Novel Treatments for Melanoma Brain Metastases
Transcription
Novel Treatments for Melanoma Brain Metastases
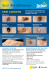
Immune-based and targeted therapy may be active in melanoma metastatic to the brain. Tenzin Norbu Lama. By Moonlight. Novel Treatments for Melanoma Brain Metastases Rajappa S. Kenchappa, PhD, Nam Tran, MD, PhD, Nikhil G. Rao, MD, Keiran S. Smalley, PhD, Geoffrey T. Gibney, MD,Vernon K. Sondak, MD, and Peter A. Forsyth, MD Background: The development of brain metastases is common in patients with melanoma and is associated with a poor prognosis. Treating patients with melanoma brain metastases (MBMs) is a major therapeutic challenge. Standard approaches with conventional chemotherapy are disappointing, while surgery and radiotherapy have improved outcomes. Methods: In this article, we discuss the biology of MBMs, briefly outline current treatment approaches, and emphasize novel and emerging therapies for MBMs. Results: The mechanisms that underlie the metastases of melanoma to the brain are unknown; therefore, it is necessary to identify pathways to target MBMs. Most patients with MBMs have short survival times. Recent use of immune-based and targeted therapies has changed the natural history of metastatic melanoma and may be effective for the treatment of patients with MBMs. Conclusions: Developing a better understanding of the factors responsible for MBMs will lead to improved management of this disease. In addition, determining the optimal treatments for MBMs and how they can be optimized or combined with other therapies, along with appropriate patient selection, are challenges for the management of this disease. From the Departments of Neuro-Oncology (RSK, NT, PAF), Cutaneous Oncology (GTG, VKS), Radiation Oncology (NGR), and Molecular Oncology (KSS) at the H. Lee Moffitt Cancer Center & Research Institute, Tampa, Florida, and the Departments of Oncologic Sciences (NT, NGR, KSS, GTG, VKS, PAF) and Surgery (NT, VKS) at the University of South Florida College of Medicine, Tampa, Florida. Submitted February 11, 2013; accepted May 21, 2013. Address correspondence to Peter A. Forsyth, MD, Department of Neuro-Oncology, Moffitt Cancer Center, 12902 Magnolia Drive, Tampa, FL 33612. E-mail: [email protected] No significant relationship exists between the authors and the companies/organizations whose products or services may be referenced in this article. 298 Cancer Control Introduction Twenty years ago, treatment options for patients with melanoma brain metastases (MBMs) were limited, and these patients would generally die of the disease within 2 months.1 Because of today’s rapid progress of effective surgical, radiation, and systemic therapies for metastatic melanoma, hope exists for these patients: MBMs respond to treatment, and patients are living longer. New treatment paradigms are emerging, possibly translating into longer survival rates and better quality of life. Clinical trials may now include patients with MBMs, including those with active MBMs on systemic therapy trials. Furthermore, trials are being specifically designed for patients with MBMs rather than “lumping” them together with all types of brain metastases. We believe this approach will accelerate the discovery of October 2013, Vol. 20, No. 4 effective and personalized therapy for MBMs. In this review of MBMs, we discuss the underlying biology, patient outcomes, and existing treatment strategies, with a focus on emerging therapeutic approaches. Epidemiology The incidence of metastatic brain tumors is 200,000 cases per year in the United States, which is 10 times higher than the incidence of primary brain tumors.2 Melanoma is the third most common cancer that metastasizes to the brain (5% to 10%, compared with 20% to 30% of breast cancer and 40% to 50% of lung cancer), with a reported average survival of less than 9 months.1,3-6 A total of 50% to 75% of the patients diagnosed with melanoma revealed brain metastases at autopsy, and approximately 60% of these patients were diagnosed with brain metastases before death.7 However, studies on the molecular mechanisms underlying MBMs are limited and current treatments are palliative. An understanding of the biological mechanisms of MBMs is critical to devising effective treatment strategies. Biology and Treatment Opportunities The metastatic process, particularly as it applies to the brain, is complex and poorly understood. It involves an orchestrated series of events that require cancer cells to escape from the primary tumor site, invade, intravasate into the circulation, extravasate into the brain parenchyma, survive, induce angiogenesis, and proliferate by responding to the brain microenvironment (Fig 1). Each step is regulated by various genes Primary Metastatic Melanoma (A) Intravasation [STAT3 + Neurotrophin Signaling] Melanoma Cells (B) Arrest in Capillary Bed (C) Extravasation [STAT3 + Neurotrophin Signaling] (D) Blood Vessel Co-Option [Beta 1-Integrin + VEGF-A] Activated Microglia Tumor Cell Survival, Colonization + Proliferation (E) Angiogenesis [VEGF-A] Brain Parenchyma Activated Astrocytes Fig 1A-E. — Schematic illustration of melanoma brain metastases. (A) Once metastatic tumor cells escape from the primary melanoma site, they will intravasate into circulation, (B) they arrest in the capillary bed, and (C) then extravasate into the brain parenchyma. STAT3 and neurotrophin signaling mediate melanoma cell intravasation and extravasation. Following the extravasation into the brain parenchyma, (D) melanoma cells either grow along blood vessels (co-option) and/or (E) establish angiogenesis; beta 1-integrin and VEGF-A are associated in this process. STAT3 = signal transducer and activator of transcription 3, VEGF = vascular endothelial growth factor. October 2013, Vol. 20, No. 4 Cancer Control 299 and signaling pathways. Metastases are inefficient and uncommon from a cellular perspective; only a small number of cells (0.01% to 0.03%) in a primary tumor metastasize to target organs.7-11 Tumors are also biologically heterogeneous, and certain tumor types show a remarkable predilection for some organs but not others. For example, melanoma metastasizes to the brain in about 40% of patients, while brain metastases are rare in patients with prostate cancer. Paget’s “seed” (ie, tumor cell) and “soil” (ie, target organ) model remains useful.12 Ewing13 proposed that circulatory patterns between the primary tumor and its target organ also determine the tissue specificity. A modern refinement of Paget’s theory suggests that tumor cell “seeds” carry their own “soil” (secreting extracellular matrix [ECM] components) to the target site.14 Although the metastatic cascade of MBMs is poorly understood, several pathways are available as therapeutic targets (Fig 1). Successful metastasis depends on the survival of tumor cells in the circulation and the successful communication of cancer cells with the brain microenvironment. Once primary malignant melanomas advance to the aggressive stage, tumor cells invade or migrate and intravasate. These processes are dependent on their capacity to degrade ECM components and invade endothelial cells or tight junctions that underlie the tumor–blood barrier through proteolysis. Melanoma cells achieve this via two or more different pathways: via neurotrophin signaling15 and through activation of plasmin and matrix metalloproteinases (MMPs).16,17 MMPs break down the ECM, particularly type 1 collagen, fibronectin, and laminin in the ECM.16 Malignant melanoma cells derived embryologically from the neuroectoderm also express neurotrophins and their receptors; in turn, the activation of these receptors (via ligand binding or cleavage and regulated intramembrane proteolysis in a manner analogous to Notch expression) increases heparinase production, an ECM proteolytic enzyme, which cleaves the heparin sulfate chain of the ECM. Heparinase degrades not only ECM but also the basement membrane of the blood–brain barrier (BBB),15-18 facilitating the extravasation of MBMs. A specific melanoma cell antigen called melanotransferrin is present that activates plasminogen that, when targeted using a monoclonal antibody (L235) in animal models, reduces MBMs. This is an important example of targeting a mechanistic pathway to prevent experimental brain metastases. The mechanism behind the tropism of melanomas for the brain is unknown. Although the process is unproven, it is likely that the brain secretes neurotrophins or other factors that act as chemokines and interact with receptors on melanoma cells that modulate survival in the blood stream and tropism to the brain. Once the metastatic melanoma cells 300 Cancer Control reach the brain circulation, they arrest in the capillary bed on the basis of tumor/vessel size. Some of these will cross the vascular endothelial cells of the brain, a process that may support metastatic tumor proliferation and invasion.19,20 After the tumor cells have crossed the endothelial cells, they encounter several host stromal cell types in the brain parenchyma, such as astrocytes and microglia, and begin establishing vascular connections necessary for sustained tumor growth and invasion. The response of the host stromal cells within the brain microenvironment is a critical component for tumor growth. For example, activated microglias make the brain microenvironment favorable for tumor growth and invasion.21 In addition, astrocytes may protect metastatic melanoma cells from cytotoxic agents.22 Neurotropic factors and their receptors are also implicated in melanoma growth in the brain parenchyma.14,23 These observations suggest that astrocytes and microglia stimulate metastatic tumor growth through direct contact and release of trophic factors. Two tumor suppressor genes are known to act on proliferation of melanoma cells when they metastasize: NM23 and BrMS1. NM23 controls cell growth by encoding for a nucleotide diphosphate protein kinase, whose reduced levels in melanoma are associated with enhanced brain metastases.24 BrMS1 prevents tumor cell growth, and its functional reduction is associated with the increased metastatic capacity of melanomas.25 Studies have indicated that the altered microenvironment and compromised tumor suppressor genes help facilitate MBMs.24,25 Another important factor in metastatic tumor growth is the establishment of a sufficient blood supply. Experimental models of MBMs have shown that the recruitment of blood vessels occurs through different mechanisms: (a) utilizing the existing blood vessels by cancer cells for growth (co-opting), (b) extending vascular budding from existing blood vessels (angiogenesis), and (c) establishing a new vascular system (vasculogenesis).26 Studies using real-time imaging through the cranial windows of MBM in mice reveal that a metastatic tumor can grow up to 3 mm through co-opting pre-existing blood vessels.10,27 Following extravasation, metastatic melanoma cells have a close association with pre-existing blood vessels10 and may remain dormant for long periods of time unless they establish a blood supply. Several molecules exist that mediate the establishment of blood supply to metastatic tumors cells. Beta 1-integrin expressed by tumor cells is an important molecule and facilitates the co-opting of tumor cells to blood vessels.28 Vascular endothelial growth factor (VEGF)-A is also vital for co-opting of pre-existing brain blood vessels in MBMs.29 When tumors grow beyond a microscopic size (~ 3 mm), they develop vasculature via angiogenesis, which also involves the VEGF pathway.30 AberOctober 2013, Vol. 20, No. 4 rant VEGF activity can occur as a hypoxic response in the tumor31 or through dysregulated tumor suppressors and oncogene activation.32,33 Therefore, blocking angiogenesis may be a promising approach to MBMs but will not affect their initial extravasation or affect them in their dormant or quiescent state. Others have studied the roles of suppressor of cytokine signaling 1 (SOCS-1) and signal transducer and activator of transcription 3 (STAT3) in the promotion of MBMs. In this paradigm, loss of SOCS-1 (the negative regulator of STAT3) leads to STAT3 activation and favors MBM development.34,35 The loss of SOCS-1 and the activation of STAT3 were confirmed in MBM tissue from patients. STAT3 may have several prometastatic roles by promoting tumor cell migration, evading anoikis, and promoting angiogenesis, the latter via the expression of basic fibroblast growth factor, MMP2 and VEGF.35 Hence, targeting STAT3 may be a useful target to prevent MBMs. Some melanomas metastasize to the brain parenchyma while others still metastasize to the leptomeninges or dura. To address this experimentally in syngeneic mouse models of melanoma, Fidler et al36 used two different melanoma cell lines in two different mouse strains (K-175 melanoma line in C3H/HeN mice and B-16 melanoma line in C57BL/6 mice). Mice were injected with these cells either intra-arterially via the carotid artery or directly inoculated into the cerebrum. Independent of the route of administration, K-175 preferentially produced parenchymal tumors and B16 preferentially produced tumor in the leptomeninges and ventricles. They implicated gelatinase-A as predisposing to parenchymal metastases and responsible for extravasation. Transforming growth factor-beta 2 (TGFβ2) is highly expressed in the brain and inhibits MBM growth in the brain parenchyma,37-39 thus highlighting the targeted approach of chemokines and immunomodulatory proteins (eg, TGFβ2) to prevent or inhibit the growth of MBMs. Identifying subsets of patients with melanoma at high risk for MBMs may justify a therapeutically preventive approach. Standard Treatment Important contributions to optimizing care for patients with MBMs include multidisciplinary care through specialized MBM clinics, comprehensive tumor board meetings, and the availability of clinical trials specifically for this subset of patients. Multidisciplinary care should include medical and surgical oncologists who specialize in melanoma, as well as clinical pharmacists, radiation oncologists, neurosurgeons, and neuro-oncologists. Surgical Treatment The decision to resect brain metastasis is a complex process that depends on the need to establish a hisOctober 2013, Vol. 20, No. 4 tologic diagnosis, lesion size and accessibility, the presence of neurological symptoms, the performance status of the patient, and the overall status of the systemic disease.40 Per guidelines from the National Comprehensive Cancer Network (NCCN), when possible, surgery is the first step for the management of MBM in patients with a reasonable prognosis, particularly in those with one to three brain metastasis,41 a recommendation that predates the evidence of efficacy of v-raf murine sarcoma viral oncogene homolog B1 (BRAF) inhibitors in MBM. This rule should be heavily qualified. For example, in patients with extensive extracranial metastases, initial treatment with BRAF or other targeted treatments may take priority, with surgery for MBM reserved for salvage.42 Resection eliminates tumor-associated edema and obviates the need for sustained corticosteroid therapy and associated adverse events, which represents an important consideration in melanoma metastases because corticosteroids may impair the effectiveness of immunotherapeutic treatment regimens. For small, asymptomatic MBM lesions (< 2 cm) in deep locations, stereotactic radiosurgery (SRS) may be considered as first-line treatment over surgery. However, SRS is associated with vasogenic edema, radiation necrosis, and hemorrhage.43 Radiotherapy Surgical treatment alone is not sufficient for prolonging survival.41 Historically, whole-brain radiotherapy (WBRT) has been used to augment surgical resection,44-46 and it is the only form of radiotherapy (RT) for brain metastases that has category 1 evidence. However, SRS is the treatment of choice for patients who are not surgical candidates (eg, > 3 brain metastases, 1 lesion > 3 cm in diameter, or other reasons) in an effort to “save the brain.” Melanomas are less sensitive to RT than, for example, lymphomas are, so the efficacy of WBRT is lower. Moreover, the longterm cognitive “costs” are high. Focused RT minimizes neurocognitive deficits. Hence, whenever possible, WBRT should be delayed or avoided if other treatment options are available. The advent of effective systemic treatments (eg, ipilimumab, vemurafenib) to treat MBMs may further change the cost–benefit ratio in favor of SRS. A new paradigm might include SRS to provide local control followed by systemic therapy to treat and prevent “micro”-brain metastases. Chemotherapy The results of standard chemotherapy for the treatment of MBMs are disappointing. This may be due to poorly understood factors such as inadequate BBB penetration, drug efflux pumps, intrinsic resistance, or astrocyte protection against chemotherapy-induced apoptosis via paracrine signaling, among others.36,47 Cancer Control 301 Because the BBB is disrupted when tumors grow beyond 1 to 2 mm,48 it is unknown whether the BBB is clinically important for the treatment of established metastases. Temozolomide and fotemustine, both of which do penetrate the BBB, have the best response rates among conventional chemotherapies; however, even they are associated with low response rates and short durations of response (Table).49-57 A phase II trial of temozolomide revealed an objective response rate in MBMs of 7%.49 Other phase II temozolomide studies were performed in combination with thalidomide, WBRT, or both and had similar response rates to temozolomide alone.3,50,51 Clinical activity with fotemustine in MBMs was initially suggested in a phase III study of fotemustine compared with dacarbazine.58 A subsequent phase III study of fotemustine produced a response rate of 7.4% when used as monotherapy vs 10% when combined with WBRT,59 but no improvement was seen in overall survival. A retrospective study found that combining WBRT with temozolomide was safe and possibly prolonged survival in some patients.60 some patients with MBMs, raising the possibility of changing future treatment approaches for patients with MBMs. Antiangiogenics Although antiangiogenics are commonly studied in systemic cancer, few clinical trials study them in MBMs because of their tendency to produce hemorrhage (up 20% in some series). Although melanoma is a highly vascular tumor that secretes VEGF and preclinical models show that angiogenesis is required for “dormant” MBMs to grow,28 a small phase II trial using bevacizumab did not show benefit in systemic melanoma (MBMs were excluded).61 No trial of antiangiogenics has been conducted for isolated MBMs. The approach of targeting angiogenesis may be important because its efficacy could be independent of the mutational status of the tumor (eg, BRAF or NRAS mutations) or the patient’s ability to mount an antitumor immune response. A theoretical disadvantage of antiangiogenic therapies is the potential development of a proinvasive phenotype as observed in patients with glioblastoma multiforme. Other drugs that are also antiangiogenic but not antibody-based and have acceptable BBB penetration include the receptor tyrosine inhibitors sunitinib and cediranib, which, although interesting and potentially important, have not been evaluated in clinical trials for MBMs. Novel Treatments The use of selective BRAF inhibitors (eg, vemurafenib, dabrafenib) or immunotherapies (eg, ipilimumab) has indicated that these agents are safe, have significant activity in systemic melanoma, and are active in Table. — Selected Prospective Clinical Trials of Systemic Therapies for MBMs Study Regimen Phase No. of Patients Response Rate (%) Long52 Dabrafenib II Cohort Aa: 24 Cohort Bb: 17 33 OIRR (81 V600E, 7 V600K) 50 OIRR (89 V600E, 22 V600K) Mornex56 Fotemustine III 39 7.4 Mornex56 Fotemustine + WBRT III 37 10.0 Margolin53 Ipilimumab II Cohort Ac: 51 Cohort Bd: 21 10 (0 CR, 5 PR) 5 (0 CR, 1 PR) Ipilimumab + fotemustine II 86 50 (6 iCR, 19 iPR) Lomustine + temozolomide I/II 26 0 Temozolomide II 117 7 (1 CR, 7 PR) Thalidomide II 35 0 Temozolomide + thalidomide II 15 12 (2 CR, 1 PR) Temozolomide + thalidomide + WBRT I/II 39 7.6 (1 CR, 2 PR) Temozolomide + WBRT I/II 31 9.7 (1 CR, 2 PR) Di Giacomo54 Larkin57 Agarwala49 55 Vestermark Hwu51 Atkins50 Margolin53 a No corticosteroids. Corticosteroids required. c No prior brain therapy. d Prior brain therapy. CR = complete response, iCR-iPR = immune-related complete response or partial response, OIRR = overall intracranial response rate, PR = partial response, WBRT = whole-brain radiation therapy. b 302 Cancer Control October 2013, Vol. 20, No. 4 BRAF Pathway Inhibitors With the remarkable responses of BRAF V600-mutant melanomas to selective inhibitors of the mutant BRAF protein,62,63 these BRAF inhibitors are now being investigated in patients with BRAF V600-mutant MBMs. One of these agents, dabrafenib, was studied in a small cohort of patients with untreated MBMs.64 Nine of 10 patients with MBM responded to therapy in this trial. Following that trial, BREAK-MB, a phase II study of dabrafenib, was conducted and reported promising results.52 A total of 172 patients with between 1 to 4 active MBMs (0.5 to 4 cm in diameter) were stratified into two cohorts: no prior brain therapy (cohort A) or prior brain therapy (cohort B). The overall intracranial response rates in patients with BRAF V600Emutant melanoma were 39% and 31% in cohorts A and B, respectively. The median progression-free survival was 16.1 for cohort A and 16.6 months for cohort B, while the median overall survival rates were 33.1 and 31.4 months, respectively. A lower response rate was seen in patients with BRAF V600K-mutant melanoma. The drug was well tolerated, and common Pretreatment 3 Months on Vemurafenib adverse events were limited to fatigue, nausea, and pyrexia. Data have also been presented for the BRAF inhibitor vemurafenib in previously treated patients with MBM,65 and a phase II trial of vemurafenib in patients with BRAF V600E-mutant MBMs is underway (NCT01378975). An example of a patient with a V600E-mutant MBM who responded to vemurafenib is shown in Fig 2. Overall, the response rates seen in MBMs with BRAF inhibitors mirror extracranial response rates. Combination therapy strategies with targets downstream of BRAF, such as combination BRAF/mitogen-activated protein kinase inhibitors, have shown promise in patients with extracranial BRAF V600E-mutant melanoma.66 However, it remains to be seen whether combination BRAF-targeted strategies will be more effective for MBMs. Patients with BRAF V600E-mutant melanoma may develop brain metastases while taking BRAF inhibitor therapy, with the central nervous system (CNS) as the only site of disease progression in 19% of patients.67 It will be important to investigate the underlying mechanisms involved in this paradoxical phenomenon and the potential strategies to prevent it. Multiple mechanisms may be responsible, including involvement of the tumor microenvironment and evolving melanoma phenotype/resistance during drug treatment. Currently, patients who develop MBMs while experiencing control of their extracranial disease on BRAF inhibitor therapy can be managed with RT or surgery while temporarily withholding the drug. An intact BBB may also protect small micrometastases from the effects of systemic BRAF inhibitors until these micrometastases grow large enough to damage or destroy that barrier, in which case some MBMs that develop while patients are on BRAF inhibitor therapy may retain a degree of sensitivity to the drugs. Immunotherapies High-Dose Interleukin Reports exist of complete response (CR) in patients with MBMs who were treated with high-dose interleukin 2 (HD IL-2). A retrospective analysis of 1,069 patients with either metastatic melanoma or renal cell carcinoma found that 7 patients had untreated brain metastases and 2 had a brain response.68 Another retrospective review of 15 patients with MBMs treated with HD IL-2 reported that 2 patients had a CR.69 Given the nature of this treatment, it is unlikely to be used routinely for this indication, and a theoretical risk of excessive inflammation and vasogenic edema could occur in MBMs responding to IL-2. Fig 2. — Example of a patient with melanoma brain metastases (MBMs) who responded to systemic vemurafenib therapy. Previous treatment for the MBMs included surgery and radiotherapy. In these active MBMs within the brainstem/posterior fossa (upper figures) and right temporal lobe (lower figures), modest regression was seen over the course of 3 months on vemurafenib. October 2013, Vol. 20, No. 4 Adoptive Cell Therapy A retrospective analysis was performed in 26 patients with untreated MBMs discovered incidentally in a cohort undergoing immunodepletion (with chemotherCancer Control 303 apy or total body irradiation) followed by autologous tumor-infiltrating lymphocytes (TILs) or autologous peripheral lymphocytes transduced with a T-cell receptor (TCR) to recognize MART-1 or gp100.70 Of the 17 patients who received adoptive cell therapy with TILs, 7 (41%) had a CR in the brain and 6 (35%) achieved an overall partial response (PR). Similarly, 2 (22%) of 9 patients who received TCR-transduced lymphocytes had a CR in the brain. Therefore, aggressive immunotherapy used in patients with small asymptomatic MBMs may produce CRs. These results also suggest that activated T cells traffic into MBMs in the CNS. Ipilimumab Another strategy that has changed the treatment of melanoma is the use of drugs to upregulate T-cell function using antibodies to block the cytotoxic T-lymphocyte antigen (CTLA) 4, which potentiates antitumor immune responses. Ipilimumab, the anti–CTLA-4 monoclonal antibody, prolonged overall survival rates in patients with metastatic melanoma in two phase III studies.71,72 Although traditional radiographic responses were seen in a small percentage of patients (~ 10% to 15%), many of these responses lasted months to years. These first pivotal studies excluded patients with MBMs. Retrospective analyses of phase II studies with ipilimumab that did not exclude patients with small asymptomatic brain metastases showed evidence of clinical activity in patients with MBM.73,74 These reports led to a prospective phase II study of ipilimumab specifically to treat MBMs (CA184-042).53 Patients were stratified into two cohorts based on symptoms and current corticosteroid use. Patients in cohort A were neurologically asymptomatic and not receiving corticosteroids, whereas those in cohort B were symptomatic and taking a stable dose of corticosteroids. Overall, there was CNS disease control (broadly defined as a CR, PR, or stable disease) in approximately 25% of patients in cohort A and approximately 10% of patients in cohort B. One patient (5%) in cohort B had a CR and 8 patients (16%) in cohort A had a PR. The only grade 3 CNS adverse events were headaches in 4% of patients and confusion in 1%, although these events may have been attributed to the disease. One patient had a grade 4 intratumoral hemorrhage attributed to the disease, not the treatment. The results from this study revealed that ipilimumab has activity in MBMs, particularly when patients are asymptomatic and not receiving corticosteroids. Moreover, the treatment appeared safe in the small number of patients treated. However, it is unknown whether the treatment was more effective in larger MBMs in which the highly permeable BBB may allow a greater ingress of activated cytotoxic T cells. A degree of patience and optimism to monitor clinical benefit is required when using immunothera304 Cancer Control pies. Early in the course of treatment, lesions may grow and become symptomatic, which is a pattern seen in systemic melanoma lesions treated with ipilimumab, even though a significant clinical response will ultimately occur. In general, we attempt to wait until the end of the induction phase of ipilimumab (4 doses given every 3 weeks) before concluding the presence of progressive disease. One retrospective review sought to answer whether combination therapy with CTLA-4 antibodies was effective.75 Control rates using SRS combined with ipilimumab were reported and favorable survivals and response rates were seen. In this context, SRS may synergistically work with ipilimumab by lysing tumor cells and presenting a broader antigen repertoire to primed T cells. A prospective phase II combination trial of ipilimumab with fotemustine, an alkylating agent with BBB penetration, was conducted in Europe.54 The trial included 20 patients with asymptomatic MBMs and found that one-half of participants had stable disease or a response following treatment. A phase III study is planned. Data suggest that ipilimumab produces antitumor T-cell responses in the brain without significantly different response rates or toxicities from extracranial melanoma. It is not clear whether activity seen in small or asymptomatic brain lesions (such as those detected in routine magnetic resonance imaging) will apply to larger symptomatic lesions. Whether response rates are lower in patients who require corticosteroids for symptom control remains undetermined. It is possible that surgery, SRS, or a BRAF inhibitor may be used first, followed by an immunological therapy to provide sustained and longterm responses. Similarly, strategies to promote BBB breakdown (eg, mannitol, small molecule inhibitors) may improve the efficacy of ipilimumab by enhancing T-cell migration to MBMs. Programmed Death 1 Inhibitors Programmed death (PD) 1 is an inhibitory co-receptor on antigen-activated T-cells. Activated T cells may be suppressed by ligands PD-L1 (B7-H1) and PD-L2 (B7DC), which are expressed by tumor or stromal cells. Activated PD-1 inhibits T-cell activation and effector function by suppressing phosphatidylinositide 3-kinase/Akt activation in T cells as well as by many other unknown mechanisms. Inhibiting the PD-1 receptor with a blocking antibody enhances T-cell responses and antitumor activity. Response rates have been reported in melanoma and in non–small-cell lung cancer.76,77 Patients with radiographically stable (≥ 8 weeks) brain metastases were enrolled in these trials, but results in MBMs were not separately reported. Clinical trials are in progress for systemic melanoma that may include patients with small asymptomatic MBMs. October 2013, Vol. 20, No. 4 Special Challenges in Novel Therapies in Patients With MBM The use of immune modulators (eg, CTLA-4, PD-1 antibodies) poses several challenges in the setting of brain metastases. Thus far, no direct evidence exists of the enhancement of T-cell function by ipilimumab within the MBM tumor tissue. Although T cells pass through an intact BBB, which is disrupted in MBMs, the brain remains “immunoprivileged” and is deficient in adoptive immune responses.78 This suggests that strategies to increase BBB penetration may increase the efficacy of immune-based approaches. Curiously, no clear report has addressed CNS inflammation in or around the brain metastases associated with CTLA-4 antibody treatment, nor has there been a clear association with the immune-related hypophysitis, which occurs in 1% to 6% of patients treated with ipilimumab with activity against MBMs (assuming that the occurrence of hypophysitis is a surrogate for T-cell trafficking into the CNS and possibly a predictor of response).79 However, some CNS inflammation may be necessary for efficacy. Response criteria for CNS lesions are challenging because no criteria have been developed specifically for brain metastases. The concept of delayed response — for example, with anti-CTLA-4 antibody treatment — is important to avoid discontinuing a drug too early because of changes in volume enhancement unrelated to tumor growth. Uniform criteria for measuring MBM responses to immunotherapy and corticosteroid management are needed to compare therapies across different studies. Clinical trial design, particularly tissue interrogation of MBMs following an experimental treatment, is an important issue. For example, small phase 0 clinical trials that study the surgical resection of MBMs following treatment with an experimental agent may help determine drug penetration, target modulation (eg, BRAF, mitogen-activated protein kinase, extracellular signal-regulated kinase inhibition), immunological response, and phenotypic changes (eg, necrosis, relative T-cell populations, cytokine activation). Doing so may help clarify the biology, develop new therapies, and prioritize agents to take to phase II studies. However, many of these trials were performed on atypical patients with MBMs. Most of the patients treated had small asymptomatic lesions that did not require corticosteroids. Therefore, future clinical trials should investigate the usefulness of these therapies in patients with large symptomatic lesions, which represent those more typically encountered in real practice. Conclusions New treatment approaches show real activity in melanoma brain metastases that will likely change current treatment. Whole-brain radiotherapy and its cognitive October 2013, Vol. 20, No. 4 adverse events may be avoided by using cytoreductive-targeted therapies followed by immunological therapy for long-term control or cure. How to improve these treatments and how or when to combine them are important questions. Patients at high risk for melanoma brain metastases should be identified and strategies should be developed to prevent these metastases. Finally, clinical trials that incorporate tissue interrogation following exposure to a novel treatment in melanoma brain metastases may accelerate drug discovery and improve patient care. References 1. Fife KM, Colman MH, Stevens GH, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7): 1293-1300. 2. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533-540. 3. Hofmann M, Kiecker F, Wurm R, et al. Temozolomide with or without radiotherapy in melanoma with unresectable brain metastases. J Neurooncol. 2006;76(1):59-64. 4. Sampson JH, Carter JH Jr, Friedman AH, et al. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11-20. 5. Davies MA, Liu P, Mclntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687-1696. 6. Wen PY, Loeffler JS. Brain metastases. Curr Treat Options Oncol. 2000;1(5):447-458. 7. McWilliams RR, Rao RD, Buckner JC, et al. Melanoma-induced brain metastases. Expert Rev Anticancer Ther. 2008:8(5):743-755. 8. Yano S, Shinohara H, Herbst RS, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000;60(17):4959-4967. 9. Shaffrey ME, Mut M, Asher AL, et al. Brain metastases. Curr Probl Surg. 2004;41(8):665-741. 10. Kienast Y, Von Baumgarten L, Fuhrmann M, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1): 116-122. 11. Kang M, Fujimaki T, Yano S, et al. Biology of brain metastases. In: Ali-Osman F, ed. Brain Tumors. Totowa, NJ: Humana Press Inc; 2005:91-106. 12. Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989:8(2):98-101. 13. Ewing J. Neoplastic diseases: a treatise on tumors. Br J Surg. 1928;16(61):174-175. 14. Duda DG, Duyverman AM, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107(50):21677-21682. 15. Denkins Y, Reiland J, Roy M, et al. Brain metastases in melanoma: roles of neurotrophins. Neuro Oncol. 2004;6(2):154-165. 16. Perides G, Zhuge Y, Lin T, et al. The fibrinolytic system facilitates tumor cell migration across the blood-brain barrier in experimental melanoma brain metastasis. BMC Cancer. 2006;6:56. 17. Hotary K, Li XY, Allen E, et al. A cancer cell metalloprotease triad regulates the basement membrane transmigration program [published correction appears in Genes Dev. 2007;21(9):1139]. Genes Dev. 2006;20(19):26732686. 18. Nathoo N, Chahlavi A, Barnett GH, et al. Pathobiology of brain metastases. J Clin Pathol. 2005;58(3):237-242. 19. Marchetti D, Denkins Y, Reiland J, et al. Brain metastatic melanoma: a neurotrophic perspective. Pathol Oncol Res. 2003;9(3):147-158. 20. Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176(6):2958-2971. 21. Carbonell WS, Ansorge O, Sibson N, et al. The vascular basement membrane as “soil” in brain metastasis. PLoS One. 2009;4(6):e5857. 22. Fitzgerald DP, Palmieri D, Hua E, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis. 2008;25(7):799-810. 23. Lin Q, Balasubramanian K, Fan D, et al. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia. 2011;12(9): 748-754. 24. Menter DG, Herrmann JL, Nicolson GL. The role of trophic factors and autocrine/paracrine growth factors in brain metastasis. Clin Exp Metastasis. 1995;13(2):67-88. 25. Sarris M, Scolyer RA, Konopka M, et al. Cytoplasmic expression of nm23 predicts the potential for cerebral metastasis in patients with primaCancer Control 305 ry cutaneous melanoma [published correction appears in Melanoma Res. 2004;14(3):239]. Melanoma Res. 2004;14(1):23-27. 26. Seraj MJ, Samant RS, Verderame MF, et al. Functional evidence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer Res. 2000;60(11):2764-2769. 27. Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610-622. 28. Küsters B, Westphal JR, Smits D, et al. The pattern of metastasis of human melanoma to the central nervous system is not influenced by integrin alpha(v)beta(3) expression. Int J Cancer. 2001;92(2):176-180. 29. Küsters B, Leenders WP, Wesseling P, et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002;62(2):341-345. 30. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27-31. 31. Neufield G, Cohen T, Gengrinovitch S. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9-22. 32. Blancher C, Moore JW, Robertson N, et al. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3’-kinase/Akt signaling pathway. Cancer Res. 2001;61(19):7349-7355. 33. Zundel W, Schindler C, Haas-Kogan D, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14(4):391-396. 34. Huang FJ, Steeg PS, Price JE, et al. Molecular basis for the critical role of suppressor of cytokine signaling-1 in melanoma brain metastasis. Cancer Res. 2008;68(23):9634-9642. 35. Xie TX, Huang FJ, Aldape KD, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66(6):3188-3196. 36. Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21(2):107-112. 37. Schackert G, Fidler IJ. Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 1988;48(12):3478-3484. 38. Schackert G, Price JE, Zhang RD, et al. Regional growth of different human melanomas as metastases in the brain of nude mice. Am J Pathol. 1990;136(1):95-102. 39. Fujimaki T, Fan D, Staroselsky A, et al. Critical factors regulating site-specific brain metastasis of murine melanomas. Int J Oncol. 1993;3(5): 789-799. 40. Ewend MG, Morris DE, Carey LA, et al. Guidelines for the initial management of metastatic brain tumors: role of surgery, radiosurgery, and radiation therapy. J Natl Compr Canc Netw. 2008;6(5):505-514. 41. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers. V.2.2013. http://www. nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed June 17, 2013. 42. Carlino MS, Fogarty GB, Long GV. Treatment of melanoma brain metastases: a new paradigm. Cancer J. 2012;18(2):208-212. 43. Mathieu D, Kondziolka D, Cooper PG, et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery. 2007;60(3):471-482. 44. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489. 45. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322(8):494-500. 46. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141. 47. Rades D, Panzner A, Dziggel L, et al. Dose-escalation of whole-brain radiotherapy for brain metastasis in patients with a favorable survival prognosis. Cancer. 2012;118(15):3852-3859. 48. Eichler AF, Chung E, Kodack DP, et al. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344-356. 49. Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22(11):2101-2107. 50. Atkins MB, Sosman JA, Agarwala S, et al. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer. 2008;113(8):2139-2145. 51. Hwu WJ, Lis E, Menell JH, et al. Temozolomide plus thalidomide in patients with brain metastases from melanoma: a phase II study. Cancer. 2005;103(12):2590-2597. 52. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095. 53. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465. 306 Cancer Control 54. Di Giacomo AM, Fonsatti PA, Pittiglio E, et al. A phase II study combining ipilimumab and fotemustine in patients with metastatic melanoma: the NIBIT-M1 trial. J Clin Oncol. 2011;29:TPS230. 55. Vestermark LW, Larsen S, Lindeløv B, et al. A phase II study of thalidomide in patients with brain metastases from malignant melanoma. Acta Oncol. 2008;47(8):1526-1530. 56. Mornex F, Thomas L, Mohr P, et al. A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma. Melanoma Res. 2003;13(1):97-103. 57. Larkin JM, Hughes SA, Beirne DA, et al. A phase I/II study of lomustine and temozolomide in patients with cerebral metastases from malignant melanoma. Br J Cancer. 2007;96(1):44-48. 58. Margolin K, Atkins B, Thompson A, et al. Temozolomide and whole brain irradiation in melanoma metastatic to the brain: a phase II trial of the Cytokine Working Group. J Cancer Res Clin Oncol. 2002;128(4):214-218. 59. Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22(6):1118-1125. 60. Devito N, Yu M, Chen R, et al. Retrospective study of patients with brain metastases from melanoma receiving concurrent whole-brain radiation and temozolomide. Anticancer Res. 2011;31(12):4537-4543. 61. Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluation the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol. 2012;30(1):34-41. 62. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516. 63. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358-365. 64. Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893-1901. 65. Dummer R, Hauschild A, Guggenheim M, et al. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012(23 suppl 7):86-91. 66. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107-114. 67. Kim KB, Flaherty KT, Chapman PB, et al. Pattern and outcome of disease progression in phase 1 study of vemurafenib in patients with metastatic melanoma (mm). J Clin Oncol. 2011(29 suppl):8519. 68. Guirguis LM, Yang JC, White DE, et al. Safety and efficacy of highdose interleukin-2 therapy in patients with brain metastases. J Immunother. 2002;25(1):82-87. 69. Powell S, Dudek AZ. Single-institution outcome of high-dose interleukin-2 (HD IL-2) therapy for metastatic melanoma and analysis of favorable response in brain metastases. Anticancer Res. 2009;29(10):4189-4193. 70. Hong JJ, Rosenberg SA, Dudley ME, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16(19):4892-4898. 71. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8): 711-723. 72. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011; 364(26):2517-2526. 73. Weber JS, Amin A, Minor D, et al. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21(6):530-534. 74. Schartz NE, Farges C, Madelaine I, et al. Complete regression of a previously untreated melanoma brain metastasis with ipilimumab. Melanoma Res. 2010;20(3):247-250. 75. Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227-233. 76. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):24432454. 77. Brahmer JR, Tykodi SS, Chow L, et al. Safety and activity of antiPD-L1- antibody in patients with advanced cancer. N Engl J Med. 2012; 366(26):2455-2465. 78. Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122(4):1164-1169. 79. Weber JS, Kahler KC, Hauschild A, et al. Management of immunerelated adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. October 2013, Vol. 20, No. 4