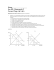CHM 1046 Homework #2
Transcription
CHM 1046 Homework #2
CHM 1046 Homework #2 3.1 What is the law of mass action? 3.2. Write Kc and Kp if applicable for each of the following: a. 2 NO2 (g) + 7H2 (g) ÅÆ 2NH3 (g) + 4H2O (l) b. 2 ZnS (s) + 3 O2 (g) ÅÆ2ZnO (s) + 2 SO2 (g) c. C (s) + CO2 (g) ÅÆ2CO (g) d. C6H5COOH (aq) ÅÆC6H5COO- (aq) + H+ (aq) 3.3. Write the relationship between Kc and Kp and define the terms. 3.4. The following diagram represents 3 equilibrium reactions: a. Which has the largest K? Explain your answer. b. Which has the smallest K? Explain your answer. 3.5. Ammonium carbamate NH4CO2NH2 decomposes as follows: NH4CO2NH2 (s) ÅÆ2 NH3 (g) + CO2 (g) Starting with only the solid, it is found that at 40 oC the total gas pressure is 0.363 atm. Calculate Kp 3.6. The following equilibrium constants have been determined for oxalic acid: What is the equilibrium constant for the following reaction? 3.7. The dissociation of molecular iodine into iodine atoms is as follows: I2 (g) ÅÆ2I (g) At 1000 K, Kc = 3.8x10-5. Suppose you start with 0.0456 mole of molecular iodine in a 2.3 L flask. What is the concentration of the gases at equilibrium? 3.8. The equilibrium constant for the dissociation of phosgene COCl2 is 4.63x10-3 at 527oC. Caclculate the equilibrium partial pressure of all the components if you start with pure phosgene at 0.760 atm? 3.9. Consider the equilibrium: 2I (g) ÅÆ I2 (g) What would be the effect of: a. increasing the total pressure on the system by decreasing the volume b. adding I2 to the reaction? c. decreasing the temperature? 3.10. The decomposition of ammonium hydrogen sulfide is endothermic. A 6.1589 g sample of the solid is placed in an evacuated 4.000L vessel at 24oC. After the equilibrium is reached, the total pressure is 0.709 atm. Some solid NH4HS remains. a. What is value of Kp? b. What percentage of the solid has decomposed? c. If the volume of the vessel were doubled at constant temperature, what would happen to the amount of the solid remaining? 3.11. At 1130oC the equilibrium constant Kc for the reaction is 2.25x10-4. If the concentration of H2S is 4.48x10-3 M, and the concentration of H2 is 1.5x10-3 M, what is the concentration of S2? 3.12. A mixture containing 3.9 moles of NO and 0.88 mole of CO2 was allowed to react in a flask at a certain temperature: At equilibrium, 0.11 mole of CO2 was present. Calculate Kc for this reaction. 3.13. The equilibrium constant Kc for the following reaction is 1.2 at 375oC: a. What is Kp for the reaction? b. What is the value of Kc for the reverse reaction ? c. What is the value of the equilibrium constant if the reaction is run as follows? d. What are the values of Kp for the reactions in b and c? 3.14. A sealed glass bulb contains a mixture of nitrogen dioxide (brown) and dinitrogen tertroxide (colorless) gases. Describe what happens to the following properties of the gases when the bulb is heated from 20 to 40oC: a. color b. pressure c. average molar mass d. degree of dissociation (N2O4 decomposing) e. density (assume volume is constant) Lecture 4 – Acid Base Equilibria 4.1. Write the formulas of the conjugate bases for the following acids: a. HNO2 b. H2SO4 c. H2S d. HCN e. HCOOH 4.2. Identify the acid-base pairs in each of the following reactions: a. CH3COO- + HCN ÅÆCH3COOH + CNb. HCO3- + HCO3- ÅÆ H2CO3 + CO32c. H2PO4- + NH3 ÅÆ HPO42- + NH4+ d. HClO + CH3NH2 ÅÆ CH3NH3+ + ClOe. CO32- + H2O ÅÆ HCO3- + OH4.3. Oxalic acid has the following structure: An oxalic acid solution contains the following species in varying concentrations: C2H2O4, C2HO4-, C2O42and H+. a. Draw Lewis structures for the anions. b. Which of the above 4 can act only as acids? As only bases? As both acid and base? 4.4. Define pOH. Write the equation relating pH and pOH. 4.5. Calculate the concentration of H+ ions in a 0.62 M NaOH solution. 4.6. Calculate the pH of each of the following solutions: a. 0.0010M HCl b. 0.76 M KOH 4.7. Which of the following diagrams represents a solution of a weak diprotic acid? Which are not chemically possible situations? 4.8. The Ka for benzoic acid is 6.5x10-5. Calculate the pH of a 0.10 M benzoic acid solution. 4.9. Calculate the percent ionization of benzoic acid at the following concentrations: a. 0.20 M b. ).00020 M 4.10. Calculate the pH for each of the following solutions: b. 0.050 M C5H5N (pyridine) a. 0.10 M NH3 4.11. Write the equation relating Ka for a weak acid and Kb for its conjugate base. Use NH3 and its conjugate acid NH4+ to derive the relationship between Ka and Kb. 4.12. Calculate the concentrations of all major species in a 0.025M H2CO3 4.13. Consider the following compounds: Experimentally, phenol is found to be the stronger acid. Using the structures, explain why this is the case using the differences between the conjugate bases. (Hint: Only one of the conjugate bases undergoes resonance). 4.14. Calculate the pH of a 0.36 M CH3COONa solution. 4.15. Classify the following as Lewis Acids or Bases: a. SO2 b. CO2 c. H2O f. OH- g. H+ h. BCl3 d. I- e. NH3 4.16. The reaction between an antiacid and the gastric juice in the stomach is as follows: NaHCO3 (s) + HCl (aq) ÅÆ NaCl (aq) + H2O (l) + CO2 (g) Calculate the volume (in L) of CO2 generated from 0.350 g NaHCO3 and excess gastric juice at 1.00 atm and 37.0 oC. 4.17. How many grams of NaCN would you need to dissolve in enough water to make exactly 250.0 mL of solution with a pH of 10.00? Lecture 5 – Solubility Equilibria 5.1. Use Le Chatlier’s principle to explain how the common ion effect affects the pH of a solution. 5.2. Define pKa for a weak acid. What is the relationship between the value of the pKa and the strength of the acid? Repeat this for pKb. 5.3. What is a buffer solution? What constitutes a buffer solution? 5.4. The pH of blood plasma is 7.40. Assuming the principle buffer system is HCO3-/H2CO3, calculate the ratio of their concentration in blood. Is this buffer more effective towards acid or base? 5.5. Calculate the pH of the 0.20M NH3/0.20M NH4Cl buffer. What is the pH of the buffer after the additions of 10.0 mL of 0.10M HCl to 65.0 mL of the buffer? 5.6.A 0.2688 g sample of a monoprotic acid neutralizes 16.4 mL of 0.08133 M KOH. Calculate the molar mass of the acid. 5.7. In a titration, 12.5 mL of 0.500 M sulfuric acid neutralizes 50.0 mL of NaOH. What is the concentration of the NaOH? 5.8. A 10.0 mL solution of ammonia is titrated with a 0.100 M HCl. Calculate the pH of the solution after adding the following amounts of HCl: a. 0.00 mL b. 10.00 mL c. 20.00 mL d. 30.00 mL e. 40.00 mL 5.9. The Ka of an indicator is 2 x 10-6. HIn is green, and In- is red. When HCl is titrated with NaOH in the presence of the indicator, at what pH will the color change? 5.10. The solubility of an ionic compound M2X3 (molar mass = 288 g) is 3.5 x 10-17 g/L. What is the Ksp? 5.11. Calculate the molar solubility of AgCl in a 1.00 L solution containing 10.0 g of CaCl2. 5.12. Calculate whether a precipitate will form if 2.00 mL of 0.60 M NH3 are added to 1.0 L of 1 x 10-3 M Fe2SO4. 5.13. If 2.50 g of CuSO4 are dissolved in 9.0 x 102 mL of 0.30M NH3, what are the concentrations of Cu2+, Cu(NH3)2+ and NH3 at equilibrium. 5.14. If NaOH is added to 0.010 M Al3+, which will be the predominant species at equilibrium: Al(OH)3 or Al(OH)4-? pH = 14 and Kf for Al(OH)4- is 2 x 1033. 5.15. Solid NaBr is slowly added to a solution that is 0.010 M Cu+, and 0.010 Ag+. a. Which compound will begin to precipitate first? b. Calculate the [Ag+] when CuBr starts t precipitate. c. What percent of the silver ion is present at this point? 5.16. Penicillin G is a common antibiotic. It is a weak monoprotic acid with the structure: Ka = 1.64 x 10‐3. It is removed from molds that grow at 25oC and a pH range of 4.5‐5.0. a. Identify the acidic proton. Explain your answer. b. In the purification process, the molecule is treated with a 6.50 pH buffer. What would be the ratio of the conjugate base to that of the acid? Would you expect the conjugate base to be very soluble in water? Why or why not? c. Penicillin G is not suitable for a pill, and must be converted into a sodium salt NaPen. Calculate the pH of a 0.12 M NaPen solution.









![HA[ ]A][OH[ ]HA[ ]A][H[ :]B[ ] OH][ BH[ HA[ ]A][OH[ ]HA[ ]A][H[ :]B[ ] OH][ BH[](http://s1.doczz.net/store/data/006647807_1-336b0f130c0c1ff3e680087349653784-70x70.png)