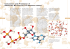Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism
Transcription
Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism
Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism (1) The mitochondrial membrane is densely packed with protein. Categorize each of the three independent, active transport proteins in the diagram below. (2) Identify the general type of transport which is described in the following examples. (a) Chloride ions pass through protein channels into cells under conditions where the extracellular chloride concentration exceeds the intracellular concentration. (b) Lactose is transported into cells through integral membrane proteins under conditions in which the intracellular concentration of lactose exceeds the extracellular concentration. (3) Identify the type of active transport which is describe in the following examples: (a) The Ca2+-ATPase pumps Ca2+ out of the cytosol as ATP is hydrolysed. (b) The (H+-K+)-ATPase of the gastric pumps protons out of the cell. Each proton is accompanied by the transport of a K+ into the cell. (c) Subsequently to the action of the describe (H+-K+)-ATPase, the K+ is again transported out of the cell in parallel to the export of Cl- by a different transporter. (4) Draw the structures of the following carbohydrates: (a) As many D-aldopentoses as possible in the linear form (b) A ketohexose in the linear and cyclic, α-anomeric form (c) A disaccharide of two riboses connected by a (β1-α1) glycosidic bond. (d) A disaccharide building block of the polysaccharide amylose. (5) The transformed, standard free energy change for the formation of glucose-6phosphate from glucose and phosphate is +13.8 kJ/mol. Assuming T=37º C, are there any conditions under which this reaction will occur? If so, quantitatively describe the conditions. 1 Biochemistry 2000 (6) Sample Questions 5 Transport, Carbohydrates, Metabolism What is the chemical phosphoenolpyruvate (PEP)? basis of the high energy compound, (7) Fill in the blanks in the following statements regarding glycolysis. (a) Fructose-1,6-bisphosphate, a six carbon sugar is split into two three carbon sugars by the enzyme ____________________. (b) Enzymes that generate reduced electron carriers (ie. NADH) are called ____________________. (c) Each molecule of glyceraldehyde-3-phosphate generates ________ ATP and ________ NADH. (d) The energetically least favourable reaction in glycolysis is catalyzed by ________________. (8) Glucose-6-phosphate isomerase catalyzes the interconversion of glucose-6phosphate (an aldose) and fructose-6-phosphate (a ketose). Draw the structure(s) of the reaction intermediate(s). (9) Which glycolytic reactions are energetically most favorable and why? (10) In the overall reaction describing glycolysis, 2 H2O are released. Which enzyme(s) are responsible for the dehydration reaction(s)? (11) You have provided ATP containing a radioactively labelled terminal phosphate to a system undergoing glycolysis. Which of the glycolytic intermediates will be radioactively labelled? (12) A certain metabolic reaction takes the form A -> B. Its standard transformed free energy change is 7.5 kJ mol-1. (R = 8.3145 J K-1 mol-1) (a) Calculate the equilibrium constant for the reaction at 25°C. (b) Calculate ∆G at 37°C when the concentration of A is 0.5 mM and the concentration of B is 0.1 mM. Is the reaction spontaneous under these conditions? (c) How might the reaction proceed in the cell? 2 Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism (13) Choose the best definition for a near-equilibrium reaction: (a) always operates with a favorable free energy change (b) has a free energy change near zero (c) is usually a control point in a metabolic pathway (d) operates very slowly in vivo. (14) Creatine kinase catalyzes the following reaction: ATP + creatine phosphocreatine + ADP, ∆G°’ = + 12.6 kJ mol-1 Predict wether creatine kinase will operate in the direction of ATP synthesis or phosphocreatine synthesis at 25°C when [ATP] = 4 mM, [ADP] = 0.15 mM, [phosphocreatine] = 2.5 mM, and [creatine] = 1 mM. (15) Which reaction in glycolysis is catalyzed by the following enzyme? Provide names of all reactants and products (no structures required). (a) Aldolase (b) Hexokinase (c) Pyruvate Kinase (16) Glycolytic Enzymes II: (a) List the glycolytic enzymes which consume ATP. (b) List the glycolytic enzymes which produce ATP. (17) What is the net reaction of glycolysis for 2 glucoses? (18) Phosphohexose isomerase converts Glucose-6-phosphate to Fructose-6phosphate. Write down the structures of both substrate and product. (19) Energetics of glycolysis: Explain in general words which reactions in glycolysis are favourable, which operate at equilibrium and which are unfavourable. (20) ∆G°’ for the aldolase reaction is 22.8 kJ mol-1. In the cell at 37°C, there is the following ratio [DHAP]/[GAP] = 5.5. Calculate the equilibrium ratio of [FBP]/[GAP] when [GAP] = 10-4 M. (abbreviations: DHAP = dihydroxyacetone phosphate, GAP = glyceraldehyde3-phosphate, FBP = fructose-1,6-bisphosphate) (21) Put the following events in the correct order of their occurrence: Citric acid cycle, digestion, oxidative phosphorylation, glycolysis (22) Give brief definitions or unique descriptions of the following terms: (a) Pyranose (b) Mutarotation (c) anabolism (d) ∆G°' (e) substrate level phosphorylation (f) energy coupling (g) Kinase 3 Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism (23) Answer each of the following questions about carbohydrates: (a) Classify or give the general name of the following carbohydrate based upon its functional group, number of C-atoms and stereochemistry. CH2OH O HO H H OH CH2OH (b) Draw the linear structure of L-glucose (the enantiomer of D-glucose). (c) Draw the cyclic, β-anomeric structure of L-glucose. 4 Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism Answers: (1) (Left) Electrogenic uniporter, (Middle) Electrogenic antiporter, (Right) Electroneutral symporter (2) Transport (a) Mediated, passive transport (b) Mediated, active transport Active Transport (1) electrogenic uniport (2) electroneutral antiport (3) electroneutral symport (3) Carbohydrates a. D-Aldopentoses b. Linear and cyclic ketohexose (α-anomeric form), e.g. fructose c. two riboses connected by a (β1-α1) glycosidic bond HO O α-D ribose HO HO OH O O β-D ribose HO OH d. Amylose 5 Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism (4) Yes as ∆G’ not ∆Gº’ determines whether a reaction proceeds. ∆G' = ∆G'º + RT ln ([products]/[reactants]) 0 > 13.8 kJ/mol + 2.57 kJ/mol ln ([products]/[reactants]) -5.35 > ln ([products]/[reactants]) [products]/[reactants] < 4.7 x 10-3 Provided the reactants are present at greater than 213 fold excess the reaction will proceed. (5) The chemical basis of the high energy of PEP is the same as for ATP - the relief of charge repulsion - resonance stabilization of released Pi (and pyruvate) - solvation energy difference (7) Fill in the blanks: (a) Aldolase (b) Dehydrogenases (c) 2 ATP and 1 NADH (d) Aldolase (~23 kJ/mol) (8) (9) The reactions catalyzed by kinases all have large negative standard transformed free energies due to the hydrolysis of high energy bonds. These are reactions Reaction 1 3 7 10 Enzyme hexokinase phosphofructokinase phosphoglycerokinase pyruvate kinase Substrate (sugar) glucose F6P G3P PEP Product (sugar) G6P F1,6BP 1,3BPG pyruvate (10) Enolase (11) The phosphate added to glucose in step 1 of glycolysis is removed in step 10. As a result all intermediates in glycolysis, except glucose and pyruvate, will contain radioactive phosphate. 6 Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism (12) Standard free energy and equilibrium constants: a. Since ∆G°’ = -RT ln K, K = e (-∆G°’ / RT) , T = 298 K (25 °C) K = e -7500 J mol-1 / (8.3145 J K-1 mol-1) (298K) = 0.048 b. ∆G = ∆G°’ + RT ln [B]/[A] = 7500 J mol-1 + (8.3145 J K-1 mol-1) (310 K) ln (0.0001/0.0005) = 7500 J mol-1 – 4150 J mol-1 = 3.35 kJ mol-1 The reaction is not spontaneous since ∆G >0. c. The reaction can proceed in the cell if the product B is the substrate for a second reaction such that the second reaction continually draws off B, causing the first reaction to continually produce more B from A. (13) (b) has a free energy change near zero (14) ∆G = ∆G°’ + RT ln ([phosphocreatine] [ADP] / [creatine] [ATP]) = 12.5 kJ mol-1 + (8.3145 J K-1 mol-1) (298K) ln (2.5mM * 0.15 mM / 1 mM * 4 mM) = 12.6 KJ mol-1 – 5.9 kJ mol-1 = 6.7 kJ mol-1 Since ∆G >0, the reaction will proceed in the opposite direction as written in the quesiton, that is, in the direction of ATP synthesis. (15) Glycolytic enzymes: (a) Aldolase catalyzes the cleavage of fructose-1,6-bisphosphate to dihydroxyacetone phosphate and glyceraldehyde-3-phosphate. (b) Hexokinase catalyzes phosphorylation of Glucose at C6. Thus, it uses glucose and ATP to produce Glucose-6-phosphate and ADP. (c) Pyruvate kinase uses phosphoenolpyruvate (PEP) and ADP to generate pyruvate and ATP (16)Glycolytic enzymes II: (a) Hexokinase and Fructokinase consume ATP. (b) Phosphoglyceratekinase and Pyruvate Kinase generate ATP. (17) 2 glucose + 4 NAD+ + 8 ADP + 8 Pi -> 4 pyruvate + 4 NADH + 4 H+ + 8 ATP + 4 H2O (18) Phosphohexose isomerase: 7 Biochemistry 2000 Sample Questions 5 Transport, Carbohydrates, Metabolism (19) All reactions catalyzed by kinases, i.e. all phosphotransferyl reactions are highly favorable since the phosphate is transferred from a high energy donor to an acceptor. All isomerizations work at equilibrium, i.e. ∆G’o is close to zero as both substrate and product have comparable free energy. The three “special” reactions catalyzed by aldolase, glyceraldehyde-3-phosphate dehydrogenase and enolase are unfavorable. These are driven by removal of the product by the favorable phosphoryltransfer reactions. (20) When [GAP] = 10-4 M, [DHAP] = 5.5 x 10-4 M. ∆G°’ = -RT ln K, thus K = e (-∆G°’/RT) = [GAP] [DHAP] / [FBP] = e (– 22,800 J mol-1) / 8.3145 J K-1 mol-1) (310K) = 1.4 x 10-4 Thus: (10-4) (5.5 x 10-4) / [FBP] = 1.4 x 10-4 Thus: [FBP] = 3.8 x 10-4 M And [FBP]/[GAP] = (3.8 x 10-4 M) / (10-4 M) = 3.8 (21) (1) digestion, (2) glycolysis, (3) citric acid cycle, (4) oxidative phosphorylation (22) Definitions: (a) A pyranose is a cyclic monosaccharide with a 6-membered ring. (b) Mutarotation is the interconversion of the α- and β-anomeric forms of a cyclic monosaccharide. (c) The synthesis of complex molecules from simple building blocks and energy. (d) Standard transformed free energy change (e) Phosphoryl transfer reactions that generate ATP in glycolysis. (f) Sequential reactions sharing common intermediates have additive standard free energy changes (g) Kinases are enzymes that transfer a phosphoryl group from a high energy donor to an acceptor (i.e. consume or produce ATP). (23) (a) D-ketopentose (b) H (c) O C HO HO HO H H OH HO H HO H HO CH2OH 8 O OH HO