Integra LifeSciences
Transcription
Integra LifeSciences
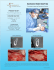
Integra ™ su rgic al T echni que MBA Titanium Subtalar Implant ™ PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY Integra™ MBA™ Titanium Subtalar Implant Table of contents Implant description�������������������������������������������������������������������������������������������������03 The subtalar MBA™ system offers these unique features����������������������������������03 Indications����������������������������������������������������������������������������������������������������������������03 Contraindications����������������������������������������������������������������������������������������������������03 Surgical technique 04 1. Incision and dissection�������������������������������������������������������������������������������������� 04 2. Probe insertion��������������������������������������������������������������������������������������������������� 04 3. Guide pin insertion��������������������������������������������������������������������������������������������� 05 4. Sizer insertion����������������������������������������������������������������������������������������������������� 05 5. Trial implant insertion���������������������������������������������������������������������������������������� 05 6. Intra-operative radiographs����������������������������������������������������������������������������� 05 7. Implantation�������������������������������������������������������������������������������������������������������� 06 8. Guide pin removal and closure������������������������������������������������������������������������� 06 Post-op/Follow-up06 Instructions for use 07 References08 Features08 Component material 2 Surgical technique Integra™ MBA™ PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY 08 Implant Description The subtalar MBA™ titanium implant is the original, time-tested metallic arthrodesis implant for the correction of pediatric pes valgus and adult posterior tibial tendon dysfunction. The subtalar MBA™ system offers these unique features Contraindications • Barrel-shaped implant provides optimal medial support. • Vertical calcaneus. • Uniform implant diameter minimizes lateral discomfort. • Rigid flatfoot (unreducible). • Exclusive slotted design helps prevent implant extrusion. • Rearfoot varus. • Cannulated, color-coded instrumentation simplifies surgical procedure. • Peroneal spasm. • Ankle joint valgus. • Excessive ligamentous laxity. • Degenerative joint disease of the subtalar joint. Indications • Severe obesity. • Pediatric pes valgus. • Asymptomatic flatfoot condition. • Adult posterior tibial tendon dysfunction. • Patients less than 3 years of age. Pre-op lateral view Post-op lateral view Surgical technique Integra™ MBA™ PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY Post-op ap view 3 Integra™ 05-1002 Yellow probe MBA™ Titanium Subtalar Implant Surgical Technique 1. Incision and dissection 2. Probe insertion Make a 1-3 cm incision over the sinus tarsi along the relaxed skin tension lines (Fig. 1). • The intermediate dorsal cutaneous nerves should course superior to the incision. • The sural nerve should course inferior to the incision. • Insert the yellow probe instrument through the sinus tarsi into the sinus canalis from lateral to medial until tenting is noted on the medial aspect of the foot (Fig. 3). • The probe should be positioned perpendicular to the lateral wall of the calcaneus, angled slightly posterior and superior. • Incise the deep fascia. The anterior lateral edge of the posterior facet of the calcaneus should be palpable with instrumentation upon completing the dissection into the tarsal canal (Fig. 2). • Move the probe in a clockwise and counter clockwise direction to slightly dilate the tarsal canal. • Remove the probe. incision Fig. 1 Probe instrument Fig. 2 4 Surgical technique Integra™ MBA™ PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY Fig. 3 05-1006 05-1008 05-1009 05-1010 05-1012 Sizers 05-0017 Guide pin 05-1020 3.5 mm hex 45° 3. Guide pin insertion • Insert the guide pin and make a small incision medially to allow the guide pin to exit through the medial aspect of the foot (Fig. 4). 15° Guide pin • The distal aspect of the pin should exit the skin just inferior to the tibialis posterior tendon and anterior and slightly inferior to the medial malleolus. 0° Fig. 4 4. Sizer insertion • Place the cannulated 6 mm sizer over the guide pin, and insert it through the sinus tarsi into the sinus canalis from lateral to medial (Fig. 5). 6 mm sizer • Assess the range of motion of the subtalar joint. • Continue to insert the remaining sizers (8, 9, 10, 12 mm) until proper correction is achieved. • The appropriate size will allow approximately 2-4 degrees of subtalar joint eversion. Fig. 5 5. Trial implant insertion • After the appropriate size is determined, remove the sizer. 3.5 mm hex Trial • Insert the corresponding trial implant using the cannulated 3.5 mm hex insertion tool (Fig. 6). • Asses the range of motion of the subtalar joint. • Use intra-operative imaging to evaluate the degree of correction and placement of the trial implant. Fig. 6 6. Intra-operative radiographs • To determine the correct position on AP (anteroposterior) view, the leading edge of the trial implant should approach, but not cross, the longitudinal bisection of the talus (Fig. 7). • The trailing edge of the implant should be at least 5 mm medial to the lateral wall of the calcaneus. Guide pin 5 mm • Examining the lateral view, the trial implant should be angled posterior, and the implant should not be sitting on the floor of the calcaneus. Longitudinal bisection of the talus Fig. 7 Surgical technique Integra™ MBA™ PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY 5 05-1002 Probe instrument 05-1020 Insertion Device 05-0017 Guide Pin 2 mm 7. Implantation • Once the appropriate sized trial implant and placement are determined, remove the trial implant. Insertion tool • The equivalent size sterile implant is placed onto the insertion tool over the guide pin and threaded in a clockwise direction until clinical correction is noted (Fig. 9). • Intra-operative imaging is essential to verify proper positioning of the implant (Refer to Step 6). Fig. 9 • Once the implant has been properly positioned, assess the range of motion of the subtalar joint. • The appropriate size will allow 2-4 degrees of subtalar joint eversion. 8. Guide pin removal and closure • Remove the guide pin medially when satisfactory positioning of the MBA™ implant is obtained (Fig. 10). • Copiously irrigate the area with saline and reevaluate subtalar joint motion. • Close the deep tissue, fascia, and subcutaneous and skin layers. • Place the foot in a mildly compressive dressing. Fig. 10 Post-op / Follow-up • Restrict ambulation for the first 48 hours followed by protective weight-bearing in a removable, below-the-knee, walking cast for two weeks. • Allow a gradual return to activity over the course of the next month. • Typically, adjunctive procedures are performed, so the appropriate post-operative care should be followed for these procedures. 6 Surgical technique Integra™ MBA™ PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY Instruction for Use Subtalar implant MBA™ 1. Description: The Subtalar MBA System consists of a soft threaded implant, designed to be inserted between the posterior and middle facets of the subtalar joint and corresponding instrumentation to facilitate insertion. It is important that the instruments and trial implants used are those specifically designed for this device to ensure accurate insertion. The Subtalar MBA implant is cylindrical in shape and incorporates a center cannula designed for use with a guide wire to facilitate proper placement of the implant. An internal hex-head allows for maximum torque with minimal risk of stripping. External rounded threads increase ease of insertion, while slotting of the implant absorbs peak shock and allows fibrous in-growth. 2. Indications: The Subtalar MBA System is indicated for use in treatment of the hyperpronated foot and stabilization of the subtalar joint. It is designed to block the posterior and inferior displacement of the talus, thus allowing normal subtalar joint motion while blocking excessive pronation and the resulting sequela. --Severely pronated foot --Walking intemperance --Calcaneal stance position greater than 5° --Manually correctable deformities --Mid-tarsal breech (arch pain) --Forefoot varus greater than 10° 3. Contraindications: The Subtalar MBA implant is contraindicated for use in patients with the following conditions: --Active local infection. (Any evidence of infection) --Metal sensitivity or allergic reaction to foreign bodies --Poor or insufficient bone stock --The presence of any clinical or functional abnormalities that would preclude the potential of achieving a good result for the patient --Other conditions that may place the patient at risk (Physiologically) 4. Warnings: For safe and effective use of this implant system, the surgeon should be familiar with the recommended surgical procedure for this device (see the Surgical Protocol, 81-0006). Improper selection, placement, positioning, or seating of the implant may result in unusual loading conditions which could affect the long-term service life of the implant. In every case, accepted surgical practices should be followed in post-operative care. The patient should be made aware of the limitations of the subtalar implant and that physical activity and full weight bearing have been implicated in premature failure of similar devices. Patient sensitivity to implant materials should be considered and assessed prior to surgery. 5. Adverse effects: The following are specific adverse effects, which should be understood by the surgeon and explained to the patient. These do not include all adverse effects, which can occur with surgery in general, but are important considerations specific to metallic internal stabilization devices. General surgical risks should be explained to the patient prior to surgery. --Infection --Pain, discomfort, or abnormal sensations due to presence of the implant --Metal sensitivity, or allergic reaction to a foreign body --Migration of the implant, loosening of the implant --Delayed correction in alignment --Decrease in bone density due to stress shielding --Bursitis 6. Implant materials: The Subtalar MBA implant is manufactured from titanium alloy (Ti-6Al-4V ELI, ASTM F 136, ISO 5832-3). 7. Packaging and sterility: All implants have been sterilized with a minimum dose of 2.5 Mrads of gamma irradiation, sterility assurance level 10 (E-6). Prior to use, inspect package for damage which may compromise sterility. If damaged, the product must be assumed to be non-sterile. If a sterile metal implant package is opened but the device is not used, it should be resterilized according to the hospital’s standard practice. The Subtalar MBA implants and instruments may be steam sterilized using the following process parameters: Method Cycle Temperature Exposure time Steam Gravity 270°F (132°C) 30 Minutes Steam Vacuum 270°F (132°C) 4 Minutes Surgical implants should not be reused. Any implant once used should be discarded. Even though it may appear undamaged, it may have small defects or internal stress patterns which may lead to failure. 8. Instructions for reprocessing: The instrument tray and its contents are provided nonsterile and must be sterilized prior to surgery. The following instructions should be used for cleaning and decontaminating non-sterile product. All products should be cleaned, decontaminated and sterilized before use. Always immediately clean and decontaminate all devices that have been soiled. dispose of implants that exhibit surface or configuration damage. Contouring or clamping of implants should be avoided if possible. It is recommended that implants should not be cut, sharply bent or re-bent, notched, or scratched. These alterations can produce defects or stresses, which may lead to failure of the implant. 10. Caution Federal Law (USA) restricts this device to sale by or on the order of a physician. ©2007 *Patented. Integra and the wave logo are trademarks of Integra LifeSciences Corporation or its subsidiaries. ©2007 Integra LifeSciences Corporation. All rights reserved. 82-0003J Manual Cleaning Procedure: --Prepare a neutral pH enzymatic detergent. Enzol®, as per the manufacturer’s recommendation at 1 oz. per gallon using lukewarm tap water. --Fully immerse each device in the prepared detergent and allow them to soak for a minimum of two minutes. --After soaking the devices, scrub them using a soft bristle brush and circular strokes to remove any visible soil. Pay particular attention to all the areas where the soil could be imbedded (i.e. grooves, crevices, lumens, blind holes.) Use a syringe to flush lumens and a pipe cleaner to clean lumens and holes. Perform cleaning under the water surface to limit aerosolization of the cleaning fluid and soil, as well as for worker and environmental safety. --Rinse devices in lukewarm deionized water for a minimum of one minute to remove any detergent residuals. --Prepare a neutral pH enzymatic detergent, Enzol®, in a sonicator as per the manufacturer’s recommendation at 1 oz. per gallon using lukewarm tap water. Fully immerse the devices in the detergent and sonicate for 10 minutes. --After sonication, rinse the devices with lukewarm reverse osmosis/deionized water for one minute. --Dry the devices using a clean cloth. Automated Cleaning Procedure: --Prepare an enzymatic detergent (Klenzyme®) using lukewarm tap water as per the manufacturer’s recommendation. --Fully immerse the devices and allow to soak for a minimum of two minutes. --Following the soak time, flush any lumens of the device using a syringe. --Rinse the devices under lukewarm running tap water for a minimum of one minute. --Place the devices into an automated washer (Steris 444 or equivalent). The washer cycle parameters are: Phase Pre-Wash 1 Recirculation Water Time (Min.) Temperature Cold Tap 02:00 Water Enzyme Wash 01:00 Wash 1 02:00 Rinse 1 05:00 Detergent NA KlenHot Tap Water zyme®, 1 oz/gal Renu60°C Klenz™ 1/2 oz/gal Hot Tap Water NA --Dry the devices with a clean cloth and visually examine to determine if all adherent visible soil has been removed. Thermal Disinfection Cycle for Automated Cleaning Procedure: Thermal disinfection rinse/cycle should be used at 82.2°C for 1 minute. 9. Product handling: Store implants unopened in their respective protective packages until use. When removing the implant from its packaging, observe all relevant aseptic instructions. Protect the prosthesis from contact with objects, which may damage the surface finish. Inspect each implant prior to use and Surgical technique Integra™ MBA™ PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY 7 Integra™ MBA™ Titanium Subtalar Implant References Instrument tray # Reference Instrument tray Description # Implants Reference Description Instruments 05-0106 MBA™ 6 mm Implant 3 05-0016 Guide Pin Insert Holder 05-0108 MBA 8 mm Implant 4 05-0206 Trial 6 mm 05-0109 MBA™ 9 mm Implant 4 05-0208 Trial 8 mm 05-0110 MBA 10 mm Implant 4 05-0209 Trial 9 mm 05-0112 MBA™ 12 mm Implant 4 05-0210 Trial 10 mm 4 05-0212 Trial 12 mm 1 05-1002 Yellow Probe 2 05-1006 6 mm Sizer 2 05-1008 8 mm Sizer 2 05-1009 9 mm Sizer 2 05-1010 10 mm Sizer 2 05-1012 12 mm Sizer 5 05-1020 Insertion Device ™ ™ Disposables 05-0017 Guide pin 2 mm Features • The Subtalar MBA™ implant system has the longest history of successful clinical outcomes.* • Barrel-shaped implant provides optimal medial support. • Uniform implant diameter minimizes lateral discomfort. • Slotted design helps prevent extrusion. • Simple, minimally-invasive surgical procedure. 1 * Data available upon request 5 2 4 Component material 3 6 • Subtalar implant: Titanium alloy. Related documents ILS Xx-XX-XX DVD Flatfoot treatment Integra™ Flatfoot treatment XXXXXXXX English version xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx Integra LifeSciences Services (France) SAS Sales & Marketing EMEA Immeuble Séquoia 2 97 allée Alexandre Borodine Parc technologique de la Porte des Alpes 69800 Saint Priest FRANCE +33 (0)4 37 47 59 00 fax +33 (0)4 37 47 59 99 [email protected] integralife.com Distributed by Customer Services International: +33 (0)4 37 47 59 50 +33 (0)4 37 47 59 25 (Fax) [email protected] United Kingdom: [email protected] France: +33 (0)4 37 47 59 10 +33 (0)4 37 47 59 29 (Fax) [email protected] Benelux: +32 (0)2 257 4130 +32 (0)2 253 2466 (Fax) [email protected] Switzerland: +41 (0)2 27 21 23 30 +41 (0)2 27 21 23 99 (Fax) [email protected] Integra LifeSciences Corp (Integra Ohio) 4900 Charlemar Drive Cincinnati OH 45227 USA ©2011 Integra LifeSciences Corporation. All rights reserved. ILS 08-07-089-02-11 PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY. Availability of these products might vary from a given country or region to another, as a result of specific local regulatory approval or clearance requirements for sale in such country or region. ▪ Always refer to the appropriate instructions for use for complete clinical instructions. ▪ Non contractual document. The manufacturer reserves the right, without prior notice, to modify the products in order to improve their quality. ▪ WARNING: Applicable laws restrict these products to sale by or on the order of a physician. ▪ Klenzyme, Enzol and Renuklenz are trademarks or registered trademarks of their respective owners. MBA, Subtalar MBA, Integra and the Integra logo are trademarks of Integra LifeSciences Corporation.