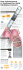Cavotricuspid isthmus angiography predicts atrial
Transcription
Cavotricuspid isthmus angiography predicts atrial
Clinical research European Heart Journal (2006) 27, 1833–1840 doi:10.1093/eurheartj/ehl121 Arrhythmia/electrophysiology Cavotricuspid isthmus angiography predicts atrial flutter ablation efficacy in 281 patients randomized between 8 mm- and externally irrigated-tip catheter ´cile Romeyer-Bouchard1, Virginie Dauphinot2, Damien Lipp1, Antoine Da Costa1*, Ce 1 ´ro ˆme The ´venin1, Jean-Claude Barthe ´le ´my2, and Karl Isaaz1 Loucif Abdellaoui , Marc Messier3, Je 1 Department of Cardiology, Faculty of Medicine J. Lisfranc, The Jean Monnet University, 42 055 Saint-Etienne, Cedex 2, France; 2 Clinical Physiology and Exercise Laboratory, The Jean Monnet University, 42000 Saint-Etienne, France; and 3 The Bakken Research Center, 5 Endepolsdomein, 6229 GW Maastricht, The Netherlands Received 3 March 2006; revised 27 May 2006; accepted 1 June 2006; online publish-ahead-of-print 28 June 2006 KEYWORDS Aims Radiofrequency ablation (RFA) of cavotricuspid isthmus (CTI)-dependent atrial flutter can be performed using various types of ablation catheters. Recent evaluations comparing externally cooledtip RFA (ecRFA) catheters and large-tip (8 mm) catheters found that ecRFA catheter may have a higher efficacy for CTI ablation. The aim of this prospective study was to compare both catheters by stratifying on CTI morphology in order to explain, in part, the discrepancies between previous randomized studies, and to validate predictive factors of difficult CTI ablation on clinical, echocardiographic, and angiographic data. Methods and results Over a period of 24 months, 281 patients were included and stratified on CTI morphology: ‘straight’, ‘concave’, and ‘pouch-like recess’. In straight CTI (n ¼ 150), the duration of application time with a median of 6 min [interquartile range (IQR) 4–9] vs. a median of 12 min (IQR 16–19; P , 0.0001) and the duration of X-ray exposure with a median of 6 min (IQR 4.4–9.7) vs. a median of 10.4 min (IQR 7–17; P , 0.0001) were significantly lower with an 8 mm-tip when compared with ecRFA catheter. In contrast, in concave CTI (n ¼ 95), a trend towards both shorter application time with a median of 12.5 min (IQR 6–23) vs. a median of 19 min (IQR 7–28; P ¼ 0.08) and X-ray duration exposure with a median of 10.4 min (IQR 6–20) vs. a median of 13 min (IQR 8–24; P ¼ 0.08) with an ecRFA catheter when compared with 8 mm-tip catheter were evidenced. No significant difference was shown between 8 mm-tip and ecRFA catheters in the pouch-like recess group (n ¼ 36). Predictive factors of difficult ablation include right CTI length and morphology. Conclusion This study demonstrates that the 8 mm-tip catheter is more effective for ablation in case of a straight angiographic isthmus morphology and that the ecRFA catheter tends to be more effective in case of concave angiographic isthmus morphology. Thus, angiographic isthmus evaluation may predict both the effectiveness of an RF catheter, and the risk of an expensive crossover. These data may explain, in part, the discrepancies of previous studies comparing both catheters. Introduction Radiofrequency catheter ablation (RFA) of the cavotricuspid isthmus-dependent atrial flutter (CTI-AFL) is the optimal treatment from the point of view of its high efficacy.1–4 Despite this high success rate, ablation of the CTI can be extremely difficult.5–7 Multiple factors that affect the lesion size8–16 include tissue contact,8,9 impedance,10 temperature at the tissue–electrode interface,10 blood flow at the catheter–tissue interface,14 power and duration of * Corresponding author. Tel: þ33 4 77 82 83 40; fax: þ33 4 77 82 84 51. E-mail address: [email protected] energy application,13 catheter orientation (perpendicular or parallel),13,15 intracavitary blood flow,16 electrode–target distance,16 electrode tip size, irrigation design, and more recently CTI morphology.5–7 The 8 mm-tip and the externally cooled-tip radiofrequency ablation (ecRFA) catheters are most widely used to treat AFL.17–23 Unfortunately, studies comparing cRFA and 8 mm-tip catheters showed questionable results because of small cohorts,19 irrigation design that may influence the results,17,19 endpoint differences,22 and CTI anatomy.5–7 A recently published meta-analysis demonstrating the equivalence between 8 mm-tip and cRFA catheters disregarded the influence of ecRFA catheters.20 Open irrigated catheters seem to have greater & The European Society of Cardiology 2006. All rights reserved. For Permissions, please e-mail: [email protected] Downloaded from by guest on October 15, 2014 Atrial flutter; Catheter ablation; Externally cooled-tip catheter; Large-tip catheter; Angiography; Structure; Anatomy 1834 efficacy than solid large 8 mm electrode, in which they dissociate the power produced from the local convective effect, thus allowing the delivery of higher and more stable power, causing larger and deeper lesions.15,24,25 In contrast, experimental studies with large electrodes (8 mm-tip) showed an increase in both the cooling and the electrode–tissue interface that induced larger and deeper lesions, with this effect dependent on catheter orientation.21 Several studies have highlighted the influence of anatomy on RFA parameters, yet, catheter assessments have not stratified on tissue morphology.5–7 There was thus a need for a prospective randomized comparison of the efficacy and safety of ecRFA and 8 mm-tip catheters with randomized CTI anatomy to complement our study with long CTIs.7 No large prospective study is available evaluating predictive factors of difficult CTI RFA. The aim of this prospective study was two-fold: (1) to compare the efficacy of an 8 mm-tip and an ecRFA by considering CTI morphology and (2) to evaluate predictive factors of difficult CTI AFL RFA. Methods Study population The methodology of the right atrial angiography Biplane angiography was performed after mapping, shortly before RF energy delivery. An isthmogram was made by positioning a 5-F pigtail catheter in the IVC.5–7 Contrast solution (50 cc) was injected for 3–5 s (right anterior view at 258). The angiograms were digitally acquired, allowing replay and storage. Measurements were calibrated by inter-electrode spaces projecting perpendicular to the given cine-view. The length of the CTI was obtained at 258 in the RAO projection between the IVC and the lower hinge point of the tricuspid valve.5–7 CTI length measurements and morphology analyses were made on the last atrial diastolic frame (confirmed by tricuspid valve opening on the next frame). The perpendicular distance between the line connecting the IVC (A) and the lower hinge point of the tricuspid valve (B) with the deepest point of the isthmus was measured.6 Patient groups were classified by CTI length (short 32 mm or long .32 mm)5,6 and further sub-divided in CTI morphology as straight, concave, or with a pouch-like recess.6,7 The straight aspect was defined as a maximal distance between A and B with an isthmus depth 2 mm (Figure 1). The concave aspect was defined as a maximal distance between A and B with an isthmus depth .2 mm with a concave CTI aspect (Figure 2). When the isthmus could be divided into a recess (inferoposterior to the CS ostium) and a flat vestibule (between this recess and the tricuspid annulus), the CTI aspect was defined as a pouch-like recess (Figure 3).5,6 An independent operator (BS) performed all measurements and analyses. Catheter ablation Catheter randomization was performed on the entire population after right atrium angiography. A radiofrequency current (un-modulated, sine wave) was delivered in the unipolar mode between the distal ablation catheter tip and a cutaneous patch electrode placed over the left scapula. RF delivery was applied point-by-point by the same operator (ADC) and was started at the ventricular aspect of the tricuspid annulus when a stable electrogram with a small atrial and large ventricular amplitude was observed. The central part of the isthmus was considered the Electrophysiological study Two catheters were introduced percutaneously through the right femoral vein into the right atrium. A 6-F quadripolar catheter with an inter-electrode distance of 5 mm (Bard Electrophysiology, Tewksbury, MA, USA) was advanced to the His-bundle position; then a dodecapolar catheter with a 5 mm bipolar separation (Bard) was positioned in the coronary sinus (CS). The distal tip was placed in the CS ostium with electrodes 1,2 (H1), 3,4 (H2), 5,6 (H3), and 7,8 (H4) close to the IVC and tricuspid isthmus, while electrodes 9,10 (H5) and 11,12 (H6) recorded the low and high right atrial activation, respectively. All measurements were performed with the Cardiolab system (Prucka Engineering). Figure 1 Short straight isthmus. Angiographic visualization of isthmus in RAO during contrast injection. Isthmus width measured between IVC (A) and lower hinge point of tricuspid valve (B). Figure shows an isthmus with straight appearance (maximal distance between line A and B and the isthmus is 2 mm) of average width 25.8 mm. Downloaded from by guest on October 15, 2014 All patients provided written informed consent. From November 2003 to October 2005, 332 consecutive patients with CTI AFL were referred for RFA. AFL was diagnosed when: (1) the surface electrocardiogram (EKG) showed flutter waves that were predominantly negative in leads II, III, aVF, and positive in lead V1 (counterclockwise AFL) or positive in leads II, III, aVF, and negative in lead V1 (clockwise AFL), with a regular atrial rate between 240 and 340 bpm; (2) the intracardiac EKG displayed the following activation sequence for counterclockwise AFL: high right atrium, low right atrium, a counterclockwise inferior vena cava (IVC)-tricuspid isthmus activation sequence followed by left atrial activation established with a dodecapolar lead. The opposite activation sequence applied to clockwise AFL.3 Isthmus involvement of the arrhythmic circuit was demonstrated by entrainment manoeuvres (concealed entrainment in the isthmus). Exclusion criteria were described elsewhere.10 Catheter randomization between 8 mm-tip and ecRFA catheters was performed, with stratification, in three isthmus anatomic groups: ‘straight’, ‘concave’, and ‘pouch-like recess’.6,7 The prospective study was designed to demonstrate a 40% reduction in fluoroscopy time exposition comparing externally irrigated and 8 mm-tip catheter ablation according to the anatomical status, with 90% power. This required, for each anatomical category, 44 patients for each catheter ablation type, thus giving 88 patients in each anatomical CTI. The size (means difference expected 7 min) and the standard deviation (10 min) of the fluoroscopy time exposition, used for the sample calculation, were chosen using published data in similar studies.7,17,19 A. Da Costa et al. AFL ablation catheters Figure 2 Short concave isthmus aspect; illustrates a concave appearance (maximal distance between line A and B and the isthmus is .2 mm) of average width 26 mm and 4.2 mm depth. 1835 Echocardiographic measurements Transthoracic Doppler Echocardiography (TTE) was performed within 24 h after the electrophysiological procedure by an observer (DL) blinded to the patients’ electrophysiological status. Ultrasound studies were performed with a Vivid 7 imaging system using a 2.5-MHz transducer (second harmonic activation). M-mode and bidimensional measurements were made according to the recommendations of the American Society of Echocardiography. The left ventricular (LV) ejection fraction was calculated by the modified Simpson rule. Statistical analysis Figure 3 Short pouch-like recess isthmus; shows a flat vestibular structure against the tricuspid annulus and a pouch-like recess long near the IVC. optimal site to ablate (inferior isthmus). This site of placement was chosen to avoid the septal portion of the isthmus to preclude both the AV block risk and very difficult ablation in a subset of patients with pouches. RF delivery was applied until a bidirectional isthmus block was obtained. Two types of catheters were used: either an 8-F quadripolar bidirectional deflectable catheter with an 8 mm-tip electrode (BLAZER II large curve XP 4500 TK2 Boston EP-Technologies, San Jose, USA) used with a maximum power output of 70 W and a maximum target temperature of 608 for 60 s or an externally irrigated 5 mm-tip thermocouple catheter (Cordis-Biosense-Webster Thermocool-F-curve, Diamond Bar, USA) with a temperature-controlled RFA delivery with a maximum power output of 50 W and temperature limit of 45–508C applied for 60 s at each point. A saline solution (0.9%) was infused through the irrigated catheter with a Gemini pump (battery powered to Statistical analysis was performed using the software packages SPSS 12.OF (SPSS Inc., Chicago, IL, USA). Values were given as mean + SD, if a normal distribution was validated. Otherwise median and IQR were used. To take into account the randomization and the stratification, the interaction between the two was assessed, validating the stratification. If the distribution of the values were skewed, the values were log-transformed before statistical analysis. We tested systematically normality distribution by evaluating the heteroscedasticity of variance using the Levene test. If normality was proven, the differences among groups were analysed using MANOVA for effectiveness variables: the number of applications, the X-ray exposure, and the procedure duration. A two-sided probability value of P , 0.05 was defined as statistically significant. Clinical, TTE, and right atrial angiographic variables were tested to predict difficult ablation procedure pre-defined by greater than 20 RFA unsuccessful applications (cumulated time of 1200 s). Predictive factors of difficult ablation procedure were assessed by unpaired t-test. Results Study population During 24-months, 332 consecutive patients were considered eligible, 51 patients had an exclusion criteria and Downloaded from by guest on October 15, 2014 avoid 50 Hz line noise) at a mean rate of 17 mL/min during RFA delivery (20–40 mL/min). Between applications, a flow rate of 3 mL/min was used to maintain patency.17 When over 20 RFA applications (cumulating 1200 s) were unsuccessful, the alternative catheter was used. The procedure endpoint was defined as a complete bidirectional isthmus block as described elsewhere.1,2 Reversal of the right atrial depolarization sequence was established by a complete cavotricuspid map using a multipolar mapping catheter straddling the line of block,3 and by recording widely separated local double potentials along the ablation line during atrial pacing.26 A differential pacing was performed to differentiate complete or incomplete bidirectional isthmus block.27 When CTI block was not achieved after the first ablation line, the conduction gaps were mapped and ablated by searching for single or narrow-split potentials with the ablation catheter moving along the original ablation line. When signs of conduction block were observed during RF application with proximal CS pacing, one extra RF application lasting 1 min was performed at that site. Pacing was performed at a cycle length of 600 ms from the proximal CS and the low lateral right atrium. The status of the bidirectional block was assessed continuously over the 30 min period following bidirectional block occurrence. On occasion, conduction resumed after ablation, which re-initiated a complete RF ablation sequence until the bidirectional block could be observed again, resetting the 30 min waiting period. Cumulative time of RF delivery was recorded and fluoroscopy time was calculated as total fluoroscopy time used for catheter positioning and RF ablation, including the time to reach bidirectional block. 1836 A. Da Costa et al. Table 1 Stratification of the randomization by CTI groups 8 mm-tip vs. ecRFA catheter Straight CTI group (n ¼ 150) (n ¼ 78 vs. n ¼ 72) Concave CTI group (n ¼ 95) (n ¼ 50 vs. n ¼ 45) Pouch-like recess CTI group (n ¼ 36) (n ¼ 20 vs. n ¼ 16) P-value (8 mm vs. ecRFA tip catheters) Age (year + SD) 67 + 12 vs. 68 + 12 (P ¼ 0.8) 15/78 (19%) vs. 21/72(29%) (P ¼ 0.2) 59 + 10 vs. 56 + 13 (P ¼ 0.2) 32/78 (41%) vs. 33/72 (46%) (P ¼ 0.7) 37/78 (47%) vs. 36/72 (50%) (P ¼ 0.9) 4.4 + .7 vs. 4.4 + .6 (P ¼ 0.9) 25+6 vs. 26+6 (P ¼ 0.3) 10/77 (13%) vs. 11/68 (16%) (P ¼ 0.8) 31 + 11 vs. 30 + 10 P ¼ 0.6 33/78 (42%) vs. 32/72 (44%) (P ¼ 0.9) 68 + 10 vs. 69 + 10 (P ¼ 0.7) 15/50 (30%) vs. 10/45 (22%) (P ¼ 0.5) 59 + 12 vs. 56 + 11 (P ¼ 0.3) 26/50 (52%) vs. 17/45 (38%) (P ¼ 0.2) 25/50 (50%) vs. 20/45 (55%) (P ¼ 0.7) 4.4 + .6 vs. 4.6 + .7 (P ¼ 0.2) 31+7 vs. 30+7 (P ¼ 0.5) 11/48 (23%) vs. 9/41 (22%) (P ¼ 0.9) 36 + 12 vs. 36 + 11 P ¼ 0.8 22/50 (44%) vs. 12/45 (27%) (P ¼ 0.1) 68 + 10 vs. 63 + 14 (P ¼ 0.2) 5/20 (25%) vs. 1/16 (7%) (P ¼ 0.3) 62+8 vs. 58+8 (P ¼ 0.1) 11/20 (55%) vs. 7/16 (47%) (P ¼ 0.7) 10/20 (50%) vs. 11/16 (68%) (P ¼ 0.3) 4.7+1 vs. 4.7 + .7 (P ¼ 0.8) 30+5 vs. 30+4 (P ¼ 0.9) 6/20 (30%) vs. 3/15 (20%) (P ¼ 0.8) 32 + 11 vs. 30+9 P ¼ 0.6 11/20 (55%) vs. 2/16 (13%) (P ¼ 0.01) 0.4 Gender (% women) LVEF Pre-ablation history of Afib Structural heart disease (%) Left atrial size (mm) CTI (mm) Tricuspid regurgitation 3 Systolic pulmonary pressure (mmHg) Anti-arrhythmic drugs discharge 0.9 0.1 0.5 0.8 0.5 0.3 0.9 0.5 0.1 LVEF, left ventricular ejection fraction. 8 mm-tip vs. ecRFA: No difference were found for baseline characteristics between the randomized Irrigated-tip and 8 mm-tip group LVEF. AFL ablation results Angiographic analyses Anatomic morphology was assessed: 150 patients had a straight CTI, 95 patients had a concave CTI with a mean depth of 6.7 + 2.7 mm (from 2.6 to 11.7 mm), 36 patients presented a pouch-like recess with a mean depth of 8.2 + 2.4 mm (from 2.1 to 12.4 mm) (Table 2). X-ray exposure (P , 0.0001) were significantly lower with an 8 mm-tip catheter when compared with an ecRFA catheter in straight CTI (n ¼ 150) (Figures 4 and 5). In contrast, in concave CTI (n ¼ 95), a trend toward both shorter application time and X-ray exposure (P ¼ 0.08) with an ecRFA catheter was evidenced (Figures 4 and 5). No significant difference was shown between 8 mm-tip and ecRFA catheters in the pouch-like recess group (n ¼ 36). The number of audible ‘pop’ did not differ between 8 mm and ecRFA groups in both straight (n¼6 vs. n ¼ 7; P ¼ 0.7) and pouchlike recess CTI (n¼1 vs. n ¼ 2; P ¼ 0.2), but were significantly greater in concave CTI with an 8 mm group when compared with the ecRFA catheter group (n¼9 vs. n ¼ 2, P ¼ 0.03). Long isthmii (n ¼ 73) required similar RF application time (23 + 16 vs. 23 + 22) and X-ray exposure (19 + 13 vs. 21 + 21) for the ecRFA (n ¼ 36) and 8 mm-tip (n ¼ 37) catheters (Table 2). Predictive factors of difficult CTI RFA RF ablation results Bidirectional block was obtained in 99% of cases with a mean RF application time of 15 + 15 min and mean fluoroscopic time of 14 + 13 min. There were two significant procedure-related complications: one false arterial femoral aneurysm requiring surgical treatment and a stroke with incomplete recovery in a 78-year-old woman because of an AF episode 12 h after the RFA despite anticoagulation. RF results with 8 mm-tip and ecRFA catheters The Levene test was significant for straight (P ¼ 0.03) and concave CTI (P ¼ 0.01) but not significant for pouch-like recess group (P ¼ 0.3). When target tissue morphology was considered, the total RF applications and the length of Difficult ablation requiring .20 min of application appeared in 66 patients (Tables 3 and 4). Predictive factors include CTI length (32+7 vs. 27 + 6 mm; P , 0.0001), concavity depth (7.4+3 vs. 6.4 + 2.5 mm; P ¼ 0.04), longitudinal (5.7 + 0.8 vs. 5.3 + 0.7; P ¼ 0.003) and transversal (4.5 + 0.8 vs. 4.1 + 0.7; P ¼ 0.005) RA dimensions, LVEF (54.5 + 11 vs. 60 + 11; P ¼ 0.008), systolic arterial pulmonary pressure (37 + 11 vs. 32 + 11 mmHg; P ¼ 0.005), isthmus morphology [‘straight’ (11%) vs. ‘concave’ (39%) or ‘pouch-like recess’ (33%); P , 0.0001], short (,32 mm) or long CTI (32 mm) (17% vs. 42%; P , 0.0001) and evidence of grade 3 or 4 tricuspid regurgitation (46 vs. 19%, P ¼ 0.0002). In the subset with ecRFA catheter (n ¼ 33), these difficult ablations showed a trend towards both Downloaded from by guest on October 15, 2014 281 gave their written consent. Patients’ characteristics are summarized in Table 1. At the beginning of the procedure, 208 patients exhibited AFL, 60 sinus rhythm, and 13 atrial fibrillation (AF). Patients showing sinus rhythm underwent burst pacing in the CS or the low right lateral atrium at cycle lengths as short as 180 ms to induce a flutter. Sinus rhythm was restored by electric cardioversion (internal or external) for patients in AFib (n ¼ 13) and the decision to ablate these was based on a documented EKG of typical AFL. AFL ablation catheters 1837 Table 2 RFA results of the MANOVA comparing 8 mm-tip to ecRFA on CTI groups Appl. duration time for validated RFA (min) Straight CTI group Concave CTI group Pouch CTI group (n ¼ 36) X-ray exposure (min) Straight CTI group Concave CTI group Pouch CTI group (n ¼ 36) Procedure duration (min) Straight CTI group Concave CTI group Pouch CTI group (n ¼ 36) 8 mm-tip catheter group Irrigated tip catheter group X + SD (range) Median (IQR) X + SD (range) Median (IQR) P-value 8 + 8 (2–60) 24 + 22 (3–86) 15 + 18 (4–80) 6 (4–9) 19 (7–28) 9 (6–16) 14 + 10 (3–56) 16 + 13 (3–56) 22 + 19 (3–60) 12 (6–19) 12.5 (6–23) 18.5 (5–36) ,0.001 0.08 0.3 9+9 (1–51) 20 + 19 (2–85) 15 + 19 (3–75) 6 (4.4–9.7) 12.5 (8–24) 8.4 (6–12) 13 + 9 (2–47) 14 + 10 (2–40) 21 + 15 (4–51) 10.4 (7–17) 10.4 (6–20) 20 (6–31) ,0.001 0.08 0.2 61 + 16 (31–120) 83 + 33 (35–180) 72 + 30 (48–155) 55 (50–71) 75 (55–103) 62 (53–81) 71 + 21 (40–150) 74 + 23 (50–141) 86 + 35 (45–140) 70 (57–80) 70 (57–92) 71 (60–126) ,0.001 0.1 0.2 8 mm-tip vs. ecRFA. Values are presented as mean + SD (X + SD) and median (IQR). Significant difference (P-value 0.05) found between groups were calculated on log-transformed values. Figure 5 This box plot shows that a greater effect exists with the 8 mm-tip catheter in straight CTI morphology when compared with the externally cooled-tip catheter based on X-ray exposure. In contrast, there is a trend towards a greater effect of the externally cooled-tip catheter in concave CTI when compared with the 8 mm-tip catheter. Moreover, the cooling catheter seems to be less influenced by CTI anatomy. shorter RF application time (33 + 11 vs. 40 + 21, P ¼ 0.1) and shorter X-ray exposure (28 + 10 vs. 33 + 22, P ¼ 0.2) when compared with the 8 mm-tip catheter group (n ¼ 33). Role of 8 mm-tip catheter specificities in various CTI anatomies Discussion Major findings Our study demonstrates that the 8 mm-tip catheter is more effective for ablation in case of a straight angiographic isthmus morphology allowing to predict the effectiveness of a catheter, which is cheaper and easier to use and may prevent the risk of an expensive crossover to a second ablation catheter. In contrast, a trend towards a greater effectiveness was evidenced in concave CTI with an ecRFA catheter (P ¼ 0.08). These results may explain the controversial data of previous comparative studies on the efficacy of these two catheter types7,17–20,22,23 as CTI morphology was not taken into account then. Thus, angiographic isthmus evaluation may predict the effectiveness of an RF catheter. However, difficult AFL ablation occurs in approximately one-fourth of the patients, which is related to anatomic and haemodynamic factors. Previous randomized studies comparing large (8 mm) and cooled tip catheters in CTI AFL RFA have shown either equivalence or slightly superior results for ecRFA catheters.7,17–20,22,23 Some factors may explain these: sample sizes too small to highlight differences, catheter specificities that may have been different in various clinical situations, different endpoint definitions,22 and the impact of CTI anatomy.5–7 Multiple factors affecting the lesion size have been identified in experimental studies.8–16,21,24,25,28–36 Considering all these factors, we assumed that RFA lesions were expected to be deeper and larger with both cRFA and 8 mm-tip catheters, with variable results depending on CTI anatomy. Experimental studies had shown that the 8 mm-tip was the most effective and that even better results could be obtained in specific clinical situations.8–11,21 Two mechanisms were described to account for deeper RF lesions produced by larger electrodes in experimental studies:8–11,21 . an increase in convective cooling due to the larger surface exposed to the blood flow,21 which maintains a lower Downloaded from by guest on October 15, 2014 Figure 4 This box plot shows that a greater effect exists with the 8 mm-tip catheter in straight CTI morphology when compared with the externally cooled-tip catheter based on duration application time. In contrast, there is a trend towards a greater effect of the externally cooled-tip catheter in concave CTI when compared with the 8 mm-tip catheter. Moreover, the cooling catheter seems to be less influenced by CTI anatomy. 1838 A. Da Costa et al. Table 3 Difficult ablation procedure No. of appl. To interrupt AFL (min) No. of appl. for validated RFA [.300 ] (min) Number of conduction recurrence within 300 X-ray exposure (min) Procedure duration (min) Difficult procedure (n ¼ 66) (24%) Normal procedure (n ¼ 215) (76%) P-value 19 + 15 36.5 + 17 16/66 (24%) 30.3 + 17 103 + 27 4.8 + 4.4 8.3 + 5 31/215 (14%) 9+6 62 + 15 ,0.001 ,0.001 ¼0.001 ,0.001 ,0.001 Difficult ablation procedure defined as .20 RFA unsuccessful applications cumulating .1200 s (P , 0.05). Table 4 Significant predictive factors of difficult ablation procedure [defined by .20 RFA unsuccessful applications (cumulating 1200 s)] [P , 0.05] Difficult procedure (n ¼ 66) . 66 + 10 14/66 (21%) 28/66 (42%) 35/66 (53%) 4.6 + 0.7 32 + 7 7.4 + 3 54 + 11 5.7 + 0.8 4.5 + 0.8 23/65 (35%) 37 + 11 17/150 (11.3%) 37/95 (39%) 12/36 (33.3%) electrode–tissue interface temperature, thus allowing greater power to be delivered resulting in higher tissue current density and deeper direct resistive heating;21 an increase in electrode–tissue interface area.21 In both proposals the volume of resistive heating and lesion depth is increased.21 These may be reduced owing to the position of the catheter being perpendicular or parallel to the target tissue.12–16,21 The precise orientation of the ablation catheter (relative to the tissue) determines the size of the surface area in contact with the tissue and influences the geometry and size of the RFA lesions created.15 In straight CTI, creating a bidirectional block with an 8 mm-tip catheter, rapidly, can be related to the parallel positioning of the large electrode tip lying on its side, thus optimizing the electrode–tissue interface with a larger volume of resistive heating, and with a convective cooling effect improving the power delivered.12–14,16,21 Other factors seem to contribute to a better result in this subset, namely a constant high blood flow in the CTI region across the ablation electrode and a continuous sliding of the ablation electrode during RF application, which allows for continuous linear lesion.12–14,21,35 In such situations, the smaller electrode tip found on irrigated catheters may offset the potential advantages of irrigation. P-value 66 + 11 54/215 (25%) 98/215 (46%) 104/215 (48%) 4.4 + 0.7 27 + 6 6.4 + 2.5 60 + 11 5.3 + 0.7 4.1 + 0.7 27/204 (13%) 32 + 11 0.3 0.7 0.8 0.6 0.2 ,0.001 0.03 0.01 0.003 0.005 0.0001 0.005 133/150 (88.7%) 58/95 (61%) 24/36 (66.7%) ,0.0001 In contrast, the potential advantages of externally irrigated catheters had been demonstrated in cases of low local convective cooling where power output is reduced. It may be the case in concave CTIs.6,7 These advantages include a higher electrode resolution improving mapping accuracy and subsequently allowing gap detection particularly in difficult CTIs.36–38 This factor increases ablation efficacy and decreases the number of RF applications in concave CTIs. A shorter catheter tip increases catheter flexibility and mobility allowing access to difficult areas.15 All these factors contribute to a more stable level of power with the creation of larger lesions.15–17,19,20,24–29 When using cooled-tip electrodes, the duration of the application is a major determinant of the size of the lesion. This is important in difficult isthmii ablations, such as concave CTIs, where the required duration of RFA application is recognized to be longer than in straight CTIs.6,7 As electrode cooling is provided by irrigation in ecRFA, the voltage or power can be chosen and maintained independent of the local blood flow (‘extrinsic cooling’), leading to a more consistent and predictable lesion size.15,27 In contrast, the power delivered and the lesion depth in the temperature-control mode, without irrigation, varies greatly with local blood flow.15,27 In low-flow areas, such as inert concavities, the RFA delivery power is considerably Downloaded from by guest on October 15, 2014 Age year + SD Gender (% female) AFib history (%) Structural heart disease Left atrial size (mm) CTI length (mm) Concavity depth (mm) LVEF (%) Longitudinal right atrial dimension (mm) Transversal right atrial dimension (mm) Grade 3 or 4 tricuspid regurgitation Systolic pulmonary pressure (mmHg) Isthmus morphology Straight Concave Pouch-like recess Non-difficult procedure (n ¼ 215) AFL ablation catheters reduced, producing small non-transmural lesions.15,27 In concave CTIs, the advantages of an 8 mm-tip catheter may be lessened by a reduction in electrocardiographic resolution from the ablation electrode, whereas with a smaller catheter tip, the identification of gaps is enhanced.17,34,36,37 Another factor is a greater variability in electrode–tissue contact, depending on catheter tip orientation relative to the endocardium, likely to cancel the potential benefit of using a larger-tip catheter.15,28 In this complex isthmus architecture, the strictly parallel orientation to the endocardial surface for efficient contact pressure is almost impossible along the entire CTI and a long application using a point-by-point technique in the absence of optimal electrode–tissue contact may be counterproductive.34 Predictive factors of difficult CTI ablation Study limitations The main limitation of this study might be due to the absence of significant difference between both catheters when the isthmus had pouch-like recess. In contrast, when pouches occur, they are often found in the proximity to the thebesian valve and in the septal portion of the isthmus,5,6,31 while in our initial methodology, the site of placement of the ablation line was the inferior isthmus avoiding the septal zone. On another explanation, may be because of the absence of study power achievement in the subset of pouches with only 36 patients included, while 88 patients were expected. In addition, pouches form only a third part of the isthmus, the other part constituted by the vestibule is a straight area.5,6,31 Right atrial angiography provides a guide to CTI anatomy, but no detailed information on the thickness of the isthmus and the local vasculature.33,39 An RF application time at each point of maximal 60s is a clear disadvantage for the cooled-tip catheter, as it lasts much longer for the ecRFA catheter than for the 8 mm-tip catheter to reach the maximal power output. In case of straight CTI, the results for an ecRFA catheter might be expected to be better with a continuous application and the dragging method. This point should be taken into account in further studies evaluating ecRFA catheters. Furthermore, the different catheter designs between cooled-tip and 8 mm-tip catheters (size of the deflectable curve, rotation stability, and size of the distal non-steerable catheter part) might influence the comparison results. Perhaps, a better choice for evaluating both techniques would have been a comparison of 8 mm-tip and ecRFA tip catheters from the same company. The clinical implication may be an angiographic isthmus evaluation that may predict the effectiveness of an RF catheter, which is cheaper and easier to use (8 mm-tip catheter in the case of a straight CTI). However, this clinical implication is still hampered by the question, whether an individual angiographic evaluation is really superior to an empirical use of ablation catheters. A more relevant design would have been a catheter choice based on angiography in one set of patients when compared with a ‘controlled set’ with no angiography. Conclusions This study demonstrates that the 8 mm-tip catheter is more effective for ablation in case of a straight angiographic isthmus morphology and that the ecRFA catheter tends to be more effective in case of concave angiographic isthmus morphology. Angiographic isthmus evaluation may predict both the effectiveness of an RF catheter and the risk of an expensive crossover. These data may explain, to a certain extent, the discrepancies of previous studies comparing both catheters. Conflict of interest: none declared. References 1. Poty H, Saoudi N, Nair M, Anselme F, Letac B. Radiofrequency catheter ablation of atrial flutter. Further insights into the various types of isthmus block: application to ablation during sinus rhythm. Circulation 1996;94:3204–3213. 2. Cauchemez B, Haı¨ssaguerre M, Fischer B, Thomas O, Clementy J, Coumel P. Electrophysiological effects of catheter ablation of inferior vena cavatricuspid annulus isthmus in common atrial flutter. Circulation 1996;93:284–294. 3. Chen J, De Chillou C, Basiouny T, Sadoul N, Da Silva Filho J, Magnin-Poull I, Messier M, Aliot E. Cavotricuspid isthmus mapping to assess bi-directional block during common atrial flutter radiofrequency ablation. Circulation 1999;100:2507–2513. 4. Natale A, Newby KH, Pisano E, Leonelli F, Fanelli R, Potenza D, Beheiry S, Tomassoni G. Prospective randomized comparison of antiarrhythmic therapy vs. first line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol 2000;35:1898–1904. 5. Cabrera JA, Sanchez-Quintana D, Ho SY, Medina A, Wanguemert F, Gross E, Grillo J, Hernandez E, Anderson RH. Angiographic anatomy of the inferior right atrial isthmus in patients with and without history of common atrial flutter. Circulation 1999;99:3017–3023. 6. Heidbu ¨chel H, Willems R, Van Rensburg H, Adams J, Ector H, Van de Werf F. Right atrial angiographic evaluation of the posterior isthmus. Relevance for ablation of typical atrial flutter. Circulation 2000;101:2178–2184. 7. Da Costa A, Faure E, Thevenin J, Messier M, Bernard S, Abdel K, Robin C, Romeyer C, Isaaz K. Effect of isthmus anatomy and ablation catheter on radiofrequency catheter ablation of the cavotricuspid isthmus. Circulation 2004;110:1030–1035. 8. Hoyt RH, Huang SKS, Marcus FI. Factors influencing trans-catheter radiofrequency ablation of the myocardium. J Appl Cardiol 1986;1:469–485. 9. Haines DE. Determinants of lesion size during radiofrequency catheter ablation: the role of electrode tissue contact pressure and duration of energy delivery. J Cardiovasc Electrophysiol 1991;2:509–515. 10. Haines DE, Verow AF. Observations on electrode tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation 1990;82:1469–1474. 11. Haverkamp W, Hindricks G, Gulker H, Rissel U, Pfennings W, Borggrefe M, Breithardt G. Coagulation of ventricular myocardium using radiofrequency alternating current: biophysical aspects and experimental findings. Pace 1989;12:187–195. Downloaded from by guest on October 15, 2014 A landmark study disclosed the existence of a highly variable isthmus anatomy and its impact on ablation tactics.6 We had already demonstrated that CTI morphology affects RFA procedures in long CTI.7 No large prospective study had, yet, evaluated the impact of all anatomic factors while stratifying on CTI morphology independent of length. Other clinical data such as right atrial dimensions, concavity depth, and LVEF confirm the role of various isthmus morphologies (straight vs. complex). Haemodynamic factors such as tricuspid regurgitation and high systolic pulmonary pressure qualified difficult ablations. In this subset of difficult ablations, an intention-to-treat analysis showed a trend towards both shorter RFA application (26.5 min) and X-ray exposure (25 min) with the ecRFA catheter when compared with the 8 mm-tip catheter and highlights the role of external irrigation in difficult cases where longer application and greater cooling effect are necessary. 1839 1840 26. Anselme F, Savoure ´ A, Cribier A, Saoudi N. Catheter ablation of typical atrial flutter. A randomized comparison of two methods for determining complete bi-directional isthmus block. Circulation 2001; 103:1434–1439. 27. Nakagawa H, Yamanashi WS, Pitha JV, Arruda M, Wang X, Ohtomo K, Beckman KJ, McClelland JH, Lazzara R, Jackman WM. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline irrigated electrode vs. temperature control in a canine thigh muscle preparation. Circulation 1995;91:2264–2273. 28. Petersen HH, Roman-Gonzalez J, Johnson SB, Hastrup Svendsen J, HaunsO S, Packer DL. Mechanisms for enlarging lesion size during irrigated tip radiofrequency ablation: is there a virtual electrode effect? J Interv Cardiol 2004;17:171–177. 29. Yamane T, Jais P, Shah DC, Hocini M, Peng JT, Deisenhofer I, Clementy J, Haissaguerre M. Efficacy and safety of an irrigated-tip catheter for the ablation of accessory pathways resistant to conventional radiofrequency ablation. Circulation 2000;102:2565–2568. 30. Nakao M, Saoudi N. More on isthmus anatomy for safety and efficacy. J Cardiovasc Electrophysiol 2005;16:409–410. 31. Cabrera JA, Sanchez-Quintana D, Farre J, Rubio JM, Ho SY. The inferior right atrial isthmus: further architectural insights for current and coming ablation technologies. J Cardiovasc Electrophysiol 2005;16: 402–408. 32. Cabrera JA, Sanchez-Quintana D, Ho SY, Medina A, Anderson RH. The architecture of the atrial musculature between the orifice of the inferior vena caval vein and the tricuspid valve: the anatomy of the isthmus. J Cardiovasc Electrophysiol 1998;9:1186–1195. 33. Morton JB, Sanders P, Davidson NC, Sparks PB, Vohra JK, Kalman JM. Phased-array intracardiac echocardiography for defining cavotricuspid isthmus anatomy during radiofrequency ablation of typical atrial flutter. J Cardiovasc Electrophysiol 2003;14:591–597. 34. Kasai A, Anselme F, Teo WS, Cribier A, Saoudi N. Comparison of effectiveness of an 8 mm vs. a 4-mm tip electrode catheter for radiofrequency ablation of typical flutter. Am J Cardiol 2000;86:1029–1032. 35. Feld G, Wharton M, Plumb V, Daoud E, Friehling T, Epstein L, EPT-1000 XP Cardiac Ablation System Investigators. Radiofrequency catheter ablation of type 1 atrial flutter using large-tip 8-or 10-mm electrode catheters and high-output radiofrequency energy generator. J Am Coll Cardiol 2004;43:1466–1472. 36. Jais P, Hocini M, Gillet T, Shah DC, Haissaguerre M, Yamane T, Deisenhofer I, Garrigue S, Le Metayer P, Roudaut R, Clementy J. Effectiveness of irrigated tip catheter ablation of common atrial flutter. Am J Cardiol 2001;88:433–435. 37. Shah DC, Takahashi A, Jais P, Hocini M, Peng JT, Clementy J, Haissaguerre M. Tracking dynamic conduction recovery across the cavotricuspid isthmus. J Am Coll Cardiol 2000;35:1478–1484. 38. Shah DC, Jais P, Haissaguerre M, Chouairi S, Takahashi A, Hocini M, Garrigue S, Clementy J. Simplified electrophysiologically directed catheter ablation of recurrent common atrial flutter. Circulation 1997;96:2505–2508. 39. Fuller IA, Wood MA. Intramural coronary vasculature prevents transmural radiofrequency lesion formation. Implications for linear ablation. Circulation 2003;107:1797–1803. Downloaded from by guest on October 15, 2014 12. Chugh SS, Chan RC, Johnson SB, Packer DL. Catheter tip orientation affects radiofrequency ablation size in the canine ventricule. Pace 1999;22:413–420. 13. Chan RC, Johnson SB, seward JB, Packer DL. The effect of ablation electrode length and catheter tip to endocardial orientation on radiofrequency lesion size in the canine right atrium. Pace 2002;25:4–13. 14. Petersen HH, Chen X, Pietersen A, Svendsen JH, Haunso S. Lesion dimension during temperature-controlled radiofrequency catheter ablation of left ventricular porcine myocardium. Impact of ablation site, electrode size, and convective cooling. Circulation 1999;99:319–325. 15. Nakagawa H, Wittkampf FH, Yamanashi WS, Pitha JV, Imai S, Campbell B, Arruda M, Lazzara R, Jackman WM. Inverse relationship between electrode size and lesion size during radiofrequency ablation with active electrode cooling. Circulation 1998;98:458–465. 16. Simmers TA, de Bakker JM, Coronel R, Wittkampf FH, van Capelle FJ, Janse MJ, Hauer RN. Effects of intracavitary blood flow and electrodetarget distance on radiofrequency power required for transient conduction block in a langendorff-perfused canine model. J Am Coll Cardiol 1998;31:231–235. 17. Jaı¨s P, Shah DC, Haı¨ssaguere M, Hocini M, Garrigue S, Le Metayer P, Cle ´menty J. Prospective randomized comparison of irrigated-tip vs. conventional-tip catheters for ablation of common atrial flutter. Circulation 2000;101:772–776. 18. Schreieck J, Zrenner B, Kumpmann J, Ndrepepa G, Schneider AE, Deissenhofer I, Schmitt C. Prospective randomized comparison of closed cooled tip ablation vs. conventional 8 mm tip radiofrequency ablation of common atrial flutter. J Cardiovasc Electrophysiol 2002; 13:980–985. 19. Scavee C, Jais P, Hsu LF, Sanders P, Hocini M, Weerasooriya R, Macle L, Raybaud F, Clementy J, Haissaguerre M. Prospective randomised comparison of irrigated-tip and large-tip catheter ablation of cavo-tricuspid isthmus-dependent atrial flutter. Eur Heart J 2004;25:963–969. 20. Da Costa A, Cucherat M, Pichon N, Messier M, Laporte S, RomeyerBouchard C, Mismetti P, Lopez M, Isaaz K. Comparison of the efficacy of cooled-tip and 8 mm-tip catheters for radiofrequency catheter ablation of the cavo-tricuspid isthmus: meta-analysis. Pace 2005;28:1081–1087. 21. Otomo K, Yamanashi WS, Tondo C, Antz M, Bussey J, Pitha JV, Arruda M, Nakagawa H, Wittkampf FH, Lazzara R, Jackman WM. Why a large tip electrode makes a deeper radiofrequency lesion: effects of increase in electrode cooling and lectrode tissue interface area. J Cardiovasc Electrophysiol 1998;9:47–54. 22. Ventura R, Klemm H, Lutomsky B, Demir C, Rostock T, Weiss C, Meinertz T, Willems S. Pattern of isthmus conduction recovery using open cooled and solid large-tip catheters for radiofrequency ablation of typical flutter. J Cardiovasc Electrophysiol 2004;15:1126–1130. 23. Calkins H. Catheter ablation of atrial flutter: do outcomes of catheter ablation with ‘large-tip’ vs. ‘cooled-tip’ catheters really differ. J Cardiovasc Electrophysiol 2004;15:1131–1132. 24. Demazumder D, Mirotznik MS, Schwartzman D. Comparison of irrigated electrode designs for radiofrequency ablation of myocardium. J Interv Card Electrophysiol 2001;5:391–400. 25. Demazumder D, Mirotznik MS, Schwartzman D. Biophysics of radiofrequency ablation using an irrigated electrode. J Interv Card Electrophysiol 2001;5:377–389. A. Da Costa et al.