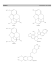PERIPHERAL MECHANISMS OF INTESTINAL DYSMOTILITY IN
Transcription
PERIPHERAL MECHANISMS OF INTESTINAL DYSMOTILITY IN
JOURNAL OF PHYSIOLOGY AND PHARMACOLOGY 2006, 57, 1, 7382 www.jpp.krakow.pl T. BANACH, D. ¯UROWSKI, K. GIL, N.W. WEISBRODT1, G. ROSENFELD1, P.J. THOR PERIPHERAL MECHANISMS OF INTESTINAL DYSMOTILITY IN THE MORPHINE TOLERANT AND DEPENDENT RATS 1 Dept. of Pathophysiology, Jagiellonian University Medical College, Cracow, Poland Dept. of Integrative Biology and Pharmacology, University of Texas Medical School, Houston, USA Changes of intestinal motility and transit produced by tolerance to and dependence upon morphine have been partly attributed to peripheral mechanisms. We evaluated the effect of chronic peripheral morphine administration and peripheral µ-receptor blockade on vagal afferent activity (VAA) and c-Kit positive intramuscular cells of Cajal (ICCs). Ten rats were subjected to chronic subcutaneous morphine infusion for 72 h with subsequent VAA recording. Potential frequency was evaluated within recordings before and after µ receptor blockade by D-Phe -Cys -Tyr -D-Trp -Orn -Thr -Phe -Thr (CTOP) i.p. injections. Afterwards the rats were sacrificed and intramuscular c-Kit antigen expression was assessed by image analysis within removed fragments of duodenum and ascending colon. An equal group of rats served as a control for VAA and c-Kit expression. Analysis of VAA revealed similar frequencies of potentials in morphine tolerant / dependent rats before CTOP and in the controls. CTOP increased potential frequency in the morphine group which effect was visible mostly within the first 20 minutes (p=0.01). The morphine infused animals presented also higher c-Kit expression in both the duodenum (p<0.001) and the ascending colon (p<0.001) in comparison to the control group. Results of our study may indicate the involment of both the intestinal wall and the long vago-vagal reflexes in tolerance to and dependence upon opioids. K e y w o r d s : c-Kit antigen, morphine dependence, morphine tolerance, vagal afferent activity INTRODUCTION Opioids are the principal agents in treatment of severe acute and chronic pain. Their antinociceptive effect is produced by activation of the µ receptors located 74 not only in the central nervous system (CNS) but also in the peripheral tissues (1). Unfortunately gastrointestinal GI dysmotility and constipation are one of main side effects of the opioid therapy. Several studies have supported the hypothesis of involvement of both the CNS and the enteric nervous system (ENS) receptors in the attenuation of gastrointestinal (GI) myoelectrical activity, motility and transit by morphine (2, 3, 4, 5). Therefore the central and peripheral receptors have been also suspected in development of the intestinal tolerance and dependence. Both phenomena have been frequently reported as the effects of chronic administration of morphine. GI tolerance has been defined as the decrease overtime of the motility response to the chronically administered drug whereas dependence was assessed by the generation of characteristic withdrawal syndrome due to interruption of the medication. In the experimental studies use of suitable µ receptor antagonists develops the clinical syndrome of withdrawal with increase of motility and intense diarrhoeas. Previous studies on morphine revealed mutual dependence of both phenomena based on the mechanisms of intracellular adaptation to the administered drug including the receptor quantitative and structural changes, second messengers changes and alterations of neuronal membrane potential (6). In the current study we have been interested in the involvement of peripheral ENS µ2 receptors in mechanisms of the intestinal tolerance to and dependence upon morphine. We have hypothesised that if the peripheral receptors are involved the development of tolerance / dependence would produce changes in both the intestinal wall and the long vago-vagal reflex activity. Our purpose was the evaluation of the influence of chronic, peripheral administration of morphine and peripheral injections of µ-receptor antagonist D-Phe -Cys -Tyr -D-Trp -Orn Thr -Phe -Thr (CTOP) on tyrosine kinase receptor (c-Kit antigen) and vagal afferent activity (VAA). According to the previous studies we have presumed that intramuscular c-Kit expression could reflect activity of the interstitial cells of Cajal (ICCs), which generate the intestinal base electrical rhythm (BER) (7, 8). Peripheral administration of CTOP, the agent that doesn't cross the blood-brain barrier revealed the peripheral mechanisms of the induced morphine withdrawal. MATERIAL AND METHODS Animals Twenty male Wistars rats, weighting 200 g were used in our experiments. During the study animals were housed in single cages in the temperature and humidity controlled room with 12-hour light-dark cycle (light on at 7 a.m.). Food and water were available ad libitum. The rats were divided into 2 equal group. One group was continuously infused with morphine for 72 h, for the development of tolerance and dependence, whereas the other served as controls. All animals underwent the recordings of VAA in left vagus nerve and the assessment of c-Kit antigen expression in duodenum and ascending colon. All experimental protocols were approved by Local Bioethical Committee of the Polish Board of Scientific Investigations in Cracow. 75 Surgery procedures Placement of osmotic minipumps For the procedure the animals were anaesthetised by intraperitoneal (i.p.) injections of ketamine - Ketamina 10% (Biowet, Poland), 100 mg/kg and xylasine - Sedazin (Biowet, Poland), 2 mg/kg. No information about the prolonged influence of the drugs applied on the GI motility has been available so far. The osmotic minipumps (Alzet, model 2001, Durect Corporation, USA) were filled with 200 µl of morphine solution and then placed subcutaneously (s.c.) in the lumbar region of the animal's back. The procedure was performed to produce 72 h of the continuous s.c. administration of morphine, 1 mg/kg/h The dose was chosen to establish blood concentration of morphine that resulted in decrease of the intestinal transit by 50% (9). Placement of electrodes on left vagus nerve The procedure was performed in the rats under the ketamine / xylasine anaesthesia after 72 h of continuous morphine administration. During the surgery and the subsequent recordings a thermostatically controlled heating pad maintained rectal temperature at 37 ± 1 °C. The left vagus nerve was uncovered on the animal neck, isolated from the cervical artery and cut possibly proximally for the access to the distal nerve trunk of 1.5 cm length. The cuff electrode consisted of the silver wire of diameter 75 µm (A-M Systems, Carlsborg, USA) and the elastic tube 1,5 mm of diameter and 1,5 cm of length was installed in the distal end of the nerve trunk accordingly to the data previously published (10). In order to avoid postoperative damage response the latency period of 15 min before the commencement of the VAA recording was applied. Experimental procedures VAA evaluation VAA in the left vagus nerve was recorded under the ketamine / xylasine anaesthesia subsequently to the placement of the cuff electrode. Both groups of animals the morphine and the control were subjected to the 60-min VAA recordings. In the morphine group initial 30 min of fasting recording was followed by i.p. CTOP (Sigma, USA) injection (1 mg/kg) and another 30 min of recording. Use of CTOP, specific µ receptor antagonist, allowed evoking the morphine withdrawal syndrome (11). The potentials were amplified by the BIO Amp (ADInstruments, Australia) amplifier and analysed spectrally using the Spike Histogram (ADInstruments) software. The recordings obtained were divided into 10-min intervals. Four subsequent 10-min intervals, one before and three after the CTOP injection were subjected to the analysis, which allowed for better exploration of vagal response to CTOP. Frequencies of the potentials were compared between the controls and morphine animals before CTOP injections for evaluation of the tolerance. Dependence upon morphine was evaluated by comparison of VAA frequency before and after CTOP. After the procedure animals were killed by overdosage of the anaesthetics. Fragments of the duodenum and the ascending colon were removed for intramuscular c-Kit assessment. Intramuscular c-Kit assessment The intestinal fragments removed were fixed with alcohol and immersed with paraffin. The 45 µm thick slices prepared, containing longitudinal sections of the bowel wall, were deparaffinated and marked using the rabbit monoclonal antibodies anty-CD117 (c-Kit Antibody C-19, Santa Cruz Biotechnology, USA) and the dye set En Vision (DAKO Corporation, USA) for presentation of the c-Kit antibodies. The entire surface of c-Kit antigen expression [mm2] per 1 mm of the section was 76 evaluated using the optical microscope Axiophot (Zeiss, Germany), co-operating with the software applied for the morphometrical measurement. Statistical analysis The results were performed as mean values ± standard deviations (SD). The results obtained from the same group of the animals were subjected to the ANOVA analysis of variance with the A B Rat TB M14 nerve X (mV) -1,0 -0,5 0,0 1 :18:20 1:20:00 1:21:40 1:23:20 1:25:00 1:26:40 1:28:20 1:30:00 1:31:40 1:33:20 1:35:00 1:36:40 1:38:20 1:40:00 1:41:40 1:43:20 1:45:00 1:46:40 Fig.1. Vagal afferent activity (VAA) recorded (Spike Histogram, ADInstruments) in left vagus nerve of the control (A) and the morphine tolerant and dependent rat (B). [1] - i.p. injection of CTOP. 77 "post hoc" t-Student test for multiple comparisons. The t-Student test for two populations of images was applied for the analysis of the results obtained from two compared groups. For each test p<0.05 was considered as statistically significant. RESULTS Effect of chronic morphine administration on VAA Frequency of VAA recorded in the rats influenced by morphine for 72 h was similar to those obtained from the control animals (0.45±0.2 vs. 0.58±0.3 Hz, p>0.05). Analysis of the left vagus nerve afferent activity in the morphine infused animals revealed short lasting, significant response to i.p. injection of µ-receptor antagonist. After CTOP VAA frequency increased, which was visible within the first (0.78±0.3, p=0.02) and second (0.7±0.3, p=0.01) 10-min. interval. Frequency of VAA recorded within the last period, over 20 min after CTOP administration, was similar to those obtained prior to the m-receptor blockade (Fig. 1, 2). Effect of chronic morphine administration on c-Kit Period dominant freguency [Hz In the control group comparison of c-Kit expression areas in the duodenum and the ascending colon revealed the prevalence of their expression in the large bowel (105 ± 15 vs. 78 ± 16 10-4 mm2, p<0.001). Significant differences of cKit expression were also visible between the compared groups. The animals tolerant to and dependent upon morphine presented bigger than the controls areas of c-Kit expression in the examined bowel fragments. Such differences were found in both duodenum (97 ± 15 vs. 78 ± 16 10-4 mm2, p<0.01) and ascending colon (160 ± 65 vs. 105 ± 15 10-4 mm2, p<0.01) and confirmed by the applied analysis of variance (Fig. 3, 4). 1,2 p=0.01 1 p=0.02 0,8 0,6 0,4 0,2 0 control before CTOP 0-10 min. 10-20 min. 20-30 min. after 1 h Fig.2. Quantification of VAA period dominant frequency in the controls and the morphine tolerant / dependent rats, before and after i.p. injections of CTOP. Values plotted as means ± standard deviations. 78 Fig.3. Areas of c-Kit antigen expression in longitudinal sections of duodenum (A) and ascending colon (B) of morphine tolerant and dependent rat (magnification - 360 x). DISCUSSION Chronic administration of morphine inhibits GI motility and transit, which effect induces constipation. Previous observations suggest that intestinal dysmotility may result from both central and peripheral action of morphine (4, 12). Simultaneously tolerance and dependence develop due to chronic morphine administration. Both phenomena are clinically independent however they possess common mechanisms based on receptor dysfunction and changes of neuronal 79 Area of c-Kit expression [1 x 10 -4 mm2 x mm-1] 200 p<0.01 180 160 p<0.01 140 120 100 duodenum 80 colon 60 40 20 0 control morphine Fig.4. Quantification of c-Kit antigen expression in duodenum and ascending colon of the controls and the morphine tolerant / dependent rats. Values plotted as means ± standard deviations. membrane potential (6). Suggestion of the central mechanisms of tolerance to and dependence upon morphine is based on the well-described opioid action on CNS (13). However regarding the intestinal tolerance / dependence the involvement of the peripheral mechanisms located within the ENS or the intestinal wall can not be excluded. Our purpose in the current study was the evaluation of the long vago-vagal reflexes and the intramuscular c-Kit expression in the small and large bowel in rats tolerant to and dependent upon morphine. We hypothesised that the coexistence of changes in both evaluated parameters might indicate the involvement of peripheral mechanisms in intestinal response to chronic morphine administration. Evaluation of the long vago-vagal reflexes was based on VAA measurement. Vagal afferent fibres serve as the afferent branch of the reflex and their activity depends on stimulation of receptors located in the intestinal wall (14,15). During the study we applied chronic peripheral infusions of morphine from the subcutaneously implanted osmotic pumps. Our previous results showed that the time of tolerance development depends on the opioid dose (16). In our study we applied the standard D50 dose of morphine that results in 50% inhibition of the transit. For the experimental development of withdrawal syndrome we used the i.p. injections of CTOP, a µ receptor antagonist that doesn't cross the bloodbrain barrier (6). The dose of CTOP was matched with dose of morphine based on our previous observations. According to the data available intensity of the dependence symptoms may depend on the dose of antagonist which interacts competitively with the agonist and the receptor (17). The results obtained by Williams et al. (9) who evaluated the influence of morphine on the colon contractility and transit suggested that the intestinal tolerance could be visible on the first day of the chronic drug infusion. Physical dependence and intestinal dysmotility in reaction to the applied naloxone were observed after 72 h of the morphine infusion. Therefore in our study we haven't 80 expected changes of the fasting VAA after 72 h of morphine administration. Decrease of the fasting VAA after 72 h would have meant that tolerance still hadn't developed. However we expected the response of vagal activity to the peripheral µ receptors blockade by CTOP. Our expectations were based on the other results that confirmed the coexistence between GI dysmotility and changes of VAA. Blackshaw (18) observed excitation of the vagal afferent fibres during gastric or oesophageal distension in the anaesthetised ferrets. Similarly Thor et al. (19) revealed increased vagal afferent response to gastric distension (GD) in rats, whereas Ozaki et al. (20) didn't observe decreased VAA reaction to GD after morphine. Those results may suggest that morphine doesn't affect the vagal afferent fibres directly. On the contrary Kaczyñska et al. (21) suggested that opioid - receptor activity on vagal afferents maight have been involved in apnoea an hypotension due to morphine administration. Regarding these expectations in our study we haven't observed changes of VAA frequency due to the development of tolerance to morphine. No significant differences were visible between the recordings obtained in the controls and the morphine rats before CTOP. Intraperitoneal injection of CTOP produced strong increase of the VAA frequency in the rats suspected of dependence upon morphine. Peripheral action of the injected µ-receptor antagonist suggested that the observed withdrawal response was located inside the intestinal (ENS) µ2 receptors because their blockade revealed transient hyperactivity of the long vago-vagal reflex. The observed changes of VAA were compared to the expression of the intramuscular c-Kit antigen in the duodenum and colon. We presumed that the intestinal c-Kit expression might reflect the ICCs activity and the intestinal ability to generate BER (7). Changes in c-Kit expression seem to play the fundamental role in the intestinal dysmotility related to chronic morphine administration. It has been previously evidenced that c-Kit antigen blockade decreases the capability of BER generation by ICCs and facilitates their transformation to typical muscular cells (22). In our study we evaluated expression of the intramuscular c-Kit, situated on the border of the longitudinal and circular musculature of the duodenal and the colon wall. Our study showed significantly bigger areas of c-Kit expression in both small and large intestine, in the rats chronically infused with morphine. Such increase of c-Kit expression may be responsible not only for the development of tolerance to the administered opioid but also for the excessive myoelectrical and motility withdrawal response due to rebound effect after peripheral µ2-receptor blockade. In summary in the examined animals we observed the involvement of the long vago-vagal reflexes in tolerance to and dependence upon chronically administered morphine. Changes of the vago-vagal reflex activity coexisted with increased expression of c-Kit antigen in both the duodenum and the ascending colon. Our results suggest the involvement of peripheral µ2 receptors located in ENS in development of tolerance to and dependence upon morphine. 81 REFERENCES 1. Bates JJ, Foss JF, Murphy DB. Are peripheral opioid antagonists the solution to opioid side effects. Anest Analg 2004; 98: 116-122. 2. Gelot A, Fioramonti J, Zaj¹c JM, Bueno L. Central effects of neuropeptide FF on intestinal motility in naive and morphine-dependent rats. Neuropeptides 1995; 29: 245-250. 3. Stewart JJ, Weisbrodt NW, Burks TF. Centrally mediated intestinal stimulation by morphine. J Pharmacol Exp Ther 1977; 202: 174-181. 4. Stewart JJ, Weisbrodt NW, Burks TF. Central and peripheral actions of morphine on intestinal transit. J Pharmacol Exp Ther 1978; 205: 547-555. 5. Kurz A, Sessler DI. Opioid induced bowel dysfunction: pathophysiology and potential new therapies. Drugs 2003; 63: 649-671. 6. Johnson S, Fleming W. Mechanisms of cellular adaptive sensitivity changes: applications to opioid tolerance and dependence. Pharmacol Rev 1989; 41: 435-488. 7. Hanani M, Freund HR. Interstitial cells of Cajal - their role in pacing and signal transmission in the digestive system. Acta Physiol Scand 2000; 170: 177-190. 8. Maeda H, Yamagata A, Nishikawa S. Requirement of c-kit for development of interstitial pacemaker system. Development 1992; 116: 369-375. 9. Williams CL, Bihm CC, Rosenfeld GC, Burks TF. Morphine tolerance and dependence in the rat intestine in vivo. J Pharmacol Exp Ther 1996; 280: 656-663. 10. Fenik V, Fenik P, Kubin L. Simple cuff electrode for nerve recording and stimulation in acute experiments on small animals. J Neurosci Meth 2001; 106: 147-151. 11. Shook JE, Pelton JT, Lemcke PK, Porreca F, Hruby VJ, Burks TF. Mu opioid antagonist properties of a cyclic somatostatin octapeptide in vivo: identification of mu receptors related functions. J Pharmacol Exp Ther 1987; 242: 1-7. 12. Burks TF, Long JP. Responses of isolated dog small intestine to analgesic agents. J Pharmacol Exp Ther 1967; 158: 264-271. 13. Parolarlo D, Sala M, Gori E. Effect of intracerebroventricular administration of morphine upon intestinal motility in rat and its antagonism with naloxone. Eur J Pharmacol 1977; 46: 329-338. 14. Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition 2000; 16: 866-873. 15. Schwartz GJ, Moran TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am J Physiol 1998; 274 (5 Pt. 2): 1236-1242. 16. Weisbrodt NW, Thor PJ, Copeland EM, Burks TF. Tolerance to the effects of morphine on intestinal motility of unanesthetized dogs. J Pharmacol Exp Ther 1980; 215: 515-521. 17. Shook JE, Pelton JT, Wire WS, Hirning LD, Hruby VJ, Burks TF. Pharmacologic evaluation of a cyclic somatostatin analog with antagonist activity at mu opioid receptors in vitro. J Pharmacol Exp Ther 1986; 240: 772-777. 18. Blackshaw LA. Gastro-oesophageal afferent and serotonergic inputs to vagal afferent neurones. J Auton Nerv Syst 1994; 49: 93-103. 19. Królczyk G, ¯urowski D, Sobocki J, Laskiewicz J, Thor PJ. Encoding meal in integrated vagal afferent discharge. J Physiol Pharmacol 2004; 55: 99-106. 20. Ozaki N, Sengupta JN, Gebhart GF. Differential effects of mu-, delta- and kappa-opioid receptor agonists on mechanosensitive gastric vagal afferent fibers in the rat. J Neurophysiol 2000; 83: 2209-2216. 21. Kaczynska K, Szereda-Przestaszewska M. Involvement of vagal opioid receptors in respiratory effects of morphine in anaesthetized rats. J Physiol Pharmacol 2005; 56: 195-203. 82 22. Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and intestinal electrical rhythmicity in steel mutants. J Physiol (Lond) 1994; 480: 91-97. R e c e i v e d : October 4, 2005 A c c e p t e d : February 3, 2006 Author's address: Tomasz Banach M.D., Ph.D., Dept. of Pathophysiology Jagiellonian University Medical College, ul. Czysta 18, 31-543 Kraków, Poland