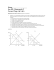Revised (12 Sept 2009) Topic: Chemical Equilibrium
Transcription
Revised (12 Sept 2009) Topic: Chemical Equilibrium
Revised (12 Sept 2009) Sample Teaching Sequence (Hong Kong Secondary 4 – 6 Chemistry) Topic: Chemical Equilibrium Teaching Sequence Reversible reactions Content Examples of reversible reaction; forward reaction; reverse reaction; importance of reversible reactions. 1.2 Characteristics of chemical equilibrium Dynamic equilibrium; changes in reactant and product concentration with time; changes in the rates of forward and reverse reactions with time; equilibrium state. 1.3 The equilibrium law The equilibrium law; equilibrium constant; equilibrium position; determination of equilibrium constants. 1.4 Predicting the direction of a reversible reaction Use the equilibrium constant and reaction quotient to predict the direction in which a reversible reaction will proceed to reach equilibrium. 1.5 Factor affecting the position of equilibrium Use the equilibrium constant and reaction quotient to analyze the effects of changing concentrations: adding or removing reactants and products; changing volume or pressure in gaseous equilibria; adding inert gases to gaseous equilibria. 1.1 z Changing concentrations of reactants and/or products z Adding a catalyst Effect of catalysts on the rates of forward reaction and reverse reaction; effect of catalysts on equilibrium constant and equilibrium position. z Changing temperature Apply van’t Hoff law qualitatively to predict the effect of changing temperature on equilibrium constant and equilibrium position. 1.6 Controlling reversible reactions Maximum yield; cost-effectiveness; the Haber process. For illustrative purposes, a sample of teaching and learning materials for Parts 1.4 – 1.6 is presented on pp. 2 -13. © Derek Cheung 2009 1 1.4 Predicting the Direction of a Reversible Reaction The equilibrium constant, Kc, is 138 at a given temperature for the reaction of iron(III) ions with thiocyanate ions. Fe3+(aq) + NCS−(aq) ⇌ Fe(NCS)2+(aq) In a certain experiment, 0.0240 mole of Fe3+(aq), 0.0460 mole of NCS−(aq), and 0.0880 mole of Fe(NCS)2+(aq) are mixed in a 2.0-Litre flask. Is the system at equilibrium? If it is not, in which direction will the system proceed to reach equilibrium? If the initial concentrations of Fe3+(aq), NCS−(aq), and Fe(NCS)2+(aq) are substituted into the equilibrium constant expression, the value obtained is greater than the equilibrium constant. ⎛ 0.0880 ⎞ ⎜ ⎟ [ Fe( NCS ) ] 2 ⎠ ⎝ = = 159 [ Fe3+ ][ NCS − ] ⎛ 0.0240 ⎞⎛ 0.0460 ⎞ ⎜ ⎟⎜ ⎟ ⎝ 2 ⎠⎝ 2 ⎠ 2+ This indicates that the concentration of Fe(NCS)2+(aq) is bigger than its concentration at equilibrium and the reversible reaction is not at equilibrium. In order to reach equilibrium, the value must be restored to 138. This can be achieved if some Fe(NCS)2+(aq) ions react (making the numerator smaller) to produce Fe3+(aq) and NCS−(aq) ions (making the denominator bigger). Thus, although both forward and reverse reactions occur simultaneously, the reverse reaction will predominate to decrease the Fe(NCS)2+(aq) concentration until equilibrium is established. When the reverse reaction occurs to a greater extent than the forward reaction, we say that the reversible reaction proceeds from right to left to reach equilibrium. The ratio of concentrations shown above is called reaction quotient, Qc. The reaction quotient expression has the same mathematical form as the equilibrium constant expression but involves specific concentrations that are not necessarily equilibrium concentrations. The subscript c indicates that the reaction quotient is a ratio of concentrations. We can apply the reaction quotient to predict the direction of a reversible reaction. When a mixture contains only reactants, Qc is equal to zero. When only products are present, Qc is equal to an infinitely large value. When both reactants and products are present and their concentrations remain constant, Qc is equal to Kc. The direction in which a reversible reaction will proceed is shown in the following figure: Reactants only Qc = 0 At equilibrium Qc = Kc Reaction proceeds to the right to reach equilibrium z z z Products only Qc = ∞ Reaction proceeds to the left to reach equilibrium If Qc is less than Kc, the reversible reaction will proceed from left to right to form more products until equilibrium is achieved. If Qc is greater than Kc, the reversible reaction will proceed from right to left to form more reactants until equilibrium is achieved. If Qc is equal to Kc, the reversible reaction is already at equilibrium and the concentrations of reactants and products will not change. © Derek Cheung 2009 2 Consider the reaction: PCl5(g) ⇌ Cl2(g) + PCl3(g) Kc = 12.5 at 60 oC If a 2.0-Litre container is found to contain 3.2 moles of Cl2(g), 1.5 moles of PCl3(g), and 2.0 moles of PCl5(g), is the system at equilibrium? If it is not, in which direction will the system proceed to reach equilibrium? The concentration of a gas can be expressed as mol/L; that is, moles of the gas per litre occupied. The reaction quotient expression for PCl5(g) ⇌ Cl2(g) + PCl3(g) is ⎛ 3.2 ⎞⎛ 1.5 ⎞ ⎜ ⎟⎜ ⎟ [Cl2 ][ PCl3 ] ⎝ 2 ⎠⎝ 2 ⎠ Qc = = = 1.2 [ PCl5 ] ⎛ 2.0 ⎞ ⎜ ⎟ ⎝ 2 ⎠ Because Qc is less than Kc, the concentrations of Cl2(g) and PCl3(g) are not as large as they would be at equilibrium. Thus, the system is not at equilibrium. Although both forward and reverse reactions occur simultaneously, the forward reaction will predominate until equilibrium is established. In other words, the reversible reaction will proceed from left to right to reach equilibrium. Qc = 1.2 Kc = 12.5 Reaction proceeds to the right to reach equilibrium SAMPLE EXERCISE At 25 oC, Kc is 25 for the reaction H2(g) + I2(g) ⇌ 2HI(g). Predict how the reversible reaction proceeds if [H2] = 0.12 M, [I2] = 0.14 M, and [HI] = 0.82 M. ANSWER The reaction quotient expression is Qc = [ HI ]2 (0.82) 2 = = 40 [ H 2 ][ I 2 ] (0.12)(0.14) Because Qc is greater than Kc, the reversible reaction is not at equilibrium and the reverse reaction will predominate until equilibrium is established. Thus, the reversible reaction will proceed from right to left to form more H2 and I2 molecules until equilibrium is achieved. © Derek Cheung 2009 3 1.5 Factors Affecting the Position of Equilibrium Once a reversible reaction has reached equilibrium, it remains at equilibrium until it is disturbed by changes in concentration and/or temperature. 1.5.1 Changing Concentrations of Reactants and/or Products Adding or Removing Reactants and Products The way in which the position of equilibrium alters by changing concentration can be directly predicted from the properties of equilibrium constant. Consider the following reversible reaction in a test tube: 2 H+(aq) + 2 CrO42−(aq) ⇌ Cr2O72−(aq) + H2O(l) yellow orange In aqueous solution, chromate ions, CrO42-, and dichromate ions, Cr2O72-, exist. The equilibrium constant expression for the reversible reaction is 2− Kc = [Cr2O7 ]eq 2− 2 2 [ H + ]eq [CrO4 ]eq For a dilute solution, water concentration is not significantly changed by the reaction and does not appear in the equilibrium constant expression. Imagine that the reaction mixture is allowed to reach equilibrium at a given temperature. Suppose we now add a few drops of dilute H2SO4(aq) to the mixture and keep the temperature constant. How will an increase in H+(aq) concentration affect the equilibrium position? Immediately after adding the extra H+(aq) ions, before any chemical changes occur, Qc is less than Kc due to an increase in [H+] (making the denominator of the Qc expression bigger). 2− Qc = [Cr2O7 ] 2− + 2 [ H ] [CrO4 ]2 Because Qc is less than Kc, the reaction mixture is no longer at equilibrium. The reversible reaction will proceed from left to right to increase the product concentrations until equilibrium is re-established. Therefore, although both forward and reverse reactions occur, the forward reaction will predominate until equilibrium is re-established (i.e., the forward reaction and the reverse are occurring at equal rates). In the new equilibrium state, the concentration of dichromate ions, Cr2O72-, are greater than it was initially. This indicates that the position of equilibrium has shifted to the product side (i.e., to the right). The mixture becomes more orange in the new equilibrium state. (Carry out a demonstration experiment or insert a photo here) Atmospheric carbon dioxide reacts with water in rain to form carbonic acid (H2CO3), which ionizes into hydrogen ion and hydrogen carbonate ion: H2CO3(aq) ⇌ H+(aq) + HCO3−(aq) That is why normal rainwater is slightly acidic. The pH of natural, unpolluted rainwater is about 5.6, but rainwater with a pH below 5.6 is called acid rain. The equilibrium constant expression for the ionization reaction is Kc = [ H + ]eq [ HCO3− ]eq [ H 2CO3 ]eq Imagine that this reversible reaction is allowed to reach equilibrium in a container at a given temperature. © Derek Cheung 2009 4 Suppose we now add a few drops of NaOH(aq) to the mixture and keep the temperature constant. How will addition of NaOH(aq) affect the equilibrium position? The reversible reaction, H2CO3(aq) ⇌ H+(aq) + HCO3−(aq), does not involve NaOH, but the OH−(aq) ions from NaOH can react with H+(aq) ions to form H2O. Immediately after adding NaOH(aq), the H+(aq) concentration in the mixture will decrease. As a result, Qc is less than Kc due to a decrease in [H+] (making the numerator of the Qc expression smaller). Qc = [ H + ][ HCO3− ] [ H 2CO3 ] Because Qc is less than Kc, the reaction mixture is no longer at equilibrium. The reversible reaction will proceed from left to right to increase the product concentrations until equilibrium is re-established. Therefore, although both forward and reverse reactions occur, the forward reaction will predominate until equilibrium is re-established (i.e., the forward reaction and the reverse are occurring at equal rates). In the new equilibrium state, the concentration of HCO3-(aq) ions is greater than it was initially. This indicates that the position of equilibrium has shifted to the product side (i.e., to the right). SAMPLE EXERCISE Consider the equilibrium 2NO(g) ⇌ N2(g) + O2(g) in a sealed container at a given temperature. Suppose that more oxygen gas is suddenly added to the equilibrium system at constant temperature. How will an increase in oxygen concentration affect the position of equilibrium? ANSWER The equilibrium constant expression for the reversible reaction is Kc = [ N 2 ]eq [O2 ]eq 2 [ NO2 ]eq Immediately after adding the extra oxygen, before any chemical changes occur, Qc is greater than Kc due to an increase in [O2] (making the numerator of the Qc expression bigger). Qc = [ N 2 ][O2 ] [ NO2 ]2 Because Qc is greater than Kc, the reaction mixture is no longer at equilibrium. The reverse reaction will predominate until equilibrium is re-established. The position of equilibrium will shift to the reactant side (i.e., to the left) and more NO molecules will be formed. © Derek Cheung 2009 5 For gaseous equilibrium systems, we need to pay special attention to the conditions under which the change in concentration of reactants and/or products is made because volume of the container will also affect concentration of a gaseous species. However, the container volume is not important if the number of moles of gaseous products in the balanced equation is equal to the number of moles of gaseous reactants in the balanced equation. An example is N2(g) + O2(g) ⇌ 2NO(g). Consider the reaction CO(g) + 3H2(g) ⇌ CH4(g) + H2O(g) at equilibrium in a container fitted with a movable piston (i.e., the piston can move inwards and outwards). If a small amount of CO(g) is suddenly added to the equilibrium mixture at constant pressure and temperature, what will happen to the number of H2O(g) molecules when equilibrium is re-established? Atmospheric pressure Temperature regulator Mixer Constant temperature fluid The Qc expression for this reversible reaction is ⎛ nCH 4 ⎞⎛ nH 2 O ⎞ ⎜ ⎟⎜ ⎟ 2 [CH 4 ][ H 2O] ⎜⎝ V ⎟⎠⎜⎝ V ⎟⎠ (nCH 4 )(nH 2 O )(V ) = Qc = = 3 [CO][ H 2 ]3 (nCO )(nH 2 )3 ⎛ nCO ⎞⎛ nH 2 ⎞ ⎟⎟ ⎜ ⎟⎜⎜ ⎝ V ⎠⎝ V ⎠ where [CH4] is the concentration of CH4, nCH4 is the number of moles of CH4, V is the container volume, and so on. Immediately after adding the extra CO(g), before any chemical changes occur, the number of moles of CO, nco, will increase. The container volume, V, also increases because the piston is movable and the change is made at constant pressure. But the numbers of moles of CH4, H2O, and H2 remain unchanged. Therefore, the new position of equilibrium will depend upon the ratio V2/nco in the above reaction quotient expression. If the new ratio in the Qc expression > the original ratio in the Kc expression, then Qc > Kc; the equilibrium position will shift to the reactant side (i.e., to the left). If the new ratio < the original ratio, then Qc < Kc; the equilibrium position will shift to the product side (i.e., to the right). Thus, the position of equilibrium can be shifted to the left or right, depending upon the initial amount of CO in the equilibrium mixture. © Derek Cheung 2009 6 SAMPLE EXERCISE At a particular temperature, the reaction N2(g) + 3H2(g) ⇌ 2NH3(g) is at equilibrium in a sealed container with fixed volume. The equilibrium mixture contains 0.45 M N2, 1.12 M H2, and 0.28 M NH3. (a) Calculate Kc. (b) Calculate Qc and predict what will happen if 0.30 mol/dm3 N2 is suddenly injected into the container at constant temperature. ANSWER 2 [ NH 3 ]eq Kc = (b) Immediately after adding the extra N2 gas, before any chemical changes occur, the concentration of N2 becomes (0.45 + 0.30) = 0.75 M. The reaction quotient expression is [ N 2 ]eq [ H 2 ]3eq = (0.28) 2 = 0.12 (0.45)(1.12)3 (a) [ NH 3 ]2 (0.28) 2 Qc = = = 0.074 [ N 2 ][ H 2 ]3 (0.75)(1.12) 3 Because Qc < Kc, the equilibrium position will shift to the product side (i.e., to the right) to form more NH3 molecules. SAMPLE EXERCISE The reaction N2(g) + 3H2(g) ⇌ 2NH3(g) is at equilibrium in a container fitted with a movable piston. Predict the direction of the shift in equilibrium position if more N2 gas is suddenly added to the mixture at constant pressure and temperature. ANSWER The piston is movable. We need to consider the change in container volume, V, when writing the reaction quotient expression. 2 ⎛ n NH 3 ⎞ ⎟ ⎜⎜ 2 V ⎟⎠ [ NH 3 ] ⎝ Qc = = [ N 2 ][ H 2 ]3 ⎛ n N ⎞⎛ nH ⎜⎜ 2 ⎟⎟⎜⎜ 2 ⎝ V ⎠⎝ V ⎞ ⎟⎟ ⎠ 3 = (n NH 3 ) 2 (V ) 2 (n N 2 )(nH 2 ) 3 Immediately after adding the extra N2 gas, before any chemical changes occur, the number of moles of N2 and the container volume must increase. The number of moles of H2 and the number of moles of NH3 remain unchanged. Therefore, the new position of equilibrium will depend upon the ratio, V2/nN2, in the above reaction quotient expression. If the new ratio, V2/nN2, in the Qc expression is greater than the original ratio in the Kc expression, then Qc is greater than Kc and the position of equilibrium must shift to the left. If the new ratio is less than the original ratio, then Qc is less than Kc and the position of equilibrium must shift to the right. Thus, the position of equilibrium can be shifted to the left or right, depending upon the initial amount of N2 in the equilibrium mixture. © Derek Cheung 2009 7 Changing Volume or Pressure in Gaseous Equilibria The concentrations of reactants and products in a gaseous equilibrium system can be changed by changing the volume of the reaction container, without adding or removing any reactants and products. Imagine that the following reversible reaction is at equilibrium in a container. How will an increase in container volume affect its equilibrium position? 2 SO2(g) + O2(g) ⇌ 2 SO3(g) We need to consider the change in container volume, V, when writing the reaction quotient expression. The reaction quotient expression is 2 ⎛ nSO3 ⎞ ⎜⎜ ⎟ 2 V ⎟⎠ [ SO3 ] ⎝ = Qc = [ SO2 ]2 [O2 ] ⎛ nSO ⎞ 2 ⎛ nO ⎜⎜ 2 ⎟⎟ ⎜⎜ 2 ⎝ V ⎠ ⎝V ⎞ ⎟⎟ ⎠ = (nSO3 ) 2 (V ) (nSO2 ) 2 (nO2 ) The effect of an increase in container volume on this system can be directly predicted from the properties of equilibrium constant and reaction quotient. Immediately after the container volume is increased and before any chemical changes occur, the value of V becomes bigger. Because V increases, the numerator of the Qc expression becomes bigger. Qc is greater than Kc. The equilibrium position will shift to the left to form more SO2 and O2 molecules. However, consider the following reversible reaction: H2(g) + Br2 ⇌ 2 HBr(g) Imagine that this reversible reaction is at equilibrium in a container. How will an increase in container volume affect its equilibrium position? The Qc expression is 2 ⎛ n HBr ⎞ ⎜ ⎟ 2 [ HBr ] V ⎠ ⎝ Qc = = [ H 2 ][ Br2 ] ⎛ n H 2 ⎞⎛ n Br2 ⎜⎜ ⎟⎟⎜⎜ ⎝ V ⎠⎝ V (n HBr ) 2 = ⎞ (n H 2 ) 2 (n Br2 ) ⎟⎟ ⎠ Because equal numbers of moles of gaseous substances appear on both sides of the balanced equation, the container volume, V, in the numerator and denominator cancels out. Thus, changing the container volume does not affect the position of equilibrium. When the container volume increases, Qc is still equal to Kc. That is, the system is still at equilibrium, and no shift in equilibrium position occurs. Therefore, changing container volume will shift an equilibrium system only if the sum of the coefficients for gaseous reactants in the balanced equation is different from the sum of the coefficients for gaseous products. SAMPLE EXERCISE The reaction Cl2(g) + PCl3(g) ⇌ PCl5(g) is at equilibrium in a syringe. If the volume is decreased at constant temperature by forcing the plunger inwards, will the equilibrium shift to the left or right? © Derek Cheung 2009 8 ANSWER The Qc expression for the reaction is ⎛ nPCl5 ⎞ ⎜⎜ ⎟⎟ V [ PCl5 ] ⎝ ⎠ Qc = = [Cl2 ][ PCl3 ] ⎛ nCl2 ⎞⎛ nPCl3 ⎜⎜ ⎟⎟⎜⎜ ⎝ V ⎠⎝ V ⎞ ⎟⎟ ⎠ = (nPCl5 )(V ) (nCl2 )(nPCl3 ) When the volume, V, is decreased at constant temperature, Qc is less than Kc and thus the equilibrium must shift to the right to form more PCl5 molecules. SAMPLE EXERCISE The reaction CH4(g) + Cl2(g) ⇌ CH3Cl(g) + HCl(g) is at equilibrium in a container. Predict whether this equilibrium system will shift to the right, left, or not be affected by an increase in the container volume. ANSWER Changing the container volume will not affect the equilibrium position because the sum of the coefficients for gaseous reactants in the balanced equation is equal to the sum of the coefficients for gaseous products. This prediction is confirmed by the Qc expressions; the volume, V, cancels out in the expression. ⎛ nCH 3Cl ⎞⎛ nHCl ⎞ ⎜ ⎟⎜ ⎟ [CH 3Cl ][ HCl ] ⎜⎝ V ⎟⎠⎝ V ⎠ (nCH 3Cl )(nHCl ) Qc = = = [CH 4 ][Cl2 ] (nCH 4 )(nCl2 ) ⎛ nCH 4 ⎞⎛ nCl 2 ⎞ ⎜⎜ ⎟⎟⎜⎜ ⎟⎟ ⎝ V ⎠⎝ V ⎠ SAMPLE EXERCISE The reaction N2O4(g) ⇌ 2NO2(g) is at equilibrium in a syringe. If the volume is decreased at constant temperature by forcing the plunger inwards, will the concentration of NO2(g) be higher or lower than the original concentration when equilibrium is re-established? ANSWER The Kc and Qc expressions are 2 Kc = 2 [ NO 2 ] eq [ N 2 O4 ]eq ⎛ n NO2 ⎞ ⎟⎟ ⎜⎜ 2 (n NO2 ) 2 V [ NO2 ] ⎠ ⎝ = Qc = = [N 2O4 ] ⎛⎜ nN 2O4 ⎞⎟ (nN 2O4 )(V ) ⎜ V ⎟ ⎠ ⎝ When the volume, V, is decreased at constant temperature, Qc is greater than Kc and thus the equilibrium must shift to the left. As a result, the number of N2O4 molecules will increase. Because the syringe volume is reduced, the concentration of N2O4 in the new equilibrium mixture must increase. To keep Kc constant, the concentration of NO2 in the new equilibrium mixture must be higher than that in the initial equilibrium mixture. (Carry out a demonstration experiment or insert a photo here) © Derek Cheung 2009 9 Adding Inert Gases to Gaseous Equilibria If the sum of the coefficients for gaseous reactants in the balanced equation for a reversible reaction is different from the sum of the coefficients for gaseous products, the position of equilibrium can be shifted by adding an inert gas at constant pressure and temperature. As an example, consider the following reversible reaction: CO(g) + Cl2(g) ⇌ COCl2(g) Suppose that this reaction is at equilibrium in a container fitted with a movable piston. What will happen if some argon gas is added to the mixture at constant pressure and temperature? The Qc expression for the reaction is ⎛ nCOCl2 ⎞ ⎜⎜ ⎟ V ⎟⎠ ⎝ Qc = ⎛ nCO ⎞⎛ nCl2 ⎟⎜⎜ ⎜ V ⎠⎝ V ⎝ ⎞ ⎟⎟ ⎠ = ( nCOCl2 )(V ) (nCO )(nCl2 ) Because the piston is movable, the container volume, V, will increase after argon gas is added. Thus, Qc is greater than Kc. The equilibrium position will shift to the left to form more CO and Cl2 molecules. If the reaction CO(g) + Cl2(g) ⇌ COCl2(g) is at equilibrium in a sealed container with fixed volume, then adding an inert gas will not affect the value of any of the terms in the above Qc expression. Thus, adding an inert gas at constant volume and temperature will not affect the position of equilibrium. SAMPLE EXERCISE The reaction 2CO(g) + O2(g) ⇌ 2CO2(g) is at equilibrium in a container fitted with a movable piston at room temperature. What will happen if some nitrogen gas is added to the equilibrium mixture at constant pressure and temperature? ANSWER The Qc expression for the reaction is 2 ⎛ nCO2 ⎞ ⎜⎜ ⎟⎟ V Qc = ⎝ 2 ⎠ ⎛ nCO ⎞ ⎛ nO2 ⎜ ⎟ ⎜⎜ ⎝ V ⎠ ⎝ V ⎞ ⎟⎟ ⎠ = (nCO2 ) 2 (V ) (nCO ) 2 (nO2 ) Nitrogen does not react with any of the three gases at room temperature. Because the piston is movable, the container volume, V, will increase after nitrogen gas is added. Thus, Qc is greater than Kc. The equilibrium position will shift to the left to form more CO and O2 molecules. 1.5.2 Adding a Catalyst A reversible reaction can reach equilibrium more quickly in the presence of a catalyst. But addition of a catalyst to an equilibrium system does not affect its equilibrium constant and equilibrium position because the catalyst affects the activation energy of both forward and reverse reactions equally. © Derek Cheung 2009 10 1.5.3 Changing Temperature Although the values of Kc are unaffected by changes in concentration or by adding catalysts, chemists have found that when temperature changes, the values of most Kc also change. The values of Kc at different temperatures for two reversible reactions are shown in the table below. The corresponding enthalpy changes for the forward reactions are also shown. N2O4(g) ⇌ 2NO2(g) ∆H0 = 57 kJ mol-1 Temperature/K 298 350 600 Kc 5.9 x 10-3 1.3 x 10-1 4.6 x 102 2NO(g) ⇌ N2(g) + O2(g) ∆H0 = -181 kJ mol-1 Temperature/K 298 900 2300 Kc 2.2 x 1030 1.5 x 109 5.9 x 102 In the equilibrium system N2O4(g) ⇌ 2NO2(g), colourless dinitrogen tetraoxide (N2O4) molecules dissociate to form brown nitrogen dioxide (NO2) molecules and this forward reaction is endothermic (∆H0 > 0). The intensity of brown colour can be used to measure the concentration of nitrogen dioxide in the equilibrium mixture. The equilibrium constant increases as the temperature increases from 298 K to 600 K. This indicates that increasing temperature can shift the equilibrium position to the right to form more NO2 molecules. Thus, if we allow this reversible reaction to reach equilibrium in a sealed flask, increasing the reaction temperature will make the equilibrium mixture appear darker. (Carry out a demonstration experiment or insert a photo here) In the equilibrium system 2NO(g) ⇌ N2(g) + O2(g), the forward reaction is exothermic (∆H0 < 0). The equilibrium constant decreases as the temperature increases from 298 K to 2300 K. This indicates that increasing temperature can shift the equilibrium position to the left to form more NO molecules. The equilibrium mixture will contain more NO molecules at a higher temperature. Jacobus Henricus van’t Hoff, a Dutch chemist and the winner of the first Nobel Prize in chemistry, discovered the effects of changing temperature on equilibrium position: Increasing temperature will shift the equilibrium position of a reversible reaction in the endothermic direction, whilst decreasing temperature will shift the equilibrium position of a reversible reaction in the exothermic direction. van’t Hoff (1852 – 1911) SAMPLE EXERCISE The reaction below is at equilibrium in a beaker. What will happen to the equilibrium position if the temperature of the mixture is increased? Co(H2O)62+(aq) + 4 Cl-(aq) ⇌ CoCl42-(aq) + 6 H2O(l) pale pink deep blue ∆H0 > 0 ANSWER Due to the presence of pink and blue ions, the equilibrium mixture is usually violet in colour. However, an increase in temperature will shift the equilibrium position of the reversible reaction in the endothermic direction. The above thermochemical equation indicates that the forward reaction is endothermic. Thus, © Derek Cheung 2009 11 the equilibrium position will shift to the right when temperature is increased. The equilibrium mixture will appear dark blue at a higher temperature. (Carry out a demonstration experiment or insert a photo here) SAMPLE EXERCISE The reaction below is at equilibrium in a sealed flask. What will happen to the equilibrium position if the temperature of the mixture is increased? 2 SO2(g) + O2(g) ⇌ 2 SO3(g) ∆H0 = -197 kJ mol-1 ANSWER The thermochemical equation indicates that the reverse reaction is endothermic. Thus, the equilibrium position will shift to the left when temperature is increased. The equilibrium mixture will contain more SO2 and O2 molecules at a higher temperature. 1.6 Controlling Reversible Reactions: The Haber Process In 2009, the world population is 6.79 billion. To make the large quantities of fertilizers needed for present-day agriculture, chemists synthesize ammonia from its elements: N2(g) + 3 H2(g) ⇌ 2 NH3(g) ∆H0 = -92 kJ mol-1 This famous reversible reaction is known as the Haber process. How can chemists obtain the maximum yield of ammonia in a reasonable time and at a reasonable cost? The forward reaction is exothermic. Therefore, decreasing temperature can shift the equilibrium position to the right to yield more NH3 molecules. However, the reaction is slow at low temperatures. To increase the reaction rate, a catalyst may be used. An effective catalyst for the Haber process is Fe3O4 mixed with KOH, Al2O3, and SiO2. Because the catalyst does not work below 400 oC, the operating temperature is usually about 450 oC. Should chemists use low or high pressure to increase the yield of NH3? We can apply the concept of reaction quotient to predict the pressure that we should use. The reaction quotient expression for the Haber process is 2 ⎛ n NH 3 ⎞ ⎟⎟ ⎜⎜ V [ NH 3 ]2 ⎠ Qc = = ⎝ 3 [ N 2 ][ H 2 ] ⎛ n N 2 ⎞⎛ nH 2 ⎟⎟⎜⎜ ⎜⎜ V ⎠⎝ V ⎝ ⎞ ⎟⎟ ⎠ 3 = (n NH 3 ) 2 (V ) 2 (n N 2 )(nH 2 ) 3 To give the maximum yield of NH3, we need to make Qc smaller than Kc so that the equilibrium position shifts to the right. Since the total volume, V, is in the numerator, an increase in pressure of the gaseous system will reduce V and thus make Qc smaller than Kc. Therefore, the Haber process is carried out at high pressure. But the higher the pressure, the greater the cost will be. The operating pressure is usually about 200 atm. The boiling point of NH3 (-33 oC) is much higher than that of N2 (-196 oC) and H2 (-253 oC). To further shift the equilibrium position to the right, the product gas is chilled to liquefy and remove NH3 gas from the reactor. As a result, the number of moles of NH3, nNH3, decreases and Qc is less than Kc. © Derek Cheung 2009 12 To summarize, the following conditions are used by chemists to produce ammonia by the Haber process: z z z z Fe3O4 is used as a catalyst A relatively low temperature (450 oC) High pressure (200 atm) NH3 is continuously liquefied and removed compressed N2 and H2 hot N2 and H2 heating coil reactor with catalyst NH3 and unreacted N2 and H2 recycling pump unreacted N2 and H2 cooling coil Liquid NH3 storage tank © Derek Cheung 2009 13