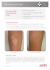
National Medical Policy Subject: Vitiligo
Transcription
National Medical Policy Subject: Vitiligo
National Medical Policy Subject: Vitiligo (click on hyperlink Phototherapy and Photochemotherapy (PUVA) for Dermatological Conditions for related policy) Policy Number: NMP32 Effective Date*: October 2003 Updated: July 2014 This National Medical Policy is subject to the terms in the IMPORTANT NOTICE at the end of this document For Medicaid Plans: Please refer to the appropriate Medicaid Manuals for coverage guidelines prior to applying Health Net Medical Policies The Centers for Medicare & Medicaid Services (CMS) For Medicare Advantage members please refer to the following for coverage guidelines first: Use Source National Coverage Determination (NCD) National Coverage Manual Citation Local Coverage Determination (LCD)* Article (Local)* Other Reference/Website Link X None Use Health Net Policy Instructions Medicare NCDs and National Coverage Manuals apply to ALL Medicare members in ALL regions. Medicare LCDs and Articles apply to members in specific regions. To access your specific region, select the link provided under “Reference/Website” and follow the search instructions. Enter the topic and your specific state to find the coverage determinations for your region. *Note: Health Net must follow local coverage determinations (LCDs) of Medicare Administration Contractors (MACs) located outside their service area when those MACs have exclusive coverage of an item or service. (CMS Manual Chapter 4 Section 90.2) Vitiligo Jul 14 1 If more than one source is checked, you need to access all sources as, on occasion, an LCD or article contains additional coverage information than contained in the NCD or National Coverage Manual. If there is no NCD, National Coverage Manual or region specific LCD/Article, follow the Health Net Hierarchy of Medical Resources for guidance. Current Policy Health Net, Inc. considers any of the following medically necessary for the treatment of vitiligo: 1. 2. 3. 4. 5. Topical and systemic corticosteroids Topical calcineurin inhibitors [e.g., tacrolimus ointment (Protopic), pimecrolimus (Elidel)] when HNPS criteria is met. Criteria is available at: Pharmacy Prior Authorization Criteria Ultraviolet B (UVB) radiation (phototherapy) Topical or oral psoralen ultraviolet A (photochemotherapy or PUVA) Excimer laser (e.g. 308 Excimer System, XTRAC Excimer Laser) Health Net, Inc. considers any of the following investigational modalities of treatment because there is inadequate scientific evidence of effectiveness from randomized controlled clinical trials in the peer-reviewed medical literature: 1. 2. 3. 4. Autologous skin grafting Blister grafting Autologous melanocyte transplants Topical calcipotriol (topical vitamin D analogs) Health Net, Inc. considers any of the following cosmetic in nature: 1. 2. 3. 4. 5. Micropigmentation (tattooing) Depigmentation with monobenzylether of hydroquinone Sunscreens Camouflage products Cosmetics Codes Related To This Policy NOTE: The codes listed in this policy are for reference purposes only. Listing of a code in this policy does not imply that the service described by this code is a covered or noncovered health service. Coverage is determined by the benefit documents and medical necessity criteria. This list of codes may not be all inclusive. On October 1, 2015, the ICD-9 code sets used to report medical diagnoses and inpatient procedures will be replaced by ICD-10 code sets. Health Net National Medical Policies will now include the preliminary ICD-10 codes in preparation for this transition. Please note that these may not be the final versions of the codes and that will not be accepted for billing or payment purposes until the October 1, 2015 implementation date. ICD-9 Codes 709.01 Vitiligo Vitiligo Jul 14 2 ICD-10 H02.73-H02.739 L80 Vitiligo of eyelid and periocular area Vitiligo CPT Codes 96900 Actinotherapy (Ultraviolet light) 96910 Photochemotherapy; tar and ultraviolet B (Goeckerman treatment) or petroleum and ultraviolet B 96912 Photochemotherapy; psoralens and ultraviolet A 96913 Photochemotherapy, (Goekerman and/or PUVA) for severe photoresponsive dermatoses requiring at least four to eight hours of care under direct supervision of the physician (includes application of medication and dressing) HCPCS Codes N/A Scientific Rationale – Update January 2014 Bansal et al (2013) evaluated the efficacy of psoralen-NBUVB (P-NBUVB) vs. NBUVB in vitiligo in a randomized study. 45 Indian patients (age above 13 years) with vitiligo involving more than 5% body surface area were randomly allocated to receive either NBUVB or P-NBUVB treatment. Both groups received NBUVB exposure thrice weekly, with a total of 60 sessions. The extent of repigmentation achieved was calculated on the basis of Vitiligo Area Severity Index (VASI) scoring. Forty patients were available for analysis at the end of the study. The extent of repigmentation in the P-NBUVB group was statistically significantly greater in face and neck (P=0.006, t-test) and hands (P=0.007, t-test) in comparison with the NBUVB group (t-test). Percentage reduction in VASI scores was statistically significantly greater in the PNBUVB group (29.2% vs. 21.7%, P=0.043, t-test). The response to P-NBUVB therapy started earlier than the response to NBUVB. After excluding sunlight as a confounding factor, treatment response was also significantly better in the P-NBUVB group (P=0.005). Investigators concluded addition of psoralen increased the extent of repigmentation due to NBUVB therapy in vitiligo. Further studies are required to determine the long-term efficacy and safety of P-NBUVB. Matsuzaki and Kumagai (2013) examined 27 patients who underwent vitiligo treatment using autologous cultured keratinocytes. The study comprised 20 patients with segmental vitiligo and seven patients with generalized vitiligo, and they were followed up for at least 1 year postoperatively. In all 27 cases, topical steroid or ultraviolet therapy had been previously performed by dermatologists, but this treatment had been ineffective. The patients' vitiligo had stabilized. The patients were treated using keratinocytes obtained from primary culture using Green's techniques or from first passage. Dispase treatment was used to detach the stratified cultured epithelial sheets from their culture dishes. The detached sheets shrank to approximately one half to two thirds of their original size on the culture dish. After the recipient site was completely epithelialized, the skin was exposed to sunlight. For patients with segmental vitiligo, 12 had a good therapeutic outcome (90 % or more repigmentation) after the first surgery. This number increased to 14 when patients with multiple surgeries were included. There were six patients with fair outcomes (50-90 % repigmentation), and no patients with poor outcomes (50 % or less repigmentation). For patients with generalized vitiligo, no patients had a good outcome despite multiple surgeries. There were three patients with fair outcomes, Vitiligo Jul 14 3 and four patients with no change outcomes. Investigators concluded cultured keratinocyte grafting was a more effective treatment for segmental vitiligo than for generalized vitiligo. Scientific Rationale – Update January 2013 Ghosh et al (2012) evaluated the clinical effectiveness of a cultured graft consisting of autologous cultured melanocytes on a poly (dl-lactic acid) (PLA) film in subjects with stable vitiligo in a prospective open-label, randomized, multicenter clinical trial conducted with 22 patients. Each subject was treated with cultured graft and polyurethane dressing (control arm) after epidermal ablation and followed for up to 9 months. The extent of repigmentation in the treated sites was compared with that control sites at days 90, 180, and 270. In the treatment arm, a minimum of 70% repigmentation was observed in five subjects at day 90; nine at day 180, and 10 at day 270. In the control arm, only one subject showed repigmentation until day 270. None of the test sites reported any recurrence of vitiliginous patches by the end of the study. Investigators concluded cultured melanocytes delivered on PLA film were efficacious and safe when applied on patients with stable vitiligo. Sapam et al (2012) compared the efficacy and adverse effects of narrowband UVB (NBUVB) with oral psoralen UVA (PUVA) therapy in the treatment of vitiligo in a parallel-group, assessor blinded, randomized, controlled trial. Patients aged 13-70 years with vitiliginous lesions involving more than 5% body surface area were eligible for the study. In total, 56 patients were randomized in a 1:1 ratio to oral PUVA or NBUVB phototherapy groups. Patients were assessed for the percentage of repigmentation over the depigmented areas as the primary outcome measure at each visit during the first three months and then monthly within the next three months. The incidence of adverse effects was also noted during the study period as the secondary outcome measure. The median repigmentation achieved at the end of the six-month therapy course was 45% in the NBUVB group and 40% in the oral PUVA group. Focal vitiligo had the best response in both treatment groups. There were lesser adverse effects within the NBUVB (7.4%) than in the PUVA (57.2%) group. Two PUVA patients discontinued therapy due to severe dizziness. There was no significant difference in the mean degree of repigmentation; however, NBUVB carried a greater response rate and might be superior to oral PUVA with better tolerance and color match with the surrounding normal skin, as well as fewer side effects in the treatment of vitiligo. Budania et al (2012) compared autologous noncultured epidermal cell suspension (NCES; a cellular grafting technique) and suction blister epidermal grafting (SBEG; a tissue grafting technique) for producing repigmentation in patients with stable vitiligo. 41 patients with 54 stable vitiligo lesions were randomized into two groups. Patients in group 1 were treated with NCES, and those in group 2 with SBEG. Each were evaluated 16 weeks postsurgery for the extent of repigmentation, color match, change in Dermatology Life Quality Index (DLQI) score and patient satisfaction. The extent of repigmentation was excellent (showing 90-100% repigmentation) in 71% of lesions in the NCES group and 27% of lesions in the SBEG group (P=0·002). Repigmentation ≥ 75% (good repigmentation) was observed in 89% of lesions in the NCES group and 85% of lesions in the SBEG group (P=0·61). There was a significant decline in DLQI score in both the groups; the mean decline among groups differed significantly (P=0·045). No significant difference was seen in color match and pattern of repigmentation. Adverse effects were minimal. Investigators concluded NCES is significantly better than SBEG and should be the preferred treatment for patients with stable vitiligo. Vitiligo Jul 14 4 Hallaji et al (2012) evaluated the influence of disease duration on its clinical response to NB-UVB phototherapy in a open and uncontrolled study. Vitiligo was considered 'recent' when the duration of disease was less than or equal to 4 years and 'long standing' when it was greater than 4 years. The patients received NB-UVB thrice weekly with an initial dose of 200 mJ/cm(2) and 10% increments at each subsequent treatment. After categorizing the clinical response to four groups (mild, moderate, good, and excellent), duration of disease and clinical response to NB-UVB were correlated statistically using the t-test. There were 63 patients: 34 women and 29 men, aged 6-60 years. The mean of disease duration was 10.13 ± 9.1 years. Vitiligo was 'recent' in 26 and 'long standing' in 37 patients. The mean of overall response was 51.94 ± 18.48%. Higher grades of response were more prevalent in patients with recent vitiligo than those with long-standing disease, and there was also statistically significant difference in overall response between these two groups of disease duration (P = 0.023). Investigators concluded the early treatment of generalized vitiligo may enhance the chance of successful repigmentation. El-Zawahry et al (2012) investigated forty patients with vitiligo comparing ultraviolet A1 (UVA1) phototherapy to narrow-band ultraviolet B (NB-UVB). Twenty patients received NB-UVB and 20 received UVA1 three times weekly for 12 weeks. The UVA1 group was divided into two subgroups. Ten patients received moderate and 10 received low dose of UVA1. Serum samples were collected before and after 36 sessions to assess soluble interleukin 2 receptor level. Patients were clinically evaluated before therapy then monthly according to Vitiligo Area Scoring Index (VASI) and Vitiligo European Task Force (VETF) scores. In addition, extent of response was determined by a blinded dermatologist comparing before and after therapy photographs. Pattern of response and side effects were recorded. NB-UVB was superior to UVA1 with a significant difference in blinded dermatological assessment (P<0.001), percentage change in VASI score (P<0.001) and percentage change in VETF area score (P=0.001). No significant difference in side effects was observed between both groups. Comparing UVA1 subgroups, better response in moderate-dose group was found as regard to percentage change in VASI (P<0.001) and percentage change in VETF area score (P=0.001), while no significant difference was found in blinded dermatological assessment (P=0.121). Investigatros concluded NB-UVB phototherapy remains to be an effective and safe therapeutic option in vitiligo. Response to UVA1 in vitiligo seems to be dose dependent and seems to be of limited value in treatment of vitiligo as a monotherapy. Further studies combining it with other lines of therapy such as systemic steroids may prove beneficial. Huggins et al (2012) sought to determine the efficacy and safety of the melanocytekeratinocyte transplantation procedure (MKTP) in an academic dermatology department in the United States. The prospective, uncontrolled, open-label study enrolled patients aged 18 years or older with a self-reported history of vitiligo and no new or expanding lesions for at least 6 months before surgery. Patients with a history of koebnerization or keloid formation were excluded. Patients underwent autologous MKTP. Repigmentation during a 3- to 6-month follow-up period was assessed categorically and by modified Vitiligo Area Scoring Index. Safety was assessed by frequency of adverse events. Of the 28 patients who underwent 36 procedures, 23 patients who underwent 29 procedures completed the 3- to 6-month follow-up period. Data for these 29 procedures show excellent repigmentation (ie, 95%-100%) after the MKTP in 17%, and good repigmentation (ie, 65%-94%) in 31%. Fair (64%-25%) and poor (24%-0%) repigmentation were achieved in 10% and 41% of patients, respectively. Average percent change in Vitiligo Area Scoring Vitiligo Jul 14 5 Index was -45% (95% confidence interval -64% to -26%), signifying an improvement in pigmentation. Investigators concluded MKTP is an effective and well-tolerated procedure based upon categorical and Vitiligo Area Scoring Index assessments of repigmentation. The study was limited by its small sample size and lack of a control group. Scientific Rationale – Update December 2009 Vitiligo is an acquired skin depigmentation that produces white patches and can affect any part of the body. Generalized vitiligo is the most common and usually involves the face, lips, hands, arms, legs, and genital areas. Both sides of the body are usually affected. Vitiligo can be psychologically devastating, especially when present on visible areas of the body, such as the face and hands. The etiology of vitiligo is unclear although it is believed to be an autoimmune disorder. The goal of treatment is to restore the skin's color by restoring healthy melanocytes to the skin (repigmentation) allowing the skin to regain its normal appearance. Individuals with vitiligo should always protect their depigmented skin against excessive sun exposure by wearing protective clothing, applying a UVA/UVB sunscreen daily, and avoid prolonged sun exposure. Treatments have highly variable results, therefore, treatment modalities should be selected for maximum benefit and minimum risk, with consideration for the body surface area involved. Topical corticosteroids are often used as first-line therapy. Topical tacrolimus has shown efficacy in some trials and has the advantage over topical corticosteroids as it does not cause skin atrophy. Tacrolimus often works best when combined with NB-UVB light. Topical and oral psoralen ultraviolet A (PUVA) and photochemotherapy or phototherapy with Ultraviolet A or B therapy have good success rate. PUVA is effective for the face, trunk, upper arms, and upper legs. NBUVB requires two to three treatment sessions per week for several months. Another source of NB-UVB is excimer lasers. Phototherapy with the 308-nanometer wavelength xenon chloride excimer laser produces high ultraviolet B energy that is delivered precisely to vitiligo patches (depigmented areas), with the goal of achieving more rapid repigmentation than is achieved with standard phototherapy. While there are different regimens, excimer laser therapy is generally given twice weekly, with or without topical drugs, up to 6 months. Each treatment takes only a few minutes. There are several manufacturers of excimer lasers, including some specifically approved for the treatment of vitiligo by the Food and Drug Administration. Excimer laser therapy is intended for any individual with vitiligo, especially those with moderate-to-severe disease, although it appears to be most efficacious on the face and neck than on other areas of the body. Many studies of treatments for vitiligo are of poor quality and so evidence is limited, particularly for the long-term benefits and safety of therapies. A Cochrane database systematic review (2006) identified nineteen randomized trials with a total of 1350 participants assessing the effects of interventions used to manage vitiligo. Most of the studies identified had low numbers of participants. In one study, potent topical steroids resulted in better repigmentation than placebo and they were also better than oral psoralens plus sunlight in another study although their long-term use is limited by adverse effects. Two studies suggested that topical calcipotriol enhanced repigmentation rates from PUVA and PUVA when compared with placebo. Another two studies showed higher repigmentation rates with oral PUVA versus placebo plus sunlight. They noted that the safety of the interventions were poorly described and none of the studies were able to demonstrate long term benefits. Vitiligo Jul 14 6 Radakovic et al (2009) investigated seventeen patients with generalized vitiligo. In each patient, two lesions were selected and randomized to treatment with either once- or twice-daily application of 0.1% tacrolimus for a total period of 6 months. In 10 patients, a third patch was left untreated to serve as a control. Fifteen patients with 40 target lesions completed the study. Twice-daily treatment induced excellent (> 75%) repigmentation in two lesions, moderate (> 25-50%) and poor (1-25%) repigmentation in four lesions each, and no response in five lesions. Once-daily treatment resulted in moderate repigmentation in two lesions and poor repigmentation in five lesions, whereas no effect was observed in the remaining eight lesions. One out of 10 control lesions developed moderate spontaneous repigmentation, the other nine remained unchanged. Besides the frequency of tacrolimus application, the treatment outcome was determined by the localization of the affected areas with the facial region showing the best response. The authors concluded that Tacrolimus ointment appears to be an effective treatment option for facial vitiligo. Treatment must be applied twice daily for optimum response. Hui-Lan Y et al (2009) investigated forty-nine patients in a single-blinded, randomized, comparing 308-nm excimer laser therapy together with topical 1% pimecrolimus cream twice daily (group A) with excimer laser therapy twice per week (group B). Of 48 patients evaluated after 30 weeks of treatment, 71% of patients from group A achieved Grade 3 or 4 repigmentation compared with 50% in group B. Significant difference was found between group A and B at the end of 30 weeks of treatment Sassi et al (2008) designed a parallel-group randomized controlled trial to compare the effectiveness of 308-nm excimer laser alone or in combination with topical hydrocortisone 17-butyrate cream in patients with vitiligo unresponsive to previous treatment with topical steroids or narrow-band ultraviolet (UV) B phototherapy. 84 patients with nonsegmental vitiligo localized on the face and/or neck were randomized to 308-nm excimer laser phototherapy twice weekly alone or in combination with topical hydrocortisone 17-butyrate cream twice daily for three periods of 3 weeks followed by a 1-week steroid-free interval. The primary outcome was a reduction of at least 75% of the overall lesional areas as judged by automatic image analysis on reflected UV photographs, conducted blind to treatment assignment, at 12 weeks compared with baseline. Secondary outcomes were clearance, and improvements on Physician's Global Assessment (PGA) and Skindex29 scores. 76 (90%) patients completed the study. In an intention-to-treat analysis, seven patients in the excimer monotherapy arm and 18 in the combination arm showed > 75% reduction of vitiligo lesions at 12 weeks. Clearance was observed in two and nine patients, respectively. A significant difference also emerged for PGA scores, while no difference was documented for Skindex-29. The author concluded that recalcitrant vitiligo of the face and neck may benefit from the combination of excimer laser phototherapy with topical hydrocortisone 17-butyrate cream. Cassaci et al (2007) compare the effectiveness of NB-UVB phototherapy and 308-nm monochromatic excimer light (MEL) in vitiligo patients. The study was done in a randomized, investigator-blinded and half-side comparison design. Twenty-one subjects with symmetrical vitiligo lesions were enrolled in this study. Vitiligo lesions on one body side were treated twice weekly for 6 months with 308-nm MEL, while NB-UVB phototherapy was used to treat lesions on the opposite side. At the end of the study six lesions (37.5%) treated with 308-nm MEL and only one lesion (6%) treated with NB-UVB achieved an excellent repigmentation (score 4) while four Vitiligo Jul 14 7 lesions (25%) treated with 308-nm MEL and five lesions (31%) treated with NB-UVB showed a good repigmentation (score 3). The investigator concluded that 308-nm MEL is more effective than NB-UVB in treating vitiligo lesions and it induces repigmentation more rapidly. Scientific Rationale – Update December 2008 Current treatments for vitiligo, a common pigmentary disorder with cosmetic and psychological morbidity, are largely unsatisfactory. No treatment available is a definitive cure. Systemic psoralen and ultraviolet A (PUVA) has been the mainstay of treatment. Narrowband ultraviolet B (NB-UVB) has been more recently introduced, and seems to be more effective and less dangerous. Although a few retrospective comparative studies of systemic PUVA and NB-UVB for the treatment of vitiligo have been published, they have been very small, with no long-term efficacy or safety demonstrated. Intralesional steroids have been used in vitiligo to specifically deliver the drug to the Vogt-Koyanagi damaged dermal and epidermal structures, with contradictory results. Because of the high risk of side effects and the inconsistent results, this procedure is not recommended. It has been proposed that the use of systemic corticosteroids for months or years could stop vitiligo progression, depending on their anti-inflammatory and immunosuppressive properties. However, poor or contradictory results parallel a long list of side effects, including gastrointestinal symptoms, Cushing's syndrome and Cushing-like symptoms, cutaneous atrophy, acneiform eruptions, weight increase, osteoporosis, menstrual disturbances, and avascular necrosis of bones. Whitton et al. (2008) sought to report a Cochrane review of all interventions for the treatment of vitiligo. The authors systematically searched a range of databases for randomized controlled trials. Nineteen trials were included, which found moderate evidence of the benefit of topical steroids. The search uncovered limited to moderate evidence for various types and regimens of phototherapy (ultraviolet [UV] A and UVB) used alone or in combination with oral and topical treatments. Topical khellin combined with UVA should be questioned in view of the lack of available evidence of benefit. (Khellin is a furanochromone derivative isolated from seeds of the plant Ammi visnaga, with a chemical structure similar to that of psoralens. It has been reported that khellin is a useful alternative to psoralens when used in combination with UVA (KUVA), being less phototoxic, mutagenic and carcinogenic than psoralens). Studies generally were poorly designed and reported. Variations in study design and different outcome measures limit the evidence for the different therapeutic options. The best evidence from individual trials showed short-term benefit from topical steroids and various forms of UV light with topical preparations. Long-term follow-up and patient-centered outcomes should be incorporated in study design and psychologic interventions need more attention. Hercogová et al. (2007) Topical steroids are still considered by most dermatologists to be the first line of treatment for vitiligo, and for patients suffering from hypopigmentation with a consistent inflammatory component, such as pityriasis alba. Advantages of topical corticosteroid therapy for vitiligo include ease of application, and ability to use at home. Unlike phototherapy, topical corticosteroids are considered a treatment option for children of all ages on treatment of limited areas, and they are most effective for lesions on the face (excluding eyelids), elbows, and Vitiligo Jul 14 8 knees; the proximal extremities and trunk also respond fairly well, while the distal extremities respond poorly. Bhatnagar et al. (2007) completed a randomized, open, prospective study of 50 patients with vitiligo, divided equally in PUVA and NB-UVB groups. The study period was from January 2004 to June 2005. The mean degree of repigmentation attained in the NB-UVB group was 52.24% over a mean treatment period of 6.3 months, whereas in the PUVA group it was 44.7% in a mean period of 5.6 months (P= 0.144). After excluding the results of therapy-resistant sites, that is, hands and feet, the mean degree of repigmentation in the NB-UVB group was 67.57%, whereas in the PUVA group it was 54.2% (P = 0.007). NB-UVB performed better in comparison to TMP PUVA in terms of mean total repigmentation when traditionally considered therapy-resistant sites were excluded. However, this study was small, with no longterm efficacy or safety noted. In summary, vitiligo is a cosmetic problem and does not affect the individual’s health directly. The condition cannot be cured at present, but treatments are available that may be helpful. Medical treatments target the immune system and try to reverse the destruction. Surgical treatments are less commonly done, in which healthy melanocytes are transplanted from other areas. Both treatments may be difficult and prolonged. Depigmentation involves fading the rest of the skin on the body to match the already whitened areas, however, this process is permanent and cannot be reversed. The major side effects of depigmentation therapy are inflammation of the skin, and an abnormal sensitivity to sunlight. This form of therapy is cosmetic in nature. In addition, the published data are limited, the study population numbers are low and the long-term outcomes of these procedures are not known. Scientific Rationale – Update October 2008 Treatments used for vitiligo include medicines (such as steroid creams), light and laser therapy, and surgery (skin grafting). An excimer laser is a highly concentrated beam of UV light directed at vitiligo spots or patches. The targeted delivery spares the adjacent skin from UV exposure. Some evidence points to the synergistic activity of combination therapy with topical tacrolimus and UVB phototherapy (either NB-UVB or excimer laser). However, this combination may increase the risk of skin carcinogenesis. Laser therapy for treatment of vitiligo is a procedure and is, therefore, not subject to regulation by the FDA. However, the devices used to laser the vitiligo are regulated by the FDA. A xenon chloride (XeCl) excimer laser is a laser surgical instrument for use in general and plastic surgery and in dermatology. It is a Class II device. There are a number of laser devices that have been approved by the FDA for use in general and plastic surgery and dermatology. An example of a recently approved (K062963) excimer laser for the treatment of vitiligo is the Pharos Excimer Laser System, Model EX-308 (Ra Medical Systems Inc.), approved on April 3, 2007. The Pharos is a self-contained ultraviolet (UV) laser light source that uses an XeCl gas mixture to generate a selected dose and targetspecific UV light at a wavelength of 308 nm. The intended use of the Pharos laser is for UVB phototherapy of targeted skin for treatment of psoriasis, vitiligo, atopic dermatitis, and leukoderma. Vitiligo Jul 14 9 510(k) clearance has subsequently been obtained for a number of targeted UVB lamps and lasers, including the XTRAC XL and VTRAC lamp (PhotoMedex), the BClear lamp (Lumenis), and the European manufactured Excilite and Excilite µ XeCL lamps. The indicated use of these devices is targeted UVB phototherapy for treatment of skin conditions including psoriasis, vitiligo, atopic dermatitis, and leukoderma. Narrow band UVB microphototherapy, monochromatic excimer light, and excimer lasers grew out of NB-UVB therapy. Many vitiglio patients undergoing phototherapy wanted to avoid the obvious increase in chromatic contrast between normal and lesional skin, caused by the tanning of healthy skin under UV-ray stimulation, which can be seen before total repigmentation might be obtained. Moreover, considering that different body sites require different doses of phototherapy to repigment (ie, the eyelids need less exposure than the feet to repigment), the patients undergoing cabin UVB treatments could receive a high cumulative dose of radiation, leading to the excessive risk of sunburns in areas such as the eyelids, the face, and genital areas, thus limiting the compliance to therapy regulations. Furthermore, there is a risk of secondary cutaneous disorders occurring, such as photoaging, teleangectasias, and skin neoplasms. The BIOSKIN microphototherapy concept has been recently achieved by the development of two different ways of delivering, respectively, a UVB peak light and a laser coherent emission of a similar wavelength. The former is a monochromatic excimer light (MEL) device, which can generate and selectively deliver a 308-nm peak UVB light; the latter is an excimer laser (XeCl), which delivers monochromatic rays very similar to “classical” narrowband radiation. Both MEL and XeCl are capable of selectively treating single hypopigmented patches, sparing nonaffected areas. There is a clinical trial regarding Excimer Lamp Versus Excimer Laser in Vitiligo Treatment. This study is currently recruiting participants. The ClinicalTrial Identifier # is NCT00696358. The aim of this study is to make a prospective comparison between the two phototherapies of 308nm excimer lamp and 308nm excimer laser. Both have provided interesting results in treating vitiligo. They have the same wavelengh but the type of emission of the photons is different. To date there is no direct comparative data concerning these two devices in this indication. There is another clinical trial on excimer laser for vitiligo, which is completed. This is on the Optimal Treatment Frequency of 308-Nm Excimer Laser for Vitiligo on the Face and Neck. The ClinicalTrial Identifier # is NCT00368407. The purpose of this study was to determine the optimal treatment frequency of 308-nm excimer laser for vitiligo and identify the key clinical variable(s) associated with the treatment efficiency under the optimal treatment frequency. Prospective, randomized, comparative study among groups of vitiligo patients treated with 308-nm excimer laser, with the limitation of no follow-up. Shen et al. (2007) A total of 187 patients were treated with the 308-nm excimer laser for 20 sessions at different frequencies (0.5, 1.0, 2.0, and 3.0 per week). Repigmentation occurred fastest with treatment frequencies of 2.0 and 3.0 and there was no statistically significant difference between them. The 308-nm excimer laser is effective for therapy to treat vitiligo on the face and neck. It appears that the onset of repigmentation correlates with the total area of vitiliginous patches and the optimal treatment frequency. Monitored studies on a larger population with longterm follow-up would be needed to confirm and extend our findings. Vitiligo Jul 14 10 There are also no Position Statements from professional groups on this technology. To date, only five small, randomized, controlled studies were done on excimer laser for vitiligo. The other studies were prospective or retrospective. Some of the studies do note that recent evaluation of excimer laser approaches reported good results in terms of repigmentation, alone or in association with other agents (ie, topical steroids or tacrolimus ointment), but the results of clinical trials are necessary. Treatment periods of more than 12 weeks may be necessary to obtain a satisfactory clinical repigmentation, particularly when vitiligo lesions are treated only once or twice compared with 3 x weekly. Some of the studies done on individuals treated with excimer laser for vitiligo had the limitation of no follow-up. This technique of using excimer laser for vitiligo, which is considered a newer procedure for this diagnosis, requires additional, larger, published, peer-reviewed, randomized controlled studies, with long-term outcomes to determine the efficacy and safety. Scientific Rationale – Update November 2007 A Medline search was performed to review more recent studies regarding Vitiligo. There continues to be inadequate evidence in the most recent literature to support treatment for vitiligo for Commercial members. However, phototherapy and photochemotherapy (PUVA) for the treatment of vitiligo is a local Medicare covered service as dictated by specific local Medicare carriers; it must therefore be covered for all Medicare Advantage members who reside in the local area in which coverage is applicable, subject to the relevant Medicare criteria and/or guidelines. Brazzelli et al. (2007) completed a study of sixty patients (23 male and 37 female), aged 6 to 70 years, with vitiligo, who were treated with UVB-NB therapy over a maximum period of 2 years. The lesions located on the face obtained a complete repigmentation in 68% of the patients, on the neck in 57.89%, and on the trunk in 50% within the first year of the therapy. In young patients vs. adults patients, the lesions located on the neck obtained a complete repigmentation in 83.33% vs. 46.15%, on the upper limbs in 28.57% vs. 9.52%, and on the lower limbs in 25% vs. 16.67%. In patients with vitiligo of recent onset, the lesions located on the neck obtained a complete repigmentation in 83.33%, on the upper limbs in 33.33%, and on the lower limbs in 28.57%. Hands did not give a positive response in either groups. This study shows that certain body sites respond better than others to the UVB-NB therapy; patients, aged less than 20 years, with recent vitiligo, achieve more repigmentation; the duration of the therapy can influence the response of the lesions over hands and lower limbs, showing only mild repigmentation. However, this study is small, and there is insufficient peer-reviewed scientific literature to support the safety and efficacy of this therapy. Radmanesh and Saedi (2006) randomly assigned 60 patients with vitiligo to PUVA plus azathioprine therapy (group 1, n=30) or to oral PUVA therapy alone (group 2, n=30). Following twice-weekly PUVA therapy, progress was followed based upon a graph transparency of the affected lesions. Comparison of the lesions prior to treatment and four months following treatment revealed that group 1 had nine patients with excellent results compared to no patients in group 2. Four patients in group 1 showed weak results compared to 14 patients in group 2. Overall, group 1 demonstrated 23.6% repigmentation compared to 8.7% in group 2. The authors concluded that azathioprine may potentiate repigmentation in patients with vitiligo when used in combination with PUVA. However, since this was a very small study, and published data is limited, treatment for vitiligo with PUVA is not supported at this time. Vitiligo Jul 14 11 El-Mofty et al. (Feb 2006) reported on two independent studies evaluating nbUVB 311 for the treatment of vitiligo. One study included 15 patients treated with UVB 311 on the left half of the body and PUVA on the right half of the body. The difference in improvement of the vitiligo between the two sides was insignificant. A second study included 20 patients treated with UVB 311 on the left side of the body compared to nbUVB 311 with psoralen (PUVB) on the left side of the body. Based upon the percentage of repigmentation, both sides showed equal clinical improvement. Although not statistically significant, the cumulative dose needed to achieve the same response was lower when used in combination with PUVA. Scientific Rationale - Update June 2005 Vitiligo is an acquired skin disorder that continues to have great social impact. Treatment continues to include medical, surgical and adjunctive therapies. Topical corticosteroids are indicated for the treatment of limited areas of vitiligo. A pilot study was performed to evaluate the efficacy of the 0.05% clobestasol propionate (Temovate) as compared to 1% pimecrolimus, which has been recently shown to be effective in the treatment of vitiligo. Ten patients with bilateral symmetrical lesions were treated twice daily. Lesions on right side of the body were treated with the 0.05% clobestasol propionate, while the opposite side was treated with the 1% pimecrolimus. It was determined that both treatments resulted in comparable rate of repigmentation, although the response to treatment varied with the anatomical location treated. The author notes that further studies involving larger groups of patients are warranted. A recent study in 2005 suggests that the sympathetic nervous system might have a particular role in the pathogenesis of vitiligo. This study examined 14 healthy subjects and 14 patients with generalized vitiligo before and after PUVA therapy in an attempt to determine how Electrodermal Activity (EDA) parameters were affected from vitiligo illness before PUVA therapy and whether any electrophysiological gains acquired from PUVA therapy would influence the progression of the condition. Before treatment, skin conductance level (SCL) and habituation number (HN) was higher in vitiligo group than the control groups, however after treatment, SCL and HN were decreased nearly to normal value. The author suggests that patients with vitiligo may have changes to EDA parameters that are reversible to great extent with PUVA therapy. Narrowband phototherapy has been effective in the treatment of psoriasis. A metaanalysis on the role of Narrow Band UVB phototherapy seems to be less clear in the management of skin conditions other than psoriasis. The article references randomized controlled trials, open prospective studies, and retrospective observations on Narrow Band UVB in chronic atopic dermatitis, generalized vitiligo, early stages of cutaneous T-cell lymphoma, chronic urticaria, lichen planus, pruritus associated with polycythemia vera, seborrheic dermatitis, actinic prurigo, and acquired perforating dermatosis. The best current data exists for treatment with Narrow Band UVB for atopic dermatitis and widespread vitiligo. In the treatment of most other nonpsoriatic conditions, NB UVB appears to be effective, however, there are no definitive conclusions as to preference over current options, such as UVA1 phototherapy and psoralen-UVA regimens. Another recent study compared the clinical efficacy of 308-nm excimer laser with that of narrow band UVB phototherapy. Eight patients with symmetrical patterns of Vitiligo Jul 14 12 vitiligo were studied. The patches on one side of the body were treated with the laser, while the opposing side was treated with narrow band UVB phototherapy. After 20 sessions, higher repigmentation was noted on the side treated with the 308nm excimer laser. Additional studies of the 308-nm excimer laser for the treatment of vitiligo propose that this type of treatment should be proposed for limited vitiligo and of the “UV sensitive” areas. This treatment is promising however, additional studies are needed to ensure the absence of the median and long term side effects. Autologous skin grafting must still be viewed as investigational and experimental because its effectiveness and side effects remain to be fully defined. Scientific Rationale –Initial Vitiligo is a common medical condition of uncertain etiology characterized by loss of skin pigment secondary to destruction of melanocytes in the skin, the mucous membranes and the retina. One theory is that vitiligo may be an autoimmune disease associated with antibodies to melanocytes. Studies also suggest that there is some genetic mechanism involved since there is a positive family history in at least 30% of cases. As a result of the depigmentation, white patches of skin and hair appear on different parts of the body. The distribution of the lesions can be remarkably symmetrical. Initially the disease is limited; it then progresses slowly over years. Commonly involved sites include the backs of the hands, the face, and body folds, including axillae and genitalia. Hypo pigmentation areas are common around body openings such as the eyes, nostrils, mouth, nipples, umbilicus, and anus. Vitiligo can also occur at sites of trauma, such as around the elbows and in previously sunburned skin. The disorder affects all races and both sexes equally. Approximately 1% of the population is affected; 50% of cases begin before age 20. Although most patients with vitiligo are healthy, there is an increased association with certain autoimmune conditions such as thyroiditis, hyperthyroidism, Addison's disease, pernicious anemia, and diabetes mellitus. The change in appearance caused by vitiligo can affect a person's emotional and psychological well being, especially if it involves the face. The choice of therapy for this condition depends on the number of white patches, how widespread they are and on the patient's preference for treatment. Each patient responds differently to therapy, and a particular treatment may not work for everyone. Current treatment options for vitiligo include medical, surgical, and adjunctive therapies. Steroids may be helpful in repigmenting the skin, particularly if started early in the disease. It is the simplest and safest treatment but not as effective as psoralen photochemotherapy, but may take at least 3 months before seeing any results. For limited lesions, a trial of high-potency topical corticosteroids such as 0.05% clobetasol cream (Temovate) applied twice daily for at least 3 months is indicated. For facial lesions, less potent corticosteroids such as 0.05% fluocinonide cream (Lidex) are safer but can still produce cutaneous atrophy, skin striae and steroidinduced rosacea if used for a prolonged period. If after 4 to 6 months of continuous topical corticosteroid therapy no repigmentation is noted, this therapy should be discontinued. Topical psoralens may also have some success in patients with limited areas of pigmentation, but some dermatologists believe that the potential for phototoxicity to produce severe burns is too great. Systemic corticosteroids can arrest the progression of vitiligo and lead to repigmentation in a significant proportion of patients, but may also produce unacceptable side effects. Oral mini-pulse therapy with 5 mg Vitiligo Jul 14 13 betamethasone/dexamethasone has been reported to arrest the progression and induce spontaneous repigmentation in some vitiligo patients. In one study, betamethasone as a single oral dose was taken after breakfast on 2 consecutive days per week to minimize the side effects. The progression of the disease was arrested in 89% with active disease, whereas some patients needed an increase in the dosage to 7.5 mg per day to achieve a complete arrest of lesions. Within 2 to 4 months, 80% of the patients started having spontaneous repigmentation of the existing lesions that progressed with continued treatment. Depigmented skin is devoid of melanocytes in the epidermis. Repigmentation is caused by activation and migration of melanocytes from a melanocytic reservoir located in the hair follicles. Therefore, skin with little or no hair (hands and feet) or with white hair responds poorly to treatment. When a vitiliginous spot repigments, it repigments from the follicle and spreads outward. Melanocytes divide rapidly after any inflammatory process or following ultraviolet (UV) radiation. Psoralen ultraviolet A (PUVA) photochemotherapy produces inflammation in the skin at the depth of the hair follicle. Cytokines released by the inflammatory process may stimulate melanocytes to proliferate and migrate out. UVA is one type of radiation that is part of sunlight and reaches the earth's surface. Exposure to UVA can cause the skin to tan. The most effective therapy for vitiligo is photochemotherapy using topical psoralen plus ultraviolet A (PUVA). Psoralens are drugs that contain chemicals that react with ultraviolet light to cause darkening of the skin. Psoralen photochemotherapy often is used for people with a small number of depigmented patches (affecting less than 20 percent of the body). Patients should be selected carefully for psoralen photochemotherapy. They should be informed that in approximately 50% of patients there may be some repigmentation following a long protracted period of 150 to 200 PUVA treatments over 12 to 24 months. Best results are obtained on the face and neck. The face usually begins to respond after 25 treatments, other areas after 50. Vitiligo of the backs of the hands almost never responds. Repigmentation may not be complete, and the partially treated areas may appear more bizarre than they did initially. The response is slow. PUVA is not indicated for children younger than 10 years or for those with lesions predominately over the hands, elbows, and knees, as these sites rarely repigment with any treatment. In those with localized disease, the new micronized crystalline form of methoxsalen (Oxsoralen) is the preferred drug because it is consistently absorbed and may be applied in a 0.1% concentration in Cetaphil lotion 30 minutes before UVA exposure. Patients usually receive treatments in their doctors' offices once or twice a week so they can be carefully watched for any side effects. Patients must minimize exposure to sunlight at other times. There are two major potential side effects of topical PUVA therapy: (1) severe sunburn and blistering and (2) too much repigmentation or darkening of the treated patches or the normal skin surrounding the vitiligo (hyperpigmentation). Oral PUVA is easier to administer in patients with widespread disease (affecting greater than 20% of the body) and has somewhat less phototoxicity associated with it. Oral psoralen is not recommended for children under 10 years of age because of an increased risk of damage to the eyes, such as cataracts; instead, a mild topical corticosteroid cream is often prescribed. Oral PUVA may be used for people who do not respond to topical PUVA therapy. A dose of 0.6 mg/kg is administered 90 minutes before UVA exposure. Treatments are given two to three times per week, never on successive days. Patients should also wear protective UVA sunglasses for 18 to 24 hours after each treatment to avoid eye damage, particularly cataracts. Vitiligo Jul 14 14 Follicular repigmentation usually begins after 15 or 20 treatments. Significant repigmentation may occur after 50 treatments. Patients may be given a trial off of therapy for a couple of months. They may continue to repigment during this period. Patients who respond usually keep their pigment, however, one of the drawbacks is an increased risk of skin cancer. Patients who have actively spreading vitiligo should not be treated; treatment does not halt the spread of the disease. Maintenance therapy is not required. While a minimum of 100 treatments, given two to three times a week over many months, is necessary before any repigmentation response is seen, less than 50 percent of the patients have a positive response to this therapy. An emerging new therapy for extensive vitiligo is narrow-band UVB phototherapy. It involves highintensity exposure to a single wavelength in the UVB spectrum. One does not need to ingest methoxsalen or other photoactive agents for the treatment to be effective. A number of recently published studies have demonstrated that narrow-band UVB is an effective treatment for vitiligo, and compares favorably to PUVA. Narrow-band UVB is typically administered two to three times per week for several months. Given the proximity of the wavelengths of UVB (311 nm) and the excimer laser (308 nm), remarkable success in the repigmentation of skin patches has been shown to occur using UVB laser light which allows deeper penetration of the synthetic UVB light to reach any surviving pigment producing cells in the vitiliginous area. In a recent study, vitiligo patients, most of whom had failed conventional therapies, were treated with the excimer laser three times a week. Fifty-seven percent of the vitiligo patches treated in the study experienced partial to complete repigmentation in only two weeks. The face demonstrated the most rapid repigmentation. As no side-effects have been observed, the researchers concluded that the XTRAC laser could represent the treatment of choice for vitiligo limited to less than 30% of the skin surface. Depigmentation involves fading the rest of the skin on the body to match the already white areas. For people who have vitiligo on more than 50 percent of their bodies, depigmentation may be the best treatment option. Patients apply the drug monobenzylether of hydroquinone (monobenzone or Benoquin) twice a day to pigmented areas until they match the already depigmented areas. The major side effect of depigmentation therapy is inflammation of the skin. Depigmentation is permanent and cannot be reversed. In addition, a person who undergoes depigmentation will always be abnormally sensitive to sunlight. This form of therapy is purely cosmetic in nature. Several surgical procedures have been developed for treating depigmented skin. These include grafting suction-blistered epidermis, minigrafts, and transplantation of in vitro-cultured epidermis bearing melanocytes. Autologous skin grafting must still be viewed as investigational and experimental because its effectiveness and side effects remain to be fully defined. There are several possible complications of autologous skin grafting. Infections may occur at the donor or recipient sites. The recipient and donor sites may develop scarring, a cobblestone appearance, or a spotty pigmentation, or may fail to repigment at all. Treatment with grafting takes time, and most people find it unacceptable. Another method of skin grafting is performed by creating blisters on the patient's pigmented skin by using heat, suction, or freezing cold. The tops of the blisters are then cut out and transplanted to a depigmented skin area. The risks of blister grafting include the development of a cobblestone appearance, scarring, and lack of repigmentation. However, there is less Vitiligo Jul 14 15 risk of scarring with this procedure than with other types of grafting. This method is also considered investigational at this time. In performing autologous melanocyte transplants, a sample of the patient's normal pigmented skin is removed and placed it in a laboratory dish containing a special cell culture solution to grow melanocytes. When the melanocytes in the culture solution have multiplied, they are transplanted to the patient's depigmented skin patches. This procedure is currently experimental and is impractical for the routine care of people with vitiligo. Micropigmentation (tattooing) implants pigment into the skin with a special surgical instrument. This procedure works best for the lip area, particularly in people with dark skin; however, it is difficult for the doctor to match perfectly the color of the skin of the surrounding area. Tattooing tends to fade over time. In addition, tattooing of the lips may lead to episodes of blister outbreaks caused by the herpes simplex virus. This method is also considered investigational in nature. Review History October 2003 June, 2005 November 2006 November 2007 August 2008 October 2008 December 2008 July 2009 December 2009 February 2010 April 2011 January 2012 January 2013 January 2014 July 2014 Medical Advisory Council Medical Advisory Council, Update – no revisions Update – no revisions Update. Phototherapy and Photochemotherapy (PUVA) for the treatment of vitiligo is a local Medicare covered service as dictated by specific local Medicare carriers, therefore, it must be covered for all Medicare Advantage members who reside in the local area in which coverage is applicable, subject to the relevant Medicare criteria and/or guidelines. Update for Commercial members remains unchanged. CA reconstructive surgery law added to Disclaimer Update. Excimer laser for vitiligo is considered investigational since there is a lack of well designed, randomized, controlled clinical trials with adequate follow-up to support the efficacy and safety of this technique. Update. No revision. Codes reviewed. Update. Added photochemotherapy or phototherapy with Ultraviolet A or B therapy as cosmetic for vitiligo. Added medically necessary treatment options for vitiligo of the face and hands. Added link to HNPS to access Protopic and Elidel criteria. Updated policy- treatment no longer restricted to face and hands. Update. Added Medicare Table. No revisions Update – no revisions Update – no revisions. Code updates. Update – no revisions. Update – no revisions. Code updates. References – Update July 2014 1. Akdeniz N, Yavuz IH, Bilgili SG, et al. Comparison of efficacy of narrow band UVB-alone, combination of calcipotriol-narrow band UVB, and combination betamethasone-calcipotriol-narrow band UVB therapies in vitiligo. J Dermatolog Treat. 2013 Feb 26. [Epub ahead of print] Vitiligo Jul 14 16 2. 3. 4. 5. Dayel SB, Alghamdi K. Failure of alefacept in the treatment of vitiligo. J Drugs Dermatol. 2013;12(2):159-161. Goldstein BG, Goldstein AO. Vitiligo. Last reviewed February 2013. UpToDate Inc. Waltham, MA. Grimes PE, Hamzavi I, Lebwohl M, et al. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol. 2013;149(1):68-73. Wong R, Lin AN. Efficacy of topical calcineurin inhibitors in vitiligo. Int J Dermatol. 2013;52(4):491-496. References – Update January 2014 1. 2. 3. 4. 5. 6. 7. Al Jasser MI, Ghwish B, Al Issa A, Mulekar SV. Repigmentation of vitiligoassociated leukotrichia after autologous, non-cultured melanocyte-keratinocyte transplantation. Int J Dermatol. 2013 Nov;52(11):1383-6. Bansal S, Sahoo B, Garg V. Psoralen-narrowband UVB phototherapy in treatment of vitiligo in comparison to narrowband UVB phototherapy. Photodermatol Photoimmunol Photomed. 2013 Sep 5. Matsuzaki K, Kumagai N. Treatment of vitiligo with autologous cultured keratinocytes in 27 cases. Eur J Plast Surg. 2013;36:651-656. Mulekar SV, Isedeh P. Surgical interventions for vitiligo: an evidence-based review. Br J Dermatol. 2013 Oct;169 Suppl 3:57-66. Ramos MG, Ramos DG, Gontijo G, et al. Non-cultured melanocyte/keratinocyte transplantation for the treatment of stable vitiligo on the face: report of two cases. An Bras Dermatol. 2013 Oct;88(5):811-3. Sharma S, Garg VK, Sarkar R, Relhan V. Comparative study of flip-top transplantation and punch grafting in stable vitiligo. Dermatol Surg. 2013 Sep;39(9):1376-84. Sharquie KE, Noaimi AA, Al-Mudaris HA. Melanocytes transplantation in patients with vitiligo using needling micrografting technique. J Drugs Dermatol. 2013 May;12(5):e74-8. References – Update January 2013 1. 2. 3. 4. 5. 6. 7. Alhowaish AK, Dietrich N, Onder M, Fritz K. Effectiveness of a 308-nm excimer laser in treatment of vitiligo: a review. Lasers Med Sci. 2012 Aug 15. Budania A, Parsad D, Kanwar AJ, Dogra S. Comparison between autologous noncultured epidermal cell suspension and suction blister epidermal grafting in stable vitiligo: a randomized study. Br J Dermatol. 2012 Dec;167(6):1295-301. Cheng YP, Chiu HY, Jee SH, Tsai TF. Excimer light photototherapy of segmental and non-segmental vitiligo: experience in Taiwan. Photodermatol Photoimmunol Photomed. 2012 Feb;28(1):6-11. Colucci R, Lotti T, Moretti S. Vitiligo: an update on current pharmacotherapy and future directions. Expert Opin Pharmacother. 2012 Sep;13(13):1885-99. El-Zawahry BM, Bassiouny DA, Sobhi RM, et al. A comparative study on efficacy of UVA1 vs. narrow-band UVB phototherapy in the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2012 Apr;28(2):84-90. Ghosh D, Kuchroo P, Viswanathan C, et al. Efficacy and Safety of Autologous Cultured Melanocytes Delivered on Poly (dl-Lactic Acid) Film: A Prospective, Open-Label, Randomized, Multicenter Study. Dermatol Surg. 2012 Dec;38(12):1981-90. Hallaji Z, Ghiasi M, Eisazadeh A, Damavandi MR. Evaluation of the effect of disease duration in generalized vitiligo on its clinical response to narrowband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2012 Jun;28(3):115-9. Vitiligo Jul 14 17 8. 9. 10. 11. 12. 13. 14. 15. 16. Huggins RH, Henderson MD, Mulekar SV, et al. Melanocyte-keratinocyte transplantation procedure in the treatment of vitiligo: the experience of an academic medical center in the United States. J Am Acad Dermatol. 2012 May;66(5):785-93. Maleki M, Banihashemi M, Sanjari V. .Efficacy of suction blister epidermal graft without phototherapy for locally stable and resistant vitiligo. Indian J Dermatol. 2012 Jul;57(4):282-4. Nisticò S, Chiricozzi A, Saraceno R, et al. Vitiligo treatment with monochromatic excimer light and tacrolimus: results of an open randomized controlled study. Photomed Laser Surg. 2012 Jan;30(1):26-30. Percivalle S, Piccinno R, Caccialanza M, Forti S. Narrowband ultraviolet B phototherapy in childhood vitiligo: evaluation of results in 28 patients. Pediatr Dermatol. 2012 Mar-Apr;29(2):160-5. Sapam R, Agrawal S, Dhali TK. Systemic PUVA vs. narrowband UVB in the treatment of vitiligo: a randomized controlled study. Int J Dermatol. 2012 Sep;51(9):1107-15. Shin J, Lee JS, Hann SK, Oh SH. Combination treatment by 10 600 nm ablative fractional carbon dioxide laser and narrowband ultraviolet B in refractory nonsegmental vitiligo: a prospective, randomized half-body comparative study. Br J Dermatol. 2012 Mar;166(3):658-61. Singh S, Khandpur S, Sharma VK, Ramam M. Comparison of efficacy and sideeffect profile of oral PUVA vs. oral PUVA sol in the treatment of vitiligo: a 36week prospective study. J Eur Acad Dermatol Venereol. 2012 Oct 16. Vázquez-Martínez OT, Martínez-Rodríguez HG, Velásquez-Arenas L, et al. Treatment of vitiligo with a melanocyte-keratinocyte cell suspension versus dermabrasion only: a pilot study with a 12-month follow up. J Drugs Dermatol. 2011 Sep;10(9):1032-6. Verhaeghe E, Lodewick E, van Geel N, Lambert J. Intrapatient comparison of 308-nm monochromatic excimer light and localized narrow-band UVB phototherapy in the treatment of vitiligo: a randomized controlled trial. Dermatology. 2011;223(4):343-8 References – Update January 2012 1. 2. 3. 4. 5. 6. Gan EY, van Geel N, Goh BK. Repigmentation of leukotrichia in vitiligo with noncultured cellular grafting. Br J Dermatol. 2011 Jul 25. doi: 10.1111/j.13652133.2011.10540.x. Hossani-Madani A, Halder R. Treatment of vitiligo: advantages and disadvantages, indications for use and outcomes. G Ital Dermatol Venereol. 2011 Oct;146(5):373-95. Jin Y, Xu A, Wang P, Song X, Liu X. Long-term follow-up and correlated factors of vitiligo following autologous epidermal transplantation. Cutis. 2011 Mar;87(3):137-41. Jill Feetham H, Chan JL, Pandya AG. Characterization of Clinical Response in Patients with Vitiligo Undergoing Autologous Epidermal Punch Grafting. Dermatol Surg. 2011 Sep 27 Li J, Fu WW, Zheng ZZ, Zhang QQ, Xu Y, Fang L. Suction blister epidermal grafting using a modified suction method in the treatment of stable vitiligo: a retrospective study. Dermatol Surg. 2011 Jul;37(7):999-1006. doi: 10.1111/j.1524-4725.2011.01966.x. Linthorst Homan MW, Spuls PI, Nieuweboer-Krobotova L, et al. A randomized comparison of excimer laser versus narrow-band ultraviolet B phototherapy after punch grafting in stable vitiligo patients. J Eur Acad Dermatol Venereol. 2011 Jun 29. doi: 10.1111/j.1468-3083.2011.04147.x. Vitiligo Jul 14 18 7. 8. 9. Mouzakis JA, Liu S, Cohen G. Rapid response of facial vitiligo to 308nm excimer laser and topical calcipotriene. J Clin Aesthet Dermatol. 2011 Jun;4(6):41-4. Sameem F, Sultan SJ, Ahmad QM. Split thickness skin grafting in patients with stable vitiligo. J Cutan Aesthet Surg. 2011 Jan;4(1):38-40 Sahni K, Parsad D, Kanwar AJ. Noncultured epidermal suspension transplantation for the treatment of stable vitiligo in children and adolescents. Clin Exp Dermatol. 2011 Aug;36(6):607-12. doi: 10.1111/j.13652230.2011.04065.x. References – Update April 2011 1. 2. Asawanonda P, Klahan SO. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: a preliminary randomized controlled study. Photomed Laser Surg. 2010 Oct; 28(5): 679-84. El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo (chemo) therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010 Jul-Aug;23 (4):428-34. References – Update December 2009 1. Asawanonda P, Kijluakiat J, Korkij W, Sindhupak W. Targeted broadband ultraviolet b phototherapy produces similar responses to targeted narrowband ultraviolet B phototherapy for vitiligo: a randomized, double-blind study. Acta Derm Venereol. 2008;88(4):376-81 2. Casacci M, Thomas P, Pacifico A, Bonnevalle, et al. Comparison between 308nm monochromatic excimer light and narrowband UVB phototherapy (311-313 nm) in the treatment of vitiligo--a multicentre controlled study. J Eur Acad Dermatol Venereol. 2007 Aug;21(7):956-63 3. Dawid M, Veensalu M, Grassberger M, Wolff K. Efficacy and safety of pimecrolimus cream 1% in adult patients with vitiligo: results of a randomized, double-blind, vehicle-controlled study. J Dtsch Dermatol Ges. 2006 Nov;4(11):942-6. 4. Esfandiarpour I, Ekhlasi A, Farajzadeh S, Shamsadini S. The efficacy of pimecrolimus 1% cream plus narrow-band ultraviolet B in the treatment of vitiligo: a double-blind, placebo-controlled clinical trial. J Dermatolog Treat. 2009;20(1):14-8. 5. El-Mofty M, Mostafa W, Youssef R, et al. Ultraviolet A in vitiligo. Photodermatol Photoimmunol Photomed. 2006 Aug;22(4):214-6. 6. Forschner T, Buchholtz S, Stockfleth E. Current state of vitiligo therapy-evidence-based analysis of the literature. J Dtsch Dermatol Ges. 2007 Jun;5(6):467-75 7. Goldinger SM, Dummer R, Schmid P, et al. Combination of 308-nm xenon chloride excimer laser and topical calcipotriol in vitiligo. J Eur Acad Dermatol Venereol. 2007 Apr;21(4):504-8. 8. Groysman V, Sami N. Vitiligo. eMedicine. Aug 2008. Available at: http://emedicine.medscape.com/article/1068962-overview 9. Lu-yan T, Wen-wen F, Lei-hong X, et al. Topical tacalcitol and 308-nm monochromatic excimer light: a synergistic combination for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2006 Dec;22(6):310-4. 10. Middelkamp-Hup MA, Bos JD, Rius-Diaz F, et al. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2007 Aug;21(7):942-50 Vitiligo Jul 14 19 11. Radakovic S, Breier-Maly J, Konschitzky R, et al. Response of vitiligo to oncevs. twice-daily topical tacrolimus: a controlled prospective, randomized, observer-blinded trial. J Eur Acad Dermatol Venereol. 2009 Aug;23(8):951-3 12. Rath N, Kar HK, Sabhnani S. An open labeled, comparative clinical study on efficacy and tolerability of oral minipulse of steroid (OMP) alone, OMP with PUVA and broad / narrow band UVB phototherapy in progressive vitiligo. ndian J Dermatol Venereol Leprol. 2008 Jul-Aug;74(4):357-60 13. Sassi F, Cazzaniga S, Tessari G, et al. Randomized controlled trial comparing the effectiveness of 308-nm excimer laser alone or in combination with topical hydrocortisone 17-butyrate cream in the treatment of vitiligo of the face and neck. Br J Dermatol. 2008 Nov;159(5):1186-91 14. Yones SS, Palmer RA, Garibaldinos TM, Hawk JL. Randomized double-blind trial of treatment of vitiligo: efficacy of psoralen-UV-A therapy vs Narrowband-UV-B therapy. Arch Dermatol. 2007 May;143(5):578-84. References – Update December 2008 1. 2. 3. 4. 5. 6. 7. Gawkrodger DJ, Ormerod AD, Shaw L, et al. Guideline for the diagnosis and management of vitiligo. : Br J Dermatol. 2008 Nov;159(5):1051-76. Whitton ME, Ashcroft DM, González U. Therapeutic interventions for vitiligo. J Am Acad Dermatol. 2008 Oct;59 (4):713-7. Goldstein BG, Goldstein AO. Vitiligo. UpToDate. September 22, 2008. Available at: http://www.uptodate.com/online/content/topic.do?topicKey=pri_derm/13979&s electedTitle=1~150&source=search_result#7 Szczurko O, Boon HS. A systematic review of natural health product treatment for vitiligo. BMC Dermatol. 2008 May 22; 8:2. Hercogová J, Buggiani G, Prignano F, et al. A Rational Approach to the Treatment of Vitiligo and Other Hypomelanoses. Dermatologic Clinics - Volume 25, Issue 3 (July 2007). Forschner T. Current state of vitiligo therapy. Evidence-based analysis of the literature. J Dtsch Dermatol Ges. 01-JUN-2007; 5(6): 467-75. Bhatnagar A, Kanawar AJ, Parsad D. Comparison of systemic PUVA and NB-UVB in the treatment of vitiligo: an open prospective study. Journal of the European Academy of Dermatology and Venereology, ISSN 0926-9959. 2007, vol. 21, no5, pp. 638-642. References – Update October 2008 1. Clinical Trials.gov. Excimer Lamp Versus Excimer Laser in Vitiligo Treatment. Identifier # NCT00696358. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00696358?term=excimer+laser+AND +treatment+of+vitiligo&rank=1 2. Chimento SM, Newland M, Ricotti C, et al. A pilot study to determine the safety and efficacy of monochromatic excimer light in the treatment of vitiligo. J Drugs Dermatol. 2008; 7(3): 258-63 (ISSN: 1545-9616). 3. Sassi F, Cazzaniga S, Tessari G, et al. Randomized controlled trial comparing the effectiveness of 308-nm excimer laser alone or in combination with topical hydrocortisone 17-butyrate cream in the treatment of vitiligo of the face and neck. Br J Dermatol. 2008 Aug 19. 4. Mahmoud BH, Hexsel CL, Hamzavi IH. An Update on New and Emerging Options for the Treatment of Vitiligo. 05/14/2008. Skin Therapy Letter. 5. Shen Z, Gao TW, Chen L, et al. Optimal frequency of treatment with the 308-nm excimer laser for vitiligo on the face and neck. Photomed Laser Surg. 2007 Oct; 25(5): 418-27. Vitiligo Jul 14 20 6. Casacci M, Thomas P, Pacifico A, et al. Comparison between 308-nm monochromatic excimer light and narrowband UVB phototherapy (311-313 nm) in the treatment of vitiligo--a multicentre controlled study. J Eur Acad Dermatol Venereol. 2007 Aug; 21(7): 956-63. 7. Forschner T, Buchholtz S, Stockfleth E. Current state of vitiligo therapy-evidence-based analysis of the literature. J Dtsch Dermatol Ges. 2007 Jun; 5 (6): 467-75. 8. Hercogova J, Buggiani G, Prignano F, et al. A Rational Approach to the Treatment of Vitiligo and Other Hypomelanoses. Dermatologic Clinics - Volume 25, Issue 3 (July 2007). 9. Lotti T, Prignano F, Buggiani G. New and Experimental Treatments of Vitiligo and Other Hypomelanoses. Dermatologic Clinics - Volume 25, Issue 3 (July 2007). 10. Goldinger SM, Dummer R, Schmid P, et al. Combination of 308-nm xenon chloride excimer laser and topical calcipotriol in vitiligo. J Eur Acad Dermatol Venereol. 2007 Apr; 21(4): 504-8. 11. Hadi S, Tinio P, Al-Ghaithi K, et al. Photomedicine and Laser Surgery. Treatment of Vitiligo Using the 308-nm Excimer Laser. June 1, 2006, 24(3): 354-357. doi:10.1089/ pho.2006.24.354. 12. Hong SB, Park HH, Lee MH. Short-term effects of 308-nm xenon-chloride excimer laser and narrow-band ultraviolet B in the treatment of vitiligo: a comparative study. J Korean Med Sci. 2005 Apr; 20 (2): 273-8. 13. Passeron T, Ortonne JP. [The 308 nm excimer laser in dermatology] Presse Med. 2005 Feb 26; 34(4): 301-9. 14. Clinical Trials.gov. Optimal Treatment Frequency of 308-Nm Excimer Laser for Vitiligo on the Face and Neck. ClinicalTrials Identifier # is NCT00368407. Available at: http://clinicaltrials.gov/ct2/show/NCT00368407 15. Hofer A, Hassan AS, Legat FJ, et al. Optimal weekly frequency of 308-nm excimer laser treatment in vitiligo patients. Br J Dermatol. 2005 May; 152(5): 981-5. 16. Hadi SM, Spencer JM, Lebwohl M. The Use of the 308-nm Excimer Laser for the Treatment of Vitiligo. Dermatologic Surgery. Volume 30 Issue. 7, Pages 983 – 986. June 22, 2004. 17. Passeron T, Ostovari N, Zakaria W, et al. Topical tacrolimus and the 308nm excimer laser: a synergistic combination for the treatment of vitiligo. Arch Dermatol 140(9): 1065-9 (2004 Sep). 18. Baltas E, Csoma Z, Ignacz F, et al. Treatment of vitiligo with the 308-nm xenon chloride excimer laser. Arch Dermatol. 2002 Dec; 138(12): 1619-20. References – Update November 2007 1. 2. 3. 4. Brazzelli V, Antoninetti M, Palazzini S, et al. Critical evaluation of the variants influencing the clinical response of vitiligo: study of 60 cases treated with ultraviolet B narrow-band phototherapy. Journal of European Academy Dermatol Venereol. 2007 Nov;21(10):1369-74. National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). People With Vitiligo at Increased Risk for Other Autoimmune Diseases. August 2006. Available at: http://www.niams.nih.gov/News_and_Events/Spotlight_on_Research/2006/vitili go_risk.asp Radmanesh M, Saedi K. The efficacy of combined PUVA and low-dose azathioprine for early and enhanced repigmentation in vitiligo patients. J Dermatolog Treat. 2006;17(3):151-3. El-Mofty M, Mostafa W, Youssef R, et al. Ultraviolet A in vitiligo. Photodermatol Photoimmunol Photomed. 2006 Aug;22(4):214-6. Vitiligo Jul 14 21 5. 6. 7. 8. 9. Leone G, Pacifico A, Iacovelli P, et al. Tacalcitol and narrow-band phototherapy in patients with vitiligo. Clin Exp Dermatol. 2006 Mar;31(2):200-5. Mehrabi D, Pandya AG. A randomized, placebo-controlled, double-blind trial comparing narrowband UV-B Plus 0.1% tacrolimus ointment with narrowband UV-B plus placebo in the treatment of generalized vitiligo. Arch Dermatol. 2006 Jul;142(7):927-9. Rusfianti M, Wirohadidjodjo YW. Dermatosurgical techniques for repigmentation of vitiligo. Int J Dermatol. 2006;45(4):411-417. van Geel N, Ongenae K, Vander Haeghen Y, et al. Subjective and objective evaluation of noncultured epidermal cellular grafting for repigmenting vitiligo. Dermatology. 2006;213(1):23-29. Whitton ME, Ashcroft DM, Barrett CW, Gonzalez U. Interventions for vitiligo. Cochrane Database Syst Rev. 2006;(1):CD003263. References - Update June 2005 1. Hong SB, Park HH, Lee MH. Short-term effects of 308-nm xenon-chloride excimer laser and narrow-band ultraviolet B in the treatment of vitiligo: a comparative study. J Korean Med Sci. 2005 Apr; 20 (2): 273-8. 2. Berneburg M, Rocken M, Benedix F. Phototherapy with narrowband vs broadband UVB. Acta Derm Venereol. 2005; 85(2): 98-108. 3. Passeron T, Ortonne JP. [The 308 nm excimer laser in dermatology] Presse Med. 2005 Feb 26; 34(4): 301-9. 4. Dolu N, Ferahbas A, Ozesmi C, et al. Effect of PUVA therapy on electrodermal activity parameters in vitiligo patients. Auton Neurosci. 2005 Mar 31; 118(1-2): 102-107. 5. Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis.J Am Acad Dermatol. 2005 Apr; 52(4): 660-70. 6. Pianigiani E, Risulo M, Andreassi A, et al. Autologous epidermal cultures and narrow-band ultraviolet B in the surgical treatment of vitiligo. Dermatol Surg. 2005 Feb; 31(2): 155-9. 7. Coskun B, Saral Y, Turgut D. Topical 0.05% clobetasol propionate versus 1% pimecrolimus ointment in vitiligo. Eur J Dermatol. 2005 Mar-Apr; 15(2): 88-91. 8. Ada S, Sahin S, Boztepe G, Karaduman A, Kolemen F. No additional effect of topical calcipotriol on narrow-band UVB phototherapy in patients with generalized vitiligo. Photodermatol Photoimmunol Photomed. 2005 Apr; 21(2): 79-83. 9. Kostovic K, Pasic A. Related Articles, New treatment modalities for vitiligo: focus on topical immunomodulators. Drugs. 2005; 65(4): 447-59. 10. Kanwar AJ, Dogra S, Parsad D, Kumar B. Narrow-band UVB for the treatment of vitiligo: an emerging effective and well-tolerated therapy. Int J Dermatol. 2005 Jan; 44(1): 57-60. 11. Tjioe M, Otero ME, van de Kerkhof PC, Gerritsen MJ. Quality of life in vitiligo patients after treatment with long-term narrowband ultraviolet B phototherapy. J Eur Acad Dermatol Venereol. 2005 Jan; 19(1): 56-60. References - Initial 1. 2. 3. Taneja A, Trehan M, Taylor CR. 308-nm excimer laser for the treatment of localized vitiligo. Int J Dermatol. 2003 Aug; 42(8): 658-62. Arroyo MP, Tift L. Vitiligo therapy: where are we now? J Drugs Dermatol. 2003 Aug; 2(4): 404-8 Gupta S, Kumar B. Epidermal grafting in vitiligo: influence of age, site of lesion, and type of disease on outcome. J Am Acad Dermatol. 2003 Jul; 49(1): 99-104. Vitiligo Jul 14 22 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. Roelandts R. Photo(chemo) therapy for vitiligo. Photodermatol Photoimmunol Photomed. 2003 Feb; 19(1): 1-4. Menchini G, Tsoureli-Nikita E, Hercogova J. Narrow-band UV-B microphototherapy: a new treatment for vitiligo. J Eur Acad Dermatol Venereol. 2003 Mar; 17(2): 171-7. Dogra S, Parsad D. Combination of narrowband UV-B and topical calcipotriene in vitiligo. Arch Dermatol. 2003 Mar; 139(3): 393 Baltas E, Csoma Z, Ignacz F, et al. Treatment of vitiligo with the 308-nm xenon chloride excimer laser. Arch Dermatol. 2002 Dec; 138(12): 1619-20. Grimes PE, Soriano T, Dytoc MT. Topical tacrolimus for repigmentation of vitiligo. J Am Acad Dermatol. 2002 Nov; 47(5): 789-91. Chiaverini C, Passeron T, Ortonne JP. Treatment of vitiligo by topical calcipotriol. Eur Acad Dermatol Venereol. 2002 Mar; 16(2): 137-8. Taneja A. Treatment of vitiligo. J Dermatolog Treat. 2002 Mar; 13(1): 19-25. Spencer JM, Nossa R, Ajmeri J. Treatment of vitiligo with the 308-nm excimer laser: a pilot study. J Am Acad Dermatol. 2002 May; 46(5): 727-31. Kwok YK, Anstey AV, Hawk JL. Psoralen photochemotherapy (PUVA) is only moderately effective in widespread vitiligo: a 10-year retrospective study. Clin Exp Dermatol. 2002 Mar; 27(2): 104-10. Njoo MD, Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol. 2001; 2(3): 167-81. Scherschun L, Kim JJ, Lim HW. Narrow-band ultraviolet B is a useful and welltolerated treatment for vitiligo. J Am Acad Dermatol. 2001; 44(6): 999-1003. Phillips J, Gawkrodger DJ, Caddy CM, et al. Keratinocytes suppress TRP-1 expression and reduce cell number of co-cultured melanocytes - implications for grafting of patients with vitiligo. Pigment Cell Res. 2001; 14(2): 116-125. Halder RM. New and emerging therapies for vitiligo. Dermatol Clin. 2000; 18(1): 79-89, ix. Chen YF, Chang JS, Yang PY, et al. Transplant of cultured autologous pure melanocytes after laser-abrasion for the treatment of segmental vitiligo. J Dermatol. 2000; 27(7): 434-439. Sachdev M, Shankar DS. Dermatologic surgery: pulsed erbium:YAG laserassisted autologous epidermal punch grafting in vitiligo. Int J Dermatol. 2000; 39(11): 868-871. Njoo MD, Bos JD, Westerhof W. Treatment of generalized vitiligo in children with narrow-band (TL-01) UVB radiation therapy. J Am Acad Dermatol; 2000; 42(2 Pt 1): 245-253. Kim HY, Kang KY. Epidermal grafts for treatment of stable and progressive vitiligo. J Am Acad Dermatol. 1999; 40(3): 412-417. Kim SM, Lee HS, Hann SK. The efficacy of low-dose oral corticosteroids in the treatment of vitiligo patients. Int J Dermatol. 1999; 38(7): 546-550. Njoo MD, Westerhof W, Bos JD, et al. The development of guidelines for the treatment of vitiligo. Arch Dermatol. 1999; 135:1514-1521. Jimbow K. Vitiligo. Therapeutic advances. Dermatol Clin. 1998; 16(2): 399-407. Olsson MJ, Juhlin L. Epidermal sheet grafts for repigmentation of vitiligo and piebaldism, with a review of surgical techniques. Acta Derm Venereol. 1997; 77(6): 463-466. Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs topical psoralen plus UV-A. Arch Dermatol. 1997; 133(12): 1525-1528. Grimes PE. Vitiligo. An overview of therapeutic approaches. Dermatol Clin. 1993; 11(2): 325-338. Park HS, Lee YS, Chun DK. Squamous cell carcinoma in vitiligo lesion after longterm PUVA therapy. J Eur Acad Dermatol Venereol. 2003 Sep; 17(5): 578-80. Vitiligo Jul 14 23 28. Schallreuter KU, Behrens-Williams S, Khaliq TP, Picksley SM, Peters EM, Marles LK, Westerhof W, Miehe B, Fanghanel J. Increased epidermal functioning wildtype p53 expression in vitiligo. Exp Dermatol. 2003 Jun; 12(3): 268-77. 29. Schallreuter KU, Tobin DJ, Panske A. Decreased photodamage and low incidence of non-melanoma skin cancer in 136 sun-exposed caucasian patients with vitiligo. Dermatology. 2002; 204(3): 194-201. 30. Le Gal FA, Avril MF, Bosq J, Lefebvre P, Deschemin JC, Andrieu M, Dore MX, Guillet JG. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001 Dec; 117(6): 1464-70. 31. Seo SL, Kim IH. Squamous cell carcinoma in a patient with generalized vitiligo. J Am Acad Dermatol. 2001 Dec; 45(6 Suppl): S227-9. 32. Njoo MD, Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol. 2001; 2(3): 167-81. 33. Harrist TJ, Pathak MA, Mosher DB, Fitzpatrick TB. Chronic cutaneous effects of long-term psoralen and ultraviolet radiation therapy in patients with vitiligo. Natl Cancer Inst Monogr. 1984 Dec; 66:191-6. 34. Buckley DA, Rogers S. Multiple keratoses and squamous carcinoma after PUVA treatment of vitiligo. Clin Exp Dermatol. 1996 Jan; 21(1): 43-5. 35. Yashiro K, Nakagawa T, Takaiwa T, Inai M. Actinic keratoses arising only on sunexposed vitiligo skin. Clin Exp Dermatol. 1999 May; 24(3): 199-201. Important Notice General Purpose. Health Net's National Medical Policies (the "Policies") are developed to assist Health Net in administering plan benefits and determining whether a particular procedure, drug, service or supply is medically necessary. The Policies are based upon a review of the available clinical information including clinical outcome studies in the peer-reviewed published medical literature, regulatory status of the drug or device, evidence-based guidelines of governmental bodies, and evidence-based guidelines and positions of select national health professional organizations. Coverage determinations are made on a case-by-case basis and are subject to all of the terms, conditions, limitations, and exclusions of the member's contract, including medical necessity requirements. Health Net may use the Policies to determine whether under the facts and circumstances of a particular case, the proposed procedure, drug, service or supply is medically necessary. The conclusion that a procedure, drug, service or supply is medically necessary does not constitute coverage. The member's contract defines which procedure, drug, service or supply is covered, excluded, limited, or subject to dollar caps. The policy provides for clearly written, reasonable and current criteria that have been approved by Health Net’s National Medical Advisory Council (MAC). The clinical criteria and medical policies provide guidelines for determining the medical necessity criteria for specific procedures, equipment, and services. In order to be eligible, all services must be medically necessary and otherwise defined in the member's benefits contract as described this "Important Notice" disclaimer. In all cases, final benefit determinations are based on the applicable contract language. To the extent there are any conflicts between medical policy guidelines and applicable contract language, the contract language prevails. Medical policy is not intended to override the policy that defines the member’s benefits, nor is it intended to dictate to providers how to practice medicine. Policy Effective Date and Defined Terms. The date of posting is not the effective date of the Policy. The Policy is effective as of the date determined by Health Net. All policies are subject to applicable legal and regulatory mandates and requirements for prior notification. If there is a discrepancy between the policy effective date and legal mandates and regulatory requirements, the requirements of law and regulation shall govern. * In some states, new or revised policies require prior notice or posting on the website before a policy is deemed effective. For information regarding the effective dates of Policies, contact your provider representative. The Policies do not include definitions. All terms are defined by Health Net. For information regarding the definitions of terms used in the Policies, contact your provider representative. Policy Amendment without Notice. Health Net reserves the right to amend the Policies without notice to providers or Members. In some states, new or revised policies require prior notice or website posting before an amendment is deemed effective. No Medical Advice. Vitiligo Jul 14 24 The Policies do not constitute medical advice. Health Net does not provide or recommend treatment to members. Members should consult with their treating physician in connection with diagnosis and treatment decisions. No Authorization or Guarantee of Coverage. The Policies do not constitute authorization or guarantee of coverage of particular procedure, drug, service or supply. Members and providers should refer to the Member contract to determine if exclusions, limitations, and dollar caps apply to a particular procedure, drug, service or supply. Policy Limitation: Member’s Contract Controls Coverage Determinations. The determination of coverage for a particular procedure, drug, service or supply is not based upon the Policies, but rather is subject to the facts of the individual clinical case, terms and conditions of the member’s contract, and requirements of applicable laws and regulations. The contract language contains specific terms and conditions, including pre-existing conditions, limitations, exclusions, benefit maximums, eligibility, and other relevant terms and conditions of coverage. In the event the Member’s contract (also known as the benefit contract, coverage document, or evidence of coverage) conflicts with the Policies, the Member’s contract shall govern. Coverage decisions are the result of the terms and conditions of the Member’s benefit contract. The Policies do not replace or amend the Member’s contract. If there is a discrepancy between the Policies and the Member’s contract, the Member’s contract shall govern. Policy Limitation: Legal and Regulatory Mandates and Requirements. The determinations of coverage for a particular procedure, drug, service or supply is subject to applicable legal and regulatory mandates and requirements. If there is a discrepancy between the Policies and legal mandates and regulatory requirements, the requirements of law and regulation shall govern. Reconstructive Surgery California Health and Safety Code 1367.63 requires health care service plans to cover reconstructive surgery. “Reconstructive surgery” means surgery performed to correct or repair abnormal structures of the body caused by congenital defects, developmental abnormalities, trauma, infection, tumors, or disease to do either of the following: (1) To improve function or (2) To create a normal appearance, to the extent possible. Reconstructive surgery does not mean “cosmetic surgery," which is surgery performed to alter or reshape normal structures of the body in order to improve appearance. Requests for reconstructive surgery may be denied, if the proposed procedure offers only a minimal improvement in the appearance of the enrollee, in accordance with the standard of care as practiced by physicians specializing in reconstructive surgery. Policy Limitations: Medicare and Medicaid. Policies specifically developed to assist Health Net in administering Medicare or Medicaid plan benefits and determining coverage for a particular procedure, drug, service or supply for Medicare or Medicaid members shall not be construed to apply to any other Health Net plans and members. The Policies shall not be interpreted to limit the benefits afforded Medicare and Medicaid members by law and regulation. Vitiligo Jul 14 25