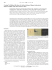Chem. Commun. 48, 5913 (2012). - Lasun
Transcription
Chem. Commun. 48, 5913 (2012). - Lasun
View Online / Journal Homepage / Table of Contents for this issue ChemComm Dynamic Article Links Cite this: Chem. Commun., 2012, 48, 5913–5915 www.rsc.org/chemcomm COMMUNICATION Highly efficient SERS test stripsw Downloaded by Jilin University on 22 May 2012 Published on 25 April 2012 on http://pubs.rsc.org | doi:10.1039/C2CC31604H Ran Zhang,a Bin-Bin Xu,a Xue-Qing Liu,a Yong-Lai Zhang,a Ying Xu,a Qi-Dai Chen*a and Hong-Bo Sun*ab Received 3rd March 2012, Accepted 24th April 2012 DOI: 10.1039/c2cc31604h We present a facile production approach to highly efficient SERS test strips by physical vapor deposition of silver on paper, which contains natural wrinkle and fibril structures. The SERS test strips open the door to highly sensitive (e.g., 10 10 M) SERS detection in a convenient fashion. In recent years, surface enhanced Raman scattering (SERS) spectroscopy combining molecular fingerprint specificity with potential single-molecule sensitivity1 has seen thorough development in chemical2 and biological analysis.3 It is attractive for its enhancement, which allows quick detection of low-concentration analytes.4 For SERS detection, micronanostructured metallic substrates that give rise to localized surface plasmon resonances (LSPRs) are widely used in both theoretical research5 and experimental analysis.6 To date, the strong interaction between LSPR and analytes has been used to boost the efficiency of various analytical techniques, especially for SERS.7 Currently, the reported SERS substrates are generally based on various metallic substrates, such as rough metallic surfaces,8 metal colloidal solutions,9 fractal metal films,10 periodic nanostructures11 and so on. Generally, the SERS substrates could be readily produced through complex procedures including classical ‘‘bottom–up’’ and ‘‘top–down’’ approaches,12 represented by lithography13 and self-assembly,14 respectively. Despite the fact that these SERS-active substrates produce tremendous SERS signal enhancement, even single molecule detection, their complicated synthesis or fabrication procedures make them inconvenient in common analysis. Moreover, their high cost and storage problems further limit their commercial uses, especially for routine laboratory analysis and on-site analysis in the common medical care and public safety fields. a State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, 2699 Qianjin Street, Changchun, 130012, P. R. China. E-mail: [email protected], [email protected]; Fax: +86-431-85168281; Tel: +86-431-85168281 b College of Physics, Jilin University, 119 Jiefang Road, Changchun, 130023, P. R. China w Electronic supplementary information (ESI) available: Experimental details; SERS spectrum of p-MA; SERS spectrum of R6G excited by a laser of 785 nm; SEM images of silver of different thicknesses; SERS spectra of paper with silver thicknesses from 10 nm to 90 nm; 1360 cm 1 and 1507 cm 1 peak intensity relation; TEM of nanofibrils; EX distribution simulations; SERS activity spectra; EDX spectrum and maps of the silver covered paper; calculation of SERS enhancement factor. See DOI: 10.1039/c2cc31604h This journal is c The Royal Society of Chemistry 2012 As a convenient analysis and detection medium, test strips such as pH test strips, glucose test strips, urine test strips and lipolysis test strips are widely used in both research fields and daily life because of their distinct convenience and high efficiency. Especially in various biological and chemical detections, test strips also demonstrate many unique advantages including low-cost, easy handling, portability, high sensitivity and less analyte consumption. However, for SERS spectroscopy, there is still a lack of generally available and highly efficient substrates, just like test strips, which significantly limits its wide applications and makes SERS analysis an ‘‘in-lab-only’’ technique. At present, to prepare and use a high-performance SERS substrate in a similar manner to test strips, is still not only a dream but also a scientific challenge. Paper, which is widely used in our daily life, is considered to be a cheap and flexible substrate. Nowadays, the use of paper has been extended beyond its general applications, for instance in electronic displays15 and sensors.16 Fang et al. demonstrated the use of Ag/Au nanoparticle-coated filter paper in a variety of SERS measurements.17 In this communication we propose convenient and efficient SERS test strips by simple physical vapor deposition (PVD) coating of a silver layer on paper for the first time. With the help of our SERS test strips, sensitive and reproducible SERS tests are realized in a low cost and facile fashion. Taking advantage of the connatural hierarchical micro/nanostructures of paper constructed from irregular stacking of cellulose microfibers which have abundant nanofibrils and wrinkles on the surface, a deposited silver nanolayer could be arranged into a flexible and wrinkled plasmonic nanostructure,18 which therefore, forms a large-area SERS ‘‘hot spot’’. As a representative illustration, Rhodamine 6G is used as a probing molecule and the limit of detection (LOD) of our SERS test strip is at the level of 10 10 M. Based on our experiments, we find that the microfibers of the paper play a main role in the formation of SERS ‘‘hot spots’’. So we first screened 6 kinds of candidate papers, including filter paper, napkin paper, sulfate paper, kraft paper, printing paper and newspaper. Fig. 1 shows scanning electron microscope (SEM) images of the different kinds of papers. We find that the fibers of the filter paper, napkin paper and sulfate paper are relatively pure and the fibers of the kraft paper, printing paper and newspaper are filled with some fragments. Interestingly, the microfiber networks of the filter paper and napkin paper are very loose, which could render them with abundant porosity to ensure a hydrophilic and soft performance Chem. Commun., 2012, 48, 5913–5915 5913 Downloaded by Jilin University on 22 May 2012 Published on 25 April 2012 on http://pubs.rsc.org | doi:10.1039/C2CC31604H View Online Fig. 1 SEM images of different kinds of papers: (a) filter paper; (b) napkin paper; (c) sulfate paper; (d) kraft paper; (e) printing paper and (f) newspaper. The insets are the fiber structures of the corresponding papers. The scale bars of the outset and inset panels are 100 and 5 mm, respectively. for their lab and life usage, respectively. Sulfate paper, which is produced from pure cellulose fibers by using various sulfurous salts to extract the lignin from wood chips in large pressure vessels, shows a relatively smooth surface. Despite these papers showing distinct differences in surface topography, magnified images (see the insets of each SEM image) show that there are abundant wrinkles and hierarchical structures on the microfiber surfaces, which contribute to a significant roughness for SERS substrates. We fabricated the SERS test strips by decorating a certain thickness of silver on the rough surfaces of the papers by PVD. The different surface topography would have considerable influence on the formation of SERS ‘‘hot spots’’. The SERS test strips were versatile materials for different molecules and excitation wavelengths, and we took Rhodamine 6G (R6G) excited by a laser of 514 nm as an example for this communication (the Raman spectrum of p-mercaptoaniline (p-MA) is available in the ESIw, Fig. S1 and the SERS spectrum of R6G excited by a laser of 785 nm is shown in the ESIw, Fig. S2). Fig. 2a shows the Raman spectra tested over different kinds of silver coated papers. Notably, all the listed Ag/papers show strong SERS activity due to the hierarchical roughness derived from the natural wrinkles of the microfibers. To investigate the trace detection capabilities of the SERS test strips, the Ag/printing paper substrate was used as a Fig. 2 Raman spectra of (a) different kinds of Ag/papers, R6G 10 6 M; (b) 0.1 nM of the R6G collected from the Ag/printing paper substrate; (c) different concentrations of R6G, inset is a semi-log plot of the concentration versus 1651 cm 1 peak intensity; (d) 25 different spots on the same fiber, R6G 10 6 M. 5914 Chem. Commun., 2012, 48, 5913–5915 representative example for the detection of R6G with a concentration of 0.1 nM. Obviously, even down to such a low concentration, strong SERS signals could still be identified clearly (Fig. 2b), indicating the excellent enhancement of our Ag/paper substrates. To avoid any undesired influence from the additives in the paper, the PVD silver coating layer should cover the entire surface without any exposure. Fig. S3 (ESIw) shows SEM images of paper coated with silver of different thicknesses. We found that the fibers were entirely covered by silver when the thickness reached 20 nm (monitored by the film thickness meter). SERS spectra of paper substrates with silver thicknesses ranging from 10 nm to 90 nm all showed great enhancement, in which the highest enhancement was found at 50 nm of silver coating (ESIw, Fig. S4). Fig. 2c shows Raman spectra for different concentrations of R6G. All the characteristic bands of the R6G exhibited a monotonic decrease in intensity with decreasing concentration. A semi-log plot of the concentration versus the 1651 cm 1 peak intensity showed a monotonic increase in the Raman intensity with increasing R6G concentration (inset of Fig. 2c). The 1360 cm 1 and 1507 cm 1 peak intensity relations are shown in Fig. S5 (ESIw). The SERS enhancement relied on ‘‘hot spots’’ and they were often scattered in the normal substrate, which brought a lot of troubles to the test and significantly reduced their reproducibility. However, in the paper-based SERS strips, the microfibers which contain abundant rough wrinkles cover B80% of the paper surface. Such a high coverage of microfibers made the Ag/paper substrate full of ‘‘hot spots’’, which imparted superior SERS enhancement to the Ag/paper composites, thus they could work as highly efficient SERS test strips. To test the reproducibility of our SERS strips, 25 spots were randomly chosen on one microfiber, and the SERS signals were almost consistent, which confirmed the homogeneity (Fig. 2d). To gain further insight into the mechanism of the high SERS enhancement of our test strips, we systematically studied the structure of the paper. It was known that the cellulose fibers in paper were wood cells which are usually a few millimetres long, and several to tens of micrometres wide depending on the origin. On the surface of the fibers, there are secondary micro/ nanofibrils that form wrinkles, as shown in Fig. 3a and b. The wrinkles distributed in an approximately parallel arrangement with an interval of about 500 nm and a height of about 100 nm (Fig. 3c). Fig. 3 SEM image of (a) a fiber with wrinkles distributed in an approximately parallel arrangement with an interval of about 500 nm; (b) the silver nanoparticles compactly accumulated on the wrinkles corresponding to the rectangular region in (a); AFM image and the cross section of (c) wrinkles; (d) nanofibrils. This journal is c The Royal Society of Chemistry 2012 Downloaded by Jilin University on 22 May 2012 Published on 25 April 2012 on http://pubs.rsc.org | doi:10.1039/C2CC31604H View Online Fig. 4 (a) SERS test strips simulated the commercial pH test strips which were made from silver coated paper; (b) the flexible and wellencapsulated SERS test strip; (c) the concentration process of the analyte dropped on the test strips. From the magnified image (Fig. 3b), we could observe that the silver nanoparticles compactly accumulated on the wrinkles, which further increased the surface roughness. The wood fibers would be mechanically separated; the TEM image of a single microfiber shows nanofibrils with diameters between 20 nm and 100 nm (ESIw, Fig. S6). The height of the tightly arranged nanofibrils was about 10 nm, as shown in the section profile of the atomic force microscopy (AFM) image (Fig. 3d). The electric field component EX distribution simulations of the enhancements caused by the periodic wrinkles and nanofibril structures were implemented by the finite-difference timedomain (FDTD) method (ESIw, Fig. S7), which showed 104 and 400 times enhancements to the SERS signal, respectively. Compared with chemical silver plating and PVD of silver film on glass (ESIw, Fig. S8), we find a tremendous contribution of the cellulose fibers to the SERS intensity. Fig. 4a and b shows photographs of our SERS test strips. Similar to pH test strips, the silver test papers could be easily tailored into strips. The test strips can remain active for 13.5 h, and we can place them in an airtight N2 atmosphere for long term storage (ESIw, Fig. S9 and 10). Elemental maps of Ag, C and O show clear boundaries on paper with and without silver coating, as well as the homogeneous Ag distribution (ESIw, Fig. S11). Compared with other SERS substrates, our SERS test strips also exhibit the advantages of flexibility, which allows bending without any desquamation, and enrichment of analytes due to the hydrophobicity. Fig. 4c shows the contact angles (CAs) of a R6G aqueous droplet during the evaporation process. The starting CA was about 1201, indicating the hydrophobic feature. With the evaporation of water, the droplet became smaller and smaller without diffusion. In this regard, the analyte (here R6G) could be enriched in a defined region, which further lowered detection concentration and improved its sensitivity significantly. In conclusion, we have developed a facile method for the production of SERS test strips by PVD coating of silver nanolayers on various kinds of paper, including filter paper, napkin paper, sulfate paper, kraft paper, printing paper and newspaper. This journal is c The Royal Society of Chemistry 2012 Typically, the wrinkles and nanofibrils of the natural fiber on paper formed a rough surface with strong LSPR, which could serve as SERS ‘‘hot spots’’. In addition, our SERS test strips also showed unique advantages of flexibility and hydrophobicity. All of these features made our SERS test strips a promising platform for highly efficient SERS detection down to very low analyte concentration (10 10 M). The novel SERS test strips open the way for convenient SERS detection and exhibit broad commercial prospects in the near future. This work was supported by the National Science Foundation of China (Grant Nos. 90923037, 61137001, 61008014 and 60978048), as well as the China Postdoctoral Science Foundation (Grant No. 20110490156). The experimental help and useful discussion with Mr. Wen-Yi Zhang is also greatly acknowledged. Notes and references 1 R. Liu, J.-f. Liu, X.-x. Zhou, M.-T. Sun and G.-b. Jiang, Anal. Chem., 2011, 83, 9131. 2 B.-B. Xu, R. Zhang, X.-Q. Liu, H. Wang, Y.-L. Zhang, H.-B. Jiang, L. Wang, Z.-C. Ma, J.-F. Ku, F.-S. Xiao and H.-B. Sun, Chem. Commun., 2012, 48, 1680. 3 (a) J. Ando, K. Fujita, N. I. Smith and S. Kawata, Nano Lett., 2011, 11, 5344; (b) Y. He, S. Su, T. Xu, Y. Zhong, J. A. Zapien, J. Li, C. Fan and S.-T. Lee, Nano Today, 2011, 6, 122. 4 M.-W. Shao, L. Lu, H. Wang, S. Wang, M.-L. Zhang, D.-D.-D. Ma and S.-T. Lee, Chem. Commun., 2008, 2310. 5 F. De Angelis, F. Gentile, F. Mecarini, G. Das, M. Moretti, P. Candeloro, M. L. Coluccio, G. Cojoc, A. Accardo, C. Liberale, R. P. Zaccaria, G. Perozziello, L. Tirinato, A. Toma, G. Cuda, R. Cingolani and E. Di Fabrizio, Nat. Photonics, 2011, 5, 682. 6 V. Canpean and S. Astilean, Lab Chip, 2009, 9, 3574. 7 C. Tian, C. Ding, S. Liu, S. Yang, X. Song, B. Ding, Z. Li and J. Fang, ACS Nano, 2011, 5, 9442. 8 (a) P. Kao, N. A. Malvadkar, M. Cetinkaya, H. Wang, D. L. Allara and M. C. Demirel, Adv. Mater., 2008, 20, 3562. 9 (a) L.-L. Qu, D.-W. Li, J.-Q. Xue, W.-L. Zhai, J. S. Fossey and Y.-T. Long, Lab Chip, 2012, 12, 876; (b) S. Liu and Z. Tang, J. Mater. Chem., 2010, 20, 24; (c) Y. Gao and Z. Tang, Small, 2011, 7, 2133. 10 (a) J. Zhu, M. Xue, D. Zhao, M. Zhang, L. Duan, Y. Qiu and T. Cao, Angew. Chem., Int. Ed., 2011, 50, 12478; (b) M. Zamuner, D. Talaga, F. Deiss, V. Guieu, A. Kuhn, P. Ugo and N. Sojic, Adv. Funct. Mater., 2009, 19, 3129. 11 Z. Zhu, H. Meng, W. Liu, X. Liu, J. Gong, X. Qiu, L. Jiang, D. Wang and Z. Tang, Angew. Chem., 2011, 123, 1631. 12 M. Rycenga, C. M. Cobley, J. Zeng, W. Li, C. H. Moran, Q. Zhang, D. Qin and Y. Xia, Chem. Rev., 2011, 111, 3669. 13 B.-B. Xu, Z.-C. Ma, H. Wang, X.-Q. Liu, Y.-L. Zhang, X.-L. Zhang, R. Zhang, H.-B. Jiang and H.-B. Sun, Electrophoresis, 2011, 32, 3378. 14 B.-B. Xu, Z.-C. Ma, L. Wang, R. Zhang, L.-G. Niu, Z. Yang, Y.-L. Zhang, W.-H. Zheng, B. Zhao, Y. Xu, Q.-D. Chen, H. Xia and H.-B. Sun, Lab Chip, 2011, 11, 3347. 15 D. Tobjörk and R. Österbacka, Adv. Mater., 2011, 23, 1935. 16 B. Yoon, D.-Y. Ham, O. Yarimaga, H. An, C. W. Lee and J.-M. Kim, Adv. Mater., 2011, 23, 5492. 17 (a) Z. Luo and Y. Fang, J. Colloid Interface Sci., 2005, 283, 459; (b) D. Wu and Y. Fang, J. Colloid Interface Sci., 2003, 265, 234. 18 L. Zhang, X. Lang, A. Hirata and M. Chen, ACS Nano, 2011, 5, 4407. Chem. Commun., 2012, 48, 5913–5915 5915