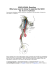The vasovagal response
Transcription
The vasovagal response
575 Clinical Science (1991) 81,575-586 Editorial Review The vasovagal response JOHANNES J. VAN LIESHOUT’.*, WOUTER WIELING‘, JOHN M. KAREMAKER3 AND DWAIN L. ECKE3ERG4*5 Departments of ‘Medicine, 21ntensiveCare and 3Physiology,Academic Medical Centre, University of Amsterdam, The Netherlands, and Departments of 4Medicine and 5Physiology,Hunter Holmes McGuire Department of Veterans, Affairs Medical Center and Medical College of Virginia, Richmond, Virginia, U.S.A. INTRODUCTION PHYSIOLOGY OF THE VASOVAGAL RESPONSE The vasovagal response is the development of arteriolar dilatation (‘vaso’) and inappropriate cardiac slowing (‘vagal’)leading to arterial hypotension with loss of consciousness [ l , 21. The syndrome is called vasovagal (vasodepressor) syncope or fainting. Although it may occur in response to a relative or absolute loss of blood, it also may develop in response to strong emotions. The surgeon John Hunter (1728-1793) wrote [3]: “I bled a lady but she fainted and while she continued in the fit the colour of the blood that came from the vein was a fine scarlet. The circulation was then very languid”. We now think that Hunter observed the effects of vasodilatation during fainting: the scarlet appearance of the blood can be ascribed to the small arteriovenous oxygen difference [4]. In 1895, Leonard Hill [ 5 ] suggested that emotional fainting results from withdrawal of vasomotor neural traffic. In 1932, Thomas Lewis introduced the term ‘vasovagal’ to stress that both blood vessels and the heart are involved. He showed that the abrupt slowing of the heart rate is vagally mediated; however, although atropine prevented bradycardia, it had little o r no effect on hypotension [6]. The finding in Chagas’s heart disease that vasodepressor syncope occurs in patients with defective cardiac vagal control supports the view that hypotension in vasovagal fainting is not a heart-rate-dependent phenomenon [7]. The first section of this review summarizes much of the physiology of the cardiovascular events in vasovagal fainting, and the second part focuses on the diagnostic and therapeutic approach to the patient with recurrent vasovagal syncope. Neural control of the circulation and vasovagal fainting Correspondence: Dr J. J. van Lieshout, Academic Medical Centre, Department of Intensive Care - C3, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands. Simultaneous vagal activation and sympathetic inhibition during fainting can be regarded as the neural mechanism of a vasovagal response which can be activated along at least two different neural pathways (Fig. 1) [S]. T h e first pathway descends from cortico-hypothalamic centres to medullary cardiovascular centres, hence emotional events can evoke vasovagal fainting (central type; Fig. 1) [9]. In susceptible human subjects, severe emotional stress and severe pain (visceral or deep somatic) may lead to vasovagal syncope; the bradycardia and hypotension are usually preceded by moderate tachycardia and hypertension [lo]. The neural centres involved in humans are not known. In animal experiments two higher centres have been identified as important in cerebral circulatory control: the ‘defence reaction area’ in the limbic lobe, and the limbic sympatho-inhibitory center [ l l ] . Stimulation of the former increases heart rate and blood pressure and provokes reactions resembling the human reaction to anxiety and anticipation of muscular exercise (‘fight o r flight’ response). Stimulation of the latter causes hypotension and bradycardia [ 111. A second afferent pathway involved in the vasovagal response is thought to originate in the heart itself (peripheral type) [l,2, 12-16]. This mechanism was clarified in animal experiments by ThorCn and co-workers [S, 17, 181. In anaesthetized cats, the slowing of the heart rate, elicited by rapid haemorrhage or pooling of blood, was preceded by an increased activity in about 20% of nonmyelinated vagal afferent C-fibres from the left ventricle [ 181. Increased receptor activity preceded the slowing of the heart [17]. The main determinants of neuronal firing in these fibres seem to be wall deformation and the inotropic state of cardiac muscle [1S, 191. The combination of an increased inotropic stimulus to the heart and a 576 J. J. van Lieshout et al. CENTRES AFFERENT Glossopharyngeal nerv A EFFERENT Carotid afferent -W Blood vessels Fig. 1. Cardiovascular part of the autonomic nervous system. Schematic representation of the two different pathways which may elicit the depressor response: the baroreceptor- and depressor-reflex arcs and the ‘central command’ to the vasomotor centres (VMC).(Reproduced from [106].) decreased volume of the left ventricle is thought to give rise to a powerful contraction around an almost empty heart chamber, leading to deformation and activation of ventricular mechanoreceptors [8, 17,20-221. The evidence for this sequence is as follows. Gauer [15, 221 induced hypovolaemic shock in dogs and gave them adrenaline. From the moment the animals developed circulatory shock a great disparity ensued between aortic pressure, which fell below 50 mmHg, and intraventricular pressure, which fluctuated between 120 and 200 mmHg. Cineangiography showed that the almost emptied ventricle continued to contract isometrically. The subendocardial haemorrhages which develop in severe and longer-lasting hypovolaemic shock [23] probably result from the same mechanism: trauma resulting from heavy mechanical forces, acting on the inner wall of the emptied left ventricle. These haemorrhages are comparable with the lesions evoked by exposure to centrifugal force in hypovolaemic hypotensive animals [ 16,221. However, not all investigators accept the notion that the signal which causes vasodilatation comes solely from cardiac receptors. Morita & Vatner [24] found that the loss of sympathetic drive to the vessels during haemorrhage in conscious dogs was not prevented by surgical denervation of the heart. There are also data from animal experiments indicating that when arterial baroreceptor areas become shrunken by volume depletion, they may paradoxically increase their firing rates at low blood pressure levels [25,26]. The contribution of baroreceptors to the vasovagal response in humans remains, however, unclear. The recent finding in a cardiac transplant patient of vasodilator-induced withdrawal of sympathetic nerve activity with syncope supports the view that ventricular baroreceptor activation is not the exclusive cause of vasovagal syncope [27]. The term ‘vasodepressor syncope’ has been used to describe both vasovagal syncope [4, 28-32] and carotid sinus syncope [33, 341. In both conditions a depressor reflex is triggered, which leads to vasodilatation and reflex bradycardia. However, an important difference is that in carotid sinus syncope, carotid sinus baroreceptor circulatory control is thought to be abnormal. Another clinically important depressor reflex originating from the heart itself can be observed in myocardial ischaemia or infarction [35], coronary reperfusion [36] and during coronary angiography [37]. It is comparable with the Von Bezold-Jarisch reflex as produced experimentally in laboratory animals by topical application of chemical stimulants on the heart or their injection into the coronary circulation [12, 19, 35, 381. Reflex bradycardia and hypotension may also be observed in glossopharyngeal neuralgia [39] as an example of reflex syncope involving afferent pathways which do not originate from the heart or hypothalamic centres [40]. It may be impossible to discriminate sharply between the central and peripheral types of fainting [40]. It is conceivable that interactions between the two types take place; for example, primary vasodilatation initiated by central factors (e.g. seeing one’s own blood) may lead to a fall in ventricular filling pressure which stimulates ventricular mechanoreceptors. Vasodilatation: active or passive, neural or humoral? Vasovagal fainting sometimes develops in healthy subjects in a laboratory environment; this permits study of the circulatory transients (Fig. 2) [28, 31, 41, 421. It has also been observed in the dental chair [l] and has been induced deliberately by vasodilating agents [43, 441, passive head-up tilt [45, 461, subatmospheric pressure applied to the lower part of the body [32, 46-49], bleeding and/or application of venous occluding tourniquets [13, 14,501 and mental stress [51,52]. From thesediverse experiments the following data have been obtained. Roddie [53], Weiss et al. [43] and Barcroft et al. [13, 141 studied the forearm muscle blood flow in fainting humans and found vasodilatation to be confined to muscle. Debate concerning the nature of these vascular changes in the forearm has focused on whether the response is neural or humoral and, if neural, whether it is active or passive [40, 541. Data on the mechanisms of vasodilatation are not easy to interpret: the cardiovascular effects of the 577 The vasovagal response 0 1 2 6 5 (b) 7 11 10 Time (min) I Time (min) Fig. 2. Vasovagal syncope. In two healthy male subjects without a history of previous fainting, the blood pressure and heart rate responses on standing are normal. Both subjects fainted after a comparable time in the upright position (indicated by arrows). The subject in ( a ) shows a predominantly vasodilatory type, whereas the subject in ( b )shows a more cardio-inhibitory type of vasovagal fainting with a cardiac asystole of 10 s. Note the interruptions in the time scale. (Reproduced from [106].) various methods used to induce vasovagal syncope in healthy subjects [40,51,52] differ considerably. A n active cholinergic vasodilatory mechanism was suggested to be causative in vasovagal fainting, instead of the reflex inhibition of tonic sympathetic discharge [9, 13, 14, 51-54]. There is evidence for a sympathetic cholinergic vasodilator mechanism in the cutaneous circulation in humans [40, 541. Active vasodilatation in skeletal muscle was shown in the cat [54]. Although data in primates are not uniform [40, 541, Sanders et al. [55] recently provided evidence for cholinergically mediated vasodilatation in humans during isometric exercise by sustained handgrip [55]. Nevertheless, the overall cardiovascular effect of handgrip is an elevated arterial pressure caused by a rise in cardiac output [56]. With respect to the vasovagal response in humans, the evidence for the operation of active vasodilator nerves remains limited to two findings: ( 1 ) after cervical sympathectomy, vasodilatation is absent 'during post-haemorrhagic fainting [14], and (2) at the time of a faint, blood flow in forearms with intact vasomotor innervation is higher than after neural blockade [57].These findings document vasodilatation but d o not provide information on its nature: active or passive [38, 401. Forearm blood flow increases during mental stress [51, 581. Barcroft et al. [58] and Blair et al. [51] showed that vasodilatation during mental stress remains present, although to a lesser extent, after cholinergic blockade. T h e finding that adequate intra-arterial cholinergic blockade fails to prevent vasodilatation [58] does not tally with the supposed involvement of a cholinergic sympathetic vasodilatory system in the genesis of vasovagal syncope. T h e evidence for passive vasodilatation in fainting as a result of decreased sympathetic drive is much stronger. A n important argument is the finding that in fainting humans plasma noradrenaline levels are relatively low despite severe hypotension [28, 31, 451. Eckberg et al. [59] showed that the noradrenaline response was already blunted at a mild to moderate fall in blood pressure induced by nitroprusside. There is also evidence for a sudden decrease in cardiac and renal sympathetic nervous activity during vasovagal fainting: Esler et al. [60] found a severely reduced cardiac and renal noradrenaline spillover in patients who fainted during cardiac catheterization. T h e disappearance of secondary reflected arterial pressure waves at fainting also suggested a decrease in sympathetic tone [28]. Direct evidence for the withdrawal of vasoconstrictor activity was provided by the demonstration of a sudden cessation of peroneal muscle sympathetic bursts at the onset of a vasovagal faint in healthy subjects [42,61,62]and the disappearance and reappearance of muscle sympathetic nerve activity coinciding with 578 J. J. van Lieshout et al. the onset and termination of vasovagal attacks [63]. An example of a reduction in sympathetic nerve activity during a nitroprusside-induced vasovagal reaction is shown in Fig. 3. There is also evidence for a humoral vasodilating mechanism in the vasovagal response. Adrenaline has been suggested as the humoral agent involved: plasma levels of adrenaline are elevated in fainting humans [45, 48, 641 (see below) and this catecholamine produces /?adrenergic dilatation in both skeletal muscle and splanchnic resistance vessels at concentrations measured in humans under stress [9, 48, 491. Increased adrenaline levels would oppose the vasoconstriction needed to maintain blood pressure during hypotension [48]. The finding of less vasodilatation after adrenalectomy [58] also supports a contributory role for adrenaline in the mechanism of vasovagal fainting. Haemodynamics response before and during the vasovagal The interpretation of the literature on central haemodynamic changes preceding and during vasovagal fainting is hampered by the lack of beat-to-beat data on changes in cardiac output and peripheral vascular resistance. Cardiac output, as measured by single-indicator-dilution and Fick techniques, either remains unaltered or falls below control values with a fall in total peripheral resistance in the early phase of spontaneous, post-haemorrhagic or head-up-tilt-induced syncope [ 13,23,29,65]. In healthy subjects, induction of fainting by lower-body negative pressure in the sitting position elicits an initial rise in heart rate and a decline in blood pressure, followed by a marked drop in heart rate and further fall in blood pressure in the last minute before the faint [30, 471. The fall in stroke volume, cardiac output and arterial pressure usually occurs before the bradycardia [l11. The fall in peripheral vascular resistance in vasovagal syncope is apparently not compensated for by a rise in cardiac output. During prolonged lower-body negative pressure renal vascular resistance does not change, but splanchnic vasoconstriction increases [66], which is consistent with the observation of cyanosis and pallor of the colonic mucosa during syncope in a patient with a colostomy [67]. The behaviour of the veins in human subjects during fainting has been studied almost exclusively in limbs, although they almost certainly make no important contribution to cardiovascular control [68]. Epstein et al. [65] showed that veins retain the ability to constrict during vasovagal syncope. Nevertheless, in the majority of studies on vasovagal syncope, usually induced by withdrawal of blood in volunteers, a drop in central venous pressure was found to precede syncope [ l , 13, 32, 691. The fall in central venous pressure induced by lowerbody negative pressure was shown to level off in the presyncopal phase. It might even rise again but to values below control level [32], probably by venoconstriction without full restoration of central blood volume [32]. Measurements of central venous pressure in vasovagal fainting remain, however, difficult to interpret. On the one hand, a reduction in cardiac output at the onset of syncope (see below) would tend to increase central venous pressure and thereby mask the effects of peripheral venodilatation [70]. On the other hand, some contribution towards the recorded fall in atrial pressure could be made by yawning and the increase in depth of breathing which precede fainting (Fig. 3) [ll].The hypocapnia associated with pre-syncopal hyperventilation might amplify the fainting reaction per se by cerebral vasoconstriction and systemic vasodilatation [ 11,23,38]. The magnitude of blood volume reduction and its distribution between central and peripheral vascular compartments are independent denominators of the underlying haemodynamics in vasovagal syncope. A large fraction of the calf volume change during venous occlusion is attributable to filling of the deep venous spaces [71]. Since their compliance is determined primarily by the surrounding skeletal muscle [711, these findings indicate that decreased skeletal muscle tone increases orthostatic venous pooling. This is in agreement with the finding of Mayerson & Burch [72], who showed that gastrocnemius intramuscular pressures during passive head-up tilt were lower in fainters. Although postural changes in plasma volume or capillary filtration rate in fainting head-up-tilted healthy subjects are not different from non-fainters [73], a smaller blood volume is found in fainters [69]. The pre-syncopal chronotropic response during tilt in subjects who develop vasovagal syncope is, however, independent of blood volume [74]. Redistribution of blood volume between central and peripheral vascular compartments may be more important than total blood volume. This view is supported by the finding that selective restoration of central blood volume in vasovagal syncope, by inflation of an anti-gravity suit, results in a dramatic resolution of symptoms by rapidly elevating central venous and arterial pressure [29, 751. The beneficial circulatory effects of ‘re-transfusion’ point to the paramount role of central blood volume in the circulatory adaptation to orthostatic stress [4,13,29,32,69]. Hypovolaemic hypotension elicits an antagonism between cardiovascular reflexes A drop in blood pressure as a result of a fall in venous return on assumption of the upright posture, or as a result of loss of blood, may set in motion two opposing reflexes: the arterial baroreflex and the depressor reflex. The former is activated by the fall in arterial blood pressure, and the latter by a decrease in ventricular filling volume (Fig. 1)[21]. A n antagonism between the control of filling pressure and volume of the heart, and the control system of arterial pressure has been suggested [69, 761. When syncope ensues, the balance may be directed one-way in that the depressor reflex overrides the baroreflex; the latter is still functionally intact [21] but switched off [771. This shift from pre-syncopal tachycardia to bradycardia and/or arterial hypotension during syncope [ 111has been referred to as the ‘biphasic response’ (Fig. 2) [22, 78,793. The sympathetic ‘fight or flight’ response (moderate tachycardia, hypertension) shifts to a state analogous to R-R interval (ms) ECG Muscle sympathetic activitv Tidal volume Nitroprusside dose (pg min-’ kg-’) 149180 Blood pressure (mmHg) 600 0 The vasovagal response 146165 1.0 100140 2.0 579 78/41 0 Fig. 3. Vasovagal reaction during sodium nitroprusside infusion in a healthy subject. Muscle sympathetic bursts increase when arterial blood pressure declines, followed by ‘sympathetic silence’ at the verge of vasovagal syncope with a further fall in arterial blood pressure and an abrupt increase in R-R interval. Note the change in depth of breathing preceding the faint as reflected by the increase in tidal volume. (Reproduced from [62]with the permission of the publisher.) the ‘playing dead‘ reaction seen in young animals placed in a dangerous situation (bradycardia, hypotension) [5, 22,531. A possible relationship between psychological stress, vasovagal syncope and sudden death was hypothesized by Engel [SO]. The concept of sudden death was developed in which emotional fainting is held potentially lethal. Although the majority of subjects with sudden cardiac death have evidence of recent clotting in their coronary arteries [81] or hypertrophic cardiomyopathy [82], emotional fainting could interact with pre-existing cardiovascular disease to cause death [35, 53, SO]. Both cardiovascular collapse associated with prolonged cardiac asystole and syncope or neurally mediated origin may be elements of the spectrum of the hypotension-bradycardia syndrome [83]. Milstein et al. [83] recently showed that patients with suspected or documented cardiac asystole and normal conventional electrophysiological evaluation exhibit a susceptibility to tilt-induced, neurally mediated, hypotension-bradycardia (see below). Maloney et al. [84] found in an otherwise healthy man who developed vasovagal syncope on head-up tilting with asystole for 73 s no abnormalities on subsequent electrophysiological testing [84]. Although these data do not provide conclusive evidence that vasovagal syncope is life threatening, they suggest that the vasovagal reflex may induce life-threatening cardiac asystole mimicking sudden cardiac death [SO, 83-85]. The teleological significance of the vasovagal response is problematic. Is the vasovagal response in humans an appropriate adjustment to hypovolaemic stress or an inconvenient remnant of the ‘playing dead reaction’ in animals? It may be disadvantageous in severe haemorrhagic shock to antagonize the arterial baroreceptor reflex by accentuating hypotension [86]. However, it may possess cardiac protective properties by rapidly reducing myocardial oxygen demand when cardiac strain is excessive [20]. When someone faints as a result of a critically reduced venous return, the induced reflex bradycardia together with the increase in venous return by the supine position will allow for a better diastolic filling of the heart and restoration of arterial blood pressure [87, 881. From this speculative viewpoint the vasovagal response may be regarded as the body’s compromise for the antagonism between the control of systemic arterial pressure and of volume and pressure of the heart itself. Neuroendocrine aspects of the vasovagal response Cardiovascular reflexes, although of decisive importance in short-term circulatory control, are only part of an elaborate design of servo-control loops [89].In maintaining orthostasis, intact volume control is of vital importance. The neuroendocrine regulation of water and salt balance is embodied in the long-term control of body fluids, with the kidney as the main effector organ regulated by catecholamines, the renin-angiotensin-aldosterone system and antidiuretic hormone (vasopressin) [90]. Since a normal regulatory role has not been established for atrial peptides they will not be discussed. Catecholamines. The shift from a pre-syncopal moderate tachycardia to bradycardia and/or arterial hypotension (biphasic response) in vasovagal fainting [22,78,79] is reflected by an initial elevation of the plasma noradrenaline concentration in the minutes preceding syncope followed by a stable, or possibly declining, level during the vasovagal response [28,31,64,91].In contrast to the blunted noradrenaline response, an increase in plasma adrenaline concentration was reported in most but not all subjects during vasovagal fainting induced by nitroglycerine [44], head-up tilt [45, 48, 491 and central hypovolaemia induced by venous tourniquets [92]. This discrepancy in the behaviour of adrenaline and noradrenaline seems characteristic of the vasovagal response [28, 31, 45, 641. The role of adrenaline as a vasodilating hormone in the pathogenesis of vasovagal fainting is discussed above. Renin. The secretion of renin is partially controlled by 580 J. J. van Lieshout et al. efferent adrenergic renal nerve fibres innervating juxtaglomerular cell P-adrenoceptors [93]. When humans change from the supine to the upright posture, plasma catecholamine, renin and aldosterone concentrations normally rise within several minutes. Elevation of the plasma renin levels is subsequently followed by increases in the plasma angiotensin I1 concentration [93, 941. The magnitude of the rise in the plasma renin concentration strongly depends on the effective circulating volume [95]. The decrease in efferent sympathetic nerve traffic during vasovagal fainting is not restricted to the limb blood vessels but involves the kidney as well [60, 94, 961. In some (but not all) studies in humans, plasma renin activity was reported to fall just before vasovagal fainting induced by head-up tilt, and to rise again only after the subjects had returned to the supine position [28,94,97]. Vasopressin. The secretion of vasopressin is influenced primarily by plasma osmolality, and secondarily by changes in blood pressure [90, 981 and by nausea [99, 1001. A rise in plasma vasopressin concentration was found in conscious rabbits during gradual haemorrhage at the point that blood pressure suddenly fell. Wang et af. [loll in conscious dogs, prevented the fall in blood pressure and the rise in vasopressin by pharmacological blockade of the cardiac nerves, thus substantiating the role of a signal originating in the cardiac ventricles. The situation in humans, however, is more complex; the vasopressin response to acute blood volume shifts in cardiac transplant patients is similar to that in normal cardiacinnervated control subjects [ 1021. This implies that in humans isosmotic vasopressin release is not exclusively mediated by cardiac receptor reflexes. In normal humans, slight hypotension does not stimulate vasopressin secretion [46, 1031. The level of vasopressin increases sharply up to that which has a direct vasoconstrictor action, but only with a marked fall in blood pressure such as exists during haemorrhage or vasovagal fainting [45, 64, 991. In animals, ventricular receptors [8, 1041 send impulses along afferent C-fibres which activate the vomit centre and result in a general vagal discharge with reflex relaxation of the stomach [99, 1051. In addition, nausea itself is a potent direct stimulus of vasopressin secretion by activation of central cholinergic pathways [99, 1001. Since nausea as a typical premonitory symptom is often associated with vasovagal fainting, a rise in plasma vasopressin concentration in fainting humans does not permit further discrimination between central and ventricular reflex release of vasopressin. Our observations in patients with pure autonomic failure that very low upright blood pressure is accompanied by a rise in vasopressin, but not by sensations of nausea [ 1061, support a causal relationship between hypotensive arterial baroreceptor inhibition and vasopressin release. Interactions between vasopressin and the other neural control systems involved in vasovagal fainting seem relevant, since vasopressin sensitizes cardiac vagal afferent nerves [20] and probably modulates arterial baroreflex gain [ 1071. The observation of reduced diuresis in the first hour after vasovagal fainting is explained by the rise in vasopressin [ l , 4,411. Pancreatic polypeptide. The hormone pancreatic poly- peptide controls exocrine pancreas secretion and influences the motility of the biliary ducts [108].The stimuli for its secretion are hypoglycaemia and the presence of food in the stomach, mediated by cholinergic fibres in the vagus nerve [108]. Impaired secretion in diabetic patients probably indicates early autonomic neuropathy [ 1091. The drop in blood pressure and heart rate in healthy subjects on syncope provoked by 60" head-up tilt is accompanied by a rapid increase in pancreatic polypeptide concentration, indicating an increase in tone of the abdominal part of the vagus nerve [45,86]. It seems likely that a general vagal discharge underlies both the bradycardia [45] and the increase in pancreatic polypeptide concentration [ 1081. Opiates. Opiates may also be involved in the vasovagal response, since endogenous opiate mechanisms are implicated in the vasodilator response to acute blood loss: pharmacological doses of the opiate antagonist naloxone in conscious animals attenuate the fall in blood pressure that arises when a large volume of blood is rapidly withdrawn [46, 77, 1lo]. The dose of naloxone delivered into the fourth ventricle that prevents vasoconstriction is 90-900 times less than a corresponding intravenous dose, indicating that the endogenous opioid mechanisms act in the central nervous system rather than peripherally [46, 771. CLINICAL EXPRESSION OF THE VASOVAGAL RESPONSE The conimon faint The relative extent to which blood pressure and heart rate fall during true vasovagal fainting may vary from severe hypotension with only a small drop in heart rate (Fig. 2a) [ 13, 441 to a predominantly cardio-inhibitory type (sometimes with clear sinus arrest) (Fig. 2b) [6, 31, 64, 84, 86, 92, 11I]. Although vasovagal fainting may develop when subjects are supine [63, 1111, as a rule it happens in the upright position. Vasovagal responses are relatively common in daily life: up to 20% of 'normal' subjects lose consciousness at some time in their lives [112, 1131 with a 5-15% rate in healthy blood donors [79]. Vasovagal syncope provoked by passive head-up tilt has a comparable incidence of about 15% [ 114-1 161. There may be a constitutional predisposition: fainting develops more frequently in slender subjects [5,69, 1171; this might be related to a larger leg compliance when muscle mass is less [118]. True vasovagal fainting is rare in the elderly compared with young subjects [119-1211. It is not known why patients with orthostatic hypotension owing to autonomic neuropathy or high spinal cord lesions do not develop premonitory symptoms of vasovagal fainting [50, 1221. Van Lieshout and co-workers [106, 1231 found in two patients with hypoadrenergic orthostatic hypotension who had intact vagal cardiac control, that vasovagal bradycardiac responses were surprisingly absent despite a profound fall in arterial pressure on standing. A possible explanation might be their inability to release adrenaline, which protects the heart from too The vasovagal response vigorous contractions and thereby prevents triggering of ventricular receptors. Relatively slow heart rate in hypovolaemic arterial hypotension An unexpectedly low heart rate was found in patients in shock during a study of air-raid casualties in World War I1 [124, 1251, in volunteers bled large amounts experimentally [126] and in patients with acute hypotensive intraperitoneal bleeding [ 1271. Sander-Jensen et af. [86] found in patients with serious bleeding from various causes during the phase of hypovolaemic shock, a mean heart rate of only 73 beats/min. Resuscitation with fluids increased both blood pressure and heart rate. Thus a relatively low heart rate may be a clinical sign of severe hypovolaemic shock. It has even been argued that the widespread belief that haemorrhage is easily diagnosed by a rapid pulse and a low blood pressure is based on a historical misconception [ 126, 1271. A relative bradycardia accompanying an important reduction of venous return seems a paradox and points to an apparently inappropriate increase in vagal activity [4, 32,861. Patients with leftsided congestive heart failure show the opposite phenomenon: they tolerate orthostatic stress during passive head-up tilt remarkably well and are reported to be free of vasovagal syncope [2, 221. The elevated central blood volume and heightened venous pressure may prevent a fall in upright cardiac filling pressures which results in improved orthostatic tolerance [ 1281. An alternative explanation is that decreased /3-adrenoceptor density in patients with congestive heart failure [129] interferes with the increased contractility that is thought to increase ventricular receptor firing and provoke vasovagal responses in healthy subjects. Factors predisposing to vasovagal syncope Exercise. Decreased orthostatic tolerance is found in healthy subjects after exhaustive exercise [ 1301. Blunting of the vasoconstrictive responses in the exercised muscles by hyperthermia and local lactic acidaemia are postulated to underlie the failure in maintaining arterial pressure in the upright position. Also the fall in arterial pressure which reduces left ventricular afterload is thought to allow a more complete emptying of the ventricle, thereby eliciting a vasodepressor reflex [88]. Aortic and subaortic stenosis. Patients with aortic or subaortic stenosis may suddenly develop syncope during or shortly after critical levels of exercise [131]. Recently, the exertion-syncope of aortic valve stenosis was suggested not to be solely due to flow obstruction but also to be of reflex origin [22, 132, 1331. During leg exercise there is a fall in forearm vascular resistance, rather than the increase that occurs in normal subjects [132]. Reflex vasodilatation by the sudden large elevation in left ventricular pressure in the face of an obstructed outflow tract [54, 1331, together with fixed cardiac output, has been suggested as the most common pathogenic mechanism of syncope in aortic stenosis [134]. Exertion syncope in 581 aortic stenosis patients is an example of vasodepressor syncope not primarily elicited by venous pooling. Cardiovascular drugs. The instantaneous increase in venous capacitance by drugs, such as organic nitrates and frusemide, and by alcohol predisposes to vasovagal responses. Vasodepressor reflex activation due to reduced cardiac filling sensitizing ventricular receptors seems a common contributor to postural syncope in patients who use these drugs (for a review, see [19]). Diagnosis of vasovagal syncope The diagnostic work-up of syncope is difficult because of its intermittent nature and the frequent absence of diagnostic abnormalities [113]. An approach to diagnosis of a patient presenting with syncope commences with a thorough history, taken with particular attention to the following diagnostic clues: age (recent onset of syncope in the elderly is most commonly due to cardiac disease),premonitory symptoms (profuse sweating, pallor, nausea, yawning, hyperventilation, pupillary dilation), the conditions provoking syncope (emotion, fear, pain, hot environment, use of medication, prolonged standing, exercise), and the estimated duration of a prodromal period (usually longer in vasovagal than cardiac syncope [ 1351).Physical examination should include recording of blood pressure and pulse rate in the supine position and after several minutes upright, looking for hypotension and/or bradycardia, and a neurological examination. Electrocardiographic abnormalities are found in 5-10°/0 of the patients with syncope [112]. In case of a normal ECG, prolonged ambulatory electrocardiographic monitoring seems justified in the light of incidence of unsuspected rhythm disturbances [ 1361. The main problem with interpreting Holter data is the high incidence of false-positive results [ 1121. Electrophysiological testing has been advocated to exclude undetected vagally mediated brady-arrhythmia [40]. In young children [137, 1381 and in trained athletes [134, 1391with a history of cardiac syncope, resting sinus bradycardia and varying degrees of sino-atrial and atrio-ventricular block have been considered to indicate increased vagal activity as a cause of the syncope. Atropine returns these functional conduction abnormalities to normal [137, 140, 1411. The absence, however, of hypotension during the spells of bradycardia [ 1381 and the beneficial effects of atropine [137, 1381 throw doubt on its true vasovagal nature. Because of the arbitrary definition of excessive vagal tone, its unclear prognostic importance [ 1361 and the low sensitivity, specificity and predictive accuracy of electrophysiological testing in patients with syncope caused by transient bradycardia [134, 1421, such testing should be offered only to patients with structural heart disease and recurrent unexplained syncope [112, 113,134,1431. Prolonged standing and passive head-up tilt induce venous pooling to a variable extent. Although the tiltboard was already used in 1945 by Allen et al. [144] to study orthostatic hypotension, the growing literature on the use of postural change as a promising diagnostic test in the assessment of recurrent syncope with reproduction 582 J. J. van Lieshout et al. of symptoms is of recent onset [83, 84, 113, 134, 145-1481. Vasovagal responses in the cardiovascular laboratory are, however, not uncommon in healthy subjects who otherwise never faint (Fig. 2) [ l l , 116, 122, 1491. In addition, the pre-test probability of a positive test result, i.e. syncope during passive head-up tilt, depends especially on two independent variables: age and nature of instrumentation (invasive versus non-invasive). Susceptibility to vasovagal syncope during tilt is common in the young but is reduced in the old [119-1211. In addition, use of intravascular instrumentation increases the incidence of fainting up to 90% [41, 114, 1501.The diagnostic value of vasovagal fainting as induced by head-up tilt in a non-invasively instrumented older subject with unexplained syncope is probably considerable. However, with invasive instrumentation, the value of a tilt test is still unknown in the elderly, and is low in the young [150]. Although the addition of intravenous isoprenaline to passive head-up tilt increases the incidence of bradycardia, hypotension and syncope in patients with unexplained syncope and negative electrophysiological tests [ 147, 151, 1521, false-positive test results have been reported [ 1511. Therapeutic approach to the habitual vasovagal fainter In 1826, Piorry [153] suggested that syncope be treated by having the patient lie down and by lowering the head even further and raising the legs, using gravity to beneficial effect. Although patients suffering from true vasovagal syncope in general have a benign prognosis [ 1541, extreme episodes of reflex asystole can cause major disability [84]. Muscle-tensing manoeuvres of the lower limbs alone [155] or in combination with abdominal muscles (anti-G manoeuvre) [47] may be successful in preventing syncope. In more serious recurrent vasovagal syncope we propose the following approach. First, the use of measures to counteract pooling of blood in the legs and the abdomen such as elastic stockings and abdominal binders. Secondly, isometric exercise of the muscles of the legs and abdominal wall may be helpful in improving orthostatic tolerance [28, 1561. There is also evidence that moderate endurance training improves orthostatic tolerance in deconditioned subjects; the resulting increment in plasma volume might contribute to this effect [ 157- 1591. However, the relation between degree of aerobic fitness and orthostatic tolerance remains controversial: both reduced and normal orthostatic tolerance have been reported in endurance-trained subjects [ 158, 1601 (for a review see [156]). Orthostatic tolerance in individuals who undergo strength training differs from those who undergo endurance-type training [ 1561. Muscle mass seems to be the more important factor contributing to the prediction of leg compliance independent of aerobic fitness [118, 1581; a larger muscle mass of the lower extremities might restrict orthostatic venous pooling [ 1561. If true, a training programme should ideally be aimed at minimizing the blood volume change in the leg veins under similar pressure changes. A purely cardio-inhibitory type of vasovagal syncope (Fig. 1) should respond to either cardiac pacing or anti- cholinergic drugs. Permanent cardiac pacing, beneficial in the cardio-inhibitory type of carotid-sinus hypersensitivity, has not been of help in its vasodepressor variant [33, 341. Permanent pacing has been recommended to prevent bradycardia in those patients who develop long periods of asystole during their vasovagal attacks [84]. In vasovagal fainting, cardiac pacing has, however, never proved to prevent hypotension [161, 1621. The finding that atropine neither influences the degree of hypotension [6, 13, 28-30] nor the time until the onset of vasovagal syncope during orthostatic stress [163] does not justify anti-cholinergic drug treatment. Nevertheless, some patients with recurrent vasovagal syncope who were treated with transdermal scopolamine remained free of symptoms for as long as they received the drug [84]. Although its parasympatholytic effects are probably significant [84], administration transdermally leads to low serum levels in the parasympathomimetic range [113, 1641. The reported efficacy of scopolamine in vasovagal syncope might be related to modulatjon of cerebral autonomic outflow, since scopolamine, unlike atropine, has a central depressant effect [113]. Propranolol, in pharmacological doses, was suggested to decrease the activity of ventricular mechanoreceptors [165]. Although this was not confirmed in a recent study [ 1201, cardioselective P,-adrenoceptor blockade was reported earlier to diminish susceptibility to vasovagal responses during passive head-up tilt [91, 1521. If true, B-adrenoceptor blockade might be of help in preventing vasovagal syncope by modifying neural traffic from and to the heart, blocking both afferent and efferent pathways [201. Normovolaemic patients with debilitating recurrent vasovagal syncope may improve with measures which increase blood volume, such as generous salt diets and mineralocorticoids [84]. We suggest an attempt to increase the patients’ blood volume by the use of head-up tilt at night. This was earlier proven to be effective in patients with orthostatic hypotension due to the postural tachycardia syndrome [166] and in patients with autonomic circulatory failure [106,166, 1671. SUMMARY The vasovagal response is the development of inappropriate cardiac slowing and arteriolar dilatation. Vasovagal responses reflect autonomic neural changes: bradycardia results from sudden augmentation of efferent vagal activity, and hypotension results from sudden reduction or cessation of sympathetic activity and relaxation of arterial resistance vessels. Two different neural pathways are thought to be involved, one originating in the hypothalamus, the other in the heart. Direct hypothalamic activation of the medullary cardiovascular centres triggered by emotional stress or pain causes a vasovagal response (central type). The combination of a reduced central blood volume secondary to venous pooling or blood loss, and an increased inotropic state of the heart, may stimulate ventricular mechanoreceptors and provoke vasodila- The vasovagal response tation and bradycardia (peripheral type). Cardiovascular afferents originating from stretch receptors in various parts of the vascular tree sometimes induce opposite reflexes when compared with those from ventricular afferents. T h e depressor reflex involved in the peripheral type of vasovagal response originates i n the heart itself and overrides normal baroreflex circulatory control; an antagonism between the control of volume and pressure on the filling side of the heart and the control system of arterial pressure becomes apparent. Vasovagal responses are not necessarily abnormal; the neural pathways involved in the vasovagal response are probably present in all healthy subjects who individually mainly differ in susceptibility. REFERENCES 1. Sharpey-Schafer, E.P. Emergencies in general practice. Syncope. Br. Med. J. 1956; i, 506-9. 2. Sharpey-Schafer, E.P., Hayter, C.J. & Barlow, E.D. Mechanism of acute hypotension from fear or nausea. Br. Med. J. 1958; ii, 878-80. 3. Palmer, J.F. Works of John Hunter 1837. London, vol. 3. 4. Weissler, A.M. & Warren, J.V. Vasodepressor syncope. Am. Heart J. 1959,40,786-94. 5. Hill, L. The influence of the force of gravity on the circulation of the blood. J. Physiol. (London) 1895; 18,15-53. 6. Lewis, T. Vasovagal syncope and the carotid sinus mechanism with comments on Gowers’s and Nothnagel’s syndrome. Br. Med. J. 1932; i, 873-6. 7. Marin Neto, J.A., Gallo, L., Manco, J.C., Rassi, A. & Amorim, D.S. Postural reflexes in chronic Chagas’s heart disease. Cardiology 1975; 60,343-57. 8. Thortn, P. Depressor reflex from the heart during severe haemorrhage. In: Hainsworth, R., McWilliam, P.N. & Mary, D.A.S.G., eds. Cardiogenic reflexes. Oxford: Oxford University Press: 1987,389-401. 9. Shepherd, J.T. & Vanhoutte, P.M. The human cardiovascular system. Facts and concepts. New York Raven Press, 1980. 10. Wahbha, M.M.A.E., Morley, C.A., Al-Shamma, Y.M.H. & Hainsworth, R. Cardiovascular reflex responses in patients with unexplained syncope. Clin. Sci. 1989; 77,547-53. 1 1 . Smith, J.J. Circulatory response to the upright posture. Boca Raton: CRC Press: 1990,30-3. 12. Jarisch, A. Vagovasale Synkope. Z. Kreislaufforsch. 1941; 23.267-79. 13. Bakroft, H., Edholm, O.G., McMichael, J. & SharpeySchafer, E.P. Posthaemorrhagic fainting. Study by cardiac output and forearm flow. Lancet 1944; i, 489-91. 14. Barcroft, H. & Edholm, O.G. On the vasodilatation in human skeletal muscle during post-haemorrhagic fainting. J. Physiol. (London) 1945; 104, 161-75. 15. Gauer, O.H. Evidence in circulatory shock of an isometric phase of ventricular contraction following ejection. Fed. Proc. Fed. Am. SOC.Exp. Biol. 1950; 9,47. 16. Gauer, O.H. & Hen?y, J.P. Negative ( - Gz) acceleration in relation to arterial oxygen saturation, subendocardial haemorrhage and venous pressure in the forehead. Aerosp. Med. 1964; 35,533-45. 17. Oberg, B. & Thortn, P. Increased activity in left ventricular receptors during haemorrhage or occlusion of caval veins in the cat - a possible cause of the vaso-vagal reaction. Acta Physiol. Scand. 1972; 85,164-73. 18. Thortn, P., Skarphedinsson, J.O. & Carlsson, S. Sympathetic inhibition from vagal afferents during severe haemorrhage in rats. Acta Physiol. Scand. 1988; 133 (SUPPI.571), 97-105. 19. Semple, P.F., ThorCn, P. & Lever, A.F. Vasovagal reactions 583 to cardiovascular drugs: the first dose effect. J. Hypertens. 1988; 6,601-6. 20. Abboud, EM. Ventricular syncope: is the heart a sensory organ? N. Engl. J. Med. 1989; 320,390-2. 21. Blomqvist, C.G. & Stone, H.L. Cardiovascular adjustments to gravitational stress. In: Shepherd, J.T. & Abboud, EM., eds. Handbook of physiology. American Physiological Society, Washington, D.C.: 1984, 1025-63. (The cardiovascular system, section 2.) 22. Henry, J.P. On the triggering mechanism of vasovagal syncope. Psychosom. Med. 1984; 46,91-3. 23. Sheehan, H.L. The pathology of obstetric shock. J. Obstet. Gynecol. 1939; 46,218-31. 24. Morita, H. & Vatner, S.F. Effects of haemorrhage on renal nerve activity in conscious dogs. Circ. Res. 1985; 57, 788-93. 25. Landgren, S. On the excitation mechanism of the carotid baroreceptors. Acta Physiol. Scand. 1952; 26,l-34. 26. Angel1 James, J.E. The responses of aortic arch and right subclavian baroreceptors to changes of non-pulsatile pressure and their modification by hypothermia. J. Physiol. (London) 1971; 214,201-23. 27. Schemer, U., Vissing, S., Morgan, B.J., Hanson, P. & Victor, R.G. Vasovagal syncope after infusion of a vasodilator in a heart-transplant recipient. N. Engl. J. Med. 1990; 332,602-4. 28. Goldstein, D.S., Spanarkel, M., Pitterman, A. et al. Circulatory control mechanisms in vasodepressor syncope. Am. HeartJ. 1982; 104,1071-5. 29. Weissler, A.M., Warren, J.V., Estes, E.H., McIntosh, H.D. & Leonard, J.J. Vasodepressor syncope. Factors influencing cardiac output. Circulation 1957; 15,875-82. 30. Murray, R.H. & Shropshire, S. Effect of atropine on circulatory responses to lower body negative pressure and vasodepressor syncope. Aerosp. Med. 1970; 41,717-22. 31. Ziegler, M.G., Echon, C., Wilner, K.D., Specho, P., Lake, C.R. & McCutchen, J.A. Sympathetic nervous withdrawal in the vasodepressor (vasovagal) reaction. J. Auton. Nerv. Syst. 1986; 17,273-8. 32. Murray, R.H., Thompson, L.J., Bowers, J.A. & Albright, C.D. Hemodynamic effects of graded hypovolemia and vasodepressor syncope induced by lower body negative pressure. Am. Heart J. 1968; 76,799-81 1. 33. Almquist, A., Gornick, C., Benson, D.W., Dunnigan, A. & Benditt, D.G. Carotid sinus hypersensitivity: evaluation of the vasodepressor component. Circulation 1985; 71, 927-36. 34. Zee-Cheng, C. & Gibbs, H.R. Pure vasodepressor carotid sinus hypersensitivity.Am. J. Med. 1986; 81, 1095-7. 35. Robertson, D., Hollister, AS., Forman, M.B. & Robertson, R.M. Reflexes unique to myocardial ischemia and infarction. J. Am. Coll. Cardiol. 1985; 5,99-104B. 36. Wei, J.Y., Markis, J.E., Malagold, M. & Braunwald, E. Cardiovascular reflexes stimulated by reperfusion of ischemic myocardium in acute myocardial infarction. Circulation 1983; 67,796-801. 37. Eckberg, D.L., White, C.W., Kioschos, J.M. & Abboud, EM. Mechanisms mediating bradycardia during coronary arteriography. J. Clin. Invest. 1974; 54,1455-61. 38. Hainsworth, R. Fainting. In: Bannister, R., ed. Autonomic failure, a textbook of clinical disorders of the autonomic nervous system. Oxford: Oxford University Press, 1988: 142-58. 39. Wallin, B.G., Westerberg, C.E. & Sundlof, G. Syncope induced by glossopharyngeal neuralgia: sympathetic outflow to muscle. Neurology 1984; 34,522-4. 40. Rowell, L.B. Active neurogenic vasodilatation in man. In: Vanhoutte, P.M. & Leusen, I., eds. Vasodilatation. New York: Raven Press, 1981: 1-17. 41. Glick, G. & Yu, P.N. Hemodynamic changes during spontaneous vasovagal reactions. Am. J. Med. 1963; 34, 42-51. 42. Wallin, B.G. & Sundlof, G. Sympathetic outflow to muscles 584 J. J. van Lieshout et al. during vasovagal syncope. J. Auton. Nerv. Syst. 1982; 6, 287-91. 43. Weiss, S., Wilkins, R.W. & Haynes, F.W. The nature of circulatory collapse induced by sodium nitrite. J. Clin. Invest. 1937; 16.73-84. 44. Robertson, D., Johnson, G.A., Robertson, R.M., Nies, S.A., Shand, D.G. & Oates, J.A. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation 1979; 59,637-43. 45. Sander-Jensen, K., Secher, N.H., Astrup, A. et al. Hypotension induced by passive head-up tilt: endocrine and circulatory mechanisms. Am. J. Physiol. 1986; 251 (Regul. Integr. Comp. Physiol. 22), R742-8. 46. Schadt, J.C. & Ludbrook, J. Hemodynamic and neuro humoral responses to acute hypovolemia in conscious mammals. Am. J. Physiol. 1991;260 (Heart Circ. Physiol. 29). H305-18. 47. Newberry, P.D., Hatch, A.W. & MacDonald, J.M. Cardiorespiratory events preceding syncope induced by a combination of lower body negative pressure and head-up tilt. Aerosp. Med. 1970; 41,373-8. 48. Rowell, L.B. & Blackmon, J.R. Hypotension induced by central hypovolemia and hypoxemia. Clin. Physiol. 1989; 9,269-77. 49. Rowell, L.B. & Seals, D.R. Sympathetic activity during graded central hypovolemia in hypoxemic humans. Am. J. Physiol. 1990; 259 (Heart Circ. Physiol. 28), H1197-206. 50. Warren, J.V., Brannon, E.S., Stead, E.A. & Merrill, A.J. The effect of venesection and the pooling of blood in the extremities on the atrial pressure and cardiac output in normal subjects with observations on acute circulatory collapse in three instances. J. Clin Invest. 1945; 24,337-44. 51. Blair, D.A., Glover, W.E., Greenfield, A.D.M. & Roddie, I.C. Excitation of cholinergic vasodilator nerves to human skeletal muscles during emotional stress. J. Physiol. (London) 1959; 148,633-47. 52. Greenfield, A.D.M. An emotional faint. Lancet 1951; i, 1302-3. 53. Roddie, I.C. Human responses to emotional stress. Ir. J. Med. Sci. 1977; 395-417. 54. Shepherd, J.T. Circulation to skeletal muscle. In: Shepherd, J.T. & Abboud, EM., eds. Handbook of physiology. American Physiological Society, Washington, D.C.: 1984, 3 19-70. (The cardiovascular system, section 2.) 55. Sanders, J.S., Mark, A.L. & Ferguson, D.W. Evidence for cholinergically mediated vasodilatation at the beginning of exercise in humans. Circulation 1989; 79,815-24. 56. Lind, A.R., Taylor, S.H., Humphreys, P.W., Kennelly, B.M. & Donald, K.W. The circulatory effects of sustained voluntary muscle contraction. Clin. Sci. 1964; 27,229-44. 57. Greenfield, A.D.M. Survey of the evidence for active neurogenic vasodilatation in man. Fed. Proc. Fed. Am. SOC. Exp. Biol. 1966; 25, 1607-10. 58. Barcroft, H., Brod, J., He$, Z., Hirsjarvi, E.A. & Kitchin, A.H. The mechanism of the vasodilatation in the forearm muscle during stress (mental arithmetic). Clin. Sci. 1960; 19,577-86. 59. Eckberg, D.L., Harkins, S.W., Fritsch, J.M., Musgrave, G.E. & Gardner, D.F. Baroreflex control of plasma norepinephrine and heart period in healthy subjects and diabetic patients. J. Clin. Invest. 1986; 78,366-74. 60. Esler, M., Jennings, G., Lambert, G., Meredith, I., Horne, M. & Eisenhofer, G. Overflow of catecholamine neurotransmitters to the circulation: source, fate and functions. Physiol. Rev. 1990; 70,963-85. 61. Burke, D., Sundlof, G. & Wallin, B.G. Postural effects o n muscle nerve sympathetic activity in man. J. Physiol. (London) 1977; 272,399-414. 62. Eckberg, D.L. & Sleight, P. Human baroreflexes in health and disease. Oxford: Oxford University Press, 1991. (In press). 63. Yatomi, A., Iguchi, A., Uemura, K., Sakamoto, N., Iwase, S. & Mano, T. A rare case of recurrent vasodepressive 64. 65. 66. 67. 68. 69. 70. 71. 72. attacks of 2-hours duration: analysis of the mechanisms by muscle sympathetic nerve activity recording. Clin. Cardiol. 1989; 12,164-8. Robinson, B.J. & Johnson, R.H. Why does vasodilatation occur during syncope? Clin. Sci. 1988; 74,347-50. Epstein, S.E., Stampfer, M. & Beiser, G.D. Role of the capacitance and resistance vessels in vasovagal syncope. Circulation 1968; 37,524-33. Hirsch, A.T., Levenson, D.J., Cutler, S.S., Dzau, VJ. & Creager, M.A. Regional vascular responses to prolonged lower body negative pressure in normal subjects. Am. J. Physiol. 1989; 257 (Heart Circ. Physiol. 26), H219-55. Grayson, J. & Swan, H.J.C. Intestinal blood-flow changes in man during fainting. J. Physiol. (London) 195 1; 112,44. Hainsworth, R. The importance of vascular capacitance in cardiovascular control. News Physiol. Sci. 1990; 5,250-4. Bergenwald, L., Freyschuss, U. & Sjostrand, T. The mechanism of orthostatic and haemorrhagic fainting. Scand. J. Clin. Lab. Invest. 1977; 37,209-16. Rothe, C.F. Venous system: physiology of the capacitance vessels. In: Shepherd, J.T. & Abboud, EM., eds. Handbook of physiology. American Physiological Society, Washington, D.C.: 1984, 397-453. (The cardiovascular system, section 2.) Buckey, J.C., Peshock, R.M. & Blomqvist, C.G. Deep venous contribution to hydrostatic blood volume change in the human leg. Am. J. Cardiol. 1988; 62,449-53. Mayerson, H.S. & Burch, G.E. Relationships of tissue (subcutaneous and intramuscular) and venous pressure to svncoDe induced in man bv gravitv. Am. J. Phvsiol. 1940: i z s , i58-69. Tarazi. R.C.. Melsher. HJ.. Dustan. H.P. & Frohlich. E.D. Plasma volume changes with upright tilt: studies in hypertension and in syncope. J. Appl. Physiol. 1970; 28, 121-6. Foud-Tarazi, EM. & Maloney, J.D. Vasovagal syncope: lack of relationship between baseline blood volume and presyncopal chronotropic response. Clin. Res. 1989; 37, 880A. Kravik, S.E., Keil, L.C., Geelen, G. et al. Effect of antigravity suit inflation on cardiovascular, PRA, and PVP responses in humans. J. Appl. Physiol. 1986; 62,766-74. Tarazi, R.C. & Fouad, EM. Circulatory dynamics in progressive autonomic failure. In: Bannister, R., ed. Autonomic failure, a textbook of clinical disorders of the autononic nervous system. Oxford: Oxford University Press, 1983: 109-14. Ludbrook, J. Faint heart. Br. Med. J. 1989; 298, 1053-4. Vingerhoets, A.J.J.M. Biochemical changes in two subjects succumbing to syncope. Psychosom. Med. 1984; 46, 96-103. Graham, D.T. Prediction of fainting in blood donors. Circulation 1961; 23,901-6. Engel, G.L. Psychologic stress, vasodepressor (vasovagal) syncope, and sudden death. Ann. Intern. Med. 1978; 89, 403-12. Davies, M.J. & Thomas, A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N. Engl. J.Med. 1984;310,1137-40. McKenna, W.J. & Camm, A J . Sudden death in hypertrophic cardiomyopathy. Assessment of patients at high risk. Circulation 1989; 89, 1489-92. Milstein, S., Buetikofer, J., Lesser, J. et al. Cardiac asystole: a manifestation of neurally mediated hypotensionbradycardia. J. Am. COIL Cardiol. 1989; 14, 1626-32. Maloney, J.D., Jaeger, F.J., Fouad-Tarazi, EM. & Morris, H.H. Malignant vasovagal syncope: prolonged asystole provoked by head-up tilt. Cleveland Clin. J. Med. 1988; 55,542-8. McIntosh, H.D. The stabilizing and unstabilizing influences of neurogenic and vascular activities of the heart as related to sudden cardiac death. J. Am. Coll. Cardiol. 1985; 5,105-10B. Sander-Jensen, K., Secher, N.H., Bie, P., Warberg, J. & .- 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. I , I , < The vasovagal response Schwartz, T.W. Vagal slowing of the heart during haemorrhage: observations from 20 consecutive hypotensive patients. Br. Med. J. 1986; 292,3646. 87. Oberg, B. & White, S. The role of vagal cardiac nerves and arterial baroreceptors in the circulatory adjustments to haemorrhage in the cat. Acta Physiol. Scand. 1970; 80, 395-403. 88. Bjurstedt, H., Rosenhamer, G., Balldin, U. & Katkov, V. Orthostatic reactions during recovery from exhaustive exercise of short duration. Acta Physiol. Scand. 1983; 119,25-31. 89. Guyton, A.C., Cowley, A.W., Norman, R.A., Coleman, T.G. & Samar, R.E. The arterial baroreceptor reflex, a pressure buffering system. In: Guyton, A.C., ed. Circulatory physiology 111. Arterial pressure and hypertension. Philadelphia: W.B. Saunders, 1980,248-65. 90. Bennett, T. & Gardiner, S.M. Involvement of vasopressin in cardiovascular regulation. Cardiovasc. Res. 1985; 19, 57-68. 91. Goldenbeg, I.F., Almquist, A., Dunbar, D.N., Milstein, S., Pritzker, M.R. & Benditt, D.G. Prevention of neurallymediated syncope by selective beta-1 adrenoceptor blockade. Circulation 1987; 76 (Suppl.),IV-133. 92. Sander-Jensen, K., Mehlsen, J., Secher, N.H. et al. Progressive central hypovolaemia in man resulting in a vasovagal syncope? Haemodynamic and endocrine variables during venous tourniquets of the thighs. Clin. Physiol. 1987; 7, 231-42. 93. Zanchetti, AS. Neural regulation of renin release. Circulation 1977; 56,691-8. 94. Oparil, S., Vassaux, C., Sanders, C.A. & Haber, E. Role of renin in acute postural homeostasis. Circulation 1970; 41, 89-95. 95. Tuck, M.L., Dluhy, R.G. & Williams, G.H. Sequential responses of the renin-angiotensin-aldosterone axis to acute postural change: effect of dietary sodium. J. Lab. Clin. Med. 1975; 86,754-63. 96. Morganti, A., Lopez-Ovejero, J.A., Pickering, T.G. & Laragh, J.H. Role of the sympathetic nervous system in mediating the renin response to head-up tilt. Am. J. Cardiol. 1979; 43,600-4. 97. Shvartz, E., Convertino, V.A., Keil, L.C. & Haines, R.F. Orthostatic fluid-electrolyte and endocrine responses in fainters and nonfainters. J. Appl. Physiol. 1981; 51, 1404-10. 98. Goldsmith, S.R. Baroreflex control of vasopressin secretion in normal humans. In: Cowley, A.W., Liard, J.F. & Ausiello, D.A., eds. Vasopressin. Cellular and integrative functions. New York: Raven Press 1988,389-97. 99. Sander-Jensen, K., Mehlsen, J., Stadeager, C. et al. Increase in vagal activity during hypotensive lower-body negative pressure in humans. Am. J. Physiol. 1988; 253 (Regul. Integr. Comp. Physiol. 24), R149-56. 100. Verbalis, J.G., Richardson, D.W. & Stricker, E.M. Vasopressin release in response to nausea-producing agents and cholecystokinin in monkeys. Am. J. Physiol. 1987; 252 (Regul.Integr. Comp. Physiol. 23), R749-53. 101. Wang, B.C., Flora-Ginter, G., Leadley, R.J. & Goetz, K.L. Ventricular receptors stimulate vasopressin release during hemorrhage. Am. J. Physiol. 1988; 254 (Regul. Integr. Comp. Physiol. 23), R204-11. 102. Convertino, V.A., Thompson, C.A., Benjamin, B.A. et al. Haemodynamics and ADH responses to central blood volume shifts in cardiac-denervated humans. Clin. Physiol. 1990; 10,55-67. 103. Goldsmith, S.R. The effect of moderate hypotension on vasopressin levels in normal humans. Am. J. Med. Sci. 1989; 298,295-8. 104. Abrahamsson, H. & ThorCn, P. Vomiting and reflex vagal relaxation of the stomach elicited from heart receptors in the cat. Acta Physiol. Scand. 1973; 88,433-9. 105. Sleight, P. Cardiac vomiting. Br. Heart J. 1981; 46,5-7. 585 106. Van Lieshout, J.J. Cardiovascular reflexes in orthostatic disorders. Amsterdam: Rodopi, 1989. 107. Cowley, A.W., Quillen, E.W. & Skelton, M.M. Role of vasopressin in cardiovascular regulation. Fed. Proc. Fed. Am. SOC.Exp. Biol. 1983; 42,3170-6. 108. Schwartz, T.W. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology 1983; 85,1411-25. 109. Krarup, T., Schwartz, T.W., Hilsted, J., Madsbag, S., Verlaege, 0. & Sestoft, L. Impaired response of pancreatic polypeptide to hypoglycaemia: an early sign of autonomic neuropathy. Br. Med. J. 1979; 2, 1544-6. 110. Ludbrook, J. & Rutter, P.C. Effect of naloxone on haemodynamic responses to acute blood loss in unanaesthetized rabbits. J. Physiol. (London) 1988; 400, 1-14. 1 1 1 . Verrill, P.J. & Aellig, W.H. Vasovagal faint in the supine position. Br. Med. J. 1970; iv, 348. 112. Day, S.C. & Eisenberg, J.M. Syncope. In: Griner, P.F., Panzer, J.R. & Greenland, P., eds. Clinical diagnosis and the laboratory. Chicago: Year Book Medical Publishers Inc., 1980: 107-22. 1 1 3. Abi-Samra, F., Maloney, J.D., Fouad-Tarazi, EM. & Castle, L.W. The usefulness of head-up tilt testing and hemodynamic investigations in the workup of syncope of unknown origin.Pace 1988; 11,1202-14. 114. Stevens, P.M. Cardiovascular dynamics during orthostasis and the influence of intravascular instrumentation. Am. J. Cardiol. 1966; 17,211-18. 1 1 5. Shvartz, E., Meroz, A., Magazanik, A., Shoenfeld, Y. & Shapiro, Y. Exercise and heat orthostatism and the effect of heat acclimation and physical fitness. Aviat. Space Environ. Med. 1977; 48,836-42. 116. Graybiel, A. & McFarland, R.A. The use of the tilt-table test in aviation medicine. Aviat. Med. 1941; 11, 194-21 1 . 117. Ogata, H., Iinuma, N., Nagashima, K. & Akabane, T. Vasovagal reactions in blood donors. Transfusion 1980; 20,679-83. 118. Convertino, V.A., Doerr, D.F., Flores, J.F., Hoffler, G.W. & Buchanan, P. Leg size and muscle functions associated with leg compliance. J. Appl. Physiol. 1988; 64, 1017-21. 119. Dambrink, J.H.A., Imholz, B.P.M., Karemaker, J.M. & Wieling, W. Circulatory adaptation to orthostatic stress in healthy 10- 14-year-old children investigated in a general practice. Clin. Sci. 1991; 81,51-8. 120. Lipsitz, L.A., Marks, E.R., Koestner, J., Jonsson, P.V. & Wei, J.Y. Reduced susceptibility to syncope during postural tilt in old age. Arch. Intern. Med. 1989; 149, 2709-12. 121. Imholz, B.P.M., Dambrink, J.H.A., Karemaker, J.M. & Wieling, W. Orthostatic circulatory control in the elderly evaluated by non-invasive continuous blood pressure measurement. Clin. Sci. 1990; 79,73-9. 122. Wagner, H.N. Orthostatic hypotension. Bull. Johns Hopkins Hosp. 1959; 105,322-59. 123. Van Lieshout, J.J., Wieling, W., Wesseling, K.M. & Karemaker, J.M. Pitfalls in the assessment of cardiovascular reflexes in patients with sympathetic failure but intact vagal control. Clin. Sci. 1989; 76,523-8. 124. Grant, R.T. & Reeve, E.B. Clinical observations on air-raid casualties. Br. Med. J. 1941; 2,293-7 and 329-32. 125. McMichael, J. Clinical aspects of shock. J. Am. Med. ASSOC. 1944; 124,275-81. 126. Shenkin, H.A., Cheney, R.H., Govons, S.R., Hardy, J.D. & Fletcher, A.G. On the diagnosis of hemorrhage in man. A study of volunteers bled large amounts. Am. J. Med. Sci. 1944; 208,421-36. 127. Jansen, R.P.S. Relative bradycardia: a sign of acute intraperitoneal bleeding. Aust. N. Z. J. Obstet. Gynaecol. 1978; 18,206-8. 128. Abelmann, W.H. Alterations in orthostatic tolerance after myocardial infarction and in congestive heart failure. Cardiology 1976; 61 (Suppl. l),236-48. 129. Bristow, M.R., Ginsburg, R., Minobe, W. et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor J. J. van Lieshout et al. 586 density in failing human hearts. N. Engl. J. Med. 1982; 307,205-1 1. 130. Fleg, J.L. & Lakatta, E.G. Prevalence and significance of postexercise hypotension in apparently healthy subjects. Am. J. Cardiol. 1986; 57,1380-4. 131. Braunwald, E., Lambrew, C.T., Rockoff, S.D., Ross, J. & Morrow, A.G. Idiopathic hypertrophic subaortic stenosis. Circulation 1964; 30 (Suppl. 4), 1-1 15. 132. Mark, A.L., Kioschos, J.M., Abboud, EM., Heistad, D.D. & Schmid, P.G. Abnormal vascular responses to exercise in patients with aortic stenosis. J. Clin. Invest. 1973; 52, 1138-46. 133. Richards, A.M., Nicholls, M.G., Ikram, H., Hamilton, E.J. & Richards, R.D. Syncope in aortic valvular stenosis. Lancet 1984; ii, 1 1 13-6. 134. Manolis, AS., Linzer, M., Salem, D. & Estes, N.A.M. Syncope: current diagnostic evaluation and management. Ann. Intern. Med. 1990; 112,850-63. 135. Martin, GJ., Adams, S.L., Martin, H.G., Mathews, J., Zull, D. & Scanlon, P.J. Prospective evaluation of syncope. Ann. Emerg. Med. 1984; 13,499-504. 136. Noble, R.J. The patient with syncope. J. Am. Med. Assoc. 1977; 237,1372-6. 137. Sapire, D.W., Shah, J.J. & Black, I.F.S. Prolonged atrioventricular conduction in young children and adolescents. S . Afr. Med. J. 1979; 55,669-73. 138. Sapire, D.W., Casta, A., Safley, W., ORiordan, A.C. & Balsara, R.K. Vasovagal syncope in children requiring pacemaker implantation. Am. Heart J. 1983; 106, 1406-1 1. 139. Ector, H., Verlinden, M., Vanden Eynde, E. et al. Bradycardia, ventricular pauses, syncope, and sports. Lancet 1984; ii, 591-4. 140. Mehta, A.V., O'Riordan, A.C., Sanchez, G.R., Balsara, R.K. & Black, I.F.S. Mobitz Type I second degree atrioventricular block in children: clinical and electrophysiological findings and long-term follow-up. J. Am. Coll. Cardiol. 1983; 1,613. 141. Schlesinger, Z., Barzilay, J., Stryjer, D. & Almog, C.H. Life-threatening vagal reaction to emotional stimuli. Isr. J. Med.Sci. 1977; 13,59-61. 142. Fujimura, O., Yee, R., Klein, G.J., Sharma, A.D. & Boahene, K.A. The diagnostic sensitivity of electrophysiologic testing in patients with syncope caused by transient bradycardia. N. Engl. J. Med. 1989; 321, 1703-7. 143. Gulamhusein, S., Naccarelli, G.V., KO, P.T. et al. Value and limitations of clinical electrophysiological study in assessment of patients with unexplained syncope. Am. J. Med. 1982; 73,700-5. 144. Allen, S.C., Taylor, C.L. & Hall, V.E. A study of orthostatic insufficiencv bv the tiltboard method. Am. J. Phvsiol. 1945; 143,-11-d20. 145. Kennv. R.A.. Bavliss. J.. Ingram. A. & Sutton. R. Head-uD tilt: a useful test for investigating unexplained syncope. Lancet 1986; i, 1352-4. 146. Fitzpatrick, A. & Sutton, R. Tilting towards a diagnosis in recurrent unexplained syncope. Lancet 1989; i, 658-69. 147. Chen, M.Y., Goldenberg, I.F., Milstein, S . et al. Cardiac electrophysiologic and hemodynamic correlates of neurally mediated syncope. Am. J. Cardiol. 1989; 63, 66-72. 148. Strasberg, B., Rechavia, E., Sagie, A. et al. The head-up tilt table test in patients with syncope of unknown origin. Am. Heart J. 1989; 118,923-7. 149. Scott, A. Head-up tilt test for unexplained syncope. Lancet 1986; i, 169. I , I 150. Imholz, B.P.M., Wieling, W., Langewouters, G.J. & Van Montfrants, G.A. Continuous finger arterial pressure: utility in the cardiovascular laboratory. Clin. Auton. Res. 1991; 1,43-53. 151. Almquist, A., Goldenberg, I.F., Milstein, S. et al. Provocation of bradycardia and hypotension by isoproterenol and upright posture in patients with unexplained syncope. N. Engl. J. Med. 1989; 320,346-51. 152. Waxman, M.B., Yao, L., Cameron, D.A., Wald, R.W. & Roseman, J. Isoproterenol induction of vasodepressortype reaction in vasodepressor-prone persons. Am. J. Cardiol. 1989; 63,58-65. 153. Piorry, P.A. Recherches sur I'influence de la pesanteur sur le cours du sang; diagnostic de la syncope et de I'apoplexie; cause et traitement de la syncope. Arch. GCn. MCd. 1826; 12,527-44. 154. Eagle, K.A., Black, H.R., Cook, E.F. & Goldman, L. Evaluation of prognostic classifications for patients with syncope. Am. J. Med. 1985; 79,455-60. 155. Smith, M.L., Hudson, D.L. & Raven, P.B. Effect of muscle tension on the cardiovascular responses to lower body negative pressure in man. Med. Sci. Sports Exercise. 1987; 19,436-42. 156. Ebert, T.J. & Barney, J.A. Physical fitness and orthostatic tolerance. In: Smith, J.J., ed. Circulatory response to the upright posture. Boca Raton: CRC Press, 1990: 47-63. 157. Frey, M.A.B. Considerations in prescribing preflight aerobic exercise for astronauts. Aviat. Space Environ. Med. 1987; 58,1014-23. 158. Convertino, V.A. Aerobic fitness, endurance training, and orthostatic intolerance. Exercise Sport Sci. Rev. 1987; 15, 223-59. 159. Ten Harkel, A.D.J., Baisch, F., Becj, L. & Karemaker, J.M. The autonomic nervous system in blood pressure regulation during 10 days 6" head down tilt. The Physiologist 1990; 33, S178-9. 160. Convertino, V.A., Sather, T.M., Goldwater, D.J. & Alford, W.R. Aerobic fitness does not contribute to prediction of orthostatic intolerance. Med. Sci. Sports Exercise 1986; 18,551-6. 161. Fitzpatrick, A., Travill, C.M., Vardas, P.E. ct al. Recurrent symptoms after ventricular pacing in unexplained syncope. Pace 1990; 13,619-24. 162. Kust, T., Lalonde, G., de Champlain, J. & Shenas, M. Vasovagal syncope: management with atrioventricular sequential pacing and beta-blockade. Can. J. Cardiol. 1989; 5,375-8. 163. Sander-Jensen, K., Mehlsen, J., Stadeager, C. et al. Increased cholinergic activity during gravitation-induced pre-syncope in man. The Physiologist 1986; S-74. 64. Dibner-Dunlap, M.E., Eckberg, D.L., Magid, N.M. & Cintron-Trevino, N.M. T h e long-term increase of baseline and reflexly augmented levels of human vagal-cardiac nervous activity induced by scopolamine. Circulation 1985; 71,797-804. 65. Ferguson, D.W., Thames, M.D. 6r Mark, A.L. Effects of propanolol on reflex vascular responses to orthostatic stress in humans. Circulation 1983; 67, 802-7. 166. MacLean, A.R. & Allen, E.V. Orthostatic hypotension and orthostatic tachycardia. Treatment with the head-up tilt bed. J. Am. Med.Assoc. 1940; 115,2162-7. 167. Bannister, R. & Mathias, C. Management of postural hypotension. In: Bannister, R., ed. Autonomic failure, a textbook of clinical disorders of the autonomic nervous system. 2nd ed. Oxford: Oxford University Press, 1988: 569-95.