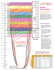Gait dysfunctions are commonly encountered in the pri-
Transcription
Gait dysfunctions are commonly encountered in the pri-
CASE REPORT Editor’s Note: Corrections to this article were published in the March 2010 issue of JAOA—The Journal of the American Osteopathic Association (2010;110[3]:210). The corrections have been incorporated in this online version of the article, which was posted January 2011. An explanation of these changes is available at http://www.jaoa .org/cgi/content /full/110/3/210-a. Use of Osteopathic Manipulative Treatment to Manage Compensated Trendelenburg Gait Caused by Sacroiliac Somatic Dysfunction Adam C. Gilliss, DO; Randel L. Swanson, II, OMS III; Deanna Janora, MD; and Venkat Venkataraman, PhD Gait dysfunctions are commonly encountered in the primary care setting. Compensated Trendelenburg gait is a gait dysfunction that was originally described in patients with weakness of ipsilateral hip abduction. This condition is thought to result from neuronal injury or myopathy. No treatment modalities currently exist for compensated Trendelenburg gait. The authors present a case in which osteopathic manipulative treatment may have improved a Trendelenburg gait dysfunction in a man aged 65 years with multiple sclerosis. Evidence of this improvement was obtained with the GaitMat II system for measuring numerous gait parameters. Based on the results reported in the present case, the authors propose that compensated Trendelenburg gait may arise from somatic dysfunction and may be corrected by osteopathic manipulative treatment. [Published correction appears in J Am Osteopath Assoc. 2010;110(3):210.] J Am Osteopath Assoc. 2010;110(2):81-86 P hysicians routinely encounter gait dysfunctions in the primary care setting. Abnormal gait patterns have many causes and may represent either a primary or secondary (ie, compensatory) gait dysfunction. Compensated Trendelenburg gait, also known as compensated gluteus medius gait, was originally described in the late 1800s by German surgeon Friedrich Trendelenburg in patients with weakness of ipsilateral hip abduction.1 This weakness is thought to typically result from injury to the superior gluteal nerve or from a pathologic condition affecting proximal hip abductor muscles, primarily the gluteus medius. Currently, no treatment modalities exist for patients with compensated Trendelenburg gait. From the Department of Osteopathic Manipulative Medicine (Dr Gilliss) and the Department of Cell Biology (Student Doctor Swanson and Dr Venkataraman) at the University of Medicine and Dentistry of New JerseySchool of Osteopathic Medicine in Stratford; and the JFK Johnson Rehabilitation Institute (Dr Janora) at the University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School in Edison. This study was supported by intramural grants from the Osteopathic Heritage Foundations (V.V., R.L.S.). Financial Disclosures: None reported. Address correspondence to Adam C. Gilliss, DO, 27 E Chestnut Ave, Merchantville, NJ 08109-2504. E-mail: [email protected] Submitted August 22, 2008; final revision received April 13, 2009; accepted April 22, 2009. Gilliss et al • Case Report In the present case report, we provide evidence that compensated Trendelenburg gait may represent a secondary gait dysfunction stemming from somatic dysfunction of the sacroiliac joints. We also describe evidence of osteopathic manipulative treatment (OMT) resulting in quantitative improvements in the gait cycle. Traditional and Osteopathic Gait Theory The gait cycle is divided into two main phases—stance and swing, each consisting of numerous subphases.2,3 Traditionally, the human gait cycle is considered to have six determinants that function independently, yet simultaneously, to generate the normally fluid, continuous movements of ambulation.4 The first two determinants of the gait cycle—pelvic rotation and pelvic tilt—involve the pelvis, including the right and left innominate bones and the sacrum. During the swing phase of the gait cycle, the pelvis rotates forward on the side of the swinging leg about a transverse plane, with the hip joint of the stance leg serving as the axis of rotation. In addition to this forward rotation, Trendelenburg tilt occurs on the side of the swinging leg, with the pelvis dropping by approximately 5 degrees.2 The degree of tilt is limited by contraction of the hip abductor muscles of the stance limb, primarily the gluteus medius. Also during Trendelenburg tilt, there is a lateral shift of the pelvis toward the stance leg, resulting in a shift in the center of gravity.2 In traditional gait theory, the pelvis is thought to function and move as a single unit, with the individual components immobile relative to one another.2,4 Osteopathic theory, however, holds that individual motions occur between the right and left innominate bones and the sacrum.5-7 These individual motions arise when torsion forces are created within the pelvis during the gait cycle, leading to variations in the timing of rotation, tilt, and lateral shift between the sacrum and innominates. Studies have documented the range of motion and the degree of rotation in these bones.8,9 Thus, clinical gait analysis must incorporate both traditional and osteopathic gait theory to arrive at the correct diagnosis. Physicians typically rely on visual observation alone to assess both normal and pathologic gait. Gait disturbances may arise from pathologic conditions of the spinal cord and lower body, as well as from cognitive and neurologic disorders of the brain.10-12 Gait analysis systems, such as GaitMat II (EQ Inc, Chalfont, Pennsylvania), are now available for making reliable JAOA • Vol 110 • No 2 • February 2010 • 81 CASE REPORT measurements of numerous spatial and temporal gait parameters as a patient walks across a mat, 12 feet in length, that has electronic sensors connected to a computer. These systems, which can aid physicians in diagnosing gait pathologic conditions,13 are increasingly used in the clinical setting.1,14-15 Although the GaitMat II system measures only foot contact patterns recorded on the mat, these patterns arise from the collective motions of the individual musculoskeletal components that contribute to bipedal locomotion.16 Therefore, GaitMat II can be used to assess normal and abnormal gait patterns, as well as the effects of somatic dysfunction and OMT on gait. We used GaitMat II in the present case report to evaluate a patient with Trendelenburg gait before and after OMT. Report of Case A man aged 65 years with a history of relapsing and remitting multiple sclerosis presented to the office of an osteopathic physician (A.C.G.) in 1999 with a chief complaint of back and hip pain. He had been diagnosed as having multiple sclerosis in 1991 based on findings from a magnetic resonance imaging (MRI) scan. From 1991 to 1999, he was treated with standard medications for multiple sclerosis, including interferon beta1a and glatiramer acetate. The patient sought care from the osteopathic physician when he experienced marked deterioration in ambulation in March 1999 resulting in the need for a walker and motorized wheelchair. The patient’s comorbid conditions at presentation included rheumatoid arthritis and benign prostatic hyperplasia. The patient’s surgical history was unremarkable. The osteopathic physician (A.C.G.) initiated OMT in 1999 to manage somatic dysfunctions detected throughout the patient’s body. This treatment has continued to the present day. For the present case, we focus on two OMT sessions conducted in September and October 2006, during which gait analysis and videotaping of the patient were conducted. Medications used by the patient at the time of the present study included vitamin B12 injections (1000 g, intramuscular, once weekly); tamsulosin hydrochloride (0.4 mg daily) and dutasteride (0.5 mg daily) for benign prostatic hyperplasia; celecoxib (200 mg daily) and hydroxychloroquine sulfate (200 mg daily) for rheumatoid arthritis; and alprazolam (0.5 mg three times daily, as needed) for anxiety. Gait Analysis Before and After OMT In the present study, the patient had two appointments (ie, trials)—one in September 2006 and the other 1 month later, in October 2006—during which his gait was analyzed as he walked across the GaitMat II mat.13 (Weekly OMT sessions occurred between these two appointments as part of the patient’s regular treatment.) Videotaped recording of the patient during ambulation was also performed. In each appointment, the GaitMat II analysis and video recording 82 • JAOA • Vol 110 • No 2 • February 2010 occurred once before and once after OMT was administered by the osteopathic physician. Values derived from the GaitMat II analysis for step length, stride length, stance time, double support time, number of steps, and velocity were recorded for the left and right sides, and the averages of these values were calculated for pretreatment and posttreatment in both trials. We analyzed the following four parameters: ▫ step length—distance along the mat from the “first switch closure” of one footprint to the first switch closure of the next footprint on the contralateral side, typically the distance from the heel of one foot to the heel of the opposite foot ▫ stride length—distance along the mat from the first switch closure of one footprint to the first switch closure of the next footprint on the ipsilateral side, typically the distance from the heel of one foot to the heel of the same foot where it falls next ▫ stance time—time during which a foot is in contact with the mat ▫ double support time—time during which both feet are in contact with the mat Statistical analyses of all values were conducted initially with InStat software (version 4.0; GraphPad Software Inc, La Jolla, California) and were repeated with SigmaStat (version 4.0; Systat Software Inc, San Jose, California) and Excel (Office Professional 2007; Microsoft Corp, Redmond, Washington) software. Diagnosis of Somatic Dysfunction Video analysis of the patient demonstrated an antalgic gait with left lateral trunk lean and hip hiking to compensate for decreased clearance of the right leg in the swing phase of the gait cycle. This pretreatment video was shown separately to three physicians—two physiatrists (including D.J.) and one osteopathic family physician who was board certified in neuromusculoskeletal medicine and osteopathic manipulative medicine. Based on this video, all of these physicians diagnosed the patient as having a compensated Trendelenburg gait. Biomechanical examination of the patient during the OMT sessions revealed somatic dysfunction within the sacroiliac joints, in addition to other somatic dysfunctions throughout the body. The sacroiliac diagnoses in both trials were right-onright sacral torsion and posterior rotation of the left innominate. OMT Application Osteopathic manipulative treatment was administered to the patient in each of the two trials. For the right-on-right sacral torsion and the posterior rotation of the left innominate, the patient was treated with standard OMT muscle energy techniques.16-18 Gilliss et al • Case Report CASE REPORT Trial 1 Trial 2 Pretreatment (18) (18) Posttreatment (8) (7) Left Right Direction of Motion Figure 1. Step patterns of the patient in the present case—a 65-yearold man with compensated Trendelenburg gait—as traced by the GaitMat II (EQ Inc, Chalfont, Pennsylvania) gait analysis system before and after osteopathic manipulative treatment in trial 1 and—1 month later—in trial 2. The number of steps is shown within parentheses next to each tracing. This analysis revealed a 58% decrease in the number of steps after treatment, indicative of an improvement in the patient’s compensated Trendelenburg gait. Briefly, the sacral torsion was addressed by manipulating the left gluteus maximus muscle in an isometric contraction to pull the left sacral base posteriorly, and the posterior rotation of the left innominate was counteracted by manipulating the iliopsoas and rectus femoris muscles to rotate the innominate anteriorly during an isometric contraction. This difference vanished after treatment (left, 0.50 [0.05] m; right, 0.51 [0.03] m; P=.691). These results suggest that analysis of step length variation may serve as a diagnostic tool for Trendelenburg gait, as well as an evaluation method for gait improvement after OMT. The Table provides numerical values for two additional parameters—stance time and double support time. Results showed statistically significant decreases after OMT for both the left and right sides in stance time (left, -33%; right, -24%; P⬍.001) and double support time (left, -44%; right, -48%; P⬍.001). These results are consistent with the observed increase in velocity and demonstrate that the OMT sessions resulted in improvements in ambulation. Results Analysis of the posttreatment video showed substantial improvement in the patient’s gait, with a dramatic decrease in the compensated gluteus medius pattern. Specifically, left lateral trunk lean and hip hiking were noticeably decreased, indicating that the OMT was effective. Posttreatment analyses using the GaitMat II system provided quantitative evidence that supported the video-based conclusions. Figure 1 shows the GaitMat II tracing of the patient’s steps from both trials before and after OMT. This analysis revealed a 58% decrease after treatment in the number of steps taken across the mat, with a mean of 18 steps in the pretreatment analysis and 7.5 steps in the posttreatment analysis (P⬍.01). The changes in mean step length, stride length, and velocity after OMT in both trials are provided in the Table and Figure 2. After OMT, analyses revealed significant increases in both left and right step lengths (left, +85%; right, +216%; P⬍.001 for each) and left and right stride lengths (left, +136%; right, +128%; P⬍.001 for each). An increase of 193% was observed in the patient’s velocity after OMT, with a pretreatment value of 0.21 m per second and a posttreatment value of 0.81 m per second (P⬍.01). Consistent with a compensated Trendelenburg gait, a statistically significant difference was observed between the mean (SD) right and left step lengths before treatment (Figure 3), with the right step length 41% shorter than the left step length (left, 0.27 [0.04] m; right, 0.16 [0.06] m; P⬍.05). Gilliss et al • Case Report Follow Up The patient has not needed a walker or wheelchair or used any multiple sclerosis–related medication since 2001. The patient’s gait improvement was such that his neurologist questioned the original 1991 diagnosis of multiple sclerosis and referred the patient to a multiple sclerosis specialist, who ordered a new MRI in 2006. Both the neurologist and a radiologist reviewed the results from this MRI and confirmed the diagnosis of multiple sclerosis. Thus, despite having multiple sclerosis, the patient appeared to benefit from OMT in terms of managing his compensated Trendelenburg gait. Comment The normal gait of the patient in the present case had been altered primarily through dysfunctions in the sacroiliac joint. The diagnoses for this patient included right-on-right sacral torsion and left posterior innominate somatic dysfunctions. The sacrum and innominate bones are in these positions when the left leg is moved forward during ambulation—providing an explanation of why the patient’s left stride was longer at presentation. JAOA • Vol 110 • No 2 • February 2010 • 83 CASE REPORT Table Gait Parameters of Patient With Compensated Trendelenburg Gait Before and After Osteopathic Manipulative Treatment Step Length, m Left Right Stride Length, m Left Right Stance Time, s Left Right Double Support Time, s Left Right 0.29 (0.04) 0.15 (0.05) 0.44 (0.06) 0.45 (0.07) 1.29 1.25 (0.41) (0.18) 0.43 (0.03) 0.25 (0.05) 0.17 (0.06) 0.43 (0.07) 0.44 (0.07) 1.15 1.06 (0.31) (0.11) 0.27 (0.04) 0.16 (0.06) 0.44 (0.06) 0.45 (0.06) 1.22 (0.37) 0.47 (0.04) 0.51 (0.04) 0.98 (0.02) 1.00 (0.06) 0.53 (0.05) 0.50 (0.03) 1.07 (0.05) 0.50 (0.05) 0.51 (0.03) 0.23 85.19 <.001 0.35 215.63 <.001 Steps, No. Velocity, m/s 0.47 (0.21) 18 0.26 0.36 (0.02) 0.39 (0.04) 18 0.29 1.15 (0.18) 0.40 (0.05) 0.43 (0.16) 18 0.28 0.84 (0.08) 0.87 (0.07) 0.23 (0.04) 0.23 (0.01) 8 0.80 1.03 (0.03) 0.80 (0.03) 0.89 (0.11) 0.21 (0.05) 0.22 (0.03) 7 0.81 1.02 (0.05) 01.01 (0.05) 0.82 (0.06) 0.88 (0.09) 0.22 (0.04) 0.23 (0.02) 7.5 0.81 0.59 135.63 <.001 0.57 128.09 <.001 -0.18 -44.30 <.001 -0.21 -47.67 <.001 -10.50 -58.33 <.01 䡲 Pretreatment, Mean (SD)* ▫ Trial 1 ▫ Trial 2 ▫ Both trials, average 䡲 Posttreatment, Mean (SD)* ▫ Trial 1 ▫ Trial 2 ▫ Both trials, average 䡲 Difference Between Averages ▫ Units ▫ Percent ▫ P value -0.40 -0.28 -32.79 -23.81 <.001 <.001 0.53 192.73 <.01 * All values in mean (SD) except for steps and velocity. The right-on-right sacral torsion occurred in this patient with the left stride. With the left sacral base stuck forward in a rightward rotation about a right oblique axis, it was not able to return to a neutral position. Because of this inability, the left sacroiliac joint could not lock into position, and the right sacral base could not move forward in a leftward rotation about a left axis—as is required for efficient forward movement of the right leg. Thus, a problem with the right stride resulted. Similarly, the left innominate in this patient was restricted in anterior rotation and, therefore, it was unable to allow the left sacroiliac joint to close so that the patient’s weight could be balanced effectively on the left leg while the right leg was swung forward. The patient compensated for this restricted motion by laterally flexing his trunk to the left. This flexing balanced the weight of the body on top of the femoral head, through the acetabulum of the innominate. As a result, the right leg could be swung forward without striking the ground and without the patient falling. After OMT, improved motion of the pelvis was observed, including a more normal gait pattern with less evidence of 84 • JAOA • Vol 110 • No 2 • February 2010 lateral trunk lean and hip hiking. The observed decrease in right-left variation and the observed increases in step and stride lengths provided quantitative measures of the beneficial results of OMT. Furthermore, energy expenditure during gait was reduced after treatment, allowing the patient to increase his speed of walking. Consistent with this observation, an increase in velocity and decreases in stance time and double support time were measured. Conclusion For the patient in the present case, OMT resulted in improved gait parameters that could be measured with the use of the GaitMat II system. Further research is being carried out (by A.C.G.) to clarify the relationship between somatic dysfunctions and gait deviations, as well as to investigate the effects of OMT in alleviating these conditions. References 1. Pomeroy VM, Chambers SH, Giakas G, Bland M. Reliability of measurement of tempo-spatial parameters of gait after stroke using GaitMat II. Clin Rehabil. 2004;18(2):222-227. Gilliss et al • Case Report CASE REPORT Figure 2C Figure 2B 1.0 1.0 0.8 0.8 0.8 0.6 0.4 0.2 Velocity, m/s 1.0 Stride Lenght, m Step Lenght, m Figure 2A 0.6 0.4 0.0 0.0 Left 0.4 0.2 0.2 0.0 0.6 Right Left Right Pretreatment Posttreatment Pretreatment Posttreatment Figure 2. Step length, stride length, and velocity of the patient in the present case—a 65-year-old man with compensated Trendelenburg gait—as revealed by the GaitMat II (EQ Inc, Chalfont, Pennsylvania) gait analysis system. After osteopathic manipulative treatment, analyses found significant increases in the left and right step lengths (P⬍.001 for both) (A) and left and right stride lengths (P⬍.001 for both) (B). An increase of 193% was observed in the patient’s velocity (C) after osteopathic manipulative treatment (P⬍.01). The bars indicate the mean for each parameter, and the error bars denote the standard error of the mean, calculated from the mean for the two trials. Figure 3. The step length and stride length of the patient in the present case—a 65-year-old man with compensated Trendelenburg gait—as revealed by the GaitMat II (EQ Inc, Chalfont, Pennsylvania) gait analysis system. Consistent with a compensated Trendelenburg gait, a statistically significant difference was observed between the mean right and mean left step lengths before osteopathic manipulative treatment, with the right step length 41% shorter than the left step length (P⬍.05). The pretreatment right stride length was slightly longer than the pretreatment left stride length. The difference was not statistically significant posttreatment. 2. Pease W, Bowyer B, Kadyan V. Human walking. In: DeLisa JA, Gans BM, Walsh NE, Bockenek WL, Frontera WR, eds. Physical Medicine and Rehabilitation: Principles and Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:156-167. 3rd ed. Philadelphia, PA: Butterworth-Heinemann; 2002:42-86. 3. Whittle M. Normal gait. In: Whittle MW. Gait Analysis: An Introduction. 5. Heinking K, Kappler R. Pelvis and sacrum. In: Ward RC, ed. Foundations for Gilliss et al • Case Report 4. Saunders JB, Inman VT, Eberhart HD. The major determinants in normal and pathological gait. J Bone Joint Surg Am. 1953;35(3):543-558. JAOA • Vol 110 • No 2 • February 2010 • 85 CASE REPORT Osteopathic Medicine. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:762-783. 6. Mitchell FL. Structural pelvic function. In: Barnes M, ed. 1965 Year Book of Selected Osteopathic Papers. Vol 2. Carmel, CA: Academy of Applied Osteopathy; 1965:178-199. 7. Schiowitz S, DiGiovanna EL, DiGiovanna JA. Gait and postural considerations. In: DiGiovanna EL, Schiowitz S, Dowling DJ, eds. An Osteopathic Approach to Diagnosis and Treatment. Philadelphia, PA: Lippincott Williams & Wilkins; 2004:293-303. 14. Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1380-1388. http://biomed.gerontologyjournals.org/cgi/content/full/63/12/1380. Accessed December 10, 2009. 15. Rosano C, Brach J, Longstreth Jr WT, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults [published online ahead of print October 25, 2005]. Neuroepidemiology. 2006;26:52-60. 8. Kissling RO, Jacob HA. The mobility of sacroiliac joint in healthy subjects. Bull Hosp Jt Dis. 1996;54(3):158-164. 16. Craik RL, Dutterer L. Spatial and temporal characteristics of foot fall patterns. In: Craik RL, Oatis CA, eds. Gait Analysis: Theory and Application. 1st ed. St Louis, MO: Mosby; 1995:143-158. 9. Jacob HA, Kissling RO. The mobility of the sacroiliac joints in healthy volunteers between 20 and 50 years of age. Clin Biomech (Bristol, Avon). 1995;10(7):352-361. 17. Apley G. Apley’s System of Orthopaedics and Fractures. 6th ed. London, England: ELBS; 1986:243. 10. Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait [review]. Mov Disord. 2008;23(3):329-342. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2535903/?tool=pubmed. Accessed December 10, 2009. 11. Boonstra TA, van der Kooij H, Munneke M, Bloem BR. Gait disorders and balance disturbances in Parkinson’s disease: clinical update and pathophysiology. Curr Opin Neurol. 2008;21(4):461-471. 12. Scherder E, Eggermont L, Swaab D, van Heuvelen M, Kamsma Y, de Greef M, et al. Gait in ageing and associated dementias; its relationship with cognition [published online ahead of print February 15, 2007]. Neurosci Biobehav Rev. 2007;31(4):485-497. 18. Dowling DJ. Muscle energy of the lower extremity. In: DiGiovanna EL, Schiowitz S, Dowling DJ, eds. An Osteopathic Approach to Diagnosis and Treatment. Philadelphia, PA: Lippincott Williams & Wilkins; 2004:506-513. Editor’s Note: Video clips of the patient described in the present case walking before and after osteopathic manipulative treatment have been posted online to the JAOA‘s Web site at http://www.jaoa.org/cgi/content/full/110/2/81. 13. Barker S, Craik R, Freedman W, Herrmann N, Hillstrom H. Accuracy, reliability, and validity of a spatiotemporal gait analysis system [published online ahead of print August 24, 2005]. Med Eng Phys. 2006;28(5):460-467. JAOA Call for Case Reports To advance the scholarly evolution of osteopathic medicine, JAOA—The Journal of the American Osteopathic Association invites osteopathic physicians, researchers, and others in the healthcare professions to submit case reports relevant to osteopathic medicine. In preparing submissions, authors should adhere to the JAOA’s “Information for Contributors,” which is available online at http://www.jaoa.org/misc/ifora.shtml. Submissions should be e-mailed to [email protected] with the subject heading “JAOA Call for Case Reports.” 86 • JAOA • Vol 110 • No 2 • February 2010 Gilliss et al • Case Report