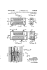How does a Battery work?
Transcription
How does a Battery work?
How does a Battery work? There are a lot of different kinds of batteries, but they all function based on the same concept. A battery is a device that is able to store electrical energy in the form of chemical energy, and convert that energy into electricity. You cannot catch and store electricity, but you can store electrical energy in the chemicals inside a battery. There are three main components of a battery: two terminals made of different chemicals (typically metals), the anode and the cathode; and the electrolyte, which separates these terminals. The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode. When a device is connected to a battery—a light bulb or an electric circuit—chemical reactions occur on the electrodes that create a flow of electrical energy to the device. The extra electrons at the anode want to flow to the cathode because it has fewer electrons and they’re trying to balance out the voltage. More specifically: during a discharge of electricity, the chemical on the anode releases electrons to the negative terminal and ions in the electrolyte through what’s called an oxidation reaction. Meanwhile, at the positive terminal, the cathode accepts electrons, completing the circuit for the flow of electrons. The electrolyte is there to put the different chemicals of the anode and cathode into contact with one another. The ions created in the oxidation reaction transport current through the electrolyte while the electrons flow in the external circuit, and that’s what generates an electric current. If the battery is disposable, it will produce electricity until it runs out of reactants. These batteries only work in one direction, transforming chemical energy to electrical energy. But in other types of batteries, the reaction can be reversed. Rechargeable batteries (like the kind in your cellphone or in your car) are designed so that electrical energy from an outside source (the charger that you plug into the wall or the dynamo in your car) can be applied to the chemical system, and reverse its operation, restoring the battery’s charge. Build your own battery! 1. 2. 3. 4. 5. 6. 7. 8. In a small bowl, mix together ¼ cup of vinegar (electrolyte) and 1 tbsp of salt Using scissors, cut up a paper towel into small squares, approximately 1in X 1in Place the small squares to soak in the bowl of salt-vinegar solution and set aside Gather some pennies and nickels and wash them with dish soap to remove dirt and grime Start building your stack on a dry paper towel on a plate. Put down a penny first, then place a square of vinegar soaked paper towel on top, and then add a nickel. Keep repeating the layers until you have a stack of four coins (alternating pennies, wet paper towel pieces, and nickels), making sure you end with a nickel on top. Attach the leads of a multimeter to the ends of the battery by touching one lead to the penny on the bottom and the other to the nickel on the top. Measure the voltage produced by the battery. Increase the stack height by adding more pennies, nickels and paper towels to see what happens to the voltage. Try to add an LED to the leads and see if you can light it up! Since the penny is made of 95% copper, it will oxidize more easily than the nickel, which is made of 75% nickel because of the acidic vinegar solution. This causes the electrons to flow from the penny, through the electrolyte and towards the nickel which creates a voltage. When you stack up several of these combinations, their voltage adds up just like with alkaline batteries!
Similar documents
Batteries and Energy Storage Craig B. Arnold Department of Mechanical and Aerospace Engineering

Electrochemistry Oxidation-Reduction: Half Reactions Method for Balancing Redox Equations:

Silicon Anode Battery Market : Share, Size, Competitive Strategies and Forecast 2016 - 2026
 Silicon anodes can improve the capacity of standard graphite anodes 10 times. However the limiting factor is for cathode capacity and not for Si anode capacity, as the overall capacity of the battery is only increased by 35% even if the Si anode reach its maximum capacity level.
Silicon anodes can improve the capacity of standard graphite anodes 10 times. However the limiting factor is for cathode capacity and not for Si anode capacity, as the overall capacity of the battery is only increased by 35% even if the Si anode reach its maximum capacity level.
Global Silicon Anode Battery Market Growth
 Overview of expected Y-o-Y growth comparison of Lithium ion battery market and Silicon anode battery market along with the production scenario of developed and developing countries for silicon anode battery market is discussed in the report.
Overview of expected Y-o-Y growth comparison of Lithium ion battery market and Silicon anode battery market along with the production scenario of developed and developing countries for silicon anode battery market is discussed in the report.