01 - Mongal - Human Subjects Approvall
Transcription
01 - Mongal - Human Subjects Approvall
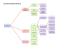
Training Workshop on Household Air Pollution and Monitoring March 21-25, 2016 Hotel Holiday Home Paro, Bhutan Human Subjects Approval Mongal Singh Gurung Member Secretary Research Ethics Board of Health Ministry of Health Content Contributions from: Lee See Muah, Ruth Macklin with other SERG members, Sheryl Vanderpoel, Dr Lungten Z Wangchuk and Dr Pakila Drukpa PresentationOutline Need for Ethical Review Basic Ethical Principles Research Ethics Board of Health (REBH) WHYISETHICALREVIEWOF RESEARCHNEEDED? THETUSKEGEEEXPERIMENT Ò From 1932-1972, about 400 African American participants diagnosed with syphilis were medically followed as part of a US Public Health Service research study. The participants were provided: É free medical examinations É free transport to the clinic É free hot meals on examination days É free treatment for minor ailments É free burial What’s the catch? TheTuskegeeExperiment Ò Ò Ò Ò Palliative treatments were provided initially because no cure was available. Only after penicillin became available was there a possibility of real treatment. Yet, once available, although in the middle of the study, penicillin was still not provided to the participants! The experiment was established and conducted in order to study the natural medical history of syphilis. Important knowledge was obtained and published in reputable journals, one such article was titled: “Tuskegee Study of Untreated Syphilis in the Negro Male” DoingBadintheNameofGood v v Not until 1997, did the US government offer an official apology. It was President Clinton who formally apologized to the surviving participants and their descendants. Earlier a Commission had been established to study the episode, and recommended monetary compensation to the men who were still alive, and to the families of those who had died. THENAZIEXPERIMENTS High Altitude Experiments March 1942 – August 1942 Experiments conducted at a concentration camp for the benefit of the German Air Force Experimental subjects were placed in the low-pressure chamber and thereafter the simulated altitude therein was raised Many victims died Freezing Experiments August 1942 – May 1943 Experiments conducted at a concentration camp for the benefit of the German Air Force Experimental subjects were forced to remain in a tank of ice water for periods up to 3 hours Many victims died Sea Water Experiments July 1944 – September 1944 Experiments conducted at a concentration camp for the benefit of the German Air Force and Navy Experimental subjects were deprived of all food and given only chemically processed sea water The experiments caused great pain and suffering and resulted in serious bodily injury to the victims WhatHappenedtotheNazis? • Some escaped to other countries including the United States. Many fled to Brazil. • 22 Nazi officers were arrested and tried at the Nuremburg Trials. 12 were sentenced to death by hanging The Nuremberg Code “It took the cruelty described at Nuremberg to make the world realize it had to do something that would protect human subjects from inhumane research.” John Bryant (2000), President, CIOMS AtRavensbruck • Women were shot or slashed on the legs. • The wounds stuffed with glass, dirt, and bacteria cultures and sewn shut • Then treated with experimental antiinfective agents. UNIT731*DURINGTHESECONDSINO-JAPANESEWAR ANDWORLDWARII Ò Ò "Unit 731 was a covert biological and chemical warfare research and development unit of the Imperial Japanese Army that undertook lethal human experimentation from 1937 until 1945. It was responsible for some of the most notorious war crimes carried out by Japanese personnel." UNIT731*DURINGTHESECONDSINO-JAPANESEWARAND WORLDWARII Ò ü ü ü ü ü Vivisection Vivisection without anesthesia, after infecting them with various diseases. limbs amputated to study blood loss. Removed limbs were sometimes re-attached to the opposite sides. Some prisoners' limbs were frozen and amputated Limbs frozen then thawed to study the effects of the resultant untreated gangrene and rotting. Stomachs surgically removed and the esophagus reattached to the intestines. Parts of the brain, lungs, liver, etc. were removed from some prisoners”* *Last modified on 18 March 2014 from https://www.mtholyoke.edu/~kann20c/classweb/dw2/page1.html Vivisectionprocedurewhilepatientisalive, performedbyShiro Ishiihimself UNIT731*DURINGTHESECONDSINO-JAPANESEWARAND WORLDWARII Ò ü ü ü Germ warfare attacks “Prisoners injected with disease, disguised as vaccinations, to study their effects. To study the effects of untreated venereal diseases, male and female prisoners were deliberately infected, often by rape, with syphilis and gonorrhea, then studied. Plague fleas, infected clothing, and infected supplies encased in bombs were dropped on various targets. The resulting cholera, anthrax, and plague were estimated to have killed around and possibly more than 400,000 Chinese civilians”* *Last modified on 18 March 2014 from https://www.mtholyoke.edu/~kann20c/classweb/dw2/page1.html "Because of their brutality, Unit 731's actions have since been declared by the United Nations to have been crimes against humanity." *Last modified on 28 April 2010 THETHALIDOMIDESTORY Ò Thalidomide was first synthesized in 1954 Ò Thalidomide was used as a sedative for symptoms of morning sickness: Ò Thalidomide was marketed in Europe in 1957, however never approved by the US federal authorities É “drug of choice for pregnant women” É “completely safe’” WHATWENTWRONG? Thousands of babies were born with multiple deformities… The drug had been marketed without completing tests in participants, never including pregnant women in initial pilot studies and without completing appropriate tests in animal studies. 20th CenturyResearchEthicsMilestones ICH GCP Common Rule 1991 CIOMS 1982 Consolidated HHS/FDA Regulations Belmont Report Declaration of Helsinki 1964 1981 1979 1972 Syphilis Study Exposed 1966 The Beecher Article (NEJM) Nuremberg Code 1947 The Thalomide Tragedy US Human Radiation Experiments The Nazi Experiments 1932 The Syphilis Study Begins Trigger Events Milgram Study Kefauver-Harris Amendments Food, Drug and Cosmetic Act 1962 Do we really need to do research on humans? Do we really need to do research on humans? Ò Is our knowledge of the human body complete ? Ò Is our understanding of human disease comprehensive ? Ò Is medical treatment perfect ? Ò Is disease prevention absolute ? Yes,asresearchis designedto… …lead to generalizable new knowledge which will increase our understanding of ourselves, our relationship with others as well as with the natural world. Researchisdesignedto: v enhance the quality of life (including being free of disease) v alleviate suffering caused by disease and disability Hasthependulumswungtoofar? People are now demanding the right to be a research participant. v HIV has changed the face of research ethics and participation in research studies v Hasthependulumswungtoofar? Ò Ò From burden to benefit From sacrifice to promise “I believe the current tendency to see research as largely beneficial and benign is just as erroneous as the earlier tendency to view it primarily dangerous and exploitative” Robert J Levine, Keynote address, The XXVIth CIOMS Conference, February 1992 The Ethical Dilemma..…… Knowledge Risks Complexity in the Guidance of Research Conduct: Deconstructing that complexity with the Belmont Principles A. Respect for persons – Informed Consent , Access to information – Privacy of participants, protection of confidential information B. Beneficence C. Justice A. Respect for person Information: Moral implications in research Information revealed may be extremely disturbing for the participant (e.g., minors, violence) Research may include vulnerable groups, adolescents, trafficked women Information about sexual matters are deeply personal and private Information received may be deeply distressing for the research worker Respect for the person: Maintaining Confidentiality: Legal implications Sensitive information about collateral illegal activities, human trafficking, drug use may be obtained Will the information gathered harm the participants or/and researchers? Statutory duty to inform the authorities Court order to compel disclosure B. Beneficence: Harm and Benefits Does the research help participants? Can the research be used against participants? How is the research beneficial? – Can the identified gaps be addressed? Paragraph 5, Helsinki Declaration: …wellbeing of the participants should take precedence over the interests of science and society. Beneficence: not just to participants but to groups (communities, schools, workplace, hospitals…) Harm from Stigmatization: A stigma is a mark of shame or discredit Double stigmatizations: Association of AIDS with homosexuality Association of AIDS and/or STIs with sex workers Association of a husband with sexual violence if wife is interviewed on the subject Association of risky behaviour of teens to a particular school or community Association of limitations of employees to a hospital Researchers must evaluate whether or not their research could result in such group harms and, if this is a possibility, minimize this risk C. Justice • People should be treated fairly. Selection of research participants, must be unbiased and fair. Examples of injustices: • Potentially risky research for undesirable vulnerable groups • Potentially beneficial research for favoured groups The prospect of gaining new scientific knowledge need not and should not be pursued at the expense of human rights and dignity. གསོ་བའི་ ཞིབ་འཚ*ལ་ ,ན་.ོད་0མ་གཞག་ བཀོད་ཚ*གས།། Research Ethics Board of Health (REBH) HISTORY • 1960s ...Department of Health Services • • two basic hospitals [31 hospitals (including 1 indigenous hospital)] 11 dispensaries [235 Basic Health Units and Sub-posts, 52 indigenous units, and 562 Outreach clinics] • • • • 1984… Health Information Unit 1995 …Health Research and Epidemiology Unit 1999 …Bhutan HMIS 2001…Health Research Technical and Ethical Committee • 2009 … REBH (Officially – January 26, 2009) ORGANOGRAM Minister BMHC InternalAudit Secretary ResearchEthicsBoardof Health(REBH) BhutanHealthTrustFund AFD ICT QASD PPD DoPH HealthResearch& EpidemiologyUnit (secretariattoREBH) DMS March 24, 2016 39 AffiliationofREBH 1. 2. SIDCER/FERCAP • NAREC FWA(OHRP) ProtocolManagementinREBH March 24, 2016 41 StandardOperatingProcedures(SOPs)of REBH 28 Sops 5th Revision Available online TypesofReview Full Board Review Expedited Review Emergency Board Meeting March 24, 2016 43 MeetingSchedules • Board meetings • February, April, June, August, October and December (6/year) • Protocol assessment time – one month • Expedited reviews – two weeks • Emergency meetings (as and when) Listofdocumentsrequiredtobesubmitted forinitialreview Application Form for Initial Review (It can be downloaded from http://www.health.gov.bt/357-2/ Also, it’ll be provided on request) Research protocol/proposal including the itemized budget and research project schedule or timeline (final version) – Please write dated version number on it Research/study tools (e.g. final version of questionnaire, forms, guides. etc.) – Please write dated version number on it Listofdocumentsrequiredtobesubmitted forinitialreview…continued Curriculum Vitae of all investigator(s). Informed Consent Form and Information Sheet both in Dzongkha and English languages – Please write dated version number on it. For minor subjects (less than 18 years): Informed Assent Form and Information Sheet both in Dzongkha and English languages, and Informed Consent Form and Information Sheet both in Dzongkha and English languages for their parents/legal guardians What is the role of IRB/EC? THANKYOU