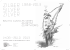van Geel workbook 2012
Transcription
van Geel workbook 2012
Chemistry
Workbook
2012
T. van Geel
Wellesley High School
1
Chapter 1: Classifying Matter 1
Match the descriptions to the substances below.
____
____
____
____
____
____
____
____
____
1.
2.
3.
4.
5.
6.
7.
8.
9.
An atomic element
A molecular element
A compound
A homogenous mixture of a compound and an atomic element
A homogenous mixture of a compound and a molecular element
A homogenous mixture of two compounds
A heterogeneous mixture of two compounds
A heterogeneous mixture of a compound and a molecular element
A heterogeneous mixture of an atomic element and a molecular element
A.
B.
C.
D.
E.
F.
G.
H.
I.
© 2012 Thomas van Geel
2
Chapter 1: Classifying Matter 2
Fill in the following chart. The first problem has been done for you.
Chemical formulas of
particles
Classification
Sketch all particles in
proper arrangement
(draw 6 particles)
1.
H
H
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
H
CH4
C
H
H
molecular
H
H
C
H
C
H
H
H
C
C
H
H
H
H
H
H
H
H
H
H
H
C
H
H
2.
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
O2
molecular
3.
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
CO2, N2
molecular
4.
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
H2 , C
molecular
5.
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
H2, O3
molecular
6.
matter
pure substance
element
atomic
compound
mixture
homogeneous
heterogeneous
N2O2
molecular
© 2012 Thomas van Geel
3
7.
H
H
H
H
N
matter
pure substance
element
atomic
H
H
homogeneous
H
N
H
H
N
mixture
compound
N
H
H
H
H
N
H
H
heterogeneous
H
H
N
H
H
H
N
molecular
H
H
H
N
H
N
H
H
H
8.
H
H
H
N
H
H
matter
pure substance
element
atomic
H
H
H
N
H
O
mixture
compound
H
N
O
H
homogeneous
H
H
heterogeneous
H
H
N
H
H
H
H
H
H
O
H
molecular
O
O
H
N
H
H
H
H
N
H
9.
H
H
H
H
H
H
H
H
H
element
10.
atomic
H
H
H
O
H
O
H
homogeneous
H
O
H
O
H
O
H
H
H
H
heterogeneous
C
pure substance
homogeneous
C
heterogeneous
C
C
C
C
mixture
compound
C
C
matter
element
H
H
H
H
O
molecular
atomic
H
H
H
mixture
compound
H
H
O
O
matter
pure substance
H
H
H
H
H
H
H
H
C
C
C
C
C
C
C
C
C
molecular
C
C
C
11.
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
HCN
molecular
12.
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
O3
molecular
13.
matter
pure substance
element
atomic
compound
mixture
homogeneous
heterogeneous
C2H4, O2
molecular
© 2012 Thomas van Geel
4
14.
O
O
C
O
matter
pure substance
element
atomic
C
O
homogeneous
C
O
C
O
O
O
O
O
C
O
C
C
heterogeneous
O
O
C
O
mixture
compound
C
O
O
O
C
O
O
O
O
molecular
C
15.
O
O
C
O
C
O
O
N
N
N
N
N
O
N
O
matter
pure substance
element
atomic
N
mixture
compound
N
N
O
N
N
N
homogeneous
N
N
N
N
O
N
N
N
heterogeneous
N
N
N
N
N
O
O
molecular
N
N
O
N
N
N
N
16.
He
matter
pure substance
element
atomic
homogeneous
He
He
He
mixture
compound
He
He
He
heterogeneous
He
He
He
He
He
molecular
He
He
He
17.
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
H2O2
molecular
18.
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
H2, O2
molecular
19.
H
H
O
H
O
H
H
pure substance
element
atomic
H
mixture
compound
H
H
C
matter
homogeneous
H
H H
H
O
H
C
H
H
H H
H
H
H
H
O
H
H
O
C
H
C
H
H
H
H
H
H
H
H
C
H
H
H
H
H
O
C
H
H
H
H
molecular
H
H
C
O
heterogeneous
O
H
H
O
H
H
O
O
H
H
H
20.
matter
pure substance
element
atomic
compound
mixture
homogeneous
heterogeneous
H2, O2, N2
molecular
© 2012 Thomas van Geel
5
Chapter 1: Chemical Formulas
Write the chemical formulas for the following molecules.
1. _______________
2. _______________
H
O
N
C
C
H
C
H
C
O
H
H
N
H
C
C
H
O
C
C
H
H
H
N
H
H
H
H
O
C
O
H
3. _______________
H
4. _______________
H
H
H
H
O
H
H
H
H
O
H
C
C
C
C
C
N
O
H
O
C
N
C
C
C
H
N
C
H
C
O
O
O
H
O
H
H
H
C
H
H
H
H
O
H
5. _______________
H
H
H
H
H
O
O
C
H
C
C
H
H
C
H
C
H
C
O
H
H
H
O
C
C
C
H
H
H
H
C
O
H
H
H
© 2012 Thomas van Geel
6
Chapter 1: Organic Chemical Formulas
Write the chemical formulas for the following molecules.
1. _______________
2. _______________
3. _______________
4. _______________
5. _______________
O
H2 N
OH
N
H
O
6. _______________
NH2
N
N
HN
N
© 2012 Thomas van Geel
7
Chapter 1: Chemical and Physical Changes
Classify each of the changes shown as chemical or physical. If the change is a
phase change, name the phase change.
1.
2.
___________________________
3.
___________________________
4.
___________________________
5.
___________________________
6.
___________________________
7.
___________________________
8.
___________________________
9.
___________________________
10.
___________________________
___________________________
© 2012 Thomas van Geel
8
Chapter 1: Chemical and Physical Properties
Classify the following as physical or chemical properties.
____________ 1.
When 17 g of ammonia (NH3) are formed from its individual
elements, 46.1 kJ of heat are released.
____________ 2.
Diamond has a hardness of 10 on the Mohs scale.
____________ 3.
When heated, limestone (CaCO3) decomposes into quicklime
(CaO) and carbon dioxide (CO2).
____________ 4.
Silicon is a mediocre conductor of electricity.
____________ 5.
The density of lead is 11.34 g/cm3.
____________ 6.
Pure sodium reacts vigorously with water.
____________ 7.
Oxygen freezes at -219°C.
____________ 8.
Molasses flows very slowly.
____________ 9.
Magnesium combines with chlorine in a 1:2 ratio.
____________ 10. Nitroglycerine will detonate when it receives a physical
shock.
© 2012 Thomas van Geel
9
Chapter 2: Taking Measurements
Measure the length of the blacks bars, using the proper number of digits.
1. __________ cm
1 cm
2 cm
3 cm
4 cm
2 cm
3 cm
4 cm
2 cm
3 cm
4 cm
20 cm
30 cm
40 cm
20 cm
30 cm
40 cm
2. __________ cm
1 cm
3. __________ cm
1 cm
4. __________ cm
10 cm
5. __________ cm
10 cm
© 2012 Thomas van Geel
10
Chapter 2: Taking Measurements 2
Measure the volumes, using the proper number of digits.
1. __________ mL
2. __________ mL
3. __________ mL
2 mL
2 mL
20 mL
1 mL
1 mL
10 mL
4. __________ mL
5. __________ mL
6. __________ mL
2 mL
2 mL
20 mL
1 mL
1 mL
10 mL
© 2012 Thomas van Geel
11
Chapter 2: Counting Sig Figs
Determine the number of sig figs in the following numbers.
1. 0.005687
_____ sig figs
2. 124
_____ sig figs
3. 54.802
_____ sig figs
4. 5030.00
_____ sig figs
5. 2.30 x 104
_____ sig figs
6. 300.
_____ sig figs
7. 0.00020500
_____ sig figs
8. 4100
_____ sig figs
9. 202.2
_____ sig figs
10. 24.5620
_____ sig figs
© 2012 Thomas van Geel
12
Chapter 2: Calculations with Sig Figs
Perform the following calculations using the correct number of sig figs.
1. 250. + 750. =
2. 250 + 750. =
3. 250 + 750 =
4. 250. + 753 =
5. 250 + 753 =
6. 250. + 758 =
7. 250 + 758 =
8. 84100. – 4100. =
9. 84100 – 4100. =
10. 84100 – 4100 =
11. 8.410 × 104 – 4100. =
12. 8.410 × 104 – 4100 =
13. 50 × 40 =
14. 50. × 40. =
15. 50.0 × 40.0 =
16. 50.00 × 40.00 =
17. 909 × 11 =
18. 909 × 11.0 =
19. 96000 / 1000 =
20. 6699 / 33 =
© 2012 Thomas van Geel
13
Chapter 2: Scientific Notation
1. Express each of the following numbers in scientific notation:
a. 6,700.0 = ______________________
b. 0.000,000,000,015,05 = ______________________
c. 805,000. = ______________________
d. 0.00040400 = ______________________
2. Express each of the following numbers in ordinary notation:
a. 8.2 x 108 = ______________________
b. 1.50 x 10-6 = ______________________
c. 1.775 x 10-7 = ______________________
d. 7.060 x 103 = ______________________
© 2012 Thomas van Geel
14
Chapter 2: Precision and Accuracy
Four students measure the length of the same bullfrog multiple times. The
actual length of the frog is 10.24 cm long. The students’ results are shown
below.
Student A
10.26
10.30
10.21
10.39
Student B
11.05
9.56
10.01
10.35
Student C
12.03
12.06
12.01
12.05
Student D
12.66
8.65
10.24
11.09
1. Calculate the average for each student.
A: ________
B: ________
C: ________
D: ________
2. Using the averages, calculate the percent error for each student.
A: ________
B: ________
C: ________
D: ________
3. Calculate the average deviation for each student.
A: ________
B: ________
C: ________
D: ________
4. Rank the students from best to worst in accuracy:
____ ____ ____ ____
5. Rank the students from best to worst in precision:
____ ____ ____ ____
© 2012 Thomas van Geel
15
Chapter 2: Metric-to-Metric Conversions
Convert the following. Use the proper number of sig figs.
1. 0.22 g = __________ mg
2. 14.3 dm = _________ cm
3. 202 mg = _________ kg
4. 0.225 kg = ________ cg
5. 58 hg = _________ cg
6. 5.65 m = _________ mm
7. 0.375 ml = ________ L
8. 9.0 hm = __________ cm
9. 0.095 mm = ________ m
10. 124 mm = _________ km
© 2012 Thomas van Geel
16
Chapter 2: Length Conversions
Use the proper number of sig figs to solve the following problems.
1 league
1 mile
1 foot
1 hand
1 furlong
1 rod
1 yard
=
=
=
=
=
=
=
3 miles
5280 feet
12 inches
4 inches
40 rods
5.5 yards
3 feet
1. How many feet are in 2.0 leagues?
2. 6,000. feet is how many furlongs?
3. 5.68 leagues is how many furlongs?
Because we Americans don’t use the metric system, getting a feel for meters
takes a little time. One inch is 2.54 cm. Do the following conversions:
4. 1 m = ______________ feet
5. 1 km = ______________ miles
6. 1 mm = ______________ inches
© 2012 Thomas van Geel
17
For the following lengths, what is the best metric unit to use, km, m, cm, or mm?
7. the length of a pencil ________
8. the distance from Wellesley to Boston ________
9. the length of the school ________
10. the length of your pinky fingernail ________
11. the size of a TV screen ________
12. your height ________
13. the diameter of a dime ________
14. the distance from earth to the moon ________
© 2012 Thomas van Geel
18
Chapter 2: Mass Conversions
When buying groceries in China, the following units of mass are used:
1 dan = 100 jin
1 jin = 10 liang
1 liang = 10 qian
For comparison, 1 jin is about 1 pound.
Make the following conversions, paying attention to sig figs:
1. 400 liang is how many jin?
2. 4.500 jin is how many qian?
3. How many qian are in 0.035 dan?
To get a better feel for how much grams, milligrams, and kilograms weigh, do
the following conversions.
1 kg
1 pound
=
=
2.2 pounds
16 ounces
4. 12 ounces (a can of soda) = ______________ g
5. 0.200 ounces (mass of a quarter) = ______________ g
6. 0.00046 ounces (mass of a grain of sand) = ______________ mg
© 2012 Thomas van Geel
19
7. 15.00 pounds (mass of a bowling ball) = ______________ kg
8. 155 pounds (weight of average person) = ______________ kg
© 2012 Thomas van Geel
20
Chapter 2: Area and Volume Conversions
Convert the following metric area and volume measurements.
1. 1.34 cm2 = _________ mm2
2. 450 km2 = _________ cm2
3. 35 mm2 = __________ dm2
4. 2.31 dm2 = _________ mm2
5. 54.4 cm3 = _________ mm3
6. 0.222 mm3 = ________ hm3
7. 9.98 m3 = __________ km3
God said to Noah, "The end of all flesh has come before me, for the earth is filled with violence through them.
Behold, I will destroy them with the earth.
Make a ship of gopher wood. You shall make rooms in the ship, and shall seal it inside and outside with pitch.
This is how you shall make it. The length of the ship will be three hundred cubits, its width fifty cubits, and its
height thirty cubits.
-Genesis 6:13-15
The exact size of the cubit that Noah would have used is difficult to determine.
The size of a cubit was defined as the distance between a man’s elbow and the
tips of his fingers. Different ancient civilizations standardized the cubit at
different lengths (between 17 and 25 inches), so for now we’ll assume that:
1 cubit = 20 inches
(To simplify our calculations, we’ll assume that the ark was shaped like a
rectangular box.)
8. What is the volume of the ark in cubits3? (V = l × w × h)
© 2012 Thomas van Geel
21
9. Convert this value to inches3 (12 inches = 1 foot)
10. Convert this value to yards3 (3 feet = 1 yard)
(By the way, nobody really knows what “gopher wood” is, either.)
To get a feel for metric volume, here are some conversions to units we use
everyday:
1 cup = 200 mL
1 gallon = 3.75 L
1 teaspoon = 5 mL
What is the most appropriate metric unit for the following amounts, L or mL?
11. The amount of gas needed to fill a car’s tank ________
12. A can of soda ________
13. One dose of liquid cough medicine ________
14. The volume of a refrigerator ________
© 2012 Thomas van Geel
22
Chapter 2: Conversions with Density
Use the following information to answer the questions. Pay attention to sig figs.
Material
gasoline
mercury
platinum
uranium
Density (g/mL)
0.70
13.6
21.4
18.7
1. 5.0 kg uranium = ______________ cL
2. 3.50 kL gasoline = ______________ kg
3. 51.65 mm3 mercury = _______________ mg (remember that 1 mL = 1
cm3)
4. 343 cg platinum = ______________ km3
5. You have 2.00 kg of gasoline. What is the mass of an equal volume of
platinum?
© 2012 Thomas van Geel
23
Chapter 2: Conversions with Percent
1. A certain soda is 9.00% sugar by mass. If you drink 0.500 kg of soda,
how many grams of sugar have you consumed?
14K yellow gold is an alloy (a homogenous mixture of metals) which is 58.0%
gold by mass, and the rest is copper.
2. If you have 5.95 g of 14K gold, how many mg of pure gold do you have?
3. If you have 5.95 g of 14K gold, how many L of pure copper do you have?
(The density of pure copper is 8.96 g/cm3.)
© 2012 Thomas van Geel
24
Chapter 2: Converting Compound Units
Convert the following metric compound units.
1. 5.5 g/mL = _______ kg/cL
2. 7.66 mg/mL = ______ g/dL
3. 4.4 kg/L = _______ mg/kL
Useful information for the following problems:
1 mile = 5280 feet
1 gallon = 3.79 L
1 gallon = 8 pints
1 inch = 2.54 cm
1 kg = 2.2 pounds
1 pound = 16 ounces
4. 30 miles/gallon = _______________ km/L
5. 77 g/L = _______________ pounds/pint
© 2012 Thomas van Geel
25
6. 65.3 miles/hour = _______________ m/second
7. 4.80 g/mL = _______________ ounces/L
8. 10.0 pounds/inch2 = _______________ g/cm2
© 2012 Thomas van Geel
26
Chapter 2: Fenceposting Word Problems
Each of the following should be done with a single fenceposting sequence!
1. Dr. Frankenstein decides to go into mass production. He wants to build an
army of 300 zombies from spare parts. He sees an infomercial on The
Graverobber Channel selling pickled eyeballs; each jar contains 13 eyeballs. If
each jar costs $19.95, how much will Dr. F have to spend?
2. Dr. Frankenstein needs to feed his zombie army. Zombies love brains, but
Dr. F needs to save his supply of brains to make more Zombies! The next best
thing is scrambled eggs. Each zombie needs to eat a breakfast containing 666
calories. Each egg contains 70.0 calories. How many cartons of eggs does he
need? (1 carton contains 12 eggs.)
3. The maximum speed of a zombie on foot is 2.00 miles per hour. The nearest
village is 20.0 km away. For each hour of travel, a zombie burns 50.0 calories.
If a zombie eats one omelet that morning, will it be able to reach the town? Will
it be able to get back? (0.62 miles = 1 km, one omelet has 3 eggs)
© 2012 Thomas van Geel
27
Chapter 2: Converting Temperature
Convert the following temperatures.
Celsius
1.
101
2.
3.
298
-78
4.
5.
6.
Kelvin
20,000
1,500
4
© 2012 Thomas van Geel
28
Chapter 3: Ionic Formulas
Cl
CO3-2
OH-
SO4-2
PO4-3
NO3-
Na
NH4+
K
Ca
Mg
Zn
Fe+3
Al
Co+3
Fe+2
H
© 2012 Thomas van Geel
29
Chapter 3: Ionic Nomenclature
Fill in the blanks.
Formula
Name
1.
CaCO3
________________________________
2.
KCl
________________________________
3.
FeSO4
________________________________
4.
LiBr
________________________________
5.
MgCl2
________________________________
6.
FeCl3
________________________________
7.
Zn3(PO4)2
________________________________
8.
NH4NO3
________________________________
9.
Al(OH)3
________________________________
10. ______________ copper(I) acetate
11. ______________ iron(III) oxide
12. ______________ ammonium phosphate
13. ______________ copper(II) sulfate
14. ______________ sodium bicarbonate
15. ______________ nickel(III) bromide
16. ______________ beryllium nitrate
17. ______________ zinc sulfate
18. ______________ gold(III) chloride
© 2012 Thomas van Geel
30
Chapter 3: Molecular Nomenclature
Fill in the blanks.
Formula
Name
1.
SO2
________________________________
2.
__________________ Carbon tetrachloride
3.
NCl3
4.
__________________ Boron triiodide
5.
SF6
6.
__________________ Dinitrogen pentaoxide
7.
N2 O
8.
__________________ Silicon tetrachloride
9.
NI8
________________________________
________________________________
________________________________
________________________________
10. __________________ Iodine heptachloride
11. C2Cl2
________________________________
12. __________________ Xenon hexafluoride
© 2012 Thomas van Geel
31
Chapter 4: Moles Worksheet
Complete the following table:
Formula
C
Molar Mass
Moles
Grams
2.5
O2
200
4.5 x 1024
Mg3(PO4)2
NaOH
Mg(OH)2
K2CO3
Particles
7.7
0.034
5.5 x 1020
1. 4 moles of a certain compound has a mass of 50 g. What is the molar
mass of the compound?
2. 3.5 moles of a certain compound has a mass of 70 g. What is the molar
mass of the compound?
3. 4 moles of a certain ionic compound has a mass of 552.8 g. The formula
of the compound is X2CO3. What is element X?
© 2012 Thomas van Geel
32
Chapter 4: Mole/Mass/Particle Conversions
1.
5.67 g C = ________ mol C
2.
8,330 g N = _________ mol N
3.
80 mol Au = ________ g Au
4.
3.02 mol Xe = ________ g Xe
5.
0.25 mol Sr = _________atoms
Sr
6.
2.01 x 1023 atoms He = _______ mol
He
7.
19
9.55 x 10
8.
atoms Al = _____ mol Al
34.9 moles Cl = ___________
atoms Cl
9. 0.51 g Ag = ________ atoms Ag
10. 3.011 x 1023 atoms of K = ___________ g K
11. 103 g P = ________ atoms P
© 2012 Thomas van Geel
33
12. 8.31 x 1023 atoms of S = ___________ g S
13. If you have 3 mol Ca, how many moles of Cl do you need to combine with
the Ca to make CaCl2?
14. Mg and F will combine to form the ionic compound ________. If you have
8.0 g Mg, how many grams of F do you need to make this compound?
15. Al and O will combine to form the ionic compound ________. If you have
12 g Al, how many grams of O do you need to make this compound?
© 2012 Thomas van Geel
34
Chapter 4: % Composition
Determine the % composition of each of the compounds below:
1. KMnO4
2. HCl
3. Mg(NO3)2
4. (NH4)3PO4
5. Al2(SO4)3
6. How many grams of oxygen are there in 100 g of KClO3?
7. How many grams of iron are there in 25 g of Fe2O3?
8. How many grams of silver are there in 125 g of Ag2S?
© 2012 Thomas van Geel
35
Chapter 4: Empirical and Molecular Formulas
Find the empirical formulas for the following compounds.
1. C2H2 ____________________
2. C6H12O6 ____________________
3. C3H4 ____________________
4. C4H6 ____________________
5. C12H22O11 ____________________
6. The empirical formula of a compound is NO2, and its molar mass is about
90 g/mol. Find the molecular formula.
7. The empirical formula of a compound is CH2, and its molar mass is about
67 g/mol. Find the molecular formula.
8. A certain compound is found to be 50.35% C, 4.940 % H, and 44.71% O,
and its molar mass is between 250 and 300 g/mol. Find its empirical and
molecular formulas.
© 2012 Thomas van Geel
36
9. The lactate ion is 40.45% C, 5.670% H, and 53.88% O, and its molar
mass is found to be less than 100 g/mol. Find its empirical and molecular
formulas.
10. The cough medicine guaifenesin is 60.58% C, 7.13% H, 32.28% O, and
its molar mass is about 200 g/mol. Find its empirical and molecular
formulas.
11. Aspirin is 59.99% C, 4.485% H, 35.52% O, and its molar mass is between
150 and 200 g/mol. Find its empirical and molecular formulas.
© 2012 Thomas van Geel
37
Chapter 5: Balancing Chemical Equations
Rewrite and balance the following equations.
1. N2 + H2 → NH3
______________________________________________________
2. KClO3 → KCl + O2
______________________________________________________
3. NaCl + F2 → NaF + Cl2
______________________________________________________
4. H2 + O2 → H2O
______________________________________________________
5. AgNO3 + MgCl2 → AgCl + Mg(NO3)2
______________________________________________________
6. AlBr3 + K2SO4 → KBr + Al2(SO4)3
______________________________________________________
7. CH4 + O2 → CO2 + H2O
______________________________________________________
8. C3H8 + O2 → CO2 + H2O
______________________________________________________
9. C8H18 + O2 → CO2 + H2O
______________________________________________________
© 2012 Thomas van Geel
38
10. FeCl3 + NaOH → Fe(OH)3 + NaCl
______________________________________________________
11. P + O2 → P2O5
______________________________________________________
12. Na + H2O → NaOH + H2
______________________________________________________
13. Ag2O → Ag + O2
______________________________________________________
14. S8 + O2 → SO3
______________________________________________________
15. CO2 + H2O → C6H12O6 + O2
______________________________________________________
16. HCl + CaCO3 → CaCl2 + H2O + CO2
______________________________________________________
© 2012 Thomas van Geel
39
Chapter 5: Wacky Balancing
Rules: { } indicates a missing coefficient. [ ] indicates a missing element symbol
or subscript. All the missing coefficients and subscripts are greater than 1. All
numbers should be as low as possible.
1. NiS + { }O2 + { }HCl → [ ]Cl2 + H2SO4
2. V2[ ]5 + { }Zn → V[ ]O2 + { }[ ]O
3. { }P4[ ]3 + { }Br2 → 3P4S5 + 8P[ ]3
4. 3[ ] + { }I2 → Nb[ ][ ]8
5. CO([ ]H2)2 + { }HOCl → 2NCl3 + CO[ ] + { }H2O
6. { }NaOH + Cl[ ] → NaCl + NaClO + H2[ ]
7. [ ]C2O4 → Fe[ ] + C[ ]2 + [ ]O
8. { }[ ]Cl[ ] + [ ]H3 → N[ ]3 + 3NaO[ ]
9. Na[ ][ ]O[ ] + 2HCl → H2C[ ]3 + 2[ ]Cl
10. { }Ca3([ ][ ]4)2 + { }SiO2 + 10C →
P4 + { }CaSiO[ ] + 10[ ]O
© 2012 Thomas van Geel
40
Chapter 5: Mole-to-Mole Stoichiometry
1. N2 + 3H2 → 2NH3
How many moles of H2 are needed to react with two moles of N2?
2. 2KClO3 → 2KCl + 3O2
How many moles of O2 are produced by six moles of KClO3?
3. Zn + 2HCl → ZnCl2 + H2
How many moles of H2 are produced from three moles of Zn?
4. C3H8 + 5O2 → 3CO2 + 4H2O
How many moles of O2 are needed to react with four moles of C3H8?
5. K3PO4 + Al(NO3)3 → 3KNO3 + AlPO4
How many moles of KNO3 are produced when two moles of K3PO4 react?
For the remaining questions, you must balance the equation before
answering.
6.
H3PO4 + NaOH → Na3PO4 + H2O
How many moles of NaOH would you need to react completely with 0.44
moles of H3PO4?
7. Mixing bleach (NaClO) and ammonia (NH3) produces toxic NCl3 and
NaOH. Write the chemical equation for this reaction and balance it.
How many moles of NCl3 would you make if you mixed 9.9 mol bleach with
ammonia?
© 2012 Thomas van Geel
41
Chapter 5: Mass-to-Mass Stoichiometry
1. 2KClO3 → 2KCl + 3O2
How many grams of KCl are produced if 25.0 g of KClO3 react?
2. N2 + 3H2 → 2NH3
How many grams of H2 are needed to react with 50.0 g of N2?
3. How many grams of NH3 would be produced in problem 2?
4. 2AgNO3 + BaCl2 → 2AgCl + Ba(NO3)2
How many grams of AgCl are produced from 5.00 g of AgNO3?
5. How much BaCl2 is needed to react with the AgNO3 in problem 4?
6. Zn + 2HCl → ZnCl2 + H2
How many grams of H2 are produced from 2.50 g Zn?
7. How many grams of HCl are needed to react in problem 6?
© 2012 Thomas van Geel
42
Chapter 5: Advanced Stoichiometry
1. 10.00 g of an unknown carbonate (XCO3) reacts with HCl in the following
reaction, producing 4.397 g of CO2. What is the identity of element X?
XCO3 + 2HCl → XCl2 + H2O + CO2
2. 3.000 g of an unknown metal (Y) react with water in the following
reaction, producing 0.07749 g of H2 gas. What is the unknown metal?
2Y + 2H2O → 2YOH + H2
3. 5.000 g of an unknown element (Z) react with oxygen in the following
reaction, producing 11.46 g of Z2O5. What is element Z?
4Z + 5O2 → 2Z2O5
© 2012 Thomas van Geel
43
4. 2.000 g of an unknown diatomic element (X2) react with NaOH in the
following reaction, producing 1.648 g of NaX and 2.100 g of NaXO. What
is element X?
X2 + 2NaOH → NaX + NaXO + H2O
6. 10.00 g of an unknown fuel (CxH6) is burned in the following unbalanced
combustion reaction, producing 6.921 g H2O. What is the fuel?
CxH6 + O2 → CO2 + H2O
7. 6.000 g of an unknown fuel (C2Hx) is burned in the following unbalanced
combustion reaction, producing 7.709 g H2O. What is the fuel?
C2Hx + O2 → CO2 + H2O
© 2012 Thomas van Geel
44
Chapter 5: Limiting Reagent Stoichiometry
1. N2 + 3H2 → 2NH3
a. If 20.0 g of N2 and 25.0 g of H2 are mixed together, what is the
limiting reagent?
b. How many grams of NH3 will be formed? Use one fencepost to
solve.
c. How many grams of everything will there be once the reaction is
complete? Use an ICE chart to solve.
© 2012 Thomas van Geel
45
2. Mg + 2HCl → MgCl2 + H2
a. If 50.0 g Mg and 75.0 g of HCl are mixed together, what is the
limiting reagent?
b. How many grams of H2 will be formed? Use one fencepost to solve.
c. How many grams of everything will there be once the reaction is
complete? Use an ICE chart to solve.
© 2012 Thomas van Geel
46
3. 3AgNO3 + Na3PO4 → Ag3PO4 + 3NaNO3
a. If 200. g of AgNO3 and 200. g of Na3PO4 are mixed together, what
is the limiting reagent?
b. How many grams of Ag3PO4 will be formed? Use one fencepost to
solve.
c. How many grams of everything will there be once the reaction is
complete? Use an ICE chart to solve.
© 2012 Thomas van Geel
47
4. SO2 + Ca(OH)2 → CaSO3 + H2O
a. If 12.2 g of SO2 and 84.3 g of Ca(OH)2 are mixed together, what is
the limiting reagent?
b. How many grams of water will be formed? Use one fencepost to
solve.
c. How many grams of everything will there be once the reaction is
complete? Use an ICE chart to solve.
© 2012 Thomas van Geel
48
For the following problems you must first balance the equation.
5.
C2H2 + O2 → H2O + CO2
a. If 2.30 g C2H2 and 3.90 g of O2 are mixed together, what is the
limiting reagent?
b. How many grams of CO2 will be formed? Use one fencepost to
solve.
c. How many grams of everything will there be once the reaction is
complete? Use an ICE chart to solve.
© 2012 Thomas van Geel
49
6.
Cu + AgNO3 → Cu(NO3)2 + Ag
a. If 30.0 g of Cu and 90.0 g of AgNO3 are mixed together, what is
the limiting reagent?
b. How many grams of Ag will be formed? Use one fencepost to solve.
c. How many grams of everything will there be once the reaction is
complete? Use an ICE chart to solve.
© 2012 Thomas van Geel
50
7.
H2 O +
CO2 →
H2CO3
a. If 11.1 g of H2O and 88.8 g of CO2 are mixed together, what is the
limiting reagent?
b. How many grams of H2CO3 will be formed? Use one fencepost to
solve.
c. How many grams of everything will there be once the reaction is
complete? Use an ICE chart to solve.
© 2012 Thomas van Geel
51
8.
Al(NO3)3 +
Na →
NaNO3 +
Al
a. If 344 g sodium and 657 g aluminum nitrate are mixed together,
what is the limiting reagent?
b. How many grams of aluminum will be formed? Use one fencepost
to solve.
c. How many grams of everything will there be once the reaction is
complete? Use an ICE chart to solve.
© 2012 Thomas van Geel
52
Chapter 5: Limiting Reactants and Percent Yield
1. The fizz produced when an Alka-Seltzer tablet is dissolved in water is due
to the reaction between sodium bicarbonate, NaHCO3, and citric acid,
H3C6H5O7:
3NaHCO3(aq) + H3C6H5O7(aq) → 3CO2(g) + 3H2O(l) + Na3C6H5O7(aq)
In a certain experiment, 1.00 g of sodium bicarbonate and 1.00 g of citric
acid react.
a. What is the theoretical yield of CO2 in grams?
b. If only 0.25 g of CO2 are actually produced, what is the percent
yield?
© 2012 Thomas van Geel
53
2. When hydrogen sulfide gas is bubbled into a solution of sodium hydroxide,
the reaction forms sodium sulfide and water.
a. Write a balanced equation for this reaction.
b. What is the theoretical yield of sodium sulfide if 2.50 g of hydrogen
sulfide is bubbled into a solution containing 1.85 g of sodium
hydroxide?
c. If only 1.05 g of sodium sulfide is formed, what is the percent
yield?
3. A student reacts benzene, C6H6, with bromine, Br2, to prepare
bromobenzene, (C6H5Br and H2).
a. Write the balanced equation.
b. What is the theoretical yield of bromobenzene in this reaction when
30.0 g of benzene reacts with 65.0 g of bromine?
c. If the actual yield of bromobenzene was 56.7 g, what was the
percent yield?
© 2012 Thomas van Geel
54
4. Hydrogen sulfate and lead(II) acetate react to form solid lead(II) sulfate
and aqueous hydrogen acetate. Write the balanced equation. 10.0 g of
hydrogen sulfate and 10.0 g of lead(II) acetate are mixed, and the
percent yield is 70%. How much of everything is there once the reaction
is done?
© 2012 Thomas van Geel
55
Chapter 5: Classifying Reactions
Classify the following reactions as synthesis, decomposition, single replacement,
double replacement, or combustion.
1. 2H2 + O2 → 2H2O
2. Zn + H2SO4 → ZnSO4 + H2
3. C2H6O + 3O2 → 2CO2 + 3H2O
4. 2H2O → 2H2 + O2
5. CH4 + 2O2 → CO2 + 2H2O
6. 2HgO → 2Hg + O2
7. 2KBr + Cl2 → 2KCl + Br2
8. CaO + H2O → Ca(OH)2
9. 2C2H2 + 5O2 → 4CO2 + 2H2O
10. AgNO3 + NaCl → AgCl + NaNO3
11. 2H2O2 → 2H2O + O2
12. Ca(OH)2 + H2SO4 → CaSO4 + 2H2O
13. 2Hg + O2 → 2HgO
14. Na + Ca(OH)2 → NaOH + Ca
15. MgCl2 + KNO3 → Mg(NO3)2 + KCl
© 2012 Thomas van Geel
56
Chapter 5: Predicting Products
Predict the products of the following reactions, then balance the equations.
Synthesis Reactions—pattern:
1. Ca + I2 →
2. Al + F2 →
3. Na + Br2 →
Decomposition Reactions—pattern:
1. K2O →
2. H2O →
3. NaCl →
Single Replacement Reactions—pattern:
1. Na + Cu(NO3)2 →
2. Al + AuNO3 →
3. H2 + CuO →
Double Replacement Reactions—pattern:
1. K2S + H2SO4 →
2. FeSO4 + BaCl2 →
3. ZnCl2 + Na2S →
Combustion Reactions—pattern:
1. C3H6 + O2 →
2. CH4 + O2 →
3. CH4O + O2 →
© 2012 Thomas van Geel
57
Determine the type of reaction, predict the products, then balance the
equation.
1. P4 + O2 →
(P will be +5 in the product)
2. Na + H2O →
3. CH2O + O2 →
4. Li + HCl →
5. H2 + Cl2 →
6. Cl2 + KI →
7. HC2H3O2 + Ca(OH)2 →
8. Si + F2 →
(Si will be +4 in the product)
9. KBr + AgNO3 →
10. C2H4 + O2 →
11. AlI3 →
12. Fe + S8 →
(Fe will be +3 in the product)
13. BaCl2 + NaNO3 →
14. HCl →
15. C3H8 + O2 →
16. CrBr3 + NaNO3 →
17. Ag2O →
18. Mg + FeCl3 →
19. Na + O2 →
20. Fe2O3 →
© 2012 Thomas van Geel
58
Chapter 6: Atomic Models
1. Draw a diagram of a cathode ray tube, and label all the parts.
2. In a cathode ray tube,
a. If particle mass increases, deflection (increases/decreases).
b. If particle charge increases, deflection (increases/decreases).
c. If particle velocity increases, deflection (increases/decreases).
d. If the electric field increases, deflection (increases/decreases).
3. Draw and label the atomic models developed by the following scientists:
Dalton/Proust
Thomson
Rutherford
Bohr
© 2012 Thomas van Geel
59
4. Draw and label the parts of Rutherford’s gold foil experiment.
5. Rank the following types of electromagnetic waves in terms of energy,
wavelength, and frequency (1 is the highest, 7 is the lowest).
EM wave
Energy
Wavelength
(1 = longest, 7 = shortest)
Frequency
X-ray
Visible light
Gamma
Radio
Microwaves
Ultraviolet
Infrared
6. Rank the colors of visible light from lowest energy to highest:
7. The atom below has 4 energy levels, and gives off six colors of light: red,
orange, yellow, green, blue, and purple. Draw six arrows on the diagram below
to represent the electron transitions that would create these colors. Label each
arrow with the proper color.
© 2012 Thomas van Geel
60
Chapter 6: Energy, Frequency, and Wavelength
For light:
"=
c
#
E = h"
E=
hc
"
where ν is frequency in seconds-1, λ is wavelength in meters, c is the speed of light (3 ×
108 m/s), h is Planck’s!constant (6.63 × 10-34 J⋅s), and E is energy in Joules
!
Fill in the following chart.
Energy (J)
!
Frequency (s-1)
Wavelength (m)
1.79 × 10-18
5.48 × 1013
2.50 × 10-7
2.60 × 10-19
3.53 × 1015
1.03 × 10-5
© 2012 Thomas van Geel
61
Chapter 6: Bohr’s Model
For an electron in hydrogen:
E = "R H
1
n2
$1
1'
"E = R H && 2 # 2 ))
% ni n f (
where RH is Rydberg’s constant (2.18 × 10-18 J), n is energy level, E is energy in Joules,
∆E is change in energy!in Joules, ni is initial energy
! level, and nf is final energy level.
1. Calculate the energy of an electron in energy level 3.
2. A certain electron has an energy of -1.362 × 10-19 J. What energy level is
it in?
3. An electron drops from level 5 to level 2. How much energy is released?
4. An electron in energy level 1 absorbs 2.119 × 10-18 J. What level will it
rise to?
© 2012 Thomas van Geel
62
Chapter 6: Light and the Hydrogen Atom
1. When an electron drops from level 3 to level 1, what is the wavelength of
light that will be emitted?
2. An electron in energy level 3 absorbs light with a frequency of 2.74 × 1014
s-1. What level will the electron rise to?
3. If an electron drops from level 5 to level 4, what is the frequency of light
that will be emitted?
4. After absorbing some light with a wavelength of 4.657 × 10-6 m, an
electron ends up in level 7. What level did it start in?
© 2012 Thomas van Geel
63
Chapter 6: Isotopes
1. What is the most common isotope of the following elements?
Ar:
U:
K:
2. Given the following isotope abundance data for imaginary element X:
X-233: 90%
X-234: 7%
X-235: 3%
Which of the following would be a reasonable atomic mass for element X?
(Don’t calculate it, just use common sense.)
a. 233.0
b. 233.1
c. 234.3
d.234.0
e. 235.3
3. Use the following information to calculate the atomic mass of Silicon.
Isotope
28
Si
Si
30
Si
29
Mass
27.98
28.98
29.97
Abundance
92.2297
4.6832
3.0872
4. Use the following information to calculate the atomic mass of Germanium.
Isotope
70
Ge
Ge
73
Ge
74
Ge
76
Ge
72
Mass
69.92
71.92
72.92
73.92
75.92
Abundance
20.84
27.54
7.73
36.28
7.61
© 2012 Thomas van Geel
64
5. Complete the following chart.
Isotope
14
6
Atomic #
# Neutrons
Mass
Number
C
Charge
-2
92
30
1
129
53
# Electrons
2
92
238
23
56
10
29
+5
2
I
0
77
48
36
46
75
193
36
85
46
110
+1
36
© 2012 Thomas van Geel
65
Chapter 7: Balancing Nuclear Equations
1. ________ + 9Be4 → 6Li3 + 4He2
2. 9Be4 + 4He2 →
3.
238
12
C6 + ________
U92 → ________ + 4He2
4. 1H1 + 3H1 → ________
5.
27
Al13 + ________ →
24
Na11 + 4He2
6. 9Be4 + 1n0 → 2 ________ + 2 1n0
7. ________ + 2H1 →
8.
23
Na11 + 1n0 →
9.
246
Cm96 +
12
24
C6 →
22
Na11 + 4He2
Na11 + ________
254
No102 + 4 ________
10. 6Li3 + 1n0 → 0e-1 + 4He2 + ________
11.
12.
241
214
Am95 + 4He2 → 2 1n0 + ________
Po84 + 2 4He2 + 2 0e-1 → ________
© 2012 Thomas van Geel
66
Write the nuclear equations for the following processes.
13. Alpha decay of Pa-231
14. Beta decay of Fr-223
15. Electron capture of Al-26
16. Positron emission of F-18
17. Gamma emission of Ba-137*
18. Bombardment of U-238 with N-14 yields five neutrons and another
isotope. Write the complete nuclear equation.
19. Bombardment of U-235 with a neutron yields three neutrons, Sr-90, and
another isotope. Write the complete nuclear equation.
20. Bombardment of Li-6 with a neutron produces an alpha particle and
another isotope. Write the complete nuclear equation.
© 2012 Thomas van Geel
67
Chapter 7: Nuclear Instability
Explain why the following nuclei are unstable, and predict what type of nuclear
decay they are likely to undergo:
1. U-235
2. C-10
3. C-14
4. Ne-17
5. Be-11
6. Np-225
© 2012 Thomas van Geel
68
Chapter 7: Half-life, Chart Method
Use charts to solve the following problems.
1.
Element X has a half-life of 4 years. If you start with 600. g, how much
will be left after 20 years?
2.
Exactly two days ago, you had 500. g of element Y. You now have 62.5
g left. What is the half-life of element Y?
3.
You have 30.0 g of element Z, which has a half-life of 3 hours. How
much of element Z did you have 15 hours ago?
4.
Element A has a half-life of 5 minutes. If you start with 200. g, how
much will be left after half an hour?
5.
Exactly four years ago, you had 200. g of element B. You now have
12.5 g left. What is the half-life of element B?
6.
You have 85.0 g of element C, which has a half-life of 12 hours. How
much of element C did you have 3 days ago?
© 2012 Thomas van Geel
69
Chapter 7: Half-life, Equation Method
Use the following equation to solve these problems:
At = A0 " (0.5)
1.
!
t
t1
2
Element X has a half-life of 15 years. If you start with 100. g, how much
will be left after 20 years?
2.
You have 50.0 g of element Y, which has a half-life of 4 hours. How
much of element Z did you have 13 hours ago?
3.
Element Z has a half-life of 8 minutes. If you start with 150. g, how
much will be left after 30 minutes?
4.
You have 88.0 g of element Q, which has a half-life of 11 hours. How
much of element Q did you have 3 hours ago?
© 2012 Thomas van Geel
70
Chapter 8: Electron Configurations
Write the long form of the electron configurations of the following elements:
1. sodium
________________________________________________
2. iron
________________________________________________
3. bromine
________________________________________________
4. copper
________________________________________________
5. einsteinium ________________________________________________
Write the condensed electron configurations of the following:
6. cobalt
________________________________________________
7. technetium
________________________________________________
8. tellurium
________________________________________________
9. radium
________________________________________________
10. europium
________________________________________________
Write the long form of the electron configuration for the following ions:
11. F-1
________________________________________________
12. B+3
________________________________________________
13. P-3
________________________________________________
14. Cr+1
________________________________________________
15. Hg+2
________________________________________________
Identify the following elements (assume they are neutral atoms) and determine
if they are in the ground or excited state:
Element
G/E state
16. 1s22s22p63s23p34s1
____________________
17. 1s22s22p63s23p64s23d104p65s1
____________________
18. [Kr] 5s24d105p26s1
____________________
19. [Xe] 6s24f145d6
____________________
20. [Rn] 7s25f11
____________________
© 2012 Thomas van Geel
71
Determine which of the following electron configurations are not valid, and
rewrite them correctly (maintain the same number of electrons).
21. 1s22s22p63s23p64s24d104p5 _________________________
22. 1s22s22p63s33d5 _________________________
23. [Ra] 7s25f9 _________________________
24. [Kr] 5s24d105p5 _________________________
25. [Xe] _________________________
© 2012 Thomas van Geel
72
Chapter 8: Schrödinger/Heisenberg
1. How many electrons can the
following subshells/shells
contain?
2. How many orbitals are
contained in the following
subshells?
# electrons
# orbitals
s subshell
s
p subshell
p
d subshell
d
f subshell
f
Shell 1
Shell 2
Shell 3
Shell 4
3. Which subshells are present in the following shells (s, p, d, f)?
Subshells present
Shell 1
Shell 2
Shell 3
Shell 4
4. List all the subshells from lowest to highest energy (1s, 2s, etc…).
low
high
© 2012 Thomas van Geel
73
Chapter 9: The Periodic Table
1. Fill in the following chart.
Atom
Electron configuration (long form)
# valence
electrons
# core
electrons
Zeff
Mg
1s2 2s2 2p5
B
1s2 2s2 2p6 3s2 3p6 4s2 3d10
2. Write down the properties of metals, and contrast these properties with
nonmetals and metalloids.
© 2012 Thomas van Geel
74
3. Name the shaded regions of the periodic tables below.
__________________________
__________________________
__________________________
__________________________
__________________________
__________________________
__________________________
__________________________
__________________________
© 2012 Thomas van Geel
75
4. On the periodic table below, draw one arrow to indicate the trend in Zeff; the
end of the arrow is where Zeff is low, the point is where Zeff is large.
5. On the periodic table below, draw one arrow to indicate the trend in atomic
radius; the end of the arrow is where the radii are small, the point is where the
radii are large.
Explain this trend in complete sentences.
6. On the periodic table below, draw one arrow to indicate the trend in
ionization energy; the end of the arrow is where the I.E.’s are low, the point is
where the I.E.’s are high.
Explain this trend in complete sentences.
© 2012 Thomas van Geel
76
7. On the periodic table below, draw two arrows to indicate the trends in
reactivity for metals and for nonmetals. The end of the arrows are where
reactivity is low, the point is where the reactivity is high.
Explain these trends in complete sentences.
8. Circle the atom with the highest Zeff.
As
Na
Xe
I
9. Circle the atom with the largest atomic radius.
Cl
P
Sb
I
10. Circle the atom with the lowest I.E.
K
Mg
Cs
Sr
11. Circle the element that is the most reactive.
Al
Na
Mg
Rb
12. Circle the element that is the most reactive.
Ne
Cl
Br
S
13. Based on your knowledge of reactivity trends, which of the following
reactions will occur, and which will not?
Yes/No
Ca(s) + MgCl2(aq) → CaCl2(aq) + Mg(s)
2Li(s) + BeCl2(aq) → 2LiCl(aq) + Be(s)
I2(s) + MgCl2(aq) → MgI2(aq) + Cl2(g)
O2(g) + 2CaF2(aq) → 2CaO(aq) + 2F2(g)
© 2012 Thomas van Geel
77
Chapter 10: Drawing Lewis Structures
Draw the Lewis structures for the following molecules.
1. HF
2. H2O
3. CCl4
4. NH3
5. NH4+
© 2012 Thomas van Geel
78
6. SO2
7. C2H6
8. C2H2
9. HCN
10. H3O+
© 2012 Thomas van Geel
79
11. CO2
12. CO
13. BeF2
14. BF3
15. NO2-
© 2012 Thomas van Geel
80
16. NO3-
17. SF3+
18. PH2-
© 2012 Thomas van Geel
81
Chapter 10: Lewis Structures with Resonance
All of the following compounds have resonance. Draw the Lewis structures.
1. O3
2. CO3-2
3. NO2-
4. NO3-
© 2012 Thomas van Geel
82
5. HNO3
(N is the central atom, surrounded by the O’s. The H is bound to an O.)
6. CH3CO2-
7. C6H6 (ring structure)
8. N2O4
© 2012 Thomas van Geel
83
Chapter 10: Molecular Geometry
For each molecule draw the Lewis structure and the 3-D drawing. Name the
electron geometry and the molecular geometry.
CO2
Lewis Structure
3-D Drawing
Electron geometry:
Molecular geometry:
ONF
Lewis Structure
Lewis Structure
3-D Drawing
Electron geometry:
Molecular geometry:
NCl3
3-D Drawing
Electron geometry:
Molecular geometry:
© 2012 Thomas van Geel
84
Lewis Structure
Lewis Structure
Lewis Structure
NO2-
3-D Drawing
Electron geometry:
Molecular geometry:
OF2
3-D Drawing
Electron geometry:
Molecular geometry:
PCl4+
3-D Drawing
Electron geometry:
Molecular geometry:
© 2012 Thomas van Geel
85
Lewis Structure
Lewis Structure
Lewis Structure
BBr3
3-D Drawing
Electron geometry:
Molecular geometry:
AsBr3
3-D Drawing
Electron geometry:
Molecular geometry:
SiF4
3-D Drawing
Electron geometry:
Molecular geometry:
© 2012 Thomas van Geel
86
Chapter 10: Polarity of Molecules
The Lewis structures for a number of molecules have been provided for you.
Draw the molecules in 3D in the second column. Pay close attention to the bond
angles in the 3D drawings; the will not necessarily be the same as in the Lewis
structure! On the first 3D drawing, indicate the polarity of all the bonds with
arrows. Then determine if the molecule is polar; if it is, draw the molecule again
in 3D in the third column and indicate the overall polarity of the molecule with
one arrow. If the molecule is nonpolar, write “NONPOLAR” in the second box;
you do not need to draw it again.
For determining bond polarity, the electronegativity values are given below:
Si: 1.9
C: 2.55
Lewis
Structure
H
H
O
Si
H: 2.2
O: 3.44
N: 3.04
3D drawing showing
bond polarity
Cl: 3.16
S: 2.58
F: 3.98
3D drawing showing
overall polarity
H
N
© 2012 Thomas van Geel
87
F
F
C
F
F
O
Si
O
F
N
F
F
Cl
H
Si
Cl
H
© 2012 Thomas van Geel
88
F
C
F
C
F
C
F
O
S
O
O
H
C
H
O
H
C
O
© 2012 Thomas van Geel
89
O
O
C
O
Cl
Cl
C
C
H
H
H
Cl
C
C
H
Cl
H
Cl
C
C
Cl
H
© 2012 Thomas van Geel
90
Chapter 11: IMF intro
Using the table of electronegativity values in your book, draw arrows to indicate
bond polarity on the following molecules. Determine if the molecules are polar
or nonpolar, and if they are capable of hydrogen bonding.
Is this molecule polar or
nonpolar?
H
H
A. C2H6
Is this molecule capable
of H-bonding?
H
C
C
H
H
H
H
B. CH3Cl
C
H
Cl
H
C. NH3
N
H
H
H
-2
O
D. CO3-2
C
O
O
P
Cl
Cl
E. PCl3
Cl
H
F. CH3OH
C
H
H
H
O
© 2012 Thomas van Geel
91
Circle the type of IMF that would hold the following pairs of molecules together.
A and another A
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
B and another B
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
C and another C
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
D and another D
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
E and another E
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
F and another F
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
A and B
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
C and D
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
E and F
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
C and F
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
A and E
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
© 2012 Thomas van Geel
92
Chapter 11: Strength of IMF’s
Determine which of the following substances will have stronger IMF’s, and briefly
explain why. You may draw Lewis/3-D structures as part of your explanation.
1. CH4 vs. C2H6
2. HF vs. HCl
3. CH4 vs. CH3F
4. CH3CH2OH vs. CH3OCH3
5. HOCH2CH2OH vs. CH3CH2CH2OH
6. NO2 vs. CO2 (The nitrogen only has 7 electrons in the Lewis structure.)
7. NO2 vs. SO2 (The nitrogen only has 7 electrons in the Lewis structure.)
© 2012 Thomas van Geel
93
Chapter 11: Types of Solids
1. My particles are arranged in a repeating structure called a crystal lattice.
a. What kind of solid am I?
b. Draw my crystal lattice.
c. Can I conduct electricity as a solid? As a liquid? When I’m
dissolved in water?
2. I am a good conductor of electricity in the solid phase.
a. What kind of solid am I?
b. Draw my atomic structure.
c. Do I have a high melting point or a low melting point?
3. I don’t conduct electricity and I don’t dissolve in anything.
a. What kind of solid am I?
b. Do I have a high melting point or a low melting point?
c. Are my particles held together with IMF’s, ionic bonds, or
covalent bonds?
4. I have a low melting point and dissolve in water.
a. What kind of solid am I?
b. Can I conduct electricity as a solid? As a liquid? When I’m
dissolved in water?
c. As a solid, are my particles held together with IMF’s, ionic bonds,
or covalent bonds?
© 2012 Thomas van Geel
94
Chapter 11: Heating Curves
Temperature (K)
300
250
200
150
100
0
5
10
15
20
25
30
35
40
Energy input (kJ)
The heating curve for 50 g of substance X is shown above. The molar mass of
substance X is 12.5 g/mol.
Useful equations: q = mcΔT
q = nΔHfus
q = nΔHvap
(Remember, for the first equation, q is in J. For the other two, q is in kJ.)
1. What is the melting point of substance X?
2. What is the boiling point of substance X?
3. Calculate the specific heat (c) of all phases of substance X:
Solid:
Liquid:
Gas:
4. If 75 g of solid substance X at 50K absorbs 900 J, what will the new
temperature be?
© 2012 Thomas van Geel
95
5. Calculate the ΔHfus and ΔHvap of substance X:
ΔHfus =
ΔHvap =
6. Question 6 has multiple parts:
a. How much energy, in J, would it take to heat up 400 g of solid X
from 135 K to 190 K?
b. If you have 400 g of X, how many moles of X do you have?
c. How much energy, in kJ, would it take to melt 400 g of X?
d. How much energy, in J, would it take to heat 400 g of liquid X from
190 K to 240 K?
e. How much energy, in J, would it take to heat up 400 g of X from
135 K to 240 K?
7. How much energy, in J, would it take to heat up 200 g of X from 20 K to
300 K?
© 2012 Thomas van Geel
96
Chapter 11: Heat Transfer Problems
1. An insulated beaker contains 150 g of water at 24.6°C. A 110-g piece of
molybdenum metal is heated to 100°C and placed in the water. The
temperature of the water rises until it plateaus at 28.0°C. Calculate the
specific heat of molybdenum metal. (The specific heat of water is 4.184
J/g⋅K.)
2.
50 g of marble chips (heat capacity = 0.94 J/g⋅K) are to 200°C. The hot
marble chips are placed in 500 g of water at 10°C. How high will the
temperature of the system go? (The specific heat of water is 4.184
J/g⋅K.)
© 2012 Thomas van Geel
97
3. 30.00 g of ice at 0.0°C are placed in an insulated cup containing 150.00 g
of warm water at 60.0°C. The ice melts, and the temperature of the
water comes to 36.7°C. What is value of the heat of fusion (∆Hfus) of ice?
4. You are given 50.0 g of water at 5°C and a piece of aluminum weighing
25 g. The specific heat of water is 4.18 J/g⋅K. The specific heat of
aluminum is 0.9 J/g⋅K. The heat of fusion of water is 6.02 kJ/mol.
a. Could you freeze the water by chilling the aluminum to a very low
temperature and dropping it into the water? How cold would the
aluminum need to be?
b. If you could chill the aluminum all the way to absolute zero, how
much aluminum would you need to freeze the water?
© 2012 Thomas van Geel
98
Chapter 11: Phase Diagrams
1. Draw a phase diagram for a substance with the following characteristics:
Normal boiling point = 350 K
Normal melting point = 260 K
Triple point: 230 K, 0.5 atm
Critical point: 500 K, 2 atm
2. If you have some of this substance in the solid phase, could you melt it by
increasing the pressure? Why or why not?
3. If you have some of this substance in the liquid phase, could you boil it by
decreasing the pressure? Why or why not?
© 2012 Thomas van Geel
99
Chapter 12: Solubility Curves
Use this graph to answer the following questions.
Solubility Curves
150
140
130
KNO3
Pb(NO3)2
120
110
g solute per 100 g water
100
90
Al2(SO4)3
80
70
60
HCl
50
NaCl
40
30
20
NH3
HgCl2
10
0
0
10
20
30
40
50
60
70
80
90
100
Temperature (°C)
1. What mass of KNO3 must be dissolved in 100 g of water at 60°C to make
a saturated solution?
2. You have a solution containing 90 g of Pb(NO3)2 in 100 g water at 90°C.
As the solution cools, at what temperature will a precipitate start to
appear?
© 2012 Thomas van Geel
100
3. If you continued cooling the solution in question 2 down to 10°C, what
mass of Pb(NO3)2 would precipitate out?
4. Classify the following solutions as saturated, unsaturated, or
supersaturated.
a. 50 g NH3 at 10°C
b. 10.5 g HgCl2 at 40°C
c. 110 KNO3 at 50°C
5. If you disturb the solution in #4c, how much KNO3 will precipitate out?
6. In your own words, explain why the solubility of solids increases with
temperature.
7. In your own words, explain why the solubility of gases decreases with
temperature.
8. In your own words, explain the differences between solutions, colloids,
and suspensions.
© 2012 Thomas van Geel
101
Chapter 12: Solubility Rules
Using the following solubility rules, determine which of the following ionic
compounds are soluble in water.
1. Compounds containing a group 1 cation or ammonium (NH4+) are soluble.
2. Compounds containing nitrates (NO3-) or acetates (C2H3O2-) are soluble.
3. Compounds containing halogen anions (other than F-) are soluble, except
when paired with Ag, Hg(I), and Pb.
4. Sulfate (SO4-2) compounds are soluble, except those with Ba, Sr, Ca, Pb,
Ag, and Hg(I).
5. Carbonates (CO3-2), hydroxides (OH-), oxides (O-2), silicates (SiO3-2), and
phosphates (PO4-3) are insoluble, except for group 1 cations and
ammonium.
1. LiOH
2. CaCO3
3. AgCl
4. CuSO4
5. MgO
6. (NH4)3PO4
7. PbSO4
8. AgNO3
© 2012 Thomas van Geel
102
Chapter 12: Molarity
1. You have 7 mol BaCl2 dissolved in 5 L of solution.
a. What is the molarity of BaCl2?
b. If the BaCl2 dissociates completely, what is the molarity of Ba+ ions
and Cl- ions?
2. You have 4 mol Al2(SO4)3 dissolved in 2 L of solution.
a. What is the molarity of Al2(SO4)3 in this solution?
b. If the Al2(SO4)3 dissociates completely, what is the molarity of Al+3
ions and SO4-2 ions?
3. If you have 3 L of 2 M HCl, how many moles of HCl do you have?
4. If you have 0.4 L of 0.5 M KNO3, how many moles of KNO3 do you have?
5. A sample of KI solution contains 1 mol KI and has a concentration of 0.25
M. What is the volume of the sample?
6. A sample of sugar solution contains 0.75 mol sugar and has a
concentration of 2 M. What is the volume of the sample?
© 2012 Thomas van Geel
103
7. To make 2.0 liters of a 3.5 M sodium nitrate (NaNO3) solution, how many
grams of NaNO3 do you need?
8. If you have 12 g of lithium hydroxide (LiOH) in 0.75 L of solution, what is
the molarity?
9. A 1.5 M solution contains 85 g of silver nitrate (AgNO3). What is the
volume of the solution?
10. Use the following balanced equation to solve.
Ca(OH)2(aq) + H2SO4(aq) → CaSO4(s) + 2H2O(l)
If the H2SO4 has a concentration of 0.82 M, how many liters of it are
needed to react with 5.5 moles of calcium hydroxide?
11. Use the following balanced equation to solve.
2NH4Cl(aq) + Ca(OH)2(aq) → CaCl2(aq) + 2NH3(g) + 2H2O(l)
How many grams of calcium chloride will be made when 1.2 L of a 0.05 M
calcium hydroxide solution react?
© 2012 Thomas van Geel
104
Chapter 12: Dilutions
1. How much 2.0 M NaCl solution would you need to make 250 mL of 0.15 M
NaCl solution?
2. What would be the concentration of a solution made by diluting 45.0 mL
of 4.2 M KOH to 250 mL?
3. What would be the concentration of a solution made by adding 250 mL of
water to 45.0 mL of 4.2 M KOH?
4. How much 0.20 M glucose solution can be made from 50.0 mL of 0.50 M
glucose solution?
5. To how much water should 100 mL of 18 M H2SO4 be added to prepare a
1.5 M solution?
6. To what volume should 25 mL of 15 M HNO3 be diluted to prepare a 3.0 M
solution?
© 2012 Thomas van Geel
105
Chapter 12: Freezing Point Depression
and Boiling Point Elevation
1. Determine the molality of a solution made from 8 g AlCl3 and 200 g water.
2. Determine the freezing point and boiling point of the solution in the
previous question.
3. A solution of NaCl boils at 101 °C. Determine the molality of the solution.
4. A solution of K2CO3 freezes at -5.9 °C. Determine the boiling point of the
solution.
5. 67.6 g of an unknown molecular substance is dissolved in 300 g of
water. The resulting solution has a boiling point of 102.3 °C. Determine
the molar mass of the substance.
© 2012 Thomas van Geel
106
6. Sodium aluminum chloride, NaAlCl4, is soluble in water. One chemist
suggests that, on dissolving, it completely dissociates into six individual
ions:
NaAlCl4(s) → Na+(aq) + Al+3(aq) + 4Cl–(aq) (proposal A)
Another chemist believes that it dissociates into only two ions:
NaAlCl4(s) → Na+(aq) + AlCl4–(aq) (proposal B)
When 6.00 grams of NaAlCl4 are dissolved in 100 grams of pure water, the
freezing point of the resulting solution is –3.5 °C. Do the results of this
experiment support proposal A, proposal B, or neither? Provide
calculations to support your choice.
7. An unknown ionic substance contains a metal and chlorine. When 0.25
moles of this substance are dissolved in 150 g of water, the resulting
solution has a freezing point of -9.3 °C. Is the metal in this compound a
alkaline metal or an alkaline earth metal?
© 2012 Thomas van Geel
107
Chapter 12: Net Ionic Equations
Predict the products of the following reactions, then balance the equations.
Then write their complete ionic equations and their net ionic equations.
1.
AgNO3(aq) +
2.
Fe(s) +
3.
K(s) +
4. Cl2(g) +
5.
KI(aq) +
NaCl(aq) →
CuSO4(aq) →
AlPO4(aq) →
CaBr2(aq) →
Pb(NO3)2(aq) →
6. Ba(NO3)2(aq) + H2SO4(aq) →
© 2012 Thomas van Geel
108
Chapter 13: Ideal Gas Law (PV=nRT)
1. How many moles of oxygen will occupy a volume of 2.5 L at 1.2 atm and 25°C?
2. What volume will 2.0 moles of nitrogen occupy at 760 mm Hg and 20°C?
3. What pressure will be exerted by 25 g of CO2 at a temperature of 25°C and a
volume of 0.500 L?
4. At what temperature will 5.0 g of Cl2 exert a pressure of 900 mm Hg at a
volume of 0.750 L?
© 2012 Thomas van Geel
109
5. How many moles of nitrogen gas will occupy a volume of 0.347 L of 6680
mmHg and 27°C?
6. What volume will 454 grams of hydrogen (H2) occupy at 1.05 atm and 25°C?
7. Find the number of grams of CO2 that exert a pressure of 785 mmHg at a
volume of 32.5 L and a temperature 32°C.
8. An elemental gas has a mass of 10.3 g. If the volume is 58.4 L and the
pressure is 758 mmHg at a temperature of 2.5°C, what is the gas?
© 2012 Thomas van Geel
110
Chapter 13: Combined Gas Law
1. A sample of gas occupies 0.075 L at 0.97 atm and 18°C. Calculate its
volume at 1.052 atm and 150°C.
2. A balloon of helium occupies 2.30 L at 825 mm Hg and 70°C. What is its
volume at STP?
3. 0.200 L of a gas are at a temperature of 450°C and a pressure of 800 mm
Hg. What would be the volume of the gas at STP?
4. At STP, the volume of a gas is 1.050 L. What would be the volume of the
gas at 2.6 atm and 500 Kelvin?
5. 350 L of a gas are at a temp of 567°C and a pressure of 2.4 atm. What
would be the pressure of the gas if the temp went to 700 K and the
volume went to 200 L?
© 2012 Thomas van Geel
111
6. A cylinder of oxygen exerts a pressure of 2.0 atm at 20°C. At what
temperature will the pressure become 2.5 atm?
7. A soccer ball contains a confined sample of air. The pressure of the air is
1350 mm Hg at 23°C. What will be the pressure in the ball at 40°C (a
very hot afternoon!)?
8. A gas occupies a volume of 0.0284 L at 725 mmHg. What will be the
volume of this gas at 800 mmHg?
9. A gas occupies a volume of 0.0359 L at a temperature of 22°C. What will
the same gas occupy at a temperature of 28°C?
© 2012 Thomas van Geel
112
Chapter 13: Gas Stoichiometry
Use the following equation for questions 1-3: N2(g) + 3H2(g) → 2NH3(g)
1. What volume of nitrogen at STP would be required to react with 0.100 mol of
hydrogen?
2. What volume of nitrogen at 215°C and 715 mm Hg would be required to
react with 0.100 mol of hydrogen?
3. What volume of nitrogen at 215°C and 4.56 atm would be required to
produce 75.3 g of NH3?
4. What volume of NO at STP could be produced by reacting 8.74 g of Cu?
3Cu(s) + 8HNO3(aq) → 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l)
5. What volume of hydrogen, at 35°C and 0.965 atm, would be required to
produce 0.400 mol of HCl?
H2(g) + Cl2(g) → 2HCl(g)
6. If 0.500 mol of carbon disulfide reacts, what would be the total volume of the
products at 25°C and 4.23 atm?
CS2(l) + 3O2(g) → CO2(g) + 2SO2(g)
© 2012 Thomas van Geel
113
7. If 13.5 g of aluminum reacts in a 2.0 L bottle at 26°C, what will the pressure
be?
2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)
8. When 10.7 g of Al react, what volume of H2 will be produced at 47°C and 725
mm Hg?
2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)
9. Trinitrotoluene (TNT) undergoes the following reaction when it explodes:
2C7H5N3O6(s) → 3N2(g) + 7CO(g) + 5H2O(g) + 7C(s)
If 10 g of TNT are detonated inside a vessel with a volume of 0.5 L, what will
the pressure be inside the vessel? (Assume the temperature is 1000 K.)
Ammonium nitrate undergoes the following reaction when it explodes:
2NH4NO3(s) → 2N2(g) + 4H2O(g) + O2(g)
If 10 g of ammonium nitrate are detonated inside a vessel with a volume of
0.5 L, what will the pressure be inside the vessel? (Assume the temperature
is 1000 K.)
Gram for gram, is TNT or ammonium nitrate a more powerful explosive?
© 2012 Thomas van Geel
114
Chapter 13: Partial Pressure
1. A 5-L vessel contains 1 mol O2 and 2 mol N2. The temperature is 300 K.
a. What is the total number of moles of gas?
b. What is the total pressure inside the vessel?
c. What is the mole fraction of each gas?
d. What are the partial pressures of each gas?
2. A 10-L vessel has a total pressure of 2 atm. The temperature is 300 K.
The vessel contains:
He with a mole fraction of 0.50
Ne with a mole fraction of 0.40
Ar with a mole fraction of 0.10
a. What is the partial pressure of each gas?
b. How many moles of each gas are in the container?
c. How many total moles of gas are in the container?
© 2012 Thomas van Geel
115
3. You collect 38 mL of H2 over water in a
eudiometer. The temperature is 292 K and the
pressure is 1.05 atm. How many moles of H2
did you collect?
1
Vapor
Pressure
(atm)
0.0065
21
Vapor
Pressure
(atm)
0.0246
2
0.0070
22
0.0261
3
0.0075
23
0.0277
4
0.0080
24
0.0294
5
0.0086
25
0.0313
6
0.0092
26
0.0332
7
0.0099
27
0.0351
8
0.0106
28
0.0373
9
0.0113
29
0.0395
10
0.0121
30
0.0418
11
0.0129
31
0.0443
12
0.0138
32
0.0469
13
0.0148
33
0.0496
14
0.0158
34
0.0525
15
0.0168
35
0.0555
16
0.0180
36
0.0586
17
0.0191
37
0.0619
18
0.0203
38
0.0653
19
0.0217
39
0.0690
20
0.0231
40
0.0727
Temp
(°C)
Temp
(°C)
4. You collect 45 mL of an unknown gas over water in a eudiometer. The
temperature is 24°C and the pressure is 1 atm. How many moles of the
gas did you collect?
5. If the gas you collected has a mass of 0.100 g, what is the molar mass of
the gas?
© 2012 Thomas van Geel
116
Chapter 13: Graham’s Law of Diffusion
1. At the same temperature and pressure, which gas moves faster, He or N2?
What is the ratio of their velocities (He to N2)?
2. If a balloon of CH4 takes 2 days to deflate by leakage, how long would it
take an identical balloon of H2 to deflate?
3. If it takes gas X 1.5 times longer to diffuse than O2 gas, what is the molar
mass of gas X?
4. If it takes gas Y 1.41 times longer to diffuse than SO2 gas, what is the
molar mass of gas Y?
© 2012 Thomas van Geel
117
Chapter 14: Kinetics
Reaction A
Reaction B
iii
ii
E
i
E
iv
v
progress of reaction
progress of reaction
1. Label the parts of graph A:
i.
ii.
iv.
v.
iii.
2. Which reaction is endothermic, and which is exothermic? Explain.
3. What is happening to the bonds at point iii?
4. Explain the following in terms of reaction rates.
a. Why do many animals chew their food instead of swallowing it whole?
b. Why do we refrigerate food?
c. Why does rubbing apple slices with lemon juice prevent them from
browning?
d. Why do oxygen tanks in hospitals have warning labels?
© 2012 Thomas van Geel
118
5. Draw two energy diagrams for an exothermic reaction; one without a catalyst
and one with a catalyst. Explain the differences.
6. Surprisingly small amounts of catalysts are needed to cause rapid increases in
reaction rates. Why are such small amounts so effective?
© 2012 Thomas van Geel
119
Chapter 14: Equilibrium Problems
1. Write the K expressions for the following equations.
a. HF(aq) ⇄ H+(aq) + F-(aq)
b. Cu+2(aq) + 4NH3(aq) ⇄ Cu(NH3)4+2(aq)
c. H2O(l) + CO2(aq) ⇄ H2CO3(aq)
d. BaF2(s) ⇄ Ba+2(aq) + 2F-(aq)
© 2012 Thomas van Geel
120
2. When the equilibrium in 1c is established, the following concentrations are
measured. What is the K value for the reaction?
[H2CO3] = 7.7 x 10-6 M
[CO2] = 4.5 x 10-3 M
3. When the equilibrium in 1b is established, the following concentrations are
measured. What is the K value for the reaction?
[Cu+2] = 3.8 x 10-4 M
[NH3] = 9.1 x 10-5 M
[Cu(NH3)4+2] = 2.9 x 10-7 M
4. When the equilibrium in 1a is established, the following concentrations are
measured. What is the K value for the reaction?
[H+] = 2.5 x 10-4 M
[F-] = 0.011 M
[HF] = 0.0044 M
© 2012 Thomas van Geel
121
5. The equilibrium in 1c is established (K = 1.7 x 10-3). The concentration of
CO2 is 0.38 M. What is the concentration of H2CO3?
6. The equilibrium in 1a is established (K = 6.3 x 10-4). The concentrations
of H+ and HF are 4.8 x 10-5 M and 6.3 x 10-4 M, respectively. What is the
concentration of F-?
7. A solution contains the reaction in 1b (K = 1.1 x 1013). At equilibrium, the
Cu+2 concentration and the NH3 concentration are found to be 1.5 x 10-4
M and 3.2 x 10-5 M, respectively. What is the concentration of
Cu(NH3)4+2?
© 2012 Thomas van Geel
122
8. A saturated solution of BaF2 is made. The Ba+2 concentration is measured
as 3.58 x 10-3 M. What is the K value for reaction 1d?
9. A 0.1 M solution of HF is made and allowed to come to equilibrium. The
equilibrium concentration of H+ is 0.0076 M. What is the K value of the
reaction?
10. A 0.05 M solution of Cu(NH3)4+2 is made and allowed to reach equilibrium.
The equilibrium concentration of Cu+2 is measured to be 4.5 x 10-4 M.
What is the K value for equation 1b?
© 2012 Thomas van Geel
123
11. A saturated solution of BaF2 is made. The equilibrium in 1d is established
(K = 1.84 x 10-7). Calculate the concentrations of Ba+2 and F- in the
solution.
12. A 0.300 M solution of HF is made, and the HF dissociates according to the
reaction in 1a, which has a K value of 6.3 x 10-4. What are the
concentrations of all ions once equilibrium has been reached?
13. A 0.0200 M solution of CO2 is made and the equilibrium in 1c is
established (K = 1.7 x 10-3). What are the equilibrium concentrations of
CO2 and H2CO3?
© 2012 Thomas van Geel
124
14. A solution is made where the initial concentrations of HF, H+ and F- are
0.03, 4.1 x 10-6 M, and 8.9 x 10-8 M respectively. Calculate Q and predict
which way the reaction will shift to reach equilibrium. (K = 6.3 × 10-4)
15. A solution is made where the initial concentrations of CO2 and H2CO3 are
0.045 M and 0.0022 M, respectively.
a. Calculate Q and predict which way the reaction will shift to reach
equilibrium. (K = 1.7 × 10-3)
b. Calculate the concentrations of the ions once equilibrium has been
reached from the starting concentrations.
© 2012 Thomas van Geel
125
16. Consider reaction 1d, which is an endothermic reaction.
What will happen to the concentration of Cl- when each of the following
changes is made?
a. Add Ag+ to the solution
b. Remove some AgCl(s) from the container
c. Heat the solution
17. Consider the following equilibrium (the reaction is endothermic):
N2O4(g) ⇄ 2NO2(g)
In what direction will the equilibrium shift when each of the following
changes is made?
a. Add N2O4
b. Remove NO2
c. Increase the volume
d. Decrease the temperature
e. Add a catalyst
© 2012 Thomas van Geel
126
Chapter 15: pH and pOH
1. Fill in the following chart.
[H+]
[OH-]
pH
pOH
Acidic or Basic?
3.4 × 10-5
9.3 × 10-2
11.3
13.1
1.3 × 10-10
7.7 × 10-13
2.4
1.8
2. Calculate the pH of the following strong acids and bases.
a. 0.05 M HCl
b. 0.003 M HNO3
c. 0.02 M NaOH
d. 0.00035 M Mg(OH)2
© 2012 Thomas van Geel
127
3. Calculate the pH of the following weak acids and bases.
a. 0.15 M HF (Ka = 6.3 × 10-4)
b. 0.5 M HOCl (Ka = 3.5 × 10-8)
c. 0.8 M NH3 (Kb = 1.8 × 10-5)
d. 0.56 M NaHCO3 (Kb = 2.4 × 10-8)
© 2012 Thomas van Geel
128
Chapter 15: Finding Ka and Kb
1. The pH of a 0.100 M solution of formic acid (HCHO2) is 2.38. What is the
Ka of formic acid?
2. The pH of a 0.200 M solution of HCN is 5.00. What is the Ka of HCN?
3. The pH of a 0.500 M solution of Na2CO3 is 12.01. What is he Kb of CO3-2?
4. The pH of a 0.300 M solution of N2H4 is 8.73. What is he Kb of N2H4?
© 2012 Thomas van Geel
129
Chapter 15: Acid-Base Definitions
1. Identify the following as an Arrhenius Acid, Arrhenius Base, or neither:
HCl
HClO4
Fe(OH)3
NH3
BF3
H2SO3
NaOH
C6H12O6
2. Fill in the following chart:
Conjugate Acid
Conjugate Base
H2CO3
HCO3H2 O
HBr
H2 O
NH3
PO4-3
I3. Connect the conjugate acid-base pairs with brackets and label each species
as an acid or a base:
a. HC2H3O2 + NH3 ⇄ NH4+ + C2H3O2b. NH2- + H2O ⇄ NH3 + OHc. H2O + H2O ⇄ H3O+ + OHd. NH3 + H2O ⇄ NH4+ + OHe. HBr + H2O ⇄ H3O+ + Br-
© 2012 Thomas van Geel
130
5. Write an equation for the reaction of H2SO3 with water in which the
molecule acts as a Brønsted-Lowry acid.
6. Write an equation for the reaction of SO3-2 with water in which the ion
acts as a Brønsted-Lowry base.
7. Write an equation for the reaction of H2PO3- with water in which the ion
acts as an acid.
8. Write an equation for the reaction of CH3NH2 with water in which the
molecule acts as a base.
© 2012 Thomas van Geel
131
Chapter 15: Indicators
Use the following table to solve the problems.
Indicator
Thymol Blue
Methyl yellow
Bromphenol blue
Alizarin sodium sulfonate
Bromcresol green
Methyl red
Bromcresol purple
Phenol red
Thymol blue
Phenolphthalein
Thymolphthalein
Alizarin yellow
Trinitrobenzoic acid
Color
changes
at pH of:
2
3.5
3.8
4.5
4.8
5.3
6
7.2
8.8
9
10
11
12.7
Color on
the Acid
Side
red
red
yellow
yellow
yellow
red
yellow
yellow
yellow
colorless
colorless
yellow
colorless
Color on
the Base
Side
yellow
yellow
blue-violet
violet
blue
yellow
purple
red
blue
red
blue
lilac
orange-red
1. A solution is tested with multiple indicators, yielding the following results:
Phenolphthalein
Thymol blue
Thymolphthalein
Alizarin yellow
Trinitrobenzoic acid
red
blue
blue
yellow
colorless
Estimate the pH of the solution.
2. A solution is tested with multiple indicators, yielding the following results:
Methyl yellow
Thymol Blue
Bromphenol blue
Bromcresol green
Alizarin sodium sulfonate
yellow
yellow
blue-violet
yellow
violet
Estimate the pH of the solution.
© 2012 Thomas van Geel
132
Chapter 15: Titrations
1. 10 mL of HCl of unknown concentration is titrated with 0.100 M NaOH. It
requires 25.0 mL of base to reach the equivalence point. What is the
concentration of the acid?
2. 15 mL of H2SO4 of unknown concentration is titrated with 0.200 M KOH.
It requires 21.0 mL of base to reach the (second) equivalence point.
What is the concentration of the acid?
3. 12 mL of H3PO4 of unknown concentration is titrated with 0.100 M NaOH.
It requires 82.8 mL of base to reach the (third) equivalence point. What
is the concentration of the acid?
4. 10 mL of Ca(OH)2 of unknown concentration is titrated with 0.100 M HCl.
It requires 18.0 mL of acid to reach the equivalence point. What is the
concentration of the base?
5. 25 mL of Mg(OH)2 of unknown concentration is titrated with 0.050 M
H3PO4. It requires 9.67 mL of acid to reach the equivalence point. What
is the concentration of the base?
© 2012 Thomas van Geel
133
Chapter 15: Buffers
The following buffer system helps maintain the pH of human blood:
H2CO3(aq) ⇄ H+(aq) + HCO3-(aq)
1. If something acidic is added to the bloodstream, which way would the
equilibrium shift?
2. If something basic is added to the bloodstream, does the concentration of
bicarbonate rise or fall?
Another way your body controls the pH of your blood is by changing your
breathing rate. Note the following facts:
The following equilibrium also exists in blood:
CO2(aq) + H2O(l) ⇄ H2CO3(aq)
CO2 is removed from the blood by breathing. The faster you
breath, the faster CO2 leaves the blood.
3. If something acidic is added to the blood, would your body respond by
breathing faster or slower?
4. If something basic is added to the blood, would your body respond by
breathing faster or slower?
5. If you make yourself breath faster, would the pH of your blood increase or
decrease?
6. If you held your breath, would the pH of your blood increase or decrease?
© 2012 Thomas van Geel
134
7. How many different buffers could be made by combining pairs of the
following compounds in solution? (Making roughly equal concentrations
of each compound.)
Na3PO4
Na2SO4
NH4Cl
NaOH
H3PO4
NH3
Na2HPO4
NaHSO4
NaH2PO4
H2SO4
HCl
© 2012 Thomas van Geel
135
Chapter 16: Enthalpy of Reaction
1. 7 moles of an unknown substance are dissolved in 200 g of water. The
temperature of the water decreases 4 degrees. What is the ∆H for the
dissolving of this substance? (specific heat of the solution is 4.18 J/g-K.)
2. 4 moles of the same substance in the previous problem are dissolved in
100 g of water at 25 °C. What will the temperature be after the
substance has dissolved?
3. The ΔH for the combustion of CH4 is -890 kJ/mol. How much heat is
released when 80.0 g is burned?
© 2012 Thomas van Geel
136
4. The combustion of a certain gas has a ∆H = -241.8 kJ/mol. When 40 g of
the gas are burned, 4836 kJ are given off. Identify the gas.
5. When a student mixes 50 mL of 1.0 M HCl and 50 mL of 1.0 M NaOH in a
coffee-cup calorimeter, the temperature increases from 21.0 to 27.5 °C.
Calculate the ΔH for the reaction. (specific heat of solution = 4.18 J/g-K)
© 2012 Thomas van Geel
137
Chapter 16: Bond Enthalpies
Use the following table of bond enthalpies to estimate the enthalpies of reaction.
Bond
C-C
C=C
C≡C
C-N
C=N
C≡N
C-Br
N=N
N≡N
N-O
Bond Enthalpy (kJ/mol)
347
598
813
285
616
866
285
418
946
222
Bond
C-H
C=O
O=O
H-O
H-Cl
H-N
H-H
Br-Br
Cl-Cl
N=O
Bond Enthalpy (kJ/mol)
413
805
498
464
431
391
436
193
242
590
1. H2 + Cl2 → 2HCl
2. 2C2H2 + 5O2 → 4CO2 + 2H2O
3. C2H4 + Br2 → C2H4Br2
4. 4CH3NH2 + 9O2 → 4CO2 + 10H2O + 2N2
© 2012 Thomas van Geel
138
Chapter 16: Hess's Law
1. The ∆H of the following reaction is -242 kJ/mol.
2CO(g) → 2C(s) + O2(g)
Determine the ∆H values for the following reactions:
a. 2C(s) + O2(g) → 2CO(g)
b. CO(g) → C(s) + ½O2(g)
c. 6C(s) + 3O2(g) → 6CO(g)
2. Given the following information:
S(s) + O2(g) → SO2(g)
∆H = -297 kJ/mol
2SO3(g) → 2SO2 + O2(g)
∆H = +198 kJ/mol
Determine the ∆H of the reaction:
2S(s) + 3O2(g) → 2SO3(g)
© 2012 Thomas van Geel
139
3. Given the following information:
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(l)
∆H = -1332 kJ/mol
C(graphite) + O2(g) → CO2(g)
∆H = -236 kJ/mol
3C(graphite) + 4H2(g) → C3H8(g)
∆H = -62 kJ/mol
Determine the ∆H of the reaction:
H2O(l) → H2(g) + ½O2(g)
4. Given the following information:
H2(g) + Br2(g) → 2HBr(g)
∆H = -72 kJ/mol
H2(g) → 2H(g)
∆H = 436 kJ/mol
Br2(g) → 2Br(g)
∆H = 224 kJ/mol
Determine the ∆H of the reaction:
H(g) + Br(g) → HBr(g)
© 2012 Thomas van Geel
140
Chapter 16: Standard Enthalpies of Formation
1. For which of the following reactions would the enthalpy change represent
a standard enthalpy of formation? For those where it does not, what
changes would need to be made in the reaction conditions?
a. 2Na(s) + 1/2O2(g) → Na2O(s)
b. 2K(l) + Cl2(g) → 2KCl(s)
c. C6H12O6(s) → 6C(diamond) + 6H2(g) + 3O2(g)
2. Write the equation corresponding to the standard enthalpy of formation of
liquid carbon tetrachloride.
For the following problems, use the standard enthalpies of formation on the
following page.
3. Calculate the ΔH for the dissolving of ammonium nitrate in water:
NH4NO3(s) → NH4NO3(aq)
4. Calculate the enthalpy change for the detonation of nitroglycerin:
2C3H5(NO3)3(l) → 3N2(g) + 1/2O2(g) + 6CO2(g) + 5H2O(g)
5. Calculate the ΔH for the following reaction:
C6H6(l) +
15
/2O2(g) → 6CO2(g) + 3H2O(l)
© 2012 Thomas van Geel
141
Standard Enthalpies of Formation
C6H6(l)
∆H°f = 48.95 kJ/mol
C3H5(NO3)3(l)
∆H°f = -364 kJ/mol
CO2(g)
∆H°f = -393.5 kJ/mol
H2O(g)
∆H°f = -241.8 kJ/mol
H2O(l)
∆H°f = -285.8 kJ/mol
NH4NO3(s)
∆H°f = -365.56 kJ/mol
NH4NO3(aq)
∆H°f = -339.87 kJ/mol
© 2012 Thomas van Geel
142
Chapter 16: Entropy and Gibb’s Free Energy
1. Identify each situation below as being an example of either increasing or
decreasing entropy.
a. water freezing
b. separating two liquids by distillation
c. KNO3 dissolving
d. coal burning
e. mixing two solutions to make a precipitate
f. sugar melting
increase
increase
increase
increase
increase
increase
decrease
decrease
decrease
decrease
decrease
decrease
2. If the spontaneity depends on temperature: At high temperatures, does ∆H
or ∆S predominate and determine if the reaction is spontaneous or not?
3. If the spontaneity depends on temperature: At low temperatures, does ∆H or
∆S predominate and determine if the reaction is spontaneous or not?
4. Fill in the following chart:
Sign of ∆H
Sign of ∆S
+
+
+
-
-
+
-
-
Always spontaneous?
If temperature dependent:
Never spontaneous?
spontanenous at high or low
Depends on temperature?
temperature?
© 2012 Thomas van Geel
143
5. Identify the signs of ∆H and ∆S for each reaction.
∆H
+
or
a.
∆S
+
or
-
If temperature
Always spontaneous?
dependent:
Never spontaneous?
spontanenous at
Depends on temperature?
high or low
temperature?
A(s) → B(g) + energy
b.
A(l) → B(s)
(exothermic)
c.
A(s) + B(g) → B(s) + energy
d.
A(s) → B(aq) + energy
e.
A(s) → B(g) + C(g)
(endothermic)
f.
A(l) + energy → B(g)
g.
AB(s) → A+(aq) + B-(aq) + energy
h.
A(l) + energy → B(s)
6. When ∆G = 0, the reaction is stuck in the middle between spontaneous and
non-spontaneous; in other words, it is at equilibrium.
a. For a reaction with ∆H = -40000 J/mol and ∆S = -34 J/mol-K, at what
temperature will this reaction be at equilibrium?
b. At higher temperatures will this reaction be spontaneous or
nonspontaneous? Explain your reasoning.
© 2012 Thomas van Geel
144
Chapter 17: Oxidation Numbers and Redox Reactions
Assign oxidation numbers to all the atoms in the following:
1. He
2. P4
3. Al2O3
4. H2O
5. PO4-3
6. MnO4-
7. H2O2
8. NH4+
9. NaH
10. Cr2O7-2
11. Cl2O
12. NO3-
© 2012 Thomas van Geel
145
Determine the oxidizing agent and the reducing agent in the following redox
reactions.
13. Fe + Zn+2 → Fe+2 + Zn
14. Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
15. 2H2 + O2 → 2H2O
16. Mg + Br2 → MgBr2
© 2012 Thomas van Geel
146
Chapter 17: Voltaic Cells
1. A standard voltaic cell is set up using Ag metal in Ag+ solution and Cu
metal in Cu+2 solution.
a. Which half cell will be the anode and which will be the cathode?
b. Write the two half reactions in this cell.
c. Write the overall (balanced) reaction in this cell.
d. What voltage will the cell generate?
2. A standard voltaic cell is set up using Fe metal in Fe+3 solution and Zn
metal in Zn+2 solution.
a. Which half cell will be the anode and which will be the cathode?
b. Write the two half reactions in this cell.
c. Write the overall (balanced) reaction in this cell.
d. What voltage will the cell generate?
© 2012 Thomas van Geel
147
3. A standard voltaic cell is set up using Al metal in Al+3 solution and Cu
metal in Cu+ solution.
a. Which half cell will be the anode and which will be the cathode?
b. Write the two half reactions in this cell.
c. Write the overall (balanced) reaction in this cell.
d. What voltage will the cell generate?
4. A standard voltaic cell is set up using Cr metal in Cr+3 solution and Mg
metal in Mg+2 solution.
a. Which half cell will be the anode and which will be the cathode?
b. Write the two half reactions in this cell.
c. Write the overall (balanced) reaction in this cell.
d. What voltage will the cell generate?
© 2012 Thomas van Geel
148
Chapter 18: Organic Functional Groups
On the following compounds, circle and label all functional groups.
O
OH
OH
OH
O
O
HO
H
O
OH
aspirin (a pain reliever)
OH
glucose (a simple sugar)
O
H
N
O
O
OH
diphenhydramine (an antihistamine)
vanillin (the flavor of vanilla)
OH
H
H
O
H
OH
O
O
testosterone (a male hormone)
H
N
pyruvic acid (a metabolic intermediate)
O
O
O
CF3
H 2N
fluoxetine (an antidepressant)
benzocaine (a topical anesthetic)
© 2012 Thomas van Geel
149
KEY
Chapter 1: Classifying Matter 1
1.
2.
3.
4.
5.
6.
7.
8.
9.
F
C
A
B
D
H
G
I
E
© 2012 Thomas van Geel
150
Chapter 1: Classifying Matter 2
Chemical formulas of
particles
Classification
Sketch all particles in
proper arrangement
(draw 6 particles)
1.
H
H
matter
pure substance
element
atomic
mixture
compound
H
homogeneous
heterogeneous
H
C
CH4
H
H
H
H
H
H
H
molecular
C
C
H
H
H
C
C
H
H
H
H
H
H
H
H
C
H
H
2.
O
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
O
O2
O
O
O
O
O
O
O
O
molecular
O
O
C
O
3.
N
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
CO2, N2
N
O
O
C
O
N
N
molecular
N
N
O
C
O
4.
matter
pure substance
element
atomic
H
mixture
compound
homogeneous
heterogeneous
H2 , C
H
H
H
H
H
C
C
C
molecular
5.
O
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
H2, O3
H
O
O
H
H
O
H
O
O
O
molecular
O
O
H
H
6.
pure substance
element
atomic
molecular
compound
mixture
homogeneous
heterogeneous
N2O2
O
N
N
O
N
N
O
N
O
N
O
O
N
O
N
O
O
N
O
N
matter
O
N
O
N
© 2012 Thomas van Geel
151
7.
H
H
H
H
N
matter
pure substance
element
atomic
mixture
compound
N
H
homogeneous
heterogeneous
H
H
NH3
H
N
H
H
N
H
H
H
N
H
H
molecular
H
H
N
H
8.
H
H
element
atomic
mixture
compound
H
H
matter
pure substance
H
N
O
homogeneous
heterogeneous
H
H2O, NH3
H
H
H
H
N
molecular
O
O
H
H
H
H
N
H
9.
H
H
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
H 2 , H2 O
H
H
H
H
H
H
O
O
H
H
molecular
H
O
H
10.
C
matter
pure substance
element
atomic
mixture
compound
C
C
homogeneous
heterogeneous
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
molecular
C
C
C
11.
matter
pure substance
element
atomic
H
mixture
compound
homogeneous
heterogeneous
HCN
C
H
H
N
C
C
H
N
C
H
molecular
H
C
N
N
C
N
N
12.
O
pure substance
element
atomic
homogeneous
heterogeneous
O3
O
O
O
O
O
O
H
compound
mixture
homogeneous
heterogeneous
H
C
H
H
H
H
C
H
C
C
H
C2H4, O2
H
H
C
matter
pure substance
molecular
O
O
O
13.
atomic
O
O
O
molecular
element
O
O
mixture
compound
O
O
O
matter
H
O
O
C
O
O
H
O
O
© 2012 Thomas van Geel
152
14.
O
C
matter
pure substance
element
atomic
mixture
compound
C
O
homogeneous
heterogeneous
O
O
O
CO2
O
C
C
O
O
O
molecular
C
O
C
O
O
15.
N
matter
pure substance
element
atomic
mixture
compound
N
N
O
N
homogeneous
heterogeneous
O
N 2 , N2 O
N
N
N
N
N
N
N
O
molecular
N
16.
He
matter
pure substance
element
atomic
mixture
compound
He
He
homogeneous
heterogeneous
He
He
He
He
molecular
17.
O
H
H
O
matter
pure substance
element
atomic
compound
H
H
mixture
homogeneous
heterogeneous
H2O2
O
O
O
O
O
H
H
O
O
molecular
H
H
H
H
O
O
H
H
O
18.
O
matter
pure substance
element
atomic
mixture
compound
homogeneous
heterogeneous
H
H2, O2
H
H
H
H
H
matter
compound
homogeneous
heterogeneous
H
C
H
H H
mixture
H
H
O
H
pure substance
O
O
molecular
element
H
O
O
19.
atomic
O
H H
H
C
H2O, CH4
O
H
H
C
H
H
H
H
molecular
O
H
20.
O
matter
pure substance
element
atomic
molecular
compound
mixture
homogeneous
heterogeneous
H2, O2, N2
H
O
N
H
O
N
N
O
N
H
H
© 2012 Thomas van Geel
153
Chapter 1: Chemical Formulas
1.
2.
3.
4.
5.
C5H8NO2
C4H8N2O3
C6H12O6
C6H9N3O3
C10H20O5
Chapter 1: Organic Chemical Formulas
1.
2.
3.
4.
5.
6.
C4H10
C3H6
C6H8
C5H6
C4H8N2O3
C5H5N5
Chapter 1: Chemical and Physical Changes
1. physical, boiling
2. chemical
3. physical, sublimation
4. chemical
5. physical, deposition
6. chemical
7. chemical
8. physical, freezing
9. physical, melting
10. physical, condensation
Chapter 1: Chemical and Physical Properties
1. chemical
2. physical
3. chemical
4. physical
5. physical
6. chemical
7. physical
8. physical
9. chemical
10. chemical
© 2012 Thomas van Geel
154
Chapter 2: Taking Measurements
1.
2.
3.
4.
5.
2.76 cm
2.30 cm
1.00 cm
28 cm
30. cm
Chapter 2: Taking Measurements 2
1.
2.
3.
4.
5.
6.
0.78 mL
1.00 mL
10.0 mL
1.50 mL
1.5 mL
17 mL
Chapter 2: Counting Sig Figs
1. 4
2. 3
3. 5
4. 6
5. 3
6. 3
7. 5
8. 2
9. 4
10. 6
Chapter 2: Calculations with Sig Figs
1. 1000.
2. 1.00 × 103
3. 1.0 × 103
4. 1000.
5. 1.00 × 103
6. 1008
7. 1010
8. 80,000.
9. 8.00 × 104
10. 8.00 × 104
11. 8.000 × 104
12. 8.000 × 104
© 2012 Thomas van Geel
155
13. 2000
14. 2.0 × 103
15. 2.00 × 103
16. 2000.
17. 1.0 × 104
18. 1.00 × 104
19. 1 × 102
20. 2.0 × 102
Chapter 2: Scientific Notation
1. a. 6.7000 × 103
b. 1.505 × 10-11
c. 8.05000 × 105
d. 4.0400 ×10-4
2. a. 820,000,000
b. 0.00000150
c. 0.0000001775
d. 7060.
Chapter 2: Precision and Accuracy
1.
2.
3.
4.
5.
A: 10.29
A: 0.488
A: 0.0550
BADC
CABD
B: 10.24
B: 0.0244
B: 0.458
C: 12.04
C: 17.6
C: 0.0175
D: 10.66
D: 4.10
D: 1.22
Chapter 2: Metric-to-Metric Conversions
1. 220 mg
2. 143 cm
3. 0.000202 kg
4. 22.5 cg
5. 580 cg
6. 5650 mm
7. 0.000375 L
8. 0.90 cm
9. 0.0000095 m
10. 0.000124 km
© 2012 Thomas van Geel
156
Chapter 2: Length Conversions
1. 31680 ft
2. 9.09 furlongs
3. 48 furlongs
4. 39.37 feet
5. 7.46 miles
6. 0.039 inches
7. cm
8. km
9. m
10. mm
11. cm
12. m
13. mm
14. km
Chapter 2: Mass Conversions
1.
2.
3.
4.
5.
6.
7.
8.
40 jin
450 qian
350 dan
341 g
5.68 g
13.1 mg
6.82 kg
70.5 kg
Chapter 2: Area and Volume Conversions
1. 134 mm2
2. 4.5 x 1012 cm2
3. 3.5 x 10-7 dm2
4. 23100 mm2
5. 54400 mm3
6. 2.22 x 10-16 hm3
7. 9.98 x 10-9 km3
8. 450,00 cubits
9. 2,080,000 in3
10. 77,200 yd3
11. L
12. mL
13. mL
14. L
© 2012 Thomas van Geel
157
Chapter 2: Conversions with Density
1.
2.
3.
4.
5.
26.7 cL
2100 kg
680 mg
1.6 x 10-16 km3
61.1 kg
Chapter 2: Conversions with Percent
1. 4.5 g sugar
2. 2600 mg gold
3. 0.000234 L copper
Chapter 2: Converting Compound Units
1.
2.
3.
4.
5.
6.
7.
8.
0.055 kg/cL
0.766 g/dL
4,400,000,000 mg/kL
12.7 km/L
0.08 pounds/pint
29.1 m/second
140.8 ounces/L
704.5 g/cm2
Chapter 2: Fenceposting Word Problems
1. $920.77
2. 178.6 cartons
3. Can go 13.5 km, so can’t reach the town
Chapter 2: Converting Temperature
1.
2.
3.
4.
5.
6.
374 K
25°C
195 K
19727°C
1773 K
-269°C
© 2012 Thomas van Geel
158
Chapter 3: Ionic Formulas
Cl
CO3-2
OH-
SO4-2
PO4-3
NO3-
Na
NaCl
Na2CO3
NaOH
Na2SO4
Na3PO4
NaNO3
NH4+
NH4Cl
(NH4)2CO3
NH4OH
(NH4)2SO4
(NH4)3PO4
NH4NO3
K
KCl
K2CO3
KOH
K2SO4
K3PO4
KNO3
Ca
CaCl2
CaCO3
Ca(OH)2
CaSO4
Ca3(PO4)2
Ca(NO3)
Mg
MgCl2
MgCO3
Mg(OH)2
MgSO4
Mg3(PO4)2
Mg(NO3)
Zn
ZnCl2
ZnCO3
Zn(OH)2
ZnSO4
Zn3(PO4)2
Zn(NO3)
Fe+3
FeCl3
Fe2(CO3)3
Fe(OH)3
Fe2(SO4)3
FePO4
Fe(NO3)3
Al
AlCl3
Al2(CO3)3
Al(OH)3
Al2(SO4)3
AlPO4
Al(NO3)3
Co+3
CoCl3
Co2(CO3)3
Co(OH)3
Co2(SO4)3
CoPO4
Co(NO3)3
Fe+2
FeCl2
FeCO3
Fe(OH)2
FeSO4
Fe3(PO4)2
Fe(NO3)
H
HCl
H2CO3
HOH
H2 O
H2SO4
H3PO4
HNO3
© 2012 Thomas van Geel
159
Chapter 3: Ionic Nomenclature
1. Calcium carbonate
2. Potassium chloride
3. Iron(II) sulfate
4. Lithium bromide
5. Magnesium chloride
6. Iron(III) chloride
7. Zinc phosphate
8. Ammonium nitrate
9. Aluminum hydroxide
10. CuC2H3O2
11. Fe2O3
12. (NH4)3PO4
13. CuSO4
14. NaHCO3
15. NiBr3
16. Be(NO3)2
17. ZnSO4
18. AuCl3
Chapter 3: Molecular Nomenclature
1. Sulfur dioxide
2. CCl4
3. Nitrogen trichloride
4. BI3
5. Sulfur hexafluoride
6. N2O5
7. Dinitrogen monoxide
8. SiCl4
9. Nitrogen octaiodide
10. ICl7
11. Dicarbon dichloride
12. XeF6
© 2012 Thomas van Geel
160
Chapter 4: Moles Worksheet
Formula
Molar Mass
Moles
Grams
Particles
C
12.01
2.5
30.0
1.51 × 1024
O2
32.00
6.25
200
3.76 × 1024
Mg3(PO4)2
262.86
7.48
1960
4.5 x 1024
NaOH
40.00
7.7
308
4.64 × 1024
Mg(OH)2
58.32
0.000583
0.034
3.51 × 1020
K2CO3
138.12
0.000914
0.126
5.5 x 1020
4. 12.5 g/mol
5. 20.0 g/mol
6. potassium
Chapter 4: Mole/Mass/Particle Conversions
1. 0.472 mol C
2. 595 mol N
3. 15800 g Au
4. 396 g Xe
5. 1.51 × 1023 atoms Sr
6. 0.334 mol He
7. 0.000159 mol Al
8. 2.10 × 1025 atoms Cl
9. 2.85 × 1021 atom Ag
10. 19.6 g K
11. 2.00 × 1024 atoms P
12. 44.3 g S
13. 6 mol Cl
14. MgF2, 12.5 g F
15. Al2O3, 10.7 g O
© 2012 Thomas van Geel
161
Chapter 4: % Composition
1.
2.
3.
4.
5.
6.
7.
8.
24.74% K, 34.76% Mn, 40.50% O
2.76% H, 97.24% Cl
16.39% Mg, 18.89% N, 64.72% O
28.19% N, 8.11% H, 20.78% P, 42.93% O
15.77% Al, 28.11% S, 56.11% O
39.17 g O
17.5 g Fe
190 g Ag
Chapter 4: Empirical and Molecular Formulas
1. CH
2. CH2O
3. C3H4
4. C2H3
5. C12H22O11
6. N2O4
7. C5H10
8. empirical C6H7O4,
9. empirical C3H5O3,
10. empirical C5H7O2,
11. empirical C9H8O4,
molecular
molecular
molecular
molecular
C12H14O8
C3H5O3
C10H14O4
C9H8O4
Chapter 5: Balancing Chemical Equations
1. 2N2 + 3H2 → 2NH3
2. 2KClO3 → 2KCl + 3O2
3. 2NaCl + F2 → 2NaF + Cl2
4. 2H2 + O2 → 2H2O
5. 2AgNO3 + MgCl2 → 2AgCl + Mg(NO3)2
6. 2AlBr3 + 3K2SO4 → 6KBr + Al2(SO4)3
7. CH4 + 2O2 → CO2 + 2H2O
8. C3H8 + 5O2 → 3CO2 + 4H2O
9. 2C8H18 + 25O2 → 16CO2 + 18H2O
10. FeCl3 + 3NaOH → Fe(OH)3 + 3NaCl
11. 4P + 5O2 → 2P2O5
12. 2Na + 2H2O → 2NaOH + H2
13. 2Ag2O → 4Ag + O2
14. S8 + 12O2 → 8SO3
15. 6CO2 + 6H2O → C6H12O6 + 6O2
16. 2HCl + CaCO3 → CaCl2 + H2O + CO2
© 2012 Thomas van Geel
162
Chapter 5: Wacky Balancing
1. NiS + {2}O2 + {2}HCl → [Ni]Cl2 + H2SO4
2. V2[O]5 + {3}Zn → V[2]O2 + {3}[Zn]O
3. {5}P4[S]3 + {12}Br2 → 3P4S5 + 8P[Br]3
4. 3[Nb] + {4}I2 → Nb[3][I]8
5. CO([N]H2)2 + {6}HOCl → 2NCl3 + CO[2] + {5}H2O
6. {2}NaOH + Cl[2] → NaCl + NaClO + H2[O]
7. [Fe]C2O4 → Fe[O] + C[O]2 + [C]O
8. {3}[Na]Cl[O] + [N]H3 → N[Cl]3 + 3NaO[H]
9. Na[2][C]O[3] + 2HCl → H2C[O]3 + 2[Na]Cl
10. {2}Ca3([P][O]4)2 + {6}SiO2 + 10C → P4 + {6}CaSiO[3] + 10[C]O
Chapter 5: Mole-to-Mole Stoichiometry
1.
2.
3.
4.
5.
6.
7.
6 mol H2
9 mol O2
3 mol H2
20 mol O2
6 mol KNO3
H3PO4 + 3NaOH → Na3PO4 + 3H2O, 1.32 mol NaOH
3NaClO + NH3 → NCl3 + 3NaOH, 3.3 mol NCl3
Chapter 5: Mass-to-Mass Stoichiometry
1.
2.
3.
4.
5.
6.
7.
15.2 g
10.8 g
60.8 g
4.22 g
3.06 g
0.0771 g
2.79 g
Chapter 5: Advanced Stoichiometry
1.
2.
3.
4.
5.
6.
Ca
K
P
Cl
C6H6
C2H4
© 2012 Thomas van Geel
163
Chapter 5: Limiting Reagent Stoichiometry
1. a. N2
b. 34.1 g
c. N2 0 g, H2 18.9 g, NH3 34.1 g
2. a. HCl
b. 2.08 g H2
c. Mg 25.0 g, HCl 0 g, MgCl2 97.8 g, 2.08 g H2
3. a. AgNO3
b. 164.3 g
c. AgNO3 0 g, Na3PO4 135.7 g, Ag3PO4 164.3 g, NaNO3 100.0 g
4. a. SO2
b. 3.43 g
c. SO2 0 g, Ca(OH)2 70.2 g, CaSO3 22.9 g, H2O 3.43 g
5. 2C2H2 + 5O2 → 2H2O + 4CO2
a. O2
b. 4.29 g
c. C2H2 1.67 g, O2 0 g, H2O 0.243 g, CO2 4.29 g
6. Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
a. AgNO3
b. 57.1 g
c. Cu 13.2 g, AgNO3 0 g, Cu(NO3)2 49.7 g, Ag 57.1 g
7. H2O + CO2 → H2CO3
a. H2O
b. 38.2 g
c. H2O 0 g, CO2 61.7 g, H2CO3 38.2 g
8. Al(NO3)3 + 3Na → 3NaNO3 + Al
a. Al(NO3)3
b. 83.2 g
c. Al(NO3)3 0 g, Na 131.3 g, NaNO3 786.5 g, Al 83.2 g
Chapter 5: Limiting Reactants and Percent Yield
1. a. 0.687 g
b. 36.4%
2. a. H2S + 2NaOH → Na2S + 2H2O
b. 1.81 g
c. 58.2%
3. a. 2C6H6 + Br2 → 2C6H5Br + H2
b. 60.3
c. 94.0%
4. H2SO4 + Pb(C2H3O2)2 → PbSO4 + 2HC2H3O2
7.89 g H2SO4, 3.00 g Pb(C2H3O2)2, 6.53 g PbSO4, 2.58 g HC2H3O2
© 2012 Thomas van Geel
164
Chapter 5: Classifying Reactions
1. synthesis
2. single replacement
3. combustion
4. decomposition
5. combustion
6. decomposition
7. single replacement
8. synthesis
9. combustion
10. double replacement
11. decomposition
12. double replacement
13. synthesis
14. single replacement
15. double replacement
Chapter 5: Predicting Products
Synthesis Reactions
1. Ca + I2 → CaI2
2. 2Al + 3F2 → 2AlF3
3. 2Na + Br2 → 2NaBr
Decomposition Reactions
1. 2K2O → 4K + O2
2. 2H2O → 2H2 + O2
3. 2NaCl → 2Na + Cl2
Single Replacement Reactions
1. 2Na + Cu(NO3)2 → 2NaNO3 + Cu
2. Al + 3AuNO3 → Al(NO3)3 + 3Au
3. H2 + CuO → H2O + Cu
Double Replacement Reactions
1. K2S + H2SO4 → K2SO4 + H2S
2. FeSO4 + BaCl2 → FeCl2 + BaSO4
3. ZnCl2 + Na2S → ZnS + 2NaCl
Combustion Reactions
1. 2C3H6 + 9O2 → 6CO2 + 6H2O
2. CH4 + 2O2 → CO2 + 2H2O
3. 2CH4O + 3O2 → 2CO2 + 4H2O
1. P4 + 5O2 → 2P2O5 synthesis
2. 2Na + 2H2O → 2NaOH + H2 single replacement
3. CH2O + O2 → CO2 + H2O combustion
© 2012 Thomas van Geel
165
4. 2Li + 2HCl → 2LiCl + H2 single replacement
5. H2 + Cl2 → 2HCl synthesis
6. Cl2 + 2KI → 2KCl + I2 single replacement
7. 2HC2H3O2 + Ca(OH)2 → Ca(C2H3O2) + 2H2O double replacement
8. Si + 2F2 → SiF4 synthesis
9. KBr + AgNO3 → KNO3 + AgBr double replacement
10. C2H4 + 3O2 → 2CO2 + 2H2O combustion
11. 2AlI3 → 2Al + 3I2 decomposition
12. 16Fe + 3S8 → 8Fe2S3 synthesis
13. BaCl2 + 2NaNO3 → Ba(NO3)2 + 2NaCl double replacement
14. 2HCl → H2 + Cl2 decomposition
15. C3H8 + 5O2 → 3CO2 + 4H2O combustion
16. CrBr3 + 3NaNO3 → Cr(NO3)3 + 3NaBr double replacement
17. Ag2O → Ag + O2 decomposition
18. 3Mg + 2FeCl3 → 3MgCl2 + 2Fe single replacements
19. 4Na + O2 → 2Na2O synthesis
20. Fe2O3 → 2Fe + 3O2 decomposition
Chapter 6: Atomic Models
1.
cathode (-)
anode (+)
+ plate
— plate
—
+
power
source
phosphorescent
paint
2. a. decreases
b. increases
c. decreases
d. increases
© 2012 Thomas van Geel
166
3.
Dalton/Proust
Thomson
e-
+
Rutherford
+
e-
+
e-
e-
+
e-
+
e-
+
+
e-
+
+
e-
e-
Bohr
e-
e-
e-
e-
e-
e-
+
+
ee-
eee-
e-
4.
alpha
particle
emitter
gold foil
fluorescent
screen
© 2012 Thomas van Geel
167
5.
Energy
Wavelength
Frequency
X-ray
2
6
2
Visible light
4
4
4
Gamma
1
7
1
Radio
7
1
7
Microwaves
6
2
6
Ultraviolet
3
5
3
Infrared
5
3
5
EM wave
(1 = longest, 7 = shortest)
6. red orange yellow green blue violet
7.
Colors, from left to right, are purple, blue, green, yellow, orange, red.
Chapter 6: Energy, Frequency, and Wavelength
Energy (J)
Frequency (s-1)
Wavelength (m)
1.79 × 10-18
2.70 × 1015
1.11 × 10-7
3.63 × 10-20
5.48 × 1013
5.47 × 10-6
1.01 × 10-18
1.52 × 1015
2.50 × 10-7
2.60 × 10-19
3.92 × 1014
7.65 × 10-7
2.34 × 10-18
3.53 × 1015
8.50 × 10-8
1.93 × 10-20
2.91 × 1013
1.03 × 10-5
© 2012 Thomas van Geel
168
Chapter 6: Bohr’s Model
1.
2.
3.
4.
-2.42 × 10-19 J
level 4
4.52 × 10-19 J
level 4
Chapter 6: Light and the Hydrogen Atom
1.
2.
3.
4.
1.03 × 10-7 m
level 6
7.40 × 1013
level 5
Chapter 6: Isotopes
1.
2.
3.
4.
Ar: 40, U: 238, K: 39
b. 233.1
28.09
72.61
© 2012 Thomas van Geel
169
5.
Atomic #
# Neutrons
# Electrons
Mass
Number
Charge
C
6
8
8
14
-2
U92
92
146
92
238
0
Fe26
26
30
23
56
+3
P15
15
14
10
29
+5
H1
1
2
2
3
-1
I
53
76
53
129
0
Ir77
77
116
75
193
+2
Rb37
37
48
36
85
+1
Ag47
47
63
46
110
+1
Kr82
36
46
36
82
0
Isotope
14
6
238
56
29
3
129
53
193
85
110
36
Chapter 7: Balancing Nuclear Equations
1. 1H1 + 9Be4 → 6Li3 + 4He2
2. 9Be4 + 4He2 → 12C6 + 1n0
3. 238U92 → 234Th90 + 4He2
4. 1H1 + 3H1 → 4He2
5. 27Al13 + 1n0 → 24Na11 + 4He2
6. 9Be4 + 1n0 → 2 4He2 + 2 1n0
7. 24Mg12 + 2H1 → 22Na11 + 4He2
8. 23Na11 + 1n0 → 24Na11 + 0γ0
9. 246Cm96 + 12C6 → 254No102 + 4 1n0
10. 6Li3 + 1n0 → 0e-1 + 4He2 + 3He2
11. 241Am95 + 4He2 → 2 1n0 + 243Bk97
12. 214Po84 + 2 4He2 + 2 0e-1 → 222Rn86
© 2012 Thomas van Geel
170
13. 231Pa91 → 227Ac89 + 4He2
14. 223Fr87 → 223Ra88 + 0e-1
15. 26Al13 + 0e-1 → 26Mg12
16. 18F9 → 18O8 + 0e+1
17. 137Ba56* → 137Ba56 + 0γ0
18. 238U92 + 14N7 → 5 1n0 + 247Es99
19. 235U92 + 1n0 → 3 1n0 + 90Sr38 +
20. 6Li3 + 1n0 → 4He2 + 3H1
143
Kr54
Chapter 7: Nuclear Instability
1. U-235 is too large for the strong force to keep it together; it will likely
undergo alpha decay.
2. C-10 has a neutron-to-proton ratio of 0.667. For a small nucleus such as
this, the most stable ratio is 1. To reach a more stable ratio, C-10 will
probably undergo electron capture or positron emission.
3. C-14 has a neutron-to-proton ratio of 1.33. For a small nucleus such as
this, the most stable ratio is 1. To reach a more stable ratio, C-14 will
probably undergo beta decay.
4. Ne-17 has a neutron-to-proton ratio of 0.7. For a small nucleus such as
this, the most stable ratio is 1. To reach a more stable ratio, N-17 will
probably undergo electron capture or positron emission.
5. Be-11 has a neutron-to-proton ratio of 1.75. For a small nucleus such as
this, the most stable ratio is 1. To reach a more stable ratio, Be-11 will
probably undergo beta decay.
6. Np-225 is too large for the strong force to keep it together; it will likely
undergo alpha decay.
Chapter 7: Half-life, Chart Method
1.
2.
3.
4.
5.
6.
18.75 g
16 hours
960 g
3.125 g
1 year
5440 g
Chapter 7: Half-life, Equation Method
1.
2.
3.
4.
59.5 g
476 g
11.1 g
106 g
© 2012 Thomas van Geel
171
Chapter 8: Electron Configurations
1. 1s22s22p63s1
2. 1s22s22p63s23p64s23d6
3. 1s22s22p63s23p64s23d104p5
4. 1s22s22p63s23p64s13d10
5. 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s25f11
6. [Ar]4s23d7
7. [Kr]5s24d5
8. [Kr]5s24d105p4
9. [Rn]7s2
10. [Xe]6s24f7
11. 1s22s22p6
12. 1s2
13. 1s22s22p63s23p6
14. 1s22s22p63s23p63d5
15. 1s22s22p63s23p64s23d104p65s24d105p64f145d10
16. S, excited
17. Rb, ground
18. Sb, excited
19. Os, ground
20. Es, ground
21. 1s22s22p63s23p64s23d104p5
22. 1s22s22p63s33p5
23. [Rn]7s25f11
24. correct
25. [Kr]5s24d105p6
© 2012 Thomas van Geel
172
Chapter 8: Schrödinger/Heisenberg
1.
2.
# electrons
# orbitals
s subshell
2
s
1
p subshell
6
p
3
d subshell
10
d
5
f subshell
14
f
7
Shell 1
2
Shell 2
8
Shell 3
18
Shell 4
32
3.
Subshells present
Shell 1
s
Shell 2
s and p
Shell 3
s, p, and d
Shell 4
s, p, d, and f
4.
1s
2s
2p
3s
3p
4s
3d
4p
5s
4d
5p
6s
4f
5d
6p
7s
5f
6d
© 2012 Thomas van Geel
173
Chapter 9: The Periodic Table
1.
Atom
Electron configuration (long form)
# valence
electrons
# core
electrons
Zeff
Mg
1s2 2s2 2p6 3s2 3p6 4s2
2
18
2
Cl
1s2 2s2 2p5
7
2
7
B
1s2 2s2 2p1
3
2
3
Zn
1s2 2s2 2p6 3s2 3p6 4s2 3d10
2
28
2
2.
Metals
Good electrical conductors
Good heat conductors
Malleable
Ductile
Has luster
Silver color
High melting point
Metalloids
Intermediate
properties
Nonmetals
Bad electrical conductors
Bad heat conductors
Brittle
Brittle
Dull surface
Colors vary
Low melting point
© 2012 Thomas van Geel
174
3.
Transition metals
Alkali metals
Halogens
Noble gases
Inner transition metals
Metals
Nonmetals
Metalloids
Alkaline earth metals
4. Zeff
5. Atomic radius
© 2012 Thomas van Geel
175
The radii are large near the bottom of the table because there are more
shells of electrons. The radii are large on the left side of the table
because Zeff is low.
6. Ionization energy
The I.E. is high near the top of the table because the radii are smaller.
The I.E. is high on the right side of the table because Zeff is larger.
7. Reactivity
Metals that hold on to their valence electrons weakly are more reactive. The
elements on the bottom left of the table have the weakest hold because the
radii are larger and the Zeff is smaller. Nonmetals that hold on to their
valence electrons strongly are more reactive. The elements on the top right
of the table have the strongest hold because the radii are smaller and the Zeff
is larger.
8. Xe
9. Sb
10. Cs
11. Rb
12. Cl
13. yes
yes
no
no
© 2012 Thomas van Geel
176
Chapter 10: Drawing Lewis Structures
1.
H
F
H
O
2.
H
Cl
Cl
C
Cl
Cl
3.
H
N
4.
H
H
+
H
H
N
H
H
5.
S
O
O
6.
H
H
C
C
7.
H
H
8. H
C
C
9. H
C
N
H
H
H
+
H
10.
O
H
H
© 2012 Thomas van Geel
177
O
C
12. C
O
F
Be
11.
13.
O
F
F
B
14.
F
F
N
O
O
15.
O
N
O
16.
O
+
F
S
F
F
17.
H
18.
P
H
© 2012 Thomas van Geel
178
Chapter 10: Lewis Structures with Resonance
O
O
O
1.
O
O
O
2-
O
C
C
C
O
O
O
O
N
O
O
O
O
-
-
O
O
N
N
O
4.
O
N
O
O
O
N
H
O
N
O
O
-
H
H
6.
C
H
O
O
O
5.
O
O
H
O
-
N
3.
2-
O
O
2.
2-
O
H
O
C
H
O
C
H
O
C
O
© 2012 Thomas van Geel
179
H
H
C
H
C
C
O
H
N
N
O
O
O
O
O
N
O
O
C
H
N
O
N
H
O
O
O
C
C
O
C
C
O
N
8.
H
H
O
N
H
C
H
H
C
C
C
7.
H
N
O
© 2012 Thomas van Geel
180
Chapter 10: Molecular Geometry
CO2
Lewis Structure
O
C
3-D Drawing
Electron geometry: linear
Molecular geometry: linear
O
O
C
O
ONF
Lewis Structure
3-D Drawing
Electron geometry: trigonal planar
Molecular geometry: bent
N
F
O
N
O
F
NCl3
Lewis Structure
Cl
N
Cl
3-D Drawing
Electron geometry: tetrahedral
Molecular geometry: trigonal pyramidal
Cl
Cl
Cl
N
Cl
© 2012 Thomas van Geel
181
Lewis Structure
NO2-
3-D Drawing
Electron geometry: trigonal planar
Molecular geometry: bent
N
N
O
O
O
Lewis Structure
F
O
OF2
3-D Drawing
Electron geometry: tetrahedral
Molecular geometry: bent
O
F
F
O
F
Lewis Structure
PCl4+
3-D Drawing
Electron geometry: tetrahedral
Molecular geometry: tetrahedral
Cl
Cl
Cl
P
Cl
Cl
Cl
Cl
P
Cl
© 2012 Thomas van Geel
182
BBr3
Lewis Structure
3-D Drawing
Electron geometry: trigonal planar
Molecular geometry: trigonal planar
Br
Br
B
Br
B
Br
Br
Lewis Structure
Br
As
Br
AsBr3
3-D Drawing
Electron geometry: tetrahedral
Molecular geometry: trigonal pyramidal
Br
Br
Br
Br
Lewis Structure
As
Br
SiF4
3-D Drawing
Electron geometry: tetrahedral
Molecular geometry: tetrahedral
F
F
F
Si
F
F
Si
F
F
F
© 2012 Thomas van Geel
183
Chapter 10: Polarity of Molecules
Lewis
Structure
3D drawing showing
bond polarity
3D drawing showing
overall polarity
H
H
O
H
H
O
O
H
H
Si
N
H
Si
C
F
F
Si
Si
N
F
F
O
O
NONPOLAR
C
F
O
H
F
F
F
N
H
Si
O
NONPOLAR
© 2012 Thomas van Geel
184
F
N
F
F
F
Cl
Cl
H
Si
Cl
H
C
F
C
F
O
C
F
S
F
Si
H
Cl
H
F
Cl
Si
H
F
F
F
F
N
F
N
Cl
H
F
C
C
NONPOLAR
C
F
F
S
O
S
O
O
O
O
© 2012 Thomas van Geel
185
O
H
C
H
O
O
C
C
H
O
H
C
O
H
H
O
O
C
C
H
H
O
O
O
O
O
H
C
O
Cl
Cl
C
C
H
H
NONPOLAR
C
O
O
Cl
Cl
C
H
Cl
C
Cl
C
H
H
C
H
© 2012 Thomas van Geel
186
H
Cl
C
C
H
Cl
H
Cl
C
C
Cl
H
H
Cl
C
Cl
H
Cl
C
C
H
H
C
Cl
Cl
C
Cl
H
NONPOLAR
C
H
© 2012 Thomas van Geel
187
Chapter 11: IMF intro
H
H
A. C2H6
Is this molecule polar or
nonpolar?
Is this molecule capable
of H-bonding?
nonpolar
no
polar
no
polar
yes
nonpolar
no
polar
no
polar
yes
H
C
C
H
H
H
H
B. CH3Cl
C
H
Cl
H
C. NH3
N
H
H
H
-2
O
D. CO3-2
C
O
O
P
Cl
Cl
E. PCl3
Cl
H
F. CH3OH
C
H
H
H
O
© 2012 Thomas van Geel
188
Circle the type of IMF that would hold the following pairs of molecules together.
A and another A
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
B and another B
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
C and another C
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
D and another D
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
E and another E
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
F and another F
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
A and B
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
C and D
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
E and F
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
C and F
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
A and E
ion-dip
H-bonding
dip-dip
dip-indip
indip-indip
© 2012 Thomas van Geel
189
Chapter 11: Strength of IMF’s
1. Both molecules are nonpolar; the main type of IMF for both is indip-indip.
C2H6 has more “sloshing” electrons, giving it the stronger IMF.
2. Both are polar. HF has hydrogen bonding; HCl has dip-dip. Hydrogen
bonding is stronger.
3. CH4 is nonpolar and therefore has indip-indip IMFs. CH3F is polar and has
dip-dip IMFs. Dip-dip is stronger.
4. Both are polar. The bonding in the first molecule is CH3-CH2-OH; it has
hydrogen bonding because a hydrogen is directly bound to the oxygen.
The bonding in the second molecule is CH3-O-CH3; none of the hydrogens
are bound directly to the oxygen, so it can not hydrogen bond. It has dipdip IMFs, which are weaker.
5. Both are capable of hydrogen bonding, but the first can hydrogen bond at
both ends, making its IMFs stronger.
6. NO2 is bent, making the molecule polar and giving it dip-dip IFMs. CO2 is
linear, making it nonpolar and giving it indip-indip IMFs.
7. Both molecules are polar and have dip-dip IMFs. The N-O bonds are more
polar than the S-O bonds, so NO2 has stronger IMFs.
Chapter 11: Types of Solids
1. a. ionic
b.
c. Conducts as a liquid and when dissolved in water, not as a solid.
2. a. metallic
b.
c. high melting point
3. a. network covalent
b. high melting point
c. covalent bonds?
4. I have a low melting point and dissolve in water.
a. polar covalent
b. Can never conduct electricity
c. IMF’s
© 2012 Thomas van Geel
190
Chapter 11: Heating Curves
1. 190 K
2. 250 K
3. Solid: 1.11 J/g⋅K
Liquid: 0.667 J/g⋅K
Gas: 3.667 J/g⋅K
4. 60.8 K
5. ΔHfus = 0.75 kJ/mol
ΔHvap = 5.00 kJ/mol
6. a. 24,400 J
b. 32 mol
c. 24 kJ
d. 13,300 J
e. 61,700 J
7. 174,400 J
Chapter 11: Heat Transfer Problems
1.
2.
3.
4.
0.269 J/g⋅K
14.2°C
6.02 kJ/mol
a. No; the aluminum would have to be below absolute zero (-515 K).
b. 72.1 g
© 2012 Thomas van Geel
191
Chapter 11: Phase Diagrams
1.
Pressure (atm)
2.00
1.00
0.5
230
260
350
500
Temperature (K)
4. No. Increasing the pressure on the solid does not cross the solid-liquid
phase boundary; the phase boundary slopes to the right.
5. Yes. Decreasing the pressure on the liquid would cross the liquid-gas
phase boundary.
Chapter 12: Solubility Curves
1.
2.
3.
4.
116 g
58°C
43 g
a. unsaturated
b. saturated
c. supersaturated
5. 26 g
6. For a solid to dissolve into a liquid, its particle must separate; the IMF’s
holding the solid together must be overcome. At higher temperatures, it
is easier for the IMF’s to be overcome.
7. Gases have a natural tendency to expand and leave the solution, but
IMF’s between gas particles and solvent particles holds them in the
© 2012 Thomas van Geel
192
solution. At higher temperatures, these IMF’s are more easily overcome,
and the gas can escape the liquid.
8. The size of solute particles increases from solutions, to colloids, to
suspensions. Solutions are clear; colloids and suspensions tend to be
cloudy. Suspensions tend to settle out over time; solutions and colloids
do not.
Chapter 12: Solubility Rules
1.
2.
3.
4.
5.
6.
7.
8.
soluble
insoluble
insoluble
soluble
insoluble
soluble
insoluble
soluble
Chapter 12: Molarity
1. a. 1.4 M
b. Ba+ 1.4 M, Cl- 2.8 M
2. a. 2 M
b. Al+3 4 M, SO4-2 6 M
3. 6 mol
4. 0.2 mol
5. 4 L
6. 0.375 L
7. 595 g
8. 0.668 M
9. 0.334 L
10. 6.71 L
11. 6.66 g
Chapter 12: Dilutions
1.
2.
3.
4.
5.
6.
18.8 mL
0.756 M
0.641 M
125 mL
1100 mL
125 mL
© 2012 Thomas van Geel
193
Chapter 12: Freezing Point Depression and Boiling Point Elevation
1.
2.
3.
4.
5.
6.
8.
0.300 m
-2.23°C, 100.61°C
0.98 m
101.62
50 g/mol
proposal A
alkaline earth metal
Chapter 12: Net Ionic Equations
1.
2.
3.
4.
5.
6.
Ag+(aq) + Cl-(aq) → AgCl(s)
Fe(s) + Cu+2(aq) → Cu(s) + Fe+2(aq)
3K(s) + Al+3(aq) → 3K+(aq) + Al(s)
Cl2(g) + 2Br-(aq) → 2Cl-(aq) + Br2(g)
2I-(aq) + Pb+2(aq) → PbI2(s)
Ba+2(aq) + SO4-2(aq) → BaSO4(s)
Chapter 13: Ideal Gas Law (PV=nRT)
1.
2.
3.
4.
5.
6.
7.
8.
0.123 mol
48.1 L
27.8 atm
-120°C
0.124 mol
5240 L
59.0 g
He
Chapter 13: Combined Gas Law
1.
2.
3.
4.
5.
6.
7.
8.
9.
0.101 L
1.99 L
0.0795 L
0.740 L
3.5 atm
93.3°C
1430 mm Hg
0.0257 L
0.0366 L
© 2012 Thomas van Geel
194
Chapter 13: Gas Stoichiometry
1.
2.
3.
4.
5.
6.
7.
8.
9.
0.747 L
1.42 L
58.3 L
2.05 L
5.24 L
8.68 L
9.21 atm
10.9 L
Ammonium nitrate
Chapter 13: Partial Pressure
1. a. 3 mol
b. 14.8 atm
c. 0.333 O2, 0.667 N2
d. 4.92 atm O2, 9.88 atm N2
2. a. 1.00 atm He, 0.80 atm Ne, 0.20 atm Ar
b. 0.616 mol He, 0.483 mol Ne, 0.123 mol Ar
c. 1.23 mol
3. 0.00163 mol
4. 0.00179 mol
6. 55.9 g/mol
Chapter 13: Graham’s Law of Diffusion
1.
2.
3.
4.
He is faster, ratio is 2.65:1
0.71 days
36 g/mol
127.4 g/mol
Chapter 14: Kinetics
1. i. reactants ii. activation energy
iii. activated complex
iv. ∆H
v. products
2. B is endothermic, A is exothermic. In endothermic reactions, the products
are higher than the reactants. In exothermic reactions, the products are
lower than the reactants.
3. Old bonds are breaking, and new bonds are forming.
4. a. To increase the surface area, making digestion happen faster.
b. At colder temperatures, the chemical reactions that cause spoilage
happen slower.
© 2012 Thomas van Geel
195
c. The browning of apples is caused by an enzyme (a catalyst). The acidic
lemon juice deactivates the enzyme, so the browning reactions happen
slower.
d. Normal air is about 21% oxygen. The highly concentrated air in
oxygen tanks is a fire hazard because it can cause combustion reactions
to be much faster.
5.
Because the reactions are exothermic, the products are lower than the
reactants. The first reaction is not catalyzed, the second one is catalyzed,
and therefore has lower activation energy.
6. Catalysts are not consumed by a chemical reaction. Because they are not
used up, only small amounts of catalyst are needed.
Chapter 14: Equilibrium Problems
1. a.
!
!
b.
[H ][F ]
+
"
[HF ]
+2 %
"
$#Cu(NH3 ) 4 '&
[ ][ ]
Cu+2 NH3
[H CO ]
[CO ]
d. [Ba ][F ]
c.
2
3
2
+2
2. 1.71
3. 1.11
4. 6.25
!
5. 6.46
6. 8.27
7. 1.73
8. 1.84
9. 6.25
10. 1.05
!
4
"
2
× 10-3
× 1013
× 10-4
× 10-4 M
× 10-3 M
× 10-9 M
× 10-7
× 10-4
× 1013
© 2012 Thomas van Geel
196
11. Ba+2 3.58 × 10-3 M, F- 7.16 × 10-3 M
12. HF 0.286 M, H+ 0.0137 M, F- 0.0137 M
13. CO2 0.0017 M, H2CO3 0.0183 M
14. 1.22 × 10-11, shifts to the right
15. a. 4.89 × 10-2, shifts to the left
b. CO2 0.0471 M, H2CO3 0.00011 M
16. a. Decrease
b. No change
c. Increase
17. a. Right
b. Right
c. Right
d. Right
e. No shift
Chapter 15: pH and pOH
1.
[H+]
[OH-]
pH
pOH
Acidic or Basic?
3.4 × 10-5
2.94 × 10-10
4.47
9.53
Acidic
1.08 × 10-13
9.3 × 10-2
12.97
1.03
Basic
5.01 × 10-12
2.00 × 10-3
11.3
2.7
Basic
0.126
7.94 × 10-14
0.9
13.1
Acidic
1.3 × 10-10
7.69 × 10-5
9.89
4.11
Basic
1.30 × 10-2
7.7 × 10-13
1.89
12.11
Acidic
3.98 × 10-3
2.51 × 10-12
2.4
11.6
Acidic
6.3 × 10-13
1.58 × 10-2
12.2
1.8
Basic
© 2012 Thomas van Geel
197
2. a. 1.30
b. 1.52
c. 12.3
d. 10.8
3. a. 2.01
b. 3.88
c. 11.58
d. 10.06
Chapter 15: Finding Ka and Kb
1.
2.
3.
4.
1.75
5.00
2.12
9.61
×
×
×
×
10-5
10-10
10-4
10-11
Chapter 15: Acid-Base Definitions
1. HCl: acid
HClO4: acid Fe(OH)3: base
BF3: neither H2SO3: acid NaOH: base
NH3: neither
C6H12O6: neither
2.
Conjugate Acid
Conjugate Base
H2CO3
HCO3-
HCO3-
CO3-2
H2 O
OH-
HBr
Br-
H3O+
H2 O
NH4+
NH3
HPO4-2
PO4-3
HI
I-
acid
base
acid
base
3. a. HC2H3O2 + NH3 ⇄ NH4+ + C2H3O2-
base
acid
acid
base
b. NH2- + H2O ⇄ NH3 + OH© 2012 Thomas van Geel
198
base
acid
acid
base
acid
acid
+
base
c. H2O + H2O ⇄ H3O + OH-
base
d. NH3 + H2O ⇄ NH4+ + OH-
acid
base
acid
+
base
-
e. HBr + H2O ⇄ H3O + Br
5. H2SO3 + H2O ⇋ HSO3- + H3O+
6. SO3-2 + H2O ⇋ HSO3- + OH-
7. H2PO4- + H2O ⇋ HPO4-2 + H3O+
8. CH3NH2 + H2O ⇋ CH3NH3+ + OHChapter 15: Indicators
1. Between 10 and 11
2. Between 4.5 and 4.8
Chapter 15: Titrations
1.
2.
3.
4.
5.
0.25 M
0.14 M
0.23 M
0.090 M
0.029 M
Chapter 15: Buffers
1.
2.
3.
4.
5.
6.
Left
Rise
Faster
Slower
Decrease
Increase
© 2012 Thomas van Geel
199
7. Na2HPO4/Na2HPO4
NaHSO4/Na2SO4
H3PO4/NaH2PO4
Na2HPO4/Na3PO4
NH4Cl/NH3
Chapter 16: Enthalpy of Reaction
1.
2.
3.
4.
5.
+0.478 kJ/mol
20.43°C
4430 kJ
H2
-54.3 kJ/mol
Chapter 16: Bond Enthalpies
1.
2.
3.
4.
-184 kJ/mol
-2528 kJ/mol
-126 kJ/mol
-3906 kJ/mol
Chapter 16: Hess's Law
1. a. +242 kJ/mol
b. -121 kJ/mol
c. +726 kJ/mol
2. -792 kJ/mol
3. +171.5 kJ/mol
4. -366 kJ/mol
Chapter 16: Standard Enthalpies of Formation
1. a. The reaction is correct as written.
b. K(s) + ½Cl2(g) → KCl(s)
c. 6C(graphite) + 6H2(g) + 3O2(g) → C6H12O6(s)
2. C(graphite) + 2Cl2(g) → CCl4(l)
3. +25.69 kJ/mol
4. -2842 kJ/mol
5. -3267 kJ/mol
© 2012 Thomas van Geel
200
Chapter 16: Entropy and Gibb’s Free Energy
1. a. decrease
b. decrease
c. increase
d. increase
e. decrease
f. increase
2. ∆S
3. ∆H
4.
Sign of ∆H
Always spontaneous?
If temperature dependent:
Never spontaneous?
spontanenous at high or low
Depends on temperature?
temperature?
Sign of ∆S
+
+
depends on T
spontaneous at high T
+
-
never spontaneous
N/A
-
+
always spontaneous
N/A
-
-
depends on T
spontaneous at low T
5.
a.
A(s) → B(g) + energy
(exothermic)
∆H
+
or
-
∆S
+
or
-
-
+
always
N/A
-
-
depends
low T
If temperature
Always spontaneous?
dependent:
Never spontaneous?
spontanenous at
Depends on temperature?
high or low
temperature?
b.
A(l) → B(s)
c.
A(s) + B(g) → B(s) + energy
-
-
depends
low T
d.
A(s) → B(aq) + energy
-
+
always
N/A
e.
A(s) → B(g) + C(g)
(endothermic)
+
+
depends
high T
f.
A(l) + energy → B(g)
+
+
depends
high T
g.
AB(s) → A+(aq) + B-(aq) + energy
-
+
always
N/A
h.
A(l) + energy → B(s)
+
-
never
N/A
6. a. 1176 K
b. Both ΔH and ΔS are negative, so the reaction will be spontaneous at
temperatures below 1176 K.
© 2012 Thomas van Geel
201
Chapter 17: Oxidation Numbers and Redox Reactions
1. He: 0
2. P: 0
3. Al: +3, O: -2
4. H: +1, O: -2
5. P: +5, O: -2
6. Mn: +7, O: -2
7. H: +1, O:-1
8. N: -3, H: +1
9. Na: +1, H: -1
10. Cr: +6, O: -2
11. Cl: +1, O: -2
12. N: +5, O: -2
13. oxidizing agent:
14. oxidizing agent:
15. oxidizing agent:
16. oxidizing agent:
Zn+2, reducing agent: Fe
AgNO3, reducing agent: Cu
O2, reducing agent: H2
Br2, reducing agent: Mg
Chapter 17: Voltaic Cells
1. a. anode Cu, cathode Ag
b. Cu → Cu+2 + 2e-, Ag+ + e- → Ag
c. Cu + 2Ag+ → 2Ag + Cu+2
d. 0.65 V
2. a. anode Zn, cathode Fe
b. Zn → Zn+2 + 2e-, Fe+3 + 3e- → Fe
c. 3Zn + 2Fe+3 → 2Fe + 3Zn+2
d. 0.72 V
3. a. anode Al, cathode Cu
b. Al → Al+3 + 3e-, Cu+ + e- → Cu
c. Al + 3Cu+ → 3Cu + Al+3
d. 2.18 V
4. a. anode Mg, cathode Cr
b. Mg → Mg+2 + 2e-, Cr+3 + 3e- → Cr
c. 3Mg + 2Cr+3 → 2Cr + 3Mg+2
d. 1.63 V
© 2012 Thomas van Geel
202
Chapter 18: Organic Functional Groups
carboxylic acid
O
OH
alcohol (5)
OH
OH
aldehyde
O
O
HO
ester
H
O
OH
aspirin (a pain reliever)
glucose (a simple sugar)
O
ether
OH
aldehyde
H
N
O
amine
O
alcohol
ether
OH
diphenhydramine (an antihistamine)
vanillin (the flavor of vanilla)
OH
alcohol
H
H
ketone
H
OH
ketone
O
testosterone (a male hormone)
H
N
carboxylic acid
O
O
pyruvic acid (a metabolic intermediate)
ether
O
ester
O
amine
O
CF3
amine
fluoxetine (an antidepressant)
H 2N
benzocaine (a topical anesthetic)
© 2012 Thomas van Geel