from intertidal meadows at Cockle Bay and Picnic Bay (19u10.889S, 146u50.639E), Magnetic Island, northern Great Barrier Reef, in September 2009. At this site, daily mean water temperature ranges from 19uC to 31.5uC throughout the year (C. Collier unpubl. data), maximum monthly temperature is 29.6uC to 30.5uC (Fig. 1), and light intensity is 15.2 mol m22 d21 (annual average; C. Collier unpubl. data). The experiments were run for 1 month to align with maximum monthly temperature data (current and future predicted) used to establish the treatment levels (Fig. 1). Three temperature treatments were applied in a large-scale aquarium experiment: 27uC, 30uC, and 33uC. The light treatments were ‘‘high light’’ (400 mmol quanta m22 s21) and ‘‘low light’’ (40 mmol quanta m22 s21), set on a 12-h night and day cycle. The half-saturation constant (Ek) was derived from rapid light curves measured with a pulse amplitude modulated fluorometer at time 1, 2, and 3 and was on average 102 mmol quanta m22 s21 and 40 mmol quanta m22 s21 in high and low light for H. uninervis and was 138 mmol quanta m22 s21 and 90 mmol quanta m22 s21 for Z. muelleri. Therefore, the high-light treatment was well above saturating irradiance, and the low-light treatment was below saturating irradiance. Plugs of seagrass were collected using a 10-cm (internal diameter) polyvinyl chloride (PVC) corer, which was pushed into the sediment to a depth of 10 cm, and the intact plug of seagrass and sediment was placed in a plastic pot and lined with a plastic bag, which was pulled up and secured over the seagrass to retain humidity during transport to the aquaria. The seagrass was kept in an outdoor flow-through aquarium for 2 weeks prior to the experiment. Two weeks of ‘‘acclimation’’ were provided to allow recovery from any possible ‘‘shock’’ associated with harvesting of plants, including severing of rhizomes and possible disruption to roots in the sediment. The experiment was conducted in an indoor flow-through aquarium system at the Australian Institute of Marine Sciences. For each temperature treatment, there was a single header tank in which the water was heated and then dispersed. A computer-controlled thermometer regulated temperature to within 0.5uC of the target temperature. Water was supplied by a nearby coastal intake as a continuous through-flow (i.e., not recycled), and flow was maintained at a rate of , 9 mL s21 with complete exchange of the water in the treatment aquaria every 45 min. Light was supplied from a single halogen lamp mounted over the top of each tank, and the low-light treatment involved placing light-reducing black shade-cloth between the light source and the water surface. There were four replicate 50-liter tanks for each temperature treatment. Each tank was divided into a high-light and a low-light treatment using Perspex dividers, which were randomly assigned within the tanks. A separate inflow tap led to each side of the Perspex divider, and for outflow, the water from one section passed through a small mesh window (1-mm mesh) at the top of the divider into the other section. In each light and temperature treatment, there were two subreplicate pots of each species and the data from these subreplicates were averaged (mean) for later analysis. The experiment was run for a total of 35 d from 10 October 2009. Ambient temperature was , 27uC, and the temperature treatments were initiated on 10 October at 11:00 h, with the target temperatures of 27uC, 30uC, and 33uC reached at 05:00 h on 11 October. During the experiment, measurements were made in four blocks of time: days 26 to 21 (time 0), days 1–6 (time 1), days 17–23 (time 2), and days 28–34 (time 3). Growth was measured at each time according to Short and Duarte (2001). Ten shoots from each pot were marked at the top of the sheath with a needle. The length of growth (mm) was measured after 5 to 7 d on the shoots without removing them from the pots. Photosynthetic rates and respiration rates were measured at time 1 and time 3 on the upper 20–40 mm of the youngest mature leaf of a random shoot from each subreplicate pot using optical oxygen sensors (‘‘optodes,’’ PreSens, Sensor spots-Pst3) and a PreSens Oxy 4 four-channel fiber-optic oxygen meter. Small custom-made glass chambers (6.62 mL) were set in an array of four (i.e., four separate chambers allowing four parallel measures) and incubated at the treatment water temperature (27uC, 30uC, 33uC) using a flow-through water system connected to a water bath (Lauda, Ecoline RE 106). Each chamber was stirred with a glass-coated magnetic stirrer bar, and in each chamber, there was a perforated plastic shelf separating the stirrer from the seagrass material. The leaves were placed on top of the shelf in a U-shape, which resulted in minimal contact of the leaf with the glass chamber or plastic shelf. Oxygen consumption (dark respiration) was measured over a 20- to 30-min period in the dark. Photosynthetic rates were then measured on the same leaf fragment in the light (treatment light intensity, either 40 or 400 mmol quanta m22 s21) over 20 to 30 min. Oxygen concentration data in the chambers were logged every 30 s, and respective respiration and production rates were calculated by fitting a linear regression to the data. Regressions omitted the initial period of incubation (, 5 to 15 min) until rates had stabilized. Respiration rates of belowground rhizomes were also measured, but at time 3 only, because removal of the rhizome is destructive. A small piece of rhizome (5–10 mm) and associated roots were removed from the sediment, rinsed at treatment temperature, and then transferred to incubation chambers. Each optode was calibrated prior to initial measurements using a two-point method (100% O2 in air: using a wet sponge in the respiration chamber to saturate air; 0% using a 1% Na2SO3 solution). Individual chambers were cleaned with ethanol between incubations to prevent biofilm buildup, and at least 1 blank chamber was run on each measuring day to test for blank respiration. At the end of the experiment, the remaining biomass was collected, separated into above- and belowground biomass, dried at 80uC, and weighed. Metabolic rates were calculated according to the following: Lf PhsG mmol O2 g{1 dry weightðdry wtÞh{1 ~Lf PhsN {Lf Resp ð1Þ where Lf PhsG is the gross photosynthetic rate of leaves, Seagrass thermal tolerance i.e., with loss from respiration removed, Lf PhsN (mmol O2 g21 dry wt h21) is the net rate of O2 production of the leaf material (measured value, with respiration included), Lf Resp (mmol O2 g21 dry wt h21) is the respiration rate in the leaves, and dry wt is the mass of leaf material in dry weight. Total C budget of the shoot was calculated for time 3 only when biomass measures were also made. Firstly, biomass per shoot (WtShoot, g shoot21) was calculated from the sum of: WtLf ~ biomassA=Gr shoots ð2Þ biomassB=Gr shoots ð3Þ and Wtrhiz ~ where leaf weight (WtLf, g shoot21) is the weight of leaf material in each shoot, biomassA/Gr is the biomass of aboveground material (leaves and live sheath, g dry wt pot21), rhizome weight (Wtrhiz, g shoot21) is the weight of rhizome in each shoot, biomassB/Gr is the biomass of belowground parts (rhizome and roots, g dry wt pot21), and shoots is the number of shoots per pot (shoots pot21). Total leaf photosynthetic O2 production for a 24-h period was then calculated: Prod24 ~ðLf PhsG |12hÞ{ðLf Resp|24Þ ð4Þ where Prod24 is the rate of photosynthetic O2 production of the leaves after respiration rates of the leave are subtracted for 24 h (mmol O2 g dry wt21 d21). The respiration rate of the rhizome for a 24-h period was then calculated: RespRhiz24 (mmol O2 g dry wt{1 d{1 )~RespRhiz |24 ð5Þ where RespRhiz24 is respiration rate in the rhizome over 24 h, and RespRhiz is the hourly rate of respiration in the rhizome (mmol O2 g dry wt21 h21). Finally, the total production of carbon for the entire shoot (shoot, leaf + rhizome) over a 24-h period (C ProdN mg C shoot21 d21) was calculated from: C ProdN (mg C shoot{1 d{1 )~½ðProd24 {Resp24 Þ=P:Q: |(Mol WtC =1000)|Wtshoot ð6Þ where P.Q. is the photochemical quotient of tropical seagrasses (P.Q. 5 1; Pollard and Greenway 1993) and Mol WtC is the molecular weight of carbon (12). Statistical analysis—Metabolic data for time 1 and Time 3, and biomass data for time 3 were analyzed separately for each species using a two-way analysis of variance (ANOVA) testing for the fixed effects of temperature (three levels) and light (two levels). Repeated measures ANOVA (RM ANOVA) was used for growth rates, with temperature and light as fixed effects (between-subjects effects) over time (within-subjects effects). The variance–covariance 2203 matrices were tested using Mauchly’s test of sphericity, and if the assumption was not met, the Greenhouse-Geisser epsilon adjustment was applied to the degrees of freedom. Temperature effects were interpreted using Tukey’s posthoc analysis with light levels combined if no temperature and light interaction was observed or at each separate light level if an interaction occurred. Data were checked for homogeneity of variance using Levene’s test, and if they failed (p . 0.05), data were square-root or log transformed. If the transformation was not successful at improving the variance in the data, the ANOVA was performed but with significant p values set to 0.01 to minimize the risk of a type 1 error (Underwood 1997). Transformations and p values are shown for all significant results. Results Metabolic rates and whole plant carbon budgets—For the tropical species H. uninervis, the effects of light and temperature on leaf photosynthesis (Lf PhsG mmol O2 g21 dry wt h21; Eq. 1) and leaf respiration (Lf Resp mmol O2 g21 dry wt h21) were similar after 5 d (time 1) and 30 d (time 3). There was no effect of temperature or light on Lf Resp at time 1 or time 3 (Fig. 2A,B). For Lf PhsN, however, there was a significant temperature and light interaction (time 1 temperature 3 light p , 0.05; time 3 temperature 3 light p , 0.05; Table 1). Post-hoc analysis indicated that: in high light, Lf PhsG was significantly higher at 33uC than 27uC (time 1 Tukey’s p , 0.05; time 3 p , 0.01), but there was no difference between temperatures in low light, and Lf PhsG was faster in high light than low light at all temperatures at time 1 but only at 30uC (p , 0.05) and 33uC (p , 0.01) at time 3 (Fig. 2A,B). In summary, the photosynthesis to respiration ratio (P : R) of leaves only of H. uninervis was 5.6, 8.8, and 11.2 at 27uC, 30uC, and 33uC in high light, but it showed less variation in low light at 4.9, 6.5, and 7.0 at time 3. Lf Resp in Z. muelleri was affected by temperature at both time 1 and time 3 (two-way ANOVA time 1, temperature p , 0.05; time 3 temperature p , 0.01; Table 1) with Tukey’s post-hoc analysis revealing higher Lf Resp at 33uC compared to the 30uC at time 1 (p , 0.05) and at time 3 compared to both 27uC (p , 0.01) and 30uC (p , 0.05) (Fig. 2C,D). Lf PhsG was significantly affected by light at time 1 (two-way ANOVA p , 0.001) and was less than half the rate in low light compared to high light for all temperatures (Fig. 2C). At time 3, there was a significant interaction between temperature and light on Lf PhsG (twoway ANOVA p , 0.01): Tukey’s post-hoc analysis indicated that Lf PhsG was reduced at 33uC compared to 27uC (p , 0.01), but only in high light, and Lf PhsG was significantly faster in high light than low light at 27uC (p , 0.01) and 30uC (p , 0.01), but at 33uC, there was no difference between light treatments. In summary, the P : R ratios of Z. muelleri decreased with temperature: 14.1, 8.5, and 2.1 at 27uC, 30uC, and 33uC in high light and 8.3, 3.8, and 3.7 in low light at time 3. Respiration rates in the rhizomes (RespRhiz) were measured at time 3 only, and were, on average, 22 and 19 mmol O2 g21 dry wt h21 in H. uninervis and Z. muelleri, 2204 Collier et al. Fig. 2. Gross photosynthetic rate of leaves (Lf PhsG) and respiration rate of leaves (Lf Resp) for H. uninervis at (A) time 1 (5 d) and (B) time 3 (30 d) and Z. muelleri at (C) time 1 and (D) time 3 in high light and low light at 27uC (light gray), 30uC (medium gray), and 33uC (dark gray). Letters indicate significant differences between temperature treatments based on Tukey’s post-hoc analysis; asterisks indicate significant differences between light treatments based on ANOVA results where there was no interaction between temperature and light or based on Tukey’s post-hoc analysis performed at each temperature if an interaction occurred (** p , 0.01, *** p , 0.001); n 5 4 6 SE. respectively (Fig. 3). This was substantially lower than the respiration rate of leaves, which were, on average, three times and two times faster at 71 and 41 mmol O2 g21 dry wt h21 in H. uninervis and Z. muelleri, respectively. Respiration was highly variable, but it was not significantly affected by temperature or light for either species (Table 1). Net C production (C ProdN, mg C shoot21 d21; Eq. 6) of H. uninervis was affected by a temperature and light interaction (two-way ANOVA temperature 3 light p , 0.001; Table 1). Tukey’s post-hoc analyses reveal that in high light only, C ProdN was lower at 27uC than at 30uC (53 lower; p , 0.05) or 33uC (103 lower; p , 0.001), and C ProdN was greater in high light than low light at 30uC (p , 0.001) and 33uC (p , 0.01; Fig. 4A). For Z muelleri, there was a significant effect of temperature on C ProdN (twoway ANOVA temperature p , 0.001), with Tukey’s posthoc analysis indicating that C ProdN was lower at 33uC than at 27uC (p , 0.01) or 30uC (p , 0.01). There was also an effect of light (two-way ANOVA light p , 0.001) on C ProdN, which was reduced in low light compared to high light (Fig. 4B). Growth and biomass—There was a significant effect of light on growth rates of H. uninervis, but it depended on Seagrass thermal tolerance 2205 Table 1. Results of two-way ANOVA testing for the fixed effects of temperature (27uC, 30uC, 33uC) and light level (high, 400 mmol m22 s21 and low, 40 mmol m22 s21) on gross leaf photosynthesis (Lf PhsG), leaf respiration (Lf Resp), rhizome respiration (RespRhiz), and net carbon production (C ProdN) of the seagrasses Halodule uninervis and Zostera muelleri. Transformations are indicated where applied. MS, mean square. * p , 0.05, ** p , 0.01, *** p , 0.001; n 5 4 6 SE. H. uninervis Factor df Temperature Light Temp3light 2 1 3 Temperature Light Temp3light 2 1 3 Temperature Light Temp3light 2 1 3 Lf Resp (mmol O2 g21dry wt h21) Temperature Light Temp3light 2 1 3 RespRhiz (mmol O2 g21dry wt h21) Temperature Light Temp3light 2 1 3 C ProdN (mg C shoot21 d21) Temperature Light Temp3light 2 1 3 Time 1 Lf PhsG (mmol O2 g21dry wt h21) Lf Resp (mmol O2 g21dry wt h21) Time 3 Lf PhsG (mmol O2 g21dry wt h21) MS F Z. muelleri p 31,016.933 4.405 550,349.090 78.159 31,930.389 4.535 No transformation p,0.05 1371.941 1.534 2307.545 2.580 116.242 1.534 No transformation p,0.05 * *** * 182,586.281 10.204 570,818.231 31.902 77,885.366 4.353 No transformation p,0.05 202.423 0.181 336.002 0.300 342.615 0.306 No transformation p,0.05 0.397 1.761 0.025 0.111 0.712 3.159 Ln transformation p,0.05 0.258 17.439 0.813 54.843 0.212 14.314 Ln transformation p,0.01 ** *** * *** *** *** MS F 4151.520 1.032 457,310.877 1.137 53.487 0.013 No transformation p,0.05 1230.272 4.398 217.999 0.779 953.263 3.408 No transformation p,0.05 36,961.325 6.636 101,572.708 18.237 35,136.168 6.309 No transformation p,0.05 1360.479 7.867 566.516 3.276 246.063 1.423 No transformation p,0.05 0.690 1.855 0.035 0.095 0.067 0.181 Ln transformation p,0.05 0.258 10.953 0.492 20.897 0.029 1.230 No transformation p,0.05 p *** * ** *** ** ** *** *** Fig. 3. Respiration rates in the rhizomes in high light (left-hand bars) and low light (right-hand bars) for (A) Halodule uninervis and (B) Zostera muelleri at 27uC (light gray), 30uC (medium gray), and 33uC (dark gray); n 5 4 6 SE. 2206 Collier et al. Fig. 4. Net shoot production (C ProdN mg C shoot21 d21) for (A) Halodule uninervis and (B) Zostera muelleri in high light and low light at time 3 (30 d) at 27uC (light gray), 30uC (medium gray), and 33uC (dark gray). Letters indicate significant differences between temperature treatments based on Tukey’s post-hoc analysis; asterisks indicate significant differences between light treatments based on ANOVA results where no interaction occurred between temperature and light or based on Tukey’s post-hoc analysis if an interaction occurred (** p , 0.01, *** p , 0.001); n 5 4 6 SE. time (RM ANOVA time 3 light p , 0.001; Table 2). Tukey’s post-hoc analysis indicated that there was no difference between light treatments at time 0, but at times 1 (p , 0.05), 2 (p , 0.001), and 3 (p , 0.001), growth rate was significantly faster in high light compared to low light (Fig. 5A). For Z. muelleri, there was a highly significant effect of temperature on growth rates that was affected by time (RM ANOVA time 3 temperature p , 0.001; Table 2), and post-hoc analyses indicated no significant difference between temperature treatments at time 0, but on all other days, growth in the 33uC treatments was slower than all other treatments (Tukey’s p , 0.001). There was also a significant effect of light in the between-subjects test (RM ANOVA light p , 0.001; Table 2), where leaf growth was slower in the low-light compared to the high-light treatments. Biomass was measured only at the conclusion of the experiment (time 3). For H. uninervis, there was a significant effect of the light treatment (two-way ANOVA light p , 0.001; Table 3) on aboveground biomass, with less biomass in low light than in high light for all temperatures (Fig. 6A). There was no significant effect of temperature or light on the belowground biomass of H. uninervis. In Z. muelleri, there was a significant effect of temperature (two-way ANOVA temperature p , 0.001; Table 3) on the aboveground biomass, with Tukey’s posthoc analysis indicating that biomass was reduced at 33uC compared to both 27uC and 30uC (Tukey’s post-hoc p , 0.001; Fig. 6B). There was also a significant effect of light on the aboveground biomass (two-way ANOVA light p , 0.001; Table 3) and on the belowground biomass (two-way ANOVA light p , 0.01; Table 3), with reduced biomass in low light compared to high light. The belowground biomass was considerably larger than the aboveground biomass, ranging from 3.73 (33uC high light) to 253 more (30uC low light) in H. uninervis and from 2.13 (27uC high light) to 213 (33uC low light) more in Z. muelleri Table 2. Results of two-way RM ANOVA testing for the fixed effects of temperature (27uC, 30uC, 33uC) and light level (high, 400 mmol m22 s21 and low, 40 mmol m22 s21) on leaf growth (mm d21) of the seagrasses Halodule uninervis and Zostera muelleri. Transformations are indicated where applied. MS, mean square; Sqrt, square root. *** p , 0.001. H. uninervis Test Effects df Within-subjects effects Time Time3temperature Time3light Time3temperature3light Temperature Light Temperature3light 3 6 3 6 2 1 2 Between-subjects effects MS F4 Z. muelleri p 1.280 31.897 *** 0.094 2.344 1.194 29.748 *** 0.057 1.414 0.051 1.548 6.714 204.977 *** 0.091 2.772 Ln transformation p,0.01 MS 5.572 1.913 0.120 0.075 5.538 0.845 0.021 F4 p 90.058 *** 30.916 *** 1.945 1.209 121.594 *** 18.551 *** 0.461 Sqrt transformation p,0.05 Seagrass thermal tolerance 2207 Fig. 5. Leaf growth rate (mm d21) of (A) Halodule uninervis and (B) Zostera muelleri at 27uC (circles), 30uC (square), and 33uC (triangle) in high light (white fill) and low light (dark fill) at time 0 (T0, 0 d) through to time 3 (T3, 32 d) . Significant (p , 0.05) results of RM ANOVA and post-hoc analyses are shown. Asterisks indicate significant differences between light treatments (* p , 0.05, ** p , 0.01, *** p , 0.001); n 5 4 6 SE. (Fig. 6A,B). Changes to morphological characteristics of the shoots, including a reduction in leaves per shoot and leaf length, were large contributors to the reduction in aboveground biomass; there was little difference in shoot density among treatments (C. Collier unpubl.). Discussion This study has demonstrated that H. uninervis and Z. muelleri, although overlapping in their distribution throughout the GBR, have distinctly different temperature thresholds, which could affect their distributions and relative dominance if sea temperatures increase according to projections. H. uninervis can be considered a truly tropical species: its photosynthetic rates, overall net carbon production, and consequently growth and biomass increased with temperature from 27uC to 33uC. These responses are consistent with those of other higher plants following increases in temperature that fall within their physiological optimum temperature range (Berry and Bjorkman 1980; McDonald 2003). However, photosynthetic sensitivity to high temperature is greatest at saturating light levels (Berry and Bjorkman 1980; Bulthius 1987), as demonstrated in this study by the response of H. uninervis. In contrast, at low and limiting light levels, photosynthetic rates of H. uninervis were drastically reduced, and temperature had little effect on net photosynthesis. As a result, in limiting light, there was no difference in overall carbon (C) production with increasing temperature and no temperature effect on growth and biomass, which were both reduced compared to the high-light treatments. In low and limiting light, photosynthesis and respiration rates are expected to have a lower threshold temperature than in high light (Berry and Bjorkman 1980), but this threshold temperature was not reached in these experiments. Habitat conditions that provide saturating light levels to H. uninervis enable faster rates of photosynthesis, growth, Table 3. Results of a two-way ANOVA testing for the fixed effects of temperature (27uC, 30uC, 33uC) and light level (high, 400 mmol m22 s21 and low, 40 mmol m22 s21) on aboveground and belowground biomass of the seagrasses Halodule uninervis and Zostera muelleri. Transformations are indicated where applied. MS, mean square; Sqrt, square root. ** p , 0.01, *** p , 0.001. H. uninervis Factor df pot21) Light Temperature Light3temperature 1 2 2 Belowground biomass (g pot21) Light Temperature Light3temperature 1 2 2 Aboveground biomass (g MS F Z. muelleri p 0.150 64.730 *** 0.003 1.202 0.003 1.330 Sqrt transformation p,0.05 0.118 4.327 0.069 2.530 0.004 0.135 No transformation p,0.05 MS F p 0.055 11.253 ** 0.143 29.045 *** 0.003 0.545 Sqrt transformation p,0.01 0.076 11.869 ** 0.007 1.171 0.005 0.797 No transformation p,0.05 2208 Collier et al. Fig. 6. Aboveground biomass (top) and belowground biomass (bottom) in high light (lefthand bars) and low light (right-hand bars) for (A) Halodule uninervis and (B) Zostera muelleri at 27uC (light gray), 30uC (medium gray), and 33uC (dark gray). Letters indicate significant differences between temperature treatments based on Tukey’s post-hoc analysis; asterisks indicate significant differences between light treatments based on ANOVA results where no interaction occurred between temperature and light or based on Tukey’s post-hoc analysis if an interaction occurred (** p , 0.01, *** p , 0.001); n 5 4 6 SE. and biomass production and should enable it to not only survive future increases in water temperature up to 33uC, but may also result in an increased abundance of this species in the GBR. Sea-surface temperature throughout the range of H. uninervis reaches 33–34uC (Fig. 1). The threshold temperature for H. uninervis is evidently greater than 33uC, since the upper threshold was not reached in this study. This would imply that the upper thermal limit of this species is not currently exceeded, and future temperature increases will lead to an increase in growth and biomass throughout its distributional range until its thermal limit is reached. However, in shallow nearshore habitats, water-temperature increases of more than 6uC have been observed, leading to temperatures in seagrass habitats over 40uC caused by high air temperatures and the high conductivity of water (Campbell et al. 2006; Anthony and Kerswell 2007; Massa et al. 2009). These temperature spikes typically only last a few hours at low tide, but the effect can be significant; photosynthetic impairment and ‘‘burning’’ of leaves can ensue for species that are not adapted to it. Increases in the severity and frequency of high-temperature extremes are likely to occur as the climate changes (IPCC 2007; Lough 2007), thus increasing the frequency with which physiological optima are exceeded in shallow-water habitats. Therefore, predictions of future seagrass distribution based on Seagrass thermal tolerance SST will be complex, and responses in shallow habitats will deviate from those predicted in less dynamic water bodies. At the sampling site, water temperature can reach 30– 30.5uC in summer (December–February). A maximum increase of 3uC is projected by 2100 under the A2 scenario, taking mean water temperatures to 33uC (Lough 2007), and, therefore, H. uninervis should remain within its physiological optimum temperature range within the GBR. We might expect this species to colonize areas further south along the east Australian coast as warmer temperatures extend to the south, expanding its distribution to central New South Wales; however, this assumes that its southern distribution is not constrained by other habitat conditions, including appropriate substrate, light, salinity, nutrient availability, and suitable disturbance regimes. In contrast to the responses of H. uninervis, Z. muelleri was severely affected by the 33uC temperature treatment; its photosynthetic rates plummeted and respiration rates increased at 33uC compared to 30uC. Plants can withstand negative carbon (C) balances for short durations by drawing on storage reserves, but Z. muelleri also made growth and structural modifications at 33uC, which reduced the energetic costs of maintenance of the plant (Fourqurean and Zieman 1991). These responses are consistent with other higher plants when physiological optimum temperatures have been exceeded (Berry and Bjorkman 1980). The photosynthetic mechanisms became impaired to such a degree at 33uC that Z. muelleri could not benefit from the saturating light levels of the high-light treatment. This change in photosynthetic rate was not immediate, however; within the first 5 d (time 1), this 30– 33uC threshold was absent for photosynthesis, and only a small reduction in respiration was observed. The Z. muelleri plants used in these experiments were collected a considerable distance from their northern distributional limit; however, summer maximum sea-surface temperature varies only slightly (0.5–1uC) over this distance (Fig. 1). The region where samples were collected occurs towards the northern, tropical limit of this species distribution, and thus they are less likely to have broad phenotypic tolerances compared with that of populations situated within the center of the species distribution. The genetic diversity within the region is likely to be reduced due to the central-marginal theory, where edge of range populations often exhibit reduced genetic diversity and narrower phenotypic tolerances to environmental stressors (Vucetich and Waite 2003). However, specific testing of plants from extreme northern populations (i.e., those exposed to higher water temperature) is needed to evaluate if they are better adapted to higher temperatures. The ecological roles of H. uninervis and Z. muelleri are not interchangeable (Carruthers et al. 2002), and displacement of Zostera from northern seagrass ecosystems would affect the overall functioning of the seagrass communities it occupies. Projected increases in water temperature for the GBR (up to 3uC by 2100 under A2 scenario; Lough 2007) could see maximum monthly water temperature in the GBR from the Torres Strait to Mackay increase to well over 33uC (Fig. 1). This could potentially result in a contraction of the distribution of Z. muelleri by more than 2209 1000 km and result in local extinction in the northern GBR. Such a loss could have ecologically significant ramifications because this species tends to occupy habitats that few other species can inhabit—it is typically found in shallow, muddy estuarine waters (Lee Long et al. 1993; Carruthers et al. 2002). In these habitats, there may be no equivalent species that can act as a functional replacement amongst the tropical species because it often forms monospecific meadows, tolerating conditions that are not suitable for other seagrass species. The environmental factors that currently limit the distribution of the tropical estuarine and coastal species (e.g., Enhalus acroides) are not known, making it difficult to predict their responses to future environmental change and their potential to act as a functional replacement for Z. muelleri. The two species studied here—Halodule uninervis, which is predominantly tropical, and Zostera muelleri, which is predominantly temperate—exhibited contrasting responses to increasing water temperatures. They also represent members of different seagrass families, possessing different evolutionary histories. Their responses may reflect these different histories, and different physiological strategies for survival in the marine environment, which are as yet unresolved for these taxa. In addition, light levels were also critical to their response to increasing temperature: Saturating light was required for H. uninervis to respond positively to increasing temperature, whereas Z. muelleri responded by going into carbon deficit at limiting light levels, although it maintained a positive balance under saturating light. This has important implications for the management of these species into the future, as maintaining and improving water quality—and thus maximizing light levels—will enhance resilience of both species to elevated temperatures. Acknowledgments We thank A. Negri and R. Berkelmans for assistance running the aquaria systems, and A. Giraldo Ospina and D. Tracey for assistance with seagrass collection and seagrass measurement throughout. We are grateful to S. Kininmonth for his assistance in preparing temperature Geographical Information Systems maps based on the data provided at http://www.oracle.ugent.be/. We also thank the reviewers of this manuscript for their feedback. This work was funded by the Marine and Tropical Sciences Research Facility, Department of Environment, Heritage and the Arts (Australian government). References ANTHONY, K. R. N., AND A. P. KERSWELL. 2007. Coral mortality following extreme low tides and high solar radiation. Mar. Biol. 151: 1623–1631, doi:10.1007/s00227-006-0573-0 BERRY, J., AND O. BJORKMAN. 1980. Photosynthetic response and adaptation to temperature in higher plants. Ann. Rev. Plant Physiol. 31: 491–543, doi:10.1146/annurev.pp.31.060180. 002423 BULTHIUS, D. A. 1987. Effects of temperature on photosynthesis and growth of seagrasses. Aquat. Bot. 27: 27–40, doi:10.1016/ 0304-3770(87)90084-2 CAMPBELL, S. J., L. J. MCKENZIE, AND S. P. KERVILLE. 2006. Photosynthetic responses of seven tropical seagrasses to elevated seawater temperature. J. Exp. Mar. Biol. Ecol. 330: 455–468, doi:10.1016/j.jembe.2005.09.017 2210 Collier et al. CARRUTHERS, T. J. B., W. C. DENNISON, B. J. LONGSTAFF, M. WAYCOTT, E. G. ABAL, L. J. MCKENZIE, AND W. J. LEE LONG. 2002. Seagrass habitats of northeast Australia: Models of key processes and controls. Bull. Mar. Sci. 71: 1153–1169. CHAPIN, F. S., A. J. BLOOM, C. B. FIELD, AND R. H. WARING. 1987. Plant responses to multiple environmental factors. Bioscience 37: 49–57, doi:10.2307/1310177 COLLIER, C. J., P. S. LAVERY, R. J. MASINI, AND P. J. RALPH. 2009. Shade-induced response and recovery of the seagrass Posidonia sinuosa. J. Exp. Mar. Biol. Ecol. 370: 89–103, doi:10.1016/ j.jembe.2008.12.003 DE’ATH, G., AND K. FABRICIUS. 2010. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl. 20: 840–850, doi:10.1890/08-2023.1 DENNISON, W. C. 1987. Effects of light on seagrass photosynthesis, growth and depth distribution. Aquat. Bot. 27: 15–26, doi:10.1016/0304-3770(87)90083-0 ENRı́QUEZ, S. 2005. Light absorption efficiency and the package effect in the leaves of the seagrass Thalassia testudinum. Mar. Ecol. Prog. Ser. 289: 141–150, doi:10.3354/meps289141 FOURQUREAN, J. W., AND J. C. ZIEMAN. 1991. Photosynthesis, respiration and whole plant carbon budget of the seagrass Thalassia testudinum. Mar. Ecol. Prog. Ser. 69: 161–170, doi:10.3354/meps069161 HECK, K. L., T. J. B. CARRUTHERS, C. M. DUARTE, A. R. HUGHES, G. KENDRICK, R. J. ORTH, AND S. W. WILLIAMS. 2008. Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems 11: 1198–1210, doi:10.1007/ s10021-008-9155-y INTERGOVERNMENTAL PANEL ON CLIMATE CHANGE (IPCC) 2007. Summary for policymakers, p. 12–17. In S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, and H. L. Miller [eds.], p. 12–17. Climate change 2007: The physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. The Intergovernmental Panel on Climate Change. LEE LONG, W. J., R. G. COLES, AND L. J. MCKENZIE. 2000. Issues for seagrass conservation management in Queensland. Pac. Conserv. Biol. 5: 321–328. ———, J. E. MELLORS, AND R. G. COLES. 1993. Seagrasses between Cape York and Hervey Bay, Queensland, Australia. Aust. J. Mar. Freshw. Res. 44: 19–31. LOUGH, J. 2007. Climate and climate change on the Great Barrier Reef, p. 15–50. In J. E. Johnson and P. A. Marshall [eds.], Climate change and the Great Barrier Reef. Great Barrier Reef Marine Park Authority. MASSA, S., S. ARNAUD-HAOND, G. PEARSON, AND E. SERRÃO. 2009. Temperature tolerance and survival of intertidal populations of the seagrass Zostera noltii (Hornemann) in southern Europe (Ria Formosa, Portugal). Hydrobiologia 619: 195–201, doi:10.1007/s10750-008-9609-4 MCDONALD, M. S. 2003. Photobiology of higher plants. John Wiley and Sons. ORTH, R. J., AND OTHERS. 2006. A global crisis for seagrass ecosystems. Bioscience 56: 987–996, doi:10.1641/00063568(2006)56[987:AGCFSE]2.0.CO;2 POLLARD, P. C., AND M. GREENWAY. 1993. Photosynthetic characteristics of the seagrasses (Cymodocea serrulata, Thalassia hemprichii and Zostera capricorni) in a low-light environment with a comparison of leaf-marking and lacunal-gas measurements of productivity. Aust. J. Mar. Freshw. Res. 44: 127–139. RALPH, P. J., M. J. DURAKO, S. ENRIQUEZ, C. J. COLLIER, AND M. A. DOBLIN. 2007. Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 350: 176–193, doi:10.1016/ j.jembe.2007.06.017 RASHEED, M. A., AND R. K. F. UNSWORTH. 2011. Long-term climate-associated dynamics of a tropical seagrass meadow: Implications for the future. Mar. Ecol. Prog. Ser. 422: 93–103, doi:10.3354/meps08925 SCHULZE, E. D., E. BECK, AND K. MÜLLER-HOHENSTEIN. 2002. Plant ecology. Springer. SHORT, F. T., AND C. M. DUARTE. 2001. Methods for the measurement of seagrass growth and production, p. 155–182. In F. T. Short and R. Coles [eds.], Global seagrass research methods. Elsevier Science. UNDERWOOD, A. J. 1997. Experiments in ecology: Their logical design and interpretation using analysis of variance. Cambridge Univ. Press. UNSWORTH, R. K. F., AND L. C. CULLEN. 2010. Recognising the necessity for Indo-Pacific seagrass conservation. Conserv. Lett. 3: 63–73, doi:10.1111/j.1755-263X.2010.00101.x VUCETICH, J. A., AND T. A. WAITE. 2003. Spatial patterns of demography and genetic processes across the species’ range: Null hypotheses for landscape conservation genetics. Conserv. Genet. 4: 639–645, doi:10.1023/A:1025671831349 WAYCOTT, M., K. MCMAHON, J. MELLORS, A. CALLADINE, AND D. KLEINE. 2004. A guide to tropical seagrasses of the Indo-West Pacific. James Cook Univ. ———, AND OTHERS. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 106: 12377–12381, doi:10.1073/pnas.0905620106 Associate editor: Anthony W. D. Larkum Received: 07 May 2011 Accepted: 04 August 2011 Amended: 09 August 2011