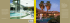Full Text PDF - Herald International Research Journals
Transcription
Full Text PDF - Herald International Research Journals
Herald Journal of Microbiology and Biotechnology Vol. 1 (1): 001 - 009 August, 2014 Available online http://www.heraldjournals.org/hjmb/archive.htm Copyright © 2014 Herald International Research Journals Full Length Research Paper Microbiological assessment and physiochemical parameters of palm oil mill effluent collected in a local mill in Ovia North East area of Edo State, Nigeria Okogbenin O.B*1, Anisiobi G.E2, Okogbenin E.A3, Okunwaye T3 and Ojieabu. A1 1 Plant Pathology Division, Nigerian Institute for Oil Palm Research (NIFOR), P.M.B. 1030. Edo State. Nigeria. 2 Chemistry Division, Nigerian Institute for Oil Palm Research (NIFOR), P.M.B. 1030. Edo State. Nigeria. 3 Biochemistry Division, Nigerian Institute for Oil Palm Research (NIFOR), P.M.B. 1030. Edo State. Nigeria. Received May 21, 2014 Abstract Accepted June 24, 2014 Changes in microbial community as well as physio-chemical properties of Palm oil mill effluent before and after two weeks were determined using standard procedures. The results showed that the sample contained a variety of microorganisms which include Bacillus sp. Pseudomonas sp. Micrococcus sp, Corynebacterium sp, Apergillus niger, Penicillium sp, Candida sp. and Geothricium candidum. The viable count of bacteria at the initial time of sample collection was 1.3 x 101 CFU/g while that of fungi was 1.9 × 102 CFU/g. The microbial load for the final analysis of bacteria and fungi were 4.5 x 10 3 CFU/g and 5.5 x102 CFU/g respectively. From the results, it was observed that after the duration of two weeks, there was a reduction in all the elements from the initial reading with a percentage of 74.88% for Calcium, 50.66% for Magnesium, 53.73% for sodium, 79.15% for phosphorus, 31.17% for Nitrogen and 10.40% for carbon. Free fatty acid content and organic matter content increased from 11.06 to 17.00% and 0.55 to 10.42 respectively while the oil content in the samples reduced from 15.17% to a value of 10.04%. The reduction in oil content observed is an indication of the lipolytic effect by the microbial isolates identified in this study and possible sources for bioremediation purposes both in small and large scale. Keywords: Palm oil mill effluent, Bacteria, Fungi, Physio-chemical, Bioremediation. INTRODUCTION Oil palm (Elaeis guineensis), the most productive oil producing plant in the world is of great economic importance in Nigeria, Okechalu, (2011); SMA Tagoe, (2012). Palm trees may grow up to 60 feet or more in height. The trunks of young and adult palms are wrapped in fronds which give them a rough appearance. Each tree produces compact bunches of fruitlets weighing about 10 kg to 25 kg with 1000 to 3000 fruits per bunch. Each fruit which is spherical or elongated in shape turns from a dark purple color to orange red when ripe Panapanaan et al., (2009) and ready for milling. In general, the palm oil milling process can be categorized into a dry and wet (standard) process. The wet process of milling palm oil is the most common. It is estimated that 0.5-0.75 tonnes of *Corresponding Author: [email protected] Email: Palm oil mill effluent can be discharged from every tonne of oil palm fresh fruit Wakil et al., (2013). The process of extracting palm oil requires significant large quantities of water to steam and sterilize the palm fruit bunches, clarify the extracted oil and the cleaning processes in the mill Nwoko and Ogunyemi, (2010). It is estimated that for each tonne of crude palm oil (CPO) that is produced, 5.0 tonnes-7.5 tonnes of water are required and more than 50% of this water ends up as waste water sludge commonly referred to as palm oil milling effluents POME Ahmad et al. (2003). Phaik, Wei-Jin and Mei, (2010). Palm oil mill effluent is an acidic, thick, brownish, colloidal suspension with 95% to 96% of water, 0.6% to 0.7% of oil and 2% to 4% total suspended solids (TSS) Wakil et al., (2013). The continuous collection of POME in particular locations can bring about undesirable changes in soil pH, increase in salinity Kittikun et al., (2000), soil microbial properties and biological activities Herald J. Microbiol. and Biotech. 002 (Thirukkumaran, and Parkison, (2000); Paredes et al., (2005). Soil microbiological and biochemical properties have been considered early and sensitive indicators of soil changes and can be used to predict long term trends in the quality of soil Ros et al., (2003). Due to the daily release of large amounts of POME generated, annual disposal remains a challenge to the environment and as such bioremediation has been considered as an alternative for this pollution control Hipolito et al., (2008). There is therefore need to evaluate the occurrence of microbes as possible candidates for bioremediation/bioconversion processes in small and large scale. This in vitro research work was aimed at evaluating the changes in microbial and physicochemical properties of POME exposed under room conditions over a period of two weeks (14days) in order to undergo biodegradation. MATERIALS AND METHODS Sample collection Palm oil mill effluent samples were collected from an oil mill processing unit located in Ovia North East area of Edo state on latitude 6.462861 and longitude, 5.597825. The sludge was freshly collected at the point of discharge during oil mill processing into pre sterilized 1000ml conical flasks and covered with cotton wool. The samples were left to stand on a sterile bench in the laboratory overnight to cool and for further analysis. The following initial physicochemical parameters were carried out; pH, Temperature, Conductivity, Nitrogen, Carbon, Organic matter, Phosphorus, BOD, COD, Calcium, Magnesium, Sodium. Biochemical parameters assayed were: Moisture content, free fatty acids (FFA), Dirt content and Oil content. Biodegradation of POME This was carried out by exposing the effluent under room temperature in sterile containers. It was stirred every four days to allow for free flow of air inside the sample. The mixture was allowed to stand to degrade for 14 days after which samples for analysis were drawn. Preparation of Media In this study, the two different media used were potato dextrose agar (PDA) and nutrient agar (NA). The media were prepared aseptically following manufacturer’s instructions while autoclavable materials such as glassware were sterilized at 121oC for 15 minutes at 15psi. Isolation and Identification present in sample of microorganisms The initial and final (after two weeks) isolation of micro flora on the sample was carried out as thus; stock solutions of the sample at day one (initial) was made by weighing one gram of the thick sludge into sterile MacCokney bottles containing 9ml of sterile distilled water. A tenfold serial dilution was prepared with the aid of sterile syringes into labeled MaCokney bottles. One (1) ml of each dilution of 10-1 –10-4 and 10-5 – 10-8 was pipetted with sterile syringes and plated using the pour plate method Pepper and Gerba, (2004) into sterile disposable Petri dishes. In the isolation of fungi, prepared potato dextrose agar medium (PDA) was allowed to cool to a temperature of about 35oC and 250mg/250ml of chloramphenicol was added to the medium to inhibit the growth of any possible bacteria, and then gently dispensed into the plates containing the samples. The bacteria isolations were also carried out by adopting the pour plate method Pepper and Gerba, (2004) using sterile prepared Nutrient agar medium. The mixtures in the plates were carefully swirled so as to allow for even distribution of possible colonies. The experiment was carried out in four replicates and observations were made on a daily basis. Bacteria colonies were enumerated using a colony counter and values expressed as CFU/g of the sample while the isolates were identified and characterized to the species level based on reactions observed during gram staining and biochemical tests carried out. The characteristics of the bacteria isolates were also compared with those of known taxa, using the Bergey’s manual of determinative bacteriology and the scheme of Cheesbrough, (2004). The biochemical tests carried out on the isolates include; coagulase, catalase, indole, urease, citrate utilization test, utilization of carbohydrates such as glucose and lactose. The spores of the bacterial colonies isolated and their gram staining characteristics were observed under microscope. Total viable count of the colonies was carried out according to Collins et al., (1989) using the formula: Number of colonies X dilution factor/ Inoculum volume and the values expressed as colony forming units per gram (CFU/g) of the effluent. The yeast isolates were characterized using the procedures described by Barnett et al., (2000) while Fungal isolates were examined macroscopically and microscopically using the needle mounts technique. Their identification was performed according to the scheme of Barnett and Hunter (1972). The most predominant fungi isolate was further sent to CABI, Surrey, United Kingdom where the isolates were confirmed using molecular techniques and given ascension number. The pictomicrograph of the spores were taken using a 9MP Amscope motic camera attached to one of the eye piece of the microscope. Okogbenin et al. 003 Physiochemical analysis and biochemical tests All biochemical parameters were determined according to the Malaysian Palm Oil Board (MPOB) test methods (2004) and the analysis was carried out in triplicates. Free Fatty Acid = 25.6 × Molarity of KOH × Volume of KOH /mass of sample. Where 25.6 in the formular for FFA determination is equivalence factors (e) for palmitic acid; the predominant fatty acid in palm oil. Determination of Moisture and Volatile Content Determination of temperature A mass of 10grams of thoroughly mixed POME sample was weighed into a known mass of clean Petri dish which had been previously dried and cooled in a desicator. It was placed in the oven for four (4) hours, allowed to cool to room temperature in a dessicator for 45mins and further weighed. This was repeated till a constant weight was obtained. The moisture and volatile matter content was expressed in percentage by mass using the formula: % Moisture and Volatile content= (Mb-Md / Mb-M) ×100 Where M=Mass (g) of Petri dish Mb = Mass (g) of Petri dish +sample Md = Mass (g) of Petri dish + test sample after drying. The initial reading was determined at the point of sample collection, while the final reading was carried out after fourteen days. This was done by dipping the bulb of mercury-in-glass thermometer into the POME samples using 76MM immersion MEDALCARD thermometer and the readings recorded. Determination of pH. The pH was determined with the aid of a pH meter. Statistical Analysis Determination of Oil Content: Ten grams (10g) of dry POME sample was weighed into a thimble and extraction was done with the aid of a Soxhlet extractor for 6 hours using 150 ml of Hexane (analytical grade) as extracting solvent. The oil extract was dried at atmospheric pressure at 103oC for 2 hours, cooled for 30mins and weighed. The oil content was expressed as percentage by mass using this formula: Mass (g) of oil in the flask after drying / mass (g) of sample×100%. Determination of Dirt content The dirt content of the sample was analyzed as thus; 8.50grams of the sludge was weighed into a conical flask, 100ml of hexane was added, swirled and carefully filtered through a glass fiber filter paper in a crucible and dried in the oven at 103oC for 1 hour. Percentage dirt content was expressed as: (Mass of crucible +filter paper + dirt) – (Mass of crucible + filter paper) / Mass of sample ×100%. Determination of Free Fatty Acid (FFA) A mass of 2.5grams of the sample was weighed into a conical flask, 50ml of neutralizing solvent was carefully added to the weighed sample in the conical flask then the flask was placed on a hot plate at 40oC, swirled gently and titrated with standard potassium hydroxide (0.5M). Values were calculated using the formula: SPSS software version 19 was used to analyze the results statistically. A one-way analysis of variance was carried out at α = 0.05 RESULTS Figure 1; Shows the POME collected during milling. It was perceived to have a smell characteristic of freshly milled palm oil. However, during the days when it was left to stand, it started deteriorating thereby giving an offensive odor. A total of eight (8) microorganisms were isolated and identified as Pseudomonas sp., Bacillus sp, Micrococcus sp, Corynebacterium sp., Aspergillus niger, Penicillium sp, Candida sp. and Geothricium candidum. (Table 1). The Geothricium candidum (IMI Number; 503814) was identified by ITS DNA sequence analysis using the FASTA algorithm with the Fungus database from EBI. The result was confirmed using the online CBS yeast database. The initial physio-chemical characteristics of the effluent collected and analyzed were found to have a pH of 5.10, organic matter content of 0.55, BOD value of 502.93mg/l and COD value of 821.45 mg/l. The percentage oil content was 10.04%, percentage dirt content; 6.73% , nitrogen content; 3.24mg/l, carbon content; 32.70mg/l, calcium content; 605.50mg/l, magnesium content; 193.50mg/l, phosphorus; 17.80mg/l, conductivity (dsm-1); 137.34, Free fatty acids (%);11.06±0.01 , temperature of 32.80oC (Table 3). Cultural characteristics of the bacteria isolates were observed and recorded based on their elevation, margin, colour and shape (Table1). The morphological Herald J. Microbiol. and Biotech. 004 Figure 1. Freshly collected POME from milling site Table 1. Cultural, morphological and biochemical characteristics of bacteria isolated from Palm Oil Mill Effluent. Characteristics Cultural Elevation Margin Color Shape Morphological Gram staining Cell type Cell arrangement Spore staining Biochemical Test Catalase Oxidase Coagulase Urease Indole Citrate Glucose Lactose Isolates Isolate 1 Isolate 2 Isolate 3 Isolate 4 Low convex Entire Green Circular Convex Entire Orange Circular Flat Entire Cream Circular Convex Entire Cream Circular Rod Single - + Cocci Single - + Rod Chains + + Rod Single - + + + + + Pseudomonas sp. + + + + + Micrococcus sp. + + + + + Bacillus sp. + + + + + Corynebacterium sp. Key= + Positive, - negative characteristics were recorded using gram staining techniques as described by Christopher K and Bruno E (2003). The gram staining was aimed at differentiating gram reactions, sizes, shapes and arrangement of cells of each bacteria. The results are shown in table 1. Confirmation of the isolates were carried using the biochemical tests Christopher K and Bruno E, (2003) as shown in table 1. Okogbenin et al. 005 Table 2: Microscopic and cultural characteristics of fungi isolated from unsterilized freshly milled palm oil Fungi Isolates Aspergillus sp. Candida sp. Penicillium sp. Geotrichium candidum (IMI Number; 503814) Microscopic Morphology Presence of septate hyphae; long and smooth conidiophores, long unbranded sporangiospores with large, round head. Cultural characteristics Brownish-black mycelium with dark spores on the surface Microscopic morphology shows spherical to subspherical Colonies are white to cream colored, budding yeast-like cells or blastoconidia, 2.0-7.0 x 3.0-8.5 smooth, glabrous and yeast-like in um in size. Long branched pseudohyphas were also seen. appearance. Presence of septate and fruity mycelium and branched conidiophores. A rapid dark green colored growth with the edge surrounded by whitish margin was observed on the surface of the organism and pale yellow on the reverse side. They are either rectangular in shape or rounded at the produce rapidly growing, white, dry, ends resembling the barrel shape. They posses powdery to cottony colonies Arthroconidia which are unicellular, in chains, hyaline, and result from the fragmentation of undifferentiated hyphae by fission through double septa. Figure 2: Penicillium sp. grown on PDA medium At the initial stage of day one, the microbial load was very low with a few bacteria species isolated with a load of 1.25 x 103 CFU/g. The isolation after the fourteen days period, revealed the predominance of Geotrichum candidum (IMI Number; 503814) which dominated and covered the entire plates. The initial result could be due to the nature of the sludge as it was freshly collected in sterile beakers and hadn’t started undergoing biodeterioration as the initial time of analysis while the later could be attributed to the fact that yeast are more Figure 3. G.candidum grown on PDA fastidious than fungi and hence the multiplication and total colonization of the plates. (table 2) The plates below show some of the fungi isolated from POME and grown on PDA medium (Figure 2-5) Physio-chemical parameters including BOD measurements were prepared according to the modified method explained in American Public Health Association APHA (1992). COD determination was based on closed reflux dichromate oxidation colorimetric method and was read out in DR 2000 Spectrophotometer as explained in Herald J. Microbiol. and Biotech. 006 Figure 4: Aspergillus niger isolated from POME Figure 5: Pictomicrograph of A.niger (x40) captured using a motic camera Table 3. Physio-chemical parameters of the sample at the initial and final stage (after fourteen days) under the same conditions of room temperature. PARAMETERS BOD (mg/L) COD (mg/L) Electrical Conductivity (ds m-1) pH Organic Matter Moisture content (%) Oil content (%) Dirt content (%) Free fatty acids (%) Temperature (o C) INITIAL READING 502.93±0.04 821.45±0.65 137.34±0.00 5.10±0.05 0.55±0.02 77.98±0.00 15.17±0.02 6.73±0.00 11.06±0.01 32.80±0.00 FINAL READING 741.98±0.00 1186.22±0.5 93.70±0.00 5.70±0.04 10.42±0.00 72.00±0.02 10.04±0.2 6.08±0.10 17.00±0.08 30.50±0.00 *Values are means of triplicates ± SEM APHA (1998). The electrical conductivity (1:5w/v) was measured according to Jackson, (1962) while pH of the sample was determined using Hach-one pH meter in a ratio of 1:2. Total organic carbon was determined by oxidation with K2Cr2O7 in a concentrated H2SO4 medium followed by measurements of dichromate excess using (NH4)2Fe(SO4)2 Yeomans and Bremmer,(1989). From the results in table 3, it was observed that there was an increase in some physicochemical parameters of the sludge from the initial experiment and the final experiment. The BOD and COD content of the POME increased tremendously, this could be an indication of serious pollution when the POME is actually left on d soil. This finding is similar to work done by Dhouib et al., (2006), who suggested that the use of anaerobic digestion with white rot fungus could decrease COD and BOD levels in POME. The organic content of the sludge increased from 0.55 to 10.42 this confirms the findings of Logan et al., (1997). A slight increase in pH from the initial experiment and the final experiment showed that the medium was still acidic. The results in Table 4 show the initial and final elements present in the sample. From the results, it was observed that after the duration of two weeks, there was a reduction in all the elements from the initial reading and the final reading with following percentages 74.88% for Calcium, 50.66% for Magnesium, 53.73% for sodium, 79.15% for phosphorus, 31.17% for Nitrogen and 10.40% for carbon. Okogbenin et al. 007 Table 4. Elements at the initial and final experimental period ELEMENTS Calcium Magnesium Sodium Phosphorus Nitrogen Carbon INITIAL READING(mg/l) 605.50±0.05 193.50±0.02 255.50±0.01 17.80±0.03 3.24±0.05 32.70±0.65 FINAL (mg/l) 151.80±0.08 95.76±0.04 118.49±0.07 3.71±0.03 2.23±1.00 29.30±0.8 READING DISCUSSION Fresh POME collected from the palm oil mill was thick yellow in color, colloidal suspension, oily and viscous with a sweet smell. The isolation of lipase producing bacteria; Bacillus sp. from the initial microbial analysis of this study, revealed that the oily nature of POME serves as a good medium for lipolytic microorganisms to thrive Mukesh et al., (2012), this is in agreement with the work of Gowland et al., (1987) who also isolated lipase producing Bacillus sp. from POME. The presence of this organism in POME is most likely an indication that the microorganisms can be isolated, multiplied and applied to POME contaminated sites, as it will aid in bioremediation of the contaminated site. Studies by Kanokrat et al., (2013), revealed that some bacteria such as Bacillus sp, Pseudomonas sp, Corynebacterium sp. and some others isolated from palm oil contaminated soils had the ability to produce biosurfactants. Biosurfactants are amphiphilic (containing both hydrophilic and hydrophobic moieties) surface active agents produced by microorganisms Kanokrat et al., (2013) that have the ability to reduce surface and interfacial tensions by accumulating at the interface between two immiscible fluids such as oil and water Nitschke and Coast, (2007). The presence of biosurfactants can increase the solubility of oil and hence potentially increase their bioavailability for use as carbon and energy sources Mulligan, (2009) in soils. The ability of the biosurfactant producers to reduce interfacial surface tension in oil contaminated sites that are most important in tertiary oil recovery and bioremediation processes Lin, (1996); Rouse et al., (1994), Volkering et al., (1998) and many of the known biosurfactant producers are also hydrocarbon-degrading organisms Rouse et al., (1994); Willumsen and Karlson, (1997); Volkering et al., (1998). The bacteria isolated from this work may have the potential of producing biosurfactants, hence, consideration should be given to this area of research as it will help reduce the continuous use of chemical surfactants as well as aid in bioremediation processes especially in Nigeria. Several lipases from Bacillus thermoleoverans, Lee et al., (2001) Markossian et al., (2000), B. stearothermophilus Sinchaikul et al., (2001), thermoacidophilic bacteria, B. acidocaldarius have been identified and characterized. Lipases (triacylglycerol acylhydrolases, EC 3.1.1.3) catalyze the hydrolysis and the synthesis of esters formed from glycerol and longchain fatty acids thereby leading to release of free fatty acids (FFA) Koshimizu et al. (1997); Onilude et al. (2010). Microbial lipases extensively applied in food and dairy industry for the hydrolysis of milk fat, cheese ripening, flavor enhancement and lipolysis of butter fat due to their shorter generation time; ease of bulk production and ease of manipulation, and are economical. This work and the works of others show that palm oil mill effluent provides a good source for abundant lipase producing bacteria for the confectionary industries. Growth and multiplication on any substrate is often considered as the first step towards its bioconversion Molla et al., (2002). The presence of Aspergillus sp. in the sample may not be unconnected with its high lipase producing ability as POME serves as a good substrate for the fungus. However, at the final (after 14days) isolation of the sample, Geotrichum candidum (IMI Number; 503814) was most predominant and had a very rapid growth over the rest fungi. The results of this work gives an indication that this rapidly growing yeast specie can be applied to palm oil mill effluent contaminated soils as they tend to breakdown oil components and utilize them rapidly as substrates for their proliferation. The increase in organic matter may be attributed to increase in biomass of resident microorganisms in the POME during the fermentation. The Organic matter from Palm oil mill effluent application can be applied to soil to help increase some beneficial soil chemical and physical characteristics such as organic carbon and some major nutrients. Free fatty acid content of fermented POME increased with fermentation days, this was probably due to the activities of lipolytic bacteria isolated from the samples and it is in agreement with Onilude et al., (2010) who had earlier made such observation in a similar study. Mineral nutrients are inorganic elements that have essential and specific functions in oil palm plant metabolism due to this reason, some of minerals content from palm fruits might have been retained in POME during the milling and extraction process Mohd Rizal Abas et al., (2013). The reduction in the levels of the nutrient elements may be due to the activities of microbes as they utilize the nutrient sources for proliferation. The low nitrogen content in POME was validated by previous literature where it was also reported that nitrogenous content in POME was significantly in low amount Chow (1991). The presence of the elements determined in this study Herald J. Microbiol. and Biotech. 008 revealed that POME is rich in nutrients; however, the amount of nutritional contents varies according to the location of palm oil plantation and milling factories Wu et al., (2009). The presence of these degrading microorganisms seemed to utilize the available nutrients during the fermentation process. The increase in pH during fermentation could be correlated with proteolytic activity which yields ammonia into the medium that causes increase in pH Parihar, (2012). This rise in pH also raises the possibility that POME may be used in the form of liming material. Shamshuddin et al., (1995) reported that when raw POME is discharged, the pH is acidic but seems to gradually increase to alkaline as biodegradation takes place as observed in this study. The reduction in the oil content of the sample was observed, this is likely due to the breakdown of oil content by microbial isolates Koshimizu et al., (1997). Reports show that, Ahmed et al., 2011 utilized the bacterium, Pseudomonas sp. in cocomposting process of Oil palm frond with Palm Oil mill anaerobic sludge. CONCLUSION The exploitation of the microorganisms isolated from palm oil mill effluent can be considered as potentials for biodegradation or bioremediation of palm oil spills. Once their remediation potential has been established on a large scale, it may serve as a cheap and efficient tool for purifying contaminated effluent water and soils. REFERENCES Ahmad A, Ismail S, Bhatia S (2003). Water Recycling from Palm Oil Mill Effluent POME) Using Membrane Technology. Desalination. 157: 8795. APHA. (1992). AWWA, WEF (1992) Standard method for the th Examination of water and waste water. 16 edn. America Public Health Asso. Washington. DC. Ainie K, Siew WL, Tan YA, Nor AI, Mohtar Y, Tang TS, Nuzil AI (2004). MPOB test method: A compendium of test on palm Oil products, palm kernel product, fatty acids, food related products and others. Published in 2005 by the Malaysian Palm Oil Board. Barnett HL, Hunter BB (1972). Illustrated genera of imperfecti fungi, (3rd ed.) Burgess Publishing Company, Minnesota, U.S.A. Barnett J, Payne R and Yarrow D (2000). Yeasts characteristics and rd identification. (3 edition). Cambridge University Press.Pp11-39. Bergey DH, Holt JG, Krieg NR, Sneath PHA (1994). Bergey's Manual of Determinative Bacteriology (9th edn.) Lippincott Williams and Wilkins. ISBN 0-683-00603-7. Cheesbrough M (2004). District Laboratory Practice in Tropical Countries. Low price Edition part 2. Cambridge press, England. Chow MC (1991). Palm oil mill effluent analysis. Palm Oil Research Institute of Malaysia. Kuala Lumpur, Malaysia. 11-18. Christopher K, Bruno E (2003). Identification of bacterial species in Tested studies for Laboratory teaching, (M. A. O’Donnell, Editor). Proceedings of the 24th Workshop/Conference of the Association for Biology Laboratory Education (ABLE). 24:103-130. Collins CH, Lyne PM, Grange GM (1989). Collins and Lyne th Microbiological Methods, 6 Ed. Butterworth, London Dhouib A, Ellouz M, Aloui F, Sayadi S (2006). Effect of bioaugmentation of activated sludge with white-rot fungi on olive mill wastewater detoxification. Lett in Applied Microbiol. 42: 405-411. Gowland P, Kernick O, Sundaron M (1987). TK. FEMS Microbiology Letters, 48(3): 339 -342. Hipolito CN, Crabbe E, Badillo CM, Zarrabal OC, Mora MA, Flores GB, Cortazar MH, Ishizaki AF (2008). Bioconversion of industrial wastewater from palm oil processing to butanol by Clostridium saccharoperbutylacetonicum N 1-4 (ATCC 13564). 16: 632-638. Holt JG, Kneg NR, Sneath PHA, Stanley JT, Williams ST (1994). Bergey’s Manual of Determinative Bacteriology. William and Wilkins Publisher, Maryland. New York. Jackson ML (1962). Soil Chemical Analysis. London, UK: Constable and Co. Kanokrat S, Suppasil M, Atipan S (2013). Isolation and characterization of biosurfactants-producing bacteria isolated from palm oil industry and evaluation for biosurfactants production using low-cost substrates. Journal of Biotechnology, Computational Biology and Bionanotechnology. 94(3):275- 284. Kittikun AH, Prasertsan P, Srisuwan G, Krause A (2000). Environmental Management for palm oil mill material flow analysisof integrated biosystems. Pp.11. Koshimizu S, Ohtake I, Yoshioka H, Saito Y, Taki H (1997). Development of kitchen wastewater treatment system using fats and oils degrading microorganisms. Kuuki-chouwa Eiseikougaku 71: 999– 1009. Lee D, Kim H, Lee K, Kim B, Hoe E, Lee H, Kim D, Pyun Y (2001). Enzyme and Microbial Technology, 29: 363-371. Lin S (1996). “Biosurfactants: Recent Reviews.” J. Chem.Tech. Biotechnol. 66:109-120. Logan TJ, Lindsay BJ, Goins LE, Ryan JA (1997). Field assessment of sludge metal bioavailability to crops: sludge rate response. Journal of Enviro. Quality. 26: 534-550. Markossian S, Becker P, Marc H, Antranikian G (2000). Extremophiles. Isolation and characterization of lipid-degrading Bacillus thermoleovorans IHI-91 from an ocelandic hot spring. Extremophiles. 4:365-371. Mohd RA, Abdul JAK, Mohd SK, Aidil AH, Mohd HMI (2013). Production of Surfactin from Bacillus subtilis ATCC 21332 by Using Treated Palm Oil Mill Effluent (POME) as Fermentation Media. International Conference on Food and Agricultural Sciences. 55(17): 1-7 Molla AH, Fakhru ‘l – Razi A, Abd- Aziz S, Hanafi MM, Roychoudhury PK, Alam MZ (2002). A potential resource for bioconversion of domestic waste water sludge. Biores. Tech. 85: 263 -272. Mukesh KDJ, Rejitha R, Devika S, Balakumaran MD, Rebecca AIN and Kalaichelvan PT (2012). Production, optimization and purification of lipase from Bacillus sp. MPTK 912 isolated from oil mill effluent. Advances in Applied Science Research, 3(2):930-938. Mulligan CN (2009).Recent advances in the environmental applications of biosurfactants. Curr. Opin. Colloid In. 14:372-378. Nitschke M, Coast SG (2007). Biosurfactants in food industry. Trends in food Sci. Technol., 18:252-259. Nwoko CO, Ogunyemi S (2010). Evaluation of Palm oil mill effluent to maize (Zea mays. L) crop: yields, tissue nutrient content and residual soil chemical properties. Australian Journal of Crop Science, 4 (1): 16 -22 Okechalu JN, Dashen MM, Lar PM, Okechalu B, Gushop T (2011). Microbiological quality and chemical characteristics of palm oil sold within Jos Metropolis,Plateau State, Nigeria. Journal of Microbiology and Biotechnology Research, 1(2): 107-112. Onilude AA, Wakil SM, Igbinadolor RO (2010). Effect of varying relative humidity on the rancidity of cashew (Anacardium occidentale L.) kernel oil by lipolytic organisms. African Journal of Biotechnology. 9 (31): 4890-4896. Panapanaan V, Helin T, Kujanpää M, Soukka R, Heinimö JJ, Linnanen L (2009). Sustainability of Palm Oil Production and Opportunities for Finnish Technology and Know-how Transfer. Pp13 - 61. Paredes C, Cegarra J, Bernal MP, Roig A (2005). Influence of olive mill waste water in composting and impact of the compost on a Swiss chard and soil properties. Environmental International. 31:305-312. and Technology.1 (3):135-143. Parihar DK (2012). Production of lipase utilizing linseed oilcake as Okogbenin et al. 009 fermentation substrate International Journal of Science, Environment Pepper IL, Gerba CP (2004). Environmental microbiology. A laboratory manual. Second edition. Elsevier academic press. Phaik EP, Wei-Jin Yong, Mei FC (2010). Palm oil Mill Effluent (POME) Characteristic in High Crop Season and the Applicability of High-Rate Anaerobic Bioreactors for the Treatment of POME, Ind. Eng. Chem.Res., 49, 11732-11740. Ros M, Hernandez MT, Garcia C (2003). Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil biology and biochemistry. 35:463-469. Rouse JD, Sabatini DA, Suflita JM, Harwell JH (1994). “Influence of Surfactants on Microbial Degradation of Organic Compounds.” Critical Reviews in Environmental Science and Technology. 24: 325370. Sinchaikul S, Sokkheo B, Phutrakul S, Pan F, Chen S (2001). “Optimization of a thermostable lipase from Bacillus Stearothermophilus P1: Overexpression, purification andcharacterization,” Protein Expression and Purification. 22:388398. Shamshuddin J, Ng GG, Ruzaini ZA (1995). Ameliorating Effects of Palm Oil Mill Effluent on the Chemical Properties of Soils and Maize Okogbenin OB, Anisiobi GE, Okogbenin EA, Okunwaye T, Ojieabu. A. (2014). Microbiological assessment and physiochemical parameters of palm oil mill effluent collected in a local mill in Ovia North East area of Edo State, Nigeria. Herald J. Microbiol and Biotech Vol. 1 (1): 001 - 009 . Wu TY, Mohammad AW, Jahim JM, Anuar N (2009). A holistic approach to managing palm oil mill effluent (POME): Biotechnological Growth in Pots. Pertanika J. Trop. Agric. Sci. 18(2): 125-133. advances in the sustainable reuse of POME. Biotechnol. Adv. 27 (1): 40-52. Tagoe SMA, Dickinson MJ, Apetorgbor MM (2012). International Food Research Journal. 9 (1): 271 – 278. Thirukkumaran, CM, Parkison, D (2000). Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodge pole pine forest floor amended with nitrogen and phosphorus fertilizer. Soil biology and Biochemistry.32:59-66. Volkering F, Breure AM, Rulkens WH (1998). “Microbiological Aspects of Surfactant Use for Biological Soil Remediation.” Biodegradation. 8: 401-417. Wakil SM, Adelabu AB, Fasiku SA, Onilude AA (2013). Production of Bioethanol from Palm Oil Mill Effluent using Starter Cultures. New York Science Journal. 6(3) :77-85. Willumsen PA, Karlson U (1997). “Screening of Bacteria, Isolated from PAH-Contaminated Soils, for Production of Biosurfactants and Bioemulsifiers.” Biodegradation, 7: 415-423. Yeomans J, Bremmer JM (1989). A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 19: 1467–1476