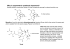Chiral Spectrometer Product Information
Transcription
Chiral Spectrometer Product Information
FT-‐MRR Spectrometers for Chiral Analysis BrightSpec is developing instruments based on Fourier transform molecular rota8onal resonance (FT-‐MRR) spectroscopy with full capabili8es for chiral analysis: • Quan'ta've determina'on of diastereomer composi'on for a molecule with mul'ple chiral centers. ©2014, BrightSpec, Inc. Differen'a'on of Diastereomers without Chromatography • Determina'on of the absolute configura'on of chiral molecules. • Quan'ta've measurement of the enan'omeric excess for a mixture of le? and right handed forms of a single, chiral diastereomer. The technique does not require chromatography to separate diastereomers and enan8omers and it does not require chiral deriva8zing agents to dis8nguish the enan8omers. Chiral analysis of molecules of interest can be performed directly on complex sample mixtures. The technique can provide real-‐'me monitoring of diastereomer composi8on and enan8omeric excess. BrightSpec FT-‐MRR spectrometers for chiral analysis are enabled by two recent technology advances in the field of molecular rota8onal spectroscopy.[1] Broadband Fourier transform rota8onal spectroscopy has been extended to low frequency opera8on so that large molecules can be studied using pulsed jet sample methods.[2,3] This advance has made it possible to determine the structure of difficult chemical systems, like water clusters [4] and molecular complexes [5]. The high spectral resolu8on of the instrument coupled with the extreme sensi8vity of the spectral paSern to small changes in the three dimensional geometry make it possible to resolve diastereomers of molecules with mul8ple chiral centers without prior separa8on. The molecular rota-onal spectrum is highly sensi-ve to the three dimensional geometry of the molecule. The simulated molecular rota-onal spectra of two artemisinin diastereomers that have been iden-fied in the natural product are shown. These two structures differ in the stereochemistry at a single asymmetric carbon (the structures are epimers). As the expanded scale view on the right makes clear, there is essen-ally no spectral overlap in the rota-onal spectra so that the diastereomers can be readily iden-fied in the FT-‐MRR spectrum. Early Access Program Analy-cal services and on-‐site spectrometers Contact BrightSpec BrightSpec is ac'vely seeking customers with challenging problems in chiral analysis who are interested in working with us to develop this new measurement technology. Please contact jus'[email protected] The New Physics of Absolute Configura'on Determina'on by FT-‐MRR Spectroscopy Analysis of the chirality, or handedness, of the dis8nct diastereomers is possible through a revolu8onary measurement technique first reported in Nature in 2013.[6] This work demonstrated that a chiral spectral signature can be measured using FT-‐MRR spectroscopy when a three-‐wave mixing scheme is employed. The key insight is that enan8omers differ in the way that the molecular dipole moment projects onto the three principal axes of molecular rota8on and that the three wave mixing method creates a signal that reflects this physical difference. The handedness of the molecule is obtained from the phase of the molecular free induc8on decay and enan8omeric excess is measured by the amplitude of the chiral signal.[7] Because the measurement uses three precisely defined resonance frequencies for the molecule, there is prac8cally no chance to record an interfering signal even in a complex sample matrix. The measurement uses the fact that enan-omers can be dis-nguished by the proper-es of the molecular dipole moment. The product of the projec-ons of the dipole moment on the three principal axes for molecular rota-on (denoted a, b, and c) changes signs for the enan-omer pair. This behavior is illustrated for solketal (C6H12O3).[8] Three Wave Mixing Measurements Determine the Absolute Configura'on Three wave mixing experiments use two, resonant excita-on pulses to generate a coherent chiral signal that is detected by FT-‐MRR spectroscopy. The chiral signal is propor-onal to the product of the dipole moment components and, therefore, has opposite sign (or phase) for the two enan-omers. The chiral signals for pure R-‐ and S-‐solketal are shown in the middle panel to illustrate this effect.[8] More generally, the amplitude of the chiral FT-‐MRR signal measures the enan-omeric excess (ee) providing way to monitor ee in real -me. Literature References (1) B.H. Pate, “Taking the Pulse of Molecular Rota8onal Spectroscopy”, Perspec8ves, Science 333, 947-‐948 (2011). (2) Cristóbal Pérez, Simon Lobsiger, Nathan A. Seifert, Daniel P. Zaleski, Berhane Temelso, George C. Shields, Zbigniew Kisiel, and Brooks H. Pate, “Broadband Fourier Transform Rota8onal Spectroscopy for Structure Determina8on: The Water Heptamer (Fron8ers Ar8cle)”, Chem. Phys. LeJ. 571, 1-‐15 (2013). (3) Steven T. Shipman, Jus8n L. Neill, Richard D. Suenram, MaS T. Muckle, Brooks H. Pate, “Structure Determina8on of Strawberry Aldehyde by Broadband Microwave Spectroscopy: Conforma8onal Stabiliza8on by Dispersive Interac8ons”, J. Phys. Chem. LeJ. 2, 443-‐448 (2011). (4) Cristóbal Pérez, MaS T. Muckle, Daniel P. Zaleski, Nathan A. Seifert, Berhane Temelso, George C. Shields, Zbigniew Kisiel, and Brooks H. Pate, “Structures of Cage, Prism, and Book Isomers of Water Hexamer from Broadband Rota8onal Spectroscopy”, Science 336, 897-‐901 (2012). (5) Seifert, N. A.; Zaleski, D. P.; Pérez, C.; Neill, J. L.; Pate, B. H.; Vallejo-‐ López, M.; Lesarri, A.; Cocinero, E. J.; Castaño, F.; Kleiner, I., “Probing the C-‐H·∙·∙·∙pi Weak Hydrogen Bond in Anesthe8c Binding: The Sevoflurane– Benzene Cluster”, Angew. Chem. 2014, 126, 3274. (6) PaSerson, D.; Schnell, M.; Doyle, J. M. Enan8omer-‐Specific Detec8on of Chiral Molecules via Microwave Spectroscopy. Nature 2013, 497, 475– 477. (7) PaSerson, D.; Doyle, J. M. Sensi8ve Chiral Analysis via Microwave Three-‐Wave Mixing. Phys. Rev. LeJ. 2013, 111, 023008. (8) Simon Lobsiger, Cristobal Perez, Luca Evangelis8, Kevin K. Lehmann, Brooks H. Pate, “Molecular Structure and Chirality Detec8on by Microwave Spectroscopy”, J. Phys. Chem. LeJ. (submiSed).