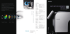Growth increments in Gomphotherium tusks and implications for late
Transcription
Growth increments in Gomphotherium tusks and implications for late
Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 www.elsevier.nl/locate/palaeo Growth increments in Gomphotherium tusks and implications for late Miocene climate change in North America David L. Fox *,1 University of Michigan, Department of Geological Sciences and Museum of Paleontology, Ann Arbor, MI 48109, USA Received 4 November 1998; received in revised form 27 September 1999; accepted for publication 28 September 1999 Abstract Changes in mammalian faunas in North America during the late Miocene are thought to have been caused by the replacement of woodland habitats with grassland or steppe. The proposed cause of this habitat shift is the transition from the relatively low-seasonality early Miocene climate to a more highly seasonal climate regime by the end of the Miocene. Tusks, which are highly modified incisor teeth, of late Miocene Gomphotherium (Mammalia, Proboscidea) were sectioned to document the nature of tusk growth in this genus and to test for patterns of seasonal growth. Both the dentin and enamel of Gomphotherium tusks preserve incremental growth lines. Dentin has increments on three scales, inferred to represent annual (first-order), weekly (second-order), and daily (third-order) periodicities. Luminance and second-order increment thickness profiles were measured on transverse thin sections of tusk dentin viewed at low magnification, and the data were examined with bivariate plots and autocorrelation. Autocorrelograms of luminance data support the consistent identification and measurement of second-order increments within and among the tusks sampled. Profiles of second-order increment thickness in three tusks from the Barstovian North America Land Mammal Age (NALMA) show no indication of seasonal patterns of growth. One tusk from the late Clarendonian and one from the early Hemphillian NALMA have patterns of growth consistent with an increase in seasonality during the late Miocene, an interpretation supported by autocorrelation of the increment thickness data from these tusks. The growth patterns are consistent with the hypothesized changes in North American climate during the late Miocene and are suggestive of an increase in aridity and development of a wet season. Tests of this hypothesis are suggested. © 2000 Elsevier Science B.V. All rights reserved. Keywords: Miocene; North America; paleoclimate; proboscidea; seasonality 1. Introduction The middle to late Miocene, from about 15 to 5 Ma, was a time of significant faunal change among North American mammals. Generic diversity declined from the Cenozoic high of approxi* Fax: +1-831-459-3074. E-mail address: [email protected] (D.L. Fox) 1 Present address: University of California, Santa Cruz, Department of Earth Sciences, Santa Cruz, CA 95064, USA. mately 140 genera (Stucky, 1990) during the Barstovian North American Land Mammal Age (NALMA; 14.5–11.5 Ma) in a series of extinctions that began in the Clarendonian NALMA (11.5– 9 Ma) and culminated in the extinctions at the end of the late Hemphillian NALMA (9–4 Ma) ( Tedford et al., 1987). This late Hemphillian extinction, which affected as many as 65 genera, was more devastating than the better known Rancholabrean event at the close of the last glacial period ( Webb, 1984a; Alroy, 1992). Extinction of 0031-0182/00/$ - see front matter © 2000 Elsevier Science B.V. All rights reserved. PII: S0 0 3 1 -0 1 8 2 ( 9 9 ) 0 0 14 8 - 0 328 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 large herbivores was dramatic. Many species with brachydont ( low-crowned) dentition typical of browsers, such as leptomerycid artiodactyls, disappeared early during the late Miocene, and almost all browsers were extinct by the end of the Clarendonian. Most lineages that survived, such as equids, had hypsodont (high-crowned ) teeth typical of grazers and locomotor adaptations for cursoriality. Thus, many of the herbivores that persisted into the Pliocene were well adapted for fibrous or gritty diets and open habitats ( Webb, 1984b). The patterns of survivorship have been inferred to result from the replacement of woodland savanna in the mid-continent with pure grassland or steppe habitats due to some combination of cooling, drying and an increase in the seasonality of temperature and/or precipitation during the late Miocene ( Webb, 1977; Janis, 1989). The changes among North American mammals during the Miocene are broadly correlative with records, both marine and terrestrial, indicating climate, habitat, and faunal changes globally. The oxygen isotope composition of benthic foraminifera dropped sharply during the middle Miocene (Miller et al., 1987; Wright et al., 1992), marking a transition from warmer climates earlier in the Cenozoic to the cold climates of the PlioPleistocene. Sea levels fluctuated widely and then fell over this same interval ( Haq et al., 1987). The East Antarctic Ice Sheet also experienced a period of fluctuation in size followed by expansion during the middle Miocene, possibly in response to changes in deep water circulation ( Woodruff and Savin, 1989; Flower and Kennett, 1994). These changes in the marine record are associated with increased turnover among planktonic foraminifera ( Wei and Kennett, 1986), and mammalian faunas from continents other than North America, particularly the well-documented faunas of Pakistan, also exhibit significant turnover during the same interval (Barry et al., 1985). Terrestrial floras from around the world indicate the geographic expansion of xeric, or dry habitat, plants during the middle and late Miocene ( Wolfe, 1985; Singh, 1988). Paralleling the paleobotanical evidence, the carbon isotope composition of paleosol carbonates and the teeth of mammalian herbivores on many, but not all, continents record marked increases in the abundance of tropical C grasses beginning 4 around 7 Ma (Cerling et al., 1997), consistent with the growth of continental grasslands during the late Miocene. While all of these records, including the succession of mammalian faunas in North America, certainly reflect climate change on some spatial and temporal scales, none records directly and unambiguously the changes in annual climatic variability postulated to have contributed to the biotic responses in the late Miocene. In this paper, I discuss a source of evidence that may be sensitive to seasonal variability in climate and hence may shed light on the nature of climate change during the Miocene: incremental growth features in the dentin of the upper tusks of the proboscidean Gomphotherium. Proboscidean tusks are highly modified incisor teeth that are composed primarily of the tissue dentin. The growth and structure of tusks have been studied in a number of proboscideans, including mastodons (Mammut americanum) and mammoths (genus Mammuthus) of the late Pleistocene of North America, as well as both extant species of elephant (Elephas maximus and Loxodonta africana) (Fisher, 1984, 1987, 1988; Koch, 1989; Fox and Fisher, 1994). The tusks of many late Pleistocene mastodons and mammoths have patterns of growth increments in tusk dentin that are consistent with seasonal changes in the rate of dentin apposition, presumably reflecting seasonality of climate and food-resource abundance in parts of North America during the late Pleistocene ( Fisher, 1987, 1990, 1996; Koch et al., 1989). As discussed below, similar phenomena have been documented in the teeth of a variety of mammals. Using such growth patterns as an analog, it is possible that gomphothere tusks may similarly record the influence of climate and habitat conditions on growth. This study has two goals. The first is to describe the internal structure of Gomphotherium tusks. The tusk dentin of proboscidean taxa that have been studied are composed of incremental laminae organized on three spatial scales. While gomphothere tusks are somewhat different from others in having a continuous band of enamel along the lateral surface, their general form suggests that gomphothere tusks should be structurally similar to those D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 of other proboscideans. The second, more significant goal is to use the pattern of variation in the thickness of subannual growth increments to infer growth rate. Analysis of a suite of gomphothere tusks from localities spanning the middle to late Miocene provides a means to detect changes in the degree of seasonal patterns of tusk growth, which provides a means of testing the hypothesized trend toward increasing annual variation in climate. 2. Growth increments in mammalian teeth The growth of the mineralized dental tissues of mammals — cementum, dentin and enamel — is an accretionary process that varies in duration from weeks to whole lifetimes among different taxa. At a gross scale, mineralization is broadly similar in all three tissues; hydroxyapatite crystals nucleate and grow in an organic matrix deposited on the growing surface of the tooth by secretory cells. Regular disturbances to or variations in physiology from both external and internal sources during deposition and mineralization of these tissues are often expressed as incremental growth banding. Incremental features appear as alternating light and dark zones in the mineralized tissue, but the contrast relationship (dark–light or light– dark) is dependent on the type of illumination (transmitted, reflected, with/without crossed Nichols), and, in the case of fossil teeth, the quality of preservation. Although growth lines in teeth have been known since the nineteenth century work of Owen (1845) and others, temporal periodicities of these features were not recognized until later (Gysi, 1931; Kimura, 1939; Schour and Hoffman, 1939; Okada, 1943; Sheffer, 1950; Laws, 1952). To date, temporally periodic increments have been identified in at least 89 species of marine and terrestrial mammals ( Fox, unpub. review). In most species, only annual periodicities have been identified, possibly stemming from the common use of incremental features by mammalogists for aging individuals (e.g. Klevezal and Kleinenberg, 1969; Perrin and Myrick, 1980). However, subannual increments with lunar monthly, fortnightly and daily periodicities, in some cases hierarchically 329 arranged, are known from several extant species (Laws, 1962; Sheffer, 1970; Koch, 1989; Klevezal and Mina, 1990; Fox and Fisher, 1994). Neither the structural basis of color banding in teeth nor the biological basis for its periodic expression is well understood. No consensus currently exists on either issue for any dental tissue. Lieberman (1994) has reviewed hypotheses for annual increments in cementum and, based on controlled experiments, proposed that incremental features in cementum result from changes in occlusal stresses associated with diets that vary seasonally in nutritional quality and physical properties. This explanation cannot apply to dentin and enamel, both of which have incremental features that are produced before tooth eruption or, in the case of proboscidean tusks, in the absence of occlusal stress. The generally accepted explanation for annual increments in dentin is that they reflect seasonal variation in the density of the mineral portion of the tissue as a result of seasonal variation in nutritional stress and growth rate of an individual (Laws, 1962; Klevezal and Kleinenberg, 1969). This idea dovetails the structural origin of annual increments with the biological basis of the periodicity. Explanations of the structural basis of subannual increments in dentin and enamel include physico-chemical factors inherent to the process of crystallite growth (Mummery, 1924), variations in the degree of mineralization, variations in the orientation of collagen fibers in the organic matrix (Schmidt and Kiel, 1971) and variations in the chemical composition of the mineral itself (Boyde, 1979). Explanations for subannual periodicities include a circadian rhythm of body-fluid alkalinity (Okada, 1943), the daily timing of feeding (Miani and Miani, 1972; Klevezal, 1980), and periodicities in the body-fluid concentration of hormones possibly related to the growth of mineralized tissues ( Rosenberg and Simmons, 1980; Halberg, 1983). Interactions of different physiological systems with circadian, circaseptan (‘about seven’ day) and other rhythms could result in features with the various periodicities that have been observed in teeth, but the identity and nature of the physiological systems remain elusive. Despite uncertainties associated with both the physical and biological genesis of incremental fea- 330 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 tures, they can still be used as sources of data on the lives of animals, provided that the temporal signal is consistently recognized. Based on the recognition of annual increments, mammalogists frequently use growth bands as a means of assessing the age structure and dynamics of wild populations in a variety of mammals, most commonly marine species (e.g. Hewer, 1964; Marsh, 1980; Slooten, 1991). Archaeologists use annual increments in teeth from archaeological sites to examine temporal patterns of site and resource utilization in past human populations (e.g. Saxon and Higham, 1969; Fisher, 1987; Lieberman, 1993; Burke and Castanet, 1995), and physical anthropologists use them to examine tooth growth and age at death of fossil hominids (e.g. Dean, 1987). Incremental features have been examined in teeth of some fossil and subfossil mammals, such as the beaver (Mayhew, 1978), but the most detailed work to date is on the tusks of late Pleistocene proboscideans. 3. Growth increments in proboscidean tusks Micro- and macroscopic analyses of the tusks of mastodons, mammoths, and both living species of elephant have identified a hierarchical system of growth increments in the dentin ( Fisher, 1984, 1987, 1988, 1990, 1996; Koch, 1989; Fox and Fisher, 1994). Growth banding occurs in the tusks of these animals on three spatial scales and is visible as alternating dark–light couplets, as is the case in the teeth of many other mammal teeth. The growth bands parallel the pulp cavity and imbricate along the length of the tusks so that the earliest deposited — hence oldest — dentin is distal, and the dentin becomes younger towards the surface of apposition in the pulp cavity. Borrowing the conventions of sequence stratigraphy, the largest and most inclusive increments are referred to as first-order increments. The thickness of these increments is on the order of several millimeters in cross-section. At the next tier in the hierarchy, second-order increments are on the scale of tenths of millimeters in thickness. Third-order increments are the smallest and are tens of microns in thickness. The hierarchy of increments is not only spatially, but also temporally, periodic. The full arguments have been presented elsewhere (Fisher, 1987; Koch, 1989; Koch et al., 1989) but will be briefly reviewed here. The strongest evidence is the consistent numerical relation between the three scales. First-order increments are composed of either 52, 26 or 13 second-order features and about 364 third-order increments. Variability in the number of second-order increments is dependent on individual and species variation and whether a sample is from molar or tusk dentin. In cases of 52 secondorder increments per first-order feature, each second-order increment is composed of about seven third-order increments; in cases of 26 secondorder increments per first-order increment, each second-order increment is composed of about 14 third-order increments; and in cases of 13 secondorder increments per first-order increment, each second-order increment is composed of about 28 third-order increments. These numbers indicate that first-order increments are annual features, second-order increments are weekly, fortnightly or monthly and third-order increments are daily. As discussed above, analogous features and periodicities are known in a variety of other mammals. This temporal interpretation of growth increments is corroborated by two lines of evidence. The first is the pattern of variation in the oxygen isotope composition of tusk dentin. The d18O values of samples removed following growth lines have a cyclic pattern in which the lowest values correspond to winter growth and the highest values correspond to summer, the season of growth being inferred from the color and thickness of the first-order increments (e.g. Koch et al., 1989, Fig. 3) and by analogy with extant mammals. The correlation between the variation in d18O and the color pattern of first-order increments strongly supports the interpretation of first-order increments as annual features. The second line of evidence is the pattern of variation in second-order increment thickness, and its correlation to both the pattern of oxygen isotope variation and the color banding of first-order increments. Plots of increment thickness versus sequential increment number (increment thickness profiles) show a characteristic rise and fall over periods of about 26 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 increments in individuals interpreted to have fortnightly increments (e.g. Koch et al., 1989, Fig. 2). Sets of narrow increments correlate with the inferred winter portions of first-order increments and are presumed to indicate slow growth that results from winter nutritional stress; the broader high growth rate periods correlate to the spring– autumn portions of the first-order increments and may result from the greater availability of food during the growing season. Thus, the cyclic patterns of oxygen isotope composition and increment thickness represent intra-annual or seasonal variation in body-water isotope composition and growth rate and, and corroborate the temporal interpretation of both first- and second-order increments. 4. Implications for Gomphotherium tusks The recognition of temporally periodic growth increments and seasonal growth patterns in the tusks of late Pleistocene proboscideans suggests that tusks of Miocene proboscideans, such as Gomphotherium, may be useful as indicators of the hypothesized climate change during the late Miocene. It must first be established that Gomphotherium tusks grew in a manner like those of later proboscideans and that the tusks have growth increments that are spatially periodic. Plots of subannual increment thickness could then be used to examine temporal periodicity and changes in tusk growth rate through time. The hypothesis of increasing seasonality during the late Miocene predicts a straightforward change in the pattern of gomphothere tusk growth, assuming a response to seasonality similar to that documented in the tusks of late Pleistocene mastodons and mammoths ( Fisher, 1987, 1990, 1996; Koch et al., 1989). Barstovian specimens should show either low-amplitude cyclicity or irregular variability with no discernible pattern in subannual increment thickness. Increasing seasonality during the Clarendonian and Hemphillian land mammal ages would be evident as increment thickness profiles with recurring, paired intervals of high and low growth with an annual periodicity inferred from the number of included subannual increments. 331 Behavioral factors, such as migration, seasonal dietary shifts, and musth and other aspects of reproductive behavior, could weaken or even mask patterns of seasonal growth. 5. Materials and methods 5.1. Specimens Seven upper tusks of the genus Gomphotherium from localities in Nebraska, Oklahoma and Texas were analyzed in this study. Unlike late Pleistocene and Recent proboscideans, gomphotheres had permanent upper and lower tusks throughout life. Only upper tusks were used because they are generally larger than the mandibular tusks, and increments might therefore be more conspicuous and easier to analyze. Also, only the upper tusks have a lateral enamel band, allowing for investigation of both enamel and dentin in each individual. The specimen numbers, localities and ages for the seven tusks are given in Fig. 1. Due to the currently confused and inexact state of gomphothere taxonomy, attribution of these specimens to species is Fig. 1. Specimen numbers and biostratigraphic position of the localities arranged by North American Land Mammal Ages (NALMA) with absolute ages indicated in millions of years (Ma). The order of specimens within subdivisions of the landmammal ages is arbitrary and should not be taken as indicative of superpositional relationships among the localities. Age assignments are based on Corner (1976) and Tedford et al. (1987); the radiometric scale is based on Woodburne (1987). 332 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 not currently possible. Six of the seven specimens have been previously assigned only to the subfamily Gomphotheriinae. One, UNSM 88511, was attributed to G. osborni by Corner (1976). However, according to Tobien (1972), all named species of Gomphotherium in North America can be accommodated within G. producutus. Although this issue awaits a rigorous analysis, the aforementioned similarities in the tusks of elephantids and mammutids suggest that analysis of closely related congeneric species is not a major concern. Specimens were selected based on quality of preservation, although judgment of condition from external appearance is difficult. Badly cracked or weathered tusks were rejected, as were extremely heavy specimens that had probably undergone substantial post-depositional alteration and permineralization. An additional criterion for inclusion was suitability for shipment to the University of Michigan, leading to exclusion of large tusks from old individuals. 5.2. Sectioning In order to examine the internal structure and pattern of growth of the enamel and dentin, it was necessary to section tusks and prepare thin sections. Tusks were embedded in plaster and then sectioned longitudinally on a rock slabbing saw using a non-aqueous cutting lubricant. Plaster was used because it provides adequate support for potentially friable specimens, yet is easily removed later. A non-aqueous lubricant was used because of the potential for water absorption to cause expansion and damage dry fossil material (Fisher, 1988). The longitudinal cut was oriented to bisect the enamel band perpendicularly and to pass just to one side of the tusk axis so that one half of the tusk actually contained the axis. Sample blocks for thin sections were removed by making a pair of transverse cuts (i.e. perpendicular to the tusk axis) approximately 1.5 cm deep in the tusk section containing the axis. These cuts were then joined by a single longitudinal cut to liberate a sample block. Thin sections on glass slides were prepared from individual sample blocks following standard thin sectioning techniques. The finished thickness of slides was not standardized. Rather, slides were thinned to achieve the optimal expression of second-order increments, generally occurring at a thickness of about 0.3 mm. Thinning beyond this weakened or eliminated the second-order features, though it did enhance third-order increments. However, these smallest scale increments are variably preserved, and second-order increments are preferred for evaluation of seasonal patterns of growth. Because the scale of first-order increments is larger than the field of view under even low magnification, optimizing their expression was not a concern in thinning. 5.3. Increment thickness As discussed below, gomphothere tusks were found to have three scales of laminar features, much like Pleistocene and Recent tusks. The pattern of variation, or lack of pattern, in secondorder increment thickness was used to assess the degree of seasonal growth in Gomphotherium tusks. This approach is based on the utility of secondorder increment thickness in Mammut and Mammuthus for identifying seasonal growth. All analyses of thin sections were carried out at a magnification of 25×. Second-order increment thickness was measured on transverse thin sections using digitized video images and Optimas image analysis software ( Vers. 4.1) from Optimas Corp. In this procedure, an image of a thin section in transmitted light was captured by a video camera mounted directly on a petrographic microscope and the contrast and brightness of the image adjusted with Optimas image-processing controls to maximize the contrast between light and dark portions of second-order increments. Luminance was then measured by Optimas along a transect perpendicular to the growth banding at 0.0071 mm intervals. Markers were placed along the transect at the midpoint of the dark parts of each increment, which are generally more discrete than the accompanying light portion ( Fig. 2A). The proper position for markers is judged both by eye from the video and microscope images and from the luminance profile measured by Optimas, which can be viewed while evaluating the transect ( Fig. 2B). Because of local changes in the preservation and prominence of growth lines, luminance D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 values along parts of the transect can be uninformative or even misleading. However, by following faint or ambiguous lines across a thin section, it is possible to mark increments accurately even when the luminance profile is not helpful. Optimas records the position of each marker along the transect and exports both luminance and increment marker data to a spreadsheet. The data from each slide were collected in a series of discrete, but overlapping, transects which were then pieced together into a continuous record. This continuous record consists of the distance, in millimeters, of each increment marker from the beginning of the first discrete transect. Increment thickness is calculated by subtracting the distance along the continuous transect of successive pairs of increment markers. These values are then plotted against increment number with the earliest-formed increment at the origin to generate an increment thickness profile ( Fig. 2C ). 5.4. Autocorrelation Autocorrelation was used both to evaluate the placement of second-order increment markers on luminance transects from individual screens and to examine the second-order thickness data for periodicities consistent with seasonal growth. Autocorrelation is a simple time series analysis in which a time series is correlated with itself after a displacement in the time dimension. The autocorrelation coefficient is calculated between pairs of values y and y , where i is the position in the i i+t time series and t, or the lag, is the number of observations by which the time series is offset from itself. The equation for the autocorrelation coefficient at a given lag, r , is t ∑ ( y y )∑ y ∑ y i i+t i i+t r= t E[∑ y2 −(∑ y )2][∑ y2 −∑ y )2] i i i+t i+t (Davis, 1986). Autocorrelation coefficients at successive lags are conventionally plotted on an autocorrelogram, which has r on the ordinate and t on the abscissa t (Fig. 2D). As with a standard correlation coefficient, r ranges from 1.0 to −1.0. The value of r t t is necessarily 1.0 at t=0, and it decreases with 333 increasing lag. For random or aperiodic data, r t will have an arbitrarily small value at t=1 and will vary randomly about 0 at increasing lag. Any smoothness in the data will result in a more gradual decline in r . Peaks (or valleys) in r after t t a short decline due to smoothness may indicate cyclicity in the data at a wavelength corresponding to the lag. A standard Z statistic can be calculated to assess the statistical significance of the autocorrelation coefficient at a given lag, and hence can be used to distinguish periodic structure in a data set from random variation in r . The equation for t the Z statistic at a given lag t is Z =r En−t+3 t t (Swan and Sandilands, 1995) where r is the autot correlation coefficient at that lag, and n is the number of observations in the data set. The critical values for Z at a=0.05 are ±1.96. All autocorrelt ograms in this paper have both the Z statistic and the critical values plotted in addition to r . Any t lag with a Z statistic that crosses the critical values is statistically consistent with a periodicity in the data at that lag. For the luminance data, r should t be high and Z statistically significant for lags that t correspond to the mean increment thickness measured for a transect. For example, in the case of the sample data in Fig. 2, the mean second-order increment thickness is 0.076 mm. Given that luminance is measured along the transect in 0.0071 mm intervals, the mean increment thickness implies that lags 10 and 11 should have a high r and t significant Z . The peak in r and Z over lags 10– t t t 14 corresponds to expectation, confirming the impression from Fig. 2A and B that the variation in luminance is regular, and supports the identification of second-order increments. For the increment thickness data, the expectation is that specimens that have observable first-order or annual increments and that have a regular variation in the size of second-order increments should have a high r and significant Z at lags correspondt t ing approximately to a year, either 13, 26 or 52, depending on the temporal value of the secondorder increments. Autocorrelation coefficients were calculated for only a subset of the individual luminance profiles 334 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 as many of the individual screens of data cross areas with either cracks or unusual luminance or contrast due to diagenesis. Such transects result in aberrant, indistinct, or inconsistent luminance profiles that would result in spurious or meaningless autocorrelograms. A total of seven luminance transects from three individuals were judged suitable for autocorrelation. The second-order increment thickness profiles from all seven tusks were analyzed with autocorrelation. Three restrictions apply to autocorrelation. First, autocorrelation should only be used on data sets with at least 50 observations, and the lag should not exceed one-quarter the length of a data set (Davis, 1986). This restriction is easily met as the shortest luminance transect examined with autocorrelation had 247 luminance measurements, and the shortest incremental thickness profile had 175 measurements. Second, any linear trend in the data should be removed prior to analysis, as such a trend will cause a steady decline in r with t increasing lag and hence will mask any pattern in the data (Swan and Sandilands, 1995). Linear trends can be introduced into the luminance data by uneven illumination and by local variability in the preservation of the sample; linear trends can be introduced into the increment thickness data by long-term trends in the growth of an individual that are distinct from and overlay sub-annual patterns. Finally, a more important restriction is that the spacing of observations be constant (Davis, 1986). For the luminance data, this is unproblematic due to the constant sampling interval (0.0071 mm) used in collecting the data with Optimas; a constant interval in the spatial domain substitutes for a constant interval in the temporal domain without violating the restriction of application to a constant interval. For the increment thickness data, the assumption is that all features marked are second-order increments 335 and hence represent the same temporal spacing. All autocorrelation coefficients and detrending (when necessary) were calculated using SYSTAT ( Wilkinson, 1990). 6. Results 6.1. Geometry of growth Longitudinally sectioned tusks, as well as analysis of thin sections, indicate that the basic mode and geometry of growth of the tusks of Gomphotherium are essentially the same as in other proboscideans (e.g. Fisher, 1987; Koch, 1989), with the exception of the continuous lateral enamel band. Fig. 3A is an idealized representation of a sectioned gomphothere tusk indicating the arrangement of the mineralized tissues. The tusk is mostly composed of dentin, which is deposited at the surface of the conical pulp cavity. Two specimens ( F:AM 129672 and 129673) preserve open pulp cavities, indicating that the tusks continued growing well into adulthood and, based on comparison with Pleistocene and Recent tusks, implying they were evergrowing. Fig. 3B illustrates the open pulp cavity of F:AM 129672, in which a plug of loose, coarse sand is visible. Growth increments in the dentin parallel the pulp cavity, as suggested by the cracks that follow the growth banding in Fig. 3B. Such cracking is common in fossil tusks and is due to differential drying and shrinking of the interior and exterior of the tusk (D.C. Fisher, pers. commun., 1999). Enamel is deposited along the lateral surface of the dentin in a band several centimeters wide, 1–3 mm thick, and continuous along the length of the tusk. The geometry of the surface of enamel apposition is inferred from the increments in the enamel, as discussed below. Several specimens preserve a thin Fig. 2. Example of a screen of data and its results. (A) Digitized image of a portion of F:AM 129670 viewed in transmitted light at 25× as it appears on the video monitor. Growth increments run vertically and the enamel dentin junction is to the right. The transect is 1.85 mm long and 24 increments are marked with Vs at the dark line of each increment. Increments thus span the distance between successive V markers. (B) Luminance profile for the transect in (A). (C ) Sequential increment thickness for the increments marked in (A), beginning with the increment furthest to the left. The average increment thickness is 0.076 mm. (D) Autocorrelogram with plot of Z statistic and critical values of Z for the luminance data plotted in (B). The peak in r and Z for t=10–14 corresponds to t t t an implied increment thickness of 0.071–0.099 mm, which includes the measured mean increment thickness for the transect in (A). 336 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 Fig. 3. (A) Schematic diagram of the proximal end of a gomphothere tusk illustrating the basic geometry of tusk growth. Only firstorder or annual increments are shown. The angle of increments in enamel, thickness of the enamel and cementum, and the degree of contrast and scale of the first-order increments are exaggerated for clarity. (B) Longitudinally cut surface of F:AM 25729. The distal end of the tusk is to the right, and the pulp cavity extends the length of this section of tusk. The figured portion of the tusk is approximately 30 cm in length. coating of cementum over the dentin proximally, but this is lost distally by abrasion in life; it is not clear if the cementum also overlapped the enamel. 6.2. Increments Growth banding is evident in both enamel and dentin. In both tissues and at all scales, the increments appear as alternating zones of light and dark mineral. Fig. 4A and B are photomicrographs (transverse and longitudinal, respectively) of enamel on the same sample block from F:AM 129673. The increments in enamel are much smaller and less regular than those in dentin. The increments are approximately planar features that parallel the irregular enamel–dentin junction in transverse section, but intersect it at an acute angle (~7°) proximally in longitudinal section. This lowangle proximal inclination of the increments relative to the enamel–dentin junction (Fig. 4B) is the basis for the orientation of the surface of enamel apposition in Fig. 3A. Evident in both photomicrographs of enamel are the faint traces of the enamel prisms, which run in curving paths from the enamel–dentin junction toward the exterior surface of the enamel at the top. In parts of both photo- D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 337 Fig. 4. Photomicrographs of the enamel of F:AM 129671. Magnification is 40×. (A) Transverse view with the undulatory enamel– dentin junction at the bottom. (B) Longitudinal view with the proximal portion of tusk to the left. Note the low inclination of the increments in the proximal direction. The scale bars are 0.5 mm. micrographs, there is the suggestion of two scales of incremental features in enamel. Due to their smaller scale and more irregular expression, the increments in enamel were not analyzed for seasonal patterns of growth. However, recognition of the pattern of growth and scale of lamination in the enamel, and its relation to the more regular incremental features in dentin, is important for planned studies of the oxygen and carbon isotope composition of gomphothere tusks. Increments occur in the dentin at three spatial scales and are arranged hierarchically ( Fig. 5A– C ). Only two specimens have portions that clearly exhibit first-order increments: F:AM 129671 (Clarendonian) and F:AM 129673 (Hemphillian). The inconsistent expression of first-order features could be a result of diagenetic alteration of the dentin. Alternatively, the degree of seasonality may have varied from year to year so that the environmental signal leading to first-order features may not have been as strong or regular from year to year. The appearance and scale of the firstorder increments are similar to those in other proboscideans, as shown by the transverse view of a sample from F:AM 129673 in Fig. 5A. Each increment is visible in transmitted light as a narrow dark zone and a broader light zone. Measured under a microscope with dial calipers, the thickness of the increments labeled ‘a’ and ‘b’ in Fig. 5A are 3.7 and 4.2 mm, respectively. The single first-order increment visible in F:AM 129671 (not figured ) measures 4.0 mm. These measurements, though few, are within the range of variation for firstorder increments in Mammut and Mammuthus (3– 8 mm; D.C. Fisher, pers. commun., 1999) and suggest that they might represent annual periodicities in dentinal deposition. A notable non-incremental feature in Fig. 5A is the checkerboard pattern of the Schreger bands that results from the undulation of the dentinal tubules and which is characteristic of proboscidean ivory. The only specimens in which the Schreger banding is not visible are UNSM 88511 and F:AM 99059. Both of these Barstovian specimens are altered by silicification. The loss of Schreger banding is somewhat puzzling, as the other Barstovian specimen, F:AM 129670, has also been heavily altered but still expresses the Schreger banding. A possible explanation is differential diagenetic infilling of the dentinal tubules, which would obscure the checkerboard pattern of the Schreger bands. At low magnification, the second-order increments within the first-order increments look like tree rings or the grooves in a vinyl record ( Fig. 5A). At higher magnification ( Figs. 2A and 5B), the character and regularity of these features are clear. All specimens examined in this study had clearly recognizable second-order increments. 338 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 Fig. 5. Examples of growth increments in Gomphotherium tusks. Specimens chosen for clarity at each scale. (A) Annual banding in a transverse surface of F:AM 129673 viewed in transmitted light. The block is from the proximal end of the tusk. The lateral enamel band is at the top and is translucent in transmitted light. The curved surface at bottom is the surface of the pulp cavity. Two complete first-order increments are labeled ‘a’ and ‘b’. The dark zone and part of the light zone of a third first-order increment are evident above ‘a’, and the light zone of a fourth is evident below ‘b’ . Cracks in the dentin are evident towards the pulp cavity and below the enamel–dentin junction. Second-order increments are visible across most of this block as roughly horizontal dark and light lines. The checkerboard pattern is Schreger banding. The scale bar is 5.0 mm. (B) Photomicrograph of a thin section of F:AM 129670 in transverse view at 40× magnification. The prominent light and dark bands running horizontally are second-order increments (18 are visible). The faint vertical lineation is caused by dentinal tubules, which run from the axial portion of the section at bottom towards the enamel–dentin junction toward the top. Third-order increments are visible within a number of the second-order increments. The scale bar is 0.5 mm. (C ) Photomicrograph of a thin section of F:AM 129671 in transverse view at 100× magnification. Growth increments run horizontally. The broad dark and light bands are second-order increments (seven are visible) and the more numerous, narrow couplets are third-order, or daily, increments. The vertical lineations are dentinal tubules. The scale bar is 0.2 mm Table 1 summarizes the second-order increment data collected from each specimen. The secondorder increments are interpreted as the result of a weekly periodicity in dentinal deposition based on three lines of evidence. First is the average thickness of second-order increments. In 13 tusks of Mammut, Mammuthus, and Elephas that have second-order increments with a fortnightly period, the average increment thickness is 0.197 mm ( Koch, 1989). As indicated in Table 1, the average second-order increment thickness in the seven Gomphotherium specimens analyzed here ranges D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 339 Table 1 Second-order increment thickness data for specimens analyzed Specimen F:AM 129673 F:AM 129672 F:AM 129671 F:AM 25729 F:AM 99059 F:AM 129670 UNSM 88511 Age E. E. L. L. L. L. L. Hemphillian Hemphillian Clarendonian Clarendonian Barstovian Barstovian Barstovian Increment thicknessa Yearsc Number Meanb S.D. 210 244 273 175 239 350 237 0.091 0.093 0.082 0.098 0.111 0.085 0.095 0.039 0.034 0.037 0.036 0.044 0.027 0.027 4.0 4.7 5.2 3.4 4.6 6.7 4.6 a Number of increments measured b Mean thickness (mm) of increments, measured as the distance between successive dark bands of second-order increments. c Calculated by dividing the number of second-order increments, which represent weeks, by 52. from 0.082 to 0.111 mm, and the average for all seven is 0.094 mm, almost half that of the other species. Next, second-order increments typically contain about seven third-order increments, with variation in number due probably to both preservation and inherent variability in the signals responsible for increments at both scales. Assuming that the third-order increments represent days (based on their size and analogy with other proboscideans), second-order increments typically represent intervals of 1 week. Perhaps the most significant evidence for weekly periodicity is the number of second-order increments that occur within the recognizable first-order features. If these features do represent weeks, then there should be approximately 52 per first-order feature. The thicknesses of the two first-order increments in F:AM 129673 ( Fig. 5A) were measured on the sample block with a dial caliper and their positions identified in the second-order increment data to determine the number of second-order increments identified within each first-order feature. The firstorder increment marked ‘a’ in Fig. 5A includes 53 second-order increments, and that marked ‘b’ contains 49 second-order increments. Similarly, the single first-order increment visible in F:AM 129671 (not figured; marked ‘c’ in Fig. 7A) includes 52 second-order increments. Thus, both specimens conform to expectation and strongly support the interpretation of second-order increments as weekly features. The evidence for the weekly periodicity of second-order increments also corrobo- rates the annual periodicity of the first-order increments. Third-order increments (Fig. 5C ) were analyzed only to assist interpretation of the second-order increments. Detailed analysis of these smallestscale features is complicated by the inconsistency of their expression both along a single transect and across a thin section. Frequently, third-order increments are only visible in the dark portion of the weekly increments. However, all specimens examined in this study had recognizable thirdorder increments in all thin sections prepared. In areas of good preservation, as in Fig. 5C, the hierarchical relationship between second- and third-order increments is evident. 6.3. Luminance transects The autocorrelation results for the seven individual luminance transects analyzed are presented in Table 2. The purpose of these autocorrelation analyses is to evaluate the identification of the second-order increments based on analysis of the luminance data from the individual, discrete sampling transects and to determine whether the features marked are reasonably the same both within and between tusks. For each transect, the mean increment thickness is based on the identification and measurement of second-order increments using Optimas. The implied lag assumes that the second-order increments were properly identified and represents the lag expected to have statistically 340 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 Table 2 Results of autocorrelation of luminance transects from three specimens Specimen Transect Mean increment thickness (mm) Implied laga Statistically significant lagsa Implied increment thickness (mm) F:AM 129670 5 7 10b 3 10b 2b 6b 0.090 0.083 0.079 0.107 0.069 0.123 0.086 12–13 11–12 11–12 15 9–10 17–18 12–13 10–14 9–14 10–11 15–17 9–17 17–24 11–19 0.071–0.099 0.064–0.099 0.071–0.078 0.107–0.121 0.064–0.121 0.121–0.170 0.078–0.135 F:AM 129671 F:AM 129673 a Lags are in number of increments. b Detrended to remove a linear trend prior to autocorrelation. significant r when the luminance data are autocort related. The implied lag is the ratio of the mean increment thickness measured along a given luminance transect to the luminance sampling interval, 0.0071 mm. The ranges for implied lags in Table 2 are for means that fall between the integer valued lags. The statistically significant lags are the result of the autocorrelation of the data for each of the seven individual luminance transects and is determined from the values of Z ; for those transects t that have more than one statistically significant peak, only that corresponding to the mean increment thickness is included. Peaks in Z that t do not correspond to mean increment thickness can occur at lags that are multiples of a base periodicity in the data or because of other potentially periodic features in the luminance data, such as Schreger banding. The implied increment thickness is the converse of the implied lag: it is the product of the statistically significant lags and the luminance sampling interval. Comparisons can be made either between the mean and implied increment thicknesses or between the implied and statistically significant lags. In six of the seven transects, the mean increment thickness and the implied lag are in the range of the statistically significant lags and the implied increment thickness. Additionally, in three of these six transects, the lag with the highest Z statistic corresponds either to one of the values of the implied lag (transect 7 of F:AM 129670, transect 6 of F:AM 129673) or exactly to the implied lag (transect 3 of F:AM 129671). For the seventh autocorrelated transect, transect 10 of F:AM 129670, the implied lag does overlap with the statistically significant lag, and the mean increment thickness is less than one 0.001 mm larger than the implied thickness for t=11. Two additional transects, not included in Table 2, have peaks in r that are not statistically significant but do corret spond exactly to the implied lag for those transects. These results indicate that the features marked as second-order increments can be objectively identified on the basis of the luminance profile given good preservation and also imply that the ‘same’ features are consistently marked. Given that no qualitative differences were evident between the seven transects in Table 2 and others from those specimens or from the remaining four specimens, it is reasonable to conclude that second-order increments were consistently and objectively identified, even in those individual luminance transects that were not checked with autocorrelation. 6.4. Second-order increment thickness profiles Measurements of second-order increment thickness are presented in Table 1 and Figs. 6–8. The similarities in the mean and standard deviation of increment thickness in Table 1 for all seven specimens further argue for the ‘sameness’ of the features measured. The autocorrelograms are based on autocorrelation of the increment thickness data. None of the Barstovian specimens has any regular or cyclic pattern in increment thickness for sections of tusk representing 4.6–6.7 years of tusk D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 341 Fig. 6. Second-order increment thickness and autocorrelograms of thickness data for late Barstovian Gomphotherium tusks. In increment thickness plots, the abscissa begins with the oldest increment measured, at the enamel–dentin junction, and moves forward in time toward the axis of the tusk or the pulp cavity surface, which represent the youngest increments in a section. (A) Plot of second-order increment thickness for F:AM 99059. The asterisks mark places where cracks in the dentin could not be bridged by luminance transects. The number of increments missing was estimated using the length of missing section and the average increment thickness for the whole slide; thus, increment numbers for F:AM 99059 are somewhat approximate. (B) Autocorrelogram, Z statistics and critical values of Z (a=0.05) for F:AM 99059. Increment thickness data were detrended prior to autocorrelation. (C ) Plot of t second-order increment thickness for F:AM 129670. (D) Autocorrelogram, Z statistics and critical values of Z (a=0.05) for F:AM t 129670. (E ) Plot of second-order increment thickness for UNSM 88511. (F ) Autocorrelogram, Z statistics and critical values of Z t (a=0.05) for UNSM 88511. Increment thickness data were detrended prior to autocorrelation. growth ( Fig. 6). The pattern for F:AM 99059 (Fig. 6A) exhibits two areas of relatively high growth, near the beginning and again at the end of the section plotted. However, given the inconsistency of the pattern, the simplest explanation is simply individual variation in growth rate. This is corroborated by the lack of a temporally significant periodicity in the autocorrelogram in Fig. 6B. Although the correlation coefficient for t=14 is significant, and 14 is close to the value expected 342 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 Fig. 7. Second-order increment thickness and autocorrelograms of thickness data for late Clarendonian Gomphotherium tusks. (A) Plot of second-order increment thickness for F:AM 129671. (B) Autocorrelogram, Z statistics and critical values of Z (a=0.05) for t F:AM 129671. (C ) Plot of second-order increment thickness for F:AM 25729. (D) Autocorrelogram, Z statistics and critical values of Z (a=0.05) for F:AM 25729. t for a lunar monthly signal for the second-order increments, the patterns in Fig. 6A and B are not consistent overall with a regular cyclicity in the data, and an alternative temporal signal (i.e. weekly) for the second-order increments is well supported. F:AM 129670 and UNSM 88511 (Fig. 6C and E) have irregularly spiky patterns and the lowest standard deviations, indicating that few increments stray far from the average thickness. (Increment thickness measurements on three additional thin sections from F:AM 129670 cover most of the life history of that animal; the increment thickness profiles, however, are not qualitatively different from Fig. 6C and are not presented here.) The autocorrelograms for F:AM 129670 and UNSM 88511 ( Fig. 6D and F ) are characteristic of an aperiodic time series. The peaks in Z that do cross the critical values are associated t with relatively short lags that do not correspond to temporal signals clearly associated with a cyclic, sub-annual pattern of variation in increment thickness. Increment thickness data from one of the two late Clarendonian specimens, F:AM 129671, exhibit a pattern that is consistent with seasonal growth ( Fig. 7A and B). The pattern of shading beneath the plot of increment thickness indicates the location and extent of the first-order increment ‘c’ discussed above, as well as the location and extent of the light part of a second first-order increment toward the enamel. The question mark in the long shaded stretch after increment 96 indicates the absence of first-order increments through that part of the section. The overall impression of Fig. 7A is that there are a number of areas of high growth rate separated by narrower zones of low growth. The most prominent areas of high growth are from increment 62 to increment 96, from 132 to about 170 and from just past 190 to about 240. A possible fourth zone of high D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 343 Fig. 8. Second-order increment thickness and autocorrelograms of thickness data for early Hemphillian Gomphotherium tusks. (A) Plot of second-order increment thickness for F:AM 129673. (B) Autocorrelogram, Z statistics and critical values of Z (a=0.05) for t F:AM 129673. (C ) Plot of second-order increment thickness for F:AM 129672. (D) Autocorrelogram, Z statistics and critical values of Z (a=0.05) for F:AM 129672. t growth extends from increments 21 to 56, which would have a corresponding zone of slow growth from increment 1 to 20. The two peaks at increments 5 and 12 are possibly erroneously large because the quality of the dentin near the enamel– dentin junction is often different, and increments there are more difficult to identify. A final area of high growth seems to commence at the end of the plot for F:AM 129671; these peaks are generally smaller than those in other zones of high growth as increments near the axis are usually somewhat thinner and often also more difficult to identify. Three of the complete zones of high growth seem to last about 35–40 weeks. The only exception to this is the zone from increments 190 to 240. The zones of high growth from increments 21 to 56 and from 62 to 96 roughly correspond to the position of the light portions of the first-order increments. The pattern of growth exhibited by F:AM 129671 seems to be periodic but does not precisely correspond to annual cycles in that pairs of high and low growth zones do not come in intervals of 52 second-order increments. This is supported by the autocorrelogram ( Fig. 7B), the overall pattern of which is indicative of cyclicity in the data, but the peak in significance is for t=57–63 (and again at t=65), too long to be annual if all secondorder increments are properly identified. However, the autocorrelation coefficient for t=53 (Z =1.90) is marginally significant. Also, the stat tistically significant negative values of r in Fig. 7B, t which result from cyclic data lagged so that it is perfectly out of phase with itself, range from t= 24 to 37. This range includes t=26, which is the lag associated with an annual cycle (52 weekly increments) that is out of phase with itself. A variety of factors could lead to an inexact correspondence of the identified cycles to annual cycles, including differences in the behavioral and physio- 344 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 logical responses of the animal from year to year, annual climate variability, and error in increment identification. However, the five identifiable zones of high growth imply approximately 5 years of growth in Fig. 7A, and the number of increments indicates that there should be just over 5 years represented in this section of the tusk ( Table 1). Thus, a reasonable conclusion is that this specimen is exhibiting a pattern of seasonal growth in which long periods of high growth are interspersed with somewhat shorter intervals of slower growth, although these periods do not seem to correlate perfectly with annual cycles. Overall, the other late Clarendonian specimen, F:AM 25729, does not appear to have a seasonal growth pattern (Fig. 7C and D). However, the first 90 or so increments are somewhat suggestive of a pattern like that of F:AM 129671. The first 34 increments are a relatively high growth period and are followed by 10 weeks of slower growth beginning with increment 35. Another high growth period commences with increment 45 and lasts until about increment 80, or 35 weeks. The remainder of the increments represent almost 2 years of lower growth without an identifiable pattern, and none of the increments over this period is as thick as the thickest increments in either of the earlier high growth periods. It is possible that the first part of this profile, up to increment 80, represents one full seasonal cycle and the high growth period of another. However, in the absence of clear firstorder increments or a more consistent growth pattern, this explanation is difficult to evaluate. The autocorrelogram has a single significant peak at t=35, which could be influenced by the pattern noted for the first 80 increments. Otherwise, the autocorrelogram does not indicate any strong cyclic behavior in the data. As with the Clarendonian specimens, only one of the early Hemphillian specimens, F:AM 129673, has a pattern that is interpretable as the result of seasonal growth (Fig. 8A and B). The shading indicates the position of the first-order increments discussed above, and the question mark again indicates the portion of the sample for which there is no evidence of first-order increments. Two prominent areas of high growth run from increment 83 to 105 and from increment 134 to 148. The first of these zones corresponds closely to the position of the dark portion of first-order increment ‘a’ ( Fig. 8A), which runs from increment 89 to 95 in Fig. 8A. The other zone of high growth is slightly broader than the dark portion of first-order increment ‘b’ (Fig. 5A), which only runs from about increment 137 to 143. However, the placement of the first-order increments is measured with dial calipers and hence is somewhat gross relative to the precision of the increment thickness data. Two other zones of high growth can also be identified. The more distinct of these is from increment 178 to increment 195. Although there are two peaks just beyond this zone, both are close to the axis of the tusk where increments are more difficult to identify, and so each may result from inaccurate identification of increments. The second, less distinct zone of high growth is from increment 32 to 43. This zone would be more pronounced but for single peaks on either side at increments 26 and 52. Both of these increments are about twice as thick as the surrounding increments, and it is possible that they are incorrectly marked. Beginning with the zone of high growth at increment 32, the pairs of high and low growth zones have a fairly close correspondence to intervals of 52 increments, as expected for a seasonal pattern of growth. The four zones of high growth separated by zones of low growth imply that this section represents about 4 years, the same number of years inferred from the increment count ( Table 1). Additionally, the autocorrelogram ( Fig. 8B) is consistent with an annual periodicity as r is statistically significant for t=43–53, though t the highest r is for t=46, a discrepancy that could t easily result from variability in tusk growth and a small number of improperly marked increments. The other Hemphillian specimen, F:AM 129672 ( Fig. 8C and D), has little evidence of a seasonal pattern. Some intervals of relatively low growth are evident, such as from increment 7 to 20 and again around increment 125, but these are not particularly distinct and do not relate to welldefined high growth zones. The most notable aspect of the profile is the extended period of very low growth from increment 195 to the end of the plot, an interval of just more than a year. The increments over that section are consistently thin- D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 ner than almost all of the preceding increments, and they represent an interval that is conspicuously different from the earlier portion of the profile. 7. Discussion The increment thickness profiles generally correspond to the expectations of an increase in seasonality during the late Miocene. However, the lack of conformity within both the Clarendonian and Hemphillian samples and the small sample size of the study suggest caution in interpreting the data. The lack of seasonal patterns in F:AM 129672 and F:AM 25729 may result from interannual or geographic variation in the degree of seasonality. Some late Pleistocene mammoths, such as those from the Dent site in Colorado, do not exhibit a high-amplitude variation in second-order growth increments, despite clearly seasonal oxygen isotope profiles ( Fisher, 1995). Additionally, the sample of tusks may contain a range of ages and a mix of tusks from males and females. Patterns of tusk growth do change with age, sex and reproductive status ( Fisher, 1996). Thus, a lack of strong patterns in two of the four more recent specimens does not necessarily invalidate the observed patterns of the remaining specimens. Notable in this regard is that the only specimens that have clear first-order increments, associated with seasonality in extant mammals ( Klevezal and Kleinenberg, 1969), are also the only specimens to exhibit seasonal patterns of growth in the second-order increments. A question that remains is whether the late Miocene was characterized by seasonality in temperature, precipitation, or both. A slight difference in the second-order increment patterns of F:AM 129671 and F:AM 129673 is suggestive of an answer. The lengths of the three most prominent high growth intervals in F:AM 129671, the late Clarendonian specimen, range from 35 to 40 weeks, and the low growth periods are briefer (Fig. 7A and C ). This pattern is similar to that of many late Pleistocene tusks, which commonly have high growth throughout most of the year and a couple of months of slow growth. As discussed, this pattern in Pleistocene tusks is interpreted to 345 result from nutritional stress associated with highly seasonal temperature distributions (Fisher, 1988; Koch, 1989), which is corroborated by the pattern of oxygen isotope variation in the tusk dentin ( Koch et al., 1989). The high growth intervals in F:AM 129673, the late Hemphillian specimen, are only 11–24 weeks long ( Fig. 8A), essentially the inverse of the late Clarendonian and late Pleistocene patterns. This implies that the growing season may have been much shorter and more distinct in the early Hemphillian than in the late Clarendonian. A possible explanation for the difference between late Clarendonian and early Hemphillian tusks is the gradual increase in aridity and the development of a single, distinct wet season. Seasonally abundant precipitation could lead to brief periods of high food abundance and high growth rates in proboscideans. 8. Conclusion This study had two main goals. The first was to describe the growth and structure of Gomphotherium tusks and to make comparisons with the tusks of other proboscideans. The specimens examined indicate that gomphotheres are similar to other proboscideans in that their tusks grew throughout life and are composed of incremental features that occur on multiple scales, both spatially and temporally. Annual and daily increments are similar among all species examined thus far, but gomphotheres are similar to some specimens of Mammuthus (unpub. data) in having intermediate-scale increments that have a weekly, rather than fortnightly or lunar monthly, period. The second goal was to use patterns of tusk growth to test the hypothesized increase in seasonality during the late Miocene in North America. The increment thickness profiles correspond to the expectations of an increase in seasonality in that only late Clarendonian and Hemphillian tusks have seasonal patterns of growth. Additionally, the differences between the late Clarendonian and Hemphillian specimens are consistent with the development of a wet season and an increase in the seasonality of precipitation. Unsurprisingly, the results also suggest that the changes in climate 346 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 may have been more complicated than a simple secular increase in seasonality. Regardless, the data presented here do support an increase in seasonality, and hence lend support to the climate change hypothesis for mammalian faunal changes in North America during the late Miocene. The interpretation of the growth patterns can be tested in three ways. The first is to increase the sample size. A number of the localities represented in this study have numerous isolated tusks; analysis of multiple specimens from the same locality would be important to demonstrate the generality and replicability of the patterns noted here. Next, tusks of extant elephants from a range of climatic regimes could be analyzed to determine the impact of different patterns of seasonality on tusk growth in a modern analog. For example, a comparison of tusks of elephants from the grasslands of East Africa with those of elephants from the forests of Central Africa could be useful in determining the effect of aridity and seasonal precipitation on tusk growth. The third test would be to examine the patterns of variation in stable isotope composition in a manner analogous to that of Koch et al. (1989). If temperature, through its effect on the availability of food resources, is a major factor in the changes in growth rate, the variation in oxygen isotope composition of tusk hydroxyapatite should correlate with the variation in increment thickness. Variation in the carbon isotope composition within individual tusks could be used to investigate changes in diet that result from shifting food resource abundances on annual time scales. Carbon isotope measurements of tusks from middle to latest Miocene localities might indicate long-term trends in diet that reflect habitat change over the course of Miocene in response to climate change. The carbon isotope composition of the teeth of Miocene herbivorous mammals from North America and elsewhere has indicated an increase in the abundance of C plants ( lowland 4 and arid grasses) relative to C plants (trees, 3 shrubs, herbs), both of which have distinct carbon isotope signatures (Cerling et al., 1997). Thus, if the type of vegetation available to gomphotheres were changing over the course of each year of an individual’s life or over the course of the late Miocene in response to changing climate and habitat, the shift might be detectable from the carbon isotope composition of the tusks. These tests, some of which are in progress, will serve to increase our understanding of the causes and dynamics of the climatic and ecological changes that occurred in North America as grassland habitats spread out over the last 10 million years of the Miocene. Acknowledgements This paper would not have been possible without the generous access to specimens provided by R. Tedford (AMNH ) and M. Voorhies ( UNSM ). The research benefited greatly from the guidance of D.C. Fisher. The manuscript was improved by the comments and advice of C. Badgley, J. Bloch, D.C. Fisher, G. Gunnell, E. Kowalski, B. Sanders, J. Trappani, and M. Uhen, though none of them should be held accountable for its final form. P. Koch and J.F. Thackeray provided helpful reviews. This research was supported by NSF grant SBR-9211984 to D.C. Fisher. References Alroy, J., 1992. Conjunction among taxonomic distributions and the Miocene mammalian biochronology of the Great Plains. Paleobiology 18, 326–343. Barry, J.C., Johnson, N.M., Raza, S.M., Jacobs, L.L., 1985. Neogene mammalian faunal change in southern Asia: correlations with climatic tectonic and eustatic events. Geology 13, 637–640. Boyde, A., 1979. Carbonate concentration crystal centers core dissolution caries cross striations circadian rhythms and compositional contrast in the SEM. J. Dent. Res. 58, 981–985. Burke, A., Castanet, J., 1995. Histological observations of cementum growth in horse teeth and their application to archaeology. J. Archaeol. Sci. 22, 479–493. Cerling, T.E., Harris, J.M., MacFadden, B.J., Leakey, M.G., Quade, J., Eisenmann, V., Ehleringer, J.R., 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389, 153–158. Corner, R.G., 1976. An early Valentinian vertebrate local fauna from southern Webster County, Nebraska. Masters thesis, University of Nebraska. Davis, J.C., 1986. Statistics and Data Analysis in Geology. second ed., Wiley, New York. D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 Dean, M.C., 1987. Growth layers and incremental markings in hard tissues, a review of the literature and some preliminary observations about enamel structure in Paranthropus boisei. J. Hum. Evol. 16, 157–172. Fisher, D.C., 1984. Taphonomic analysis of late Pleistocene mastodon occurrences: evidence of butchery by North American Paleo-Indians. Paleobiology 10, 338–357. Fisher, D.C., 1987. Mastodon procurement by Paleoindians of the Great Lakes Region: hunting or scavenging? In: Nitecki, H., Nitecki, D.V. (Eds.), The Evolution of Human Hunting. Plenum Press, New York, pp. 309–421. Fisher, D.C., 1988. Season of death of the Hiscock mastodonts, in: Laub, R.S., Miller, N.G., Steadman, D.W. (Eds.), Late Pleistocene and Early Holocene Paleoecology and Archaeology of the Eastern Great Lakes Region. Bulletin of the Buffalo Society of Natural Sciences Vol. 33., 115–125.. Fisher, D.C., 1990. Age, sex and season of death of the Grandville mastodon. Michigan Archaeol. 36, 141–160. Fisher, D.C., 1995. in: Season of Death of the Dent mammoths, Society of American Archaeologists, Abstracts for 60th Annual Meeting, 96. Fisher, D.C., 1996. Extinction of proboscideans in North America. In: Shoshani, J., Tassy, P. (Eds.), The Proboscidea. Oxford University Press, Oxford, pp. 296–315. Flower, B.P., Kennett, J.P., 1994. The middle Miocene climatic transition: East Antarctic ice sheet development, deep ocean circulation and global carbon cycling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 108, 537–555. Fox, D.L., Fisher, D.C., 1994. Tusk growth rate in Loxodonta africana as recorded by incremental laminae in tusk dentin. J. Vert. Paleontol. 14, 26A. Gysi, A., 1931. Metabolism in adult enamel. Dent. Digest 37, 661–668. Halberg, F., 1983. Quo vadis basic and clinical chronobiology, promise for health maintenance. Am. J. Anat. 168, 543–594. Haq, B.U., Hardenbol, J., Vail, P.R., 1987. Chronology of fluctuating sea levels since the Triassic. Science 235, 1156–1167. Hewer, H.R., 1964. The determination of age, sexual maturity, longevity and a life-table in the grey seal (Halichoerus grypus). Proc. Zool. Soc. Lond. 142, 593–634. Janis, C.M., 1989. A climatic explanation for patterns of evolutionary diversity in ungulate mammals. J. Palaeontol. 32, 463–481. Kimura, F., 1939. Horoshitsu ni mirareru Seicho-sen no shuki. Kobyo-shi 13, 454–455. Klevezal, G.A., Kleinenberg, S.E., 1969. Age Determination of Mammals from Annual Layers in Teeth and Bones. Israel Program for Scientific Translations, Jerusalem. translated from Russian. Klevezal, G.A., 1980. Layers in the hard tissues of mammals as a record of growth rhythms of individuals. In: Perrin, W.F., Myrick, A.C. ( Eds.), Age Determination of Toothed Whales and Sirenians. Reports of the International Whaling Commission. Cambridge, Special Issue 3, pp. 89–94. Klevezal, G.A., Mina, M.V., 1990. Daily layers and hibernation marks in incisor dentin of Sicista pseudonapaea and some biological remarks. Acta Theriol. 35 3–4, 345–356. 347 Koch, P.L., 1989. Paleobiology of Late Pleistocene Mastodonts and Mammoths from Southern Michigan and Western New York, Ph.D. thesis, University of Michigan. Koch, P.L., Fisher, D.C., Dettman, D.L., 1989. Oxygen isotope variation in the tusks of extinct proboscideans: a measure of season of death and seasonality. Geology 17, 515–519. Laws, R.M., 1952. A new method of age determination for mammals. Nature 169, 972. Laws, R.M., 1962. Age determination of pinnipeds with special reference to growth layers in teeth. Zeitschrift fur Säugetierkunde 27, 129–146. Lieberman, D.E., 1993. The rise and fall of seasonal mobility among hunter–gatherers. Curr. Anthropol. 34, 599–631. Lieberman, D.E., 1994. The biological basis for seasonal increments in dental cementum and their application to archaeological research. J. Archaeol. Sci. 21, 525–539. Marsh, H., 1980. Age determination of the dugong (Dugong dugon (Müller)) in northern Australia and its biological implications. In: Perrin, W.F., Myrick, A.C. (Eds.), Age Determination of Toothed Whales and Sirenians. Reports of the International Whaling Commission, Cambridge, Special Issue 3, pp. 181–200. Mayhew, D.F., 1978. Age structure of a sample of subfossil beavers (Castor fiber L). In: Butler, P.M., Joysey, K.A. ( Eds.), Development, Function and Evolution of Teeth. Academic Press, London, pp. 495–505. Miani, A., Miani, C., 1972. Circadian advancement rhythm of the calcification front in dog dentin. Panminerva Medica 14, 127–136. Miller, K.G., Fairbanks, R.G., Mountain, G.S., 1987. Tertiary oxygen isotope synthesis sea-level history and continental margin erosion. Paleoceanography 2, 1–19. Mummery, J.H., 1924. The Microscopic and General Anatomy of the Teeth, Human and Comparative. Oxford University Press, Oxford. 240 pp. Okada, M., 1943. Hard tissues of animal body. The Shanghai Evening Post., 15–31. date unknown. Owen, R., 1840–1845. Odontography. Hippolyte Bailliere, London. Perrin, W.F., Myrick, A.C., 1980. Age Determination of Toothed Whales and Sirenians. Reports of the International Whaling Commission. Cambridge. Special Issue 3, 229 pp. Rosenberg, G.D., Simmons, D.J., 1980. Rhythmic dentinogenesis in the rabbit incisor: circadian, ultradian, and infradian periods. Calcif. Tiss. Int. 32, 29–44. Saxon, A., Higham, C., 1969. A new research method for economic prehistorians. Am. Antiq. 34, 303–311. Schour, I., Hoffman, M.M., 1939. Studies in tooth development I: the 16 microns calcification rhythm in the enamel and dentin from fish to man. J. Dent. Res. 18, 91–102. Schmidt, W.J., Kiel, A., 1971. Polarizing Microscopy of Dental Tissues. Pergamon Press, Oxford. Sheffer, V.B., 1950. Growth layers on the teeth of Pinnepedia as an indicator of age. Science 112, 309–311. Sheffer, V.B., 1970. Growth layers in a dugong tooth. J. Mammal. 51, 187–190. 348 D.L. Fox / Palaeogeography, Palaeoclimatology, Palaeoecology 156 (2000) 327–348 Singh, G., 1988. History of aridland vegetation and climate: a global perspective. Biol. Rev. 63, 156–196. Slooten, E., 1991. Age, growth and reproduction in Hector’s dolphin. Can. J. Zool. 69, 1689–1700. Stucky, R.K., 1990. Evolution of land mammal diversity in North America during the Cenozoic, in: Genoways, H. ( Ed.), Current Mammalogy Vol. 2. Plenum, New York, pp. 375–432. Swan, A.R.H., Sandilands, M., 1995. Introduction to Geological Data Analysis. Blackwell Science, Oxford. 446 pp Tedford, R.H., Skinner, M.F., Fields, R.W., Rensberger, J.M., Whistler, D.P., Galusha, T., Taylor, B.E., Macdonald, J.R., Webb, S.D., 1987. Faunal succession and biochronology of the Arikareean through Hemphillian internal (Late Oligocene through Earliest Pliocene epochs) in North America. In: Woodburne, O. ( Ed.), Cenozoic Mammals of North America. University of California Press, Berkeley, CA, pp. 153–210. Tobien, H., 1972. Status of the genus Serridentinus Osborn 1923 (Proboscidea, Mammalia) and related forms. Mainzer Geowissenschaftliche Mitteilungen 1, 143–191. Webb, S.D., 1977. A history of savanna vertebrates in the new world — Part 1: North America. Annu. Rev. Ecol. Syst. 8, 355–380. Webb, S.D., 1984a. Ten million years of mammal extinctions in North America. In: Martin, P.S., Klein, R.G. ( Eds.), Quaternary Extinctions: A prehistoric revolution. The University of Arizona Press, Tucson, AZ, pp. 189–210. Webb, S.D., 1984b. On two kinds of rapid faunal turnover. In: Berggren, W.A., van Couvering, J. ( Eds.), Catastrophes and Earth History: The New Uniformitarianism. Princeton University Press, Princeton, NJ, pp. 417–436. Wei, K.Y., Kennett, J.P., 1986. Taxonomic evolution of Neogene planktonic foraminifera and paleoceanographic relations. Paleoceanography 1, 67–84. Wilkinson, L., 1990. SYSTAT: The System for Statistics. Systat, Evanston, IL. Wolfe, J.A., 1985. Distribution of major vegetational types during the Tertiary, in: The Carbon Cycle and Atmospheric CO Geophysical Monograph Vol. 32., 357–375. 2 Woodburne, M.O., 1987. Cenozoic Mammals of North America. University of California Press, Berkeley, CA. Woodruff, F., Savin, S.M., 1989. Miocene deepwater circulation. Paleoceanography 4, 87–140. Wright, J.D., Miller, K.G., Fairbanks, R.G., 1992. Early and middle Miocene stable isotopes: implications for deepwater circulation and climate. Paleoceanography 7, 357–389.