Petrogenesis of the volcanic suite of Bouvetøya (Bouvet Island
Transcription
Petrogenesis of the volcanic suite of Bouvetøya (Bouvet Island
R1d
Petrogenesis of the volcanic suite of Bouvetøya
(Bouvet Island), South Atlantic
TORE PRESTVIK, STEVEN GOLDBERG & GORDON G. GOLES
Prestvik, T., Goldberg, S. & Goles, G. G. Petrogenesis of the volcanic suite of Bouvetøya (Bouvet Island), South Atlantic. Norsk
Geologisk Tidsskrift, Vol. 79, pp. 205-218. Oslo 1999. ISSN 0029-196X.
Whole rock and mineral compositions of volcanic rocks collected during the Norwegian Polarsirkel expedition (1978n9) to the
volcanic istand of Bovetøya (close to the Bouvet Triple Junction) are discussed and compared with previously published data from
the island. The rock types, hawaiite, benmoreite, and peralkaline trachyte and rhyolite (comendite) are related to each other by
crystal fractionation processes. The trace element and radiogenic isotope signatures displayed by the Bouvetøya rocks are !hose of a
moderately enriched oceanic island suite. On several isotope plots Bouvetøya rocks fall on or close to mixing lines between the
euriched EM-l and HIMU mantle components. Mixing between depleted morb mantle (DMM) and euriched components is not
likely. Thus, Bouvetøya displays a typical plume signature.
T.
Prestvik, Department of Geology and Mineral Resources Engineering, Norwegian University of Science and Technology, N-7491,
Trondheim, Norway; S. Goldberg, Department of Geology, University of North Carolina, Chapel Hill, NC 27599-3315, USA.
Present address: New Brunswick Laboratory, US Department of Energy, 9800 S. Cass Avenue, Argonne,
IL 60439,
USA;
G. G. Goles, Department of Geological Sciences, University of Oregon, Eugene, OR 97403-1272, USA.
Introduction
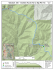
Bouvetøya (official Norwegian speiling) is located 50 km
west of the South-West Indian Ridge (SWIR) in the The
Bouvet Triple Junction (BTJ) area of the South Atlantic
(Fig. l) and consists entirely of young ( < 1 .4 Ma) volcanic
rocks. Bouvetøya is 95% covered by permanent ice and no
detailed geological map can be made. Geologic and
petrologic features have been described by several authors.
Among these are the contributions ofVerwoerd et al. ( 1 976)
and Imsland et al. ( 1 977), who described both the geology
and petrology, of Prestvik ( 1 982a), who reported on trace
element geochemical features, and of le Roex & Erlank
( 1982), who discussed the petrologic evolution of the
volcanic suite of the island. O' Nions & Pankhurst ( 1 974),
O' Nions et al. ( 1 977), and Sun ( 1 980) have published some
data on Sr, Nd, and Pb isotopes. All these contributions were
based on samples collected during short visits to the island
(which is of difficult access) from the late 1920s to 1966.
Geologic features of the island were described in consider
able detail by Prestvik & Winsnes ( 1 98 1 ), who participated
in the Norwegian Polarsirkel expedition during the
Antarctic summer of 1 978179. During this expedition 1 5
different locations o n the istand were sampled, resulting in
83 samples with good geological control. According to the
IUGS classification based on the non-genetic total alkalis
silica (TAS) diagram, the rocks plot in the fields St. S2, S 3 , T
and R (Le Bas et al. 1 986). The series is sodic (Na20 2 � K20) including a wide variety of hawaiites (grading
into mugearite) together with benmoreites and rhyolites. In
addition, we have two samples of altered mugearite (B 3 1 &
B 34) and one fresh sample of trachyte (B 1 6). Trachyte
(q < 20) has not been reported from Bouvetøya previously.
The whole series is oversaturated (q-normative), and the
trachyte and rhyolites are peralkaline (ac-normative; see
Tables l, 3). Thus, the rhyolites are comendites, a term that
is used in what follows.
The present account is based on material from the
Polarsirkel expedition. We present (l) major and trace
element composition of the various rock types, (2)
microprobe data on the minerals and trace element data
on plagioclase separates, and (3) isotope ratios of O, Sr,
Nd, and Pb. Furthermore, we (4) discuss the results of
-53'
\
Shona
. 54'
MAR
•.
/)
...."'/
..
-55'
__
--MR
-4·
/
\ (Spi6SS
)
·2'
"
w/
(:V/
<oo�/
11..
��
7-·
c_::_�?-f.!--J
/� SWIR
/
"- /'/
/
o
Bouvetøya
"�/
Ridge
o·
2'
4'
I. Schematic configuration of the Bouvet Triple Junction (BTJ), with the
location of Bouvetøya. The sketch is based on data from Simonov et al. (1996),
Ligi et al. (1997) and Mitchell & Livermore (1998). MAR= Mid-Atlantic
Ridge, AAR= American-Antarctic Ridge, SWIR=South-West Indian Ridge.
The black arrow indicates that the Spiess Ridge is propagating northwestwards.
Also, the location of enriched MAR basalts at 54°S, commonly referred to as
the 'Shona-Ridge anomaly' (Moreira et al. 1995), is indicated by the stippled
pattem of the ridge. The geographic Iocation and plate configuration of the BTJ
are shown in the lower, right-hand corner.
Fig.
206
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
T. Prestvik et al.
hydrothermal alteration, (5) present a revised petrologic
model, and (6) discuss the petrogenesis of the suite.
The petrographic features of the major rock types
(except trachyte) have been well described in several of
the previous studies (op. cit.). The newly discovered
trachyte (B 16) mainly consists of a brown glass in which
very few microphenocrysts of feldspar ( oligoclase to
anorthoclase) and almost pure hedenbergite are dissemi
nated together with crystallites of feldspar and titanomag
netite. Samples of crystalline comendite from a dome at
Kapp Valdivia in the north of the island contain alkali
feldspar, pure hedenbergite and fayalite, arfvedsonitic
amphibole, and ilmenite.
In the study of lmsland et al. ( 1 977) in particular, the rock
types are described under different names; 'transitional
basalts ' I and IT, (for hawaiites ), and 'transitional icelandite'
(for benmoreite), but these authors recognized the peralka
line nature of the rhyolites and used 'comendite' . Le Roex &
Erlank ( 1 982) used the terms 'accumulated hawaiite' ,
'hawaiite' , 'mugearite' , 'benmoreite' and 'rhyolite' .
Previous petrologic models and associated
problems
Both lmsland et al. ( 1 977) and le Roex & Erlank ( 1 982)
concluded that the rock types of the Bouvetøya suite were
related by crystal fractionation and/or accumulation pro
cesses. However, both these studies recognized 'problems '
at various model stages; these will be comrnented on below.
lmsland et al. ( 1 977) divided the basic rocks into two
groups on the basis of their K20 content and concluded
that these two groups were not related by simple
fractionation, mostly because the model required a more
sodic plagioclase than observed in abundant megacrysts.
They concluded that the benmoreites (trachytic icelan
dites) formed by open-system fractionation from evolved
hawaiites (transitional basalt Il) and that further fractiona
tion resulted in comendite. On the other hand, le Roex &
Erlank ( 1 982) suggested that porphyritic hawaiites repre
sented 'regular Bouvetøya hawaiite' with as much as 40%
accumulation of plagioclase (34-37%) and clinopyroxene
(3-6% ). The only problem with this interpretation was an
inferred Ba inconsistency (le Roex & Erlank 1 982, p. 333).
These authors rej ected a model in which comendite
(rhyolite) was formed from benmoreite, both because it
required a fractionating plagioclase of An 47 (benmoreite
plagioclase is An 33) and because there was a 'Ba and Sr
problem' , such that none of the minerals present as
phenocrysts in benmoreite could remove enough barium
(and strontium) to obtain the lower levels of these elements
observed in comendite. Instead, they suggested that
comendite was formed directly from mugearite, a very
long fractionating step ( 69% ), in which a plagioclase of An
47 was required. However, this is in conflict with the
composition of modal plagioclase in mugearite (most
calcic plagioclase in mugearite is An 45 ; le Roex & Erlank
( 1 982), Table Ill, p. 321). The fractionation model of le
Roex & Erlank ( 1 982) can be sumrnarized as follows: (l)
hawaiite � mugearite � benmoreite, (2) mugearite �
rhyolite, i.e. benmoreite is not an intermediate composition
in the formation of rhyolite.
In this study, we address several of the problems
connected with the models proposed in the two mentioned
studies. Since our radiogenic isotope data (see below) are
consistent with a model in which the various rock types at
Bouvetøya are closely related, we present a refined crystal
fractionation model, based on new mineral and chemical
data.
/
Geochemical features
Analytical methods
Major elements and the trace elements Ba, Rb, Sr, Y, Nb,
Cr, Ni, V, Cu, Zn, and Ga were analysed by XRF at
Washington State University, Pullman, Washington, USA.
Loss on ignition and volatiles on all samples as well as Co
(XRF) on samples B 55, B 57, B 58, and B 59 were measured
at NTNU, Trondheim, Norway. Scandium, Co, REE, Hf,
Ta, and Th on most samples and plagioclase separates were
analysed by INAA and Zr by XRF at the University of
Oregon, Eugene, Oregon, USA. On samples B 55, B 57, B
58, and B 59, Se, REE, Hf, Ta, Th, and U were deterrnined
by INAA at Imperial College - Reactor Centre, London,
UK. Rare earth elements, Se, Hf, Ta, and Th of sample B 3 1
were analysed by INAA at the University of Texas at El
Paso, USA. Mineral analyses were obtained at NTNU/
SINTEF, Trondheim, Norway using a JEOL microprobe.
Oxygen isotopes were measured at the University of South
Carolina, Columbia, South Carolina, USA, and some
strontium isotopes at University of Rochester, Rochester,
New York, USA, while the rest of the Sr isotopes as well as
the Nd and Pb isotopes were analysed at the University of
North Carolina, Chapell Hill, North Carolina, USA.
Chemical data of fresh whole rocks are presented in
Table l. However, before we discuss the general data we
will comment on the fact that in this study, the Na20
contents of all rock types are consistently higher than those
found in the previous studies (Table 2). Given the
considerable significance of sodium concentrations in the
model calculations, this aspect of our data has been
thoroughly studied. Our Na20 calibration is good for the
0.03-4.38 wt% calibration range (r = 0.999 16). However,
the intermediate and silicic rocks from Bouvetøya are high
in Na20. As a check of calibration at higher values, we
analysed the international standard STM- 1 and an intemal
standard TMS as unknowns and got 9.1 5 wt%, which,
relatively, is 2.3 % higher than the recomrnended value
8.94 for STM- 1 (Govindaraju 1989), and 9.52 wt% for
TMS , which, relatively, is 2.0% higher than our preferred
value of 9.33 wt% . Even though both of these results are on
the high side of recomrnended or used values, we feel that
these are acceptable data.
We have no satisfactory explanation for this interla-
Q
Ac
Y!Nb
'ZJ:!Nb
Th/U
Th
u
Hf
Ta
Cr
Co
Ni
V
Cu
Zn
Ga
Se
Eu
Tb
Yb
Lu
Sm
La
Ce
Nd
Ba
Rb
Sr
y
'lJ:
Nb
2.82
1.59
2.87
0.84
5.94
<2.63
0.88
6.03
0.88
6.23
<2.4
0.84
6.02
1.36
6.6
2.8
<2.1
0.8
14
48
8
311
39
128
19
34.6
69.8
35.8
9.2
3.0
1.3
2.4
0.43
4.60
1.86
2.25
24.2
28
32.9
14
259
32
99
22
17.2
47.0
33.0
5.7
2.3
1.18
3.12
0.39
8.2
2.8
<2.4
l.O
9
41
o
306
13
140
23
36.3
75.2
42.5
9.8
3.1
1.4
2.7
0.41
6.93
2.83
3.23
24.8
5
36.7
o
294
54
148
30
27.6
71.8
72.0
8.6
3.22
1.61
4.21
0.55
213
23
421
41
291
49
3.08
0.84
6.02
3.00
6.8
2.8
2.4
0.8
12
45
12
331
35
130
26
30.8
63.0
36.6
9.3
2.9
1.1
2.5
0.44
224
22
415
41
295
49
156
14
453
30
205
34
250
21
424
42
299
48
234
22
431
42
301
50
2.68
0.90
6.19
2.54
5.9
2.4
1.7
0.67
16
37
7
311
32
121
23
28.3
54.7
31.4
8.4
2.6
1.3
2.4
0.37
213
19
416
38
260
42
1.62
0.93
6.27
5.86
2.30
3.03
27.1
15
37.5
6
317
36
122
24
22.5
57.7
39.0
7.5
2.72
1.33
3.72
0.48
220
20
428
38
257
41
100.64
101.70
101.98
100.50
100.91
100.59
100.87
50.49
3.52
14.60
11.77
0.19
4.64
9.16
3.89
1.18
0.56
0.38
0.26
51.02
3.52
14.47
11.70
0.19
4.49
8.99
3.89
1.18
0.55
*0.87
50.98
3.89
14.16
12.05
0.19
4.04
8.60
4.04
1.39
0.67
*1.69
50.68
3.85
14.05
12.43
0.20
4.22
8.65
3.90
1.36
0.65
*1.99
50.70
2.80
16.62
10.09
0.16
4.44
10.09
3.67
0.97
0.44
0.21
0.31
51.05
3.68
14.26
12.55
0.20
4.03
8.12
4.01
1.43
0.67
*0.91
50.79
3.63
14.18
12.81
0.20
3.88
8.18
4.23
1.44
0.67
0.25
0.33
B 62
B 59
B 58
B 57
B 55
B 51
B 56
1.58
0.85
6.05
5.38
2.28
2.46
27.1
36
36.1
10
301
10
11
25
23.0
59.2
47.0
7.5
2.66
1.4
3.26
0.43
171
19
445
34
242
40
100.68
50.40
3.35
15.29
ll.l5
0.18
4.78
9.48
3.75
1.12
0.51
0.46
0.21
B 70
1.79
0.81
5.83
7.02
3.03
3.76
23.5
7
33.6
o
295
24
144
27
31.2
79.1
52.0
8.7
3.38
1.66
3.81
0.54
264
25
436
44
315
54
100.99
51.64
3.41
14.14
12.39
0.21
3.72
7.85
4.38
1.54
0.72
0.58
0.41
B 79
l. Chemical composition of fresh volcanic rocks from Bouvet Island. Major element data normalized to 100%, volatiles are added.
Si02
Ti02
Ah03
Fe0101
MnO
MgO
CaO
Na20
K20
P20s
H20+
H20c�
Total
* =L.O.I.
Table
6.70
0.70
6.25
13.5
5.28
7.68
12.9
o
3.92
2
11
9
216
31
54.4
138
84
13.5
4.56
2.50
7.03
1.00
542
56
316
69
619
99
61.14
1.16
14.72
8.76
0.24
0.95
4.01
5.66
2.97
0.38
0.49
1.10
0.04
101.62
B 28
8.89
0.75
6.31
13.8
5.38
7.86
12.5
4
4.27
o
16
o
164
29
57.2
139
80
13.2
4.52
2.52
7.2
1.02
554
52
312
74
625
99
61.86
1.14
14.68
8.56
0.20
0.88
3.89
5.44
2.97
0.37
0.41
0.79
0.32
101.51
B 67
13.07
0.68
0.62
6.86
20.2
7.19
12.5
2.43
2
0.19
9
7
7
183
35
16.7
3.47
3.12
9.43
1.14
75.4
182
701
90
89
88
981
143
68.43
0.31
14.64
4.35
0.13
0.00
1.58
6.03
4.50
0.03
1.21
0.38
0.28
101.87
B 16
17.19
5.20
0.68
7.23
26.6
8.93
15.5
0.92
o
0.31
12
o
o
195
34
89.7
228
120
19.7
2.98
3.85
11.5
1.58
312
105
22
108
1143
158
100.65
70.38
0.32
13.41
4.25
0.12
0.00
0.79
6.05
4.93
0.02
0.23
0.15
B 39
18.84
5.00
0.73
7.61
27.9
9.14
16.0
0.24
3
0.44
12
4
l
237
37
108.8
229
125
23.4
2.26
4.08
11.8
1.63
85
114
5
122
1279
168
100.26
71.30
0.23
13.35
3.63
0.08
0.00
0.60
5.96
4.83
0.01
0.10
0.17
B 44
17.07
5.02
0.64
7.57
28.8
9.32
16.7
0.25
3
o
15
o
3
233
42
122.4
238
137
24.6
2.35
3.98
12.6
1.71
74
112
6
109
1279
169
100.36
70.74
0.24
13.67
3.54
0.08
0.00
0.60
6.28
4.92
0.02
0.13
0.14
B 83
N
o
-..l
�
�
$:l
;:::
-.:::
(1:1
t::l;j
<::)
�
(")
Fi'
�
;:s
2
�
�
te
'O
�
_,
'O
::1
til
�
�
a
til
...,
�
@
til
o
gJ
t"'
�
Cl
�
"'
z
o
208
Table 2.
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
T. Prestvik et al.
Measured Na20 values for corresponding K20 contents. Before calculating the averages, all data were norrnalized to 100% volatile free and with total iron as
FeO.
Na20
K20
No. of samples
Comendite
Benmoreite
Hawaiites
(l)
(2)
(3)
(l)
(2)
(3)
(l)
(2)
(3)
3.96
1.30
7
3.66
1.32
5
3.55
1.31
3
5.55
2.97
2
4.98
2.97
3
4.77
3.01
5
6.10
4.89
3
5.93
4.83
3
5.36
4.88
2
(l) This study; XRF - lithium tetraborate:rock powder=2:1.
(2) Imsland et al. (1977); atomic absorption spectrometry.
(3) le Roex & Erlank (1982); XRF - data from Verwoerd et al. (1976).
studies. Thus, the altered rocks we have studied (volatiles
>2%) are all significantly lower in sodium than their fresh
equivalents (Fig. 3e, Table 3). It is therefore possible that
boratory discrepancy. However, we think it is partly
related to alteration (see below) and perhaps to a dilution
effect through using strongly porphyritic samples in some
Table 3.
Altered basaltic and interrnediate rocks. Major element data norrnalized to 100%, volatiles are added.
SiOz
TiOz
Alz03
FeOtot
MnO
MgO
CaO
Na20
KzO
P205
HzO+
HzOCOz
B 10
B 19
B 20
B 22
B 23
B 24
B 31
B 34
B 49
B 14
48.09
4.10
15.59
12.80
0.28
3.33
11.24
3.22
0.76
0.59
2.36
0.88
3.86
107.10
48.36
4.09
14.05
14.31
0.25
4.41
10.68
1.98
0.95
0.92
4.71
0.25
5.10
ll0.06
49.98
3.77
14.03
12.94
0.23
5.ll
9.94
2.42
0.98
0.59
5.05
0.47
4.70
110.21
45.34
3.47
18.35
10.95
0.21
3.96
12.75
2.08
2.36
0.52
5.10
0.42
6.75
ll2.26
50.72
3.54
15.14
11.99
0.14
5.76
8.43
3.19
0.53
0.55
1.97
5.55
0.76
108.27
49.81
2.03
18.53
8.33
0.13
5.81
11.87
2.71
0.50
0.28
0.99
1.98
1.00
103.97
52.44
3.38
14.66
12.54
0.23
3.29
7.44
3.62
1.50
0.90
*5.34
105.34
52.46
2.21
15.06
13.09
0.33
3.05
7.86
4.08
0.97
0.89
1.82
3.91
2.58
108.31
61.95
1.12
14.69
8.76
0.23
0.81
3.82
5.21
3.04
0.35
2.86
0.78
0.12
103.74
64.77
2.04
14.16
8.50
O.ll
2.94
3.57
0.98
2.54
0.39
2.97
0.76
2.28
106.01
165
ll
551
42
285
47
159
6
510
43
260
46
160
14
407
41
277
46
661
36
750
34
251
39
208
3
500
37
262
41
ll8
4
524
20
153
22.6
299
23
436
53
366
61
371
12
550
58
425
68
559
58
309
71
620
101
209
52
107
55
476
73
42.1
105
64
11.4
3.94
2.1
4.68
0.69
58.8
137
75
14.2
4.34
2.50
6.97
0.98
15.1
4
14.3
o
88
26
171
26
12.4
4
3.52
5
13
2
2ll
30
* = L.O.I.
Ba
Rb
Sr
y
Zr
Nb
La
Ce
Nd
Sm
Eu
Tb
Yb
Lu
Se
Cr
Co
Ni
V
Cu
Zn
Ga
27.4
63.4
42
8.79
2.77
1.38
3.44
0.53
27.7
27
30.7
5
363
25
145
27
30.5
65.6
38.8
9.2
3.23
1.38
3.75
0.44
24.9
7
38.6
o
316
14
153
22
25.5
60.8
35.9
7.92
2.68
1.23
3.5
0.43
25.8
13
34.5
2
322
38
123
20
21.5
49
31.5
7.07
2.33
1.06
2.8
0.40
25.2
31
35.2
14
301
38
ll3
27
25.4
55.4
42
8.1
2.52
1.32
3.16
0.46
27.1
28
35.7
15
293
48
122
22
13.1
32.7
26
4.7
1.6
0.67
1.9
0.25
21.9
107
32.1
49
195
53
70
21
39.5
79.0
47.7
11.3
3.01
1.74
4.20
0.64
22.6
18
29.8
o
269
6
156
23
37.0
90.5
48.5
9.9
2.76
1.67
5.1
0.72
14.7
6
16.6
8
147
12
118
21
Hf
Ta
Tb
6.02
2.45
2.58
5.36
2.27
2.64
6.13
2.38
2.77
4.95
2.26
2.06
5.49
2.18
2.47
3.21
1.24
1.38
8.36
3.49
4.25
9.20
3.46
4.98
13.6
5.14
7.93
10.7
3.60
5.64
Y/Nb
Zr/Nb
0.89
6.06
0.93
5.65
0.89
6.02
0.87
6.44
0.90
6.39
0.88
6.77
0.87
6.00
0.85
6.25
0.70
6.14
0.75
6.52
8180
+
Q
Ne
3.14
+1.9
7.90
+2.8
+1.9
7.15
+6.0
5.98
2.75
+6.2
1.60
+7.2
7.20
3.61
+5.8
9.79
+5.1
36.61
NORSK GEOLOGISK TIDSSKRIFf 79 (1999)
200
������--��--T�-�-���
a)
l
...
l
l
•
X
800
l
b)
600
•
�
209
Volcanic rocks, Bouvetøya
100
4oo
X
X
•
Æ
200
X
X
o
15
d) -
X
-
1.0
0.8
•
o
Q)
u.
•
10
0.6
•
5
o
•
0.4
...
•
��--��--���
-
5 .--.-1-.-1-.-1-.-1-.-1-,--.--,�
4
N
o
l=
xleX
-
f)
X
X
X
X
•
-X
•••
X
X
X
"
•
X
•
•
•
1
__
3
0.0
- 0.3
o
c:
:::;!
0.1
...
.
_,_L__��--�--"---��
-
__._
J
_
�-..._
a..
0.2
0.2
•
1
0
N
0.4
lf
3
2
e)
XX
LD
o
5
7
9
Ta
1
3
7
5
9
Ta
Variation diagrams of Nb, Ba, FeO, P205, Ti02 and MnO vs. Ta. Filled circles represent fresh rocks; crosses represent hydrotherrnally altered samples
(Tables l, 3).
Fig. 2.
most of the discrepancies might be due to the use of altered
and/or porphyritic samples in the previous studies.
General characteristics
Both this and the former studies show that the K20
contents of hawaiites vary considerably: from 0.75 to 1 .65
wt% (lmsland et al. 1 977), 0.72 to 1 . 3 1 wt% (le Roex &
Erlank 1 982) and from 0.97 to 1 .54 in this study. The low
values are almost always associated with high Al203
(> 15%) and CaO (> 1 0%) contents, sometimes with 'high'
MgO (samples B 24 & B 70, this study) and relatively low
values of several other elements. These are the effects of
plagioclase (and sometimes olivine) accumulation as
revealed by the porphyritic nature of many hawaiite
samples. The sparsely porphyritic or aphyric Bouvet
hawaiites, which are thought to be doser to melt com
positions, seem to vary in K20 in the range 1 . 1 2-1 .54 wt%.
They are high in Ti02 (3.35-3 .89 wt%) and total FeO
( 1 1 . 1 5-12. 8 1 wt%), and low in MgO (3.72-4.78 wt%) and
CaO (7.85-9.48 wt%). We have only used such samples in
our model calculations.
In Figs. 2 & 3 we have plotted selected major and trace
elements in variation diagrams versus Ta, which was
apparently incompatible throughout the development of
the suite and also rather immobile during alteration. Three
different pattem styles are observed: (a) Purely incompa
tible elements (vs. the observed minerals) such as Nb (Fig.
2a) vary almost linearly with Ta throughout the series. This
type of pattem is observed for Zr, Hf, Y, La, Lu, K20, and
Rb, while Ba increases linearly up to the trachyte stage (B
16) after which it drops considerably to and among the
comendites (Fig. 2b). (b) FeO (Fig 2c), P205 (Fig. 2d),
Ti02 (Fig. 2e), MnO (Fig. 2f), and Se (Fig. 3f) increase
from the most primitive rocks and reach a maximum
before decreasing in the intermediate and silicic rocks.
210
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
T. Prestvik e t al.
6
l
•
�N
•
3
o
60
X
e
It
••
X
••
X
X
15
o
X
10
• �l
.
•:s:
•
7
('\1
z
-
, .. X
e)
•
•
X
5
4
3
2
e
X
·'•
•
..
•
•
-
X
J
.'�.•
X
....
f)
X
o
30
u
10
X
l!
3
5
7
9
1
3
L
_]_
_j_
5
_l_
7
j_
�
9
o
Ta
Ta
Fig.
- 200
-
Cf)
xx
l
1
l....
Cf)
- 20
•
1
600
X
-
X
800
400
...
l
6
ro
o
X
�
0::
20
5
o
o
X
X
d) -
X
X
.c
40
IX
c)
X
X
ro
()
80
X
2
1
100
•
4
o
120
..
a)
5
3. Variation diagram for K20, Rb, CaO, Sr, Na20 and Se vs. Ta. Symbols as in Fig. 2.
Note, for example, the pattern of P205 contents, which
increases linearly to mugearite (B 3 1 & B 34), indicating
that apatite fractionation must have been rather insignif
icant up to this point, but from then on it decreases in more
evolved compositions. Ti02 reaches its maximum some
what earlier than FeO; we can also see (Fig. 2c, f) that
manganese removal is delayed compared to iron, a feature
compatible with the increased Mn/Fe ratio of the various
ferromagnesian minerals we have analysed in benmoreite
and comendite (Table 5). (c) CaO (Fig. 3c), MgO and Sr
(Fig. 3d) decrease almost linearly throughout the series.
Alteration
Nine samples are listed in Table 3, all of which are altered
by hydrothermal processes. Of these, samples B 10, B 19,
B 20, B 22, and B 23 seem to represent 'normal' to evolved
Bouvetøya type hawaiite, B 24 represents a hawaiite with
accumulated plagioclase, B 3 1 and B 34 are altered
mugearites, and B 49 is altered benmoreite. With the
exception of B 23 and B 24, the altered samples were
collected from a hydrothermally altered sequence, which is
exposed in the cliffs behind the newly formed platform
Nyrøysa (< 1 00 m a.s.l.) on the west coast of Bouvetøya.
Samples B 23 and B 24 were collected at the site of the
active fumaroles in the north of the island (see Prestvik &
Winsnes 198 1 for details).
Chemically, the alteration is characterized by large, but
variable H20+ and C02 contents in most samples. The
secondary minerals include calcite, celadonite, chlorite,
various clay minerals, pyrite, and quartz.
Elements such as Nb (Fig. 2a), Hf, Zr, Y, the REE, and
Se (Fig. 3f) were rather immobile during the alteration
(assuming that Ta was also immobile). It is mentioned
above that sodium is low in the altered samples (Fig. 3e).
With the exception of one sample (B 22), K20 and Rb have
NORSK GEOLOGISK TIDSSKRIFI' 79 (1999)
Table 4.
Trace element composition of plagioclase separates (in ppm).
Fe(%)
Se
Cr
Co
Na(%)
Ba
La
Ce
Nd
Sm
Eu
Th
Yb
Lu
Hf
Ta
Th
D(Ba) plag!melt
21 1
Volcanic rocks, Bouvetøya
B 24
Hawaiite
B 56
Hawaiite
B 49
Benmore.
0.49
0.44
1.66
1.35
2.34
57
1.46
2.72
1.13
0.20
0.528
ND
ND
ND
0.051
ND
0.027
0.28
0.44
0.30
0.97
1.23
1.97
36
1.16
2.31
ND
0.175
0.50
ND
ND
ND
0.067
ND
ND
0.18
0.38
0.14
ND
ND
5.30
397
6.5
9.00
3.7
0.646
5.78
0.061
ND
0.014
ND
ND
0.055
0.72
and +6.2%o, respectively, and +7.2o/oo in B 34. The
significantly higher values of B 23 and B 24 may be
related to their location in the fumarole area, while the
samples with the lowest 618 0 are from the hyaloclastite
section. Apparently, the fumarolic water has other isotopic
signatures than the fluids that altered the lower hyaloclas
1
tite formation of the island. Average 6 80 for five fresh
hawaiites is +5 .5%o. Comparing our data on altered rocks
with the data on Rb, Sr, Na20 and K20 published by
O' Nions & Pankhurst ( 1 974) and O' Nions et al. ( 1 977) on
hawaiite and benmoreite from Bouvetøya, we can con
clude that some of the samples analysed by these authors
represent heavily altered material. However, this alteration
does not appear to have affected the 8 7 Sr/8 6Sr and
143 Nd/144Nd ratios.
ND= not detected. Detection limit for Yb and Ta = 0.08 and 0.03 resp.
Mineral compositions
been leached during hydrothermal alteration (Fig. 3a, b).
With a few exceptions B a, P205, Ti02, and FeO appear to
have been less mobile. CaO, Sr (Fig. 3c, d) and MnO (Fig.
2t) show enrichment in most altered hawaiites. One sample
(a hawaiite sill; B 22, the most heavily altered of all
samples in terms of total volatiles) is especially notable
because it is the only sample considerably enriched in
K20, Rb, Ba, Sr and Ca. In this sample, all phenocrysts and
most groundmass plagioclase are completely replaced by
sericite + calcite ± quartz, and other phases have been
transformed into a mixture of clay minerals. Vesicles have
been filled with calcite, chlorite, various clay minerals and
sometimes quartz.
Oxygen isotope data (Table 7) show low 618 0 values in
the heavily altered samples, as low as + 1 .9o/oo in B 22 and
B 1 9. However, in samples B 23 and B 24, 6180 is +6.0%o
Several hundred mineral grains have been analysed by
electron microprobe. Some olivine, pyroxene and amphi
bole data are summarized in Table 5 and Fig. 4.
The feldspars show large compositional variation. In the
hawaiites they range from An 83 to An 45 with An 60-75
as the most common composition. It is difficult, however,
to evaluate the equilibrium plagioclase composition of the
hawaiites, but in the sparsely porphyritic samples An 5562 are common compositions. Plagioclase phenocrysts of
the benmoreites are typically around An 30, and microlites
grade into the field of anorthoclase. The feldspar of the
trachyte (B 1 6) is anorthoclase, whereas the comendites
have a very Ca-poor feldspar; the most potassium-rich
varieties are close to the alkali-feldspar minimum melt
composition, i.e. Or 35.
Plagioclase separates have been analysed for some
trace elements (Table 4). The small concentrations of Fe,
Se, Cr, and Co show that very clean separates were
Table 5.
Average composition of olivine, clinopyroxene, and amphibole.
Si02
FeO
MnO
MgO
CaO
Total
Molecular ratios:
Fo
Fa
Tf
Olivine
Benmore.
Olivine
Comendite
33.39
51.31
1.62
13.77
0.42
100.51
30.44
66.94
2.47
0.08
0.44
100.36
Si02
Ti02
Ah03
FeO
MnO
MgO
CaO
Na20
KzO
Total
Cpx
Benmore.
Cpx
Comendite
50.54
0.63
1.21
17.16
0.64
10.00
20.08
0.31
48.19
0.07
0.18
28.98
0.92
0.19
19.75
0.20
100.57
98.48
42.4
29.4
28.2
46.4
0.6
53.0
9.2
25.0
7.3
31.7
66.2
2.1
0.2
96.2
3.6
Wo
En
Fs
D(Mn)
D(Ca)
D(Fe)
7.4
O.l
5.9
24.7
0.6
16.7
D(Mn)
D(Ca)
D(Fe)
2.9
5.2
2.0
MnO/FeO
0.032
MnO/FeO
0.037
0.037
0.032
Amphib
.
Comendite
51.12
0.21
0.24
35.93
0.72
0.07
1.9
7.69
1.27
99.15
7.2
2. 4
9.0
0.020
Amphib
Cations
.
Si 7.7
Al 0.4
Ti 0.2
Mg 0.1
Fe 4.5
Mn O.O
Ca 0.3
Na 2.2
K 0.2
16.0
2 12
Table
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
T. Prestvik et al.
6. Petrogenetic models of various rock types from the Bouvet Island. A least-squares approximation method (Bryan et al. 1969) was used.
(I) Fractionation within hawaiites (B 59-B
100.00%
A. Hawaiite B 59
0.71%
-Olivine Fo 70
8.84%
-Clinopyroxene Fs 17
13.42%
-Plagioclase An 56
1.72%
-Ilmenite
0.46%
-Titanomagnetite
74.85%
=Hawaiite B 79
79)
B79 obs
51.640
3.410
14.140
12.390
0.210
3.720
7.850
4.380
1.540
0.720
B79 est
51.882
3.431
14.126
12.495
0.202
3.741
7.873
4.359
1.523
0.737
Si
Ti
Al
Fe
Mn
Mg
Ca
Na
K
p
B28 obs
61.140
1.160
14.720
8.760
0.240
0.950
4.010
5.660
2.970
0.380
B28 est
61.133
1.159
14.713
8.760
0.232
0.958
4.023
5.731
2.998
0.363
(IV) From trachyte to comendite (B16-B 39)
100.00%
Trachyte B 16
Si
3.81%
-Clinopyroxene Fs 47
Ti
8.72%
-Plagioclase An JO
Al
17.34%
-Feldspar Or�b�2
Fe
0.43%
-Titanomagnetite
Mn
69.70%
= Rhyolite B 39
Ca
Na
K
p
0.207
E�=
B39 obs
70.400
0.320
13.410
4.250
0.120
0.790
6.050
4.930
0.020
B39 est
70.404
0.291
13.575
4.254
0.164
0.779
5.630
4.904
0.043
E�=
0.073
(Il) From hawaiite to benmoreite (B 79-B
100.00%
Hawaiite B 79
3.57%
-Olivine Fo 64
15.69%
-Clinopyrox Fs 19
24.17%
-Plagioclase An 42
1.27%
-Apatite
2.91%
-Ilmenite
5.13%
-Titanomagnetite
47.26%
=Benmoreite B 28
E�=
0.006
Si
Ti
Al
Fe
Mn
Mg
Ca
Na
K
p
28)
obtained. Especially interesting is the large Ba content of
benmoreite plagioclase (397 ppm) compared to 35 and
56 ppm in plagioclase of hawaiites. This contrast shows
that the distribution coefficient of Ba (D8a plaglliquid) is
increasing from about 0.2 to 0.72 as An contents (and T
of equilibrium) decrease. The benmoreite plagioclase
(An 33) is considerably richer in the REE than are
accumulated bytownites (An 71-75) in hawaiites (see
below).
The clinopyroxene data are shown in Fig. 4, and the
averages of representative pyroxenes and olivines of the
intermediate and silicic rocks are presented in Table 5 .
Pyroxene o f benmoreite i s Fe-rich augite, and i n trachyte
and comendite almost pure hedenbergite is present.
Because most hawaiite pyroxenes are irregularly zoned,
it is difficult to estimate a reliable 'average' composition. It
is, however, important to recognize the compositional
variation, because that sheds light on the magma' s pre
eruption history.
Olivine varies from Fo 87 to Fo 45 in the hawaiites, but
compositions around Fo 70 seem to be most common.
These may represent equilibrium olivine, because a range
of Fo 75--65 would be expected for the actual whole-rock
Mg numbers (Roeder & Emslie 1970). The most magne
sium-rich olivine that occurs in the hawaiites is, however,
petrogenetically important because it shows the existence
of more primitive magmas at deeper levels. The olivine
B. Hawaiite B 59
-Olivine Fo 70
-Clinopyroxene Fs 17
-Plagioclase An 65
-Ilmenite
=Hawaiite B 79
E�=
100.00%
1.35%
6.98%
12.67%
1.82%
77.18%
1.216
(Ill) From benmoreite to trachyte (B 28-B
100.00%
Benmoreite B 28
3.29%
-Olivine Fo 32
4.24%
-Clinopyroxene Fs 28
24.13%
-Plagioclase An 31
4.57%
-Titanomagnetite
0.84%
-Apatite
61.93%
=Trachyte B 16
E�=
0.486
B79 obs
51.640
3.410
14.140
12.390
0.210
3.720
7.850
4.380
1.540
0.720
B79 est
52.679
3.418
13.951
12.704
0.202
3.722
7.847
4.416
1.525
0.721
K
B l 6 obs
68.410
0.310
14.640
4.350
0.020
1.580
6.030
4.500
BJ6 est
68.491
0.307
13.978
4.544
0.020
1.609
5.999
4.469
p
0.030
0.030
B39 obs
70.380
0.320
13.410
4.250
0.001
0.790
6.050
4.930
0.020
B39 est
70.376
0.311
13.430
4.252
0.002
0.796
6.006
4.977
0.012
Si
Ti
Al
Fe
Mn
Mg
Ca
Na
K
p
16)
Si
Ti
Al
Fe
Mg
Ca
Na
(V) From benmoreite to comendite (B 28-B39)
100.00%
B28
Si
3.55%
-Olivine Fo 32
Ti
5.10%
-Clinopyroxene Fs 33
Al
30.68%
-Plagioclase An 31
Fe
0.86%
-Apatite
Mg
-Titanomagnetite
4.64%
55.35%
Ca
=Rhyolite B 39
Na
K
p
0.005
E�=
phenocrysts of benmoreite are around Fo 30, whereas pure
fayalite (Fo 0.2, Table 5) is found in several comendite
samples.
Hedenbergite and fayalite have not been reported from
Bouvetøya previously. In both of these minerals the
MnO content is relatively large (Table 5). Thus, MnO
contents range to 2.59 wt% in olivine of comendite
sample B 83.
Imsland et al. (1977) reported rare blue-green alkali
amphibole in some comendite samples. We present
microprobe data of such amphiboles from a fresh
holocrystalline comendite (Table 5). This is arfvedsonite
according to the classification of Leake (1978).
Rare earth elements (REE).- In the petrologic models of
Imsland et al. (1977) and le Roex & Erlank ( 1982), as
well as in our own models (Table 6), plagioclase
fractionation is important. Extensive plagioclase fractio
nation is usually thought to deplete the residual melt in
Eu because this element readily replaces Ca (and Sr) in
the feldspar lattice.
Plagioclase separates of hawaiite and benmoreite all
show a significant positive Eu anomaly (Fig. 5) where Eu
is enriched about 10 times compared to the neighbour
elements in hawaiite and about 30 times in benmoreite. As
can be seen from Fig. 5 , benmoreite does not display
negative Eu anomalies despite the approximately 24%
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
Table 7.
Volcanic rocks, Bouvetøya
213
Isotope data for Bouvetøya rocks.
Sample
Hawaiite B 56
Hawaiite B 59
Hawaiite B 62
Hawaiite B 79
Benmor. B 28
Trachyte B 16
Comendite B 39
Comendite B 44
*Hawaiite B JO
*Hawaiite B 22
*Mugear. B 34
*Benmor. B 49
8 180**
87Sr/86Sr
143Nd/144Nd
206PbP04Pb
207Pb/204Pb
208PbP04Pb
+5.8
ND
+5.4
+6.0
+6.5
+7.7
+7.7
+7.4
+4.2
+1.9
+7.2
+5.8
0.703626
0.703695
0.703629
0.703575
0.703609
0.703738
0.703754
0.703615
0.703675
0.703739
0.703524
0.703665
0.512895
0.512961
0.512919
0.512891
0.512887
0.512962
0.512852
0.512900
0.512883
0.512850
0.512888
0.512845
ND
ND
ND
19.105
ND
19.535
19.606
ND
ND
19.420
ND
19.687
ND
ND
ND
15.671
ND
15.641
15.636
ND
ND
15.630
ND
15.723
ND
ND
ND
38.836
ND
39.138
39.165
ND
ND
39.053
ND
39.434
* Altered samples.
** Additional 8 180 detenninations on two fresh hawaiites are +5.0 and +5.5, and from +1.9 to +6.2 in 5 altered hawaiites (see also Table 3).
ND = not detennined.
plagioclase fractionation that is indicated from the
petrologic models (Table 6; model ll). Intuitively, and as
was stated by Dickey et al. (1977), one should expect a
considerable effect on the Eu content of the derived melt
when Eu-enriched plagioclase is removed. For the
Bouvetøya suite, therefore, two questions arise: (i) Is it
possible to fractionate a feldspar with a pronounced
positive Eu anomaly without developing a negative
anomaly in the derived melt, and/or (ii) Is the petrologic
model principally wrong, perhaps because plagioclase
fractionation was not that important? The answers appear
to be very simple: the plots in Fig. 5 also show another
important feature; the bytownites of hawaiite have very
small concentrations of REE compared to those of the
whole rock compositions. Even the apparently large Eu
values are significantly lower than in the host hawaiites,
i.e. the plot is a visual way of showing that Eu has a small
2
aggregate distribution coefficient (for Eu3+ and Eu +
combined) between basic plagioclase and a mafic melt, a
fact that has been known for many years. A similar
relationship exists between the plagioclase (An 33) and the
host benmoreite (Fig. 5) except that in this case the Eu
content is larger than in the melt the plagioclase crystal
� andesine/melt is greater than unity (about
lized from; i.e.
u
1.3), and therefore further fractionation may be very
efficient in developing negative Eu anomalies in the
more silicic melts. It is thus logical that trachyte (B 16),
which is somewhat less silicic than the comendite, displays
an REE pattern which is parallel with that of comendite,
but has smaller REE abundances and a less pronounced
negative Eu anomaly.
2
It was mentioned above that Eu + is expected to have
geochernical behaviour similar to Sr. However, Eu occurs
1000 .-------,
100
10
La Ce
En
(Fo)
Fs
(Fa)
Fig. 4. Clinopyroxene plotted in the En-Fs-Di-He quadrilateral. For simplicity,
olivine compositions (Fo-Pa) are plotted in the same diagram, along the En-Fs
join (filled circles).
Nd
Sm Eu
Tb
Yb Lu
Chondrite-normalized REE (rare earth elements) patterns of average ha
waiite, benmoreite and comendite, plotted together with patterns of plagioclase
separates from hawaiites (An71 & An 75) and benmoreite (AN 33). Values for
HREE of basic plagioclases are stipulated on the basis of data obtained for basic
plagioclase from Iceland (Prestvik l 985) which was analysed in the same
experiment.
Fig. 5.
214
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
T . Prestvik e t al.
Peter 1 . øy
:li::
(.)
o
0::
10
•
*
Ba
Bouvetøya
Bouvet ridge
Rb
Th
K
Nb
Ta
La
Ce
Sr
Nd
p
Sm
Zr
Hf
Ti
Tb
y
Yb
6. Chondrite-nonnalized minor and trace element diagram (spider diagram,Thompson et al. (1984)) of average Bouvetøya hawaiite (large filled circles). For
comparison, and at fixed (Yb)N = 10, are plotted pattems of Bouvet Ridge basaalt (Dickey et al. 1977) and alkali basalt from Peter I Øy, Bellinghausen Sea, Antarc
tica (Prestvik et al. 1990).
Fig.
in both the 2+ and the 3+ states. Thus, the aggregate
distribution coefficient for Eu depends on the prevailing
oxidation states. Distribution coefficients are also tem
perature dependent (Blundy & Wood 1 99 1 ) ; they increase
with decreasing temperature. If we apply distribution
2
coefficients between plagioclase and melt for Eu + and
Eu3 + similar to those for s�+ and REE3 + , respectively, we
can estimate the proportion of the two Eu ions in the melt.
If we distribute the observed aggregate DEu of 0.2 between
bytownite plagioclase and hawaiite with DEuZ = l and
2
+
DEuJ = 0.03, we find that the Eu3 +Æu + ratio in the melt
+
is ca. 4.7; in other words, Eu is rather oxidized. Choosing a
2
higher coefficient for Eu + (Dsllagioclaselmelt range
between l and 3) would correspond to an even more
oxidized melt. Making use of the same procedure for
andesinelbenmoreite, using aggregate DEu = 1 .3 (as
observed), DEuJ = 0.05, and DEuZ = 2.55 gives a Eu3 +/
2
+
+
Eu + ratio of 0.85, i.e. considerably more reduced than in
the basic melts. These results are in general agreement
with the conclusion drawn by Prestvik ( 1 982a) on a
qualitative basis. If we compare with the experimental data
of Weill & Drake ( 1 973), we find that f 02 for bytownite/
hawaiite at l 1 00°C is about 10-5 Pa and about 10- 8 ·5 Pa
for andesinelbenmoreite at 1 000°C. For comparison, le
Roex & Erlank ( 1 982) estimatedf02 for hawaiite at l 00°C
to be about 1 0-6·0 Pa on the basis of coexisting Ti
magnetite and ilmenite.
New petrologic model
We used a common least squares mass balance mixing
technique (Bryan et al. 1 969) to evaluate the possible
relationships between the observed rock and mineral
compositions. Before modelling, all data were normal
ized to 1 00% volatile-free, and total iron was expressed
as FeO. In models involving silicic rocks (models IV and
V), Mg was omitted in model IV, because the poor
analytical accuracy of whole rock analyses with
MgO :::; 0. 10% made error estimates meaningless. Mn
was omitted from models IV and V. Our models are
presented in Table 6.
We made the calculations in several steps. As
described above, there is considerable chemical variation
within the hawaiite group, and we wanted to include this
variation in our modelling. We chose sample B 59
(K20 = 1 . 1 8) as representative of the least evolved
hawaiites and B 79 (K20 = 1 .54 and transitional to
mugearite) as the most evolved. Both these groups are
almost non-porphyritic. Models lA and lB (Table 6)
represent variation within the group of hawaiites. Models
Il and Ill show the evolution from evolved hawaiite (B
79) to benmoreite (B 28; K20 = 2.97) and furthermore to
trachyte (B 16; K20 = 4.50). The evolution of comendite
(B 39; K20 = 4.93) is presented in two models, model IV
from trachyte and model V directly from benmoreite.
With one exception (lb), we present models with low
residuals CE� <0.5), but we also rejected models with
low residuals if the relative discrepancy for Na and K
was > 1 .5%.
Our preferred sequence of evolution of the Bouvet suite
is as follows: hawaiite � evolved hawaiite � benmoreite
� trachyte � comendite (models la, Il, Ill & IV), see
discussion below.
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
Volcanic rocks, Bouvetøya
l
1 5, 9 t-
HIMU
1 5, 8
.c
l.
a..
N
.o
,._a..
o
N
1 5,7
o
1 5, 6
-$
1 5, 5 r-
-
EM-1
1 5, 4
DMM
17
l
19
18
20
21
22
'
40
HIMU
o
EM-1
38
DMM
0, 705 -
l
l
17
18
21
22
-
EM-1
i>
::: 0 , 704
,._(/)
.�
CD
HIMU
0, 703
DMM
o, 7 0 2 ..."__.___._....____..._�_._-.._I_.___.,_...L...-J'
17
18
19
20
21
22
7. Radiogenic isotope plots of Bouvetøya rocks. Filled circles - hawaiite;
open circles - evolved rocks; stars - Ph data from Sun (1980). Locations of
DMM (depleted morb mantle), EM-l (enriched mantle l) and HlMU (hlgh
11; 11 = 238Uf041>b) fields are taken from Hofmann (1997).
Fig.
Discussion
Former models
We agree with Imsland et al. ( 1 977) that the irregularly
zoned and commonly corroded basic plagioclase mega
crysts in the hawaiites are not real phenocrysts. On the
basis of mixing calculations, le Roex & Erlank ( 1 982)
215
suggested that such phyric hawaiites represented regular
Bouvetøya hawaiite with as much as 40% accumulation of
plagioclase (34-37%) and clinopyroxene (3-6% ). As
mentioned above, the only problem with this interpretation
was an inferred Ba inconsistency (le Roex & Erlank 1 982,
p. 333). However, the very small Ba concentrations
obtained by us on hawaiite plagioclase separates (Table
4) explain and eliminate this problem.
Transitional basalts I of Imsland et al. ( 1 977) correspond
well to 'accumulated' hawaiite of le Roex & Erlank
( 1 982) . This rock type is represented by samples such as B
24 and B 56 (the hawaiite samples from which we
separated plagioclase). However, the strongly phyric
hawaiites not only have An-rich plagioclase, bot also
tend to have the most primitive clinopyroxene and olivine,
i.e. a mineral assemblage representing more primitive
melts. Our data show that Mg numbers (Mg#s) of the
hawaiites from Bouvetøya range from 36 to 55 (not
tabulated). The largest Mg#s are found in the phyric
hawaiites. If these rocks were formed by accumulation of
pheno/xenocrysts, as suggested by le Roex & Erlank
( 1 982), it would require that as much as 1 5 % cpx + ol was
added to hawaiite melt (B 59) in addition to plagioclase.
Unless significant resorption occurred, this seems to be
unlikely, based on observed hawaiite modal composition.
We favour an interpretation in which more primitive
hawaiitic magmas (than those represented by the aphyric
hawaiites) were present and fractionated at depth, and that
only porphyritic varieties are represented in our sample
collection. Sometimes, also more evolved hawaiite melts
accumulated phenocrysts (especially plagioclase) from
their basic precursors and brought these to the surface.
There is a tendency, however, for a gradual composi
tional change also within the group of sparsely phyric or
non-phyric hawaiites. Low Mg#s and generally small
concentrations of compatible elements such as Ni, Co, Se,
and Cr in these hawaiites (Table l) strongly indicate a
history in which olivine and clinopyroxene ( + spinel?)
fractionated from the more primitive magmas. Pre
fractionation of plagioclase is not documented by negative
Eu anomalies in REE pattems of the basic rocks, because
plagioclase has such small Eu concentrations (Table 4).
However, the strong, negative anomaly of strontium in the
spidergram of the Bouvetøya hawaiite (Fig. 6) indicates
that parental magmas at depth also crystallized plagio
clase, because distribution coefficients of Sr are large
enough ( 1-3 ; Henderson 1982) effectively to remove this
element during plagioclase fractionation.
New models
We present two fractionation models (Table 6, lA & lB) to
explain compositional variation in the hawaiites. Both
suggest
ca.
25%
fractionation
of ol + cpx +
plag + ilm + mt, all of which occur in hawaiite. The
difference in the two models is the plagioclase composi
tion (An 56 in lA; An 65 in lB). However, the
unacceptably high residual in model lB makes that model
216
T. Prestvik e t al.
unrealistic. Even though modal plagioclase in many
hawaiites falls in the range An 60 - 75, we did not
consider models using plagioclase more calcic than An 65 .
As was discussed above, the true equilibrium plagioclase
composition may well be lower; and we think the
plagioclase (An 56) used in model IA is a realistic choice.
The composition of olivine and clinopyroxene is as
observed in hawaiite. Thus, IA is our preferred model for
this stage. The amount of fractionation is consistent with
the observed incompatible element variation.
In model Il, benmoreite is derived from evolved
hawaiite by slightly more than 50% fractionation. This is
about 1 0-1 5% less than reported by both Imsland et al.
( 1 977) and le Roex & Erlank ( 1 982). However, the parent
hawaiites used in the models of these authors were less
evolved than those we have used. The model uses minerals
and compositions that are typical in evolved hawaiite,
except that the plagioclase is slightly more sodic than is
common in hawaiite. Since this step is so large, we tind it
reasonable that the average fractionating plagioclase is
intermediate between parent and daughter compositions.
Olivine and clinopyroxene compositions are both fairly
magnesium-rich, but that can be explained by postulating
that these minerals fractionated early in the sequence. This
model has very low residuals. Model Ill shows bow
trachyte can be formed by ca. 38% fractionation from
benmoreite. Only benmoreite mineral compositions were
used in this model, which has a somewhat high residual.
This is mainly due to misfits in the Al and Fe contents
(both ca. 4.5% relative). We still believe this is an
acceptable model for the derivation of trachyte, not least
because trachyte is intimately related to benmoreite in the
field. The trachyte is relatively enriched in incompatible
elements such as Ba, Rb, Y, il and Nb compared to
benmoreite, but strontium is considerably lower (89 vs. ca.
3 1 5 ppm). This requires bulk Dsr and DBa of ca. 3.6 and
0.5, respectively. With the actual proportion of plagioclase
a
in the fractionating assemblage, this gives D�� g of 5 .4,
which is reasonable for plagioclase An 31 (Nash &
Crecraft 1 985), and Dfl:g = 0.72, which is identical to
calculated values from the analysed plagioclase separate
from benmoreite (Table 4).
In models IV and V (Table 6), we have sought to
deterrnine bow further fractionation from trachyte (IV) or
benmoreite (V) can yield comendite. In model IV, there is
a considerable deficiency in Na content (4.49% relative) of
the derived comendite. In this model, we used mineral
compositions from the trachyte, including alkali feldspar.
In order to avoid the Na deficiency, a much less sodic and
unrealistic feldspar composition was attempted, which in
turn created problems with other elements. In model V,
mineral compositions of phenocrysts in benmoreite were
used, and the fit is almost perfect for all elements.
However, le Roex & Erlank ( 1 982) rejected their model
in which comendite could be formed from benmoreite,
because the model required a fractionating plagioclase of
An 47 (An 3 1 in our model V), and because there was a 'Ba
and Sr problem' . Instead, they suggested that comendite
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
was formed directly from mugearite, a very long fractio
nating step (69%) in which a plagioclase of An 47 was
required.
The barium (and strontium) problem
Prestvik ( 1 982a) reported Ba contents of three comendites
(1 83-2 1 6-245 ppm) from Bouvetøya. Similarly, le Roex
& Erlank ( 1 982) reported 1 80 and 298 ppm Ba in two
comendites. In this work, we obtained 3 1 2 ppm Ba in
comendite sample B 39 (obsidian). However, our crystal
line comendites are much more depleted in Ba (and Sr),
with only 80 ppm Ba. Apparently, the 'barium problem'
would have been even larger if these samples bad been
used in the models. From the whole rock data we have
calculated the value of DBa and Dsr for the fractionating
feldspar of models IV and V (the comendite models). In
model IV (formation of comendite from trachyte) we get
DBa = 3.8 and Dsr = 5 .7. The fractionating feldspar in this
model is about 2/3 alkali feldspar and 113 anorthoclase (An
1 0). The estimated D ' s are well within the possible ranges
for both elements (Henderson 1982; Blundy & Wood
1 99 1 ) . For model V (formation of comendite directly from
benmoreite) the values of DBa and Dsr for fractionating
feldspar are 2.8 and 4.2, respectively. For plagioclase (An
3 1 ) this is a plausible value, but the DBa value is much
larger than that found by us for this plagioclase when we
analysed the mineral separate (DBa = 0.72). We thus agree
with le Roex & Erlank ( 1 982) that comendite did not form
by fractionation directly from benmoreite. We disagree,
however, that the comendite was formed by fractionation
directly from mugearite. Despite the Na discrepancy of the
comendite derived by fractionation of trachyte, we think
that model IV provides a plausible explanation for the way
in which Bouvetøya comendites are derived. The very high
Ba content of trachyte - and much lower content in
comendite - explains bow the benmoreite magmas
fractionate through a trachyte stage and shows that there
is a shift from plagioclase to alkali feldspar as a
fractionating phase around the trachyte stage. Furthermore,
the ferromagnesian minerals are much more evolved in
trachyte than benmoreite. Fractionation of these minerals
not only results in comenditic composition, but also
explains the trace element data, such as the Ba and Sr
concentration. We have no valid explanation for the Na
discrepancy seen in model IV, but the model is, of course,
'vulnerable' , owing to the fact that we have only one
sample of trachyte.
We have also tried to model (not tabulated) the
relationship between comendite B 39 (glass) and crystal
line comendite. This is possible by approximately 7%
fractionation of alkali feldspar and minor amounts (ca. 2%)
of ol + cpx + mt if Dfeldspar for both Sr and B a is about 19.
This is within the published range for Sr, but higher than
the value we have seen published for Ba (up to 1 2.9;
Henderson (1 982)).
NORSK GEOLOGISK TIDSSKRIFT 79 (1999)
Mantle signature
On the basis of Y - Ti - Zr ratios Prestvik ( 1982b)
characterized the basic rocks from Bouvetøya as 'within
plate' type (i.e. influenced by a mantle plume in more
modem terms). Based on trace element ratios such as
Y/Nb, Zr/Nb, (La/Yb)N, le Roex ( 1987) characterized
them as identical to P(lume)-type MORB of the American
Antarctic Ridge (AAR) and South-West Indian Ridge,
which he suggested had formed by partial melting of
depleted astenosphere that had been veined by enriched
melt (2-4%) in a previous metting event. Our new data
show that the geochemical signatures displayed by the
Bouvetøya suite (Fig. 6) are those of a 'mildly enriched'
ocean island type (Thompson et al. 1984), with typical
high normalized abundances of the HFS elements Nb and
Ta. The average YINb and Zr/Nb ratios of Bouvetøya
hawaiites, which are 0.86 and 6.06, respectively (Table 1),
also attest to their enriched nature.
Although there is a considerable amount of Sr and Nd
isotope data for the ridge axes of the BTJ area (e.g. Dickey
et al. 1977; le Roex et al. 1983; 1985; 1987), very few such
data are published from Bouvetøya (O' Nions & Pankhurst
1974; O' Nions et al. 1977), and the only known Pb isotope
data from Bouvetøya are three determinations of Sun
( 1980). Here we report new Sr and Nd (as well as O)
isotopic data on 11 samples from Bouvetøya and Pb
isotope data on 5 samples (Table 7), all collected during
the Polarsirkel expedition. In Fig. 7 we have plotted some
of the new data together with the previously published
Bouvetøya data (O' Nions & Pankhurst 1974; O' Nions et
al. 1977; Sun 1980). The Bouvetøya rocks fall on, or very
dose to, mixing lines between EM- l and HIMU basalts in
207 P04
04
06
20 8 PbP04 Pb
both
Pb
Pb
vs? PbP Pb,
86
206 P04
206 P04
87
vs. Pb
Pb and Sr/ Sr vs.
Pb
Pb plots (Fi,f,; 7)
and very dose to such mixing trends in 143Nd/1 Nd
144
04
06
143
86
87
vs? PbP Pb and
Nd/ Nd vs. Sr/ Sr diagrams (not
shown). However, the combined isotope data (e.g. the
87
86
06
04
Sr/ Sr vs? PbP Pb plot) are not compatible with
mixing between a depleted mantle source (DMM in Fig. 7)
and enriched sources such as EM- l (and EM-2) or HIMU
sources. This implies that the model of le Roex ( 1987) has
to be modified.
Enrichment was probably integral to the plume. HIMU
signatures are primarily seen from elevated U/Pb ratios
because of loss of Pb (Vidal l992). Unfortunately, we lack
elementa! Pb data on the Bouvetøya rocks, but typical
HIMU signatures are apparent also from the Ce/Rb and Bal
Nb ratios of hawaiites (average 3. 13 and 4.78, respectively,
of 9 samples).
Dredged samples from the South-West Indian Ridge
immediately east of Bouvet (Fig. l) are isotopically almost
identical to the Bouvetøya rocks and apparently related to
the Bouvet plume as well. However, the regional influence
of the Bouvet plume is not well understood. The possible
presence of more than one plume in the BTJ area has been
discussed by several authors (i.e. le Roex 1987). Thus,
Moreira et al. ( 1995), Douglas et al. ( 1995) and Simonov et
Volcanic rocks, Bouvetøya
2 17
al. ( 1996) indicated that the anomalously enriched segment
of the southem MAR east of the Shona seamount (the
'Shona ridge-anomaly' , Fig. l ) - only about 600 km from
Bouvetøya - might represent a separate Shona plume.
Conclusions
Our study supports previously published models which
concluded that the main rock types of Bouvetøya are
interrelated by fractional crystallization. However, we find
that the best evolutionary path of the suite was as follows:
hawaiite � evolved hawaiite � benmoreite � trachyte �
comendite.
Primitive megacrysts of olivine, clinopyroxene and
plagioclase in hawaiite represent phenocrysts of more
primitive (parental) magmas that crystallized at depth,
indicating the presence of an open-system magma cham
ber(s) in which variously evolved melts and crystal
assemblages were able to mix.
Similarity in trace element and isotope ratios indicate
that the Bouvetøya suite, and the much more primitive
basalts dredged from the Bouvet Ridge east of the island,
originated from a common, enriched mantle source. The
radiogenic isotope data show that this source is complex,
consisting of several mantle components.
Acknowledgements. - We express our thanks to the Norsk Polarinstitutt for giving
T.P. the opportunity to participate in the Polarsirkel expedition to Bouvetøya in
1978/79. A grant from The Norwegian Research Council and several contributions
from the Department of Geology and Mineral Resources Engineering,
NTNU
supported the analytical work. We also extend our thanks to Drs Elizabeth E.
Anthony and Asish R. Basu for analytical assistance and to Drs Pall lrnsland, Sven
Maaløe, Calvin G. Barnes and one anonymous journal reviewer for their
constructive comments on the manuscript.
Manuscript received February 1999
References
Blundy, J. D.
& Wood, B. J. 1991: Crystal-chemical controls on the partitioning of
Sr and Ba between plagioclase feldspar, silicate melts, and hydrothermal
solutions. Geochimica et CostrUJchimica Acta 55, 193-209.
Bryan, W. B., Finger, L. W.
& Chayes, F. 1969: Estimating proportions in
petrographic mixing equations by least-squares approximation. Science 163,
926-927.
Dickey, J. S., Frey, F. A., Hart, S. R.
& Watson, E. B. 1977: Geochemistry and
petrology of dredged basalts from the Bouvet triple junction, South Atlantic.
Geochimica et Cosmochimica Acta 41, 1105-1118.
& Kingsley, R. H. 1995: Influence of the Discovery
Douglas, J., Schilling, J.-G.
and Shona mantle plumes on the southem Mid-Atlantic Ridge: Rare earth
evidence. Geophysical Research Letters 22, 2893-2896.
Govindaraju, K. (ed.) 1989: Analytical standards of minerals, ores and rocks.
Geostandards Newsletter, vol.
XIII, special issue.
Hart, S. R. 1984: A large-scale isotope anomaly in the southem hemisphere
mantle. Nature 309, 753-757.
Henderson, P. 1982: lnorganic Geochemistry, 353 pp. Pergamon Press, London.
Hofmann, A. W. 1997: Mantle geochemistry:
volcanism. Nature 385, 219-229.
Imsland, P., Larsen, J. G., Prestvik, T.
the message from oceanic
& Sigmond, E. M. 1977: The geology and
petrology of Bouvetøya, South Atlantic Ocean. Lithos JO, 213-234 .
Leake, B. E. 1978: Nomenclature of amphiboles. American Mineralogist 63,
1023-1052.
Le Bas, M. J., Le Maitre, R. W., Streckeisen, A.
& Zanettin, B. 1986: A
classification of volcanic rocks based on the total alkali-silica diagram. Journal
of Petrology 27, 745-750.
218
NORSK GEOLOGISK TIDSSKRIFf 79 (1999)
T. Prestvik et al.
le Roex, A. P. 1987: Source regions of mid-ocean ridge basalts: evidence for
enrichment processes. In Menzies, M. A. & Hawkesworth, C .1. (eds.): Mantle
Metasomiltism, 389-422. Academic Press, London.
le Roex, A. P. & Erlank, A. J. 1982: Quantitative evaluation of fractional
crystallization in Bouvet Island lavas. Journal of Vo/canology and Geothermal
Research, 13, 309-338.
le Roex, A. P., Dick, H. J. B., Er lank, A. J., Reid, A.M., Frey, F. A. & Hart, S. R.
1983: Geochemistry, mineralogy and petrogenesis of lavas erupted along the
southwest Indian Ridge between the Bouvet triple junction and Il degrees east.
Journal of Petrology 24, 267-318.
le Roex, A. P., Dick, H. J. B., Reid, A.M., Frey, F. A., Erlank, A. J. & Hart, S. R.
1985: Petrology and geochemistry of basalts from the American-Antarctic
Ridge, Southem Ocean: implications for the westward infiuence of the Bouvet
mantle plume. Contributions to Mineralogy and Petrology 90, 367-380.
le Roex, A. P., Dick, H. J. B., Gulen, L., Reid, A.M. & Erlank, A. J. 1987: Local
and regional heterogeneity in MORB from the Mid-Atlantic Ridge between
54.5 os and 51°S: Evidence for geochemical enrichment. Geochimica et
Cosmochimica Acta 51, 541-555.
Ligi, M., Bonatti, E., Bortoluzzi, G., Carrara, G., Fabretti, P., Penitenti, D., Gilod,
D., Peyve, A. A., Skolotnev, S. & Turko, N. 1997: Death and transfiguration of
a triple junction in the South Atlantic. Science 276, 243-245.
Mitchell, N. C. & Livermore, R. A. 1998: The present configuration of the Bouvet
triple junction. Geology 26, 267-270.
Moreira, M., Staudacher, T., Sarda, P., Schilling, J.-G. & Allegre, C. J. 1995: A
primitive plume neon component in MORB: the Shona ridge-anomaly, South
Atlantic (51-52°5). Earth and Planetary Science Letters 133, 367-377.
Nash, W. P. & Crecraft, H. R. 1985: Partition coefficients for trace elements in
silicic magmas. Geochimica et Cosmochimica Acta 49, 2309-2322.
O'Nions, R. K. & Pankhurst, R. J. 1974: Petrogenetic significance of isotope and
trace element variations in volcanic rocks from the Mid-Atlantic. Journal of
Petrology 15, 603-634.
O'Nions, R. K., Hamilton, P. J. & Evensen, N.M. 1977: Variations in 143Ndi1""Nd
and 87Sr/86Sr ratios in oceanic basalts. Earth and Planetary Science Letters 34,
13-22.
Prestvik, T. 1982a: Trace element geochemistry of volcanic rocks from
Bouvetøya, South Atlantic. In Craddock, C. (ed.): Antarctic Geoscience,
771-774. The University of Wisconsin Press, Madison.
Prestvik, T. 1982b: Basic volcanic rocks and tectonic setting. A discussion of the
Zr-Ti-Y discrimination diagram and its suitability for classification purposes.
Lithos 15, 241-247.
Prestvik, T. 1985: Petrology of Quatemary volcanic rocks from Oræfi, south-east
Iceland. Report 21, Geological Institute, NI'H, Trondheim, 81 pp.
Prestvik, T. & Winsnes, T. 1981: Geology of Bouvetøya, South Atlantic. Norsk
Polarinstitutt Skrifter 175, 41--{;9.
Prestvik, T., Barnes, C. G., Sundvoll, B. & Duncan, R. A. 1990: Petrology of Peter
I Øy (Peter I Island), West Antarctica. Journal of Volcanology and Geothermal
Research 44, 315-338.
Roeder, P. & Emslie, R. F. 1970: Olivine-liquid equilibrium. Contribution to
Mineralogy and Petrology 29, 275-289.
Simonov, V. A., Peyve, A. A., Kolobov, V. Y., Milosnov, A. A. & Kovyazin, S. V.
1996: Magmatic and hydrothermal processes in the Bouvet Triple Junction
Region (South Atlantic). Terra Nova 8, 415-424.
Sun, S.-S. 1980: Lead isotopic study of young volcanic rocks from mid-ocean
ridges, ocean islands and island arcs. Philosophical Transactions of the Royal
Society of London, series A 297, 409-445.
Thompson, R. N., Morrison, M. A., Hendry, G. L. & Parry, S. J. 1984: An
assessment of the relative roles of crust and mantle in magma genesis: an
elementa! approach. Philosophical Transactions of the Royal Society of
London, series A 310, 549-590.
Verwoerd, W. J., Erlank, A. J. & Kable, E. J. D. 1976: Geology and geochemistry
of Bouvet Island. In Gonzales Ferran, O. (ed.): Andean and Antarctic
Volcanology Problems, 203--237. lAVCEI, Naples.
Vida!, P. 1992: Mantle: More HIMU in the future? Geochimica et Cosmochimica
Acta 56, 4295-4299.
Weill, D. F. & Drake, M. J. 1973: Europium anomaly in plagioclase feldspar:
experimental results and semiquantitative model. Science I 80, l 059-1060.