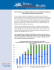
THESIS FILESObesity is a medical condition in
Transcription
THESIS FILESObesity is a medical condition in
A POLYHERBAL FORMULATION FOR HYPERLIPIDEMIA (ANCIENT MEDICINE MODERN TARGETS) Dr. Imtiaz Ahmed Channa MBBS, MS (Ortho), POA, Fellowship in Hip & Knee Replacement Surgery Dr. Kiran Saroor M.PHIL. / PHD, B-PHARM, MBA, Oncology certified, Resident in Oncology, Research Fellow at Karachi University Published by: Jabbar’s Printers Shop No. 11, Adeel Luxury Appartment, Opp. Baber Square Karimabad Karachi Pakistan OCTOBER 2015 Email: [email protected] DEDICATION THIS BOOK IS DEDICATED TO MY MOTHERS HASEENA KHATOON (LATE) & IFFAT JAHAN (LATE). WITHOUT THEIR GUIDANCE & LOVE I AM NOTHING. LOVE YOU MOM’S DR. KIRAN SAROOR TABLE OF CONTENTS Title Page No. CHAPTER 1 1.INTRODUCTION 1-3 1.1.CLASSES OF ANTI-OBESITY DRUGS 3 1.1.1.ESTABLISHED CLASSES 3 1.1.1.1.STATIN 3-4 1.1.1.2.FIBRATES 4 1.1.1.3.NIACIN 4 1.1.1.4.BILE ACID SEQUESTRANTS 4-5 1.1.1.5.EZETIMIBE 5 1.1.1.6.PHYTOSTEROL 5 1.1.1.7.ORLISTAT 5 1.1.2.INVESTIGATIONAL CLASSES 6 1.2.HERBS FOR WT LOSS AND SIDE EFFECTS 6 1.2.1.ASTRAGALUS 6 1.2.2.BEE POLLEN 7 1.2.3.BLADDER WREK 7 1.2.4.BREWERS YEAST 7 1.2.5.COCONUT OIL 7-8 1.2.6.MEDIUM CHAIN AND LONG CHAIN TRIGLYCERIDES 8-9 1.2.7.DANDELION 9-10 1.2.8.EVENING PRIMROSE 10 1.2.9.FENNEL 10 1.2.10.FENU GREEK 10 1.2.11.GREEN TEA 10 1.2.12.KELP 10-11 1.2.13.LICORICE 11 1.2.14.MALABAR TAMARIND 12 1.2.15.PINRAPPLE 12 1.2.15(a).PLANTAIN OR PSYLLIUM 13 1.2.16.RED PEPPER AND OTHER SPICES 13 1.2.17.SIBERIAN GINSENG 13-14 1.2.18.WALNUT 14 1.2.19.BOJENMI CHINESE TEA 14 1.2.20.OTHERS 15-16 1.3.MECHANISM OF ACTION OF ANTI - 17 OBESITY DRUGS 1.3.1.DRUGS FOR OBESITY CONTROL 17 1.3.1.1.NIACIN 18 1.3.1.1.1.NIACIN EFFECT ON LIPID METABOLISM 19 1.3.1.2.FIBRATES 19 1.3.1.2.1.FIBRATES EFFECT ON HDL 20 1..3.1.3.STATINS(HMG COA REDUCTASE INHIBITORS) 20 1.3.1.3.1.EFFECTS OF SIMVASTATIN ON TGRL 21 METABOLISM 1.3.1.4.OMEGA 3 FATTY ACIDS 21 1.3.1.4.1.CONVERSION OF LINOLEIC ACID TO LONG CHAIN 22 DERIVATES 1.4.MORTALITY 22-23 1.5.MORBIDITY 23 1.6.EFFECTS OF OBESITY ON DIFFERENT 24-25 BODY SYSTEMS 1.7.FACTORS OF OBESITY 26 1.7.1.FACTOR 1 (DIET) 26-27 1.7.2.FACTOR 2 (SEDENATRY LIFE STYLE) 27-28 1.7.3.FACTOR 3 (GENETICS) 28-29 1.7.4.FACTOR 4 (OTHER ILLNESS) 29 1.7.5.FACTOR 5 (SOCIAL DETERMINATION) 29-30 1.7.6.PATHOPHYSIOLOGY 30-31 1.8.PLANT DESCRIPTION 31 1.8.1.PLANT A (Cinnamomum cassia blume) 31 1.8.1.1.SCIENTIFIC CLASSIFICATION 31-32 1.8.1.2.TAXONOMICAL ENUMERATION 32 1.8.1.3.MEDICINAL USES 32-33 1.8.1.4.CHEMICAL AND BIOLOGICAL SURVEY 33 1.8.2.PLANT B(Commiphora wightii) 33 1.8.2.1.SCIENTIFIC CLASSIFICATION 33-34 1.8.2.2.TAXONOMICAL ENUMERATION 34 1.8.2.3.MEDICINAL USES 35 1.8.2.4.CHEMICAL AND BIOLOGICAL SURVEY 35 1.8.3.PLANT C (Embelica officinalis) 35 1.8.3.1.SCIENTIFIC CLASSIFICATION 35-36 1.8.3.2.TAXONOMICAL ENUMERATION 36-37 1.8.3.3.MEDICINAL USES 37 1.8.3.4.CHEMICAL AND BIOLOGICAL SURVEY 37-38 1.8.4.PLANT D (Ferula foetida L) 38 1.8.4.1.SCIENTIFIC 38-39 CLASSIFICATION,HABITAT,CULTIVATION 1.8.4.2.MEDICNAL USES 39 1.8.4.3.CHEMICAL AND BIOLOGICAL SURVEY 40 CHAPTER 2 40 2.INTRODUCTION 40-41 2.1.PARAMETERS FOR CRUDE DRUG 41-42 EVALUATION 2.1.1.QUALITATIVE CHEMICAL EVALUATION 42 2.2.IDENTIFICATION POF PLANTS 43 2.2.1.PLANT A(Cinnamomum cassia) 43 2.2.1.1.ORGANOLEPTIC EVALUATION OF PLANT A 44-45 2.2.1.2.ORGANOLEPTIC EVALUATION OF PLANT A BY 46 CHART 2.2.1.3.POWDER MICROSCOPY OF PLANT A 47 2.2.1.4.HISTOLOGY OF PLANT A 47-48 2.2.2.PLANT B (Commiphora wightii) 49 2.2.2.1.ORGANOLEPTIC EVALUATION OF PLANT B 50-51 2.2.2.2.ORGANOLEPTIC EVALUATION OF PLANT B BY 52 CHART 2.2.2.3.POWDER MICRSCOPY OF PLANT B 53 2.2.2.4.HISTOLOGY OF PLANT B 54-55 2.2.3.PLANT C (Embelica officinalis) 56 2.2.3.1.ORGANOLEPTIC EVALUATION OF PLANT C 57-58 2.2.3.2.ORGANOLEPTIC EVALUATION OF PLANT C BY 59 CHART 2.2.3.3.POWDER MICROSCOPY OF PLANT C 60 2.2.3.4.HISTOLOGY OF PLANT C 61-63 2.2.4.PLANT D(Ferula foetida L) 64 2.2.4.1.ORGANOLEPTIC EVALUATION OF PLANT D 65-66 2.2.4.2.ORGANOLEPTIC EVALUATION OF PLANT D BY 67 CHART 2.2.4.3.IDENTIFICATION TESTS OF PLANT D 68 2.2.5.REDUCIN 69 2.2.5.1.POWDER MICROSCOPY OF REDUCIN 69-70 CHAPTER 3 71 3.EXPERIMENTAL STUDIES 71 3.1.APPARATUS 71-73 3.2.MATERIAL AND METHOD 73 3.3.SOLVENTS 74 3.3.1.SOLVENT SYSTEM USED IN CHROMATOGRAPHY 75 3.4.BEHAVIOUR OF POWDER 76 3.4.1.BEHAVIOR OF POWDER WITH DIFFRENT CHEMICAL 76 REAGENTS (PLANT B 3.4.2.BEHAVIOR OF POWDER WITH DIFFRENT CHEMICAL 77 REAGENTS (PLANT C) 3.4.3.BEHAVIOUR OF POWDER WITH DIFFRENT 78 CHEMICAL REAGENTS (PLANT D) 3.5.PHYTOCHEMICAL ANALYSIS OF PRIMARY 79 AND SECONDRY METABOLITES (PLANTS A-D) 3.5.1.PHYTOCHEMICAL ANALYSIS OF PLANT A 79 3.5.2.PHYTOCHEMICAL ANALYSIS OF PLANT B 80 3.5.3.PHYTOCHEMICAL ANALYSIS OF PLANT C 81-82 3.5.4.PHYTOCHEMICAL ANALYSIS OF PLANT D 82 3.6.EXTRACTION AND ISOLATION 83 PROCEDURES 3.6.1.EXTRACTION AND ISOLATION OF REDUCIN POWDER 83 3.6.2.EXTRACTION OF PLANT DRUG A-D FOR 84 PHYTOCHEMICAL AND CHROMOGENIC TESTING 3.6.3.CHROMATOGRAM DEVELOPMENT FOR TLC 85-89 CHROMATOGRAPHY CHAPTER 4 90 4.CLINICAL STUDIES OF REDUCIN 90 4.1.INTRODUCTION 90 4.1.1.CONSEQUENCES OF OBESITY 91 4.1.2.SCREENING AND DIAGNOSIS 92 4.1.3.WAIST CIRCUMFERENCE 92 4.1.4.MEDICATION PROMOTE WT GAIN 93 4.1.5.CLASSIFICATION OF OBESITY AND DISEASES 94 4.1.6.METABOLIC SYNDROME 94-95 4.1.6.1.DIAGNOSTIC CRITERIA 95-96 4.2.MANAGMENT OF OBESITY 96 4.2.1.THE 5As FOR TREATMENT OF OBESITY 96 4.2.2.PHARMACOTHERAPY 97 4.2.3.BARIATRIC SURGERY 98 4.3.CLINICAL STUDIES OF REDUCIN 98 4.3.1.PROVEN THERAPEUTIC EFFICENCY OF HERBAL DRUGS (A-D)IN LITERATURE 98 4.3.1.1.PLANT A (Cinnamomum cassia) 98 4.3.1.2.PLANT B (Commiphora wightii) 98-99 4.3.1.3.PLANT C (Embelica officinalis) 99 4.3.1.4.PLANT D (Ferula foetida) 99-100 4.3.2.ANIMAL TRIALS 100 4.3.2.1.AIMS AND OBJECTIVES 100 4.3.2.2.CONDUCTION AND EVALUATION OF STUDIES 100-101 4.3.2.3.TRIAL I 101 4.3.2.4.TRIAL II 101-102 4.3.2.5.RESULTS 103 4.3.2.5.1.EFFECTS OF REDUCIN ON SERUM LIPID PROFILE 104 OF ADULT MALE RATS (A V/S B) 4.3.2.5.2.EFFECTS OF FATTY MIXTURE FEEDING DIET ON SERUM MALE RATS(A V/S C) 105 4.3.2.5.3.EFFECTS OF REDUCIN ON LIPID PROFILE (C V/S D) 106 4.3.2.6.DISCUSSION 107-108 4.4.EXPERIMENTAL CONDITIONS OF CLINAICAL TRIALS OF REDUCIN 108 4.4.1.CONDUCTION AND EVALUATION OF CLINICAL TRIALS 108 4.4.1.1.PARTICIPANTS 108 4.4.1.2.DOSAGE PROTOCOL 109 4.4.1.3.MEASUREMENT OF NEW OUT COMES 109 4.4.1.4.METHOD 109-110 4.4.1.5.DIAGNOSTIC CRITERIA 110 4.4.1.5.1.TYPES OF OBESITY 110-111 4.4.1.5.2.SIGN AND SYMPTOMS OF OBESITY 112-114 4.4.1.5.3.GENERAL PATIENT CRITERIA 115 4.4.1.6.COMPARITIVE STUDIES OF GENERAL PATIENT CRITERIA 115=116 4.4.1.7(a).GROUP A NORMAL DIET GENERAL HEALTH EXISTING DISEASE 117 4.4.1.7(b).GROUP B RICH FAT DIET HEALTH EXISTING DISEASE 118 4.5.DIAGNOSIS 119 4.5.1.BASIS OF DIAGNOSIS 119 4.5.2.ASSESMENT OF CHOLESTEROL LEVEL BEFORE AND AFTER TREATMENT OF REDUCIN 120 4.5.2.1.ASSESMENT OF CHOLESTEROL LEVEL BEFORE AND 120 AFTER TREATMENT OF REDUCIN IN NORMAL DIET GP. 4.5.2.2.ASSESMENT OF CHOLESTEROL LEVEL BEFORE AND 121 AFTER TREATMENT OF REDUCIN IN RICH FAT DIET GP 4.5.3.REDUCE HDL LEVELS BEFORE AND AFTER TREATMENT 122 4.5.3.1.REDUCED HDL LEVELS BEFORE AND AFTER TREATMENT OF REDUCIN IN MEN 122 4.5.3.2.REDUCED HDL LEVELS BEFORE AND AFTER TREATMENT OF REDUCIN IN WOMEN 123 4.5.4.REDUCED WAIST CIRCUMFERENCE BEFORE AND AFTER TREATMENT OF REDUCIN 124 4.5.4.1.REDUCED WAIST CIRCUMFERENCE AFTER TREATMENT OF REDUCIN IN MEN 124 4.5.4.2.REDUCED WAIST CIRCUMFERENCE AFTER TREATMENT OF REDUCIN IN WOMEN 125 CHAPTER 5 126 5.PHYTOCHEMICAL STUDIES 126 5.1.FORMULATION OF ORAL DOSAGE FPRM OF REDUCIN 126 5.2.MATERIAL AND METHOD 126 5.2.1.AUTHENTICATION OF DRUG 126 5.2.2.CLEANSING AND DRYING 127 5.2.3.GRINDING 127 5.2.4.PERCOLATION AND LYOPHILIZATION 127 5.2.5.FLOW ABILITY OF POWDER 128 5.2.6.ANGLE OF REPOSE OF POWDER 128-129 5.2.7.POROSITY OF POWDER 129-132 5.3.PHYSIOCHEMICAL FEATURES OF REDUCIN 132 5.3.1.WT VARIATION 132-136 5.3.2.DISINTEGRATION 137-138 5.3.3.DISSOLUTION 139-140 CHAPTER 6 141 6.RESULTS AND DISCUSSION 141-142 6.1.PHARMACOGNOSTIC EVALUATION OF PLANT DRUG (A-D) 142 6.1.1.CHARACTERISTIC FEATURES OF PLANT A 143 6.1.2.CHARACTERISTIC FEATURES OF PLANT B 144-145 6.1.3.CHARACTERISTIC FEATURES OF PLANT C 145-146 6.1.4.CHARACTERISTIC FEATURES OF PLANT D 146-147 6.1.5.CHARACTERISTIC FEATURES OF REDUCIN 147 6.2.PHYTOCHEMICAL EVALUATION OF PLANT DRUGS (A- 148-149 D) 6.3.CLINICAL STUDIES ON REDUCIN 150-151 6.4.PHYTOPHARMACEUTICAL STUDIES OF REDUCIN 152 REFRENCES 153210 CHAPTER 1.INTRODUCTION: Obesity is a medical condition in which excess body fat has accumulated to the extent that it may have a negative effect on health leading to reduce life expectancy and or increase health problems. Obesity increases the occurrence of various diseases especially heart diseases, type 2 diabetes, obstructive sleep apnea, certain type of cancer and osteoarthritis . Obesity is most commonly caused by combination of excessive food, energy intake, lack of physical activity and genetic susceptibility. A few cases are caused by genes, endocrine disorders, medications and psychiatric illness, evidence to support the view that some obese people eat little yet, gain weight due to a slow metabolism is limited. Dieting and exercising are the main treatment for obesity. Diet quality can be improved by reducing the consumption of energy dense foods such as those high in fat and sugars and by increasing the intake of dietary fiber with a suitable diet. Anti-obesity drugs may be taken to reduce appetite or decrease fat absorption. If diet, exercise and medications are not effective, a gastric balloon may assist with weight loss or surgery may be performed to reduce stomach volume or bowel length, leading to feeling full earlier and a reduce ability to absorb nutrient foods. Basically obesity is a medical condition in which excess body fat has accumulated to the extent that it may have an adverse effect on health. It is define by body mass index. BMI is defined as the subject weight divided by the square of their height and is calculated as follows:- BMI = m h2 Where m and h are the subjects wt and height respectively. BMI is usually expressed in Kg per square meter, resulting when wt is measured in kilograms and height in meters. To convert from pounds per square inch multiply by 703 (kg/m2) (1b/sq in) . The most commonly used definition established by the WHO in 1997 and published in 2000, provides the value listed in the table. BMI From (kg/m2) Upto 18.5 Classification 18.5 25.0 Normal wt 25.0 30.0 Over wt 30.0 35.0 Class I obesity 35.0 40.0 Class II obesity 40.0 Under wt Class III obesity Obesity has harmful effect on health leading to various types of diseases like cardiovascular diseases, diabetes mellitus type 2, and obstructive sleep apnea, certain type of cancers, osteoarthritis and asthma. 1.1. Classes of Anti-Obesity Drugs: There are several classes of hypolipidemic drugs. They may differ in both their impact on the cholesterol profile and adverse effects. For example, some may lower the "bad cholesterol" low density lipoprotein (LDL) more so than others, while others may preferentially increase high density lipoprotein (HDL), "the good cholesterol". Clinically, the choice of an agent will depend on the patient's cholesterol profile, cardiovascular risk, and the liver and kidney functions of the patient, evaluated against the balancing of risks and benefits of the medications. 1.1.1. Established Classes: 1.1.1.1. Statins: Statins are particularly well suited for lowering LDL, the cholesterol with the strongest links to vascular diseases. In studies using standard doses, statins have been found to lower LDL-C by 18% to 55%, depending on the specific statin being used. There is a risk of severe muscle damage (myopathy & rhabdomyolysis) with statins. Hypercholesterolemia is not a risk factor for mortality in persons older than 70 years and risks from statin drugs are more increased after age 85. 1.1.1.2. Fibrates: Fibrates are indicated for hypertriglyceridemia. Fibrates typically lower triglycerides by 20% to 50%. Level of the good cholesterol HDL is also increased. Fibrates may decrease LDL, though generally to a lesser degree than statins. Similar to statins, there is a risk of severe muscle damage (myopathy & rhabdomyolysis). 1.1.1.3. Niacin: Niacin like fibrates, is also well suited for lowering triglycerides by 20–50%. It may also lower LDL by 5–25% and increase HDL by 15–35%. Niacin may cause hyperglycemia and may also cause liver damage. The niacin derivative acipimox is also associated with a modest decrease in LDL. 1.1.1.4. Bile Acid Sequestrants: They are particularly effective for lowering LDL-C by sequestering the cholesterolcontaining bile acids released into the intestine and preventing their reabsorption from the intestine. It decreases LDL by 15–30% and raises HDL by 3–5%, with little effect on triglycerides but can cause a slight increase. Bile acid sequestrants may cause gastrointestinal problems and may also reduce the absorption of other drugs and vitamins from the gut. 1.1.1.5. Ezetimibe (Zetia): It is a selective inhibitor of dietary cholesterol absorption. Lomitapide (Juxtapid) is a microsomal triglyceride transfer protein (MTP) inhibitor. 1.1.1.6. Phytosterols: It may be found naturally in plants. Similar to ezetimibe, phytosterols reduce the absorption of cholesterol in the gut. Hence, they are most effective when consumed with meals. However, the precise mechanism of action of phytosterols differs from ezetimibe. 1.1.1.7. Orlistat (Xenical): Its primary function is to prevent the absorption of about 30% of fats from the human diet, thereby reducing caloric intake. A drug designed to treat obesity, it works by inhibiting pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. 1.1.2. Investigational Classes: CETP inhibitors (cholesteryl ester transfer protein). It is expected that these drugs will mainly increase HDL while lowering LDL; Squalene synthase inhibitor. ApoA-1 Milano. Hccinobucol(AGI-1067), a novel antioxidant, failed a phase 3 trial Apoprotein-B inhibitor Mipomersen (approved by the FDA in 2013 homozygous familial hypercholesterolemia). PCSK9 Monoclonal antibody inhibitors. 1.2. Herbs for Weight Loss & Side Effects: There are many herbal plants used as antilipidemic agents,some of them are as follow 1.2.1. Astragalus (Astragalus gummifer): Astragalus increases energy and improves nutrient absorption. SIDE EFFECTS: Do not use this herb in the presence of a fever. 1.2.2. Bee pollen: Bee pollen stimulates the metabolism and helps to curb appetite. Take up to 1 teaspoon daily.But reports of over stimulation are here. 1.2.3. Bladderwrack (Fucus vesiculosus): Bladderwrack contain iodine, which helps to enhance thyroid function. Dosage: Take 150 milligrams at breakfast and another 150 milligrams at lunch for two months. SIDE EFFECTS: Check with your doctor before taking this herb if you have a thyroid disorder, high blood pressure, or heart problems. If you are allergic to shellfish and/ or sensitive to iodine, do not take this herb. Also do not take kelp and bladderwrack at the same time. 1.2.4. Brewer’s yeast&Chick Weed: Brewer's yeast will help to reduce various cravings for food and drink. Chickweed (Stellaria media): This herb a great folk reputation for shedding weight. 1.2.5. Coconut oil: Coconut oil, extracted from coconuts, is a rich source for medium chain triglycerides. Medium chain triglycerides (MCTS) are special types of saturated fats separated out from coconut oil that range in length from six to twelve carbon chains. Unlike regular fats, MCTs do not appear to cause weight gain; they actually promote weight loss. 1.2.6. Medium Chain Triglycerides and Long Chain Triglycerides: Medium chain triglycerides (MCTS) are special types of saturated fats separated out from coconut oil. It range in length from six to twelve carbon chains. The long-chain triglycerides (LCTS) are the most abundant fats found in nature. LCTs are the storage fat for both humans and plants. They range in length from eighteen to twenty-four carbons. MCTs are used by the body differently than LCTs. This is because of the difference in length of carbon chains. The larger LCTs are difficult for the body to metabolize. So the body tends to store these fats. MCTS, on the other hand, are easy to metabolize. So, they are rapidly burned as energy. They also promote the burning of LCTs. Because of the way body handles MCTs, MCTs do not appear to cause weight gain like the conventional fats do. They, in fact, actually promote weight loss. LCTs are usually stored in the fat deposits. Since their energy is conserved, a high fat diet decreases the metabolic rate. Lower the metabolism, higher the weight gain. Scientists suggest that medium-Chain Triglycerides promote weight loss by increasing thermogenesis (heat production). Thermogenesis is the method by which the body "wastes" calories. There is evidence that the level of diet-induced thermogenesis is what determines whether an individual is likely to be overweight. In lean individuals, a meal may stimulate up to a forty-percent increase in heat production. In contrast, overweight individuals often experience only a ten-percent or less increase in heat production. The food energy is stored instead of being converted to heat. In actuality, the food is more efficiently converted in case of LCTs. So, there is less need for them to obtain the same energy. (LCTs is like a fuel efficient car. It needs less fuel to go to the same distance as compared to a fuel guzzling car (like MCTs). So, for the same food consumption, LCTs will have more fat stored in the body promoting weight gain. Researchers concluded that substituting MCTs for LCTs in your diet would produce weight loss as long as the calorie level remained the same. Recommended Dosage: 1 to 2 tablespoons per day. 1.2.7.Dandelion (Taraxacum officinale): Dandelion may flush out the kidneys, boost metabolism, and off- set a craving for sweets. Eat the leaves raw in a salad or make a tea by boiling 2 to 3 tsp of the root in a cup of water for I 0 to 15 minutes. Drink three times a day. 1.2.8. Evening primrose (Oenothera biennis): This herb is a good source for tryptophan which is believed to help in weight loss. Take a half-teaspoon of evening primrose oil three times a day. 1.2.9. Fennel (Foeniculum vulgare): Fennel removes mucus and fat from the intestinal tract, and is a natural appetite suppressant. 1.2.10. Fenugreek (Trigonella foenum-graecum): Fenugreek is useful for dissolving fat within the liver. 1.2.11. Green Tea (Camellia sinensis): Green tea enhances the ability of the body to burn fat. Choose a standardized extract containing 50 percent catechin and 90 percent total polyphenols and take 300 milligrams thirty minutes before breakfast and another thirty minutes before lunch. Do not take more. 1.2.12. Kelp (Fucus spp.): Kelp is a type of seaweed that’s rich in antioxidant vitamins and iodine. It is believed to stimulate a hormone produced by the thyroid gland that’s responsible for boosting metabolism, so you’ll burn more calories by the hour. You can also get other kinds of seaweed in your diet by adding them to soups and salads. Kelp is very useful for thyroid-related obesity. Dosage: Take 300-1,500 mg daily as directed on the label. SIDE EFFECTS: Check with your doctor before taking kelp if you have a thyroid disorder, high blood pressure, or heart problems. 1.2.13.Licorice (Glycyrrhiza glabra): Licorice root strengthens the adrenal glands, thus helping to sustain a regulated blood-sugar level and reduce cravings for sweets. Licorice tastes sweet. Dose: Take a cup of licorice daily, one week out of every month for up to three months. Licorice can also be added to other teas to sweeten them. SIDE EFFECTS: Do not take licorice by itself on a daily basis for more than five days at a time, as it can elevate blood pressure. Do not take it at all if you have high blood pressure. This herb should be used with caution. Check the herbal database for other important safety information. 1.2.14.Malabar tamarind: The Malabar tamarind is a yellowish fruit that is about the size of an orange, with a thin skin and deep furrows similar to an acorn squash. It is native to southern India, where it is dried and used extensively in curries (especially fish). It looks black when dried. The dried fruit of Malabar Tamarind contains about thirty percent hydroxycitric acid. It is a powerful lipogenic inhibitor. (Lipogenic inhibitor is a substance which helps prevent the production of fat). In addition to inhibiting the production of fat, hydroxycitrate may also suppress appetite. Note that hydroxycitrate only inhibits the conversion of carbohydrates into fat. It will have no effect if a high-fat diet is consumed. Recommended Dosage: 500 mg three times per day. Take it along with a supplement of Chromium for best results. 1.2.15. Pineapple (Ananas comosus): Pineapple contains an enzyme called bromelain, which helps digest both proteins and fats. 1.2.15(a).Plantain or psyllium (Plantago): Psyllium is the seed of Plantain. Metamucil is a commercial product that contain psyllium. Herbalists say that the weight-loss effect of plantain and psyllium is related to the spongy fiber (mucilage) in the seeds and to specific chemicals (polyphenols) in the leaves. Dose: Take a teaspoonful of psyllium mixed well with a glass of juice or water. Take it before each meal. SIDE EFFECTS: If you are allergic to this herb, stop its use immediately. 1.2.16. Red pepper (Capsicum), Hit Mustard, and other hot spices: Scientists have found that people who took hot spicy foods (adding a teaspoon of red-pepper sauce and a teaspoon of mustard to meal) raised their metabolic rates by as much as 25 percent. The hot spice also stimulates thirst, so you drink more liquids that also helps in gaining less weight. 1.2.17. Siberian Ginseng (Eleutherococcus Senticosus): Siberian ginseng helps to stabilize blood sugar and reduce cravings for sweets. It is also a natural energizer. Dose: Choose a standardized extract containing 0.5 percent eleutheroside E and take 100 milligrams daily, two weeks out of every month, for up to three months. SIDE EFFECTS: Do not use if you have high blood pressure. 1.2.18. Walnut (Juglans): A study of more than 25,000 Seventh-Day, Adventists showed that those who ate the most nuts were the least obese. Walnuts are rich in serotonin. Serotonin is shown to make us feel full; so we eat less as a result. 1.2.19. Bojenmi Chinese Tea (Reduce Fat Tea): This Chinese herbal formula is useful for combating weight gain and obesity. It has beneficial effects on the Stomach and Spleen, dispels Dampness and phlegm, invigorates Qi, promotes urination, and reduces fat. Dosage: Steep one bag per cup of water; drink up to three cups a day. Alisma, astragalus, and atractylodes is a Chinese herbal combination that helps to encourage weight loss. Alisma is a diuretic; astragalus enhances energy; atractylodes helps the digestion of carbohydrates. These three herbs work best when taken together. Select a combination formula and take one dose, as directed on the product label, two to three times daily. 1.2.20. Other Herbs: Combine 1 tbsp. of yarrow and 1 tbsp. of sea weed. Pour 1 cup of boiling water over 1 tsp. of this mix, steep for eight minutes, strain and drink 1 cup three times daily for one to two weeks. Other slimming herbs often included in herbal tea mixtures include kelp, chickweed, dandelion, sage, hawthorn berries, licorice root, papaya leaves, anise, wormwood, black alder bark, lovage and saffron. Alfalfa, com silk, dandelion, gravel root, horsetail, hydrangea, hyssop, juniper berries, oat straw, parsley, seawrack, thyme, uva ursi, white ash, and yarrow can be used in tea form for their diuretic properties. Butcher's broom, cardamom, cayenne, cinnamon, Garcinia cambogia, ginger, green tea, and mustard seed are thermogenic herbs that improve digestion and aid in the metabolism of fat. SIDE EFFECTS: Do not use cinnamon in large quantities during pregnancy. Bladderwrack, borage seed, hawthorn berry, licorice root, and sarsaparilla stimulate the adrenal glands and improve thyroid function. SIDE EFFECTS: If overused, licorice can elevate blood pressure. Do not use this herb on a daily basis for more than seven days in a row. Avoid it completely if you have high blood pressure. Ephedra,guarana and kola nut are appetite suppressants. SIDE EFFECTS:Do not use ephedra if you suffer from anxiety,glaucoma, Heart disease,high blood pressure ,insomnia or if you take MAO inhibitor Drug for depression. Note: to the high incidence of abuse of the ephedra and the resultant deaths and serious injuries, FDA has banned the sale of ephedra in the US. A number of other countries have banned the use of ephedra too. We recommend that you do not use or buy any products with ephedra. Siberian ginseng aids in moving fluids and nutrients throughout the body, and reduces the stress of adjusting to new eating habits. SIDE EFFECTS: Do not use this herb if you have hypoglycemia, high blood pressure, or a heart disorder. As we discuss above all herbal drugs have mild to severe side effects. Now we take a look towards mechanism of action of antilipidemic agents. 1.3. Mechanism of Action of Anti obesity Drugs: These are 4 classes of FDA-approved drugs, all of which have an indication for reducing triglyceride. 1.4. Mortality: Obesity is one of the leading causes of death worldwide. Large scale American and European studies have found that mortality risk is lowest at BMI of 20-25 kg/m2 in now smokers and at 24-27 kg/m2 in Asia risk begin to increase b/w 22-25 kg/m2 . A BMI above 32 kg/m2 has been associated with a double mortality rate among women over a 16 years period. In the USA, obesity in estimate to cause 111,909 to 365,000 deaths per year, while 1 million (7.7%) of death in Europe are attributed to excess (wt [3/32]) on average obesity reduce life expectancy by 6 to 7 years, a BMI of 30-35 (kg/m2) reduce life expectancy by 2 to 4 years while severe obesity (BMI > 40 kg/m2) reduces life expectancy by 10 years. 1.5. Morbidity: Obesity increases the risk of many physical and mental conditions. These co morbidities are combination of medical disorder which includes diabetes mellitus type 2, H.B.P, H.B.C and high triglycerides levels. Health consequences fall in to two broad categories, those attributable to the effects of increased fat mass (such as osteoarthritis, obstructive sleep apnea, and social stigmatization) and those due to increased no. of fat cells (diabetes, cancer, cardiovascular disease, non- alcoholic fatty liver diseases. Increase in body fat alter the body response to insulin, potentially leading to insulin resistance. Increased fat also creates a pro inflammatory state and a prothrombic state. The table given below shows the effect of obesity on different body systems. 1.6.EFFECT of Obesity on diffrent Body Systems: Medial field Cardiology • Condition Coronary Medical field Dermatology heart disease angina Endocrinolog y • and reproductive • medicine • Condition Stretch marks • Acanthosis and nigricans myocardial • Lymphedema infarction • Cellulitis • Hirsutism • Intertigo • Gastroesophageal Diabetes Gastrointestin mellitus al reflux disease Polycystic • Fatty liver disease ovarian • Cholelithiasis syndrome • (gallstones) Menstrual disorders • Infertility • Complications during pregnancy • Birth defects • Intrauterine fetal death Neurology • Stroke • Meralgia Oncology • Esophageal • Cobrectal paresthetica • Pancreatic • Migarines • Gall bladder • Carpal • Endometrial syndrome • Kidney • Dementia • Leukemia • Idiopathic • Malignant tunnel intracranial melanoma hypertension • Multiple sclerosis Psychiatry • Depression in Respirology • women • Obstructive sleep apnea Social • stigmatization Obesity hypoventilation syndrome • Asthma • Increased complications during general anaesthesia Rheumatolog • Gout y • Poor mobility Urology and • Erectile dysfunction • Urinary incontinence 1.7. Factors: There are many factors which involve to produce obesity. 1. Factor 1 (Diet) 2. Factor 2 (Sedentary Life Style) 3. Factor 3 (Genetics) 4. Factor 4 (Other illness) 5. Factor 5 (Social Determinants) 1.7.1. Factor 1 (Diet) Dietary energy supply per capita varies markedly between different regions and countries. It has also changed significantly overtime. From the early 1970s to the late 1990s the average food energy available per person per day (the amount of food bought) increased in all parts of the world except Eastern Europe. The United States had the highest availability with 3, 654 calories (15,290 kJ) per person in 1996. This increased further in 2003 to 3,75 calories (15,710) kJ). During the late 1990s Europeans had 3,394 calories (14,200 kJ) per person, in the developing areas of Asia there were 2,648 calories (11,080 kJ) per person, and in sub-Saharan Africa people had 2,176 calories (9,100) kJ) per person. Total food energy consumption has been found to be related to obesity. The widespread availability of nutritional guidelines has done little to address the problem of overeating and poor dietary choice. From 1971 to 2000, obesity rates in the United States increased from 14.5% to 30.9%. During the same period, an increase occurred in the average amount of food energy consumed. For women the average increase was 335 calories (1,400 kJ) per day (1,542 calories (6,450 kJ) in 1971 and 1,877 calories (7,850 kJ) in 2004, while for men the average increase was 168 calories (700 kJ) per day (2,450 calories (10,300 kJ) in 1971 and 2,618 calories (10,950 kJ) in 2004). Most of this extra food energy came from an increase in carbohydrate consumption rather than fat consumption. The primary sources of these extra carbohydrates are sweetened beverages, which now account for almost 25 percent of daily food energy in young adults in America, and potato chips. Consumption of sweetened drinks such as soft drinks, fruit drinks, iced tea, and energy and vitamin water drinks is believed to be contributing to the rising rates of obesity and to an increased risk of metabolic syndrome and type 2 diabetes. 1.7.2.Factor 2: Sedentary Life Style: A sedentary life style plays a significant role in obesity. Worldwide there has been a large shift towards less physical demanding work and currently atleast 30% of the world population gets in sufficient exercise gets in sufficient exercise. This is primarily due to increasing use of mechanized transportation and a greater prevalence of labour saving tech in home. In children these appear to be declines in levels of physical activity due to less walking and physical education. In both children and adults, there is an association b/w television viewing time and the risk of obesity. A review found 63 of 73 studies 86% showed an increased rate of childhood. Obesity with increased media exposure without increasing proportionally to time spent watching television. 1.7.3.Factor 3: Genetics: Obesity is actually a result of an interplay b/w genetic and environmental factors. Polymorphisms in various genetic controlling appetite and metabolism predispose to obesity, when sufficient food energy is present. As of 2006, more than 41 of these sites on human genome have been linked to the development of obesity when a favourable environment is present. Obesity is a major feature in several syndromes, such as prader- willi syndrome, Bardet-Biedl syndrome, Cohen syndrome and Momo syndrome. In people with early onset severe obesity (defined by on onset before 10 years of age and body mass index over three standard deviations & above normal 7% harbor a single point DNA mutation). Studies have focused on inheritance patterns rather than on specific genes have found that 80% of the off spring of two obese parents were also obese, in contrast to less than 10% of the off spring of 2 parents who were of normal wt , different people exposed to the some environment have different risk of obesity due to their underlying genetics . 1.7.4.Factor 4: Other Illness: Certain physical and mental illnesses and the pharmaceutical substances use to treat them can increase risk of obesity. Medical illnesses that increases obesity risk include several rare genetic syndrome and some congenital conditions:- hypo thyroidism, Cushing’s syndrome, growth hormones deficiency and the eating disorders:- binge eating disorder and night eating syndrome . The risk of over wt and obesity is higher in patients with psychiatric disorders than in persons without psychiatric disorder. 1.7.5.Factor 5: Social determination: Many explanations have been put fourth for link b/w BMI and social class. In undeveloped countries the ability to afford food, high energy expenditure with physical labour and cultural values favouring a large body size are believe to contribute to the obesity . A correlation in BMI change overtime has been found among friends, siblings and spouses . Stress and perceived low social status appear to increase risk of obesity. Smoking has a significant effect on an undivided wt. Those who quit smoking gain an average of 4.4 kilo (9.7 lb) for men and 5 kilogram (11.0 lb) for women over 10 years. In developing world urbanization is playing a role in increasing rate of obesity. In china overall rates of obesity are below 5%, in some cities obesity rates greater than 20% . 1.7.6.Pathophysiology:There are many possible pathophysiological mechanism involved in the development and maintenance of obesity. The leptin gene was discovered in 1994 and it was a satiety factor. Since leptin discovery ghrelin, insulin, orexin, pyy-3-36 and other medication have been studied. The leptin and ghrelin are considered to be complement in their influence on appetite with ghrelin produced by the stomach modulating short term appetite control. Leptin is produced by a dispose tissue to signal fat storage reserves in the body and mediates long term appetitive control. . For preparation of a perfect antilipidemic drug which has no side effects, we make a formulation named as REDUCIN. In prepraration of this wt reducing drug we consider four plants, which description is as follows: 1.8.PLANT DESCRIPTION 1.8.1. PLANT A:Cinnamomum cassia Blume: 1.8.1.1.Scientific Classification: • Kingdom Plantae • Unranked angiosperms • Unranked magnolids • Order Laurales • Family Lauraceae • Genus Cinnamomum • Species C. Cassia • Binomial name • Cinnamomum cassia • Synonyms • Camphorina cassia (Nees + T. Nees) • Cinnamomum longifolium Lukman • Cinnamomum medium Lukman • Cinnamomum nitidum hook.nom.illeg 1.8.1.2.Taxonomical Enumeration: Habitat: Cinnamomum cassia called Chinese cassia or Chinese is an evergreen tree originated in southern china. The tree grow to 10-15m tall, with grayish bark and hard elongated leaves that are 10-15 cm long and have a dediedly reddish colour when young . Cultivation: It is widely cultivated in Southern China and in Southern and Eastern Asia (India, Indonesia, Laos, Malaysia, Taiwan, Thailand and Vietnam). It is one of Soul species of cinnamomum, used for their aromatic bark, with is used as a spice. In use Chinese cassia is the most common type of Cinnamomum used. 1.8.1.3.Medicinal uses: Cassia bark both powdered and in whole or stick form used as a flavouring agent for confectionary, desert, pastries and meat. It is specified in many recipies. Cassia is sometimes added to Ceylon cinnamon but is as much tricker, coarser product. Cassia buds resemble cloves in appearance and have a mild, flowering Cinnomon flavour. Cassia buds are primarily used in old fashioned pickling recipies, marindas and yeast. 1.8.1.4.Chemical and biological survey : The main component of Cassia oil are Cinnamic aldehyde, cinnamyl acetate, benzaldehyde, dinatal and charicol, coumarin with constituents of sugar, protein, crude fats, pectin and others. It has following biological activity, It is used as antibacterial, antiinflammatory, antidiabetic, gene expression and immune response. It also exert antioxidant, anti-tumor, anti-thrombotic and other activities. 1.8.2.Plant B:Commiphora wightii(AM) Bhandari: 1.8.2.1.Scientific Classifications: • Kingdom plantae • Unranked angiosperms • Unranked Eudicots • Unranked rosids • Order Sapindales • Family Burseraceae • Genus Commiphora • Species C. Wightii • Binomial name • Commiphora wightii (Am) Bhandari • Synonyms • Commiphora mukul (stooks hook) 1.8.2.2.TAXONOMICAL ENUMERATION: HABITAT: Commiphora wightii plant may be found from Northern Africa to Central Asia but is most common in Northern India. Cultivation: It is a shrub or small shrub reaching a max height of 4m within papary bark. The branches are throny, the leaves are simple or trifoliate, the led of orate, 1-5cm long, 0.52.5cm broad, irregularly throned. Guggul is sought for its gummy resin, in India and Pakistan guggul is cultivated commercially. The resin of the guggul plant known as gum guggul has a fragrance similar to treat of myrrh. 1.8.2.3.Medicinal uses: Guggul produces a resinous sap known as gum guggul. The extract of this gum called guggullipid, which used in unani and ayurvedic medicine, a traditional unani med for nearly 3000 yrs in India, also used for reducing obesity, rheumatoid arthritris, osteoarthritis and sociatica . The gum guggul has a fragnance similar to that of myrrh and is commonly used in perfumes. Guggul strengthen the neuron, often it promote digestion and good for liver functioning, and it is antihemorrhoid in action. It also act on urinary system help in dysurea and renal calculi. It also act as aphrodisiac agent and help in retaining menstrual problems. 1.8.2.4.Chemical and biological survey: It contain an oleogum resin in that contain myrcene, dimyrcene and polymyrcene. As per constituent it has 6.9% moisture, 0.6% volatile oil, resin 61% ,gum 29.6% and insoluble substance 3.2% beside all it also contain alkoloids in low amounts. . 1.8.3.Plant C :Emblica officinalis L: 1.8.3.1.Scientific Classifications: • Kingdom Plantae • Unranked Angiosperms • Unranked Eudicots • Unranked Rosids • Order Malpighiales • Family Phyllanthaceae • Tribe Phyllanthaceae • Subtribe Flueggeinae • Genus Phyllantus • Species P. Emblica • Binomial name • Phyllantus emblica • Synonyms • Diasperus emblica (L) Kuntze • Dichelactira nodicaqulis Hance • Emblica arborea Raf • Phyllanthus glomeratus • Phyllanthus taxifolius 1.8.3.2.TAXONOMAICAL ENUMERATION: Habitat: It grows in Pakistan, throughout India, Sri lanka and East to South China, West Malaysia and Nepal, found wild in the foot hills of Himalayas and cultivated in plains. Cultivation: Riping in autumn, the berries are harvested by hard after climbing to upper branches bearing the fruits. 1.8.3.3.Medicinal uses: Fruit is astringent, antipyretic, expectorant, diuretic, alterative, laxative, refrigerant and carminative. The bark and root are astringent whereas flowers are aparent and refrigerant. Aqeuous extract of fruit prevents the hepatotoxic and neurotoxic effects induced by lead and aluminum in mice. The fruit has been observed to reduce cytotoxic effects of zinc chloride, ethyl parathion and matanil yellow when administer to mice. The seeds are regarded as aphrodisiac and antipyretic generally Emblica officinalis fruit is used in forms of decotion, infusion of leaves and seeds, liquour, emulsion, oil, confection, powder, paste and pickles, fruit juice with honey is used as vermifuge and fresh fruit in the form of pickle . 1.8.3.4.Chemical and biological survey : Biological survey of Emblica officinalis had shown it is a rich source of vit C, a good antioxidant, can be used as long term contraceptive, cardio protective, anticancer and antibacterial and immune modulator. Along with improving vigor and bone strength, it is also used for treating menstrual disorders. It also has a mild depresent action on the CNS. It is used as oral contracepire and anti mutagenic effect . Skin cosmetics contains powder of E. officinalis (b/c of its antioxidant properties). It also use for improvement of brain functions for prevention and treatment of dementia and alzheimer’s diseases. It has anticancer and antibacterial activity, use for prophylactic treatment of migraine and contain antistress, antioxidant, immunomodulator and adaptogenic properties . 1.8.4.Plant D:Ferula foetida L: 1.8.4.1.Scientific Classifications: • Kingdom Plantae • Unranked Angiosperms • Unranked Eudicots • Unranked Asterids • Order Apiales • Family Apiaceae • Genus Ferula L • Synonyms • Lady ginia lipsky • Schumannia Kuntze • Scorodosma Bunge • Soranthus Ledeb Habitat: Ferula is a genus of about 170 species of flowering plants in the carrot family, native to the mediteran near region East to Central Asia. Mostly growing in arid climate. Cultivation: It is cultivated from East region to Central Asia. They are hebaceous perennial plants, glowing to 1-4m tall, with stout, hollow somewhat succulent stems. The leaves tripinnate or even more finely divided with a stout basal sheath clasping the stem. The flowers are usually yellow, rarely white, produced in large umbels. Many plants of this genus, are referred to as giant fennel, although they are not fennel in the strict sense. 1.8.4.2.Medicinal uses: It is used to make spice asafoetida or hira hing , the Roman called the hollow light rod made from this plant a ferula, such rods were used for walking sticks, splints, for stirring boiling liquids and for punishment. The led aqueous ethanol extract of ferula foetida has shown antioxidant and antihemolytic activities. It has anti-cancer activity, gene expression, and use in woman ailments. Also used in blood pressure, vasodilator has effect on blood & blood vessel, it has chemotherapy usage used for lipid profile reduction, help to protect CNS, heart, control sugar levels. 1.8.4.3.Chemical and biological survey : An analysis of ferula foetida describes it consist of CHO of 67.8% per kg moisture 16.0% ,proteins 4%, ,fat 11%, mineral 7.0% and fiber 4.4%. Its mineral and vitamin content include substantial calcium besides phosphorus ion, carotene, riboflavin and niacin. Its clarific value is 297, contains 40.64% resinous material comprised of ferulic acid, umbeliferone , asaresinotannols, fernesiferols A, B and C . CHAPTER .2: GENERAL INTRODUCTION The use of herbs as medicine is the best oldest remedy of health care,and one known to humanity and has been used in all cultural, throughout history. Early humans recognized their dependence on nature for a healthy life and since that time humanity has depended on the diversity of plant resources for food, shelter and medicines to cure myriads of ailments. Led by instinact taste and experience, men and women previously treated illness by using plants, animal parts and minerals treated illness. A herb is a plant or a part of a plant valued for its medicinal aromatic or saromy qualities. Herbs can be viewed as biosynthetic chemical in laboratories, producing a no. of chemical compounds, herbal remedies or medicines consist of portion of plants of unpurfieid plant extracts cotntaining several constituents, which often work together synergistically. Herbal medicine or herbalism is the use of herbs or herbal products for their therapeutic or medicinal value. They may came from leaves, roots, bark, seeds and flowers. Chemicals known to have medicinal benefits are referred to as active ingredients. A/c to WHO(1996 a and b, 1992), standardization and quality control of herbs is the process involved in the physiochemical evaluation of crude drug covering aspects such as selection and handling of crude drug, safety, efficiency and stability and amount of finished product. Alteration is normally paid to such quality parameters such as: 2.1.Parameters for Crude Drug Evaluation: Macro and Microscopic Examination: (for identification of right variety). Foreign Organic Matter: This involves removal of matter other than source plant to get the drug in pure form. Ash values: These are criteria to judge the identity and purity of crude drug. Total ash, sulphate & ash, water soluble and acid in soluble ash etc. Moisture Content: checking moisture content helps to reduce error in the estimation of the actual no of drug material, low moisture suggest better stability against degradation of product. Extractive Value: These are indicative wts of the extractable chemical constituent’s crude drug under diff. solvent environment. Crude Fibre: This help to determine the woody material component and it is a criteria for judging purity. 2.1.2.Qualitative Chemical Evaluation: This covers identification and characterization of crude drug with respect to phytochemical constituents. It employs different analytical tech to detect and isolate the active constituent. Phytochemical screening technique are involve botanical identification, extraction with suitable solvent, purification and characterization of the active constituent of pharmaceuticals importance. • Chromatographic Examination: include identification of crude drug based on the use of major chemical constituent. • Qualitative Chemical Evaluation: To estimate the amount of the major classes of constituents. • Toxicological Studies: This helps to determine the pesticides, potentially toxic elements, safety studies in animals like LD50 and microbial among to establish the absence or presence of potentially harmful organism. 2.2. IDENTIFICATION OF PLANTS: 2.2.PLANT A: 2.2.1.1.ORGANOLEPTIC EVALUATION OF PLANT A: a)Macroscopic Evaluation: • Bark about 0.5 mm thick,brittle,occur as single or double. • Closely packed compound quills up to a meter or more in length and about km in diameter. • Outer surface is dull yellowish brown,marked with pale waxy longitudinal lines with occasional small holes. • Inner surface dark in colour,strated with longitudinally elongated reticulation. • fracture-splintery • Odour-fragment • Taste-sweet,aromatic with sensation of warmth. b) Identity, purity and strength: • Foreign matter → not more than 2%. • Total ash → not more than 3%. • Acid insoluble → not more than 2%. • Alcohol soluble extraction not less than 2%. • Water soluble extraction not less than 3%. • Volatile oil not more than 1%. c) Constituents: • Essential oil, tannins and mucilage . 2.2.1.3.POWDER MICROSCOPY OF PLANT A: Diagnostic features Explanations • Stone Cells Stone cells are occassionally found. • Paranchymatous cells Paranchymatous cells are found. • Phloem Phloem fibers has thick wall. • Starch grains Starch grains small in quality. • Ca- oxalate Minute occular crystal of ca- oxalate found. • Medullary rays are of isodiametric cells. 2.2.1.4.HISTOLOGY OF PLANT A: Transverse section of bark, devoid of cork and cortex show except at certain places. • Oxalate present. • Paricyclic sclerychyma. • 3 or 4 rows of isodiametric cells. • Starch grains. • Small gps of pericyclic fibers embeded at intervals in the sclernchyma. • Phloem of tangential band of tissues altering with parenchyma and containing axillary elongated secretary cells. • Secretary cells containing volatile oiler mucilage. • Phloem fibers with thick wall isolated in short tangential rows. • Medullay ray of isodiametric cells, mostly 2 cells wides.Corticle, parenchyma and medullary ray containing small starch grains, minute a circular crystals of Calcium. • • 2.2.2.PLANT B: Leaves and Flowers of Plant B Gum of Commiphora wightii 2.2.2.1.ORGANOLEPTIC EVALUATION OF PLANT B: a)Macroscopic evalution: • It is translucent, tears of varying sizes. • Reddish yellow or brown in colour more often in resinous lump when the darker in colour on long storage. • Fracture is brittle. • Exposing a rough or waxy surface having moist uncutanious appearance. • Odour is bashmic • Taste is Bitter, acrid, aromatic • Makes milky emulsion in hot water and easily burn when fresh, viscid and golden colour. b) Identity, purity and strength: • Foreign matter not more than 4%. • Total ash not more than 5%. • Acid insoluble ash not more than 1%. • Alcohol soluble extraction not less than 27%. • Water soluble extraction not less than 53%. • Volatile oil not more than 1 %. c) Constituents: • Essential oil, gum, resin, steroids. 2.2.2.3. Powder Microscopy of Plant B: Diagnostic features Explanations • Spiral vessels the spiral vessels found abundant. • Starch grain starch grain are oval like in shape. • Stone cells stone cells of diff. shapes are found. • Cork There was cork in surface view. • Isolated scleroids isolated scleroids of rob like shape. • Fibers longitudinal fibers found in microscopy. 2.2.2.4.Histology of Plant B: Transverse section of the Plant B shows: • A daxial epidermis: A large vascular bundle of adaxial epidermis. • Hypodermis: • Parenchyma: Spongy, few oleo resin located. • Midrib: Midrib with vascular bundle found. • Collenchyma: Mid rib count of thin 20mm of collenchymas. • Secretary ducts: Oleoresin ducts are covered by small rectangular Consist of single layer of coloumnar plasades. parenchymatous epithelium cells. • Xylem: Xylem shows, situated towards. • Phloem: Phloem situated upwards. 2.2.3:PLANT C: 2.2.3.1.ORGANOLEPTIC EVALUATION OF PLANT C: a)MACROSCOPIC EVALUATION: • Macroscopic evaluation shows heavily wrinkled fruit deep impression in the center having net like appearence. • Has internally triangular strong seed having sharp margin with 3 stiff fibers. • Texture is rough. • Fracture is hard. • Shape somewhat reni form. • Size is 2 – 3 cm. • Colour is blackish and dusty. • Odour is sweet & sour • Taste is sour or slightly acidic. b)Identity purity and strength: • Foreign mater not more than 3%. • Total ash not more than 7%. • Acid insoluble ash not more than 2%. • Alcohol soluble extraction not less than 40%. • Water soluble extraction not less than 50%. c) Constituents: Ascorbic acid and gallotannins. 2.2.3.3:Powder Microscopy of Plant C: Diagnostic features Explanations • Ca - oxalate Cubical shape ca-oxalate are most frequently found. • Phloem Heavy group of phloem fibers observed. • Tannin Cells Thick deposition of hydrolysalide tannin in hexagonal cell. • Stone Cell Narrow luman stone cells also seen. • Starch grain Simple, small, rounded starch grains were abundant. • Single phloem fiber Single strand were found which rich in nos. • Epidermal cells epidermal cells in a big fragment which are centrally empty. • Lignified and bast Rows of lignified cells found along with bast cells. • Cortical cells thin walled oval chain like cortical cells are found. 2.2.3.4:Histology of Plant C: • Diagnostic features Explanations • Cuticle Most prominent out most layer of the section having sunken stomata. • Epidermis Layers of barrel shape cell last beneath the stomata. • Cortical region Composition of parenchyma cells in cortical region. • Cortical cells thickly lignified rounded cells are occupied 3-5 layers in the cortical region. • Ca-oxalate Cubical Ca-oxalate are scattered in the section especially in cortical region. • Phloem vessels corticle region. thin elongated phloem fibers are arranged in a group under the • 2.2.4:PLANT D 2.2.4.1. ORGANOLEPTIC EVALUATION OF PLANT D: a)Macroscopic evaluation of Plant D: • Rounded, flatttened or masses of agglutinated tears. • Grayish white to dull yellow. • Mostly 12-25 mm in diameter, 1-4m tall. • Freshly exposed surface yellowish and translucent or milky white. • Opaque. • Slowly become finally reddish brown. • Odour is strong. • Taste: bitter and acrid • Stout hollow, succulent stems. b)Identity purity and strength: • Foreign mater not more than 2%. • Total ash not more than 15%. • Acid insoluble ash not more than 3%. • Alcohol soluble extraction not less than 50%. • Water soluble extraction not less than 50%. c) Constituents: It contains 40.64% resinous material comprised of ferulic acid, umbeliferone , asaresinotannols, fernesiferols A,B and C. 2.2.4.3. Identification Tests: • Freshly broken surface when touched with sulphuric acid, reddish brown colour produced changes to violet. • Boil 0.2 gm with 2ml of HCl acid for about 1min, cool and dilute with an equal amout of water and filter to 3ml of dilutes, chromic florescene is produced. • Titrate with H2O → Milky white • Treatment with 50% nitric acid → Green Colour. • Treatment with fractured dark surface with H2SO4 → Reddish Brown • Combined umbeliferone → Dark Blue Colour. • On burning → Yellow Flame 2.2.5.1. Powder Microscopy of REDUCIN: Diagnostic features • Stone Cells explanations Rounded rhombohedral shaped stone cells are found. • Fibers Elongated fibers of two types, phloem and lignified. • Oil Cells Thin and thick walled almost oral in shape. • Ca - oxalate various types, Ca- oxalates are found. • Tannin Cells Thick deposition of hydrolysalide tannin in hexagonal cells. • Mucilage cell Most of the spherical shapes cell contain mucilage. • Starch grain Simple type of starch grain found. • sclerieds Thickly lignified fibers. CHAPTER 3 3. EXPERIMENTAL STUDIES: The phytochemical and pharmacological work was performed at Research Institute of Pharmaceuticals Sciences, Department of Pharmacognosy, Faculty of Pharmacy, and University of Karachi. 3.1. Apparatus: Following apparatus were used for experimental purpose: • Rotary Evaporator Eyela (Japan) was used for extraction and evaporation of the solvent from the extract under reduced pressure and controlled temperature. • Lyophilizer Freeze dryer model FDI, (Eyela, Tokoio Rikakekai Co. Ltd. Japan) was used for the removal of water from the extract under controlled temperature. • Electronic Microscope Laboval-4, Germany, was used in microscopic examination of plants powder. • Stereomicroscope (Wolfe, Carolina, 59.18.18, Burlington, North Carolina 2715) was used for organoleptic evaluation of plant drugs in a magnified from. • Ultraviolet Lamp (Max 254 and 366) was used for the detection of chromatographic plates (for suspected chromophores in chemical constituents) • Water Bath Thermostatic water bath Model No. HH. S21.8, (Jiangsu Medical Instrument Factory China) was used to maintain the temperature. • Physical Balance Below mentioned physical balance were used for weighing extracts during experiments. • Libro AEG – 120 Shimadzu (Japan) • Libro EB – 3200D, Shimadzu (Japan) • Grinder West point automatic series TSK-333 France (National) was used for grinding of herbs. 3.2. MATERIAL AND METHODS: For Chromatographic Procedures Silica Gel for TLC Plate • 20x20 cm silica gel 60 florescence at 254 nm (Merck, Germany). • 5x10cm gel 60T (0.2mm thick) pre coated TLC plates, florescence at 254nm (Merck, Germany). • Pre coated Silica gel F254 TLC plate (E-Merck) was used to check the purity of compounds. Rotary evaporator The following materials were used for chemical work during experiments. • Plant material (about 2,000gm of each plant A-D) were purchased and collected from the standard shop of herbal market (address given in chapter II) and were identified by Prof. Dr. Ghazala H. Rizwani, Chairperson Department of Pharmacognosy, Faculty of Pharmacy, University of Karachi. Specimen bottles of the plants (0040, 0041, 0042, and 0043 for plant drugs A-D respectively) were deposited in the Pharmacognosy Herbal Museum, Department of Pharmacognosy, and University of Karachi. • For checking the solubility of drugs in different reagents by color reaction; lead acetate, ferric chloride, n-hexane, and Iodine solution were obtained from Merck, Germany / BDH, UK. 3.3.Solvents: • N-Hexane (Merck, Germany) • Methanol (Merck, Germany) • Ethyl acetate (Merck, Germany) • Chloroform (Merck, Germany) • Ether (Merck, Germany) • N-Butanol (Merck, Germany) • Acetone (Merck, Germany) • Ferric chloride (Merck, Germany) • Lead acetate (Merck, Germany) • Iodine solution (Merck, Germany) • Sulphuric acid (Merck, Germany) • Hydrochloric acid (Merck, Germany) • Nitric acid (Merck, Germany) • Distilled water 3.3.1. Solvents systems Used in Chromatography: Following solvent systems were used for chromatography: • Ethyl Acetate – Methanol – Water (9:1:0:1) • Hexane (100%) • Hexane – Chloroform (7:3) • Chloroform (100%) – Water 9:1) • Chloroform – Methanol – Water (8:2:0.2) • Butanol – Ethanol – Water (8:2:0.2) TABLE 1 3.4.1.Behaviour of powder with different Chemical regents (Plant B) S.No. Treatment Observation 1 Powder + 1 N NaOH Yellowish brown 2 Powder + Saturated Sulphuric acid Yellowish green 3 Powder + Acetic acid Orange yellow 4 Powder + Conc. Hcl. Reddish brown 5 Powder + Conc. HNo3 Chocolate brown 6 Powder + Iodine (5%) Blackish brown 7 Powder + Sakuwabiff’s Reagent Yellowish brown 8 Powder + Ferric chloride (5%) Dark Blackish brown 9 Powder + 40% NaOH+Few drops of 10% Blackish brown Lead acetate 10 Powder + Sudan III (ALCHOHOLIC) Dark reddish orange 11 Powder + Conc. HNo3 + Ammonia Yellowish orange 12 Powder + 35% HCI Brownish 13 Powder + 5% KOH Yellowish green 14 Powder + Phloroglucinol: HCI (1:1) Yellowish brown with purple spots TABLE 2 3.4.2Behavior of powder with different Chemical Reagents {Plant C} S.No. Treatment Observation 1 Powder + Iodine sol Dark brown 2 Powder + Chloroform Light Brown 3 Powder + Hexane Very Light Brown 4 Powder + Methanol Light brown 5 Powder + Acetone Light brown 6 Powder + Ferric Chloride Bluish Black 7 Powder + Lead Acetate White ppt with clear sol TABLE 3 3.4.3. Behavior of powder with differencial reagent (Plant D) S.No. Treatment Observation 1 Powder + H2O Milky White 2 Powder + 50% nitric acid Green 3 Powder + H2SO4 Dark Reddish Brown 4 Powder + Umbeliferone Dark blue 5 Powder on burning Yellow Flame 3.5.Phytochemical Analysis for Primary & Secondary Metebolites of Plant A-D: TABLE 4 3.5.1:Phytochemical Analysis of Plant A Tests Carbohydrates : Molish test Fehling test Proteins : Biuret test Steroids : Salkowski test Alkaloids: Dragendorff’s Saponin test: Froth test Glycosides test: Legal’s test (for cardenoloids) Lekellar killani test Starch: Tannic acid test Flavanoids : Shinoda test Tannins &Phenols: FeCI3 test Reagents Conc. Hcl Conc Hcl+Mg turnnings 4% NaOH, 1% CuSO4 Chloroform and conc. H2SO4 Positive result violet ring yellow and red ppt Violet or pink color Chloroform layer appeared red and acid layer show greenish yellow inflorescence Dragendorff’s regt. Orange ppt. H2O Honeycomb froth Pyridine, Sodiumnitroprusside Pink to red color. Reddish brown color &bluish green color Glacial acetic acid, 5% FeCI3 20% Tannic acid White ppt 95% ethanol, HCI, Magnesium Pink Color FeCI3 Intense green colour TABLE 5 3.5.2:Phytochemical Analysis of Plant B Tests ALKALOIDS : Mayers Test Wagners Reagent FLAVONOIDS: NaoH and HCL TEST H2SO4 TEST PROTEIN: Milton Test Ninhydrin Test Reagents 1.36 gm Mercuric Chloride 5gm of Potaasium Iodide Positive result Cream Colour PPT 1.27 gm of iodine and 2g of Potassium Iodine +VE NaoH and HCL H2SO4 Yellow Orange colour Orange colour 2 ml of milton reagent 2 ml of ninhydrin White Colour Blue+Purple colour TANNINS: 10%of lead acetate Lead Acetate STEROIDS: Libermanburchard test 2-3 ml of acetic anhydrate glacial acid+2 drops of H2SO4 White Colour PPT Bluish green colour TABLE 6 3.5.3:Phytochemical Analysis Of Plant C Tests Carbohydrates : Molish test Fehling test Reagents Few drops of alcohalic alpha nephtohol+2ml of conc sulfuric acid Positive result violet ring fehling sol Brick Red PPT Bendict reagent Brown PPT GLYCOSIDES: Legals test sod.nitroprusside+sod hydroxide Colour Change Libermanburchard test Libermanburchard reagent Bendict test Fixed oils: Spot test Sponification test Colour change oil statin Sample press b/w two filter papers pot.hydroxide+phenolpthalei sponification indicated n Alkaloids: Dragendorff’s Dragendorff’s regt. Brown colour Mayers test mayers reagent cream colour Wagner test wagners reagent Reddish Brown PROTEIN: Ninhydrin test Miltons test Tannins: FeCI3 test Ninhydrin reagent Miltons reagent Blue White FeCI3 Intense green colour TABLE 7 3.5.4:Phyochemical Analysis of Plant D Tests Carbohydrates : Molish test Fehling test Proteins : Biuret test Steroids : Salkowski test Alkaloids: Dragendorff’s Saponin test: Froth test Glycosides test: Legal’s test (for cardenoloids) Lekellar killani test Starch: Tannic acid test Flavanoids : Shinoda test Tannins &Phenols: FeCI3 test Reagents Positive result Conc. hcl Conc hcl+mag turnnings Absent 4% NaOH, 1% CuSO4 Absent Chloroform and conc. H2SO4 Absent Dragendorff’s regt. Brown colour H2O Absent Pyridine, Sodiumnitroprusside Absent Glacial acetic acid, 5% FeCI3 Absent Absent 20% Tannic acid Absent 95% ethanol, HCI, Magnesium Absent FeCI3 Absent 3.6. Extraction and Isolation Procedures: 3.6.1: Extraction and Isolation of Reducin Powder: For the extraction of REDUCIN powder, dry drugs of all four plants (i.e. Plant A,B,C and D; approximately 2,000gm each) were first cleaned, garbled, washed with clean water and when dried (separately). Then the dried materials of plant drugs were grinded in to coarse powder with the help of a grinder. After getting coarse powder (about 7kg) of all plant drugs, about 2kg was kept for getting the individual MeOH extract of plant drugs for phytochemical and chromogenic testing of all plant drugs respectively; whereas remaining 5kg was mixed well and then grinded again to again fine powder of REDUCIN .This fine powder was percolated in 80% methanol at room temperature for 15 days. The percolate was then filtered through Whatman Filter Paper No.1. This process was repeated for three times. The methanolic extract of REDUCIN was then evaporated under reduced pressure and controlled temperature at 400C.A reddish brown gummy residue was obtained, which was then lyophilized and finally we obtained brown powder of REDUCIN about 4kg powder was kept for clinical studies, whereas about 1kg powder was kept for comparative chromatographic analysis of all plant drugs . 3.6.2: Extraction of Plant Drugs (A-D) for Phytochemical and Chromogenic Testing: Same procedure was adopted for extraction of all plant drugs (i.e. Plant A,B,C, and D). The plant drugs (about 2kg) were first cleaned, garbled, washed with clean water and then dried. Then the dried materials of plant drugs were grinded in to coarse powder with the help of grinder. The coarse powder of plant drugs were percolated in 80% Methanol for 15 days at room temperature separately. The percolate was then filtered through Whatman Filter Paper. No.1. This process was repeated for three times, and finally we got first methanolic extract of all plant drugs. This extract was evaporated under reduced pressure and controlled temperature at 400C to get second extract, which was then again reconstituted in methanol to get the third extract. The third extract was lastly used for phytochemical and chromogenic testing of individual plant drugs. 3.6.3:Chromatogram Development for TLC Chromatography: The methanolic extracts (of reducin and individual plant drugs) so obtained were analyzed on TLC plates by using different solvent systems. These plates were first observed under ultraviolet lamp at wavelength of 254 nm and 366nm. Then the iodine vapors were used (the chromatogram was introduced in to a closed vessel on the bottom of which some crystals of iodine have been placed) for making unstable complexes with the compounds for identification purposes. Afterwards Ceric Ammonium Sulphate reagent, Dragondroff’s reagent were used for the detection of chemical compounds of different nature found in the methanolic fraction of plant drugs and compared them with Reducin fraction. CHAPTER 4 4. CLINICAL STUDIES OF REDUCIN: 4.1. Introduction: In 2012, the U.S. Preventive Services Task Force (USPSTF) issued the recommendation that all adults be screened for obesity, and that patients with a body mass index (BMI) of 30 kg/m2 or greater be offered intensive, multi-component behavioral interventions. The American Academy of Family Physicians has endorsed the USPSTF recommendation, which is based on evidence that intensive counseling can promote modest sustained weight loss and improved clinical outcomes. The prevalence of obesity exceeds 30% in adults and is associated with increased risk of such serious health problems as cardiovascular disease, type 2 diabetes, and various types of cancer. These co morbid conditions are associated with greater use of health care services among obese patients. Obesity is also associated with an increased risk of premature death in adults younger than 65. 4.1.1:Consequences of Obesity Physical Psychosocial Functional Cancer Depression Absenteeism from school Cardiovascular disease Discrimination or work Cholestasis Low self-esteem Disability Dyslipidemia Negative body image Disqualification from Gallbladder disease Negative stereotyping active military/fire/ police Glucose intolerance and Social marginalization services insulin resistance Stigma Low physical fitness Hepatic steatosis Teasing and bullying Mobility limitations Hypertension Reduced academic Hyperuricemia and gout performance Menstrual abnormalities Reduced productive Orthopedic problems Unemployment Reduction of cerebral blood flow Sleep apnea Type 2 diabetes 4.1.2. Screening and Diagnosis: BMI is recommended for use in clinical practice as a practical way to identify individuals who are overweight or obese. Furthermore, calculating BMI is still a good way to evaluate changes over time, because incremental increases most likely represent gains in body fat. Recognizing that BMI is just one indicator of potential health risks associated with being overweight or obese, the National Heart, Lung and Blood Institute (NHLBI) recommends that physicians also look at the following factors, • Risk factors for diseases associated with obesity, such as high blood pressure and physical inactivity. • Waist circumference as a measure of abdominal adiposity 4.1.3.Waist Circumference: Abdominal adiposity is an important independent risk factor for cardiovascular disease, type 2 diabetes, dyslipidemia, and hypertension. The NHLBI defines abdominal obesity as • Waist circumference greater than 40 in (102 cm) in men • Waist circumference greater than 35 in (88 cm) in women Individuals with larger waist circumferences have more than a fivefold greater risk of multiple cardio metabolic risk factors, even after adjusting for BMI, compared with individuals with waist measurements in the normal range 4.1.4.Medications That Promote Weight Gain Anticonvulsants Antihypertensives Antipsy hotics Valproic acid Clonidine Chlorpro Corticosteroid s Psychotropics mazine Carbamazepine Guanabenz Thiothixene Lithium Antidepressants Methyldopa Haloperidol Sulfonylureas Amitriptyline Prazosin Olanzapine Glipizide Imipramine Terazosin Clozapine Glyburide Phenelzine Propranolol Risperidone Nisoldipine Quetiapine Abdominal obesity is also one of five diagnostic criteria for metabolic syndrome. Approximately 34% of adults meet the criteria for metabolic syndrome, and the risk increases with age. Men ages 60 years or older are more than four times as likely and women ages 60 years and older are more than six times as likely to be diagnosed with metabolic syndrome compared with younger adults (ages 20 to 39 years 4.1.5: Classification of Overweight and Obesity, and Associated Disease Risk: Disease Risk (Relative to Normal Weight and Waist Circumference)t Classification* BMI Obesity Waist Waist (kg/m2) Stage Circumference Circumference Men: <40 in (102) Men: >40 in (102 cm) cm) Women: <35 in (88 Women: >35 in (88 cm) cm) Underweight <18.5 - - - Normal 18.5 to 24.9 - - - Overweight 25.0 to 29.9 - Increased High Obesity 30.0 to 34.9 I High Very high 35.0 to 39.9 II Very high Very high 40.0 III Extremely high Extremely high Extreme obesity 4.1.6: Metabolic Syndrome: Metabolic syndrome is a constellation of risk factors, including abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, and elevated plasma glucose levels, that increase the risk of cardiovascular disease. The predominant underlying risk factors for metabolic syndrome are abdominal obesity and insulin resistance. Although many patients may be genetically susceptible to metabolic syndrome, it rarely develops in the absence of obesity and physical inactivity 4.1.6.1: Diagnostic Criteria for Metabolic Syndrome: Measure (any 3 of 5 criteria constitute diagnosis of metabolic syndrome) Categorical Cut Points Elevated waist circumference >102 cm (>40 in ) in men >88 cm (>35 in) in women Elevated TG >150 mg/dL (1.7 mmol/L) or drug treatment for elevated TG Reduced HDL-C <40 mg/dL (1.03 mmol/L) in men <50 mg/dL (1.3 mmol/L) in women or drug treatment for reduced HDL-C Elevated BP >130 mm Hg systolic >85 mm Hg diastolic Elevated fasting glucose{or treatment for elevated fasting glucose} or drug treatment for hypertension >100mg/dl{5.6mmol/L},or drug treatment for elevated glucose. 4.2: Management of Obesity: 4.2.1:The 5 A's for Evaluation and Treatment of Obesity: Assess Severity of obesity with calculated BMI, waist circumference, and comorbidities Food intake and physical activity in context of health risks and appropriate dietary approach Medications that affect weight or satiety Readiness to change behavior and stage of change. Diagnosis Advise of overweight, obese, or severe obesity Caloric deficit needed for weight loss Various types of diets that lead to weight loss and ease of adherence Appropriateness, cost, and effectiveness of meal replacements, dietary supplements, over-thecounter weight aids, medications, surgery Importance of selfmonitoring Agree If patient is not ready, discuss at another visit. If patient is motivated and ready to change, develop treatment plan. If patient chooses diet, physical activity, and/or medication, set weight-loss goal at 100/0 from baseline. If patient is a potential candidate for surgery, review options Assist Provide a diet plan, physical activity guide, and behaviormodification guide. Provide Web resources based on patient interest and need Identify method for self-monitoring (e.g., diary) Review food and activity diary on follow-up (reassess if initial goal is not met) Arrange Follow-up appointments to meet patient needs Referral to registered dietitian and/or behavioral specialist for individual counseling/monitoring or weight-management class Referral to surgical program Maintenance counseling to prevent relapse or weight regain 4.2.2: Pharmacotherapy: Drug Mechanism of Action Possible Adverse Effects Lorcaserin (Belviq) Decreases appetite, Headache, dizziness, fatigue, nausea, Orlistat (Xenical) increases dry mouth, constipation Phentermine and feeling of fullness, Intestinal cramps, gas, diarrhea, oily topi Blocks absorption of fat, spotting. ramate extended- Decreases appetite, Increased heart rate, birth defects, release (Qsymia) increases tingling of hands and feet, insomnia, feeling of fullness izziness, constipation, dry mouth 4.2.3: Bariatric Surgery: Bariatric surgery may be considered in adults who have not achieved weight loss with dietary or other treatments and who have a BMI of 40 kg/m2 or greater, or for those who have a BMI of 35 kg/m2 or greater with significant obesity-related comorbidities (e.g., severe hypertension, type 2 diabetes, obstructive sleep apnea).Bariatric surgery may also benefit patients with obesity-related comorbidities who have a BMI of 35 kg/m2 or lower, but it is not routinely recommended for these patients 4.3: Clinical Studies of Reducin:4.3.1: Proven Therapeutic Efficiency of Herbal Drugs (A-D) in Literature:4.3.1.1:Plant A:- (Cimamomum cassia blume): Cinnamomum cassia blume have a direct role in lipid metabolism and prevent hyper cholesterolemia and hyperlipidemia and lower free fatty acid by its strong lipolytic activity. Dietry cinnamate inhibits the hepatic HMG COA reductase activity resulting in lower hepatic cholesterol-content. Suppress lipid preoxidation via enhancement of hepatic antioxidant activity.Cinnamomum cassia may have an effect on treating hyper lipidemia and there may be responsible for the prevention of consequences of aging process, from hypertension to heart failure, cardiovascular disease and myocardial infarction. 4.3.1.2:Plant B:- (Commiphora wightii): Guggul an oleogum resin is plant exudates of Commiphora wightii, has been use medicinally since vesdic period for treatment of no of diseases such as hyper cholesterolemia, artherosclerosis, obesity, liver disorders, digestive problem and menstural irregulaties. Guggul of Commiphora wightii possess lipid lowering effect, in various studies it has shown to decrease atherosclerosis, lower serum cholesterol and triglycerides and also decrease HDL and cholesterol. 4.3.1.3:Plant C:- [Embelica officinalis): Although Embelica officinalis does not have a direct effect on reproductive organs but it exhibits effects on total body hormonal system. Some effects are:It is nutritional power house and used to boost immunity and restore body immunity, provides energy to vital organs and used in chronic illness recovery. Its help in regulating blood glucose level in diabetic pts, improve hb level and is an imperative eye and liver toner. It possess ant inflammatory action and in various GIT inflammation such as gastritis, it has been used. It contains natural digestion enzyme and therefore used in indigestion. It lowers serum cholesterol and reduce body heat / wt naturally. 4.3.1.4:Plant D:- (Ferule foetida): Ferule foetida used to make spice as asafoetida or hing. It has many marvelous effects on the human body. Which are:It has ant-oxidant, anti-hemolytic activity. It has anticancer activity, gene expression, use in women ailments. It is also used in blood pressure, vasodilator, has effect on blood & blood vessels. It has chemotherapy usage, used for lipid profile reduction, help to protect CNS & Heart. It also control blood sugar levels. 4.3.2:Animal trials: 4.3.2.1:Aims and objectives:By conducting these trials, our main objective to establish the incidence of obesity in pts with high T.Gs levels, to assess the age and severity of obesity and to establish the efficacy of formulated drug with in the pts presented with obesity problem. 4.3.1.2:Conduction and Evaluation of studies: The research was conducted and evaluated by the ethical committee of Dr. Zia Uddin hospital Block B, North Nazimabad and Murshid Hospital and health care center hub-river road Karachi. • Participants: The study was performed on total nos of 30 adult long Evans (Rattus Rattus) rats weighing b/w 200 to 210 gm collected from pharmacology Dept of SON of Murshid Hospital. The chosen animals were housed in cages separately at normal atmospheric temp b/w 25 to 290 C, under good ventilation and were given water & standard balanced diet. Animals were randomly distributed in to 4 gps each cage was labeled for identification of different gps. 4.3.1.3:Trial 1: Trial 1 was conducted to demonstrate the effect of Reducin on normal adult male rats, for this purpose a total no of 12 rats were taken and was divided in to two gps, six animals in each gp. • GP A: Each animal received laboratory diet 20g/day and distilled water for 35 days. • GP B: Each animal receive 15% powder of Reducin mixed with laboratory diet /day for 35 days. 4.3.1.4:Trial II: Trial II was conducted to demonstrate the effect of Reducin on hyper cholestrolemic rats. For this purpose a total no of 18 rats were taken and divided in to 3 gps containing 6 animals in each gp. • GP C: Hypercholestrolemic gp that received fatty mixture diet (normal lab diet plus 1% cholesterol with 0.3% cholic acid) for 35 days. • GP D: This gp receive fatty mixture diet with 15% of powder of Reducin for 35 days. • Collection of Blood Specimens: The rats were kept fasted over night before taking the blood samples. All the animals were anesthetized with diethyl ether and blood samples were collected in test tubes and allow to coagulate at room temp. It was then centrifuged at 3000 rpm for 30 min in a centrifuge machine. The clear non hemolysed supernatant sera was quickly removed and stored at -200C for biochemical analysis of serum lipid profile at the murshid hospital laboratory. • Biochemical Analysis: After collection of all blood & specimens, serum total cholesterol (TC), serum high density lipoprotein cholesterol (HDL-C) and serum triglycerides (TGS) were measured by enzymatic calorimetric (CHOD-PAP) method. Low density lipoprotein cholesterol (LDL-C) was calculated by frie dewards formula. • Statistical Analysis: The data were analyzed using unpaired t test. Results were expressed as meant ± SE and p value <0.05 and <0.01 were considered statistically significant and highly significant especially. 4.3.1.5:Results: Table shows the effect of Reducin on serum lipid profile of adult male rats. Serum total cholesterol, serum LDL cholesterol, HDL cholesterol and serum triglycerides level shows some difference b/w the Reducin gp (B) and the control gp (gpA). 4.3.2.5.1: Effects of Reducin on Serum lipid Profile of Adult Male Rats (AV/SB): Parameters GPA GPB N=6 mean ± SE N=6 mean ± SE 71.17 ± 1.59 69.83±3.04 >0.05 Serum TG (mg/dl) 78.10 ± 2.45 76.33±1.93 >0.05 Serum HDL 31.50±93 30.67±4.87 >0.05 Serum total P value Cholesterol mg/dl Cholesterol mg/dl Serum LDL 26.61±2.23 23.90±6.00 >0.05 4.3.2.5.2:Effects of Fatty Mixture Feeding Diet on Serum Male Rats [A V/S C]: Parameters Group A (n=6) Group B (n=6) Mean ± SE Mean ± SE serum total cholesterol P VALUE 136 ±3.74 <0.01 78.1+2.45 106.5 ± 2.85 <0.01 31.50 ± 0.93 30.81 ± 2.73 >0.05 76.61 ± 2.23 83.81 ± 5.6 >0.05 71.17 +1.59 [mg/ml] Serum T.G (mg/dl) Serum HDL Cholesterol (mg/dl) Serum LDL Cholesterol (mg dl) 4.3.2.5.3: Effects of Reducin on Serum lipid profile of adult male rats fed with fatty mixture diet (C V/S D): Parameters Serum total GPC GPD N=6 mean ± SE N=6 mean ± SE 136 ± 3.74 119.5±2.68 P value <0.01 Cholesterol mg/dl Serum TG (mg/dl) 106.5 ± 2.85 92.13±3.02 <0.01 Serum HDL 30.81±2.78 29.17±1.73 >0.05 83.81±5.61 71.91±1.88 Cholesterol mg/dl Serum LDL <0.05 4.3.2.6:Discussion: A proving body of research demonstrated that the commonly used herbs & spices such as garlic, cumin, cloves cinnamomum, thyme, guggul, fine spices, bay leaves, hing, amla can be used therapeutically in some cases. Medicinal plants play a vital role for the development of new drugs. Almost 70% modern medicines in Asia are derived from natural products. Medicinal plants play a central role not only as traditional medicines but also as trade commodities, mentioned the demand of distant markets. In experiments I & II all conventionally measured indicators (serum cholesterol, T.G, LDL and HDL levels) of lipid profile were slightly arranged in gp B as compare to these in control gp A. In trial II the effect of Reducin was observed on serum lipid profile of hyperlipidemia in long Evan rats on describing the fatty diet gp showed significant increase in serum lipid profile parameters of (T.C, T.G, LDLC) as compared to these of control (gpA). This finding indicates that fatty mixture diet treatment is used to evaluate the serum lipid profile parameters was able to evaluate all parameter except HDLC measured in this trial. It was found in this study that 15% Reducin, significantly decreases the serum TC, T.G, LDLC approximately 12% 11.% and 14% respectively (P<0.01, P<0.01 and P<0.05) of fatty mixture diet fed rats, as shown in table. 4.4. Experimental Conditions of Clinical Trials of Reducin: • AIMS AND OBJECTIVES: By conducting these clinical trials our main objectives was, to establish the incidence of obesity in pts presenting with high cholesterol levels & to establish the efficacy of formulated drug in pts with obesity 4.4.1: CONDUCTION AND EVALUATION OF CLINICAL TRIALS: This research was conducted & evaluate & by the ethical committee of Murshid. Hospital and health care center hub river road Karachi. 4.4.1.1: PARTICIPANTS:Hundred patients randomly selected for these trials age was (18-65) were observed and evaluated. 4.4.1.2:DOSAGE PROTOCOL: Reducin powdered capsules, 3 caps of 250mg were given for at least one month (30 days). Dose regime was that one cap had to be taken in the morning with water after breakfast, 2nd are has to be taken after lunch & third one had to be taken at night before joining to bed. 4.4.1.3: MEASUREMENT OF MAIN OUT COME: Main efficiency variable, T.G levels was evaluate ,pts sign & symptoms noted which included irritability, mood alteration , insomnia, lethargy, fatigue, rest lessens, chest heaviness was also observed. we evaluate the effects of Reducin in a large, perspective, randomized controlled study over 30 days. The study and analysis were planned & conducted under strict methodology. 4.4.1.4: Method: All patients were out pts attending general clinic from the 2013 to august 2015 were divided in two gps. The consultant gp which conducted the experiment under supervision of Dr. Imtiaz Ahmed Channa underwent training before the study on the seek to enhance consistency with in and b/w clinics. In each OPD clinic all assessment were made the respective nursing staff and fill up the chart about the pts signs & symptoms. These pts went for consultant checkup and advised cap Reducin 250mg in soft gelation cap with pink and black color combination of zero size a/c to pharmaceutical standards. The caps were gave tds for 30 days. The studies were perform a/c to the amount WHO guide lines on good clinical practice. All the pts age b/w (18-65) year were Grouped in to two divisions. Group A includes patients containing cholesterol diet, Group B containing patients maintained on simple diet. All the pts have obesity symptoms a/c to WHO guide lines about obesity like increase in cholesterol, increase in wt, sweeting, lethargy, fatigue, restlessness etc. 4.4.1.5:Diagnostic Citenia for Pts:The criteria for diagnosis of problematic conditions were based on 3 main factors. 4.4.1.5.1:Types of obesity:All the pts have previous history of obesity. Types of obesity was define as by estimating their obesity by using BMI index. BMI = m/h2 Where m is for wt & h for height respectively. 4.4.1.5.2:Sign & Symptoms of Obesity:• Depression • Disability • Low physical fitness • Negative body image • Negative stereotyping • Stigma • Absenteeism from work. • Mobility limitations • Reduced productivity 4.4.1.5.3: General Patients Criteria:General patient criteria established for this research in which general heath / condition & socioeconomic status of the pts were also explained for determine the effect of drugs on obesity. • Existing disease condition:-Hypertension, Diabetes, Anemia, GIT upset, Constipation, Orthopedic Problem. • Daily deity pattern: Such as low cholesterol diet and high cholesterol diet. • Sleep pattern: Sleep pattern and length of sleep cycle of pts were also integrated, whether it is normal or disturbed. • Socio economic status: As it exerts major effects on an in dividual life, socio economic status of pts was also kept in consideration. 4.4.1.7(a):Group A (Normal diet) General Health (existing disease) DISEAESES POOR GOOD Normal B.P 0% 0% 12% Diabetes 0% 0% 08% Anemia 0% 0% 04% Constipation 12% 18% 15% G.I.T Up 0% 04% 12% Orthopedic 2% 10% 18% Head Ache 04% 06% 10% SET 4.4.1.7(b):Group B(Rich Fat Diet) General Health (existing disease) DISEAESES POOR GOOD Normal B.P 6% 10% 22% Diabetes 04% 8% 14% Anemia 04% 04% 04% Constipation 12% 18% 20% G.I.T Up 08% 14% 22% 05% 15% 25% SET Orthopedic 4.2: DIAGNOSIS:The diagnosis of obesity was based on two main factors, clinical and instrumental factors, clinical and instrumental (cholesterol levels & T.G levels). Basis of Diagnosis: Diagnosis of obesity was made only on clinical finding in 58 of pts in remaining 42% pts diagnosis was made on clinical findings as well as labtest as shown on table:- 4.2.1: • • Basis of diagnosis Clinical finding lab Test No of cases % 58 58% 42 42% 4.2.2: Assessment of Cholesterol level before and after the Treatment:We evaluate the cholesterol level, BP and TGS of the pts by dividing the pt of each gp in sub gp of ten pts.T.G. 4.2.2.1: Assesment of Cholesterol before and after treatment of REDUCIN in Normal Diet Gp: GPA Sub gp No of gps T. G levels {Before} (Normal diet) T.G Levels {After} I 10 155±2 140±2 II 10 159±1 146±2 III 10 156±3 139±3 IV 10 161±2 142±1 V 10 154±4 141±5 (Mean ) 157 141.6 (S.D) 2.915± 2.701± 4.2.2.2: Assesment of Cholesterol before and after treatment of REDUCIN in (Rich Fat Diet Gp): GP B Sub gp No of pts (Rich diet) T.G Level T.G Levels {Before} {After} 152+2 1 10 161+1 II 10 162±3 153±3 III 10 165±2 154±2 IV 10 167±5 152±5 V 10 169±4 156±6 Mean 166.8 155.4 S.D 3.397 3.820 4.2.3: Reduced HDLD Levels Before and After Treatment: 4.2.3.1: Reduced HDLD levels before and after treatment of REDUCIN in Men Gp A (Men) Subgp No.of pts before after 1 10 40±1 38±1 II 10 42±2 37±1 III 10 45±1 39±2 IV 10 43±1 37±1 Mean 42.5 37.75 SD 2.236± 1.1169± REDUCED HDLD: Normal values are: • 40mg / dl in men 4.2.3.2: Reduced HDLD Levels before and after treatment of REDUCIN in Women: GP B Subgp (Women) No. of Pts before after I 10 52±2 48±1 II 10 54±1 47±2 III 10 53±2 46±1 IV 10 58±2 51±1 V 10 56±1 52±2 VI 10 55±2 48±1 (Mean) 54±66 48.66 (S.D) 2.160± 2.337± 4.2.3.2: Reduced HDLD levels before and after Treatment of REDUCIN in Women REDUCED HDLD: Normal values are: • 50 mg / dl in women 4.2.4: Reduced waist circumference before and after Treatment: 4.2.4.1: Reduced Waist Circumference before and after Treatment of REDUCIN in Men: GpA Sup gp No of pts before after Men I 10 104cm 99cm II 10 100cm 100cm III 10 107cm 101cm IV 10 107cm 101cm V 10 108cm 102cm Mean 106.25± 100.25± S.D 1.707± 1.322± 4.2.4.1: Reduced Waist Circumference before and after Treatment of REDUCIN in Men 4.2.4.2: Reduced Waist Circumference before and after Treatment of REDUCIN in Women: Gp B Subgp no of pts before (Women) I 10 90cm 85cm II 10 95cm 91cm III 10 94cm 90cm IV 10 96cm 91cm V 10 98cm 92cm VI 10 92cm 88cm Mean 94.1 after 89.5cm 4.2.4.2: Reduced Waist Circumference before and after Treatment of REDUCIN in Women CHAPTER 5 5. Phytopharmaceutical Studies:5.1. Formulation of Oral dosage form of REDUCIN: Phytopharmaceutical studies of powder of our product Reducin was also carried out. In this studies a 250 mg caps was prepared according to pharmaceutical standards. 5.2. Material and Method:5.2.1. Authentication of Drug:The drug parts of individual herbs used, appox 08 kgs of all herbs was purchased from the herbal market sadder Karachi and identified by Dr. Ghazala.H. Rizwani (Professor, Dept of Pharmacognosy, Faculty of pharmacy, University of Karachi). Formulation of oral dosage form of Reducin powder in such way that 4 kg was prepared in 250 mg cap capacity, soft gelatin cap were purchase from standard drug store (shakil trader, cutchi gali No, 1 Marriott road Karachi. Many steps were attempted for the formulation are given below:- 5.2.2. Cleansing and drying:- The sample (herbal drugs) was quickly transferred in to a big sieve after washing with running clear tape water, then to absorb excessive water, samples was spread on filter paper (Whats Man No. 1) finally the sample was dried under fan air / open air. This step has been performed on individual herbs separately. 5.2.3.Grinding: After complete drying the drugs were grinded in to coarse powdered from with the help of grinder (national) first individually, then after getting a coarse powdered form of each drug, all drugs were grinded together to get fine powder of Reducin. 5.2.4. Percolation and lyophilization: This fine powder was percolated in 80% methanol on room temp for 15 days. The percolate was than filtered through what man filter paper No. 1. The process was repeated for 3 times. Methanolic extract of Reducin was then evaporated under reduced pressure and controlled temp at 400C. A brown gummy residue was obtained which was then lyophilized and finally we obtained brown powder of Reducin, having acrid taste, and typical herbal odour. 5.2.5. Flow ability of powder: Powders are generally composed of solid particles of the same/ diffrent composition having equivalent diameter, less than 1000 um along with mixing and compaction properties, flow ability of the powder is very useful in clinical applications of drugs when it is used in pharmaceutical dosage form. Flow ability of powder can be checked by 2 different methods, which we used in this research. 5.2.6.Angle of repose of powder: For the determination of cohesiveness and non-cohesiveness of the powder of Reducin was measured by fixed cone method. For this purpose 3 gm of powdered material was used and then the calculation of angle of repose was measured by following equation. Angle of repose:Tan = h/d = tan-1 h/d h = heigh of powder. d = diameter of Petri dish. S.No. height of conical powder Radius of petridish 1. 2.3 9.9 2. 2.5 9.9 3. 2.4 9.9 Mean 2.4 9.9 Angle of repose: = tan-1 h/d = tan-1 2.4/9.9 = 23.77 5.2.7. Porosity of Powder:To check the porosity of powdered drug, first a cylinder was roughly filled up to 100 ml with powdered drug (that is called bulk volume of powder). Then the cylinder was tapped for 100 times on smoothen surface in order to obtain the pack volume of powder. Following observations were made during the process:- S. No. Bulk vol. of powder (ml) Pack vol. of powder (ml) Volume Mean € % • 100 78 • 100 77 • 100 76 77 23% Powder porosity is another method useful in characterization of packing geometry which linked to the bulk density of powder. The powder porosity is expressed in % and can be calculated by two diff method. We used both methods for cross confirmation state as under:- Method 1 by volume: Variable used are:Vb = bulk volume of powder Vp = pack volume of powder e= porosity of powder. Formula:€ = vb – vp/vp = 100 – 77/100 = 0.23 %€ = € x100 = 0.23 x 100 = 23% Method 2 by density:- Variable used are:Vb = bulk volume of powder vp = pack volume of powder pb = density of bulk volume pp = density of pack powder m = wt of powdered body. € = porosity of powder. M = wt of filled (with drug) cylinder Wt of empty cylinder = 159.23 / 11.279 m = 46.44 Pb = m/Vp = 46.44 / 77 = 0.6031 € – pp – pb / pp = 0.6031 – 0.4644 / 0.6031 € = 0.1387/0.6031 = 0.23 %€= € x 100 = 0.23 x 100 = 23% Results:- Before filling the caps we have checked the flow property of the powder (angle of repose and porosity). The result of flow ability and porosity of Reducin shown that the flow was up to standard limits. 5.3. Physio chemical features: In order to determine physio chemical feature of our product Reducin below mentioned exercises were also performed. • Wt variation • Disintegration • Dissolution • Stability 5.3.1.Wt variation: To check the uniformity of wt of filled caps, the powdered material of REDUCIN was filled in zero size caps (soft gelation) having pink and black color. For the estimation of wt uniformity, wt variation was checked along with diameter and length of caps. All caps came under the upper and lower limits of wt variation. Cap Cap Cap Cap No. wt length mm length mm Diameter mm 1 254 24.10 7.20 2 251 24.00 7.25 3 255 23.95 7.10 4 235 23.75 7.35 5 200 24.15 7.31 6 259 23.80 7.10 7 260 23.95 7.20 8 248 24.00 7.45 9. 200 24.05 7.25 10. 255 23.70 7.20 11. 250 23.85 7.25 12. 245 23.70 7.30 13. 258 23.95 7.30 14 245 24.10 7.10 15 256 24.05 7.20 16 255 23.75 7.45 17 265 23.95 7.25 18 250 24.15 7.35 19. 250 24.00 7.30 20 255 24.05 7.26 (Mean) 253.25 23.95 7.25 (S.D ) 6.41 0.246 0.1060 5.3.2. Disintegration: At least 10 caps should be used to determine the disintegration time of caps. It was noted within a specified period when 10 caps were placed in liq medium under the prescribed experimental condition. The minimum and maximum time was observed b/w 8-15 mins respectively. Thus showing the disintegration time of all 10 caps within 15 mins. S.No Cap Disintegration Mean No Time(min) & S.D 1 1 8 2 2 11 3 3 12 4 4 9 11.4 5 5 13 & 6 6 15 2.221 7 7 14 8 8 10 9 9 12 10 10 10 RESULT: The max time for disintegration was 15min and the min time was 8 mins. When the mean time of disintegration of 10 caps with ± S.D was is 2.221. 5.3.3. Dissolution: Like disintegration, for dissolution we also took 10 caps for taking observations and determine the rate at which the active drug substance dissolved in the fluid of git. This is most suitable study (in vitro) for determining the release of drugs. S.No Extent Mean No of & Dissolution (%) S.D 1 1 97 2 2 98 3 3 94.85 4 4 92.02 95.362 5 5 98.9 & 6 6 93.85 1.594 7 7 91 8 8 94 9 9 98 10 10 95 Results: mins. Cap All the caps containing powdered drug were start dissolving in 30 CHAPTER 6 6. Result and Discussion: In modern era, herbal medicines occupy an important and significant role for the treatment of various diseases, although synthetic drugs and antibiotics are also used for different diseases along with a large no of hormone balancing steroids, but herbal medicines can be used more safely and conveniently in various regions of the world. Keeping all factors in mind, we have focused our research on wt reducing herbal preparation of four plants i.e plant A: Cinnamomum cassia blume,PLANT B:- Commiphora wightii, plant C:- Emblica officinalis plant D:- Ferula foetida against obesity Although all best plants anciently been used for treating different disease, especially obesity complaints and cancer complaints but they have never been used in this combination for treating obesity. As majority of persons suffer from obesity and its related problems we targeted our research towards the significant effects of the herbal preparation made from the mixed powder of all four above mentioned plants. We named this herbal formulation Reducin. Our research has not only been focused on the dosage formulation of REDUCIN, but also towards the authentication, standardization and phyto chemical investigation of compound present in these four plants (A-D). we divided this in to four main phases given below:- 6.1. Pharmacognostic Evaluation of Plant Drugs (AD):The establishment of identification protocol or mainting is of prime importance for herbal entity. To achieve this goal we had performed the evaluation of plant by organoleptic, macroscopic and microscopic methods. From the former method we got information about the sensory organs, such as external marking, internal marking, texture, fracture color, taste, shape, size and odour of the herbal drug while the later method revealed the presence of cellular structure and their shape and size, if the drug is in powdered form. However the cellular arrangement of the plant cells was determined by histological exam through microscope. In systemic histological exam permanent slides were made of the transverse section of the active material of the plants (A-D) used in formulation. 6.1.1. Characteristic Features of Plant A: Pharmacognostic Featuresof Drug: Scientific name Cinnamomum cassia Family laurance English name Chinese cassia Parts used Bark, bud, flowers Active constituents Cinnamic aldehyte, Cinnamyl acceate, benzaldehyde, dinatal, charicol, essential oil, coumarin with constituents of sugar fractions, crudefats and pectin, tannins and mucilage. Action and Uses: used as flavoring agent for confectionery, deserts, pastries and meat, old fashion pickling recipes and marinades. Organoleptic Features:• Texture is brittle. • Fracture is splintery. • Odour is fragment. • Taste is sweet, aromatic. Microsocopic Features: stone cells, parenchymatous cells, phloem, starch grains, ca- oxalate & medullay rays. 6.1.2. Characteristic Features of Plant B:Pharmacognostic Features of Drug: Scientific name Commiphora wigtii Family Burseraceae English name Commiphora roxburghill Parts used leaves, gum Active constituent’s essential oil, gum, resin, steroids, polymyrcene, dimyercene, myrcene. Action and uses: use for decrease obesity, rheumatoid arthritis, sciatica, anti hemorrhidal, aphrodisiac agent, in menstrual problems, for treatment of cancer and thyroid problems. Organoleptic Features:• Fracture is brittle. • Odour is bashmic. • Taste is bitter, acrid. • Texture is translucent. • Color is Reddish Brown. Microscopic Features: - Spiral vessel, starch grains, stone cells, isolated scleroids and fibers. 6.1.3. Characteristic Features of Plant C: Pharmacognestic features of Drug: Scientific name Embelica officindis Family Euphorbiaceae English name Indian gooseberry Part used Ripe & dried fruit, seeds leafss, root, bark and flower. Active constituent’s Ascorbic acid, gallo tannins, mucic acid, phyllembicacid, ellagic acid, B site sterol. Action and uses:-used for jaundice, gonorrhea, frequent mensturation,diabetes, poultice for skin ulcers, sores, swelling and itchness. Organoleptic features: • Texture is rough. • Fracture is hard. • Color is Blackish. • Odour is sweet & sour. • Taste is sour or slightly acidic. Microscopic Features of Drug: Gp of phloem fibers, tannin cells, starch grain, lignified cells, ca -oxalate and phloem vessels. 6.1.4. Characteristic Features of Plant D:Pharmacognostic Features of Drug: Scientific name Ferula foetida Family Apiaceae English name Lady ginia lipsky Part used Stems, leafs, flowers Active constituet Ferulic acid, umbelifarone, asaresino tannols, fernesiferols A,B,C. Action and use: Antioxidant, anti-hemolytic, anticancer, control B.P, lipid lowering agent, help to protect CNS, heart, sugar levels, it has chemotherapy usage. Organoleptic Features: • Texture is rough. • Fracture is hard. • Odour is bitter and acrid. Powder Microscopy of Drug: Powder microscopy not done b/c it is inorganic in nature. 6.1.5. Characteristic Features of REDUCIN: Pharmacognostic Features of Drug: Organoleptic features: • Color is brown powder. • Odour is fragment. • Taste is acrid taste. Microscopic Features of Powder Drug: stone cells, fiber cells, caoxalate, tannin cells, starch grains and scleried. [ Action and Uses: used successfully for true treatment of all types of obesity as well as ovarian cysts in some obese patients. 6.2. Phytochemical Evaluation of Plant Drugs (A-D): Then in second phase, we has performed the chromogenic testing for knowing the existence of the naturally occurring compounds in Meoh extract of all four plant drugs (A-D) and matched with the literature reported compounds in all four plants. The observation was made on the basis of different coloration by chromogenic reagent by TLC procedures for identification of different classes of compounds such as Molish test & fehling test for carbohydrate, for protein Bioret test, for steroids salkowski test and libermanburchad test, for alkaloids Dragendroffs & Mayers test, for saponins froth test, for glycosides Legals test & Lekellar killani test, for starch tannic acid test, for flavonids shinoda test, for tannins & phenols fecl3 test, for protein Milton test & Ninhydin test and for reducing sugar Benedicts test. A positive responds against the specific reagent of each chemical type was observed. Phytochemical investigations of plant drug was started from extraction, fractionation, isolation & detection followed by chromatographic operation i.e thin layer chromatography. In TLC the spots given by different chromogenic reagents was noted. Like dragendroffs reagent has given orange color spots on the plate, Cerum Amonium sulphate reagent give light green bands and light pink spots appeared when TLC plate was observed under UV lamp. The results obtained by phytochemical analysis of all four medicinal plants are given below: 6.2.1 CHEMICAL COMPOSITION carbohydrate sugar steroids protein lipid alkaloids tannin saponin Plant A + + + + + + + + Plant B + + + + + + + + Plant C + + + + + + + + Plant D - - - - - + - - 6.2.1: Chemical Composition of plant (A-D) used in the preparation of REDUCIN 6.3. Clinical Studies on REDUCIN: It was the third and most important phase of the research studies in which Reducin was undergone clinical trials on human subject in obesity & its related disorders. The study was conducted and approved by the ethical committee of Murshid hospital and health care center hub river road Karachi. Various parameters were set for diagnostic protocol such as Depression, low physical fitness, absenteeism from work, reduced producing. Although all plants used in Reducin are previously used as traditional medicine, but they have never been used before together for the treatment of obesity. Reducin played a important and remarkable role in this regard by producing excellent results in obese pts. The drug given as 250 mg TDS to the patients who suffered from obesity and its related disorders like orthopedic problem, anemia, cysts etc. The trials was conducted on two gps A and B. group A is comprise on simple diet gp and gp B is comprises on rich fat diet gp. Each gp had 50 participant and all had obesity & its related disorders. Diagnosis was made by strict experimental protocol. Prior to experimental trials we also conducted the comparative studies of pts among gp A and B, in this study we had set the criteria for the general health condition of participating patients by inquiring about the existing disease [increase B.P, diabetes, anemia, GIT upset, constipation head ache], dietary pattern (i.e simple diet, rich fat diet) and sleep pattern. (Normal or disturbed). In this study we also kept in consideration the socioeconomic status of participants as it exerts major effect on an individual life. The most important analysis was that out of loo, 85 patient belongs to poor economic class and came to patient welfare clinic (PWA) of Murshid hospital. The symptoms which were measured are depression, disability, low physical fitness, stigma, absent from work, reduced productivity. After 21 days medical examination was carried out, checks complaints, and any adverse effect and lab tests. Assessments was made on the basis of T.G and HDLC levels of gp A and B. the results shows a significant decrease in rich fat diet gp, T.G & HDLC levels. Gp A also shows reduction in TGS & HDLC levels. As well as the waist circumference which show a remarkable decrease after treatment of Reducin in obese pts (both male & females). 6.4. Phytopharmaceutical Studies of REDUCIN: This was the fourth phase of our research work. In this, a study of product formulation a/c to pharmaceutical standards in oral dosage form was carried out. During this course of study of physiochemical features of product by wt variation, dissolution and disintegration along with stability studies were performed. The powdered material of Reducin was filled in zero sized caps having pink and black color combination by manual method. Prior to filling of powder in caps, its flow property was also checked (ie. Angle of repose and porosity) and it was found that flow was up to standard limits. In physiochemical features of powdered caps wt variation along with diameter and length of caps were checked. And it was observed that the product is of standard quality and efficacy both. REFRENCES: 1. WHO 2000 p.6 2. Haslam DW, James WP (2005). "Obesity". Lancet (Review) 366 (9492): 1197–209. doi:10.1016/S0140-6736(05)67483-1. PMID 16198769. 3. WHO 2000 p.9 4. Kushner, Robert (2007). Treatment of the Obese Patient (Contemporary Endocrinology). Totowa, NJ: Humana Press. p. 158. ISBN 1-59745-400-1. Retrieved April 5, 2009. 5. Adams JP, Murphy PG (July 2000). "Obesity in anesthesia and intensive care". Br J Anaesth 85 (1): 91–108. doi:10.1093/bja/85.1.91. PMID 10927998. 6. NICE 2006 p.10–11 7. Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J (July 2008). "Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis". Obes Surg 18 (7): 841–6. doi:10.1007/s11695-007-9331-8. PMID 18459025. 8. Dibaise JK, Foxx-Orenstein AE (July 2013). "Role of the gastroenterologist in managing obesity". Expert Review of Gastroenterology & Hepatology (Review) 7 (5): 439–51. doi:10.1586/17474124.2013.811061. PMID 23899283. 9. Woodhouse R (2008). "Obesity in art: A brief overview". Front Horm Res. Frontiers of Hormone Research 36: 271–86. doi:10.1159/000115370. ISBN 978-3-8055-8429-6. PMID 18230908. 10.Pollack, Andrew (June 18, 2013). "A.M.A. Recognizes Obesity as a Disease". New York Times. Archived from the original on June 18, 2013. 11.Weinstock, Matthew (June 21, 2013). "The Facts About Obesity". H&HN. American Hospital Association. Retrieved June 24, 2013. 12."Obesity". Blausen Medical. Retrieved 4 November 2015. 13.Sweeting HN (2007). "Measurement and Definitions of Obesity In Childhood and Adolescence: A field guide for the uninitiated". Nutr J 6 (1): 32. doi:10.1186/1475-2891-6-32. PMC 2164947. PMID 17963490. 14.NHLBI p.xiv 15.Gray DS, Fujioka K (1991). "Use of relative weight and Body Mass Index for the determination of adiposity". J Clin Epidemiol 44 (6): 545–50. doi:10.1016/0895-4356(91)90218-X. PMID 2037859. 16."Healthy Weight: Assessing Your Weight: BMI: About BMI for Children and Teens". Center for disease control and prevention. Retrieved April 6, 2009. 17.Flegal KM, Ogden CL, Wei R, Kuczmarski RL, Johnson CL (June 2001). "Prevalence of overweight in US children: comparison of US growth charts from the Centers for Disease Control and Prevention with other reference values for body mass index". Am. J. Clin. Nutr. 73 (6): 1086–93. PMID 11382664. 18.1 (lb/sq in) is more precisely 703.06957964 (kg/m2). 19."BMI classification". World Health Organization. Retrieved 15 February 2014. 20.Sturm R (July 2007). "Increases in morbid obesity in the USA: 2000–2005". Public Health 121 (7): 492–6. doi:10.1016/j.puhe.2007.01.006. PMC 2864630. PMID 17399. 21.Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S (December 2002). "Criteria and classification of obesity in Japan and Asia-Oceania". Asia Pac J Clin Nutr. 11 Suppl 8: S732–S737. doi:10.1046/j.14406047.11.s8.19.x. PMID 12534701. 22.Bei-Fan Z (December 2002). "Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults". Asia Pac J Clin Nutr. 11 Suppl 8: S685–93. doi:10.1046/j.1440-6047.11.s8.9.x. PMID 12534691. 23.Poulain M, Doucet M, Major GC, Drapeau V, Sériès F, Boulet LP, Tremblay A, Maltais F (April 2006). "The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies". CMAJ 174 (9): 1293– 9. doi:10.1503/cmaj.051299. PMC 1435949. PMID 16636330. 24.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, ZeleniuchJacquotte A, Willett WC, Thun MJ (2010). "Body-mass index and mortality among 1.46 million white adults". The New England Journal of Medicine 363 (23): 2211–9. doi:10.1056/NEJMoa1000367. PMC 3066051. PMID 21121834. 25.Barness LA, Opitz JM, Gilbert-Barness E (December 2007). "Obesity: genetic, molecular, and environmental aspects". American Journal of Medical Genetics 143A (24): 3016–34. doi:10.1002/ajmg.a.32035. PMID 18000969. 26.Mokdad AH, Marks JS, Stroup DF, Gerberding JL (March 2004). "Actual causes of death in the United States, 2000" (PDF). JAMA 291 (10): 1238–45. doi:10.1001/jama.291.10.1238. PMID 15010446. 27.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB (October 1999). "Annual deaths attributable to obesity in the United States". JAMA 282 (16): 1530–8. doi:10.1001/jama.282.16.1530. PMID 10546692. 28.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R (March 2009). "Body-mass index and causespecific mortality in 900 000 adults: collaborative analyses of 57 prospective studies". Lancet 373 (9669): 1083–96. doi:10.1016/S0140-6736(09)60318-4. PMC 2662372. PMID 19299006. 29.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW (October 1999). "Body-mass index and mortality in a prospective cohort of U.S. adults". N. Engl. J. Med. 341 (15): 1097–105. doi:10.1056/NEJM199910073411501. PMID 10511607. 30.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjønneland A; et al. (November 2008). "General and abdominal adiposity and risk of death in Europe". N. Engl. J. Med. 359 (20): 2105–20. doi:10.1056/NEJMoa0801891. PMID 19005195. 31.WHO Expert, Consultation (Jan 10, 2004). "Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies.". Lancet 363 (9403): 157–63. doi:10.1016/s0140-6736(03)152683. PMID 14726171. 32.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE (1995). "Body weight and mortality among women". N. Engl. J. Med. 333 (11): 677–85. doi:10.1056/NEJM199509143331101. PMID 7637744. 33.Tsigos C, Hainer V, Basdevant A, Finer N, Fried M, Mathus-Vliegen E, Micic D, Maislos M, Roman G, Schutz Y, Toplak H, Zahorska-Markiewicz B (April 2008). "Management of Obesity in Adults: European Clinical Practice Guidelines" (PDF). The European Journal of Obesity 1 (2): 106–16. doi:10.1159/000126822. PMID 20054170. 34.Fried M, Hainer V, Basdevant A, Buchwald H, Deitel M, Finer N, Greve JW, Horber F, Mathus-Vliegen E, Scopinaro N, Steffen R, Tsigos C, Weiner R, Widhalm K (April 2007). "Inter-disciplinary European guidelines on surgery of severe obesity". Int J Obes (Lond) 31 (4): 569–77. doi:10.1038/sj.ijo.0803560. PMID 17325689. 35.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L (January 2003). "Obesity in adulthood and its consequences for life expectancy: A life-table analysis". Annals of Internal Medicine 138 (1): 24– 32. doi:10.7326/0003-4819-138-1-200301070-00008. PMID 12513041. 36.Grundy SM (2004). "Obesity, metabolic syndrome, and cardiovascular disease". J. Clin. Endocrinol. Metab. 89 (6): 2595–600. doi:10.1210/jc.20040372. PMID 15181029. 37.Seidell 2005 p.9 38.Bray GA (2004). "Medical consequences of obesity". J. Clin. Endocrinol. Metab. 89 (6): 2583–9. doi:10.1210/jc.2004-0535. PMID 15181027. 39.Shoelson SE, Herrero L, Naaz A (May 2007). "Obesity, inflammation, and insulin resistance". Gastroenterology 132 (6): 2169–80. doi:10.1053/j.gastro.2007.03.059. PMID 17498510. 40.Shoelson SE, Lee J, Goldfine AB (July 2006). "Inflammation and insulin resistance". J. Clin. Invest. 116 (7): 1793–801. doi:10.1172/JCI29069. PMC 1483173. PMID 16823477. 41.Dentali F, Squizzato A, Ageno W (July 2009). "The metabolic syndrome as a risk factor for venous and arterial thrombosis". Semin. Thromb. Hemost. 35 (5): 451–7. doi:10.1055/s-0029-1234140. PMID 19739035. 42.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated, Effects); Lu, Y; Hajifathalian, K; Ezzati, M; Woodward, M; Rimm, EB; Danaei, G (15 March 2014). "Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants.". Lancet (London, England) 383 (9921): 970–83. doi:10.1016/S0140-6736(13)61836-X. PMID 24269108. 43.Darvall KA, Sam RC, Silverman SH, Bradbury AW, Adam DJ (February 2007). "Obesity and thrombosis". Eur J Vasc Endovasc Surg 33 (2): 223–33. doi:10.1016/j.ejvs.2006.10.006. PMID 17185009. 44.Yosipovitch G, DeVore A, Dawn A (June 2007). "Obesity and the skin: skin physiology and skin manifestations of obesity". J. Am. Acad. Dermatol. 56 (6): 901–16; quiz 917–20. doi:10.1016/j.jaad.2006.12.004. PMID 17504714. 45.Hahler B (June 2006). "An overview of dermatological conditions commonly associated with the obese patient". Ostomy Wound Manage 52 (6): 34–6, 38, 40 passim. PMID 16799182. 46.Arendas K, Qiu Q, Gruslin A (2008). "Obesity in pregnancy: pre-conceptional to postpartum consequences". Journal of Obstetrics and Gynaecology Canada : JOGC = Journal D'obstétrique Et Gynécologie Du Canada : JOGC 30 (6): 477–88. PMID 18611299. 47.Harney D, Patijn J (2007). "Meralgia paresthetica: diagnosis and management strategies". Pain Med (Review) 8 (8): 669–77. doi:10.1111/j.1526-4637.2006.00227.x. PMID 18028045. 48.Bigal ME, Lipton RB (January 2008). "Obesity and chronic daily headache". Curr Pain Headache Rep (Review) 12 (1): 56–61. doi:10.1007/s11916-0080011-8. PMID 18417025. 49.Sharifi-Mollayousefi A, Yazdchi-Marandi M, Ayramlou H, Heidari P, Salavati A, Zarrintan S, Sharifi-Mollayousefi A (February 2008). "Assessment of body mass index and hand anthropometric measurements as independent risk factors for carpal tunnel syndrome". Folia Morphol. (Warsz) 67 (1): 36–42. PMID 18335412. 50.Beydoun MA, Beydoun HA, Wang Y (May 2008). "Obesity and central obesity as risk factors for incident dementia and its subtypes: A systematic review and meta-analysis". Obes Rev (Meta-analysis) 9 (3): 204–18. doi:10.1111/j.1467-789X.2008.00473.x. PMID 18331422. 51.Wall M (March 2008). "Idiopathic intracranial hypertension (pseudotumor cerebri)". Curr Neurol Neurosci Rep (Review) 8 (2): 87–93. doi:10.1007/s11910-008-0015-0. PMID 18460275. 52.Munger KL, Chitnis T, Ascherio A (2009). "Body size and risk of MS in two cohorts of US women". Neurology (Comparative Study) 73 (19): 1543–50. doi:10.1212/WNL.0b013e3181c0d6e0. PMC 2777074. PMID 19901245. 53.Basen-Engquist, Karen; Chang, Maria (16 November 2010). "Obesity and Cancer Risk: Recent Review and Evidence". Current Oncology Reports 13 (1): 71–76. doi:10.1007/s11912-010-0139-7. PMID 21080117. 54.Aune, D; Norat, T; Vatten, LJ (December 2014). "Body mass index and the risk of gout: a systematic review and dose-response meta-analysis of prospective studies.". European journal of nutrition 53 (8): 1591–601. doi:10.1007/s00394-014-0766-0. PMID 25209031. 55.Tukker A, Visscher TL, Picavet HS (April 2008). "Overweight and health problems of the lower extremities: osteoarthritis, pain and disability". Public Health Nutr (Research Support) 12 (3): 1–10. doi:10.1017/S1368980008002103. PMID 18426630. 56.Molenaar EA, Numans ME, van Ameijden EJ, Grobbee DE (November 2008). "[Considerable comorbidity in overweight adults: results from the Utrecht Health Project]". Ned Tijdschr Geneeskd (English abstract) (in Dutch) 152 (45): 2457–63. PMID 19051798. 57.Corona, G; Rastrelli, G; Filippi, S; Vignozzi, L; Mannucci, E; Maggi, M (2014). "Erectile dysfunction and central obesity: an Italian perspective.". Asian Journal of Andrology 16 (4): 581–91. doi:10.4103/1008-682X.126386. PMID 24713832. 58.Hunskaar S (2008). "A systematic review of overweight and obesity as risk factors and targets for clinical intervention for urinary incontinence in women". Neurourol. Urodyn. (Review) 27 (8): 749–57. doi:10.1002/nau.20635. PMID 18951445. 59.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O (2006). "Obesity and risk for chronic renal failure". J. Am. Soc. Nephrol. (Research Support) 17 (6): 1695–702. doi:10.1681/ASN.2005060638. PMID 16641153. 60.Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R (September 2005). "Hypogonadism and metabolic syndrome: Implications for testosterone therapy". J. Urol. (Review) 174 (3): 827–34. doi:10.1097/01.ju.0000169490.78443.59. PMID 16093964. 61.Pestana IA, Greenfield JM, Walsh M, Donatucci CF, Erdmann D (October 2009). "Management of "buried" penis in adulthood: an overview". Plast. Reconstr. Surg. (Review) 124 (4): 1186–95. doi:10.1097/PRS.0b013e3181b5a37f. PMID 19935302. 62.Schmidt DS, Salahudeen AK (2007). "Obesity-survival paradox-still a controversy?". Semin Dial (Review) 20 (6): 486–92. doi:10.1111/j.1525139X.2007.00349.x. PMID 17991192. 63.U.S. Preventive Services Task Force (June 2003). "Behavioral counseling in primary care to promote a healthy diet: recommendations and rationale". Am Fam Physician (Review) 67 (12): 2573–6. PMID 12825847. 64.Habbu A, Lakkis NM, Dokainish H (October 2006). "The obesity paradox: Fact or fiction?". Am. J. Cardiol. (Review) 98 (7): 944–8. doi:10.1016/j.amjcard.2006.04.039. PMID 16996880. 65.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F (2006). "Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies". Lancet (Review) 368 (9536): 666–78. doi:10.1016/S0140-6736(06)69251-9. PMID 16920472. 66.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA (July 2008). "Body mass index and mortality in heart failure: A meta-analysis". Am. Heart J. (Meta-analysis, Review) 156 (1): 13–22. doi:10.1016/j.ahj.2008.02.014. PMID 18585492. 67.Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K (February 2008). "Effect of obesity on short- and long-term mortality postcoronary revascularization: A meta-analysis". Obesity (Silver Spring) (Meta-analysis) 16 (2): 442–50. doi:10.1038/oby.2007.36. PMID 18239657. 68.Diercks DB, Roe MT, Mulgund J, Pollack CV, Kirk JD, Gibler WB, Ohman EM, Smith SC, Boden WE, Peterson ED (July 2006). "The obesity paradox in nonST-segment elevation acute coronary syndromes: Results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative". Am Heart J (Research Support) 152 (1): 140–8. doi:10.1016/j.ahj.2005.09.024. PMID 16824844. 69.^ Jump up to: a b c d e Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E (April 2007). "2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children summary". CMAJ (Practice Guideline, Review) 176 (8): S1–13. doi:10.1503/cmaj.061409. PMC 1839777. PMID 17420481. 70.Bleich S, Cutler D, Murray C, Adams A (2008). "Why is the developed world obese?". Annu Rev Public Health (Research Support) 29: 273–95. doi:10.1146/annurev.publhealth.29.020907.090954. PMID 18173389. 71.Drewnowski A, Specter SE (January 2004). "Poverty and obesity: the role of energy density and energy costs". Am. J. Clin. Nutr. (Review) 79 (1): 6–16. PMID 14684391. 72.Nestle M, Jacobson MF (2000). "Halting the obesity epidemic: a public health policy approach". Public Health Rep (Research Support) 115 (1): 12– 24. doi:10.1093/phr/115.1.12. PMC 1308552. PMID 10968581. 73.James WP (March 2008). "The fundamental drivers of the obesity epidemic". Obes Rev (Review) 9 (Suppl 1): 6–13. doi:10.1111/j.1467789X.2007.00432.x. PMID 18307693. 74.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine KR, Wang C, Aronne LJ, Wright SM, Baskin M, Dhurandhar NV, Lijoi MC, Grilo CM, DeLuca M, Westfall AO, Allison DB (2006). "Putative contributors to the secular increase in obesity: Exploring the roads less traveled". Int J Obes (Lond) (Review) 30 (11): 1585– 94. doi:10.1038/sj.ijo.0803326. PMID 16801930. 75."EarthTrends: Nutrition: Calorie supply per capita". World Resources Institute. Archived from the original on 2011-06-11. Retrieved Oct 18, 2009. 76."USDA: frsept99b". United States Department of Agriculture. Retrieved January 10, 2009. 77."Diet composition and obesity among Canadian adults". Statistics Canada. 78.National Control for Health Statistics. "Nutrition For Everyone". Centers for Disease Control and Prevention. Retrieved 2008-07-09. 79.Marantz PR, Bird ED, Alderman MH (March 2008). "A call for higher standards of evidence for dietary guidelines". Am J Prev Med 34 (3): 234– 40. doi:10.1016/j.amepre.2007.11.017. PMID 18312812. 80.Flegal KM, Carroll MD, Ogden CL, Johnson CL (October 2002). "Prevalence and trends in obesity among US adults, 1999–2000". JAMA 288 (14): 1723– 1727. doi:10.1001/jama.288.14.1723. PMID 12365955. 81.Wright JD, Kennedy-Stephenson J, Wang CY, McDowell MA, Johnson CL (February 2004). "Trends in intake of energy and macronutrients—United States, 1971–2000". MMWR Morb Mortal Wkly Rep 53 (4): 80–2. PMID 14762332. 82.Caballero B (2007). "The global epidemic of obesity: An overview". Epidemiol Rev 29: 1–5. doi:10.1093/epirev/mxm012. PMID 17569676. 83.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB (23 June 2011). "Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men". The New England Journal of Medicine (Meta-analysis) 364 (25): 2392–404. doi:10.1056/NEJMoa1014296. PMC 3151731. PMID 21696306. 84.Malik VS, Schulze MB, Hu FB (August 2006). "Intake of sugar-sweetened beverages and weight gain: a systematic review". Am. J. Clin. Nutr. (Review) 84 (2): 274–88. PMC 3210834. PMID 16895873. 85.Olsen NJ, Heitmann BL (January 2009). "Intake of calorically sweetened beverages and obesity". Obes Rev (Review) 10 (1): 68–75. doi:10.1111/j.1467-789X.2008.00523.x. PMID 18764885. 86.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB (November 2010). "Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis". Diabetes Care (Meta-analysis, Review) 33 (11): 2477–83. doi:10.2337/dc10-1079. PMC 2963518. PMID 20693348. 87.Rosenheck R (November 2008). "Fast food consumption and increased caloric intake: a systematic review of a trajectory towards weight gain and obesity risk". Obes Rev (Review) 9 (6): 535–47. doi:10.1111/j.1467789X.2008.00477.x. PMID 18346099. 88.Lin BH, Guthrie J and Frazao E (1999). "Nutrient contribution of food away from home". In Frazão E. Agriculture Information Bulletin No. 750: America's Eating Habits: Changes and Consequences. Washington, DC: US Department of Agriculture, Economic Research Service. pp. 213–239. 89.Pollan, Michael (22 April 2007). "You Are What You Grow". New York Times. Retrieved 2007-07-30. 90.Kopelman and Caterson 2005:324. 91.Metabolism alone doesn't explain how thin people stay thin. John Schieszer (The Medical Post). 92.Seidell 2005 p.10 93."WHO: Obesity and overweight". World Health Organization. Archived from the original on December 18, 2008. Retrieved January 10, 2009. 94."WHO | Physical Inactivity: A Global Public Health Problem". World Health Organization. Retrieved February 22, 2009. 95.Ness-Abramof R, Apovian CM (February 2006). "Diet modification for treatment and prevention of obesity". Endocrine (Review) 29 (1): 5–9. doi:10.1385/ENDO:29:1:135. PMID 16622287. 96.Salmon J, Timperio A (2007). "Prevalence, trends and environmental influences on child and youth physical activity". Med Sport Sci (Review). Medicine and Sport Science 50: 183–99. doi:10.1159/000101391. ISBN 9783-318-01396-2. PMID 17387258. 97. Borodulin K, Laatikainen T, Juolevi A, Jousilahti P (June 2008). "Thirty-year trends of physical activity in relation to age, calendar time and birth cohort in Finnish adults". Eur J Public Health (Research Support) 18 (3): 339– 44. doi:10.1093/eurpub/ckm092. PMID 17875578. 98. Brownson RC, Boehmer TK, Luke DA (2005). "Declining rates of physical activity in the United States: what are the contributors?". Annu Rev Public Health (Review) 26: 421–43. doi:10.1146/annurev.publhealth.26.021304.144437. PMID 15760296. 99. Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH (April 1996). "Television viewing as a cause of increasing obesity among children in the United States, 1986–1990". Arch Pediatr Adolesc Med (Review) 150 (4): 356–62. doi:10.1001/archpedi.1996.02170290022003. PMID 8634729. 100. Vioque J, Torres A, Quiles J (December 2000). "Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain". Int. J. Obes. Relat. Metab. Disord. (Research Support) 24 (12): 1683–8. doi:10.1038/sj.ijo.0801434. PMID 11126224. 101. Tucker LA, Bagwell M (July 1991). "Television viewing and obesity in adult females" (PDF). Am J Public Health 81 (7): 908–11. doi:10.2105/AJPH.81.7.908. PMC 1405200. PMID 2053671. 102. "Media + Child and Adolescent Health: A Systematic Review" (PDF). Ezekiel J. Emanuel. Common Sense Media. 2008. Retrieved April 6, 2009. 103. Mary Jones. "Case Study: Cataplexy and SOREMPs Without Excessive Daytime Sleepiness in Prader Willi Syndrome. Is This the Beginning of Narcolepsy in a Five Year Old?". European Society of Sleep Technologists. Retrieved April 6, 2009. 104. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH (May 2006). "Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight 968–76. loss". Arterioscler. Thromb. Vasc. Biol. (Review) 26 (5): doi:10.1161/01.ATV.0000216787.85457.f3. PMID 16627822. 105. Loos RJ, Bouchard C (May 2008). "FTO: the first gene contributing to common forms of human obesity". Obes Rev (Review) 9 (3): 246–50. doi:10.1111/j.1467-789X.2008.00481.x. PMID 18373508. 106. Yang W, Kelly T, He J (2007). "Genetic epidemiology of obesity". Epidemiol Rev (Review) 29: 49–61. doi:10.1093/epirev/mxm004. PMID 17566051. 107. Walley AJ, Asher JE, Froguel P (June 2009). "The genetic contribution to non-syndromic 431–42. human obesity". Nature Reviews Genetics (Review) 10 (7): doi:10.1038/nrg2594. PMID 19506576. 108. Farooqi S, O'Rahilly S (December 2006). "Genetics of obesity in humans". Endocr. Rev. (Review) 27 (7): 710–18. doi:10.1210/er.2006-0040. PMID 17122358. 109. Kolata,Gina (2007). Rethinking thin: The new science of weight loss – and the myths and realities of dieting. Picador. p. 122. ISBN 0-312-42785-9. 110. Walley, Andrew J., Asher, Julian E., Froguel, Philippe (July 2009). "The genetic contribution to non-syndromic human obesity.". Nat Rev Genet. (Review) 10 (7): 431–42. doi:10.1038/nrg2594. PMID 19506576. However, it is also clear that genetics greatly influences this situation, giving individuals in the same 'obesogenic' environment significantly different risks of becoming obese. 111. Chakravarthy MV, Booth FW (2004). "Eating, exercise, and "thrifty" genotypes: Connecting the dots toward an evolutionary understanding of modern chronic diseases". J. Appl. Physiol. (Review) 96 (1): 3–10. doi:10.1152/japplphysiol.00757.2003. PMID 14660491. 112. Wells JC (2009). "Thrift: A guide to thrifty genes, thrifty phenotypes and thrifty norms". International Journal of Obesity (Review) 33 (12): 1331–1338. doi:10.1038/ijo.2009.175. PMID 19752875. 113. Wells JC (2011). "The thrifty phenotype: An adaptation in growth or metabolism?". American Journal of Human Biology (Review) 23 (1): 65–75. doi:10.1002/ajhb.21100. PMID 21082685. 114. Rosén T, Bosaeus I, Tölli J, Lindstedt G, Bengtsson BA (1993). "Increased body fat mass and decreased extracellular fluid volume in adults with growth hormone deficiency". Clin. Endocrinol. (Oxf) 38 (1): 63–71. doi:10.1111/j.13652265.1993.tb00974.x. PMID 8435887. 115. Zametkin AJ, Zoon CK, Klein HW, Munson S (February 2004). "Psychiatric aspects of child and adolescent obesity: a review of the past 10 years". J Am Acad Child Adolesc Psychiatry (Review) 43 (2): 134–50. doi:10.1097/00004583200402000-00008. PMID 14726719. 116. Chiles C, van Wattum PJ (2010). "Psychiatric aspects of the obesity crisis". Psychiatr Times 27 (4): 47–51. 117. Yach D, Stuckler D, Brownell KD (January 2006). "Epidemiologic and economic consequences of the global epidemics of obesity and diabetes". Nat. Med. 12 (1): 62–6. doi:10.1038/nm0106-62. PMID 16397571. 118. Sobal J, Stunkard AJ (March 1989). "Socioeconomic status and obesity: A review of the literature". Psychol Bull (Review) 105 (2): 260–75. doi:10.1037/0033-2909.105.2.260. PMID 2648443. 119. McLaren L (2007). "Socioeconomic status and obesity". Epidemiol Rev (Review) 29: 29–48. doi:10.1093/epirev/mxm001. PMID 17478442. 120. Wilkinson, Richard; Pickett, Kate (2009). The Spirit Level: Why More Equal Societies Almost Always Do Better. London: Allen Lane. pp. 91–101. ISBN 978-184614-039-6. 121. Christakis NA, Fowler JH (2007). "The Spread of Obesity in a Large Social Network over 32 Years". New England Journal of Medicine (Research Support) 357 (4): 370–379. doi:10.1056/NEJMsa066082. PMID 17652652. 122. Björntorp P (2001). "Do stress reactions cause abdominal obesity and comorbidities?". Obesity Reviews 2 (2): 73–86. doi:10.1046/j.1467- 789x.2001.00027.x. PMID 12119665. 123. Goodman E, Adler NE, Daniels SR, Morrison JA, Slap GB, Dolan LM (2003). "Impact of objective and subjective social status on obesity in a biracial cohort of adolescents". Obesity Reviews (Research Support) 11 (8): 1018–26. doi:10.1038/oby.2003.140. PMID 12917508. 124. Flegal KM, Troiano RP, Pamuk ER, Kuczmarski RJ, Campbell SM (November 1995). "The influence of smoking cessation on the prevalence of overweight in the United States". N. Engl. J. Med. 333 (18): 1165–70. doi:10.1056/NEJM199511023331801. PMID 7565970. 125. Chiolero A, Faeh D, Paccaud F, Cornuz J (1 April 2008). "Consequences of smoking for body weight, body fat distribution, and insulin resistance". Am. J. Clin. Nutr. (Review) 87 (4): 801–9. PMID 18400700. 126. Weng HH, Bastian LA, Taylor DH, Moser BK, Ostbye T (2004). "Number of children associated with obesity in middle-aged women and men: results from the health and retirement study". J Women's Health (Larchmt) (Comparative Study) 13 (1): doi:10.1089/154099904322836492. PMID 15006281. 127. Bellows-Riecken KH, Rhodes RE (February 2008). "A birth of inactivity? A review of physical activity and parenthood". Prev Med (Review) 46 (2): 99–110. doi:10.1016/j.ypmed.2007.08.003. PMID 17919713. 128. "Obesity and Overweight" (PDF). World Health Organization. Retrieved February 22, 2009. 129. Caballero B (March 2001). "Introduction. Symposium: Obesity in developing countries: biological and ecological factors". J. Nutr. (Review) 131 (3): 866S–870S. PMID 11238776. 130. Smith E, Hay P, Campbell L, Trollor JN (2011). "A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment". Obesity Reviews (Review) 12 (9): 740–755. doi:10.1111/j.1467-789X.2011.00920.x. PMID 21991597. 131. DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE (April 2008). "Gut microbiota and its possible relationship with obesity". Mayo Clinic proceedings. Mayo Clinic (Review) 83 (4): 460–9. doi:10.4065/83.4.460. PMID 18380992. 132. Falagas ME, Kompoti M (July 2006). "Obesity and infection". Lancet Infect Dis (Review) 6 (7): 438–46. doi:10.1016/S1473-3099(06)70523-0. PMID 16790384. 133. Flier JS (2004). "Obesity wars: Molecular progress confronts an expanding epidemic". Cell (Review) 116 (2): 337–50. doi:10.1016/S0092-8674(03)01081-X. PMID 14744442. 134. Zhang, Y; Proenca, R; Maffei, M; Barone, M; Leopold, L; Friedman, JM (Dec 1, 1994). "Positional cloning of the mouse obese gene and its human homologue.". Nature (Research Support) 372 (6505): 425–32. doi:10.1038/372425a0. PMID 7984236. 135. Considine, RV; Considine, EL; Williams, CJ; Nyce, MR; Magosin, SA; Bauer, TL; Rosato, EL; Colberg, J; Caro, JF (Jun 1995). "Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity.". The Journal of Clinical Investigation (Research Support) 95 (6): 2986–8. doi:10.1172/jci118007. PMC 295988. PMID 7769141. 136. Hamann A, Matthaei S (1996). "Regulation of energy balance by leptin". Exp. Clin. Endocrinol. Diabetes (Review) 104 (4): 293–300. doi:10.1055/s-0029- 1211457. PMID 8886745. 137. Boulpaep, Emile L.; Boron, Walter F. (2003). Medical physiology: A cellular and molecular approach. Philadelphia: Saunders. p. 1227. ISBN 0-7216-3256-4. 138. Loscalzo, Joseph; Fauci, Anthony S.; Braunwald, Eugene; Dennis L. Kasper; Hauser, Stephen L; Longo, Dan L. (2008). Harrison's principles of internal medicine. McGraw-Hill Medical. ISBN 0-07-146633-9. 139. Satcher D (2001). The Surgeon General's Call to Action to Prevent and Decrease Overweight and Obesity. U.S. Dept. of Health and Human Services, Public Health Service, Office of Surgeon General. ISBN 978-0-16-051005-2. 140. Moyer VA (4 September 2012). "Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement". Annals of Internal Medicine (Practice Guideline) 157 (5): 373–8. doi:10.7326/0003-4819-157-5-201209040-00475. PMID 22733087. 141. Brook Barnes (2007-07-18). "Limiting Ads of Junk Food to Children". New York Times. Retrieved 2008-07-24. 142. "Fewer Sugary Drinks Key to Weight Loss - healthfinder.gov". U.S. Department of Health and Human Services. Retrieved Oct 18, 2009. 143. Brennan Ramirez LK, Hoehner CM, Brownson RC, Cook R, Orleans CT, Hollander M, Barker DC, Bors P, Ewing R, Killingsworth R, Petersmarck K, Schmid T, Wilkinson W (December 2006). "Indicators of activity-friendly communities: An evidence-based consensus process". Am J Prev Med (Research Support) 31 (6): 530–32 doi:10.1016/j.amepre.2006.07.026. PMID 17169714. 144. National Heart, Lung, and Blood Institute (1998). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (PDF). International Medical Publishing, Inc. ISBN 1-58808-002-1. 145. Storing up problems; the medical case for a slimmer nation. London: Royal College of Physicians. 2004-02-11. ISBN 1-86016-200-2. 146. Great Britain Parliament House of Commons Health Committee (May 2004). Obesity – Volume 1 – HCP 23-I, Third Report of session 2003–04. Report, together with formal minutes. London, UK: TSO (The Stationery Office). ISBN 978-0-215-01737-6. Retrieved 2007-12-17. 147. "Obesity: guidance on the prevention, identification, assessment and management of overweight and obesity in adults and children" (PDF). National Institute for Health and Clinical Excellence(NICE). National Health Services (NHS). 2006. Retrieved April 8, 2009. 148. Wanless, Sir Derek; Appleby, John; Harrison, Anthony; Patel, Darshan (2007). Our Future Health Secured? A review of NHS funding and performance. London, UK: The King's Fund. ISBN 1-85717-562-X. 149. Sacks G, Swinburn B, Lawrence M (January 2009). "Obesity Policy Action framework and analysis grids for a comprehensive policy approach to reducing obesity". Obes Rev 10 (1): 76–86. doi:10.1111/j.1467-789X.2008.00524.X. PMID 18761640. 150. Strychar I (January 2006). "Diet in the management of weight loss". CMAJ (Review) 174 (1): 56–63. doi:10.1503/cmaj.045037. PMC 1319349. PMID 16389240. 151. Shick SM, Wing RR, Klem ML, McGuire MT, Hill JO, Seagle H (April 1998). "Persons successful at long-term weight loss and maintenance continue to consume a low-energy, low-fat diet". J Am Diet Assoc 98 (4): 408–13. doi:10.1016/S0002-8223(98)00093-5. PMID 9550162. 152. Tate DF, Jeffery RW, Sherwood NE, Wing RR (1 April 2007). "Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain?". Am. J. Clin. Nutr. (Randomized Controlled Trial) 85 (4): 954–9. PMID 17413092. 153. Johnston, Bradley C.; Kanters, Steve; Bandayrel, Kristofer; Wu, Ping; Naji, Faysal; Siemieniuk, Reed A.; Ball, Geoff D. C.; Busse, Jason W.; Thorlund, Kristian; Guyatt, Gordon; Jansen, Jeroen P.; Mills, Edward J. (3 September 2014). "Comparison of Weight Loss Among Named Diet Programs in Overweight and Obese Adults". JAMA 312 (9): 923. doi:10.1001/jama.2014.10397. 154. Naude, CE; Schoonees, A; Senekal, M; Young, T; Garner, P; Volmink, J (2014). "Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis.". PLOS ONE (Research Support) 9 (7): e100652. doi:10.1371/journal.pone.0100652. PMID 25007189. 155. Wing RR, Phelan S (2005). "Long-term weight loss maintenance". The American Journal of Clinical Nutrition (Review) 82 (1 Suppl): 222S–225S. PMID 16002825. 156. Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, Kunz R, Mol BW, Coomarasamy A, Khan KS (16 May 2012). "Effects of interventions in pregnancy on maternal weight and obstetric outcomes: metaanalysis of randomised evidence". BMJ (Clinical research ed.) (Meta-analysis) 344: e2088. doi:10.1136/bmj.e2088. PMC 3355191. PMID 22596383. 157. LeFevre, Michael L. (26 August 2014). "Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults With Cardiovascular Risk Factors: U.S. Preventive Services Task Force Recommendation Statement". Annals of Internal Medicine 161: 587. doi:10.7326/M14-1796. 158. Yanovski SZ, Yanovski JA (Jan 1, 2014). "Long-term drug treatment for obesity: a systematic and clinical review.". JAMA: the Journal of the American Medical Association (Review) 311 (1): 74–86. doi:10.1001/jama.2013.281361. PMC 3928674. PMID 24231879. 159. Rucker D, Padwal R, Li SK, Curioni C, Lau DC (2007). "Long term pharmacotherapy for obesity and overweight: updated meta-analysis". BMJ (Meta-analysis) 335 (7631): 1194–99. doi:10.1136/bmj.39385.413113.25. PMC 2128668. PMID 18006966. 160. Wood, Shelley. "Diet Drug Orlistat Linked to Kidney, Pancreas Injuries". Medscape. Medscape News. Retrieved 26 April 2011. 161. Wolfe SM (21 August 2013). "When EMA and FDA decisions conflict: differences in patients or in regulation?". BMJ (Clinical research ed.) 347: f5140. doi:10.1136/bmj.f5140. PMID 23970394. 162. Bays HE (March 2011). "Lorcaserin: drug profile and illustrative model of the regulatory challenges of weight-loss drug development". Expert review of cardiovascular therapy (Review) 9 (3): 265–77. doi:10.1586/erc.10.22. PMID 21438803. 163. Bays HE, Gadde KM (December 2011). "Phentermine/topiramate for weight reduction and treatment of adverse metabolic consequences in obesity". Drugs Today (Review) 47 (12): 903–14. doi:10.1358/dot.2011.47.12.1718738. PMID 22348915. 164. Colquitt, JL; Pickett, K; Loveman, E; Frampton, GK (Aug 8, 2014). "Surgery for weight loss in adults". The Cochrane database of systematic reviews (Meta- analysis, Review) 8: CD003641. doi:10.1002/14651858.CD003641.pub4. PMID 25105982. 165. Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA (2014). "The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012". JAMA Surgery (Meta-analysis, Review) 149 (3): 275– 87. doi:10.1001/jamasurg.2013.3654. PMID 24352617. 166. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM (August 2007). "Effects of bariatric surgery on mortality in Swedish obese subjects". N. Engl. J. Med. (Research Support) 357 (8): 741–52. doi:10.1056/NEJMoa066254. PMID 17715408. 167. Weintraub, Karen. "New allies in war on weight". The Boston Globe. The Boston Globe. Retrieved 30 June 2014. 168. "Global Prevalence of Adult Obesity" (PDF). International Obesity Taskforce. Retrieved January 29, 2008. 169. Haslam D (March 2007). "Obesity: a medical history". Obes Rev (Review). 8 Suppl 1:31–6. doi:10.1111/j.1467-789X.2007.00314.x. PMID 17316298. 170. "Obesity and overweight". World Health Organization. Retrieved April 8, 2009. 171. Seidell 2005 p.5 172. Howard NJ, Taylor AW, Gill TK, Chittleborough CR (2008). "Severe obesity: Investigating the socio-demographics within the extremes of body mass index". Obesity Research & Clinical Practice 2 (1): I–II. doi:10.1016/j.orcp.2008.01.001. PMID 24351678. 173. Tjepkema M (2005-07-06). "Measured Obesity–Adult obesity in Canada: Measured height and weight". Nutrition: Findings from the Canadian Community Health Survey. Ottawa, Ontario: Statistics Canada. 174. "Online Etymology Dictionary: Obesity". Douglas Harper. Retrieved December 31, 2008. 175. "Obesity, n". Oxford English Dictionary 2008. Retrieved March 21, 2009. 176. Zachary Bloomgarden (2003). "Prevention of Obesity and Diabetes". Diabetes Care (Review) 26 (11): 3172–3178. doi:10.2337/diacare.26.11.3172. PMID 14578257. 177. "History of Medicine: Sushruta – the Clinician – Teacher par Excellence" (PDF). Dwivedi, Girish & Dwivedi, Shridhar. 2007. Retrieved 2008-09-19. 178. Theodore Mazzone; Giamila Fantuzzi (2006). Adipose Tissue And Adipokines in Health And Disease (Nutrition and Health). Totowa, NJ: Humana Press. p. 222. ISBN 1-58829-721-7. 179. Keller p. 49 180. Breslow L (September 1952). "Public Health Aspects of Weight Control". Am J Public Health Nations Health 42 (9): 1116–20. doi:10.2105/AJPH.42.9.1116. PMC 1526346. PMID 12976585. 181. Puhl R, Brownell KD (December 2001). "Bias, discrimination, and obesity". Obes. Res. (Review) 9 (12): 788–805. doi:10.1038/oby.2001.108. PMID 11743063. 182. Rubinstein S, Caballero B (2000). "Is Miss America an undernourished role model?". JAMA (Letter) 283 (12): 1569. doi:10.1001/jama.283.12.1569. PMID 10735392. 183. Johnson F, Cooke L, Croker H, Wardle J (2008). type = Comparative Study "Changing perceptions of weight in Great Britain: comparison of two population surveys" Check |url= value (help). BMJ 337: a494. doi:10.1136/bmj.a494. PMC 2500200. PMID 18617488. 184. Fumento, Michael (1997). The Fat of the Land: Our Health Crisis and How Overweight Americans Can Help Themselves. Penguin (Non-Classics). p. 126. ISBN 0-14-026144-3. 185. Puhl R., Henderson K., and Brownell K. 2005 p.29 186. Johansson E, Böckerman P, Kiiskinen U, Heliövaara M (2009). "Obesity and labour market success in Finland: The difference between having a high BMI and being fat". Economics and Human Biology 7 (1): 36–45. doi:10.1016/j.ehb.2009.01.008. PMID 19249259. 187. Cawley J, Meyerhoefer C (January 2012). "The medical care costs of obesity: An instrumental variables approach". Journal of Health Economics 31 (1): 219– 230. doi:10.1016/j.jhealeco.2011.10.003. PMID 22094013. 188. Finkelstein EA, Fiebelkorn IA, Wang G (1 January 2003). "National medical spending attributable to overweight and obesity: How much, and who's paying". Health Affairs. Online (May). doi:10.1377/hlthaff.w3.219. 189. "Obesity and overweight: Economic consequences". Centers for Disease Control and Prevention. 22 May 2007. Retrieved 2007-09-05. 190. Colagiuri S, Lee CM, Colagiuri R, Magliano D, Shaw JE, Zimmet PZ, Caterson ID (2010). "The cost of overweight and obesity in Australia". The Medical Journal of Australia (Comparative Study) 192 (5): 260–4. PMID 20201759. 191. Cummings, Laura (5 February 2003). "The diet business: Banking on failure". BBC News. Retrieved 25 February 2009. 192. van Baal PH, Polder JJ, de Wit GA, Hoogenveen RT, Feenstra TL, Boshuizen HC, Engelfriet PM, Brouwer WB (February 2008). "Lifetime Medical Costs of Obesity: Prevention No Cure for Increasing Health Expenditure". PLoS Med. (Comparative Study) 5 (2): e29. doi:10.1371/journal.pmed.0050029. PMC 2225430. PMID 18254654. 193. Bakewell J (2007). "Bariatric furniture: Considerations for use". Int J Ther Rehabil 14 (7): 329–33. 194. Neovius K, Johansson K, Kark M, Neovius M (January 2009). "Obesity status and sick leave: a systematic review". Obes Rev (Review) 10 (1): 17–27. doi:10.1111/j.1467-789X.2008.00521.x. PMID 18778315. 195. Ostbye T, Dement JM, Krause KM (2007). "Obesity and workers' compensation: Results from the Duke Health and Safety Surveillance System". Arch. Intern. Med. (Research Support) 167 (8): 766–73. doi:10.1001/archinte.167.8.766. PMID 17452538. 196. "Alabama "Obesity Penalty" Stirs Debate". Don Fernandez. Retrieved April 5, 2009. 197. Puhl R., Henderson K., and Brownell K. 2005 p.30 198. Lisa DiCarlo (2002-10-24). "Why Airlines Can't Cut The Fat". Forbes.com. Retrieved 2008-07-23. 199. Dannenberg AL, Burton DC, Jackson RJ (2004). "Economic and environmental costs of obesity: The impact on airlines". American journal of preventive medicine (Letter) 27 (3): 264. doi:10.1016/j.amepre.2004.06.004. PMID 15450642. 200. Lauren Cox (July 2, 2009). "Who Should Pay for Obese Health Care?". ABC News. Retrieved 2012-08-06. 201. "109th U.S. Congress (2005–2006) H.R. 554: 109th U.S. Congress (2005– 2006) H.R. 554: Personal Responsibility in Food Consumption Act of 2005". GovTrack.us. Retrieved 2008-07-24. 202. Basulto, Dominic (June 20, 2013). "A changing battlefield in the fight against fat". The Washington Post. Retrieved June 20, 2013. (WebCite archive) 203. "Obesity can be deemed a disability at work - EU court". Reuters. December 18, 2014. Retrieved December 18, 2014. 204. "What is NAAFA". National Association to Advance Fat Acceptance. Retrieved February 17, 2009. 205. "ISAA Mission Statement". International Size Acceptance Association. Retrieved February 17, 2009. 206. Pulver, Adam (2007). An Imperfect Fit: Obesity, Public Health, and Disability Anti-Discrimination Law. Social Science Electronic Publishing. Retrieved January 13, 2009. 207. Neumark-Sztainer D (March 1999). "The weight dilemma: a range of philosophical perspectives". Int. J. Obes. Relat. Metab. Disord. (Review). 23 Suppl 2: S31–7. doi:10.1038/sj.ijo.0800857. PMID 10340803. 208. National Association to Advance Fat Acceptance (2008). "We come in all sizes". NAAFA. Retrieved 2008-07-29. 209. "International Size Acceptance Association – ISAA". International Size Acceptance Association. Retrieved January 13, 2009. 210. Flynn MA, McNeil DA, Maloff B, Mutasingwa D, Wu M, Ford C, Tough SC (February 2006). "Reducing obesity and related chronic disease risk in children and youth: a synthesis of evidence with 'best practice' recommendations". Obes Rev (Review). 7 Suppl 1: 7–66. doi:10.1111/j.1467-789X.2006.00242.x. PMID 16371076. 211. Dollman J, Norton K, Norton L (December 2005). "Evidence for secular trends in children's physical activity behaviour". Br J Sports Med (Review) 39 (12): 892–7; discussion 897. doi:10.1136/bjsm.2004.016675. PMC 1725088. PMID 16306494. 212. Metcalf B, Henley W, Wilkin T (2012). "Effectiveness of intervention on physical activity of children: systematic review and meta-analysis of controlled trials with objectively measured outcomes (EarlyBird 54)". BMJ (Clinical Research Ed.) (Review, Meta-analysis) 345: e5888. doi:10.1136/bmj.e5888. PMID 23044984. 213. Lund Elizabeth M. (2006). "Prevalence and Risk Factors for Obesity in Adult Dogs from Private US Veterinary Practices" (PDF). Intern J Appl Res Vet Med 4 (2): 177–86. 214. McGreevy PD, Thomson PC, Pride C, Fawcett A, Grassi T, Jones B (May 2005). "Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved". Vet. Rec. 156 (22): 695–702. doi:10.1136/vr.156.22.695. PMID 15923551. 215. Nijland ML, Stam F, Seidell JC (June 2009). "Overweight in dogs, but not in cats, is related to overweight in their owners". Public Health Nutr 13 (1): 1–5. doi:10.1017/S136898000999022X. PMID 19545467. 216. Bhargava A, Guthrie JF (2002). "Unhealthy eating habits, physical exercise and macronutrient intakes are predictors of anthropometric indicators in the Women's Health Trial: Feasibility Study in Minority Populations". British Journal of Nutrition (Randomized Controlled Trial) 88 (6): 719–728. doi:10.1079/BJN2002739. PMID 12493094. 217. Bhargava A (2006). "Fiber intakes and anthropometric measures are predictors of circulating hormone, triglyceride, and cholesterol concentration in the Women's Health Trial". Journal of Nutrition (Research Support) 136 (8): 2249– 2254. PMID 16857849. 218. Jebb S. and Wells J. Measuring body composition in adults and children In:Peter G. Kopelman, Ian D. Caterson, Michael J. Stock, William H. Dietz (2005). Clinical obesity in adults and children: In Adults and Children. Blackwell Publishing. pp. 12–28. ISBN 1-4051-1672-2. 219. Kopelman P., Caterson I. An overview of obesity management In:Peter G. Kopelman, Ian D. Caterson, Michael J. Stock, William H. Dietz (2005). Clinical obesity in adults and children: In Adults and Children. Blackwell Publishing. pp. 319–326. ISBN 1-4051-1672-2. 220. National Heart, Lung, and Blood Institute (NHLBI) (1998). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (PDF). International Medical Publishing, Inc. ISBN 1-58808-002-1. 221. "Obesity: guidance on the prevention, identification, assessment and management of overweight and obesity in adults and children" (PDF). National Institute for Health and Clinical Excellence(NICE). National Health Services (NHS). 2006. Retrieved April 8, 2009. 222. Puhl R., Henderson K., and Brownell K. Social consequences of obesity In:Peter G. Kopelman, Ian D. Caterson, Michael J. Stock, William H. Dietz (2005). Clinical obesity in adults and children: In Adults and Children. Blackwell Publishing. pp. 29–45. ISBN 1-4051-1672-2. 223. Seidell JC. Epidemiology — definition and classification of obesity In:Peter G. Kopelman, Ian D. Caterson, Michael J. Stock, William H. Dietz (2005). Clinical obesity in adults and children: In Adults and Children. Blackwell Publishing. pp. 3–11. ISBN 1-4051-1672-2. 224. World Health Organization (WHO) (2000). Technical report series 894: Obesity: Preventing and managing the global epidemic. (PDF). Geneva: World Health Organization. ISBN 92-4-120894-5. 225. Effect of hydroxycitrate on fattyy acid synthesis by rat liver in vivo''.The journel of biological chemistry,vol 216,no.3,issue of feb 10,pp.629-632,1971. 226. ''Safety and mechanism of appetite suppression by a novel hydroxy citric acid extract HCA-SX '' by Ohia SE,Opere ca,Leday AM,Bgehi M,Stohs SJ, Mol QU Biochem,2002 sep,238,89-103,PMID:12349913. 227. ''A double blinded randomized clinical study with wt level.a combination of medicinal plants by Omair Said,Khaled Khalil,Stephen Fulder,Yousuf marie at the open complementery medicine journel,2012,2,pp:1-6. 228. Normal caffeine consumption influence on thermogenesis and daily energy expenditure in lean and postobase human volunteers",by AG Dullo,CA Geisser,T Horton,A Collins,DS Miller,The American Journel of Clinical Nutritions,USA,1989,pp:44-50. 229. HTTP://authority nutrition.com/12-weight loss pills-received/by Kris Gunnars,bsc,dec 2015. Raspberry ketone increases both lipolysis and fatty acid oxidation'',by Park KS,Planta Med 2010,oct ,pp:1654-58. 230. "Identification,expression and immunoreactivity of the first coffee allergen'', by Manavski N,Baur x,Bitner c,Int Arch Allergy Immunol,2012,159,pp:235-42. 231. ''Dietry fiber and energy regulation",by Britt Burton-Free Man,The American Society For Nutitional Sciences,online ISSN-1541-6100. 232. "Anti obesity mechanishm of action of conjugated linoleic acid", J Nutri Biochem,2010 march,vol 21{3},pp:171-179. 233. "The effects of forskolin on cAMP,cGMP and free fatty acid in diet induced obesity",by Doesyici S,Toker A,Biochem Histochem,,2014 jul,pp:388-92. 234. "Effect of p synephrine", by Stons SJ,Keith PL,Int J.M,2011 apr,pp:295-301. 235. "The Plant List". 236. Xi-wen Li, Jie Li & Henk van der Werff. "Cinnamomum cassia". Flora of China. Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA. Retrieved 28 March 2013. 237. "Cassia: A real spice or a fake cinnamon". China Business Limited as Regency. 238. "Cassia". theepicentre.com. 239. Wong, Ming (1976). La Médecine chinoise par les plantes. Le Corps a Vivre series. Éditions Tchou. 240. NPR: German Christmas Cookies Pose Health Danger 241. High daily intakes of cinnamon: Health risk cannot be ruled out. BfR Health Assessment No. 044/2006, 18 August 2006 15p. 242. Yao, Ying (Mar 13, 2015). "Cinnamic aldehyde treatment alleviates chronic unexpected stress-induced depressive-like behaviors via targeting cyclooxygenase2 in mid-aged rats". BIOSIS 162: 97–103. doi:10.1016/j.jep.2014.12.047. Retrieved Apr 27, 2015. 243. "Tropicos.org". Retrieved June 6, 2014. 244. "USDA GRIN Taxonomy". Retrieved 13 September 2014. 245. Sultanul Abedin And S.I. Ali. "Commiphora wightii". Flora of Pakistan 26. 246. Indian herb can reduce cholesterol, BBC NEWS, 2 May 2002 247. Murray (2012). Joseph E. Pizzorno, Jr., Michael T., ed. Textbook of natural medicine (4th ed.). Edinburgh: Churchill Livingstone. p. 691. ISBN 9781437723335. 248. Szapary, PO; Wolfe, ML; Bloedon, LT; Cucchiara, AJ; Dermarderosian, AH; Cirigliano, MD; Rader, DJ (2003). "Guggulipid Ineffective for Lowering Cholesterol". JAMA 290 (6): 765–772. doi:10.1001/jama.290.6.765. PMID 12915429. 249. Sahni, S; Hepfinger, CA; Sauer, KA (2005). "Guggulipid Use in Hyperlipidemia". Am J Health-Syst Pharm 62 (16): 1690–1692. doi:10.2146/ajhp040580. PMID 16085931. 250. "Commiphora wightii". IUCN Red List of Threatened Species. Retrieved 12 January 2012. 251. Maheshwari, D V (8 January 2008). "Kutch to house Centre’s Rs 8-cr Guggal conservation project". The Indian Express. 252. Paliwal, Ankur (31 July 2010). "Guggal faces sticky end". Down to Earth: Science and Environment Online. Retrieved 12 January 2012. 253. "Education and Awareness in the 'Save Guggul Movement'". IUCN News. 31 July 2010. Retrived jan 2012. 254. "Monographs of unani medicine",vol 1,by drug control and tradational medicinal division,National Institute of Health,ISBD,PP:411-412,485-486,514-515. 255. "Flora of west Pakistan",no 172,pp:22-23,35-36. 256. DebJC and Chandrasekhara N,"FOOD SCI"mysore 9,1960,pp:306-7. 257. Iftikhar.A.Muhammad,KBK and Muhammad,west regional labs lahore,PAKISTAN,Pak.j.sci,1960,research 12,pp:71-73. 258. Rajeranna rao.Mr and Siddiqui HH,Indian.J.Exp.Biol,2,1964,pp:29-31. 259. Das,Phatic Chandra Brit,1976,pp:445-559. 260. Rani.G.Bala,Saroj and Grover,J.Plant Sciences,1994,pp:1-4. 261. Hu,Titanxi,Cao,Meizhen,Chen and Jiwu,Nanyuan yong fang developement co,ltd,1994,vol 86,pp:532 262. Sagkaguchi,Noboru,Theertham.p,Jpn,Kokai,Tokkyo koho JP,2006,28,91. 263. Sharma,SK,Chandra,R,Arora,SK and Joshi,Journel of verteniary pharmacology and toxicology,5,pp:22-25. 264. Agarwal,Ravindr kumarand Amit,Indian herbs research and pvt ltd,app 1,996 de 02,941,2005. 265. The Ayurvedic Pharmacopeia of India,vol 1,pp:43,113. 266. "Tropicos.org". Retrieved 13 September 2014. 267. Altervista Flora Italiana, genere Ferula 268. Flora of Pakistan, Ferula Linn 269. Plants for a Future, Ferula assa-foetida L., Asafoetida - Devil's Dung. Hing 270. Graham White, National Institute of Medical Herbalists, Ferula hermonis "The Lebanese Viagra" by Stuart FitzSimmons 271. Nabavi SM. Ebrahimzadeh MA. Nabavi SE. Eslami B. Dehpour AA (2011). "Antioxidant and antihaemolytic activities of Ferula foetida regel (Umbelliferae)". European Review for Medical & Pharmacological Sciences 15 (2): 157–64. 272. Ackerknecht EH (1973) Therapeutics: from the Primitives to the Twentieth Century. Hafner Press, New York. 273. AOAC (2005). Official Methods of Analysis of AOAC International, 18th edn. AOAC International, Gaithersburg, MD. 274. Barnes J, Anderson LA, Phillipson JD (2007). Herbal medicine. 3rd Edition, Pharmaceutical Press, London. pp 1-23. 275. Bauer R (1998). Quality criteria and standardization of phytopharmaceuticals: Can acceptable drug standards be achieved? Drug Inform. J., 32: 101–110. 276. Bisset NG (1994). Herbal Drugs and Phytopharmaceuticals. CRC Press, Boca Raton, FL. 277. Blumenthal M (2000) Ephedra update: industry coalition asks FDA to adopt national labeling guidelines on ephedra; offers co-operative research with NIH. Herbal Gram., 50: 64-65. 278. Blumenthal M, Brusse WR, Goldberg A, Gruenwald J, Hall T, Riggins CW, Rister RS (1998). The Complete German Commission E Monographs. Therapeutic Guide to Herbal Medicines. The American Botanical Council, Austin, TX. 279. Bodeker C, Bodeker G, Ong CK, Grundy CK, Burford G, Shein K (2005). WHO Global Atlas of Traditional, Complementary and Alternative Medicine. World Health Organization, Geneva. 280. De Smet PAGM (2004). Health risks of herbal remedies: An update. Clinical Pharmacology Therapeutics, 76(1): 1–17. 281. De Smet, PAGM (1995). Health risks of herbal remedies. Drug Safety, 13: 81-93. 282. De Smet PAGM, Keller K, Hansel R, Chandler RF (1992). Aristolochia species In: Adverse Effects of Herbal Drugs, Springer-Verlag, Heidelberg. 1. 283. De Smet PAGM, Keller K, Hansel R, Chandler RF (1997). Adverse Effects of Herbal Drugs, (eds), Springer-Verlag, Heidelberg. 129(3): 137145. 284. De Smet PAGM (1999). Overview of herbal quality control. Drug Inform. J., 33: 717-724. 285. Duke JA, Martinez RV (1994). Handbook of Ethnobotanicals (Peru). CRC Press, Boca Raton, FL. 286. EMEA (1998). Quality of Herbal Medicinal Products. Guidelines. European Agency for the Evaluation of Medicinal Products (EMEA), London. 287. EMEA (2002). Points to Consider on Good Agricultural and Collection Practice for Starting Materials of Herbal Origin. 288. EMEA/HMPWP/31/99 Review. European Agency for the Evaluation of Medicinal Products (EMEA), London. 289. EMEA (2005). Guidelines on Quality of Herbal Medicinal Products/Traditional Medicinal Products, EMEA/CVMP/814OO Review. European Agency for the Evaluation of Medicinal Products (EMEA), London. 290. Eskinazi D, Blumenthal M, Farnsworth N, Riggins CW (1999). Botanical Medicine: Efficacy, Quality, Assurance, and Regulation. Mary Ann Liebert, New York. 291. Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z (1985). World Health Organ., 63: 965. 292. Gaedcke F, Steinhoff B (2003) Quality assurance of herbal medicinal products. In: Herbal Medicinal Products. Stuttgart: medpharm GmbH Scientific Publishers. pp. 37-66, 81-88. 293. Houghton P (1998). Establishing identification criteria for botanicals. Drug Inform. J., 32: 461-469. 294. Majno GM (1975). Healing Hand: Man and Wound in the Ancient World. Harvard University Press, Cambridge, MA. 295. Mukherjee PW (2002). Quality Control of Herbal Drugs: An Approach to Evaluation of Botanicals. Business Horizons Publishers, New Delhi, India. 296. Phillipson JD (1993). Quality assurance of medicinal plants. In: Franz C et al. First World Congress on medicinal and aromatic plants for human welfare, WOCMAP, quality, phytochemistry, industrial aspects, economic aspects. Acta Horticult., 333: 117-122. 297. Roberts JE, Tyler VE (1997). Tyler’s Herbs of Choice. The Therapeutic Use of Phytomedicinals. The Haworth Press, New York. 298. Solecki RS (1975). Standardized product as well as the quality of the consumer information on the herbal remedy. hanidar IV. Science, 190: 880. 299.2 Int. J. Biodvers. Conserv. 300. (2007). Herbal medicine and its standardization. Pharma. info., 1: 6. 301.Watson DG (1999). Pharmaceutical Analysis. Churchill Livingstone, Edinburgh. 302.WHO (1979). The International Pharmacopeia, General Methods of Analysis, 3rd edn. World Health Organization, Geneva. 1. 303.WHO (1988a). The International Pharmacopeia,Quality Specifications for Pharmaceutical Substances, Excipients, and Dosage Forms, 3rd edn. World Health Organization, Geneva. 3. 304.WHO (1988b). Quality Control Methods for Medicinal Plant Materials. World Health Organization, Geneva. 305 WHO (1990). The Use of Essential Drugs. Eighth report of the WHO Expert committee. World Health Organization, Geneva. 306 WHO (1992). Quality Control Methods for Medicinal Plant Materials. World Health Organization, Geneva. 307 WHO (1996a). Quality Assurance of Pharmaceuticals: A Compendum of Guidelines and Related Materials, Good Manufacturing Practices and Inspection. World Health Organization, Geneva. 2. 308 WHO (1996b). Guidelines for the Assessment of Herbal Medicines. WHO Technical Report Series, World Health Organization, Geneva. 863. 309.WHO (1998a). Quality Control Methods for Medicinal Plant Materials, World Health Organization, Geneva. 310. WHO (1998b). Guidelines for the Appropriate Use of Herbal Medicines. WHO Regional Publications, Western Pacific Series. WHO Regional office for the Western Pacific, Manila. 3. 311. WHO (1998c). Basic Tests for Drugs, Pharmaceutical Substances, Medicinal Plant Materials and Dosage Forms. World Health Organization, Geneva. 312. WHO (1999a). Quality Control Methods for Medicinal Plant Materials. World Health Organization, Geneva. 313. WHO (1999b). WHO Monographs on Selected Medicinal Plants, World Health Organization, Geneva. 1. 314. WHO (2000). General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. World Health Organization, Geneva . 315. WHO (2000). The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2000–2002 (WHO/PCS/01.5). International Programme on Chemical Safety, World Health Organization, Geneva. 316. WHO (2002a). WHO Monographs on Selected Medicinal Plants, World Health Organization, Geneva. 2. 317. WHO (2002b). Ancient Remedies, New Disease. World Health Organization, Geneva. 318. WHO (2002c). General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. World Health Organization, Geneva. 319. WHO (2003). WHO Guidelines on Good Agricultural and Collection Practices (GACP). World Health Organization, Geneva. 320. WHO (2004). Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants. World Health Organization, Geneva. 321. WHO (2005). WHO Global Atlas of Traditional, Complementary and Alternative Medicine. World Health Organization, Geneva. 1and 2. 322. U.S. Preventive Services Task Force. Screening for an dmanagement of obesity in adults. Ann Intern Med. 2012;157(5):373-378. 323. . American Academy of Family Physicians. Obesity in adults (screening for and management). www.aafp.org/online/en/home/clinical/exam/obesity.html. Accessed Jan. 20, 2013. 324. Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. Asilent response to the obesity epidemic: Decline in U.S. physicianweight counseling. Med Care. 2013;51(2):186-192. 325. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence 330. report. Bethesda, Md.: National Heart, Lung and BloodInstitute, 1998; NIH 98-4083. 326. Smith AW, Borowski LA, Liu B, et al. U.S. primary carephysicians’ diet-, physical activity-, and weight-related careof adult patients. Am J Prev Med. 2011;41(1):33-42. 327. Potter MB, Vu JD, Croughan-Minihane M. Weight management:What patients want from their primary care physicians.J Fam Pract. 2001;50(6):513518 328. Sesselberg TS, Klein JD, O’Connor KG, et al. Screening and counseling for childhood obesity: Results from a nationalsurvey. J Am Board Fam Med. 2010;23(3):334-342 . . 329. . Bleich SN, Bennett WL, Gudzune KA, et al. Impact of physician BMI on obesity care and beliefs. Obesity (SilverSpring). 2012;20(5):999-1005. 330. Post RE, Mainous AG 3rd, Gregorie SH, et al. The influence of physician acknowledgment of patients’ weight statuson patient perceptions of overweight and obesity in the United States. Arch Intern Med.2011;171(4):316321. 331. Gaglioti A, Petterson SM, Bazemore AW, et al. Primarycare’s ecologic impact on obesity. Am Fam Physician. 2009;79(6):446. . 332. . Centers for Disease Control and Prevention. Obesity andoverweight for professionals: Adult obesity facts. www.cdcgov/obesity/data/adult.html. Accessed Jan. 22, 2013 333. . Centers for Disease Control and Prevention. Obesity andoverweight for professionals: Childhood data. www.cdc.gov/obesity/data/childhood.html. Accessed Jan. 22, 2013. 334. Pan L, Blanck HM, Sherry B, et al. Trends in the prevalence of extreme obesity among U.S. preschool-agedchildren living in low-income families, 19982010. JAMA.2012308(24):2563-2565 335. . Centers for Disease Control and Prevention. Obesity andoverweight for professionals: Adult causes. www.cdc.gov/obesity/adult/causes/index.html. Accessed Jan. 22, 2013 336. Karasu SR. Of mind and matter: Psychological dimensionsin obesity. Am J Psychother. 2012;66(2):111-128. 337. . Goacher PJ, Lambert R, Moffatt PG. Can weight-relatedhealth risk be more accurately assessed by BMI, or by genderSpecific calculations of percentage body fatness? MedHypotheses. 2012;79(5):656-662. 338. Romero-Corral A, Somers VK, Sierra-Johnson J, et al.Accuracy of body mass index in diagnosing obesity in theadult general population. Int J Obes (Lond). 2008;32(6): 356. 959-966. 339. Institute for Clinical Systems Improvement. Preventionand management of obesity (mature adolescents andadults). Bloomington, Minn.: Institute for Clinical SystemsImprovement, 2011 340. Ghandehari H, Le V, Kamal-Bahl S, et al. Abdominalobesity and the spectrum of global cardiometabolic risks inU.S. adults. Int J Obes (Lond). 2009;33(2):239-248. 341. . Dunkley AJ, et al. Waist circumference measurement:Knowledge, attitudes and barriers in patients and practitionersin a multi-ethnic population. Fam Pract. 2009;26(5):365-371 342. . Bray GA. Screening for and clinical evaluation of obesity inadults.www.UpToDate.com. Accessed Jan. 30, 2013. 343. Centers for Disease Control and Prevention. Healthyweight: Assessing your weight www.cdc.gov/healthyweight/assessing/index.html. Accessed Feb. 3, 2013. 344. Bosy-Westphal A, Booke CA, Blocker T, et al. Measurementsite for waist circumference affects its accuracy as anindex of visceral and abdominal subcutaneous fat in a Caucasianpopulation. J Nutr. 2010;140(5):954-961. 345. . E rvin RB. Prevalence of metabolic syndrome among adults20 years of age and over, by sex, age, race and ethnicity, andbody mass index: United States, 2003–2006. Natl HealthStat Report. 2009 May 5;(13):1-7. 346. . Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis andmanagement of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientificstatement. Circulation. 2005;112(17):2735-2752. 347. Armstrong C. AHA and NHLBI review diagnosis andmanagement of the metabolic syndrome. Am Fam Physician.2006;74(6):1039-1047. 348. Kolasa KM, Collier DN, Cable K. Weight loss strategiesthat really work. J Fam Pract. 2010;59(7):378-385. 349. . Diabetes Prevention Program (DPP). National DiabetesInformation Clearinghouse. diabetes.niddk.nih.gov/dm/pubs/preventionprogram. Accessed Feb. 12, 2013. 350. . Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction metformin. N Engl J Med. in the incidence of type 2 diabetes with lifestyle interventionor2002;346(6):393-403. 351. . Bray GA. Behavioral strategies in the treatment of obesity.www.UpToDate.com. Accessed Jan. 15, 2013. 352. LeBlanc ES, O’Connor E, Whitlock EP,et al. Effectivenessof primary carerelevant treatments for obesity in adults: ATask Force. Ann Intern Med. 2011;15(7)5:434-447. 353. Rao G. Office-based strategies for the management of obesity.Am Fam Physician. 2010;81(12):1449-1456. 354. Poston WS, Foreyt JP. Successful management of the obesepatient. Am Fam Physician. 2000;61(12):3615-3622. 355. . Searight HR. Realistic approaches to counseling in theoffice setting. Am Fam Physician. 2009;79(4):277-284. 356. Pollak KI, Alexander SC, Tulsky JA, et al. Physician empathyand listening: Associations with patient satisfaction and autonomy. J Am Board Fam Med. 2011;24(6):665-672. 357. Teixeira PJ, et al. Motivation, self-determination, and longtermweight control. Int J 358. Behav Nutr Phys Act. 2012 Mar 2;9:22 Shick SM, Wing RR, Klem ML, et al. Persons successfulat long-term weight loss and maintenance continue toconsume a low-calorie, low-fat diet. J Am Diet Assoc. 1998;98(4):408-413 359. Klem ML, Wing RR, McGuire MT, et al. A descriptivestudy of individuals successful at long-term maintenance ofsubstantial weight loss. Am J Clin Nutr. 1997;66(2):239-246. 360. Wadden TA, Volger S, Sarwer DB, et al. A two-year randomizedtrial of obesity treatment in primary care practice.N Engl J Med. 2011;365(21):1969-1979. 361. Department of Health and Human Services. DietaryGuidelines for Americans 2010. www.cnpp.usda.gov/DGAs2010-PolicyDocument.htm. Accessed Feb. 13, 2013. 362. Casazza K, Fonatine KR, Astrup A, et al. Myths, presumptions,and facts about obesity. N Engl J Med. 2013;368(5): 446-454. . 363. Heymsfield SB, van Mierlo CA, van der Knaap HC, et al.Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes RelatMetab Disord. 2003;27(5):537-549. 364. Haas WC, Moore JB, Kaplan M, et al. Outcomes from amedical weight loss program: Primary care clinics versusweight loss clinics. Am J Med. 2012;125(6):603-611. 365. Sacks FM, Bray GA, Carey VJ, et al. Comparison ofweight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859-873. 366. Hall KD, Sacks G, Chandramohan D, et al. Quantificationof the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826-837. 367. Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes:A systematic review and meta-analysis of weight-loss(10):175clinical trials with a minimum 1-year follow-up. J Am DietAssoc. 2007;1075-1767. 368. Bray GA. Dietary therapy for obesity www.UpToDate.com.eAccessd Jan. 15, 2013. 369. Leibel RL, Rosenbaum M, Hirsch J, et al. Changes inenergy expenditure resulting from altered body weight.N Engl J Med. 1995;332(10):621-628. 370. Hemmingsson E, Johansson K, Eriksson J, et al. Weightloss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, orrestricted normal food: Observational cohort study. AmJ Clin Nutr. 2012;96(5):953-961. 371. . Heshka S, Anderson JW, Atkinson RL, et al. Weight losswith self-help compared with a structured commercial program: A randomized trial. JAMA. 2003;289(14):1792-1798. 372. . Rock CL, Flatt SW, Sherwood NE, et al. Effect of a freeprepared meal and incentivized weight loss program onweight loss and weight loss maintenance in obese andoverweight women: A randomized controlled trial. JAMA. 2010;304(16):1803-1810. 373. Tsai AG, Wadden TA. Systematic review: An evaluationof major commercial weight loss programs in the United States. Ann Intern Med. 2005;142(1):56-66. 374. . Department of Health and Human Services. 2008 PhysicalActivity Guidelines for Americans. www.health.gov/PAGUIDELINES/guidelines/default.aspx. Accessed Feb.13, 2013. 375. . Bray GA. Role of physical activity and exercise in obesity.www.UpToDate.com. Accessed Feb. 13, 2013. 376. Coleman KJ, Ngor E, Reynolds K, et al. Initial validationof an exercise ‘vital sign’ in electronic medical records. Med Sci Sports Exerc. 2012;44(11):2071-2076. 377. Laskowski ER. The role of exercise in the treatment of obesity.of an exercise ‘vital sign’ in electronic medical records. MedPM R. 2012;4(11):840-844. 378. Ferrari CKB. Metabolic syndrome and obesity: Epidemiologyand prevention by physical activity and exercise. J Exer Sci Fit. 2008;6(2):87-96. 379. National Institute of Diabetes and Digestive and KidneyDiseases. Prescription medications for the treatment of obesity. win.niddk.nih.gov/publications/prescription.htm#meds. Accessed July 18, 2012. 380. . Bray GA. Drug therapy of obesity. www.UpToDate.com.Accessed Jan. 15, 2013. 381. Taylor JR, Dietrich E, Powell JG. New and emerging pharmacologictherapies for type 2 diabetes, dyslipidemia, and obesity. Clin Ther. 2013;35:A3-17. 382. Carter R, Mouralidarane A, Ray S, et al. Recent advancementsin drug treatment of obesity. Clin Med. 2012; 12(5):456-460. 383. Ioannides-Demos LL, Piccenna L, McNeil JJ. Pharmacotherapiesfor obesity: Past, current, and future therapies. J Obes. 2011;2011:179674. Epub 2010 Dec 12. 384. Wilbert B, Mohundro BL, Shaw V, et al. Appetite suppressantsas adjuncts for weight loss. Am Fam Physician. 2011; 83(7):1-2. 385. Orlistat (Xenical) prescribing information. San Francisco,Calif.: Genentech, Inc., 2012. 386. E pocrates Online. www.Epocrates.com. Accessed Feb. 25,2013. 387. Lorcaserin (Belviq) prescribing information. WoodcliffLake, N.J.: Arena Pharmaceuticals, 2012. 388. U.S. Food and Drug Administration. Consumer updates:Medication target long-term weight control. www.fda.gov/ForConsumers/ConsumerUpdates/ucm312380.htm.Accessed Feb. 13, 2013 . 389. Arena Pharmaceuticals provides corporate updateand reviews fourth quarter and full year 2012 financial results. invest.arenapharm.com/releasedetail.cfm?ReleaseID=744786. Accessed March 7, 2013. 390. Federal Register. Schedules of Controlled Substances:Placement of Lorcaserin into Schedule IV. federalregister. gov/a/2012-30531. Accessed March 7, 2013. 391. Phentermine and topiramate extended-release (Qsymia)prescribing information.Mountain View, Calif.: Vivus, Inc., 2012.. 392. U.S. Food and Drug Administration. FDA approvesweightmanagementQsymiwww.fda.gov/NewsEvents/Newsroom/PressAnnouncements/uc m312468.htm.Accessed Feb. 14, 2013. 393. Andrews RA, Lim RB. Surgical management of severe obesity.Accessed Feb. 13, 2013. 394. Sjostrom L, Narbro K, Sjostrom D, et al. Effects of bariatricsurgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741-752. 395. Adams TD, Gress RE, Smith SC, et al. Long-term mortalityafter gastric bypass surgery. N Engl J Med. 2007;357(8): 753-761. 396. . SAGES Guidelines Committee. SAGES guideline for clinicalapplication of laparoscopic bariatric surgery. Surg Obes Relat Dis. 2009;5(3):387-405. 397. . DeMaria EJ. Bariatric surgery for morbid obesity. N Engl JMed. 2007;356(21):2176-2183. 398. O’Brien PE, MacDonald L, Anderson M, et al. Long-termoutcomes after bariatric surgery: Fifteen-year follow-up ofadjustable gastric banding and a systematic review of thebariatric surgical literature. Ann Surg. 2013;257(1):87-94. 399. Vest AR, Heneghan HM, Aqarwal S, et al. Bariatric surgeryand cardiovascular outcomes: A systematic review. Heart. 2012;98(24):1763-1777. 400. Buchwald H, Estok R, Fahrbach K, et al. Weight and type2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med. 2009;122(3):248-256. 401. Sjostrom L. Review of the key results from the SwedishObese Subjects (SOS) trial: A prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219-234. 402. . Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity.Cochrane Database Syst Rev. 2009;2:CD003641. . 403. Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastricbanding and conventional therapy for type 2 diabetes: A randomized controlled trial. JAMA. 2008;299(3):316-323. 404. Flum DR, Belle SH, King WC, et al. Perioperative safety inthe longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445-454. 405. . de Zwaan M, Hilbert A, Swan-Kremeier, et al. Comprehensiveinterview assessment of eating behavior 18-35 months after gastric bypass surgery for morbid obesity. Surg ObesRelat Dis. 2010;6(1):79-85. 406. Sarwer DB, Dilks RJ, West-Smith L. Dietary intake andeating behavior after bariatric surgery: Threats to weight loss maintenance and strategies for success. Surg Obes RelatDis. 2011;7(5):644-651. 407. U.S. Preventive Services Task Force. Screening for obesityin children and adolescents: Recommendation statement Am Fam Physician. 2011;83(6):739-740. 408. Waters E, de Silva-Sanigorski A, Hall BJ, et al. Interventionsfor preventing obesity in children. Cochrane Database Syst Rev. 2011;12:CD001871. 409. Centers for Disease Control and Prevention. Basics aboutchildhood obesity www.cdc.gov/obesity/childhood/basics.html. Accessed Feb. 15, 2013. 410. American Academy of Pediatrics. Prevention of pediatricoverweight and obesity. Pediatrics. 2003;112(2):424-430. 411. American Academy of Pediatrics. Healthy Active LivingFor Families (HALF). www2.aap.org/obesity/HALF.html. Accessed Feb. 14, 2013. 412. American Academy of Pediatrics. Media use by childrenyounger than 2 years. Pediatrics.2011;128(5);1040-1045. 413. American Academy of Family Physicians joins First LadyObama’s effort to end childhood obesity. www.aafp.org/ online/en/home/media/releases/2010b/lets-movchilhoodobesity.html. Accessed Feb.14,2013. 414. Bambs C, Reis SE. Embracing primordial prevention forideal cardiovascular health. Future Cardiol. 2011;7(4):447-450. 415. . Weintraub WS, Daniels SR, Burke LE, et al. Value of primordialand primary prevention for cardiovascular disease: and primary prevention for cardiovascular disease:A policy statement from the American Heart Association.Circulation. 2011;124(8):967-990. 416. . Institute of Medicine. Accelerating progress in obesity prevention:Solving the weight of the nation. Washington, D.C.: National Academies Press, 2012.24 Diagnosis and Management of Obesity 417.Miller Keone Encyclopedia and Dictionary of Medicine,7th Edition 2003 by Saunders. 418.Mobsby Medical Dictionay, 9TH Edition 2009@Elsevier. 419.Herbs for Obesity@Histolic online.com, wt control info center 2013 ICBS.Inc. 420.AMDA The Society for Post-Acute and Long-Term Care Medicine (February 2014), "Ten Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation (AMDA – The Society for Post-Acute and LongTerm Care Medicine), retrieved 20 April 2015. 421.Pollack, Andrew (29 January 2013) F.D.A. Approves Genetic Drug to Treat Rare Disease The New York Times, Retrieved 31 January 2013. 422.Staff (29 January 2013) FDA approves new orphan drug Kynamro to treat inherited cholesterol disorder U.S. Food and Drug Administration, Retrieved 31 January 2013. 423. Koren MJ, Scott R, Kim JB et al Lancet 2012; 380:1995-2006. 424.Gugliano RP, Desai NR, Kohli P et al Lancet 2012; 380:2007-17. 425.Antonio M Gotto,jr,MD Mphil,Omega 3 and Other Novel Pharmacological Approches in Management of Hyperlipidemia@Multidisciplinary 1994-2016.