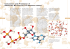The parental couple relationship in child and adolescent mental health
Transcription
The parental couple relationship in child and adolescent mental health
Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 rsif.royalsocietypublishing.org Constructing and characterizing a bioactive small molecule and microRNA association network for Alzheimer’s disease Fanlin Meng1, Enyu Dai1, Xuexin Yu1, Yan Zhang1, Xiaowen Chen1, Xinyi Liu1, Shuyuan Wang1, Lihua Wang2 and Wei Jiang1 Research Cite this article: Meng F, Dai E, Yu X, Zhang Y, Chen X, Liu X, Wang S, Wang L, Jiang W. 2014 Constructing and characterizing a bioactive small molecule and microRNA association network for Alzheimer’s disease. J. R. Soc. Interface 11: 20131057. http://dx.doi.org/10.1098/rsif.2013.1057 Received: 15 November 2013 Accepted: 3 December 2013 Subject Areas: bioinformatics, computational biology, systems biology Keywords: Alzheimer’s disease, bioinformatics, drug, microRNA, small molecule, therapeutics Authors for correspondence: Wei Jiang e-mail: [email protected] Lihua Wang e-mail: [email protected] Electronic supplementary material is available at http://dx.doi.org/10.1098/rsif.2013.1057 or via http://rsif.royalsocietypublishing.org. 1 College of Bioinformatics Science and Technology, Harbin Medical University, 194 Xuefu Road, Harbin 150081, People’s Republic of China 2 Department of Neurology, The Second Affiliated Hospital, Harbin Medical University, 246 Xuefu Road, Harbin 150081, People’s Republic of China Alzheimer’s disease (AD) is an incurable neurodegenerative disorder. Much effort has been devoted to developing effective therapeutic agents. Recently, targeting microRNAs (miRNAs) with small molecules has become a novel therapy for human diseases. In this study, we present a systematic computational approach to construct a bioactive Small molecule and miRNA association Network in AD (SmiRN-AD), which is based on the gene expression signatures of bioactive small molecule perturbation and AD-related miRNA regulation. We also performed topological and functional analysis of the SmiRN-AD from multiple perspectives. At the significance level of p 0.01, 496 small molecule–miRNA associations, including 25 AD-related miRNAs and 275 small molecules, were recognized and used to construct the SmiRN-AD. The drugs that were connected with the same miRNA tended to share common drug targets ( p ¼ 1.72 1024) and belong to the same therapeutic category ( p ¼ 4.22 1028). The miRNAs that were linked to the same small molecule regulated more common miRNA targets ( p ¼ 6.07 1023). Further analysis of the positive connections (quinostatin and miR-148b, amantadine and miR15a) and the negative connections (melatonin and miR-30e-5p) indicated that our large-scale predictions afforded specific biological insights into AD pathogenesis and therapy. This study proposes a holistic strategy for deciphering the associations between small molecules and miRNAs in AD, which may be helpful for developing a novel effective miRNA-associated therapeutic strategy for AD. A comprehensive database for the SmiRN-AD and the differential expression patterns of the miRNA targets in AD is freely available at http://bioinfo.hrbmu.edu.cn/SmiRN-AD/. 1. Introduction Alzheimer’s disease (AD) is an ageing-related degenerative brain disease of unknown aetiology. This disease is prevalent in the elderly population and is the most common form of dementia. This debilitating disorder affects more than 24 million people worldwide today [1]. Currently, there are five prescription drugs approved by the US Food and Drug Administration to temporarily relieve the relevant symptoms, but there are no cures for AD according to the Alzheimer’s Association [2]. AD is characterized by the presence of amyloid plaques and neurofibrillary tangles and the loss of neurons and synapses in brain regions, resulting in the death of neurons, memory impairment and cognitive deficits. The well-known AD-associated genes include the amyloid precursor protein (APP) gene, the presenilin 1 (PSEN1) gene and the presenilin 2 (PSEN2) gene [3,4]. In the normal state, the APP gene is a key player in neuronal survival, synaptic plasticity, learning and memory [5]. Mutations relevant to the APP and PSEN genes can lead to an increase in amyloid b-peptide (Ab) deposits around neurons & 2013 The Author(s) Published by the Royal Society. All rights reserved. Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 2. Material and methods 2.1. Data source 2.1.1. Alzheimer’s disease-related microRNA and mRNA expression profiles We obtained four AD-related gene expression profiles from Gene Expression Omnibus, including GSE16759, GSE12685, GSE5281 and GSE1297. All of the four expression profiles provided mRNA expression measurements and GSE16759 also involved miRNA expression levels (detailed clinical information of the samples in the electronic supplementary material, S1). AD is a complex disease, and many factors may affect the gene expression patterns, such as brain region, age as well as the degree of brain destruction (Braak and Braak (B&B) stage). However, several studies have suggested that certain important common features could be shared among different regions in AD brain [29–31]. In this study, to investigate the commonality among AD subjects and to obtain stable and consistently differentially expressed genes (CDGs), we used meta-analysis to detect differential 2 J. R. Soc. Interface 11: 20131057 Computational approaches have been more and more widely used in drug discovery [22,23]. Bork and co-workers [24] reviewed that computational approaches contribute to tackle complex problems in drug discovery, including largescale prediction of drug target or drug repurposing. Many studies have been conducted based on the hypothesis that gene expression profiles of perturbations (gene, drugs, diseases or miRNAs) could characterize the corresponding effects to some extent, and the perturbations could be associated based on the similarity of their induced expression profiles. For example, Butte’s group confirmed known effective drug–disease pairs and predicted new indications for already approved agents by comparing the expression profile similarity [25,26]. Iorio et al. [27] constructed a drug network based on similar expression profiles perturbed by various compounds and identified drug communities to predict modes of action for compounds that are still being studied or to discover previously unreported modes of action for well-known drugs. Our previous research investigated the potential connections between small molecules and miRNAs across 23 human cancers based on transcriptional responses similarity. We constructed a complex small molecule and miRNA network among 23 cancers and explored the molecular and functional features for small molecule modules, as well as miRNA modules in each cancer type [28]. In this study, we effectively extended and supplemented our previous approach to identify the positive and negative associations between small molecules and AD-related miRNAs (ADMs) by incorporating miRNA expression profiles. The differentially expressed target genes, which were regulated by the aberrantly expressed miRNAs, were defined as the gene expression signatures of ADM regulation. By comparing these signatures with transcriptional responses to drug treatment, we constructed the small molecule and miRNA association network in AD (SmiRN-AD), which was further analysed with respect to its topological characteristics and functional properties. Lastly, a web server was built for querying and visualizing the predicted associations between small molecules and ADMs and the differentially expressed targets of ADMs. Thus, our method and its application provided a novel viewpoint with respect to the treatment of AD. rsif.royalsocietypublishing.org [6]. The intracellular neurofibrillary tangles result from the accumulations of the microtubule-associated protein Tau and lead to apoptosis of the neuron. miRNAs are non-coding short RNA molecules that are involved in post-transcriptional gene regulation. Generally, these RNA molecules operate as guides for RISC to cleave a target mRNA or to block the target mRNA translation [7]. Certain analyses have shown the spatial or temporal distribution of miRNAs in the nervous system and have indicated that miRNAs may be involved in the fine-tuning of neuronal gene expression. MiRNA-mediated post-transcriptional regulations in cellular pathways and functions have effects on dendrite spine development, neuronal differentiation, memory and synaptic plasticity [8]. Moreover, accumulating evidence suggests that the alterations in miRNAs expression have potential contributions to AD pathophysiology [9]. Hebert et al. investigated alterations in miRNA expression profiles of patients with AD and recognized the miR-29a/ 29b-1 cluster as a potential suppressor of BACE1, based on theoretical predictions and in vitro validation in cells. They proposed that the loss of specific miRNAs could be responsible for the elevations of BACE1 and Ab levels in patients with AD [10]. Wang et al. [11] examined miRNA expression levels of the brain tissue of patients with AD and detected approximately 70 differentially expressed miRNAs. They suggested that miR-107 might contribute to AD progression by the regulation of BACE1, which was validated using Northern blot and target prediction algorithm. In addition, miRNAs have recently elicited a growing interest as new potential therapeutic targets and anti-cancer agents [12]. Reconstitution of underexpressed miRNAs or restriction of overexpressed miRNAs could become treatments for human diseases [13]. On the one hand, miRNAs have the potential to shift the philosophy of drug design from ‘one drug, one target’ to ‘one drug, multiple targets’ owing to their ability to target multiple genes and regulate a variety of cellular functions [14]. The approaches to inhibit the function of oncogenic miRNAs include the use of anti-miRNA oligonucleotides, miRNA sponges, miRNA masking and small molecule inhibitors [15], in which small molecules are inclined to possess ideal drug properties, including good solubility, bioavailability and metabolism [16]. Gumireddy et al. [17] identified diazobenzene and its derivatives as inhibitors of miR-21 after screening approximately 1000 compounds for increases in the luminescent signalling of luciferase expression and after performing RT-PCR studies. Similar virtual screening and RT-PCR experiments were conducted against liver-specific miR-122. Young et al. [18] reported compounds NSC158959 and NSC5476 to be miR-122 inhibitors and identified compound NSC308847 to be an activator of this miRNA. On the other hand, as many miRNAs are implicated in tumour suppressive effects, restoring their expression (endogenously or exogenously) may yield therapeutic effects [15,19]. Wiggins et al. [20] demonstrated that synthetic miR34a (a tumour-suppressor miRNA) is effective against tumours by restoring normal levels of miR-34a. The formulated synthetic miR-34a can be delivered in tumour xenografts, and also does not produce toxicity in mice. Reid et al. [21] verified that miR-16 mimics could be effectively delivered using EGFR-targeted minicells resulting in tumour regression in vivo, which represented a novel therapeutic approach for malignant pleural mesothelioma. Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 Table 1. Information on AD-related gene expression profiles the no. normal samples the no. AD samples array type 4 4 mRNA GSE1297 9 22 miRNA mRNA GSE12685 8 6 mRNA GSE5281 13 13 10 10 mRNA 12 13 16 9 11 23 12 95 19 119 Total expression in nine case–control studies that correspond to different microarray studies (table 1). 2.1.2. microRNA target genes We integrated the miRNA target gene dataset from seven prediction algorithms [32], which involved DIANA-microT, miRanda, RNA22, RNAhybrid, TargetScan, miRBase Targets and Pictar. In total, 289 469 miRNA–target mRNA pairs including 776 miRNAs and 15 185 miRNA target genes were predicted by at least two of the seven algorithms. 2.1.3. mRNA expression profiles perturbed with small molecules The connectivity map (cMap, build 02) provides mRNA expression profiles of four cell lines that were treated with 1309 bioactive small molecules (potential drugs or compounds) [33]. For each small molecule, we could obtain the differential gene expression levels based on the instances of the small molecule. Each instance comprises a pair of treatment and vehicle expression profiles for one small molecule. 2.1.4. Chemical structures of small molecules PubChem is an accessible repository for biological information of small molecules [34]. We searched chemical structures of small molecules and downloaded two-dimensional structure information in the form of ‘sdf’ files from PubChem Compound. 2.2. Data analysis We presented a computational pipeline to investigate the connections between small molecules and miRNAs in AD through comparative analysis of transcriptional responses of small molecule perturbation and miRNA regulation in AD. Compared with our previous study [28], we here introduced miRNA expression profiles to gain insights into the biological effects of small molecules and miRNAs more deeply in this study. The workflow of our approach is shown in the electronic supplementary material, figure S1. (See details in the electronic supplementary material, S2.) 2.2.1. Differential expression analysis To obtain the CDGs, we conducted meta-analysis to merge the nine case–control microarray studies related to AD. We first separately normalized the nine sets of mRNA expression data using 2.2.2. Alzheimer’s disease-related microRNA signatures and small molecule signatures In order to identify the transcriptional responses of miRNA regulations in AD, the gene expression profiles of miRNA perturbation should be used. However, there were no sufficient microarray data of miRNA perturbation. Here, we simulated the presence and absence of miRNA in miRNA perturbation as significantly upregulated and downregulated miRNAs in AD. Thus, for each ADM, we produced miRNA signatures through intersecting the CDGs and corresponding target gene sets. One ADM signature represented the genome-wide expression pattern regulated by the miRNA. For each small molecule, the corresponding signatures comprised all of the genes that were ranked in descending order according to their differential expression levels in expression profiles after perturbation by the small molecule. We obtained the drug signatures based on the connectivity map (cMap, http:// www.broadinstitute.org/cmap/) which contains drug perturbed and vehicle-treated (as control) gene expression profiles. Therefore, the small molecule signatures denoted the genome-wide expression alterations after small molecule perturbation. The set of small molecule signatures (as a reference set) collectively characterize the transcriptional responses of corresponding small molecules, which were applied to compare with the miRNA signatures through statistics test. 2.2.3. Associations between small molecules and microRNAs in Alzheimer’s disease We performed a rank-based, non-parametric test to associate small molecules with ADMs based on their gene expression signatures. For each ADM, if the upregulated target genes of the ADM tended to appear at the bottom of the ranked small molecule signatures, while the downregulated target genes tended to appear at the top of the ranked signatures, we inferred that the ADMmediated expression alterations were opposite to that of the small molecule perturbation. In this situation, a negative similarity score was calculated to measure the connection between the small molecule and the ADM (termed as ‘negative connection’), which might be helpful in the development anti-miRNA drugs because the gene expression pattern of corresponding ADM was opposite to that of small molecule as above. On the contrary, if the upregulated target genes fell near the top of the ranked small molecule signature, and the downregulated appeared near the bottom of the ranked small molecule signature, the small molecule and ADM pair was assigned with a positive similarity score representing similar transcriptional responses from the small molecule treatment and the miRNA perturbation. The small molecule and ADM connection that acquired a positive similarity score is called a ‘positive connection’, which may be helpful in developing miRNA mimic drugs. The similarity (S) score of one small molecule and ADM pair was calculated as follows. First, we calculated the Kolmogorov– Smirnov (KS) values for the upregulated (KSup) and downregulated J. R. Soc. Interface 11: 20131057 GSE16759 3 rsif.royalsocietypublishing.org GSE accession number median normalization via ArrayTools [35]. We next used the R package of ‘metaMA’ to implement meta-analysis based on the theory of effect size combination, in which the ‘SMVar’ method was applied to calculate the gene differential expression levels for each case–control study (details in the electronic supplementary material, S3). For miRNAs, the differentially expressed miRNAs were selected based on the empirical Bayes procedure, which was performed in Nunez-Iglesias et al. [36]. We then reserved the miRNAs, which were licensed by miRBase (v. 9.1) and provided targets in our integrated miRNAs target genes dataset. The differentially expressed miRNAs were considered to be ADMs. Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 2.2.4. The evaluation of common characteristics of pairwise small molecules We attempted to detect common characteristics with respect to chemical structures, targets and therapeutic categories between all of the possible small molecule pairs. We first evaluated the structural similarity between any two small molecules in the SmiRN-AD. The Tanimoto coefficient was used to detect the structural similarity for all possible small molecule pairs, which was implemented using small molecule subgraph detector (SMSD) software [37]. We then applied the unpaired t-test to evaluate the differences in the Tanimoto scores between small molecule pairs that were connected with the same ADM and small molecule pairs that were linked with different ADMs in the SmiRN-AD. Second, we detected the common characteristics of drug targets between all possible small molecule pairs. Information regarding the small molecules’ drug targets and therapeutic categories was obtained from DrugBank [38]. On the one hand, all of the possible small molecule pairs were divided into pairs that shared and did not share common targets. On the other hand, all of the possible small molecule pairs were divided into pairs that were connected and not connected to the same ADM. We hypothesized that the proportion of small molecule pairs that shared common drug targets was higher in pairwise small molecules that were connected with the same ADM. We attempted to test whether any difference in the proportions was significant by Fisher’s exact test. Similarly, we also performed the same statistical analysis of the pairwise small molecules on the therapeutic categories. 2.2.5. The evaluation of the common target similarities of pairwise Alzheimer’s disease-related microRNAs We also evaluated the proportions of common target genes between two miRNAs in the SmiRN-AD. For miRNA i and miRNA j, their differentially expressed target gene sets were denoted as target(i) and target( j), respectively. The ‘Meet/Min’ score was calculated as follows: jtargetsðiÞ > targetsð jÞj : minðjtargetsðiÞj; jtargetsð jÞjÞ The unpaired t-test was used to evaluate the difference in the ‘Meet/Min’ scores between ADM pairs that were connected to the same small molecule and ADMs pairs that were linked to different small molecules. 4 3.1. Differentially expressed mRNAs and microRNAs We collected AD-related gene expression profiles that involved GSE16759, GSE1297, GSE12685 and GSE5281, from the Gene Expression Omnibus. Using the R package of ‘metaMA’, we performed meta-analysis and identified 3506 CDGs that corresponded to 4503 differentially expressed probes (FDR 0.05). The details of CDGs are presented in the electronic supplementary material, S4. The CDGs represented the common characteristics that were potentially shared by different AD patients. Moreover, we determined 26 differentially expressed miRNAs (hsa-miR-15a, -20b, -29b, -29c, -30e-5p, -95 –101, -130a, -134, -148b, -181c, -185, -188, -320, -376a, -368, -374, -382, -432, -494, -572, -575, -598, -601, -617, -765) among AD and normal subjects (FDR 0.05) based on the miRNA expression profiles from GSE16759 (details in the electronic supplementary material, S5). 3.2. Bioactive small molecule and microRNA association network in Alzheimer’s disease To identify the relationships between small molecules and miRNAs in AD, we conducted a KS statistics test based on the signatures of 1309 bioactive small molecules (also known as potential drugs or compounds) and ADMs. As the implementation of cMap requires both up- and downregulated genes for each ADM, we excluded one ADM (hsa-miR-374) from the list of 26 ADMs, which has no upregulated genes. Thus, the final 25 ADMs were applied to identify the associations with small molecules. For each ADM, we calculated the similarity scores for all of the possible small molecule– miRNA connections and reserved only the significant associations at p 0.01. As a result, 496 small molecule–miRNA connections including 25 ADMs and 275 small molecules were recognized and used to construct the SmiRN-AD (figure 1), in which the ADMs and small molecules were represented as triangles and circles, respectively. Grey and red lines in the online version indicate positive and negative similarity scores, respectively. We next investigated the topological properties of the SmiRN-AD. The degree distribution of the small molecules is displayed in figure 2a, which shows that the majority of the small molecules (263/275, 95.6%) were connected with only a small number of ADMs (less than or equal to 5), which was consistent with the observations in a previous study of 23 types of cancers [28]. Here, 189 out of 275 small molecules were linked with only one ADM alone. That is, most of the small molecules were associated with the ADMs in a specificmiRNA manner. However, the degree distribution of miRNA was different. The degrees of miRNAs ranged from 12 to 28 (figure 2b). There were no miRNAs that associated with only one or even a small number of the small molecules, suggesting that miRNA may participate in multiple drug-related pathways. In addition, according to the integrated miRNAs’ target predictions, 12 out of the 25 ADMs regulated the wellknown AD genes (APP and PSEN2) and beta-site APP cleaving enzyme 1 (BACE1), in which BACE1 is involved in two sequential cleavages of the APP gene and play key roles in forming of the Ab deposits [39]. Highly connected nodes are listed in table 2. J. R. Soc. Interface 11: 20131057 Here, both KSup and KSdown are in the range [21,1]. The variable t represents the number of genes in the ADM signature, j represents the jth gene based on the rank of the differential expression in the ADM signature, N denotes the magnitude of the ranked small molecule signature and the element V( j ) of a vector V is the position of the jth target gene in the ordered small molecule signature. Second, the corresponding KSup and KSdown generated were integrated into the S score, as follows: S is equal to 0 when KSup and KSdown have the same sign and is equal to KSup 2 KSdown otherwise. In this study, we used cMap to implement the procedure. Based on the identified associations between small molecules and miRNAs, we constructed the SmiRN-AD. 3. Results rsif.royalsocietypublishing.org (KSdown) target genes of ADM, respectively. t j Vð jÞ ; a ¼ Max j¼1 t N t VðjÞ ðj 1Þ b ¼ Max j¼1 N t a; ða . bÞ and KSup=down ¼ b; ðb . aÞ: Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 nordihydroguaiaretic sulfacetamide thiostrepton acid clonidine nifenazone glimepiride solanine tacrolimus meptazinol tolmetin benzonatate clopamide levodopa emetine vincamine griseofulvin tribenoside clorsulon tolnaftate sulfaquinoxaline Prestwick-642 nomegestrol iocetamic acid betazole MG-262 mometasone josamycin practolol anisomycin astemizole Prestwick-984 rotenone mifepristone terfenadine dilazep proadifen gelsemine ivermectin mir-188 cefalexin CP-944629 clemizole cefuroxime clotrimazole proxyphylline myosmine nisoxetine mir-598 prenylamine cyclizine mir-130a triamterene trifluridine thioperamide bumetanide benzamil dobutamine lomustine mir-134 levonorgestrel 0316684-0000 acetylsalicylsalicylic niclosamide oxolamine zimeldine tanespimycin propranolol alsterpaullone metitepine atractyloside acid mir-765 semustine camptothecin alfaxalone methylbenzethonium terguride pramocaine wortmannin 3-acetamidocoumarin chloride terazosin perhexiline Prestwick-1082 mebeverine CP-690334-01 diperodon quinisocaine quinostatin ergocalciferol bezafibrate mir-494 apigenin protoveratrine A ampicillin trimethobenzamide cephaeline NS-398 niflumic acid flucytosine ceforanide propantheline bromide mir-181c luteolin harmol meclofenamic acid zidovudine SR-95639A Gly-His-Lys mir-368 chenodeoxycholic mir-101 0198306-0000 bepridil probenecid vorinostat acid thioridazine molindone ketotifen tetrandrine pseudopelletierine 3-hydroxy-DL-kynurenine vigabatrin mir-382 thioproperazine levothyroxine sodium novobiocin 0225151-0000 LY-294002 PNU-0230031 metacycline nicotinic acid nitrofural buspirone 15-delta ouabain triflupromazine desipramine metrizamide disopyramide resveratrol prostaglandin J2 ifenprodil nocodazole acenocoumarol estropipate etidronic acid moroxydine amoxapine MS-275 remoxipride spaglumic acid colchicine Prestwick-857 amikacin vinburnine mir-601 indapamide clobetasol mir-20b tonzonium bromide thapsigargin guanadrel ketorolac mafenide trichostatin A mexiletine mir-95 penbutolol pivmecillinam antazoline mir-432 aceclofenac terbutaline mebendazole lanatoside C mir-185 isoxicam tolfenamic acid meglumine prochlorperazine mevalolactone harmine PHA-00851261E fusaric acid etacrynic acid mir-575 flecainide carbimazole 5279552 mebhydrolin paromomycin amantadine melatonin ciclosporin sanguinarine captopril dinoprost digoxin beta-escin sirolimus rottlerin kaempferol 5230742 mir-376a puromycin alvespimycin lovastatin ethosuximide pimozide iproniazid 5707885 fenofibrate SC-58125 tetroquinone cefepime pyrazinamide STOCK1N-28457 gemfibrozil harmalol suloctidil syrosingopine fluphenazine mir-29b articaine Prestwick-675 hexylcaine hydrastine mir-30e-5p pilocarpine Y-27632 clemastine trifluoperazine atropine pentoxyverine pyrvinium tropine hydrochloride citalopram 15(S)-15-methylprostaglandin Prestwick-967 hydrocortisone diphemanil geldanamycin 6-bromoindirubin-3'-oxime 0317956-0000 acacetin mir-617 E2 hycanthone Prestwick-860 mir-572 metilsulfate promazine helveticoside H-7 16,16-dimethylprostaglandin amiodarone mir-320 digoxigenin quipazine trimethoprim idoxuridine E2 lasalocid Prestwick-685 loxapine colforsin SR-95531 mycophenolic acid mir-29c mir-15a IC-86621 mesalazine TTNPB proscillaridin sulfamethizole ursodeoxycholic acid estrone chlorzoxazone Prestwick-1080 rilmenidine phenindione latamoxef monensin 1,4-chrysenequinone nifuroxazide mir-148b tridihexethyl dorzolamide quercetin cobalt chloride sulindac methoxamine staurosporine loperamide cefotetan 5182598 dosulepin deptropine digitoxigenin 4-hydroxyphenazone amprolium oxymetazoline fenbendazole cefmetazole 5 hecogenin (b) 3 180 140 no. miRNAs no. small molecules 160 120 100 80 60 2 1 40 20 0 1 2 3 4 5 6 7 8 9 1213 small molecule degree 21 0 10 12 14 16 18 20 22 24 26 28 30 miRNA degree Figure 2. Degree distribution of small molecule and miRNA nodes. (a) Degree distribution of the small molecule nodes. (b) Degree distribution of miRNA nodes. 3.3. Functional characteristics of small molecule and microRNA association network in Alzheimer’s disease To investigate the characteristics of small molecules (or miRNAs) that were associated with the same miRNAs (or small molecules) in the SmiRN-AD, we performed comparative analysis for small molecule (or miRNA) pairs. For ADM pairs, we introduced the ‘Meet/Min’ score to evaluate the ratio of the ADM pairs’ common target genes. We obtained a significant difference ( p ¼ 6.07 1023) between ADM pairs that were linked to the same small molecule and that were connected with different small molecules. For small molecule pairs, we observed that small molecule pairs that connected with the same miRNA tended to share common drug targets ( p ¼ 1.72 1024) and tended to belong to the same therapeutic category ( p ¼ 4.22 1028). In addition, we calculated the similarity of two-dimensional chemical structures for each small molecule pair, which was measured via the Tanimoto coefficient provided by the SMSD software. The results indicated that small molecule pairs that were associated with the same miRNA in SmiRN-AD had moderate structure similarity ( p-value ¼ 0.27). The distribution of the small molecules in our predicted results across different classes according to the anatomical, therapeutic and chemical (ATC) classification system is shown in figure 3. Notably, we predicted significantly more small molecules that belong to the ATC classes of the cardiovascular system (C) and the nervous system (N) compared with the other classes. We also found evidence to support an insidious and likely causal association between cardiovascular disease (CVD) and AD [40]. According to autopsy reports on AD brains, approximately 60 –90% of the cases exhibited variable cerebrovascular pathology that was analogous to CVD [41]. Furthermore, a series of studies revealed that the very same risk factors for heart disease also put J. R. Soc. Interface 11: 20131057 (a) 200 rsif.royalsocietypublishing.org Figure 1. Small molecules – miRNAs network in AD. Network organization of miRNA and small molecule associations. Triangles indicate miRNAs and circles signify small molecules. (Online version in colour.) Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 V 2% R 7% S 9% A 9% C 17% N 16% D 9% J 12% H 2% L 4% G 4% Figure 3. Distribution of ATC top-level classes among small molecules. Alimentary tract and metabolism (A), blood and blood-forming organs (B), cardiovascular system (C), dermatologicals (D), genito-urinary system and sex hormones (G), systemic hormonal preparations, excluding sex hormones and insulins (H), antiinfectives for systemic use (J), antineoplastic and immunomodulating agents (L), musculo-skeletal system (M), nervous system (N), antiparasitic products, insecticides and repellents (P), respiratory system (R), sensory organs (S) and various (V). (Online version in colour.) Table 2. Highly connected small molecules and miRNAs in the SmiRN-AD small molecule nodes degree miRNA nodes degree thioridazine quinostatin 21 13 hsa-miR-148b hsa-miR-617 28 26 perhexiline metrizamide 12 9 hsa-miR-368 hsa-miR-181c 25 24 MS-275 9 hsa-miR-382 24 digoxin lanatoside C 8 8 hsa-miR-188 hsa-miR-376a 23 23 melatonin ouabain 8 8 hsa-miR-765 hsa-miR-95 23 22 beta-escin prochlorperazin 7 6 hsa-miR-432 hsa-miR-601 21 21 vorinostat 6 hsa-miR-320 20 individuals at greater risk of developing AD. For example, de Toledo Ferraz Alves et al. [42] reported that cardiovascular risk factors (such as hypertension, heart failure and hypercholesterolemia) could trigger AD pathology. All of the above evidence generally supports our predictions. In total, the majority of the small molecules in the SmiRN-AD belonged to ATC classes of the nervous system (N) and cardiovascular system (C), suggesting that these small molecules should be further examined for extended indications, especially for AD. 3.4. Biological insights into small molecule and microRNA association network in Alzheimer’s disease In the SmiRN-AD, we observed several ‘AD or neuron system-specific’ miRNAs through a literature review, such as miR-134, miR-101 and miR-15a. By both northern blotting and an RNase protection assay, Schratt et al. revealed that miR-134 was specially expressed in the brain and had a specific expression pattern in the hippocampus. MiR-134 levels gradually increased with development, peaking when synaptic maturation occurs [43]. Long and Lahiri applied qPCR to assay miR-101 levels, finding that miR-101 appears to be preferentially expressed in model CNS neurons [44]. At the same time, in additional research on rat hippocampal neurons that were cultured in vitro, Vilardo et al. [45] demonstrated that miR-101 negatively tuned the APP gene in hippocampal neurons in a luciferase assay. Thus, overexpressed miR-101 could reduce the APP gene levels and decrease the abundance on Ab deposits. Additionally, miR-15a is a Parkinson-related miRNAs according to Kim et al. study [46]. Moreover, miR-15a often results in a severe dementia that is similar to AD, when Parkinson’s disease progresses [2]. Furthermore, we concentrated on several positive and negative small molecule–miRNA connections in the SmiRNAD. Quinostatin is known to have an antiproliferative effect and is named after its quinoline core structure. Yang et al. screened a list of small molecule modulators of the mammalian target of rapamycin (mTOR) signalling network from a compound library of approximately 20 000 molecules. These authors identified quinostatin as a novel mTOR inhibitor through a high-throughput and cell-based assay [47]. The mTOR signalling pathway is relevant to various human diseases, including AD [48]. In vitro studies showed that Ab was an activator of the PI3K/AKT pathway, which in turn activated mTOR [49]. Caccamo et al. proposed that the interplays between mTOR signalling and Ab impinged on cognitive disturbances. A reduction in the mTOR signalling caused positive regulation of autophagy, which decreased the accumulation of Ab [50]. Thus, as an inhibitor of mTOR, quinostatin has a potential effect on AD via the regulation of the mTOR pathway. In our SmiRN-AD, miR-148b has a positive connection with quinostatin. MiR-148b is located at chromosome 12q13. Recent studies have found that it is downregulated in multiple human diseases using microarray analysis [51,52]. Using TaqMan low density miRNA arrays, Schonrock et al. observed that miR-148b was downregulated in subjects with AD, as well as another 19 downregulated miRNAs. The authors also verified the downregulation of miR-148b with in vivo analysis of APP23 hippocampus [53]. Based on enrichment analysis, we also found that the differential target mRNAs of miR-148b were overrepresented in the mTOR signalling pathway (hsa04150, figure 4) in the KEGG database ( p ¼ 0.042). Pink genes in the online version of the figure denote the differential targets of miR-148b that are overrepresented in the mTOR signalling pathway. 6 J. R. Soc. Interface 11: 20131057 M 6% alimentary tract and metabolism blood and blood-forming organs cardiovascular system dermatologicals genito-urinary system and sex hormones systemic hormonal preparations, excluding sex hormones and insulins anti-infectives for systemic use antineoplastic and immunomodulating agents musculo-skeletal system nervous system antiparasitic products, insecticides and repellents respiratory system sensory organs various rsif.royalsocietypublishing.org P 1% B 2% Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 7 rsif.royalsocietypublishing.org J. R. Soc. Interface 11: 20131057 Figure 4. MiR-148b in the mTOR signalling pathway. The mTOR signalling pathway from KEGG. (Online version in colour.) Given that our strategy reflected biological consequences by comparing the gene expression patterns of miRNA regulation and the transcriptional responses to small molecules, the positive connection indicated that miR-148b regulation and quinostatin treatment could lead to similar biological changes. That is, miR-148b potentially has degradative effects on Ab that are similar to quinostatin. Both miR-148b and quinostatin may become therapy for AD or other nervous system disorders based on the underlying molecular machinery. Especially, administration of synthetic miR-148b mimics might become a therapeutic treatment through ameliorating influence induced by AD-specific miR-148b downregulation. Another positive linkage is the amantadine and miR-15a connection. Generally, amantadine acts as an N-methyl-D-aspartate receptor antagonist and a dopamine agonist. Many researchers have observed that amantadine is associated with the treatment of behavioural disturbances, synaptic and cholinergic receptors, neurotransmitter deficits and improving neurorecovery [54,55]. MiR-15a was identified as an SA-miRNA (senescence-associated miRNA) and was downregulated in AD patients’ brains [56]. This miRNA is a member of the miR15/107 group, which has been implicated in neuronal degenerative diseases, including AD [57]. Moreover, the differentially expressed target genes of miR-15a are involved in AD pathway (hsa05010, figure 5) in the KEGG database. Five pink (online version) genes are miR-15a-targeted differentially expressed genes which are annotated in the hsa05010 pathway. Therefore, the amantadine-miR-15a positive connection we predicted also gives biological insights into AD therapeutics. When formulated miR-15a is administrated, the symptoms of AD subjects might be relieved, which achieve similar affects to amantadine. In previous investigations, researchers have proposed analogous therapeutic function in cancers. For example, Nora et al. showed that miR-15a was frequently deleted or downregulated in squamous cell carcinomas. They indicated that overexpression of miR-15a could induce cell cycle arrest in G1 –G0 [58]. Another study suggested that administrating a combination of miR-15a and miR-34a may also potentiate their therapeutic effects based on the demonstration that formulated miR-34a blocked tumour growth in vivo [59]. Additionally, melatonin has a negative connection with miR-30e-5p. Melatonin defects have been reported to occur in neurodegenerative disorders [60]. Moreover, as a known CNS depressant, melatonin is also implicated in the regulation of cognitive (e.g. learning and memory) impairment [61] and is associated with Ab [62]. Rosales-Corral et al. [63] suggested that melatonin could present multiple potential functions that correspond to several possible pathological mechanisms including the cholinergic hypothesis. Moreover, ‘melatoninergic’ or melatonin-type drugs may relieve dementia-related cognitive deficits and other syndromes [64,65]. Although melatonin has not been applied in treating AD in the clinical setting, we propose melatonin as a repositioning candidate to maximize its potential. Furthermore, Xu et al. demonstrated that the expression level of miR-30e in peripheral leucocytes was significantly higher comparing schizophrenia patients with the control group, and a genetic polymorphism (ss178077483) in the genes encoding miR-30e increased vulnerability to depression [66]. Cogswell et al. [67] concentrated on the miRNA changes in AD brains and found the overexpression of miR-30e-5p in the hippocampus by qRT-PCR. Melatonin has the potential to treat AD phenotype by reverting the gene expression pattern of miR-30e-5p ectopic regulation in AD patients. 3.5. Online database We constructed an online database to query and visualize the identified small molecule and miRNA associations. The Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 8 rsif.royalsocietypublishing.org J. R. Soc. Interface 11: 20131057 Figure 5. MiR-15a in the AD pathway. The AD pathway from KEGG. (Online version in colour.) database has a user-friendly interface to retrieve information according to the miRNA or the small molecule. Through the miRNA query, users can obtain the stem– loop structure of the primary miRNAs, the consistently differentially expressed target genes, the associated small molecules and the corresponding p-values. In addition, for each ADM, we illustrated the expression profiles of their consistently differentially expressed target genes using a heat map and the expression levels of each consistently differentially expressed target gene using a bar chart. This was done separately for nine case –control studies. Through small molecule queries, we provided the two-dimensional chemical structure, the associated miRNAs and the corresponding p-values. Most importantly, either query can return an illustration of the small molecule and miRNA associations for the user submitted small molecule or miRNA. The database is freely available at http://bioinfo.hrbmu.edu.cn/SmiRN-AD/. We anticipate that users can gain insights into screening drug candidates, drug repurposing and experimental designs when using our online database. 4. Discussion In this study, we effectively extended our previous study to identify the associations between small molecules and ADMs through comparative analysis of the transcriptional responses (or gene expression signatures) of small molecules perturbation and miRNA regulation in AD. We first conducted a meta-analysis to identify the CDGs by combining nine case–control AD-related microarray studies. The ADMs were selected from the Nunez-Iglesias’s microarray study. Second, we created the gene expression signatures for each ADM, which were attained by intersecting the ADM target gene set with CDGs. Lastly, through the cMap web server, we executed the query with each of the ADM signatures against the transcriptional responses of each small molecule, which represented the differentially expressed levels of the genes, comparing mRNA expression profiles in treatment versus non-treatment with small molecules. The aim was to return a similarity score of the small molecule–miRNA connections. According to the similarity scores, we evaluated the significance of the association. As a result, we constructed the SmiRN-AD including 25 ADMs and 275 small molecules at a significance level of 0.01. Through topological and functional analysis of the SmiRN-AD, we found that the small molecules (or miRNAs) that were connected with the same miRNA (or small molecule) shared common targets or therapeutic categories. Furthermore, we also investigated the biological insights afforded by the SmiRN-AD. We found that amantadine and quinostatin had positive connections with miR-15a and miR-148b in the SmiRN-AD, respectively. Moreover, melatonin had a negative connection with miR-30e-5p. The results might be facilitated to make references to molecular therapy for Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 extracted from the miRNA affected expression profile, the applicability of our method will broaden. In total, we propose a scheme for molecular signature-based inference of small molecule and miRNA associations in AD that is capable of guiding both drug repositioning and drug candidate screening from thousands of compounds. We also suggest that the analysis strategy that we used is not only appropriate for AD, but also appropriate for other complex diseases. References 1. Goedert M, Spillantini MG. 2006 A century of Alzheimer’s disease. Science 314, 777–781. (doi:10.1126/science.1132814) 2. Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 8, 131–168. (doi:10.1016/j.jalz.2012.02.001) 3. Scheuner D et al. 1996 Secreted amyloid betaprotein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 2, 864–870. (doi:10.1038/nm0896-864) 4. De Strooper B, Iwatsubo T, Wolfe MS. 2012 Presenilins and gamma-secretase: structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2, a006304. (doi:10.1101/ cshperspect.a006304) 5. Mattson MP. 2004 Pathways towards and away from Alzheimer’s disease. Nature 430, 631–639. (doi:10. 1038/nature02621) 6. Hardy J, Selkoe DJ. 2002 The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353 –356. (doi:10.1126/science.1072994) 7. Bartel DP. 2004 MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281– 297. (doi:10.1016/S0092-8674(04)00045-5) 8. Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. 2006 Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 124, 191–205. (doi:10.1016/j.cell.2005.12.017) 9. Delay C, Mandemakers W, Hebert SS. 2012 MicroRNAs in Alzheimer’s disease. Neurobiol. Dis. 46, 285–290. (doi:10.1016/j.nbd.2012.01.003) 10. Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. 2008 Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc. Natl Acad. Sci. USA 105, 6415–6420. (doi:10.1073/pnas.0710263105) 11. Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, Rigoutsos I, Nelson PT. 2008 The expression 12. 13. 14. 15. 16. 17. 18. 19. 20. of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 28, 1213–1223. (doi:10.1523/JNEUROSCI.5065-07.2008) Garzon R, Marcucci G, Croce CM. 2010 Targeting microRNAs in cancer: rationale, strategies and challenges. Nat. Rev. Drug Discov. 9, 775–789. (doi:10.1038/nrd3179) Ling H, Fabbri M, Calin GA. 2013 MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 12, 847 –865. (doi:10.1038/nrd4140) Tagliaferri P, Rossi M, Di Martino MT, Amodio N, Leone E, Gulla A, Neri A, Tassone P. 2012 Promises and challenges of MicroRNA-based treatment of multiple myeloma. Curr. Cancer Drug Targets 12, 838 –846. (doi:10.2174/156800912802429355) Bhardwaj A, Singh S, Singh AP. 2010 MicroRNAbased cancer therapeutics: big hope from small RNAs. Mol. Cell Pharmacol. 2, 213 –219. (doi:10. 4255/mcpharmacol.10.27) Zhang S, Chen L, Jung EJ, Calin GA. 2010 Targeting microRNAs with small molecules: from dream to reality. Clin. Pharmacol. Ther. 87, 754 –758. (doi:10. 1038/clpt.2010.46) Gumireddy K, Young DD, Xiong X, Hogenesch JB, Huang Q, Deiters A. 2008 Small-molecule inhibitors of microRNA miR-21 function. Angew. Chem. Int. Ed. Engl. 47, 7482–7484. (doi:10.1002/anie. 200801555) Young DD, Connelly CM, Grohmann C, Deiters A. 2010 Small molecule modifiers of microRNA miR-122 function for the treatment of hepatitis C virus infection and hepatocellular carcinoma. J. Am. Chem. Soc. 132, 7976–7981. (doi:10.1021/ja910275u) Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. 2008 Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152. (doi:10. 1038/nature06487) Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. 2010 Development 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 70, 5923– 5930. (doi:10.1158/0008-5472.CAN-10-0655) Reid G et al. 2013 Restoring expression of miR-16: a novel approach to therapy for malignant pleural mesothelioma. Ann. Oncol. (doi:10.1093/ annonc/mdt412) Marhofer RJ, Oellien F, Selzer PM. 2011 Drug discovery and the use of computational approaches for infectious diseases. Future Med. Chem. 3, 1011– 1025. (doi:10.4155/fmc.11.60) Ou-Yang SS, Lu JY, Kong XQ, Liang ZJ, Luo C, Jiang H. 2012 Computational drug discovery. Acta Pharmacol. Sin. 33, 1131 –1140. (doi:10.1038/aps. 2012.109) Iskar M, Zeller G, Zhao XM, van Noort V, Bork P. 2012 Drug discovery in the age of systems biology: the rise of computational approaches for data integration. Curr. Opin. Biotechnol. 23, 609–616. (doi:10.1016/j.copbio.2011.11.010) Sirota M, Dudley JT, Kim J, Chiang AP, Morgan AA, Sweet-Cordero A, Sage J, Butte AJ. 2011 Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci. Transl. Med. 3, 96ra77. (doi:10.1126/scitranslmed.3001318) Dudley JT et al. 2011 Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci. Transl. Med. 3, 96ra76. (doi:10. 1126/scitranslmed.3002648) Iorio F et al. 2010 Discovery of drug mode of action and drug repositioning from transcriptional responses. Proc. Natl Acad. Sci. USA 107, 14 621 –14 626. (doi:10.1073/pnas.1000138107) Jiang W et al. 2012 Identification of links between small molecules and miRNAs in human cancers based on transcriptional responses. Sci. Rep. 2, 282. (doi:10.1038/srep00282) Liang D, Han G, Feng X, Sun J, Duan Y, Lei H. 2012 Concerted perturbation observed in a hub network in Alzheimer’s disease. PLoS ONE 7, e40498. (doi:10. 1371/journal.pone.0040498) Liang WS et al. 2007 Gene expression profiles in anatomically and functionally distinct regions J. R. Soc. Interface 11: 20131057 Funding statement. This work was supported by the National Natural Science Foundation of China (grant no. 30900837), the Heilongjiang Postdoctoral Funds for Scientific Research Initiation (grant no. LBHQ11042), the Graduate Innovation Fund of Heilongjiang Province (grant no. YJSCX2012-249HLJ) and the Program for Young Talents of Science and Technology in Harbin (grant no. 2013RFQXJ057). 9 rsif.royalsocietypublishing.org AD in the future. We lastly constructed a free online database to help researchers easily access our predictions. Here, our main goal is to identify the associations between small molecules and miRNAs in AD. To this end, we focused on the common gene expression signatures across AD patients with different conditions, such as AD severity and brain regions. Furthermore, we integrated multiple microarray data to identify the consistently differently expressed genes by meta-analysis. The cross-study integration could not only contribute to high statistical power, but also make our predictions stable to some extent if we introduce additional new microarray data. Additionally, the lack of genome-wide data regarding the effects of miRNA on gene expression might place limits on the reliability of our results. Once we incorporate the miRNA-specific signatures that are Downloaded from http://rsif.royalsocietypublishing.org/ on July 7, 2015 32. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. J. Head Trauma Rehabil. 17, 300 –313. (doi:10. 1097/00001199-200208000-00004) Hebert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, Sergeant N, Buee L, De Strooper B. 2010 Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum. Mol. Genet. 19, 3959–3969. (doi:10.1093/hmg/ddq311) Finnerty JR, Wang WX, Hebert SS, Wilfred BR, Mao G, Nelson PT. 2010 The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J. Mol. Biol. 402, 491–509. (doi:10.1016/j.jmb.2010.07.051) Bandi N, Zbinden S, Gugger M, Arnold M, Kocher V, Hasan L, Kappeler A, Brunner T, Vassella E. 2009 miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 69, 5553–5559. (doi:10.1158/0008-5472.CAN-08-4277) Bandi N, Vassella E. 2011 miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol. Cancer 10, 55. (doi:10. 1186/1476-4598-10-55) Hardeland R. 2012 Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Scientific World Journal 2012, 640389. (doi:10.1100/2012/640389) Tuzcu M, Baydas G. 2006 Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur. J. Pharmacol. 537, 106–110. (doi:10.1016/j.ejphar.2006.03.024) Ionov M, Burchell V, Klajnert B, Bryszewska M, Abramov AY. 2011 Mechanism of neuroprotection of melatonin against beta-amyloid neurotoxicity. Neuroscience 180, 229 –237. (doi:10.1016/j. neuroscience.2011.02.045) Rosales-Corral SA et al. 2012 Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. J. Pineal Res. 52, 167–202. (doi:10. 1111/j.1600-079X.2011.00937.x) Furio AM, Brusco LI, Cardinali DP. 2007 Possible therapeutic value of melatonin in mild cognitive impairment: a retrospective study. J. Pineal Res. 43, 404–409. (doi:10.1111/j.1600-079X.2007.00491.x) Wu YH, Swaab DF. 2007 Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 8, 623–636. (doi:10.1016/j.sleep.2006.11.010) Xu Y et al. 2010 MicroRNAs and target site screening reveals a pre-microRNA-30e variant associated with schizophrenia. Schizophr. Res. 119, 219–227. (doi:10.1016/j.schres.2010.02.1070) Cogswell JP et al. 2008 Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimers Dis. 14, 27 –41. 10 J. R. Soc. Interface 11: 20131057 33. 44. microRNA regulates dendritic spine development. Nature 439, 283–289. (doi:10.1038/nature04367) Long JM, Lahiri DK. 2011 MicroRNA-101 downregulates Alzheimer’s amyloid-beta precursor protein levels in human cell cultures and is differentially expressed. Biochem. Biophys. Res. Commun. 404, 889–895. (doi:10.1016/j.bbrc.2010. 12.053) Vilardo E, Barbato C, Ciotti M, Cogoni C, Ruberti F. 2010 MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J. Biol. Chem. 285, 18 344–18 351. (doi:10.1074/jbc.M110. 112664) Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. 2007 A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317, 1220–1224. (doi:10.1126/ science.1140481) Yang J, Shamji A, Matchacheep S, Schreiber SL. 2007 Identification of a small-molecule inhibitor of class Ia PI3Ks with cell-based screening. Chem. Biol. 14, 371–377. (doi:10.1016/j.chembiol.2007.02.004) Ma T et al. 2010 Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS ONE 5, e12845. (doi:10.1371/journal.pone.0012845) Oddo S. 2012 The role of mTOR signaling in Alzheimer disease. Front. Biosci. 4, 941– 952. (doi:10.2741/S310) Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. 2010 Molecular interplay between mammalian target of rapamycin (mTOR), amyloidbeta, and Tau: effects on cognitive impairments. J. Biol. Chem. 285, 13 107–13 120. (doi:10.1074/ jbc.M110.100420) Bloomston M et al. 2007 MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 297, 1901–1908. (doi:10.1001/jama. 297.17.1901) Ueda T et al. 2010 Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 11, 136–146. (doi:10.1016/S1470-2045 (09)70343-2) Schonrock N, Ke YD, Humphreys D, Staufenbiel M, Ittner LM, Preiss T, Gotz J. 2010 Neuronal microRNA deregulation in response to Alzheimer’s disease amyloid-beta. PLoS ONE 5, e11070. (doi:10.1371/ journal.pone.0011070) Huey ED, Putnam KT, Grafman J. 2006 A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 66, 17 –22. (doi:10.1212/01.wnl.0000191304.55196.4d) Meythaler JM, Brunner RC, Johnson A, Novack TA. 2002 Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: a pilot double-blind randomized trial. rsif.royalsocietypublishing.org 31. of the normal aged human brain. Physiol. Genomics 28, 311– 322. (doi:10.1152/physiol genomics.00208.2006) Liu ZP, Wang Y, Zhang XS, Chen L. 2010 Identifying dysfunctional crosstalk of pathways in various regions of Alzheimer’s disease brains. BMC Syst. Biol. 4(Suppl. 2), S11. (doi:10.1186/1752-0509-4S2-S11) Li X et al. 2012 Dissection of human MiRNA regulatory influence to subpathway. Brief Bioinform. 13, 175–186. (doi:10.1093/bib/bbr043) Lamb J et al. 2006 The connectivity map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935. (doi:10.1126/science.1132939) Wang Y, Xiao J, Suzek TO, Zhang J, Wang J, Bryant SH. 2009 PubChem: a public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 37, W623 –W633. (doi:10.1093/nar/ gkp456) Zhao Y, Simon R. 2008 BRB-ArrayTools data Archive for human cancer gene expression: a unique and efficient data sharing resource. Cancer Inform. 6, 9– 15. Nunez-Iglesias J, Liu CC, Morgan TE, Finch CE, Zhou XJ. 2010 Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer’s disease cortex reveals altered miRNA regulation. PLoS ONE 5, e8898. (doi:10.1371/journal.pone.0008898) Rahman SA, Bashton M, Holliday GL, Schrader R, Thornton JM. 2009 Small molecule subgraph detector (SMSD) toolkit. J. Cheminform. 1, 12. (doi:10.1186/1758-2946-1-12) Knox C et al. 2011 DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res. 39, D1035–D1041. (doi:10.1093/nar/gkq1126) Vassar R et al. 1999 Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741. (doi:10.1126/science.286.5440.735) Stampfer MJ. 2006 Cardiovascular disease and Alzheimer’s disease: common links. J. Intern. Med. 260, 211–223. (doi:10.1111/j.1365-2796.2006. 01687.x) Obisesan TO, Gillum RF, Johnson S, Umar N, Williams D, Bond V, Kwagyan J. 2012 Neuroprotection and neurodegeneration in Alzheimer’s disease: role of cardiovascular disease risk factors, implications for dementia rates, and prevention with aerobic exercise in African Americans. Int. J. Alzheimers Dis. 2012, 568382. (doi:10.1155/2012/568382) de Toledo Ferraz Alves TC, Ferreira LK, Wajngarten M, Busatto GF. 2010 Cardiac disorders as risk factors for Alzheimer’s disease. J. Alzheimers Dis 20, 749–763. (doi:10.3233/JAD-2010-091561) Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. 2006 A brain-specific