Solutions Worksheet 1 -- Chapter 13 Part III Coming to Terms with
Transcription
Solutions Worksheet 1 -- Chapter 13 Part III Coming to Terms with
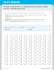
Name: Chapter 14 Solutions Section: Date: Solutions Worksheet 1 Calculating Solution Concentrations A solution is a physical mixture of at least two pure substances. Any of the three phases can be mixed in each other to achieve a solution. Give an example of a solid/liquid solution Give an example of a gas/liquid solution Define Solute – Define Solvent – Define Solution – Draw a picture of solute + solvent = solution Molarity = moles of solute Liters of solution + = Molarity Problems: List given information, use conversion factor, include units. 1. What would be the molarity of a solution if you dissolved 2 moles of NaCl in 6 liters of solution? 2. How many moles of HCl are present in 10 liters of 2.0M HCl? 3. What volume of calcium sulfate, CaSO4, would you need if you dissolved 4 moles of it to make a 0.1M solution? 4. What is the mass of solute in 61.8 ml of 1.03M LiCl? 1 Name: Chapter 14 Solutions Section: Date: 5. Calculate the mass of solute required to make 1 liter of each of the following: a.) 0.925 M NaNO3 b.) 2.12 M NaOH c.) 0.965 M LiNO3 6. Calculate the concentration of each of the following solutions using Molarity. a.) 200 ml of solution containing 8.31 g of LiNO3 b.) 750 ml of solution containing 0.00641 g of AgNO3 7. 3 grams of potassium sulfate is dissolved into 2 liters of solution. What is the molarity? 8. What would be the molar mass of an unknown salt if 11.70 grams dissolved in 2 liters of solution give a 0.1 M solution? 2 Name: Chapter 14 Solutions Section: Date: Refer to this Solubility Chart when answering the solubility questions in Worksheet #2 3 Name: Chapter 14 Solutions Section: Date: Solutions Worksheet 2 -- Chapter 14 Coming to Terms with Solutions - Using a solubility chart provided answer each of the following: 1. What solvent is used to dissolve the four compounds? 2. Are KNO3, NaCl, NaNO3 and NH4Cl miscible with water at temperatures from 0C to 100C? 3. What is the solubility of KNO3 at 0C? (make sure to report units) 4. What is the solubility of NaNO3 at 10C? 5. If a solution at 50C contains 50g of the solute NH4Cl in 100g of water, is the solution saturated, unsaturated, or supersaturated? 6. What would you see happening if a solution containing 80g of KNO3 in 100g of water at 50C cooled to 30C? 7. Describe the cooled solution of KNO3 as saturated, unsaturated, or supersaturated. 8. Which of the four substances has the greatest solubility at 0C? 9. Which substance, Li2SO4 or KNO3 has the greater solubility at 10C? Which has the greater solubility at 70C? 10. What two processes take place when each of the four substances dissolves in water? 11. Diagram (draw a picture of) each of the processes for NaCl in water: 4 Name: Chapter 14 Solutions Section: Solutions Worksheet 3 Date: Using a Solubility Table This activity will help you to become efficient in using the solubility table to determine whether an ionic compound is soluble or insoluble. To determine the solubility of an ionic compound, locate the compound’s negative ion in the first column of the solubility table. Find the positive ion or the group in which the positive ion belongs. Determine whether the compound is soluble or insoluble. Practice using the chart by determining if each of the following compounds will dissolve in water. Then write the correct form for the compound in water: If a compound dissolves, write the ions formed (in aqueous solution) If it does not dissolve, write in molecular formula for the compound Example: 1. NaCl (s) Na+1(aq) + Cl-1(aq) 2. KCH3COO(s) 3. AgNO3(s) 4. Fe(OH)2(s) 5. SnBr4(s) 6. Na2CrO4(s) 7. ZnS(s) 8. (NH4)2CO3(s) 9. Li(OH)(s) 10. PbCl2(s) 5 Name: Chapter 14 Solutions Section: Date: Solutions Worksheet 4 - Writing Net Ionic Equations (you will need to be able to do this on the test) A complete ionic equation shows all of the reactants and products in correct solution form. A net ionic equation shows o Only the reacting species in a chemical reaction, and o Does not include spectator ions. First write a balanced complete equation, showing all reactants and products in correct form. Then write a balanced net ionic equation. Cross out the spectator ions from the complete equation. Include only the reacting species on the reactant and product sides. 1. Sodium hydroxide and iron (III) chloride, give sodium chloride and iron (III) hydroxide. Complete ionic equation: Net ionic equation: 2. Potassium sulfide and copper (II) nitrate, give potassium nitrate and copper (II) sulfide. Complete ionic equation: Net ionic equation: Determine the correct precipitate formed for each of the following reactions: 3. Ammonium sulfate reacts with barium chloride. Complete ionic equation: Net ionic equation: 4. Lithium phosphate reacts with calcium bromide. Complete ionic equation: Net ionic equation: 6 Name: Chapter 14 Solutions Section: Solutions Worksheet 5 Molality and Colligative Properties The addition of a(n) and Date: to a pure liquid solvent changes the of the liquid. The vapor pressure, boiling point, and freezing point of a solution are properties and depend upon the rather than on their The of solute particles . of a liquid is related to the tendency of the molecules to escape from a solution. For example, the proportion and escaping tendency of water molecules is when a solute is dissolved in pure water, and the vapor pressure of the solution is therefore than that of pure water. What is the equation for calculating boiling point elevation? What is the equation for calculating freezing point depression? Determine the molality, and freezing point of the following solutions: 1. 50 g sucrose, C12H22O11, in 200 g water. 2. 100 g glycerol, C3H8O3, in 200 g water 3. 35 g sodium chloride, NaCl, in 200 g water 4. 18 g ammonium phosphate, (NH4)3PO4 200 g water. 7 Name: Chapter 14 Solutions Section: Date: Solutions Worksheet 6 -- Converting Concentrations Mass of solvent Mass of solute MolaLity Molar mass Moles of solute Mass of solution = solute + solvent Density of solution MolaRity Volume of solution 5. Describe what you would do to prepare 100.0 gram of a 3.5% solution of ammonium sulfate in water. 6. What is the Molarity of this solution? Assume the density is 1.0 g/ml. 7. What is the molality, and boiling point of this solution? Dilution Problems: Are based on the fact that the original moles of solute are equal to the final moles of solute. Rearrange the equation to solve for the missing quantity. M1V1 = M2V2 8. What is the molarity of a solution of ammonium chloride prepared by diluting 50.00 mL of a 3.79 M NH4Cl solution to 2.00 L? 9. To what volume should 1.19 mL of an 8.00 M acetic acid solution be diluted in order to obtain a final solution that is 1.5 M? 10. A 25.00 mL sample of ammonium nitrate solution produces a 0.186 M solution when diluted with 50.00 mL of water. What is the molarity of the stock solution? 8