Downloaded from by guest on September 5, 2014 ajcn.nutrition.org
Transcription
Downloaded from by guest on September 5, 2014 ajcn.nutrition.org
Vitamin B12 bloavailability from egg yolk and egg
white: relationship to binding proteins13
AS Levine,
PhD
and
A Doscherholmen,
MD
ABSTRACT
Egg yolk has been reported
to inhibit
B,2 absorption
less than egg white
that different
vitamin
B,2 binding
proteins
may be present in egg white and egg yolk.
Using gel-exclusion chromatography
we found that the mean MR for the B,2 binding
protein
derived
from egg yolk was 125,000, whereas
that derived
from egg white was 97,750. Heat
treatment
of the apoprotein
differentially
reduced
the binding
capacity
of egg yolk and egg white
in a time-dependent
manner
with the greatest
decrease
in binding
capacity
occurring
with egg
white. In contrast,
heat treatment
of the holoenzyme
delineated
the egg yolk as the more labile.
These studies suggest that egg yolk and egg white contain
distinct
R binders which could explain
the differential
B,2 absorption
from egg yolk and egg white.
Am
J C/in Nuir
1983:38:436439.
suggesting
WORDS
Cobalophilin,
vitamin
Introduction
Presently
there
is little
knowledge
about
the bioavailability
of vitamin
B12 from various food sources.
The absorption
by normal
subjects
of radio-B32
in vivo labeled
mutton
( 1) and chicken
meat
(2) is at least as good
as that from crystalline
radio-B12,
whereas
in vivo (3) or in vitro radio-B12
(4) labeled
eggs are poorly absorbed.
The reason
for the
great difference
in the absorption
of vitamin
B32 from
meat and eggs is unknown.
One
possible
explanation
would
be the presence
of a potent
B12-binding
substance
in eggs.
B12-binding
proteins
such as intrinsic
factor
and serum
transcobalamin
II are involved
in the absorption
and transport
of vitamin
B,2 (5). A gastric nonintnnsic
factor vitamin
B12-binding
protein
with rapid
electrophorelic mobility
was also described
and termed
R protein
(for rapid mobility)
(6). Proteins
with similar immunological
properties
to the
latter have also been identified
in saliva (7),
erythrocytes
(7), granulocytes
(8), cerebrospinal fluid (7) and tears (9), amniotic
fluid
(10), milk (12), and cord blood and renamed
by Stenman
to cobalophilins
(13). This is a
more appropriate
name as R proteins
from
various
sources
have varying
electrophoretic
mobilities
(cf with R for rapid mobility).
436
The American
Journal
of Clinical
Nutrition
B,2, eggs, binding proteins, bioavailability
We have previously
similation
of vitamin
whole
reported
that the asB12 from scrambled
eggs is inferior
to that
of boiled
and
fried eggs and egg yolk (3). This may be
explained
by the fact that egg yolk has less
inhibitory
effect on B12 absorption
than egg
white, whether
expressed
as absolute
values
or as a percentage
of absorption
of a com-
parable
amount
of crystalline
57Co-vitamin
B12 (4). This suggested
that different
vitamin
B12 binding
proteins
may be present
in egg
white and egg yolk. In the present
study, we
have attempted
to identify R binders in eggs
and to differentiate
between
the binders
present
in egg white and egg yolk by means
of molecular
weight determination
and heat
lability
of the proteins.
‘ From
the Neuroendocrine
and Department
of Medicine
ministration
Medical Center,
ments
of Food Science
and
University
of Minnesota,
St.
2Supported
by the Veterans
Center.
Address
reprint
requests
Neuroendocrine
apolis
VA
55417.
Received
Accepted
38:
Research
Medical
Center,
Research
Laboratory
(SDTU),
Veterans
AdMinneapolis,
and DepartNutrition
and Medicine,
Paul-Minneapolis,
MN.
Administration
Medical
to: Allen
S Levine,
PhD,
(11 1P), MinneMinneapolis,
Minnesota
Laboratory
March
1, 1983.
for publication
May
3, 1983.
SEPTEMBER
1983, pp 436-439.
Printed
in USA
© 1983 American
Society for Clinical
Nutrition
Downloaded from ajcn.nutrition.org by guest on September 5, 2014
KEY
B,2 BINDING
BY
EGGS
Materials
and methods
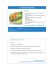
5000
Gel-exclusion
chronatograp/zy
4000
Q
0
-
EGG WHITE
?I
I
:i,
.1
BlueDex
I
fran
‘kI
:
x,
0
5
17
19
21
23252729
FRACTION
FIG I. Sephadex
G-200
B,2 saturated
chicken
white.
mm
31
434547
NUMBER
fractionation
of 57Co-vitaserum,
egg yolk, and egg
before
binding
studies
with
labeled
57Co-
vitamin
B32 differentially
reduced
the binding capacity
of egg yolk,
egg white,
and
chicken
serum
in a time-dependent
manner
(Fig 2). The greatest
decrease
in binding
capacity
occurred
with egg white
and the
least with egg yolk. It should
be noted
that
the initial
rates of inactivation
of the egg
yolk and egg white are similar
and therefore
it is possible
that each crude extract
contains
more
than
one binder.
In contrast,
heat
treatment
of the holoenzyme
(ie, after
B32
labeling
ofthe binders)
clearly delineated
the
egg yolk as the most labile and the egg white
and chick serum
being equally
effected
(Fig
3). In this case each crude extract could also
contain
more than one binder.
Heat treatment
of the holoprotein
affected
binding
much
less than the apoprotein
(Figs 2 and
3).
Discussion
The present
study indicates
that vitamin
B12-binding
proteins
are present
in eggs and
that the binding
proteins
in egg white and
egg yolk are distinct
proteins.
The molecular
weight of the binding
protein
from egg yolk
was determined
to be 125,000
compared
with a molecular
weight
of 97,750
for the
binding
protein
from egg white as estimated
by gel filtration.
Furthermore,
neuroaminidase had no significant
effect on the MR of
the egg white binding
protein,
whereas
the
Downloaded from ajcn.nutrition.org by guest on September 5, 2014
suggests that the proteins
from egg white and
egg yolk are similar
but the egg yolk binder
is modified
by the addition
of carbohydrate.
Heat treatment
of the apoprotein
(80#{176}C)
-
.
2000
000
and heat treatment
The MR ofthe
B,2 binding
protein
derived
from egg yolk was 125,000
± 0 (n = 6, three
calibrations),
that from egg white was 97,750
± 35 1 3 (n = 8, four calibrations)
and that
from chicken
serum
was 125,000
± 500 (n
=
8, four calibrations).
Representative
plots
of the gel filtration
results
are presented
in
Figure
1 The addition
of neuroaminidase
altered
the MR of egg yolk, egg white,
and
chicken
serum
to 107,333
± 4627,
102,000
± 7071,
and 123,000
± 0, respectively.
This
A
A
Holoprotein
assay.
Ten milliliters
of diluted
and
extracted
egg yolk, egg white, or chicken
serum
(1:4
dilution)
was added to 10 ml “Co-vitamin
B,2 (50,000
pg/mI). Thirty minutes
later an equal volume
of bovine
serum
albumin-coated
charcoal
was added
to absorb
free “Co-vitamin
B,2, the charcoal
spun down, and 4
ml of supernatant
was added
to I ml of “cold”
B,2
(1000
zg/ml) plus 45 ml phosphate
buffer (pH 7.4).
This mixture
was then placed in a shaking
water bath
at 80#{176}C
for the appropriate
time period (0, 30, 60, 120,
180, 240, 300, and 360 mm).
Apoprotein
assay.
Binder alone (egg yolk, egg white,
or chicken
serum)
was placed in a shaking
water bath
at 80#{176}C
for the approximate
time period.
After this a
standard
binding
study was run as described
previously
(14).
Results
SERUM!/\#{176}’
I
treatment
studies
CHICKEN
YOLK
3000
Extracts
of vitamin
B,2 binders
were prepared
from
egg white and egg yolk. The egg white was separated
from egg yolk by simple
mechanical
means.
One egg
white was diluted to 100 ml with normal
saline dilution
and one egg yolk was diluted
to 100 ml with normal
saline and extracted
with anaesthetic
ether. This extract
was further
diluted
1:10 with saline. Ten milliliters
of
diluted
egg white (pH 5) or diluted
and extracted
egg
yolk (pH 5) was incubated
with 5.85 mg neuroaminidase (Cl perfringens,
Sigma Chemical
Co. St Louis,
MO) for 4 h at 37#{176}C
and the reaction
was stopped
by
placing mixtures
on ice.
Binding
EGG
I
Sephadex
G-200 (Pharmacia,
Uppsala,
Sweden)
was
packed
in columns
2.5 x 45 cm. The flow rate was 15
ml/h and fractions
(4 ml) were collected
at 37#{176}C.
The
buffer was 0.04 M phosphate
(pH 7.4) containing
150
mmol/l
NaC1. In each run the void volume
(Vol) was
determined
with Blue Dextran
2000 (Pharmacia).
To
estimate
“molecular
weight,”
crystalline
bovine
albumm and ribonuclease
A, Aldolase,
ovalbumin,
and
chymotrypsinogen
A were used as markers.
It was assumed that Ve/Vo is a linear function
of the logarithm
ofthe
molecular
weight.
Neuroaminidase
437
438
LEVINE
AND
DOSCHERHOLMEN
z
2
4
Li
60
z
40
a:
xEGGYOLK
0
2
CHICKEN
20
SERUM
EGG WHITE
0
I
.3-
.L_
2
3
2. Effect
2
4
Li
a:
z
‘EE
6
(hr)
of the apoprotein
on 57Co-vitamin
B,2 binding
x
x
x
x
EGGY0LK
2
3
4
5
6
capacity.
40
2
20
0
I
0
1
TIME
FIG
3. Effect
of 80#{176}C
treatment
(hr)
of the holoprotein
MR ofthe
binding
protein
from egg yolk was
reduced
by approximately
1 5%. In addition,
heat treatment
differentially
affected
the B32
binding
proteins
from egg yolk and white.
Heat treatment
of the holoprotein
(which
is
analogous
to cooking
an egg) had a more
marked
effect on the B32 binding
capacity
of
the egg yolk when
compared
to the egg
white.
Individuals
consuming
a cooked
egg
white 57Co-vitamin
B32 mixture,
excrete
less
urinary
radioactivity
(indicating
less B32 absorption)
then those consuming
an egg yolk
57Co-vitamin
B12 mixture,
which
also mdicates that the egg white B12 binding
capacity
is greater
than that of egg yolk (4).
The reason
for the inhibition
of absorption of vitamin
B32 by egg white or egg yolk
thus appears
to be due to the presence
of
cobalaphilins.
The molecular
weights
of the
binding
proteins
present
in eggs are similar
to the molecular
weights
reported
for other
cobalaphilins
when determined
by gel filtration (5). Also, we have recently
found (Levine AS, Doscherholmen
A, unpublished
re-
on 57Co-vitamin
B,2 binding
capacity.
suits) that ingestion
ofpresaturated
egg white
(2.5 mg) or egg yolk ( 14 mg) with unlabeled
vitamin
B32 results
in normal
absorption
of
the vitamin,
further
implicating
binding
as
the cause
of poor absorption
of B12 from
eggs. Eggs are also known
to impair
the
absorption
of iron (1 5) and a substance
in
egg white, avidin,
inhibits
absorption
of biotin (16).
The binding
capacity
of the heat treated
egg yolk and egg white is markedly
diminished
after heat treatment.
Therefore,
the
danger
of cooked
eggs inhibiting
binding
of
other food sources
of B12 is not as great as
the B32 present
in the egg itself.
This
is
consistent
with our previous
finding
that
cooked
egg white had no effect on the absorption
of crystalline
57Co-vitamin
B,2 (4).
The form of vitamin
B,2 naturally
occurring in eggs is unknown.
The naturally
occurring
coenzymes
of B,2, methylcobalamin,
and 5 ‘deoxyadenosylcobalamin,
are not as
well absorbed
as hydroxycobalamin
and
cyanocobalamin
(17, 18). It is possible
that
Downloaded from ajcn.nutrition.org by guest on September 5, 2014
z
of 80#{176}C
treatment
5
4
TIME
FIG
J
liii DINLIIINU
DI tIIO
the vitamin
B32 in eggs is present
in the
coenzyme
form(s)
and that little or no conversion
to hydroxycobalamin
occurs during
the food preparation,
thus reducing
the absorption
rate.
However,
it seems
more
likely
that avid binding
of the B,2 occurs
in the
egg. Gullberg
(19) has postulated
that the
cobalophilins
might
have a selective
antimicrobial
function,
particularly
in the colon
of humans.
It seems
reasonable
to suggest
that the cobalophilins
present
in eggs might
also provide
antimicrobial
protection
to the
environment
of the ovum.
El
1 . Heyssel
RM, Bozian
RC, Darby
Wi, Bell MC.
Vitamin
B,2 turnover
in man. The assimilation
of
vitamin
B,2 from natural
food stuff by man and
estimatesofminimal
daily requirements.
Am J Clin
Nutr 1966;18: 176-84.
2. Doscherholmen
A, McMahon
i, Ripley D. Vitamin
B,2 absorption
from chicken
meat. Am I Clin Nutr
1978:31:825-30.
3. Doscherholmen
A, McMahon
I, Ripley D. Vitamin
B,2 absorption
from eggs (38940).
Proc Soc Exp
Biol Med 1975:149:987-90.
4. Doscherholmen
A, McMahan
i, Ripley D. Inhibitory effect of eggs on vitamin
B,2 absorption:
description
of a simple ovalbumin
57Co-vitamin
B,2
absorption
test. Br J Haematol
l976;33:261-72.
5. Carmel
R. Cobalamin-binding
proteins
of man. In:
Silber R, ed. Contempory
hematology/oncology.
Vol 2. New York, NY: Plenum
Press, 1981.
6. Grasbeck
R, Simons
K, Sinkkonen
I. Purification
of intrinsic
factor and vitamin
B,2 binders
from
human
gastric
juice.
Ann
Med
Exp
Fenn
l962;(suppl
6):40.
7. Simons
K. Vitamin
B,2 binders
in human
body
fluids and blood cells. Soc Sci Fennica
Comment
Biol l964;27:fasc 5.
Downloaded from ajcn.nutrition.org by guest on September 5, 2014
References
Beard
MF, Pitney
WR, Sanneman
El-I. Serum
concentrations
of vitamin
B,2 in patients
suffering
from leukemia.
Blood 1954;9:789-94.
9. Carmel
R. The vitamin
B,2-binding
proteins
of
saliva and tears and their relationship
to other
vitamin
B,2 binders.
Biochim
Biophys
Acta
1972:263:747-52.
10. Stenman
U-H. Amniotic
fluid vitamin
B,2-binding
protein.
Purification
and characterization
with isoelectric
focusing
and other techniques.
Biochim
Biophys
Acta 1974:342:173-84.
I 1 . Hurlimann
I, Zuber
C. Vitamin
B,2-binders
in
human
body fluids. II. Synthesis
in vitro. Clin Exp
Immunol
1969;4: 141-8.
12. Kumento
A. Studies on the serum binding
of vitamm B,2 in the newborn
human
infant.
Acta PaediatrScand
Suppl l969;l94:l-55.
13. Stenman
U-H. Characterization
of R-type vitamin
B,2-binding
proteins
by isoelectric
focusing.
II.
Comparison
ofcobalophilin
(R proteins)
from different
sources.
Scand
I
Clin
Lab
Invest
1975:35:147-55.
14. Gottlieb
C, Lau KS, Wasserman
LR, Herbert
V.
Rapid charcoal
assay for intrinsic
factor(IF),
gastric
juice unsaturated
B,2 binding
capacity,
antibody
to
IF, and serum
unsaturated
B,2 binding
capacity.
Blood 1965:25:875-84.
15. Chodos
RB, Ross iF, Apt L, Pollycove
M, Halkett
JAE. The absorption
ofradioiron
labeled foods and
iron salts in normal
and iron deficient
subjects
and
in idiopathic
hemochromatosis.
I Clin
Invest
1957:36:314-26.
16. Langer
W, Gyorgy
P. Biotin,
active compounds
and antagonists.
In: Sebrell WH, Harris
RD. eds.
The vitamins.
New York,
NY: Academic
Press,
1968.
17. Adams iF, Kennedy
EH, Thompson
i, Williamson
J. The effect of acid peptic digestion
on free and
tissue-bound
cobalamins.
Br J Nutr 1968:22:11114.
18. Herbert
V, Sullivan
LW. Activity
of coenzyme
B,2
in man. Ann NY Acad Sci 1964;I 12:855-70.
19. Gullberg
R. Possible
antimicrobial
function
of the
large molecular
size vitamin
B,2-binding
protein.
Scand i Gastroenterol
1974:9:19-21.
8.