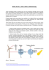ABSTRACT HETEROGENEOUS CATALYSTS FOR HYDROGEN
Transcription
ABSTRACT HETEROGENEOUS CATALYSTS FOR HYDROGEN
ABSTRACT HETEROGENEOUS CATALYSTS FOR HYDROGEN PRODUCTION FROM METHANE AND CARBON DIOXIDE By Julia M. Pusel May 2015 Several heterogeneous catalysts were studied for synthesis gas production through dry reforming of methane (DRM). This process uses carbon dioxide in lieu of the steam that is traditionally used in conventional methane reforming to produce hydrogen that can then be repurposed in more chemical processes. The monometallic catalysts explored were Ni/Al2O3 and Ni/CeZrO2 followed by their bimetallic versions PtNi/Al2O3 and PtNi/CeZrO2 at 800°C. In addition to these catalysts, platinum supported Zeolitic Imidazolate Framework (ZIF)-8 was also investigated in comparison with PtNi/CeZrO2 at 490°C. The studies suggest that these catalysts are suitable for promoting the dry reforming of methane for hydrogen production. Keywords: Pt/ZIF-8, PtNi/Al2O3, PtNi/CeZrO2, Ni/Al2O3, Ni/CeZrO2, hydrogen, carbon dioxide, methane, methane reforming, dry reforming, carbon dioxide reforming, synthesis gas, gas chromatography, catalysts, plug flow reactor, greenhouse gases. HETEROGENEOUS CATALYSTS FOR HYDROGEN PRODUCTION FROM METHANE AND CARBON DIOXIDE A THESIS Presented to the Department of Chemical Engineering California State University, Long Beach In Partial Fulfillment of the Requirements for the Degree Master of Science in Engineering Committee Members: Sepideh Faraji, Ph.D. (Chair) Gregory Smith, Ph.D. Ted Yu, Ph.D. College Designee: Antonella Sciortino, Ph.D. By Julia M. Pusel B.S., 2007, Rose-Hulman Institute of Technology May 2015 UMI Number: 1585646 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMI 1585646 Published by ProQuest LLC (2015). Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, MI 48106 - 1346 ACKNOWLEDGEMENTS I would like to thank California State University, Long Beach for giving me the opportunity to study Chemical Engineering at the graduate level. I would also like to thank all of the professors that have shared their knowledge with me and helped to make me a better engineer. I would especially like to thank my Research Advisor, Dr. Sepideh Faraji, for her patience with me as I transitioned into a new job and by supporting my research and my thesis through the challenges of living outside the local area and balancing a full time job. I would also like to acknowledge the department’s Graduate Advisor, Dr. Roger C. Lo and the chairman of the Department of Chemical Engineering, Dr. Larry Jang for their continued support as I pursued my degree. Finally, I would like to recognize and thank my family: my mother for always demanding that I do my best and instilling in me the love for higher education since I was a child; my father for always supporting my mother and I during our pursuit of education; and my husband for his love, support, and engineering perspective during late night study sessions. iii TABLE OF CONTENTS Page ACKNOWLEDGEMENTS ......................................................................................... iii LIST OF TABLES ....................................................................................................... v LIST OF FIGURES ..................................................................................................... vi LIST OF ABBREVIATIONS ...................................................................................... viii LIST OF EQUATIONS ............................................................................................... ix CHAPTER 1. INTRODUCTION ............................................................................................ 1 2. BACKGROUND INFORMATION ................................................................. 4 The Utility of Hydrogen Production .......................................................... Hydrogen Production ................................................................................. Catalyst Selection....................................................................................... 4 5 6 3. EXPERIMENTAL ............................................................................................ 8 Setup .......................................................................................................... Gas Chromatograph ................................................................................... Procedure ................................................................................................... Test Runs ................................................................................................... Calculations................................................................................................ 8 10 12 16 20 4. RESULTS AND DISCUSSION ....................................................................... 22 Four Catalysts in Equal Quantities at the Same Temperature ................... The Effect of Catalyst Mass ....................................................................... New Catalyst, Pt/ZIF, Compared to Traditional Bimetallic Catalyst ........ 22 24 31 5. CONCLUSIONS............................................................................................... 35 REFERENCES ............................................................................................................ 36 iv LIST OF TABLES TABLE Page 1. First Run............................................................................................................ 16 2. Second Run ....................................................................................................... 17 3. Third Run .......................................................................................................... 17 4. Fourth Run ........................................................................................................ 17 5. Fifth Run ........................................................................................................... 18 6. Sixth Run .......................................................................................................... 18 7. Seventh Run ...................................................................................................... 18 8. Eighth Run ........................................................................................................ 19 9. Ninth Run .......................................................................................................... 19 10. Tenth Run........................................................................................................ 19 11: Eleventh Run................................................................................................... 20 12: Side-by-Side Comparison of All Test Runs ................................................... 21 v LIST OF FIGURES FIGURE Page 1. Land air temperature anomalies, for global, north hemisphere and south hemisphere for the period of 1850 to 2007 ................................................ 3 2. Schematic of experimental setup consisting of plug-flow reactor, gas chromatograph, fume hood, and a variety of gas feeds ............................. 9 3. Image of laboratory setup at Cal State - Long Beach ....................................... 10 4. Temperature profile for plug-flow reactor furnace ........................................... 14 5. Methane conversion of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC ................ 23 6. Methane conversion of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC ............... 24 7. Carbon Dioxide conversion of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC ..... 25 8. Carbon Dioxide conversion of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC.... 25 9. Hydrogen to Carbon Monoxide ratio of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC ......................................................................................... 26 10. Hydrogen to Carbon Monoxide ratio of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC ......................................................................................... 26 11. Methane conversion of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC .............................. 28 12. Methane conversion of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC ............................ 28 vi FIGURE Page 13. Carbon Dioxide conversion of Ni/Al2O3, in different quantities, at 800°C through plug flow reactor utilizing the FID sensor in the GC ................... 29 14. Carbon Dioxide conversion of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC ............... 29 15. Hydrogen to Carbon Monoxide ratio of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC ..... 30 16. Hydrogen to Carbon Monoxide ratio of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC.... 30 17. Methane conversion of 2 catalysts, in equal quantities, at 490°C through a plug flow reactor utilizing both the FID and TCD sensor in the GC ......... 33 18. Carbon Dioxide conversion of 2 catalysts, in equal quantities, at 490°C through a plug flow reactor utilizing both the FID and the TCD sensor in the GC .................................................................................................... 33 19. Hydrogen to Carbon Monoxide ratio of 2 catalysts, in equal quantities, at 490°C through a plug flow reactor utilizing both the FID and TCD sensor in the GC ......................................................................................... 34 vii LIST OF ABBREVIATIONS CH4 Methane CO Carbon Monoxide CO2 Carbon Dioxide DRM Dry Reforming of Methane FID Flame Ionization Detector GC Gas Chromatograph H Hydrogen MOF Metal Organic Framework Syngas Synthesis Gas TCD Thermal Conductivity Detector ZIF Zeolitic Imidazolate Framework viii LIST OF EQUATIONS Page DRY REFORMING OF METHANE .......................................................................... 2 STEAM REFORMING OF METHANE ..................................................................... 2 MOLAR FR FACTOR ................................................................................................ 20 CONVERSION OF METHANE ................................................................................. 20 METHANE DECOMPOSITION ................................................................................ 27 ix CHAPTER 1 INTRODUCTION Hydrogen is a valuable gas for use in chemical processes across a number of different industries. Because of this, efficient hydrogen production methods are of substantial interest. Additionally, increased awareness in the rising concentrations of greenhouse gases in the Earth’s atmosphere encourages the utilization and research of methods that will help mitigate environmental impact. The greenhouse gas effect is a phenomenon where atmospheric gases, namely water vapor, carbon dioxide, and methane, absorb outgoing infrared radiation that results in the rising of the earth’s temperature [1]. Catalytic reforming of methane with carbon dioxide, also known as dry reforming, is one potential solution to these challenges to the industry because it utilizes some of the most abundant greenhouse gases in our atmosphere as reactants to produce hydrogen. Dry reforming of methane (1) utilizes carbon dioxide and methane and converts them into carbon monoxide and hydrogen gas, also known as synthesis gas (syngas) [2]. Dry reforming is preferable over traditional steam reforming (2) for two reasons. First, dry reforming is preferable because it utilizes the carbon dioxide present in natural gas fields and lowers the CO/H2 syngas ratio for the subsequent reaction to the desired products [3]. Second, there is no need to separate CO2 from the product as would be needed during traditional steam reforming. During steam reforming, the two products resulting from the reaction are CO2 and H2 and therefore the CO2 would need to be 1 removed from the hydrogen before the hydrogen was usable. The separation of CO2 is a costly and energy intensive process and therefore not desirable [4]. CH4 + CO2 ↔ 2 CO + 2 H2 (∆Hº = 247 kJ/mol) (1) CH4 + 2 H2O ↔ CO2 + 4 H2 (∆Hº = 165 kJ/mol) (2) Commercialization of dry reforming would be an ideal solution to help mitigate the greenhouse gases in the atmosphere while promoting industrial chemical processes. One challenge to this solution has been finding a catalyst that has both high activity and high stability. Various studies exploring different types of catalysts that could be suitable for dry reforming of methane have been conducted [5]. Carbon dioxide is not only naturally occurring in the atmosphere but also a result of the burning of fossil fuels, changes in land-use, and other industrial processes. It is the principal anthropogenic gas that is thought to affect the Earth’s radiative balance and because of this there is thought to be a close correlation between carbon dioxide and the rise in the Earth’s temperature [1]. 2 FIGURE 1. Land air temperature anomalies, for global, north hemisphere and south hemisphere for the period of 1850 to 2007. 3 CHAPTER 2 BACKGROUND INFORMATION The Utility of Hydrogen Production Hydrogen, in its monatomic form, is the most abundant chemical element in the universe and contributes to approximately 75% of normal matter by mass [6]. Despite its abundance, naturally occurring monatomic hydrogen is relatively rare on Earth. This is because the gas is so light that it gains enough velocity to leave our atmosphere from collisions with other gases [7]. In hydrogen’s elemental form, however, it is a versatile and valuable substance that can be used in a variety of chemical processes, many of which have industrial application. Hydrogen’s versatility comes from the fact that it contains only one proton and one electron and is therefore able to readily either donate or accept an electron in order to react with or saturate other molecules. In the petrochemical industry, hydrogen is used to process lower grade hydrocarbons into more valuable hydrocarbons through hydrodealkylation (removal of functional groups), hydrodesulfurization (removal of sulfur), and hydrocracking (molecule saturation) [8]. Often, refineries have their own supplementary supply of hydrogen from the hydrogen plant in order to provide for the aforementioned processes [9]. Industrially produced hydrogen can also be reacted with elemental nitrogen in order to produce ammonia, which is a valuable substance to the agricultural and chemical industries. Furthermore, the creation of saturated fats, such as margarine, is made possible through the 4 hydrogenation of unsaturated fats and oils. These applications illustrate the demand in today’s industry for an economical solution to hydrogen production [7]. Hydrogen Production A common method for the production of hydrogen in today’s industry is through reforming. Reforming is a technique used in chemistry or chemical engineering to rearrange the molecular structure of lower grade or undesirable hydrocarbons in order to alter their properties into a more desirable state. There are two types of reforming: thermal and catalytic. Thermal reforming achieves results by exposing the reactants to high temperatures and pressures. In catalytic reforming, similar results are achieved using a catalyst. A catalyst is a substance that improves the reaction rate of a chemical reaction without itself being changed or consumed [10]. The process of catalytic reforming is a strongly endothermic conversion, meaning the process requires a great amount of heat in order for the reaction to proceed [11]. During the reaction, the hydrocarbons are converted with either carbon dioxide (dry reforming as seen in reaction 1) or steam (steam reforming as seen in reaction 2) in order to produce syngas [2]. Syngas is simply a mixture of carbon monoxide and hydrogen. The product of interest in the syngas production is hydrogen [12]. It should be noted that dry reforming presents a more economical solution than steam reforming which also includes added environmental benefits such as upgrading of biogas (a renewable resource composed mostly of methane and carbon dioxide) and the utilization of two greenhouse gases [4]. The catalysts and energy required to perform dry reforming can become costly when used industrially. The goal of this research was both to test the hydrogen yields of different catalysts as well as to explore the possibility of producing hydrogen with a 5 catalyst at lower temperatures to determine the feasibility of significantly reduced production costs when applied commercially. Catalyst Selection For dry reforming the Noble Metals (Rh, Ru, Pt, and Pd) in addition to the nonnoble metal, Ni, are most commonly used for active components. In addition, the catalyst supports used are: Al2O3, SiO2, ZrO2, and La2O3 [5]. Supported noble metal catalysts exhibit considerable catalytic activity and are less sensitive to carbon deposition but nonnoble metal catalysts are more available and cost effective [5]. Carbon deposits present a problem where the catalytic pores are blocked leading to the deactivation of the catalyst through active site blockage. Fortunately, by using adequate promoters and modifying catalysts supports, carbon formation upon the catalyst can be mitigated. Various promoters and supports have been investigated [13]. As previously mentioned, it is commonly known that noble metals inhibit coke formation. Furthermore, it was found that adding noble metals to Nickel based catalysts can promote the reducibility of Ni and stabilize its degree of reduction during the catalytic process [13]. Additionally, it was found that the catalytic activity for the auto thermal reforming of methane was increased when small amounts of Platinum (Pt) were added to the catalyst Ni/Al2O3 [13]. A catalyst with increased stability, activity, and reduced carbon formation is a feasible choice for utilization during hydrogen production by dry methane reforming [13]. The two monometallic Nickel based catalysts chosen for this experiment were Ni/Al2O3 and Ni/CeZrO2. Additionally, their bimetallic versions were also investigated: PtNi/Al2O3 and PtNi/CeZrO2 . 6 A fifth catalyst based on a new support, Zeolitic Imidazolate Framework (ZIF)-8, was also investigated (Pt/ZIF). Zeolites, an aluminosilicate-based material, are an essential synthetic product with valuable uses as catalysts in the petroleum refining industry. ZIFs are a new class of porous crystals whose three-dimensional structure consist of tetrahedral metal ions connected by imidazolates at a 145° angle [14]. ZIFs, although a subfamily of metal-organic frameworks (MOFs), are quite chemically stable. ZIF-8, in particular, is both chemically and thermally stable. ZIF-8 can withstand boiling in water, alkaline solutions, and refluxing in organic solvents without losing crystallinity or porosity. Furthermore, ZIF-8 is thermally stable up to 500°C [14]. Additionally, ZIFs have an affinity for capturing carbon dioxide and can separate carbon dioxide from gas mixtures and store carbon dioxide within the framework [14]. 7 CHAPTER 3 EXPERIMENTAL Setup The laboratory and equipment used during this experiment were acquired and set up by Dr. Sepideh Faraji and her students during Summer 2011. A plug flow reactor in which the reagents flow at an assumed constant velocity through the reactor with no upstream or downstream mixing was used [15]. The reactor received a feed from up to four gas lines: methane, carbon dioxide, argon, and mix gas. The methane and carbon dioxide feeds were the reactants during the experiment. These two lines were fed in equal proportions through the plug flow reactor, in which the gases passed through the catalyst at a constant temperature. The purpose of the argon gas line was to purge the system of residual gases before starting the calibration and test runs. Finally, the mix gas feed was used to calibrate the gas chromatograph (GC) before each test run. For this reason, the mix gas consisted of set percentages of each of the gases that would be measured by the GC during the reaction: the two reactant gases, methane and carbon dioxide; and the two product gases, carbon monoxide and hydrogen. The gases used during this experiment were obtained from Airgas and the flow rates of the different feeds were controlled with a Porter Model CM-400 Mass Flow Controller manufactured by Parker Hannifin Corporation. An Omega model CN7200 microprocessor-based temperature process control unit controlled and monitored the reactor’s furnace temperature. 8 The resulting product exiting the reactor consisted of a mix of carbon monoxide and hydrogen gas. The carbon monoxide and hydrogen gas products passed through a valve where the gas stream was routed directly to the fume hood or to the GC, where the percentage of each gas in the stream was measured. The GC utilized for this experiment was a Model 8610C Gas Chromatograph manufactured by SRI Instruments. The GC worked in conjunction with PeakSimple, a computer program, to sample and record the data from the gas injections. After leaving the GC, the gas stream flowed to the fume hood for proper ventilation. This process is shown in Figure 2. FIGURE 2. Schematic of experimental setup consisting of plug-flow reactor, gas chromatograph, fume hood, and a variety of gas feeds. Additionally, an image of the actual laboratory setup at California State University, Long Beach can be seen in Figure 3. 9 Mass Flow Controller Feed Valves GC display and control computer with PeakSimple Plug-Flow Reactor Gas Chromatograph Temperature Controller FIGURE 3. Image of laboratory setup at Cal State - Long Beach. Gas Chromatograph Chromatography is a laboratory technique used to separate mixtures. The product of interest is mixed with a fluid, known as the mobile phase, and travels through the stationary phase where the elemental structures in the product of interest separate because their different physical properties cause them to travel at different speeds [16]. In gas chromatography (GC), the mobile phase is a gas. It is known as the carrier gas and is usually helium or another type of inert gas [17]. For the purpose of this work, however, helium as the carrier gas may have caused difficulties with the GC. This difficulty is caused because the product of interest in our reaction is hydrogen and the thermal conductivity of hydrogen is very close to that of helium. The fidelity of the GC may not 10 be sufficient to determine the hydrogen and helium as two separate gases leading to false positives for hydrogen production. In order to avoid this, the carrier gas chosen was ultra-high purity argon. The carrier gas then moves the products through the stationary phase, or a packed column in the case of a GC. The compounds in the product interact with the walls of the column and elute at different times. This is known as the retention time for each compound and will correlate to a peak location on the resulting graph that is produced by the GC. The retention time, or location, of the peak is what differentiates compounds and allows one to determine which peak represents each compound. The areas under each peak are then calculated to find the percent composition of each compound in the feed [16]. Gas Chromatographs typically have two types of detectors, a flame ionization detector (FID) and a thermal conductivity detector (TCD) [16]. An FID is more sensitive to hydrocarbons and is compatible with helium, nitrogen, and argon as carrier gases [18]. TCDs are universal detectors that can detect nearly any compound such as air, hydrogen, carbon monoxide, and many other compounds. TCDs tend to work best with helium, nitrogen, argon, or even hydrogen as carrier gases [19]. For these reasons, during the experiment the FID will be used as the primary detector with TCD data collected as a backup for data comparison. The GC was set to take a sample of the product gas every thirty minutes. This injection was analyzed by both detectors and then translated into PeakSimple Chromatography Software that displays the amount of a component versus retention time, graphically. This information was used to determine the percent composition of the syngas produced. 11 Procedure The first run was conducted using the catalyst Ni/Al2O3. A personal gas monitor was used to ensure the laboratory conditions were safe. Then, the hood was turned on. A glass tube approximately 18” in length was cleaned on both the inside and the outside with acetone and then blown dry with clean air. The mass balance was tared with a weigh paper and then 0.0045 g of catalyst was weighed onto the paper. The glass tube was packed with quartz wool and centered in the tube. The pre-weighed catalyst was loaded into the tube so that it was suspended in the middle of the tube upon the quartz wool. The glass tube was carefully placed through the reactor so that the catalyst would be situated in the center of the reactor. The tube was then fixed to the copper tubing on each side of the reactor. The glass tube was sealed with rubber O-rings and the fixtures were tightened carefully so as to not exceed a pressure that would crack the glass tube. Each side of the reactor (top and bottom) was packed with fiberglass around the openings, with the exception of the glass tube near the catalyst to ensure that minimal heat would escape from the furnace while also not insulating the catalyst bed from the heat. The ultra-high purity argon cylinder and hydrogen cylinder were opened so that they may flow into the GC. This is needed for proper operation of the GC. The reason argon was chosen over helium as the carrier gas is due to the difficulty of hydrogen detection in the GC in the presence of helium versus argon. The GC was then turned on. 12 The computer and Peak Simple software were turned on to ensure proper connection to the GC. The mix gas cylinder was opened and the mix valve on the front of the instrument was routed to the bypass line, which feeds directly to the GC for calibration prior to execution of the reaction. The regular argon cylinder was also opened and the argon valve on the front of the instrument was routed to the reactor. The argon was used to purge the system of any residual gases prior to the test. Thus far, ultra high purity argon and hydrogen were going directly to the GC, mix gas was flowing through the bypass line into the GC, and the regular argon was flowing through the reactor and into the hood in order to purge the system without disrupting the GC calibration. The system was checked for leaks using gas leak detection fluid and any questionable joints were tightened. The flow rates were then adjusted using a Porter Model CM-400 Mass Flow Controller. The mix gas and argon flow rates were set to 5.00 mL/min. The gas factors were also set on the flow controller as follows: Ar = 1.443, CO2 = 0.745, CO = 1.001, CH4 = 0.731, H2 = 1.021. The GC was checked to ensure the Carrier 1 and Hydrogen 1 lights were green indicating positive gas flow to the GC. The carrier was set to 30 psi and the hydrogen was set to 20 psi. The mix gas was set to flow for 20 minutes to allow the percentages of each gas to reach equilibrium before they were injected into the GC. The reactor’s furnace was then turned on using the Omega CN 7200 temperature process control unit. The controller was set to the following temperature profile: ramp up 13 from ambient temperature (25°C) to 800°C over 2 hours, hold 800°C for 15 hours, then shut off. The temperature profile can be seen in Figure 4. FIGURE 4. Temperature profile for plug-flow reactor furnace. The GC was programmed to take an injection every 30 minutes. Four injections were needed of the mix gas for calibration and so the reactor’s furnace was set to heat to the desired temperature over a span of two hours so that the GC could be calibrated in the same amount of time. The regular argon flushed the reactor during the time that the furnace was heating and the GC was calibrating. Once the calibration was complete, the reaction began. The mix gas cylinder was turned off and mix valves were closed on the instrument. The regular argon cylinder was 14 turned off and the argon valves were closed on the instrument. The three-way valve on the exiting stream of the reactor was switched to route the gas to the GC. The methane and carbon dioxide cylinders were turned on and their corresponding valves on the instrument were opened. Both the methane and carbon dioxide feeds were routed to the reactor. The methane and carbon dioxide flows were adjusted on the flow controller to 5.00 mL/min. The flow measurements taken at the hood were 4.52 mL/min for methane and 4.63 mL/min for carbon dioxide. Although the flow controller was set to 5 mL/min, the actual flow rates measured at the hood were slightly less than 5 mL/min. This could have been due to imperfections in the joints of the laboratory setup resulting in some loss of flow. The GC was set up to take twenty-eight injections over the next fourteen hours or one injection every thirty minutes. Peak Simple was closely monitored to ensure that the program was recording the areas of the correct curves during each injection. An FID was used to measure the amount of methane, carbon dioxide, and carbon monoxide in the stream. A TCD was used to measure the hydrogen gas in the stream. After the twenty-eighth injection, the GC was set to automatically stop. After the GC was turned off, the ultra-high purity argon and hydrogen cylinders were closed. The temperature controller for the furnace was then turned off and the reactor was allowed to cool. The remaining cylinders were closed and the valves on the instrument were also closed so that nothing more would flow into the reactor. After the reactor was cooled to room temperature, the fiberglass was unpacked, and the glass tube was removed. The catalyst and quartz wool were removed and 15 disposed of properly. The glass tube was cleaned inside and out with acetone in preparation for the next run. All steps were repeated for each catalyst run. The catalysts, amounts, and flow rates for each run are recorded in the tables below. Test Runs A total of eleven runs were performed during the course of this research. The first four were to compare two monometallic catalysts to their bimetallic versions. A crack developed in the glass tube during the course of the third run that resulted in a leak. This catalyst had to be re-run. In addition, the effects of varying the amount of Ni/Al2O3 were to be investigated. Four additional runs were performed to investigate this concept. The data from the initial four milligram run would be used. In addition, a second run was performed with four milligrams of catalyst, followed by two runs with eight milligrams of catalyst, and one run with ten milligrams of catalyst. Finally, PtNi/CeZrO2 was compared to Pt/ZIF at the reduced temperature of 490°C. As mentioned previously, ZIF8 is thermally stable only up to a temperature of 500°C [14]. In order to ensure that the catalyst was acting at its full potential during the test, 490°C was not exceeded. TABLE 1. First Run Catalyst Ni/Al2O3 Mass 0.0045 g Flow Rate of CH4 4.52 mL/min Flow Rate of CO2 4.63 mL/min Reaction Temperature 800°C 16 TABLE 2. Second Run Catalyst PtNi/Al2O3 Mass 0.0042 g Flow Rate of CH4 4.61 mL/min Flow Rate of CO2 4.69 mL/min Reaction Temperature 800°C TABLE 3. Third Run Catalyst Ni/CeZrO2 Mass 0.0040 g Flow Rate of CH4 4.58 mL/min Flow Rate of CO2 4.65 mL/min Reaction Temperature 800°C (Leak during run) TABLE 4. Fourth Run Catalyst PtNi/CeZrO2 Mass 0.0040 g Flow Rate of CH4 4.63 mL/min Flow Rate of CO2 4.67 mL/min Reaction Temperature 800°C 17 TABLE 5. Fifth Run Catalyst Ni/Al2O3 Mass 0.0080 g Flow Rate of CH4 4.50 mL/min Flow Rate of CO2 4.57 mL/min Reaction Temperature 800°C TABLE 6. Sixth Run Catalyst Pt/ZIF Mass 0.0115 g Flow Rate of CH4 4.52 mL/min Flow Rate of CO2 4.59 mL/min Reaction Temperature 490°C TABLE 7. Seventh Run Catalyst Ni/Al2O3 Mass 0.0039 g Flow Rate of CH4 3.38 mL/min Flow Rate of CO2 3.40 mL/min Reaction Temperature 800°C 18 TABLE 8. Eighth Run Catalyst Ni/Al2O3 Mass 0.0080 g Flow Rate of CH4 3.50 mL/min Flow Rate of CO2 3.20 mL/min Reaction Temperature 800°C TABLE 9. Ninth Run Catalyst Ni/Al2O3 Mass 0.0101 g Flow Rate of CH4 3.30 mL/min Flow Rate of CO2 3.10 mL/min Reaction Temperature 800°C TABLE 10. Tenth Run Catalyst Ni/CeZrO2 Mass 0.0040 g Flow Rate of CH4 4.58 mL/min Flow Rate of CO2 4.65 mL/min Reaction Temperature 800°C (re-do of leaky run) 19 TABLE 11. Eleventh Run Catalyst PtNi/ CeZrO2 Mass 0.0102 g Flow Rate of CH4 4.63 mL/min Flow Rate of CO2 4.67 mL/min Reaction Temperature 490°C Calculations The first calculation performed for each test run is the calibration of the gas chromatograph. Four calibration injections were taken prior to each test run. As with the actual run, the first injection is disregarded. The average area under the curves for the second through fourth injection is calculated for each compound. The average area is then divided by its corresponding known percentage from the mix gas tank to find the slope. The values for each curve obtained during the test runs are divided by the slope in order to ensure the results are calibrated. From this result the molar FR factor (3) can be calculated and then used to calculate the methane conversion (4) value. 20 (3) (4) TABLE 12. Side-by-Side Comparison of All Test Runs Run Ni/Al2O3 1 4 mg 800°C PtNi/Al2O3 Ni/CeZrO2 4mg (leak) 800°C 3 4 mg 800°C 4 8 mg 800°C 11.5 mg 490°C 6 7 4 mg 800°C 8 8 mg 800°C 9 10 mg 800°C 10 Pt/ZIF 4 mg 800°C 2 5 PtNi/ CeZrO2 4 mg (redo) 800°C 10.2 mg 490°C 11 21 CHAPTER 4 RESULTS AND DISCUSSION Four Catalysts in Equal Quantities at the Same Temperature The first evaluation compared the methane conversion results of the four different catalysts at reaction temperatures of 800°C. Logically, a methane conversion of 100%, or 1.00, would be ideal. The conversion rate provides an idea of how much of the methane feed was successfully converted into product during the reaction. The hydrogen to carbon monoxide ratio was evaluated. As seen in Equation 1, a 1:1 ratio of hydrogen to carbon monoxide was expected. For the first evaluation, two monometallic catalysts, Ni/Al2O3 and Ni/CeZrO2, and their bimetallic versions, PtNi/Al2O3 and PtNi/ CeZrO2, were used. Each run was performed at 800°C with approximately four milligrams of catalyst at atmospheric pressure. For specific details on the amounts of catalysts used in each of these test runs, refer to Chapter 3. Because of the FID’s sensitivity for hydrocarbons and the TCD’s ability to detect hydrogen, both the FID and the TCD were utilized to record data. To ensure accurate data collection, data acquired by both sensors was compared. The first one or two injections into the gas chromatograph were removed from data of interest. These first injections were ignored due to inaccuracies of these first measurements compared to the remainder of the experimental measurements. 22 Figure 5 and Figure 6 show the results for the methane conversion of the four catalysts as measured by the FID sensor and TCD sensor, respectively. Both sensors reveal that the NiAl2O3 had the highest methane conversion value of the four catalysts. 1.0000 0.9000 Fractional Conversion 0.8000 0.7000 0.6000 Ni/Al2O3 0.5000 PtNi/Al2O3 0.4000 Ni/CeZrO2 0.3000 PtNi/CeZrO2 0.2000 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.1000 Time (hours) FIGURE 5. Methane conversion of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC. Figures 7 and 8 below show the carbon dioxide conversions from the FID and TCD sensors respectively. Similar to the methane conversion charts, the carbon dioxide chart from the FID sensor shows the catalyst NiAl2O3, as having the highest carbon dioxide conversion rate. As expected, the hydrogen to carbon monoxide ratio was approximately 1:1 slightly favoring hydrogen. Figure 9 and Figure 10 below show that both FID and TCD sensors measured similar values for hydrogen to carbon monoxide 23 1.2000 Fractional Conversion 1.0000 0.8000 Ni/Al2O3 0.6000 PtNi/Al2O3 Ni/CeZrO2 0.4000 PtNi/CeZrO2 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.2000 Time (hours) FIGURE 6. Methane conversion of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC. ratio. This 1:1 ratio means that the amount of hydrogen produced by the reaction was equivalent to the amount of carbon monoxide produced. Since hydrogen is the product of interest, finding a catalyst whose results favor hydrogen would be preferred. The Effect of Catalyst Mass The second evaluation was performed to determine if increasing the mass of a single catalyst while holding the flow rates of the reactants and temperature constant would result in improved conversion. Four, eight, and ten milligrams of catalyst were used for the tests. Given the results from the previous experiment, the catalyst chosen was Ni/Al2O3 at 800°C. The results demonstrated no significant difference in the methane conversion percentages between the varying amounts of catalyst as seen in Figure 11. This could mean one of two things. First, the reaction might have reached an 24 1.0000 0.9000 Fractional Conversion 0.8000 0.7000 0.6000 Ni/Al2O3 0.5000 PtNi/Al2O3 0.4000 Ni/CeZrO2 0.3000 PtNi/CeZrO2 0.2000 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.1000 Time (hours) FIGURE 7. Carbon Dioxide conversion of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC. 1.2000 Fractional Conversion 1.0000 0.8000 Ni/Al2O3 0.6000 PtNi/Al2O3 Ni/CeZrO2 0.4000 PtNi/CeZrO2 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.2000 Time (hours) FIGURE 8. Carbon Dioxide conversion of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC. 25 4.50 4.00 H2/CO Ratio 3.50 3.00 2.50 NiAl2O3 2.00 Pt/NiAl2O3 1.50 NiCeZrO2 1.00 Pt/NiCeZrO2 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.00 0.0 0.50 Time (hours) FIGURE 9. Hydrogen to Carbon Monoxide ratio of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC. 5.00 4.50 4.00 H2/CO Ratio 3.50 3.00 Ni/Al2O3 2.50 PtNi/Al2O3 2.00 Ni/CeZrO2 1.50 PtNi/CeZrO2 1.00 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.00 0.0 0.50 Time (hours) FIGURE 10. Hydrogen to Carbon Monoxide ratio of 4 different catalysts, in equal quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC. 26 equilibrium point at 800°C; or second, given that the flow rates of the reactants were held constant, it is probable that the limiting factor in this test was the amount of reactant provided and increasing the amount of catalyst did not increase the efficiency of the conversion. It should be noted that the ratio of hydrogen to carbon monoxide is greater than one for all ratios for all catalysts demonstrating each catalysts’ propensity of hydrogen production over carbon monoxide. This also indicates that there were other reactions occurring as well that were contributing to the hydrogen production, such as steam reforming of methane (2) or methane decomposition (5) [4]. This means the reaction mechanism is not as simple as what was expected. CH4 + H2O ↔ CO + 3 H2 (∆Hº = 206 kJ/mol) (2) CH4 ↔ C + 2 H2 (∆Hº = 75 kJ/mol) (5) Additionally, as the amount of catalyst is increased from four to eight milligrams, the hydrogen to carbon monoxide ratio also increases. This is demonstrated in both the FID and TCD sensors as seen in Figures 15 and 16. Furthermore, when the amount of catalyst is increased from eight to ten milligrams, the ratio of hydrogen to carbon monoxide production increases yet again when analyzing the TCD data as seen in Figure 16. Figure 15 shows conflicting data from the FID sensor, which actually shows the hydrogen to carbon monoxide ratio decreasing with the increase to ten milligrams of catalyst. However, this could be due to the fact that the FID is not as suitable a detector for measuring hydrogen as the TCD [19]. 27 1.0000 0.9000 Fractionial Conversion 0.8000 0.7000 0.6000 4mg Run 1 0.5000 8mg Run 1 0.4000 4 mg Run 2 0.3000 8 mg Run 2 0.2000 10 mg Run 1 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.1000 Time (hours) FIGURE 11. Methane conversion of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC. 0.9000 0.8000 Fractional Conversion 0.7000 0.6000 4 mg Run 1 0.5000 8 mg Run 1 0.4000 4 mg Run 2 0.3000 8 mg Run 2 0.2000 10 mg Run 1 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.1000 Time (hours) FIGURE 12. Methane conversion of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC. 28 1.0000 0.9000 Fractionial Conversion 0.8000 0.7000 0.6000 4mg Run 1 0.5000 8mg Run 1 0.4000 4 mg Run 2 0.3000 8 mg Run 2 0.2000 10 mg Run 1 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.1000 Time (hours) FIGURE 13. Carbon Dioxide conversion of Ni/Al2O3, in different quantities, at 800°C through plug flow reactor utilizing the FID sensor in the GC. 1.2000 Fractional Conversion 1.0000 0.8000 4 mg Run 1 0.6000 8 mg Run 1 4 mg Run 2 0.4000 8 mg Run 2 10 mg Run 1 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.2000 Time (hours) FIGURE 14. Carbon Dioxide conversion of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC. 29 2.50 H2/CO Ratio 2.00 1.50 4mg Run 1 8mg Run 1 1.00 4 mg Run 2 8 mg Run 2 0.50 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 0.00 10 mg Run 1 Time (hours) FIGURE 15. Hydrogen to Carbon Monoxide ratio of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the FID sensor in the GC. 3.00 H2/CO Ratio 2.50 2.00 4 mg Run 1 1.50 8 mg Run 1 4 mg Run 2 1.00 8 mg Run 2 10 mg Run 1 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.00 0.0 0.50 Time (hours) FIGURE 16. Hydrogen to Carbon Monoxide ratio of Ni/Al2O3, in different quantities, at 800°C through a plug flow reactor utilizing the TCD sensor in the GC. 30 New Catalyst, Pt/ZIF, Compared to Traditional Bimetallic Catalyst The third experiment utilized a different type of catalyst support than used in the previous two experiments, zeolitic imidazolate framework (ZIF). The platinumsupported ZIF is utilized at a lower temperature than the usual 800 °C because ZIFs are only thermally stable up to 500 °C [14]. However, this upper reaction temperature limitation could be considered a positive characteristic when evaluated with the significant cost savings potential when applied commercially. Methane conversion and hydrogen-to-carbon monoxide ratios of the reaction utilizing platinum supported ZIF-8 would be compared to the bimetallic catalyst, PtNi/CeZrO2. The catalysts were used in equal amounts and were processed through the plug-flow reactor at the reduced temperature of 490°C. Our goal is to find a low temperature catalyst with high surface area for dry reforming of methane. The methane conversion results of both PtNi/CeZrO2 and Pt/ZIF catalysts were significantly lower than the percent conversion in any of the previous tests. The temperature, which was reduced by 38.8%, yielded methane conversion values for PtNi/CeZrO2 that were on average 62.2% lower than the conversion values for tests ran at the full 800°C as seen in Figure 17. Because of the endothermicity of the dry reforming reaction, an increase in temperature leads to an increase in methane conversion and therefore a decrease in methane conversion at a lower temperature was expected [20]. However, it is important to note that while the overall conversion rate was lower due to the lower temperature of the tests, the percent methane conversion from the ZIF catalyst is very similar to the percent conversion of the PtNi/CeZrO2. The same observation is made when comparing the percent carbon dioxide conversion of ZIF and PtNi/CeZrO2. 31 Figure 19 details FID sensor data demonstrating hydrogen-to-carbon monoxide ratios that greatly favor hydrogen production in the reaction using ZIF-8 as the catalyst. Additionally the hydrogen-to-carbon monoxide ratio for the PtNi/CeZrO2 yielded similar results to all of the previous test runs of one-to-one. These results imply that even at significantly lower production temperatures, ZIF is capable of converting methane and favoring hydrogen production over carbon monoxide by an average factor of 2.4. There are several reasons why achieving results with the ZIF-8 catalyst is favorable. First, the Pt/ZIF reaction can be ran at a lower temperature resulting in lowered production costs. Second, despite lower temperatures, the Pt/ZIF reaction favors the production of hydrogen, the desirable product, over carbon monoxide. Lastly, although less of the methane was converted during the run, the remaining methane is not lost. It can be salvaged and continued to be fed through the reaction resulting in even more hydrogen production. 32 1.0000 0.6000 Pt/ZIF FID Pt/ZIF TCD 0.4000 PtNi/CeZrO2 FID PtNi/CeZrO2 TCD -0.2000 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 0.0000 1.0 0.2000 0.0 Fractional Conversion 0.8000 Time (hours) FIGURE 17. Methane conversion of 2 catalysts, in equal quantities, at 490°C through a plug flow reactor utilizing both the FID and TCD sensor in the GC. 0.8000 Fractional Conversion 0.7000 0.6000 0.5000 Pt/ZIF FID 0.4000 Pt/ZIF TCD 0.3000 PtNi/CeZrO2 FID 0.2000 PtNi/CeZrO2 TCD 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0000 0.0 0.1000 Time (hours) FIGURE 18. Carbon Dioxide conversion of 2 catalysts, in equal quantities, at 490°C through a plug flow reactor utilizing both the FID and the TCD sensor in the GC. 33 6.00 H2/CO Ratio 5.00 4.00 Pt/ZIF FID 3.00 Pt/ZIF TCD PtNi/CeZrO2 FID 2.00 PtNi/CeZrO2 TCD 13.0 12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.00 0.0 1.00 Time (hours) FIGURE 19. Hydrogen to Carbon Monoxide ratio of 2 catalysts, in equal quantities, at 490°C through a plug flow reactor utilizing both the FID and TCD sensor in the GC. 34 CHAPTER 5 CONCLUSIONS Carbon dioxide reforming, or dry reforming, is an essential technique that should be further utilized in today’s industry to not only synthesize a valuable substance, but simultaneously present a potential carbon footprint reduction by using a greenhouse gas as a reagent. Furthermore, several monometallic and bimetallic catalysts have been shown to be suitable for the production of hydrogen through dry reforming. Finally, zeolitic imidazolate frameworks show great promise as a next-generation catalyst for use in carbon dioxide reforming. Not only does the lower reaction temperature result in significant production savings when applied industrially, but the resulting product ratio greatly favors hydrogen and should be exploited for commercial use in dry reforming. 35 REFERENCES 36 REFERENCES [1] G. A. Florides, and P. Chrisodoulides. "Global warming and carbon dioxide through sciences." Environment International, Aug 2008. [2] M. M. Halmann. Chemical Fixation of Carbon Dioxide: Methods for Recycling CO2 Into Useful Products. Boca Raton, FL: CRC Press, 1993. [3] K. Takanabe. "Catalytic Conversion of Methane: Carbon Dioxide Reforming and Oxidative Coupling." Journal of the Japan Petroleum Institute, vol. 55, pp. 1-12, 2012. [4] A. Serrano-Lotina, and L. Daza. "Long-term stability test of Ni-based catalyst in carbon dioxide reforming of methane." Applied Catalysis A: General, vol. 474, pp. 107-113, 2013. [5] D. Liu, R. Lau, A. Borgna, and Y. Yang. "Carbon Dioxide Reforming of Methane to Synthesis Gas over Ni-MCM-41 Catalysts." Applied Catalysis A: General, vol. 358, pp. 110-118, 2009. [6] B. Mattson, M. Gibb, and P. Newman (2004, Dec. 30). Imagine the Universe Dictionary. [Online]. Available: http://imagine.gsfc.nasa.gov/docs/dict_ei.html# hydrogen. [7] R. Husted (1992). Hydrogen. [Online]. Available: http://periodic.lanl.gov/1.shtml. [8] S. Matar, M. Mirbach, and H. Tayim. Catalysis in Petrochemical Processes. Norwell, MA: Kluwer Academic Publishers, 1989. [9] W. L. Leffler. Petroleum Refining. Tulsa, OK: PennWell Publishing Company, 1985. [10] Catalyst. Merriam-Webster. [Online]. Available: http://www.merriam-webster.com/ dictionary/catalyst (accessed May 6, 2014). [11] L. S. Neiva, and L. Gama (2010). The Importance of Natural Gas Reforming. InTech. [Online]. Available: http://www.intechopen.com/books/natural-gas/theimportance-of-natural-gas-reforming. [12] S. Yanpeng, N. Yong, W. Angshan, J. Dengxiang, Y. Fengwen, and J. Jianbing. "Carbon Dioxide Reforming of Methane to Syngas by Thermal Plasma." Plasma Science and Technology, vol. 14, no. 3, pp. 252-256, 2012. 37 [13] S. R. De Miguel, I. M. J. Vilella, S. P. Maina, D. San Jose-Alonso, M. C. Roman Martinez, and M. J. Illan-Gomez. "Influence of Pt addition to Ni catalysts on the catalytic performance for long term dry reforming of methane." Applied Catalysis A: General, vols. 435-436, pp. 10-18, 2012. [14] A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe, and O. M. Yaghi. "Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks." Accounts of Chemical Research, vol. 43, no. 1, pp. 58-67, January 2010. [15] U. Mann. "Plug-flow reactor," in Principles of Chemical Reactor Analysis and Design, by Uzi Mann. Hoboken, NJ: Wiley, 2009, pp. 239-316. [16] D. L. Pavia, G. M. Lampman, G. S. Kriz, and R. G. Engel. Organic Laboratory Techniques. Belmont, CA: Brooks / Cole, 2005. [17] The Linde Group. Specialty Gases & Specialty Equipment. 2014. [Online]. Available: http://hiq.linde-gas.com/en/analytical_methods/gas_chromatography/ index.html. [18] Linde Gases Division. Flame Ionisation Detector, Data Sheet. Pullach, Germany: Linde AG, 2015. [19] Linde Gases Division. Thermal Conductivity Detector, Data Sheet. Pullach, Germany: Linde AG, 2015. [20] A. Serrano-Lotina, and L. Daza. "Influence of the operating parameters over dry reforming of methane to syngas." International Journal of Hydrogen Energy, vol. no. 39, pp. 4089-4094, 2014. 38