Hali Steinmann (ENSTU 444, Advisor: Kirk Zigler)
Transcription
Hali Steinmann (ENSTU 444, Advisor: Kirk Zigler)
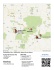
Biological Survey of Coon’s Labyrinth Cave, a Pennington Forma<on Cave on the University Domain Hali Steinmann (ENSTU 444, Advisor: Kirk Zigler) Introduc<on Ceuthophilus cave cricket undergoing a molt in CLC (2/20/15 Mean (n=2) Number of Species per Transect Despite the level and complexity of research in a handful of well-‐studied caves, there remain major knowledge gaps in the overall biodiversity of karsted regions and individual karst systems. Even in Mammoth Cave, Kentucky, the best-‐studied cave system in North America, there is reason to believe that species are yet to be discovered and described (Culver & Pipan 2009). In an effort to quan<fy and be_er understand the fauna of a Pennington Forma<on cave on the Domain of the University of the South in Sewanee, Tennessee, we systema<cally surveyed four selected cave passages in February and April for two consecu<ve years (2014-‐2015) and produced a map showing the cave’s morphology, including hydrologic sources, springs, and areas of biologic interest. Caves present a challenge for researchers interested in quan<fying biodiversity. Cave fauna are mostly small and inconspicuous, occupying habitats that are difficult and some<mes impossible for humans to access. Addi<onally, the sensi<vity of cave obligate organisms to disturbance of physical habitat, for example, increased sediment content in streams due to foot traffic, raises concern for the conserva<on of cave fauna during sampling a_empts. Repeated visita<on to Coon’s Labyrinth Cave and the slow pace of travel through the cave during the 2015 tape-‐and-‐compass survey ensured that all the accessible passage in the cave (~730 meters) was observed with care. This resulted in a number of exci<ng biological observa<ons that would have otherwise been missed by mul<ple <med surveys of short (10 meters) sec<ons of passage. We determined a high poten<al for future study in this cave, with special regard to: a) recurrence interval and community consequences of mammalian guano subsidies, b) popula<on dynamics and ecology of the beetle genus Ptomaphagus (Order Coleoptera), and c) assessment of nutrient and sediment cycling dynamics in a sandstone-‐capped, Pennington Forma<on cave. Table 1. Fauna (scien<fic name, common name, and ecological status – troglobiont (TB), troglophile (TP) and trogloxene (TX)) found during the 2014-‐2015 biological surveys. Methods Coon’s Labyrinth Cave was chosen for this study because of its unique geologic posi<on, in the ecotone between upland and cove habitats, and because of the lack of biological data for caves on the University Domain, especially in this geologic unit. The cave entrance is around 1800’ eleva<on, in the Upper Pennington Forma<on where Mississippian-‐aged limestone directly underlies Pennsylvanian-‐aged Warren Point sandstone (the Raccoon Mountain Forma<on is absent in this loca<on). The cave was visited a total of eleven <mes, twice in spring of 2014 and nine <mes in spring of 2015. Biological surveying transects were established in February 2014 in four 10-‐meter stretches of cave passage, designated as “entrance terrestrial,” “entrance stream,” “deeper terrestrial,” and “deeper stream.” All surveyed passages were in the dark zone of the cave and ranged from 1 to 2 meters high and from 1 to 2.5 meters wide. Biological surveys occurred on the following dates: 2/19/14, 4/9/14, 2/9/15, and 4/13/15. Two to five researchers (depending on year/ availability) conducted visual surveys ten to twelve minutes in length, working together to find and iden<fy fauna (see “Special Thanks”). In each passage, all accessible rock, sediment, and organic debris on the cave floor, walls, and ceiling was searched and fauna were reported as a tally. Species were iden<fied using Niemiller et al.’s Cave Life of TAG (2013) and the World Wide Web. Very few specimens were removed from the cave for the purpose of iden<fica<on. The 2015 general cave survey was conducted with a one hundred meter tape, compass, inclinometer, protractor, pencil, and paper (see “Special Thanks”). Nancy Lilly (C’2015) drew the map and cross-‐ sec<onal views for 2415 feet (736 m) of passage. Entrance Terrestrial February Entrance Stream Deeper Terrestrial Deeper Stream April 0 5 10 15 Figure 1. Mean (n=2) number of species found in each of four transects in Coon’s Labyrinth Cave in February and April of 2014-‐2015. Stream passage in CLC showing maximum ver<cal and horizontal extent In total, thirty species were observed within Coon’s Labyrinth Cave (Table 1). Ten species were considered to be troglobionts (cave-‐obligate residents), while the remaining twenty were considered troglophiles or trogloxenes (faculta<ve or accidental cave residents). Tree roots penetra<ng the cave ceiling could not be iden<fied, but were noted for their use as mol<ng sites by cave crickets (observed 2/20/15 and 4/13/15). On average, the greatest transect species diversity over both months was found in the terrestrial transect nearest the cave entrance (Figure 2). The entrance terrestrial transect had an even 13 to 14 species throughout February and April, while the deeper terrestrial transect underwent a shih from 10.5 species on average in February to 8.5 in April. More data is needed to determine whether this reflects seasonal movement of fauna throughout the cave. Organic carbon enters Coon’s Labyrinth Cave via percola<ng water (ceiling drips), flowing water (the cave serves as a spring for water trapped in the above sandstone layer), and from energy subsidies brought in from aboveground by roots, crickets, bats, mammals, and other animals. Guano is by far the most easily observable source of energy in caves, and harbored the vast majority of pseudoscorpions (H. mirabilis), beetles (Ptomaphagus and Pseudanophthalmus), and millipedes (Tetracion and Pseudotremia) observed in the cave. Weekly visita<on to the cave in 2015 allowed us to observe the progression of guano decomposi<on, from fresh guano with a diverse community of arthropods and fungi to highly decomposed guano with only mold colonies remaining. Using evidence in the form of tracks and scat piles, we determined that at least one raccoon (Procyon lotor) uses the cave frequently, especially in the winter when the cave serves as a warm retreat from harsh winter condi<ons. Li_le is known about the ecological consequences of frequent mammalian guano subsidies to resource-‐poor cave environments, but the subject warrants further inves<ga<on if a complete picture of nutrient dynamics in caves is to be had. Discussion For those concerned with the conserva<on of subterranean life, the establishment of rou<ne biological surveys in representa<ve caves should be a major priority. Ten thousand or more caves have been documented in the state of Tennessee, yet only a small frac<on of these have been sampled biologically. Cave fauna are threatened by direct human impacts, such as introduc<on to or removal of species from the cave, as well as indirect impacts from surficial ac<vi<es like mining, pollu<on, and habitat fragmenta<on. In order for land managers and policy-‐makers to protect sensi<ve cave ecosystems, we must have a complete picture of which species occupy caves, when, and in what ways scarce resources are u<lized by cave inhabitants. The results of this survey suggest that mul<ple cave visits are necessary to acquire a complete list of fauna for one cave, meaning there is much work to be done if a representa<ve number of Tennessee caves are to be thoroughly surveyed. Addi<onally, researchers must give equal a_en<on to faculta<ve and obligate/endemic cave fauna, since sources of energy necessary for the la_er are derived from the surface and are transported by troglophiles or trogloxenes. In Coon’s Labyrinth Cave, mammalian guano serves as the primary energy source for a diverse community of decomposers, several of which never leave the cave. Literature Cited Culver, D.C., & Pipan, T. 2009. The Biology of cave and other subterranean habitats. Oxford University Press. Niemiller, M.L., Zigler, K.S., & Fenolio, D.B. 2013. Cave Life of TAG: a guide to commonly encountered species in Tennessee, Alabama and Georgia. The Biology Sec<on of the Na<onal Speleological Society, Huntsville, Alabama, USA. 45 pp. Special Thanks to Nancy Lilly, Kelly Smallwood, Jason Hardy, Kris<ne Medlen, Anne Grindle, Lorna Harkey, and Peter Davis Results & Observa<ons Roots on cave floor (March 2015) Bishopella laciniosa (Bishop’s Harvestman) 3/30/15 Raccoon scat with white fungus (February 2015)