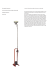Experiment05
Transcription
Experiment05
Name: Laboratory Section: Laboratory Section Date: Partners’ Names: Grade: Last Revised on January 6, 2016 EXPERIMENT 5 - New Part 1: Equivalence of Energy: Heat, Mechanical Part 2: Equivalence of Energy: Heat, Electrical, Light 0. Pre-Laboratory Work [2 pts] 1. A 90kg person jumps from a 30m tower into a tub of water with a volume of 5m3 initially at 20°C. Assuming that all of the work done by the person is converted into heat to the water, what is the final temperature of the water? It’s helpful to first find the work done by the person to the water tub and then the amount of heat equivalent to that work. Make sure you have the correct value for the mass of the water. Include units. [1pt] 2. In both Section 3.1 (part 1) and Section 3.2.1 (part 2) you are asked to continue taking temperature measurements even after the heat source has been turned off. What effect are we trying to observe and how do we use this effect in our data analysis? [1pt] 1 of 20 Name: Laboratory Section: Laboratory Section Date: Partners’ Names: Grade: Last Revised on January 6, 2016 EXPERIMENT 5 - New Part 1: Equivalence of Energy: Heat, Mechanical Part 2: Equivalence of Energy: Heat, Electrical, Light 1. Introduction and Purpose Conservation of Energy is one of the foundational principles in Physics. As a consequence of this principle, we expect that when energy changes forms, there should be the same amount of energy before and after that change. Different forms of energy relevant in this experiment include mechanical work, electrical work, heat (thermal energy), and light. The purpose of this experiment is to observe the conversion of energy from one form to another. Mechanical and electrical work are generally measured in Joules, whereas thermal energy (heat) is usually measured in Calories. Calories and Joules should be proportional to one another, since they are just different units of measure for the same physical quantity (energy). In part Ι, you will measure that proportionality constant between Joules and Calories (i.e. the proportionality between mechanical work and heat), known as Joule’s constant. The accepted value of Joule's constant is 4.19 J/cal. In part ΙΙ, your TA will assign you one of two possible procedures: (1) You will use a light bulb to convert electrical energy into either heat, from which you will again determine Joule’s constant, or (2) you will convert electrical energy into heat and light, allowing the light energy from the light bulb to escape. By comparing your results with another group, you will be able to calculate the efficiency of the conversion of electrical energy to light. The efficiency of a light-bulb at producing light would be determined by how much of the energy put into the bulb is converted into light: a high-efficiency bulb converts most of the electrical energy it is given into light, whereas a low-efficiency bulb only converts a small fraction of that energy to light, and wastes the rest in other forms (nominally heat). In summary, you will do the following: Ι. Conversion of Mechanical Energy into Heat ΙΙ. Conversion of Electrical Energy (TA will assign 1 or 2 below) 1. Conversion of Electrical Energy into Heat 2. Conversion of Electrical Energy into Heat and Light 2 of 20 2. Theory 2.1 Conversion of Mechanical Energy into Heat (Part 1) In this experiment, a measurable amount of work is performed by turning a crank. The crank drives the rotation of an aluminum cylinder, which is subject to friction from a rope looped around the cylinder several times, supporting a mass. When the system is set up correctly, turning the crank will just lift that supported mass off the ground—when this occurs, we know that the force of friction between the aluminum cylinder and the rope is equal to the gravitational force F = Mg on the mass. If we know the average force, and we know the number of turns of the crank (there is a counter on the hardware), then we can compute how much work we have put into the system. We assume that all of this work is converted to heat through friction, and that we should subsequently be able to make a connection between the amount of work put into the system and the temperature of the aluminum cylinder over time. Specifically, we expect that the mechanical work performed and the thermal energy gained by the cylinder will be proportional. There is a thermistor embedded in the aluminum. By measuring the resistance of the thermistor using a multimeter, we can monitor the temperature change of the cylinder (and thus compute the thermal energy transferred to the cylinder). Finally we calculate the ratio of mechanical work performed (in Joules) to heat gained by the cylinder (in Calories), in order to compute Joule's Constant Jmechanical = 4.19 J/Cal, or the mechanical equivalence of heat. (Note that we notate Jmechanical = Jm throughout the manual, in order to contrast this result with the corresponding one in the second half of the lab, which will be notated Jelectrical = Je). We go through the process for computing the amount of mechanical work performed by turning the crank. The torque required to support a mass M is given by Equation 5.1 τ = MgR where g is the graviational accelerating near Earth's surface, and R is the radius of the aluminum cylinder being cranked. The work performed by this torque is given by W = τθ, where θ is the angle through which the cylinder has been rotated. Each complete turn of the crank adds 2π to θ. It then follows that if we have performed a total of N turns of the crank in the experiment, the total mechanical work must equal to: W = τθ = ( 2πN ) MgR Equation 5.2 This completes the calculation of the mechanical work we put into the system. Next we consider how to compute the heat Q imparted to the cylinder from the measured temperature change. The general formula to compute the heat required to change the temperature of an object by a certain amount is given by: Equation 5.3 Q = mcΔT The mass of the object being heated is m, and c is the specific heat of the material. For us, the object being heated is the aluminum cylinder. Its mass m can be measured (it should be about 200 g), and the specific heat of aluminum is 0.220 (cal/g oC). ΔT is the change in temperature experienced by the object being heated, and is a measured quantity. We will calculate this a few different ways, discussed in the post-lab. We can then finally find Joule's Constant: W J m = ( J / cal ) Equation 5.4 Q Any remaining details in the calculations are discussed in section 3.1. 3 of 20 2.2 Conversion of Electrical Energy into Heat and Light (Part 2) Another form of energy that we use on a daily basis is electrical energy. When a light switch is flipped on, electrons flow through the filament of the lamp. Electrons collide with the nuclei of the lattice of the lamp’s filament, causing them to vibrate with larger amplitude. The energy due to the vibrating electrons is radiated away as electromagnetic waves called photons (light particles). This radiation is what people identify as “light” and/or “heat.” A regular incandescent light bulb produces a light spectrum consisting of the infrared, the visible and the ultraviolet. If the light bulb is submerged under water, the infrared and ultraviolet parts of the spectrum are absorbed by the water causing the water temperature to increase. The visible part of the spectrum passes through water1. The amount of electrical energy consumed by the light bulb, EConsumed , is calculated by multiplying the power P used, by the amount of time Δt the light bulb is on. The power P is found by multiplying the voltage V with the electric current I through the light bulb— P = IV . Assuming that the light bulb used the entire electrical energy it consumed to produce the radiation energy E Produced , we have E Produced = EConsumed = PΔt = IVΔt . Equation 5.5 If V is in Volts and I in Amps, then P is in Watts and the energy E is in Joules. If the light bulb is surrounded by material that will absorb almost all of the photons and whose specific heat is known, the total heat can be found again by using Equation 5.3. In this experiment, the materials that surround the light bulb are the water, water jar and thermometer. The amount of heat ΔQ associated with the temperature change ΔT is Equation 5.6 ΔQ = DΔT , where for this setup D is given by cal ⎞ ⎛ cal ⎞ ⎛ D = mwater cwater +23 ⎜ o ⎟ +Vthermometer ⎜ 0.46 3 o ⎟ . Equation 5.7 ⎝ C⎠ ⎝ cm C ⎠ This is a generalization of equation 5.3; instead of one term mcΔT, we now have several terms playing the role of mc for each of the different substances and objects in the water. In Part 3.2.1 of the experiment (Conversion of Electrical Energy into Heat), a small amount of India ink is added to the water to capture the otherwise escaping visible spectrum of light. In order to further reduce the heat lost to the air, the water jar is inserted into a Styrofoam Calorimeter insulator. By adding India ink and insulating the jar, one can assume the entire electrical energy produced by the light bulb is converted to heat and no heat is lost to the air. The electrical Joule’s constant J electrical = J e can then be calculated based on Equ. 5.5 through 5.8, E IV Δt J e = Produced = . Equation 5.8 DΔT ΔQ In Part 3.2.2 of the experiment (Conversion of Electrical Energy into Heat and Light) no India ink is added and the jar is not insulated. Because the visible part of the spectrum is not absorbed in this case, all the radiation does not go into producing heat and thus Equation 5.8 is not applicable in this case. However, the visible light producing efficiency can be calculated from the following, − Eabsorbed E E , Equation 5.9 VisibleLightProducingEfficiency = visible = produced E produced E produced 1 If you consider the composition of your eyes, you can understand why the light that passes through water is “visible”. 4 of 20 where Evisible is the energy of the visible light and Eabsorbed is the radiation energy absorbed by the water. Since the absorbed radiation energy Eabsorbed is the heat absorbed by the water, Eabsorbed can be calculated according to Eabsorbed = JΔQ . Equation 5.10 where J = 4.19 J is the accepted value of Joule’s constant. The amount of heat ΔQ cal transferred to the water must be found using Equations 5.6 and 5.7. 3. Laboratory Work 3.1 Conversion of Mechanical Energy (Part 1) Preparing the Apparatus The apparatus for this lab must be set up carefully in order to obtain a good result. The overall apparatus is shown in Fig. 5.1. A multimeter (ohmmeter) will be used to determine the temperature of the cylinder as shown in Fig. 5.2 and described below. We convert mechanical work into heat through friction between a nylon rope and aluminum cylinder, as described in section 2.1. The source of mechanical energy will be provided by you—the aluminum cylinder will be turned by a crank. You should do the following to ensure your hardware is set up correctly. 1. You should have the crank apparatus set up on the table top as shown in Fig. 5.3. Measure the mass of the aluminum cylinder, and replace it by screwing in the knob (see Fig 5.3). There are two brushes on the crank apparatus—make sure that they in contact with the side of the aluminum cylinder with the brass slip rings exposed, as shown in Fig. 5.4. The brushes establish an electrical contact with the thermistor inside the cylinder, which is used to monitor the cylinder's temperature. 2. Spray some powdered graphite on the cylinder. This acts as a lubricant. The graphite is harmless so long as it is not inhaled (so avoid spraying it near your face). 3. Mass the bucket and whatever masses have been placed in it. The total M = Mbucket + Min is taken to be the mass supported by the rope. We neglect the rope's mass. A total M of 23 kg is recommended. 4. Tie the nylon rope to the bucket, leaving relatively little extra rope hanging down below the bucket. (You will need as much of the rope's length above as possible.) 5. Align the bucket with the slot on the edge of the table-top crank apparatus, such that the nylon rope passes vertically through the slot. Wrap the rope several times around the aluminum cylinder (4-5 turns recommended), keeping some tension in the rope as it is wrapped. (It should be wrapped tightly). 6. Tie the rope to rubber band anchored to the base-plate of the crank, as shown in Fig. 5.1. The rubber band should be through the hook in such a way that it creates two loops. (One loop is not strong enough to maintain proper tension in the rope for most rubber bands— loop it through so that it is doubled up. Ask you TA/TI for help if needed.) Pull the rubber band's loops towards the aluminum cylinder before tying that end of the rope off, so that when you are not cranking the rubber band maintains some tension in the rope. Make sure the rope does not cross over itself anywhere on the cylinder. 7. Turn the crank a few times. How much does the mass rise off the floor? The amount of friction between the rope and cylinder is determined by the tension in the rope, and the number of turns of the rope around the cylinder. If the mass rises more than 3cm from the floor, there is too much friction between the rope and aluminum cylinder. In this case 5 of 20 either re-tie the string to the rubber band such that it is looser, or unwind one turn of the rope around the cylinder. If the mass does not entirely leave floor, there is not enough friction, and you should either add a turn or re-tie the rope to the rubber band to make it tighter. To correctly calculate the force of the hanging mass, all of the mass must leave the floor when you are cranking. 8. Ideally the mass will just leave the floor when you crank, and fall back to the floor if you stop cranking but hold on to the crank handle. Keep playing with step 7 until this happens. 9. Use the banana-plug connectors to attach the ohmmeter (see Figs. 5.2 and 5.4), and set it to the 200 kΩ setting or similar resistive range. Your apparatus is ready to go! Some tips about setting up multimeters are provided at the end of the instructions (p. 10). Using the Apparatus (Data Collection) We now describe the experimental procedure which uses the apparatus described above. 1. Make sure the turn counter for the crank is reset to zero. (Turn the knob of the counter to reset it.) 2. Make sure your Ohmmeter is on, and record your starting resistance R for time t = 0, in 6 of 20 3. 4. 5. 6. 7. table 5.1. Note that a table and function for converting the resistance measured across the thermistor to a temperature can be found below. It is most efficient to record all of the resistances in the experiment and then make conversions at the end. Start your hand timer, and begin cranking the apparatus. Every thirty seconds you should briefly stop cranking in order to record the resistance and number of revolutions in table 5.1. (Note that the thermistor's reading will vary while the apparatus is being cranked, but will quickly settle to a steady value when it is not being cranked. The person cranking should stop for less than five seconds at each thirty second interval, just long enough for a lab partner to record N and R, and then resume.) Continue performing step three for thirty second intervals until your recorded temperature has risen 10-12 oC. You can eyeball this from the table above (or the reduced table on the apparatus itself) while doing the experiment, and then do more careful temperature calculations once data collection is over. A total cranking time of about 5 minutes (300 seconds), in which 500-700 revolutions of the crank are performed would be typical. At a thirty second interval at which you have achieved the temperature change of approximately 10-12 oC, stop cranking. Mark this time as tstop. However long you were cranking the system, continue to monitor it for that long again every thirty seconds. (Continue taking data without cranking until the time reaches 2tstop.. Clearly N no longer changes, but the resistance should rise slowly as the temperature of the aluminum cylinder decreases as it gradually tries to return to equilibrium with the environment.) This step is in place in order to make a rough estimate of how much energy was lost as heat dissipating into the environment. A change of 1-4 oC in this step would be typical. Talk to your TA if you observe something outside of this range. Convert all of your resistance data to temperatures using the table and/or function below. The table will allow you to convert to temperatures with an acceptable degree of precision in the absence of a good calculator for evaluating the function. If you are able to use the functional form to get data however, your results will be much nicer. The function is: T ( R ) = ( 67.03) − ( 0.7136 ) R + (3.801∗10 −3 ) R 2 − (8.680 ∗10 −6 ) R 3 Equation 5.11 This function requires input of R in kΩ in order to obtain a result in degrees Celsius. Figure 5.1 Figure 5.2 7 of 20 Figures 5.5 and 5.6 visualize the data from the table below. (Equation 5.11 is an approximation of the actual curve, but we see from figure 5.6 that it is a good one over the temperature range of interest. The function is shown in green, and the table data below in blue.) 8. Follow the instructions and questions in the post-lab in order to complete the analysis of the data. 3.2 Conversion of Electrical Energy (Part 2) To be able to compare more data in the allotted time, you will be collecting data for only half this section and will be utilizing results from other groups to complete the analysis. Before you begin this section, the Lab TA or TI will assign your group to complete EITHER Part 3.2.1 OR Part 3.2.2. DO NOT DO BOTH. You are asked to share your data with the laboratory section by writing your results on the chalk board as soon as you have them available, and in turn you will be using results of others to answer some of the questions. 8 of 20 Introduction Introduction (Part 2) In In Part Part 3.2.1, 3.2.1, Conversion Conversion of of Electrical Electrical Energy Energy into into Heat Heat (With (With Ink Ink & & Insulator), Insulator), we we are are assuming assuming all all the the energy energy produced produced by by the the light light bulb bulb is is absorbed absorbed by by the the water water when when calculating calculating JJElectrical Electrical .. A A 35-Watt 35-Watt incandescent incandescent lamp lamp is is immersed immersed in in aa known known quantity quantity of of water water with with aa small small amount of India ink added to make it opaque to the visible light, so as to absorb the visible light. amount of India ink added to make it opaque to the visible light, so as to absorb the visible light. The The water water jar jar is is inserted inserted into into aa Styrofoam Styrofoam Calorimeter Calorimeter insulator insulator to to prevent prevent heat heat from from escaping escaping to to the air. The temperature of the water is measured with a thermometer. By monitoring the water the air. The temperature of the water is measured with a thermometer. By monitoring the water temperature, temperature, the the heat heat produced produced by by the the lamp lamp can can be be calculated. calculated. The The ratio ratio between between the the electrical electrical energy that flows into the lamp and the heat produced by the lamp determines the energy that flows into the lamp and the heat produced by the lamp determines the Joule’s Joule’s constant constant for for the the electrical electrical energy. energy. In In Part Part 3.2.2, 3.2.2, Conversion Conversion of of Electrical Electrical Energy Energy into into Heat Heat and and Light Light (Without (Without Ink Ink & & Insulator), Insulator), the the efficiency efficiency of of the the incandescent incandescent lamp lamp is is measured. measured. The The details details are are similar similar to to the the first first part, part, but but no no India India ink ink is is added added to to the the water water and and the the jar jar is is not not insulated. insulated. Without Without the the ink, ink, some some (not (not all) all) of of the the energy energy from from the the lamp lamp is is absorbed absorbed into into the the water, water, but but the the visible visible light light energy energy escapes. escapes. To To determine determine the the amount amount of of visible visible light light energy, energy, the the heat heat transferred transferred into into the the water water is is subtracted subtracted from from the the total total energy energy produced produced by by the the light light bulb, bulb, which which is is the the same same as as the the total total electrical electrical energy energy itit consumed. consumed. The The ratio ratio between between the the light light energy energy and and the the electrical electrical energy energy gives gives the the light light producing efficiency of the bulb. producing efficiency of the bulb. 3.2.1 3.2.1 Conversion Conversion of of Electrical Electrical Energy Energy into into Heat Heat (With (With Ink Ink & & Insulator) Insulator) (Part 2) Procedure Procedure 1. 1. Measure Measure the the room room temperature. temperature. 2. Weigh Weigh the the jar jar assembly assembly including including the the lid lid and and record record its its mass mass m mJar 2. inSection Section4.2.1. 4.2.1. Jar in 3. 3. Remove Remove the the lid lid of of the the jar jar and and fill fill itit to to the the indicated indicated water water level. level. Do not overfill. Filling beyond beyond this this level level can can significantly significantly reduce reduce the the life life of of the the lamp. lamp. Close Close the the lid. lid. 4. 4. Make Make the the electrical electrical connections connections with with aa DC DC power power supply, supply, two two multimeters multimeters (one (one acting acting as as aa voltmeter voltmeter and and the the other other as as an an ammeter) ammeter) and and wires wires with with banana banana plug plug connectors. connectors. The The voltmeter voltmeter is is to to measure measure the the voltage voltage difference difference between between the the two two terminals terminals of of the the lamp lamp and the the ammeter ammeter is is to to measure measure the the current current through through the the lamp. lamp. (See (See Figures 5.7, 5.8) Figure 5.8 Figure 5.7 5. 5. Turn Turn on on the the power power supply supply and and quickly quickly adjust adjust the the power power supply supply voltage voltage to to about about 9.8 9.8 volts. volts. At At this this voltage voltage the the ammeter ammeter should should read read about about 2.2 2.2 amps. amps. Shut Shut the the power power off off right right away. away. Do Do not not leave leave the the power power on on long, long, otherwise otherwise itit will will raise raise the the water water temperature temperature before before the the = VI VI .).) measurement P= measurement takes takes place place later. later. Do Do not not let let power power exceed exceed 35 35 watts! ( P 99 of of 20 20 6. Add enough India ink to the water, so the lamp filament is just barely visible when the lamp is illuminated. 7. Insert the jar into a Styrofoam Calorimeter insulator. 8. Insert a thermometer through the hole in the top of the jar. You may want to swirl the jar slightly while in contact with table to reach an equilibrium temperature (room temperature). When swirling, hold the rubber part of the insulator in order to reduce the heat going into the jar from your hand. 9. When ready, turn the power supply on and start the timer. 10. On Table 5.2, record the current, voltage, and temperature of water with respect to time in constant intervals of 60 seconds. Keep an eye on the ammeter and voltmeter throughout the measurement to be sure these values do not change significantly. Continually swirl the jar gently the whole time! As in Step 8, hold the rubber part of the insulator. 11. When the temperature increases by about 8°C, shut off the power but do not stop the timer. Record the time tstop and temperature. 12. As done in Section 3.1, continue to take temperature readings in 60-seconds intervals until the timer reads 2t stop . Continue swirling the water gently. 13. Remove the jar from the insulator. Note how much of the thermometer is immersed in the water. Remove the thermometer from the jar. Calculate Vthermometer —an estimate volume of the portion of the thermometer that was immersed in the water. Record it in Section 4.2.1. 14. Weigh the jar assembly including the water m jar +mwater = m j+w and record it in Section 4.2.1. Discard the water. 3.2.2 Conversion of Electrical Energy into Heat and Light (Without Ink & Insulator) Procedure (Part 2) 1. Without the India ink and Styrofoam Calorimeter insulator, follow Steps 3 – 14 of Section 3.2.1. You wish to allow visible light to escape. Since you will not be using the Styrofoam calorimeter insulator hold the lid of the jar when swirling instead. Record your data in Table 5.3. Instruction Appendix: Notes on Wiring Multimeters (Part 2) 1. One wire always goes to the common (COM) port on a multimeter. 2. Where the second wire goes depends on the quantity you want to measure. The other choices may include any of the following: (A), (mA), (V and/or Ω). 3. In order to measure voltage (voltmeter), the second wire goes to the (V) port. The multimeter should then be turned to an appropriate voltage reading setting, depending on the size of the voltage being measured. Take care to note whether the device is set to measure a voltage for an alternating source (AC or ~), or direct source (DC or –). There are no alternating sources in this lab. 4. Voltages are always measured across two different points in the circuit, and a voltmeter should consequently be wired in parallel with the circuit element(s) of interest. 5. In order to measure a current the second wire should go either to the (mA) or (A) port. (Some multimeters may not have both). The (mA) port is more sensitive than the (A) port, and therefore has higher numerical resolution / precision for small currents. Putting too much current through a (mA) port however will blow the fuse, and then that port will no longer work until the fuse is replaced. For larger currents (closer in order of magnitude 10 of 20 to an amp as opposed to a milliamp), always use the (A) port. The currents in this lab are large enough that you should not ever plug into a (mA)-only port today. Once again turn the device to an appropriate setting for current readout to make measurements. 6. Currents travel through loops in a circuit. An ammeter should therefore be placed within the loop of interest (or in series with the circuit element(s) of interest). 7. On devices set up to measure resistances, you would generally use the same port as for voltage. Turn the device to an appropriate Ω setting and proceed with measurements once it is wired. This is the case you are interested in for the first half of the lab, where you are measuring a resistance across the aluminum cylinder's thermistor to get a temperature reading. 11 of 20 Name: Laboratory Section: Laboratory Section Date: Partners’ Names: Grade: Last Revised on January 6, 2016 EXPERIMENT 5 - New Part 1: Equivalence of Energy: Heat, Mechanical Part 2: Equivalence of Energy: Heat, Electrical, Light For Mechanics courses (PHY113, PHY121) do only part 1 4. Post-Laboratory Work [20 pts] 4.1 Conversion of Mechanical Energy into Heat [10pts] Time (sec) 0 30 60 90 120 150 180 210 240 270 300 330 360 390 420 450 480 510 540 570 600 630 660 690 720 750 780 R (kΩ) N (Revs) Temp (°C) Time (sec) R (kΩ) N (Revs) Temp (°C) Time (sec) R (kΩ) N (Revs) Temp (°C) Table 5.1 Convert R into T using T ( R ) = ( 67.03) − ( 0.7136 ) R +( 3.801∗10 −3 ) R 2 − ( 8.680 ∗10 −6 ) R 3 where R must be entered in kΩ , or use the table on page 8. 12 of 20 1. Plot temperature versus time of the data from Table 5.1 on Graph 5.1. Draw two best-fit straight lines—one for the time between 0 and t stop and the other between t stop and 2t stop . As shown on Figure 5.7, mark on the y -axis the initial ( Tinitial ), peak ( T peak ) and final ( T final ) temperatures. These three temperatures must be based on the two best-fit straight lines, not the data Figure 5.3: Temperature vs. Elapsed Time. points themselves. The initial temperature Tinitial is at the y-intercept of the first line; the peak temperature T peak is at the intersection of the two lines; the final temperature T final is when the time is 2t stop . Include title and axis labels with units. [2pts] Graph 5.1 13 of 20 2. The peak temperature in Graph 5.1 may not be exactly the same as the temperature when you stopped cranking. Why might it be possible to for the temperature reading to rise a bit more after you stop putting energy into the system? [1pt] 3. Calculate the work done to lift the mass while cranking, using equation 5.2. Next compute what we will call ( ΔT )Uncorrected = ΔTu , which is the temperature change between your starting temperature at t=0 and peak temperature. (If your peak temperature and stop temperature are different, as discussed in question 2, use the peak). Then compute Qu using ΔTu and equation 5.3. Finally, compute Jmechanical,uncorrected = Jmu using the above quantities and equation 5.4. [2 pt] Total Number of Revolutions N = __________ Hanging Mass M = __________ Aluminum Mass m = Radius of Aluminum cylinder R = ΔTu (°C) = TPeak − TInitial = __________ 4. While cranking, some heat was likely lost to the air due to ambient cooling. Estimate the temperature change due to this effect by computing ΔTambient = ΔTa = Tpeak − Tfinal . Then compute a corrected temperature change ΔTcorrected = ΔTc = ΔTu + ΔTa . Repeat the calculation from question 3 above to obtain J mechanical,corrected = J mc . [1 pt] 14 of 20 5. Which of the two values Jmu or Jmc is closer to the accepted value of J = 4.2 J/cal? (The value which corrects for ambient cooling may not always be better.) Explain what you observe. (Hint: Why might measuring ambient cooling relative to the peak over-estimate the amount of heat lost to the environment during cranking?) [2pt] 6. Compute the average of Jmu and Jmc. You can call the average Jm. Compute the standard deviation based on those two numbers, and the error in the mean Jm. Does your average agree with the accepted value? [1pt] 7. Discuss one major source of error in this experiment besides the ambient/radiant cooling, and how it may have affected the measured Joule’s constant. Had that error been removed, would it have increased the measured Joule’s constant or decreased it? Explain. [1pt] 15 of 20 4.2 Conversion of Electrical Energy [9pts] (Part 2) CIRCLE WHICH SECTION YOU ARE ASSIGNED: m Jar (grams) = m Jar + mWater (grams) = mWater (grams) = Section 3.2.1 With India Ink Section 3.2.2. Without India Ink __________ __________ __________ VThermometer (cm3) = __________ (Estimate Vthermometer as a cylinder, making appropriate measurements with the calipers. The relevant volume is the portion of the thermometer that is submerged.) Time (sec) Temperature (°C) Voltage (V) Current (A) Time (sec) Table 5.2 16 of 20 Temperature (°C) Voltage (V) Current (A) 8. Plot the water temperature versus time of the data from Table 5.2 on Graph 5.2, as was done in Question 1. Referring to Figure 5.4, draw two best-fit straight lines and mark on the y axis the initial, peak and final temperatures ( TInitial , TPeak and TFinal , respectively). Include title, axis labels with units. What is the uncorrected temperature change ΔTUncorrected = TInitial − TPeak ? [2pt] Graph 5.2 9. As done as in Question 4, find ( ΔT )Corrected based on the Question 8’s graph. Record your answer below and in Table 5.3. Include units and show calculation work. [1pt] 17 of 20 10. Calculate the quantity D using Equation 5.7. Record your answer below and in Table 5.3. Include units and show calculation work. [1pt] 11. Using Equation 5.5, calculate the total amount of electrical energy consumed by the light bulb, EConsumed . If the voltage and current changed, use an average value of the power P = VI in your calculations. Record your answer below and in Table 5.3. Include units and show calculation work. [1pt] Once you have completed Questions 9, 10, & 11, place your values for (ΔT )Corrected , D , and EConsumed on the board in the appropriate section (either “Section 3.2.1 Results” or “Section 3.2.2 Results”). Then, by selecting a set of data that is consistent with the majority of the class’s data, complete the second half of Table 5.3 using data from another group. (ΔT )Corrected Section 3.2.1: Conversion of Electrical Energy into Heat using Ink and Insulator D EConsumed Table 5.3 18 of 20 Section 3.2.2: Conversion of Electrical Energy into Heat and Energy (No Ink or Insulator) 12. By applying D and ( ΔT )Corrected from Section 3.2.1 to Equation 5.6, calculate the total heat ΔQ1 absorbed by the water containing the India ink, the jar and the thermometer. Then, using D and (ΔT )Corrected from section 3.2.2, calculate the total heat ΔQ2 absorbed by the set-up that does not include the India ink. Include units and show calculation work. [1pt] 13. Calculate Joule’s constant J e by applying ΔQ1 from Question 12 and EProduced = EConsumed from Section 3.2.1 to Equation 5.8. Include units and show calculation work. [1pt] 14. By what fraction of the accepted Joule’s constant J = 4.19 J does your J e differ from it, cal ⎛ J −J⎛ i.e. what is ⎜ e ? What is the more accurate of J mu and J mc you found in Question 5? ⎝ J ⎜⎝ Compare: which one of J m and J e is closer to the accepted value J ? Explain. [1pt] 19 of 20 15. Find EAbsorbed by the water without the ink, the jar, and the thermometer by expressing the heat ΔQ2 found in Question 12 in units of Joules using Equation 5.10. [1pt] 16. Calculate the efficiency of the lamp in producing light using Equation 5.9. Is the incandescent lamp more efficient as a light producing source or as a heat source? Explain. (You have access to data in which the visible spectrum is allowed to escape / is not absorbed into the water as heat, and another data set in which the entire spectrum is absorbed. Think about how these should differ.) [1pt] 20 of 20