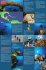Dissertation - RC Lab - University of California, Santa Cruz
Transcription
Dissertation - RC Lab - University of California, Santa Cruz
UNIVERSITY OF CALIFORNIA
SANTA CRUZ
SPECIES INTERACTIONS AFFECTING CORALS AND RECRUITMENT
ON A PROTECTED, HIGH-LATITUDE REEF: HERBIVORY, PREDATION,
AND COMPETITION BY FISHES, URCHINS, MACRO ALGAE AND
CYANOBACTERIA
A dissertation submitted in partial satisfaction
of the requirements for the degree of
DOCTOR OF PHILOSOPHY
in
ECOLOGY AND EVOLUTIONARY BIOLOGY
by
Wendy A. Cover
June 2011
The Dissertation of Wendy A. Cover
is approved:
Professor Donald C. Potts, Chair
Professor Mark Carr
Professor James Estes
Professor Michael Graham
Tyrus Miller
Vice Provost and Dean of Graduate Studies
UMI Number: 3471811
All rights reserved
INFORMATION TO ALL USERS
The quality of this reproduction is dependent upon the quality of the copy submitted.
In the unlikely event that the author did not send a complete manuscript
and there are missing pages, these will be noted. Also, if material had to be removed,
a note will indicate the deletion.
UMI
Dissertation Publishing
UMI 3471811
Copyright 2011 by ProQuest LLC.
All rights reserved. This edition of the work is protected against
unauthorized copying under Title 17, United States Code.
ProQuest LLC
789 East Eisenhower Parkway
P.O. Box 1346
Ann Arbor, Ml 48106-1346
Copyright© by
Wendy A. Cover
2011
TABLE OF CONTENTS
LIST OF FIGURES AND TABLES
iv
ABSTRACT
vi
INTRODUCTION
1
CHAPTER 1. Coral recruitment on Midway Atoll: settlement patterns,
cyanobacterial blooms, and grazing effects on a high-latitude reef
6
Abstract
Introduction
Methods
Results
Discussion
6
7
13
20
26
CHAPTER 2. Differentiating impacts of fish and urchin grazing on algal
growth and coral recruitment
Abstract
Introduction
Methods
Results
Discussion
57
57
58
61
65
71
CHAPTER 3. Direct, species-specific impacts of sea urchins on live corals
Abstract
Introduction
Methods
Results
Discussion
87
87
88
92
98
102
LITERATURE CITED
118
in
LIST OF FIGURES AND TABLES
CHAPTER 1
Table 1. Total counts and mean numbers of coral recruits per tile
Table 2. One-way ANOVA for numbers of recruits per tile among six sites,
and a matrix of pairwise comparison probabilities
Table 3. Numbers of recruits on forward and rear tiles and two-way ANOVA
Table 4. Mean numbers of coral recruits per m2 on the three tile surfaces and
their subcategories (zones)
Table 5. Numbers and percent of coral recruits settling on different substrates
Table 6. Condition of recruits based on amount of overgrowth by other
organisms
Table 7. Frequency of recruit overgrowth by various organisms
Figure 1. Study sites on Midway Atoll, Northwestern Hawaiian Islands
Figure 2. Tile surfaces and zones used when scoring data
Figure 3. Examples of each taxon recorded
Figure 4. Mean coral recruitment for three treatments and by site
Figure 5. Mean numbers of recruits per tile pair for each coral taxon at each
site
Figure 6. Proportion of total benthic cover comprised of adult coral colonies
at four sites
Figure 7. Frequency distributions of coral recruits by a) size, and b) number
of corallites
Figure 8. Relationship between colony size and number of corallites in
colony
Figure 9. Total numbers of recruits in grooved vs. non-grooved (exposed)
zones of outer tile surfaces at each site
Figure 10. Numbers of recruits in three coral taxa that settled on the four
most common substrates
Figure 11. Numbers of recruits that were Overgrown and Not Overgrown at
each site
Figure 12. Examples of various organisms overgrowing coral recruits
Figure 13. Numbers of recruits overgrown by CCA or by Other Algae
Appendix. Additional organisms noted on settlement tiles
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
CHAPTER 2
Table 1. 2-factor ANOVA comparing algal wet weights in factorial
treatments exposed to fishes and urchins
IV
75
Table 2. Effect of urchins and fishes on species composition of algae in each
treatment
Table 3. 2-factor ANOVA comparing sediment wet weights in factorial
treatments exposed to fishes and urchins
Table 4. 2-factor ANOVA comparing the number of coral recruits in factorial
treatments exposed to fishes and urchins
Figure 1. Diagram of the 2 x 2 factorial design
Figure 2. Effects of fishes and urchins on the mean biomass of algae on
experimental tiles
Figure 3. Effect of urchin density on algal biomass
Figure 4. Biomass of turf algae in the four treatments
Figure 5. Mass of sediments on tiles in the four treatments
Figure 6. Numbers of coral recruits per tile (± 1SE) in the four treatments
Figure 7. Number of coral recruits as a function of algal biomass
Figure 8. Quantitative spectra of light under three treatments (open, full cage,
and half cage) and a natural rock overhang
76
77
78
79
80
81
82
83
84
85
86
CHAPTER 3
9
1
1
Table 1. Mean daily rates (cm urchin" day") of live coral tissue loss from
corals exposed to E. mathaei
Table 2. 2-factor ANOVA results for rate of coral tissue removal by site and
coral genus
Table 3. 2-factor ANOVA results for day of first damage by site and coral
genus
Figure 1. Study sites on Midway Atoll
Figure 2. Numbers of undamaged Pocillopora ligulata nubbins per plot
Figure 3. Area (mean ± 1SE) of Pocillopora ligulata tissue removed by E.
mathaei on days 1,3, and 9
Figure 4. Two Pocillopora ligulata nubbins, one exposed to E. mathaei and
the other in a control plot without E. mathaei
Figure 5. Numbers of damaged and undamaged coral nubbins by treatment
(E. mathaei removed or present) for two coral taxa after 116 days
Figure 6. Examples of coral nubbins (with and without E. mathaei) on three
dates for a) Montipora flabellata, and b) Porites lobata
Figure 7. Number of days (mean ± 1SD) for nubbins to be damaged by E.
mathaei
v
108
109
110
111
112
113
114
115
116
117
ABSTRACT
SPECIES INTERACTIONS AFFECTING CORALS AND RECRUITMENT
ON A PROTECTED, HIGH-LATITUDE REEF: HERBIVORY, PREDATION,
AND COMPETITION BY FISHES, URCHINS, MACRO ALGAE AND
CYANOBACTERIA
Wendy A. Cover
Interactions between species can affect major processes that shape community
structure in many systems. Both fishes and urchins are important herbivores on
corals reefs, maintaining resilience through their grazing activities and preventing
coral-macroalgal phase shifts. Despite their importance, little is known about the
relative importance of these different herbivore guilds and of the scale and magnitude
of their positive and negative effects on corals. First, I investigated coral recruitment
at six backreef sites on Midway Atoll, including two anthropogenically-impacted
sites with metal debris that have periodic blooms of the benthic cyanobacterium
Hormothamnion enteromorphoides. Contrary to expectations, coral recruitment was
significantly higher at the two cyanobacterial bloom sites than at the four control
sites. The proportion of recruits on exposed surfaces was higher at bloom sites than
at controls, indicating that cyanobacteria indirectly enhanced recruitment by
inhibiting fish grazers that usually remove small corals from exposed surfaces. Next,
I conducted a factorial field experiment to quantify the relative effects of herbivorous
fishes and urchins (Echinometra mathaei) on macroalgal growth and coral
recruitment. Fish grazing effectively limited algal biomass, which was >50 times
higher in treatments without fishes. Coral recruitment was >2X higher in treatments
exposed to fish grazing, indicating that algae inhibit coral recruitment more than do
fishes. Algal biomass was negatively correlated with coral recruitment, suggesting
that management to increase herbivorous fishes and grazing levels is likely to benefit
coral recruitment. Finally, I investigated direct, species-specific effects of urchins on
corals. Most fragments of all three coral species exposed to E. mathaei were
damaged within days and the damage increased over several weeks to months, often
ending with complete removal of all coral tissue and skeleton. Fragments in plots
without E. mathaei, and all fragments exposed to H. mammillatus plots were
unaffected. These studies demonstrate a number of positive and negative, direct and
indirect effects of herbivores on corals. Negative effects of urchins are lessened by
their spatial restrictions, and negative effects of fishes are outweighed by the positive
effects of algae removal, clearing space for coral recruits and enhancing the resilience
of coral reef communities.
INTRODUCTION
Interactions between species can sometimes be so profound that they shape
the entire structure of the community (Terborgh & Estes 2010). Massive changes in
communities are often referred to as "phase shifts" or "regime shifts", where the
foundational species in the community change dramatically in abundance, usually due
to changes in top-down forcing (Scheffer et al. 2001, Folke et al. 2004). Some of the
most striking examples in both terrestrial and aquatic systems come from trophic
cascades, where a top predator has cascading effects through multiple trophic levels,
indirectly controlling the abundance of lower levels, and often affecting the
foundational species (Terborgh & Estes 2010). Freshwater lakes are a well-known
example of such control: in the absence of a piscivore, small fish consumers dominate
and consume zooplankton, which allows phytoplankton to proliferate, coloring the
lake green. With the addition of a top predator, such as the largemouth bass, there are
cascading effects through each trophic level, reducing small fish and increasing
zooplankton abundance, which then controls phytoplankton and results in a clear,
blue lake (Mittelbach et al. 1995).
There are also important species interactions on coral reefs, which provide the
basis of what I studied. Fishes and urchins are the primary herbivores, consuming
algae, or seaweeds. Algae compete with corals for space on the reef, preventing
corals from occupying new spaces, and sometimes causing coral death (McCook et al.
2001a, Jompa & McCook 2003, Smith et al. 2006). This means that herbivores
1
indirectly benefit corals by removing their algal competitors. On a healthy reef with
abundant herbivore populations, algal densities are kept low, allowing corals to
proliferate and form the framework of the reef ecosystem (Mumby & Harborne
2010). But many reefs worldwide are overfished, resulting in reduced herbivore
densities and lower grazing rates (Jackson et al. 2001). When grazers are effectively
removed from the system, and a disturbance, such as a hurricane or a bleaching event,
kills corals, macroalgae can take over the reef, limiting space for new corals to recruit
and reducing coral growth, ultimately limiting the cover of corals on the reef
(Knowlton 2004).
The importance of these interactions was starkly illustrated in Jamaica, where
rising human populations contributed to overfishing of reef fishes, notably herbivores,
and a disease killed 99% of the primary herbivorous urchins, Diadema antillarum,
throughout the Caribbean (Lessios et al. 1984). Following hurricane Allen, which
destroyed much of the coral cover in the region, and with the last functional
herbivores effectively removed, there was a phase-shift from a coral-dominated to a
macroalgal-dominated state (Hughes 1994). Coral cover declined from over 50% to
around 5% cover, and macroalgae increased to over 90% cover, preventing recovery
of coral populations and leaving the reefs with lower diversity and reduced habitat for
fish and other organisms.
Hurricanes are a natural disturbance from which reefs normally recover, but
instead of recovery, Jamaican reefs experienced a phase shift due to an underlying
loss of resilience in the system. Resilience is the ability of a system to recover from
2
disturbance and return to its original state (Gunderson 2000). As Jamaica lost its
herbivores, it lost the capacity of the reef to prevent macroalgal overgrowth and
recover from disturbance (Scheffer et al. 2001).
One process that is particularly important to the resilience, or recovery
potential, of a system is coral recruitment. Corals have a bipartite life cycle, with
adults living on the benthos and larvae suspended in the water column, transported by
currents until they settle onto the reef and begin to accrete a skeleton, becoming a
new recruit into the coral population of that reef. Recruitment is an important process
that not only replenishes coral populations on healthy reefs, but also fuels recovery on
reefs that have suffered a disturbance and loss of adult corals (Williams et al. 2008,
Done etal. 2010).
Coral reefs are facing increasing threats to their persistence worldwide, with
overfishing, pollution, and climate change among the key issues contributing to reef
loss (Pandolfi et al. 2003). Overfishing not only reduces food supplies, it also lowers
the resilience of the reef by limiting the grazing function of herbivorous fishes
(Hughes et al. 2007a). Pollution can cause many problems, including macroalgal
proliferation via nutrient inputs, but healthy herbivore populations can limit
macroalgal growth despite increased nutrient loads (Smith et al. 2010). Climate
change contributes to warming oceans and coral bleaching events, which are largely
caused by global factors beyond the scope of local reef managers (Baker et al. 2008).
However, resilient reefs with many herbivores are more likely to recover from a
bleaching event (Arthur et al. 2006), causing many to advocate maintaining the
3
resilience of reefs through protection of herbivorous fishes (Hughes et al. 2003).
Fishing regulations and no-take areas (marine protected areas, or MP As) are
important tools for boosting fish populations and maintaining the resilience of reefs to
many perturbations (Hughes et al. 2007a). Several conservation organizations, such
as The Nature Conservancy, have major initiatives aimed at promoting coral reef
resilience.
Despite the importance placed on protecting herbivores and keeping algae at
bay, we know very little about the specific roles of the great diversity of herbivores
on the reef, nor of the strength of their interactions. In addition to their more wellknown positive effects, herbivores are also capable of negatively affecting corals
through direct removal while grazing (Sammarco 1980, Mumby 2009a), but few
studies have investigated the relative importance of positive and negative effects. My
work investigated some of the details of these interactions, as well as trying to assess
the relative importance of dominant reef herbivores.
I conducted my research on Midway Atoll in the Northwestern Hawaiian
Islands (NWHI), an archipelago that is fully protected from fishing and other
extractive activities under the Papahanaumokuakea Marine National Monument, one
of the largest marine protected areas in the world. As a result of its isolation and
fishing restrictions, the NWHI have a relatively intact trophic structure, with high
densities of top predators and large, abundant herbivores (Friedlander & DeMartini
2002). Since most other reefs in the world have reduced herbivore densities due to
4
exploitation, Midway is an ideal location to study herbivore interactions on an intact
reef.
5
CHAPTER 1
Coral recruitment on Midway Atoll: settlement patterns,
cyanobacterial blooms, and grazing effects on a high-latitude reef
Wendy A. Cover
Abstract:
Larval recruitment fuels the resilience and replenishment of coral reef ecosystems. I
investigated coral recruitment at six backreef sites on Midway Atoll (Northwestern
Hawaiian Islands), including two anthropogenically-impacted sites with metal debris
that have periodic blooms of the benthic cyanobacterium Hormothamnion
enteromorphoides. These blooms can cover 85% of the substrate, deter herbivorous
fishes, and grow over live corals. To test the hypothesis that cyanobacterial blooms
inhibit coral recruitment, I measured recruitment on terra cotta tiles at two degraded
(cyanobacterial) sites, two nearby unimpacted control sites, and two distant control
sites. I also examined spatial patterns of coral settlement (rugosity, orientation, and
exposure effects), substrate associations, organisms overgrowing recruits, and the
relationship between adult cover and recruitment. Of the 743 coral recruits, 63%
were Pocillopora. There was no association between recruitment and adult coral
cover, largely because the most abundant coral genus, Montipora, had only a single
6
recruit. The majority of corals (53%) settled on crustose coralline algae (CCA), but
many settled directly on the tile (16%) or on partial tile-partial CCA (24%). Many
recruits (44%) had evidence of overgrowth by CCA and non-CCA algae in roughly
equal frequencies. Contrary to expectations, coral recruitment was significantly
higher at the two cyanobacterial bloom sites than at the four control sites, indicating
that Hormothamnion enhanced coral settlement or survival. Recruit densities were
higher in grooves than on exposed surfaces overall, but the proportion of recruits on
exposed surfaces was higher at bloom sites than at controls. I propose that the
cyanobacteria indirectly enhanced recruitment by inhibiting fish grazers that usually
remove small corals from exposed surfaces.
Introduction:
Coral recruitment is one measure of the capacity of reef-building corals to
replenish their populations, and is critical in their recovery from degradation and
disturbance, a fundamental aspect of reef resilience (Hughes & Tanner 2000). There
are many natural and anthropogenic perturbations affecting coral reefs, but we have
limited understanding of how they may influence the spatial patterns or rates of larval
recruitment.
Benthic cyanobacterial blooms are an anthropogenically-influenced
phenomenon that may affect coral recruitment. Cyanobacteria contain numerous
toxic compounds known to negatively impact humans and marine organisms
7
(Osborne et al. 2001, Codd et al. 2005, Pittman & Pittman 2005). Blooms of the
benthic cyanobacterium Lyngbya are now common off the coast of Florida, where
they are associated with elevated iron and nutrient levels (N and P) and grow over
gorgonian corals (Paul et al. 2005). In Moreton Bay, Australia, river outflows
containing iron, phosphorus, and organic carbon stimulated blooms of Lyngbya
majuscula, with associated losses of seagrasses and reduced fish densities (Albert et
al. 2005, Pittman & Pittman 2005). Benthic cyanobacterial blooms, like other harmful
algal blooms (HABs), are increasing in frequency and severity, and this trend is
predicted to continue due to increasing temperatures from global climate change (Van
Dolah 2000).
On Pacific atolls, blooms of filamentous cyanobacteria have been observed
around iron shipwrecks, on larger spatial scales than that reported from continental
and volcanic island reefs (J. E. Maragos, pers. comm.). It is possible that iron is a
particularly important limiting resource for cyanobacteria on atolls, which are
naturally iron-free, carbonate systems in iron-depleted waters. Cyanobacteria require
iron for the nitrogenase enzyme that fixes nitrogen. The well-known iron-addition
experiments in the South Pacific triggered blooms of planktonic cyanobacteria (Rue
& Bruland 1997, Hyenstrand et al. 2000) and iron has also been implicated in
stimulating a benthic cyanobacterial bloom (Arthur et al. 2009). Due to the potential
negative effects from toxicity and competition, iron debris and associated benthic
cyanobacterial blooms are a growing management concern. For example, a benthic
8
cyanobacterial bloom associated with a shipwreck on Rose Atoll prompted the
USFWS to remove all shipwreck debris (Green et al. 1997, Schroeder et al. 2008).
Like other macroalgae, benthic cyanobacteria can compete with corals for
space, but they also have the potential to cause more damage than other macroalgal
species via allelochemical effects; Titlyanov (2007) found that Lyngbya bouillonii
reduced coral growth, lowered densities of symbiotic dinoflagellates, induced
bleaching, and ultimately resulted in coral tissue death. In Florida, the toxic Lyngbya
majuscula smothered octocorals and other invertebrates (Paul et al. 2005), while
Lyngbya reduced survival and recruitment of three species of coral larvae in
experiments on Guam (Kuffner and Paul 2004, Kuffner and Walters 2006).
Coral recruitment is often variable both within sites (1-5 m) and between sites
(0.5- 3 km) across broad latitudinal gradients (Hughes et al. 1999). Despite the
importance of recruitment for reef recovery after large disturbances, the link between
recruitment levels and adult coral cover at a site is often tenuous (Hughes et al. 1999),
with a few exceptions (Penin et al. 2007).
Larger-scale variation in larval recruitment can reflect small-scale habitat
features. Many studies have shown strong influences of small-scale habitat features
on local patterns of coral recruitment. In particular, the composition (e.g. bare
substrate, live coral, dead coral, coralline algae) and physical structure (e.g. rugosity,
orientation, light exposure) of the reef surface can influence larval settlement choices
or post-settlement survival, ultimately affecting the density of juvenile corals
(Carleton & Sammarco 1987, Maida et al. 1994, Harrington et al. 2004). One
9
common approach for examining the role of these features is with ceramic tiles that
can be manipulated to reflect these habitat features. For example, studies of
settlement locations generally find more recruits on the inner surfaces of paired
settlement plates and on the undersides of single plates (Carleton & Sammarco 1987,
Maida et al. 1994). These patterns are often attributed to active larval choice, in
which larvae responding to environmental cues such as light, substrate composition,
or orientation, tend to settle selectively on more protected surfaces (Babcock &
Mundy 1996, Mundy & Babcock 1998, Raimondi & Morse 2000).
Alternatively, patterns of recruit location may result from spatially variable,
post-settlement mortality. Although mechanisms of early post-settlement mortality
are poorly understood, likely mortality factors include such ecological interactions as
competition with other organisms for space, direct predation, or indiscriminate
grazing by herbivorous fishes or sea urchins. Direct predators of recruits are largely
unknown but may include mollusks (e.g. Drupella), and other mobile invertebrates
(e.g. flatworms, crabs). Incidental removal through the grazing of herbivorous fishes
or urchins is likely to play a large role in the post-settlement mortality of coral
recruits (Sammarco 1980, Baria et al. 2010). In French Polynesia, recruit mortaility
and the local density of parrotfishes were positively correlated (Penin et al. 2010).
Several studies have found more recruits (Gleason 1996, Adjeroud et al. 2007,
O'Leary & Potts 2011) and higher survival of recruits (Nozawa 2008, Christiansen et
al. 2009, Baria et al. 2010) on surfaces protected from grazers, as well as evidence of
mortality and damage to recruits exposed to fish grazers (O'Leary & Potts 2011,
10
Penin et al. 2011). I used paired tiles with grooved outer surfaces to provide variable
exposure to grazers and to test hypothesis that more recruits survive in spaces
protected from grazers.
Few studies have looked at competitive effects at the scale of a coral recruit,
but major competitors are likely to be rapidly-growing sessile invertebrates (e.g.
bryozoans, tunicates, sponges) and algae (fleshy macroalgae, turfs, or crustose
coralline algae). Macroalgae may preempt space from coral recruits, and sponges
may overgrow recruits (Vermeij 2006). Macroalgae can also reduce survivorship of
juvenile corals through interference competition (Box & Mumby 2007), while both
turfs and macroalgae can inhibit coral recruitment (McCook et al. 2001b).
In contrast, other organisms have been shown to enhance rates of larval
recruitment of corals. In particular, many coral larvae are known to settle
preferentially on crustose coralline algae (CCA), especially particular CCA taxa, like
Titanoderma and Hydrolithon (Morse et al. 1996, Raimondi & Morse 2000,
Harrington et al. 2004). However, some taxa are not strongly selective in substrate,
notably Pocilloporidae (Baird & Hughes 2000, Baird & Morse 2004), which is often
the most common family recruiting in high latitudes (Harriott & Banks 1995,
Glassom et al. 2004). I documented settlement substrate (CCA or other), as well as
which organisms were overgrowing individual coral recruits, and to what extent, to
gain insight into settlement preferences and the organisms and mechanisms
responsible for post-settlement mortality via competition.
11
High-latitude (subtropical) coral reefs survive at the extremes of coral
distribution where they withstand larger temperature fluctuations than their lowerlatitude counterparts. Two primary factors implicated in limiting coral growth at
high-latitudes are: colder annual temperatures with larger seasonal differences, and a
lower aragonite saturation state (Kleypas et al. 1999). This means that high-latitude
reefs may be indicators of coral reef responses to climate changes that are predicted to
increase temperature fluctuations and lower carbonate concentrations. Recruitment
levels on latitudinally marginal reefs are usually much lower and have higher
proportions of brooding corals than their tropical counterparts (Harriott 1999). The
extreme environment of high-latitude reefs may provide insight into upcoming
changes in coral recruitment patterns under the broad-scale stresses of climate change
and ocean acidification.
I investigated coral recruitment across subtropical Midway Atoll to better
understand its recovery potential across sites; to compare its recruitment to the
neighboring Hawaiian Islands and other high-latitude reefs; to gain insight into
possible causes of post-settlement mortality, especially grazer impacts, and to
investigate the role of anthropogenically-induced cyanobacterial blooms on coral
recruitment rates. Midway is part of the Northwestern Hawaiian Islands, an isolated
and protected archipelago characterized by >50% offish biomass as large carnivores
(e.g. sharks, jacks, grouper) that are rare (<3%) in the main Hawaiian Islands, and a
higher biomass and mean weight of herbivores such as parrotfish and surgeonfish
12
(Friedlander & DeMartini 2002), making this an ideal location to study the
functioning of a trophically intact coral reef ecosystem.
I studied two degraded, iron-impacted sites on Midway which have periodic,
ephemeral blooms of the cyanobacterium Hormothamnion enteromorphoides. While
the ecology of//, enteromorphoides is little-studied, it is known to contain toxins that
deter fish and gastropod herbivores (Wylie & Paul 1988, Gerwick et al. 1992,
Pennings & Paul 1992, Pennings et al. 1997). Given the toxicity of many
cyanobacteria and prior studies showing reduced coral recruitment rates, I
hypothesized that benthic cyanobacterial blooms have negative impacts on coral
recruitment at the focal degraded sites. I expected fewer coral recruits would settle
on tiles placed within bloom sites than on tiles in nearby control sites.
Methods
Study system
Midway Atoll is a high-latitude (28°N), ecologically marginal environment for
corals, lying at or near the proposed Darwin Point, where reef accretion matches
erosion and subsidence (Grigg 1982). It lies near the center of the North Pacific
Ocean (179°W), 141 km NW of Pearl and Hermes Atoll, and 87 km SE of Kure, the
last atoll in the Hawaiian archipelago. Lagoon temperatures range from a late winter
low of 19°C to a late summer high of 28°C.
Midway is a National Wildlife Refuge (established in 1996), and is part of the
Papahanaumokuakea Marine National Monument which protects the entire
13
Northwestern Hawaiian Islands (NWHI), a chain of 10 emergent reefs and islands
extending 2400km beyond the main Hawaiian Islands. It is one of the most isolated
reef systems in the world. Historically, there has been little to no fishing, leaving an
intact food chain with 260% higher fish biomass than in the main Hawaiian Islands
(Friedlander & DeMartini 2002).
Although reef fishing has been prohibited on Midway for at least 20 years,
and probably much longer under the Navy, the atoll has experienced extensive
anthropogenic influence. For over 60 years, beginning as a Pan-American seaplane
base in 1935 and then a U.S. Navy base with up to 5000 residents, the island and reef
were extensively modified, including removal of patch reefs, dredging and
dynamiting a deep channel through the southern reef margin, dredging a ship basin in
the lagoon, construction of harbors and airstrips, and clearing a reef-free region in the
lagoon for seaplane landing strips. Dredged material was used to add substantially to
the land area and elevation of Sand Island: approximately one quarter of the current
island area was filled in to create a larger runway and a protected harbor. The filled
region is retained by a sheet metal sea wall stretching around half of the island's
circumference. Environmental consequences of these WWII and Cold War actions
are poorly documented, but almost certainly resulted in large-scale mobilization of
sediments within the lagoon and major changes in lagoonal circulation, with water
now exiting through the new opening in the southern reef crest. Many contaminants,
such as petroleum, lead, and PCBs, were widely used on the island, and for many
14
years raw sewage was released into the lagoon off the West Beach, finally ending in
the 1980's.
Before the Navy left Midway in 1996, they cleaned up many of the land-based
contaminants and dump sites but left several shallow-water scrap metal sites,
including three areas known locally as: Rusty Bucket, a nearshore patch reef where
debris was bulldozed off the end of the airport runway; Reef Hotel, the remains of a
former building on steel pilings on the northern backreef; and Bulky Dump, a
peninsula on the south side of the island created from large debris and covered with
cement blocks and soil.
Periodic benthic cyanobacterial blooms occur at these sites,
lasting for a few weeks and recurring episodically during the year over a broad
temperature range (21.5 - 27.5°C), with densities ranging from isolated thalli to 85%
cover on rocky surfaces.
Effect of cyanobacteria on coral recruitment
To determine the effect of cyanobacteria on coral recruitment rates, I
compared sites that characterized three treatment levels: cyanobacterial bloom sites,
nearby controls, and more distant controls. I selected two replicate sites for each
treatment level, with replicates on opposite sides of the atoll to avoid
pseudoreplication (Fig. 1). The two bloom sites, Reef Hotel (RH) and Rusty Bucket
(RB), have extensive subtidal metal debris and experience periodic blooms of
cyanobacteria (Hormothamnion enteromorphoides). Adjacent to each of these, I
identified a nearby control, Reef Hotel Control (RHC) and West Beach Control
15
(WBC), neither of which have iron debris and do not experience cyanobacterial
blooms, but have similar geomorphology, species composition, and oceanographic
influences as their matched treatment sites. Two additional "far control" sites, North
Reef Far (NRF) and The Hook Far (HKF), are distant from known human impacts, do
not experience cyanobacterial blooms, and provide a broader view of recruitment
between the northern and southern halves of the atoll.
The northern sites (RH, RHC, and NRF) are backreef habitats protected by a
raised crest and consisting of scattered patch reefs with relatively high coral cover
(-35%) of predominantly Montiporaflabellata. The southern sites (RB, WBC, and
HKF) have much lower coral cover (-3%), primarily Pocillopora. HKF is in a region
with high water flow, RB and WBC are large patch reefs close to Sand Island (Fig.
1). All sites are shallow (1-2 m) reefs within the lagoon. Sites were chosen to cover
a range of backreef habitats to give a broader picture of recruitment patterns across
the atoll, including regions with high and low coral cover.
I used terra cotta tiles as standardized settlement habitats to sample coral
recruitment across the three treatment levels. Two terra cotta edging tiles (~14 x 15 x
1.4 cm) were used to form a "sandwich" spaced ~3cm apart by curved edges along
one side of each tile (Fig. 2a). Paired tiles provide a wider range of microhabitats for
settlement. Each tile sandwich was attached to a vertical rock surface on a raised reef
structure, using a 10 cm stainless steel lag-screw inserted through a hole in the center
of each tile and screwed into a plastic wall anchor in a hole drilled into the rock.
Tiles were placed vertically to minimize accumulation of sediments and maximize
16
recruitment (Babcock & Mundy 1996). The tiles were close to, but not touching, live
corals. The outer surface of each tile had grooves (1.5 across x 2.5 mm deep)
providing spaces protected from grazers alternating with non-grooved surfaces
exposed to grazers. The smooth inner surfaces of the tiles faced toward each other,
providing further space protected from fish grazers. Within each pair, the tile
touching the rock was designated the "rear" tile; the one facing open water was the
"forward" tile.
I deployed ten replicated tile "sandwiches" at each site in June 2006 and
collected them 13 months later in July 2007. I photographed each tile in the field
after removal. After removing fleshy macroalgae and loose sediments manually, the
tiles were bleached for 24 hrs to remove remaining soft tissues and to expose coral
skeletons, then air dried.
Each tile was searched completely under a dissecting microscope by two
independent observers. All coral recruits were identified to the lowest possible taxon
using reference photos and descriptions from recruitment studies in Hawaii (Brown
2004, Basch & White 2008), the Great Barrier Reef (Babcock et al. 2003), and Kenya
(Mangubhai et al. 2007). Both Pocilloporidae and Poritidae have only one known
genus in the NWHI, so identifications were to genus level for these families. All
recruits were identified, except those that were too small (i.e. a very early
developmental stage with only partial accretion of the skeleton), too overgrown, or
too severely damaged (rare). I was able to identify Pocillopora recruits at a much
earlier developmental stage than most of the other taxa. Many recruits were
17
photographed for reference vouchers (Fig. 3). Data comparing density of recruits
(mean number per replicate tile pair) between sites and taxa were analyzed using
ANOVA with Tukey post-hoc tests and general linear models (GLM) with hypothesis
tests, after checking for conformance with the assumptions of normality and of
homoscedacity of variances. To compare with other studies, the overall number of
9
1
9
recruits was converted into recruits m" yr" using the tile area of 0.05963 m and the
number of weeks each tile was deployed.
Effects of substratum features on coral recruitment
For each coral, I recorded the colony size (as number of corallites, and as
maximum diameter in mm); condition (5 categories from no overgrowth to fully
overgrown) and identity of organisms overgrowing it; substrate association (what the
coral was growing on); tile position (rear, forward); tile surface (inner, outer, edge);
and zone on tile (subcategories of surface; Fig. 2a and b). Zones were delineated to
distinguish specific settlement locations of recruits for comparisons with other studies
that have shown more recruits on protected surfaces and edges. On the outer surfaces
of tiles, where light conditions were comparable, rugosity effects and exposure to
grazers were represented by "groove" and non-groove, or "exposed", surfaces (Fig.
2a). The edges of the tiles provided a means to explore orientation effects,
distinguishing between the "top", "bottom", and "sides" of tiles (Fig. 2a). The inner
surfaces differ in light availability based on their proximity to the open edge of the
tile; I delineated four bands that were horizontal to the open edges (A, B, C, and D;
18
Fig. 2b). Recruits on surfaces where the tiles touched each other were included in the
total inner surface count but not in the horizontal band counts. The upper and lower
bands, A and D, were 3.4 cm wide to include the typical growth region of crustose
coralline algae; intermediate bands B and C were 4.2 cm wide. For comparison
between tile surfaces and zones with different areas, the number of recruits was
converted to recruits m" using the area of each surface and zone. These data were
analyzed using GLM hypothesis tests after squareroot transformation.
Substrate association was usually determined by looking down through the
primary calyx; this is possible up to a colony size of several corallites, before the
calices develop an opaque base. This technique was used on most recruits, but when
not possible, usually due to large size, the association was inferred from the
surrounding substrate. The latter method may be unreliable because CCA often
grows around the primary corallite after it settles. I confirmed questionable
settlement substrates by removing corals to check their underlying association.
Condition of each coral recruit was assigned to one of five categories based on
the proportion of the recruit had been overgrown by other organisms: No
Overgrowth, Slight Overgrowth (an edge of the coral had some overgrowth, 1- 9%>),
Partially Overgrown (10-50%) overgrown), Mostly Overgrown (51-90%) overgrown),
and Fully Overgrown (completely covered or nearly so, 91-100%). For coarser
distinctions, these categories were pooled into two: Not Overgrown (0-9%>
overgrown) and Overgrown (10-100%) overgrown). Frequency data on coral recruit
condition, substrate association, and locations were analyzed using Chisquared tests.
19
Relationship between coral recruitment and adult coral cover
Surveys of benthic cover were conducted at four sites, Reef Hotel
(encompassing both RH and RHC), North Reef Far, Hook Far, and Rusty Bucket,
using six haphazardly placed, 10 m long, line intercept transects at each site. I scored
all corals, algae, and other invertebrates >3 cm, and identified them to the lowest
possible taxon. Data were used to estimate percent cover of adult coral taxa for
comparison with recruit taxon densities.
Results
Corals recruited to 52 of the 55 tile pairs, with a total of 743 recruits across
the 6 sites (Table 1). Recruitment ranged from 0 to 42 recruits per tile pair and
averaged 13.4 ± 6.0 SD. The mean recruitment rate was 101 recruits m"2 yr"1, and site
means ranged from 9 to 151 recruits m" yr" (Table 1).
Pocillopora was the most common taxon at 63.1% of recruits, followed by
Pontes (15.7%), Faviidae (7.4%), and Montipora (0.4%), with 13.3% that could not
be identified (Table 1; Fig. 3). Only one of three Montipora recruits established from
a settling larva, the other two Montipora colonies came from the horizontal spread of
adjacent colonies of Montipora flabellata onto the tile.
Recruit size was assessed in two ways: mean diameter and number of
corallites (Fig. 7A). Sizes ranged from 0.7 to 6 mm diameter, with a median of 2 mm
(Fig. 7A). The average size of a recruit with one corallite was 1.4mm ± 0.5 SD.
20
Over half the recruits (57%) had only one corallite, while the largest colony had 67
corallites (Fig. 7B). The number of corallites in a colony tended to increase with
increasing colony diameter (Fig. 8).
Effect of cyanobacteria on coral recruitment
There were significant differences in the numbers of recruits per site (Table 2;
ANOVA, pO.OOl). Contrary to the initial hypothesis that cyanobacteria inhibit
settlement and/or survival, the two sites that experience cyanobacterial blooms, Rusty
Bucket (RB) and Reef Hotel (RH), had the highest, not the lowest, numbers of
recruits (Table 1, Fig. 4). Recruitment at these cyanobacterial bloom sites was
significantly higher than at nearby control sites (RHC and WBC) (GLM hypothesis
test, p < 0.001) and at the far control sites (NRF and HKF) (GLM Hypothesis test, p =
0.032). The two cyanobacterial sites in different regions of the lagoon had very
similar recruitment rates (p = 0.999; Table 2). Recruitment rates at the four control
sites were much more variable (Fig. 4B), with the pooled far control sites
intermediate between the treatment sites and the nearby controls (Fig. 4A).
Distributions of coral recruit taxa by site are shown in Fig. 5 and Table 1. The
two Reef Hotel sites (RH and RHC) had the highest taxon richness, with all four taxa
present (Pocillopora, Pontes, Faviidae, and Montipora). Pocillopora is the only
taxon that was present at all sites, and it was the sole taxon identified at two sites (RB
and WBC). Relationships between taxon and all other variables (position, substrates,
21
condition) were tested, but only the results that were either statistically significant or
notable are described below.
Relationship between coral recruitment and adult coral cover
There was little relationship between relative adult coral cover (Fig. 6) and
recruitment of coral taxa at each site (Fig. 5, Table 1). Adult coral cover did not
predict recruitment densities, especially for Montipora, which was by far the most
abundant coral at the three northern sites (RH, RHC, NRF) but had almost no
recruitment (and only one by a larva). Excluding Montipora, there was some
concordance between the species composition at a site and the species composition of
recruits at that site. The adult colonies at RB and WBC consisted solely of
Pocillopora, and this was reflected in the recruit composition. Adults at the other
sites were more diverse, with the addition of Pontes and Faviids, and this was
reflected in the diversity of recruits at these sites. Note that although the surveys at
NRF did not record Pontes or Faviids on the transects, these taxa were present as
adults at this site.
Three coral families are present but uncommon to rare on the Midway
backreef: Agariciidae (Pavona and Leptoseris), Fungiidae, and Siderastreidae
(Psammocora). None of these were identified as recruits, but a few of the small
recruits that I could not identify may have been from these families. The most
common species of Faviidae on Midway are in the genus Cyphastrea (as indicated in
the surveys), but colonies of Leptastrea are present in some habitats. It is likely that
22
Cyphastrea ocellina, the most common faviid on Midway, also makes up most (if not
all) of the faviid recruits, but since there are no detailed descriptions of the structure
of early settlers of this genus, I was unable to confirm this.
Effects of substratum features on coral recruitment
Orientation effects. Recruitment rates on the three main tile surfaces, outer,
inner, and edges, were compared using data expressed as density m"2 to account for
differences in area between the three surfaces (Table 4). There were more recruits on
the outer surfaces than on the edges (marginally significant, GLM hypothesis test df =
1, F = 3.582, p = 0.051) and significantly more on the edges than the inner surfaces
(Table 4; GLM hypothesis test df = 1, f = 6.023, p = 0.015).
There were
significantly fewer coral recruits on the top edges of tiles than on the sides and
bottom edges (Table 4; x2=29.68, df=l, pO.OOl).
Rugosity effects / exposure to grazers. On outer surfaces, there were
significantly more recruits in grooves than on exposed areas (353 vs. 155; Table 4;
2x2 contingency table, x =77.17, df=l, pO.OOl), and this pattern was consistent for
all coral size classes. This pattern also held for all sites except Rusty Bucket (RB),
which had the opposite pattern of more recruits on the exposed areas (Fig. 9). Reef
Hotel (RH), the other cyanobacterial site, had relatively more recruits on exposed
surfaces than at the four remaining sites, but when RB and RH were removed from
the analysis, the %2 was not significant. When the sites were grouped by treatment,
the cyanobacterial sites had significantly more recruits on exposed areas than did the
23
two control treatments (x2=3.97, df=l, p=0.046). For each taxon there were more
recruits in grooves than in exposed zones (Pocillopora: 197 vs. 121, Pontes: 52 vs.
14, Faviidae: 45 vs. 2).
Light availability. Recruitment was significantly different among the four
inner surface bands of the tiles (Table 4), and this appeared to correspond directly to
the presumed amount of light reaching each band, based on the vertical orientation of
the sandwiched tiles with openings at the top and bottom but closed sides (band A
high light, band D medium light, band B medium-low light, and band C low light).
Correspondingly, band A had the most recruits, followed by band D, then band B,
then band C. The four bands were significantly different, with non-overlapping
confidence intervals (%2=66.30, df=3, pO.OOl). The high light bands near the edges
(A and D) had significantly more recruits than the low light bands toward the inside
(B and C; x2=45.37, df=l, pO.OOl).
Proximity to reef. There were roughly equal numbers of recruits on rear and
forward tiles (364 vs. 379), but considerable variation among sites (Table 3A,B).
Reef Hotel had many more recruits on rear tiles than forward tiles, three sites (HKF,
RB, RHC) had the opposite pattern, and two sites (NRF and WBC) had roughly equal
distribution (Table 3A). This variation contributed to a significant interaction
between site and tile position (rear or forward tile) (Table 3B; ANOVA, p=0.027).
There was a significant difference between sites when Reef Hotel was included (%2 =
27.405, df = 5, p < 0.001), but sites were independent when it was excluded (% =
3.166, df = 4, p = 0.531). There were also some taxon differences, with Pontes and
24
faviids more abundant on rear tiles (76 vs. 41 and 33 vs. 22, respectively), and
Pocillopora significantly more abundant on forward tiles (210 vs. 259) (%2 = 17.776,
df=2,p<0.001).
Use of settlement substrates
Coral recruits settled on a variety of substrates on the tiles, including crustose
coralline algae (CCA), partial crustose coralline algae (PCCA), other algae, tile, tube
worms, vermetid snails, bryozoans, and coral (Table 5). I defined PCCA as small
patches of CCA interspersed with open tile at a scale smaller than a single corallite
such that the coral was on both CCA and tile. Associations with tube worms and
vermetid snails were pooled due to their low frequencies, and were scored if the coral
was partially touching the organism; coral recruits usually nestled in crevices of the
tubes while also growing on CCA, and therefore could have been included in the
CCA category.
Although the relative proportion of settlement substrates varied somewhat by
site, the same rankings generally applied. Pocillopora had a relatively higher
proportion of recruits associated with PCCA and tile than did both Porites and faviids
(Fig. 10; x2=18.38, df=6, p=0.005).
Recruit condition and overgrowth
Coral condition was assigned one of five categories based on the proportion of
the recruit that was overgrown by other organisms. The majority of corals were in the
25
No Overgrowth category (53%), but 43% had some form of overgrowth (Table 6).
16% of coral recruits had >50% of their skeletons covered by other organisms.
After grouping the condition data into two broad categories, Not Overgrown
and Overgrown (Fig. 11), Rusty Bucket (RB) had significantly fewer overgrown
corals (x2=36.46, df=5, pO.OOl) than all other sites (for which condition and site
were statistically independent). The broad condition categories were independent of
taxon, tile surface, zone, tile position, and recruit size.
While a variety of organisms overgrew coral recruits, the main one was CCA
(46%), followed by four groups of non-CCA algae (totaling 50%), PCCA (2.3%), and
a few invertebrates (1.3%) (Fig. 12; Table 7). There were no significant differences
among sites in the numbers of recruits overgrown by CCA, algal filaments, and algal
films (x =15.41, df=8, p=0.052). Several coral recruits seemed to be resisting CCA
overgrowth by extending their epitheca upwards, with CCA growing up to the top
edge of the epitheca while the remainder of the recruit remained untouched inside this
barrier. When algae were grouped into CCA vs. non-CCA, there were significantly
more recruits overgrown by non-CCA algae at sites NRF and RHC (x2=9.69, df=4,
p=0.046) (Fig. 13). The remaining sites were independent of overgrowth category, as
were coral taxa and groove vs. non-groove zones.
Discussion:
Settlement Densities
26
9
1
The coral recruitment rate of 121 recruits m" y" (13 recruits per tile pair) on
Midway Atoll (28° N) was higher than reported for some high-latitude reefs in
southwestern Japan (2 recruits m"2y~', 32° N) and the Solitary Islands, Australia (17
recruits m"2y"!, 30° S) (Harriott & Banks 1995, Harriott 1999, Nozawa et al. 2006).
Rates from my study represent lagoonal habitats and include some sites with much
higher coral diversity and cover than occurs on the more exposed forereef habitats.
Therefore, these rates may be higher than the average atoll-wide recruitment. Rates I
measured fall within the range of recruitment rates in the more tropical, main
Hawaiian Islands (Maui: 41-415 recruits m"2y-1 (Brown 2004); Kaneohe Bay, Oahu:
8 recruits m^y'^Demers 1996)), indicating that Midway has a reasonable capacity
for reef replenishment and recovery via recruitment. Most of the Hawaiian rates,
however, are much lower than reported for the Great Barrier Reef (4258 recruits m"
y"1 (Hughes et al. 1999); 489 recruits m"2y_1 (Fisk & Harriott 1990), and elsewhere in
the Indo-Pacific (Fiji: 734 recruits m"2y"' (Kojis & Quinn 2001); Zanzibar: 594
recruits m"2y"' (Franklin et al. 1998)).
Over half the recruits (63%) were Pocillopora, which is consistent with other
studies showing a preponderance of Pocillopora and brooding taxa over broadcast
spawning species at high latitude (Harriott 1992, Harriott & Banks 1995, Harriott
1999, Glassom et al. 2004). The nearly nonexistent recruitment of Montipora despite
its high cover at many sites, is also consistent with the same studies which
documented reduced recruitment of Acroporidae at high latitudes. Studies in the
main Hawaiian Islands have much higher proportions of Montipora recruits (approx.
27
75% in Maui) but these sites are characterized primarily by Montipora capitata
(Brown 2004), whereas M. flabellata (cf. turgescens) is the dominant member of the
genus on Midway. This species is common only in the Northwestern Hawaiian
Islands, and its reproductive biology is unknown, so I cannot evaluate whether its
recruitment is always low or is reduced with latitude. M. flabellata is an encrusting to
foliaceous species that spreads quickly and may rely on this strategy rather than larval
recruitment. Alternatively, it may prefer a different settlement substrate, experience
disproportionately high post-settlement mortality or have infrequent recruitment
events.
Adult coral abundance was a poor predictor of recruitment, a pattern that also
is consistent with other studies, and probably reflects high post-settlement mortality,
episodic recruitment, and the long life of adult corals. The three sites with the highest
coral cover (Fig. 6) had only the second, fourth, and fifth highest recruitment rates
(Table 1). The site with the highest mean recruitment (RB) has the lowest adult coral
cover (<3%) of all the sites. Species richness patterns between adults and recruits
were more similar: all taxa present as recruits are present as adults at each site. The
converse is not true, as several less common taxa were not seen as recruits (Pavona,
Leptoseris, Psammocora, Fungiidae) and the common taxon, Montipora, was
represented by only one larval recruit and two "recruitment" events via lateral growth
onto the tile from a nearby colony. The lack of Montipora recruits, but dominance of
adult Montipora at the northern sites was the primary driver of the dichotomy in
recruit and adult abundances. At both RB and WBC the only adults are Pocillopora,
28
and this was the only taxon found recruiting at these sites. Concordance between
richness of adults and recruits at a site may indicate some degree of local retention of
recruits.
Site Differences and Cyanobacteria
Intra-atoll variation in recruitment was very high on Midway, with a 19-fold
difference in recruit densities between the highest and lowest sites. High variation
among sites is common in recruitment studies (Hughes et al. 1999, Glassom et al.
2004, Adjeroud et al. 2007), making recruitment a difficult metric to compare sites
with and without anthropogenic impacts. Nevertheless, due to the high cover and
toxicity of cyanobacterial blooms, I initially hypothesized that there would be lower
recruitment at sites affected by iron debris and benthic cyanobacterial blooms.
Contrary to my expectations, the highest recruitment was at the more impacted sites
(Fig. 4A). While it seems unlikely that either cyanobacteria or metal debris would be
attracting recruits, high recruitment at these sites indicates they are not substantially
reducing recruitment.
There are several possible explanations for the high recruitment at
cyanobacterial bloom sites. The pattern may simply be a coincidence reflecting
variation among sites in other characteristics. Hormothamnion may not have direct
negative effects on recruits, or the ephemeral blooms may be too short to have major
negative effects, or any signal of negative effects may have been overwhelmed by
other, spatially covarying positive effects. Alternatively, the cyanobacteria may deter
29
predators that would otherwise reduce recruitment (in the absence of blooms).
Predation on coral recruits is poorly documented, but many herbivores, especially
parrotfishes, are believed to remove recruits incidentally while grazing (Box &
Mumby 2007, Penin et al. 2010). The primary cyanobacterium forming blooms on
Midway, Hormothamnion enteromorphoides, is known to deter herbivorous fishes
(Gerwick et al. 1989), and this may have contributed to reduced grazing during
blooms and consequently reduced mortality of coral recruits. Despite high densities
of herbivorous fish at Midway, we did not observe any grazing on or around
Hormothamnion blooms, which suggests it is effective at deterring herbivores which
graze heavily in the absence of cyanobacteria.
When the tiles were collected at Rusty Bucket, they were covered in a thick
mat of turf-like algae, which has been shown to deter recruitment in other localities
(Birrell et al. 2005), so the high RB recruitment was unexpected. WBC had by far the
lowest recruitment (a fifth of the next lowest site), but may have been influenced by
prolonged local water retention or by hidden debris, such as a large piece of lead
found later near the study site.
Recruit Orientation and Substrates
There were substantial differences in the numbers of recruits on different
surfaces of the tiles, indicating larval settlement preferences and/or post-settlement
mortality were acting. On outer tile surfaces, most (69%) recruits were in grooves
rather than on exposed regions (Table 4, Fig. 9), which is consistent with previous
30
studies (Carleton & Sammarco 1987, Nozawa 2008). Rusty Bucket had the opposite
pattern; recruits were much more evenly distributed, with slightly more recruits in the
exposed zone than the protected zone. Since Rusty Bucket also has very low
herbivorous fish densities compared to the other sites, reduced grazing may allow
recruits to survive on exposed surfaces, whereas they would be removed at highherbivory sites. At the other cyanobacterial bloom site, Reef Hotel, approximately
one third of recruits were in exposed than in grooved zones, which is still a much
higher proportion than other sites (Fig. 9). It is possible that the cyanobacterial
blooms deterred the usually common fish herbivores, reducing incidental take of
recruits and increasing the proportion seen in exposed regions. If the effect of
grazing is removed by only considering recruitment into grooves, mean recruitment at
Rusty Bucket would fall from first to third among sites, and Reef Hotel would move
from second to fourth (Table 1).
Unlike many studies in which more recruits were on the protected undersides
of tiles (Fisk & Harriott 1990, Maida et al. 1994, Tioho et al. 2001), there were
significantly more recruits on the exposed outer surfaces than on the protected inner
surfaces (Table 4). This may be because the grooves on the outer surfaces may have
attracted recruits or enhanced their survival by protecting them from incidental
removal by grazers (Carleton & Sammarco 1987, Nozawa 2008). The inner surfaces
were also protected from large grazers the close spacing between tiles reduced light
on the innermost surfaces, which may explain the lower recruitment on these
protected surfaces, especially since 82% of recruits on inner surfaces were in the
31
outer (higher light) zones (A and D; Table 4). Although small invertebrate predators
or competition with other sessile organisms may also reduce recruitment on inner
surfaces, these scenarios seem less likely than responses to light, because recruitment
to the inner surface zones corresponded to the order of declining light intensity, with
the highest recruitment near the brightly illuminated opening at the top of the vertical
sandwich (zone A), followed by the region near the bottom (zone D), then the uppermiddle (zone B), and the lowest recruitment in the lower-middle zone C, which
presumably receives the least light. Several other studies have found larval
settlement to be positively correlated with light, as well as species-specific responses
to light (Maida et al. 1994, Mundy & Babcock 1998). Well-lit zones also had higher
cover of crustose coralline algae (CCA), some of which are known to stimulate coral
larvae to settle (Harrington et al. 2004), and so may have induced higher recruitment
in these zones.
There were strong orientation effects, with the highest densities of recruits on
the edges of tiles rather than on the larger inner and outer surfaces. Most edge
recruits were on the sides and bottom edge, with very few on the top edge, which is
consistent with studies finding low recruitment on upward-facing surfaces (Carleton
& Sammarco 1987, Maida et al. 1994).
Although certain CCA are inductive, stimulating larvae to settle, and although
the majority of recruits settled on CCA (53%), a sizable number of them (16%), from
all taxa represented, settled on bare tile, and even more (24%) on partial tile (PCCA),
indicating that CCA is not essential for coral settlement on Midway. This agrees with
32
several studies in which Pocilloporidae larvae did not show a strong preference for
CCA (Baird & Hughes 2000, Baird & Morse 2004). Among the coral taxa,
Pocillopora had the highest settlement rates on tile and PCCA, consistent with it
being less selective in substrates during settlement.
Tile Community Interactions
I recorded the amount of overgrowth of each recruit at the time of tile
collection as an indicator of its condition and of its competitive interactions with
other benthic organisms. A substantial number of recruits (36%) were at least slightly
overgrown by another organism. While overgrowth probably leads to underestimates
of the total number of recruits because many may have been overgrown beyond the
point where they could be detected, it does provide an index of the proportion of
corals involved in competitive interactions at around the time the tiles were removed.
A smaller number of recruits (7%) were completely overgrown, presumably having
already succumbed to post-settlement mortality due to competition. The organisms
overgrowing recruits were almost entirely non-CCA algae (50%) and CCA and
PCCA (48%o; Table 7), although complete overgrowth by invertebrates such as
bryozoans would prevent detection of the coral recruit, and so was likely
underestimated. The pattern of overgrowth indicated that it was often a non-CCA
alga that was the first to overgrow, and then CCA followed, possibly after tissue
stress or mortality in that region.
33
Conclusions
Recruitment patterns on Midway were variable among sites, but were
comparable to rates from the tropical main Hawaiian Islands. Pocillopora, a brooder,
was the most common taxon and Montipora the least common, despite its high adult
cover. Recruit densities did not reflect adult cover at a site, nor did recruit species
richness match that of adults, although the recruit taxa were a subset of adult taxa at
each site. Recruits settled on a variety of substrates and showed overgrowth
indicative of competitive interactions with other benthic organisms.
While blooms of the benthic cyanobacterium, Hormothamnion
enteromorphoides, may have other negative effects on reef ecosystems, they do not
appear to significantly reduce recruitment, since recruit densities were highest at sites
with Hormothamnion blooms. Most sites had lower recruitment on exposed surfaces
than in protected grooves, but sites with cyanobacterial blooms had a higher
proportion of recruits on exposed surfaces, reinforcing the suggestion that the
cyanobacteria deterred herbivorous fishes that removed exposed recruits, reducing
recruitment at the other sites.
Acknowledgements
Thanks to Jamie Barlow and Don Potts for field assistance in both years; Mitsubishi
volunteers Derek Jones, Vanessa Melgar, Brian Navarro and Michael Hojnacki for
34
tile preparation in 2006; Barbara Pimentel, Becky Ingold, Luis Alvarez, and Steve
Miller for field processing of tiles in 2007; Brittany Schlotfeldt, Nick Bers, Aiko
Watanabe, Eleanor Gilbert, Piara Sandhu, Gillian Parcells for scoring and measuring
coral recruits; and Pete Raimondi for statistical advice. Eric Brown and Andrew
Baird provided help with identifications. USFWS and Midway Atoll National
Wildlife Refuge staff Barry Christenson, John Klavitter and John Miller provided
access to Midway Atoll, use of its facilities, and other in-kind support. The Mitsubishi
Corporation (Tokyo) provided the primary funding as part of their Global Coral Reef
Conservation Project. Work was conducted under permit PMNM-2007-013.
35
Table 1. Total counts and mean numbers of coral recruits per tile, classified by site and taxon.
unknown
Total
Mean
recruits/tile
pair, ±SD
3
184
20.4±5.8
157
41
193
19.3±7.6
142
10
1.0±0.8
9
32
136
13.6+7.3
100
15
15
165
18.3±10.3
141
3
5
8
55
7.9±4.2
59
469
117
55
3
99
743
13.4±6.0
101
63.1%
15.7%
7.4%
0.4%
13.3%
100%
Taxon:
Site
N tile pairs
Pocillopora
RB
9
181
RH
10
53
WBC
10
10
RHC
10
60
32
11
HKF
9
126
9
NRF
7
39
55
Total /
Mean
% Recruits
Pontes
73
Faviidae
24
Montipora
2*
1
These two Montipora were from existing colonies that spread onto the tile.
Mean
recruits/
m2/yr
Table 2. One-way ANOVA for numbers of recruits per tile among six sites, and a
matrix of pairwise comparison probabilities from Tukey's tests. Significant
probabilities in bold font.
Source
Sum-of-Squares
df
Mean-Square
F-ratio
P
1383.083
5
276.617
13.809
<0.001
2083.290
104
20.032
Site
Err0r
Matrix of pairwise comparison probabilities:
North Reef
„
Far
West Beach
„ ^ .
Control
„ , _,
Hook Far
_ ^ _, . .
Rusty Bucket
Reef Hotel
_ ^ .
Control
North Reef Far
1.000
West Beach
Control
0.247
1.000
Hook Far
0.017
<0.001
1.000
Rusty Bucket
0.002
<0.001
0.981
1.000
Reef Hotel Control
0.444
<0.001
0.582
0.183
1.000
Reef Hotel
0.005
<0.001
0.999
0.999
0.342
37
Table 3. a) Numbers of recruits on forward and rear tiles at six sites, b) Two-way
ANOVA for numbers of recruits per tile among six sites and two tile positions
(forward, rear).
A.
Tile Position
Rusty
Bucket
Reef
Hotel
West
Beach
Control
Reef
Hotel
Control
Hook
Far
North
Reef Far
Total
Forward tile
106
69
6
80
93
25
379
78
124
4
56
72
30
364
Rear tile
B.
Source
Site
Tile Position
Site * Tile
Position
Error
Sum-of-Squares
df
Mean-Square
F-ratio
P
1383.083
5
276.617
14.788
O.001
2.518
1
2.518
0.135
0.714
248.046
5
49.609
2.652
0.027
1833.198
98
18.706
38
Table 4. Mean numbers of coral recruits per m on the three tile surfaces and their
subcategories (zones). N = 59 tile pairs for each category (surfaces and zones).
Tile
Surface
Total #
Recruits
Mean #
Recruits/m2
508
146
16
124
Groove
353
216
28
211
Non-groove
155
84
13
102
125
52
9
72
A
59
89
22
167
B
18
33
10
80
C
1
2
2
14
D
30
45
10
78
106
118
18
136
2
9
6
49
Bottom Edge
29
133
28
216
Sides
75
161
28
216
Zone
Outer
Inner
Edges
Top Edge
39
SE
SD
Table 5. Numbers and percent of coral recruits settling on different substrates
(ranked by abundance of recruits). CCA = crustose coralline algae; PCCA = partial
crustose coralline algae.
Settlement Substrate
Recruit
Percent
Count
CCA
392
52.8%
PCCA
178
24.0%
Tile
116
15.6%
32
4.3%
Algae
2
0.3%
Bryozoan
2
0.3%
Coral
1
0.1%
20
2.7%
743
100%
Vermetid and Tube worm
Can't determine
Grand Total
40
Table 6. Condition of recruits based on amount of overgrowth by other organisms.
Broader grouping totals are in bold type.
Total
Recruits
Percent of
Recruits
No Overgrowth
397
53%
Slight Overgrowth
104
14%
Recruit Condition
Total Not Overgrown
401
67%
Partially Overgrown
99
13%
Mostly Overgrown
66
9%
Fully Overgrown
52
7%
Total Overgrown
217
29%
Can't be determined
25
3%
Grand Total
743
100%
41
Table 7. Frequency of recruit overgrowth by various organisms.
Number of
Recruits
Relative
Percent
CCA
143
46.0%
other algae
48
15.4%
algal filaments
45
14.5%
algal film
34
10.9%
golden algae
29
9.3%
PCCA
7
2.3%
bryozoan
3
1.0%
tube worm
1
0.3%
sediment
1
0.3%
Overgrown by
Not overgrown
446
Total
757
100%
42
I
r
II
60
Midway
Atoll
N A U T I C A L MILES
©
60
N
fl
KILOMETER"
•>r
it ortHReef
fV»I -Tfl-V ' • -
I N JL'r.
m
fe;Bi-j
"•» ^ h » j I
GO
l|l
«#
W"«l l i »1
©
•
k
4
I*,'1JJ?» , « • • '•' •
• %
s
•-.?•-
iwpt
Hit
a*
N
©
60
IN
(lit-.,W V
'*^--' V ^ T •-*->«
*» .4 * -
4:ww-—^-^^^^i^p-';-^
177°24'W
177°21*W
177°t8,W
Figure 1. Study sites on Midway Atoll, Northwestern Hawaiian Islands. IKONOS
satellite image; NOAA Atlas 2003.
43
A. Outer surface of tile
B. Inner surface of tile
Figure 2. Tile surfaces and zones used when scoring data, (a) Outer surface of paired
tile "sandwich" in situ showing forward and rear tiles, central attachment (lagscrew),
grooved and non-grooved zones, and three edge zones (top, bottom, and sides), (b)
Inner surface of tile after bleaching, showing horizontal zones A, B, C, and D.
44
A Poallopora
E Could not be determined
i
Tr
•'- * * -
1
•
v
-i • ~%"
-„
*
:
v ••
Figure 3. Examples of each taxon recorded. Scale bars are lmm. (a) PociUopora,
(b) Pontes, (c) Favudae, (d) Montipora, (e) could not be identified.
45
A
w
-*^
o
30
(D
a:
"CO
0
!=
Q
20
-
1 S. 10
3
c
05
0)
Cyano
Sites
Nearby
Controls
Far
Controls
Treatment
B
Cyano
Sites
Nearby
Controls
Far
Controls
U
Rusty
Bucket
Reef
Hotel
West
Beach
Control
Reef
Hotel
Control
Hook
Far
North
Reef
Far
Site
Figure 4. a) Mean coral recruitment (± 1 SE) for three treatments, b) Mean
recruitment per tile pair (± 1 SE) at six experimental sites. Treatment categories
distinguished by shading.
46
Pocillopora
Pontes
Faviidae
•
Rusty
Bucket
Reef
Hotel
(9)
(10)
West
Beach
Control
Reef
Hotel
Control
(10)
(10)
Hook
Far
(9)
Montipora
North
Reef
Far
(7)
Figure 5. Mean numbers of recruits per tile pair (± SE) for each coral taxon at each
site. Number of tile pairs is in parentheses.
47
(/>
U.4
H Pocillopora
2
o
ED Pontes
o
5
E3 Favndae (Cypftasfrea)
0.3
•D
<
O
fc
>
0.2 -
j
•
Montipora
- •»
H Pavona
i
•
*
Other
p
-»•
•
p^ra
d
p
Proportion
o
Rusty
Bucket
(9)
Reef
Hotel
Region
(13)
Hook
Far
<9>
North
Reef
Far
(6)
Figure 6. Proportion of total benthic cover comprised of adult coral colonies (± 1 SE)
at four sites. Number of transects is in parentheses. Reef Hotel Region encompasses
both the Reef Hotel and Reef Hotel Control sites.
48
30
(A
"I 20h
CD
•
CD
E 10--
r—
Z3
•
•
If
.[
I
V- 1 ••(• 1
1 2
3
4
5
6
Colony Diameter (mm)
7
20 t
w
•5 15 +
i_
n
o
<o
a:
2 10 +
a>
E
z
5-
O-M
0
..LLP
5
10
XL
15
20
25
30
35
Number of Corallites in Colony
Figure 7. Frequency distributions of coral recruits by a) size, and b) number of
corallites.
49
>s 3 5 1
c
o
0 30
O
i
251
1
20
o
2 15
o
I 10
9 9 €®
#Gt>©
E
1
•
®
5+
0
3m§§isni
0
H
h
1 2
3
4
5 6
Colony Diameter (mm)
Figure 8. Relationship between colony size and number of corallites in colony.
50
I^U
100
Groove
Non-groove
•4—*
80
Z3
O
60
O
*
40 -
20
n
MM-
Rusty
Bucket
Reef
Hotel
West
Beach
Control
Reef
Hotel
Control
Hook
Far
North
Reef
Far
Figure 9. Total numbers of recruits in grooved vs. non-grooved (exposed) zones of
outer tile surfaces at each site.
51
300
Pocillopora
Pontes
Faviidae
200
100
» _
CCA
PCCA
Tile
Tube worm
or vermetid
Substrate
Figure 10. Numbers of recruits in three coral taxa that settled on the four most
common substrates.
52
Not Overgrown
150
Overgrown
100
1P&&
^i^ip
50
Rusty
Bucket
Reef
Hotel
West
Beach
Control
Reef
Hotel
Control
Hook
Far
North
Reef
Far
Figure 11. Numbers of recruits that were Overgrown and Not Overgrown at each
site.
53
Figure 12. Examples of various organisms overgrowing coral recruits, (a) Porites
colony mostly overgrown by CCA, (b) algal filaments, (c) recruit fully overgrown by
algal film, (d) mostly overgrown by algal film, (e) golden algae, (f) bryozoan
overgrowing Pocillopora. Scale bars are 1mm.
54
50
\J\J
.
Organism Overgrowing
40
1
_, CCA
1
[
I
L <
C/3
3
m All Other Algae
1
30
1-
O
0
"5
,
1
j
20
•
fcrt
10 —
1
-t
'
,-.
1
-~J
*
'*
.'•,
1.
c
1
1—rm
1 ._
'
J
FRusty
Resef
West
Reef
Hook
North
Bucket
Hote
Beach
Hotel
Far
Reef
Control
Control
Far
Figure 13. Numbers of recruits overgrown by CCA or by Other Algae. The All
Other Algae category includes algal filaments, film, golden, PCCA, and other (Table
7).
55
Appendix. Additional organisms noted on settlement tiles.
Organism
amphipods
crab
goby
E. mathaei urchin
turban snail
numerous invertebrates, esp.
crabs
oysters (3)
cowrie (Cypraea)
brittle star
E. mathaei urchin
thick algal mats
Site
RHC
RHC
RHC
RHC
RHC
WBC
WBC
WBC
HKF
HKF
RB
Notes
Small; in between tiles. Grazing marks
visible on CCA.
Mid-sized
On outer surfaces of most tiles
56
CHAPTER 2
Differentiating Impacts of Fish and Urchin Grazing on
Algal Growth and Coral Recruitment
Wendy A. Cover
Abstract
Both fishes and urchins can be important herbivores on corals reefs, maintaining
resilience through their grazing activities and preventing coral-macroalgal phase
shifts. Despite their importance, little is known about the relative importance of these
different herbivore guilds. I conducted a factorial field experiment to quantify the
relative effects of herbivorous fishes and urchins (Echinometra mathaei) on
macroalgal growth and coral recruitment in backreef habitats of Midway Atoll, a
protected Northwestern Hawaiian Island reef with largely intact fish assemblages and
abundant urchins. Algal growth and coral recruitment were measured on ceramic
tiles that were open, partially caged, caged with urchins, or fully caged. Fish grazing
effectively limited algal biomass over 10-11 months (0.5 ± 1.3g dry wt. per tile).
Algal biomass was >50 times higher in treatments with no grazers (69.8 ± 81.9 g) and
treatments exposed only to urchins (53.5 ± 63.7g). Urchin grazing reduced algal
biomass only at higher urchin densities (3 or 4 per tile). Coral recruitment was >2X
57
higher in treatments exposed to fish grazing than those exposed to urchin grazing or
no grazers, indicating that algae inhibit coral recruitment much more than does fish
grazing. Herbivorous fishes apparently have a strong positive but indirect effect on
coral recruitment by reducing algal growth. Algal biomass was negatively correlated
with coral recruitment, suggesting that management to increase herbivorous fishes
and grazing levels is likely to benefit coral recruitment. This study underscores the
importance offish herbivores in removing macroalgae and promoting coral
recruitment, enhancing the resilience of reef communities.
Introduction
Herbivores play important, often critical roles in the structure, functions, and
dynamics of benthic community dynamics in aquatic systems. On coral reefs,
herbivores consume algae that compete for space with the framework-building corals,
and by allowing space for corals to recover following disturbances (e.g. hurricanes or
bleaching events), grazing promotes resilience in reef communities (Nystrom et al.
2000, Hughes et al. 2007b). Beneath a critical threshold of grazing intensity, reefs
loose their resiliency and can shift to an alternate, coral-impoverished state
characterized by much higher densities of macroalgae (Mumby et al. 2007b, Mumby
2009b). Promoting healthy herbivore populations through protection, as in no-take
marine reserves, has been shown to enhance coral recovery (Mumby & Harborne
2010) and is increasingly a focus of management efforts.
58
Given the primary role of herbivores in maintaining resilience on reefs facing
numerous natural and anthropogenic threats, management efforts should be enhanced
by understanding the relative grazing contributions of different herbivore guilds, as
well as any negative effects that may result from their grazing activities. Fish and
urchins are the dominant reef herbivores, but species composition and abundances of
herbivore guilds varies with habitat (e.g. forereef vs. backreef), region (e.g. Diadema
antillarum is the dominant urchin herbivore in the Caribbean, but does not have an
equivalent in the Indo-Pacific), fishing method (e.g. spearfishing may preferentially
target parrotfish), and fisheries management (e.g. certain urchins common in fished
areas in Kenya are uncommon in marine reserves with high fish biomass
(McClanahan et al. 1994)). Because of this variability, it is important to understand
the contribution of particular species or guilds present at a given site, since not all
herbivores have equivalent impacts (Burkepile & Hay 2008).
Most experimental comparisons of fish and urchin grazing on coral reefs have
been conducted in the Caribbean, where the urchin Diadema antillarum has a greater
impact than herbivorous fishes on shallow reefs (Carpenter 1986, Foster 1987,
Morrison 1988). However, these studies were all in heavily fished regions with
relatively few herbivorous and predatory fishes, and high urchin densities; on lessimpacted reefs with few urchins and more fish, different outcomes have been seen
(Hay 1984). When abundant, Diadema is a formidable herbivore that is credited with
maintaining resilience and preventing phase shifts on overfished Caribbean reefs until
its populations collapsed from disease in 1983. After the collapse, many coral
59
populations failed to recover from hurricane damage and these reefs shifted to a
macroalgal-dominated state (Hughes 1994). Temperate urchins on the Pacific coast
of North America also exert strong effects on kelp communities (Estes & Palmisano
1974, Harrold & Reed 1985), but no other tropical urchin has been shown to have an
impact equivalent to that of Diadema antillarum. Although most urchin species are
herbivores, they vary in their feeding preferences and ability to reduce macroalgal
biomass (McClanahan et al. 1994).
The positive effect of grazing on corals is indirect, and mediated via
competitive interactions between corals and algae. Contact with algae (perhaps
mediated by microbes (Smith et al. 2006, Vermeij et al. 2009)) can cause bleaching
and mortality in adult corals (Jompa & McCook 2002, Rasher & Hay 2010) and has
triggered coral disease (Nugues et al. 2004). Coral recruits can also be negatively
affected by turf algae and macroalgae, which may prevent access to suitable surfaces
for larval settlement or initiate avoidance behaviors in larvae, as well as inhibiting
growth and increasing mortality of settled recruits (Kuffner et al. 2006, Box &
Mumby 2007, Arnold et al. 2010). In some studies, reduction in macroalgal cover
due to recovery of fish and urchin grazers, facilitated coral recruitment (Carpenter &
Edmunds 2006, Mumby et al. 2007a).
While grazers may indirectly help coral recruitment by removing competitive
algae, they may also harm recruits directly by consuming them during grazing
activities. Several studies have found more coral recruits on surfaces protected from
grazers (Gleason 1996, Adjeroud et al. 2007, Nozawa 2008)(Chapter 1), and Penin et
60
al. (2010) found a positive correlation between recruit mortality and the local density
of parrotfishes (Scaridae). The hard dentition of parrotfishes makes them more likely
than many other fishes to remove coral recruits incidentally while feeding, but even
small grazing blennies can reduce survival of single-polyp recruits (Christiansen et al.
2009). Sea urchins can have strong, calcareous mouthparts and some species have
been implicated in consumption of adult coral tissues in some locations (Bak & van
Eys 1975, Glynn et al. 1979), and survival of coral recruits can be suppressed under
high Diadema densities (Sammarco 1980). In one study starting with six-week old
coral spat, survival after three months was three times higher in caged than uncaged
treatments, likely due to exclusion of herbivores and corallivores (Baria et al. 2010).
While herbivores can have both positive and negative effects on corals and
coral recruits, it is unknown in most cases whether consumption or competition is
more important in determining recruit survival, and whether fishes or urchins have
the major impact. This study examines the relative roles of herbivorous fishes and
urchins {Echinometra mathaei) in controlling the growth of algae and the recruitment
of corals on settlement tiles by employing a caging experiment with orthogonal
treatments including and excluding fishes and urchins.
Methods
Location
The study was conducted in the shallow backreef (1 - 2 m) habitat of Midway
Atoll, an isolated, high-latitude (28°N) atoll in the Northwestern Hawaiian Islands,
61
that is part of the Papahanaumokuakea Marine National Monument. Midway has
been protected from reef fishing during its 23 years as a National Wildlife Refuge,
and the U.S. Navy is reputed to have prohibited reef fishing during most of its tenure
from 1940 - 1993 due to a high incidence of ciguatera fish poisoning. Consequently,
Midway has an intact trophic structure with high densities of herbivorous fishes
(Friedlander & DeMartini 2002), primarily parrotfishes and surgeonfishes, making it
an ideal place to study grazing impacts. The study was conducted at a site in the
north backreef (28.27448°N, 177.35363°W) with high coral cover (36%) compared to
the southern backreef (3%), and moderate urchin densities (4 m" ). The primary coral
genera are Montipora, Pocillopora, and Pontes, with colonies largely restricted to
patch reefs (1 - 3 m wide) separated by sand and hard substrate. An uplifted ancient
reef crest protects the site from heavy waves, but currents can sometimes be strong.
Experimental Design
A balanced 2 x 2 factorial design was used to differentiate between the
impacts of herbivorous fish and herbivorous urchins on algal growth and coral
recruitment (Fig. 1). Ceramic tiles were deployed as replicate settlement substrates
for algal and coral propagules, and different kinds of cages around each tile included
or excluded fish and/or urchins. The four treatments were: Fish & Urchin - no cage,
allowing free access by both fishes and urchins; Fish Only - a half cage, with a fence
preventing urchin entry but allowing fishes access from above; Urchin Only - a full
cage enclosing 4 urchins (Echinometra mathaei) while excluding fishes; and No
62
Herbivore - a full cage preventing access by both fishes and urchins. The half-cage
design of the Fish Only treatment also served as a cage control, although a pilot
experiment with a cage control that had a partial top and two sides showed no
difference from the uncaged treatment, and other caging studies in Hawaii have also
shown no effect of the cage on algal growth (Smith et al. 2001). There were 20
replicates of each treatment, for a total of 80 settlement tiles.
The tiles were unglazed, terra-cotta flooring tiles (~14 x 15 x 1.4 cm). One
surface had 1.5 x 2.5 mm grooves alternating with non-grooved surfaces, while the
other surface was smooth. Tiles were deployed with the grooved surfaces facing
upwards and the smooth surfaces facing the natural substrate. Similar amounts of the
grooved upper surfaces consisted of groove (138 cm ) and exposed portions (157
cm2). The grooves provided spaces where recruits might be protected from
herbivores.
Full cages (Urchin Only and No Herbivore treatments) were supported by a
hard PVC plastic frame (40 cm tall and 30 cm diameter). The frame was made in one
piece by cutting three panels from a section of PVC sewer pipe, leaving three upright
lengths connecting an upper and lower ring. The sides and top of the frame were
covered with separate pieces of green, plastic, garden fencing (2.5 cm square holes)
attached with small cable ties (Fig. 1). Plastic mesh was used to prevent
cyanobacterial growth that can occur on metal mesh hardware cloth (Ch. 1). Half
cages (Fish Only treatment) had a partial PVC frame consisting of a 30 cm diameter
~3 cm high ring supporting a wall of plastic fencing mesh approximately 8 cm tall.
63
The Fish and Urchin treatment had no frame. Each tile was attached with cable ties
to a plastic mesh base (25 x 25 cm with 2.5 cm holes). The full and half cages were
attached to their mesh bases with cable ties, and the entire unit was secured to a hard
rocky substrate using large stainless steel lag screws inserted into plastic anchors in
holes drilled into the rock (Fig. 1). Each unit was placed in close proximity (0.7 ± 0.7
m) to small patch reefs with live coral and algae to ensure adequate supply of algal
and coral recruits. Tiles were assigned randomly to treatments and positions within
sites.
Rock-boring urchins (E. mathaei) were collected from the reef near the
experimental sites, and four urchins were enclosed in each Urchin Only replicate. For
comparison of urchin densities between the cages and natural densities seen on the
reef, the area of the base of the circular cages was calculated using a radius of 15 cm.
All tiles and cages was deployed between 8 Oct and 13 Oct 2007, left to gather
recruits for 10-11 months, and retrieved between 18 Aug and 16 Sep 2008.
Retrieval and Processing
Cages and tiles were retrieved by clipping the cable ties attaching them to the
lag screws, and placing the entire unit in a large plastic garbage bag for protection and
to prevent loss of algae while transferring them onto the boat and back to shore. In
the lab, the cages were clipped open, tiles were clipped off of the base mesh, and both
sides of the tile were photographed.
64
All macroalgae inside the cages and on the tiles were removed, separated
while fresh into genera or morphospecies, hand dried with a towel, weighed, and then
dried in a domestic oven (32 - 60°C). Sediments and small turf algae were scraped
from the tiles and wet weights taken. Turf algae were defined as any small, finely
filamented alga. Tiles were then rinsed and dried in the sun. Samples and tiles were
later transported to U. C. Santa Cruz where the algae were further dried in an oven at
60°C for at least 24 hrs, cooled in a vacuum dessicator, and weighed on an analytical
balance.
Each tile was searched completely for coral recruits under a dissecting
microscope at 6x - 25x power. Records included the position of each recruit (top,
bottom, or sides of tile), and, for recruits on the tops of tiles, whether it was on an
open surface or in a groove.
Light Measurements
Because algal growth requires adequate light, spectral quality and light
intensity reaching the tiles were measured at the end of the experiment using a
spectroradiometer (GER 1500, Spectra Vista Corporation; 1.5 nm band widths) in an
underwater housing. Data were taken from two full cages (with minimal macroalgal
growth), one half cage, two open tiles, and under one natural overhang to provide
comparison with naturally low light levels in which algae were present. Reference
(incident irradiance) and target (reflected radiance) data were visualized using the
65
9
1
1
program Gerplot (NASA), and the integrated radiance values (W cm" nm~ sr" )
across the spectrum were used for comparisons.
Analyses
Algal wet weight data was analyzed using one- and two-factor ANOVAs with
General Linear Models. A log (x+1) transformation aided with normality and
homoscedacity of variances when comparing total algal biomass among all
treatments. Transformation was not needed when comparing the Urchin Only with
No Herbivore treatments. Wet weight data was used instead of dry weights because
some dry samples were lost. Turf biomasses were square root transformed. Further
analyses with GLM hypothesis tests were used to make more specific comparisons
between treatments. Numbers of coral recruits were analyzed using ANOVA (GLM)
with hypothesis tests, after square root transforming the data to conform to statistical
assumptions.
Results
Within a week of deployment, all tiles in both full-cage treatments (with and
without urchins) had grown a uniform layer of bright green turf algae which was
absent in open and half-caged treatments; this turf layer persisted for at least another
week (after which cages were left to overwinter).
66
Between October 2007 and August 2008, one Urchin Only treatment (n = 19)
cage and two No Herbivore (n = 18) cages were lost, and all tiles and half cages
remained from the Fish & Urchin (n = 20) and Fish Only (n = 20) treatments.
Some urchins chewed through the plastic mesh caging material and escaped.
When cages were retrieved in August 2008, ten of the cages in the Urchin Only
treatment retained at least one urchin inside; only the cages with urchins still present
(n = 10) were used to represent this treatment. One cage retained all four urchins,
three cages held three urchins, two cages held two urchins, and four cages held one
urchin.
Algal Growth
Algal biomass (wet weights) differed significantly among treatments
(ANOVA df = 3, F = 269.134, p < 0.001). Treatments protected from fish grazing
(Urchin Only & No Herbivore) had 50- to 70-fold more algae than those exposed to
fish (Fish Only and Fish & Urchin) (Fig. 2; Table 1; 2-factor ANOVA, df = 1, F =
737.646, p < 0.001). The presence of urchins also significantly reduced algal biomass
(2-factor ANOVA, df = 1, F = 6.160, p = 0.016), but to a lesser degree than for fishes.
Urchins added a smaller, but not quite statistically significant reduction in algal
biomass in Urchin Only cages compared to No Herbivore cages (GLM hypothesis
test, df = 1, F = 269.134, p = 0.056), while algal biomass was very similar between
the two treatments exposed to fish (Fish Only and Fish & Urchin) (Fig. 2; GLM
hypothesis test, df = 1, F = 2.364, p = 0.129).
67
The three most abundant algal types, Gracilaria, Lobophora, and turf/ thin
red branching accounted for over 97% of all algal biomass (Table 2). Most algal
types had similar proportions between the No Herbivore and Urchin Only treatments,
except for turf/ thin red branching algae which were significantly more abundant in
No Herbivore cages than in Urchin Only cages (Fig. 4; one-way ANOVA, df = 3, F =
34.241, p < 0.001; GLM hypothesis test, df = 1, F = 9.988, p = 0.002). There was
significantly more turf in Urchin Only cages than in both treatments exposed to fish
(GLM hypothesis tests; Fish Only: df = 1, F = 15.638, p < 0.001; Fish & Urchin: df=
1, F = 17.279, p < 0.001). Three morphotypes of cyanobacteria (green, tan, and
general) were grouped together in the "cyanobacteria" category. Cyanobacterial
biomass may have been overestimated because it was difficult to distinguish what
was growing outside vs. inside the cages, and some external cyanobacteria may have
been included.
Cages in the Urchin Only treatment contained from zero to four urchins at the
end of the experiment. Algal biomass declined with increasing urchin density only
after passing a threshold of two urchins (Fig. 3 or Table Z). Algal biomass was
significantly lower in the cages with three or four urchins than in cages with two or
one urchins (ANOVA, df = 4, F = 4.774, p = 0.012; GLM hypothesis test, df = 1, F =
13.778, p = 0.002), and lower in cages with three urchins than with two urchins
(GLM hypothesis test, df = 1, F = 8.984, p = 0.010).
The amount of sediment on tiles also varied significantly among treatments
(Fig. 5; one-way ANOVA, df = 3, F = 20.763, p O.001), with 2-3 times as much on
68
the two treatments exposed to fish than on the Urchin Only and No Herbivore tiles
(Table 3; 2-way ANOVA; df = 1, F = 52.802, p < 0.001). These later two treatments
did not differ significantly in their sediment loads (GLM hypothesis test, df = 1, F =
1.935, p = 0.169).
Cages with high algal biomass were usually characterized by dense clumps of
macroalgae that had many attachments to the cage itself rather than to the settlement
tile: the macroalgae were attached to the tile in only one of 32 cages with macroalgal
clumps. Gracilaria was unusual because many of its attachments were directly to
Lobophora. In a few cages, the macroalgae formed free-floating masses that were not
attached to either the cage or the tile.
The area of the base of the cages and half-cages was 707 cm (0.07 m ). At
the average density of urchins at this site (4 m" ), this equates to 3/10 of an urchin per
cage. The highest average density of urchins at a site, 34 m" in the southwest
backreef, equates to 2.4 urchins per cage. The highest density in this experiment, 4
urchins per cage, is equivalent to 57 urchins m" , which is sometimes seen in the most
dense patches.
Coral Recruitment
A total of 159 coral recruits were recorded from 59 of 77 tiles. Overall coral
recruitment was low (2.1 ± 2.0 SD recruits per tile) but the number of recruits
differed significantly among treatments (Fig. 6; one-way ANOVA, df = 3, F = 2.990,
p = 0.037), with more recruits in treatments exposed to fish (Fish Only and Fish &
69
Urchin) than in treatments protected from fish (Urchin Only & No Herbivores) (Table
4; 2-way ANOVA; df = 1, F = 8.082, p = 0.006). There were no significant
differences between the two treatments exposed to fish (GLM hypothesis test, df = 1,
F = 0.010, p = 0.919) or between the two treatments protected from fish (df = 1, F =
0.332, p = 0.567).
On the upper surfaces of tiles, there were significantly more recruits in
grooves (0.35 ± 0.89 SD recruits per tile) than on non-grooved surfaces (0.06 ± 0.29
SD recruits per tile) (one-way ANOVA, df = 1, F = 9.985, p = 0.002), despite the
slightly lower area of grooved surfaces (138 cm vs. 157 cm ). Treatment did not
significantly affect the number of recruits in grooves or non-grooves. There were a
total of 31 recruits on upper tile surfaces, 49 on lower (smooth) surfaces, and 79 on
the edges.
Recruitment was particularly low in the No Herbivore (1.4 ± 1.3 SD) and
Urchin Only (1.2 ± 1.8 SD) treatments. There was no obvious relationship between
the number of urchins and the number of recruits in the Urchin Only cages (one-way
ANOVA, df = 4, F = 0.621, p = 0.652).
While most recruits on tiles were very small (0.5 - 2.0 mm), 25 larger colonies
(approx. 25 to 75 mm) recruited to the mesh underneath the tiles or comprising the
sides of cages on 22 different replicates. Eighteen colonies were Montipora (14 of
them clearly M. flabellata [cf. turgescens]); the others were growing under very low
light conditions and had atypical morphologies, making positive identification
difficult, but are also likely Montipora.
70
Relationship Between Algal Biomass and Coral Recruitment
The number of coral recruits was significantly negatively correlated with the
wet biomass of algae (Fig. 7; Linear regression, R2 = 0.157, df = 1, F = 14.017, p <
0.001).
Light Reaching Tiles
The amount of light reaching the tiles with and without cages, in half cages,
and under a natural overhang was measured and integrated across spectra (300 - 1100
nm). The lowest spectral readings for uncaged tiles were very similar to the highest
readings for fully caged tiles (Fig. 8). Integrated values and variances were highest
among the open (uncaged) readings, which ranged from 11.3 x 106 to 36.8 x 106 W
cm"2nm_1 sr"1. Beneath full cages, values ranged from 7.6 x 106 to 10.6 x 106 W cm"2
nm"1 sr"1. While readings for half cages were lower than this (5.9 x 106 W cm"2 nm"1
sr"1), they were not as low as under the natural rock overhang (2.0 x 106 W cm"2 nm"1
sr"1) (Fig. 8).
Cage Observations
At the end of the experiment, urchins in the Urchin Only treatment were
usually located on the tile or around its edges, but in a few cases, urchins were living
on the upright side ribs of the cage and had cleared out urchin-sized "tunnels"
(approx. 30 cm long) in the dense macroalgae filling the rest of the cage.
71
Numerous other organisms were inside cages, including many small
crustaceans, a small sea cucumber, a juvenile wrasse, a juvenile lobster, two juvenile
Echinometra mathaei urchins underneath a tile, a cone shell and eggs, and a brittle
star. Two cages contained a Hawaiian whitespotted toby (Canthigaster jactator) that
was much larger than the 2.5 cm mesh size, and evidently had entered the cages as
small juveniles and grown to a size at which they could not escape. These fish are
reported to consume primarily zoobenthos (78%) with some algae and detritus (22%)
(Randall 1985).
Discussion
In this experiment, fishes had a > 50-fold larger impact on macroalgal
biomass than did urchins {Echinometra mathaei). Tiles exposed to fish grazing had
little to no macroalgal growth after a year of deployment, whereas tiles protected
from fish had variable, but often substantial macroalgal growth, whether urchins were
present or not (Fig. 2). Final urchin densities varied within the Urchin Only
treatment, and the cages with more urchins had significantly less macroalgae and turf
(Fig. 3), indicating that higher urchin densities have a greater impact on macroalgae.
Urchin effects were not detectable until densities reached three or four urchins per
cage, which equate to higher densities than average at sites with the most urchins, but
are within the range observed on the backreef That urchins had an effect only at
72
higher than average densities indicates that fish have a greater effect than urchins in
this system.
Large-bodied spectacled parrotfish (Chlorurus perspicillatus) and big schools
of surgeonfishes (various spp.) are common on the backreef and were observed
grazing on exposed tiles and cages. After many years of complete protection from
reef fishing, Northwestern Hawaiian Island reefs have fish stocks 260% greater than
in the main Hawaiian Islands (Friedlander & DeMartini 2002) and are therefore
should reflect how these systems function in their more natural, undisturbed state.
Large-bodied fishes tht could not enter cages presumably have a bigger grazing effect
than their smaller-bodied counterparts. While juvenile parrotfish were observed
swimming through the 2.5 cm mesh of the cages and grazing on the tiles, these
juveniles did not graze enough to prevent macroalgal growth in cages.
Urchin impacts were more spatially limited than for fishes. In some cages, the
only areas clear of macroalgae were in the area immediately surrounding an urchin.
E. mathaei is a bioeroder that carves out "channels" in the rock surfaces:
opportunities to carve channels did not exist within the experiment, but some urchins
created a different sort of channel by clearing urchin-sized tunnels in the algae along
the side of the cage.
Urchin grazing did not seem to affect the taxonomic composition of
macroalgae growing inside cages; both cages with and without urchins had very
similar types and amounts of macroalgae (Table 2).
73
The full cage treatments not only had much higher macroalgal biomass, but
also had significantly lower coral recruitment than treatments exposed to fish,
regardless of the presence of urchins (Fig. 6). Macroalgae are known to inhibit coral
larval settlement and decrease survival of coral recruits, but the grazing activity that
limits macroalgae has also been implicated as a mechanism for removing recruits. In
this study, grazing by fishes may have removed some coral recruits, but any negative
effects offish grazing were completely overshadowed by the negative impacts of
macroalgal growth when fishes were excluded. Coral recruitment was negatively
related to algal biomass across all treatments and replicates (Fig. 7). The macroalgae
may have deterred settling larvae by physically preventing access to the tiles, through
chemical or microbial means (Smith et al. 2006, Vermeij et al. 2009) or by slowing
water flow regimes and limiting the supply of larvae to the tile (cite). Alternatively,
larvae may have settled then died post-settlement. However, this seems less likely
since coral skeletons should persist after death, and would have been seen when tiles
were examined.
Coral recruitment is notoriously variable, even within a site (Hughes et al.
1999), but in this study, the variation in coral recruitment declined with increasing
macroalgal cover, i.e. as macroalgae increased, the maximum number of coral
recruits declined (Fig. 7). This suggests that with more macroalgae present on a reef,
there will be less recruitment; therefore, any increase in grazing intensity is likely to
have a positive effect on recruitment.
74
While tiles exposed to fish grazing had less algae, they also had higher
sediment loads. Sediment can hinder settlement (Hodgson 1990, Birrell et al. 2005),
but it apparently was less inhibiting than macroalgae, since recruitment was higher on
these tiles.
In this experiment E. mathaei did not seem to affect coral recruits, since coral
numbers in Urchin Only and No Herbivore treatments were similar. However, not all
urchins moved across the entire surface of the tiles (some made tunnels in macroalgae
along the cage side), so their behavior may have lessened the impact of urchins on
coral recruits.
In some respects, the caging mesh may have been a better recruitment
substrate than the terra-cotta tiles, since large colonies of Montipora grow on the
mesh in all treatments, and macroalgal clumps were often attached to the mesh rather
than to the tiles. The largest coral recruits (>25mm) were all Montipora colonies
growing on the mesh caging material; no recruits on the tiles reached nearly the same
size (all < 3mm).
Since light levels influence macroalgal growth (Coutinho & Zingmark 1993),
we measured the reflectance of light from tiles under open, half caged, and caged
scenarios. Light levels varied more due to temporary changes in cloud cover and
focused light from surface ripples than the from the cage material itself. The large
difference between high and low reflectance measurements from uncaged tiles (Fig.
8), along with the very low reflectance from the half caged tile, indicate the broad
range of natural light levels on a typical, partly-cloudy day in the shallow backreef.
75
The high values under the caging material were very similar to the lowest reading on
open tiles, and the low value under a full cage was still higher than the reading for the
half cage, further emphasizing the natural variability in light levels. All reflectances
from tiles were higher than that recorded under a small rock overhang nearby that had
macroalgae present, so light should not be limiting for macroalgal growth.
In summary, in an intact Hawaiian reef system, fishes are the dominant
herbivores, greatly outgrazing the abundant Echinometra mathaei urchins, and largely
preventing growth of macroalgae. In addition to being highly effective grazers, these
fishes also promote coral recruitment, probably by limiting competition with
macroalgae. The direct negative relationship between algal density and coral recruits
indicates that any reduction in algal cover will assist coral recruitment and facilitate
reef recovery from natural and anthropogenic perturbations.
76
Table 1. 2-factor ANOVA comparing algal wet weights in factorial treatments
exposed to fishes and urchins.
Source
Type III SS
Fishes
472.754
I
Urchin
3.948
Urchin*Fishes
0.200
Error
df Mean Squares
F-ratio
p-value
472.754
737.646
0.000
1
3.948
6.160
0.016
1
0.200
0.312
0.579
41.017 64
0.641
77
Table 2. Effect of urchins and fishes on species composition of algae in each treatment; mean wet weight (g ± SD),
ranked by overall abundance.
Algal Type
Gracilaria
Lobophora
Turf/Thin red branching
Cyanobacteria
Hydroclathrus
•Hypnea '
Codium
Dictyota
Ulva
Cladophora
Cryptonemia
Total
Percent
Fishes and Urchins
Mean
SD
0.4 0.09
7.5 0.91
0 0.00
3.1 0.69
0 0.00
0 0.00
0 0.00
0 0.00
0 0.00
0 0.00
0.1 0.02
11.1 1.72
0.12
Fishes
Mean
0
52.8
10.8
0.5
0
0
0
0
0
0.1
0
64.2
0.68
Only
SD
0.00
6.65
2.35
0.11
0.00
0.00
0.00
0.00
0.00
0.02
0.00
9.13
Urchins Only
Mean
SD
1492.1 163.73
702.2 42.86
240.6 28.00
17.8
5.09
46
8.00
0.11
0.5
2.4
0.76
0.8
0.25
0
0.00
0
0.00
0
0.00
2502.4 248.80
26.56
No Herbivores
Mean
SD
3164.1 156.35
2366.3 51.09
1133.6 49.32
100.2 16.31
58.2
6.44
18.1 2.00
0.3
0.07
0.24
1.4
0.40
1.9
1.2
0.26
0.00
0
6845.3 282.49
72.64
Total
4656.6
3128.8
1385
121.6
104.2
18.6
2.7
2.2
1.9
1.3
0.1
9423
100.00
SD
129.11
66.67
38.15
8.76
4.77
1.10
0.29
0.16
0.21
0.13
0.01
249.35
Percent
49.42
33.20
14.70
1.29
1.11
0.20'
0.03
0.02
0.02
0.01
0.00
Table 3. 2-factor ANOVA comparing sediment wet weights in factorial treatments
exposed to fishes and urchins.
Source
Type III SS
Urchin
3.884
1
Fishes
139.277
1
2.359
1
2.359
166.175 63
2.638
Urchin*Fishes
Error
df Mean Squares F-ratio
3.884
p-value
1.473
0.229
139.277 52.802
0.000
79
0.894
0.348
Table 4. 2-factor ANOVA comparing the number of coral recruits in factorial
treatments exposed to fishes and urchins.
Source
Type III SS
Urchin
0.206
1
0.206
0.368
0.546
Fishes
4.514
1
4.514
8.082
0.006
Urchin*Fishes
0.054
1
0.054
0.097
0.757
35.190 63
0.559
Error
df Mean Squares F-ratio
80
p-value
Urchins
'» ^
No Urchins £*V *
LV 6 *I ' " ' " I
a
m
^
^
v
w
JIT W
».w
W —- w
-
-•
m
££$
LIU*.****
Figure 1. Diagram of the 2 x 2 factorial design with four treatments: a) Fish and
Urchin, b) Urchin Only, c) Fish Only, and d) No Herbivore (No Grazers).
Photographs were taken just before cage removal.
81
100
D)
*
I
50
IFish &
Urchin
Fish
Only
Urchin
No
Only Grazers
Treatment
Figure 4. Biomass of turf algae (wet weight ± ISE) in the four treatments. Letters
indicate significant differences in GLM hypothesis tests (a:b, p <0.001; b:c, p =
0.002).
82
Fish &
Urchin
Fish
Only
Urchin
Only
No
Grazers
Treatment
Figure 5. Mass (wet weight ± 1 SE) of sediments on tiles in the four treatments.
Letters indicate significant difference in GLM hypothesis tests (p < 0.001).
83
4-i
3-
(fi
o
-i—•
«-
-
1 -
0
Fish&
Urchin
Fish
Only
Urchin
Only
No
Grazers
Treatment
Figure 6. Numbers of coral recruits per tile (± ISE) in the four treatments. Letters
indicate significant differences in GLM hypothesis tests (p = 0.004).
84
0
200
400
600
Algal wet weight (g)
Figure 7. Number of coral recruits as a function of algal biomass (wet weight). The
red line is the best fit estimate, blue lines are control limits, and black lines are
probability limits (R2 = 0.157, p < 0.001).
85
250000
Open (High)
Open (Low)
Cage (High)
Cage (Low)
Half Cage
Overhang
(/>
200000
CNJ
E
o
150000
0
O
100000
c
CO
CO
50000
a:
<& <& t^ & & <&tfPoP°N^V°
Wavelength (nm)
Figure 8. Quantitative spectra of light under three treatments (open, full cage, and
half cage) and a natural rock overhang. High and Low refer to the highest and lowest
readings for this category. Key is listed in decreasing order of magnitude.
86
CHAPTER 3
Direct, species-specific impacts of sea urchins on live corals
Abstract
Interactions between species can shape major processes that influence coral
cover and community structure on coral reefs. Grazing by sea urchins on macroalgae
has strong indirect benefits on coral cover and reef resilience. While there is much
research investigating urchin bioerosion of rock, direct impacts of urchins on live
coral tissue are rarely studied. I conducted two experiments on the backreef of
Midway Atoll (NW Hawaiian Islands) investigating direct, species-specific impacts
of two abundant, bioeroding urchins (Echinometra mathaei and Heterocentrotus
mammillatus) on three coral species {Pocillopora ligulata, Montipora c.fturgescens,
and Porites lobatd). I epoxied nubbins of each coral species inside bioeroded
channels of E. mathaei and the eroded surfaces of//, mammillatus after removing
urchins from half the plots. Most fragments of all three coral species exposed to E.
mathaei were damaged within days and the damage increased over several weeks to
months, often ending with complete removal of all coral tissue and skeleton.
Fragments in plots without E. mathaei, and all fragments exposed to H. mammillatus
plots were unaffected. These results indicate that grazing by E. mathaei can have
direct negative impacts on live corals, while H. mammillatus has little or no effect.
87
This differential grazing has important implications for recruitment success of corals,
community structure and reef growth.
Introduction
Consumers may shape community structure directly through consumption of
their prey, or indirectly, as in trophic cascades, where predators benefit primary
producers by consuming their herbivores (Terborgh & Estes 2010). Some consumers
may have mixed effects: causing direct harm to a foundational species while
indirectly benefiting it via effects on a third species.
In the ecological literature, interactions between sea urchins and corals are
often treated only as indirect processes, mediated through urchin herbivory on
macroalgae, a primary competitor with corals (Edmunds & Carpenter 2001, McCook
et al. 2001b). By preventing macroalgae from establishing after a disturbance that
causes coral mortality (e.g. bleaching, hurricane), herbivores allow coral to recover,
enhancing resilience of the reef (Diaz-Pulido et al. 2009). It is widely accepted that
grazing by diademnid urchins maintained the resilience of Caribbean reefs that had
few remaining herbivorous fishes by the late 1970's. In 1983, a mass-mortality event
decimated the urchin populations, reducing the resilience of the reefs, and the
subsequent loss of coral cover from a hurricane resulted in a shift to a macroalgaldominated state (Lessios et al. 1984, Hughes 1994).
88
Most geological literature treats urchins as bioeroders, carving away the
limestone foundation of reefs. Urchin bioerosion rates are measured by the amount of
carbonate material passing through the gut (Bak 1994, Asgaard & Bromley 2008),
sometimes giving estimates much higher than reef accretion rates and leading to
predictions of collapse of the reef structure (Bak 1990). Bioeroding urchins consume
a high proportion of CaCCb; in one study, Echinometra mathaei gut contents
contained 73% CaCCh, 20% organic matter, and 7% refractory organic matter (Mills
et al. 2000), and Carreiro-Silva and McClanahan (2001) concluded that urchin
bioerosion in Kenya was greater than herbivory for all four urchin species studied,
including E. mathaei.
The rock-boring urchin, Echinometra mathaei, has a broad tropical IndoPacific distribution and is often very abundant. Each individual resides in a
"channel" (about 10 - 40 cm long and the width of its test and spines), created by
scraping into the rock with its mouthparts (Aristotle's lantern), and variously referred
to as a "boring", "groove", "burrow", "crevice", or "cavity" (Hart & Chia 1990,
McClanahan & Kurtis 1991, Asgaard & Bromley 2008). Although sometimes treated
solely as an herbivore (Ogden et al. 1989, Mills et al. 2000), E. mathaei also
consumes floes of aggregate organic matter from the water column (making it a
detritivore as well as an herbivore), "farms" algae within its boring, and traps bits of
drift algae (Huettel et al. 2006, Asgaard & Bromley 2008). Another important
bioeroding species is the red pencil urchin, Heterocentrotus mammillatus, which does
not create channels for itself but does bioerode by scraping at the rock surface (Dart
89
1972). Bioerosion by urchins on dead carbonate substrates may be intense, and is
well documented, but direct impacts of urchins on live coral tissue are rarely reported.
However, the bioerosional capabilities of these urchins make them likely candidates
to be predators of live corals.
Ecologists usually treat urchins as herbivores or detritivores (Asgaard &
Bromley 2008), but three studies have described predation of live tissues on adult
coral colonies. Bak and van Eys (1975) described a site with high coral cover and
unusually high densities of Diadema antillarum (8.5 individuals m" ) where 8% of the
urchins were feeding on live coral; Sammarco (1982) described both D. antillarum
and Echinometra viridis damaging larger coral colonies; and in the Galapagos, where
predators are rare and Eucidaris thouarsii occurs at high population densities ( 1 0 - 5 0
individuals m"2), it feeds heavily on live pocilloporid corals, unlike its mainland
counterparts that eat marine plants and sponges (Glynn et al. 1979).
Urchins may also directly affect coral recruits through what is generally
thought to be incidental take during grazing activities, rather than by deliberate acts of
predation. Coral recruitment may be optimized at intermediate densities of Diadema,
with urchins at high densities consuming coral recruits, and macroalgae hindering
recruitment at low urchin densities (Sammarco 1980, 1982). These observations are
consistent with the larger conceptual framework of the Intermediate Disturbance
Hypothesis (Connell 1978), where high disturbance by Diadema reduces recruitment
directly, and low disturbance by Diadema allows competitive exclusion of recruits by
macroalgae. More recent studies following recovery of Diadema populations in the
90
Caribbean support this idea: coral recruitment and growth of juveniles appears higher
in regions where Diadema has recovered to what were previously considered
"intermediate" densities than where Diadema remains rare or absent (Edmunds &
Carpenter 2001, Macintyre et al. 2005, Carpenter & Edmunds 2006, Idjadi et al.
2010).
Direct and indirect interactions between urchins, algae, and corals can be
species-specific, but have not been studied extensively in urchins other than
Diadema. McClanahan and Muthiga (1988) reported lower coral cover on Kenyan
reefs with high densities of Echinometra mathaei, implying that heavy grazing
reduces coral cover. Conversely, Dart (1972) suggested that E. mathaei and H.
mammillatus indirectly benefit recruitment of corals in the Red Sea by removing turf
algae that inhibit settlement (Arnold et al. 2010). Others have suggested the
bioeroded channels created by E. mathaei may facilitate establishment of recruits
(Birkeland & Randall 1981). In a specific experimental comparison of D. antillarum
and E. viridis, high densities of D. antillarum suppressed coral recruitment, while
high densities of E. viridis did not affect recruitment (Sammarco 1982). Speciesspecific effects have also been noted in the Florida Keys where sites with recovering
D. antillarum populations were associated with lower macroalgae and higher coral
cover, but sites with high densities of E. lucunter had high macroalgal cover and few
corals (Furman & Heck 2009).
Reports of direct impacts of urchins on live corals are mixed, and estimates of
the amount of coral tissue removed over time have not been reported. Given that
91
corals have potential defenses against predators and competitors, e.g. nematocysts,
symbiotic crustaceans (Glynn 1980, Bruno & Witman 1996), it is possible that many
urchins are deterred from consuming or even moving over live corals and that
qualitative accounts of urchins feeding on corals do not translate into substantial
quantitative impacts. This study quantifies both the frequency of direct interactions
between urchins and corals, and the rates of removal of coral tissues by urchins. I
investigated two main hypotheses: 1) urchins (Echinometra mathaei and
Heterocentrotus mammillatus) are capable of removing live coral tissue from three
coral species (Pocillopora ligulata, Montipora c.fturgescens, and Pontes lobata);
and 2) urchin impacts are species-specific (i.e. vary by species of urchin and species
of coral). To test these hypotheses, I transplanted coral fragments, also called
"nubbins", of three coral species into the bioeroded grooves of E. mathaei and into
the grazing zones of// mammillatus at two sites on the shallow backreef of Midway
Atoll.
Methods
Location
This study was conducted on Midway Atoll, a National Wildlife Refuge
within the Papahanaumokuakea Marine National Monument, which encompasses the
Northwestern Hawaiian Islands. Midway is a high latitude, subtropical (28°N,
179°W) atoll near the limits of reef growth in the North Pacific Ocean (Grigg 1982).
92
All experiments were conducted on the shallow backreef (1 - 2 m depth) at two sites
(Fig. 1): one in the southwest (28.25104 °N, 177.32757 °W), where benthic surveys
documented low coral cover (3%) and high urchin densities (35 m"2), the other in the
northeast (28.20326 °N, 177.41882 °W), characterized by high coral cover (36%) and
much lower urchin densities (6 m~2). The southwest site is a rocky, subtidal reef flat
extending a few hundred meters behind the reef crest, terminating in a vertical zone
(1 - 2 m) with high densities of Heterocentrotus mammillatus that adjoins a large
region of lagoonal sediments. Echinometra mathaei is abundant on all rocky
substrates (34 m"); the overall density ofH. mammillatus is 0.3 m" at this site. The
northeast site is more protected from ocean waves by uplifted portions of an ancient
reef. The site has a narrower region of subtidal reef flat that gives way to coralcovered patch reefs separated by sandy areas. The interface between the reef and
lagoonal sand flat is also heavily populated with Heterocentrotus mammillatus on
vertical rock surfaces (0.3 m"), and Echinometra mathaei occurs throughout (6 m" )
on hard substrates. A third urchin, Echinostrephus aciculatus, is common at both
sites (0.7 m"2 in the southwest and 0.1 m"2 in the northeast), but because it does not
leave its deep, cup-shaped boring (Asgaard & Bromley 2008), it is unlikely to have
any impact on corals, and it was not considered in this study. A few other urchin
species are present on Midway (e.g. Echinothrix spp., Diadema spp., Tripneustes
gratilla), but they are rare.
Urchin damage on live coral
93
Impacts of the urchin Echinometra mathaei on live tissue of the coral
Pocillopora ligulata were quantified at the southwest backreef site in an experiment
using 12 plots (each 1 m2). Urchins were removed from six plots (selected randomly)
to serve as controls. An additional 0.5 m buffer zone was cleared of urchins around
each control plot to ensure no encroachment from the periphery. Urchins remained
undisturbed in the other six plots.
Small, 5-8 cm long, fragments (hereafter called nubbins) of Pocillopora
ligulata were collected from live colonies at the site, transported underwater in plastic
bags to the experimental plots, and epoxied into bioeroded channels created by
Echinometra mathaei. There were ten coral nubbins per plot, for a total of 120
transplanted nubbins. Either Z-Spar epoxy or Sea Goin' Poxy Putty was used to
attach the coral nubbins to the substrate; neither had a detectable negative impact on
the corals, as evidenced by live tissue that maintained its color even directly adjacent
to the epoxy. Each nubbin was identified by numbered, plastic swine ear tags
attached to the nearby substrate with cable ties.
The experiment began in mid-August 2006 and all coral nubbins were
monitored on 1, 3, 6, 9, and 32 days following attachment to the substrate. On each
date, the number of nubbins damaged by urchins was noted, and each nubbin was
photographed with a scale. Only substantial and sustained tissue loss consistent with
that caused by urchins was counted as damage; temporary nicks were not included in
this category.
94
Species-specific effects and site differences
This experiment expanded the design of the first experiment to investigate
species-specific impacts by including an additional urchin species and two new coral
species, and to investigate site-specific effects by adding an additional site. The
urchin species used were Echinometra mathaei and Heterocentrotus mammillatus,
with 30 plots (1 m2) at each site, for a total of 60 plots between two sites. Within a
site, 20 plots were assigned to E. mathaei and 10 plots to H. mammillatus. Half the
plots for each urchin were then selected randomly for removal of urchins, including a
0.25 m buffer zone around each plot. The plots were spread across the rocky
backreef, with Heterocentrotus plots closer to the inner margin of the backreef and
the sandy lagoon where Heterocentrotus is most abundant.
Two coral species were exposed to urchin grazing: Montipora flabellata [c.f.
turgescens, (James Maragos pers. comm.)] and Porites lobata, both common corals
on the north backreef. A third coral, Pocillopora ligulata, was originally included in
this experiment (as in the first experiment), but apparently due to handling stress
(transporting corals by boat in plastic bags) most nubbins of Pocillopora bleached
and died shortly after placement; therefore, only the data from Pocillopora in the first
experiment are presented and discussed. Nubbins of each coral species were
collected from the northeast site and transported by boat to both sites, protected by
water-filled plastic bags placed in large coolers. All coral nubbins were subjected to
the same transport time (approx. 25 min); immediately after arrival, the bags were
placed underwater. Four coral nubbins were epoxied to the substrate in each plot: one
95
Porites, one Montipora, and two Pocillopora. The Porites and Montipora nubbins
survived transport and transplant with no ill-effects, unlike most of the Pocillopora
nubbins. Porites and Montipora nubbins were placed within areas of obvious urchin
activity (i.e. a bioeroded channel in Echinometra plots and relatively bare, grazed
substrate in Heterocentrotus plots) to ensure exposure to urchins. One Pocillopora
nubbin was placed within a visible urchin grazing area, and the other outside the
visibly grazed area, to test for differential impacts over space. Because the
Pocillopora nubbins were lost, this experiment could not determine whether
Echinometra mathaei ever leave their channels to graze and so might impact corals
over broader areas during nocturnal feeding activities, but a separate study of urchin
movements was conducted to address this question (see below). The final
experimental design contained two nubbins in each plot (one Porites, one Montipora)
for a total of 80 nubbins exposed to Echinometra and 40 nubbins exposed to
Heterocentrotus.
The experiment began in early July 2007, and nubbins were checked for
damage and photographed with a scale at increasing intervals following attachment.
At the northeast site, nubbins were checked on days: 1, 2, 6, 13, 34, 57, and 116. At
the southwest site, nubbins were checked on days: 1, 3, 9, 25, 54, and 111.
"Damage" was more broadly defined than in the first experiment to include many
types of tissue loss, including that which may have been caused by fishes or other
sources. Each nubbin was identified by a numbered, plastic swine ear tag attached to
the nearby substrate.
96
Spatial effects of urchins
In order to understand the spatial influence of urchins across the reef,
movement patterns were observed in both species. A total of 384 Echinometra
mathaei were observed during their active period at night (two hours after sunset,
with no moonlight) on a nearshore patch reef at Rusty Bucket (28.215647 °N,
177.387943 °W) on September 21, 2010. Two observers swam haphazard transects
and recorded the position (inside or outside its bioeroded channel) of every E.
mathaei visible with a dive light.
Heterocentrotus mammillatus positions were monitored overnight in October
2007 at two sites: one in the southwest backreef (28.21431 °N, 177.42216 °W) and
one in the north backreef (28.27482 °N, 177.35451 °W). Ten urchins at each site
were tagged with marked surgical tubing slipped over a spine and their current
position marked using cable ties. Their positions were checked the next day and the
distance from the last location measured.
Any urchin movements observed during the day were also noted throughout
the course of the experiment, as well as during other activities.
Surveys
The surface area of reef affected by E. mathaei grazing and bioerosion was
estimated from surveys at both sites in November 2007, using 10 m long lineintercept transects, randomly placed and oriented within a 100 m region. The length
97
(in cm) under the line that consisted of urchin channels was recorded along ten
replicate transects at each site.
Analyses
Sequential nubbin photographs were analyzed for area of tissue lost, using the
software Coral Point Count with Excel extensions (CPCe; available as freeware).
The area of tissue removed from nubbins in the first experiment was analyzed using a
Repeated Measures analysis after square root-transforming the data to conform to
assumptions of normality and homoscedacity of variances. Estimates of damage to
Pocillopora nubbins did not require statistical analysis because no controls were
damaged.
For the second experiment, damage data were analyzed using Log linear
models with three terms: treatment (urchins present or urchins removed), coral genus
(Montipora or Porites), and nubbin status (damaged or undamaged). Rates of
removal were compared by coral genus and site using a 2-way ANOVA on square
root-transformed data.
Time from start to first damage was square root transformed and the effects of
site and coral taxon were evaluated in a 2-way ANOVA.
Results
Echinometra impacts on Pocillopora
98
Within 24 hours of exposure to E. mathaei, 90% of Pocillopora nubbins were
damaged (9.0 ± 1.4 SD out of 10 nubbins per plot), but none of the nubbins in control
plots sustained urchin damage (Fig. 2). These trends were consistent over time; all
remaining nubbins in urchin plots were damaged by day 32, while 100% of control
nubbins remained free of urchin damage. In a few cases, urchins were able to pry the
nubbin off the substrate and evict it from the channel. These were considered
"damaged", since they were affected by urchins; measurement of the area damaged
was not applicable in this case.
The area of damage increased over time as urchins gradually removed more
and more coral tissue and skeleton (Fig. 3). On day one, just 0.21 ± 0.19 SD cm2 was
missing, but by day nine, that amount had increased 12-fold to 2.56 ± 2.07 cm (Fig.
4; Repeated Measures, SS = 22.277, F = 47.566, df = 2, p <0.001)
Over the first nine days, the average rate of tissue loss from Pocillopora
nubbins was 0.32 ± 0.23 cm2 day"1 (Table 1).
Species-specific effects: corals
Over the three month duration of the second experiment, nearly all nubbins of
both Montipora (95%) and Pontes (97%) were damaged by E. mathaei in the plots
with urchins present (Fig. 5). By contrast, only a small proportion, 6% of Pontes and
11% of Montipora nubbins were damaged in control plots without E. mathaei. In a
Log linear model analysis of treatment, coral genus, and nubbin status, the only
significant term was treatment (urchins present or urchins removed) by nubbin status
99
(damaged or undamaged), indicating that treatment had a highly significant effect on
the number of damaged nubbins for both Montipora and Porites (x = 123.30, df = 4,
p < 0.0001), with more nubbins damaged in urchin plots than in plots with urchins
removed (Fig. 5).
As with Pocillopora, E. mathaei urchins removed increasing amounts over
time from both Montipora (Fig. 6a) and Porites (Fig. 6b). Coral removal rates were
calculated after the first day of exposure for Porites and Montipora at both sites
(Table 1). Removal rates were significantly higher at the southwest site than at the
northeast site (Table 2; 2-way ANOVA, df = 1, F = 5.414, p = 0.030), but did not
differ between coral genera (df = 1, F = 0.044, p = 0.836) or in the interaction term
(df= 1, F = 0.114, p = 0.340).
The time to first damage by E. mathaei differed significantly between sites,
with the average time until damage taking more than twice as long at the northeast
than at the southwest site (Fig. 7; Table 3; 2-way ANOVA, site effects, df = 1, F =
6.844, p = 0.011).
While there was an apparent tendency for Montipora nubbins to be grazed
down more quickly than Porites nubbins (Fig. 7), this was not significant (Table 3; 2way ANOVA, taxon effects, df = 1, F = 0.350, p = 0.556).
Species-specific effects: urchins
In plots with Heterocentrotus urchins at both sites, there was no damage to
any coral that could be definitively attributed to urchins. Despite the fact that
100
Heterocentrotus were often seen on top of and even completely obscuring nubbins,
such nubbins always remained undamaged upon later checks. One Porites nubbin in
a Heterocentrotus removal plot did lose most of its tissue between the second and
fourth days after deployment, but the surrounding damage marks were linear, and so
unlikely to be made by urchins, which tend to produce star-shaped marks. Two
Montipora nubbins in the northeast (one in an urchin plot and one in a removal plot)
were damaged by day 14, but neither seemed caused by urchins.
By day 14, a few Heterocentrotus had moved into removal plots in the
northeast site, and they were removed upon discovery. Because Heterocentrotus has
no visible impacts, those urchins that invaded removal plots after day 14 were no
longer removed, effectively ending the treatment differences at this point. Between
day 4 and day 10, nearly all Montipora in both treatments in the south acquired pale
spots, but no Montipora at the northeast site developed this condition. Three months
after deployment, most nubbins in Heterocentrotus plots at the northeast site,
regardless of species or treatment, looked healthy and undamaged, but after the same
period of time most nubbins of both species at the southwest site appeared stressed,
with lighter color and dead patches.
Urchin movements
Of 384 Echinometra mathaei observed after dark on a moonless night, all but
two clearly remained in their channels. One exception was on sand underneath an
overhang, where the sand may have encroached over or filled its channel, and the
101
other was not in a channel but immediately adjacent to an octopus which appeared to
be interacting with it, and may have extracted it from its channel.
Tagged Heterocentrotus mammillatus urchins, ten at each site, moved an
average of 42 ± 31 cm SD overnight at the northern site and 46 ± 35 cm at the
southwest site. Two urchins at the southwest site remained in the same daytime
resting spot (i.e. moved 0 m), while the farthest distance traveled was 100 cm.
Reef area affected by E. mathaei
In ten surveys at the southwest site, E. mathaei urchin channels covered
13.2% ± 4.5 SD of the reef substrate, with the highest value reaching 18.5%. This
was almost five times the area covered by channels at the northwest site 2.8% ±1.7
SD.
Discussion.
This study experimentally demonstrated the direct and sustained removal of
live coral tissue by the sea urchin Echinometra mathaei but not by Heterocentrotus
mammillatus. E. mathaei removed both live tissue and skeletal material from three
coral species, supporting the hypothesis that intensive removal of coral tissue is
possible and consistent across coral species tested, but varies by urchin species. E.
mathaei acted quickly, with 90% of Pocillopora nubbins sustaining urchin damage
within the first 24 hrs of exposure (Fig. 2). The urchins continued removing coral
102
tissue over subsequent days (Fig. 3), gradually reducing the coral nubbins to small
remnant pieces, and often removing the entire nubbin (Figs. 4, 6). Controls (without
E. mathaei) remained undamaged over one month for Pocillopora, and the small
amounts of damage sustained over three months on a few Porites and Montipora
nubbins were not consistent with urchin damage. The overwhelming majority of
Porites and Montipora nubbins exposed to E. mathaei were damaged (Fig. 5).
Indirect positive effects of tropical sea urchins on live corals are welldocumented mainly for Diadema antillarum, whose grazing activities in the
Caribbean have either prevented or reversed phase shifts to low-coral, high-algal
states (Hughes 1994, Edmunds & Carpenter 2001). In contrast, direct negative
effects of urchins on live corals are rarely reported, but they are not necessarily less
common, since some species are both abundant and strong bioeroders of carbonate
substrates (Sammarco 1980, Carreiro-Silva & McClanahan 2001, Asgaard &
Bromley 2008)
The results of this study support the idea that, in addition to its known diet of
algal films and flocculant organic matter (Asgaard & Bromley 2008), E. mathaei may
also consume invertebrates, such as corals that it may encounter as recruits or adult
colonies attempting to grow into its burrow. This pattern on Midway Atoll also
appears to be more pervasive than in some other studies of coral predation by urchins,
in which only a fraction of the population were damaged and only at exceptionally
high urchin densities (Bak & van Eys 1975) or at sites with high urchin densities and
under low predation pressure due to few predatory fishes (Glynn et al. 1979). Given
103
the evidence of herbivory on algae, detritivory on organic aggregates, and, at times,
predation on corals (and probably other invertebrates), E. mathaei are perhaps best
described as opportunistic omnivores, capable of feeding at multiple trophic levels on
whatever enters (or grows within) their grooves.
Species-specific differences in grazing effect of urchins on algae and corals
are likely to be common but they are rarely investigated directly (Sammarco 1982,
Furman & Heck 2009). In contrast to the behavior of E. mathaei, Heterocentrotus
mammillatus did not damage any live corals in these experiments, despite multiple
occasions where urchins were seen resting directly on top of one of the coral nubbins.
H. mammillatus is also a bioeroder, but is more mobile than E. mathaei, grazing over
a broader region and creating zones of intensive grazing characterized by reduced turf
and macroalgae, rather than creating distinct bioeroded channels like E. mathaei. H.
mammillatus is active at night when tagged individuals moved an average of 44 ± 32
SD cm between daytime resting places.
Rates and outcomes of grazing by E. mathaei grazing did not differ
significantly among three coral species (Pocillopora ligulata, Montipora flabellata,
and Porites lobata), indicating that coral identity did not substantially influence the
behavior of E. mathaei or its effects on coral mortality. All coral taxa tested were
susceptible to urchin grazing at similar rates.
Understanding the movement patterns of urchins is critical for interpreting
their broader influences on the reefs. Nighttime observations were consistent with
daytime observations on E. mathaei behavior, confirming that E. mathaei, although
104
more active at night, does not leave its channels. The common observation that small
macroalgae and corals often live right up to the edges of, but not within, E. mathaei
channels further indicates that urchin activity is restricted to the channels. Behavior
of E. mathaei seems more variable in Kenya, where it uses crevices on the outer reef
but remains on exposed surfaces on the inner reef (Khamala 1971). Similar to the
behavior we observed for E. mathaei, the congeneric E. leucunter apparently does not
leave its borings (Hoskin et al. 1986, Asgaard & Bromley 2008). Such restricted
movement severely limits Echinometra's sphere of influence, and while its impacts
on corals and algae may be intense within channels, they are highly localized and
patchy in nature.
The borings of E. mathaei have been described as traps for drift algae or as
algal "farming" areas (Asgaard & Bromley 2008), but it is likely that they also play a
large role in protection from predators. During our urchin removals, any individual
taken out of its groove and left in the open was snatched up almost immediately by
nearby wrasses (Labridae), notably including the Hawaiian Hogfish, (Bodianus
albotaeniatus) which consumed smaller urchins whole, and readily ingested pieces of
larger urchins, including the test and spines. Elsewhere, the primary predators of
urchins are usually triggerfishes, e.g. on Kenyan reefs (McClanahan & Shafir 1990),
but these fishes are rare on the shallow backreef of Midway Atoll where Echinometra
is most abundant; triggerfishes are common only on the forereef where urchins are
less dense. E. mathaei seems to have a strong channeling instinct; urchins in a
105
separate caging experiment created channels in the dense fleshy macroalgae growing
within the cages but did not consume macroalgae beyond these channels (Chapter 2).
Because Echinometra does not leave its channels, and because channels are
non-overlapping, each coral nubbin was exposed to only one urchin, and site-specific
impacts related to differences in urchin densities were not expected. However, there
were site differences, with urchins acting more quickly and removing a greater area in
the higher density southwest site than in the lower density northeast site (Fig. 7, Table
1). This suggests that there are other characteristics of the site itself that may affect
urchin behavior and grazing rate. For example, strong water movement is linked to
maintaining refuge in crevices (Tsuchiya & Nishihira 1984), and current velocities or
temperature differences may also influence urchin behavior. Previous exposure to
corals may also have a role; the northeast site had much higher coral densities and
many urchin channels were immediately adjacent to a coral colony, so previous
experience with corals may have made urchins initially more reluctant to attack the
corals.
Direct removal of coral tissue by urchins has strong implications for coral
recruitment. Some studies suggest that urchin grazing is detrimental to recruitment of
corals (Sammarco 1980, 1982), while others provide evidence of indirect benefits for
coral recruitment (Edmunds & Carpenter 2001, Carpenter & Edmunds 2006, Idjadi et
al. 2010). Channels and grazed zones are largely turf-free spaces that seem ideal for
coral recruitment. While this study looked at juvenile- to small adult-sized corals,
rather than recruits, the intensive removal of larger colonies by E. mathaei implies
106
that recruits would not survive within E. mathaei channels. It is less clear whether
Heterocentrotus, which did not seem to harm the experimental coral nubbins, also
avoids tiny recruits, or whether recruits would be removed incidentally during its
grazing activities. It is also possible that E. mathaei inhibits recruitment within its
channel, but facilitates some recruitment along the edges of its channel, where
recruits could establish in a cleared area before expanding laterally away from the
channel (Birkeland & Randall 1981), but I did not see evidence supporting this
hypothesis.
Whether by removing recruits or by limiting growth of adult colonies, the
spatial influence of E. mathaei is mostly limited to the area of reef covered by its
channels. On the northeast backreef, this averaged 3% of the reef surface area, but it
was four times higher in southwest backreef, where an average 13% of the reef
consisted of urchin channels—a substantial area over which E. mathaei can directly
reduce coral cover and influence community structure.
107
Table 1. Mean daily rates (cm2 urchin"1 day"1) of live coral tissue loss from corals
exposed to E. mathaei, calculated across the cumulative number of days exposed.
Site
Coral
Pocillopora
ligulata
Pocillopora
ligulata
Pocillopora
ligulata
Montipora
flabellata
Porites
lobata
Days exposed
Southwest
SD
mean
N
mean
Northeast
SD
N
1
0.28
0.17
33
n/a
n/a
0
3
0.45
0.08
30
n/a
n/a
0
9
0.32
0.23
30
n/a
n/a
0
1
0.34
0.72
11
0.03
0.12
4
1
0.16
0.42
7
0.05
0.21
3
108
Table 2. 2-factor ANOVA results for rate of coral tissue removal by site and coral
genus.
Source
Site
Coral genus
Site*Coral genus
Error
Type III SS
0.646
0.005
0.114
2.507
df
1
1
1
21
109
Mean Squares
0.646
0.005
0.114
0.119
F-ratio
5.414
0.044
0.954
p-value
0.030
0.836
0.340
Table 3. 2-factor ANOVA
for day of first damage by site and coral genus
Source
Sum-ofSquares
df
Coral genus
14.473
1
Site
28.522
Site*Coral
genus
Error
Mean-Square
F-ratio
P
14.473
3.473
0.067
1
28.522
6.844
0.011
1.459
1
1.459
0.350
0.556
254.210
61
4.167
110
m
Midway
Atoll
WAUTICAl MILES
o
KILOMETERS
^ 0 H
Northeast SMS<
HI
o
* * i*+
-**"*•-*•,
«
Ik
GO
-
+ f -w
* if
^SEbn&fe^^svinMtt'
J . _ . ~ Q _ . |
j[77024'w
Il«f
,£ M«|tQ,| Q'VA/
Figure 1. Study sites on Midway Atoll, Northwestern Hawaiian Islands. IKONOS
satellite image; NOAA Atlas 2003.
Ill
*-*-
"" Urchins removed
"" Urchins present
1.
• * — * • -
T
o
5
10
15
20
25
30
as
Day
Figure 2. Numbers of undamaged Pocillopora ligulata nubbins per plot (mean ± SE)
over 32 days (n = 6 plots per treatment).
112
O T1
a- c
>-»
•
CD
3
HW
o
o
<—*
s
&
en
en
CD
•1
ft
3
o
<
n
a.
cr
t*3
Coral tissue lost (sq. cm)
Day 0
Day 1
Day 9
Figure 4. Two Pocillopora ligulata nubbins, one exposed to E. mathaei and the other
in a control plot without E mathaei. White epoxy surrounds each coral nubbin; coral
tissue lost is visible as a change in color from tan to white.
114
Urchins removed
1
1
Urchins present
1
Montipora
1
H
- 30
H
- 20
H
1
1
1
Pontes
•v
*«»
1
H
- 30
H
- 20
H
-10
>
*&,
Figure 5. Numbers of damaged and undamaged coral nubbins by treatment {E.
mathaei removed or present) for two coral taxa after 116 days.
115
A. Montipora
Dayo
Day 13
with
urchins: ]&
Day 57
*«vv
-»•*(•»»*•'«•
*!<*!!—ftfcdrWwww •
without
urchins:
i
B. Pontes
DayO
Day 13
#r »-* *•«• *••
f
•
4
Day 57
•
with
urchins:
t
* , _ . » »
' - . ' . , 7.-: .., A
bflf'*
without
urchins:
.• '
' i
i.&4LJLZ
Lv *afc-*
.
Figure 6 Examples of coral nubbins (with and without E mathaei) on three dates for
a) Montipora flabellata, and b) Pontes lobata
116
CO
Montipora
20
Pontes
a>
en
cc
E
(0
T3
(0
£
10
l
0
E
0
Northeast
Southwest
Site
Figure 7. Number of days (mean ± ISD) for nubbins to be damaged by E. mathaei.
117
LITERATURE CITED
Adjeroud M, Penin L, Carroll A (2007) Spatio-temporal heterogeneity in coral
recruitment around Moorea, French Polynesia: Implications for population
maintenance. Journal of Experimental Marine Biology and Ecology 341:204218
Albert S, O'Neil JM, Udy JW, Ahern KS, O'Sullivan CM, Dennison WC (2005)
Blooms of the cyanobacterium Lyngbya majuscula in coastal Queensland,
Australia: disparate sites, common factors. Marine Pollution Bulletin 51:428437
Arnold SN, Steneck RS, Mumby PJ (2010) Running the gauntlet: inhibitory effects of
algal turfs on the processes of coral recruitment. Marine Ecology Progress
Series 414:91-105
Arthur KE, Paul VJ, Paerl HW, x, Neil JM, Joyner J, Meickle T (2009) Effects of
nutrient enrichment of the cyanobacterium Lyngbya sp. on growth, secondary
metabolite concentration and feeding by the specialist grazer Stylocheilus
striatus. Marine Ecology Progress Series 394:101-110
Arthur R, Done T, Marsh H, Harriott V (2006) Local processes strongly influence
post-bleaching benthic recovery in the Lakshadweep Islands. Coral Reefs
25:427-440
Asgaard U, Bromley RG (2008) Echinometrid sea urchins, their trophic styles and
corresponding bioerosion. In: Wisshak M, Tapanila L (eds) Current
Developments in Bioerosion. Springer, p 279-303
Babcock R, Mundy C (1996) Coral recruitment: Consequences of settlement choice
for early growth and survivorship in two scleractinians. Journal of
Experimental Marine Biology and Ecology 206:179-201
Babcock RC, Baird AH, Piromvaragorn S, Thomson DP, Willis BL (2003)
Identification of scleractinian coral recruits from Indo-Pacific reefs.
Zoological Studies 42:211-226
Baird AH, Hughes TP (2000) Competitive dominance by tabular corals: an
experimental analysis of recruitment and survival of understorey assemblages.
Journal of Experimental Marine Biology and Ecology 251:117-132
Baird AH, Morse ANC (2004) Induction of metamorphosis in larvae of the brooding
corals Acropora palifera and Stylophora pistillata. Mar Freshw Res 55:469472
Bak RPM (1990) Patterns of echinoid bioerosion in two Pacific coral reef lagoons.
Marine Ecology Progress Series 66:267-272
Bak RPM (1994) Sea-Urchin Bioerosion on Coral-Reefs - Place in the Carbonate
Budget and Relevant Variables. Coral Reefs 13:99-103
Bak RPM, van Eys G (1975) Predation of the sea urchin Diadema antillarum Philippi
on living coral. Oecologia 20:111-115
118
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: An
ecological assessment of long-term impacts, recovery trends and future
outlook. Estuarine, Coastal and Shelf Science 80:435-471
Baria MVB, Guest JR, Edwards AJ, Alino PM, Heyward AJ, Gomez ED (2010)
Caging enhances post-settlement survival of juveniles of the scleractinian
coral Acropora tenuis. Journal of Experimental Marine Biology and Ecology
394:149-153
Basch L, White J (2008) Hawaiian coral recruit reference photos Unpublished work.
National Park Service, Honolulu, Hawaii
Birkeland C, Randall RH (1981) Facilitation of coral recruitment by echinoid
excavations Proceedings of the Fourth International Coral Reef Symposium.
Marine Sciences Center, University of the Philippines, Manila, Philippines
Birrell CL, McCook LJ, Willis BL (2005) Effects of algal turfs and sediment on coral
settlement. Marine Pollution Bulletin 51:408-414
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival
of juvenile Caribbean corals. Marine Ecology Progress Series 342:139-149
Brown E (2004) Reef coral populations: Spatial and temporal differences observed
on six reefs off West Maui Dissertation, University of Hawai'i
Bruno JF, Witman JD (1996) Defense mechanisms of scleractinian cup corals against
overgrowth by colonial invertebrates. Journal of Experimental Marine
Biology and Ecology 207:229-241
Burkepile DE, Hay ME (2008) Herbivore species richness and feeding
complementarity affect community structure and function on a coral reef.
Proceedings of the National Academy of Sciences of the United States of
America 105:16201-16206
Carleton JH, Sammarco PW (1987) Effects of Substratum Irregularity on Success of
Coral Settlement: Quantification by Comparative Geomorphological
Techniques. Bulletin of Marine Science 40:85-98
Carpenter RC (1986) Partitioning Herbivory and Its Effects on Coral Reef Algal
Communities. Ecological Monographs 56:345-364
Carpenter RC, Edmunds PJ (2006) Local and regional scale recovery of Diadema
promotes recruitment of scleractinian corals. Ecology Letters 9:271-280
Carreiro-Silva M, McClanahan TR (2001) Echinoid bioerosion and herbivory on
Kenyan coral reefs: The role of protection from fishing. Journal of
Experimental Marine Biology & Ecology 262:133-153
Christiansen N, Ward S, Harii S, Tibbetts I (2009) Grazing by a small fish affects the
early stages of a post-settlement stony coral. Coral Reefs 28:47-51
Codd GA, Morrison LF, Metcalf JS (2005) Cyanobacterial toxins: risk management
for health protection. Toxicology & Applied Pharmacology 203:264-272
Connell JH (1978) Diversity in Tropical Rain Forests and Coral Reefs. Science
199:1302-1310
119
Coutinho R, Zingmark R (1993) Interactions of light and nitrogen on photosynthesis
and growth of the marine macroalga Ulva curvata (Kiitzing) De Toni. Journal
of Experimental Marine Biology and Ecology 167:11-19
Dart JKG (1972) Echinoids, algal lawn and coral recolonization. Nature 239:50-51
Demers P (1996) The "edge effect" on a submerged coral reef: Sedimentation as
controlling factor for coral survival, growth, and recruitment. Masters Thesis,
University of Hawaii
Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, Kline DI, Weeks S,
Evans RD, Williamson DH, Hoegh-Guldberg O (2009) Doom and Boom on a
Resilient Reef: Climate Change, Algal Overgrowth and Coral Recovery. PLoS
ONE 4:e5239
Done T, DeVantier L, Turak E, Fisk D, Wakeford M, van Woesik R (2010) Coral
growth on three reefs: development of recovery benchmarks using a space for
time approach. Coral Reefs 29:815-833
Edmunds PJ, Carpenter RC (2001) Recovery of Diadema antillarum reduces
macroalgal cover and increases abundance of juvenile corals on a Caribbean
reef. Proceedings of the National Academy of Sciences of the United States of
America 98:5067-5071
Estes JA, Palmisano JF (1974) Sea Otters: Their Role in Structuring Nearshore
Communities. Science 185:1058-1060
Fisk DA, Harriott VJ (1990) Spatial and temporal variation in coral recruitment on
the Great Barrier Reef: Implications for dispersal hypotheses. Marine Biology
107:485-490
Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS
(2004) Regime shifts, resilience, and biodiversity in ecosystem management.
Annual Review of Ecology Evolution and Systematics:557-581
Foster SA (1987) The Relative Impacts of Grazing by Caribbean Coral Reef Fishes
and Diadema Effects of Habitat and Surge. Journal of Experimental Marine
Biology & Ecology 105:1-20
Franklin H, Muhando CA, Lindahl U (1998) Coral Culturing and Temporal
Recruitment Patterns in Zanzibar, Tanzania. Ambio 27:651-655
Friedlander AM, DeMartini EE (2002) Contrasts in density, size, and biomass of reef
fishes between the northwestern and the main Hawaiian islands: The effects of
fishing down apex predators. Marine Ecology-Progress Series 230:253-264
Furman B, Heck KL (2009) Differential impacts of echinoid grazers on coral
recruitment. Bulletin of Marine Science 85:121-132
Gerwick WH, Jiang ZD, Agarwal SK, Farmer BT (1992) Total structure of
hormothamnin A, A toxic cyclic undecapeptide from the tropical marine
cyanobacterium Hormothamnion enteromorphoides. Tetrahedron 48:23132324
Gerwick WH, Mrozek C, Moghaddam MF, Agarwal SK (1989) Novel cytotoxic
peptides from the tropical marine cyanobacterium Hormothamnion
enteromorphoides 1. Discovery, isolation and initial chemical and biological
120
characterization of the hormothamnins from wild and cultured material.
Cellular and Molecular Life Sciences 45:115-121
Glassom D, Zakai D, Chadwick-Furman NE (2004) Coral recruitment: a spatiotemporal analysis along the coastline of Eilat, northern Red Sea. Marine
Biology 144:641-651
Gleason MG (1996) Coral recruitment in Moorea, French Polynesia: The importance
of patch type and temporal variation. Journal of Experimental Marine Biology
& Ecology 207:79-101
Glynn PW (1980) Defense by symbiotic Crustacea of host corals elicited by chemical
cues from predator. Oecologia 47:287-290
Glynn PW, Wellington GM, Birkeland C (1979) Coral reef growth in the Galapagos:
limitation by sea urchins. Science 203:47-49
Green A, Burgett J, Molina M, Palawski D, Gabrielson P (1997) The impact of a ship
grounding and associated fuel spill at Rose Atoll National Wildlife Refuge,
American Samoa, US Fish and Wildlife Service
Grigg RW (1982) Darwin Point: A threshold for atoll formation. Coral Reefs 1:29-34
Gunderson LH (2000) Ecological Resilience—In Theory and Application. Annual
Review of Ecology and Systematics 31:425-439
Harrington L, Fabricius K, De'ath G, Negri A (2004) Recognition and selection of
settlement substrata determine post-settlement survival in corals. Ecology
85:3428-3437
Harriott VJ (1992) Recruitment patterns of scleractinian corals in an isolated subtropical reef system. Coral Reefs 11:215-219
Harriott VJ (1999) Coral recruitment at a high latitude Pacific site: A comparison
with Atlantic reefs. Bulletin of Marine Science 65:881-891
Harriott VJ, Banks SA (1995) Recruitment of scleractinian corals in the Solitary
Islands Marine Reserve, a high latitude coral-dominated community in
Eastern Australia. Marine Ecology Progress Series 123:155-161
Harrold C, Reed DC (1985) Food Availability, Sea Urchin Grazing, and Kelp Forest
Community Structure. Ecology 66:1160-1169
Hart LJ, Chia F-s (1990) Effect of food supply and body size on the foraging behavior
of the burrowing sea urchin Echinometra mathaei (de Blainville). Journal of
Experimental Marine Biology and Ecology 135:99-108
Hay ME (1984) Patterns of Fish and Urchin Grazing on Caribbean Coral Reefs Are
Previous Results Typical. Ecology 65:446-454
Hodgson G (1990) Sediment and the settlement of larvae of the reef coral Pocillopora
damicornis. Coral Reefs 9:41-43
Hoskin CM, Reed JK, Mook DH (1986) Production and off-bank transport of
carbonate sediment, Black Rock, southwest Little Bahama Bank. Marine
Geology 73:125-144
Huettel, Markus, Wild, Christian, Gonelli, Sabine (2006) Mucus trap in coral reefs :
formation and temporal evolution of particle aggregates caused by coral
mucus, Vol 307. Inter-Research, Oldendorf, ALLEMAGNE
121
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a
Caribbean coral reef. Science 265:1547-1551
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R,
Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom
M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate
change, human impacts, and the resilience of coral reefs. Science 301:929-933
Hughes TP, Baird AH, Dinsdale EA, Mohschaniwskyj NA, Pratchett MS, Tanner JE,
Willis BL (1999) Patterns of recruitment and abundance of corals along the
Great Barrier Reef. Nature (London) 379:59-63
Hughes TP, Bellwood DR, Folke CS, McCook LJ, Pandolfi JM (2007a) No-take
areas, herbivory and coral reef resilience. Trends in Ecology & Evolution
22:1-3
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook
L, Mohschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007b) Phase
Shifts, Herbivory, and the Resilience of Coral Reefs to Climate Change.
Current Biology 17:360-365
Hughes TP, Tanner JE (2000) Recruitment failure, life histories, and long-term
decline of Caribbean corals. Ecology 81:2250-2263
Hyenstrand P, Rydin E, Gunnerhed M (2000) Response of pelagic cyanobacteria to
iron additions—enclosure experiments from Lake Erken. J Plankton Res
22:1113-1126
Idjadi JA, Haring RN, Precht WF (2010) Recovery of the sea urchin Diadema
antillarum promotes scleractinian coral growth and survivorship on shallow
Jamaican reefs. Marine Ecology Progress Series 403:91-100
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ,
Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange
CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner
RR (2001) Historical overfishing and the recent collapse of coastal
ecosystems. Science 293:629-638
Jompa J, McCook LJ (2002) Effects of competition and herbivory on interactions
between a hard coral and a brown alga. Journal of Experimental Marine
Biology & Ecology 271:25-39
Jompa J, McCook LJ (2003) Coral-algal competition: Macroalgae with different
properties have different effects on corals. Marine Ecology-Progress
Series:87-95
Khamala CPM (1971) Ecology of Echinometra mathaei (Echinoidea :
Echinodermata) at Diani Beach, Kenya. Mar Biol 11:167-172
Kleypas JA, McManus JW, Menez LAB (1999) Environmental limits to coral reef
development: Where do we draw the line? American Zoologist 39:146-159
Knowlton N (2004) Multiple "stable" states and the conservation of marine
ecosystems. Progress in Oceanography 60:387-396
122
Kojis BL, Quinn NJ (2001) The importance of regional differences in hard coral
recruitment rates for determining the need for coral restoration. Bulletin of
Marine Science 69:967-974
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006)
Inhibition of coral recruitment by macroalgae and cyanobacteria. Marine
Ecology-Progress Series 323:107-117
Lessios HA, Robertson DR, Cubit JD (1984) Spread of Diadema Mass Mortality
Through the Caribbean. Science 226:335-337
Macintyre IG, Glynn PW, Hinds F (2005) Evidence of the role of Diadema antillarum
in the promotion of coral settlement and survivorship. Coral Reefs 24:273-273
Maida M, Coll JC, Sammarco PW (1994) Shedding new light on scleractinian coral
recruitment. Journal of Experimental Marine Biology and Ecology 180:189202
Mangubhai S, Harrison PL, Obura DO (2007) Patterns of coral larval settlement on
lagoon reefs in the Mombasa Marine National Park and Reserve, Kenya.
Marine Ecology-Progress Series 348:149-159
Maragos JE Taxonomy of Montipora c.f. turgescens. Personal communication
McClanahan TR, Kurtis JD (1991) Population regulation of the rock-boring sea
urchin Echinometra mathaei (de Blainville). Journal of Experimental Marine
Biology & Ecology 147:121-146
McClanahan TR, Muthiga NA (1988) Changes in Kenyan Coral Reef Community
Structure and Function Due to Exploitation. Hydrobiologia 166:269-276
McClanahan TR, Nugues M, Mwachireya S (1994) Fish and sea urchin herb ivory and
competition in Kenyan coral reef lagoons: The role of reef management.
Journal of Experimental Marine Biology & Ecology 184:237-254
McClanahan TR, Shafir SH (1990) Causes and consequences of sea urchin abundance
and diversity in Kenyan coral reef lagoons. Oecologia 83:362-370
McCook, McCook L, Jompa, Jompa J, Diaz P, Diaz-Pulido G (2001a) Competition
between corals and algae on coral reefs: a review of evidence and
mechanisms. Coral Reefs 19:400-417
McCook LJ, Jompa J, Diaz-Pulido G (2001b) Competition between corals and algae
on coral reefs: A review of evidence and mechanisms. Coral Reefs 19:400417
Mills SC, Peyrot-Clausade M, France Fontaine M (2000) Ingestion and
transformation of algal turf by Echinometra mathaei on Tiahura fringing reef
(French Polynesia). Journal of Experimental Marine Biology and Ecology
254:71-84
Mittelbach GG, Turner AM, Hall DJ, Rettig JE, Osenberg CW (1995) Perturbation
and Resilience: A Long-Term, Whole-Lake Study of Predator Extinction and
Reintroduction. Ecology 76:2347-2360
Morrison D (1988) Comparing Fish and Urchin Grazing in Shallow and Deeper Coral
Reef Algal Communities. Ecology 69:1367-1382
123
Morse ANC, Iwao K, Baba M, Shimoike K, Hayashibara T, Omori M (1996) An
Ancient Chemosensory Mechanism Brings New Life to Coral Reefs. Biol Bull
191:149-154
Mumby P (2009a) Herbivory versus corallivory: are parrotfish good or bad for
Caribbean coral reefs? Coral Reefs 28:683-690
Mumby P (2009b) Phase shifts and the stability of macroalgal communities on
Caribbean coral reefs. Coral Reefs 28:761-773
Mumby PJ, Harborne AR (2010) Marine Reserves Enhance the Recovery of Corals
on Caribbean Reefs. PLoS ONE 5:e8657
Mumby PJ, Harborne AR, Williams J, Kappel CV, Brumbaugh DR, Micheli F,
Holmes KE, Dahlgren CP, Paris CB, Blackwell PG (2007a) Trophic cascade
facilitates coral recruitment in a marine reserve, p 8362-8367
Mumby PJ, Hastings A, Edwards HJ (2007b) Thresholds and the resilience of
Caribbean coral reefs. Nature 450:98-101
Mundy CN, Babcock RC (1998) Role of light intensity and spectral quality in coral
settlement: Implications for depth-dependent settlement? Journal of
Experimental Marine Biology and Ecology 223:235-255
Nozawa Y (2008) Micro-crevice structure enhances coral spat survivorship. Journal
of Experimental Marine Biology and Ecology 367:127-130
Nozawa Y, Tokeshi M, Nojima S (2006) Reproduction and recruitment of
scleractinian corals in a high-latitude coral community, Amakusa,
southwestern Japan. Marine Biology 149:1047-1058
Nugues MM, Smith GW, van Hooidonk RJ, Seabra MI, Bak RPM (2004) Algal
contact as a trigger for coral disease. Ecology Letters 7:919-923
Nystrom M, Folke C, Moberg F (2000) Coral reef disturbance and resilience in a
human-dominated environment. Trends in Ecology & Evolution 15:413-417
O'Leary J, Potts D (2011) Using hierarchical sampling to understand scales of spatial
variation in early coral recruitment. Coral Reefs: 1-11
Ogden NB, Ogden JC, Abbott IA (1989) Distribution, Abundance and Food of Sea
Urchins on a Leeward Hawaiian Reef. Bulletin of Marine Science 45:539-549
Osborne NJT, Webb PM, Shaw GR (2001) The toxins of Lyngbya majuscula and
their human and ecological health effects. Environment International 27:381392
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle
D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC
(2003) Global trajectories of the long-term decline of coral reef ecosystems.
Science 301:955-958
Paul V, Thacker R, Banks K, Golubic S (2005) Benthic cyanobacterial bloom impacts
the reefs of South Florida (Broward County, USA). Coral Reefs 24:693-697
Penin L, Adjeroud M, Pratchett MS, Hughes TP (2007) Spatial distribution of
juvenile and adult corals around Moorea (French Polynesia): implications for
population regulation. Bulletin of Marine Science 80:379-389
124
Penin L, Michonneau F, Baird AH, Connolly SR, Pratchett MS, Kayal M, Adjeroud
M (2010) Early post-settlement mortality and the structure of coral
assemblages. Marine Ecology Progress Series 408:55-64
Penin L, Michonneau F, Carroll A, Adjeroud M (2011) Effects of predators and
grazers exclusion on early post-settlement coral mortality. Hydrobiologia
663:259-264
Pennings SC, Pablo SR, Paul VJ (1997) Chemical Defenses of the Tropical, Benthic
Marine Cyanobacterium Hormothamnion enteromorphoides: Diverse
Consumers and Synergisms. Limnology and Oceanography 42:911-917
Pennings SC, Paul VJ (1992) Effect of Plant Toughness, Calcification, and Chemistry
on Herbivory by Dolabella Auricularia. Ecology 73:1606-1619
Pittman S J, Pittman KM (2005) Short-term consequences of a benthic cyanobacterial
bloom (Lyngbya majuscula Gomont) for fish and penaeid prawns in Moreton
Bay (Queensland, Australia). Estuarine Coastal & Shelf Science 63:619-632
Raimondi PT, Morse ANC (2000) The consequences of complex larval behavior in a
coral. Ecology 81:3193-3211
Randall JE (1985) Guide to Hawaiian reef fishes, Vol. Harrowood Books Newtown
Square, PA 19073, USA
Rasher DB, Hay ME (2010) Chemically rich seaweeds poison corals when not
controlled by herbivores. Proceedings of the National Academy of Sciences
107:9683-9688
Rue EL, Bruland KW (1997) The Role of Organic Complexation on Ambient Iron
Chemistry in the Equatorial Pacific Ocean and the Response of a Mesoscale
Iron Addition Experiment. Limnology and Oceanography 42:901-910
Sammarco PW (1980) Diadema and its relationship to coral spat mortality: grazing,
competition, and biological disturbance. Journal of Experimental Marine
Biology and Ecology 45:245-272
Sammarco PW (1982) Echinoid grazing as a structuring force in coral communities:
Whole reef manipulations. Journal of Experimental Marine Biology and
Ecology 61:31-55
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in
ecosystems. Nature 413:591-596
Schroeder RE, Green AL, DeMartini EE, Kenyon JC (2008) Long-Term Effects of a
Ship-Grounding on Coral Reef Fish Assemblages at Rose Atoll, American
Samoa. Bulletin of Marine Science 82:345-364
Smith J, Hunter C, Smith C (2010) The effects of top-down versus bottom-up control
on benthic coral reef community structure. Oecologia 163:497-507
Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S,
Hatay M, Rohwer FL (2006) Indirect effects of algae on coral: algaemediated, microbe-induced coral mortality. Ecology Letters 9:835-845
Smith JE, Smith CM, Hunter CL (2001) An experimental analysis of the effects of
herbivory and nutrient enrichment on benthic community dynamics on a
Hawaiian reef. Coral Reefs 19:332-342
125
Terborgh J, Estes JA (eds) (2010) Trophic Cascades: Predators, Prey, and the
Changing Dynamics of Nature, Vol. Island Press, Washington, D.C.
Tioho H, Tokeshi M, Nojima S (2001) Experimental analysis of recruitment in a
scleractinian coral at high latitude. Marine Ecology Progress Series:79-86
Titlyanov EA, Yakovleva IM, Titlyanova TV (2007) Interaction between benthic
algae (Lyngbya bouillonii, Dictyota dichotoma) and scleractinian coral Porites
lutea in direct contact. Journal of Experimental Marine Biology and Ecology
342:282-291
Tsuchiya M, Nishihira M (1984) Ecological distribution of two types of the seaurchin, Echinometra mathaei (Blainville), on Okinawan reef flat. Galaxea
3:131-143
Van Dolah FM (2000) Marine algal toxins: Origins, health effects, and their increased
occurrence. Environmental Health Perspectives 108:133-141
Vermeij M, Smith J, Smith C, Vega Thurber R, Sandin S (2009) Survival and
settlement success of coral planulae: independent and synergistic effects of
macroalgae and microbes. Oecologia 159:325-336
Vermeij MJA (2006) Early life-history dynamics of Caribbean coral species on
artificial substratum: the importance of competition, growth and variation in
life-history strategy. Coral Reefs 25:59-71
Williams D, Miller M, Kramer K (2008) Recruitment failure in Florida Keys
Acropora palmata, a threatened Caribbean coral. Coral Reefs 27:697-705
Wylie CR, Paul VJ (1988) Feeding preferences of the surgeonfish Zebrasoma
flavescens in relation to chemical defenses of tropical algae. Marine EcologyProgress Series 45:23-32
126