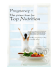Effect of multivitamin and vitamin A supplements on weight gain
Transcription
Effect of multivitamin and vitamin A supplements on weight gain
Effect of multivitamin and vitamin A supplements on weight gain during pregnancy among HIV-1-infected women1–3 Eduardo Villamor, Gernard Msamanga, Donna Spiegelman, Gretchen Antelman, Karen E Peterson, David J Hunter, and Wafaie W Fawzi Maternal HIV infection also contributes to LBW resulting from preterm delivery and intrauterine growth retardation (IUGR) (13), particularly in sub-Saharan Africa, where > 13 million women of childbearing age are infected (14). Progression of HIV disease is usually accompanied by opportunistic infections (15), diminished dietary intake (16), nutrient malabsorption (17), and metabolic and hormonal alterations (18–21) that lead to depletion of both body fat and fat-free compartments (22–25), resulting in weight loss. A part of the adverse effect of HIV disease on pregnancy outcomes is most likely mediated through the changes in maternal body composition and the weight loss induced by the infection. However, the magnitude and determinants of these changes remain virtually unknown. It was shown that daily consumption of multivitamin supplements by HIV-infected pregnant women resulted in significant reductions in the risk of LBW [relative risk (RR): 0.56; 95% CI: 0.38, 0.82], severe preterm birth (RR: 0.61; 95% CI: 0.38, 0.96), IUGR (RR: 0.57; 95% CI: 0.39, 0.82), and fetal loss (RR: 0.61; 95% CI: 0.39, 0.94) and also improved the immunologic profile of the mothers and increased their hemoglobin concentrations (26). In another study, vitamin A supplementation during pregnancy was found to reduce the risk of preterm delivery among HIV-infected women (27). If a positive effect of vitamin supplementation on gestational weight gain is also found, this could constitute a mechanistic explanation for the reduced risk of adverse pregnancy outcomes. We examined this question in the context of a randomized trial of multivitamin and vitamin A supplementation among HIV-infected pregnant women in Tanzania. KEY WORDS Weight gain, pregnancy, HIV infection, AIDS, multivitamins, vitamin A, sub-Saharan Africa, maternal health, prenatal nutrition 1 From the Departments of Nutrition (EV, GA, KEP, DJH, and WWF), Epidemiology (DS, DJH, and WWF), Biostatistics (DS), and Maternal and Child Health (KEP), Harvard School of Public Health, Boston, and the Department of Community Health, Muhimbili University College of Health Sciences, Dar es Salaam, Tanzania (GM). 2 Supported by the National Institute of Child Health and Human Development (NICHD R01 32257) and the Fogarty International Center (NIH D43 TW00004). The National University of Colombia, the Fulbright Program, and the Colombian National Science Foundation “Colciencias” provided partial support to EV. 3 Address reprint requests to E Villamor, Department of Nutrition, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115. E-mail: [email protected]. Received July 23, 2001. Accepted for publication December 7, 2001. INTRODUCTION Maternal nutritional status before and during gestation is one of the strongest determinants of pregnancy outcomes. Gestational weight gains below recommended amounts are common in developing countries (1–6) and account for a significant proportion of the risk of low birth weight (LBW) (7–9) and preterm delivery (10–12). Identifying potential interventions for improving gestational weight gain may be important in reducing the incidence of adverse pregnancy outcomes. 1082 Am J Clin Nutr 2002;76:1082–90. Printed in USA. © 2002 American Society for Clinical Nutrition Downloaded from ajcn.nutrition.org by guest on June 11, 2014 ABSTRACT Background: The pattern of weight gain during pregnancy among HIV-infected women is largely unknown. Multivitamin supplementation was shown to be effective in preventing adverse pregnancy outcomes among HIV-positive women. These protective effects could be mediated in part by an improvement in the pattern of gestational weight gain. Objective: We examined the effects of multivitamin and vitamin A supplements on weight gain during the second and third trimesters of pregnancy among HIV-infected women. Design: We enrolled 1075 pregnant, HIV-1-positive women from Dar es Salaam, Tanzania, in a randomized, placebo-controlled trial. Using a 2-by-2 factorial design, we assigned each woman to 1 of 4 regimens: multivitamins (thiamine, riboflavin, niacin, folic acid, and vitamins B-6, B-12, C, and E), vitamin A, multivitamins including vitamin A, or placebo. The women took these oral supplements daily and were weighed monthly until the end of pregnancy. Results: The mean rate of weight gain was 306 g/wk during the second trimester and 247 g/wk during the third trimester. During the third trimester, average weight gain was significantly greater (by 304 g; 95% CI: 17, 590; P = 0.04) and the risk of low rate of weight gain (≤ 100 g/wk) was significantly lower (relative risk: 0.73; 95% CI: 0.58, 0.93) in women who received multivitamins than in women who did not. Multivitamins including vitamin A were protective against low weight gain during the second trimester compared with multivitamins alone. Conclusion: Multivitamin supplementation during pregnancy improves the pattern of weight gain among HIV-infected women. Am J Clin Nutr 2002;76:1082–90. VITAMINS AND PREGNANCY WEIGHT GAIN IN HIV INFECTION SUBJECTS AND METHODS Study design and population Laboratory investigations Baseline tests were carried out to obtain a complete blood profile and routine urine and stool examinations and to test for malaria and sexually transmitted diseases. Hemoglobin was measured by using the CBC5 Coulter Counter (Coulter Corporation, Miami) or the cyanmethemoglobin colorimetric method (Corning Inc, Corning, NY). Absolute counts of CD3+, CD4+, and CD8+ T lymphocyte subsets were obtained with the FACS count system (Becton Dickinson, San Jose, CA). Serum retinol and vitamin E concentrations were measured with HPLC, and serum selenium was measured with atomic absorption analysis. Active syphilis was diagnosed if the subject had positive results for sera antibodies on 2 tests: the VDRL (Murex Diagnostic, Dartford, United Kingdom) and Treponema Pallidum Hemagglutination (Fujirebio, Tokyo). Infections caused by Neisseria gonorrhea, Candida albicans, and Trichomonas vaginalis were identified from vaginal and cervical swabs. The presence of malaria parasites was ascertained from giemsa-stained thick- and thin-smear blood films. Virtually all malaria infections were caused by Plasmodium falciparum. Parasite density per cubic millimeter was calculated from the number of malaria parasites identified per 200 leukocytes and each subject’s total leukocyte count/mm3 in peripheral blood. Stool specimens were examined for the presence of helminths (Ascaris lumbricoides, hookworm, Trichuris trichiura, Strongyloides stercoralis, and Schistosoma mansoni) and protozoans (Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum). Samples were examined macroscopically for worms and microscopically for eggs, larvae, trophozoites, and cysts by using saline and iodine wet mount and the formalin-ether concentration technique. A urine sample was examined for the presence of Schistosoma haematobium. Data analyses Analyses of weight gain during pregnancy were limited to women who had: 1) a singleton pregnancy, 2) a known date of pregnancy outcome, and 3) ≥ 2 weight measurements between enrollment and the end of gestation. Data from women with adverse outcomes, such as abortion or stillbirth, were not excluded from the analyses. The distribution of baseline characteristics was compared across treatment groups by using the Wilcoxon rank sum and KruskalWallis tests for continuous variables and 2 tests for proportions. Intent-to-treat analyses were carried out to examine treatment effects on both continuous and categorical weight gain outcomes. We examined the overall effect of multivitamin supplements by comparing women who received multivitamins alone or in combination with vitamin A with women who did not receive multivitamins, ie, those who received only vitamin A or placebo. Similarly, to assess the overall effect of vitamin A supplements, we compared women who received vitamin A alone or in combination with multivitamins with women who did not receive vitamin A, ie, those who received only multivitamins or placebo. We assessed the joint effect of multivitamins and vitamin A according to the methods described below. Continuous outcomes The continuous outcomes included overall and trimester-specific total weight gain and the rate of weight gain at various points during pregnancy. We calculated overall total weight gain as the arithmetic difference between the last weight recorded before the end of pregnancy and weight at randomization. Trimester-specific total weight gain was estimated separately for the subset of women contributing ≥ 2 weight measurements between weeks 12 and 26 (second trimester) and for those with ≥ 2 weight measurements after week 26 (third trimester), as the difference between the last and first weight measurements available during the interval. The effect of the supplements was then calculated as the difference in average weight change between treatment arms, overall and by trimesters. The 95% CI for the treatment effect was estimated from a two-way analysis of variance model with robust estimators of variance (30). We also examined the effect of the supplements on the weekly rate of weight gain by using a mixed-effects regression model for repeated measures (PROC MIXED; SAS Institute Inc, Cary, NC): WT ij = (0 + b i0) + 1MV i + 2VA i + (3 + b i1) GA ij + 4GA 2ij + 5MV i GA ij + 6VA i GA ij + 7MV i GA 2ij + 8VA i GA 2ij + 9MV i VA i + 10MV i VA i GA ij + 11MV i VA i GA 2ij + e ij (1) Downloaded from ajcn.nutrition.org by guest on June 11, 2014 Between April 1995 and July 1997, pregnant women who were receiving prenatal care and tested positive for HIV infection at 1 of 4 clinics in Tanzania were invited to participate in a randomized clinical trial. The study aim was to test the effect of micronutrient supplements on vertical transmission and various health and pregnancy outcomes. Detailed descriptions of the trial design were published previously (26, 28). In brief, women eligible for enrollment were between 12 and 27 wk gestation according to the date of the last menstrual period; they resided in Dar es Salaam and intended to stay in the city until delivery and for ≥ 1 y thereafter. As part of the prenatal screening, consent was sought for HIV-1 testing. Pretest and posttest counseling sessions were provided. We tested HIV-1 serostatus by using an enzyme-linked immunosorbent assay (Wellcozyme; Murex Biotech Ltd, Dartford, United Kingdom) and confirmed positive results with Western blot (Bio-Rad Laboratories Ltd, Hertfordshire, United Kingdom). HIV-1-infected women who consented to participate in the trial were randomly assigned in a 2-by-2 factorial design to receive a daily oral dose of 1 of 4 regimens. The 4 regimens were as follows: multivitamins (20 mg thiamine, 20 mg riboflavin, 25 mg vitamin B-6, 100 mg niacin, 50 g vitamin B-12, 500 mg vitamin C, 30 mg vitamin E, and 0.8 mg folic acid), vitamin A alone (30 mg -carotene plus 5000 IU preformed vitamin A), multivitamins including vitamin A, and placebo. Subjects consumed the supplements or placebo from enrollment until the end of the study (after delivery). The supplement and placebo tablets were indistinguishable. In addition, all subjects received 120 mg ferrous iron and 5 mg folate daily. Weekly malaria prophylaxis was also provided in accordance with the standard of prenatal care in Tanzania. Prenatal care and counseling were provided throughout the study period. Antiretroviral medications were unavailable in this setting at the time of the study. Information on subject age, education, socioeconomic and marital status, and obstetric history was obtained at the first study visit by trained personnel. Study physicians performed a complete medical examination and collected blood, urine, stool, and vaginal specimens. Height, weight, and midupper arm circumference were measured in light clothing by trained personnel using standardized methods and calibrated instruments (29). At every monthly visit after enrollment, study personnel conducted a physical examination and obtained anthropometric measurements. 1083 1084 VILLAMOR ET AL Mixed effects models allow the use of all available measurements during follow-up ( j) for every subject ( i), adjusting for the within-person correlation of measurements in the estimation of the variance (31). The effect of multivitamins (MV) at any given week of gestation (GA) was estimated as the difference ( 5 + 7 ) in the rate of weight (WT) gain between women who received multivitamins and those who did not. The effect of vitamin A (VA) was estimated as ( 6 + 8 ), whereas a potential joint effect of multivitamins and vitamin A was assessed from a three-way interaction term between multivitamins, vitamin A, and gestational age. A quadratic term for time (GA 2) has been found appropriate to describe nonlinear patterns of weight gain during pregnancy (32), and was statistically significant in this population. CIs for the treatment effect were computed by using robust estimates of variance. Average weight gain curves were fitted by using restricted cubic splines (33). Categorical outcomes We considered the effect of supplements on 1) the risk of low total weight gain, 2) the risk of low rate of weight gain, and 3) the risk of weight loss. Low total weight gain, overall and by trimesters, was defined as a weight difference between the last and first measurements that was below the 25th percentile of the distribution for total or trimester-specific weight gain, respectively. For the definitions of low rate of weight gain and weight loss as categorical outcomes, the rate of weight gain was estimated separately for the second and third trimesters as the regression slope of every individual’s set of weight measurements on week of gestation during the trimester-specific interval. Low rate of weight gain was defined as a regression slope below the 25th percentile of the trimester-specific distribution, and weight loss was defined as a slope ≤ 0. To test the effect of supplements on these categorical outcomes, we calculated RRs with 95% CIs from 2 n tables, and we used the chi-square test. We also assessed whether the effect of supplements was modified by baseline characteristics, including midupper arm circumference, CD4+ and CD8+ T cell counts, clinical stage of HIV disease according to World Health Organization criteria (34), hemoglobin concentration, malaria infection, intestinal parasitoses, sexually transmitted diseases, season at conception, and serum retinol, vitamin E, and selenium concentrations. The statistical significance of all interactions was tested with the likelihood ratio test. Analyses were carried out with Statistical Analyses System software, version 8 (SAS Institute Inc, Cary, NC). The study protocol was approved by the Research and Publications Committee of Muhimbili University College of Health Sciences, the Ethical Committee of the National AIDS Control Program of the Tanzanian Ministry of Health, and the Institutional Review Board of the Harvard School of Public Health. All subjects gave their informed consent to participate in the study. RESULTS A total of 1075 HIV-infected, pregnant women were randomly assigned to a treatment group; 6 of the women died before delivery. Twin pregnancies (n = 24), women with unknown date of pregnancy outcome (n = 42), and mothers with < 2 weight measurements during pregnancy (n = 46) were excluded from the analyses of weight gain; thus, results are shown for 957 women (Figure 1). The distribution of baseline covariates, including sociodemographic characteristics of the mother, indicators of Downloaded from ajcn.nutrition.org by guest on June 11, 2014 FIGURE 1. Profile of the study population in the Tanzania Trial of Vitamins. VITAMINS AND PREGNANCY WEIGHT GAIN IN HIV INFECTION 1085 TABLE 1 Maternal characteristics at baseline according to treatment assignment1 Characteristic Vitamin A only (n = 239) Multivitamins only (n = 237) 20.4 ± 3.4 24.8 ± 4.8 85.9 [201] 90.2 [211] 25.4 [59] 30.6 [71] 517 ± 260 20.4 ± 3.4 24.6 ± 5.0 87.9 [210] 86.2 [206] 25.2 [60] 37.2 [89] 581 ± 324 20.8 ± 3.1 24.6 ± 4.7 83.6 [198] 88.6 [210] 26.6 [63] 36.0 [85] 495 ± 243 20.4 ± 3.2 24.9 ± 4.6 88.7 [219] 88.3 [218] 24.1 [60] 31.3 [77] 510 ± 250 9.4 [22] 20.5 [48] 56.8 [133] 13.3 [31] 156.4 ± 5.9 56.3 ± 8.7 25.4 ± 2.9 0.90 ± 0.41 9.8 ± 3.0 0.130 ± 0.03 449 ± 263 767 ± 366 97 ± 17 23.1 [54] 13.0 [31] 16.7 [40] 53.1 [127] 17.2 [41] 156.7 ± 6.2 57.6 ± 9.7 25.7 ± 3.0 0.85 ± 0.33 9.8 ± 3.2 0.127 ± 0.02 440 ± 273 751 ± 343 96 ± 18 28.4 [68] 13.5 [32] 16.0 [38] 54.0 [128] 16.5 [39] 156.8 ± 5.4 58.2 ± 9.5 25.8 ± 2.9 0.84 ± 0.33 9.7 ± 3.0 0.125 ± 0.02 451 ± 255 723 ± 324 95 ± 17 20.7 [49] 13.8 [34] 17.4 [43] 56.3 [139] 12.6 [31] 156.5 ± 5.9 57.3 ± 8.4) 25.6 ± 2.7 0.86 ± 0.29 10.4 ± 3.2 0.126 ± 0.02 438 ± 242 764 ± 330 94 ± 17 24.7 [61] 15.8 [37] 4.3 [9] 11.5 [24] 5.3 [11] 6.2 [14] 25.9 [60] 18.5 [44] 4.3 [9] 9.3 [19] 3.9 [8] 4.7 [11] 21.0 [50] 17.8 [42] 5.7 [12] 12.5 [26] 4.8 [10] 8.8 [20] 27.5 [65] 22.7 [56] 7.9 [17] 13.0 [28] 4.2 [9] 5.0 [12] 25.2 [62] Completed ≥ 8 y of formal schooling. Mothers who provided part or all of the houshold income; those who did not were solely supported by the partner or another person. 4 Average household income spent on food per person per day, estimated by dividing the total amount of money spent on food by the number of household members. The exchange rate for 500 shillings was US$0.62 at the time of data collection. The difference in median household income spent on food per person per day between treatments was significant, P = 0.05 (Wilcoxon rank-sum test). 5 The month at conception was estimated from the reported date of the last menstrual period plus 14 d. 6 Women in stages 2 or 3 at recruitment according to the World Health Organization staging system of HIV disease. 2 3 nutritional and immunologic status, stage of HIV disease, and presence of selected infectious diseases did not differ significantly across treatment regimens (Table 1). For each treatment arm, mean gestational age at randomization was 20 wk, and the last visit occurred at week 36, on average. The mean number of weight measurements per woman was 4.7, independent of treatment assignment, and 94% of the mothers had ≥ 3 measurements. We reported previously that compliance with treatment during pregnancy was high, as assessed by pill count (median: 90% by the time of delivery) (26). In addition, in a subset of 125 women, evidence of high compliance was reported. In the group that received vitamin A, the plasma -carotene concentration increased significantly from 0.23 mol/L at baseline to 1.71 mol/L at delivery, whereas the values were 0.22 mol/L at baseline and 0.25 mol/L at delivery in the group not given vitamin A (28). Total weight gain from randomization to the last visit during pregnancy was 4.0 ± 3.2 kg. The estimated average rate of weight gain during the second trimester, 306 g/wk (95% CI: 283, 329), was significantly higher than that during the third trimester, 247 g/wk (95% CI: 230, 263). This indicates that the pattern of weight gain in this population did not follow a linear trend. The incidence of weight loss was 14.2% (n = 94) during the second trimester and 15.7% (n = 131) during the third trimester. The subsets of women with ≥ 2 weight measurements in the second and third trimesters were comparable to each other with respect to baseline characteristics. Supplementation with multivitamins significantly increased weight gain during the third trimester (Table 2). The average total effect was 304 g (95% CI: 17, 590; P = 0.04). We also observed a positive effect on the rate of weight gain, particularly after week 26 of gestation (Figure 2A). By week 36, mothers receiving multivitamins were gaining an average of 39 g/wk more than women not receiving multivitamins. During the third trimester, multivitamin supplements resulted in significantly reduced risks of low total weight gain (RR: 0.70; 95% CI: 0.55, 0.90; P = 0.005), weight loss (RR: 0.69; 95% CI: 0.50, 0.95; P = 0.02), and low rate of weight gain (RR: 0.73; 95% CI: 0.58, 0.93; P = 0.01). Downloaded from ajcn.nutrition.org by guest on June 11, 2014 Gestational age at recruitment (wk) Age (y) Completed primary school (%)2 Lives with partner (%) Contributes to household income (%)3 Nulliparous (%) Money spent on food (Tanzanian shillings)4 Season at conception (%)5 January–February (Dry) March–May (Long rains) June–October (Dry) November–December (Short rains) Height (cm) Weight (kg) Midupper arm circumference (cm) Serum retinol (mol/L) Serum vitamin E (mol/L) Serum selenium (ppm) CD4+ cell count (cells/mm3) CD8+ cell count (cells/mm3) Hemoglobin (g/L) Symptomatic HIV disease (%)6 Infectious diseases (%) Malaria Ascaris Hookworm Cryptosporidium Syphilis Trichomonas vaginalis 1– x ± SD; n in brackets. Multivitamins and vitamin A (n = 247) Placebo (n = 234) 1086 VILLAMOR ET AL TABLE 2 Effects of multivitamins and vitamin A supplements on gestational weight gain outcomes Outcome Vitamin A only 3914 ± 3474 3962 ± 3134 1953 ± 1886 1793 ± 1766 1855 ± 2187 1939 ± 2021 Multivitamins Multivitamins only and vitamin A Difference, MV vs no MV1 Difference, VA vs no VA2 4041 ± 2975 4048 ± 3221 107 (299, 512) 1613 ± 1961 1802 ± 1697 161 (117, 438) 2272 ± 1954 2131 ± 2302 304 (17, 590) 28 (434, 377) 13 (292, 266) 31 (256, 319) 295 ± 31 320 ± 30 309 ± 30 283 ± 28 13 (71, 46) 3 (62, 55) 254 ± 14 198 ± 21 260 ± 12 216 ± 20 266 ± 12 247 ± 19 262 ± 12 245 ± 21 7 (17, 32) 39 (1, 79) 1 (25, 24) 9 (49, 31) 28.6 [67]8 20.9 [34] 29.2 [59] 25.1 [60] 26.1 [42] 28.5 [59] 22.8 [54] 29.6 [47] 18.9 [40] 25.9 [64] 21.1 [38] 21.8 [47] 0.91 (0.73, 1.13) 1.07 (0.82, 1.40) 0.70 (0.55, 0.90) 0.99 (0.80, 1.23) 0.93 (0.71, 1.22) 1.05 (0.83, 1.33) 11.7 [19] 18.8 [38] 15.5 [25] 18.4 [38] 19.5 [31] 9.4 [20] 10.6 [19] 16.2 [35] 1.09 (0.75, 1.58) 0.69 (0.50, 0.95) 0.83 (0.57, 1.21) 1.23 (0.90, 1.69) 22.7 [37] 28.7 [58] 24.8 [40] 30.0 [62] 30.2 [48] 20.3 [43] 22.2 [40] 22.7 [49] 1.09 (0.84, 1.42) 0.73 (0.58, 0.93) 0.89 (0.68, 1.16) 1.08 (0.85, 1.36) 1 Mean difference between women receiving multivitamins (MV; multivitamins only or multivitamins plus vitamin A) and women not receiving multivitamins (vitamin A only or placebo); 95% CI in parentheses. 2 Mean difference between women receiving vitamin A (VA; vitamin A only or multivitamins plus vitamin A) and women not receiving vitamin A (multivitamins only or placebo); 95% CI in parentheses. 3– x ± SD. 4 From 20 to 36 wk of gestation, on average. 5 From a repeated-measures mixed-effects model that included weight as the dependent variable and time, treatment, and their interaction term as predictors. A quadratic term for week of gestation was included to account for the nonlinearity of the weight gain pattern. 6– x ± SE. 7 Defined as below the 25th percentile of the weight change distribution. 8 n in brackets. 9 Subject-specific slope of the regression of weight on gestational age ≤ 0. 10 Subject-specific slope of the regression of weight on gestational age < 25th percentile of the trimester-specific distribution of slopes. Vitamin A supplements did not have a significant effect on weight gain outcomes overall or during the third trimester (Figure 2B). However, there was a positive, significant interaction between vitamin A supplements and multivitamins during the second trimester (P = 0.04). Women receiving both vitamin A and multivitamins had a 29% lower risk of low total weight gain than did women who received multivitamins alone (RR: 0.71; 95% CI: 0.49, 1.03). There was no effect of vitamin A alone compared with placebo (RR: 1.25; 95% CI: 0.84, 1.86). We also examined whether the protective effect of multivitamins during the third trimester differed between strata of certain baseline characteristics that were potential effect modifiers (eg, stage of HIV disease; Table 3). The protective effect tended to be greater among women in stage 1 of HIV disease compared with women in stage 2 or higher (P for interaction = 0.12). The protective effect also tended to be greater when the approximate date of conception coincided with the first dry season of the year (P for interaction = 0.11). During the second trimester, vitamin A supplementation was associated with a significant reduction in the risk of low rate of weight gain among mothers with hemoglobin concentration ≥ 110 g/L (RR: 0.31; 95% CI: 0.14, 0.68) but not among mothers with hemoglobin < 110 g/L (RR: 1.08; 95% CI: 0.81, 1.45) (P for interaction = 0.001). There were no significant interactions between the treatments and other potential effect modifiers including the mother’s level of education, CD4+ or CD8+ cell counts, malaria infection, intestinal parasites, sexually transmitted diseases, and plasma concentrations of vitamin A, vitamin E, or selenium. DISCUSSION We described the pattern of weight gain during pregnancy among HIV-infected women in Tanzania within the context of a randomized clinical trial. Multivitamin supplementation resulted in a small increase in maternal weight gain during the third trimester of pregnancy (304 g; 95% CI: 17, 590). Multivitamins were also associated with a significant 30% reduction in the risk of weight loss or low weight gain after week 27 of gestation. It is not likely that these results are attributable to either chance or confounding, given the large sample size and the randomized nature of the trial, respectively. The distribution of baseline covariates in each trimester subset was homogeneous across treatment arms, implying an absence of selection bias. Little information is available on the relation between the micronutrient status of the mother and weight gain during pregnancy, especially among HIV-infected women. Some evidence of a beneficial association has been reported from an observational study conducted in the United States (35) and from trials performed in Greece (36) and Chile (37), all presumably among HIVnegative women. In the US study of prenatal micronutrient supplements, the proportion of mothers with inadequate weight gain was significantly higher among women who did not take prenatal Downloaded from ajcn.nutrition.org by guest on June 11, 2014 Total weight gain (g)3 Overall: from baseline to last visit4 Second trimester: weeks 12 to 26 Third trimester: week 27 to delivery Estimated rate of weight gain (g/wk)5,6 Second trimester (week 17) Third trimester Week 27 Week 36 Risk of low total weight gain (%)7 Overall: from baseline to last visit (< 1.8 kg)4 Second trimester: weeks 12 to 26 (< 0.7 kg) Third trimester: week 27 to delivery (< 0.7 kg) Risk of weight loss (%)9 During second trimester During third trimester Risk of low rate of weight gain (%)10 During second trimester (≤ 130 g/wk) During third trimester (≤ 100 g/wk) Placebo VITAMINS AND PREGNANCY WEIGHT GAIN IN HIV INFECTION supplements (28.7%) than among those who did (22.0%) (35). The mean rate of weight gain increased monotonically and significantly according to the total amount of folate consumed during pregnancy (38). In a trial conducted in Greece, pregnant women assigned to receive biweekly counseling about selecting foods with high nutrient value and using techniques for reducing nutrient loss during preparation had higher total weight gain than did the control subjects (11.3 and 10.3 kg, respectively; P < 0.05) (36). This coincided with increases in serum -carotene concentrations during late pregnancy and serum vitamin C during early and late pregnancy in the intervention group. In a supplementation trial conducted in Santiago, Chile, mothers were randomly assigned to receive a milk-based product fortified with thiamine, pyridoxine, niacin, folate, zinc, iron, copper, iodine, and vitamins A, C, E, and D or a nonfortified supplement of powdered milk (37). Total weight gain during pregnancy was significantly greater for mothers receiving the fortified product than for those receiving unfortified powdered milk (12.3 compared with 11.3 kg; P < 0.05). The authors suggested that this effect could be attributable to the micronutrients in the fortified product, because the total energy, fat, and protein contents were higher in the unfortified powered milk. However, none of these 3 studies were designed to specifically address the effect of micronutrient supplementation on weight gain. Regarding the trials, one cannot conclude that the vitamins definitely had a causal effect, and in the observational study, only unadjusted associations were presented, opening the possibility of confounding by intakes of other nutrients or total energy, socioeconomic status, or other variables. In our study, the effect of multivitamins on maternal weight gain was limited to the third trimester and was relatively modest (300 g between week 27 and term). Although this overall effect may have limited clinical relevance, our data suggest that multivitamin supplements may be particularly efficacious in preventing severe impairments in the weight-gain patterns of HIVinfected women, including weight loss and very low weight gain. Some studies among presumably HIV-negative women have linked low weight gain or weight loss during the third trimester of pregnancy with adverse pregnancy outcomes that include low birth weight and premature birth. In the Dutch Famine Birth Cohort Study, low weight gain (< 0.5 kg/wk) or weight loss during the third trimester were related to lower birth weight, length, and ponderal index (39). In women from rural Malawi, the rate of weight gain during late pregnancy (defined as after week 32) was more strongly predictive of birth weight and length than was the weight-gain rate during early to mid pregnancy (40). In the same population, lower birth weight and length were observed among the offspring of women exposed to seasonal nutritional stress during the third trimester, but not during the second trimester (41). In a group of low-income women in the United States, the risk of preterm delivery was found to be higher in those with low weight gain during the third trimester (after week 27) but not during the first or second trimester (42). The observed protective effect of multivitamins against low maternal weight gain in our study population could be a mechanistic explanation for a protective effect of multivitamins against adverse pregnancy outcomes reported previously in this same group of mothers (26). Also, an effect of vitamin supplements on weight gain during the second trimester of pregnancy cannot be ruled out. In our study, the limitation of the beneficial effect to the third trimester suggests that it may take weeks for the correction of baseline deficiencies and the activation of regulatory mechanisms to occur; therefore, supplementation should be implemented as early as possible during pregnancy. Improvement of second trimester weight gain could also have a positive effect on birth outcomes, as was shown in various cohorts in the United States (43–45). The trimester-specific rates of weight gain in this group of HIV-infected mothers were generally below the average reported in presumably HIV-negative populations in developed countries (8, 46, 47) but were comparable to those documented in undernourished women of undetermined HIV status in developing countries (5, 6). HIV infection may be related to poor patterns of gestational weight gain in many present-day populations, although the evidence is limited. One small study performed in Rwanda showed that total weight gain between the first visit and the last visit before delivery was lower for HIV-positive women Downloaded from ajcn.nutrition.org by guest on June 11, 2014 FIGURE 2. Effect of multivitamin (A) and vitamin A (B) supplements on weight gain during the third trimester of pregnancy. The average trends of weight gain were estimated by using cubic splines (33). The multivitamin group (n = 473) included those subjects assigned to receive multivitamins only or multivitamins plus vitamin A. In this group, the rate of weight gain became progressively higher over time (P = 0.06 for the rate difference at week 36 according to the Wald test). The “no multivitamins” group included those subjects assigned to receive placebo or vitamin A only (n = 484). The vitamin A group (n = 471) included those subjects assigned to receive vitamin A only or vitamin A plus multivitamins. The “no vitamin A” group included those subjects assigned to receive placebo or multivitamins only (n = 486). No significant effect was observed. 1087 1088 VILLAMOR ET AL TABLE 3 Effects of multivitamins (MV) on the risk of low rate of weight gain (≤ 100 g/wk) during the third trimester of pregnancy, by strata of potential effect modifiers at baseline1 Modifier Relative risk3 P for interaction4 0.11 13/44 22/77 71/228 14/60 5/62 19/69 57/234 11/63 0.27 (0.10, 0.71) 0.96 (0.57, 1.62) 0.78 (0.58, 1.05) 0.75 (0.37, 1.52) 88/310 32/99 63/337 29/91 0.66 (0.50, 0.87) 0.99 (0.65, 1.49) 24/51 95/354 14/54 78/370 0.55 (0.32, 0.94) 0.79 (0.51, 1.02) 62/218 28/108 53/232 24/117 0.80 (0.59, 1.10) 0.79 (0.49, 1.28) 41/152 50/175 43/187 34/162 0.85 (0.59, 1.23) 0.73 (0.50, 1.07) 20/80 100/327 8/65 84/362 0.49 (0.23, 1.04) 0.76 (0.59, 0.97) 98/336 22/72 69/346 23/82 0.68 (0.52, 0.89) 0.92 (0.56, 1.50) 0.12 0.24 0.96 0.70 0.27 0.30 1 Cutoffs for CD4+ cell counts, serum retinol, and hemoglobin were established according to commonly used references. The median of the distribution was used to determine the cutoff for serum vitamin E. WHO, World Health Organization. 2 No MV includes women assigned to vitamin A only or placebo. MV includes women assigned to multivitamins plus vitamin A or multivitamins only. 3 95% CI in parentheses. 4 Likelihood ratio test for interaction. 5 Malaria parasites were present in peripheral blood at prenatal visit. than for HIV-negative women, but the slope of weight gain was not significantly different (48). In a study conducted in Italy, total weight gain among opiate addicts was slightly higher for HIVpositive women than for HIV-negative women, but among nonaddicts, HIV-negative women gained significantly more weight than did HIV-positive women (49). Poor gestational weight gain among HIV-infected women could be explained by an HIV-related impairment of fetal and placental growth (13, 50) or by an effect of the infection on maternal body composition (51). Multivitamin supplements could ameliorate these adverse effects through several mechanisms. Regular use of multivitamins or B vitamins and higher dietary intakes of riboflavin, thiamin, and niacin were linked to slower progression of HIV disease (52–54). This effect may be mediated by an improvement in immune status, as documented in the Tanzania trial (26), which would in turn reduce the incidence and severity of secondary infections. In animal and human studies, vitamin B-6 deficiency was associated with alterations in the differentiation of lymphocytes, decreased antibody production, and delayed hypersensitivity responses (55), and serum concentrations correlated well with natural killer cell cytotoxicity (56). In animals, riboflavin and vitamin B-12 deficiencies are related to impaired production of antibodies, and low vitamin B-12 concentrations are associated with diminished expression of T cells and decreased neutrophil function (55, 57). Administration of vitamins C and E reduced HIV viral load and oxidative stress in a supplementation trial (58). Secondary infections among HIV-infected patients may lead to wasting through various mechanisms including increased secretion of cytokines, faster viral replication, decreased intake and absorption of nutrients, and increased nutrient losses and utilization (59). Enhancement of immune function mediated by micronutrient supplementation might lessen the burden of this cascade by improving the immune response against opportunistic infections. In conclusion, HIV infection is likely to impair the pattern of weight gain during pregnancy in women from sub-Saharan Africa. Multivitamin supplementation could be a useful method of ameliorating this effect and, consequently, improving pregnancy outcomes. Additional research is needed to investigate the effects that HIV-induced changes in body composition may have on fetal loss, prematurity, intrauterine growth retardation, vertical transmission, and infant and maternal mortality. Also, the ways that pregnancy may affect HIV-related wasting deserve further examination. Studies should also investigate the effect of reducing weight loss on the postpartum health and survival of HIV-infected women. Finally, the potential beneficial effects of micronutrient supplementation on gestational weight gain should be examined in women who are not infected with HIV. We thank the women who participated in this project; the study coordinator, Illuminata Ballonzi; the study physicians, Heavington Mshiu, Josephine Ballat, and Vensesla Sakwari; and the research assistants, laboratory technicians, Downloaded from ajcn.nutrition.org by guest on June 11, 2014 Season at conception January–February (Dry) March–May (Long rains) June–October (Dry) November–December (Short rains) WHO stage of HIV disease Asymptomatic (stage 1) Symptomatic (stage ≥ 2) CD4+ cell count 0–199 cells/mm3 ≥ 200 cells/mm3 Serum retinol ≥ 0.70 mol/L < 0.70 mol/L Serum vitamin E ≥ 9.7 mol/L < 9.7 mol/L Hemoglobin ≥ 110 g/L < 110 g/L Malaria No Yes5 No. of subjects with low rate of weight gain/total subjects at risk No MV2 MV2 VITAMINS AND PREGNANCY WEIGHT GAIN IN HIV INFECTION nurses, midwives, and administrative staff who made the study possible. We also acknowledge the valuable input from various colleagues including Ellen Hertzmark, Michele Dreyfuss, and Miguel Hernán. 22. REFERENCES 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. and weight loss in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Metabolism 1993;42:1270–6. Mulligan K, Tai VW, Schambelan M. Cross-sectional and longitudinal evaluation of body composition in men with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 1997;15:43–8. Macallan DC, Noble C, Baldwin C, Foskett M, McManus T, Griffin GE. Prospective analysis of patterns of weight change in stage IV human immunodeficiency virus infection. Am J Clin Nutr 1993;58:417–24. Paton NIJ, Macallan DC, Jebb SA, et al. Longitudinal changes in body composition measured with a variety of methods in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol 1997;14: 119–27. Kotler DP, Thea DM, Heo M, et al. Relative influences of sex, race, environment and HIV infection on body composition in adults. Am J Clin Nutr 1999;69:432–9. Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomized trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998;351:1477–82. Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother-to-child HIV-1 transmission in Durban, South Africa. South African Vitamin A Study Group. AIDS 1999;13:1517–24. Fawzi WW, Msamanga G, Hunter D, et al. Randomized trial of vitamin supplements in relation to vertical transmission of HIV-1 in Tanzania. J Acquir Immune Defic Syndr Hum Retrovirol 2000;23:246–54. Lohman TG, Roche AF, Martorell R, eds. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books, 1988. White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817–30. Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. New York: Oxford Science Publications, 1996. Villamor E, Gofin R, Adler B. Maternal anthropometry and pregnancy outcome among Jerusalem women. Ann Hum Biol 1998;25: 331–43. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. The WHO International Collaborative Group for the Study of the WHO Staging System. Proposed ‘World Health Organization Staging System for HIV Infection and Disease’: preliminary testing by an international collaborative cross-sectional study. AIDS 1993;7:711–8. Scholl TO, Hediger ML, Bendich A, Schall JI, Smith WK, Krueger PM. Use of multivitamin/mineral prenatal supplements: influence on the outcome of pregnancy. Am J Epidemiol 1997;146:134–41. Kafatos AG, Vlachonikolis IG, Codrington CA. Nutrition during pregnancy: the effects of an educational intervention program in Greece. Am J Clin Nutr 1989;50:970–9. Mardones-Santander F, Rosso P, Stekel A, et al. Effect of a milkbased food supplement on maternal nutritional status and fetal growth in underweight Chilean women. Am J Clin Nutr 1988;47:413–9. Scholl TO, Hediger ML, Schall JI, Khoo C, Fischer RL. Dietary and serum folate: their influence on the outcome of pregnancy. Am J Clin Nutr 1996;63:520–5. Stein AD, Ravelli ACJ, Lumey LH. Famine, third-trimester pregnancy weight gain, and intrauterine growth: the Dutch Famine Birth Cohort Study. Hum Biol 1995;67:135–50. Neufeld L, Pelletier DL, Haas JD. The timing of maternal weight gain during pregnancy and fetal growth. Am J Hum Biol 1999;11:627–37. Neufeld L, Pelletier DL, Haas JD. The timing hypothesis and body proportionality of the intra-uterine growth retarded infant. Am J Hum Biol 1999;11:638–46. Hickey CA, Cliver SP, McNeal SF, Hoffman HJ, Goldenberg RL. Prenatal weight gain patterns and spontaneous preterm birth among nonobese black and white women. Obstet Gynecol 1995;85:909–14. Abrams B, Selvin S. Maternal weight gain pattern and birth weight. Obstet Gynecol 1995;86:163–9. Downloaded from ajcn.nutrition.org by guest on June 11, 2014 1. Huddle JM, Gibson RS, Cullinan TR. Is zinc a limiting nutrient in the diets of rural pregnant Malwian women? Br J Nutr 1998;79: 257–65. 2. Jansen AAJ, Kusin JA, Thiuri B, Lakhani SA, t’Mannetje W. Machakos project studies no. XXIV. Anthropometric changes during pregnancy in rural African women. Trop Geogr Med 1984;36:91–7. 3. Kusin JA, Kardjati S, Renqvist U, Goei K. Reproduction and maternal nutrition in Madura, Indonesia. Trop Geogr Med 1992;44:248–55. 4. Achadi EL, Hansell MJ, Sloan NL, Anderson MA. Women’s nutritional status, iron consumption and weight gain during pregnancy in relation to neonatal weight and length in West Java, Indonesia. Int J Gynaecol Obstet 1995;48(suppl):S103–19. 5. Moller BO, Gebre-Medhin M, Lindmark G. Maternal weight, weight gain and birthweight at term in the rural Tanzanian village of Ilula. Br J Obstet Gynaecol 1989;96:158–66. 6. Siega-Riz AM, Adair LS. Biological determinants of pregnancy weight gain in a Filipino population. Am J Clin Nutr 1993;57: 365–72. 7. Maternal anthropometry and pregnancy outcomes: a WHO Collaborative Study. Bull World Health Organ 1995;73(suppl):1–98. 8. Institute of Medicine. Nutrition during pregnancy. Part I: Weight gain. Washington, DC: National Academy Press, 1990. 9. Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ 1987;65: 663–737. 10. Abrams B, Newman V. Small-for-gestational-age birth: maternal predictors and comparison with risk factors for spontaneous preterm delivery in the same cohort. Am J Obstet Gynecol 1991;164: 785–90. 11. Wen SW, Goldenberg RL, Cutter G, Hoffman HJ, Cliver SP. Intrauterine growth retardation and preterm delivery: prenatal risk factors in an indigent population. Am J Obstet Gynecol 1990;162:213–8. 12. Kramer MS, McLean FH, Eason EL, Usher RH. Maternal nutrition and spontaneous preterm birth. Am J Epidemiol 1992;136: 574–83. 13. Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol 1998;105:836–48. 14. UNAIDS. AIDS epidemic update: December 2000. Geneva: UNAIDS, 2000. 15. Grunfeld C, Pang M, Shimizu L, Shigenaga JK, Jensen P, Feingold KR. Resting energy expenditure, caloric intake, and short-term weight change in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Am J Clin Nutr 1992;55:455–60. 16. Kotler DP, Reka S, Orenstein JM, Fox CH. Chronic idiopathic esophageal ulceration in the acquired immunodeficiency syndrome. Characterization and treatment with corticosteroids. J Clin Gastroenterol 1992;15:284–90. 17. Kotler DP. Human immunodeficiency virus-related wasting: malabsorption syndromes. Semin Oncol 1998;25(suppl):70–5. 18. Mulligan K, Tai V, Schambelan M. Energy expenditure in human immunodeficiency virus infection. N Engl J Med 1997;336:70–1. 19. Suttmann U, Selberg O, Gallati H, Ockenga J, Deicher H, Muller MJ. Tumour necrosis factor receptor levels are linked to the acute-phase response and malnutrition in human-immunodeficiency-virusinfected patients. Clin Sci 1994;86:461–7. 20. Christeff N, Gharakhanian S, Thobie N, Rozenbaum W, Nunez EA. Evidence for changes in adrenal and testicular steroids during HIV infection. J Acquir Immune Defic Syndr 1992;5:841–6. 21. Grunfeld C, Pang M, Doerrler W, et al. Indices of thyroid function 1089 1090 VILLAMOR ET AL 52. Tang AM, Graham NMH, Saah AJ. Effects of micronutrient intake on survival in human immunodeficiency virus type 1 infection. Am J Epidemiol 1996;143:1244–56. 53. Kanter AS, Spencer DC, Steinberg MH, Soltysik R, Yarnold PR, Graham NM. Supplemental vitamin B and progression to AIDS and death in black South African patients infected with HIV. J Acquir Immune Defic Syndr Hum Retrovirol 1999;21:252–3. 54. Tang AM, Graham NMH, Kirby AJ, McCall AD, Willett WC, Saah AJ. Dietary micronutrient intake and risk progression to acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men. Am J Epidemiol 1993;138:1–15. 55. Bendich A, Cohen M. B vitamins: effects on specific and nonspecific immune responses. In: Chandra RK, ed. Nutrition and immunology. New York: Alan R Liss, 1988:101–23. 56. Beach RS, Mantero-Atienza E, Shor-Posner G, et al. Specific nutrient abnormalities in asymptomatic HIV-1 infection. AIDS 1992;6:701–8. 57. Funada U, Wada M, Kawata T, et al. Changes in CD4+CD8/ CD4CD8+ ratio and humoral immune functions in vitamin B12deficient rats. Int J Vitam Nutr Res 2000;70:167–71. 58. Allard JP, Aghdassi E, Chau J, et al. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV-infected subjects. AIDS 1998;12:1653–9. 59. Kotler DP. Nutritional alterations associated with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 2000;25(suppl):S81–7. Downloaded from ajcn.nutrition.org by guest on June 11, 2014 44. Strauss RS, Dietz WH. Low maternal weight gain in the second or third trimester increases the risk for intrauterine growth retardation. J Nutr 1999;129:988–93. 45. Luke B, Min SJ, Gillespie B, et al. The importance of early weight gain in the intrauterine growth and birth weight of twins. Am J Obstet Gynecol 1998;179:1151–61. 46. Abrams B, Altman SL, Pickett KE. Pregnancy weight gain: still controversial. Am J Clin Nutr 2000;71(suppl):1233S–42S. 47. Abrams B, Carmichael S, Selvin S. Factors associated with the pattern of maternal weight gain during pregnancy. Obstet Gynecol 1995; 86:170–6. 48. Ladner J, Castetbon K, Leroy V, et al. Pregnancy, body weight and human immunodeficiency virus infection in African women: a prospective cohort study in Kigali (Rwanda), 1992–1994. Int J Epidemiol 1998;27:1072–7. 49. Mauri A, Piccione E, Deiana P, Volpe A. Obstetric and perinatal outcome in human immunodeficiency virus-infected pregnant women with and without opiate addiction. Eur J Obstet Gynecol Reprod Biol 1995;58:135–40. 50. Weng S, Bulterys M, Chao A, et al. Perinatal human immunodeficiency virus-1 transmission and intrauterine growth: a cohort study in Butare, Rwanda. Pediatrics [serial online] 1998;102:e24. Internet: http://www.pediatrics.org/cgi/content/full/102/2/e24 (accessed 19 August 2002). 51. Macallan DC. Wasting in HIV infection and AIDS. J Nutr 1999; 129(suppl):S238–42.