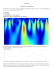
Gamna-Gandy Bodies in Surgical Neuropathology Specimens
Transcription
Gamna-Gandy Bodies in Surgical Neuropathology Specimens
Journal of Neuropathology and Experimental Neurology Copyright q 2004 by the American Association of Neuropathologists Vol. 63, No. 2 February, 2004 pp. 106 112 Gamna-Gandy Bodies in Surgical Neuropathology Specimens: Observations and a Historical Note B. K. KLEINSCHMIDT-DEMASTERS, MD Key Words: noma. Cavernous angioma; Cholesterol granuloma of bone; Hemosiderin; Pituitary adenoma; Sacral cyst; Schwan- INTRODUCTION Stedman’s Medical Dictionary, 27th edition, defines Gamna-Gandy bodies (synonyms: Gandy-Gamna bodies, Gamna-Gandy nodules, tobacco flecks, siderotic nodules) as ‘‘small, firm, spheroidal or irregular foci that are yellow-brown, brown, or rusted color, occurring chiefly in the spleen in such conditions as congestive splenomegaly and sickle cell disease, and consisting of relatively dense fibrous tissue with collagenous fibers impregnated with iron pigment and calcium salts; probably result from organization and scarring of sites where small perivascular hemorrhages occurred’’ (1). Still described under this eponymic designation in several general (2–8) and surgical (9) pathology texts published between 1965 and 1995, the entity, under this name, has all but disappeared from several other well-known contemporary general pathology books (10) and surgical pathology texts (11), including more recent editions of the same texts where it was formerly mentioned (12). In some cases, textbooks have eliminated the term in their indices and it is only by careful scrutiny of the section on atrial myxomas that one finds the term ‘‘G-G body’’ inconspicuously hiding in the middle of a paragraph (13). Few texts provide even black and white illustrations of the G-G body (2–4), although a beautiful color photomicrograph of a G-G body in an atrial myxoma can be found in the C. D. M. Fletcher’s, Diagnostic Histopathology of Tumors (14). From Departments of Pathology, Neurology, and Neurosurgery, University of Colorado Health Sciences Center, Denver, Colorado. Correspondence to: B. K. Kleinschmidt-DeMasters, MD, University of Colorado Health Sciences Center, 4200 E. Ninth Ave., Denver, CO 80262. E-mail: [email protected] Whither goest the Gamna-Gandy body? It was still a viable term taught during my pathology residency training in the early 1980s, but hard to find in textbooks even then. As noted above, the eponymic designation has now either been discarded altogether or the description has been simplified to a more mundane ‘‘multiple brown scars containing hemosiderin’’ (8). Indeed, even in the few instances where the term has been retained, the emphasis over the years has shifted away from the collagenous scar aspects of the G-G body (as described in spleen) to the mineralization of collagenous fibers by iron, and to a lesser extent, calcium salts (as described in atrial myxoma). However, going back to Gamna’s original description, and older papers in the literature, this mineralization, although always present and the essential component of G-G bodies, was not the histological feature of the nodules that captured the imagination of multiple investigators in the late 1920s and 1930s. What then did Carlos Gamna, an Italian physician (1866–1950), describe? Between 1921 and 1927 he published 3 articles (15–17) giving a detailed histological description of the lesion that bears his name. It should be acknowledged that others had described and even illustrated similar findings earlier, including the French physician Charles Gandy (1872–1943) in 1902 (18, and again in 1906 (19), Giovanni Marini in 1902 (20), and Stengel in 1904 (21). Schuippisser provided a particularly beautiful color drawing of the entity in his 1922 publication (22) (Fig. 1A). Nevertheless, Gamna gave one of the best descriptions and was given credit soon after his publications. Gamna described ‘‘disseminated granulomata in the pulp of the spleen composed of foreign-body giant cells, hemosiderin deposits, calcium precipitate, hyalinized connective tissue fibers, and curious, straw-colored, 106 Downloaded from http://jnen.oxfordjournals.org/ by guest on October 24, 2016 Abstract. Gamna-Gandy (G-G) bodies are classically defined as spheroidal yellow-brown foci consisting of dense fibrous tissue and collagenous fibers encrusted with iron pigments and calcium salts. These siderotic nodules were first described in the spleen early in the twentieth century and for a short time were considered to be caused by fungal infection due to the presence of unusual ‘‘bamboo-like and articulated’’ fibers in the lesions that vaguely mimicked mycelia forms. This notion was proven to be incorrect in the 1930s and G-G bodies are now considered to result from organization of small hemorrhages. Although originally reported in splenomegaly, G-G bodies are well-recognized findings in atrial myxomas where they form linear arrays of mineral-encrusted fibers, often at the edge of resolving hemorrhages. They rarely have been reported in lymph nodes, thymoma, thyroid adenoma, and renal cell carcinoma. Curiously, published examples of G-G bodies in central nervous system (CNS) neoplasms or vascular malformations have not appeared, despite the known tendency for bleeding, even recurrent episodes of bleeding, in several types of these lesions. Since 1999 I have accrued all the examples of G-G bodies that I have observed in my practice of surgical neuropathology. These cases are presented here and the historical aspects of the entity are reviewed. GAMNA-GANDY BODIES same year, however, more rigorous scientific studies were published that proved fungal infection to be incorrect. Fasiani and Oselladore in 1929 reported being able to experimentally produce G-G bodies in cats by the injection of alcohol (27). However, it wasn’t until 2 years later, in 1931, that definitive microbiology studies were published that completely dispelled a fungal causation for GG bodies. Reimann and Kurotchkin studied the spleen in 6 cases of primary and cirrhotic splenomegaly and found no correlation between whether G-G bodies were present and whether fungus could be cultured from that spleen (29). Even after selectively dissecting out the G-G bodies that they could grossly identify in 1 spleen and spreading the material directly onto agar slants and 30 agar tubes, no fungus could be cultured from any of the siderotic nodules. They even went further and proved that the organisms that they did recover were completely nonpathogenic by inoculating 12 monkeys with the fungus; none died from fungal infection nor showed splenic abnormalities when they were killed 12 to 18 months later (29). Reimann and Kurotchkin astutely pointed out that G-G bodies could be found in several different diseases of the spleen (tuberculosis, kala-azar, and syphilis), making it unlikely that there was a single infectious cause for G-G bodies. They even mentioned briefly that they had seen G-G bodies in an adenomatous thyroid gland, foreshadowing reports that would appear 40 years later. With the loss of a possible fungal etiology, the emphasis on the mycelial-like, waxy forms in the lesions declined and the recognition increased that iron, and to a lesser extent calcium, encrustation of degenerated tissue fibers was the essential component of the G-G bodies. As is often the case with diseases bearing eponymic designations, a gain in the understanding of the etiology parallels a decline in the need for an eponym. Hence, today a simple descriptor of ‘‘iron encrustation’’ or ‘‘mineral impregnation of degenerated tissue fibers’’ has replaced ‘‘Gamna-Gandy’’ body and is all that is necessary to recognize this phenomenon. Coupled with the fact that pathologists and physicians in general have advocated decreased usage of eponymic designations, the term ‘‘Gamna-Gandy body’’ seldom appears in our more recent pathology literature or reference tomes. Interestingly, despite this decline of the use of the term by pathologists there appears to be renewed interest in the entity by radiologists. As the resolution of imaging studies became greater, more radiologists have recognized the characteristic features of these lesions on imaging studies, especially magnetic resonance imaging (MRI). If the G-G nodules contain a significant amount of calcium, they can be seen on plain radiographs and computerized tomographic (CT) images (30). However, MRI best defines them, and G-G nodules appear as spotlike, low signal intensity lesions on all pulse sequences (30). They are described as ‘‘best seen on gradient-echo J Neuropathol Exp Neurol, Vol 63, February, 2004 Downloaded from http://jnen.oxfordjournals.org/ by guest on October 24, 2016 waxy formation, spheroid, semilunar, or bamboo-shaped, resembling mycelian structures’’ (quoted by Tedeschi et al [23]). Gamna termed this lesion that he found in cases of splenomegaly unassociated with cirrhosis, ‘‘siderotic splenogranulomatosis,’’ clearly suspecting that the iron pigment was an important component. What piqued the interest of him and others, however, were the straw colored, waxy, bamboo-shaped structures noted in the nodules. Years earlier, Marini appears to have considered these waxy mycelial-like structures to be degenerated elastic fibers and not infectious agents (20). Nevertheless, others, including Oberling in 1928 (24) and Jaffe and Hill in 1928 (25), favored fungal infection as the cause. Given the fact that the cause was debated, a noncommittal eponymic designation became quickly attached to these lesions with ‘‘waxy mycelial-like structures’’ and multinucleated giant cells. Indeed, within only a couple of years both Oberling (24) in 1928 and Fasiani and Oselladore in 1929 (27) had used the term ‘‘Gandy-Gamna’’ nodules in their publications. Obviously these 2 authors gave pre-eminence to the even earlier work of Gandy (18, 19), while DeVecchi et al in a 1929 article described ‘‘aree di Gamna’’ in the spleen and ascribed the finding solely to Gamna (26). However, according to Morehead the appellation ‘‘Gamna-Gandy body’’ had achieved popularity even earlier, beginning in 1926 (2), and the hyphenated version won out. In 1929, Hu et al published an observational study of ‘‘Gandi-Gamna’’ bodies (sic, note the permutations the spelling these gentlemen’s names suffered even 75 years ago!) They made the point that neither the ‘‘wavy, branching fibers’’ in siderotic nodules of the spleen (Fig. 1B, left) nor the ‘‘brittle, rod-like fibers’’ (Fig. 1B, middle) resembled the fungi they had recovered by culture from 3 cases of splenomegaly that they had studied (Fig. 1B, right) (28). Parenthetically, these authors were actually more struck by the similarity between the ‘‘wavy, branching fibers’’ (Fig. 1B, left) and any possible fungus and stated that in their opinion the ‘‘brittle, rod-like’’ (waxy, refractile) fibers they found ‘‘were less likely to be mistaken for mycelium’’ (28). Nevertheless, Hu et al recognized that neither type of filament found in siderotic nodules (Fig. 1B, left and center) resembled mycotic hyphae since they looked and stained entirely differently than the fungi they had recovered from spleens (Fig. 1B, right). They also noted that on close observation, collagen fibers in G-G bodies could be seen to show a gradation from normal to pigment encrustation, all within the same fiber. Then, as now, however, purely morphological studies failed to convince those who held contrary convictions. Given the presence of multinucleated giant cells and septated branching fibers in the lesions, it is perhaps somewhat understandable, in retrospect, why some authors believed so strongly in an infectious etiology. Within the 107 Downloaded from http://jnen.oxfordjournals.org/ by guest on October 24, 2016 J Neuropathol Exp Neurol, Vol 63, February, 2004 KLEINSCHMIDT-DEMASTERS 108 GAMNA-GANDY BODIES cell carcinoma, with a brief report in Archives of Pathology and Laboratory Medicine, signals a resurgence of interest by pathologists in these forgotten structures (43). The paper certainly contributes beautiful color pictures of G-G bodies to the contemporary literature. Probably due to diminished interest in the historical aspects of the entity, G-G bodies have not been reported in other organs and tumor types. Certainly other disease entities provide the hemorrhages, especially recurrent hemorrhages, which seem to be the requisite substrate on which G-G bodies occur. After encountering a particularly prominent example of a G-G body in 1999, I have made a concerted effort to seek out these structures over the past several years in my surgical neuropathology material. I have accrued 10 examples in CNS and PNS neoplasms and vascular malformations, most of which have a well-recognized tendency for spontaneous or repetitive hemorrhage. The Table provides a listing of the lesions seen, and I have attempted to place them in descending order of prominence, based on size and how conspicuous they were on a given slide and the number of slides involved. Certainly G-G bodies containing waxy, refractile fibers or a well-developed and focal collagenous scar are the most eye-catching. However, some cases with copious iron-encrusted fibers arranged around the edge of large resolving clots occasionally are also able to get one’s attention! Cases 1 to 10 listed in the Table could be considered ‘‘true’’ G-G bodies in that they were obvious enough to be found, even if the observer were not specifically searching for them. Cases 1 through 5 were ‘‘case report’’ quality. However on a much smaller and more focal scale the identical process happens in some CNS lesions that are ← Fig. 1. A: Color drawing of Gamna-Gandy body illustrated from the 1922 article by Schuppisser (22) shows the linear blueblack encrusted fibers as well as the waxy septate degenerating collagen fibers that caught the eye of early workers in this field. B: Black and white drawings from the 1929 article by Hu et al (28) that showed that there was no morphological identity between the wavy encrusted fibers (left) or the waxy, septate, bamboo-like fibers (center) seen in Gamna-Gandy bodies and the fungi that could be cultured from spleens (drawn by the authors at right). These authors argued strongly against an infectious fungal etiology for Gamna-Gandy bodies. C: One type of Gamna-Gandy body, here seen adjacent to a large resolving clot in a metastatic melanoma contains linear blue-black iron-encrusted fibers that are easily detected on hematoxylin and eosin but are in their full glory as robin’s egg blue fingers admixed with macrophages on Gomori’s iron stain. This is a nearly identical illustration to that show in reference 14 as a Gamna-Gandy body in an atrial myxoma. Original magnification: 3200. D, E: The other type of Gamna-Gandy body, most similar to that described in the spleen, is illustrated in this photomicrograph taken from a cystic ‘‘baseball-sized’’ ulnar nerve schwannoma. Note the numerous multinucleated giant cells (panel D, inset) and linear blue-black delicate wavy encrusted fibers (seen at higher power in panel E) that overshadow the collagenous background. H&E, original magnifications: 3100, 3200. F: A similar ‘‘spleen-type’’ Gamna-Gandy body in a pituitary adenoma shows more collagen and demonstrates thicker, chunkier, encrusted collagen fibers. H&E, original magnification: 3200. G: On higher power, these thicker encrusted fibers seen in panel F are associated with waxy, refractile, yellow, bamboo-like, ‘‘septate’’ degenerating collagen fibers and gradations of encrustation along the same fiber can be identified (inset, white arrowhead). Original magnification: 31000. H: A semilunar waxy, bamboo-like degenerating fiber encircles a small blood vessel in a cavernous angioma (case 6) H&E, original magnification: 31000. I: Delicate septate encrusted fibers seen focally in a supratentorial primitive neuroectodermal tumor (PNET) can cause the pathologist to stop and ponder their origin. Original magnification: 3100. J, K: A minute swirl of encrusted fibers seen near the meninges in an anaplastic meningioma showed strong histochemical reaction with Gomori’s stain for iron (panel J) and almost no reaction (i.e. black staining) with Von Kossa stain for calcium. Original magnifications: 3200. J Neuropathol Exp Neurol, Vol 63, February, 2004 Downloaded from http://jnen.oxfordjournals.org/ by guest on October 24, 2016 T2 sequences, due to the blooming artifact, caused by the superparamagnetic effects of hemosiderin within those hemorrhagic remnants ’’ (30). Several CT and MRI reports of G-G bodies in the spleen have recently appeared (31–35). MRI has allowed rediscovery of what pathologists already knew 75 years ago, namely that G-G bodies are very frequent when specifically looked for in spleen. Oberling found G-G bodies in 10% (24 of 200) spleens and DeVecchi et al in 18% (30 of 169) spleens (quoted by Tedeschi et al [23]). A more contemporary morphological study demonstrated that 44% of spleens contained G-G bodies in cases of portal hypertension (36). Imaging techniques have also identified G-G bodies in cardiac myxomas and have verified that they are not rare in this site either (37, 38). Basso et al found that 10% of 102 cardiac myxomas were heavily calcified (‘‘cardiac lithomyxoma’’) on x-ray and gross inspection; half of heavily calcified myxomas showed Gamma-Gandy (sic) body formation (38). G-G bodies in cardiac myxomas have been reported several times by pathologists (39–41), but are obviously seen much more frequently than the few case reports in the literature would suggest. Indeed the beautiful example of a G-G body illustrated in the Fletcher surgical pathology text noted above comes from a cardiac myxoma (14). The existence of single case reports of G-G nodules in thymoma (23) and follicular adenoma of the thyroid (42) are also likely misleading. Reimann and Kurotchkin noted G-G in adenomatous thyroid gland and several early authors (Gamna and Schuppisser) mention finding G-G bodies in retroperitoneal lymph nodes near cirrhotic livers (quoted by Tedeschi et al [23]). Perhaps the recent 2003 sighting of G-G bodies in a renal 109 No Yes, 11 Large clot No No Yes, 1 Moderate clot Yes, 1 Small focal clot No 9. Myxopapillary ependymoma, 1 of 2 10. Cavernous angioma, 1 of 1 11. Supratentorial PNET 12. Anaplastic oligodendroglioma 13. Brain abscess with hemorrhage 14. Anaplastic oligodendroglioma 15. Anaplastic meningioma Yes, 1 Focal process but no collagen scar No, focal linear encrusted fibers near meninges No, focal encrusted degenerating collagen fibers near vessels No, focal, minute focus of linear encrusted fibers No, focal linear encrusted fibers near meninges No No Yes, 11 Yes, 1 Yes, 1 No No Yes, 1 Yes, 11 No Yes, 111 Yes, 111 No No Yes, 111 Yellow, refractile ‘‘articulated fibers’’ Yes, 11 Yes,1 No Yes, 1 No Yes, 1 No Yes, 1 No No No No No Yes, 11 No Yes, 111 but most associated with cholesterol clefts No No No No No No No Yes, 1 No No No No Iron-encrusted fibers adjacent to macrophages No Yes, 111 Multinucleated giant cells Key: PNET 5 primitive neuroectodermal tumor; subjective grading of process with 1 5 feature present but not prominent; 11 5 moderate prominence; and 111 5 feature very prominent. Yes, 11 Large clot No 7. Benign sacral cyst, 2 of 3 8. Cavernous angioma, 2 of 3 Yes, 111 Yes, 11 Moderate size clot with numerous cholesterol clefts Yes, 111 Large clot No No Yes, 111 Yes, 11 Also numerous encrusted vessels No Yes, 11 Several nodules Yes, 11 No Yes, 111 Yes, 111 Large clot No G-G body within focal hyalinized nodule Downloaded from http://jnen.oxfordjournals.org/ by guest on October 24, 2016 J Neuropathol Exp Neurol, Vol 63, February, 2004 4. Benign sacral cyst, 2 of 12 5. Pituitary adenoma, 2 of 3 6. Cavernous angioma, 3 of 3 1. Metastatic melanoma, 3 of 3 2. Schwannoma of ulnar nerve, 1 of 7 3. Cholesterol granuloma of temporal bone, 1 of 12 Tumor type, number of slides involved Linear, iron-encrusted fibers adjacent to resolving clot TABLE 110 KLEINSCHMIDT-DEMASTERS GAMNA-GANDY BODIES too could find areas where single degenerated waxy collagen fibers showed gradations to full blue-black mineral encrustation (Fig. 1G, inset). In this series, the first type of G-G body (subacute, ‘‘atrial myxoma-like’’) was prominent around large areas of resolving hemorrhage in a metastatic melanoma (case 1), 2 congenital, non-neoplastic sacral cysts (cases 4 and 7), and a cholesterol granuloma of the temporal bone (case 3). Cases with a nodular, densely collagenous scar, linear or mycelial iron-encrusted fibers, waxy bamboolike yellow refractile material, but no clot were the most focal type of G-G body (remote, ‘‘spleen-like’’). Examples included a cystic, baseball-sized schwannoma of the ulnar nerve (case 2), a mixed growth hormone-prolactin secreting pituitary adenoma (case 5), and all 3 cavernous angiomas (cases 6, 8, and 10). A myxopapillary ependymoma (case 9) also showed a focal dense collagen deposit and linear iron-encrusted fibers, but lacked the waxy, refractile, bamboo-like degenerative collagen material. A brain abscess that manifested hemorrhage and thickened, collagenous blood vessels with mineralization and waxy refractile fibers qualified as a ‘‘miniature version’’ of the same process (case 13) due to its limited nature. More focal linear iron-encrusted fibers unassociated with waxy degenerated collagen fibers of collagen deposition were also seen in several CNS tumors that lack a collagenous background or abundant thick-walled collagenized blood vessels. These ‘‘miniature’’ examples included the supratentorial PNET (case 11, Fig. 1I). This case showed beautiful septate linear encrusted fibers (Fig. 1I) that might make the pathologist pause and ponder their origin. Given the limited amount of collagen in a supratentorial PNET, it is not surprising that these were seen adjacent to blood vessels also containing organismlike wavy linear encrustations. Wavy linear encrusted fibers were also focally present in 2 anaplastic oligodendrogliomas (cases 12 and 14) and an anaplastic meningioma (case 15). In cases where sufficient tissue on deeper levels allowed for multiple stains to be performed, the iron stain (Gomori’s iron stain) (Fig. 1J) showed considerably stronger histochemical reaction than the Von Kossa stain for calcium (Fig. 1K, note absence of black staining) in these linear encrustations. Clearly, what is described and illustrated in this review of my surgical neuropathology material is hardly unique. Virtually every reader can probably cite even better examples from their own surgical or autopsy neuropathology case files. The likely—and indeed the desired—response to the examples shown in this paper might be ‘‘I’ve seen that many times before but never knew it had a name’’ (or maybe even thought it deserved a name!) To set the record straight, G-G bodies in surgical neuropathology probably don’t deserve a special designation J Neuropathol Exp Neurol, Vol 63, February, 2004 Downloaded from http://jnen.oxfordjournals.org/ by guest on October 24, 2016 generally devoid of collagenous substrate for mineralization, save for the meninges or the hyalinized walls of blood vessels. Cases 11 to 15 are examples of this very focal and ‘‘miniature versions’’ of the same thing. Admittedly, the limited nature of the process barely deserved mention in the microscopic description of the corresponding surgical pathology report, and in most instances I found them solely because I was hunting for them. But, in this regard, the supratentorial primitive neuroectodermal tumor (PNET) (Case 11) contained enough of the segmented, septate, ‘‘fungal-like’’ encrustations that they caught the eye of the referring pathologist who submitted the case. These miniature versions really do not deserve the appellation of ‘‘Gamna-Gandy body’’ but do serve to underscore the fact that the same encrustation of degenerated collagen fibers can occur in CNS tumors, albeit to a very limited degree. Clearly, the amount of collagen available in the CNS for scarring is limited to select areas around blood vessels or in meninges, and one would not expect the process to be as well-developed in the CNS than in PNS or non-nervous system sites. Basically, G-G bodies in the CNS and PNS (and I suspect elsewhere) fall into 2 groups. The first type of G-G body represents a subacute phase of the lesion and is characterized by linear iron-encrusted fibers at the edge of a resolving clot, and is identical to what is usually illustrated in cardiac myxomas (14). The fibers are blueblack and readily detectable on hematoxylin and eosin stain, although iron stains allow full appreciation of the phenomenon (Fig. 1C). No multinucleated giant cells, waxy refractile ‘‘mycelial-like’’ fibers, or collagen scar are evident in this type of G-G body. Often the ironencrusted fibers are immediately juxtaposed to collections of macrophages. The second type of G-G body represents the remote phase of the lesion and is characterized by focal nodular collagenous scars (Fig. 1D) containing linear wavy aggregates of iron-encrusted fibers without associated clot (Fig. 1E). This second type is what is classically described in the spleen of individuals with chronic portal hypertension and best fits the Stedman’s Dictionary definition of the entity. The most prominent example (Case 2, a cystic, ‘‘baseball-sized’’ schwannoma of the ulnar nerve) also contained numerous multinucleated giant cells (Fig. 1D, inset). In several cases the collagen predominated and the encrusted fibers were thicker and chunkier (Fig. 1F, case 5, a mixed growth hormone-prolactin secreting pituitary tumor is illustrated). Several cases contained prominent waxy, yellow, refractile ‘‘bamboo-like’’ segmented degenerated collagen fibers within the dense, nodular collagenous scar (Fig. 1G), or in semilunar orientation around blood vessels (Fig. 1H), identical to those described by Gamna and illustrated so beautifully by Schuppiser (Fig. 1A). Just like Hu et al (28), I 111 112 KLEINSCHMIDT-DEMASTERS or warrant guest appearances as case reports. But the historical aspects of Gamna-Gandy bodies are worth knowing about, specifically the people involved and the beauty of their morphological descriptions, now nearly 100 years ago. When we see G-G bodies in our practices of neuropathology they now can serve as reminders of ‘‘what’s in a name.’’ ACKNOWLEDGMENTS The author thanks Dr. Robert H. Shikes, Professor of Pathology and Medical History, for his helpful insights and critical review of the manuscript, Dr. Roberto Gianani for his translation from the Italian of the article by Marini, Mr. Andrew Rex for skillful manuscript preparation, and Ms. Lisa Litzenberger for photographic expertise. REFERENCES J Neuropathol Exp Neurol, Vol 63, February, 2004 Downloaded from http://jnen.oxfordjournals.org/ by guest on October 24, 2016 1. Stedman’s medical dictionary, 27th ed. Philadelphia: Lippincott Williams & Wilkins, 2000:218 2. Morehead RP. Human pathology: An introduction to medicine. New York: McGraw-Hill, 1965:1296 3. Anderson JR, ed. Muir’s textbook of pathology, 10th ed. Glasgow: Edward Arnold, 1976:510 4. St. Clair Symmers W. The lymphoreticular system in systemic pathology, 2nd ed. Edinburgh: Churchill Livingstone, 1978:731 5. Kissane JM, ed. Anderson’s pathology, 9th ed. St. Louis: CV Mosby, 1990:1416–17 6. Underwood JCE, ed. General and systematic pathology. Edinburgh: Churchill Livingstone, 1992:605 7. Rubin E, Farber JL. Pathology, 2nd ed. Philadelphia: JB Lippincott, 1994:266, 759, 1043 8. Chandrasoma P, Taylor CR. Concise pathology, 2nd ed. Norwalk: Appleton & Lange, 1995:386 9. Coulson WF. Surgical pathology. Philadelphia: JB Lippincott, 1978; 2:940 10. Cotran RS, Kumar V, Collins T, eds. Robbin’s pathologic basis of disease, 6th ed. Philadelphia: W.B. Saunders, 1999 11. Sternberg SS, ed. Diagnostic surgical pathology, 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 1999 12. Coulson WF. Surgical pathology. Philadelphia: JB Lippincott, 1988 13. Rosai J. Ackerman’s surgical pathology. St. Louis: Mosby, 1996 14. Fletcher CDM, ed. Diagnostic histopathology of tumors, 2nd ed. Edinburgh: Churchill Livingstone, 2000;1:15 15. Gamna C. Sopra alcune lesioni dei vasi nella siderosi emolitica delle milza. Giornale della R, Accademia Med Torino 1921;84:291–97 16. Gamna C. Sopra una particolare forma di splenomegalia primitiva, Giornale Biol Med Sperimentale 1923;1:22–26 17. Gamna C. Sur la clinique et l’etiologie de la splenogranulomatose siderosique. (Etude critique) Le Sang 1927;1:610–33 18. Gandy C, Couraud FX. Melanodermie biliare a type Addisonien avec spenomegalie. Bull Mem Soc Med Hop Paris 1902;19:676–81 19. Gandy C, Bornait-Lequele: Anemie splenique, hyperplasie myeloid de la rate, hemosiderose viscerale. Bull Mem Soc Med Hop Paris 1906;23:694–717 20. Marini G. Sopra un caso di splenomegalia con cirrosi epatica. Arch Sci Med 1902;26:105–17 21. Stengel A. Varieties of splenic anaemia. Am J Med Sci 1904;128: 497–533 22. Schuppisser H. Uber Eiseninkrustation der Bindegewebssubstanzen bei Hamochromatose und bei lokalen Bluntungen. Virchow Arch Path Anat 1922;239:320–49 23. Tedeschi LG, Sherman JD, Tedeschi CG. Sclerosiderotic Granulomatosis in Thymoma. Arch Path 1965;80:235–40 24. Oberling C. Le role pathogene de la mycose splenique de nanta. Presse Med 1928;36:2–3 25. Jaffe RH, Hill LR. Splenic mycosis. Arch Pathol 1928;6:196–209 26. DeVecchi B, Picchi L, Patrassi G. Richerche sistematiche sulla genesi e sul valore delle lesioni perivasali spleniche conosciute col nome di ‘‘aree di Gamna’’. Arch Pat Clin Med 1929;8:17–49 27. Fasiani GM, Oselladore G. Essai de reproduction experimentale des nodules de Gandy-Gamna. Presse Med 1929;37:1136 28. Hu CH, Reimann HA, Kurotchkin TG. Filaments in siderotic nodules of spleen in cases of splenomegaly of unknown origin. Proc Soc Exp Biol Med 1929;6:413–16 29. Reimann, Kurotchkin TJ. The relationship of fungi to chronic splenomegaly of unknown origin. Am J Med Sci 1931;131:107–14 30. Bernaerts A, Vanhoenacker FM, Op de Beeck B, De Schepper AM. Gamna-Gandy bodies. JBR-BTR 2001;84:202 31. Dobritz M, Nomayr A, Bautz W, Fellner FA. Gamna-Gandy bodies of the spleen detected with MR imaging: A case report. Magn Reson Imaging. 2001;19:1249–51 32. Luo T, Itai Y, Yamaguchi M, Kurosaki Y, Saida Y. Gamna-gandy bodies of the spleen depicted by unenhanced CT: Report of two cases. Rad Med 1998;16:473–76 33. Roubidoux M. MR of the kidneys, liver, and spleen in paroxysmal nocturnal hemoglobnuria. Abdominal Imaging 1994;19:168–73 34. Sagoh T, Itoh K, Togashi K, et al. Gamna Gandy bodies of the spleen: Evaluation with MR imaging. Radiology 1989;172:685–87 35. Minami M, Itai Y, Ohtomo K, et al. Siderotic nodules in the spleen: MR imaging of portal hypertension. Radiology 1989;172:681–84 36. Kido C, Takagi H, Kuroyanagi Y. Clinical investigation on splenic aneurysms and Gamna-Gandy nodules. Australas Radiol 1976;20: 281–87 37. Watanabe M, Takazawa K, Wada A, et al. Cardiac myxoma with Gamna-Gandy bodies: Case report with MR imaging. J Thorac Imaging 1994;9:185–87 38. Basso C, Valente M, Casarotto D, Thiene G. Cardiac lithomyxoma. Am J Cardiology 1997;80:1249–51 39. Wang SC, Dikman SH, Goldberg SL, Deppisch LM. Gamna-Gandy body in a cardiac myxoma. Mt Sinai J Med 1974;41:524–26 40. Lie JT. Petrified cardiac myxoma masquerading as organized atrial mural thrombus. Arch Pathol Lab Med 1989;113:742–45 41. Trotter SE, Shore DF, Olsen EG. Gamna-Gandy nodules in a cardiac myxoma. Histopathology 1990;17:270–72 42. Tedeschi LG. The Gamna nodule. Hum Pathol 1971;2:182–83 43. Leroy X, Aubert S, Gosselin B. Gamna-Gandy nodules in a renal clear cell carcinoma. Arch Pathol Lab Med 2003;127:372