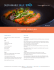
SGU Cures Index - St Georges University
Transcription
SGU Cures Index - St Georges University
SGU CURES INDEX The economic impact of diseases and their cures ! ! ! OPTION 2 ORIGINAL OPTION 1 OPTION 3 SGU CURES INDEX The economic impact of diseases and their cures 2015 ! ! ! OPTION 2 ORIGINAL Please cite this report as: Waechter, R., Akeh, Z., Angus-‐Yamada, C., Brunet, J., Cancellieri, S., Christianson, E., Cummings, J., Fritzky, K., Gibbons, A., Jalonen, T., Kangas, K., Khan, M., Kyotakoze, H., Mehta, S., Molina, A., Nalbandian, M., Pak, J., Patel, V., Russ, C., Shaffiy, OPTION S., Stever, K., Vogler, E., & Kuttner, H. 3 (2015). 1 OPTION SGU Cures Index: The Economic Impact of Diseases and their Cures. Windward Islands Research and Education Foundation -‐ St. George’s University. List of Contributors Project Lead, Author, Editor Randall Waechter School of Medicine & School of Veterinary Medicine, St. George’s University Windward Islands Research and Education Foundation True Blue, St. George’s, Grenada, West Indies, www.sgu.edu [email protected] Authors Zaina Akeh* Colleen Angus-Yamada Julia Brunet Sarah Cancellieri Eve Christianson Jason Cummings* Krysta Fritzky Ashley Gibbons* Tuula Jalonen** Kayleigh Kangas Marium Khan* Hilda Kyotakoze* Sidharth Mehta* Andres Molina* Madlena Nalbandian* Jennifer Pak* Vishal Patel* Chelsey Russ* Shervin Shaffiy* Kayla Stever Emily Vogler Project Advisor Hanns Kuttner Editor Debbi Johnson** * medical student, St. George’s University (at time of writing) ** faculty, St. George’s University (at time of writing) Table of Contents Forward……………………………………………………………......………………….………2 Purpose of the Report………………………………………………………………...…………4 Executive Summary .......................................................................................................... 5 Methods and Calculations ............................................................................................. 10 Background and Introduction ........................................................................................ 22 Results and Conclusions ................................................................................................ 34 Specific Disease Analyses A. Infectious Diseases 1. Acquired Immunodeficiency Syndrome (AIDS) ..................................... 69 2. Influenza ............................................................................................... 86 3. Poliomyelitis ....................................................................................... 108 4. Malaria ................................................................................................ 121 B. Chronic Diseases 5. Alzheimer’s Disease............................................................................. 148 6. Cardiovascular Disease ...................................................................... 173 7. Diabetes Mellitus ................................................................................ 196 8. Mood (Affective) Disorders ................................................................. 234 9. Muscular Dystrophy ............................................................................ 258 10. Sickle Cell Disease............................................................................. 271 C. Neoplasm (Cancers) 11. Child Cancers .................................................................................... 300 12. Intraepithelial Neoplasia (Anus, Cervix, Oropharynx) ...................... 320 13. Pancreatic Cancer ............................................................................. 352 Limitations of the Report ............................................................................................. 368 Appendix A – St. George’s University (SGU) ................................................................ 370 Appendix B – Windward Islands Research and Education Foundation (WINDREF) ..... 371 LIST OF ACRONYMS AD – Alzheimer’s Disease AIDS – Acquired Immunodeficiency Syndrome CDC – Centers for Disease Control and Prevention CAD – Coronary Artery Disease CVD – Cardiovascular Disease DALY – Disability Adjusted Life Year EPA – Environmental Protection Agency GDP – Gross Domestic Product HIV – Human Immunodeficiency Virus NCI – National Cancer Institute NIH – National Institutes of Health QALY – Quality Adjusted Life Year ROI – Return on Investment VMRR – Value of Mortality Risk Reduction VSL – Value of Statistical Life WHO – World Health Organization YPLL – Years of Potential Life Lost YLD – Years Lived with a Disability SGU Cures Index 1 FORWARD The cost of healthcare in the United States of America (US) is significant. According to the World Bank, the US currently spends 17.9% of Gross Domestic Product on health. This is up from 15.6% just ten years ago. Given an aging cadre of baby boomers and strong correlation between age and healthcare expenditures, the proportion of GDP spent on healthcare is likely to continue rising. For example, given current trends, the number of Americans with Alzheimer’s disease is expected to grow to 13.5 million and cost the US $20 trillion by 2050, according to the Alzheimer’s Association. Given these projections, a number of policymakers and politicians in the US have focused on the healthcare expenditure issue, and this reflects the thinking of the populace. Polls conducted by Harris Research in 2010 showed that 44% of US adults are "extremely worried" or "very worried" about paying for rising health-care costs. Policymakers generally discuss three ways to address the growing healthcare expenditure problem: (1) cut back on spending, either by limiting the amount paid for products and services, or by reducing the level of products and services being offered; (2) continue on the current path, which likely means continued increases in spending and thus, the raising of funds to support that spending through increased taxation, insurance premiums, or borrowing, (3) maintain services but limit spending increases by eliminating waste and improving efficiency. While the third is the most desirable option, it can be argued that finding efficiencies has an upper limit and may not be able to address the full scale of the problem. We believe there is a fourth option for limiting healthcare cost increases that is rarely discussed: Research and Innovation – in short, the discovery of more cures for disease. Historical “definitive cures” that prevent disease (e.g., the polio vaccine) or eliminate the impact of a disease after it has taken hold (e.g., insulin for Type I diabetes) have saved the US economy trillions of dollars, and continue to provide savings with the passage of every year. The up-front investment in basic research to understand disease processes, and applied research to intervene effectively in that process to cure the disease, is significant. While the discovery of a cure (i.e., the return) is not guaranteed, only by allocating significant resources toward the cause and distributing those resources effectively, can we have any hope of future breakthroughs. Given past experience, the potential return on those breakthroughs is staggering. Even more, the analyses included in this report suggest that preventive measures such as daily exercise and changes in diet can provide a virtually infinite return on investment and have an SGU Cures Index 2 impact across multiple health domains with essentially no unwanted side effects. Now, it is up to scientists to carry out research that will provide insights into why so few Americans follow these behavioral guidelines, and help policymakers change this reality. The US is one of only a few nations on earth with the ability to invest in the level of research that is necessary to make significant advancements in cures. First, it has the economic capacity to invest the necessary resources up-front, wherein the return on investment might be decades in the future. Second, it has a well-established education system and research base to ramp up the necessary research activity quickly. Finally, it has the culture of innovation and outside-of-the-box thinking that is so critical for cures breakthroughs. All that is needed is the cultural and political will. This is the first report to examine the burden of illness, economic impact of past cures, and potential economic impact of future cures across multiple diseases and disease categories. It is a culmination of two years’ of international collaboration, teamwork, and investigation. I would like to acknowledge the effort and dedication of the many authors on the report, and the editors who reviewed every sentence. Many of them will go on to practice medicine, conduct further research, and provide healthcare leadership in their communities. I hope that their involvement in this project has provided some insight to the importance of research and innovation for healthcare delivery not only in the US, but also around the world. This dedication to research, education, healthcare delivery, and well being from an international perspective is what drives St. George’s University and the Windward Islands Research and Education Foundation forward every day. Calum Macpherson, PhD, DIC, MEVPC, FRSPH, FSB Vice Provost for International Program Development, Dean of Graduate Studies and Director of Research, St. George’s University Director, Windward Islands Research and Education Foundation SGU Cures Index 3 PURPOSE OF THE REPORT This report examines the process of cure discovery and the economic impact of cures across varying diseases. It is the first time anyone has attempted to examine the [economic] returns across a wide spectrum of disease categories, using a common frame of reference. Where cures have already been discovered and implemented, such as the polio vaccine, we provide an historical analysis that estimates the human and economic impact of the disease prior to the introduction of the cure. We then estimate what the total impact of the disease would have been between the implementation of the cure and today, as well as an estimate of the ongoing future annual cost of the disease without the cure. Then, we estimate the current total human and economic savings associated with the implementation of the cure, while accounting for the cost of discovering the cure and implementing it on an annual basis. Using the cost and savings estimates, we can calculate a total return on investment that the cure has achieved. Where cures are nonexistent or limited, such as Alzheimer’s disease, we provide estimates of the human impact and burden of disease, as well as the overall economic cost of the disease to the US. Where possible, we break down this cost on an annualized basis, so the reader can project the future ongoing costs of the disease if no cures are developed. This provides an estimate of the potential costs savings to be achieved with the development of a cure. We then briefly discuss the current state of research into a cure for that disease, as well as the amount of public research funding currently allocated for that disease by the National Institutes of Health. Despite the heterogeneous nature of different diseases, we have attempted to categorize them and directly compare their economic impact. We have defined the following categories: (1) Infectious (e.g., influenza, HIV); (2) chronic (e.g., cardiovascular disease, diabetes); (3) Neoplasms (e.g., pancreatic cancer, childhood cancers). By examining the economic impact of these different highly burdensome diseases, and the cures that have been discovered to ameliorate that impact, within the same document, we hoped to gain fresh insight into the cures process. We further hoped to provide evidence regarding the historical and future potential economic impact of cures on the health system in the US and around the world. This will allow policymakers to make informed decisions regarding how much to invest in cures. SGU Cures Index 4 EXECUTIVE SUMMARY As the capability to alter the course of disease has grown, life has become better. Life can become better still if we pursue a cures agenda. Our motives may be selfish, wanting the risks that we ourselves face to be diminished. Or they may be altruistic, pursuing cures that will not be achieved until those who benefit are our children or their children. While there is uncertainty about when a cure can be achieved, the tremendous value created by cures that have already been accomplished shows why more cures should be pursued now. This report examines the value of cures. Cures for some diseases, like polio, have been fully achieved and some, such as heart disease, only partially. We go through life with a quiver of arrows of death and disease pointed at us. Each cure removes an arrow from the quiver or remakes an arrow so that it is less deadly or causes less injury. With fewer risks, our chances of making it to a later age in a healthier state grow larger. With arrows eliminated or made less threatening, we live longer and less afflicted lives. Cures come about through a process of scientific discovery. The accumulated stock of cures has changed what it means to live and die. While some arrows are gone and others diminished, some arrows remain in the same form they have had for millennia. In one sign of human progress, the overwhelming majority of children born today in the US [and other developed countries] live into adulthood. In one sign of how far we have to go, Alzheimer's disease has no cures, and that fact makes any step towards a cure valuable. Our analysis also indicates that cures tend to follow an “evolutionary” path, in which each generation of the cure, building on more detailed knowledge of physiological processes, provides more relief from disease, better quality of life, or more extension of life. Sometimes, a paradigm-shifting breakthrough in disease or therapeutic knowledge leads to a “leap” in cure development, significantly impacting life extension or quality of life. Ideally, as knowledge accumulates, the evolution of cures for a particular disease will make the ultimate leap from a functional (albeit, very effective) cure to a definitive cure, thereby eliminating the impact of the disease altogether. SGU Cures Index 5 Return on Investment (ROI) Multiple for Different Cardiovascular Disease Cures Over Time. Coronary Artery Bypass Graft Surgery Angioplasty & Stents Diuretics Statins Alpha/Beta-Blockers -20 -10 0 10 20 30 40 50 Cures are a gift from the past. Each cure is part of the store of knowledge the present generation will pass to the next. A cure benefits not only those in this generation but in all generations to come. This form of intergenerational generosity is a perpetual gift whose value is augmented by the passage of time. It provides a reason to believe that the future will be better than the past. Cures create large amounts of value. The stream of benefits continues on, even after a disease disappears. Consider the benefits from the polio vaccine. The number of paralytic cases of polio in the US fell from 21,269 in the early 1950's to the hundreds by 1962 and zero by 1980 (Centers for Disease Control and Prevention, 2012; Post Polio Health International, n.d.). Parents of children in 1960 knew that the chance of their children suffering from polio had declined. Even if the parents of today do not have memories of a polio outbreak, today's children benefit as well. More than fifty years have passed since the first vaccine became available, and the cumulative value of that cure to Americans now exceeds $1.3 trillion (see Poliomyelitis section of this report). This staggering amount draws attention to what a focus on cures could achieve. Cures create value in multiple ways. SGU Cures Index 6 They extend life. Life is lived with a large set of risks. Some are the risks posed by diseases than can be fatal or can diminish the quality of life. Each fatal risk that disappears or becomes weaker means each of us can expect to live longer. Longer lives mean more time to work and more time to be part of the economy. This, in turn, has a positive impact on economic measures such as the size of the workforce and the Gross Domestic Product (GDP). Longer lives also increase the time available for whatever it is that people want: connect with loved ones, enjoy life or help others. We are willing to trade other resources for the opportunity to be alive, and this willingness creates value beyond the amounts recorded in the GDP accounts. Cures increase the value of life. A cure that does not extend life can still expand the limits of what someone can do in the span of life one has. While some value of the polio cure stems from avoiding death and making the number of people alive greater than it would otherwise be, most of the polio cure’s value stems from lessening the shackles of disease that would otherwise limit lives. The polio cure means people are no longer being limited in their ability to walk and move because of polio. Some of these limits would keep people from working. Reducing these limitations can lead to increases in the measured economy by bringing people into the workforce or enabling them to stay longer. Some diseases limit or reduce how much people enjoy life. People are willing to exchange resources to have lives that are less threatened by illness and disability. Thus, a medical treatment or intervention need not fully restore a patient to perfect health in order to have value. Cures can decrease the cost of medical services. Hospitals developed iron lung units to treat those with respiratory complications due to polio. Those units no longer exist. Had a vaccine that would prevent future cases not been invented, we would continue to allocate capital to building iron lung units and pay every year to run them. In this case, a cure eliminated the need for future costs. This report shows the value of cures, realized and potential. It does this by providing an overview of the value of cures and then presents case studies that show how much value particular cures created. These case studies show the implications of different kinds of cures. Their stories show how cures have come about, and suggest how more cures could be achieved. The case studies provide a varied picture of what different kinds of cures mean. The polio vaccine brought about a definitive cure. Once immunized, an individual no longer faces the risk of experiencing paralytic polio. This definitive cure pushed the SGU Cures Index 7 riskof paralysis and death from new polio infection down to zero in America, making the polio vaccine an example of a definitive cure. The introduction of highly active antiretroviral therapy (HAART) has meant that the risk of dying from HIV is far less for those who are infected today than it was at the time that HIV first became known. As long as those additional years of life continue, an individual experiences a functional cure. The same is true of heart disease. A series of new treatments has meant that the risk of dying because of heart disease is far lower than it was 40 years ago, and as a result, many who would have died are still alive. Return on Investment (ROI) of Cures Achieved After and Prior to Disease Contact. Cures Achieved After Disease Exposure Cures Achieved Prior to Disease Exposure 90 80 70 60 50 40 30 20 10 ne Va cc i Po lio m ye l iti s- -V ac ci n en flu In -H er nc Ca Ce rv ic al za PV St oo d -M rd er e e Va cc in rs ze ab In ste rD iso Bi po la ili su nt sa be Ty pe 1 D ia nt -A n lin s e) id ep re s im st re sio Un ip ol ar D ep re s ap -P er nc Ca ic al pe up t( Te s nt -A IV H Ce rv at py ra he vi ra lT tro ire ia ar al M In flu en -A za nt -A im nt al iv ira ar ia ls ls 0 Our analysis indicates that definitive cures tend to provide a better return than functional cures, whose return can vary significantly. Polio is not the only definitive cure. Malaria was once a risk to health in the US. Today malaria in the US is known only as an SGU Cures Index 8 infrequent occurrence tied to someone who has contracted malaria prior to entering the country. The value of this cure and how it came about is the topic of another case study in this report. Together these case studies show the value of a cures strategy. Health is a form of wealth. For most people, wealth held in health exceeds the amount held financially. Wealth can increase through investment. This is a simple fact too often ignored when talking about health. This report brings together facts about past investments in cures. It does this to focus thinking about how pursuing a cures strategy can bring about higher future levels of wealth and well-being. More cures and more effective cures are the center of a cures strategy. Citations Centers for Disease Control and Prevention. (2012). Poliomyelitis. In Epidemiology and Prevention of Vaccine-Preventable Diseases (pp. 249-262). Washington DC: Public Health Foundation, Centers for Disease Control and Prevention. Post polio health international. Incidence rates of poliomyelitis in the US. Retrieved February 18, 2014 from: http://www.post-polio.org/ir-usa.html. SGU Cures Index 9 METHODS and CALCULATIONS This report examines the economic impact of cures – existing and potential – across varying diseases. Thus, it combines an historical analysis of cures already achieved, as well as an examination of the impact of current diseases that have no or limited cures. Understanding the current impact of diseases with no or limited cures provides an indication of the potential impact of an improved partial cure, or a definitive cure. Below, we provide some methodological notes regarding the completion of this report. 1) Data sources This report relies on secondary data from reliable government or institutional sources (e.g., American Cancer Society, US Census Bureau, US Centers for Disease Control and Prevention, World Health Organization) and/or peer-reviewed, published, scientific journal articles. 2) Timelines The only prediction made in this report is that there will be more cures. An historical analysis of diseases, the potential economic impact of those diseases, the cures that were discovered to treat those diseases, and the potential economic impact of those cures for the US economy shows that cures that have already been developed are the source of immense economic benefit. The present owes a large debt to the past. To be sure, we cannot say which research projects currently underway will be necessary for some future cure. Nor can anyone say in what year a particular cure will be achieved. The past suggests that many peaks get climbed only to see that there is another valley ahead. Nonetheless, the pursuit of cures itself is an important economic activity. Some of the new products from research-oriented pharmaceutical companies will be cures. Market discipline limits those companies to research projects that will provide results in the relatively near future. Public and philanthropic dollars pursue a longer timeline. Nobody can know whether spending money on future research will necessarily lead to a cure “breakthrough”, when such a thing might happen, and how much it would cost. That being said, there is strong precedence regarding the impact of spending public funds to support research initiatives. For example, the Human Genome Project returned SGU Cures Index 10 $141 for every $1 of public money invested, in terms of jobs generated and followthrough economic activity (Tripp & Grueber, 2011). There are also numerous intangible benefits associated with research funding: innovative edge in an increasingly competitive world, follow-through technologies, and the fact that “cures” can be sold not only to Americans in need, but to a world of 7 billion+ people also in need of cures. 3) Disease Categorization Disease and ill-health take many forms. Some diseases occur because a foreign entity, such as a virus, enters the body. Some diseases involve a process inside the body, such as a blocked artery. Given the varying nature of different diseases and our desire to incorporate them into a single report to compare their impact and the status of a variety of cures, we categorize them as follows: i) Infectious (transmissible / communicable) a) Viral – Diseases that spread through the population via viruses (e.g., human immunodeficiency virus, human papillomavirus, influenza, polio) b) Bacterial – Diseases that spread through the population via bacteria (e.g., tuberculosis, pneumonia syphilis, tetanus) c) Parasitic – Diseases that spread through the population via parasites (e.g., malaria, toxoplasma gondii) ii) Chronic Disease a) Slow Progression (i.e., Alzheimer’s dementia, cardiovascular disease, diabetes, mood disorders) b) Acute (i.e., scurvy, rickets, spina bifida, muscular dystrophy) iii) Neoplasms (Tumors) a) Benign Non-cancerous b) Malignant Cancerous 4) Cure Categorization Given that cures are based on fundamental knowledge of a disease process, which changes over time, we recognize that the effectiveness or impact of the cures can change over time as well. Thus, for the purposes of this report, we categorize cures as either: SGU Cures Index 11 i) Functional – the cure reduces the burden of disease as measured by either years of potential life lost (YPLL) and/or years lived with disability (DALY/QALY). However, the disease is not completely eradicated. The cure or treatment must be taken continuously, or the disease state is likely to return (e.g., Antiretrovirals for HIV, insulin for diabetes, antimalarials, stents and angioplasty for cardiovascular disease, antidepressants and mood stabilizers for bipolar disorder) ii) Definitive – the cure eliminates the burden of disease as measured by either years of potential life lost (YPLL) and/or years lived with disability (DALY/QALY). The cure may be taken one time (e.g., polio vaccine) or continuously over time (e.g., vitamin C to eliminate the risk of scurvy). Definitive cures are normally more efficient and effective than functional cures, and thus usually offer an advantage over functional cures. Prevention of disease, either through separation of the individual from the disease agent (e.g., leaving a moldinfected house, avoiding contact with infectious individuals), or through behavioral changes (e.g., limiting intact of alcohol and high-fat foods, exercising more) are particularly efficient ways of preventing disease and bringing about definitive cures. 5) Measures used to assess the value of cures Choices that involve resources require trade-offs. Something -- whether time, talent, or finances -- used to pursue one purpose is not available to pursue another. What are the possible rewards from pursuing a cures strategy? A number of tools are available to produce answers to that question, and the answers to these questions can facilitate making more informed arguments about the value of pursuing cures. i) Years of Potential Life Lost (YPLL) One of the simplest ways to measure the burden or impact of a disease is by examining mortality - that is, by estimating the average number of years a person would have lived if he or she had not died prematurely because of some disease or trauma (Gardner & Sanborn, 1990). To calculate the years of potential life lost (YPLL), the analyst has to set an upper reference age – which usually corresponds to the life expectancy of the person or population under study. For the population-based method, this means comparing the age at death with overall life expectancy. In the developed world, this is commonly set at age 75, but SGU Cures Index 12 could be as high as 83 (the overall life expectancy in Japan). Using a reference age of 83, for someone who dies at age 75; YPLL (83) = 83 – 75 = 8. For the death of an infant at age 1.5; YPLL (83) = 83 – 1.5 = 81.5. YPLL can also be calculated using remaining life expectancy; that is, how many more years can an individual of a particular age be expected to live. In this approach, the calculation sums up the years of expected life across all deaths (National Cancer Institute, 2012). Cancer currently accounts for the most YPLL of any disease in the United States, with 8,756,000 person-years lost in 2006 (National Cancer Institute, 2012). Some have argued that YPLL should be used to set priorities for health research and healthcare, to maximize impact (Burnet, Jefferies, Benson, Hunt, & Treasure, 2005). ii) Quality-Adjusted Life Years (QALY) While years of potential life lost (YPLL) measures disease burden or impact by how far below life expectancy someone dies, it does not capture the quality of life lived among those who do not die. For example, using YPLL, malaria has a significant impact in world health, with 37,448,817 years of life lost worldwide in 2001 (World Health Organization, 2001). However, unipolar depressive disorders, which significantly impact quality of life (i.e., work, social interaction, ability to enjoy life) but does not significantly reduce life expectancy, barely registers as a worldwide health problem, as measured via YPLL (177,724 years of life lost worldwide in 2001 – World Health organization, 2001). Thus, using the YPLL measure, one would conclude unipolar depression has 0.47% of malaria's impact on worldwide health. To address the shortcomings of a measure that only considers years alive, an alternate rating of the quality of life-years lived has been proposed. Entitled “quality-adjusted life years (QALY)”, this measure is based not only on the number of years of life that would be added by the intervention, but also the degree of health during those years. A year of life lived in perfect health becomes the standard of measure; "1" represents "one year of life lived in perfect health", whereas a year of life lived in less than perfect health is less than 1. Death is 0. Someone who receives a treatment that provides an extra half-year of life in perfect health would use a quality-adjusted score of 1, giving an overall QALY of: 0.5 (half-year) x 1 = 0.5 SGU Cures Index 13 Someone who receives a treatment that provides an extra 5 years of life, but perhaps with some chronic pain that can be managed, might use a quality adjusted score of 0.85, giving an overall QALY of: 5 (years) x .85 = 4.25 Someone who receives a treatment that provides an extra 5 years of life under significant duress (i.e., they are bedridden or a quadriplegic) might use a quality adjusted score of 0.4, giving an overall QALY of: 5 (years) x .4 = 2.0. This scoring system has been used by some to rank health interventions in a healthcare system that is inherently limited in resources (Nord, Pinto, Richardson, Menzel, & Ubel, 1999). If resources were available to assist only one of the patients discussed above, QALY analysis would indicate that the second patient would receive the most utility or “return” on invested resources. The most significant criticism of the QALY measurement system revolves around the determination of quality-adjusted weights (from 0 to 1). Perfect health is difficult to define, and how to weight different health scenarios is fraught with problems (Mortimer & Segal, 2007; Prieto & Sacristán, 2003). A number of methods have been developed to determine the weight values on different health scenarios, including asking people to: (a) Choose between remaining in a state of ill health for a period of time, or being restored to perfect health but having a shorter life expectancy; (b) Choose between remaining in a state of ill health for a period of time, or choosing a medical intervention which has a chance of either restoring them to perfect health, or killing them; (c) Rate a state of ill health on a scale from 0 to 100, with 0 representing being dead and 100 representing perfect health. This method has the advantage of being the easiest to ask, but is the most subjective. iii) Disability-Adjusted Life Years (DALY) Like QALYs, disability-adjusted life years (DALY) also takes into account the number of healthy years lost to disease, as well as years lived in poor health or disability. As such, DALYs are considered a measure of overall disease burden. By combining mortality and morbidity into a single metric, the World Bank and World Health Organization have used DALYs in their analyses of health investments. DALYs are calculated by summing the years of potential life lost SGU Cures Index 14 (YPLL) to a disease + years lived with a disability (YLD). One DALY is argued to be equivalent to one year of healthy life lost. Using DALYs can capture more complex disease burden than years of potential life lost on its own. For example, someone who dies at the age of 50 from a sudden and massive first heart attack, but who was relatively healthy up to that point, might add 33 to the count of DALYs (potential life span of 83 years based on Japanese life expectancy – 50 = 33). Comparatively, someone who lives to the age of 80 but suffers from severe chronic unipolar depression from the age of 20 might add 39 to the count of DALYs: (potential life span of 83 years – 80 = 3) + (quality weight of 1 [perfect health] - .4 [quality of life with depression] = .6 x 60 years living with depression = 36). 3 years short of expected life + 36 years lived with depression = 39 DALY iv) Valuing reductions in mortality through the value of mortality risk reduction achieved (VMRR) measure While human life is widely considered priceless by most societies and justice systems, in a world of limited resources, some choices cannot be resolved by appealing to this notion. For example, the risk of death from automobile accidents can only be eliminated by not traveling in automobiles. Similarly, ending the industrial processes that create emissions can only eliminate mortality and illness associated with air emissions. As long as there are automobiles and industrial processes that emit into the atmosphere, there will be risk. How willing is a society to make the trade-offs required to achieve a level of risk reduction? In economic theory, the risk/reward trade-offs that people make regarding their health, for example, taking jobs with higher and lower risks of dying on the job, provides a measure of society’s willingness to pay for one unit of fatal risk reduction (i.e., one statistical life) (Kochi, Hubbell, & Kramer, 2006). Organizations such as the Environmental Protection Agency (EPA) use estimates of values of risk reductions when conducting a benefit-cost analysis of a new policy or regulation that may affect public health. For example, many of the air and water pollution control regulations that are implemented by the EPA will reduce the risks of certain types of cancers, respiratory illnesses, and other diseases among large portions of the general public. Benefit-cost analysis compares the total willingness to pay for the health risk reductions from these policies to the additional costs that people will bear if the policies are adopted. SGU Cures Index 15 The results of a benefit-cost analysis are presented to policy-makers and the public to help inform their judgments regarding whether or not a proposed policy should be adopted (Environmental Protection Agency, 2015). Rather than the value for any particular individual’s life, measures such as the value of a statistical life (VSL) and value of mortality risk reduction (VMRR) represent what a society as a whole is willing to pay for reducing each member’s risk by a small amount rather than how much money any single individual or group would be willing to pay to prevent the certain death of any particular person (Fisher, Chestnut, & Violette, 1989). The EPA is currently looking to replace the often-misunderstood term "value of statistical life” with the term "value of mortality risk reduction”. This change in terminology will not impact the economic theory or the way that the value is calculated. 6) Cure Impact There are a number of ways to measure the impact of a cure. i) Economic output: Gross Domestic Product (GDP) is the most familiar measure of the market value of goods and services produced in the national economy. Its largest component is consumption. If more people are alive, consumption is higher and thus GDP is higher. If cures allow people to live longer and more people to participate in the labor force at their full productivity, then incomes and GDP will be higher. The creation of cures requires one-time costs. Every existing cure is a result of some past generation allocating some of its resources to pursue that cure. The pursuit of cures adds to economic output in our own generation. Cure creation has become a resource-intense activity, requiring trained scientists, ever-more sophisticated equipment, and a vast logistical support network. ii) Economic well-being: Not all well-being is reflected in the market value of goods and services. Life itself has an economic value, reflecting how much we value time and the opportunities it allows. This measure provides a way to assess whether some policy that creates costs in the economy is cost-effective. The most effective interventions and cures avoid or delay death. Definitive cures end risk. Functional cures put a disease process in abeyance, gaining time or lessening the burden of disease. Many chronic disease treatments alter the SGU Cures Index 16 disease process, slowing it or altering its course. Over the period of time they gain for an individual with the condition, they are a cure. iii) Lower health costs: Cures can reduce health care costs. This is most often true for definitive cures, where the cure keeps the need for health care from developing. Consider the example of polio. Had the polio vaccine not been developed, people would continue to be admitted to hospitals because their response to the poliovirus resulted in paralysis. After hospitalization, they would have required rehabilitation to increase their functional ability. Those with severe cases that resulted in immbolization of their chest muscles, would have required ongoing respiratory assistance, something that prior to the introduction of the polio vaccine was accomplished through a machine called an "iron lung." Today, there are no hospital admissions for polio in the US and the development of "iron lung" technology stalled out because there was no use for the technology. These savings must be weighed against the cost of continuing to administer the polio vaccine. The savings remain substantial. Other cures may not eradicate a disease but cause the disease prevalence to be lower than it would be without the cure. The lower prevalence reduces the number of people who must be treated. Even if the cost of treatment among those who have the disease remains the same, the total cost will be lower because the number treated falls. Functional cures can require more health care spending to produce longer lives or to reduce the burden of disease and disability. In these cases, the costs appear on the health care side of the ledger and the benefits are elsewhere. Some of these benefits appear in social measurements. Where the benefit is delay of death, the benefits appear in life expectancy statistics. Those years of life create new opportunities for consumption, increasing the GDP measure. The higher/lower cost analysis looks at the costs associated with a particular disease or condition. The case study of Alzheimer's disease in this report explains how the decline in the mortality rate due to other diseases has made it possible for more people to live to ages where the risk of Alzheimer's disease is higher. Someone cured from one disease is still subject to the risk of others. Our estimate of the value of a cure does not include the costs that arise from someone cured of one disease experiencing the costs of another. 7) Calculation assumptions SGU Cures Index 17 i) We use the following estimate of current US population for all calculations in the report, in millions (US Census Bureau, 2014), unless otherwise noted: 2000: 282.16 2001: 284.97 2002: 287.63 2003: 290.11 2004: 292.81 2005: 295.52 2006: 298.38 2007: 301.23 2008: 304.09 2009: 306.77 2010: 309.35 2011: 311.59 2012: 313.91 2013: 316.16 ii) We use the current US life expectancy value provided by the Centers for Disease Control, which is 78.7 years overall, breaking down to 76.4 years for males and 81.2 years for females (Centers for Disease Control, 2014). iii) In all of the calculations of aggregate social value presented in this report, we use the methodology adopted by the EPA to calculate value of mortality risk reduction (VMRR) achieved. In 2006, the EPA said that the value of lifetime mortality risk reduction was $7.4 million (2006 USD), which is $8.2 million in 2012 inflation-adjusted dollars. We obtained the annual value of mortality risk reduction used in this report by dividing the 2012 lifetime value ($8.2 million) by US life expectancy at birth (78.7 years in 2011). The result of that calculation is $104,193, and that is the value we use in this report to calculate the annual value of actual and potential cures. For example, historical analysis indicates that between 1955 and 2006, 160,000 American lives have been saved as a result of the polio vaccine. Multiplying those 160,000 lives by the current value of lifetime mortality risk reduction of $8.2 million gives a VMRR of $1.312 trillion. We present VMRR for each cure examined throughout the document. The EPA adopted the current value of lifetime mortality risk reduction based on a review of 26 studies, which appeared between 1974 and 1991, and used data from different groups of people, different times, and different places. The range SGU Cures Index 18 of estimates for lifetime mortality risk reduction in these studies ranged from $850,000 to $19.8 million (in 2006 dollars.) The EPA concluded that $7.4 million represented the best estimate of the central tendency among these studies. A majority of the studies use data from the work setting (Environmental Protection Agency, 2010, rev. 2014). Different jobs have different risks. A job at a construction site brings with it a higher risk of death than a job doing data entry in an office setting. The wages paid to workers of jobs with different risks can be compared. Data regarding on-the-job death rates can be used to measure differences in risk. With everything else being equal, the difference in wages between more and less risky jobs provides a measure of how much workers are willing to pay to have a safer job. Wages can be converted into an hourly wage; mortality risk can be calculated as the risk of dying in a jobrelated accident in an hour. The result can be scaled to calculate an annual amount. The annual amount expresses how much workers are willing to pay per year to avoid an on-the-job death. The age at death from a particular risk can lead to different approaches. One could weight the value of different years of life differently. For example, years of adulthood and old age lost when a child dies could be valued more highly than years lost by the elderly. This approach could be supported by appealing to diminishing marginal returns; that is, a year not yet lived is worth more to an individual who has lived few years than to one who has already lived many. Alternatively, one could appeal to research showing that the elderly value additional life years more than the young (Environmental Protection Agency, 2010, rev. 2014). The EPA has adopted neither approach, weighting years equally (Environmental Protection Agency, 2010, rev. 2014). This report follows the equal weighting approach, allowing evidence about life years to be aggregated into statistical lives. Even though these “statistical years” are lived across many different people, equal weighting allows for adding them and saying that the additional life years across all those involved reaches one life when the total number of years reaches the US life expectancy of 78.7 years. The EPA applies this metric to calculate valuations of changes in mortality risk. It does not assess the potential for policies to change the measures of well-being captured in QALY and DALY measures (Environmental Protection Agency, 2010, rev. 2014). However, the same conceptual approach used to value changes in mortality risk can also be applied to QALY and DALY measures. QALY and DALY create a common metric that allows changes in mortality risk to be compared SGU Cures Index 19 with changes in health state. This common metric can also be adopted to derive a value to society of changes in health state. That value can be expressed in dollar terms by the same method used to calculate the value of changes involving mortality risk. In this report, we follow the approach of multiplying the value of a statistical life year by the number of QALYs or DALYs. Citations Centers for Disease Control (2014). Life Expectancy. Retrieved on May 23, 2014 from: http://www.cdc.gov/nchs/fastats/life-expectancy.htm. Environmental Protection Agency (2010, rev. 2014). Guidelines for Preparing Economic Analyses. National Center for Environmental Economics. Retrieved on July 15, 2015 from: http://yosemite.epa.gov/ee/epa/eerm.nsf/vwAN/EE-056852.pdf/$file/EE-0568-52.pdf Environmental Protection Agency (2015). Frequently Asked Questions on Mortality Risk Valuation. National Center for Environmental Economics. Retrieved on March 8, 2015 from: http://yosemite.epa.gov/ee/epa/eed.nsf/webpages/mortalityriskvaluation.html# whatisvsl). Kochi, I., Hubbell, B., & Kramer, R. (2006). An empirical Bayes approach to combining and comparing estimates of the value of a statistical life for environmental policy analysis. Environmental and Resource Economics, 34, 385-406. National Cancer Institute (2012). Person-Years of Life Lost through 2008. Cancer Trends Progress Report 2011/2012 Update. Retrieved on March 4, 2015 from: http://progressreport.cancer.gov/doc_detail.asp?pid=1&did=2007&chid=76&coi d=730&mid=#estimate National Center for Environmental Economics (2014). Frequently asked questions on mortality risk valuation. Retrieved on May 23, 2014 from: http://yosemite.epa.gov/ee/epa/eed.nsf/webpages/mortalityriskvaluation.html# whatisvsl Tripp, S., & Grueber, M. (2011). Economic impact of the Human Genome Project. Battelle Memorial Institute. Downloaded on May 23, 2014 from: http://www.genome.gov/27544383. SGU Cures Index 20 US Census Bureau (2014). US population by year. Retrieved on May 23, 2014 from: http://www.multpl.com/united-states-population/table SGU Cures Index 21 BACKGROUND and INTRODUCTION “If you think medical innovation is expensive, try disease” - Mary Woodard Lasker Disease is one of the most costly issues faced by societies around the world. It has been this way throughout history. Not only does it rob society of its citizens through premature death, it robs individuals of quality of life, with subsequent impacts on the productivity and richness of the society. Disease also sequesters massive amounts of societal resources. Sick members of society may experience constraints in their ability to work and contribute to the society, while other healthy members of the society spend time caring for the sick, and capital resources are allocated for hospitals, clinics, ambulatory care, etc. Perhaps this is why human beings have desperately sought cures for disease throughout history. Unlike many other coordinated societal pursuits, cures relieve suffering; save lives; improve the quality of life among survivors; and save society money and resources. In short, cures are worth pursuing. Given the inherent value of cures, the quest to eliminate disease is one of the most striking examples of human scientific progress. The renaissance, industrial revolution, digital revolution, and information revolution have supported a detailed understanding of diseases and increasingly rapid development of cures. In just the last century, we have developed cures for polio, diphtheria, biotic infections, measles, mumps, rubella, tetanus, and smallpox. In the year 1900, US life expectancy at birth was 49.2 years. In 2000, that number had climbed to 76.9 years (Centers for Disease Control and Prevention, 2014a). Yet, we still have far to go. Why focus on cures? First, cures provide clear measures of advancement. Unlike those who lived a century before us, we have reason to believe that our chance of dying because of polio (and many other, mostly infectious diseases) is zero. Like those who lived a half century ago, we have good reason to fear what awaits us if we are diagnosed with Alzheimer's disease. If we are diagnosed with a soft tissue cancer such as pancreatic cancer we have little more hope of surviving five years than someone who received the same diagnosis one and two generations ago. The extent of cures defines one limit to how optimistic we can be about the future. Cures bring about more than forms of well-being whose measurement is inherently subjective. Some of that wellbeing shows up in such economic measures as GDP and employment as more people are alive and can be in the workforce. SGU Cures Index 22 The scope of benefits from cures ranges from hard costs to soft benefits: 1) Living a longer life is desirable for the individual and the society. Living people connect with loved ones, engage in meaningful work, spend money, and contribute to economic activity; 2) It is better to live life (including any extra years beyond expected death) in good health. A higher quality of life is desirable for the individual and society. Healthier people generally engage in work that is more meaningful, spend more money, and contribute more to economic activity; 3) It is better to beat than to treat disease; it can also be less expensive; 4) History demonstrates that untreated diseases and diseases with ineffective treatment have a greater impact on the economy than the cost of the cures. Thus, cures increase prosperity. Thus, new cures are needed, but what do we mean by cures? How do we “find” them? Can we actually measure their impact on human suffering and the economy? Early cures emerged from luck and careful observation. Those who contributed to the development of the cures may not have understood the biological mechanisms at work. All they knew was that they worked. Death among sailors because of scurvy ended when citrus was added to rations, long before there was an understanding of how Vitamin C deficiency could impede cellular processes to the point where death resulted. Looking back to the 1930s, when he trained to be a physician, the essayist Lewis Thomas saw much of what physicians did as "non-technology." There were routines to be followed and preparations to be administered according to the best ideas of previous generations. These non-technologies did not alter the course of a disease. They may have alleviated symptoms. They may have provided a sense of assurance or relief from knowing that all that could be done was being done. But they did not determine the outcome. "High technology," Thomas argued, requires an understanding of the mechanism of a disease. Vaccines began with a pragmatic understanding. Milkmaids were exposed to cowpox in cows but seemed unaffected by smallpox. Could exposure to cowpox keep a person from becoming infected by smallpox? This early 19th century success suggested a broader hypothesis about how a weakened version of something that caused a disease could make a person immune from the disease. This idea resulted in the development of vaccines. We now understand the mechanism: a vaccine “trains” the SGU Cures Index 23 immune system to recognize a disease-causing organism; with this training, the immune system activates and works to destroy the organism if it enters the body in the future. The organism is no longer a threat. The disease it causes has been cured. The mechanism of the cure involves augmenting the capability of the body's immune system. With an understanding of the mechanism of infectious disease, vaccines can be developed. The earliest vaccines addressed diseases more common in childhood. Diphtheria, tetanus, pertussis, measles, mumps and rubella were the cause of many childhood deaths before vaccines were developed. Now vaccines address conditions more common among those beyond childhood: hepatitis, pneumonia, and human papillomavirus (the cause of most cases of cervical cancer.) More are likely yet to come, including vaccines against herpes, chlamydia, and cytomegalovirus. Building on an understanding of the mechanism of a disease is the path to more cures. The explosion of fundamental knowledge is both opening up an understanding of more mechanisms and supporting progress towards cures in small increments. The tracing out of the human genome has opened up myriad possibilities to impact disease mechanisms and develop cures. This knowledge has also made the challenge greater by adding new and ever more complex dimensions. Sometimes a more subtle understanding results in the division of a single disease into multiple diseases. This makes the challenge greater by increasing the number of cures required. What had been termed "the war on cancer" is turning out to be part of a battle that must be fought on an increasing number of fronts. The genomic revolution has demonstrated that the challenge of cures going forward is greater than we thought. Genetic information is splitting what we thought to be one disease into many, and the appreciation of how many forms of cancer or leukemia there are both identifies new battlefields and makes progress more uneven. The variability of the territory means that new weapons that work on one battlefield may not work on another. The genomic revolution has also provided the ability to learn about more disease mechanisms. This gives new hope to how knowledge of association can be a source of cures. The development of classifications of diseases that rely on genotype has opened up more opportunities to develop cures. Human epidermal growth factor receptor 2 (HER2) is a protein encoded by a gene found on chromosome 17. Amplification or overexpression occurs in 20 to 30 percent of breast cancers (Mitri, Constantine, & O’Regan, 2012). This knowledge provided a target: find a way to interfere with the SGU Cures Index 24 receptor. Herceptin, a biotech product, was approved in the US in 1998. Early studies showed it works as a functional cure, gaining almost five months survival in the earliest studies (Genentech, n.d.). Breast cancer can be divided into two groups: those that are and those that are not HER-2 positive. For those whose cancer is not HER-2 positive, Herceptin gains nothing. Few new cures seem likely to come about by chance observation. From here on, most new cures will build from an understanding of causation. This understanding can lead to definitive cures that keep a biological process from beginning, as in the case of immunization, or functional cures that deflect the path of disease once it has begun. The fact that new cures will require deeper understanding of how the human body works shows the value of accumulating fundamental knowledge. What can be maligned today as a "curiousity study" into biological mechanisms can become the starting point for future cure development. Each additional understanding of biological processes adds to our stock of knowledge about how things work. This then allows understanding of how disease and illness differs from the normal and benign. These branching points, where things go astray, create new targets that cure strategies can pursue. Different Levels of Cures: Functional vs. Definitive Many cures that are already available involve infectious disease. This reflects our better understanding of diseases that involve external agents. We understand the one-to-one relationship between infectious organisms such as viruses and bacteria, and human disease. If the ill effect of an infectious organism can be avoided, we have a cure. Immunization is a way to put that knowledge to work. An immunized individual is no longer susceptible to that disease. Immunization is one way to bring about a cure. The capacity to do something for those with heart disease and HIV infection that adds years of life is greater now because of functional cures. The ability to lift the cloud of depression makes it possible for some to work. These functional cures reduce but do not eliminate the risk of death from a particular disease or condition. They increase the chance that someone with a disabling condition can work and participate in society. With a lower risk of death, individuals have a greater life expectancy. During the additional time that they live, they have a functional cure. Similarly, those whose treatment has made it possible to work and who otherwise would live dependent lives have a functional cure. SGU Cures Index 25 Another way to bring about a cure is to avoid contact with the agent associated with disease. When John Snow removed the pump handle on a well containing cholera on Broad Street in London, he clearly demonstrated how public health measures could reduce the spread of disease by keeping humans away from disease-causing organisms and substances. Compared to the generation who smoked at the time the Surgeon General's reports on smoking first appeared on 11 January 1964, fewer in the current generation of young people will experience lung cancer because they do not smoke tobacco. The knowledge that has brought about this change is a form of a cure. Hundreds of thousands will not die of lung cancer because their generation smokes at a lower rate than the previous generation. Knowledge has immunized them. They will experience cancer and heart disease at a lower rate than the generation in which tobacco use peaked. In this case, assessing the impact of the cure requires contemplating a counterfactual: what level of death and disease would the US have experienced had tobacco use rates remained the same? The lower rates in more recent generations can be hailed as the cure effect that came from knowledge about the risks associated with tobacco use. The path to a cure remains elusive in many domains of illness and disease. While we may know factors that are associated with a disease, none of these provide the deep knowledge required to interrupt the disease process with the definitive result of a vaccine or decreased probability of a disease starting, which is the effect of lower smoking rates. We have many treatments for conditions that threaten death or bring substantial disability that work with varying degrees of success. They are not definitive cures, as with vaccines. They do not keep a disease process from beginning. When it does, they make things different. A disease progresses more slowly. Its impact is less severe. Rather than die because of the disease, they die with it. A treatment does not lead to perfect health and the ability to participate in life activities as others do, but it makes possible living at a higher level of function than someone who is not treated. The potential for a cure depends on how close we are to a fundamental understanding of the disease process. Vaccines fit the cure paradigm. Preventing infection fits the cure paradigm. Avoiding daily habits that cause or exacerbate disease (e.g., smoking tobacco) or engaging in daily habits that reduce disease (e.g., exercise) fits the cure paradigm. Much has been written over the past five decades about the importance of behavior in determining health outcomes. Research has consistently demonstrated that daily habits (e.g., type and quantity of food consumed, activity level, amount of alcohol consumed, tobacco use) are related to a number of chronic diseases (e.g., cardiovascular disease, cancer, diabetes). Yet despite the well-established link between these behaviors and disease outcomes, millions of Americans continue, or do not SGU Cures Index 26 engage in, these behaviors. According to the Centers for Disease Control and Prevention (2015), 42.1 million people, or 18.1% of all adults aged 18 years and over in the US currently smoke cigarettes, making it the leading cause of preventable death (480,000 annually) in the US. Furthermore, 34.9% of US adults aged 18 and over are obese (i.e., a body-mass index greater than 30) (Centers for Disease Control and Prevention, 2014c). Despite widespread knowledge about the link between smoking cigarettes and chronic disease, and obesity and chronic disease, a very large number of Americans continue to smoke and take in more calories than they burn. The potential for a cure strategy depends on three factors: First, science; second, impact; third, implementation. Where we have a fundamental understanding, we have the potential for a cure. Where we may not yet have an understanding of the process that leads to the disease or the capability to interrupt that process, we may know enough to push it off its course. The impact of a cure depends on the number afflicted and the consequences of a disease. Death is the ultimate negative health care outcome; diseases that lead to death have the greatest consequences. Diseases and conditions that impair work have profound consequences both for those afflicted and society which loses some of their contribution. Finally, once we have a fundamental understanding of the disease process and an understanding of how to interrupt that process through prevention or intervention, we must implement the cure. In the case of primary prevention, we may understand the disease mechanism, as is the case with smoking cigarettes and cancer. However, the evidence indicates that we still lack a precise understanding of the mechanism by which people continue to smoke tobacco despite knowing the link with cancer. In these cases, further research is needed to effectively implement primary prevention. Together, the state of knowledge and impact on lives saved or improved help to prioritize a cures strategy. They point the way to ordering the cures agenda. This ordering takes into account both the value of a cure and the probability that a cure can be achieved based on the current state of knowledge in a given biomedical field. Measuring the Economic Impact of Cures: Cost Strategy vs. Cures Strategy Cost dominates discussions of the economic aspect of health care. This narrative focuses on how much is spent on health care in the US. Americans spend approximately $7,662 per person, per year (about 17% of GDP) on health care, which is approximately twice as much as most developed nations in the world, including relatively rich European countries like Canada, Germany, France, Sweden, Australia and the United Kingdom (OECD Health Statistics, 2014). Voices from the left and right SGU Cures Index 27 agree that spending is too high and the current path is unsustainable. Here the consensus crumbles; some see market forces at the top of the list of solutions, others technocratic steps to increase efficiency, and then some see imposition of limits through caps and controls as part of the narrative for how we make our way from our current unsustainable path to one that is sustainable. The technocratic faction has offered the most detailed analysis of the cost problem. The Institute of Medicine, an arm of the National Academy of Sciences, has said the health industry wasted $750 billion in 2009 between unnecessary services, inefficiently delivered care, excessive administrative costs, inflated prices, missed prevention opportunities and fraud. Even if waste and fraud could be eliminated and each person received the optimal care at the right time, performance improvement is a limited solution if cost trends from the past continue and US demographics unfold as projected. Even if per person costs stabilized, the impact of the aging population would push costs higher. Age is the single best predictor of health costs, and as such, the aging of the population will push costs higher. All three views leave out the value of cures. Contrast health care with the unfolding of the digital age. Spending on computers has skyrocketed. Spending on smartphones has grown infinitely because spending twenty years ago was zero since the smartphone did not exist before 1995. The spending growth rate for computers and smartphones has not produced the handwringing that higher healthcare spending has produced. In the case of computers and smartphones, we implicitly recognize that the question is not the dollars but the value obtained for those dollars. We spend more, but value is growing faster than spending. Banks understand that lower costs come from obtaining cash from ATMs rather than a human bank teller, and costs are still lower when debit or credit cards displace cash and paper checks. Anyone who has used a smartphone to consult a map or obtain real-time directions understands that spending on computers and smartphones provides access to services that previously did not exist. Smartphone users value the devices more than what they pay for them. Likewise, cures – especially definitive ones – may cost large sums to discover and roll out, but their value, measured as positive impact on human life, is potentially enormous. Furthermore, the impact of that cure on human health continues to accrue year after year. An alternative view of the relationship between health care spending and the economy focuses on the value of good health. Good health is an asset – perhaps the most important asset of all – because healthier people, blessed with longer life, contribute more to the economy than less-healthy people. As economists from Friedrich Hayek on the right to Robert Solow on the left have argued, it is human capital – including the SGU Cures Index 28 capacity for innovation – that constitutes the most important element in economic growth. Investments in physical capital such as bridges and buildings depreciate as the effects of weather and use wear them down. Critically, the investments that bring about cures do not diminish over time. The knowledge and human capital that showed that vitamin D fortification could end rickets or the knowledge of how to make a vaccine does not weather, wear out, or diminish over time. The human capital view maintains that cures, whether definitive or functional, bring about gains that far exceed the cost of developing them. A "Cure Strategy" is about maximizing well-being while a "Cost Strategy" is about minimizing cost. A "Cure Strategy" looks to the durability of the knowledge that creates a cure that can last for all time, while a "Cost Strategy" focuses on a short period of time, most often a single year. Even if minimizing cost is the goal, there are potent forces at work outside the health care delivery system that might upend a "Cost Strategy", which focuses on changes in the health care delivery system. Consider the implications of Alzheimer's disease (AD). The current lack of a cure for AD, whether dealing with treatment once the disease process is underway, or dealing with prevention before it begins, provides a clear impetus for the Cure Strategy. The current standard of care cannot alter the path that leads to many AD patients requiring 24/7 monitoring or care, month after month, year after year. Such care is inherently laborintensive and, thus, expensive. In fact, current projections from the Alzheimer’s Association (2015) suggest that in a scenario where no treatments emerge, the cumulative cost of AD could reach $1.2 trillion per year in the US by mid-century. Most of that expense will be for nursing homes and other forms of long-term care. It is difficult to see how all the effort to improve the efficiency of healthcare is going to reduce that cost by a large margin. The discovery of a cure for AD – whether knowledge of the precise causal mechanism which allows for prevention of its onset, or a vaccine that will prevent it, or a medication that will limit its symptoms – would quickly reduce that $1.2 trillion annual price tag. The potential benefit is not limited to health care savings within the US or even other developed nations. As more of the world develops economically, the health care needs of the world population increasingly resemble those of the US. As such, the world market for an effective drug with some influence on AD is vast and continues to grow. Any truly innovative and successful cures discovered and introduced in the US could be sold worldwide, supporting high-tech employment within the medical research and SGU Cures Index 29 manufacturing sector. Finally, individual patients in the US (and worldwide) benefit from longer life and better quality of life from those extra years. There is no certainty about how long, or what level of resources a Cure Strategy requires in order to succeed. As with any investment, the standard disclaimer applies: "past returns are not a guarantee of future results". Nonetheless, history shows many successes. Multiple medical research efforts that have resulted in cures – from the development of antibiotics and the polio vaccine, to the enormous progress that has been achieved against HIV/AIDS – strongly suggest that significant gains are possible in a relatively short period of time, provided there is sufficient leadership, scientific effort, and financial commitment. In that sense, how vigorously we pursue cures today determines how large a stock of cures will be passed on to the future. The case studies of past cures that are part of this report show they have given people longer lives and made the economy larger today. New cures are ever more challenging. As scientific knowledge accumulates, the biological and disease processes that it confronts are ever more complex. Complexity does not imply impossibility, but it does require patience. The Diffusion of Cures A cure embodies knowledge. The most powerful cures up to this time involve beforethe-event prevention. Keeping humans away from disease-causing organisms and substances has been central to health. John Snow's cure application following his geospatial investigation of cholera patients in London did not involve treating a particular patient. His knowledge benefited all who used the well as a source of water. His success required being able to put his knowledge to use. Had he not acted, his knowledge would not have created any benefit. Similarly, had the announcement that the polio vaccine worked not been followed by mass immunization campaigns, the number of polio cases would not have fallen precipitously. The focus of this report is the creation of knowledge that produces cures. Cures produce benefits when knowledge gets put to work. Implementation puts knowledge to work. Implementation defines the difference between what is possible and what gets achieved. This report marshals facts about cures and the story of particular cures to show the promise of a cures strategy. Some stories are already closed books. The stories of diseases already cured will not change. Other diseases addressed in this report's case studies -- heart disease, diabetes, and depression -- may someday be very different because they are still being written. How different those stories become depends on SGU Cures Index 30 decisions made today. Once a cure has been discovered and implemented, the path of discovery that led to the cure can be told neatly. One set of discoveries opened up a new set of possibilities, and the sequence to a cure seems obvious in retrospect. However, during the discovery and implementation process, the picture is far less clear. Along the way, many promising ideas will have turned out to be dead ends. An AIDS vaccine, discussed in one of this report's case studies, has proven elusive, as many ideas for the basis of an effective vaccine have fallen short. Some day there may be an effective vaccine, and the story of that vaccine will be presented as a sequence of steps that culminated in a cure. That story will give scant attention to the many ideas pursued that lead to dead ends and failure. The time when smallpox and polio were real threats has receded into the past. At some point in the future the present day will be just as far in the past. Looking backwards to the early 21st century, a future observer will surely see that the ability to cure disease has grown during the intervening time. Just how many more cures we will gain and when they come about depends on how aggressively a cures strategy is pursued between now and then. How many case studies of cures can be written in the future depends on which possibilities get pursued today. The more possibilities we pursue, the greater the probability that there will be more cures to describe. The Value of Case Studies The facts required to assess the potential of a Cures Strategy come from the case studies that follow in this report. These case studies bring together facts about previously developed cures for some of the most burdensome diseases. How many people suffered from particular diseases? What was the impact of those diseases on lifespan and quality of life? How did the cure impact lifespan and quality of life? How much did it cost to develop and how much does it cost to treat a patient? How much money was saved in caring for patients who are now cured of their disease? The answers to these questions about the value of a cure require thought experiments. What would today be like if these cures had not been developed? When carrying out this analysis, we examine only first order effects, ignoring the competing hazards to life. Someone who does not die from one cause eventually dies of another. Some among those who would be dead today if highly active antiretroviral therapy had not been developed to reduce the risk of fatal complications of HIV-infection would have died because of cancer instead. An analysis of such second, third, and fourth order effects quickly becomes complex, requiring powerful computer modeling to try and calculate comprehensive impact. Such modeling is beyond the scope of this report. Instead, we SGU Cures Index 31 focus on first-order impacts, making our estimates an upper bound to the value of particular cures. By the same token, the impact on medical expenses is a gross, not a net impact. Someone who avoids dying because of the polio vaccine lives, and in that life, may have the same risk of developing cancer or experiencing dementia in old age as someone who did not contract polio. The gross impact does not take this into account; the net impact would. The material that follows provides a further analysis of burdensome diseases, either historically or currently. We pay particular attention to the impact of these diseases on the US population: How many are affected, how drastically lives are shortened, and the quality of life lived by those with the disease. With these metrics, we can estimate the total economic impact of the diseases, both in terms of economic measures such as the size of the workforce and GDP, and also using measures of well-being embodied in the value of statistical life and full quality life-year. We use the same methodology and dollar values used by the Environmental Protection Agency in its assessment of the cost-effectiveness of its regulations. We then examine the cures that have been developed for those diseases, their impact on life expectancy and quality of life, their cost, and ultimately, their overall economic impact. Citations Alzheimer’s Association (2015). Alzheimer’s Facts and Figures. Retrieved on March 8, 2015 from: http://www.alz.org/alzheimers_disease_facts_and_figures.asp#cost Centers for Disease Control and Prevention (2014a). United States Life Tables, 2010. National Vital Statistics Reports, 63(7), p.51. Centers for Disease Control and Prevention (2014b). Leading causes of death. Retrieved on May 23, 2014 from: http://www.cdc.gov/nchs/fastats/leadingcauses-of-death.htm. Centers for Disease Control and Prevention (2014c). Adult Obesity Facts. Overweight and Obesity. Retrieved on march 8, 2015 from: http://www.cdc.gov/obesity/data/adult.html Centers for Disease Control and Prevention (2015). Current Cigarette Smoking Among Adults in the United States. Retrieved on March 8, 2015 from: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/ SGU Cures Index 32 Genentech (n.d.). Herceptin (Trastuzumab) in early-stage and advanced breast cancer. Genentech Product Information. Retrieved on March 18, 2015 from: http://www.gene.com/media/product-information/herceptin-breast OECD Health Statistics (2014). OECD Health Policies and Data. Retrieved on March 15, 2015 from: http://www.oecd.org/els/health-systems/health-data.htm. Mitri, Z., Constantine, T., & O’Regan, R. (2012). The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemotherapy Research and Practice, (2012), doi: 10.1155/2012/743193. Thacker, S. B., Stroup, D. F., Carande-Kulis, V., Marks, J. S., Roy, K., & Gerberding, J. L. (2006). Measuring the public's health. Public Health Reports, 121(1), 14-22. SGU Cures Index 33 RESULTS and CONCLUSIONS The data gathered for this report has brought together information from existing studies and data sources. It is the first time that an historical analysis of multiple disease categories, their cures, and the economic impact of those cures, has been compiled into a single document. Thus, it fosters an assessment of the general value of cures while building an appreciation of how cures have come to be. Achieved Cures The selective historical analysis conducted as part of this report indicates that the return on investment of achieved cures, while variable, is significant. At the low end, we find that the implementation of heart surgery, and specifically, coronary artery bypass grafting (CABG) provided the least return on investment (ROI). According to our estimation, CABG has actually provided a negative ROI, saving $14.07 billion in life value but costing $148.5 billion to implement. At the high end, the polio vaccine provided the greatest measurable ROI, costing $36.4 billion (2002 dollars) to discover and distribute, but saving $2.87 trillion in life value. Even more impactful is preventive medicine in the form of diet and exercise for avoiding the development of diabetes. We estimate that approximately $22.67 trillion in future life value could be saved among living pre-diabetic Americans if each of them engaged in 150 minutes of moderate exercise per week and lost 5 – 7% of their body weight. In the best-case scenario, these individuals could obtain this exercise by going for walks in their neighborhood or taking the stairs instead of the elevator, etc., which costs $0. Under this best-case scenario, the ROI is immeasurable (i.e., infinite). HIV – Antiretroviral Therapy (ART) Lifetime antiretroviral therapy (ART) for HIV infection costs approximately $354,100, 73% or $258,493 of which is related to ART (Schackman, Gebo, Walensky, Losina, Muccio, Sax, et al., 2006). It is estimated that on average, 11.8 years of life has been gained amongst HIV patients between 1996-2005 (Harrison, Song, & Zhang, 2010), mostly due to advances in ART. If 1,148,200 Americans diagnosed with HIV in 2009 gained 11.8 years of life as a result of advances in ART/HAART, the mortality reduction is worth $1.41 trillion, while the lifetime cost of ART therapy for those 1,148,200 Americans would be $296.8 billion. Thus, ART provides an ROI of 4.75. SGU Cures Index 34 $296.8 billion lifetime cost to administer antiretroviral drugs to 1,148,200 Americans diagnosed with HIV in 2009 $1.41 trillion in added life value (11.8 years) among 1,148,200 Americans diagnosed with HIV in 2009 Influenza – Antivirals With approximately 3,300 to 49,000 influenza-related deaths each year, a 25% reduction in mortality (Muthuri et al., 2014) would save between 825 and 12,250 lives each year (6,538 middle value), giving an annual value of mortality risk reduction (VMRR) of $681.21 million as a result of influenza antivirals. Zanamivir and oseltamivir were each assumed to relieve an average of 1.0 day of symptoms. From that data it was estimated that there was a gain of 0.5 workdays (Lee et al., 2002). The US labor force (among those aged 18 and older) was 155.4 million in March 2014 (Bureau of Labor Statistics, 2014). If 10% of the US working age population (approximately 193,212,000) contracts influenza annually (19.321 million individuals) and is able to gain a half workday (4 hours) from taking antivirals, that equates to 77.284 million work hours gained. When converted to dollars at an average $24.31 hourly wage, $1.879 billion dollars in efficiency is gained annually from the use of influenza antivirals. Combined with the VMRR of $681.21 million, the combined economic impact of influenza antivirals is $2.56 billion annually. Recommended antiviral treatment is five days, although longer treatment may be necessary for severely affected individuals. A five-day course of zanamivir and oseltamivir therapies costs $47.50 and $57.22 (each season or as needed), respectively. If 10% of the current US population (31.616 million) contracted influenza and experienced symptoms severe enough to warrant antiviral treatment, the total cost would be $1.81 billion at $57.22 per course. Thus, influenza antivirals provide an ROI of 1.4. SGU Cures Index 35 $1.81 billion annual cost to provide 10% of US population with influenza antivirals $681.2 million in life value saved by influenza antivirals annually, in addition to $1.88 billion in gained efficiencies by reducing number of sick days among American employees = $2.56 billion total Influenza – Vaccine We estimate that the value of the mortality gain from influenza vaccine is $76.222 billion by saving 731,542 years of potential life. (see influenza section). In line with our estimate, Duncan and colleagues (2012) estimated that total direct costs and total economic burden that could have been significantly alleviated by reducing the amount of YPLL was estimated at $10.4 billion and $87.1 billion (in 2003 dollars), respectively. We further estimate the cost of vaccinating the US population, at the current 45% coverage rate, at $1.367 billion per annum. Further, the National Institutes of Health (NIH) spends approximately $300 million per year on influenza research. If we divide the $76.222 billion in annual value by the $1.667 billion in annual research and vaccine costs, the ROI for the influenza vaccine is 45.7. SGU Cures Index 36 $1.67 billion annual influenza research and vaccine cost $76.22 billion in annual life value saved by the influenza vaccine Poliomyelitis – Vaccine In 1939, estimates of the number of polio victims – most of them confined in their homes – ranged from 100,000 to 500,000. As historian David Oshinsky reports, at that time, the expense of boarding a polio patient (about $900 a year) actually exceeded the average annual wage of $875. Financier-turned-healthcare-visionary Michael Milken, looking back to the 1950s, reminds us that it was cheaper for the USA to provide a vaccination against polio, thereby eliminating the problem, than to treat everyone who contracted the disease (Milken, 2010). The costs of immunization programs turned out to be minimal compared to the costs of wheelchairs, iron lungs, and physical therapy: “In the early 1950s, economists estimated that by the year 2000, treating polio would SGU Cures Index 37 cost the United States $100 billion annually. Today’s polio immunization programs cost one thousand times less than that and have virtually eliminated the disease” (Milken, 2010; p. 3). In other words, savings from the vaccine were a thousand-fold, an astounding economic achievement. Surveying medical progress in the USA, Murphy and Topel (2006) argue that improvements in life expectancy have added approximately $3.2 trillion to America’s wealth over the three decades beginning in 1970. Our analysis indicates that, between 1955 and 2006, the value of the mortality reduction was $1.312 trillion through the saving of 160,000 lives as a result of the polio vaccine. Further, $1.55 trillion in savings accrued because 1.1 million Americans avoided paralysis from polio between 1955 and 2006. The economic impact of the polio vaccine was approximately $2.87 trillion during that timeframe. The total estimated research cost to develop the polio vaccine and distribute it from 1955 through 2015 was $36.4 billion (in 2002 dollars). Thus, the ROI for the polio vaccine is 78.7. Critically, each year, returns from the polio vaccine continue to accrue, as more Americans are born and do not have to worry about suffering from the disease. These returns will continue to accrue indefinitely. SGU Cures Index 38 $36.4 billion cumulative cost of developing and distributing the polio vaccine $2.866 trillion accumulated return from the polio vaccine SGU Cures Index 39 Malaria – Antimalarials In 2010, 1,691 cases of malaria were reported in the US. Of those, 1,688 were classified as imported into the US after travelling abroad, one was associated with blood transfusion, and two remain classified as cryptic cases (Mali, Kachur, & Arguin, 2012). Despite global malaria control programs in place, many commonly traveled areas remain endemic and prevention measures are still problematic (Mali, Kachur, & Arguin, 2012). Approximately 3.3 billion people are at risk for malaria, with an estimated 216 million cases and 655,000 deaths worldwide in 2010 (World Health Organization, 2012). An estimated 80% of Malaria cases occurred in just 17 countries in sub-Saharan Africa (World Health Organization, 2012) costing the sub-Saharan region approximately 1.3% of its GDP ($12 billion each year) in direct losses from illness, treatment and premature death (Roll Back Malaria, 2008). Analyses indicate that 1.1 million deaths were averted from 2001 – 2011 (110,000 per annum) as a result of antimalarials (World Health Organization, 2012). If approximately 50% of the 110,000 averted annual deaths were attributable to antimalarials, while the other 50% were attributable to urbanization and overall economic development (World Health Organization, 2012), 55,000 deaths are avoided as a result of antiMalarials, equivalent to 2,713,150 life years, and a VMRR of $11.63 billion (International $). This is consistent with the estimate by Roll Back Malaria (2008). If we subtract the highest estimated cost of distributing ACT in 2001 ($3.34 billion 2014), the ROI in distributing antiMalarials is 3.5. $3.34 billion (International $) distributing antiMalarial drugs annually $11.63 billion (International $) in life value saved annually by antiMalarial drugs SGU Cures Index 40 Type 1 Diabetes Mellitus – Insulin Prior to the discovery of insulin, diabetes – especially Type 1 – was a devastating disease that killed patients at a very young age. With no effective treatment aside from a semi-starvation diet, a diabetic's outlook was grim. Before insulin, diabetic children rarely lived a year after diagnosis, five percent died within two years, and fewer than 20% lived more than ten years (Cohn, Berger, & Norton, 1968). Type 1 diabetes accounts for 5 – 10% of all diabetes cases (Daneman, 2006). Taking the middle of that range (7.5%) and multiplying it by the number of diagnosed diabetics in the US (18.8 million) gives an estimate of 1,410,000 Americans whose lives have been saved by insulin, giving a total value of mortality reduction of $11.562 trillion. The annual cost of insulin is $8,417.01 per patient (in 2013 dollars), giving a total annual cost of approximately $11.868 billion across all Type 1 diabetic patients whose lives are saved by insulin (in 2013 dollars). With a current life expectancy of about 68.8 years among Type 1 diabetics (Miller, Secrest, Sharma, Songer, & Orchard, 2012), the cost of treating all 1,410,000 Americans whose lives have been saved by insulin is $816.52 billion ($11.868 billion x 68.8 years). In this scenario, the lifetime ROI of insulin for Type 1 diabetes is 14.2. $816.52 billion in lifetime medication (insulin) costs to manage Type 1 diabetes among existing patients $11.56 trillion in total saved life value as a result of insulin SGU Cures Index 41 Type 2 Diabetes Melitus – Exercise People with a BMI greater than 35kg/m2 are 20 times more likely to develop diabetes than someone with a BMI in the normal range (18.5 to 24.9 kg/m2) (Fowler, 2007). Any person who has an impaired fasting glucose is at risk for developing the disease. Lifestyle modification, through diet and exercise, is an important factor in delaying the progression of the disease and potentially preventing it completely (Fowler, 2007). During exercise, the body can use glucose more effectively, decreasing the amount left in the blood. Thus, a sedentary lifestyle is an important modifiable risk factor for type 2 diabetes, and the overall disease burden in a given population generally undergoes a more dramatic reduction when a large segment of the population adopts small improvements in health behaviors than when a small segment of the population adopts large improvements (Bassuk & Manson, 2005). Prediabetes - defined as a blood glucose level above normal, but not yet considered to be within the range of diabetes – had a prevalence rate of 34.1% in 2007-2010 (Abraham & Fox, 2013). The Centers for Disease Control and Prevention (2011a) reports that 35% of US adults aged 20 years and older (i.e., 79 million people) have prediabetes, but only 7.3% have been told they have this diagnosis. It has been found that 8.1% of patients with impaired fasting glucose progress to diabetes within 6.3 years; this is an annual rate of 1.34%. In 2011, more than half of adults (52%) did not meet recommendations for aerobic exercise and physical activity, and 23% and 38% of adults reported consuming vegetables and fruits less than once a day, respectively. Over 100 million Americans are not following minimum recommended guidelines for physical activity and healthy diet (Centers for Disease Control and Prevention, 2014). If a pre-diabetic patient loses 5 - 7% of his/her body weight and completes at least 150 minutes of moderate exercise per week, there is a 58% reduction in risk of Type II Diabetes (Centers for Disease Control and Prevention, 2011b), as well as a 34% reduction in risk after 10 years (Tuomilehto, 2011). Thus, by following behavioral guidelines, 26.86 million pre-diabetic Americans (34% of 79 million) could avoid progressing to Type 2 diabetes over 10 years. As of the year 2000, an American male diagnosed with diabetes at age 60 years will lose 7.3 life years compared to a person of the same age without diabetes (Narayan et al., 2003). An American female diagnosed with diabetes at age 60 years will lose 8.9 life-years compared to a person of the same age without diabetes. If half of the 26.86 million Americans who could avoid progressing to Type 2 diabetes via behavioral interventions were male, 98.039 million SGU Cures Index 42 life-years could be saved over the next 3-4 decades. Likewise, 119.527 million life-years could be saved among females over the coming 3-4 decades. The total VMRR associated with this change is $22.669 trillion. In a best-case scenario, the cost of behavioral interventions to reduce the risk of Type 2 diabetes is $0 – patients can accumulate 150 minutes of moderate exercise a week at no cost by walking around their neighborhood or making choices such as taking the stairs instead of an elevator. Thus, in such a best-case scenario, the lifetime ROI of diet/exercise is infinite. Mood Disorders: Unipolar Depression – Antidepressants Self Inflicted Injuries: Given that 669,246 years of potential life were lost as a result of self-inflicted injuries in 2001 in the United States (WHO Department of Measurement and Health Information – Global Burden of Disease Study - Years of Life Lost, 2001), and 50 to 87 percent of suicides and attempted suicides are carried out by someone diagnosed with a major depressive episode (Rihmer, 2007), the potential economic impact of addressing depression, and consequently, suicide, is significant. If 50% of self-inflicted injuries in the US are directly linked with depression, 334,623 years of potential life could be saved, with an annual mortality reduction value of $34.87 billion. While Ramsberg, Asseburg, & Henriksson (2012) have shown than the SSRI antidepressant Escitalopram has an impact on QALYs of +0.6978, we take the more conservative moderate to severe depression health state preference score of 0.60, and subtracting that from 1, which is perfect health, we assume a QALY associated with an effective treatment for depression of 1 - .60 = .40 (Pyne, Fortney, Tripathi, Feeny, Ubel, & Brazier, 2009). Taking a 0.40 QALY increase x $104,193 = $41,677.20 per patient per year x 14.8 million Americans aged 18+ diagnosed with clinical depression in a given year (Kessler et al, 2005; US Census Bureau, 2005) = $616.8 billion VMRR per year. Combining this with the potential value associated with reducing suicides as a direct result of depression ($34.87 billion) gives a total VMRR of $651.67 billion associated with antidepressant use. Retail prices for commonly prescribed antidepressants range from about $20 a month to more than $400 a month (Consumer Reports Health Best Buy Drugs, 2011). Eleven percent of Americans aged 12 years and over took antidepressant medication during the period 2005-2008 (Pratt et al, 2011). There were 250 million Americans age 12 and over in 2005. Eleven percent of 250 million = 27.5 million x $200 per month (median cost) = $5.5 billion per month x 12 months = $66 billion per year (median scenario drug cost only). Thus, the annual ROI of antidepressants for mood disorder is 9.9 ($651.67 billion / $66 billion). SGU Cures Index 43 $66 billion annual (median medication) cost to distribute anti-depressants to 27.5 million Americans $34.87 billion in annual life value saved by antidepressants reducing the suicide rate by 50%, in addition to $616.8 billion in gained life value by increasing QALY ratings by 40% among 14.8 million Americans diagnosed with clinical depression in any given year = $651.67 billion total Mood Disorders: Bipolar – Mood Stabilizers As per Chisholm and colleagues (2005), taking a 0.077 disability index change for treatment of bipolar disorder with Lithium alone (.445 ---> .368) and multiplying by $104,193 = $8,022.86 per patient per year x 5.7 million Americans age 18+ diagnosed with bipolar disorder in a given year (Kessler et al, 2005; US Census Bureau, 2005) = $45.7 billion annual VMRR. The monthly cost of bipolar medications varies widely; from $12 for Lamotrigine (Lamictal) to $970 for Aripiprazole (Abilify). Using the high-end estimate for Risperidone, a commonly-used bipolar medicaton ($36) x 5.7 million Americans diagnosed with Bipolar Disorder in any given year (2005) = $205.2 million per month x 12 months = $2.46 billion per year in medication cost. Under these calculations, the annual ROI of mood stabilizers for bipolar disorders is 18.6. SGU Cures Index 44 $2.46 billion annual (median medication) cost to distribute mood stabilizers for bipolar mood disorder to 5.7 million Americans $45.7 billion in gained life value by increasing QALY ratings by 7.7% among 5.7 million Americans diagnosed with bipolar disorder Intraepithelial Neoplasia: Cervix – Pap Test Some of the most telling observational studies regarding the impact of Pap tests on cervical cancer mortality rates come from five Nordic countries before and after the introduction of screening programs (1963-1967 and 1978-1982). Mortality reductions of 8% to 73% were observed (Shingleton et al., 1995), and Pap smear screening every three to five years can reduce cervical cancer incidence by up to 80% (Arbyn et al., 2010). The percent of US women 18 years of age and over who have had a Pap test within the past 3 years is 73.2% (National Center for Health Statistics, 2013). In 2010, 3,939 women died in the US from cervical cancer (Centers for Disease Control and Prevention, 2013) and the median age at death from cervical cancer is 57 years (National Cancer Institute, 2014). If the other 26.8% of women aged 18+ in the US received a Pap test every 3 years, the maximum cost, at $101 per test in 2012 dollars (Sanders & Taira, 2003) would be (121.44 million x 26.8% = 32.546 million x $101) $3.287 billion, or $1.096 billion annually. Taking the current US female life expectancy of 81.2 years for females and subtracting the median age of death from cervical cancer of 57 gives 24.2 years of potential life lost. In line with this, Ekwueme and colleagues SGU Cures Index 45 (2008) report 21.8 as the average number of YPLL to HPV-associated cancer death. If Pap smears reduced cervical cancer mortality in the US by just 8% among the 32.546 million women 18+ who do not currently receive screening, we would expect that (3,939 x 8%), 315 fewer women would die from cervical cancer in the US each year. Given that each of those women would have died at 57 years of each, on average, (315 women x 24.2 YPLL), 7,623 years of potential life would be saved annually, giving a VMRR of $794.26 million as a result of further implementation of the Pap test. If Pap tests reduced cervical cancer mortality in the US by the upper limit in the Nordic research (73% - Shingleton et al., 1995), we would expect that (3,939 x 73%), 2,875 fewer women would die from cervical cancer in the US each year, resulting in 69,575 years of potential life saved annually, with a VMRR of $7.25 billion. To account for false negative Pap tests and other potential causes of cervical cancer, we estimate a maximum reduction in cervical cancer deaths of 73% despite 100% Pap smear screening of females 18+ in the US. In line with this, Solomon and colleagues (2007) and Diamantis, Magiorkinis, and Androutsos (2010) report that the Pap test has proven to be a model for successful cancer prevention and is largely responsible for the 70% decrease in cervical cancer mortality in the US over the last 50 years. In reality, the reduction in annual cervical cancer deaths is likely to be somewhere between 8% and 73%, which is why we have provided a range of impact in this analysis. Given an annual cost of $1.096 bilion to provide the Pap test to women who currently lack access, the ROI of the Pap Test is -1.4 at the lower estimate ($794.26 million / $1.096 billion) and 6.6 at the upper estimate ($7.25 billion / $1.096 billion). $1.1 billion annually for additional routine cervical cancer screening via Pap Test $7.25 billion in life value saved annually as a result of cervical cancer screening via Pap Test (upper estimate) SGU Cures Index 46 Intraepithelial Neoplasia: Cervix – HPV Vaccine According to the Centers for Disease Control and Prevention (2013), 50,000 girls will develop cervical cancer over their lifetime that would have been prevented had the US reached the 80% vaccination rate goal by now. In 2010, 11,818 women were diagnosed with cervical cancer, with 3,939 deaths – a 33.3% rate of all diagnoses (Centers for Disease Control and Prevention, 2013). The median age at death from cervical cancer is 57 years (National Cancer Institute, 2014). Taking the current US female life expectancy of 81.2 years for females (Centers for Disease Control and Prevention, 2014b) and subtracting the median age of death from cervical cancer of 57 gives 24.2 years of potential life lost. If vaccination could prevent 50,000 girls from developing cervical cancer, and 33.3% of those girls (16,500) would have died at a median age of 57 years, (16,500 x 24.2 YPLL) 399,300 life years would be saved by attaining an 80% vaccination rate, giving a total VMRR of $41.604 billion as a result of the HPV vaccination. The approximate cost to administer the vaccine to the 37.6% of girls who received all three doses in 2013 was $1.466 billion. The approximate cost to administer 3 doses of the vaccine to all 10 million girls aged 13-17 in 2013 would have been $3.9 billion. Thus, the ROI for the HPV vaccine is 10.7. $3.9 billion to administer the HPV vaccine to all 10 million girls aged 13-17 in the US in 2013 $41.6 billion in saved life value as a result of the HPV vaccine SGU Cures Index 47 Functional vs. Definitive Cures Our analysis indicates that definitive cures tend to provide a better return than functional cures, whose return can vary significantly. In the current report, we describe the following achieved functional cures: 1. Anti-retroviral therapy (HIV/AIDS) 2. Antivirals (Influenza) 3. Antimalarials (Malaria) 4. Heart Surgery: Coronary Artery Bypass Grafting (Cardiovascular Disease) 5. Angioplasty and Stents (Cardiovascular Disease) 6. Alpha/Beta Blockers (Cardiovascular Disease) 7. Diuretics (Cardiovascular Disease) 8. Statins (Cardiovascular Disease) 9. Insulin (Diabetes) 10. Anti-depressants (Unipolar Mood Disorder) 11. Mood Stabilizers (Bipolar Mood Disorder) 12. Corticosteroids (Duchenne Muscular Dystrophy) 13. Pap Test (Cervical Cancer) 14. Surgery, Radiation, Chemotherapy (Child Cancers) (Pancreatic Cancer) In the current report, we describe the following achieved definitive cures: 1. Vaccine (Influenza) 2. Vaccine (Poliomyelitis) 3. Vaccine (HPV-related Cervical Cancer) 4. Hematopoietic Stem Cell Transplant (Sickle Cell Disease) The past demonstrates that vaccines have provided the basis for definitive cures. A number of vaccines are being pursued in the search for future definitive cures, including for HIV and malaria. While both diseases currently have functional cures, the impact of a definitive cure for either of these diseases would be significant. The history of cures suggests that definitive cures tend to be more effective in mitigating exposure or eliminating exposure to the causal agents of a disease through preventative efforts. Vaccines prime the body’s immune system to attack and eliminate pathogens with little or no symptoms (i.e., awareness by the individual). Certain behaviors, such as washing hands with soap reduce exposure to pathogens in the first place. SGU Cures Index 48 Definitive cures are more cost-effective than functional cures. Anti-retroviral therapy (ART) provides an excellent case example. Once an individual has been infected with HIV, ART costs approximately $10,000 – $15,000 per year in high-income countries. There were an estimated 47,500 new HIV infections in the US in 2010 (Centers for Disease Control and Prevention, 2014a), resulting in approximately $712.5 million in new annual ART costs (if the average annual treatment cost is $15,000 without accounting for additional healthcare system costs). By comparison, even if an HIV vaccine cost $200 per immunization, it would cost approximately $63.8 billion to immunize every American. If the rate of new HIV infections were stable (47,500 per year), the total vaccination cost would be covered by savings in ART within approximately 13 years. This does not include the research and development costs of discovering the vaccine. The NIH spent $18.42 billion on research in HIV/AIDS between 2010 and 2015 (estimated). However, the savings from any such vaccine against HIV would continue to expand on an annual basis and accrue indefinitely, thereby recovering vaccine research and development costs within another 10-20 years. Billions of potential USD saved in ART costs as a result of HIV vaccine. Note that each year, an additional $712.5 million is saved because 47,500 new HIV infections do not occur. The total accumulated savings after 13 years is $64.8 billion (i.e., all green bars combined) – enough to vaccinate every American. $10,000,000,000.00 $9,000,000,000.00 $8,000,000,000.00 $7,000,000,000.00 $6,000,000,000.00 $5,000,000,000.00 $4,000,000,000.00 $3,000,000,000.00 $2,000,000,000.00 $1,000,000,000.00 $- Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Year 7 Year 8 Year 9 Year 10 Year 11 Year 12 Year 13 SGU Cures Index 49 Definitive cures improve quality of life more than functional cures. Functional cures address the symptoms of disease and may extend life, but the effectiveness of this impact is variable depending on the disease and the cure, and whether contact with the causative agent is inevitable. Consider ART for HIV. Patients must take medications and some can cause side effects. Medications can interact with each other. The medications must be taken consistently to be effective – the HIV could mutate and develop resistance to the drugs. Paying for the medications can be very costly. This money could be spent elsewhere (i.e., better quality food, better accommodation). A vaccine, on the other hand, would protect the individual from the HIV. Most likely, one or a few needles may be required just once, in early childhood, to protect against the virus. Consider insulin for Type 1 diabetes. Diabetics must monitor their insulin levels (usually via blood test). Diabetics must inject insulin at least once per day, and sometimes several times per day. Constantly monitoring insulin is time consuming and burdensome. Money for equipment to monitor blood glucose and inject insulin could be spent elsewhere. Growing or replacing deficient insulin-secreting islet Beta cells – possibly through stem cell therapy – would address the lack of insulin in Type 1 diabetics while eliminating the need for constant monitoring of blood glucose levels. Changing to a healthier diet and exercising daily could help those with Type 2 diabetes or prediabetes, regulate blood glucose levels. This is better than taking insulin to regulate blood glucose levels, as it is non-invasive (i.e., no needles are required), can cost virtually no money (i.e., taking a 30-45 minute walk), and has numerous beneficial side effects (i.e., improves muscle and bone condition, leads to lower BMI, improves other bodily systems such as cardiovascular health). Definitive cures tend to have fewer side effects than functional cures. In most cases, “traditional” definitive cures for infectious disease (i.e., vaccines) have minimal side effects. However, when vaccinating millions, or tens of millions of people, the law of large numbers indicates that some will experience side effects. Serious side effects do occur with vaccination, the rate of which varies widely for each vaccine. For example, the adenovirus vaccine is associated with severe problems (blood in the urine or stool, pneumonia inflammation of the stomach or intestines) for about 1:100 vaccinated individuals within six months of vaccination (Centers for Disease Control and Prevention, 2015). The anthrax vaccine causes serious allergic reaction in less than 1:100,000 vaccinated individuals (Centers for Disease Control and Prevention, 2015). The diphtheria, tetanus, and acellular pertussis (DTaP) vaccine causes a serious allergic reaction in less than 1:1,000,000 vaccinated individuals (Centers for Disease Control and Prevention, 2015). SGU Cures Index 50 Definitive Cures tend to provide a better return than Functional Cures 1. Definitive cures tend to be more effective in mitigating exposure or eliminating exposure to the causal agents of a disease (mostly through preventative efforts) 2. Definitive Cures are more cost-effective than Functional Cures 3. Definitive Cures improve quality of life more than Functional Cures 4. Definitive Cures tend to have fewer side effects than Functional Cures Most definitive cures have been developed against infectious diseases. However, the world has entered an era of chronic, or non-communicable diseases (NCDs), which now cause the majority of deaths and disability worldwide (World Health Organization, n.d.), a trend that is likely to continue into the foreseeable future. As such, the search for definitive cures for chronic diseases will also increase in importance. Unlike infectious diseases, which are often neutralized with vaccines, we do not have a “template” for chronic disease definitive cures. Behavioral interventions – and thus, prevention – hold promise for the major chronic diseases: Cardiovascular disease, diabetes, and some cancers. However, there is much to learn about human cognition, motivation, social science, and environmental context before unlocking the mysteries of why so many Americans are engaged in behaviors that are harmful to health (i.e., eating high-sugar, high-fat, nutritionally-poor foods, excessive alcohol consumption) and not engaging in behaviors that support good health (i.e., daily exercise). The Greatest Return on Investment: Prevention The greatest return on investment (ROI) is provided by cures that minimize or eliminate contact with the disease causative agent. This means that cures are generally less expensive when provided to patients earlier in the disease process – a rule that applies to both infectious and non-communicable diseases. In the specific disease sections outlined below, achieved cures that are implemented long after contact with the disease generally provide less return than cures that are implemented prior to contact with the disease. Cures Achieved After Disease Contact Influenza – Antivirals A 25% reduction in influenza mortality from antivirals would save between 825 and SGU Cures Index 51 12,250 lives each year (6,538 middle value), with an annual VMRR of $681.21 million. If 10% of the US working age population contracts influenza annually and is able to gain a half workday from taking antivirals, that equates to 77.28 million work hours gained, with a total value of $1.879 billion annually. Combined with the VMRR associated with antivirals ($681.214 million), the combined economic impact of influenza antivirals is $2.56 billion annually. The cost of treating 10% of the US population with influenza antivirals is estimated at $1.809 billion. Thus, influenza antivirals provide an ROI of 1.4. Malaria – Antimalarials If approximately 50% of the 110,000 Malaria deaths averted each year were attributable to antimalarials, while the other 50% were attributable to urbanization and overall economic development (World Health Organization, 2012), 55,000 deaths are avoided as a result of antimalarials, which is equivalent to 2,713,150 life years, and a VMRR of $11.63 billion (International $). The highest estimated cost of distributing antimalarials in 2014 dollars was $3.34 billion (international $), resulting in an ROI in distributing antimalarials of 3.5. HIV – Antiretroviral Therapy (ART) Lifetime antiretroviral therapy (ART) for HIV infection costs approximately $354,100, 73% ($258,493) of which is related to ART (Schackman, Gebo, Walensky, Losina, Muccio, Sax, et al., 2006). It is estimated that on average, 11.8 years of life has been gained amongst HIV patients between 1996-2005 (Harrison, Song, & Zhang, 2010), mostly due to advances in ART. If 1,148,200 Americans diagnosed with HIV as of 2009 gained 11.8 years of life as a result of advances in ART/HAART, the VMRR is equivalent to $1.41 trillion, and the lifetime cost of ART therapy for those 1,148,200 Americans would be $296.8 billion. Thus, ART provides an ROI of 4.75. Intraepithelial Neoplasia: Cervix – Pap Test If additional Pap testing for the 26.8% of women who do not current receive it reduced cervical cancer mortality in the US by the upper limit in the Nordic research (73% Shingleton et al., 1995), we would expect that (3,939 x 73%), 2,875 fewer women would die from cervical cancer in the US, equaling 69,575 years of potential life saved annually and a VMRR of $7.25 billion. In support of this estimate, Solomon and colleagues (2007) and Diamantis, Magiorkinis, and Androutsos (2010) report that the Pap test has proven to be a model for successful cancer prevention and is largely responsible for the 70% decrease in cervical cancer mortality in the US over the last 50 years. The annual cost of providing Pap tests for currently unscreened women every three years is estimated at $1.096 billion ($101 per test multiplied by 32.546 million women aged 18+). Thus, at SGU Cures Index 52 the upper estimate, the ROI of the Pap test is 6.6. Note that the reduction in annual cervical cancer deaths is likely to be somewhere between 8% and 73%, which is the range noted in the Nordic research (Shingleton et al, 1995). Thus, the ROI of the Pap test is likely between 1 and 6. Mood Disorders: Unipolar Depression - Antidepressants If 50% of self-inflicted injuries in the USA are directly linked with depression, 334,623 years of potential life, valued at $34.87 billion, could be saved with antidepressants. A 0.40 QALY increase among the 14.8 million Americans diagnosed with clinical depression in a given year has a value of $616.8 billion. Combining this with the potential value associated with reducing suicides as a direct result of depression ($34.87 billion) gives $651.67 billion in economic impact associated with antidepressant use. The cost of providing antidepressants for one year for the 27.5 million Americans diagnosed with clinical depression is $66 billion. Thus, the annual ROI of antidepressants for mood disorder is 9.9. Type 1 Diabetes - Insulin We calculate the total VMRR associated with insulin at $11.562 trillion. The total annual cost of insulin is $11.868 billion (2013 dllars) across all Type 1 diabetic patients whose lives have been saved by insulin. With a current life expectancy of about 68.8 years among Type 1 diabetics, the cost of treating all 1,410,000 Americans whose lives have been saved by insulin is $816.52 billion. In this scenario, the lifetime ROI of insulin for Type 1 diabetes has been 14.2. Mood Disorders: Bipolar – Mood Stabilizer A .077 QALY increase among the 5.7 million Americans diagnosed with bipolar disorder in a given year has a value of $45.7 billion. The cost of providing mood stabilizers for one year for the 5.7 million Americans diagnosed with bipolar disorder is $2.46 billion. Thus, the annual ROI of Mood Stabilizers for bipolar disorders is 18.6. Cures Achieved Prior to Disease Contact Intraepithelial Neoplasia: Cervix – HPV Vaccine If HPV vaccination could prevent 50,000 girls from developing cervical cancer, and 33.3% of those girls would have died at a median age of 57 years, 399,300 life years, with a VMRR of $41.6 billion, would be achieved by attaining an 80% vaccination rate. The cost to administer three doses of the vaccine to all 10 million girls aged 13-17 in 2013 would have been $3.9 billion. Thus, the ROI for the HPV vaccine is 10.7. SGU Cures Index 53 Influenza - Vaccine We estimate that the influenza vaccine has an annual VMRR of $76.222 billion by saving 731,542 years of potential life. We further estimate the cost of vaccinating the US population, at the current 45% coverage rate, at $1.367 billion per annum. Further, the National Institutes of Health (NIH) spends approximately $300 million per year on influenza research. If we divide $76.222 billion in annual value by $1.667 billion in annual research and vaccine costs, the ROI for the influenza vaccine is 45.7. Poliomyelitis - Vaccine Between 1955 and 2006, the polio vaccine saved 160,000 American lives, giving a VMRR of $1.312 trillion. Further, $1.55 trillion in savings accrued because 1.1 million Americans avoided paralysis from polio between 1955 and 2006. Thus, the polio vaccine produced $2.87 trillion in value during that timeframe. The total estimated research cost to develop the polio vaccine and distribute it from 1955 through 2015 was $36.4 billion (in 2002 dollars). Thus, the ROI for the polio vaccine is 78.7. Return on Investment (ROI) of Cures Achieved After and Prior to Disease Contact. Cures Achieved After Disease Exposure Cures Achieved Prior to Disease Exposure 90 80 70 60 50 40 30 20 10 ci n Va c iti s- -V ac ci n e e e za Po lio m ye l en flu nc Ca rv ic al Ce In -H er oo M r- iso rd e rD Bi po la Va cc in rs ze d PV In ste be ia D 1 Ty pe St ab ili sa su nt lin s e) nt -A n sio re s rD ep ol a Un ip id ep re s re pe up t( Te s ap -P nc er Ca al ic im st he lT ra tro vi nt ire -A IV H Ce rv at ra py ls ia ar im al -A ar ia al M In flu en za nt -A nt iv ira ls 0 SGU Cures Index 54 The evolution of cures for cardiovascular disease provides a powerful example of the importance of prevention for maximum ROI. Our analysis also indicates that cures tend to follow an “evolutionary” path, in which each generation of the cure, building on more detailed knowledge of physiological processes, provides more relief from disease, better quality of life, or further extension of life. Sometimes, a paradigm-shifting breakthrough in disease or therapeutic knowledge leads to a “leap” in cure development, significantly impacting life extension or quality of life. Ideally, as knowledge accumulates, the evolution of cures for a particular disease will make the ultimate leap from a functional (albeit, very effective) cure to a definitive cure, thereby eliminating the impact of the disease altogether. Cardiovascular disease – an ailment that was rarely seen prior to 1940 – provides an excellent case example of the evolution of cures, the importance of prevention, and the increase in ROI that is seen as a disease process is better understood. Cardiovascular Disease – Heart Surgery Cardiovascular disease (CVD), more commonly known as heart disease, encompasses a number of conditions related to the cardiovascular system. Such conditions may include coronary artery disease (CAD), myocardial infarctions (heart attacks), angina (chest pain), heart failure, and others (Centers for Disease Control and Prevention, 2009; National Heart, Lung, and Blood Institute, 2011). While CVD characterizes the nature of heart disease, there are several other types of cardiovascular conditions that may potentially result in heart disease if left unaddressed, especially hypertension. CVD is the leading cause of death among Americans, with 597,689 deaths in 2010 alone (Centers for Disease Control and Prevention, 2013b). In 2011, over 26 million Americans had heart disease, over 59 million Americans had hypertension (Schiller, Lucas, & Peregoy, 2012), and over 71 million Americans had high LDL, or “bad” cholesterol (Centers for Disease Control and Prevention, 2011c). In total, 5.02 million years of potential life were lost (YPLL) to CVD in the US in 2001 (World Health Organization, 2001). As such, any advancement in curing CVD can have a significant impact on health, well-being, extended life, and the associated economic consequences. In the early part of the 20th century, physicians from around the world started to form associations and share knowledge with the goal of helping patients with heart-related symptoms, such as chest pain and shortness of breath. By the 1960s, surgeons were SGU Cures Index 55 performing procedures on the hearts of living patients with the aid of heart and lung bypass machines (Mehta & Khan, 2002). Coronary artery bypass grafting (CABG) was one of the first procedures developed. A CABG is performed when an artery leading to the heart has been occluded, preventing adequate blood supply from reaching the heart and the rest of the body. During a CABG a healthy artery or vein from another part of the body is removed and reconnected with the coronary artery that is affected to ensure there is proper blood supply reaching the heart to circulate throughout the body (National Heart, Lung, and Blood Institute, 2007). It has been estimated that between the years of 1990 and 2000, bypass grafts added 135,000 years of potential life among those aged 25 to 84 in a study of 2,356,700 patients who underwent bypass surgery (Capewell et al., 2009), giving a VMRR of $14.07 billion. If each procedure, including post-operative care, medication, and therapy, cost $63,000 without postoperative care costs such as therapy and medications (Coronary Bypass Grafting, 2013), the total cost of the 2,356,700 procedures between 1990 and 2000 would be $148.5 billion. Thus, the ROI of coronary artery bypass grafting is -10.6. Cardiovascular Disease – Angioplasty & Stents Cardiac catheterization procedures utilize a catheter (a flexible tube) that is placed within a large artery (through the arm, thigh or groin) and traced back to the heart. The basis of the catheterization is to follow the route of blood flow in order to identify possible blockages or other causes of concern. After the first successful cardiac catheterization, angiography procedures transformed the way physicians were able to diagnose and treat patients with various heart conditions. During the 1960s, Dr. Charles Dotter combined the use of cardiac catheters to reestablish proper blood flow within blocked arteries in legs and feet. Dr. Andres Gruentzig first completed this process, known as a balloon angioplasty, on a human in 1977. He then took it another step further by performing the procedure on coronary arteries (Society for Cardiovascular Angiography and Interventions, n.d.). In 2009 the Agency for Healthcare Research and Quality (AHRQ) estimated that there were over 644,000 hospital stays for stent procedures with balloon angioplasty (Auerbach, Maeda, & Steiner, 2012). The AHRQ additionally estimated that there were 3,667 angioplasty procedures performed per one million adults in the US in 2007-2008 (Auerbach et al., 2012). Given the total US adult (18+) population of 222,722,000 in 2007 (US Census Bureau, n.d.), that amounts to 816,722 angioplasty procedures performed in the US in 2007-2008, or approximately 408,361 angioplasties per year. Recently, it was estimated that over one million angioplasty procedures were performed in the US in 2009 (Roger et al., 2013). SGU Cures Index 56 Angioplasties help to improve quality of life by reducing the risk of mortality from CVD, particularly among those at the greatest risk (Kent, Schmid, Lau, & Selker, 2002). Angioplasty procedures with stents are estimated to increase life expectancy by about 13 years (Yock et al., 2003). Multiplying 408,361 angioplasties by an average 13 years of increased life expectancy from the procedure (Yock et al., 2003) gives 5,308,693 years of life saved annually, and a VMRR of $553.13 billion. The average procedural and hospital costs for a coronary angioplasty start at about $22,000 (Coronary Angioplasty, 2013). Further, five-year follow-up costs for angioplasty were estimated at almost $20,000 (Vieira et al., 2012). This gives a total 5-year cost of approximately $50,000 USD if the average cost of angioplasty is $30,000. If we multiply that cost by 408,361 estimated angioplasties performed annually, the obtained total is $20.42 billion. The final ROI of angioplasty is 27.1. Cardiovascular Disease – Diuretics An increase in blood pressure leading to hypertension has been a known contributing factor to heart disease since the mid-nineteenth century. Since this time, various methods to control blood pressure were discovered. Diuretics were the first of these methods. By controlling the body’s salt balance, diuretics directly influence the body’s natural response to blood pressure (Hamdy, 2001). Diuretics help to lower blood pressure by changing the amount of urine leaving the body. By reducing the amount of water reabsorbed into the body, it is expelled in the urine. This mechanism signals the body to reduce blood pressure. Proper physician monitoring allows the use of diuretics to be highly beneficial in reducing patients’ blood pressure and reducing the development of heart disease. When evaluating the impact of diuretics, a recent study reported an average of 4.5 years of life gained among existing CVD patients (Banka, Heidenreich & Fonarow, 2013). Heart failure patients treated with diuretics (to control blood pressure) can potentially gain 3.5 quality adjusted life years, on average (Banka et al., 2013). The CDC reported that in 2011, 26,486,000 Americans had heart disease (Schiller et al., 2012). If all of them were prescribed diuretics, and the average increase in years of life lived among all patients were 4.5 as a result of this intervention (Banka et al., 2013), total years of potential life saved would be 119,187,000, giving a VMRR of $12.42 SGU Cures Index 57 trillion. The cost to treat all of those patients would be $12,742 (estimated lifetime healthcare costs of diuretics; Banka et al., 2013) multiplied by 26,486,000 people = $337.48 billion. In this scenario, the ROI of diuretics is 36.8. Cardiovascular Disease – Statins Ensuring adequate blood flow is critical in maintaining ideal blood pressure within the body. In the 1960s it was established that blocking the signal of certain hormones can help reduce the body’s response to constriction, but researchers sought to further discover methods to prevent the reduction in blood flow leading to hypertension and heart disease. The presence of plaques within arteries, which can cause a reduction in blood flow, was identified with the use of angiographies. This discovery prompted a search for ways to prevent plaque development in the first place (Endo, 2010). In the 1950s, John Gofman identified that heart attacks correlated with high levels of blood cholesterol, in particular, low density lipoprotein (LDL). It was also recognized that fewer heart attacks occurred in those with higher high-density lipoprotein (HDL) levels. Thus, regulation of blood cholesterol became a potential way to reduce heart attacks and heart disease risk. In the 1960s, scientists and researchers sought to identify a method to regulate the key enzyme for cholesterol synthesis in the body, HMG CoA reductase. After thorough trials the FDA approved lovastatin as the first statin to effectively lower LDL cholesterol levels in 1986 (Endo, 2010). Statin use to control high cholesterol also provides considerable economic gains in the reduction of heart disease risk. An aggressive strategy of statin prescribing, with all Americans at high risk and those with no additional risk factors besides high cholesterol, would lead to a total of 64 million Americans prescribed a daily statin and help prevent 27,000 coronary heart disease deaths per year (Lazar, Pletcher, Coxson, BibbinsDomingo, & Goldman, 2011). This mortality reduction would yield an additional 220,000 quality adjusted life years over the lifespan of those whose deaths were averted in a year, gains that would be valued at $22.9 billion dollars. Statins cost an average of $5 to $12 per month across the U.S. (www.rxpricequotes.com). Statin therapy for an estimated 64 million patients would cost approximately $620 million per year (Lazar et al., 2011). In this scenario, the annual ROI of statins is 37.0. Cardiovascular Disease – Alpha/Beta-Blockers Alpha and beta-blockers control the body’s response to hormones by influencing vasoconstriction (i.e., reduction of artery size) (Mayo Clinic, 2013). By the 1950s, scientists had established that the risk of heart disease increased when plaques reduced the area within arteries, thereby restricting adequate blood flow (Quirke, 2006). SGU Cures Index 58 Researchers sought to identify other ways to control the size of arteries, thus ensuring proper blood blow. The discovery of beta adrenergic blockers and alpha adrenergic blockers provided physicians with a way to chemically control hypertension by blocking chemical signals which would cause arteries to constrict. Alpha-blockers were found to inhibit norepinephrine from tightening the muscle of blood vessels causing constriction, while beta-blockers were found to inhibit the action of epinephrine on the same mechanism. Using a coronary heart disease (CHD) policy model and a computer-simulation Markov model of CHD in the US population, Phillips and colleagues (2000) calculated that initiating a beta-blocker regimen for all first–time myocardial infarction (MI) patients (except for those with absolute contraindications) in the year 2000, and continuing that regimen over the next 20 years, resulted in 4,300 fewer deaths and 45,000 life-years gained among this cohort, with a VMRR of $4.69 billion. The cost of taking alpha and/or beta-blockers can be as high as $240 per year (www.rxpricequotes.com). Multiplying that total amount by 603,250 first-time myocardial infarction patients in the US in 2009 (i.e., 635,000 as estimated by Go et al. [2009] minus 5% to account for the small number of patients with absolute contraindications) gives a total cost of $144.78 million. In this scenario, the ROI of beta-blockers is 32.4. Return on Investment (ROI) Multiple for Different Cardiovascular Disease Cures Over Time. Coronary Artery Bypass Graft Surgery Angioplasty & Stents Future Cures It is likely that future cures will be more difficult to achieve. Early cures, particularly Diureticsthe trait of "one cause, one effect." One snip of the causal definitive cures, shared chain was all it took. A polio vaccine provoked the immune system to respond to all polio organisms that entered the body. Scurvy results from a deficiency in one Statins molecule called "Vitamin C." While vaccines provide many of the examples of a definitive cure, the Human Immunodeficiency Virus has demonstrated an ability to mutate, leading to development of vaccines that shoot at a version of the virus that is Alpha/Beta-Blockers no more. -20 -10 0 10 20 30 40 50 SGU Cures Index 59 While science has likely plucked most low-hanging cures, other cures remain to be discovered. New cures will take more effort. One reason for more effort is more capability. Documenting the human genome has opened up a nearly infinite set of possibilities. Pursuing these possibilities is daunting, yet scientifically feasible because of techniques that have been developed that make pursuit doable. Citations Abraham, T. M. & Fox, C. S. (2013). Implications of rising prediabetes prevalence. Diabetes Care, 36(8), 2139-2141. Arbyn M., Anttila, A., Jordan, J., Ronco, G., Schenck, U., Segnan, N., et al. (2010). European guidelines for quality assurance in cervical cancer screening, second edition—summary document. Annals of Oncology, 21 (3), 448–458. Auerbach, D., Maeda, J., & Steiner, C. (AHRQ). (2012). Hospital stays with cardiac stents, 2009. (HCUP Statistical Brief #128). Agency for Healthcare Research and Quality. Retrieved from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb128.pdf Banka, G., Heindenreich, P., & Fonarow, G. (2013). Incremental cost-effectiveness of guideline-directed medical therapies for heart failure. Journal of American College of Cardiology, 61(13). Retrieved from http://dx.doi.org/10.1016/j.jacc.2012.12.022 Bassuk, S. S., & Manson, J. E. ( 2005). Epidemiological Evidence For The Role Of Physical Activity In Reducing Risk Of Type 2 Diabetes And Cardiovascular Disease. Journal of applied Physiology, 99, 1193-1204. Retrieved June 27, 2014, from: http://jap.physiology.org/content/99/3/1193 Capewell, S., Hayes D., Ford, E., Critchley, J., Croft, J., Greenlund, K., & Labarthe, D. (2009). Life-years gained among US adults from modern treatments and changes in the prevalence of 6 coronary heart disease risk factors between 1980 and 2000. American Journal of Epidemiology, 170(2), 229-236. Centers for Disease Control and Prevention. (2009). Other Related Conditions. National Center for Chronic Disease Prevention and Health Promotion: Division for Heart Disease and Stroke Prevention. Retrieved from http://www.cdc.gov/heartdisease/other_conditions.htm SGU Cures Index 60 Centers for Disease Control and Prevention. (2011a). National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Retrieved April 14, 2014, from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Centers for Disease Control and Prevention. (2011b). Diabetes. Retrieved April 27, 2014, from: http://www.cdc.gov/chronicdisease/resources/publications/aag/ddt.htm Centers for Disease Control and Prevention. (2011c). Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol. United States, 1999–2002 and 2005–2008. Morbidity and Mortality Weekly Report, 60(4), 109– 114. Centers for Disease Control and Prevention (2013). HPV-Associated Cancer Statistics. Retrieved March 5, 2014 from: http://www.cdc.gov/cancer/hpv/statistics/. Centers for Disease Control and Prevention. (2013b). QuickStats: number of deaths from 10 leading causes – national vital statistics system, United States, 2010. Morbidity and Mortality Weekly Report, 60(8), 155. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6208a8.htm Centers for Disease Control and Prevention. (2014). Chronic Disease Prevention and Health Promotion. Retrieved on July 29, 2015 from: http://www.cdc.gov/diabetes/surveillance/faq.htm#type1type2 Centers for Disease Control and Prevention. (2014a). HIV/AIDS: HIV Basics. Retrieved July 5, 2014, from: http://www.cdc.gov/hiv/basics/index.html Centers for Disease Control and Prevention (2014b). Life Expectancy. Retrieved on May 23, 2014 from: http://www.cdc.gov/nchs/fastats/lifeexpectancy.htm. Centers for Disease Control and Prevention. (2015). Possible side-effects from vaccines. Vaccines and Immunizations. Retrieved on April 5, 2015 from: http://www.cdc.gov/vaccines/vac-gen/side-effects.htm Chisholm, D., van Ommeren, M., Ayuso-Mateos, J-L., & Saxena, S. (2005). Costeffectiveness of clinical interventions for reducing the global burden of bipolar disorder. British Journal of Psychiatry, 187, 559-567. SGU Cures Index 61 Cohn, C., Berger, S., & Norton, M. (1968). Relationship between meal size and frequency and plasma insulin response in man. Diabetes, 17(2), 72-75. Coronary Angioplasty. (2013). In Healthcare Blue Book. Retrieved August 23, 2013, from http://www.healthcarebluebook.com/page_Results.aspx?id=127&dataset=hosp&g =Angioplasty Coronary Bypass Grafting. (2013). In Healthcare Blue Book. Retrieved on August 23, 2013 from http://www.healthcarebluebook.com/page_Results.aspx?id=29&dataset=hosp Daneman, D. (2006). Type 1 diabetes. Lancet, 367(9513), 847-58. Diabetes UK. (2012). Diabetes Treatments. Retrieved April 21, 2014, from: http://www.diabetes.org.uk/Guide-to-diabetes/What-is-diabetes/Diabetestreatments/ Diamantis, A., Magiorkinis, E., & Androutsos, G. (2010). What's in a name? evidence that papanicolaou, not babes, deserves credit for the pap test. Diagnostic Cytopathology, 38(7), 473-476. doi:10.1002/dc.21226 Ekwueme, D. U., Chesson, H. W., Zhang, K. B., & Balamurugan, A. (2008). Years of potential life lost and productivity costs because of cancer mortality and for specific cancer sites where human papillomavirus may be a risk factor for carcinogenesis-united states, 2003. Cancer, 113(10 Suppl), 2936-2945. doi:10.1002/cncr.23761 Endo, A. (2010). A historical perspective on the discovery of statins. Proceedings of the Japan Academy, Series B Physical and Biological Sciences, 86(5), 484-493. doi:10.2183/pjab.86.484 Fowler, M. J. (2007). Diabetes treatment, part 1: Diet and exercise. Clinical Diabetes, 25, 105-109. Go, A. S., Mozaffarian, D., Roger, V. L., Benjamin, E. J., Berry, J.D., Borden, W. B., et al. (2012). Executive summary: heart disease and stroke statistics- 2013 update. Circulation, 127(1), 143-152. doi: 10.1161/CIR.0b013e318282ab8f SGU Cures Index 62 Hamdy, R. C. (2001). Hypertension: a turning point in the history of medicine…and mankind. Southern Medical Journal, 94(11), 1045-1047. Harrison, K. M., Song, R., & Zhang, X. (2010). Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. Journal of Acquired Immune Deficiency Syndromes, 53(1), 124–130. Jamison, D., Jha, P., & Bloom, D. (2008). Disease Control. Copenhagen Consensus 2008 Diseases Challenge Paper. Retrieved September 06, 2014, from http://files.givewell.org/files/DWDA 2009/Stop TB/Copenhagen Consensus Paper-Diseases.pdf Kahn, C., & White, M. (1988). The insulin receptor and the molecular mechanism of insulin action. The American Society for Clinical Investigation, 82, 1151-1156. Kessler, R. C., Berglund, P. A., Demler, O., Jin, R., & Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Archives of General Psychiatry, 62 (6), 593-602. Lazar, L. D., Pletcher, M. J., Coxson, P. G., Bibbins-Domingo, K., & Goldman, L. (2011). Cost-Effectiveness of Statin Therapy for Primary Prevention in a Low-Cost Statin Era. Circulation, 124, 146-153. Lee, P. Y., Matchar, D. B., Clements, D. A., Huber, J., Hamilton, J. D., & Peterson, E. D. (2002). Economic Analysis of Influenza Vaccination and Antiviral Treatment for Healthy Working Adults. Annals of Internal Medicine, 137 (4), 225-231. Mali, S., Kachur, S. P., & Arguin, P. M. (2012). Malaria surveillance - United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summaries, 61(2), 1–17. Mayo Clinic. (2010). High blood pressure (hypertension). Retrieved from http://www.mayoclinic.com/health/diuretics/HI00030/NSECTIONGROUP=2 Mayo Clinic. (2013). Statin side effect: weigh the benefits and risk. Retrieved from http://www.mayoclinic.com/health/statin-side-effects/MY00205 SGU Cures Index 63 Mehta, N. J., & Khan, I. A. (2002). Cardiology’s 10 greatest discoveries of the 20th century. Texas Heart Institute Journal, 29(3), 164-171. Milken, M. (2010). Health Reform. Retrieved on July 29, 2015 from: http://www.mikemilken.com/MIR-MM1Q10.pdf Miller, R., Secrest, A., Sharma, R., Songer, T., & Orchard, T. (2012). Improvements in the Life Expectancy of Type 1 Diabetes. Diabetes, 61, 2987-2992. Murphy, K. M. & Topel, R. H. (2006). The value of health and longevity. Journal of Political Economy, 114(5), 871-904. Muthuri, S. G., Venkatesan, S., Myles, P. R., Leonardi-Bee, J., Al Khuwaitir, T. S., Al Mamun, A., Anovadiya, A. P., Azziz-Baumgartner, E., Báez, C., Bassetti, M., Beovic, B., Bertisch, B., Bonmarin, I., Booy, R., Borja-Aburto, V. H., Burgmann, H., Cao, B., Carratala, J., Denholm, J. T., Dominguez, S. R., Duarte, P. A., DubnovRaz, G., Echavarria, M., Fanella, S., Gao, Z., Gérardin, P., Giannella, M., Gubbels, S., Herberg, J., Iglesias, A. L., Hoger, P. H., Hu, X., Islam, Q. T., Jiménez, M. F., Kandeel, A., Keijzers, G., Khalili, H., Knight, M., Kudo, K., Kusznierz, G., Kuzman, I., Kwan, A. M., Amine, I. L., Langenegger, E., Lankarani, K. B., Leo, Y. S., Linko, R., Liu, P., Madanat, F., Mayo-Montero, E., McGeer, A., Memish, Z., Metan, G., Mickiene, A., Mikić, D., Mohn, K. G., Moradi, A., Nymadawa, P., Oliva, M. E., Ozkan, M., Parekh, D., Paul, M., Polack, F. P., Rath, B. A., Rodríguez, A. H., Sarrouf, E. B., Seale, A. C., Sertogullarindan, B., Siqueira, M. M., Skręt-Magierło, J., Stephan, F., Talarek, E., Tang, J. W., To, K. K., Torres, A., Törün, S. H., Tran, D., Uyeki, T. M., Van Zwol, A., Vaudry, W., Vidmar, T., Yokota, R. T., Zarogoulidis, P., PRIDE Consortium Investigators, Nguyen-Van-Tam, J. S. (2014). Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: A meta-analysis of individual participant data. The Lancet Respiratory Medicine, 14(2), 109-1182; 2(5), 395-404. Narayan, K. M., Boyle, J., Thompson, T., Sorenson, S., & Williamson, D. (2003). Lifetime risk for diabetes mellitus in the united states. Journal of the American Medical Association, 290(14), 1884-1890. National Cancer Institute (2014). A Snapshot of Cervical Cancer. Retrieved March 14, 2014 from: http://www.cancer.gov/researchandfunding/snapshots/cervical. SGU Cures Index 64 National Diabetes Information Clearinghouse. (2013). Insulin Resistance and Prediabetes. Retrieved April 13, 2014, from: http://diabetes.niddk.nih.gov/dm/pubs/insulinresistance/ National Heart, Lung, and Blood Institute (NHLBI). 2007. Shaping the future of research: a strategic plan for the National Heart, Lung, and Blood Institute. US Department of Health and Human Services. (NIH Publication No. 07-6150). Retrieved from http://www.nhlbi.nih.gov/about/strategicplan/documents/StrategicPlan_Appendix .pdf National Heart, Lung, and Blood Institute (NHLBI). (2011). How is heart disease diagnosed? US Department of Health and Human Services. Retrieved from http://www.nhlbi.nih.gov/health/health-topics/topics/hdw/diagnosis.html Phillips, K., Shlipak, M., Coxson, P., Heidenreich, P., Myriam Hunink, M. G., Goldman, P., et al. (2000). Health and economic benefits of increased b-blocker use following myocardial infarction. JAMA, 284(21), 2748-2754. Pratt, L. A., Brody, D. L., Gu, Q. (2011). US Department of Health and Human Services, Centers for Disease Control, NCHS Data Brief; 2011. Antidepressant use in persons aged 12 and over: United States, 2005–2008. Pyne, J. M., Fortney, J. C., Tripathi, S., Feeny, D., Ubel, P., & Brazier, J. (2009). How bad is depression? Preference score estimates from depressed patients and the general population. Health Services Research, 44 (4), 1406-1423. Quirke, Viviane. (2006). Putting theory into practice: James Black, receptor theory and the development of the beta-blockers at ICI, 1958-1978. Medical History (50), 6992. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1369014/pdf/medhis5001-069.pdf Ramsberg, J., Asseburg, C., & Henriksson, M. (2012) Effectiveness and Cost Effectiveness of Antidepressants in Primary Care: A Multiple Treatment Comparison Meta-Analysis and Cost-Effectiveness Model. PLoS ONE 7 (8), e42003. Rihmer, Z. (2007). Suicide risk in mood disorders. Current Opinion in Psychiatry, 20, 17-22. SGU Cures Index 65 Roger, V., Go, A., Lloyd-Jones, D., Benjamin, E., Berry, J., … Turner, M. (2012). Heart disease and stroke statistics- 2012 update: a report from the American Heart Association. Circulation, 125, e2-e220. doi: 10.1161/CIR. 0b013e31823ac046 Roll Back Malaria. (2008). The Global Malaria Action Plan For a Malaria-Free World. Retrieved September 06, 2014, from http://www.rollbackMalaria.org/gmap/gmap.pdf Schackman, B. R., Gebo, K. A., Walensky, R. P., Losina, E., Muccio, T., Sax, P. E., et al. (2006). The lifetime cost of current human immunodeficiency virus care in the United States. Medical care, 44(11), 990–997. Schiller J. S., Lucas J. W., & Peregoy J. A. (2012). Summary health statistics for US adults: national health interview survey, 2011. National Center for Health Statistics. Vital Health Statistics, 10(256). (DHHS Publication No. (PHS) 2013– 1584). Retrieved from: http://www.cdc.gov/nchs/data/series/sr_10/sr10_256.pdf Shingleton, H. M., Patrick, R. L., Johnston, W. W., & Smith, R. A. (1995). The current status of the papanicolaou smear. CA: A Cancer Journal for Clinicians, 45(5), 305320. doi:10.3322/canjclin.45.5.305 Society for Cardiovascular Angiography and Interventions (SCAI). (n.d.). History of angioplasty and stenting. Retrieved from: http://www.scai.org/SecondsCount/Resources/Detail.aspx?cid=b7b1dbfd-4f964b77-8ad8-af95f5cbb417 Solomon, D., Breen, N., & McNeel, T. (2007). Cervical cancer screening rates in the united states and the potential impact of implementation of screening guidelines. CA: A Cancer Journal for Clinicians, 57(2), 105-111. doi:10.3322/canjclin.57.2.105 Tuomilehto, J., Schwarz, P., & Lindstrom, J. (2011). Long-term benefits from lifestyle interventions for type 2 diabetes prevention. Diabetes Care, 34. Retrieved May 30, 2014, from: http://care.diabetesjournals.org/content/34/Supplement_2/S210.full US Census Bureau. (n.d.). Age and sex composition in the United States: 2007. (n.d.) Retrieved on September 28, 2014, from https://www.census.gov/population/age/data/2007comp.html SGU Cures Index 66 US Census Bureau. (n.d.). Population Estimates by Demographic Characteristics. Population Division, US Census Bureau Release Date: June 9, 2005. Retrieved on August 20, 2013 from: http://www.census/gov/popest/national/asrh/ US National Library of Medicine. (2014). Insulin Injection. Retrieved April 16, 2014, from: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a682611.html Vieira, R., Hueb, W., Hlatky, M., Favorato, D., Rezende, P., Garzillo, L., …Filho, R. (2012). Cost-effectiveness analysis for surgical, angioplasty, or medical therapeutics for coronary artery disease: 5-year follow up of medicine, angioplasty, or surgery study (MASS) II Trial. Circulation, 126. doi: 10.1161/CIRCULATIONAHA.111.084442 World Health Organization (n.d.). Global Burden of Disease (GBD) 2001 Estimates by Subregion: Years of Life Lost (YLL). Retrieved on June 4, 2014 from: http://www.who.int/healthinfo/global_burden_disease/estimates_regional_2001/ en/. World Health Organization (n.d.). Preventing Chronic Diseases: A Vital Investment. Retrieved on April 5, 2015 from: http://www.who.int/chp/chronic_disease_report/contents/part2.pdf World Health Organization (2001). Global burden of disease study. Department of Measurement and Health Information. Retrieved on April 5, 2015 from: http://www.who.int/healthinfo/global_burden_disease/en/ World Health Organization. (2012). World Malaria Report. Retrieved September 7, 2014, from: http://www.who.int/iris/bitstream/10665/78945/1/9789241564533_eng.pdf?ua= 1 Yock, C. A., Boothroyd, D. B., Owens, D. K., Garber, A. M, & Hlatky, M. A. (2003). Costeffectiveness of bypass surgery versus stenting in patients with multivessel coronary artery disease. American Journal of Medicine 11(5), 382. doi: 10.1016/S0002-9343(03)00296-1 SGU Cures Index 67 SPECIFIC DISEASE ANALYSIS A. Infectious Diseases SGU Cures Index 68 ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS/HIV) Andres Molina, Shervin Shaffiy, Vishal Patel, Julia Brunet Disease Category Infectious viral Disease Identification, Description, and Diagnostic Criteria HIV stands for Human Immunodeficiency Virus. It is a unique virus in that the human body cannot eradicate HIV once infected (Centers for Disease Control, 2014). There are many reasons why this virus cannot be eradicated by the human immune system. HIV debilitates the immune system by killing cells that protect the body against other infections and diseases, therefore, decreasing an individual’s chance in recuperating from an infection or disease. HIV harbors inside human immune cells, where it reproduces and in the process, makes the immune cell ineffective. As HIV spreads throughout the body and attacks more and more immune cells, the immune system works less efficiently, and has a difficult time fighting infections. As time increases, HIV can destroy a good percentage of the body’s important immune cells, incapacitating the immune system and leaving the body vulnerable to infection and disease. Once this “tipping point” has been passed, HIV may lead to Acquired Immunodeficiency Syndrome (AIDS). Researchers are still studying how this virus is able to survive the human immune system and how the immune system cannot fight it off (US Department of Health & Human Services, 2012). HIV is a retrovirus. Retroviruses are viruses with genetic material composed of ribonucleic acid (RNA). Once HIV infects a cell, it uses the enzyme “reverse transcriptase” to change its RNA genetic material to deoxyribonucleic acid (DNA), and then begins replicating itself using the host cell’s replicating machinery. HIV is also under the subgroup of retroviruses called “lentiviruses”, which replicate slowly. Because of this slower time frame, symptoms are not prevalent soon after infection, as is the case with most other viruses. This an important reason why many people who are infected may not know they are infected, leading to the further spread of the virus (US Department of Health and Human Services - National institute of Health, 2012). Another reason why the body cannot eradicate HIV is because of the virus’ ability to mutate and evolve in order to evade the human immune system. Mistakes in copying can arise as the virus uses the enzyme reverse transcriptase to replicate. These mistakes can lead to SGU Cures Index 69 new viral strains within the infected person, making it more difficult for the immune system to eradicate the virus and the discovery of a vaccine that will protect against infection (US Department of Health and Human Services - National Institutes of Heath, 2009). The Centers for Disease Control and Prevention (CDC) advises HIV testing for adolescents, adults, and pregnant women as part of their routine medical care. The tests are preformed using samples of blood to find human antibodies (i.e., disease fighting proteins) that are distinct to HIV. There are two types of antibody tests for HIV: the enzyme-linked immunosorbent assay (ELISA) and the Western blot. One drawback to these tests is that since they rely on identifying antibodies, an individual infected with HIV within the last three months may not show a positive test. Testing recently infected individuals requires a search for the genetic material found in HIV. The CDC advises pregnant women to get tested for HIV before and/or during delivery of the infant. If the mother is infected with HIV, physicians may be able to prevent mother-to-child transmission of the virus by administering antiviral treatment to the infected mother and the newborn infant (US Department of Health and Human Services - National Institutes of Health, 2009). Disease Etiology (Cause) HIV is transmitted from individual to individual through blood, semen, and vaginal secretions associated with sexual contact, infected blood in transfusions, infected needles, or “vertically” from mother to child during pregnancy (Centers for Disease Control, 2014). RNA found in HIV is non-infectious but is reverse-transcribed to dsDNA, which in turn results in the provirus merging into the human genome and creating viral mRNA using the host cell’s machinery. The virus then seeks CD4+ T cells and macrophages, which are essential for proper immune system function. Once the number of CD4+ T cells decreases significantly, HIV leads to AIDS. The infected individual with AIDS is at a much higher risk of death from rare infections, since AIDS patients are more prone to rare diseases or infections that are not found in otherwise healthy individuals, as a result of their weakened immune system (e.g., Kaposi’s Sarcoma, Pneumocystis Jiroveci, tuberculosis, etc.) (Douek, Roederer, & Koup, 2009). Current Prevalence/Incidence As of 2009, an estimated 1,148,200 people aged 13 and older infected with HIV in the US (Centers for Disease Control, 2014). SGU Cures Index 70 In 2010, there were an estimated 47,500 new HIV infections in the US (Centers for Disease Control, 2014). Estimated undiagnosed and At-risk As of 2009, approximately 207,600 individuals in the US were infected with HIV but were undiagnosed. The majority of these infections (69%) occurred among men who have sex with men, especially within racial and ethnic minority communities. The estimated rate of new HIV infections for African American men (103.6/100,000 population) was 7 times that of white men, twice that of Latino men, and nearly 3 times that of African American women. African Americans accounted for an estimated 44% of all new HIV infections among adults and adolescents (aged 13 years or older) in 2010, despite representing only 12% of the US population. Considering the smaller size of the African American population in the US, this represents a population rate that is 8 times that of whites overall (Centers for Disease Control, 2014). Disease Impact - Years of Potential Life Lost (YPLL) In 2005, 21.1 years of potential life were lost per infected individual, which represents an improvement of 11.8 years when compared to the figures of 1996 (32.9 YPLL) (Harrison, Song, & Zhang, 2010). When multiplied by the 1,148,200 people aged 13 and older living with HIV in the US in 2009 (Centers for Disease Control, 2014), 24,227,020 years of life will likely be lost to those currently living with HIV in the US. Disease Impact - Disability Adjusted Life Years (DALY) In 2004, 121 disability-adjusted life years (DALYs) were lost per every 100,000 people in the US (World Health Organization, 2008). Given a US population of 292.81 million people in 2004, a total of 354,300 DALYs were lost to HIV in that year. If we project the same rate (121 DALYs) forward to 2013, a total of 382,553 DALYs were lost to HIV in that more recent year. History of the Disease and Breakthroughs Simian Immunodeficiency Virus (SIV) was found to be the precursor to HIV (Plantier, Leoz, Dickerson, DeOlivera, Cordonnier, Lemee, et al., 2009), and is likely a disease over tens of thousands of years old (Worobey, Telfer, Souquière, Hunter, Coleman, Metzger, et al., 2010). The virus was able to infect humans in the 20th century, likely via animal to human, or zoonotic transmission, associated with hunting and preparation of SGU Cures Index 71 “bushmeat” (Kalish, Wolfe, Ndongmo, McNicholl, Robbins, Aidoo, et al., 2005). The first confirmed cases of HIV infection were discovered in blood samples taken in 1959. HIV is thought to have arrived in the United States in the late 1960s or early 1970s (Gilbert, Rambaut, Wlasiuk, Spira, Pitchenik, & Worobey, 2007), although it was not noticed at first. This was likely a result of its relatively long incubation period of up to 10 years or more before the emergence of symptoms, and because of the initially low incidence rate. By the time the first reported cases of AIDS were found in large United States cities, the prevalence of HIV infection in some communities had passed 5% (Jaffe, Darrow, Echenberg, O'Malley, Getchell, Kalyanaraman, et al., 1985). The AIDS epidemic officially began on June 5, 1981, when the US Centers for Disease Control and Prevention (CDC) reported unusual clusters of Pneumocystis pneumonia (PCP) in five homosexual men in Los Angeles (Centers for Disease Control, 1981). In the following 18 months, men who would have been classified as being in good health were found to have PCP, as well as other diseases that were common in immunosuppressed patients, in multiple cities in the United States (Centers for Disease Control, 1982). Further reports about gay men occurred in June 1982 in Southern California. The report implied that this disease may be a “sexually transmitted infectious agent” within the gay community (Centers for Disease Control, 1982). Because of this, the illness was called “GRID” which stood for “gay-related immune deficiency” (The New York Times, 1982). Not too long after, health officials recognized that almost half of the individuals with this illness were not homosexual men. The opportunistic infections seen in those individuals with this illness were also seen in people suffering from hemophilia (Centers for Disease Control, 1982), heterosexual individuals who used intravenous drugs, and Haitian immigrants. Thus, the term “Acquired Immune Deficiency Syndrome (AIDS)” was adopted. Current Cure Status Currently there is no cure for AIDS, only treatment through anti-viral medications. Future Cure Obstacles The nature of the HIV is the main obstacle in finding a definitive cure. Specifically, the virus is able to live in bodily “reservoirs”, such as tissues, that the immune system cannot reach. Two of these reservoirs are the brain and lymph nodes. These tissues are considered reservoirs because the HIV drug AZT is found in lower concentrations in those tissues rather than in other areas or tissues in the body. HIV is also able to resist eradication from the human body because of its ability to constantly mutate. The insufficient number of animal models to examine HIV infection restricts the amount and SGU Cures Index 72 scope of research that can be conducted (Johnston & Barre-Sinoussi, 2012). HIV hides within the cytoplasm, a gel-like substance within cells, and/or can become part of the cells’ genetic component, making it extremely difficult for the immune system to detect and destroy the virus (US Department of Health and Human Services - National Institutes of Heath, 2009b). Research Development and Treatment Costs Generally, a rigorous 24-week human study of a potential new medication including numerous tissue biopsies and administration of anti-retroviral drugs costs $15,00020,000 per subject. A non-human primate (NHP) investigation costs $20,000-30,000 per animal (Johnston & Barre-Sinoussi, 2012). Federal funding for AIDS-related research at the National Institutes of Health (NIH) began in 1983. By FY 1988, the amount allocated to the NIH for AIDS-related research reached $448 million. In December 2007, The Consolidated Appropriations Act of 2008 included the transfer of $295 million to the NIH for the Global AIDS Fund (National Institute of Health, 2013). The NIH has allocated the following funds to HIV/AIDS research over the past five years (http://report.nih.gov/categorical_spending.aspx): 2010: $3.407 billion 2011: $3.059 billion 2012: $3.074 billion 2013: $2.898 billion 2014 (estimated): $2.978 billion 2015 (estimated): $3.005 billion HIV Cures: Anti-Retroviral Therapy (ART) Cure Category Achieved, functional Cure Identification/Description Antiretroviral therapy (ART), and Highly Active Antiretroviral Therapy (HAART) consists of administering at least three antiretroviral drugs to HIV patients in combination. There are several classes of antiretroviral drugs: SGU Cures Index 73 (1) Entry/Fusion Inhibitors (e.g., Maraviroc, Enfuvirtide) interfere with binding, fusion, and entry of HIV with the host cell. (2) Reverse Transcriptase Inhibitors (e.g., Zidovudine, Abacavir, Lamivudine, Emtricitabine, Tenofovir, Nevirapine, Efavirenz) inhibit reverse transcription, wherein the genetic code within HIV, an RNA virus, is changed into DNA so that it can integrate with the DNA of the host cell. (3) Integrase Inhibitors (e.g., Elvitegravir, Dolutegravir) inhibit the viral enzyme integrase. This enzyme is crucial for integrating the viral DNA with the DNA of the infected cell. (4) Protease Inhibitors (e.g., Lopinavir, Indinavir, Nelfinavir, Amprenavir, Ritonavir, Darunavir, Atazanavir) block the viral protease enzyme necessary to produce mature virions after budding from the host cell’s membrane. Each of these classes of drugs works to inhibit the spread of HIV by blocking a key step in the viral replication process. Prior to combination therapy, individual drugs were used to block the spread of the virus. However, this lead to rapid antiretroviral drug resistance by HIV, given its very short life cycle (as short as 1.5 days from viral entry into a cell, to replication, release, and entry into other cells) (Perelson, Neumann, Markowitz, Leonard, et al., 1996), and high error rate during transcription. ART defends against resistance by suppressing HIV replication as much as possible, thus reducing the potential pool of spontaneous resistance mutations (Smyth, Davenport, & Mak, 2012). If a mutation that conveys resistance to one of the drugs being taken arises, the other drugs continue to suppress reproduction of that mutation. Cure History In 1987, the FDA approved the first anti-retroviral (ART) drug called AZT (Food and Drug Administration, 2009). In 1996, High Activity Anti-Retroviral Therapy (HAART) was introduced (Bartleet, 2006), which uses a combination of multiple antiretroviral drugs or agents to inhibit or disrupt the life cycle of HIV. HAART increases HIV patients’ lifespan and quality of life by prolonging the ability of the immune system to function properly and impeding opportunistic infections from occurring (Dybul, Fauci, Bartlett, Kaplan, & Pau, 2002). Combination therapy was then formed into fixed dose combinations in the form of one pill. This evolved and progressed during the last fifteen years leading up to the most current development, Stribild, which was approved for the US market in 2012 (Gilead Sciences, 2013). SGU Cures Index 74 Cure Science: Breakthroughs/Obstacles In 1997 the FDA approved the first HAART therapy, which was advertised under the brand name combivir (zidovudine + lamivudine), followed by Trizivir (abacavir + zidovudine + lamivudine) and Kaletra (lopinavir + ritonavir) (Kumar, Rodriguez-French, Thompson, Tashima, Averitt, Wannamaker, et al., 2006). Several drugs have been made since then, most recently, Stribild (elvitegravir + cobicistat + tenofovir/emtricitabine) from Gilead Sciences (Brinson, 2013). The success of these combination drugs critically relies on strict adherence to dosage and timing by the patients who are taking them (Bartleet, 2013). To address this issue, pharmaceutical companies have developed fixed dose combination drugs, which combine several different drugs into a single pill (Sungkanuparph, Manosuth, Kiertiburanakul, Piyavong, Chumpathat, & Chantratita, 2007). Cure Science: Future Obstacles There is an array of components that limit the effectiveness and the cure possibility of chronic suppressive therapy such as HAART. To start, HAART can cost as much as $70,000 per year (Yazdanpanah, 2004), thereby severely limiting universal access. Individuals infected with HIV in impoverished countries have much lower chances of receiving treatment. Secondly, being off a HAART regimen for even a short period of time can greatly increase the risk of an opportunistic infection and, consequently, death and morbidity (Davey, Bhat, Yoder, Chun, Metcalf, Dewar, et al., 1999). As such, to provide maximum effectiveness, patients must follow a HAART therapy regiment for their entire lives, further increasing the risk of non-adherence, at some point, to the very strict medication schedule (Sungkanuparph et al., 2007). Thirdly, suboptimal penetration of ART into the central nervous system may allow low-level replication of HIV, possibly neuropathology, and the potential formation of a viral reservoir. Fourthly, the side effects of HAART may be severe, and even moderate toxicities have long-term effects. Fifthly, HAART is not a definitive cure for HIV. Even those individuals who closely follow the HAART regimen and receive the latest anti-retroviral drugs may develop resistance and succumb to opportunistic infections (Richman, Margolis, Delaney, Greene, Hazuda, & Pomerantz, 2009). As such, there are a number of obstacles, the most critical of which is the lack of a definitive cure in the form of a vaccine, that have yet to be overcome. While a preventive vaccine would not help those already infected with HIV, it would provide a cure for those not yet infected with the virus. SGU Cures Index 75 Another example of further studies into finding a cure for HIV/AIDS is with the use of radioimmunotherapy (RIT). HAART does not kill the cells infected with HIV, nor does it kill the virus. Rather, it reduces the speed at which the virus reproduces and therefore, keeps the viral load low for the infected individual being treated with HAART therapy. Researchers also believe that given the mechanism of HAART, which reduces replication of HIV, reservoirs may exist within the individual, which would then inhibit the success of a cure. RIT uses “monoclonal” antibodies, which are cloned cells engaged from the immune system to find and counteract antigens (foreign material such as bacteria and viruses that trigger the reaction of the body’s immune system), and, once it finds the antigen, marks it radioactively. This then signals where the radiation will be administered when injected into the patient. Antibodies bind with the infected cell(s) and destroy them using radiation. Initial assessments have shown that RIT destroys HIV infected immune cells that were being treated with HAART, thereby reducing the HIV load in the blood samples collected to a level where HIV was undetected. Additionally, RIT did not negatively affect neighboring healthy cells. Researchers have also observed that RIT was capable of passing the blood-brain barrier and was able to attack infected cells within the central nervous system. Clinical RIT trials with HIV infected individuals are currently underway (Radiological Society of North America, 2013). Number of Patients Currently Being Treatment 6.65 million HIV-infected people were estimated to be receiving ART globally in 2010, which is a significant increase over the 5.26 million HIV patients receiving ART globally in 2009 (UNAIDS, 2011). Finding an exact number of infected individuals who are being treated is difficult given a number of factors. These include the slow incubation rate of the virus, resulting in a potentially large number of infected people who do not know they are infected, and individuals whose CD4+ counts are not at the required minimum level to begin antiretroviral therapy (recently raised from 350 to 500 cells/ml) (Rathbun, Liedtke, Lockhart, & Greenfield, 2013). The WHO reported that 373,733 people were receiving antiretroviral therapy in the US in 2012 (World Health Organization, 2014). It has also been reported that 426,590 people were receiving antiretroviral therapy in the US in 2011 (Quandl, 2014). Individuals who do not have health insurance and/or cannot afford the costs of antiretroviral therapy may not be receiving treatment unless they qualify for a government-run program such as Medicare, Medicaid. There are many government-run SGU Cures Index 76 programs that assist individuals infected with HIV, such as The Ryan White Program. This program is also associated with the AIDS Drug Assistance Program (ADAP), which is dedicated to individuals who cannot afford antiretroviral treatment costs. In 2010, the National HIV/AIDS Strategy observed that a third of the population in the US who tested positive for HIV were aware that they were infected but were not being treated. This may be because these individuals were not being routinely tested for their viral load count in order to monitor their health. Number of Patients Requiring Treatment In 2010, 14.2 million HIV-infected people were estimated to need ART globally, which was approximately 1 million more than the 13.3 million HIV-infected people needing ART globally in 2009 (UNAIDS, 2011). Approximately 1,148,200 people aged 13 and older were living with HIV in the US in 2009, 16% of who did not know they were infected or had not been diagnosed (Centers for Disease Control, 2014). Further, an estimated 47,500 new HIV infections occurred in the US in 2010 (Centers for Disease Control, 2014), and 49,273 new HIV infections occurred in the US in 2011 (Centers for Disease Control and Prevention, 2011). Many, if not all, of these individuals will eventually require ongoing ART/HAART to control the virus and prevent the inset of AIDS. Impact of Treatment on Years of Potential Life Lost (YPLL) As ART/HAART has advanced, 11.8 years of life have been gained on average per patient, between 1996 and 2005 (Harrison, Song, & Zhang, 2010). Impact of treatment on Disability-adjusted Life Years (DALY/QALY) Compared to no therapy, anti-retroviral drugs have shown an increase of 1.38 QALYs. The cost per quality-adjusted year gained was $23,000 as compared to no therapy. The cost-effectiveness ratio for therapy spanned from $13,000-23,000 per QALY gained (Freedberg, Losina, Weinstein, Paltiel, Cohen, Seage, et al., 2001). Cost of Treatment Using ART Per Patient ART/HAART costs approximately $10,000-15,000 per person per year in high-income countries. However, if the patient is resistant to multiple anti-retroviral drugs or is on recently developed therapeutics, the cost can be higher than $70,000 per individual per SGU Cures Index 77 year (Yazdanpanah, 2004). ART/HAART costs approximately $350 per person per year in low-income countries (Yazdanpanah, 2004). Cost of Lifetime Treatment using ART The full lifetime per-person cost associated with HIV infection is $354,100 (discounted). Around 73% of this cost is related to anti-retroviral treatment (Schackman, Gebo, Walensky, Losina, Muccio, Sax, et al., 2006). Economic Impact - Value of Life Added It is estimated that on average, 11.8 years of life has been gained amongst HIV patients between 1996-2005 (Harrison, Song, & Zhang, 2010). While a number of variables may have been involved in this increase in lifespan, the generation of current and more effective anti-retroviral drugs has without a doubt had a significant effect on the extent of survival among individuals infected with HIV. If 1,148,200 Americans diagnosed with HIV in 2009 gained 11.8 years of life as a result of advances in ART/HAART, a total of 135,478,760 years of life were gained within this patient population, giving a VMRR of $1.41 trillion, mostly attributable to ART/HAART. HIV Cures - Vaccination Cure Category Potential, Definitive Cure History Currently, no effective vaccine has been developed against HIV. Attempts to formulate a vaccine began soon after HIV was discovered in the early 1980s. Soon, researchers realized that a vaccine would be difficult to achieve given the virus’ ability to follow a very rapid process of mutation and its ability to remain latent within cell DNA reservoirs that are not easily accessed by the immune system (Johnston & Fauci, 2007). In 2005, a favorable vaccine efficacy trial began under the pharmaceutical company Merck & Co., which was based on an adenovirus synthetically altered to consist of gag, pol, and nef proteins found in HIV. Unfortunately, this vaccine demonstrated no protective effects, when compared to a placebo group, and some studies even indicated that the vaccine may have increased the risk of infection (Sekaly, 2008). In 2009 in Thailand, results for SGU Cures Index 78 RV 144 showed some favorable results and helped to model future research for a successful HIV vaccine (Haynes, Liao, & Tomaras, 2010). Promising advances were achieved in the treatment of HIV in Mississippi when a newborn infant was treated aggressively with HIV medications soon after birth. Routine blood tests continued to indicate that the infant was testing negative for HIV after three years (McNiel, 2014). A second infant was treated aggressively four hours after birth with AZT, 3TC, and nevirapine at high doses, and now tests negative for HIV in blood tests (McNiel, 2014). Unfortunately, the Mississippi infant (now four years old) recently showed signs of HIV in blood tests. After being off of antiretroviral treatment for two years, doctors have started her back on antiretroviral drugs with no plans of removing her from treatment for the rest of her life (CNN Wire, 2014). Cure Science/Cure Obstacles There are several vaccines currently in various phases of clinical trials. Phase I trials: The main focus at this level is on envelope proteins. Seen to be safe and immunogenic, a vaccine usually induces neutralizing antibodies, but on rare occasions may induce CD8+ T-cell toxicity. It is also exceedingly difficult to retain the necessarily high anti-gp120 antibody titers, which is the most easily and commonly targeted viral envelope protein, to neutralize an exposure to HIV. Additional phase I vaccines include: peptides, lipopeptides, DNA, attenuated salmonella vector, cytotoxic T-cell response and many others (Johnston & Fauci, 2007). Lastly, Spanish researchers in 2011 published data of their HIV vaccine MVA-B, which produced optimistic results (Gomez, Najera, Perdiguero, Garcia-Arriaza, Sorzano, Jimenez, et al., 2011). Phase II trials: In December 2004, a phase II clinical trial was co-funded by the National Institute of Allergy and Infectious Disease (NIAID) and by Merck & Co. This vaccine, MRKAd5 (HVTN 502 STEP study), was designed to provoke HIV-specific cellular immunity. The vaccine was deemed safe since it lacked negative side effects and increased cellular immune response against HIV to more than half of the participants (Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization, 2005). MRKAd5 includes a debilitated adenovirus that works as a carrier of three subtype B HIV genes. In September 2007, the trial for MRKAd5 was discontinued after being linked to an increased risk of HIV infection in some of the individuals who took the vaccine (National Institute of Allergy and Infectious Diseases, 2008). SGU Cures Index 79 Phase III trials: AIDSVAX was tested within a procedure called RV144 that began in Thailand with positive results. This RV144 trail in Thailand has been the largest trial yet. Its initial results were encouraging (Harmon, 2009). The vaccine showed evidence of effectiveness in 60 percent of those who received it twelve months after being vaccinated and in 31.2 percent of those vaccinated 42 months post-vaccination (Pitisuttithum, Excler and Kim, 2013.) RV144 provided a roadmap to address particular scientific challenges but has not proven to be sufficiently effective to be rolled out for wide use. Citations Bartleet, J. G. (2006). Ten Years of HAART: Foundation for the Future. Retrieved on July 5, 2014 from: http://www.medscape.org/viewarticle/523119. Bartleet, J. G. (2013). A Guide to Primary Care for People With HIV/AIDS - 2004 Edition.). United States Department of Health and Human Services. Brinson, C. (2013). Stribild, a Single Tablet Regimen for the Treatment of HIV Disease. Combination Products in Therapy. doi:10.1007/s13556-013-0001-y. Centers for Disease Control. (1982a). Opportunistic infections and Kaposi's Sarcoma among Haitians in the United States. Morbidity and Mortality Weekly Report, 31 (26), 353-354; 360-361. Centers for Disease Control. (1982b). Persistent, generalized lymphadenopathy among homosexual males. Morbidity and Mortality Weekly Report, 31 (19), 249-251. Centers for Disease Control. (1982c). Update on Kaposi's sarcoma and opportunistic infections in previously healthy persons - United States. Morbidity and Mortality Weekly Report, 31 (22), 300-301. Centers of Disease Control. (1982d). Pneumocystis carinii pneumonia among persons with hemophilia A. Morbidity and Mortality Weekly Report , 31 (27), 365-367. Centers of Disease Control. (1981). Pneumocystis pneumonia - Los Angeles. Morbidity and Mortality Weekly Report , 30 (21), 250-252. SGU Cures Index 80 Centers for Disease Control. (2011). HIV surveillance Report: Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas. Retrieved July 17, 2014, from: www.cdc.gov/hiv/statistics/basics/. Centers for Disease Control. (2014). HIV/AIDS: HIV Basics. Retrieved July 5, 2014, from: http://www.cdc.gov/hiv/basics/index.html. CNN Wire. (2014). Mississippi baby ‘cured’ of HIV now showing signs of the virus. Retrieved July 17, 2014 from: http://kdvr.com/2014/07/11/mississippi-babycured-of-hiv-is-now-showing-signs-of-the-virus/. Cohen, J. (2006). HAITI: Making Headway Under Hellacious Circumstances. Science, 313(5786), 470b-473b. Davey, R. T., Bhat, N., Yoder, C., Chun, T-W., Metcalf, J. A., Dewar, R., & Miller, K. D. (1999). HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proceedings of the National Academy of Sciences, 96(26), 15109–15114. Douek, D. C., Roederer, M., & Koup, R. A. (2009). Emerging Concepts in the Immunopathogenesis of AIDS. Annual Review of Medicine, 60(1), 471–484. doi:10.1146/annurev.med.60.041807.123549. Dybul, M., Fauci, A., Bartlett, J., Kaplan, J., & Pau. (2002). Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. Annals of Internal Medicine, 137(5 Pt 2), 381–433. Freedberg, K. A., Losina, E., Weinstein, M. C., Paltiel, A. D., Cohen, C. J., Seage, G. R., & Goldie, S. J. (2001). The cost effectiveness of combination antiretroviral therapy for HIV disease. New England Journal of Medicine, 344(11), 824–831. Gilbert, M. T., Rambaut, A., Wlasiuk, G., Spira, T. J., Pitchenik, A. E., & Worobey, M. (2007). The emergence of HIV/AIDS in the Americas and beyond. Proceedings of the National Academy of Sciences, 104(47), 18566-18570. Gilead Sciences. (2013). Highlights of prescribing information. Retrieved April 11, 2014 from: www.gilead.com/~/media/Files/pdfs/medicines/hiv/stribild_pi.pdf SGU Cures Index 81 Gomez, C. E., Najera, J. L., Perdiguero, B., Garcia-Arriaza, J., Sorzano, C. O. S., Jimenez, V., Muñoz-Fernández, M. A., López Bernaldo de Quirós, J. C., Guardo, A. C., García, F., Gatell J. M., Plana, M., Esteban, M. (2011). The HIV/AIDS vaccine candidate MVA-B administered as a single immunogen in humans triggers robust, polyfunctional, and selective effector memory T cell responses to HIV-1 antigens. Journal of Virology, 85(21), 11468–11478. doi:10.1128/JVI.05165-11. Harmon, K. (2009). Renewed hope. Scientific American , 302(1), 15-16. Harrison, K. M., Song, R., & Zhang, X. (2010). Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. Journal of Acquired Immune Deficiency Syndromes, 53(1), 124–130. Haynes, B. F., Liao, H.-X., & Tomaras, G. D. (2010). Is developing an HIV-1 vaccine possible? Current Opinion in HIV and AIDS, 5(5), 362–367. doi:10.1097/COH.0b013e32833d2e90. Food and Drug Administration. (2009). HIV/AIDS Historical Time Line 1981-1990. Retrieved on July 5, 2014 from: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAI DSActivities/ucm151074.htm. Jaffe, H. W., Darrow, W. W., Echenberg, D. F., O'Malley, P. M., Getchell, J. P., Kalyanaraman, V. S., Byers, R.H., Drennan, D.P., Braff, E.H., Curran, J.W., Francis, D.P., (1985). The acquired immunodeficiency syndrome in a cohort of homosexual men. A six-year-follow-up study. Annals of Internal Medicine , 103(2), 210-214. Johnston, M. I., & Fauci, A. S. (2007). An HIV vaccine—evolving concepts. New England Journal of Medicine, 356(20), 2073–2081. Johnston, R., & Barré-Sinoussi, F. (2012). Controversies in HIV cure research. Journal of the International AIDS Society, 15(1), 16. Joint United Nations Programme on HIV/AIDS and World Health Organization. (2005). AIDS Epidemic Update. Retrieved April 17, 2014, from: www.who.int/hiv/epiupdate2005_en.pdf. SGU Cures Index 82 Kalish, M. L., Wolfe, N. D., Ndongmo, C. B., McNicholl, J., Robbins, K. E., Aidoo, M., Fonjungo, P.N., Alemnji, G., Zeh, C., Djoko, C.F., Mpoudi-Ngole, E.; Burke, D.S., Folks, T.M. (2005). Central African hunters exposed to simian immunodeficiency virus. Emerging Infectious Diseases , 11(12), 1928-1930. Kumar, P. N., Rodriguez-French, A., Thompson, M. A., Tashima, K. T., Averitt, D., Wannamaker, P. G., Williams, V. C., Shaefer, M. S., Pakes, G. E., Pappa, K. A., ESS40002 Study Team (2006). A prospective, 96-week study of the impact of Trizivir®, Combivir®/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters and efficacy in antiretroviral-naive patients: Effect of sex and ethnicity. HIV medicine, 7(2), 85–98. McNiel, D. (2014, March 5). Early Treatment Is Found to Clear H.I.V. in a 2nd Baby. The New York Times, p. A1. National Institute of Allergy and Infectious Diseases (2008). Questions and Answers: HVTN 502 and HVTN 503 HIV Vaccine Clinical Trials. Retrieved on July 21, 2014 from: http://www.niaid.nih.gov/news/QA/Pages/step_qa.aspx. National Institute of Health. (2013a). Office of AIDS Research, NIH AIDS Research Budget. Retrieved on July 5, 2014 from: http://www.oar.nih.gov/budget/. National Institutes of Health. (2013b). The NIH Almanac. Retrieved April 10, 2014, from: www.nih.gov/about/almanac/historical/chronology_of_events.htm#nineteeneight y. Perelson, A. S., Neumann, A. U., Markowitz, M., Leonard, J. M., et al. (1996). HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science, 271(5255), 1582-1586. Pitisuttithum, P., Excler, J., & Kim, J. (2013) Beyond RV144 Efficacy Results: An Update. Procedia in Vaccionology, 7, 49-56. Plantier, J., Leoz, M., Dickerson, J., DeOlivera, F., Cordonnier, F., Lemee, V., Damond, F., Robertson, D.L., Simon, F. (2009). A new human immunodeficiency virus derived from gorillas. Nature Medicine, 15(8), 871-872 Quandl (2014). Reported number of people receiving antiretroviral therapy by country: United States. Retrieved on June 5, 2014 from: SGU Cures Index 83 http://www.quandl.com/WHO/788_USA-Reported-number-of-people-receivingantiretroviral-therapy-United-States-of-America. Radiological Society of North America. (2013, December 3). New research shows promise for possible HIV cure. Retrieved April 17, 2014, from: www.sciencedaily.com/releases/2013/12/131203091601.htm. Richman, D. D., Margolis, D. M., Delaney, M., Greene, W. C., Hazuda, D., & Pomerantz, R. J. (2009). The challenge of finding a cure for HIV infection. Science, 323(5919), 1304–1307. Schackman, B. R., Gebo, K. A., Walensky, R. P., Losina, E., Muccio, T., Sax, P. E., et al. (2006). The lifetime cost of current human immunodeficiency virus care in the United States. Medical care, 44(11), 990–997. Sekaly, R.-P. (2008). The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? Journal of Experimental Medicine, 205(1), 7–12. doi:10.1084/jem.20072681 Smyth, R. P., Davenport, M. P., & Mak, J. (2012). The origin of genetic diversity in HIV-1. Virus Research, 169(2), 415-429. Sungkanuparph, S., Manosuth, W., Kiertiburanakul, S., Piyavong, B., Chumpathat, N., & Chantratita, W. (2007). Options for a second-line antiretroviral regimen for HIV type 1-infected patients whose initial regimen of a fixed-dose combination of stavudine, lamivudine, and nevirapine fails. Clinical infectious diseases, 44(3), 447–452. The New York Times. (1982, June 18). Clue Found on Homosexuals' Precancer Syndrome. US Department of Health & Human Services. (2012). What is HIV/AIDS. Retrieved April 7, 2014, from: www.aids.gov/hiv-aids-basics/hiv-aids-101/what-is-hiv-aids/. US Department of Health and Human Services - National Institutes of Health. (2009a). Testing and Diagnosis. Retrieved April 7, 2014 from: www.niaid.nih.gov/topic/HIVAIDS/Understanding/Pages/diagnosis.aspx SGU Cures Index 84 US Department of Health and Human Services - National Institutes of Heath. (2009b). HIV Evolves to Evade the Immune System. Retrieved April 7, 2014 from: www.niaid.nih.gov/topics/hivaids/understanding/biology/Pages/evolves.aspx US Department of Health and Human Services - National institute of Health. (2012). HIV/AIDS - Biology of HIV. Retrieved April 7, 2014 from: www.niaid.nih.gov/topics/hivaids/understanding/biology/Pages/biology.aspx UNAIDS. (2011). Data Tables. Retrieved from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublic ation/2011/JC2225_UNAIDS_datatables_en.pdf. World Health Organization (2014). HIV / AIDS: Data and Statistics. Retrieved on July 5, 2014 from: http://www.who.int/hiv/data/en/. World Health Organization. (2008). The global burden of disease: 2004 update. Retrieved on July 5, 2014 from: www.who.int/evidence/bod. Worobey, M., Telfer, P., Souquière, S., Hunter, M., Coleman, C. A., Metzger, M. J., Reed, P., Makuwa, M., Hearn, G., Honarvar, S., Roques, P., Apetrei, C., Kazanji, M., Marx, P. A. (2010)). Island biogeography reveals the deep history of SIV. Science, 329(5998), 1487. Yazdanpanah, Y. (2004). Costs associated with combination antiretroviral therapy in HIVinfected patients. Journal of Antimicrobial Chemotherapy, 53(4), 558–561. doi:10.1093/jac/dkh142. SGU Cures Index 85 INFLUENZA Zaina Akeh Disease Category Infectious Disease Description, Diagnosis, and Background Influenza is a contagious virus that is classified as a respiratory illness. It is one of many viral respiratory infections, a class that includes the common cold. The onset of the illness is sudden and symptoms/complications can range from mild to fatal. Older individuals, young children, pregnant women and people with chronic illnesses, mainly respiratory related, are at high risk for complications (Centers for Disease Control and Prevention, 2014a). Morbidity rates are elevated for these high-risk groups (Thompson, Shay, Weintraub, Brammer, Cox, Anderson, & Fukuda, 2003). Understanding the etiology of influenza will help avoid the potentially devastating consequences that are associated with the virus. Incubation of the virus can last from one to four days, with an average period of about two days. Infected adults may be able to infect other people from one day prior to symptom onset and up to five to seven days after becoming sick. Children may pass the virus on to others for longer than seven days. Symptoms start one to four days after the virus enters the body (Centers for Disease Control and Prevention, 2014a). Common influenza symptoms include sore throat, headache, runny or stuffy nose and fatigue. Other symptoms include fever, chills, vomiting, and diarrhea (Centers for Disease Control and Prevention, 2014a). Complications associated with the virus include bacterial pneumonia, dehydration, fever, congestive heart failure, asthma or diabetes (Centers for Disease Control and Prevention, 2014a). The diagnosis of influenza begins by identifying the symptoms, followed by a more definitive diagnosis that can detect the virus or a rise in antibody titer (a measurement of how many antibodies are found in a blood sample) via swab analysis (Couch, 1996). The specimens are normally collected from the throat or nose by culture. However, laboratory testing is generally reserved for hospitalized individuals or those individuals who may be at high risk for influenza related complications. SGU Cures Index 86 The influenza viruses originate from the family of RNA viruses known as Orthomyxoviruses. Type A and Type B influenza viruses are the two specifically known to be infectious in humans. In types A and B the hemagglutinin and neuraminidase (which are antigen proteins found on the surface of the virus) undergo genetic variation (antigenic shift). This genetic variation is the basis for the emergence of new strains. Changes in the hemagglutinin and neuraminidase surface antigens are responsible for the appearance of novel strains that require annual reformulation of the influenza vaccine and annual vaccination with that year's vaccine. These changes also allow the newly emerging variant to evade the host’s immune system, thus causing new infections/reinfections (Couch, 1996). Disease Etiology (Cause) Influenza A and B viruses are transmitted mainly within droplets and aerosols, which originate from the respiratory secretions of infected individuals through acts such as sneezing (World Health Organization, 2012). Further, the influenza virus can infect both humans and animals, making it a zoonotic disease (i.e., a disease caused by bacteria, viruses, parasites, and fungi that are carried by animals and insects) (Centers for Disease Control and Prevention, 2014b). Antigenic shift allows flu viruses to move from animals to humans, leading to potentially novel forms of influenza circulating among the population (US Department of Health and Human Services, 2014a). Thus, the closer humans live to animals and the more contact they have, the greater the risk for spread of the flu virus. Antigenic shift can happen in three ways: 1. Genetic mixing: i. A duck or other aquatic bird passes a bird flu strain to an intermediate host such as a chicken or pig. ii. A person passes a human strain of influenza A to the same chicken or pig. iii. When the viruses infect the same cell, the genes from the bird strain mix with genes from the human strain, making a novel strain. iv. The novel strain can spread from the intermediate host to humans. 2. A bird strain of flu can jump directly from a duck or other aquatic bird to humans. 3. A bird strain of flu can jump directly from a duck or other aquatic bird to an intermediate animal host, such as a chicken or pig, and then to humans. SGU Cures Index 87 The conventional nomenclature for identification and classification of the influenza virus was established by the World Health Organization in 1979 (Centers for Disease Control and Prevention, 2014a), and uses the following criteria to name each strain: 1. The antigenic type (e.g., A, B, C) 2. The host of origin (e.g., swine, equine, chicken, etc. For human-origin viruses, no host of origin designation is given.) 3. Geographical origin (e.g., Denver, Taiwan, etc.) 4. Strain number (e.g., 15, 7, etc.) 5. Year of isolation (e.g., 2009, etc.) 6. For influenza A viruses, the hemagglutinin and neuraminidase antigen description is in parentheses (e.g., (H1N1), (H5N1)) For example, A/environment/Hong Kong/156/1997 (H5N1) is the vaccine formulation for the influenza type A virus isolated in Hong Kong, strain 156, isolated in 1997. Given that the influenza virus is constantly changing via antigenic shift, an encounter with the virus does not necessarily prime the immune system for further possible exposure in the future. Evolution is the driving force behind the novel influenza viruses that emerge. Various mutations involving the surface major glycoprotein hemagglutinin and genome RNA encoding hemagglutinin are responsible for the emergence of new strains. New subtypes may arise when the surface proteins of an antigen present with completely original surface protein receptors that are derived from hemagglutinin or neuraminidase genes from other species. The antigenic shift that occurs after coinfection within different species such as birds, pigs, and humans results in viruses with new receptors. Since these new receptor subtypes have not yet been introduced into the human population, there are no vaccinations to effectively and immediately protect against new strains. The inability to provide protection against mutated strains leaves the population vulnerable to the spread of the virus, which can lead to seasonal epidemics (Lee, Kim, Ko, Lee, Kim, Kwon et al., 2014). Current Prevalence/Incidence More than 30 million patients visit health professionals with influenza-like-illnesses (Centers for Disease Control and Prevention, 2014a), and approximately 5-20% of the US population will contract the flu annually (Centers for Disease Control and Prevention, 2014a). Of the individuals who contract the flu, more than 200,000 are hospitalized, and an estimated 3,300-49,000 of individuals will die annually as a result of influenza. However, these numbers are underestimates of the actual influenza related mortality because many deaths are caused by other secondary complications listed as cause of SGU Cures Index 88 death on death certificates, rather than influenza (Thompson et al., 2003). In 2003-2004, the CDC compiled information from 40 state health departments and reported that there were 153 influenza-associated deaths among children during that year. The median age of the children was three years, and 96 of them (63%) were younger than five years old. Bacterial co-infections were identified in 24 of the 102 children tested (24%). Thirty-three percent of the children had an underlying condition recognized to increase the risk of influenza-related complications, and 20 percent had other chronic conditions; 47 percent were previously healthy. The mortality rate was highest among children younger than six months of age (0.88 per 100,000 children; 95% confidence interval: 0.52 to 1.39 per 100,000) (Centers for Disease Control and Prevention, 2014a). Estimated Undiagnosed/At-Risk People commonly confuse influenza symptoms with that of a severe cold and do not receive a proper diagnosis. Thus, the actual numbers for individuals who are at risk or go undiagnosed are likely significantly higher than the documented influenza statistics. While influenza is common and recovery normal, there are several groups at higher risk of severe complications. Vaccination is particularly important for these high-risk individuals. This includes all children aged 6 through 59 months; all persons aged 50+ years; adults and children who have chronic pulmonary (including asthma), cardiovascular (except isolated hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus); persons who have immunosuppression (including immunosuppression caused by medications or by HIV infection); women who are or will be pregnant during the influenza season; children and adolescents (aged 6 months through 18 years) who are receiving long-term aspirin therapy and who might be at risk for experiencing Reye's syndrome after influenza virus infection; residents of nursing homes and other long-term care facilities; American Indians/Alaska Natives; and persons who are morbidly obese (BMI ≥40) (Centers for Disease Control and Prevention, 2014a). In 1999 approximately 15% of the total US population was considered high-risk influenza individuals. These groups accounted for approximately 85% of all influenza related fatalities. The majority of deaths (90%) occurred in the elderly aged 65 and over (Johns Hopkins Medicine, 2014). High-risk groups also accounted for 38% of all hospitalizations and 20% of all outpatient visits (Meltzer, Cox, & Fukuda, 1999). These percentages consist only of the individuals who presented with symptoms and were diagnosed appropriately. Disease Impact - Years of Potential Life Lost (YPLL) SGU Cures Index 89 Many deaths associated with influenza infections occur from secondary infections such as bacterial pneumonia or complications of chronic conditions such as congestive heart failure and chronic obstructive pulmonary disease (Bresee, Reed, Kim, Finelli, Fry, Chaves, & Santibanez, 2013). Therefore mortality surveillance rates incompletely reflect the severity of influenza infections. The most recent seasonal strain of influenza affecting US populations is the H1N1 strain. The average age of people in the US who died from H1N1 from April to July of 2009 was 40. The median age of death for this time period was 43. From September to October of 2009, the average age of people in the US who died from H1N1 was 41, and the median age was 45 (Centers for Disease Control and Prevention, 2014a). From 1976 to 2007, annual deaths from influenza ranged from approximately 3,300 to 49,000 per annum. Taking the US life expectancy in 2010 (78.7 years) minus the higher estimated median age at death from influenza in 2009 (45 years) gives 33.7 years of life lost. If we then multiply that figure by an approximate midrange of individuals who died of influenza annually from 1976 to 2007 (22,850), we estimate that about 770,045 life years are lost to influenza annually in the US. This is slightly higher than the estimated 610,660 undiscounted life-years lost as a result of the annual influenza epidemic (Centers for Disease Control and Prevention, 2014a). However, this estimate was based on 2003 data, so our value – based on 2009/10 data points, should be higher given an increase in population of about 20 million people during that period. Disease Impact – Disability Adjusted Life Years (DALY) According to the World Heath organization (2009), 114 DALYs per 100,000 people were lost to upper and lower respiratory infectious diseases, which includes influenza among any other viruses that can also cause flu-like symptoms, in the US in 2004. Given a total US population of 292,810,000 in 2004, that equates to 333,803 total DALYs lost to upper and lower respiratory infections that year. The DALYs lost are significantly lower than YPLL due to the fact that the results of influenza either result in death or complete recovery. Cases in which individuals suffer from chronic complications due to the influenza virus are rare in comparison to other diseases/infections. History of the Disease and Breakthroughs Infections disease outbreaks have had a significant public health impact and have been well recorded throughout history. Influenza has been a significant contributor to this SGU Cures Index 90 impact. The first outbreaks to be identified as influenza were recorded in 1918, even though the cause of the disease was not well understood at the time. It was not until Pasteur and Koch postulated the microbiological theory of disease in the 1860s - 1870s, that the path was laid to understand influenza as a microbiologically caused disease that is spread among the population. Following the discovery that viruses cause influenza, the first attempts at vaccinations were made in 1933 by Danish researchers. Novel influenza viruses that emerge through antigenic shift and lack a proper vaccination are often classified as causes of pandemics, based on the severity of the outbreak. A pandemic is an epidemic that has spread over several countries or continents, usually affecting a large number of people. An epidemic refers to an increase, often sudden, in the number of cases of a disease above what is normally expected in that population in that area. Three global influenza pandemics occurred during the 20th century, all of which were caused by H1N1 subtype viruses. The 1918 pandemic, known as the “Spanish flu” was the most severe (World Health Organization, 2012). During that pandemic, 20 to 40 percent of the world's population fell sick, and an estimated 40–60 million people died worldwide (although some estimates are as high as 100 million people), and 500,000–675,000 people died in the United States (US Department of Health and Human Services, 2014b; Thomas & Noppenberger, 2007). The influenza pandemic of 1957-58 was called the Asian flu. The illness was relatively mild compared to the Spanish flu, resulting in fewer deaths, at an estimated 2 million worldwide. Studies show that the virus responsible for this pandemic arose by genetic reassortment of a bird virus (US Department of Health and Human Services, 2014a). The 1968-70 pandemic or Hong Kong flu was also relatively mild compared to the Spanish flu. It affected mainly the elderly and is thought to have caused about 1 million deaths worldwide (Australian Office of Health Protection, 2008). A new H1N1 influenza virus derived from a combination of human, swine and avian strains was initially reported in April 2009 in Mexico and subsequently spread around the world. The 2009 pandemic was not the same as seasonal influenza, which is normally easily contained, and is an excellent example of how fast a new pandemic virus can spread in the human population once it acquires the ability to transmit among humans (Lee et al., 2014). Current Cure Status The annual influenza vaccination is the most efficient option for influenza prevention and it is now recommended for all individuals’ aged 6 months old and up in the US. All children aged 6 months to 8 years who are recommended for 2 doses should receive SGU Cures Index 91 their first dose as soon as possible after vaccine becomes available; these children should then receive the second dose no more than four weeks later (Centers for Disease Control and Prevention, 2014a). Prompt treatment with influenza antiviral medications can reduce the risk for severe illness and death among persons at increased risk for influenza or who are hospitalized with suspected or confirmed influenza (Bresee et al, 2013). Ideally these drugs should be administered within 48 hours of onset of symptoms in the disease process. Research, Development and Treatment Costs Vaccination costs increase linearly with vaccination coverage; however, the number of additional cases averted decreases with increasing coverage. The cost of averting one additional case of influenza was $9,879 when coverage increased from 60% to 70% (Ibuka, Paltiel, & Galvani, 2012). At vaccination coverage up to 40%, the medical cost savings from reduced influenza infection offset the rising costs of increased coverage. Total costs are minimized at 46% vaccination coverage (Ibuka, Paltiel, & Galvani, 2012). Seeing how symptoms of influenza are similar to those of the common cold it is important to accurately distinguish one form another. The laboratory testing, as stated before, is normally reserved for patients who are either hospitalized or at high risk for complications. When individuals who are not infected with the virus do undergo swab analysis or throat/nose cultures that need to be sent to a laboratory for diagnosis it creates an economic burden. Thus, in 2006, the CDC awarded $11.4 million in contracts to four companies working to develop low-cost, rapid diagnostic influenza tests (Centers for Disease Control and Prevention, 2014d). Establishing such rapid tests would eliminate the excessive costs of sending specimens to the laboratory for diagnosis. Funding history for National Institutes of Health (NIH) dollars directed toward influenza over the last several years were as follows (National Institutes of Health, 2014) 2015: estimated $312 million 2014: estimated $312 million 2013: $304 million 2012: $251 million 2011: $272 million Influenza Cures: Vaccinations SGU Cures Index 92 Cure Category Achieved, Definitive Cure Identification/Description The annual influenza vaccine is the most efficient option for influenza prevention and it is now recommended for all individuals’ aged 6 months and up in the United States. In an attempt to ensure a measure of efficacy, the antigenic composition of the vaccine is revised twice annually and adjusted to the antigenic characteristics of circulating influenza viruses obtained within the WHO global influenza surveillance and response system (GISRS) (World Health Organization, 2012). In addition to the injection based vaccinations there is a recently licensed nasal spray flu vaccination. Over the counter the nasal-spray flu vaccination is known as FluMist and is also referred to as Live Attenuated Influenza Vaccine (LIAV). The difference between FluMist and the injected vaccinations is that it is composed of weakened live influenza viruses rather than inactivated viruses (Centers for Disease Control and Prevention, 2014a). Cure History The influenza virus was first isolated in 1933, eventually leading to the development of the first generation of live-attenuated vaccines. The first inactivated influenza vaccine was monovalent - that is, designed to protect against only one antigen. In 1942, a bivalent vaccine was produced after the discovery of influenza B. The discovery that influenza viruses mutated, leading to antigenic changes, means that no life-long vaccination exists (Hannoun, 2013). Since 1973, the WHO has issued annual recommendations for the composition of the influenza vaccine based on results from surveillance systems that identify currently circulating strains. In 1978, the first trivalent vaccine included two influenza A strains and one influenza B strain. In the most recent WHO recommendations, it is suggested that a second B strain could be added to give a quadrivalent vaccine. The history of influenza vaccine and the associated technology shows how the vaccine is constantly evolving in order to match the evolution of influenza viruses (Hannoun, 2013). Cure Science: Breakthroughs/Obstacles In an attempt to ensure a measure of efficacy the antigenic composition of the vaccines is revised twice annually and adjusted to the antigenic characteristics of circulating SGU Cures Index 93 influenza viruses obtained within the WHO global influenza surveillance and response system (GISRS) (World Health Organization, 2012). Most vaccines manufactured since the 1970s have consisted of purified virus that is chemically inactivated. Influenza B viruses and the H1 and H3 subtypes of influenza A viruses can cause epidemic infections in the human population. Therefore, current vaccines against influenza epidemics contain two influenza A subtypes (H1N1 and H3N2) and one or two variants of influenza B virus. It is not possible to predict how well the vaccine and circulating strains will be matched in advance of the influenza season, and how this match may affect vaccine effectiveness (Centers for Disease Control and Prevention, 2014a). Research laboratories are working towards establishing live-attenuated vaccine for a broad range of influenza subtypes that have pandemic potential. Five of these vaccine candidates have currently advanced to Phase II of the clinical trial process (National Institute of Allergy and Infectious Diseases, 2014). Another platform for influenza vaccines - Live Attenuated Influenza Vaccine (LAIV) - was developed to overcome the vulnerable aspects of the previously established vaccines. Although the LAIV has the potential to be more effective than inactivated vaccinations, there are risks and restrictions when administering a live vaccination. Anyone with a weak immune system may be compromised by the live viral components of the vaccine. LAIV is administered via nasal spray (FluMist), which is less invasive than a needle. However, LAIV is less effective in adults, and thus it is not approved for use in persons over the age of 50. Currently, five seasonal LAIV backbone strains have reached regulatory approval status. Early animal experimental data suggests that a new class of 'replication-deficient vaccine' could be developed in the more distant future, with the plausibility of combining the contrasting theoretical advantages of both LAIV and the inactivated vaccines (Lee et al, 2014). The CDC is in the process of collaborating with private sector partners to test a new skin patch delivery system, which can be administered by anyone. This technology could be used in place of a traditional hypodermic needle during a pandemic. In the event of a pandemic, the skin patch could be distributed to individuals seeking vaccination, who would not need to travel to doctor’s office or medical station. Thus, it could provide an effective means of vaccinating large numbers of people quickly (Centers for Disease Control and Prevention, 2014a). Cure Science/Future Cure Obstacles and Targets SGU Cures Index 94 Vaccines against seasonal influenza do not offer protection against pandemic viruses, and vaccine efficacy against seasonal viruses is reduced in seasons when the vaccine composition is not a good match for the predominant circulating viruses (Gilbert, 2013). The key is to establish cures that will diminish the vast economic and societal destruction that could potentially be brought on by a pandemic. A highly pathogenic avian virus known as H5N1 has presented itself as a threat to society since it first killed humans in Asia in 1997. This deadly viral strain has posed the greatest risk for a new influenza pandemic over the last few decades. The H5N1 virus is not considered a pandemic virus because it does not transmit efficiently from person to person, and therefore the current H5N1 vaccine is being held in stockpiles rather than being widely used. This stockpiled vaccine aids preparedness efforts in case an H5N1 pandemic virus was to emerge. If the virus were to mutate and cause a pandemic, it is estimated that a sufficient vaccine would not be available for approximately six months after the initial wave of the outbreak (Thomas & Noppenberger, 2007). Another target for new vaccinations is a respiratory disease caused by a novel influenza virus called “H7N9” (the H7N9 flu). Human cases of H7N9 virus infection have been identified in China but there has been no sustained or documented human-to-human transmission in China. Since April 2013, no human cases of H7N9 have been confirmed in the United States. However, public health officials have determined that this virus has the potential to change in ways that could allow it to spread from human to human (Centers for Disease Control and Prevention, 2014a). Number of Patients Currently Being Treated Although individuals may acquire health complications due to influenza, it is uncommon to have individuals who require long-term treatment specifically for influenza. Instead, patients are treated when the onset of the infection occurs, or via vaccination. According to the CDC, between 24.9 and 68.1 percent of the population received an influenza vaccine in the 2012-2013 flu season (Centers for Disease Control and Prevention, 2014d), as broken down by age range below. Using a breakdown of US population by age range (US Census Bureau, 2014) and the given flu vaccination percent coverage by age groups, we estimate the number of Americans who received the flu vaccine in each age group during the 2012-13 flu season: • 56.6 percent of children 6 months to 17 years (41.945 million) • 31.1 percent of adults 18-49 years (41.322 million) • 45.1 percent of adults 50-64 years (27.215 million) SGU Cures Index 95 66.2 percent of adults 65 years (27.478 million) By summing these figures, we estimate that 137.960 million people, or 44.7 percent of the US population received the influenza vaccine in 2012-13. In line with this estimate, the CDC reports that 139.8 million people (95% CI 138.3 – 141.5 million), or 45 percent of the US population were vaccinated against seasonal flu between July 2012 and May 2013 among the civilian, non-institutionalized US population (Centers for Disease Control and Prevention, 2014d). These estimates do not include military or institutionalized persons who were vaccinated and do not count second doses given to children. • Number of People Requiring Treatment Given that only 45 percent of the US population received an influenza vaccine in 201213, approximately 170 million people still require influenza vaccine if 100% coverage is desired. If targeted vaccinations are deemed more critical, 46.3 percent of adults aged 50 years and over – 47.16 million individuals considered high risk – still require an annual influenza vaccine. Further, if 56.6 percent of children aged 6 months to 5 years old – another high risk group – receive a vaccine, 43.4 percent, or approximately 8.73 million children, still require and annual influenza vaccine. Finally, if the target vaccination rate is based on maximal health return on invested dollar, approximately 46% vaccination coverage should be achieved (Ibuka, Paltiel, & Galvani, 2012). Thus, an additional 1 percent of the US population – about 3 million people – should be receiving an annual influenza vaccine. These additional vaccinations would likely have maximal impact if targeted toward those in the high-risk groups who are not currently receiving the annual vaccine. The CDC suggests a target vaccination rate of 70% for persons 6 months through 17 years and 18 years or older (US Department of Health and Human Services, 2014d). Impact of Treatment on Years of Potential Life Lost (YPLL) From 1976 to 2007, annual deaths from influenza ranged from approximately 3,300 to 49,000 per annum. Taking the US life expectancy in 2010 (78.7 years) minus the higher estimated median age at death from influenza in 2009 (45 years) gives 33.7 years of life lost. If we then multiply that figure by an approximate midrange of individuals who died of influenza annually from 1976 to 2007 (22,850), we estimate that about 770,045 life years are lost to influenza annually in the US This is slightly higher than the estimated 610,660 undiscounted life-years lost as a result of the annual influenza epidemic (Centers for Disease Control and Prevention, 2014a). However, this estimate was based SGU Cures Index 96 on 2003 data, so our value – based on 2009/10 data points, should be higher given an increase in population of about 20 million people during that period. In 2013, the CDC published a model quantifying the annual number of influenza illnesses and hospitalizations averted by the influenza vaccination (Bresee et al, 2013). Without vaccination, 58,000 fatal influenza cases were observed, but it was estimated that 10% vaccination coverage reduced the observed cases to 10,000, or 17% of the original 58,000 documented cases. Increasing the vaccination coverage to 20%, 30%, and 40% should theoretically eliminate 36%, 63%, and 95% of the cases, respectively (Ibuka, Paltiel, & Galvani, 2012). If 95% of cases are eliminated with 40% vaccine coverage, 55,100 lives are saved. Impact of Treatment on Disability-adjusted Life Years (DALY/QALY) In 2012, the CDC estimated that annual vaccination resulted in 1.1 to 5 million fewer cases of influenza and 7,700 to 40,400 fewer hospitalizations annually during the 2005– 2011 influenza seasons (Ibuka, Paltiel, & Galvani, 2012). Recent studies from the CDC reveal that the influenza vaccine can reduce the risk of flu illness by about 60 percent among the overall population during seasons when most circulating flu viruses are similar to the viruses the flu vaccine is designed to protect against (Centers for Disease Control and Prevention, 2014a). Average Annual Total/Lifetime Cost of Vaccinations The following vaccination prices are from the CDC’s Influenza Vaccine Price List (all prices are for one vaccine dose without preservatives) (Centers for Disease Control and Prevention, 2014a): • 6 months and older $12.22 • 36 months and older $12.03 • 9 years and older $7.29 • 18 years and older $9.76 Given the current distribution of vaccine coverage among the US population, as described in the “Number of patients being treated currently” section, by the costs of vaccination within these age groups, we estimate that cost of vaccinating the US population, at the current 45% coverage rate, is $1.367 billion per annum, broken down as such: • Age 9 years and under population 40.526 million x 56.6% vaccination rate x $12.125 cost = $278,119,807 SGU Cures Index 97 • Age 10 to 17 population 33.582 million x 56.6% vaccination rate x $7.29 cost = $138,564,033 • Age 18 and older population 234.719 million x 41.5% average vaccination rate x $9.76 cost = $950,705,838 The current life expectancy of a US citizen is 78.7 years (Centers for Disease Control and Prevention, 2014c). If receiving an annual dose of vaccinations from 6 months, the lifetime cost (in current dollars), after averaging the prices of the different age groups and multiplying by the life expectancy, would be $769.81 per person. Economic impact – Value of Life Added Influenza infections are associated with substantial medical costs, hospitalizations, lost productivity, and thousands of deaths every year in the United States. The probabilistic model created by Molanari, Ortega-Sanchez, Messonnier, Thompson, Wortley, Weintraub, and Bridges (2007) estimated that 30,151,934 annual influenza cases resulted in 21,354 outpatient visits, 3,131 in-patient days, 44,003 lost productivity days, and 611 undiscounted life years lost. Total direct costs and total economic burden, that could have been significantly alleviated by reducing the amount of YPLL, in 2003 dollars was estimated as $10.4 billion [C.I., $4.1-$22.2] and $87.1 billion [C.I., $47.2-$149.5], respectively (Duncan, Taitel, Zhang, Kirkham, 2012). If 95% of cases are eliminated with 40% vaccine coverage, 55,100 lives are saved. If the median age of those individuals is 45, and life expectancy is 78.7, 33.7 years of life are saved, on average, times 55,100 lives = 1,856,870 years of potential life saved annually. If we take the 770,045 life years lost to influenza as calculated earlier, and presume that 95% of those life years could be saved with 40% vaccine coverage, which is about equivalent to our estimate of the current vaccine coverage in the US (39.4%) 731,542 years of potential life are saved in the US annually as a result of the influenza vaccine, with an annual VMRR of $76.222 billion. Economic Impact – Value of Reduction in DALYs/Increase in QALYs Given the fact that influenza usually results in either alleviation of symptoms or death, the impact of treatment on quality-adjusted life years & disability-adjusted life years post-infection are relatively insignificant. However, the quality-adjusted life years are significant in calculating other factors, for instance, values concerning the economic impact. SGU Cures Index 98 Based on 2003 US population data, it was estimated that each year the influenza epidemic results in 3.1 million total days spent in the hospital for all individuals treated for influenza (hospitalized days). Direct medical costs averaged $10.4 billion (95% confidence interval [C.I.], $4.1-$22.2) annually. Projected lost earnings due to illness and loss of life amounted to $16.3 billion (C.I., $8.7-$31.0) annually. The total economic burden of annual influenza epidemics using projected statistical life values amounted to $87.1 billion (C.I., $47.2-$149.5) (Molanari et al., 2007). During influenza season, influenza-like-illness is responsible for 45% of workdays lost and for 49% of low productivity days among working adults aged 50–64 years (Salleras, Navas, Domínguez, Ibáñez, Prat, Garrido, et al., 2009). Influenza Cures: Antivirals Cure Category Achieved, Functional Cure Identification/Description Antivirals are normally the second line of defense against influenza, the first defense being vaccination. While typically administered in clinical settings, they also have a public health role. When there is an insufficient supply of vaccines or the individual has not been vaccinated, antivirals may be used until more vaccines are available (Longini, Halloran, Nizam, Yang, 2004). Thus, anti-influenza antiviral drugs are not a substitute for vaccine. They are used in addition to vaccine in public health planning for the control of influenza (US Food and Drug Administration, 2014). Prompt treatment with influenza antiviral medications can reduce the risk of severe illness and death among persons at increased risk for influenza or who are hospitalized with suspected or confirmed influenza. (Bresee et al., 2013). Ideally these drugs should be administered within 48 hours of onset of symptoms in the disease process. There are two classes of such medicines: 1. Adamantanes (amantadine and rimantadine) 2. Inhibitors of influenza neuraminidase (oseltamivir and zanamivir; as well as peramivir and laninamivir licensed in several countries). Some influenza viruses develop resistance to theses antiviral medicines, limiting the effectiveness of treatment (WHO, 2012). SGU Cures Index 99 Cure History Influenza viruses change over time. Mutations that confer resistance can decrease drug effectiveness. Other factors (such as viral virulence) might diminish the clinical benefit of antiviral drugs (US Food and Drug Administration, 2014). Older drugs such as amantadine and rimantadine, are approved for treatment and prevention of influenza A. However, many strains of influenza, including the 2009 H1N1 influenza, are now resistant to these two older drugs. The CDC has not recommended the use of these two drugs for recently circulating influenza viruses (US Food and Drug Administration, 2014). On December 21, 2012, the US Food and Drug Administration (FDA) approved the antiviral medication oseltamivir (trade name Tamiflu®) for the treatment of influenza in people aged 14 days and older (Centers for Disease Control and Prevention, 2014a). These drugs can fight the newer, more current forms of influenza, unlike the older drugs, which are no longer effective due to viral mutations. Cure Science: Breakthroughs/Obstacles Tamiflu (oseltamivir phosphate) and Relenza (zanamivir) are the two FDA-approved influenza antiviral drugs recommended by CDC for use against recently circulating influenza viruses (US Food and Drug Administration, 2014). However, emergence of mutations that are resistant could alter the effectiveness of these drugs. Antivirals should be administered as early as possible after confirmation of influenza – ideally, within 48 hours. Delayed treatment with antiviral medication may alter its effectiveness. However, recent data does indicate that antiviral treatment might be effective in reducing morbidity and mortality in hospitalized patients, even if treatment is not started until more than 48 hours after onset of illness (Centers for Disease Control and Prevention, 2014a). Further, the CDC has played a major role in the development of novel diagnostic tests. Ongoing research aims to improve testing for H5N1 viruses in addition to assessing the probability of H1N1 changing into a pandemic flu virus. In February 2006, the FDA approved a lab test that was developed by CDC to diagnose the avian flu H5 virus. The test is now available to both public and state health laboratories (Centers for Disease Control and Prevention, 2014a). Data are limited concerning the effectiveness of antiviral treatment in preventing or reducing serious influenza-related complications and deaths. In a study combining data from 10 clinical trials, the risk of contracting pneumonia among participants with laboratory confirmed influenza who received oseltamivir treatment was approximately SGU Cures Index 100 50% less than the participants who received a placebo (Centers for Disease Control and Prevention, 2014a). Cure Science/Future Cure Obstacles and Targets For high-risk individuals, the nueraminidase inhibitors should be administered early in the course of the disease. Among the nuerminidase inhibitors, oseltamivir is the drug that is most widely used, with accumulated safety data that includes treatment in young children and pregnant women. Prophylactic uses of NA inhibitors or treatments involving immunosuppressed patients are associated with higher probability of emergence of antiviral resistance, hence careful monitoring is warranted (World Health Organization, 2012) Ideally, vaccine use would be ubiquitous enough to eliminate or drastically reduce the need for influenza antiviral medications. Number of patients being treated Antiviral treatment is recommended as soon as possible for all persons with suspected or confirmed influenza requiring hospitalization or who have progressive, severe or complicated illness regardless of previous health or vaccination status (Centers for Disease Control and Prevention, 2014a). With 15% of the US population deemed highrisk for influenza complications, 5-20% of the US population contracting influenza each year, and 45% of the population receiving an influenza vaccine in the 2012-13 flu season (Centers for Disease Control and Prevention, 2014d), approximately 170 million Americans are susceptible to influenza in any given year (i.e., no vaccine coverage) and 5-20% of those individuals may contract influenza, leaving a demand for antivirals in the potential tens of millions. Number of patients requiring antiviral treatment As of 2008, the US Department of Health and Human Services ordered more than 36 million courses of influenza antivirals for which 26 million courses were delivered to the Strategic National Stockpile for distribution to individual States in the event of a pandemic. Stockpiles are created for public health emergencies in the United States, such as an influenza pandemic, in order to ensure adequate supply of treatments (Centers for Disease Control and Prevention, 2014a). The ultimate goal is to stockpile sufficient quantities of antiviral drugs to treat 25 percent of the US population. Helping the states develop their own medical stockpiles will facilitate quicker distribution of antiviral drugs in the event of a pandemic influenza outbreak. As of 2007, 43 states SGU Cures Index 101 ordered approximately 11 million treatment courses and are committed to purchasing 30.6 million treatment courses (US Department of Health and Human Services, 2014c). Impact of Antivirals on Years of Potential Life Lost (YPLL) Data from 78 studies and 29,234 patients admitted to hospital between 2 January 2009 and 14 March 2011 demonstrated that, compared to no treatment, neuraminidase inhibitor treatment (irrespective of timing) was associated with a 25% reduction in mortality risk among adults (Muthuri, Venkatesan, Myles, Leonardi-Bee, Al Khuwaitir, Al Mamun, et al., 2014). Compared with later treatment, early treatment (i.e., within two days of symptom onset) was associated with a reduction in mortality risk (adjusted OR .48; 95% CI .41–.56; p<0·0001). Early treatment (i.e., within two days of symptom onset) versus no treatment was also associated with a reduction in mortality (adjusted OR .50; 95% CI .37–.67; p<0.0001) (Muthuri et al., 2014). However, some researchers have noted that the majority of studies involving antiviral influenza medication have significant limitations such as sample size and insufficient data (Hsu, Santesso, Mustafa, Brozek, Chen, Hopkins, et al, 2012). Thus, additional research is required to properly assess the impact of treatment with antivirals. However, the CDC does strongly recommend the use of antivirals for the treatment of severely ill patients as soon as possible (Centers for Disease Control and Prevention, 2014a). Impact of Antivirals on Disability-adjusted Life Years (DALYs/QALYs) Given the relatively short-term impact of influenza and the associated outcomes (full or near-full recovery versus death), the most logical measure of the impact of antivirals on influenza is years of potential life lost. However, some researchers have examined the impact on quality of life and/or productivity measures. Lee, Matchar, Clements, Huber, Hamilton, and Peterson (2002) report that Zanamivir and Oseltamivir each relieved an average of 1.0 day of symptoms, leading to an estimated gain of 0.5 workdays. Average Annual Cost of Antivirals Recommended antiviral treatment is five days, although longer treatment may be necessary for severely affected individuals (Centers for Disease Control and Prevention, 2014a). A five-day course of zanamivir and oseltamivir therapies costs $47.50 and $57.22 (each season or as needed), respectively. If 10% of the current US population (31.616 million people) contracted influenza and experienced symptoms severe enough to warrant antiviral treatment, the total costs would be $1.809 billion at $57.22 per course. SGU Cures Index 102 Average Lifetime Cost of Antivirals The average lifetime cost of antivirals depends on how many times an individual is infected with the flu and experiences severe complications as a result of that infection. This makes it difficult to estimate the average lifetime cost of antivirals, unlike with vaccinations, which are recommended annually regardless of whether or not the flu is contracted. Economic Impact – Value of Life Added With approximately 3,300 to 49,000 influenza-related deaths each year, a 25% reduction in mortality risk (Muthuri et al., 2014) would save between 825 and 12,250 lives each year (6,538 middle value) with a VMRR of $681.214 million. Economic Impact – Value of Reduction in DALYs/Increase in QALYs Zanamivir and oseltamivir were each assumed to relieve an average of 1.0 day of symptoms. From that data it was estimated that there was a gain of 0.5 workdays (Lee et al., 2002). According to Bureau of Labor Statistics, labor force (among those age 18 and older) was 155.4 million in March 2014 (Bureau of Labor Statistics, 2014). If 10% of the US working age population (approximately 193,212,000) contracts influenza annually (19.321 million individuals) and is able to gain a half workday (4 hours) from taking antivirals, that equates to 77.284 million work hours gained. When converted to dollars at an average $24.31 hourly wage, $1.879 billion dollars in efficiency is gained annually from the use if influenza antivirals. Citations Australian Office of Health Protection (2008). Australian Health Management Plan for Pandemic Influenza. Retrieved June 5, 2014 from: http://www.health.gov.au/internet/panflu/publishing.nsf/Content/8435EDE93CB 6FCB8CA2573D700128ACA/$File/Pandemic%20FINAL%20webready.pdf. Bresee, J., Reed, C., Kim, I., Finelli, L., Fry, A., Chaves, S. S., & Santibanez, T. A. (2013). Estimated influenza illnesses and hospitalizations averted by influenza vaccination -- United States, 2012-13 influenza season. Morbidity & Mortality Weekly Report, 62 (49), 997-1000. SGU Cures Index 103 Bureau of Labor Statistics (2014). Employment Situation Summary Table A: Household data, seasonally adjusted. Retrieved May 20, 2014, from http://www.bls.gov/news.release/empsit.a.htm. Centers for Disease Control and Prevention (2014a). Seasonal Influenza (Flu). Retrieved June 4, 2014 from: http://www.cdc.gov/flu/index.htm. Centers for Disease Control and Prevention (2014b). One Health, Zoonotic Diseases. Retrieved June 4, 2014 from: http://www.cdc.gov/onehealth/zoonoticdiseases.html. Centers for Disease Control and Prevention (2014c). FastStats: Life Expectancy. Retrieved on June 4, 2014 from: http://www.cdc.gov/nchs/fastats/lifeexpectancy.htm. Centers for Disease Control and Prevention (2014d). FluVaxView Influenza Vaccination Coverage: 2012-13 Flu Season. Retrieved on June 4, 2014 from: http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm. Couch R. B., (1996). Orthomyxoviruses. Medical Micorbiology (4th edition). Retrived from http://www.ncbi.nlm.nih.gov/books/NBK8611/ Duncan, I. (2012). Planning influenza vaccination programs: a cost benefit model. BioMed Central. Gilbert, S. (2013). Advances in the development of universal influenza vaccines. Influenza And Other Respiratory Viruses, 7 (5), 750-758. Hannoun, C. (2013). The Evolving History of Influenza Viruses and Influenza Vaccines. Expert Rev Vaccines, 12(9), 1085-94. doi: 10.1586/14760584.2013.824709. Hsu, J., Santesso, N., Mustafa, R., Brozek, J., Chen, Y. L., Hopkins, J. P., Cheung, A., Hovhannisyan, G., Ivanova, L., Flottorp, S. A., Saeterdal, I., Wong, A. D., Tian, J., Uyeki, T. M., Akl, E. A., Alonso-Coello, P., Smaill, F., Schünemann H. J. (2012). Antivirals for treatment of influenza: A systematic review and meta-analysis of observational studies. Annals of Internal Medicine, 156 (7), 512-524. SGU Cures Index 104 Ibuka Y., Paltiel A., & Galvani A. (2012). Impact of program scale and indirect effects on the cost effectiveness of vaccination programs. Medical Decision Making, 32 (3), 442-446. ohns Hopkins Medicine (2014). Influenza (Flu). Retrieved on June 4, 2014 from: http://www.hopkinsmedicine.org/healthlibrary/conditions/infectious_diseases/infl uenza_flu_85,P00625/. Lee, Y-T., Kim, K-H., Ko, E-J., Lee, Y-N., Kim, M-C., Kwon, Y-M., Tang, Y., Cho, M-K., Lee, Y-J., & Kang, S-M. (2014). New vaccines against influenza virus. Clinical and Experimental Vaccine Research, 3 (1), 12-28. Lee, P. Y., Matchar, D. B., Clements, D. A., Huber, J., Hamilton, J. D., & Peterson, E. D. (2002). Economic Analysis of Influenza Vaccination and Antiviral Treatment for Healthy Working Adults. Annals of Internal Medicine, 137 (4), 225-231. Longini, I. M., Halloran, M. E., Nizam, A., & Yang, Y. (2004). Containing Pandemic Influenza with Antiviral Agents. American Journal of Epidemiology, 159, 623-633. Meltzer, M. I., Cox , N. J., Fukuda K. (1999) The Economic Impact of Pandemic Influenza in the United States: Priorities for Intervention. Emerging Infectious Diseases 5(5). Doi: 10.321/eid0505.990507 Molinari, N. M., Ortega-Sanchez, I. R., Messonnier, M. L., Thompson, W. W., Wortley, P. M., Weintraub, E., & Bridges, C. B. (2007). The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine, 25 (27), 5086-5096. Muthuri, S. G., Venkatesan, S., Myles, P. R., Leonardi-Bee, J., Al Khuwaitir, T. S., Al Mamun, A., Anovadiya, A. P., Azziz-Baumgartner, E., Báez, C., Bassetti, M., Beovic, B., Bertisch, B., Bonmarin, I., Booy, R., Borja-Aburto, V. H., Burgmann, H., Cao, B., Carratala, J., Denholm, J. T., Dominguez, S. R., Duarte, P. A., DubnovRaz, G., Echavarria, M., Fanella, S., Gao, Z., Gérardin, P., Giannella, M., Gubbels, S., Herberg, J., Iglesias, A. L., Hoger, P. H., Hu, X., Islam, Q. T., Jiménez, M. F., Kandeel, A., Keijzers, G., Khalili, H., Knight, M., Kudo, K., Kusznierz, G., Kuzman, I., Kwan, A. M., Amine, I. L., Langenegger, E., Lankarani, K. B., Leo, Y. S., Linko, R., Liu, P., Madanat, F., Mayo-Montero, E., McGeer, A., Memish, Z., Metan, G., Mickiene, A., Mikić, D., Mohn, K. G., Moradi, A., Nymadawa, P., Oliva, M. E., Ozkan, M., Parekh, D., Paul, M., Polack, F. P., Rath, B. A., Rodríguez, A. H., SGU Cures Index 105 Sarrouf, E. B., Seale, A. C., Sertogullarindan, B., Siqueira, M. M., Skręt-Magierło, J., Stephan, F., Talarek, E., Tang, J. W., To, K. K., Torres, A., Törün, S. H., Tran, D., Uyeki, T. M., Van Zwol, A., Vaudry, W., Vidmar, T., Yokota, R. T., Zarogoulidis, P., PRIDE Consortium Investigators, Nguyen-Van-Tam, J. S. (2014). Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: A meta-analysis of individual participant data. The Lancet Respiratory Medicine, 14(2), 109-1182; 2(5), 395-404. National Institute of Allergy and Infectious Diseases (2014). Flu (influenza). Retrieved on June 4, 2014 from: http://www.niaid.nih.gov/topics/flu/research/vaccineresearch/Pages/default.asp x. National Institutes of Health (2014). Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). Retrieved on June 4, 2014 from: http://report.nih.gov/categorical_spending.aspx. Salleras, L., Navas, E., Domínguez, A., Ibáñez, D., Prat, A., Garrido, P., Asenjo, M. A., & Torner, N. (2009). Economic benefits for the family of inactivated subunit virosomal influenza vaccination of healthy children aged 3–14 years during the annual health examination in private paediatric offices. Vaccine, 27 (25-26), 3454-3458. Thomas, J. K., & Noppenberger, J. (2007). Avian influenza: A review. American Journal Of Health-System Pharmacy, 64 (2), 149-165. Thompson, W. W., Shay, D. K., Weintraub, E., Brammer, L., Cox, N. J., Anderson, L. J., & Fukuda, K. (2003). Mortality associated with influenza and respiratory syncytial virus in the United States. Journal of the American Medical Association, 289 (2), 179-186. US Census Bureau (2014). Age and Sex Composition in the United States: 2012. Table 1 retrieved on May 23, 2014 from: https://www.census.gov/population/age/data/2012comp.html. US Food and Drug Administration (2014). Influenza (Flu) Antiviral Drugs and Related Information. Retrieved February 28, 2014, from: http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm100228.htm# AntiviralMedications. SGU Cures Index 106 US Department of Health and Human Services (2014a). How the Flu Virus Changes. Retrieved on June 4, 2014 from: http://www.flu.gov/about_the_flu/virus_changes/index.html. US Department of Health and Human Services (2014b). Pandemic Flu History. Retrieved on June 4, 2014 from: http://www.flu.gov/pandemic/history/. US Department of Health and Human Services (2014c). Statement by Gerald W. Parker on Pandemic Influenza Preparedness: Update on the Development & Acquisition of Medical Countermeasures. Retrieved on June 4, 2014 from: http://www.hhs.gov/asl/testify/2007/01/t20070124c.html. US Department of Health and Human Services (2014d). Healthy People 2020: Immunization and Infectious Diseases. Retrieved June 5, 2014 from: http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid =23. World Health Organization (2009). Mortality and Burden of Disease Estimates for Member States in 2004: Age-std DALY rates for United States of America. Retrieved on June 4, 2014 from: http://apps.who.int/gho/data/node.main.1009?lang=en. November 2012. Weekly Epidemiological Record, 47, 461-476. Retrieved on June 5, 2014 from: http://www.who.int/wer/2012/wer8747/en/. SGU Cures Index 107 POLIOMYELITIS Sarah Cancellieri Disease Category Infectious Viral Disease Identification, Description, and Diagnostic Criteria The World Health Organization defines poliomyelitis (henceforth, polio) as “a highly infectious disease caused by a virus…that invades the nervous system, and can cause total paralysis in a matter of hours (World Health Organization, 2013, p.n.p.). The Centers for Disease Control and Prevention state that “infection with poliovirus results in a spectrum of clinical manifestations from inapparent infection to nonspecific febrile illness, aseptic meningitis, paralytic disease, and death” (Centers for Disease Control and Prevention, 2012; p. 1). Polio is diagnosed by viral isolation from the stool, mouth, or cerebrospinal fluid, high levels of polio-specific antibodies, or an increased number of white blood cells in the cerebrospinal fluid (Centers for Disease Control and Prevention, 2012). Disease Etiology (Cause) Polio is caused by the poliovirus, and humans are the sole reservoir for poliovirus (Robertson, 1993). There are three distinct serotypes of poliovirus, aptly named type 1, type 2, and type 3 (Robertson, 1993). The lifecycle of poliovirus is relatively straightforward. The virus enters the body through the mouth, replicates within the gastrointestinal tract, and is released back into the environment through defecation (Centers for Disease Control and Prevention, 2012). Polio is spread via the fecal-oral route or the oral-oral route (Kew, Sutter, de Gourville, Dowdle, & Pallansch, 2005). Poliovirus binds to the poliovirus receptor cells in the intestines, enters the cell, takes over the cell’s “machinery”, and proceeds to replicate itself. Eventually, the cell will lyse (i.e., break open) and release its viral contents into the body. These viruses will typically either leave the body through defecation or infect other cells in the intestines; however, sometimes the virus will enter the bloodstream via lymphatic vessels in the small intestine called lacteals. Once in the bloodstream, the virus may enter the central SGU Cures Index 108 nervous system, where it will continue its life cycle. The virus will enter a motor neuron within the spinal cord, brain stem, or motor cortex via the poliovirus receptor, replicate, lyse that neuron, and enter more neurons. It is through the destruction of motor neurons that paralytic symptoms – the hallmark of polio – occur. Nevertheless, it is important to note that paralytic polio is not the normal presentation of polio infections (Centers for Disease Control and Prevention, 2012). Classes of Polio Infections (Centers for Disease Control and Prevention, 2012) • Asymptomatic o Approximately 95% of cases o The virus is present in the host’s intestines o No clinical symptoms are presented • Abortive o 4-8% of cases o The virus is present in the peripheral nervous system o Symptoms are that of a minor, non-specific illness: sore throat, fever, nausea, vomiting, and abdominal pain o Complete recovery typically within one week • Non-Paralytic Aseptic Meningitis o 1-2% of cases o The virus is present in the central nervous system and provokes an inflammatory response o Symptoms include stiffness in the neck, back, and legs o Complete recovery typically within a few weeks • Paralytic o < 1% of cases o Virus replicates in motor neurons of the central nervous system, eventually causing those neurons to die o Symptoms include flaccid paralysis o In 5-10% of individuals with paralytic polio, lysis of motor neurons controlling the diaphragm leads to death by asphyxiation (World Health Organization, 2013) o Paralysis is typically permanent Current Prevalence/Incidence An indigenous case of paralytic polio has not been seen in the US since 1979, while the last case of vaccine associated paralytic polio (VAPP) was observed in the US in 2005 (Centers for Disease Control and Prevention, 2012). SGU Cures Index 109 Worldwide, 223 cases of paralytic polio were documented in 2012. Of these cases, the majority occurred in Afghanistan, Nigeria, and Pakistan, where polio is still endemic due to issues with vaccination programs (World Health Organization, 2013). Historically, cases of polio in the US peaked in 1952, with 57,879 reported cases (21,269 paralytic) (Post Polio Health International, n.d.). The inactivated polio vaccine was available to persons in 1955 (Centers for Disease Control and Prevention, 2011). By 1962, the number of reported cases dropped to 940 (792 paralytic), and by 1972 the number of reported cases dropped to 31 (29 paralytic) (Post Polio Health International, n.d.). In total, 457,088 cases of polio were reported in the US from 1937-1997 (postpolio health international, n.d.). While exact data on the proportion of paralytic cases is not available for all years, 104,890 cases of paralytic polio were seen in the US from 1951–1997 (Post Polio Health International, n.d.). Estimated Undiagnosed/At-Risk The 2011 National Immunization Survey in the US revealed that 93.9% of children three years of age and younger have received three doses of Inactivated Poliovirus Vaccine (IPV). The current recommended schedule is 4 shots, given at 2 months, 4 months, 6-18 months, and 4-6 years (Centers for Disease Control and Prevention, 2011). Assuming a low-end efficacy of approximately 80%, this would imply that less than 25% of the annual birth cohort is at risk (World Health Organization, 2010). In reality, this percentage is probably much smaller as the efficacy of IPV has been estimated to be as high as 96%, as well as the effects of “herd immunity” – vaccinating the majority of the population helps to protect the rest of the population since the unvaccinated group is less likely to encounter the disease. Disease Impact – Years of Potential Life Lost (YPLL) As a result of the wide use of vaccines, there is very little to no current loss of life to polio in the US It has been estimated that the polio vaccine prevented 160,000 deaths in the US between 1955 and 2006 (Thompson & Duintjer Tebbens, 2006). Disease Impact – Disability Adjusted Life Years (DALY) As a result of the wide use of vaccines, there is very little impact to no impact of polio on DALYs in the US (World Health Organization, 2009). It has been estimated that the SGU Cures Index 110 polio vaccine prevented 1.1 million cases of paralytic polio in the US between 1955 and 2006 (Thompson & Duintjer Tebbens, 2006). History of Disease and Breakthroughs Polio has been a recognized disease for centuries. Polio has been traced back to ancient Egypt, as an Egyptian stela depicts a man with a withered leg, which is thought to be the earliest representation of paralytic polio (Daniel & Robbins, 1997). Karl Landsteiner and Erwin Popper discovered the etiologic agent for polio in 1908 (Eggers, 1999). Nevertheless, polio epidemics were not observed until the late 19th century (Trevelyan, Smallman-Raynor, & Cliff, 2005). Ironically, such epidemics were mainly the result of improved sanitation (Robertson, 1993). Due to the fecal-oral transmission route of the virus, exposure to the virus early in life was a given prior to this period. Young children would still have maternal antibodies to help their immune systems fight off the virus, typically without experiencing aseptic meningitis or paralytic polio. More importantly, they were able to establish immunological memory that would protect them from the virus the rest of their lives. The rise of sanitation changed this cycle. Most notably, such advances reduced the presence of the virus in the environment and the likelihood that an individual would be exposed to the virus early in life. After this period, children who contracted the virus were older and unprotected, increasing the probability that they would contract paralytic polio. At its height in the US in 1952, over 21,000 individuals were left paralyzed by the poliovirus (Centers for Disease Control and Prevention, 2012). The outbreaks of polio in the early 20th century led to a race to find a polio vaccine. The first person to attempt to develop a vaccine was Maurice Brodie, who gave his vaccine to 3,000 children, all of whom did not develop immunity to polio (Pearce, 2004). Poliovirus was successfully cultivated in human tissue during the late 1940s and early 1950s by J. Enders, T. Weller, and F. Robbins, who were awarded the Nobel Prize in 1954 for their work. The first vaccine was developed by Jonas Salk in 1952 and announced to the world in 1955. The Salk vaccine is an inactivated poliovirus vaccine (IPV). In the late 1950s Albert Sabin developed an oral polio vaccine (OPV) using a live, but weakened virus and human trials began in 1957. The IPV and OPV are used today to combat polio around the world. Current Cure Status There are currently two different vaccines to prevent polio, however, there is no known cure for the disease after an unvaccinated individual has been infected. If the virus SGU Cures Index 111 migrates to the central nervous system of an unvaccinated individual (i.e., Non-Paralytic Aseptic Meningitis or Paralytic type), treatment focuses on providing relief of symptoms until the immune system can clear the virus. In some cases, long-term rehabilitation, such as physical therapy, braces, and corrective shoes, may be required. Survivors with respiratory paralysis need to use portable ventilators to support breathing. Future Cure Obstacles Research goals of the polio eradication initiative include optimizing oral polio vaccine efficacy and delivery, developing affordable inactivated polio vaccine; managing risks associated with vaccine-derived polioviruses, antivirals, and polio diagnostics. Research Development and Treatment Costs Thompson and Duintjer Trebbens (2006) estimated that the United States invested (2002) $35 billion (1955 net present value, discount rate of 3%) in polio vaccines research and distribution between 1955 and 2005. They also estimated that between 2006 and 2015, the US will invest an additional (2002) $1.4 billion in vaccinations (1955 present value), giving a total of $36.4 billion for the US polio vaccine campaign. Polio Cures: Vaccines Cure Category Achieved, Definitive Cure Identification/Description/Diagnostic Criteria Two different vaccines against polio were developed in the 1950s: Jonas Salk’s inactivated poliovirus vaccine (IPV), and Albert Sabin’s oral polio vaccine (OPV). While both vaccines employ all three strains of poliovirus and are successful in preventing paralytic polio, they differ in their nature, advantages, and risks. IPV falls under the category of inactivated (or whole-killed) vaccines. An enhanced potency IPV became available in the US in 1988 and has been exclusively used in the US since 2000. The US distributes one vaccine, IPOL, Sanofi Pasteur, which contains all three serotypes of polio vaccine virus (Centers for Disease Control and Prevention, 2012). As with all vaccines of this type, the viruses employed by IPV are inactivated, in SGU Cures Index 112 this case through exposure to formalin (Kew et al., 2005), and incapable of causing any form of disease. Nevertheless, the immune system still recognizes and responds to the viruses as if they were alive, leading to the formation of polio-specific antibodies and immunological memory. Because of this memory, subsequent exposure with living poliovirus would be fought off before paralytic polio set in. It is important to note that the duration of immunity is not known with certainty; however it is highly effective, with at least 99% immunity after three doses of IPV (Centers for Disease Control and Prevention, 2012). If a person immunized with IPV becomes infected with the wild poliovirus, the virus can multiply inside the intestines because IPV induces very low levels of immunity. The virus can then be shed in the feces, risking continued circulation (Polio Eradication, 2010). IPV does not stop transmission. However, OPV does and it is used during polio outbreaks. In contrast to IPV, OPV is a live-attenuated vaccine. Although the viruses in this vaccine are weakened through a technique called cold adaptation that is used to make them replicate poorly in vivo, thereby preventing nervous system involvement, they are still alive. The fact that the viruses are still alive offers a few unique advantages, and one significant risk. The first advantage is that OPV can be given orally, which is much easier for a child to tolerate and ingest than the intramuscular injection IPV requires. Secondly, the weakened viruses will replicate and spread through defecation, which means that vaccinating one child with OPV could protect an entire village. Finally, the immune system responds better to an attenuated virus than it does an inactivated one, leading to better protection. Indeed, while individuals vaccinated with IPV can still get infected with and spread the virus, those vaccinated with OPV are protected against infection. Still, there is one obvious drawback of using live viruses: they can revert to their more virulent state and cause disease, which has happened in some virus recipients. Vaccine derived poliovirus (VDPV) can cause vaccine associated paralytic poliomyelitis (VAPP) in the vaccinated individual, and can be released into circulation. Studies estimate that 1 out of every 2-3 million doses of OPV leads to VAPP, with the risk more than 7,000 times greater in immunodeficient individuals (Centers for Disease Control and Prevention, 2012). Indeed, while some fears of vaccines causing disease are immunologically inaccurate or misguided (i.e. MMR causing autism or influenza vaccines causing the flu), the risk associated with OPV is real (Centers for Disease Control and Prevention, 2012). Cure History The development of a vaccine against polio was one of the greatest medical triumphs of the 20th century. However, success did not come easily; over half a century elapsed SGU Cures Index 113 between the onset of major polio epidemics and the development of a safe and effective vaccine. Unlike previously developed viral vaccines, both IPV and OPV utilize viruses grown in cell cultures. The technique of culturing cells did not exist at the beginning of the 20th century, and even by the 1930s, it was still in its infancy. While cell culturing techniques improved in the 1930s and 1940s, significant progress in culturing viruses did not occur until 1949, when Enders et al. managed to successfully culture poliovirus in human tissue; an achievement for which they received the Nobel Prize (Enders, Weller, & Robbins, 1949). Following this breakthrough, not only did it take less than 3 years for Salk to successfully develop IPV, but within two decades measles, mumps, and rubella viruses had all been cultured and vaccines developed for each. Cure Science: Breakthroughs/Obstacles The following timeline provides a breakdown of the key milestones in the development of the polio vaccine (Chumakov & Ehrenfeld, 2008; Eggers, 1999; The College of Physicians of Philadelphia, 2013): • 1908– causative agent of polio identified • 1912 – findings detailing the possible role of small intestine infection in paralytic polio were discredited as they did not fit the existing paradigm • 1931 – multiple strains of poliovirus proposed • 1936 – virus cultured in fetal neural tissue • 1941 – findings detailing the role of small intestine infection were accepted • 1949 – virus cultured in fetal non-neural tissue • 1949 – multiple strains of poliovirus confirmed • 1951 – virus cultured in monkey kidney tissue • 1952 – inactivated poliovirus vaccine (IPV) developed • 1955 –IPV was licensed and began to be used • 1962 – oral polio vaccine (OPV) was licensed • 1970s – enhanced potency IPV was created in the Netherlands • 1988 – enhanced potency IPV available in the US • 2000 – OPV was discontinued for use in the US Based on the timeline, a critical step in the vaccine development was the ability to culture poliovirus ex vivo in non-neural tissue1. Following Enders et al.’s 1949 work the 1 Note that despite Sabin and Olitsky’s success in culturing the virus in fetal neural tissue in 1936, this was never viewed as a viable vaccine prospect as they feared the virus would cause damage to the nervous system of vaccine recipients. SGU Cures Index 114 race to discover a vaccine sped up. Salk managed to culture the virus in monkey kidney cells, and proceeded to develop and test his vaccine (The College of Physicians of Philadelphia, 2013). In the years following Salk’s success, 3 different research groups developed liveattenuated polio vaccines. Towards the end of the 1950s, efficacy and safety studies were conducted on these vaccine candidates. Based on the results, an independent NIH panel chose Sabin’s OPV as the most effective live-attenuated vaccine. Because of the lower costs and aforementioned benefits of OPV, it became the vaccine of choice for polio worldwide, with the United States making the switch in 1963 (Benison, 1982). The success associated with the polio vaccine is perhaps only paralleled by smallpox eradication. The cases of paralytic polio in the United States dropped from over 21,000 in 1952 to 2,500 in 1960, to 61 in 1965 (Centers for Disease Control and Prevention, 2012). In 1979, the last indigenous case of polio was observed. Between 1980 and 1999 only 152 cases of paralytic polio were observed, with VAPP accounting for 95% of those cases (Centers for Disease Control and Prevention, 2012). In 1996, the Advisory Committee on Immunization Practices (ACIP) recommended a joint IPV-OPV schedule be phased in, to decrease the number of cases of VAPP while maintaining the benefits associated with OPV. To eliminate the risk of VAPP, ACIP recommended the sole use of IPV in 2000 in the US. Aside from one case of VAPP acquired abroad in 2005 no cases of paralytic polio have been observed in the US (Centers for Disease Control and Prevention, 2012). Cure Science: Future Obstacles and Targets The Global Polio Eradication Initiative aim is to “deliver a polio-free world by 2018” (Global Polio Eradication Initiative, 2013, p.n.p.). Numerous groups such as Rotary International, the World Health Organization, CDC, and the Bill and Melinda Gates Foundation are attempting to help make this goal a reality. In 2012 zero cases of paralytic polio were documented in India, a country previously endemic for the disease. India is eligible to be certified polio-free as soon as 2014 (Global Polio Eradication Initiative, 2013). Unfortunately, certain hurdles still remain. Most notably, the drawbacks associated with OPV have made polio eradication elusive. For example, although the WHO declared poliovirus type 2 as being eradicated in 1999, outbreaks of vaccine-derived poliovirus 2 have been observed since. Indeed, all poliovirus 2 currently in circulation is vaccine derived rather than wild (Centers for Disease Control and Prevention, 2012). In addition, SGU Cures Index 115 as a result of VDPV outbreaks and VAPP, some communities of Afghanistan, Nigeria, and Pakistan view the vaccine as a foreign-led attack on their health rather than protection. These anti-vaccination sentiments not only make countrywide coverage incredibly difficult, but can also lead to violent backlash. Finally, the ability of the virus to continue circulating in the environment without being noticed has added to the difficulty in eradication. Not only is this due to recipients of OPV being able to spread the virus, but there have been cases – however rare – of individuals vaccinated with OPV continuously excreting VDPV for years. Most notably, a patient in the UK has been continuously excreting poliovirus 2 for 18 years (Martín, 2006). All of these obstacles have led researchers to attempt to develop a new generation of polio vaccines, one that would prevent vaccine recipients from subsequent infection such as with OPV while avoiding the risk of VDPV. Some scientists are in favor of worldwide IPV use to maintain worldwide immunity (Ehrenfeld, Glass, Agol, Chumakov, Dowdle, John, et al., 2008). However, worldwide production capacity would have to increase, and to ensure wild-type poliovirus containment top priority has been given to the development of IPV from nonpathogenic strains (Chumakov & Ehrenfeld, 2008). Number of Patients Currently Being Treated Although precise figures for vaccine coverage do not exist, combining the 2011 National Immunization Survey’s report that 93.9% of children over the age of three have received three doses of the vaccine with the annual birth cohort of 4,000,000, one can make a low-end estimate that over 3.75 million children from each birth cohort have received at least one dose of the polio vaccine (Centers for Disease Control and Prevention, 2011). Number of Patients Requiring Treatment The CDC states that all children should be vaccinated with 4 doses of IPV at ages 2 months, 4 months, 6-8 months, and a booster at 4-6 years of age (Centers for Disease Control and Prevention, 2013). Impact of Polio Vaccine on Years of Potential Life Lost (YPLL) Estimates are that over 200,000 life years are saved annually in the US as a result of vaccination. Globally, this number is 35,750,000 (Ehreth, 2003). Impact of Polio Vaccine on Disability Adjusted Life Years (DALYs/QALYs) SGU Cures Index 116 It has been estimated that 1.1 million cases of paralytic polio were prevented in the US between 1955 and 2006 (Thompson & Duintjer Tebbens, 2006). Cost of Vaccination The cost of IPV vaccination to the private sector is $27.44/dose with 4 doses being recommended. The CDC is able to secure vaccines at $12.42/dose for “state health departments, certain large city immunization projects, and current and former US territories” (Centers for Disease Control and Prevention, 2013). Note that IPV has been licensed to be combined with other vaccines such as those against diphtheria, tetanus, and pertussis (DTaP), haemophilus influenzae type B (HiB), and Hepatitis B, which lowers the overall cost of administering the individual IPV component (Centers for Disease Control and Prevention, 2013). Economic Impact – Value of Life Added If 160,000 lives have been saved in the US as a result of the polio vaccine (Thompson & Duintjer Tebbens, 2006), the total VMRR is $1.312 trillion. Economic Impact – Value of Years lived Without Disability An estimated minimal cost to care for a paralyzed 25 year-old across his or her lifespan in the US is $1,413,206 (2009 US dollars, 2% discount rate) (Cao, Chen, & DeVivo, 2011). If 1.1 million Americans avoided paralysis because of the polio vaccine (Thompson & Duintjer Tebbens, 2006), this equates to $1.554 trillion in avoided costs. Critically, these savings continue to add up yearly, in perpetuity, as paralytic cases of polio are prevented with ongoing use of the vaccine. Citations Benison, S. (1982). International Medical Cooperation: Dr. Albert Sabin, Live Poliovirus Vaccine and the Soviets. Bulletin of the History of Medicine, 56(4), 460-483. Bodian, D., & Morgan, I. (1949). Differentiation of Types of Poliomyelitis Viruses. American Journal of Hygiene, 49(2), 234-247. Burnet, F., & Macnamara, J. (1931). Immunological Differences Between Strains of SGU Cures Index 117 Poliomyelitic Virus. British Journal of Experimental Pathology, 12(2), 47-61. Cao, Y., Chen, Y., & DeVivo, M. J. (2011). Lifetime direct costs after spinal cord injury. Topics in Spinal Cord Injury Rehabilitation, 16(4), 10-16. Centers for Disease Control and Prevention. (2011). National Immunization Survey. Retrieved on July 20, 2014 from: http://www.cdc.gov/vaccines/statssurv/nis/tables/11/tab46c_3Polio_fac_iap_2011.pdf. Centers for Disease Control and Prevention. (2012). Poliomyelitis. In Epidemiology and Prevention of Vaccine-Preventable Diseases (pp. 249-262). Washington DC: Public Health Foundation, Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. (2013). CDC Vaccine Price List. Retrieved on July 20, 2014 from: http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccinemanagement/price-list/index.html Chumakov, K., & Ehrenfeld, E. (2008). New generation of inactivated poliovirus vaccines for universal immunization after eradication of poliomyelitis. Clinical Infectious Diseases, 47(12), 1587-1592. Daniel, T. M., & Robbins, C. F. (1997). A History of Poliomyelitis. In Polio (pp. 5-22). Rochester: University of Rochester Press. Eggers, H. (1999). Milestones in early poliomyelitis research (1840-1949). Journal of Virology, 73(6), 4533-4535. Ehrenfeld, E., Glass, R. I., Agol, V. I., Chumakov, K., Dowdle, W., John, T. J., Katz, S. L., Miller, M., Breman, J., G., Modlin, J., & Wright, P. (2008). Immunisation against poliomyelitis: moving forward. The Lancet, 371(9621), 1385-1387. Ehreth, J. (2003). The global value of vaccination. Vaccine, 21(7), 596-600. Enders, J., Weller, T., & Robbins, F. (1949). Cultivation of the lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science, 109(2822), 85-87. Global Polio Eradication Initiative. (2013). Polio Eradication & Endgame Strategic Plan SGU Cures Index 118 2013-2018. Geneva: World Health Organization. Kew, O. M., Sutter, R. W., de Gourville, E. M., Dowdle, W. R., & Pallansch, M. A. (2005). Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annual. Review. Microbiology, 59, 587-635. Martín, J. (2006). Vaccine-derived poliovirus from long term excretors and the end Game of polio eradication. Biologicals, 117-122. Post polio health international. Incidence rates of poliomyelitis in the US. Retrieved February 18, 2014 from: http://www.post-polio.org/ir-usa.html. Robertson, S. (1993). Module 6: Poliomyelitis. The Immunological Basis for Immunization Series. Geneva: World Health Organization. The College of Physicians of Philadelphia. (2013). History of Vaccines. Retrieved on July 20, 2014 from: http://www.historyofvaccines.org Thompson, K. M., & Duintjer Tebbens, R. J. (2006). Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Analysis, 26(6), 14231440. Trevelyan, B., Smallman-Raynor, M., & Cliff, A. (2005). The spatial dynamics of poliomyelitis in the United States: From epidemic emergence to vaccine-Induced retreat, 1910–1971. Annals of the Association of American Geographers, 95(2), 269-293. World Health Organization (2009). Mortality and Burden of Disease Estimates for Member States in 2004: Age-std DALY rates for United States of America. Retrieved on June 4, 2014 from: http://apps.who.int/gho/data/node.main.1009?lang=en. World Health Organization. (2010). Efficacy/Effectiveness of Inactivated Polio Vaccine (IPV) Against Clinical Poliomyelitis. Retrieved on July 20, 2014 from: http://www.who.int/immunization/polio_grad_ipv_effectiveness.pdf. World Health Organization. (2013). WHO Factsheet: Poliomyelitis. Retrieved on July 20, 2014 from: http://www.who.int/mediacentre/factsheets/fs114/en/. SGU Cures Index 119 World Health Organization (n.d.). Global Burden of Disease (GBD) 2001 Estimates by Subregion: Years of Life Lost (YLL). Retrieved on June 4, 2014 from: http://www.who.int/healthinfo/global_burden_disease/estimates_regional_2001/ en/. SGU Cures Index 120 MALARIA Vishal Patel Disease Category Infectious Parasitic Disease Identification, Description, and Diagnostic Criteria The Centers for Disease Control and Prevention (CDC) define Malaria as “a serious and sometimes fatal disease caused by a parasite that commonly infects a certain type of mosquito which feeds on humans.” (Centers for Disease Control, 2012a) The World Health Organization (WHO) defines Malaria as “a life-threatening disease caused by parasites that are transmitted to people through the bites of infected mosquitoes.” (World Health Organization, 2013a) The definitions from the CDC and the WHO highlight important features of Malaria, including its parasitological basis, transmission via mosquitoes, and potential to become a lethal disease (Centers for Disease Control, 2012a; World Health Organization, 2013a). The parasites, from the genus Plasmodium, mature through a complex life cycle involving human hosts and mosquito vectors. Once infected, a human host can develop symptoms as early as 10-15 days from the time of inoculation (World Health Organization, 2013a). Initial presentation often includes high fever, shaking chills, flu-like symptoms, and vomiting. If left untreated, serious complications may arise, including anemia, coma, and death. Although all ages are susceptible to the disease, populations at highest risk include young children, pregnant women, international travelers, and those with HIV/AIDS or other types of immunosuppression (World Health Organization, 2013a). Diagnosis of all suspected cases is grounded on pertinent medical and travel history, clinical findings at presentation, and thorough microscopic evaluation of blood samples. Patients who have traveled to endemic areas risk spread of the parasite to home mosquitoes which can then spread Malaria to a previously non-endemic area (See Figure 1)(World Health Organization, 2014a). Due to the advent of Rapid Diagnostic Tests (RDT), examinations for the presence of the parasite can be performed in less than 15 minutes. This information is imperative to determining treatment and it is SGU Cures Index 121 recommended that therapy should not be determined solely on the basis of a clinical diagnosis (symptom-based) but rather in conjunction with confirmatory blood evaluation, usually a peripheral blood smear (World Health Organization, 2013a). Figure 1 Disease Etiology (Cause) Four species of Malaria-causing single-celled protozoans are currently recognized to infect humans via the bite of an infected female mosquito of the Anopheline species: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium Malariae. A fifth species, Plasmodium knowlesi, typically infects macaque monkeys in Southeast Asia and can only infect humans via a zoonotic transmission (i.e., animal to human), without the mosquito vector (Centers for Disease Control, 2012a). Of the five species, P. falciparum and P. vivax account for nearly the entire global burden of Malaria, but P. falciparum is deemed to be the most deadly and dominant strain in subSaharan Africa, a recognized global hotspot of Malaria infection (Programs for Appropriate Technology in Health, 2011). Although the parasite can remain dormant in the liver for a period of time, clinical symptoms of Malaria are due to the blood stage parasites housed within red blood cells (RBC) of infected individuals (Wipasa, Elliott, Xu, & Good, 2002). The Plasmodium parasite causes lysis (rupture) of the RBC and releases toxins, waste products, and developing parasites into the bloodstream, resulting in the SGU Cures Index 122 commonly observed symptoms associated with “uncomplicated” Malaria, namely chills, general malaise, and cyclical fevers that last only a few hours but occur every 48 hours (tertian) for P. falciparum, P. vivax, and P. ovale or 72 hours (quartan) for P. Malariae. (Centers for Disease Control, 2010; Oakley, Gerald, & McCutchan, 2011; Wipasa et al., 2002). Susceptible patients (immunosuppressed or immunologically unresponsive) can experience “severe Malaria” which becomes a medical emergency and requires immediate aggressive treatment as it may involve organ-specific damage and neurological manifestations such as impaired consciousness, seizures, coma, and abnormal behavior (Centers for Disease Control, 2010). The life and transmission cycle of the parasite can be apportioned into 2 major divisions: within the Anopheles mosquito vector (approximately 5 days) and within the human host (approximately 11 days) (Centers for Disease Control, 2012c). Some differences between Plasmodium species exists in regards to these time periods, but the focus here is on the P. falciparum cycle which is the most common cause of Malaria in the United States and worldwide (Mali et al., 2012; Programs for Appropriate Technology in Health, 2011). Nevertheless, one key difference among the species is the ability of P. vivax and P. ovale to remain dormant in the host’s liver. Upon biting, the mosquito inoculates sporozoites from its salivary glands into the human blood stream (See Figure 2). These sporozoites migrate to the liver and enter a stage of dormancy as hypnozoites. An 8-day maturation period known as the Hepatic Cycle or Exoerythrocytic Cycle is required for hypnozoites to develop into liver schizonts. Once maturation and amplification is complete schizonts release merozoites into the blood stream. In the blood, merozoites infect erythrocytes to enter a 36-hour asexual multiplication stage known as the Erythrocytic Cycle. An infected erythrocyte becomes a holding cell for gametocytes and merozoites until rupture, which usually occurs after 2 Erythrocytic Cycles. It is at this stage that clinical symptoms appear, nearly 11 days after the initial bite. Merozoites can continue to infect other neighboring erythrocytes and gametocytes are released into the host’s blood stream. It is at this time that another female mosquito can take a blood meal, required for nutrition of its eggs, and ingest male and female parasitic gametocytes. These gametocytes develop into cysts in the mosquito midgut in approximately 5 days and migrate to the mosquito salivary gland to continue the cycle (Centers for Disease Control, 2012c). SGU Cures Index 123 Figure 2 (CDC, 2012c) Current Prevalence/Incidence - Global The WHO estimates that nearly 3.3 billion people, just under half of the global population, are at risk for Malaria. Over 90% of the world’s Malaria cases are likely to go unreported, given the fact that the data systems are weakest in the places where Malaria is the most common (World Health Organization, 2012). Despite this, in 2010 the WHO estimates that there were 216 million cases of Malaria worldwide, with 80% occurring in just 17 countries in sub-Saharan Africa. From these cases, it was estimated that 655,000 deaths occurred in 2010, with 80% of deaths occurring in just 14 countries (World Health Organization, 2012) and 86% of deaths occurring in children under the age of five (Birkett, Moorthy, Loucq, Chitnis, & Kaslow, 2013; The Henry J. Kaiser Family Foundation, 2013). SGU Cures Index 124 Data from 2010 also indicates that the number of reported cases worldwide, representing the sum of confirmed cases (by blood slide examination and RDT) and probable but unconfirmed cases, was approximately 23.8 million with the highest number of reported cases attributed to the Democratic Republic of Congo at 2.4 million (World Health Organization, 2011, 2013b). Between 2000 and 2010, the estimated incidence has declined by 17% and Malaria-specific mortality rates have declined by 26% (Birkett et al., 2013). This reduction is attributed to Malaria control programs, increased urbanization, and overall economic development leading to improvements in housing and nutrition (WHO, 2012). Disease prevalence on a global scale can also be gleaned from categorization of the 104 endemic countries into control phases according to WHO guidelines (See Table 1 and Figure 1). Countries in which Malaria is endemic are classified into four distinct control phases defined by interventions incorporating prevention, treatment, surveillance, monitoring, and evaluation of health systems (World Health Organization, 2013c) Phase Control Phase Pre-elimination Phase Elimination Prevention of Reintroduction Phase Total Number of Countries 79 10 10 5 104 Table 1 Current Prevalence/Incidence – US In 2010, 1,691 cases of Malaria were reported in the Unites States. Of those, 1,688 were classified as imported into the United States after travelling abroad, one was associated with blood transfusion, and two remain classified as cryptic cases (Mali, Kachur, & Arguin, 2012). The total number of cases represents a 14% increase from the 2009 surveillance data and is the highest number of reported cases since 1980, suggesting that despite the global Malaria control programs in place, many commonly traveled areas remain endemic and prevention measures are still problematic (Mali et al., 2012). Out of the 1,691 cases reported to the CDC, 176 (10%) were classified as “severe” Malaria leading to 9 deaths. Paralleling global infection patterns, P. falciparum and P. vivax together contributed to 77% of the infections (Mali et al., 2012). SGU Cures Index 125 Disease Impact – Years of Potential Life Lost (YPLL) Years of Potential Life Lost (YPLL) provide initial insight into disease burden. Using its most basic formula, YPLL corresponds to the number of deaths multiplied by the standard life expectancy at the age at which death occurs (World Health Organization, 2014b). 70 million YPLLs were associated with Malaria in 2001, representing 3.1% of the total worldwide YPLL that year. 51 million YPLLs were associated with Malaria in 2011, representing 2.5% of the total worldwide YPLL that year (World Health Organization, 2013e). The top 12 countries for Malaria-related deaths in 2009 were all located on the African continent, and together they accounted for 134,100 deaths (See Table 2). 2009 Malaria Deaths 40,079 21,168 18,156 10,530 7,892 7,522 6,527 6,296 4,943 3,862 3,747 3,378 2012 GDP per capita $1,761 $4,426 $2,039 $6,105 $1,513 $2,661 $902 $1,352 $2,342 $1,712 $1,024 $2,048 2011 Life Expectancy 60 49 56 51 56 53 58 56 53 55 53 64 Total 134,100 $27,885 664 Mean Median 11,175 7,025 $2,324 $1,900 Country Kenya Democratic Republic of Congo Côte d’Ivoire Angola Burkina Faso Nigeria Malawi Uganda Cameroon Zambia Mozambique Ghana 55.33 55.50 Table 2 Malaria in the United States today is largely limited to cases that arrive from outside the country, and thus the disease burden is relatively low. However, this has not always been the case – Malaria was endemic in some parts of the US in the past. If Malaria incidence in the US remained at the same level today as in 1933 (3.7 per 100,000 population – Linder & Grove, 1947), and given that the current average age of death of those who succumb to Malaria is 4 years (Johns Hopkins Malaria Research Institute, SGU Cures Index 126 2014), 873,841 years of potential life would be lost in the US annually [2013 population of 316,160,000 / 100,000 = 3,161.6 x 3.7 Malaria incidence rate per 100,000 = 11,698 x 74.7 (78.7 US current life expectancy - 4 years old on average at Malaria death)]. Disease Impact – Disability Adjusted Life Years (DALYs) Disease burden is also commonly represented by Disability Adjusted Life Years (DALYs), which is defined as the sum of potential life lost due to premature mortality and the years of productive life lost due to disability (World Health Organization, 2014b). In 2001, 73 million DALYs were associated with Malaria, representing 2.6% of the total worldwide DALYs. In 2011, 55 million DALYs were associated with Malaria, representing 2.0% of the total worldwide DALYs and a 25% decrease from 2001 (WHO, 2013d). It is likely that Malaria-related DALYs (55 million) are similar to Malaria-related years of potential life lost (YPLL = 51 million) because the average age of death is so young and there is minimal disability associated with Malaria. That is, patients generally recover from or succumb to the infection. History of the Disease The oldest known reference to Malaria dates back to 2700 BCE, but Hippocrates described the disease in the Book of Epidemics under the treatise “airs, waters, and places” in 400 BCE (Centers for Disease Control, 2012b; Dolby et al., 2012). The initial belief that the disease was caused by bad air (mala aria in Latin ) were abandoned in favor of a microbial causative agent following Pasteur’s Germ Theory in the late 1800s. In 1880, Charles Laveran discovered that a similar “spherical body” that he had witnessed in the gut of mosquitoes could be seen in the blood cells of a patient suffering from Marsh Fever (Centers for Disease Control, 2012b). In 1886, Camillo Golgi differentiated the species of parasite responsible for the disease and in 1890, Giovanni Grassi and Raimondo Filetti first introduced naming for P. vivax and P. Malariae (Centers for Disease Control, 2012b). Despite extensive characterization done by Golgi, Grassi, and Filetti, the scientific community would not fully accept the role of the parasite for nearly a decade. Conclusive evidence was provided in part by the collaborative efforts of Sir Ronald Ross and Patrick Manson in the early 1890s (Dolby et al., 2012). Ross was able to prove the role of the Anopheles mosquito in the role of propagating the parasite in 1897 (Centers for Disease Control, 2012b). William Welch continued describing the Malaria parasites and names P. falciparum in 1897. In 1922, John Stephens described the last of the vector-borne Malaria parasites, P. ovale (Centers for Disease Control, 2012b). SGU Cures Index 127 Current Cure Status Antimalarial drugs have been known since the 19th century and are discussed in the next section. These drugs can reduce the impact of the parasite on humans. Future Cure Obstacles Drug therapies can be undermined by the development of resistance intrinsic to the Plasmodium parasite. Thus, drug therapies will always be in danger of becoming less effective, making the development of a vaccine something that must be accomplished if the Malaria narrative is to proceed from control to cure. The challenges experienced in developing a vaccine are discussed below. Research Development Costs In 2004, the total global investment on Malaria research was approximately $323 million, with the two largest contributors being the US National Institute of Allergy and Infectious Diseases (NIAID) – one of the National Institutes of Health, and the Bill & Melinda Gates Foundation (BMGF). In 2004, $51 million (16% of total funding) was allocated toward basic Malaria research, $55 million (17% of total funding) was allocated toward implementation research, and $120 million (37% of total funding) was allocated to antiMalarial drug discovery and development (Malaria R&D Alliance, 2005). By 2009, global spending on Malaria research had expanded to $611.7 million (2007 USD), $184.3 million of which came from the BMGF (Programs for Appropriate Technology in Health, 2011). The largest public sector contributor was the US Government, with a total investment of $129 million in 2004, which comprised nearly 40% of the global investment through the NIAID, US Department of Defense, US Agency for International Development, and the CDC (Programs for Appropriate Technology in Health, 2011). Private sector contributions, including pharmaceutical and biotechnology companies, represented 12% of the total 2004 investment at $38 million. It is unknown what the individual breakdown for US private sector companies was, given that data was aggregated to maintain confidentiality (Malaria R&D Alliance, 2005). Although the US was declared Malaria-free in 1949, it continues to support research into the disease given its significant global burden. In 2007, US public spending on Malaria amounted to $129.6 million and 45.7%, or approximately $59.2 million, of this was for the development of antiMalarial drugs. The total US public funding for vaccine research in 2009 was $164.8 million (2007 USD) (Programs for Appropriate Technology in Health, 2011). SGU Cures Index 128 An analysis of the trends in funding compared the 2004 data from the Malaria R&D Alliance with 1996 data from the Wellcome Trust. Between the years of 1993 and 2004, there has been a real growth of $166 million in spending on Malaria research, with more than 80% of it coming from increased investments from the BMGF and the NIAID (Malaria R&D Alliance, 2005). The BMGF also funds diagnostic tools, mosquito-control measures, treatments, Malaria vaccines, and strategic developments (Bill and Melinda Gates Foundation, 2014). Malaria Cures: AntiMalarials Cure Category Achieved, functional Cure Identification/Description The widespread use of the first AntiMalarials paralleled the developing understanding of the Plasmodium parasite and its role as the causative agent of Malaria. It wasn’t until the 1880’s when Quinine, one of the first AntiMalarials used for the treatment of Malaria, was made affordable and deemed useful as pharmacological agent against Plasmodium species. Complete therapeutic studies were done in the 1940’s to establish Quinine’s dosage based on pharmacokinetics and pharmacodynamics (Dolby et al., 2012). Quinine-based compounds are in use today, but due to the development of drug resistance, the WHO and CDC recommends Artemisinin-based Combination Therapy (ACT) in cases of either uncomplicated or severe Malaria (Centers for Disease Control, 2013a; World Health Organization, 2010). It is important to note that the objective of antimalarial treatment differs between uncomplicated and severe Malaria. For uncomplicated Malaria, the objective is to cure the infection in order to prevent progression to severe disease. For severe Malaria, the objective is to prevent neurological decline or death (World Health Organization, 2010) In general, Antimalarials are grouped into categories based on their mechanism of action and target site. The drug Primaquine is effective against exoerythrocytic forms (liver phase) and gametocytic forms. Drugs effective against erythrocytic (blood phase) include Artemisinin, Chloroquine, Quinine, Mefloquine, and Pyrimethamine (Clark, Finkel, Rey, Whalen, & Harvey, 2012). These drugs function as schizonticides, targeting infected RBC’s before the growing numbers of parasites force rupture into the bloodstream. Quinine works by damaging the parasite membrane, whereas Artemisinin SGU Cures Index 129 works by production of free radicals, damaging specific parasitic proteins, and inhibiting parasitic virulence factors (Clark et al., 2012; Krishna, Uhlemann, & Haynes, 2004). Cure History The history of Antimalarials involves several iterations of pharmacologic design aimed to hinder rapidly developing resistance among the four Plasmodium parasites responsible for Malaria. Quinine’s origins date back to the early 1600’s when it was discovered that the bark of the Peruvian Cinchona tree was useful in the treatment of certain fevers (Centers for Disease Control, 2012b). In 1820, French chemists were able to extract the active ingredient from the tree bark for use against Malaria (Dolby et al., 2012). Quinine, and its stereoisomer, Quinidine, is used today as a powerful antimalarial, although its use is limited due to resistance and its propensity to cause adverse effects such as hypoglycemia and cardiotoxicity (Centers for Disease Control, 2013b; Krishna et al., 2004). Today’s first-line treatment includes Artemisinin, also known as Qinghao in Chinese traditional medicine, which was used for the treatment of fever since 340 BCE (Centers for Disease Control, 2012b). It was only in 1971, perhaps due to programs instituted by the Chinese Government (Krishna et al., 2004), when scientists extracted the active ingredient (Artesunate) from the sweet wormwood plant that Artemisinin was deemed to be a suitable antiMalarial (Centers for Disease Control, 2012b; Dolby et al., 2012; O’Neill, Barton, & Ward, 2010). In 1979, clinical trials of Artemisinin were published, and in 1994, first large-scale deployment of ACT was introduced (Dolby et al., 2012). Despite newly developing resistance in Southeast Asia, when paired with other antimalarial drugs, ACT is highly effective, has multifaceted Antiparasitic properties (Krishna et al., 2004), and is remarkably safe (Clark et al., 2012). It is for these reasons that the CDC and WHO have recommended use of ACT as first-line therapy (Centers for Disease Control, 2013a; World Health Organization, 2010). Cure Science – Breakthroughs/Obstacles Research on the chemical properties of Artemisinin has determined that the peroxide within the trioxane system is essential for antimalarial activity (O’Neill et al., 2010). It is proposed that the formation of Reactive Oxygen Species (ROS) via the peroxide moiety is responsible for the direct killing of parasites by overwhelming defense mechanisms (Krishna et al., 2004). Proposed parasitic molecular targets include heme, enzymes involved in calcium transport, parasitic membranes, and mitochondria. Although the molecular mechanisms are still under debate, it is suggested that the primary activator SGU Cures Index 130 of Artemisinin is an iron source. As a result, Artemisinin is effective against asexual blood stages of the Plasmodium parasite as well as the sexual gametocyte stages resulting in rapid clearance of the parasite and resolution of symptoms (O’Neill et al., 2010). Cure Science – Future Obstacles and Targets A major obstacle making the widespread use of Antimalarials unadvisable is the risk of developing parasitic resistance. In an effort to curb this risk, the WHO has recommended the use of combination therapies for uncomplicated Malaria (See Table 3). Resistance to ACT has already been documented along the Thai-Cambodian border, suggesting that Artemisinin-based therapies may face similar consequences as its predecessors, namely Chloroquine (World Health Organization, 2013a). If resistance to ACT develops in other regions, the health consequences can be significant, as no alternative Antimalarials would be available for a minimum of five years (World Health Organization, 2013a). Uncomplicated Malaria P. falciparum Complicated Malaria P. vivax Malaria Artemether + Lumefantrine Artesunate + Amodiaquine Artesunate + Mefloquine Artesunate + Sulfadoxine-Pyrimethamine Artesunate Artemisinin Arthemeter Quinine Chloroquine + Primiquine Amdiaquine + Primiquine Table 3 Additionally, not all Antimalarials affect gametocytes, thus the risk of spreading the parasite remains via usual mosquito vector transmission (Clark et al., 2012). In some areas, it is noted that patients discontinue the use of monotherapy quickly as Malarial symptoms disappear despite the presence of persistent parasitemia in the blood which can spread via mosquito vector transmission also (World Health Organization, 2013a). Ultimately, overcoming the obstacles require careful consideration of transmission, relapse, and protection from new infections (Burrows et al., 2013). As in the case of many diseases, cost and supply tend to be limiting factors that prevent patients from SGU Cures Index 131 receiving the necessary medications. This may force patients to resort to unofficial drug sellers in developing countries, undermining control programs and surveillance measures (Foster, 1991) Number of Patients Being Treated Currently In 2011, 278 million doses of ACT were delivered by public and private sectors, which is an increase from the 182 million in 2010 and 11 million in 2005 (World Health Organization, 2014c) Approximately 12 million vials of injectable Artesunate were delivered as of July 2013 (Medicines for Malaria Venture, 2013). Information about the number of individuals with confirmed Malaria who receive treatment has been limited in both household surveys and routine health information systems (World Health Organization, 2013f). As a result, numbers relating to treatment have been expressed in terms of doses delivered rather than individuals treated. Number of Patients Requiring Treatment While data is incomplete, estimates can be made based on comparing the number of ACT treatment doses distributed to the number of presumed and confirmed cases. Rates vary by region of the world, but in Africa in 2012, doses distributed did not allow 40 percent of cases to be treated (World Health Organization, 2013f). Impact of AntiMalarials on DALYs/QALYs 500,000 deaths averted (mostly child) or 7.5 million DALYs (Jamison, Jha, & Bloom, 2008). Impact of AntiMalarials on YPLL Counterfactual analyses of estimated mortality if no control program had been initiated indicate that 274 million cases of malaria and 1.1 million deaths have been averted from 2001 to 2011. Although a significant proportion is attributed to Antimalarials, progress is also attributed to urbanization and overall economic development leading to better housing and nutrition (World Health Organization, 2012). Cost of AntiMalarial Treatment Wide scale deployment of Antimalarial ACT was adopted in 1994, and in 2001 the WHO recommended a combination therapy for both P. falciparum Malaria and P. vivax SGU Cures Index 132 Malaria as first line therapy (Burrows et al., 2013; World Health Organization, 2010). It should be noted that Artemisinin-based drug therapy depends on patient presenting symptoms, region, and drug resistance, thus different sets of drugs are indicated in different scenarios. The recommended combination therapies of Artemisinin-based and Quinine-based drugs according to WHO guidelines are listed in Table 3 (World Health Organization, 2010). Studies done to investigate the cost, dosage, and application of various Antimalarials highlight the vast difference in pricing of commonly used therapies. Using data from the International Drug Price Indicator Guide in 2003 US Dollars, the cost for one adult treatment regimen of Chloroquine is $0.11, whereas that of Atovaquone-Chloroguanide is $48.00. Artemisinin-based therapies ranged from $2.00 to $9.12 per adult dose regimen (Baird, 2005) (See Table 4). Therapy Chloroquine Primaquine Quinine Mefoquine Artemether-Lumefantrine Artesunate-Amodiaquine Artesunate-Mefloquine Artesunate-SulfadoxinePyrimethamine Atovaquone-Chloroguanide Cost (US$ 2003) $0.11 $1.68 $0.97 $2.55 $9.12 $2.00 $5.00 Number of Doses 3 7-14 21 1 6 3 6 Duration of Therapy 48 hours 7 days – 8 weeks 7 days Single Dose 48 hours 48 hours 48 hours $2.40 3 48 hours $48.00 3 48 hours Table 4 Adapted from (Baird, 2005) In 2005, a costing study was done by the NIAID to determine the feasibility of rolling out ACT in Tanzania, which has a prevalence of 33% nationally (Wolf & Derriennic, 2005), and was facing increasing resistance from current protocols. Based on this analysis, the Artemether + Lumifantrine treatment had a retail price of $2.40 per adult dose with WHO-negotiated pricing in the public sector. A coforumulated Artesunate + Amodiaquine treatment had a $1.00 per adult dose and $0.50 per child dose pricing (Wolf & Derriennic, 2005). In Nigeria, the cost for the recommended Artemeter + Lumifantrine ACT ranges from $6.00 to $8.00 (Uzochukwu, Obikeze, Onwujekwe, SGU Cures Index 133 Onoka, & Griffiths, 2009). ACT treatments, even at a cost of $2.00 for an adult treatment dose, cost far more than other Antimalarials, which cost only a few cents (UNICEF, 2004). The adult dosing schedule is a three-day oral regimen of Coartem (Artemether 20mg and Lumefantrine 120mg) based on patient weight. For those 25-35kg, the complete course consists of 18 tablets, whereas for adults >35kg, the complete course consists of 24 tablets. Pediatric dosing can vary from 6 to 18 tablets per course. An estimated price for Coartem as a complete adult dose of 24 tablets is $110.86 (UpToDate, 2014). The market price for Artemisinin fluctuated widely between $120 and $1200 per kilogram from 2005 to 2008 based on current production methods and botanical sources. Synthetic and semisynthetic processes are being explored to reduce the production costs of Artemisinin, which at the present time, comes chiefly from natural sources (The Artemisinin Enterprise, 2008). Estimates based on the 278 million doses delivered in 2001 result in a total cost of ACT ranging from $556 million (low value at $2.00 per dose) to $2.53 billion (high value at $9.12 per dose). Economic Impact of Antimalarials - Value of Decrease in DALYs/YPLLs The impact of Malaria on the economies of sub-Saharan African countries is undoubtedly significant. Studies show that if Malaria had been eliminated from these countries prior to 1980, the region’s GDP in 2000 would likely have been 32% higher, an increase of about $100 billion (The Artemisinin Enterprise, 2008). Malaria is estimated to cost the sub-Saharan region approximately 1.3% of its GDP totaling about $12 billion each year in direct losses from illness, treatment and premature death (Roll Back Malaria, 2008). In endemic countries, Malaria accounts for 25-35% of outpatient visits and 25-40% of hospital admissions, which force a significant economic and human resource burden on public health systems (Malaria R&D Alliance, 2005). Using GDP per capita (in International $) (World Bank, 2014) and life expectancy, the value of a statistical life (VSL) can be calculated. VSL is approximately equal to the median GDP per capita for a given country or group of countries multiplied by a factor of 120 (Miller, 2000). For the countries impacted the most by Malaria, median GDP equals $1,905 (International $) x 120 = $228,660. Dividing by the average expected life span (53.33 years), value of a statistical life-year comes out to $4,288 per year for this region. If 110,000 deaths are averted each year (1.1 million between 2001 and 2011), SGU Cures Index 134 and 50% of that impact is attributable to antimalarials, while the other 50% is attributable to urbanization and overall economic development (World Health Organization, 2012a), 55,000 deaths are avoided each year x 49.33 (53.33 years of expected life – 4 years average age at death from Malaria) = 2,713,150 years of potential life saved by antimalarials x $4,288 VSL per year = $11.63 billion (International $) in life value saved by antimalarials every year. If we subtract the highest estimated cost of distributing ACT in 2001 ($2.53 billion) in 2014 dollars ($3.34 billion), the net gain in distributing antiMalarials would is $8.29 billion. Malaria Cures – Vaccine Cure Category Potential, Definitive Cure Identification/Description A Malaria vaccine can potentially target any one of the several stages in the life cycle of the Plasmodium parasite. As with other vaccine programs, the objective is to evoke an immunological response to an antigenic determinant of the Plasmodium parasite sufficient enough to build self-defensive mechanisms, in order to prevent disease if infection were to occur. However, unlike bacteria and viruses, a vaccine for a parasite has never been developed, partly due to the intricacy of specific immune response (Malaria Vaccine Initiative, 2014; National Institute of Allergy and Infectious Diseases, 2008). One unknown is how effective a vaccine directed at a parasite can be. Vaccine development today focuses on three main stages of the Plasmodium life cycle, the pre-erythrocytic vaccines, the blood-stage vaccines, and transmission-blocking vaccines. Pre-erythrocytic vaccines are aimed to protect against RBC infection, or in other words while the parasite is still in the liver cells or homing towards RBCs. These include recombinant proteins presented on human infected cells, DNA vaccines, or attenuated live vaccines of the whole parasite. Blood stage vaccines target parasitic replication in the RBCs, which wouldn’t prevent infection, but rather reduces numbers of parasites in the blood and boost natural immunity. Transmission-blocking vaccines would target the parasite maturation in the mosquito vector interfering with its ability to infect its next host once taking a blood meal from a vaccinated host. These vaccines would not prevent infection, but rather would be used to limit spread and hasten eradication (Malaria Vaccine Initiative, 2014). SGU Cures Index 135 Cure History Emergence of resistance in parasites to Antimalarials has pushed efforts to combat Malaria from sustained control towards eradication. Countries that had once been declared Malaria-free are now seeing reemergence, placing greater stress on Malaria control programs. Even in regions where Antimalarial drugs are available, funding gaps and cost of medications tends to inhibit widespread implementation (Wolf & Derriennic, 2005). As such, eradication of Malaria through a vaccine has gained much attention and become the focus of groups such as the Bill and Melinda Gates Foundation, PATH Malaria Vaccine Initiative, and Sanaria (Bill and Melinda Gates Foundation, 2014; Sanaria, 2012). As evidenced by the WHO’s “Malaria Vaccine Rainbow Tables,” a comprehensive database of vaccine development, there are many candidates in various phases of clinical trials (World Health Organization, 2013g). Of these, the most clinically advanced candidate is the RTS,S vaccine, which is based on the circumsporozoite protein (CSP) (Birkett et al., 2013). The discovery of CSP as a potential target dates back to 1980 when it was identified as a potential antigen useful in the development of antibodymediated immune response (Dolby et al., 2012). Vaccine trials for RTS,S entered clinical trials in 1992 and in 2012, the results of the Phase 3 trials were made available. These demonstrated a 55% overall reduction in all Malarial episodes and 47% efficacy against severe Malaria (The RTS-S Clinical Trials Partnership, 2011; World Health Organization, 2013h). While these rates are relatively low compared to viral vaccines (e.g., poliomyelitis), given the years of potential life lost to Malaria annually worldwide, the potential impact of any such vaccine will be significant. Another vaccine candidate that has recently shown high efficacy in Phase I trials is Sanaria’s PfSPZ vaccine developed from irradiated P. falciparum parasites. Preliminary results of the safety trial showed that all six patients receiving the high dose of vaccine were protected from Malaria (Butler, 2013). Of course these are very early results and clinical research continues through Phase IV where sentry studies continue to monitor safety and efficacy on a periodic basis. As for this PfSPZ vaccine, the next step is Phase 2 studies, which will establish the efficacy of the drug against a placebo, followed by Phase 3 studies, which collect final confirmation of safety and efficacy before becoming publicly available. Cure Science SGU Cures Index 136 The RTS,S vaccine is based on the CSP antigen, a four amino acid repeat known as the “repeat T epitope” (RTS), and the Hepatitis B surface (S) antigen, produced as a recombinant fusion protein in yeast (S. cerevisiae) with an AS01 adjuvant (Malaria Vaccine Initiative, 2009) The production of the vaccine candidate was through the collaborative efforts of GlaxoSmithKline (GSK) Biologicals and the PATH Malaria Vaccine Initiative (MVI). The proposed mode of action for the RTS,S/AS01 vaccine is to induce the formation of antibodies against the CSP antigen found on P. falciparum parasites. This would target the free sporozoite stage to reduce the load of sporozoites from reaching the liver if ever introduced into the blood stream by an infected mosquito. Additionally, the vaccine would stimulate a T-cell response to promote destruction of infected liver cells to impede development of any further parasites in the intra-hepatic stages. Ultimately it is intended to decrease infection rates and reduce the load of parasite emerging from liver cells to confer partial efficacy in both Malaria-naïve and Malaria-experienced adults and children. It is also important to note that RTS,S does not confer immunity to P. vivax, and it is currently aimed for use in children (Malaria Vaccine Initiative, 2009, 2011, 2013). Cure Science: Future Obstacles One of the major obstacles to the development of Malaria vaccines is the lack of a precedent in the field; there have not been any parasitic vaccines to date (National Institute of Allergy and Infectious Diseases, 2008). Furthermore, limited animal models and incomplete understanding of parasite and vector biology tend to impede successful vaccine development. The complexity of the parasitic life cycle hinders vaccine research since each stage has immunologically-distinct targets. In addition to these technical challenges, there is a perception that the Malaria vaccine will have a limited market potential which has resulted in a loss of private sector interest (National Institute of Allergy and Infectious Diseases, 2008; Programs for Appropriate Technology in Health, 2011) Five hurdles have been identified that seem to hinder vaccine development the most significantly. (1) There is poor understanding of the mechanisms of disease, immunity and correlates of protection needed to select appropriate candidates to continue into clinical trials. (2) Costs and time required for full evaluation of vaccine concepts (from inception to proof-of-concept) are often significant limiting factors. (3) The number of candidate vaccines is greater than the capacity or funding of clinical trials. (4) Although multi-antigen candidates may be highly efficacious, they cannot be evaluated quickly and require higher costs to manufacture. (5) Although whole-parasite candidates are SGU Cures Index 137 highly efficacious, they face future difficulty in the scale-up needed to meet global demand for a Malaria vaccine (Malaria Vaccine Funders Group, 2006). Several factors need to be addressed in the development of a successful Malaria vaccine. This includes the ability to accurately compare immune responses of vaccines via a standard set of immunological assays with standardized procedures. Additionally, state-of-the-art approaches including genomics should be used to characterize and identify novel potential antigen candidates. Communication and information sharing will need to be strengthened to connect the laboratory and the clinic (Malaria Vaccine Funders Group, 2006). The strategic goal is to develop and license a first-generation Malaria vaccine (with protective efficacy of over 50%) by 2015, and a second-generation vaccine (with protective efficacy of over 75%) by 2030 (Malaria Vaccine Funders Group, 2013). Recent reports also indicate that the recombinant protein-based vaccine (presumably RTS,S) has garnered private sector interest. In addition there is renewed interest in developing a whole parasitic vaccine, and perhaps future extension to other human parasitic diseases (National Institute of Allergy and Infectious Diseases, 2008). Cost of Treatment In an analysis of vaccine pipelines, it was found to take upwards of 10 years to develop a final licensed vaccine. Moreover, the cost of developing a vaccine from research to final product registration is estimated to be between $200 and $500 million (Serdobova & Kieny, 2006). Currently, there are no vaccines available for wide-spread commercial distribution. The cost of the RTS,S vaccine has not yet been determined and will depend on agreements between vaccine developers, institutions involved in distribution, governmental agencies, and international organizations (Instituto de Salud Global de Barcelona, 2011). In a recent Frequently Asked Questions statement published by GSK and MVI, it was noted that GSK’s pricing model for the RTS,S vaccine will cover manufacturing costs with a overall return of 5% which is to be reinvested in research and development of a second-generation Malaria vaccine and other tropical diseases. Moreover, it was noted that GSK and MVI hope that these vaccines, like other childhood vaccines, will be made free of charge to African communities with limited financial contributions from their respective countries (Malaria Vaccine Initiative, 2013). SGU Cures Index 138 Clinical development of the RTS,S vaccine has been funded through $200 million in grants from BMGF and $350 million by GSK. Additionally GSK plans to invest another $260 million into the project before its completion (Malaria Vaccine Initiative, 2013). Economic Impact – Value of Life Added Comparing the cost-benefit ratios for major health care interventions in sub-Saharan Africa, Malaria prevention has a cost of $2.00 to $24.00 per DALY averted, and the only intervention that had higher cost effectiveness was childhood immunization (Roll Back Malaria, 2008). This implies that each dollar spent on Malaria intervention may have potentially greater impact on region-wide health outcomes than spending on other diseases (Kiszewski et al., 2007; Roll Back Malaria, 2008). Moreover, savings from annual Malaria control can be used as reinvestment and funding of other health initiatives in endemic countries (Roll Back Malaria, 2008). Perhaps most importantly, regional economies can begin to claim back the some $12 billion estimated direct and indirect losses due to Malaria and halt the progression from “Malaria to poverty” (Rabinovich & Binka, 2005; Roll Back Malaria, 2008). The potential global impact of a vaccine with even 50% efficacy would be enormous. In 2010 alone, an estimated 655,000 people died from Malaria worldwide. Using the previously-calculated value of a statistical-life year of $4,288 (International $) x 327,000 lives saved x 49.33 years of expected life (given that Malaria mostly kills children under the age of 5) = $69.17 billion in added life value each and every year. Importantly, this added life value would continue to accrue, year after year, indefinitely. Thus, even if it cost $69 billion to develop a vaccine with 50% efficacy, and $6.9 billion to produce and distribute the vaccine annually, the initial investment would be repaid in life value gained after the first year, and would provide a 1,000% annual return on investment thereafter. In the United States, 873,841 years of potential life would currently be lost to Malaria each year if the disease had not been eradicated in 1951 (Centers for Disease Control, 2012b) presuming continuing death rates of 3.7 per 100,000 population at the peak of the disease in 1933 (Linder & Grove, 1947), the current annual VMRR associated with Malaria eradication is $91.048 billion. Importantly, the VMRR will continue to accumulate with each passing year that Malaria remains eradicated from the US. Citations SGU Cures Index 139 Baird, J. K. (2005). Effectiveness of AntiMalarial Drugs. The New England Journal of Medicine, 352(15), 1565–77. Bill and Melinda Gates Foundation. (2014). Bill and Melinda Gates Foundation Malaria. Retrieved January 26, 2014, from http://www.gatesfoundation.org/WhatWe-Do/Global-Health/Malaria Birkett, A. J., Moorthy, V. S., Loucq, C., Chitnis, C. E., & Kaslow, D. C. (2013). Malaria vaccine R&D in the Decade of Vaccines: breakthroughs, challenges and opportunities. Vaccine, 315, B233–B243. doi:10.1016/j.vaccine.2013.02.040 Burrows, J. N., Burlot, E., Campo, B., Cherbuin, S., Jeanneret, S., Leroy, D., … Willis, P. (2013). AntiMalarial drug discovery - the path towards eradication. Parasitology, 141(Special Issue 1), 128–39. doi:10.1017/S0031182013000826 Butler, D. (2013). Zapped Malaria parasite raises vaccine hopes. Nature News. Retrieved September 06, 2014, from http://www.nature.com/news/zapped-Malaria-parasiteraises-vaccine-hopes-1.13536 Centers for Disease Control and Prevention. (2010). CDC - Malaria - About Malaria Disease. Retrieved January 23, 2014, from http://www.cdc.gov/Malaria/about/disease.html Centers for Disease Control and Prevention. (2012a). CDC - Malaria - FAQs. Retrieved January 23, 2014, from http://www.cdc.gov/Malaria/about/faqs.html Centers for Disease Control and Prevention. (2012b). CDC - Malaria - About Malaria History. Retrieved January 24, 2014, from http://www.cdc.gov/Malaria/about/history/ Centers for Disease Control and Prevention. (2012c). CDC - Malaria - About Malaria Biology. Retrieved January 25, 2014, from http://www.cdc.gov/Malaria/about/biology/index.html Centers for Disease Control and Prevention. (2013a). Guidelines for treatment of Malaria in the United States. Retrieved September 06, 2014, from http://www.cdc.gov/Malaria/resources/pdf/treatmenttable.pdf SGU Cures Index 140 Centers for Disease Control and Prevention. (2013b). CDC Treatment of Malaria Guidelines For Clinicians. CDC Treatment Guidelines. Retrieved September 06, 2014, from http://www.cdc.gov/Malaria/resources/pdf/clinicalguidance.pdf Clark, M., Finkel, R., Rey, J., Whalen, K., & Harvey, R. (2012). Lippincott’s Illustrated Reviews: Pharmacology (5th ed., pp. 445–449). Baltimore, MD: Lippincott Williams & Wilkins. Dolby, K., Whitworth, J., Tufet, M., Morris, S., Burnett, J., Ickowitz-seidler, L., … Scott, J. (2012). Malaria 1990–2009. Portfolio Review. Retrieved September 06, 2014, from http://www.wellcome.ac.uk/stellent/groups/corporatesite/@policy_communications /documents/web_document/wtvm054952.pdf Foster, S. (1991). Pricing, distribution, and use of antiMalarial drugs. Bulletin of the World Health Organization, 69(3), 349–363. Instituto de Salud Global de Barcelona. (2011). RTS,S Malaria Vaccine Frequently Asked Questions. Retrieved September 07, 2014, from http://www.isglobal.org/documents/10179/25254/q%26a_rtss_eng.pdf/069144f14117-4d2c-8a77-dfa6a028b3ee Jamison, D., Jha, P., & Bloom, D. (2008). Disease Control. Copenhagen Consensus 2008 Diseases Challenge Paper. Retrieved September 06, 2014, from http://files.givewell.org/files/DWDA 2009/Stop TB/Copenhagen Consensus PaperDiseases.pdf Johns Hopkins Malaria Research Institute. (2014). Johns Hopkins Malaria Research Institute - About Malaria. Retrieved August 05, 2014, from http://Malaria.jhsph.edu/about_Malaria/ Kiszewski, A., Johns, B., Schapira, A., Delacollette, C., Crowell, V., Tan-Torres, T., … Nafo-Traoré, F. (2007). Estimated global resources needed to attain international Malaria control goals. Bulletin of the World Health Organization, 85(8), 623–630. doi:10.2471/BLT. Krishna, S., Uhlemann, A.-C., & Haynes, R. K. (2004). Artemisinins: mechanisms of action and potential for resistance. Drug Resistance Updates, 7, 233–44. doi:10.1016/j.drup.2004.07.001 SGU Cures Index 141 Linder, F. E., & Grove, R. D. (1947). Vital Statistics Rates in the United States 1900-1940. United States Bureau of the Census, 266–267. Malaria R&D Alliance. (2005). Malaria Research and Development: An Assessment of Global Investment. Retrieved January 25, 2014, from http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Malaria+Researc h+&+Development+An+Assessment+of+Global+Investment#5 Malaria Vaccine Funders Group. (2006). Malaria Vaccine Technology Roadmap. Retrieved September 06, 2014, from http://www.Malariavaccine.org/files/Malaria_Vaccine_TRM_Exec_Summary_Final_0 00.pdf Malaria Vaccine Funders Group. (2013). Malaria Vaccine Technology Roadmap. Retrieved September 06, 2014, from http://apps.who.int/immunization/topics/Malaria/vaccine_roadmap/TRM_update_n ov13.pdf?ua=1 Malaria Vaccine Initiative. (2009). RTS,S/AS01 Candidate Malaria Vaccine Summary for the SAGE Meeting. Retrieved September 06, 2014, from http://www.who.int/immunization/sage/1_SAGE_RTSS_summary_final_Malaria.pdf Malaria Vaccine Initiative. (2011). Fact Sheet: RTS,S Malaria Vaccine Candidate. Retrieved September 06, 2014, from http://www.Malariavaccine.org/files/RTSSfactsheetFINAL30September2011.pdf Malaria Vaccine Initiative. (2013). RTS,S Frequently Asked Questions. Retrieved September 06, 2014, from http://www.Malariavaccine.org/files/MVI-GSK-FAQFINAL-web.pdf Malaria Vaccine Initiative. (2014). Malaria Vaccine Approaches. Retrieved January 26, 2014, from http://www.Malariavaccine.org/malvac-approaches.php Mali, S., Kachur, S. P., & Arguin, P. M. (2012). Malaria surveillance - United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summaries, 61(2), 1–17. SGU Cures Index 142 Medicines for Malaria Venture. (2013). Artesunate Injection. Retrieved January 25, 2014, from http://www.mmv.org/achievements-challenges/achievements/artesunateinjection-32-million-vials-life-saving-medicine-deli Miller, T. R. (2000). Variations between Countries in Values of Statistical Life. Journal of Transport Economics and Policy, 34 Part 2, 169–188. National Institute of Allergy and Infectious Diseases. (2008). NIAID Research Agenda for Malaria. Retrieved September 06, 2014, from http://www.niaid.nih.gov/topics/Malaria/documents/researchagenda.pdf O’Neill, P., Barton, V., & Ward, S. (2010). The Molecular Mechanism of Action of Artemisinin - The Debate Continues. Molecules, 15(3), 1705–21. doi:10.3390/molecules15031705 Oakley, M. S., Gerald, N., & McCutchan, T. F. (2011). Clinical and Molecular Aspects of Malaria Fever. Trends in Parasitology, 27(10), 442–9. doi:10.1016/j.pt.2011.06.004 Programs for Appropriate Technology in Health. (2011). Staying the course? Malaria Research and Development in a Time of Economic Uncertainty. Seattle: PATH. Retrieved September 06, 2014, from http://www.Malariavaccine.org/files/RDreport-June2011.pdf Rabinovich, R., & Binka, F. (2005). An Assessment of Global Investment Malaria Research & Development An Assessment of Global Investment. Retrieved September 06, 2014, from http://www.Malariavaccine.org/files/MalariaRD_Report_complete.pdf Roll Back Malaria. (2008). The Global Malaria Action Plan For a Malaria-Free World. Retrieved September 06, 2014, from http://www.rollbackMalaria.org/gmap/gmap.pdf Sanaria. (2012). Malaria Eradication Through Vaccination. Retrieved January 26, 2014, from http://www.sanaria.com/index.php?s=38 Serdobova, I., & Kieny, M.-P. (2006). Assembling a global vaccine development pipeline for infectious diseases in the developing world. American Journal of Public Health, 96(9), 1554–9. doi:10.2105/AJPH.2005.074583 SGU Cures Index 143 The Artemisinin Enterprise. (2008). Meeting the Malaria Treatment Challenge: Effective Introduction of New Technologies for a Sustainable Supply of ACTs. York. Retrieved September 06, 2014, from http://www.york.ac.uk/org/cnap/artemisiaproject/pdfs/AEconference-reportweb.pdf The Henry J. Kaiser Family Foundation. (2013). The Global Malaria Epidemic. Retrieved September 07, 2014, from http://kff.org/global-health-policy/fact-sheet/the-globalMalaria-epidemic-4/ The RTS-S Clinical Trials Partnership. (2011). First Results of Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Children. The New England Journal of Medicine, 365(20), 1863–1875. UNICEF. (2004). UNICEF Fact Sheet - Malaria, A Global Crisis. Retrieved January 26, 2014, from http://www.unicef.org/media/media_20475.html UpToDate. (2014). Artemether and Lumefantrine: Drug Information. Retrieved April 24, 2014, from http://www.uptodate.com/contents/artemether-and-lumefantrine-druginformation?source=see_link&utdPopup=true Uzochukwu, B. S. C., Obikeze, E. N., Onwujekwe, O. E., Onoka, C. a, & Griffiths, U. K. (2009). Cost-effectiveness analysis of rapid diagnostic test, microscopy and syndromic approach in the diagnosis of Malaria in Nigeria: implications for scalingup deployment of ACT. Malaria Journal, 8(265). doi:10.1186/1475-2875-8-265 World Health Organization. (2010). Guidelines for the Treatment of Malaria. (P. Olumese, Ed.) (2nd ed.). Geneva: World Health Organization. World Health Organization. (2011). World Health Organization Indicator and Measurement Registry. Retrieved January 24, 2014, from http://apps.who.int/gho/indicatorregistry/App_Main/browse_indicators.aspx World Health Organization. (2012). World Malaria Report. Retrieved September 07, 2014, from http://www.who.int/iris/bitstream/10665/78945/1/9789241564533_eng.pdf?ua=1 SGU Cures Index 144 World Health Organization. (2013a). WHO Malaria Fact Sheet Number 94. World Health Organization. Retrieved January 23, 2014, from http://www.who.int/mediacentre/factsheets/fs094/en/ World Health Organization. (2013b). Global Health Observatory of the World Health Organization 2010 Malaria Reported Cases. Retrieved January 25, 2014, from http://apps.who.int/gho/athena/data/download.xsl?format=xml&target=GHO/WH S3_48&profile=excel&filter=COUNTRY:*;YEAR:2010 World Health Organization. (2013c). Overview of Malaria Elimination. World Health Organization. Retrieved January 24, 2014, from http://www.who.int/Malaria/areas/elimination/overview/en/index.html World Health Organization. (2013d). Global Health Estimates Summary Table DALY 2001-2011. Retrieved September 07, 2014, from http://www.who.int/healthinfo/global_burden_disease/estimates_regional/en/index 1.html World Health Organization. (2013e). Global Health Estimates Summary Table YLL 20012011. Retrieved January 24, 2014, from http://www.who.int/healthinfo/global_burden_disease/estimates_regional/en/index 1.html World Health Organization. (2013f). World Malaria Report. Retrieved September 07, 2014, from http://www.who.int/entity/Malaria/publications/world_Malaria_report_2013/report/ en/index.html World Health Organization. (2013g). Malaria Vaccine Rainbow Tables. Retrieved September 07, 2014, from http://www.who.int/vaccine_research/links/Rainbow/en/index.html World Health Organization. (2013h). Questions and Answers on Malaria Vaccines. Retrieved September 07, 2014, from http://www.who.int/immunization/research/development/WHO_Malaria_vaccine_q _and_a_Oct2013.pdf SGU Cures Index 145 World Health Organization. (2014a). WHO Global Health Observatory Map Gallery. Retrieved April 08, 2014, from http://gamapserver.who.int/mapLibrary/app/searchResults.aspx World Health Organization. (2014b). WHO Metrics: Disability-Adjusted Life Year (DALY). World Health Organization. Retrieved January 24, 2014, from http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/ World Health Organization. (2014c). WHO Q&A on Artemisinin Resistance. World Health Organization. Retrieved January 25, 2014, from http://www.who.int/Malaria/media/artemisinin_resistance_qa/en/index.html Wipasa, J., Elliott, S., Xu, H., & Good, M. (2002). Immunity to asexual blood stage Malaria and vaccine approaches. Immunology and Cell Biology, 80(5), 401–414. Wolf, K., & Derriennic, Y. (2005). Costing Artemisinin-based Combination Therapy for Malaria in Tanzania. Partners for Health Reform-plus Project. Retrieved January 25, 2014, from http://www.popline.org/taxonomy/term/55328?page=81 World Bank. (2014). World Bank GDP Per Capita PPP. Retrieved April 23, 2014, from http://data.worldbank.org/indicator/NY.GDP.PCAP.PP.CD SGU Cures Index 146 B. Chronic Diseases SGU Cures Index 147 ALZHEIMER’S DISEASE Sarah Cancellieri, Emily Vogler Disease Category Chronic Disease Identification, Description, and Diagnostic Criteria Alzheimer’s disease is a chronic and terminal disease of the elderly and the most common cause of dementia, accounting for 60-80% of dementia cases in the US (Plassman, Langa, Fisher, Heeringa, Weir, Ofstedal, et al, 2007; Thies & Bleiler, 2013). The majority of individuals who are diagnosed with Alzheimer’s disease are 65 years or older; rarely, people develop the disease before the age of 65 (Thies & Bleiler, 2013). Alzheimer’s disease is characterized by a wide range of cognitive and behavioral symptoms, which eventually lead to a loss of independent function. This loss of independent function can have significant financial and emotional impacts on patients, their families, and healthcare systems (Plassman et al., 2007). Physiologically, Alzheimer’s disease causes neurons (brain cells) to die or malfunction. This malfunction can impact how information is processed, ultimately leading to a constellation of behavioral symptoms such as memory loss, challenges in planning/problem solving, confusion with time or place, irritability, mood swings, trouble with language comprehension and production, and loss of control of bodily functions (Thies & Bleiler, 2013). While the pathogenesis of Alzheimer’s disease has not been fully explained, it is generally accepted that the disease has both familial (autosomal dominant) and sporadic (associated with environmental and genetic risk factors) forms. However, the familial form is rare and accounts for less than 1% of cases (Thies & Bleiler, 2013). The sporadic form usually occurs after the age of 65 (late-onset Alzheimer’s disease), however, it can also occur before the age of 65 (early-onset Alzheimer’s disease). When people develop Alzheimer’s disease before the age of 65 years, it can be the familial autosomal dominant form (Blennow, de Leon, & Zetterberg, 2006) or the sporadic earlyonset form. Alzheimer’s disease is now the sixth leading cause of death in the US and the fifth leading cause of death for those aged 65 years and older (Thies & Bleiler, 2013). SGU Cures Index 148 Individuals aged 65 and older survive an average of 4 to 8 years after diagnosis (Brookmeyer, Corrada, Currieri, & Kawas, 2002; Larson et al, 2004). The lifespan after diagnosis depends on the age of the person when Alzheimer’s disease is diagnosed (Brookmeyer et al, 2002). The diagnosis of Alzheimer’s disease is most often made by the individual’s primary care physician, and is done by obtaining medical and family history, as well as by conducting neuropsychological tests of information processing (Thies & Bleiler, 2013). In addition to these tests, the physician often performs physical and neurological examinations, as well as obtaining a CT scan or MRI (magnetic resonance imaging) of the brain to rule out other causes of dementia such as stroke or frontotemporal dementia. Guidelines for making a diagnosis of Alzheimer’s disease were set in 1984 by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s disease and Related Disorders Association (ADRDA) and were updated in 2011 (McKhann, Drachman, Folstein, Katzman, Price, Stadlan, 1984; McKhann, Knopman, Chertkow, Hyman, Jack, & Kawas, 2011). The clinical criteria for the diagnosis of Alzheimer’s disease in 1984 were divided into three categories: possible, probable, and definite. Clinical diagnosis of possible Alzheimer’s disease is characterized as having a dementia syndrome, atypical onset, and an absence of other neurologic, psychiatric, or systemic disorders sufficient to cause dementia. The clinical diagnosis of probable Alzheimer’s disease requires a clearcut history of cognitive decline and a gradual onset of dementia (established by clinical examination and confirmed by neuropsychological tests), which is defined as deficits in two or more of the following areas of cognition: Impaired ability to acquire and remember new information, impaired reasoning and handling of complex tasks, impaired visuospatial abilities, impaired language functions and changes in personality, behavior, or comportment. The criteria for definite Alzheimer’s disease include the clinical criteria for probable Alzheimer’s as well as histopathologic evidence from a biopsy or autopsy (McKhann et al., 1984). The National Institute of Aging (NIA) and Alzheimer’s Association proposed new criteria and guidelines for diagnosing the disease in 2011 (McKhann et al., 2011). The guidelines state that Alzheimer’s disease should be classified into three categories: 1) Dementia due to Alzheimer’s disease, which encompasses the stages of mild to severe Alzheimer’s disease 2) Mild cognitive impairment due to Alzheimer’s, where individuals have mild but measurable changes in thinking abilities that are noticeable, but do not affect day to day activities SGU Cures Index 149 3) Pre-clinical (pre-symptomatic) Alzheimer’s disease for individuals who have measurable changes in the brain, cerebral spinal fluid (CSF), and/or blood biomarkers, but have not developed any symptoms, such as memory loss. This category is not intended for clinical diagnosis, but to aid in research to establish which biomarkers best identify pre-clinical Alzheimer’s disease A biomarker is a biological factor that can be measured and can be used in identifying preclinical disease. Currently no biomarkers serve as a definitive diagnosis for Alzheimer’s disease, such as blood glucose and A1C levels do for Type 2 diabetes (Sunderland et al., 2003; Tarawneh et al., 2015). The most recent National Institute on Aging/Alzheimer’s Association criteria do not include biomarkers because more research needs to be done to ensure the accuracy of biomarkers, including standardization across locales and access in various community settings (McKhann et al., 2011). The primary candidates under investigation include brain imaging such as magnetic resonance imaging (MRI) (Killiany et al., 2000) or positron emission tomography (PET) (Klunk et al., 2001) to detect amyloid beta and altered levels of proteins found in cerebral spinal fluid (CSF) Biomarkers are currently used in research to detect cognitively normal people at risk of developing Alzheimer’s disease for inclusion in prevention studies and clinical trials, and significantly, in April of 2015 the Centers for Medicare and Medicaid Services (CMS) approved a four year, $100 million study to identify methods of brain imaging that will qualify for Medicare/Medicaid reimbursement for participants in research programs. There have been advances in neuroimaging techniques such as PET or Single-photon Emission Computed Tomography (SPECT), which can now differentiate between Alzheimer’s disease and other possible causes of dementia (Dougall, Bruggink, & Ebmeier, 2004). Researchers can detect β-amyloid plaques by using PET and Pittsburgh Compound B (PiB), which is an imaging agent that enters the brain through the bloodstream and attaches its self to β-amyloid plaques (Klunk et al., 2004). SPECT can differentiate between vascular dementia and frontotemporal dementia (Dougall et al, 2004). Mapstone and colleagues (2014) used a lipidoic approach to detect preclinical Alzheimer’s disease, discovering and validating a set of ten lipids from peripheral blood that predicted phenoconversion to either amnestic mild cognitive impairment or Alzheimer’s disease within a 2-3 year timeframe with over 90% accuracy. This type of biomarker panel may be sensitive to early neurodegeneration of preclinical Alzheimer’s disease (Mapstone, Cheema, Fiandaca, Zhong, Mhyre, MacArthur, et al., 2014). Symptoms of Alzheimer’s disease in the three stages according to the National Institute on Aging (2014): SGU Cures Index 150 1) Mild Alzheimer’s disease: • Getting lost • Trouble handling money • Repeating questions • Taking longer to complete daily normal tasks • Poor judgment • Losing things or misplacing them in odd places • Mood and personality changes 2) Moderate Alzheimer’s disease: • Increased memory loss and confusion • Problems recognizing family and friends • Inability to learn new things • Difficulty carrying out tasks that require multiple steps (such as getting dressed) • Problems coping with new situations • Hallucinations, delusions, and paranoia • Impulsive behavior 3) Severe Alzheimer’s disease: • Inability to communicate • Weight loss • Seizures • Skin infections • Difficulty swallowing • Groaning, moaning, or grunting • Increased sleeping • Lack of control of bowl and bladder Disease Etiology (Causes) The cause of Alzheimer’s disease is largely unknown. In less than 1% of all Alzheimer’s cases a genetic mutation is to blame (Thies & Bleiler, 2013; Blennow, de Leon, & Zetterberg, 2006), and this is the cause for the autosomal dominant inherited form of Alzheimer’s disease. The three known genetic mutations that have been found to cause Alzheimer’s disease involve the gene for the amyloid precursor protein (APP, chromosome 21) and the genes for presenilin 1 (PSEN1, chromosome 14) and presenilin 2 (PSEN2, chromosome 1) proteins (Waring & Rosenberg, 2008). The presence of these genetic mutations will almost guarantee that an individual develops SGU Cures Index 151 Alzheimer’s disease, usually before the age of 65 and sometimes as early as 30 (Thies & Bleiler, 2013). The vast majority of the population living with Alzheimer’s disease has sporadic Alzheimer’s disease (Ittner & Götz, 2011). Many experts agree that there is not a single cause for developing sporadic Alzheimer’s disease, and multiple factors may play a role in developing the disease. There are changes to the brain that can start 20 years before any symptoms appear (Thies & Bleiler, 2013), such as the accumulation of β-amyloid plaques and neurofibrillary tangles, which play a role in cell death. There is also dramatic brain shrinkage from cell loss and debris from dead and dying neurons in people with advanced Alzheimer’s disease (Wenk, 2003). β-amyloid is produced in neurons from the amyloid precursor protein by the activities of two aspartic proteases, β-secretase and γ-secretase (Ghosh, Brindisi, & Tang, 2012). Researchers have found that an accumulation of the β-amyloid protein outside of the neurons leads to the development of β-amyloid plaques (Querfurth & LaFerla, 2010), and it is thought to drive the pathologic cascade. This cascade ultimately leads to neuronal death (Cramer, Cirrito, Wesson, Lee, Karlo, Zinn, et al., 2012) by interfering with neuron-to-neuron communication at the synapse (Thies & Bleiler, 2013). This is called the “amyloid hypothesis” and is thought to drive tau aggregation (Lewis, Dickson, Lin, Chisholm, Corral, Jones, et al, 2001; Oddo, Caccamo, Shepherd, Murphy, Golde, Kayed, et al., 2003; Götz, Chen, van Dorpe, & Nitsch, 2001). Initially there was significant controversy with opposing camps promoting the “amyloid hypothesis” and the “tau hypothesis.” The tau hypothesis proposes that the pathological manifestations of Alzheimer’s disease are due to neurofibrillary tangles. Neurofibrillary tangles are composed of hyperphosphorylated forms of the protein tau (Mangialasche, Solomon, Winblad, Mecocci, & Kivipelto, 2010; Querfurth & LaFerla, 2010), and are commonly called tau tangles. Tau is normally an abundant soluble protein in axons, and promotes assembly and stability of microtubules as well as vesicle transport. However, the hyperphosphorylated tau is insoluble, lacks affinity for microtubules, and self-associates into larger structures (Querfurth & LaFerla, 2010). The hyperphosphorylated form of tau accumulates inside of the neurons and these tau tangles block the transport of nutrients in the neuron, which is thought to contribute to cell death (Thies & Bleiler, 2013). Recent research has found that both β-amyloid and tau are necessary for Alzheimer’s pathology, with β-amyloid as the likely key initiator of the pathogenic cascade leading to Alzheimer’s disease (Musiek and Holtzman, 2015). Among the beta-amyloid plaques and the neurofibrillary tangles contributing to Alzheimer’s disease, the loss of neurotrophins is another factor in Alzheimer’s disease. SGU Cures Index 152 Neurotrophins are a family of proteins, of which NGF (nerve growth factor) and BDNF (brain-derived neurotrophic factor) (Allen, Watson, & Dawbarn, 2011) are essential for the survival of neurons (Mangialasche, Solomon, Winblad, Mecocci, & Kivipelto, 2010). Neurotrophins are responsible for promoting cell proliferation, differentiation, and survival of neurons and glial cells (support cells for neurons). They also mediate learning, memory, and behavior (Querfurth & LaFerla, 2010). Researchers have found a causal link between neurotrophin imbalance, activation of the amyloidogenic pathway, and neurodegeneration in Alzheimer’s disease (Cattaneo, Capsoni, & Paoletti, 2008). In the late stages of Alzheimer’s disease, the normally high levels of neurotrophin receptors in neurons are severely reduced (Querfurth & LaFerla, 2010). Another factor contributing to the development of Alzheimer’s disease is mitochondrial dysfunction. Mitochondria act as the power plants for a cell by creating adenosine triphosphate (ATP), which is used as a source of chemical energy. In people with Alzheimer’s disease there tends to be mitochondrial dysfunction, as β-amyloid is toxic to mitochondria (Querfurth & LaFerla, 2010), and exposure to β-amyloid inhibits key mitochondrial enzymes such as cytochrome c oxidase in the brain (Caspersen et al., 2005). As a consequence, electron transport, ATP production, oxygen consumption and mitochondrial membrane potential all become impaired (Querfurth & LaFerla, 2010). Another consequence of dysfunctional mitochondria is that they release oxidizing free radicals, which in turn causes oxidative stress (Good, Werner, Hsu, Olanow, & Perl, 1996). Environmental and genetic differences may act as risk factors for developing Alzheimer’s disease. The inheritance of the ε4 allele of the apoplipoprotein E (APOE4) increases the chances an individual will develop Alzheimer's disease, but unlike the genetic mutations associated with the familial form, they do not raise the chance to an almost-certainty (Strittmatter, Saunders, Schmechel, Pericak-Vance, Enghild, Salvesen, et al., 1993; Mahley, Weisgraber, & Huang, 2006), since as many as 40-80% of people with Alzheimer’s possess at least one APOE4 allele (Farrer, Cupples, Haines, Hyman, Kukull, Mayeux, et al., 1997). Another genetic risk for Alzheimer’s disease is Down syndrome (DS), trisomy of chromosome 21, on which resides the gene for amyloid precursor protein (Rumble et al., 1989). The extra copy of this gene results in excess production of β-amyloid; consequently virtually all DS patients develop Alzheimer’s disease pathology by the age of 40 (Hartley et al., 2015). Also, insulin resistance has been shown to increase the risk of developing Alzheimer’s disease (Craft, Cholerton, & Baker, 2013). Insulin resistance occurs mainly as a result of lifestyle factors, such as a sedentary lifestyle, high caloric diets that are high in simple carbohydrates and saturated fats. SGU Cures Index 153 Current Prevalence/Incidence US Prevalence: Alzheimer’s disease was predicted to affect approximately 5.2 million Americans of all ages in 2013 (Thies & Bleiler, 2013). Of the 5.2 million Americans, 5 million are 65 years and older, which was estimated using data from the 2010 US Census and Chicago Health and Aging Project (CHAP) (Hebert, Weuve, Scherr, & Evans, 2013). The remaining 200,000 are under the age of 65 (Thies & Bleiler, 2013). The prevalence of the disease and proportion of severe Alzheimer’s disease increases with age: 17% of cases were severe in persons aged 65-74, 20% among persons aged 75-84 years, and 28% among persons aged 85 years and older (Hebert, Scherr, Bienias, Bennett, & Evans, 2003). The disease is more prevalent in females than in males, as more than two-thirds of Americans with Alzheimer’s disease are females (Plassman et al., 2007); this has been attributed to the fact that females on average live longer than males (Hebert, Scherr, McCann, Beckett, & Evans, 2001b). In 2010, the life expectancy for females in the US was 81 years, whereas for males it was 76.2 years Centers for Disease Control and Prevention (2014). Of the 5 million Americans with Alzheimer’s that are 65 years and older, 3.2 million are female and 1.8 million are male (Thies & Bleiler, 2013). However, Plassman and colleagues (2007) found no difference between the risk of males and females developing Alzheimer’s disease. More recently, age-related reduction of the steroid hormone estrogen has been linked to the increased risk for Alzheimer’s disease in women. Estrogen supports mitochondrial function in brain cells (Simpkins et al., 2010), supports neuronal substrates of learning and memory (Foy, 2011), and reduces β-amyloid accumulation and tau hypersphosphorylation (Pike et al., 2009). Studies in transgenic mouse models of Alzheimer’s disease also support a role for reduced estrogen production in cognitive impairment and increased β-amyloid production (Carroll and Pike, 2008). However, there have been mixed reports on the effects of estrogen-based hormone replacement therapy (HRT), usually for treatment of menopausal discomforts, on brain volume and risk for Alzheimer’s disease, with likelihood that beginning treatment early in menopause, before age 50 may be beneficial (Imtiaz et al., 2014) and harmful after age 65 (Espeland et al., 2015). Imtiaz and colleagues recently reported at the Alzheimer’s Association International Conference in Washington D.C. that a longitudinal study of 9000 women found that beginning HRT for more than 10 years halved the risk of developing Alzheimer’s disease in women beginning therapy between ages 47-56. US Incidence: The estimated annual incidence of Alzheimer’s disease increases dramatically with age, from about 53 new cases per 1000 people age 65-74 years, to 170 new cases per 1000 people age 75-84 years, to 231 new cases per 1000 people SGU Cures Index 154 age 85 years and older (Hebert, Beckett, Scherr, & Evans, 2001a). Hebert and colleagues (2001a) estimated that in 2010 there would be 454,000 new cases of Alzheimer’s disease and 491,000 new cases in 2020. In 2005 it was estimated that there were 468,000 newly diagnosed cases of Alzheimer’s disease (Hirtz, Thurman, GwinnHardy, Mohamed, Chaudhuri, & Zalutsky, 2007). This equates to a new diagnosis in the US every 68 seconds (Thies & Bleiler, 2013). Estimated Undiagnosed/At-Risk Individuals with dementia were identified in the CHAP and Aging, Demographics, and Memory study (ADAMS) prevalence studies. However, in the primary care populations that were studied, it was estimated that 50-66% of all cases of dementia were undiagnosed (Boustani, Peterson, Hanson, Harris, & Lohr, 2003). In addition to genetic risk factors, other risk factors for Alzheimer’s disease are: (a) advancing age, (b) family history, as individuals with a parent, brother or sister are at a greater risk (Green, Cupples, Go, Benke, Edeki, Griffith, et al., 2002; Fratiglioni, Ahlbom, Viitanen, & Winblad, 1993), (c) cardiovascular disease risk factors, such as smoking, obesity, diabetes mellitus, high cholesterol, and hypertension since the factors that increase the risk of cardiovascular disease have also been associated with a higher risk of developing Alzheimer’s disease (Fitzpatrick et al., 2004), (d) having fewer years of education (Stern, Gurland, Tatemichi, Tang, Wilder, & Mayeux, 1994; Evans, Hebert, Beckett, Scherr, Albert, Chown, et al., 1997), and (e) moderate and severe traumatic brain injury (Lye & Shores, 2000), which causes disruption of normal brain function. It has long been observed that Alzheimer’s disease patients are at increased risk for seizures, but whether seizures are a risk factor for, or the result of Alzheimer’s disease is not known. Seizures often go unrecognized as a recent study found over half of seizures in Alzheimer’s disease patients are non-convulsive (Vossel et al., 2013), and so the incidence of seizure in Alzheimer’s disease is likely underestimated. Β-amyloid induces seizure activity (Minkeviciene et al., 2009; Born et al., 2014), APOE4 has been implicated in age-dependent development of seizures (Hunter et al., 2012), and the presence of seizures predicts greater and quicker cognitive decline in both Alzheimer’s disease and Down syndrome (Lott et al., 2012; Vossel et al., 2013), suggesting that seizures contribute to Alzheimer’s disease pathology. Disease Impact – Years of Potential Life Lost (YPLL) SGU Cures Index 155 187,139 years of potential life were lost in 2001 in the US to Alzheimer's disease (WHO Department of Measurement and Health Information – Global Burden of Disease Study – Years of Life Lost, 2001). Disease Impact – Disability Adjusted Life Years (DALYs) 60 DALYs lost per 100,000 people in 2004 (WHO Department of Measurement and Health Information, 2009). Given a total population of 292,810,000 in the US in 2004, 761,306 DALYs were lost to Alzheimer’s disease in 2004 in the US Economic Impact – Years of Potential Life Lost (YPLL) 187,139 years of potential life were lost to Alzheimer’s disease in 2001, with an associated annual VMRR of $19.499 billion. This represents the potential value to be gained from further cure advancements for Alzheimer’s disease. Economic Impact – Disability Adjusted Life Years (DALYs) 761,306 DALYs were lost to Alzheimer’s disease in 2004, with an associated annual VMRR of $79.323 billion. This represents the potential value to be gained from further cure advancements for Alzheimer’s disease. History of the Disease and Breakthroughs A German psychiatrist, Alois Alzheimer, first described what is now known as Alzheimer’s disease in 1906 in a 50-year-old woman, named Auguste D. who had profound memory loss, suspicion of her family, and other psychological changes. Alois Alzheimer conducted an autopsy on Auguste D. after she died in 1906 and found dramatic brain shrinkage and abnormal deposits in and around nerve cells (Alzheimer et al., 1995). Emil Kraepelin was the first person to describe it as a distinctive disease and named it Alzheimer’s disease in his book Pysciatrie (Alzheimer’s Association, 2014). Many discoveries have occurred in the past 30 years. The β-amyloid protein was first identified in 1984 and now appears to be a central factor in the events leading to neuronal damage (Sloane, Zimmerman, Suchindran, Reed, Wang, Boustani, et al., 2002). The tau protein, which forms neurofibrillary tangles, was first identified in 1986 (Grundke-Iqbal et al., 1986). These were important discoveries because they are widely recognized as hallmarks in the understanding of Alzheimer’s brain abnormality. Developing a better understanding about these proteins can help with advances in SGU Cures Index 156 treatment and/or in detecting early brain changes during the preclinical phase. In 1987, researchers identified the first gene associated with the rare inherited form of Alzheimer’s disease (Kang et al., 1987). The gene, APP, codes for the amyloid precursor protein, which is the parent molecule from which β-amyloid is formed (Robakis et al., 1987). APOE4, which is a form of the APOE gene on chromosome 19, was the first risk factor gene to be described by researchers in 1993 (Strittmatter et al., 1993). A clinical trial of the drug tacrine started in 1987 and was approved by the FDA in 1993. It was the first drug used to target specific symptoms of the disease (Alzheimer’s association, 2014). Donepezil, galantamine, memantine, and rivastigmine were all approved in the 10 years following the approval of tacrine (Alzheimer’s Association, 2014). However, due to significant liver toxicity, tacrine has been discontinued in the United States (United States National Library of Medicine, 2014). Current Cure Status Currently, there is no cure for Alzheimer’s disease. Drug and non-drug treatments help with behavioral and cognitive symptoms, but do not alter the clinical course of the disease. Active medical management has been shown to improve the quality of life of people living with Alzheimer’s disease (Vickrey, Mittman, Connor, Pearson, Della Penna, et al., 2006; Voisin & Vellas, 2009). The types of management include: appropriate use of available treatment options, management of coexisting symptoms, coordination of care, participation in activities, and support groups (Thies & Bleiler, 2013). The drugs currently used to delay symptoms from becoming worse for a limited period of time are cholinesterase inhibitors and N-methyl D-aspartate (NMDA) antagonists (National Institute on Aging, 2008). Cholinesterase inhibitors slow the breakdown of the neurotransmitter acetylcholine by the enzyme acetylcholinesterase (National Institute on Aging, 2008; Wilkinson et al, 2004). The FDA has approved several drugs in this class: Aricept® (donepezil), Razadyne® (galantamine), and Exelon® (rivastgmine). However, as Alzheimer’s progresses, the brain produces less acetylcholine, thereby causing the inhibitors to lose their effect (National Institute on Aging, 2008). Namenda® (memantine) is an NMDA antagonist and is used in moderate to severe Alzheimer’s. Its proposed mechanism of action is to regulate glutamate in the brain (National Institute on Aging, 2008). The drug’s main effect is to delay progression of some of the symptoms, such as maintaining the ability to independently use the bathroom. It has been found to have a small positive effect on cognition at six months (McShane, Areosa, & Minakaran, 2009). However, memantine is not effective in individuals with Down SGU Cures Index 157 syndrome who develop Alzheimer’s after the age of 40 (Hanney, Prasher, Williams, Jones, Aarsland, Corbett, et al., 2012). The current best practice treatment for those with Alzheimer’s disease is environmental care and management. This can range from intermittent care in the early stages of the disease to round-the-clock nursing and intensive health care in the latter stages of the disease. Unpaid caregivers, such as family members and friends, provide 80% of care to patients suffering from Alzheimer’s disease (Institute of Medicine, 2008). In 2010 the direct cost for caring for people with Alzheimer’s disease and dementia in the US was between $159 billion and $215 billion (Hurd, Martorell, Delavande, Mullen, & Langa, 2013). The same study estimated direct health care expenses, including costs of nursing home care, Medicare, and out-of-pocket expenses, to be $109 billion in 2010 (Hurd et al., 2013). In 2012, unpaid caregivers provided an estimated 17.5 billion hours of unpaid care, which is valued at more than $216 billion (Feinberg, Reinhard, Houser, & Choula, 2011). For people with Alzheimer’s disease and other dementia related diseases, aggregate payments for health care, long-term care, and hospice care are $203 billion (Thies & Bleiler, 2013). Future Cure Obstacles While there is no cure for Alzheimer’s disease, there has been substantial research in understanding disease mechanisms and some success in symptomatic treatments. The cholinesterase inhibitors that are currently prescribed delay the progression of the disease by six to nine months (Sloane et al., 2002). Researchers believe the best response to treatment will occur in the early preclinical stages of Alzheimer’s when no symptoms are apparent. Unfortunately, there have been no significant breakthroughs in disease course-altering drugs (Mangialasche et al., 2010), however, there have been numerous discoveries of the pathophysiological processes that underlie the disease over the past decade (Kozauer & Katz, 2013). Biomarkers will be critical in diagnosing the disease at the preclinical phase for disease modifying treatments or preventative measures (Sperling, Aisen, Beckett, Bennett, Craft, Fagan, et al., 2011). There are past and present drug trials targeting all aspects of β-amyloid in the brain. These drug trials are aimed at reducing β-amyloid production, preventing β-amyloid aggregation, and promoting β-amyloid clearance. To reduce β-amyloid production, researchers are attempting to develop β-secretase inhibitors. However, β-secretase inhibitors are challenging to develop because the enzyme has many substrates and the drugs have to cross the blood-brain barrier, which resulted in few candidate drugs reaching phase III randomized controlled trials (RCT) (Mangialasche et al., 2010). SGU Cures Index 158 Developing drugs to prevent β-amyloid aggregation is an attractive approach, however, very few aggregation inhibitors have moved into clinical testing (Citron, 2010). There are immune-mediated mechanisms to promote β-amyloid removal. Active and passive immunizations have been developed to inhibit toxic β-amyloid aggregates and to remove soluble and aggregated β-amyloid (Mangialasche et al, 2010). Immunotherapy is a promising new type of therapy because it contains antibodies against β-amyloid (Relkin et al., 2009) and more than ten immunotherapeutic agents have entered clinical trials (Citron, 2010). A small trial has shown positive effects on cognition (Relkin et al, 2009), however, over 20 RCTs aimed at β-amyloid have failed through either serious adverse reactions or failure to meet target endpoints of reducing β-amyloid or improving cognition (Wischik et al., 2014) with none yet coming to market. There are two main approaches to target the tau protein: (1) compounds that inhibit tau aggregation and/or promote disassembly of tau aggregates, and (2) modulation of tau phosphorylation with inhibitors of tau-phosphorylating kinases (Mangialasche et al., 2010). Tau hyperphosphorylation and neurofibrillary tangles can be promoted by imbalanced activity of protein kinases, such as glycogen-synthase-kinase-3 (GSK3) (Mangialasche et al., 2010). Researchers are developing several GSK3 inhibitors, one of which is a thiadiazolidione-derived compound that can reduce brain concentrations of phosphorylated tau and amyloid deposition in animals (Serenó, Coma, Rodriguez, Sanchez-Ferrer, Sanchez, Gich, et al., 2009). This drug, tideglusib, has been tested in a small population of Alzheimer’s patients; the drug produced positive trends in mini mental status examinations (MMSE) without statistical significance. It is being tested in a larger clinical trial because there was insufficient evidence to support or reject the benefit of tideglusib (del Ser, Steinwachs, Gertz, Andres, Gomez-Carillo, Medina, et al., 2013). However, two different phase II trials targeting GSK3 failed to demonstrate any significant effect on cognitive decline (Wischik, Harrington, & Storey, 2014). Researchers have not been successful in designing pharmacological agents that target the tau protein, as results from trials fail to show an effect on cognition and functional status. A new approach to designing Alzheimer’s disease therapies involves targeting mitochondrial dysfunction, since there is evidence to suggest that synaptic damage and mitochondrial dysfunction play a role in aging and Alzheimer’s disease development (Reddy & Beal, 2008). Researchers are investigating a drug, Latrepirdine, which was previously used as a non-selective antihistamine and was found to increase mitochondrial membrane potential and ATP production (Zhang, Hedskog, Hansson Petersen, Winblad, & Ankarcrona, 2010). It was also found to increase mitochondrial function in both the presence and absence of stress (Zhang et al., 2010). Latrepirdine was used in Russia and in a phase II randomized trial, it significantly improved the SGU Cures Index 159 clinical course of patients with mild to moderate Alzheimer’s disease, however the results were not confirmed in a phase III CONCERT trial (Sweetlove, 2012). Another therapeutic approach to Alzheimer’s disease is targeting neurotrophins, as neurons depend on NGF and BDNF for survival. Researchers are attempting to increase NGF in the brain through implanting NGF into the cholinergic basal forebrain (Wahlberg, Lind, Almqvist, Kusk, Tornøe, & Juliusson, 2012), intracerebroventricular infusion of NGF (Eriksdotter, Nordberg, Amberla, Backman, Ebendal, Myerson, et al., 1998), gene therapy (Tuszynski et al., 2015), and intranasal delivery and application of NGF solution on the ocular surface, which are less invasive than the previously mentioned therapies (Cattaneo, Capsoni, & Paoletti, 2008). Research has found that implanting neural stem cells into transgenic mouse models of Alzheimer’s disease improves cognition by increasing BDNF and more recently it has been reported that statins, commonly used to reduce cholesterol, increase BDNF (Strom et al., 2015), which may explain why people who start taking statins in middle age have reduced risk of developing Alzheimer’s disease (Swiger et al., 2013). Research Development and Treatment Costs NIH spending on Alzheimer’s disease (National Institutes of Health Research, 2014): 2009: $457 million 2010: $450 million 2011: $448 million 2012: $503 million 2013 (Est.): $484 million 2014 (Est.): $562 million The cost of the medications for Alzheimer’s disease is between $177 and $400+ per month (Consumer Reports, 2012). In summary, lack of success in developing treatment for Alzheimer’s has been attributed to initiating treatment too late in the progression of the disease, when there is likely too much brain damage to overcome, and the possibility that the complex biology of Alzheimer’s will require more than one therapeutic intervention to prevent the cascade leading to destruction of brain cells. In recognition of this, in April 2015 the Centers for Medicare and Medicaid Services (CMS) approved a four-year, $100 million study called Imaging Dementia—Evidence for Amyloid Scanning (IDEAS) to qualify participants for Medicare/Medicaid reimbursement in research programs to investigate methods of brain imaging that will identify biomarkers for pre-clinical Alzheimer’s disease. Similarly, SGU Cures Index 160 in September 2015 the National Institute of Aging posted a call for grant proposals titled “Capturing Complexity in the Molecular and Cellular Mechanisms Involved in the Etiology of Alzheimer's Disease” to award at least $20 million annually to research institutions for multi-year projects. With annual costs currently estimated at $226 billion and projected to increase to over $1 trillion by the year 2050 (Alzheimer’s Association 2015), there is good reason to increase the amount spent on research, ($638 million in 2016 projected by NIH), to find cures for this devastating disease. Citations Allen, S. J., Watson, J. J., & Dawbarn, D. (2011). The neurotrophins and their role in Alzheimer’s disease. Current Neuropharmacology, 9, 559-573. Alzheimer’s Association. (2014). Major milestones in Alzheimer’s and brain research. Retrieved on June 12, 2014 from: http://www.alz.org/research/science/major_milestones_in_alzheimers.asp. Alzheimer, A., Stelzmann, R. A., Schnitzlein, H. N., Murtagh, F. R. (1995). An english translation of Alzheimer's 1907 paper, "Uber eine eigenartige Erkankung der Hirnrinde". Clinical Anatomy, 8, 429-431. Blennow, K., de Leon, M. J., & Zetterberg, H. (2006). Alzheimer’s disease. Lancet, 368, 387-403. Born, H. A., Kim, J. Y., Savjani, R. R., Das, P., Dabaghian, Y. A., Guo, Q., Yoo, J. W., Schuler, D. R., Cirrito, J. R., Zheng, H., Golde, T. E., Noebels, J. L., Jankowsky, J. L. (2014). Genetic suppression of transgenic APP rescues Hypersynchronous network activity in a mouse model of Alzeimer's disease. Journal of Neuroscience, 34, 3826-3840. Boustani, M., Peterson, B., Hanson, L., Harris, R., & Lohr, K. (2003). Screening for dementia in primary care: A summary of the evidence for the US Preventative Services Task Force. Annals of Internal Medicine, 138, 927-937. Brookmeyer, R., Corrada, M. M., Currieri, F.C., & Kawas, C. (2002). Survival following a diagnosis of Alzheimer disease. Archives of Neurology, 59, 1764-1767. Carroll, J. C., & Pike, C. J. (2008) Selective estrogen receptor modulators differentially SGU Cures Index 161 regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology, 149, 2607-2611. Caspersen, C., Wang, N., Yao, J., Sosunov, A., Chen, X., Lustbader, J.W., Xu, X., Stern, D., McKhann, G., & Yan, S. D. (2005). Mitochondrial Aβ: a potential point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB journal, 19, 2040. Cattaneo, A., Capsoni, S., & Paoletti, F. (2008). Towards non-invasive nerve growth factor therapies for Alzheimer’s disease. Journal of Alzheimer’s disease, 15, 255283. Centers for Disease Control and Prevention (2014). Life Expectancy. Retrieved on May 23, 2014 from: http://www.cdc.gov/nchs/fastats/life-expectancy.htm. Citron, M. (2010). Alzheimer’s disease: strategies for disease modification. Nature Reviews, 9, 387-398. Consumer Reports. (2012). Evaluating prescription drugs used to treat: Alzheimer’s disease comparing effectiveness, safety and price. Consumer Reports Health. Retrieved on January 22, 2014 from: http://www.consumerreports.org/health/resources/pdf/best-buydrugs/AlzheimersFINAL.pdf. Craft, S., Cholerton, B., & Baker, L. D. (2013). Insulin and Alzheimer’s disease: Untangling the web. Journal of Alzheimer’s Disease, 33, S263-S275. Cramer, P. E., Cirrito, J. R., Wesson, D. W., Lee, C. Y., Karlo, J. C., Zinn, A. E., Casali, B. T., Restivo, J. L., Goebel, W. D., James, M. J., Brunden, K. R., Wilson, D. A., & Landreth, G. E. (2012). ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science, 335, 1503-1506. del Ser, T., Steinwachs, K. C., Gertz, H. J., Andres, M. V., Gomez-Carillo, B., Medina, M., Vericat, J. A., Redondo, P., Fleet, D., & León, T. (2013). Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. Alzheimer’s Disease, 33, 205-215. Dougall, N. J., Bruggink, S., & Ebmeier, K. P. 2004. Systematic review of the diagnostic accuracy of 99mTc-HMPAO-SPECT in dementia. The American Journal of Geriatric Psychiatry, 12 (6), 554–70. SGU Cures Index 162 Eriksdotter J. M., Nordberg, A., Amberla, K., Backman, L., Ebendal, T., Myerson, B., Olson, L., Seiger, Å., Shigeta, M., Theodorsson, E., Viitanen, M., Winblad B., & Wahlund, L. O. (1998). Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dementia and geriatric cognitive disorders, 9, 246-257. Espeland, M. A., Brinton, R. D., Manson, J. E., Yaffe, K., Hugenschmidt, C., Vaughan, L., Craft, S., Edwards, B. J., Casanova, R., Masaki, K., Resnick, S. M., Group, W-M. S. (2015). Postmenopausal hormone therapy, type 2 diabetes mellitus, and brain volumes. Neurology. Evans, D. A., Hebert, L. E., Beckett, L. A., Scherr, P. A., Albert, M. S., Chown, M. J., Pilgrim, D. M., Taylor, J. O. (1997). Education and other measures of socioeconomic status and risk of incident Alzheimer Disease in a defined population of older persons. Archives of Neurology, 54, 1399-1405. Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., Myers, R. H., Pericak-Vance, M. A., Risch, N., van Duijn, C. M. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. Journal of the American Medical Association, 278, 1349-1356. Feinberg, L., Reinhard, S. C., Houser, A., & Choula, R. (2011). Valuing the invaluable: 2011 update: the growing contributions and costs of family caregiving. Washington, DC: AARP Public Policy Institute. Fitzpatrick, A. L., Kuller, L. H., Ives, D. G., Lopez, O. L., Jagust, W., Breitner, J. C., Jones, B., Lyketsos, C., Dulberg, C. (2004). Incidence and prevalence of dementia in the Cardiovascular Health Study. Journal American Geriatric Society, 52, 195–204. Foy, M. R. (2011). Ovarian hormones, aging and stress on hippocampal synaptic plasticity. Neurobiology of Learning and Memory, 95, 134-144. Fratiglioni, L., Ahlbom, A., Viitanen, M., & Winblad, B. (1993). Risk factors for late-onset Alzheimer’s disease: a population-based, case-control study. Annals of Neurology, 33, 258-266. SGU Cures Index 163 Ghosh, A. K., Brindisi, M., & Tang, J. (2012) Developing β-secretase inhibitors for treatment of Alzheimer’s disease. Journal of Neurochemistry, 120, 71-83. Götz, J., Chen, F., van Dorpe, J., & Nitsch, R. M. (2001) Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ42 fibrils. Science, 293, 14911495. Good, P. F., Werner, P., Hsu, A., Olanow, C. W., & Perl, D. P. (1996). Evidence for neuronal oxidative damage in Alzheimer’s disease. American journal of pathology, 149, 21-28. Green, R. C., Cupples, L. A., Go, R., Benke, K. S., Edeki, T., Griffith, P. A., Williams, M., Hipps, Y., Graff-Radford, N., Bachman, D., & Farrer, L. A. (2002). Risk of dementia among white and African American relatives of patients with Alzheimer disease. Journal of the American Medical Association, 287, 329-336. Grundke-Iqbal, I., Iqbal, K., Tung, Y. C., Quinlan, M., Wisniewski, H. M., & Binder, L. I. (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proceedings of the National Academy of Sciences USA, 83, 4913-4917. Hanney, M., Prasher, V., Williams, N., Jones, E. L., Aarsland, D., Corbett, A., Lawrence, D., Yu, L., Tyrer, S., Francis, P. T., Johnson, T., Bullock, R., Ballard, C. (2012). Memantine for dementia in adults older than 40 with Down’s syndrome (MEADOWS): A randomised, double-blind, placebo- controlled trial. Lancet, 379 (9815), 528-536. Hartley, D., et al. (2015). Down syndrome and Alzheimer's disease: Common pathways, common goals. Alzheimers Dementia, 11, 700-709. Hebert L. E., Beckett, L. A., Scherr, P. A., & Evans, D. A. (2001a). Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer disease & Associated disorders, 15, 169-173. Hebert L. E., Scherr, P. A., McCann, J., Beckett, L. A., & Evans, D. A. (2001b). Is the risk of developing Alzheimer’s disease greater for women than men? American Journal of Epidemiology,153, 132-136. SGU Cures Index 164 Hebert, L. E., Scherr, P. A., Bienias, J. L., Bennett, D. A., & Evans, D. A. (2003). Alzheimer’s disease in the US population: prevalence estimates using the 2000 census. Archives of Neurology, 60,1119-1122. Hebert, L. E, Weuve, J., Scherr, P. A., & Evans, D. A. (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80, 1778-1783. Hirtz, D., Thurman, D. J., Gwinn-Hardy, K., Mohamed, M., Chaudhuri, A. R., & Zalutsky, R. (2007). How common are the “common” neurologic disorders? Neurology, 68, 326-337. Hulstaert, F., Blennow, K., Ivanoiu, A., Schoonderwaldt, H. C., Riemenschneider, M., De Deyn, P. P., Bancher, C., Cras, P., Wiltfang, J., Mehta, P. D., Iqbal, K., Pottel, H., Vanmechelen, E., & Vanderstichele, H. (1999). Improved discrimination of AD patients using β-amyloid(1-42) and tau levels in CSF. Neurology, 52, 1555-1562. Hunter, J. M., Cirrito, J. R., Restivo, J. L., Kinley, R. D., Sullivan, P. M., Holtzman, D. M., Koger, D., Delong, C., Lin, S., Zhao, L., Liu, F., Bales, K., Paul, S. M. (2012). Emergence of a seizure phenotype in aged apolipoprotein epsilon 4 targeted replacement mice. Brain Research, 1467, 120-132. Hurd, M. D., Martorell, P., Delavande, A., Mullen, K. J., & Langa, K. M. (2013). Monetary costs of dementia in the United States. New England Journal of Medicine, 368, 1326-1334. Imtiaz, B., Tuppurainen, M., Tiihonen, M., Kivipelto, M., Soininen, H., Hartikainen, S., & Tolppanen, A. M. (2014). Oophorectomy, hysterectomy, and risk of Alzheimer's disease: A nationwide case-control study. Journal of Alzheimers Disease, 42, 575-581. Institute of Medicine. Retooling for an aging America: building the healthcare workforce. Retrieved on January 28, 2014 from: http://www.iom.edu/Reports/2008/Retooling-for-an-aging-America-Buildingthe-Health-Care-Workforce.aspx Ittner, L. M., & Götz, J. (2011). Amyloid-β and tau – a toxic pas de deux in Alzheimer’s disease. Nature Reviews Neuroscience, 12, 67-72. SGU Cures Index 165 Kang, J., Lemaire, H. G., Unterbeck, A., Salbaum, J. M., Masters, C. L., Grzeschik, K. H., Multhaup, G., Beyreuther, K., & Muller-Hill, B. (1987). The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature, 325, 733-736. Killiany, R. J., Gomez-Isla, T., Moss, M., Kikinis, R., Sandor, T., Jolesz, F., Tanzi, R., Jones, K., Hyman, B. T., & Albert, M. S. (2000). Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Annals of Neurology, 47, 430-439. Klunk, W. E., Engler, H., Nordberg, A., Wang, Y., Blomqvist, G., Holt, D., Bergström, M.,Savitcheva, I., Huang, G., Estrada, S., Ausén, B., Debnath, M. L., Barletta, J., Price, J. C., Sandell, J., Lopresti, B. J., Wall, A., Koivisto, P., Antoni, G., Mathis, C. A., & Långström, B. (2004). Imaging brain amyloid in Alzheimer’s disease with Pittsburg compound-B. Annals of Neurology, 55, 306-319. Klunk, W.E., Wang, Y., Huang, G. F., Debnath, M. L., Holt, D. P., & Mathis, C. A. (2001). Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sciences, 69, 1471-1484. Kozauer, N., & Katz, R. (2013). Regulatory innovation and drug development for earlystage Alzheimer's disease. New England Journal of Medicine, 368(13), 11691171. Larson E. B., Shadlen, M., Wang, L., McCormick, W. C., Bowen, J. D., Teri, L., & Kukull, W. A. (2004). Survival after initial diagnosis of Alzheimer Disease. Annals of Internal medicine, 140, 501-509. Lewis, J., Dickson, D. W., Lin, W., Chisholm, L., Corral, A., Jones, G., Yen, S., Sahara, N., Skipper, L., Yager, D., Eckman, C., Hardy, J., Hutton, M., & McGowan, E. (2001). Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science, 293, 1487-1491. Lott, I. T., Doran, E., Nguyen, V. Q., Tournay, A., Movsesyan, N., & Gillen, D. L. (2012). Down syndrome and dementia: seizures and cognitive decline. Journal of Alzheimers Disease, 29, 177-185. Lye, T. C., & Shores, E. A. (2000). Traumatic brain injury as a risk factor for Alzheimer’s SGU Cures Index 166 disease: a review. Neuropsychological Review, 10, 115-129. Mahley, R. W., Weisgraber, K. H., & Huang, Y. (2006). Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proceedings of National Academy of Sciences, 103 (15), 5644-5651. Mangialasche, F., Solomon, A., Winblad, B., Mecocci, P., & Kivipelto, M. (2010). Alzheimer’s disease: clinical trials and drug development. Lancet Neurology, 9, 702-716. Mapstone, M., Cheema, A. K., Fiandaca, M. S., Zhong, X., Mhyre, T. R., MacArthur, L. H., Hall, W. J., Fisher, S. G., Peterson, D. R., Haley, J. M., Nazar, M. D., Rich, S. A., Berlau, D. J., Peltz, C. B., Tan, M. T., Kawas, C. H., & Federoff, H. J. (2014). Plasma phospholipids identify antecedent memory impairment in older adults. Nature medicine Letters. McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., Stadlan, M. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939-944. McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., & Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., Mayeux, R., Mohs, R. C., Morris, J. C., Rossor, M. N., Scheltens, P., Carrillo, M. C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7, 263-269. McShane, R., Areosa, S. A., & Minakaran, N. (2009). Memantine for dementia. Cochrane database of systematic reviews, 1. Minkeviciene, R., Rheims, S., Dobszay, M. B., Zilberter, M., Hartikainen, J., Fulop, L., Penke, B., Zilberter, Y., Harkany, T., Pitkanen, A., & Tanila, H. (2009). Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. Journal of Neuroscience, 29, 3453-3462. Musiek, E. S., & Holtzman, D. M. (2015) Three dimensions of the amyloid hypothesis: time, space and 'wingmen'. Nature Neuroscience, 18, 800-806. SGU Cures Index 167 National Institute on Aging (2008). Alzheimer’s disease medications: fact sheet. NIH publication number 08-3431. National Institute on Aging (2014). About Alzheimer’s disease: symptoms. Retrieved on June 12, 2014 from: http://www.nia.nih.gov/alzheimers/topics/symptoms National Institutes of Health Research (2014). Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). Retrieved March 31, 2014, from: http://report.nih.gov/categorical_spending.aspx National Center for Health and Statistics (2013). Deaths: Final Data for 2010. Detailed Tables for the National Vital Statistics Report. Retrieved on January 13, 2014 from: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf Oddo, S., Caccamo, A., Shepherd, J. D., Murphy, M. P., Golde, P. E. Kayed, R., Metherate, R., Mattson, M. P., Akbari, Y., & LaFerla, F. M. (2003). Tripletransgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfuction. Neuron, 39, 409-421. Plassman, B. L., Langa, K. M., Fisher, G. G., Heeringa, S. G., Weir, D. R., Ofstedal, M. B., Burke, J. R., Hurd, M. D., Potter, G. G., Rodgers, W. L., Steffens, D. C., Willis, R. J., & Wallace, R. B. (2007). Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemilogy, 29(1-2), 125132. Pike, C. J., Carroll, J. C., Rosario, E. R., & Barron, A. M. (2009) Protective actions of sex steroid hormones in Alzheimer's disease. Frontiers in Neuroendocrinology, 30, 239-258. Querfurth H. W., & LaFerla, F. M. (2010). Alzheimer’s disease. New England Journal of Medicine, 362, 329-344. Reddy, P. H., & Beal, M. F. (2008). Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends in Molecular Medicine, 14, 45-53. Relkin, N. R., Szabo, P., Adamiak, B., Burgut, T., Monthe, C., & Lent, R. W. (2009). 18- SGU Cures Index 168 month study of intravenous immunoglobin for treatment of mild Alzheimer disease. Neurobiology of aging, 30, 1728-1736. Robakis, N. K., Wisniewski, H. M., Jenkins, E. C., Devine-Gage, E. A., Houck, G. E., Yao, X. L., Ramakrishna, N., Wolfe, G., Silverman, W. P., & Brown, W. T. (1987). Chromosome 21q21 sublocalisation of gene encoding beta-amyloid peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet, 1, 384-385. Rumble, B., Retallack, R., Hilbich, C., Simms, G., Multhaup, G., Martins, R., Hockey, A., Montgomery, P., Beyreuther, K., Masters, C. L. (1989). Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. New England Journal of Medicine, 320, 1446-1452. Serenó, L., Coma, M., Rodriguez, M., Sanchez-Ferrer, P., Sanchez, M. B., Gich, I., Agulló, J.M., Pérez, M., Avila, J., Guardia-Laguarta, C., Clarimón, J., Lleó, A., & Gómez-Isla, T. (2009). A novel GSK-3β inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiology of Disease, 35, 359-367. Simpkins, J. W., Yi, K. D., Yang, S. H., & Dykens, J. A. (2010) Mitochondrial mechanisms of estrogen neuroprotection. Biochimica et Biophysica Acta, 1800, 1113-1120. Sloane, P. D., Zimmerman, S., Suchindran, C., Reed, P., Wang, L., Boustani, M., & Sudha, S. (2002). The public health impact of Alzheimer’s disease, 2000-2050: potential implications of treatment advances. Annual Reviews of Public Health, 23, 213-231. Small, S. A., Perera, G. M., DelaPaz, R., Mayeux, R., & Stern, Y. (1999). Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Annals of Neurology, 45, 466-472. Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., Iwatsubo, T., Jack Jr., C. R., Kaye, J., Montine, T. J., Park, D. C., Reiman, E. M., Rowe, C. C., Siemers, E., Stern, Y., Yaffe, K., Carrillo, M. C., Thies, B., MorrisonBogorad, M., Wagster, M. V., & Phelps, C. H. (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendation from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7, 280-292. SGU Cures Index 169 Stern, Y., Gurland, B., Tatemichi, T. K., Tang, M. X., Wilder, D., & Mayeux, R. (1994). Influence of educations and occupation on the incidence of Alzheimer’s disease. Journal of the American Medical Assciation, 271, 1004-1010. Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S., & Roses, A. D. (1993). Apolipoprotein E. High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of National Academy of Sciences, 90, 1977-1981. Strom, B. L., Schinnar, R., Karlawish, J., Hennessy, S., Teal, V., & Bilker, W. B. (2015). Statin Therapy and Risk of Acute Memory Impairment. Journal of the American Medical Associatio Internal Medicine, 175, 1399-1405. Sunderland, T., Linker, G., Mirza, N., Putnam, K. T., Friedman, D. L., Kimmel, L. H., Bergeson, J., Manetti, G. J., Zimmermann, M., Tang, B., Bartko, J. J., & Cohen, R. M. (2003). Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. Journal of the American Medical Association, 289, 2094-2103. Sweetlove, M. (2012). Phase III CONCERT trial of latrepirdine: negative results. Pharmaceutical medicine, 26, 113-115. Swiger, K. J., Manalac, R. J., Blumenthal, R. S., Blaha, M. J., & Martin, S. S. (2013). Statins and cognition: a systematic review and meta-analysis of short- and longterm cognitive effects. Mayo Clinic Proceedings, 88, 1213-1221. Tarawneh, R., Head, D., Allison, S., Buckles, V., Fagan, A. M., Ladenson, J. H., Morris, J. C., & Holtzman, D. M. (2015). Cerebrospinal Fluid Markers of Neurodegeneration and Rates of Brain Atrophy in Early Alzheimer Disease. Journal of the American Medical Association Neurology, 72, 656-665. Thies, W., & Bleiler, L. (2013). 2013 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia, 208-245. Tuszynski, M. H., Thail, L., Pay, M., Salmon, D. P., Sang, H., Bakay, R., Patel, P., Blesch, A., Vahlsing, H. L., Ho, G., Tong, G., Potkin, S. G., Fallon, J., Hansen, L., Mufson, E. J., Kordower, J. H., Gall, C., & Conner, J. (2005). A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature Medicine Letters, 11, 551-555. SGU Cures Index 170 Tuszynski, M. H., Yang, J. H., Barba, D., U, H. S., Bakay, R. A., Pay, M. M., Masliah, E., Conner, J. M., Kobalka, P., Roy, S., & Nagahara, A. H. (2015). Nerve growth factor gene therapy: Activation of neuronal responses in Alzheimer disease. Journal of the American Medical Association Neurology. United States National Library of Medicine (2014). LiverTox-Tacrine. Retrieved June 12, 2014 from: http://www.livertox.nih.gov/Tacrine.htm#other_refs Vickrey, B. G., Mittman, B. S., Connor, K. I., Pearson, M. L., Della Penna, R. D., Ganiats, T. G., DeMonte Jr., R. W., Chodosh, J., Cui, X., Vassar, S., Duan, N., & Lee, M. (2006). The effect of a disease management intervention on quality and outcomes of dementia care. Annals of internal medicine, 145, 713-726. Voisin, T., & Vellas, B. (2009). Diagnosis and treatment of patients with severe Alzheimer’s disease. Drugs and Aging, 26 (2), 135-144. Vossel, K. A., Beagle, A. J., Rabinovici, G. D., Shu, H., Lee, S. E., Naasan, G., Hegde, M., Cornes, S. B., Henry, M. L., Nelson, A. B., Seeley, W. W., Geschwind, M. D., Gorno-Tempini, M. L., Shih, T., Kirsch, H. E., Garcia, P. A., Miller, B. L., & Mucke, L. (2013). Seizures and epileptiform activity in the early stages of Alzheimer disease. Journal of the American Medical Association Neurology, 70, 1158-1166. Wahlberg, L. U., Lind, G., Almqvist, P. M., Kusk, P., Tornøe, J., & Juliusson, B. (2012). Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: a technology platform for restorative neurosurgery. Journal of Neurosurgery, 117, 340-347. Waring, S. C., & Rosenberg, R. N. (2008). Genome-wide association studies in Alzheimer disease. Archives of Neurology, 65, 329-334. Wenk, G. L. (2003). Neuropathologic changes in Alzheimer’s disease. Journal of Clinical Psychiatry, 64, 7-10. Wilkinson, D. G., Francis, P. T., Schwam, E., & Payne-Parrish, J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease. (2004). Drugs and Aging, 21, 453-478. Wischik, C. M. Harrington, C. R., & Storey, J. M. D. (2014). Tau-aggregation inhibitor SGU Cures Index 171 therapy for Alzheimer’s disease. Biochemical Pharmacology, 88, 529-539. Zhang, S., Hedskog, L., Hansson Petersen, C. A., Winblad, B., & Ankarcrona, M. (2010). Dimebon (Latrepirdine) enhances mitochondrial function and protects neuronal cells from death. Journal of Alzheimer’s Disease, 21, 389-402. SGU Cures Index 172 CARDIOVASCULAR DISEASE Marium Khan, Eve Zohorsky Disease Category Chronic Disease identification/Description/Diagnostic Criteria Cardiovascular disease (CVD), more commonly known as heart disease, encompasses a number of conditions related to the cardiovascular system. Such conditions may include coronary artery disease (CAD), myocardial infarctions (heart attacks), angina (chest pain), heart failure, and others (Centers for Disease Control, 2009; National Heart, Lung, and Blood Institute, 2011). Heart disease can be diagnosed with various types of tests performed by a physician. These tests may include a stress test, an electrocardiogram (EKG), echocardiography, chest x-ray, blood tests, electron-beam computed tomography, or coronary angiography/cardiac catheterization (National Heart, Lung, and Blood Institute, 2011). Through physical examination and diagnostic tests, a physician can properly diagnose the type and severity of CVD (National Heart, Lung, and Blood Institute, 2011). While CVD characterizes the nature of heart disease, there are several other types of cardiovascular conditions that may potentially result in heart disease if left unaddressed. Hypertension is an important determinant of developing CVD. Hypertension is characterized as an increase in the pressure of blood throughout the arteries of an individual (Mayo Clinic, 2010). Blood pressure can be classified as normal, prehypertension, Stage 1 hypertension, or Stage 2 hypertension based on specific ranges of systolic and diastolic pressures (National Heart, Lung, and Blood Institute, 2011). Blood pressure readings of 120/80 and below are considered normal, while readings above this threshold place an individual into a degree of hypertension (National Heart, Lung, and Blood Institute, 2011). Hypertension may result from various causes, including diets high in sodium, physical inactivity, overweight/obesity, and tobacco use. Genetic factors including family history of heart disease, diabetes, as well as race and ethnicity can also lead to increased blood pressure (Centers for Disease Control, 2010a). Although hypertension is not a type of CVD but rather a condition, it can lead to several other heart disease-related disorders, including heart attacks or coronary heart disease. Coronary artery disease is the most common type of heart disease in the US, and is SGU Cures Index 173 caused by the buildup of a plaque within the coronary arteries (Centers for Disease Control, 2011). Atherosclerosis is a disease in which there is plaque accumulation within arteries throughout the body. Although there are many types of conditions that can be classified as CVD, there are similarities among their etiology. Disease Etiology (Cause) Cardiovascular disease is a term used to describe a number of different conditions classified as heart disease. High blood pressure is associated with older age, the presence of another medical condition (e.g., thyroid disease), family history, side effects of certain medications (e.g., birth control, corticosteroids), and/or hormone therapy in women. Other paths to cardiovascular disease involve an increased amount of cholesterol and fats circulating in the blood, an increase in sugar within the blood due to insulin resistance or diabetes and possible behavioral causes such as smoking (National Heart, Lung, and Blood Institute, 2011). Excess strain on the heart and vessels due to the presence of plaques or other causes can result in a loss of adequate blood flow throughout the body. When there is a reduction in blood flow to the heart directly, the risk of developing a myocardial infarction (heart attack) increases. A heart attack occurs when a vessel leading to the heart becomes blocked, reducing proper blood flow to the heart and possibly damaging the heart muscle (Centers for Disease Control, 2011). Similliarly, a blockage of a vessel in the brain leads to a stroke. Disease Prevalence/Incidence Cardiovascular disease is the leading cause of death among Americans, with 597,689 deaths in 2010 alone (Centers for Disease Control, 2013). This yields an age-adjusted rate of about 197.1 per 100,000 individuals (Hoyert & Xu, 2012). The Centers for Disease Control (CDC) reported that in 2011, 26,486,000 Americans had heart disease (Schiller, Lucas, & Peregoy, 2012). While CVD is the leading cause of death in the US, it is also the largest source of recent reductions in mortality. The decline began in the 1960s. Most recently, the death rate declined by 32.7 percent over the decade from 1999 to 2009 (Go et al., 2012). A myocardial infarction, or heart attack, is an acute manifestation of CVD. In 2009, approximately 635,000 Americans experienced a myocardial infarction for the first time, and 280,000 had a recurrent attack (Go et al., 2012.) SGU Cures Index 174 In addition to the number of Americans diagnosed with heart disease, the CDC estimated that an additional 59,000,000 Americans had some degree of hypertension in 2011 (Schiller, Lucas, & Peregoy, 2012). Further, 71 million American adults have high levels of low-density lipoprotein (LDL), or “bad” cholesterol, which contributes to plaque formation in the arteries (Centers for Disease Control, 2011). Estimated Undiagnosed/At-risk The National Health and Nutrition Examination Survey (NHANES) found that 33% of Americans aged 20 years or older suffered from hypertension in 2007-2008 (Go, Mozaffarian, Roger, et al., 2012). A 2007-2008 survey further identified that among those 20 years and older who were hypertensive, 81.5% were actually aware of their high blood pressure while 74.9% were taking medication to control their hypertension. However, only 53% of those taking medication had their blood pressure under control (Go et al., 2012). Disease Impact - Years of Potential Life Lost (YPLL) In 2001, a total, 5,020,000 years of potential life were lost (YPLL) due to CVD in the US from a population of 264,860,000 people (World Health Organization, 2001). There have been substantial gains in life expectancy due to the decline in CVD-related mortality over the past several decades. One analysis measured these gains in YPLL by determining what would have happened in the year 2000 had the US coronary heart disease mortality rate remained what it was in 1980. This study found that 3,147,800 life years had been gained among those aged 25 to 84; of these years, 1,092,400, or 35 percent, could be attributed to new, improved, and more widely available treatments for coronary heart disease (Capewell, Hayes, Ford, et al., 2009). Decline in the CVD mortality rate increases the number of life years lived. Within the subset of CVD mortality, the unadjusted death rate in the US declined over the period 2000 to 2008 from 183.1 to 133.3 deaths per 100,000, representing a 27.2 percent decline (National Health Lung and Blood Institute, 2012a). With the assumption that the relationship between mortality reduction and YPLL gains between 1980 and 2000 continued unchanged, the gain between 1980 and 2008 would have increased to 4,568,800 life years and the number attributed to new, improved, and more widely available treatment increased to 1,599,100 life years. SGU Cures Index 175 Disease Impact - Disability Adjusted Life Years (DALY) In total, 5,771,285 disability adjusted life years (DALYs) were lost to cardiovascular disease in the US in 2004, from a population of 292,810,000 million people (World Health Organization, n.d.). Current Cure Status The decline in mortality due to CVD reflects two phenomena. First, the prevalence of risk factors for CVD, such as smoking, has declined over time in the US (Centers for Disease Control, 2014). Second, functional cures have advanced. Longevity increases in successive cohorts because each behaves differently than earlier cohorts. Others benefit from the availability and greater use of improved treatment. A review of studies covering different countries and different time periods, albeit with data from before 2000, found that improved treatment accounted for between 23 and 47 percent of the decline in CVD mortality (Ford & Capewell, 2011.) As previously explained, CVD can present in various forms and severities. As such, the current “best practice cure” for CVD varies based on the nature of the disease. For example, the current best practice for heart failure encompasses a wide range of tests, procedures, and treatments in order to improve the health status of patients. Echocardiography is used to visualize the heart in order to identify structural damage leading to heart failure (Steimle, 2007). Additionally, B-type natriuretic peptide (BNP) assays are utilized to diagnose heart failure along with other exam diagnostic procedures indicating hypertension, oxygen saturation, respiratory distress, renal failure, cardiac arrhythmia, and others. Following diagnosis, proper medication including diuretics, ACE inhibitors, angiotensin receptor blockers, beta-blockers or others can be administered and titrated to ensure greater efficacy. Physicians may further examine the need for additional procedures to unblock or redirect arteries, use of stents, or additional invasive procedures for an effective treatment plan. In addition to improved procedural methods reducing invasiveness of risky surgeries, the discovery of simple and effective medication has allowed for the early treatment of patients at risk for developing severe heart disease (Steimle, 2007). Future Cure Obstacles Primary prevention, early detection, and screening are key to addressing CVD. However, these approaches all come with limitations. While reducing intake of highly-processed calorie-dense food, quitting smoking, and increasing physical activity have been shown SGU Cures Index 176 to reduce rates of CVD, the prevalence and incidence of CVD in America indicate that substantial opportunities for improvement remain. Once CVD risk factors such as hypertension and high LDL cholesterol levels are detected, or CVD has set in, various treatments can be recommended to reduce the risk of death. Despite all interventions, CVD remains the leading cause of death in the US. Thus, there remain additional opportunities for developing innovative approaches to treatment and prevention. The genomic revolution has yet to impact heart disease treatment. Identifying biomarkers will differentiate disease subtypes, a process that eventually implies “personalized medicine”. Second, many signaling and transcriptional pathways are not yet understood. Understanding them will open up new intracellular targets for drug development programs (National Heart, Lung, and Blood Institute, 2007). These treatment approaches will reduce future adverse cardiac events. Other approaches will be required to improve treatment for those who have already experienced adverse events. Regenerative medicine holds the promise of providing ways to improve cardiac functioning among those who have existing impairment. Tissue repair and regeneration may allow completely new treatment modalities to become common if the potential of stem cell therapies can be realized (National Heart, Lung, and Blood Institute, 2007). Research Development and Treatment Costs As CVD is the single greatest cause of death in the US, researchers and scientists are constantly seeking innovative treatment options for CVD, as well as associated conditions such as hypertension (Hoyert & Xu, 2012). In 2012, the National Institutes of Health (NIH) spent over $2 billion in researching causes, progression, and treatment of CVD (NIH, 2013). Historical NIH Spending on Heart Disease: 2009: $1.202 billion 2010: $1.329 billion 2011: $1.236 billion 2012: $1.278 billion 2013 (estimated): $1.286 billion Average Cost of Treating the Disease SGU Cures Index 177 For 2009, the National Heart, Lung, and Blood Institute, with data from the Medical Expenditure Panel Survey, estimated that the US spent approximately $312.6 billion on indirect and direct healthcare costs for cardiovascular disease (Go et al., 2012). Living with heart disease can have significant implications not only on individual health, but on quality of life as well. It has been estimated that the loss of productivity for Americans living with heart disease is about $105 billion, while those living with hypertension is $280 billion (DeVol & Bedroussian, 2007). Economic Impact - YPLL The value of improved heart disease treatment is high. Using an estimate, which puts gains due to new and improved treatment over the period 1980 to 2008 at 1,599,100 life years, the VMRR is $166.615 billion as a result of new treatments for CVD between 1980 and 2008. Cardiovascular Disease Cures: Overview Cardiovascular disease is the leading cause of death in the US. As such, treatments for heart disease and the conditions that play a role in CVD, including hypertension, have been and continue to be heavily researched. The earliest treatment options include various procedures to address the debilitating effect of CVD, while more recent treatment options seek to prevent full development of the disease by detecting and addressing signs and symptoms (i.e., hypertension) from an early stage. The historical path of cures has progressed from highly invasive and appropriate to those who have reached severe forms of disease to medications taken by large numbers of people who have risk factors but few symptoms. Seen as a pyramid, the progression has meant broader layers at the base of the pyramid and a smaller share of the population reaching the peak of the pyramid where the treatments are most expensive and most disruptive. Cardiovascular Disease Cures: Heart Surgery Cure Category Achieved, Functional Cure Identification/ Description SGU Cures Index 178 Surgery performed on the heart encompasses numerous procedures that can help alleviate conditions associated with heart disease at varying severities. Coronary artery bypass grafting (CABG) brought about a treatment for severe disease. A CABG is performed when an artery leading to the heart has been occluded, preventing adequate blood supply from reaching the heart and the rest of the body. During a CABG a healthy artery or vein from another part of the body is removed and reconnected with the coronary artery that is affected to ensure there is proper blood supply reaching the heart to circulate throughout the body (National Heart, Lung, and Blood Institute, 2007). Cure History and Science: Breakthroughs/Obstacles In the years following the development of the physician group later known as the “American Heart Association,” physicians, scientists and researchers across America sought to find innovative techniques to treat heart disease. In 1949, Wilfred Bigelow and his team performed an open-heart procedure with the use of hypothermia on animals (Mehta & Khan, 2002). In 1953, the first successful atrial septal defect procedure was completed. Physicians were now beginning to explore the patient’s heart in order to identify possible treatments. Such lengthy procedures required physicians to temporarily suspend the job of the heart during surgery. The only safe way to do so was to have a mechanism, which could run the heart’s functions separately while physicians explored. This need led to the successful invention of the heart and lung bypass machine in 1953 (Mehta & Khan, 2002). Before the bypass could be used successfully on humans, thorough testing was carried out with animals. In 1960, Robert H. Goetz performed the first successful coronary bypass procedure on canines. In 1967, Rene Favaloro completed a bypass grafting procedure at the Cleveland Clinic. Number of patients being treated Development of open-heart surgeries and bypass procedures increased life expectancy and patient quality of life significantly. Data from the National Hospital Discharge Survey shows that in 2010, over 219,000 patients throughout the US underwent 397,000 coronary artery bypass procedures (Go et al., 2012). Impact on treatment on years of potential life lost (YPLL) An analysis of life-years gained among cardiac patients and those at risk for coronary heart disease helps illustrate the impact of bypass treatment. Bypass grafts were among the interventions received by those who experienced myocardial infarction, unstable SGU Cures Index 179 angina, and chronic angina. These procedures contributed to life-year gains among those with all three conditions. Significant evidence is demonstrated from the treatment of chronic angina, as between the years of 1990 and 2000, bypass grafts added 135,000 years of potential life among those aged 25 to 84 in a study of 2,356,700 patients who underwent bypass surgery (Capewell et al., 2009). Average cost of cure per patient It is estimated that the average price for a coronary bypass, including procedural costs, hospital stay for the procedure and anesthesia, starts at about $63,000 (Coronary Bypass Grafting, 2013). However, this baseline estimate fails to include healthcare costs post-operatively, including therapy and any additional medication needed. Economic Impact - Value of Increased Years of Life Lived With 135,000 years of life saved by bypass grafts each year, the VMRR is $14.07 billion as a result of this procedure. If each procedure, including post-operative care, medication, and therapy, cost $50,000, the total cost of the 2,356,700 procedures between 1990 and 2000 would be $117.8 billion. This suggests a poor economic return for the coronary bypass procedure. Based on these numbers, each bypass procedure would need to be completed for no more than $5,970 to justify the economic gain, as measured by VMRR. Cardiovascular Disease Cures: Angioplasty Cure Category Achieved, Functional Cure Identification/ Description During percutaneous coronary intervention (PCI), formerly known as angioplasty, a balloon is inserted at the tip of a catheter, which has been inserted into an artery, and inflated. The inflation of the balloon helps to constrict the plaque blocking the artery, re-establishing proper blood flow (National Heart, Lung, and Blood Institute, 2012b). Cure History and Science Breakthroughs/ Obstacles SGU Cures Index 180 Since 1844, scientists have researched cardiac catheterization to record blood pressure in animals. Currently, cardiac catheterization procedures utilize a catheter (a flexible tube) that is placed within a large artery (through the arm, thigh or groin) and traced back to the heart. The basis of the catheterization is to follow the route of blood flow in order to identify possible blockages or other causes of concern. From here, physicians can complete additional tests such as an angiography. During an angiography, a dye is inserted into the catheter to observe any blockages within the artery caused by plaques (National Heart, Lung, and Blood Institute, 2012b). In 1929, Werner Forssmann performed the first human cardiac catheterization on himself. After the first successful cardiac catheterization, angiography procedures transformed the way physicians were able to diagnose and treat patients with various heart conditions. Between the early 1940s and into the 1960s, various types of angiography procedures were identified, making angiography an important turning point in innovative medical advancement. Small steps continued, including the addition of dyes within the catheters in 1958 to develop improved images of the heart and vessels. From the discovery of angiography, it would not be until 1977 when angioplasty, developed by Dr. Andreas Gruentzig, would change the treatment for blocked arteries (Society for Cardiovascular Angiography and Interventions, n.d.). During the 1960s, Dr. Charles Dotter combined the use of cardiac catheters to reestablish proper blood flow within blocked arteries in legs and feet. In 1977, this process, known as a balloon angioplasty, was first completed on a human by Dr. Andres Gruentzig, who took it another step further by performing the procedure on coronary arteries. Number of patients being treated In 2009 the Agency for Healthcare Research and Quality (AHRQ) estimated that there were over 644,000 hospital stays for stent procedures with balloon angioplasty, of which three fourths of the stents used were drug eluting stents (Auerbach, Maeda, & Steiner, 2012). These stents slowly release a drug that blocks cell proliferation that could otherwise block the stented artery. The AHRQ additionally estimated that there were 3,667 angioplasty procedures performed per one million adults in the US in 20072008 (Auerbach et al., 2012). Given the total US adult (18+) population of 222,722,000 in 2007 (US Census Bureau, n.d.), that amounts to 816,722 angioplasty procedures performed in the US in 2007-2008, or approximately 408,361 angioplasties per year. Impact on treatment on years of potential life lost (YPLL) SGU Cures Index 181 Angioplasties help to improve quality of life by reducing the risk of mortality from CVD, particularly among those at the greatest risk (Kent, Schmid, Lau, & Selker, 2002). Through coronary bypass grafting procedures after angioplasty procedures, it has been calculated that there is an increase in life expectancy of 13.9 years among those who undergo the procedure (Yock, Boothroyd, Owens, Garber, & Hlatky, 2003). Angioplasty procedures with stents are estimated to increase life expectancy by about 13 years (Yock et al., 2003). Impact on treatment on disability-adjusted life years (DALY/QALY) Five-year follow-ups on angioplasty demonstrated an estimated increase in 3.59 QALYs for event-free survival and about 2.77 QALYs for angina free survival (Vieira et al., 2012) Average cost of cure per patient Average procedural and hospital costs for a coronary angioplasty starts at about $22,000 (Coronary Angioplasty, 2013). Average lifetime cost of cure In 2012, it was reported that after a five year follow-up period, estimated costs for PCI (angioplasty) was estimated at almost $20,000 among patients who did not have serious complications (i.e., were “event-free”), and just over $25,800 among patients who were event-free and angina-free. This is often due to the need for re-intervention and greater costs of drug therapy following the procedure (Vieira et al., 2012). Economic Impact - Value of Increased Years of Life Lived If the 408,361 annual angioplasty procedures performed in the US increased life expectancy on average by 13 years (Yock et al., 2013), this equates to 5,308,693 years of life saved annually, with an associated VMRR of $553.13 billion. Cardiovascular Disease Cures: Stents Cure Category Achieved, Functional SGU Cures Index 182 Cure Identification/ Description After the development of angioplasty procedures, patients were able to regain proper blood flow. While these procedures were very effective in eliminating blocked arteries, physicians noticed that the same arteries would often collapse or would leave scar tissue within the artery, leading to further vessel constriction known as restenosis (Society for Cardiovascular Angiography and Interventions, n.d.) Scientists and researchers set out to improve the procedure. In 1994 the FDA approved the first stent, a metal mesh device that would be used to keep the artery open following an angioplasty procedure. These stents were known as bare metal stents (BMS) and were effective in keeping arteries open following angioplasty procedures. However, the bare metal stents were not very effective in reducing restenosis (Society for Cardiovascular Angiography and Interventions, n.d.). Researchers then set out to design a stent that would reduce the incidence of restenosis in patients. Cure History and Science: Breakthroughs/ Obstacles As physicians began performing more angioplasties in the 1980s, the incidence of restenosis among patients was a rising concern. The development of scar tissue inside arteries that were treated through angioplasty began to block blood flow once again. Seeking to improve upon the stent design, a drug eluting stent (DES) was approved by the Food and Drug Administration (FDA) in 2003. DES proved to be more effective in reducing restenosis than the original BMS as the drug eluting stents contain a drug coating that prevented the development of scar tissue within the arteries (Society for Cardiovascular Angiography and Interventions, n.d.). Number of patients being treated In 2009 the AHRQ estimated over 644,000 hospital stays for stent procedures with balloon angioplasty, of which three fourths of the stents used were drug-eluting stents (Auerbach et al., 2012). The AHRQ additionally estimated that between 2007-2008, there were 3,667 angioplasty procedures performed per one million adults across the US (Auerbach et al., 2012). Recently, it was reported that in 2009, over one million angioplasty procedures were performed in the US (Roger, Go, Lloyd-Jones, et al., 2012). Impact on treatment on disability- adjusted life years (DALY/QALY) One-year follow-ups after stent procedures estimated an increase in 0.85 (+/- 0.18) quality-adjusted life years (Cohen, Taira, Berezin, et al., 2001). SGU Cures Index 183 Average cost of cure per patient Estimated costs for stent procedures start around $22,000 as an outpatient procedure, which does not require an overnight stay (Cardiac Stent, 2013). Economic Impact - Value of Increased Quality of Life Increasing life expectancy and the quality of life illustrates the effectiveness of a cure. However, it is equally important to evaluate the cost benefit with each procedure. When evaluating the increase in QALY gained through a stenting procedure, it is observed that heart disease patients gain $189,000 per quality adjusted life year gained (Yock et al., 2003). If we multiply the 644,000 stent procedures with balloon angioplasty performed in 2009 (as estimated by the AHRQ) by 0.85 QALY gain (Cohen et al., 2001), this equates to 547,400 quality of life years gained annually. Stent procedures with balloon angioplasty in the US yield gains in quality-adjusted life years worth $57.04 billion annually. Cardiovascular Disease Cures: Diuretics Cure Category Achieved, Functional Cure Identification/ Description As long ago as the mid-19th century, an increase in blood pressure leading to hypertension has been a known contributing factor to heart disease. Since this time, various methods to control blood pressure have been discovered. Diuretics were the first of these methods. By controlling the body’s salt balance, diuretics directly influence the body’s natural response to blood pressure (Hamdy, 2001). Cure History and Science Breakthroughs/ Obstacles Diuretics were one of the earliest methods of controlling blood pressure. Sulphonamides were introduced in the 1930s, initially as antibiotics used in treating bacterial infection. These drugs were also observed to cause diuresis (water output/increased urine output) in patients (Hamdy, 2001). In 1949, Dr. William Schwarz SGU Cures Index 184 administered sulphonamides to heart failure patients and noticed an improvement in their health status. However, Dr. Schwarz further identified that large and continued doses of sulphonamides can be dangerous for patients and thus, would not serve as an effective long-term solution. Following Schwarz’s identification, Karl Beyer, a chemist, worked on adjusting the dosage and developed the diuretic chlorothiazide (Hamdy, 2001). In the 1940s, the first carbonic anhydrase inhibitor, fanilamide was discovered. Carbonic anhydrase inhibitors alter re-absorption at the level of the kidneys, leading to changes in blood pressure. However, this drug also proved to have undesired consequences and thus, scientists sought to identify similar compounds with fewer side effects (Pizzi, 2003). In the 1950s acetazolamide, also known as Dioamox, was discovered as the first successful carbonic anhydrase inhibitor diuretic (Pizzi, 2003). Diuretics help to lower blood pressure by changing the amount of urine leaving the body. Water not reabsorbed into the body is expelled in the urine. This allows for a decrease in blood volume, which in turn places a reduced load on the heart. This mechanism signals the body to reduce blood pressure. From the 1950s into the 1960s various types of diuretics were developed, all based on the mechanism of altering reabsorption of water in order to influence blood pressure (Hamdy, 2001). After their discovery, diuretics became increasingly used in treating heart disease patients by reducing hypertension (Mayo Clinic, 2010). Although highly effective in reducing blood pressure, diuretics still present with possible side effects including dizziness, headaches, increased thirst, muscle cramps, increased blood sugar, rash, gout, low sodium levels (hypoatremia), impotence, and menstrual irregularities (Bui, 2010). Proper physician monitoring allows the use of diuretics to be highly beneficial in reducing patients’ blood pressure and reducing the development of heart disease. Number of patients being treated In 2010, 131 million prescriptions were written for diuretics (Schiller et al., 2012). Impact on treatment on years of potential life lost (YPLL) When evaluating the average impact of diuretics, a recent study reported an average of 4.5 years of life gained among existing CVD patients (Banka, Heidenreich & Fonarow, 2013). Impact on treatment on disability- adjusted life years (DALY/QALY) SGU Cures Index 185 Heart failure patients treated with diuretics (to control blood pressure) can potentially gain 3.5 quality adjusted life years, on average (Banka et al., 2013). Average cost of cure per patient Diuretics cost an average of between $7 and $12 for a one-month supply across the US (www.rxpricequotes.com). Average lifetime cost of cure Estimated lifetime healthcare costs of diuretics are approximately $12,742 (Banka, et al., 2013). Economic Impact - Value of Increased Years of Life Lived The CDC reported that in 2011, 26,486,000 Americans had heart disease (Schiller et al., 2012). If only 20% of these individuals were prescribed diuretics to reduce blood pressure, 5,297,200 Americans could potentially gain 4.5 years of life as a result of this intervention (Banka et al., 2013) totaling 23,837,400 years of potential life, and a VMRR of $2.48 trillion among Americans currently living with heart disease. Cardiovascular Disease Cures: Alpha/Beta-Blockers Cure Category Achieved, Functional Cure Identification/ Description Alpha and beta-blockers control the body’s response to chemical signals referred to as neurotransmitters by influencing vasoconstriction (i.e., reduction of artery diameter) (Mayo Clinic, 2013). By the 1950s, scientists had established that the risk of heart disease increased when plaques reduced the area within arteries, thereby restricting adequate blood flow (Quirke, 2006). Researchers sought to identify other ways to control the size of arteries, thus ensuring proper blood blow. The discovery of beta adrenergic blockers and alpha adrenergic blockers provided physicians with a way to chemically control hypertension by blocking signals which would cause arteries to constrict and heart muscle to contract. Alpha-blockers were found to inhibit SGU Cures Index 186 norepinephrine from tightening the muscle of blood vessels causing constriction, while beta-blockers were found to inhibit the action of epinephrine particularly on heart muscle reducing contractility. Cure History and Science: Breakthroughs/ Obstacles While diuretics provided one mechanism to control blood pressure by altering reabsorption of water, hormone alteration proved to be another successful approach. Once the influence of different chemical signals on blood vessels was known, scientists sought to inhibit their mechanism. This inhibition would keep arteries open and ensure proper blood flow. In the 1950s, it was established that heart disease stemmed from blocked arteries and that the mechanism of the hormones or neurotransmitters epinephrine and norepinephrine acted to tighten the muscles around blood vessels causing constriction. Dr. Claude Beck, an American surgeon, had identified that increasing the amount of oxygen within the blood allowed for the blood pressure to decrease. Meanwhile, James Black approached the problem from a different angle, seeking ways to increase oxygen to the heart while decreasing the heart’s requirement for oxygen. By 1965 Black designed a medication – a beta-blocker – that counteracted the effects of the neurotransmitters epinephrine/adrenaline (Mayo Clinic, 2013; Quirke, 2006). This medication, propranol, became the first beta-blocker used in blood pressure treatment. In the following ten years, researchers continued to develop other similar medications that would block the action of hormones within the body. While beta-blockers targeted the receptors for epinephrine, scientists sought to identify a mechanism for a similar hormone, norepinephrine. In 1975, Marco Caine and his colleagues identified the effect of norepinephrine on prostate tissue. In the following year, phenozybenzamine was identified as the first alpha-blocker to be used in the treatment of benign prostatic hyperplasia (Lepor, 2006). Alpha-blockers are named due to their effect in blocking alpha-adrenergic receptors, thus blocking the effect of norepinephrine. While alphablockers were originally utilized in the treatment of benign prostatic hyperplasia, they are still effective in reducing blood pressure throughout the body and thus used in the treatment of hypertension. Similar to beta-blockers, alpha-blockers may present certain side effects among prescribed patients including a “first dose” effect (Mayo Clinic, 2010). It is possible for certain patients to experience dizziness or extremely low blood pressure leading to fainting when elevating from a laying position upon their first dosage (Mayo Clinic, 2010). SGU Cures Index 187 Number of patients being treated In 2010, almost 200 million prescriptions were filled for beta-blockers (Schiller et al., 2012). Impact on treatment on years of potential life lost (YPLL) An original study reported in the Journal of American Medical Association (JAMA) estimated the impact of beta-blocker use among survivors of myocardial infarction aged 35 to 84 years. Initiating a beta-blocker regimen following the first heart attack can result in 72,000 fewer deaths due to heart disease. Furthermore based on the population facts in the year 2000, continuing this treatment for the following 20 years would result in 4,300 fewer deaths due to heart disease, the prevention of 3,500 future heart attacks, and a gain of 45,000 life years among adults in the United States aged 35-84 years old (Phillips, Shlipak, Coxson, et al., 2000). Average cost of cure per patient Alpha and beta blockers can cost between $5 and $20 for a one month supply of medication across the country, varying by brand (www.rxpricequotes.com). Estimated beta-blocker costs per year per patient are approximately $432; however, this may vary based on the prescription brand (Phillips et al., 2000). Economic Impact - Value of Increased Years of Life Lived The use of beta-blockers is estimated to result in a gain of 45,000 life years among American adults aged 35-84 years old who had experienced a first heart attack (Phillips et al., 2000), giving a VMRR of $4.69 billion as a result of beta-blocker use from this group alone. Cardiovascular Disease Cures: Statins Cure Category Achieved, Functional Cure Identification / Description SGU Cures Index 188 Ensuring adequate blood flow is critical in maintaining ideal blood pressure within the body. In the 1960s it was established that blocking the signal of certain hormones can help reduce the body’s response to constriction, but researchers sought to further discover methods to prevent the reduction in blood flow leading to hypertension and heart disease. The presence of plaques within arteries, which can cause a reduction in blood flow, was identified with the use of angiographies. This discovery prompted a search for ways to prevent plaque development in the first place (Endo, 2010). In the 1960s, Ancel Keys identified the relationship between cholesterol and heart attack incidence. Identifying mechanisms to stop the production of excess cholesterol would soon prove to be a significant breakthrough in heart disease management. Cure History and Science: Breakthroughs/ Obstacles While the discovery and use of alpha and beta-blockers were well underway, scientists continued to research other causes of heart disease and additional treatments. In the 1950s, John Gofman identified that heart attacks correlated with high levels of blood cholesterol, in particular, low density lipoprotein (LDL). It was also recognized that fewer heart attacks occurred in those with higher high-density lipoprotein (HDL) levels. Thus, regulation of blood cholesterol became a potential way to reduce heart attacks and heart disease risk. In the 1960s, scientists and researchers sought to identify a method to regulate the key enzyme for cholesterol synthesis in the body, HMG CoA reductase. From the 1970s to the 1980s various types of medications were identified that inhibited this regulatory process, however all were found to be harmful to various organs or resulted in additional health concerns following animal testing. In the late 1970s, researchers at the pharmaceutical company Merck developed meviolin, which soon was renamed lovastatin. After thorough trials, the FDA approved lovastatin in 1986 as the first statin to effectively lower LDL cholesterol levels (Endo, 2010). Number of patients being treated In 2010, over 250 million prescriptions were filled for lipid regulators (largely statins) (Schiller, et al., 2012). Impact of treatment on years of potential life lost (YPLL) Statin use to control high cholesterol also provides considerable economic gains in the reduction of heart disease risk. An aggressive strategy of statin prescribing, with all Americans at high risk and those with no additional risk factors besides high cholesterol, would lead to a total of 64 million Americans prescribed a daily statin and help prevent SGU Cures Index 189 27,000 coronary heart disease deaths per year (Lazar, Pletcher, Coxson, BibbinsDomingo, & Goldman, 2011). This mortality reduction would yield an additional 220,000 quality adjusted life years over the lifespan if those deaths were averted in a year, with an associated VMRR of $22.9 billion. Average cost of cure per patient Statins cost an average of $5 to $12 per month across the US (www.rxpricequotes.com). Statin therapy for an estimated 64 million patients would cost approximately $620 million per year (Lazar et al., 2011). Citations Auerbach, D., Maeda, J., & Steiner, C. (AHRQ). (2012). Hospital stays with cardiac stents, 2009. (HCUP Statistical Brief #128). Agency for Healthcare Research and Quality. Retrieved July 9, 2015 from: http://www.hcupus.ahrq.gov/reports/statbriefs/sb128.pdf Banka, G., Heindenreich, P., & Fonarow, G. (2013). Incremental cost-effectiveness of guideline-directed medical therapies for heart failure. Journal of American College of Cardiology, 61(13). Retrieved June 8, 2015 from: http://dx.doi.org/10.1016/j.jacc.2012.12.022 Bui, Q. (2010). First-line treatment for hypertension. American Family Physician, 81(11), 1333-1335. Capewell, S., Hayes D., Ford, E., Critchley, J., Croft, J., Greenlund, K., & Labarthe, D. (2009). Life-years gained among US adults from modern treatments and changes in the prevalence of 6 coronary heart disease risk factors between 1980 and 2000. American Journal of Epidemiology, 170(2), 229-236. Cardiac Stent. (2013). In Healthcare Blue Book. Retrieved August 23, 2013, from: http://www.healthcarebluebook.com/page_Results.aspx?id=188&dataset=MD&g =Cardiac%20Stent Centers for Disease Control and Prevention (CDC) (2009). Other Related Conditions. National Center for Chronic Disease Prevention and Health Promotion: Division for Heart Disease and Stroke Prevention. Retrieved May 23, 2015 from: http://www.cdc.gov/heartdisease/other_conditions.htm SGU Cures Index 190 Centers for Disease Control and Prevention (CDC). (2010a). Coronary artery disease. National Center for Chronic Disease Prevention and Health Promotion: Division for Heart Disease and Stroke Prevention. Retrieved July 5, 2015 from: http://www.cdc.gov/heartdisease/coronary_ad.html Centers for Disease Control and Prevention (CDC). (2011). Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol. United States, 1999–2002 and 2005–2008. Morbidity and Mortality Weekly Report, 60(4), 109–114. Centers for Disease Control and Prevention (CDC). (2013). QuickStats: number of deaths from 10 leading causes – national vital statistics system, United States, 2010. Morbidity and Mortality Weekly Report, 60(8), 155. Retrieved July 2, 2015 from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6208a8.htm Centers for Disease Control and Prevention (CDC). (2014). Current cigarette smoking among adults — United States, 2005–2012. Morbidity and Mortality Weekly Report, 63(2), 29-34. Retrieved June 20, 2015 from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6302a2.htm?s_cid=mm6302a 2_w#tab Cohen, D., Taira, D., Berezin, R., Cox, D., Morice, M., Stone, G., & Grines, C. (2001). Cost-effectiveness of coronary stenting in acute myocardial infarction: results from the stent primary angioplasty in myocardial infarction (Stent-PAMI) trial. Circulation, 104, 3039-3045. doi: 10.1161/hc5001.100794 Coronary Angioplasty. (2013). In Healthcare Blue Book. Retrieved August 23, 2013 from: http://www.healthcarebluebook.com/page_Results.aspx?id=127&dataset=hosp&g =Angioplasty Coronary Bypass Grafting. (2013). In Healthcare Blue Book. Retrieved on August 23, 2013 from: http://www.healthcarebluebook.com/page_Results.aspx?id=29&dataset=hosp DeVol, R. & Bedroussian, A. (2007). An unhealthy America: the economic burden of chronic disease charting a new course to save lives and increase productivity and SGU Cures Index 191 economic growth (executive summary and research findings). Milken Institute. Retrieved May 20, 2015 from: http://www.milkeninstitute.org/healthreform/pdf/AnUnhealthyAmericaExecSumm. pdf Endo, A. (2010). A historical perspective on the discovery of statins. Proceedings of the Japan Academy, Series B Physical and Biological Sciences, 86(5), 484-493. doi:10.2183/pjab.86.484 Ford, E. S., & Capewell, S. (2011). Proportion of the decline in cardiovascular mortality and disease due to prevention versus treatment. Annual Review of Public Health, 32, 5-22. Go, A. S., Mozaffarian, D., Roger, V. L., Benjamin, E. J., Berry, J.D., Borden, W. B. et al. (2012). Executive summary: heart disease and stroke statistics- 2013 update. Circulation, 127(1), 143-152. doi: 10.1161/CIR.0b013e318282ab8f Hamdy, R. C. (2001). Hypertension: a turning point in the history of medicine…and mankind. Southern Medical Journal, 94(11), 1045-1047. Hoyert, D. L., & Xu, J. Q. (2012). Deaths: preliminary data for 2011. National Vital Statistics Reports, 61(6). Retrieved February 20, 2015 from: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf Kent, D. M., Schmid, C. H., Lau, J., & Selker, H. P. (2002). Is primary angioplasty for some as good as primary angioplasty for all? JGIM: Journal of General Internal Medicine, 17(12), 887-894. doi: 10.1046/j.1525-1497.2002.11232.x Lazar, L. D., Pletcher, M. J., Coxson, P. G., Bibbins-Domingo, K., & Goldman, L. (2011). Cost-Effectiveness of Statin Therapy for Primary Prevention in a Low-Cost Statin Era. Circulation, 124, 146-153. Lepor, H. (2006). The evolution of alpha blockers for the treatment of benign prostatic hyperplasia. Reviews in Urology, 8(4). Retrieved March 11, 2015 from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1765042/ Mayo Clinic. 2010. High blood pressure (hypertension). Retrieved March 12, 2015 from: http://www.mayoclinic.com/health/diuretics/HI00030/NSECTIONGROUP=2 SGU Cures Index 192 Mayo Clinic. (2013). Statin side effect: weigh the benefits and risk. Retrieved May 11, 2015 from: http://www.mayoclinic.com/health/statin-side-effects/MY00205 Mehta, N. J., & Khan, I. A. (2002). Cardiology’s 10 greatest discoveries of the 20th century. Texas Heart Institute Journal, 29(3), 164-171. National Heart, Lung, and Blood Institute (NHLBI). 2007. Shaping the future of research: a strategic plan for the National Heart, Lung, and Blood Institute. US Department of Health and Human Services. (NIH Publication No. 07-6150). Retrieved December 10, 2014 from: http://www.nhlbi.nih.gov/about/strategicplan/documents/StrategicPlan_Appendix .pdf National Heart, Lung, and Blood Institute (NHLBI). (2011). How is heart disease diagnosed? US Department of Health and Human Services. Retrieved December 10, 2014 from: http://www.nhlbi.nih.gov/health/healthtopics/topics/hdw/diagnosis.html National Heart, Lung, and Blood Institute (NHLBI). (2012a). 2012 chart book on cardiovascular, lung, and blood diseases. US Department of Health and Human Services. Retrieved December 10, 2014 from: http://www.nhlbi.gov/resources/docs/2012_ChartBook_508.pdf National Heart, Lung, and Blood Institute (NHLB). (2012b). What is cardiac catheterization? US Department of Health and Human Services. Retrieved December 10, 2014 from: http://www.nhlbi.nih.gov/health/healthtopics/topics/cath/ National Institutes of Health (NIH), US Department of Health and Human Services (2013). Estimates of funding for various research, condition, and disease category (RCDC). Retrieved June 15, 2015 from: http://report.nih.gov/categorical_spending.aspx Phillips, K., Shlipak, M., Coxson, P., Heidenreich, P., Myriam Hunink, M. G., Goldman, P., … Goldman, L. (2000). Health and economic benefits of increased b-blocker use following myocardial infarction. Journal of the American Medical Association, 284(21), 2748-2754. Pizzi, Richard. (2003). Developing diuretics. Modern Drug Discovery. Retrieved May 22, 2015 from: SGU Cures Index 193 http://pubs.acs.org/subscribe/archive/mdd/v06/i02/pdf/203timeline.pdf Quirke, Viviane. (2006). Putting theory into practice: James Black, receptor theory and the development of the beta-blockers at ICI, 1958-1978. Medical History, 50, 6992. Retrieved May 22, 2015 from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1369014/pdf/medhis5001-069.pdf Roger, V., Go, A., Lloyd-Jones, D., Benjamin, E., Berry, J., et al. (2012). Heart disease and stroke statistics- 2012 update: a report from the American Heart Association. Circulation, 125, e2-e220. doi: 10.1161/CIR. 0b013e31823ac046 Schiller J. S., Lucas J. W., & Peregoy J. A. (2012). Summary health statistics for US adults: national health interview survey, 2011. National Center for Health Statistics. Vital Health Statistics, 10(256). (DHHS Publication No. (PHS) 2013–1584). Retrieved May 22, 2015 from: http://www.cdc.gov/nchs/data/series/sr_10/sr10_256.pdf Steimle, Anthony. (2007). Clinical evidence review: best practice heart failure. The Permanente Journal, 11(2), 55-64. Society for Cardiovascular Angiography and Interventions (SCAI). (n.d.). History of angioplasty and stenting. Retrieved May 22, 2015 from: http://www.scai.org/SecondsCount/Resources/Detail.aspx?cid=b7b1dbfd-4f964b77-8ad8-af95f5cbb417 Quirke, Viviane. (2006). Putting theory into practice: James Black, receptor theory and the development of the beta-blockers at ICI, 1958-1978. Medical History (50), 6992. Retrieved December 10, 2014 from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1369014/pdf/medhis5001-069.pdf US Census Bureau. (n.d.). Age and sex composition in the United States: 2007. (n.d.) Retrieved September 28, 2014 from: https://www.census.gov/population/age/data/2007comp.html Vieira, R., Hueb, W., Hlatky, M., Favorato, D., Rezende, P., Garzillo, L. et al. (2012). Cost-effectiveness analysis for surgical, angioplasty, or medical therapeutics for coronary artery disease: 5-year follow up of medicine, angioplasty, or surgery study (MASS) II Trial. Circulation, 126. doi: 10.1161/CIRCULATIONAHA.111.084442 SGU Cures Index 194 World Health Organization (n.d.). Global burden of disease (GBD) 2001 estimates. World Health Organization Health Statistics and Health Information Systems. Retrieved December 10, 2014 from: http://www.who.int/healthinfo/global_burden_disease/estimates_regional_2001//World Health Organization Department of Measurement and Health Information (2001). Global Burden of Disease Study. Retrieved December 10, 2014 from: http://www.who.int/healthinfo/global_burden_disease/en/ Yock, C. A., Boothroyd, D. B., Owens, D. K., Garber, A. M, & Hlatky, M. A. (2003). Costeffectiveness of bypass surgery versus stenting in patients with multivessel coronary artery disease. American Journal of Medicine 11(5), 382. doi: 10.1016/S0002-9343(03)00296-1 SGU Cures Index 195 DIABETES MELLITIS Ashley Gibbons, Shervin Shaffiy, Chelsey Russ, Jason Cummings Disease Category Chronic Disease Identification, Description, and Diagnostic Criteria Glucose circulates in the blood and is used by cells as a source of energy and for biochemical processes that are critical to sustain life. Diabetes Mellitus is a disease associated with blood glucose levels above what is considered normal (Centers for Disease Control, 2011a). Exposure to higher levels of glucose damages the cells that line blood vessels. This damage leads to many of the major medical complications associated with diabetes, such as hypertension, heart disease, stroke, vision problems, kidney problems, nervous system disease, birth defects, and periodontitis. The World Health Organization (WHO) defines diabetes mellitus (hereafter referred to simply as diabetes) as either a chronic insufficiency of the pancreas to produce insulin, a peptide hormone that regulates carbohydrate metabolism (Type 1 diabetes), or when the body cannot react to high blood glucose levels effectively (Type 2 diabetes). The importance of diabetes lies in the adverse medical outcomes that are associated with it. In 2004, 68% of individuals 65 years and older who died as a result of diabetes complications, died of heart disease. Stroke was noted on 16% of diabetes-related death certificates among people in the same age group. Hypertension (blood pressure ≥ 140/90 mmHg) was found in 67% of adults (aged 20 and up) with diabetes. Diabetic retinopathy is a common complication of diabetes seen in 4.2 million people or 28.5% of diabetics aged 40 and over. Within this population, 655,000 had severe enough retinopathy to experience potential vision loss (Centers for Disease Control, 2011a), and diabetes is the most likely cause of retinopathy in adults aged 20 and up. In 2008, 44% of new diagnoses of kidney failure were due to longstanding diabetes, and in the same year 48,374 people began treatment for end-stage kidney disease. Also in 2008, 202,290 people were living on chronic dialysis or received a kidney transplant due to diabetes-related end stage renal disease. Nervous system disease is seen in 30% of people with diabetes aged 40 years or older (Centers for Disease Control, 2011a). This disease presents itself as impaired sensation or pain in the feet or hands, slowed digestion of food in the stomach, carpal tunnel syndrome, erectile dysfunction, or other SGU Cures Index 196 nerve problems (Centers for Disease Control, 2011a). Severe forms of diabetic nerve disease are a major contributing cause of the 65,700 non-traumatic lower-extremity amputations performed in 2006 – more than 60% of those amputations occurred in people with diabetes (Centers for Disease Control, 2011a). Having diabetes before conception and during the first trimester of pregnancy can cause major birth defects in 5% to 10% of pregnancies and spontaneous abortions in 15% to 20% of pregnancies (Centers for Disease Control, 2011a). Uncontrolled diabetes can lead to diabetic ketoacidosis and hyperosmolar (nonketotic) coma. Young adults with diabetes have about twice the risk of those without diabetes to have periodontitis, and adults aged 45 years or older with poorly controlled diabetes are 2.9 times more likely to have severe periodontitis (Centers for Disease Control, 2011a). People with diabetes are more susceptible to many other illnesses and often have worse prognoses than those without diabetes. Those who are 60 years or older are 2–3 times more likely to report an inability to walk one-quarter of a mile, climb stairs, or do housework compared with people without diabetes in the same age group (Centers for Disease Control, 2011a). This disease can also be cyclical - people with diabetes are twice as likely to have depression, which complicates diabetes management, and depression is associated with a 60% increased risk of developing Type II diabetes (Centers for Disease Control, 2011a). Diabetes can be diagnosed through blood tests. These tests include: (1) the level of the oxygen-transport protein Hemoglobin A1C; (2) A fasting plasma glucose test (FPG); and (3) An oral glucose tolerance test (OGTT). Hemoglobin is glycosylated in the presence of plasma glucose; once glycosylated, it remains that way. This form of hemoglobin remains steady in the body despite a recent fast. The A1C test gives an average of blood glucose from the previous 2-3 months. The FPG test measures the amount of glucose in the blood after an eight-hour fast. The OGTT is measured after an eight-hour fast and two hours after consumption of 75 grams of glucose dissolved in water. The FPG is cheaper and more convenient than the OGTT, but the OGTT is considered more sensitive. The diagnosis of gestational diabetes is only performed using the OGTT (National Diabetes Information Clearing House, 2013). The levels detected in tests place an individual into one of three categories: nondiabetic, high risk of developing diabetes (i.e., prediabetes), or diabetes. The precise clinical test values that reflect nondiabetes, prediabetes, and diabetes are set by a panel of experts (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 2002). Prediabetes is defined as a blood glucose level above normal, but not yet considered to be within the range of diabetes. Most people with SGU Cures Index 197 prediabetes develop Type 2 diabetes within 10 years of diagnosis (National Diabetes Information Clearinghouse, 2013). At the point of prediabetes, the prevention or delay of Type 2 diabetes is achievable through diet and exercise modification. Disease Etiology (Cause) When food is consumed, most of it is converted into glucose, a sugar, which the body uses as energy to sustain itself. A key step in this process is the entry of glucose into cells – a process facilitated by the hormone insulin, which is produced solely by the beta cells of the pancreas. Diabetes manifests most often in one of two ways: Type 1 diabetes, formerly known as insulin-dependent diabetes mellitus or juvenile-onset diabetes, and Type 2 diabetes, formerly known as insulin-independent diabetes mellitus or adult-onset diabetes. Type 1 diabetics simply cannot manufacture insulin as their own immune system has destroyed the pancreatic beta cells. Type 2 diabetes usually begins as insulin resistance, a disorder in which the cells do not use insulin properly. As the need for insulin rises, the pancreas gradually loses its ability to produce it (Centers for Disease Control, 2014a) In addition to type 1 and type 2 diabetes, diabetes can also present during pregnancy in the form of gestational diabetes. This often occurs around the 24th week and typically resolves upon delivery of the child. Gestational diabetes affects both mother and child, but accounts for a very small fraction of total diabetes cases. Gestational Diabetes occurs in 2% to 10% of pregnancies (Centers for Disease Control, 2013). There is another type of disease process not associated with insulin, which is diabetes insipidus. This is an uncommon disorder categorized by frequent thirst, drinking, and urination. This report will not focus on diabetes insipidus, because the two disease processes (Mellitus and Insipidus) only share a similar name, but not pathology. Further, given the relatively small impact of gestational diabetes, this report will only focus on Type 1 and Type 2 diabetes. One in every ten individuals with diabetes has Type 1. Most commonly diagnosed in young adults and children, the pancreas of a Type 1 diabetic makes very little to no insulin and therefore these diabetics are required to inject insulin into their bloodstream daily. This disease process has been studied extensively and scientists have concluded that Type 1 diabetes results from an autoimmune process. The body’s immune system attacks its own pancreas beta cells, disrupting the creation and release of insulin. Furthermore, genes have been linked to the inability of Type 1 diabetics to create insulin from these beta cells (Rich, 1990). A potential environmental trigger in utero can predispose infants to Type 1 Diabetes (Centers For Disease Control, 2012a). It is SGU Cures Index 198 thought that a maternally acquired virus causes an autoimmune attack in the fetus, leading to dysfunctional insulin secreting islet cells in the pancreas (National Diabetes Information Clearinghouse, 2014). The majority of diabetic patients are diagnosed with Type 2 diabetes. In this type, pancreas beta cells can produce insulin, at least initially. However, the body is unable to use this insulin adequately, resulting in what is known as insulin resistance. In turn, the pancreas continues to produce more insulin, as blood glucose levels remain high. Over time, this increased production “stresses” the pancreas and leads to the gradual demise of the pancreas’ insulin producing capacity. Most people are diagnosed with this type of diabetes after age 30 (Centers For Disease Control, 2012a). Genetic susceptibility plays a crucial role in the etiology and manifestation of Type 2 diabetes, with concordance in monozygotic (identical) twins approaching 100% (Adeghate, Schattner, Dunn, 2006). However, genetic factors may have to be modified by environmental factors for diabetes to become overt (Rich, 1990). A genetically susceptible individual may become diabetic if environmental factors modify the expression of these genes. These environmental factors are often linked to viruses such as Coxsackie B, Mumps, Cytomegalovirus, Rotavirus, and Rubella (World Health Organization, n.d.). Current Prevalence/Incidence US Prevalence: Currently, 25.8 million people have diabetes, which is approximately 8.3% of the US population (Centers for Disease Control, 2011a). Of these, type 1 diabetes accounts for 5 to 10% of all diagnosed cases in adults, while type 2 diabetes accounts for 90% to 95% of cases (National Diabetes Education Program, n.d.). In 2010 18.8 million people were currently diagnosed with diabetes while 7 million people were living with undiagnosed diabetes (American Diabetes Association, 2014b). Among people aged 65 and over, 10.9 million, or 26.9%, have diabetes (American Diabetes Association, 2014b). Furthermore, about 215,000 people under the age of 20 years have Type 1 or Type 2 diabetes (American Diabetes Association, 2014b). Prediabetes is more prevalent; 79 million Americans had prediabetes in 2010 (Centers for Disease Control, 2011a) – an increase of 28% from 2007. US Incidence: About 1.6 million American citizens aged 20 years or older were newly diagnosed with diabetes in 2011. The incidence of newly diagnosed diabetes aged 18 79 in 2011 was 15.4 per 1,000 people (Centers For Disease Control, 2012b). Estimated Undiagnosed / At Risk SGU Cures Index 199 Approximately 7 million Americans of all ages have diabetes but have not been diagnosed with the disease as of the year 2010 (Centers for Disease Control, 2011a). Those who are diagnosed with prediabetes are at higher risk than the rest of the population for developing Type 2 diabetes, heart disease and stroke. Approximately 35% of US adults aged 20 years or older had prediabetes (including 50% of adults aged 65 years or older) between 2005 and 2008. Disease Impact – Years of Potential Life Lost (YPLL) In 2007, diabetes was the seventh leading cause of death in the US The risk of death among people with diabetes is about twice that of people of similar age who do not have the disease (Centers for Disease Control, 2011a). As of the year 2000, the mean age at diagnosis of diabetes was 55.7 for non-Hispanic black males and females, 57.9 for Hispanic males and 57.4 for Hispanic females, 58.1 for non-Hispanic white males and 57.9 for non-Hispanic white females; and 58.8 for other males and females (Narayan, Boyle, Thompson, Sorenson, Williamson, 2003). In 2000, the total estimated years of life lost for all males diagnosed with diabetes at age 60 was 7.3, while estimated years of life lost for all females diagnosed with diabetes at age 60 was 8.9 (Narayan et al, 2003). Given that 25.8 million Americans have diabetes approximately evenly distributed between males and females, this translates to (7.3 x 12.9 million) 94.17 million potentially lost life years among currently living American males and (8.9 x 12.9 million) 114.81 million potentially lost life years among currently living American females. Disease Impact – Disability Adjusted Life Years (DALY/QALY) An estimated 449 total Disability Adjusted Life Years (DALYs) were lost to diabetes per 100,000 people in the United States in 2004 (World Health Organization, 2008). Given a total US population of 292,810,000 in 2004, 1,314,717 total DALYs were lost to diabetes Consistent with that number, Jonsson (1998) estimated that 1.3 million DALYs were lost to diabetes in the United States in 1990. As of the year 2000, an American male diagnosed with diabetes at age 60 years will lose 11.1 quality-adjusted life years (QALYs) compared to a person of the same age without diabetes. An American female diagnosed with diabetes at age 60 years will lose 14.2 QALYs (Narayan et al., 2003). Given that 25.8 million Americans have diabetes approximately evenly distributed between males and females, this translates to (11.1 x SGU Cures Index 200 12.9 million) 143.19 million potentially lost QALYs among currently living American males and (14.2 x 12.9 million) 183.18 million potentially lost QALYs among currently living American females. Disease Impact - Economic Cost of Diabetes in the US The estimated total economic cost of diagnosed diabetes in 2012 is $245 billion, which includes $176 billion in direct medical cost and $69 billion in lost productivity (American Diabetes Association, 2013). This is a 41% increase from the previous estimate of $174 billion for 2007 (in 2007 dollars), and highlights the substantial economic burden that diabetes imposes on American society. Additionally, the indirect costs of diabetic medical expenditures consist of: hospital inpatient care (43% of the total medical cost), prescription medications for diabetic complications (18%), anti-diabetic agents and supplies (12%), physician visits (9%), and health care facility stays (8%) (Yang, Dall, Halder, Gallo, Kowal, & Hogan, 2013). In order to survive a diagnosis of Type 1 Diabetes, patients accrue additional expenditures. These total expenditures in 2012 included: Insulin ($6.157 billion); Diabetic supplies ($2.296 billion); Antidiabetic agents ($12.137 billion); Prescription medications ($31.716 billion); Other supplies ($1.063 billion) (Yang, et al., 2013). History of the Disease and Cure Breakthroughs In 1910 the first physiologist to identify insulin was Sir Edward Albert Sharpey-Schafer. In 1921, Frederick Banting and his then student assistant, Charles Best, were able to isolate insulin from the pancreas of a dog. They were able to observe fluctuations in the dog’s plasma glucose levels. In that same year, James Collip purified insulin to be used in humans. Eli Lilly became the first company to manufacture insulin in 1923. Prior to that time, a diagnosis of juvenile diabetes was a death sentence. By the late 1970’s and early 1980’s, 3.5% of Type 1 diabetics died within 20 years of their diagnosis, and approximately 7% died within 25 years (Centers for Disease Control, 2011a). In 1949, Rachmiel Levine discovered the association of insulin and glucose at the cellular level. In that same year, a standardized insulin syringe was created by Beckton Dickinson and Company. Sulfonylureas (oral medications) were introduced to the public in 1955. In 1959, Solomon Berson and Rosalyn Yalow distinguished Type 1 and Type 2 diabetes (American Diabetes Association, 2014a). Genetically modified human insulin has been used as a treatment for Type 1 diabetics as either rapid, intermediate, and long acting insulin types. Insulin pumps were also introduced and widely used at this time along with HbA1c testing, which shows the average blood glucose of a patient SGU Cures Index 201 over a three month period (Rohlfing, Wiedmeyer, Little, England, Tennill, & Goldstein, 2002). Currently, there is no “definitive cure”, but theoretically a pancreatic transplant has the potential to cure Type 1 diabetes. Patients with Type 1 diabetes must inject insulin into their bloodstream daily for longevity and survival. For Type 2, if caught early on, lifestyle changes such as healthy eating and exercise can actually reverse the disease process. Tight blood sugar control has become a standard of treatment particularly with low HbA1C levels (National Diabetes Information Clearinghouse, 2013). Ultimately, both disease types require proper monitoring and control of blood glucose levels. Current Cure Status Currently, the best practice over time has been the use of insulin to prevent dangerous fluctuations in blood sugar. Monitoring diet and caloric intake as well as regular exercise has also been shown to aid in the improvement and maintenance of blood glucose levels. In economic terms, a “definitive cure” for diabetes would reduce total health care costs by about $303 billion dollars in those who currently have diabetes, and $141 billion in people who have pre-diabetes over the next 30 years (Rizza, Eddy, & Khan, 2008). The indirect costs of diabetes were estimated to be another $40 billion in 2002, due to lost workdays, restricted-activity days, mortality, and permanent disability. Additionally, there are nearly 88 million days lost to disability per year as well as 176,000 cases of permanent disability, at a cost of $7.5 billion (American Diabetes Association, 2013). Future Cure Obstacles Recently, pancreatic transplants and islet cell transplantation have begun to open the door to the possibility of a “definitive cure” for diabetes. The development of insulin producing cells has come from many different forms, including reprogramming and stem cells. Reprogramming cells means to transform non-insulin producing cells into ones that are capable of that function and stem cells are precursor cells that researchers are able to differentiate into cells that can function as insulin producers (Diabetes Research Institute Foundation, n.d.). Research Costs SGU Cures Index 202 The National Institutes of Health (NIH) provides estimates for diabetes research funding as follows (National Institutes of Health, 2014): 2010: $1.199 billion 2011: $1.076 billion 2012: $1.061 billion 2013: $1.007 billion 2014 estimated: $1.028 billion 2015 estimated: $1.039 billion Cure Category Diabetes Cures: Insulin Achieved, Functional Cure Identification / Description Insulin is the treatment prescribed for Type 1 diabetes or for those with Type 2 diabetes who cannot control their blood glucose solely with diet and/or exercise, and/or oral medication. In order to survive, a Type 1 diabetic needs insulin daily. An injection of insulin serves to replenish the body of insulin in the situation where the pancreas is unable to produce insulin and prevents the liver from producing more sugar (National Diabetes Information Clearinghouse, 2013). Insulin is the main hormone that maintains homeostatic balance of blood sugar in the body. It does this by binding to a receptor on the cell surface, which signals the nucleus of the cell to stimulate glucose, lipid, and protein metabolism (Kahn & White, 1988). Insulin is available as a solution or suspension that is injected intramuscularly several times a day. There are three types of insulin that exist, animal, human and analogues (Diabetes UK, 2012). It is contained in vials (syringes are used to inject), or prefilled disposable dosing devices and cartridges, which are placed in a dosing pen (US National Library of Medicine, 2014). About 50 – 75% of the total daily dose of insulin can be obtained in one or two daily injections, with the rest of the dosage administered as a rapid form before eating. Oral insulin is also available for diabetic patients. This class of medications, called sulfonylureas, causes the pancreas to produce and secrete insulin. Another class of medication for Type 2 diabetics, thiazolidinediones (TZD), sensitizes the body to the action of insulin. TZD’s can be combined with the sulfonylureas for the compounded effect of more insulin and higher sensitivity to it. Lastly, the third category of SGU Cures Index 203 medications used for diabetes - metformin - is not insulin-based. These stimulate the liver to produce less glucose and can also suppress the digestive enzymes that break down carbohydrates. Cure History Islets of Langerhans, found by Paul Langerhans in 1869, are the location of the beta cells that produce insulin in the pancreas. In 1889, Oskar Minkowski and Joseph von Mering demonstrated that by removing the pancreas from a dog, the animal developed diabetes (Nobel Media, 2013). Canada was the first nation to offer insulin treatment on a human diabetic patient in 1922. The patient, Leonard Thompson, aged 14, reacted positively to the treatment and thus a regimen of insulin was implemented in this disease. The Nobel Prize in Physiology or Medicine was granted to Banting and Macleod in 1923 for this discovery (Nobel Media, 2013). This was followed by the development of the long-acting insulin NPH in the 1940s (Poretsky, 2009). Cure Science: Future Obstacles The pancreas contains clusters of islet cells also known as islets of Langerhans. The normal function of islet cells is to secrete insulin upon sensing high levels of glucose in the body and to create enzymes necessary to digest food. Insulin signals cells throughout the body to absorb the glucose to use for energy. People with Type 1 diabetes have dysfunctional islet cells that are incapable of secreting insulin. In the situation of Type 2 diabetes, the body has become resistant to insulin, due to a typically high glycemic load. Both situations lead to a hyperglycemic state. A potential cure for this is allo-transplantation of pancreatic islet cells (Diabetes Research Institute, n.d.). An initial obstacle for this cure is the supply of pancreases available from deceased persons. From the 8,000 deceased organ donors in the US in 2011, only 1,562 pancreases were removed (Diabetes Research Institute, n.d.). Many of these organs do not meet the specific criteria and are unusable. One approach to solve this dilemma is to use non-human pancreatic islet cells. A secondary obstacle for this cure is ensuring that the patient’s body does not reject the human or non-human donor islet cells. To overcome this, The Diabetes Research Institute is looking at cure science through the creation of a “BioHub.” This is a mini organ that can imitate the effects of a real native pancreas. The goal of the BioHub is to recreate the natural environment of the insulin producing cells and work toward normalizing the metabolism of the host. This pancreas mini organ would use the person’s natural vasculature to supply it and the material it is made from has pores that would be conducive to function within the SGU Cures Index 204 human body. An alternative solution is to use the patient’s own stem cells to grow new islet cells. Currently, islet cell transplantation is considered experimental, meaning most healthcare and insurance companies do not cover the cost of these procedures (National Diabetes Information Clearinghouse, 2014). Number of Patients Currently Under Treatment While 17.7 million adults in the US reported taking any medication to treat their diabetes in 2011, data for 2007 to 2009 shows most people were taking only oral medications (58%), whereas 12% were only taking insulin, and 14% were taking a combination of oral medication and insulin (Centers For Disease Control, 2012b). Type 1 diabetics focus more on insulin as treatment for their disease because the pancreas is unable to produce any amount of insulin, while Type 2 diabetics start treatment with oral medications because their body still produces and responds to insulin. Number of Patients Requiring Treatment There was an estimated 3.1 million people living in the US in 2011 who were diagnosed with diabetes and who did not report taking any diabetic medication. Additionally, there are approximately 79 million people in the US who have prediabetes who may, in time, need to use insulin or some other form of diabetic medication (Centers for Disease control, 2012b; Centers for Disease Control, 2011a). Impact of insulin on Years of Potential Life Lost (YPLL) Prior to the discovery of insulin, diabetes – especially Type 1 – was a devastating disease that killed patients at a very young age. With no effective treatment aside from a semi-starvation diet, a diabetic's outlook was grim. Before insulin, diabetic children rarely lived a year after diagnosis, five percent died within two years, and fewer than 20% lived more than ten years (Cohn, Berger, & Norton, 1968). Type 1 diabetes accounts for 5 – 10% of all diabetes cases (Daneman, 2006). Taking the middle of that range (7.5%) and multiplying it by the number of diagnosed diabetics in the US (18.8 million) gives an estimate of 1,410,000 Americans whose lives have been saved by insulin over the past 6-7 decades. Impact of insulin on DALY / QALY Prior to the discovery of insulin, patients faced not only early death, but also a reduced quality of life as a result of numerous complications such as blindness, neuropathy, loss SGU Cures Index 205 of limbs, kidney failure, stroke, and heart attack. Type 1 diabetics benefit from continuous glucose monitoring (CGM), gaining an average of 0.60 QALYs. Using CGM, patients are able to maintain glycemic control using insulin injections as needed (Huang, Weinzimer, O'Grady, Wysocki, Tamborlane, Laffel, et al., 2010). Continuous subcutaneous insulin infusion (CSII) (often now just called "insulin pump therapy"), an alternative to subcutaneous insulin injections for type 1 diabetics, uses a portable electromechanical pump to help mimic nondiabetic insulin delivery. This is accomplished by infusing short-acting insulin into the subcutaneous tissue at preselected rates—essentially a slow basal rate throughout the day with patientactivated boosts at mealtimes (Pickup 2002). Charles, Lynch, Graham, and Minshall (2009) report that using CSII increased QALYs by 1.061 among adults at a cost per QALY of $16,992. CSII was also associated with lower incidence of diabetes complications: proliferative diabetic retinopathy, end stage renal disease, and peripheral vascular disease When a patient is diagnosed with type II diabetes at a young age (i.e., 15 to 24 years), this person has a RLE (Remaining Life Expectancy) of 43.09 years, as compared to a non-diabetic person with an RLE of 58.6 years. The QALY for these patients are 39.32 years using only diet to control the disease, but adding pharmacology (i.e., oral medications) to the treatment regimen adds 1.32 QALYs and 0.98 years of RLEs (Rhodes, Prosser, Hoerger, Lieu, Ludwig, & Laffel, 2012). Average Cost of Treatment using insulin People with diagnosed diabetes incur average medical expenditures of about $13,700 per year, of which about $7,900 is attributed to diabetes (American Diabetes Association, 2013). Hogan and Dall (2003) report that in 2002, per capita medical expenditures totaled $13,243 for people with diabetes versus $2,560 for people without diabetes. The lower cost in 2013 may reflect increased screening for diabetes and control of symptoms through advanced technology (i.e., more accurate homebased blood glucose monitors). Kanakis, Watts, and Leichter (2002) report that insulin injection pumps cost $6,500 per year, which is equivalent to $8,417.01 in 2013 dollars. The publication Consumer reports describes six classes of oral medication available to Type 2 Diabetics. Sulfonylureas and meglitinides act on the pancreas to increase its secretion of insulin, metformin works by decreasing insulin resistance and glucose SGU Cures Index 206 production, alpha-glucosidase inhibitors act on the intestine to slow down absorption of glucose, thiazolidinediones decrease insulin resistance and dipeptidyl peptidase 4 inhibitors stimulate the pancreas to increase insulin release after consuming food. Consumer reports recommends Metformin alone or with Glipizide or Glimepiride, Glipizide alone or with Metformin, or Glimepiride alone or with Metformin, taking into account safety, side effects, effectiveness, dosing and cost. These medications come in different forms at different prices, illustrated below in the table (Consumer Reports, n.d.). Medication and dosage Glipizide 5 mg Glipizide 10 mg Glipizide 5 mg, sustained release Glipizide 10 mg, sustained release Glimepiride 1 mg Glimepiride 2 mg Glimepiride 4 mg Metformin 500 mg Metformin 850 mg Metformin 1000 mg Metformin 500 mg, sustained release Metformin 750 mg, sustained release No. of pills per day 1 1 1 1 1 1 1 2 2 2 1 1 Total dose per day 5 mg 10 mg 5 mg 10 mg 1 mg 2 mg 4 mg 1000 mg 1700 mg 2000 mg 500 mg 750 mg Avg. cost per month $5 $4 $10 $20 $7 $8 $14 $14 $24 $35 $8 $25 Economic impact – Value of Life Added It is estimated that insulin has saved the lives of 1,410,000 Americans with Type 1 diabetes, giving a VMRR of $11.562 trillion. The annual cost of insulin is $8,417.01 per patient (2013 dollars), givig a total annual cost of approximately $11.868 billion across all Type 1 diabetic patients whose lives have been saved by insulin (2013 dollars). With a current life expectancy of about 68.8 years among Type 1 diabetics (Miller, Secrest, Sharma, Songer, & Orchard, 2012), the cost of treating all 1,410,000 Americans whose lives have been saved by insulin is ($11.868 billion x 68.8 years) $816.52 billion. Currently, 4.602 million Americans are taking insulin or a combination of insulin and oral medications to treat their diabetes (Centers for Disease Control, 2012b). At a maximum of $8,417.01 per year, the ongoing cost of insulin is $38.735 billion annually. Since injection pumps are one of the most costly forms of taking insulin, the actual annual cost of insulin across all patients is likely lower. In line with this, the American Diabetes SGU Cures Index 207 Association (2013) reports that, of the $176 billion in direct medical costs assocated with diabetes in 2013, 18% ($31.68 billion) was for prescription medications. Type 2 Diabetes Cures: Diet/Exercise Cure Type Potential, Definitive Cure Identification / Description People with a BMI greater than 35kg/m2 are 20 times more likely to develop diabetes than someone with a BMI in the normal range (18.5 to 24.9 kg/m2) (Fowler, 2007). Any person who has an impaired fasting glucose is at risk for developing the disease. Lifestyle modification, through diet and exercise, is an important factor in delaying the progression of the disease and potentially preventing it completely (Fowler, 2007). During exercise, the body can use glucose more effectively, decreasing the amount left in the blood. Cure History and Potential During the second half of the 20th century, many countries experienced a rise in fertility for two decades following the end of World War II (Centers for Disease Control, 2003). The increase in number of births at that time has resulted in a large patient population aged 65 years and older. The growth and aging of this “baby boom” population is especially important now given the strong relationship between chronic disease and age (Eyre, Kahn, Robertson, Clark, & Doyle, 2008). About 80% of older adults have one chronic condition, and 50% have at least two (Centers for Disease Control, 2011b). Lack of exercise and poor nutrition increases the risk of chronic diseases such as cardiovascular disease (CVD), cancer, obstructive pulmonary disease, and Type 2 diabetes. A second contributing factor to increased chronic disease rates since World War II is an increase in affluence (Ezzati, Hoorn, Lawes, Leach, Lopez, et al., 2005). Diseases associated with increased affluence include obesity, CVD, high blood pressure, and type 2 diabetes. Estimates of adult caloric intake in the United States, conducted by the Economic Research Service of the US Department of Agriculture, suggest that daily intake in 2000 was approximately 300 calories greater than in 1985 (Eyre et al., 2008). Dietary patterns that emphasize whole-grain foods and legumes and vegetables and SGU Cures Index 208 fruits and that limit red meat, full-fat dairy products, and foods and beverages high in added sugars are associated with decreased risk of a variety of chronic diseases, including type 2 diabetes (Eyre et al., 2008). A sedentary lifestyle should also be an important modifiable risk factor for type 2 diabetes and CVD in the general population (Bassuk & Manson, 2005). The overall disease burden in a given population generally undergoes a more dramatic reduction when a large segment of the population adopts small improvements in health behaviors than when a small segment of the population adopts large improvements (Bassuk & Manson, 2005). Cure Science The risk factors linked to affluence and age-related chronic disease such as high blood pressure, type 2 diabetes, and increased LDL cholesterol levels, are preventable. Consuming a well balanced diet and engaging in daily physical activity can control these risk factors: Observational and clinical trial data suggest that as little as 30 minutes per day of moderate-intensity physical activity can reduce the incidence of type 2 diabetes and cardiovascular events (Bassuk & Manson, 2005). In one study (JacobsVan Der Bruggen, Bos, Bemelmans, Hoogenveen, Vijgen, and Baan (2007), the most serious risk factor linked to diabetes was being overweight. With every 1 unit increase of BMI the risk of developing type 2 diabetes increased by approximately 10-30%. In a study performed by Simmons, Harding, Jakes, Welch, Wareham, and Griffin (2006), diabetes was inversely related to the number of behavioral goals met. The five goals participants were working to achieve were related to weight, diet, and physical activity. Only 20% of participants met three or more diabetes prevention goals. If the entire population were able to meet one more goal, the total incidence of diabetes was predicted to fall by 20% (Simmons et al., 2006). The NIH performed a recent study focused on Type 2 Diabetes using three separate groups; those practicing lifestyle changes, those using Metformin (oral anti-diabetic medication), and a placebo group. The lifestyle intervention group observed a 58% decreased risk of developing Type 2 Diabetes after 2.8 years (Tuomilehto, 2011). A 10year follow up found a 34% decreased risk of Type 2 diabetes in the lifestyle intervention group, as compared to the control group (Tuomilehto, 2011). The group with Metformin only saw a 31% decreased risk of developing Type 2 Diabetes compared to the control group (Tuomilehto, 2011). Critically, the lifestyle intervention group also experienced improvement in other health-related factors such as CVD, and experienced zero side effects. The direct costs in the lifestyle and Metformin groups were higher ($4,601 and $2,300 per patient) over a 10 year period compared to $769 accrued from the placebo group, however the placebo group experienced more SGU Cures Index 209 healthcare charges from hospitalizations and outpatient visits ($27,468). Healthcare charges were $24,563 for the lifestyle group and $25,616 for the Metformin group. Quality of life, gauged by mobility, level of pain, emotional outlook and other indicators, was best for the lifestyle group (National Institutes of Health, 2012a). Prediabetes is becoming more common in the US The US Department of Health and Human Services estimates that about one in four US adults aged 20 years or older—or 57 million people—had prediabetes in 2007 (National Diabetes Information Clearinghouse, 2008). Unless necessary steps such as lifestyle modifications are taken, those with prediabetes are likely to develop Type 2 diabetes within 10 years (National Diabetes Information Clearinghouse, 2008). More recent data from the CDC indicates that 79 million Americans had prediabetes in the year 2010 (Centers for Disease Control, 2011a). This is an increase of 28% over the past four years. By completing 150 minutes of moderate exercise per week and losing 5 – 7% of their body weight (National Diabetes Information Clearinghouse, 2008), 34% of 79 million (Tuomilehto 2011), or 26.86 million pre-diabetic Americans could avoid progressing to Type 2 diabetes, along with the numerous medical complications associated with such a diagnosis. Controlling blood glucose can help prevent the onset of Type II Diabetes. This can be measured via HbA1c, with a 1% reduction in glucose reducing the risk of disease to the eye, kidney and nerve by 40%. Blood pressure control results in a 33-50% reduction in stroke and heart disease, as well as a 33% reduction in eye, kidney and nerve disease. Decreasing LDL (low density lipoprotein) cholesterol results in a 20-50% reduction of risk of cardiovascular complications (Centers for Disease Control, 2011c). Cure Science: Future Obstacles The obesogenicity of an environment has been defined as the sum of influences that the surroundings, opportunities, or conditions of life have on promoting obesity in individuals or populations (Lake & Townshend, 2006). Americans live in an environment rendered unhealthful by their easy access to energy-dense foods and increasing number of devices (e.g., television remote controls) that reduce their energy expenditure (Wing, Goldstein, Acton, Birch, 2001). Type 2 diabetes has long been linked with behavioral and environmental factors such as overweight, physical inactivity, and dietary habits (Venkat Narayan, Bowman, & Engelgau, 2001). Behavioral and lifestyle interventions have numerous benefits: They are inexpensive, they often address several chronic diseases simultaneously (i.e., diabetes, overweight, CVD), they have few side effects, and they actually reverse the proximal factors SGU Cures Index 210 associated with diabetes (Venkat Narayan, Bowman, & Engelgau, 2001). They also promote health in general while making people less reliant on medicine, and improving quality of life (Venkat Narayan, Bowman, & Engelgau, 2001). However, education programs and individual-level treatments will have limited effectiveness when the environment makes it hard to follow the recommendations (Wing, Goldstein, Acton, & Birch, 2001). Differences in access to healthful foods and opportunities for physical activity may be on of the factors related to the prevalence of obesity in individuals of lower socioeconomic status (Wing et al., 2001). By influencing policies of companies, government agencies, and other organizations whose decisions influence many people, it may be possible to change the unhealthful environment and thereby change obesity at a population level (Wing et al., 2001). The future challenge is to tackle the underlying determinants of type 2 diabetes globally, which means modifying environments to make them less obesogenic (International Diabetes Federation, 2014), while addressing internal motivational and psychological factors that inhibit patients from following a behavioral health regimen. A two-pronged approach is needed, which addressed the environmental context in which people live, while examining individual decision-making, cognitive processing, emotional motivation, and the factors that impede or promote healthy decision-making internally. Making long term changes to eating and activity behaviors is extremely difficult for most patients (Klein, Sheard, Pi-Sunyer, & Daly, 2004). To manage diabetes successfully, patients must be able to set goals and make frequent daily decisions that are both effective and fit their values and lifestyle, while taking into account multiple physiological and personal psychosocial factors (Funnell & Anderson, 2004). A one-time educational program is rarely effective to sustain the types of behavior change needed for a lifetime of diabetes self-care. As a result, patients need ongoing self-management support from their providers and the entire diabetes health care team to maintain gains achieved through education (Funnell & Anderson, 2004). Number of Patients Currently Under Treatment Prediabetes prevalence was 34.1% in 2007-2010 (Abraham & Fox, 2013). The Centers for Disease Control (2011a) reports that 35% of US adults aged 20 years (i.e., 79 million people) have prediabetes, but only 7.3% have been told they have this diagnosis. It has been found that 8.1% of patients with impaired fasting glucose progress to diabetes within 6.3 years; this is an annual rate of 1.34%. Also, the more quickly the patient progresses from a normal glucose level to an impaired fasting glucose, predicts a faster onset of diabetes (Nichols, Hillier, & Brown, 2007). SGU Cures Index 211 The American diet and behavior patterns have shifted unfavorably in the past few decades. In 2011, 52% of adults 18 years or older did not meet recommendations for aerobic exercise and physical activity (Centers for Disease Control, 2014b). In terms of diet, 37.7% of US adults reported consuming fruits less than one time daily while 22.6% of adults reported consuming vegetables less than one time daily (National Center for Chronic Disease Prevention and Health Promotion, 2013). Number of Patients Requiring Treatment In 2011, more than half of adults (52%) did not meet recommendations for aerobic exercise and physical activity, and 23% and 38% of adults reported consuming vegetables and fruits less than once a day, respectively. Over 100 million Americans are not following minimum recommended guidelines for physical activity and healthy diet (Centers for Disease Control and Prevention, 2014b). There is much work to do to understand why the rates are so low, and increasing the number of Americans meeting these guidelines, which is so important for preventing chronic diseases, including Type 2 diabetes. Impact of cure on Years of Potential Life Lost (YPLL) If a pre-diabetic patient loses 5% to 7% of his/her body weight and completes at least 150 minutes of moderate exercise per week, there is a 58% reduction in risk of Type 2 Diabetes after 2.8 years (Centers for Disease Control, 2012d), and a 34% reduction in risk after 10 years (Tuomilehto, 2011). The US Department of Health and Human Services estimates that about one in four US adults aged 20 years or older—or 57 million people—had prediabetes in 2007 (National Diabetes Information Clearinghouse, 2008). Unless necessary steps, such as lifestyle modifications, are taken to prevent diabetes, those with prediabetes are likely to develop Type 2 diabetes within 10 years (National Diabetes Information Clearinghouse, 2008). More recent data from the CDC indicates that 79 million Americans had prediabetes in 2010 (Centers for Disease Control, 2011a). Thus, by completing 150 minutes of moderate exercise per week and losing 5% to 7% of their body weight, (34% of 79 million), or 26.86 million pre-diabetic Americans could avoid progressing to type 2 diabetes. As of the year 2000, an American male diagnosed with diabetes at age 60 will lose 7.3 life years compared to a person of the same age without diabetes (Narayan et al., 2003). An American female diagnosed with diabetes at age 60 years will lose 8.9 life-years compared to a person of the same age without diabetes. If half of the 26.86 million Americans who could avoid progressing to Type 2 diabetes were male, 98.039 million life-years could be saved SGU Cures Index 212 over the next 3-4 decades. Likewise, 119.527 million life-years could be saved among females over the next 3-4 decades. Impact of Diet and Exercise on DALY/QALY An estimated 449 total Disability Adjusted Life Years (DALYs) were lost to diabetes per 100,000 people in the United States in 2004 (WHO, 2008). Given a total US population of 292,810,000, that translates to 1,314,717 DALYs lost to diabetes in 2004 (WHO, 2008). Jacobs-Van Der Bruggen and colleagues (2007) compared the cost-effectiveness of lifestyle interventions for individuals at different levels of diabetes risk (communitybased lifestyle program for the general population versus an intensive lifestyle intervention for obese adults, implemented in a health care setting) using the National Institute for Public Health and the Environment (RIVM) chronic disease model (CDM). They found that community intervention increased life expectancy across the population by 0.007-0.043 (2-16 days), while the health care intervention increased the life expectancy of all the high-risk individuals selected for the intervention by 0.32-1.35 (4-16 months). Likewise, the community intervention increased quality-adjusted life years (QALYs) of the individuals in the population by 0.006-0.039 (2-15 days), while the health care intervention increased QALYs in all of the high-risk individuals selected for the intervention by 0.27-1.17 (3-14 months). While the cost associated with treating diabetes is reduced in both scenarios (-10 to -70 Euros per individual in the community intervention and -500 to -1,700 Euros per individual in the health care intervention), other healthcare costs increase as the individuals live longer, resulting in higher next healthcare costs of 20-110 Euros per individual (community intervention) and 800-3,900 Euros per individual (healthcare intervention). In spite of this, both interventions were cost effective, with the community intervention (3,900-3,100 Euros per QALY) being more so than the healthcare intervention (5,500-3,900 per QALY) (Jacobs-Van Der Bruggen et al., 2007). The authors also report that if the community intervention were able to reach 12 million adults (age 20+), it would prevent 0.4%-2.4% (8,000-43,000) of the new diabetes cases over 20 years, adding 81,000-522,000 life-years, or 76,000477,000 QALYs over that time frame, at a total cost of 40-280 million Euros. If the healthcare interventions were able to reach 12 million adults (age 20+), it would prevent 0.3%-1.6% (6,000-28,000) of the new diabetes cases over 20 years, adding 64,000271,000 life-years, or 56,000-234,000 QALYs over that timeframe, at a total cost of 200800 million Euros. From this analysis, they conclude that: "... both an intensive life-style intervention implemented in a health care setting and targeted to individuals at increased risk of developing diabetes and a community-based lifestyle intervention for SGU Cures Index 213 the general population are effective in reducing diabetes incidence. Although the average lifetime health benefit per individual for the community intervention is relatively low, health gains on a population level may be substantial when the intervention is implemented on a large scale" (Jacobs-Van Der Bruggen et al., 2007, p.132). Average Cost of Treatment via Behavioral Intervention The diabetes prevention program found that participants in the lifestyle intervention group who received intensive individual counseling and motivational support on effective diet, exercise, and behavior modification reduced their risk of developing diabetes by 58 percent after 2.8 years (National Diabetes Information Clearinghouse, 2008). The cost of behavioral interventions to reduce the risk of Type 2 diabetes was effectively $0 – patients can accumulate 150 minutes of moderate exercise a week at no cost by walking around their neighborhood or making choices such as taking the stairs instead of an elevator. Behavioral interventions may include external costs when individuals alter their diet. Research shows that the inverse relationship between energy density of foods, defined as available energy per unit weight (kilocalories per gram or megajoules per kilogram), and energy cost (dollars per kilocalorie or dollars per megajoule) means that diets based on refined grains, added sugars, and added fats are more affordable than the recommended diets based on lean meats, fish, fresh vegetables, and fruit (Drewnowski, 2004). The 'Low income diet and nutrition survey' found that, overall people on lower incomes ate similar types and quantities of food as the general population. However, they were more likely to consume a diet composed of high sugar, fats, and processed foods (Nelson, Erens, Bates, Church, & Boshier, 2007). Within the United States, higher diet quality—often indexed by higher consumption of vegetables and fruit—has been linked to higher education and incomes. Poverty and financial constraints may be one reason for low consumption of fresh produce by the poor and near poor (Drewnowski, 2010). Thus, diet-based behavioral interventions for diabetes may be associated with a cost, but it is exceedingly difficult to place a specific cost on it given the numerous and ever-shifting variables associated with it (i.e., fresh food price variation across the US, ability to grow fresh produce, access to bulk food purchases by groups of individuals, etc). Economic impact – Value of Life Added As outlined above, 79 million US adults aged 20 and older have prediabetes (Centers for Disease Control, 2011a). Lifestyle interventions seem to be at least as effective as pharmacological interventions, and can halve the risk of Type 2 diabetes in people with impaired glucose tolerance (Gillies, 2007). If a pre-diabetic patient loses 5 – 7% of SGU Cures Index 214 his/her body weight and completes at least 150 minutes of moderate exercise per week, there is a 58% reduction in risk of Type II Diabetes (Centers for Disease Control, 2011c), as well as a 34% reduction risk after 10 years (Tuomilehto, 2011). Thus, by following behavioral guidelines, 26.86 million pre-diabetic Americans (34% of 79 million) could avoid progressing to Type 2 diabetes. As of the year 2000, an American male diagnosed with diabetes at age 60 years will lose 7.3 life years compared to a person of the same age without diabetes (Narayan et al., 2003). An American female diagnosed with diabetes at age 60 years will lose 8.9 life-years compared to a person of the same age without diabetes. If half of the 26.86 million Americans who could avoid progressing to Type 2 diabetes via behavioral interventions were male, 98.039 million life-years could be saved over the next 3-4 decades. Likewise, 119.527 million life-years could be saved among females over the coming 3-4 decades. The total VMRR associated with this change is $22.669 trillion. Economic impact – Value of Added DALY/QALY As outlined above, 79 million US adults aged 20 and older have prediabetes (Centers for Disease Control, 2011a). Lifestyle interventions seem to be at least as effective as pharmacological interventions, and can halve the risk of Type 2 diabetes in people with impaired glucose tolerance (Gillies 2007). If a pre-diabetic patient loses 5 – 7% of his/her body weight and completes at least 150 minutes of moderate exercise per week, there is a 58% reduction in risk of Type II Diabetes (Centers for Disease Control, 2011c), as well as a 34% reduction risk after 10 years (Tuomilehto, 2011). Thus, by following behavioral guidelines, 26.86 million pre-diabetic Americans (34% of 79 million) could avoid progressing to type 2 diabetes. As of the year 2000, an American male diagnosed with diabetes at age 60 years will lose 11.1 quality-adjusted life years (QALYs) compared to a person of the same age without diabetes. An American female diagnosed with diabetes at age 60 years will lose 14.2 QALYs (Narayan et al., 2003). Given that 25.8 million Americans have diabetes approximately evenly distributed between males and females, this translates to 143.19 million potentially lost QALYs among currently living American males and 183.18 million potentially lost QALYs among currently living American females. If the 26.86 million Americans who could avoid progressing to Type 2 diabetes via behavioral interventions were evenly split between male and females, 326.37 million QALYs could be saved over the next 3-4 decades, with a VMRR of $34.005 trillion. Research Development and Treatment Costs SGU Cures Index 215 The National Institutes of Health (NIH) provides an annualized breakdown of all spending, organized by research, condition, and disease categories (National Institutes of Health, 2014). The following values indicate the summation of actual and expected expenditures of the “Basic Behavioral and Social Science” and “Behavioral and Social Science” spending categories: 2010 (ARRA and non-ARRA combined): $5.490 billion 2011: $4.746 billion 2012: $4.897 billion 2013: $4.707 billion 2014 estimate: $4.816 billion 2015 estimate: $4.816 billion All research projects listed under the “Basic Behavioral and Social Science” and “Behavioral and Social Science” categories for 2010-2013 were examined, resulting in the following totals of behavioral interventions for diabetes (National Institutes of Health, 2014): 2010 (ARRA and non-ARRA): $12.088 million 2011: $7.219 million 2012: $7.208 million 2013: $3.439 million Finally, all research projects listed under the “Diabetes” spending category were examined. Since the funds directed toward diabetes behavioral health interventions, as listed above, also appear in the Diabetes spending category, these were removed from the search results to obtain an idea of non-behavioral diabetes research spending (National Institutes of Health, 2014): 2010 (ARRA and non-ARRA): $1.186 billion 2011: $1.069 billion 2012: $1.054 billion 2013: $1.004 billion 2014 estimated: $1.028 billion 2015 estimated: $1.039 billion The purpose of this analysis was to gain an understanding of the proportion of public research funds allocated toward diabetes research in general, versus behavioral approaches to diabetes specifically. We estimate that the NIH allocated $29.954 million on diabetes behavioral health research, and $4.312 billion – about 155 times more than behavioral health – on a “medical-based” diabetes research, from 2010 through 2013 (National Institutes of Health, 2014). SGU Cures Index 216 Diabetes Cures: Pancreatic Transplant/Regrowth Cure Type Potential, Definitive Cure Identification/Description Since diabetes mellitus type 1 is caused by a deficiency of insulin-secreting islet Beta cells, the ideal cure for this debilitating condition would be to replace the lost or deficient Beta cells. This could be accomplished in a variety of ways: transplantation of donated islets, transplantation of the pancreas itself, or utilizing adult stem cells that appear to be precursors to islet cells or embryonic stem cells that produce insulin (Jiang & Morahan, 2014; National Institutes of Health, 2013). Similarly, patients suffering from severe diabetes mellitus type 2 who were previously able to make insulin may eventually lose this ability through a phenomenon known as Beta cell exhaustion. Current pancreatic transplant and regrowth protocols preclude type 2 diabetics, however if an effective stem cell therapy were to be found this patient population could also benefit (Robertson, Harmon, Tran, Tanaka, & Takahashi, 2003). The goal of these interventions is to restore glucose-regulated endogenous insulin secretion, arrest the progression of the complications of diabetes, and improve quality of life. Both pancreas and islet transplantation require lifelong immunosuppression to prevent rejection of the graft and recurrence of the autoimmune process. Conventional maintenance regimens consist of a combination of immunosuppressive agents that differ by mechanism of action. This strategy minimizes morbidity and mortality associated with each class of agent while maximizing overall effectiveness. However, the immunosuppressive agents used in transplantation often have side effects severe enough to adversely affect quality of life. Thus, transplantation is generally considered only in patients with serious progressive complications of diabetes in whom the quality of life is unacceptable (Robertson, 2013). The choice between islet or pancreas transplantation is a matter of age and diabetic complications given that the perioperative risk is considerably higher in pancreatic transplantation. (Lehmann, Pavilcek, Spinas & Weber, 2005). In contrast, exploiting precursor or stem cells for treatment of these patients would allow for the avoidance of inimical lifelong immunosuppression. Cure History SGU Cures Index 217 The first attempt to cure Type 1 diabetes through pancreatic transplantation took place at the University of Minnesota on December 17, 1966. This first procedure was followed by a series of whole pancreatic transplantation. However, due to the lack of potent immunosuppressive drugs, rejections and infections, these procedures were less than effective. Through the late 1970's and early 1980's, three major events boosted the development of pancreatic transplantation: first, Cyclosporine A was introduced in the clinical field, reducing the risk of rejection. Secondly, the organization of the first international meeting on Pancreas Transplantation in March of 1980, along with the first report of the International Pancreas Transplantation Registry (IPTR) was important to development. Thirdly, the organization of the first informal so-called Spitzingsee meetings, where clinicians gathered to pool their wisdom, yielded steady incremental gains. These meetings preceded the onset of IPITA (International Pancreas and Islet Transplantation Association), EuroSPK (European Study Group for simultaneous Pancreas and Kidney Transplantation) and EPITA (European Pancreas and Islet Transplantation Association (Squifflet, Gruessner & Sutherland, 2008) While initial efforts focused on whole-organ transplantation, islet transplantation has opened a separate front in the search for a cure. In the 1970s, islet transplant experiments were conducted with great success in laboratory mice. The excitement that those experiments generated soon turned to frustration, as initial attempts to reproduce that success in humans were largely disappointing. For many years progress was slow, and few transplant recipients were able to remain diabetes-free for more than a few months before the transplanted islet cells failed. However, in recent years, scientists have begun to make rapid advances in transplant technology via a new procedure dubbed the Edmonton Protocol to treat patients with Type 1 diabetes (American Diabetes Association, 2013b). The main difference between the Edmonton Protocol and previous methods of islet transplantation is a combination of steroid free immunosuppressive drugs used as well as utilizing islets from two or more donor pancreases in order to increase the number of islets cells transplanted. The Edmonton Protocol has been established as the standard for implantation of pancreatic islets for treatment for type 1 diabetes mellitus, specifically “brittle” type 1 diabetics who are prone to hypoglycemic unawareness. Realizing the continued difficulties with the aforementioned approaches, researchers have turned their attention to precursor and stem cells. Ideally, these cells should be able to multiply in culture and reproduce themselves exactly. Although emerging, SGU Cures Index 218 several investigations into multiple sources of these cells, including fetal tissue, adult tissue, and embryonic stem cells have been completed and do show promise (National Institutes of Health, 2013). Cure Science: Current In recent years there have been noticeable improvements in national pancreas transplant success rates, most noticeably in preventing early graft loss and thrombosis. The hope is that these improvements will translate into longer life expectancy and better pancreas transplant survival rates in the long term. That has certainly been the trend over the past decades as borne out by the statistics (Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients, 2010): • The national 6-month pancreas after kidney (PAK) transplant survival rate increased from 82.2% in 2008 to 88.2% in 2009. A pancreas transplant is often combined with a kidney transplant to reduce progression of the complications of diabetes (Pancreas Transplant 2014) • The national one year pancreas transplant alone (PTA) survival rate was 75.4% in 2008 • The national five-year pancreas transplant alone (PTA) survival rate was 48.3% in 2004. The previously mentioned Edmonton Protocol has restored optimism about islet transplantation as a treatment option for type I diabetes. Islet transplantation has been shown to normalize metabolic control in a way that is virtually impossible to achieve with exogenous insulin. The less invasive procedure of islet transplantation in patients with type 1 diabetes mellitus is expected to be safer and much less costly than whole pancreas transplantation (Lehmann, Pavilcek, Spinas & Weber, 2005). When using this methodology, the islet cells are mined from the donor pancreas, purified and evaluated before transplantation. When the cells have been harvested, the patient is locally anesthetized and a very thin needle and x-rays are used to locate the portal vein. The islet cells are injected into the portal vein via catheter from which they can travel to the liver, develop a blood supply and begin producing insulin, partly due to the capability of the liver to regenerate, build new blood vessels and make new supporting tissue when damaged (The Procedure, n.d.). Despite the initial success the Edmonton protocol, it will not become a widely used technique until an alternative source of cells is found to supplement the limited supply of donor tissue. Currently, the use of embryonic and adult stem cells in the Edmonton protocol is a topic of much research. Stem cells exhibit two important characteristics: SGU Cures Index 219 they are unspecialized cells that are able to proliferate over a long period of time through cell division and, under special conditions, they can be induced to differentiate into specialized cells, such as the insulin-producing cells of the pancreas. Studies are focusing on two types of stem cells: embryonic (ESC) and adult stem cells. Investigations of the ability of embryonic stem cells to differentiate into insulinproducing cells have been underway for several years. It has been shown that insulin expression occurs early in the differentiation of ESC. However, complete differentiation of ESC into mature pancreatic Beta cells has not been greatly successful. The ESC cells that have been differentiated exhibit far lower insulin content than normal Beta cells. Additionally, recent studies have shown that multiple varieties of stem cells derived from bone marrow have differentiating abilities and may be manipulated into producing insulin in the pancreas. Stem cells found in the ductal structures of adult pancreases have also exhibited self-renewing and differentiating abilities in vitro. When mouse islet cells were formed from precursor pancreatic stem cells and implanted into diabetic mice, insulin independence was achieved. There has also been strong evidence for the existence of islet precursor cells in the neonatal pig pancreas. Digestion of fetal pig pancreas in collagenase and introduction of this digest into diabetic mice has been shown to restore normal blood glucose levels (The Future). Cure Science: Future Obstacles Islet cell transplantation has led to insulin independence in 80% of patients at one year post-transplantation, but this falls to only 10% of patients at five years (Onaca, Naziruddin, Matsumoto, Noguchi, Klintmalm, & Levy, 2007). Thus, maintaining longerterm success with the transplantation is a significant obstacle. Despite this, it is important to note that persistent graft function, even without insulin independence, results in improved glucose control and avoidance of hypoglycemic events. Changes in islet processing techniques and immunosuppression regimens are currently being examined to increase the time frame of insulin independence (Onaca, Naziruddin, Matsumoto, Noguchi, Klintmalm, & Levy, 2007). Additionally, pancreatic islet transplantation use is limited due to an insufficient supply of organ-donor-provided pancreases (Anazawa, Saito, Goto, Kenmochi, Uemoto, Itoh, et al., 2014). The challenges facing pancreas transplantation essentially mirror those of islet cell transplantation, particularly with respect to viable immunosuppression regimens as well as the limited availability of donor organs and the necessity of transplantation of several pancreata in order to achieve insulin independence (Lehmann, Pavilcek, Spinas, & Weber, 2005). SGU Cures Index 220 Before stem cell-derived Beta cells can be considered for use in human trials, the cells must demonstrate normal physiologic responses to changes in glucose, must maintain genetic stability after transplantation, and must not be teratogenic (Robertson, 2013). Additionally, a renewable source of human stem cells must be developed. Although many progenitor cells have been identified in adult tissue, few of these cells can be cultured for multiple generations. Embryonic stem cells show the greatest promise for generating cell lines that will be free of contaminants and that can self renew. A potential advantage of embryonic cells is that, in theory, they could be engineered to express the appropriate genes that would allow them to escape or reduce detection by the immune system. This would overcome the biggest hurdle for both islet cell and pancreas transplants. Most researchers agree that until a therapeutically useful source of human islet cells is developed, all avenues of research should be pursued, including both adult and embryonic sources of tissue. A major consideration is whether any precursor or stem-like cells transplanted into the body might revert to a more pluripotent state and induce the formation of tumors. These risks would seemingly be lessened if fully differentiated cells were used in transplantation. Furthermore, it is not clear whether it will be desirable to produce only Beta cells or whether other types of pancreatic islet cells are also necessary. Studies have indicated that isolated Beta cells—those cultured in the absence of the other types of islet cells—are less responsive to changes in glucose concentration than intact islet clusters made up of all islet cell types (National Institutes of Health, 2013). Number of Patients Currently Under Treatment The Collaborative Islet Transplant Registry (CITR) relies on voluntary data submission, making counts from CITR data the lower bound on the actual number of transplants. The registry reports that there were 571 allogeneic islet transplant recipients [481 islet transplant alone (ITA) and 90 islet after or simultaneous with kidney (IAK/SIK)] who received 1,072 infusions from 1,187 donors between 1999 and 2009 in North America. (Collaborative Islet Transplant Registry, 2011). According to the International Pancreas Transplant Registry (IPTR), more than 30,000 pancreas transplants took place between 1966 and 2008. This included more than 22,000 in the US and more than 8,000 from outside the US Between 2004 and 2008, the most common pancreas transplant category was a combined pancreas/kidney transplant (SPK) (73%) (Gruessner & Sutherland, 2008). Number of Patients Requiring Treatment SGU Cures Index 221 Transplantation of pancreatic tissue, either the intact whole pancreas or isolated pancreatic islets, as well as interventions involving pancreatic precursor or stem cells, has become (or has the potential to become) a clinical treatment option for patients with type 1 insulin-dependent diabetes mellitus (Meloche, 2007). The approximately 1.4 million Americans who have Type 1 diabetes that could potentially benefit from this cure, should their condition progress to a state that warrants such an intervention. A successful stem cell approach could additionally benefit the 27% of persons with Type 2 diabetes who use insulin therapy, which adds another 6.4 million Americans to this total (Mayfield & White, 2004). Impact of cure on Years of Potential Life Lost (YPLL) According to Miller and colleagues (2012), the life expectancy at birth for those diagnosed with Type 1 diabetes between 1965-1980 was 68.8 years (95% CI: 64.7 – 72.8). Given the current US life expectancy of 78.7 (Centers for Disease Control, 2014), approximately 9.9 years of life are lost among Type 1 diabetics, on average. While islet cell or whole pancreas transplantation is currently reserved for the most debilitated diabetes type 1 sufferers where more traditional approaches, such as insulin therapy, have not produced desired outcomes, the procedure could be provided to all Type 1 diabetics following further advances in donor cells (most likely form stem cell-derived isolated Beta cells), and the ability of the cells to escape or reduce detection by the recipient’s immune system. While islet cell transplantation has led to insulin independence in 80% of patients at one year post-transplantation and 10% of patients at five years (Onaca et al, 2007), persistent graft function, even without insulin independence, results in improved glucose control and avoidance of hypoglycemic events. Thus, if all 1.4 million Type 1 diabetics received islet cell transplantation and experienced even a 2.5-year extension in life expectancy, total extra years of life lived would equal 3.5 million. Islet transplantations have traditionally been offered only to diabetic patients with endstage renal complications. The Edmonton Protocol has made it feasible to successfully reverse diabetes by transplanting solitary islets into patients who experience rapid disproportionate blood glucose changes despite being in good compliance with their insulin regimen. Such patients are designated as “brittle diabetics”. Although brittle diabetes is uncommon, affecting 3 in 1000 insulin-dependent diabetic patients, it can cause a considerable burden on hospital, social, and family resources due to multiple hospital admissions (Vantyghem & Press, 2008). Although most clinical experts in the management of Type 1 diabetes continue to see patients with brittle diabetes mellitus, SGU Cures Index 222 many of whom have a substantial behavioral or iatrogenic contribution to their brittle state, the modern day course of such patients has not been well described (McCulloch 2006). It has been documented, however, that these patients are at a higher risk of death, more microvascular and pregnancy complications, and a poorer quality of life (Kent, Gill, & Williams, 1994). In one study a cohort of brittle diabetics were observed to have a significantly shortened life expectancy, dying at an age ranging from 27 to 45 years (Cartwright, Wallymanhmed, Macfarlane, Wallymanhmed, Williams, & Gill, 2011). With the prevalence of brittle diabetes, which occurs virtually exclusively in type 1 diabetics (McCulloch 2006) at a rate of about 3 in 1,000, we conclude that there are approximately 4,230 brittle type 1 diabetics in the US among 1.4 million type 1 diabetics. If islet cell transplantation could extend the lives of these 4,230 brittle diabetics from a current maximum of 45 years to the current US life expectancy of 78.7 (+ 33.7), an additional 142,551 years of life could be saved. Pancreas/Islet Cell Treatment Costs The cost of isolating human pancreas islets is over $20,000 per gland. At 2-4 organs per patient, this results in over $100,000 per patient per procedure. This can be compared to the cost of a whole pancreas transplant, which averages $45,000 per procedure. Immunosuppressive regimens that are necessary after the procedure may exceed $40,000 per year in cost (The Procedure, n.d.). If 1.4 million Type 1 diabetics (including 4,230 brittle diabetics) received islet cell transplantation and needed to take a immunosuppressive regimen during the full 2.5 years of extra life lived (on average), the total maximum cost would be 1.4 million people x $100,000 (procedure) + $100,000 (2.5 years x $40,000 per year) = $280 billion. Economic Impact – Potential Value of Life Added Multiplying 3.5 million years of potential added life years via islet cell transplantation in 1.4 million Type 1 diabetics plus 142,551 potential extra years of life lived among an estimated 4,230 brittle diabetics gives a VMRR of $379.528 billion, which is $99.5 billion more than the estimated total cost to treat all 1.4 million Type 1 diabetics with islet cell transplantation and immunosuppressive regimen over 2.5 years. Citations Abraham, T. M. & Fox, C. S. (2013). Implications of rising prediabetes prevalence. Diabetes Care, 36(8), 2139-2141. SGU Cures Index 223 Adeghate, E., Schattner, P., & Dunn, E. (2006). An Update On The Etiology And Epidemiology Of Diabetes Mellitus. Annals of the New York Academy of Sciences, 1084(1), 1-29. American Diabetes Association. (2013a). Economic Costs of Diabetes in the US in 2012. Diabetes Care, 36(4), 1033-1046. Retrieved July 3, 2014, from: http://care.diabetesjournals.org/content/36/4/1033.full American Diabetes Association. (2013b). Islet Transplantation. Retrieved September 28, 2014, from http://www.diabetes.org/living-with-diabetes/treatment-andcare/transplantation/islet-transplantation.html American Diabetes Association. (2014a). History of Diabetes. Retrieved April 15, 2014 from: http://www.diabetes.org/research-and-practice/student-resources/history-ofdiabetes.html#ten American Diabetes Association. (2014b). Statistics About Diabetes. Retrieved July 4, 2014 from: http://www.diabetes.org/diabetes-basics/statistics/ Anazawa, T., Saito, T., Goto, M., Kenmochi, T., Uemoto, S., Itoh, T., Yasunami, Y., Kenjo, A., Kimura, T., Tsuchiya, T., Gotoh, M. (2014). Long-term outcomes of clinical transplantation of pancreatic islets with uncontrolled donors after cardiac death: a multicenter experience in Japan. Trasplant Procedings, 46(6), 1980-4 Bassuk, S. S., & Manson, J. E. ( 2005). Epidemiological Evidence For The Role Of Physical Activity In Reducing Risk Of Type 2 Diabetes And Cardiovascular Disease. Journal of applied Physiology, 99, 1193-1204. Retrieved June 27, 2014, from: http://jap.physiology.org/content/99/3/1193 Brown, M. M., Brown, G. C., Sharma, S., & Landy, J. (2003). Health Care Economic Analyses and Value-Based Medicine. Survey of Ophthalmology, 48(2), 204-223. Cartwright, A., Wallymanhmed, M., Macfarlane, I., Wallymanhmed, A., Williams, G., & Gill, G. (2011). The outcome of brittle type 1 diabetes--a 20 year study. QJM: An International Journal of Medicine, 104(7), 575-579. Centers for Disease Control and Prevention. (2003). Public Health and Aging: Trends in Aging --- United States and Worldwide. Retrieved June 24, 2014, from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5206a2.htm SGU Cures Index 224 Centers for Disease Control and Prevention. (2011a). National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Retrieved April 14, 2014, from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Centers for Disease Control and Prevention (2011b). Healthy Aging. Retrieved June 25, 2014, from: http://www.cdc.gov/chronicdisease/resources/publications/aag/aging.htm Centers for Disease Control and Prevention. (2011c). Diabetes. Retrieved April 27, 2014, from: http://www.cdc.gov/chronicdisease/resources/publications/aag/ddt.htm Centers for Disease Control and Prevention. (2012a). Chronic Disease Prevention and Health Promotion. Retrieved April 13, 2014, from: http://www.cdc.gov/chronicdisease/overview/index.htm#ref10 Centers for Disease Control and Prevention. (2012b). National Surveillance. Retrieved April 16, 2014, from: http://www.cdc.gov/diabetes/statistics/us/index.htm Centers for Disease Control and Prevention. (2012c). Diabetes Research and Statistics. Retrieved April 14, 2014, from: http://www.cdc.gov/diabetes/consumer/research.htm Centers for Disease Control and Prevention. (2012d). Prediabetes Facts. Retrieved June 5, 2014, from: http://www.cdc.gov/diabetes/prevention/factsheet.htm Centers for Disease Control and Prevention. (2013). Diabetes Awareness This National Diabetes Month. Retrieved March 31, 2014, from: http://www.cdc.gov/Features/LivingWithDiabetes/ Centers for Disease Control and Prevention. (2014a). Chronic Disease and Health Promotion. Retrieved June 24, 2014, from: http://www.cdc.gov/chronicdisease/overview/index.htm#ref10 Centers for Disease Control and Prevention. (2014b). Diabetes Public Health Resource. Retrieved on October 17, 2014 from: http://www.cdc.gov/diabetes/surveillance/faq.htm#type1type2 SGU Cures Index 225 Charles, M. S., Lynch, P., Graham, C., & Minshall, M. E. (2009). A cost-effectiveness analysis of continuous subcutaneous insulin injection versus multiple daily injections in type 1 diabetes patients: A third-party US payer perspective. Value in Health, 12(5), 674-686. Cohn, C., Berger, S., & Norton, M. (1968). Relationship between meal size and frequency and plasma insulin response in man. Diabetes, 17(2), 72-75. Collaborative Islet Transplant Registry. (2011). Seventh Annual Report. Rockville, MD: CITR Coordinating Center The EMMES Corporation. Consumer Reports. (n.d.). The Oral Diabetes Drugs Treating Type 2 Diabetes: Comparing Effectiveness, Safety, and Price. Retrieved March 11, 2014, from: http://www.consumerreports.org/health/resources/pdf/best-buydrugs/DiabetesUpdate-FINAL-Feb09.pdf Curry, C., De, A., Ikeda, R., & Thacker, S. (2006). Health burden and funding at the Centers for Disease Control and Prevention. American Journal of Preventive Medicine, 30(3), 269-276. Daneman, D. (2006). Type 1 diabetes. Lancet, 367(9513), 847-58. Diabetes Research Institute. (n.d.). DRI BioHub. Retrieved April 16, 2014, from: http://www.diabetesresearch.org/biohub Diabetes Research Institute Foundation. (n.d.). Cell Supply: Developing an Unlimited Supply of Insulin-Producing Cells. Retrieved April 14, 2014, from: http://www.diabetesresearch.org/cell-supply Diabetes UK. (2012). Diabetes Treatments. Retrieved April 21, 2014, from: http://www.diabetes.org.uk/Guide-to-diabetes/What-is-diabetes/Diabetestreatments/ Drewnowski, A. (2004). Obesity and the food environment: dietary energy density and diet costs. American Journal of Preventive Medicine, 27(3), 154-162. Drewnowski, A. (2010). The cost of US foods as related to their nutritive value. The American Journal of Clinical Nutrition, 92(5), 1181-1188. SGU Cures Index 226 Engelgau, M. M., Geiss, L., Imperatore, G., Narayan, V., Saaddine, J., Boyle, J., et al. (2004). The evolving diabetes burden in the United States. Annals of Internal Medicine, 140(11), 945. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care, 25(supplement 1), s5-s20. Eyre, H., Kahn, R., Robertson, R. M., Clark, N., Doyle, C., Gansler, T., et al. (2008) Preventing cancer, cardiovascular disease, and diabetes: A common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. A Cancer Journal for Clinicians, 54, 190-207. Ezzati, M., Hoorn, S. V., Lawes, C., Leach, R., James, W. P., Lopez, A., et al. (2005). Rethinking the “diseases of affluence” paradigm: Global patterns of nutritional risks In relation to economic development. PLoS Medicine, e133. Fowler, M. J. (2007). Diabetes treatment, part 1: Diet and exercise. Clinical Diabetes, 25, 105-109. Funnell, M., & Anderson, R. (2004). Empowerment and self-management of diabetes. Clinical Diabetes , 22, 123-127. Gillies, C. L., Abrams, K. R., Lambert, P. C., Cooper, N. J., Sutton, A. J., Hsu, R. T., et al. (2007). Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: Systematic review and metaanalysis. British Medical Journal, 334(7588), 299-299. Gruessner, A., & Sutherland, D. (2008). Pancreas transplant outcomes for United States (US) cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Clinical Transplants, 45-56. Downloaded on October 20, 2014 from: http://www.ncbi.nlm.nih.gov/pubmed/19708445 Hogan, P., & Dall, T. (2003). Economic Costs Of Diabetes In The US In 2002. Diabetes Care, 26(3), 917-932. SGU Cures Index 227 Huang, E. S., Weinzimer, S., O'Grady, M., Wysocki, T., Tamborlane, W., Laffel, L., et al. (2010). The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care, 33(6), 1269-1274. International Diabetes Federation (2014). The social determinants of diabetes and the challenge of prevention. Retrieved July, 2014, from: http://www.idf.org/diabetesatlas/5e/the-social-determinants-of-diabetes-and-thechallenge-of-prevention. Jacobs-Van Der Bruggen, M., Bos, G., Bemelmans, W. J., Hoogenveen, R. T., Vijgen, S. M., & Baan, C. A. (2007). Lifestyle interventions are cost-effective in people with different levels of diabetes risk. Diabetes Care, 30(1), 128-134. Jiang, F., & Morahan, G. (2014). Pancreatic Stem Cells Remain Unresolved. Stem Cells and Development, Epub 2014. Retrieved on October 20, 2014 from: http://www.ncbi.nlm.nih.gov/pubmed/25132582 Jonsson, B. (1998). The economic impact of diabetes. Diabetes Care, 21(3), C7-C10. Kahn, C., & White, M. (1988). The insulin receptor and the molecular mechanism of insulin action. The American Society for Clinical Investigation, 82, 1151-1156. Kanakis, S. J., Watts, C., & Leichter, S. B. (2002). The business of insulin pumps in diabetes care: Clinical and economic considerations. Clinical Diabetes, 20(4), 214216. Kent, L., Gill, G., & Williams, G. (1994). Mortality and outcome of patients with brittle diabetes and recurrent ketoacidosis. Lancet, 344(8925), 778-81. Klein, S., Sheard, N., Pi-Sunyer, X., Daly, A., Wylie-Rosett, J., Kulkarni, K., et al. (2004). Weight management through lifestyle modification for the prevention and management of type 2 diabetes: Rationale and strategies: A statement of the American Diabetes Association, The North American Association for the study of obesity, and the American Society for Clinical Nutrition. Diabetes Care, 27, 20672073. Retrieved July 3, 2014, from: http://care.diabetesjournals.org/content/27/8/2067.full#ref-16 SGU Cures Index 228 Krishnan, R., Alexander, M., Robles, L., Foster, C., & Lakey, J. (2014). Islet and stem cell encapsulation for clinical transplantation. The Review of Diabetic Studies, Spring 11(1), 84-101. Lake, A., & Townshend, T. (2006). Obesogenic environments: Exploring the built and food environments. The Journal of the Royal Society for the Promotion of Health, 126(6), 262-267. Lehmann, R., Pavilcek, V., Spinas, G., & Weber, M. (2005). Islet transplantation in type I diabetes mellitus. Therapeutische Umschau 6(7), 481-486. Magliocca, J., & Odorico, J. (2006). Embyonic stem cell-based therapy for the treatment of diabetes mellitus: A work in progress. Current Opinion in Organ Transplantation, 11(1):88-93. Mayfield, J., & White, R. (2004). Insulin Therapy for Type 2 Diabetes: Rescue, Augmentation, and Replacement of Beta-Cell Function. American Family Physician, 70(3), 489-500. Retrieved December 20, 2014, from http://www.aafp.org/afp/2004/0801/p489.html McCulloch, D. (2014, August 1). The adult patient with brittle diabetes melitus. Retrieved September 1, 2014 from: http://www.uptodate.com/contents/theadult-patient-with-brittle-diabetes-mellitus Meloche, R. (2007). Transplantation for the treatment of type 1 diabetes. World Journal of Gastroenterology, 13(47), 6347-55. Miller, R., Secrest, A., Sharma, R., Songer, T., & Orchard, T. (2012). Improvements in the Life Expectancy of Type 1 Diabetes. Diabetes, 61, 2987-2992. Narayan, K. M., Boyle, J., Thompson, T., Sorenson, S., & Williamson, D. (2003). Lifetime risk for diabetes mellitus in the united states. Journal of the American Medical Association, 290(14), 1884-1890. National Center for Chronic Disease Prevention and Health Promotion. (2013). State Indicator Report on Fruits and Vegetables 2013. Retrieved June 25, 2014, from: http://www.cdc.gov/nutrition/downloads/State-Indicator-Report-FruitsVegetables-2013.pdf SGU Cures Index 229 National Diabetes Information Clearinghouse. (2008). Diabetes Prevention Program. Retrieved May 30, 2014, from: http://diabetes.niddk.nih.gov/dm/pubs/preventionprogram/#results National Diabetes Information Clearinghouse. (2013). Insulin Resistance and Prediabetes. Retrieved April 13, 2014, from: http://diabetes.niddk.nih.gov/dm/pubs/insulinresistance/ National Diabetes Information Clearinghouse. (2014). Pancreatic Islet Transplantation. Retrieved April 15, 2014, from: http://diabetes.niddk.nih.gov/dm/pubs/pancreaticislet/#7 National Institutes of Health. (n.d.). The Facts About Diabetes: A Leading Cause of Death in the US Retrieved April 14, 2014, from: http://ndep.nih.gov/diabetesfacts/#age National Institutes of Health. (2012a). Diabetes Prevention: A Good Investment. Retrieved April 21, 2014, from: http://www.nih.gov/researchmatters/april2012/04022012diabetes.htm National Institutes of Health (2013). Stem cells and diabetes. Stem Cell Information. Retrieved on August 31, 2014 from: http://stemcells.nih.gov/info/scireport/pages/chapter7.aspx National Institutes of Health (2014). Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). Retrieved March 31, 2014, from: http://report.nih.gov/categorical_spending.aspx Nelson, M., Erens, B., Bates, B., Church, S., Boshier, T., (2007). Low income diet and nutrition survey: Summary of key findings. Retrieved June, 2014, from http://multimedia.food.gov.uk/multimedia/pdfs/lidnssummary.pdf Nichols, G.A., Hillier, T.A., Brown, J. B. (2007). Progression from newly acquired impaired fasting glucose to type 2 diabetes. Diabetes Care, 30(2), 228-233. Retrieved n.d., from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1851903/ Nobel Media. (2013). Diabetes and Insulin. Retrieved February 3, 2014, from http://www.nobelprize.org/educational/medicine/insulin/discovery-insulin.html SGU Cures Index 230 Onaca, N., Naziruddin, B., Matsumoto, S., Noguchi, H., Klintmalm, G., & Levy, M. (2007). Pancreatic islet cell transplantation: Update and new developments. Nutrition in Clinical Practice, 22(5), 485-93. Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients (2010). OPTN/SRTR 2010 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. Pancreas Transplant. (2014, July 12). Retrieved September 22, 2014, from http://www.nhs.uk/conditions/pancreastransplant/Pages/Introduction.aspx Pickup, J. (2002). Continuous Subcutaneous Insulin Infusion at 25 Years: Evidence Base for the Expanding Use of Insulin Pump Therapy in Type 1 Diabetes. Diabetes Care, 25(3). Poretsky, L. (2010). Principles of Diabetes Mellitus (2nd ed.). New York: Springer Verlag. Rhodes, E., Prosser, L., Hoerger, T., Lieu, T., Ludwig, D., & Laffel, L. (2012). Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabetic Medicine: A Journal Of The British Diabetic Association, 29(4), 453-463. Rich, S. (1990). Mapping genes in diabetes genetic epidemiological perspective. Perspectives in Diabetes, 39, 1315-1319. Rizza, R., Eddy, D., & Khan, R. (2008). Cure, care, and commitment: What can we look forward to? Diabetes Care, 31(5), 1051-1059. Robertson, P. (2013). Pancreas and islet transplantation in diabetes mellitus. Retrieved On September 28, 2014, from: http://www.uptodate.com/contents/pancreas-andislet-transplantation-in-diabetes-mellitus?source=search_result&search=Pancreas and islet transplantation in diabetes mellitus&selectedTitle=1~150#H1 Robertson, P., Harmon, J., Tran, P., Tanaka, Y., & Takahashi, H. (2003). Glucose Toxicity in Beta-Cells: Type 2 Diabetes, Good Radicals Gone Bad, and the Glutathione Connection. Diabetes, 52(3). Retrieved December 20, 2014, from http://www.medscape.com/viewarticle/450151 SGU Cures Index 231 Rohlfing, C. L., Wiedmeyer, H., Little, R. R., England, J. D., Tennill, A., & Goldstein, D. E. (2002). Defining the relationship between plasma glucose and HbA1c: Analysis of glucose profiles and HbA1c in the diabetes control and complications trial. Diabetes Care, 25(2), 275-278. Simmons, R. K., Harding, A.-H., Jakes, R. W., Welch, A. Wareham, N. J., & Griffin, S. J. (2006). How much might achievement of diabetes prevention behavior goals reduce the incidence of diabetes if implemented at the population level. Diabetologia, 49, 905-911. Squifflet, J., Gruessner, R., & Sutherland, D. (2008). The history of pancreas transplantation: Past, present and future. Acta Chirurgica Belgica, 108(3), 367-78. The Future. (n.d.). Retrieved September 21, 2014, from http://biomed.brown.edu/Courses/BI108/BI108_2004_Groups/Group09/fu ture.htm The Procedure. (n.d.). Retrieved September 21, 2014, from http://biomed.brown.edu/Courses/BI108/BI108_2004_Groups/Group09/pr ocedure.htm Tuomilehto, J., Schwarz, P., & Lindstrom, J. (2011). Long-term benefits from lifestyle interventions for type 2 diabetes prevention. Diabetes Care, 34. Retrieved May 30, 2014, from: http://care.diabetesjournals.org/content/34/Supplement_2/S210.full US National Library of Medicine. (2014). Insulin Injection. Retrieved April 16, 2014, from: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a682611.html US Renal Disease System (2013). USRDS Annual Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD. Vantyghem, M., & Press, M. (2006). Management for brittle diabetes. Annals of Endocrinology, 67(4), 287-296. Venkat Narayan, K. M., Bowman, B. A., & Engelgau, E. (2001). Prevention of type 2 diabetes. British Medical Journal, 323, 63. SGU Cures Index 232 Wing, R., Goldstein, M., Acton, K., Birch, L., Jackicic, J., Sallis, J., et al. (2001). Behavioral science research in diabetes: Lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care, 21, 117-123. Retrieved July 3, 2014, from: http://care.diabetesjournals.org/content/24/1/117.full World Health Organization. (n.d.). Genetics and Diabetes. Human Genetics programme. Retrieved March 1, 2014, from http://www.who.int/genomics/about/Diabetisfin.pdf World Health Organization, (2008). The global burden of disease: 2004 update. Geneva, WHO. Available at www.who.int/evidence/bod Yang, W., Dall, T., Halder, P., Gallo, P., Kowal, S., & Hogan, P. (2013). Economic costs of diabetes in the US in 2012. Diabetes Care, 36(4), 1033-1046. SGU Cures Index 233 MOOD (AFFECTIVE) DISORDERS Randall Waechter Disease Category Chronic Disease Identification, Description, and Diagnostic Criteria There are two main categories of mood disorder: Unipolar (i.e., depression), and Bipolar (i.e., swings between depression and mania). In general, those suffering from mood disorders experience more severe and longer-lasting swings in normal emotional reactions to life experiences. In some cases the swings are rapidly occurring. The line between "normal" and "abnormal" swings in mood is not always clear, especially given the lack of a bona fide objective diagnostic test (Berton & Nester, 2006). Thoughts, physiology, psychomotor functioning, behavior, motivation, and psychosocial functioning are impacted in addition to mood. Average age of onset is 30 years and mood disorders often co-occur with anxiety disorders (Kessler, Berglund, Demler, Jin, & Walters (2005). According to the American Psychiatric Association’s (APA) Diagnostic and Statistical Manual (DSM) there are seven types of bipolar and related disorders: 1. Bipolar I Disorder; 2. Bipolar II Disorder; 3. Cyclothymic Disorder; 4. Substance/Medication-Induced Bipolar and Related Disorder; 5. Bipolar and Related Disorder Due to Another Medical Condition; 6. Other Specified Bipolar and Related Disorder; 7. Unspecified Bipolar and Related Disorder Further, there are eight types of Depressive Disorders: 1. Disruptive Mood Dysregulation Disorder; 2. Major Depressive Disorder, Single and Recurrent Episodes; 3. Persistent Depressive Disorder (Dysthymia); 4. Premenstrual Dysphoric Disorder; 5. Substance/Medication-Induced Depressive Disorder; 6. Depressive Disorder Due to Another Medical Condition; SGU Cures Index 234 7. Other Specified Depressive Disorder; 8. Unspecified Depressive Disorder According to the World Health Organization’s (WHO) International Classification of Diseases (ICD-10), mood (affective) disorders are classified as: F30 (Manic Episode); F31 (Bipolar Affective Disorder); F32 (Depressive Episode); F33 (Recurrent Depressive Disorder); F34 (Persistent Mood [Affective] Disorders); F38 (Other Mood [Affective] Disorders); F39 (Unspecified Mood [Affective] Disorders). Mood (Affective) Disorders comprise a complex disease field with multiple variations in disease representation, causes and complicating environmental factors. Variations can be conceived along a broad spectrum of affective illness, with Bipolar Disorder I and a Major Depressive Episode associated with the most severe symptoms and thus, at the upper extreme of life disruption, and Bipolar II, Cyclothymic Disorder, Premenstrual Dysphoric Disorder, and Unspecified Depressive Disorder usually presenting in less disruptive forms. Diagnosis of mood (affective) disorders is based on pre-specified behavioral criteria as outlined by the APA or the ICD-10, and must be done by a qualified psychologist or psychiatrist who interviews the patient (i.e., there is no objective blood or urine test). The severity of the disorder is judged as mild, moderate or severe, based on the degree of impairment in daily occupational and social functioning (Berton & Nester, 2006). This report focuses on the more disruptive and extreme forms of Bipolar and Depressive Disorders (i.e., Bipolar I, Bipolar II, Disruptive Mood Dysregulation Disorder, Major Depressive Disorder, Persistent Depressive Disorder). Disease Etiology (Cause) The precise cause of mood disorders is unknown, but most mental health professionals agree with a general causal model known as Diathesis-Stress. The term diathesis derives from the Greek word for disposition, or vulnerability, and it can take the form of genetic, psychological, biological, or situational factors (Ingram & Luxton, 2005). The diathesis, or predisposition, interacts with the subsequent stress response of an individual to life events that disrupt a person’s psychological equilibrium (Oatley, Keltner, & Jenkins, 2006b). The diathesis-stress model asserts that if the combination of the predisposition SGU Cures Index 235 (i.e., vulnerability) and the existing stress exceeds a threshold, the person will develop a disorder (Lazarus, 1993). Consistent with the diathesis-stress model, roughly 40–50% of the risk for depression is genetic, although the specific genes that underlie this risk have not yet been identified. The remaining 50–60% of the non-genetic risk also remains poorly defined, with suggestions that early childhood trauma, emotional stress, physical illness, and even viral infections might be involved (Berton & Nestler, 2006). The effectiveness of antidepressant medications points to an imbalance of neurotransmitters as a critical component in mood disorders, specifically monoamine neurotransmitters such as dopamine, serotonin, noradrenaline, adrenaline, and histamine. However, the precise reasons for monoamine imbalance are unknown (Berton & Nestler, 2006). Numerous reductions in brain structure volumes are also seen in mood disorders, including the frontal cortex, temporal cortex, hippocampus, amygdala, caudate nucleus, thalamus, and hypothalamus (Berton & Nestler, 2006; Sheline, 2003). Current Prevalence/Incidence US Prevalence: Major Depressive Disorder affects approximately 14.8 million American adults, or about 6.7 percent of the US population age 18 and older in any given year (Kessler, Berglund, Demler, Jin, & Walters, 2005; US Census Bureau, 2005). The disorder is more prevalent in women than men (Kessler, Berglund, Demler, Jin, Koretz, Merikangas, Rush, Walters, & Wang, 2003). US Prevalence: Bipolar Disorder affects approximately 5.7 million American adults, or about 2.6 percent of the US population age 18 and older in any given year (Kessler et al, 2005; US Census Bureau, 2005). The median age of onset for bipolar disorders is 25 years (Kessler et al, 2005). Estimated Undiagnosed/At-Risk Patients with depression are most likely to present to their primary care physician with somatic complaints such as vague, nonspecific pain (e.g., headaches, muscle aches), feelings of malaise, decreased energy, insomnia, and headaches. Patients often do not volunteer information about their emotional well-being, so identification of major depressive disorder by primary care clinicians remains a challenge (Alarcon, Isaacson, Franco-Bronson, 1998). A study showing that primary care physicians missed the diagnosis of major depressive disorder in 66% of patients presenting with the illness SGU Cures Index 236 (Schwenk, 1994) demonstrates that there are opportunities to improve screening and diagnosis in the primary care setting (Lieberman, 2003). Disease Impact – Disability Adjusted Life Years (DALY) Unipolar Depression: 1,455 DALYs lost per 100,000 people in 2004 (World Health Organization, 2009). Given a total population of 292,810,000 in the USA in 2004 = 4,260,386 DALYs lost to Unipolar Depression in 2004 in the USA. Bipolar Disorder: 184 DALYs lost per 100,000 people in 2004 (World Health Organization, 2009). Given a total population of 292,810,000 in the USA in 2004 = 538,770 DALYs lost to Bipolar Depression in 2004 in the USA. Disease Impact – Years of Potential Life Lost (YPLL) Unipolar Depression: 3,565 years of potential life lost in 2001 in the United States (World Health Organization, n.d.) Bipolar Disorders: 891 years of potential life lost in 2001 in the United States (World Health Organization, n.d.). Self Inflicted Injuries: 669,246 years of potential life lost in 2001 in the United States (World Health Organization, n.d.). Fifty to eighty-seven percent of suicides and attempted suicides are carried out by someone diagnosed with a major depressive episode (Rihmer, 2007). If 50% of self-inflicted injuries in the USA are directly linked with depression, 334,623 years of potential life lost currently attributed to self inflicted injuries (669,246 total in 2001) could also be attributed to depression, raising the total years of potential life lost to depression in the USA from 3,565 (2001) to 338,188 – a 94fold increase. History of the Disease and Breakthroughs Mood disorders were described by the Greeks (i.e., Hippocrates), but were likely acknowledged prior to that time. Hippocrates accurately described mood disorders as having physiological origins and being located in the brain. For much of recorded history, mood disorders were thought to be the result of imbalances in bodily humors (bile), and were associated with the heart rather than the brain. Both the Greeks and Avicenna recognized the connection between mania and melancholia (i.e., depression). SGU Cures Index 237 At the start of the Renaissance, mania and melancholia were widely viewed as separate disorders that were chronic and deteriorating. Some French psychiatrists (i.e., Baillarger, Falret) first described a circular disorder as la folie circulaire in 1854 (Falret, 1854; Pichot, 2004). By the turn of the 20th century, Emil Kraepelin carefully distinguished psychotic disorders from mood disorders, and believed that the primary origin of psychiatric disease was biological and genetic malfunction. Unfortunately, he did not possess many effective medical treatments for the conditions, as in his words, "we were therapeutically hopeless but kind" (Jaspers, 1986; p. 5). In the early 1900s, Adolf Meyer viewed psychopathology as arising from interactions between an individual’s biological/psychological characteristics and his/her social environment (Lief, 1948). Consequently, by the 1950's, pharmaceutical research ushered in the era of antidepressants, which confirmed the importance of biophysiological factors in mood disorders (Davison, 2006; Marnaeros, 2006). In 1957, Karl Leonhard first introduced the terms bipolar (for those with accompanying mania or hypomania) and unipolar (for those with depressive episodes only) (Goodwin & Jamison, 2007). Current Cure Status Mood disorders are currently treated with a combination of pharmaceuticals such as antidepressants or mood stabilizers, talk therapy and behavioral interventions such as psychotherapy, cognitive-behavioral therapy, and/or social-environmental support such as group therapy or removing the individual from a chaotic home environment. Many patients, especially those with Bipolar Disorder, experience ongoing, chronic difficulties requiring ongoing therapy. Future Cure Obstacles Current treatments mostly target symptoms rather than underlying causes. If the diathesis-stress model is correct, predicting and pre-emptively treating those with high vulnerability either by addressing the vulnerability or minimizing environmental stressors could reduce incidence of mood disorders. A “permanent” fix for biological vulnerability (i.e., vaccine or altering genetic coding) would be most efficient. Research Costs NIH Spending on Depression research (National Institutes of Health Research, 2014): SGU Cures Index 238 2009: $450 million 2010: $470 million 2011: $426 million 2012: $429 million 2013 (Est.): $432 million 2014 (Est.): $428 million Unipolar Depression Cures: Anti-Depressants Cure Category Achieved, Functional Cure Identification/Description Antidepressants are drugs used for the treatment of depression and related psychiatric conditions such as anxiety disorders, obsessive-compulsive disorder, and eating disorders. There are currently ten classes of pharmaceutical drugs available to treat depression, most of which influence the serotonin and/or norepinephrine neurotransmitter systems in the brain: 1. Selective serotonin reuptake inhibitors (SSRI) 2. Norepinephrine reuptake inhibitors (NRI) 3. Noradrenergic and specific serotonergic antidepressants (NaSSA) 4. Serotonin-norepinephrine reuptake inhibitors (SNRI) 5. Serotonin antagonist and reuptake inhibitors (SARI) 6. Norepinephrine-dopamine reuptake inhibitors (NDRI) 7. Selective Serotonin reuptake enhancers (SSRE) 8. Norepinephrine-dopamine disinhibitors (NDDI) 9. Tricyclic antidepressants 10. Monoamine oxidase inhibitors (MAOI) Additionally, a number of non-pharmaceutical compounds such as nicotine and caffeine are recognized for their antidepressant effects. At present, SSRIs, NRIs, and SNRIs are the first drugs of choice for most psychiatrists when treating unipolar depression. Cure History The Greeks, such as Asclepiades, recognized the importance of community/environmental support in treating mental illnesses. Their motto was SGU Cures Index 239 “Curare tuto, celeriter, et jucunde” (direct quote needs a citation), meaning the cure should be safe, quick, and pleasant. This meant prescribing bathing, exercise, massages, and wine (Alexander & Selesnick, 1966). The first European hospital exclusively organized for the mentally ill was inaugurated in 1409 in the Spanish city Valencia (Alexander & Selesnick, 1966). The first North American hospital exclusively organized for the mentally ill was Eastern State Hospital in Virginia, in 1768 With the renaissance came an era of natural observation, discovery, and scientific inquiry. Throughout the 1800s and early 1900s, breakthroughs in physics and chemistry led to the synthesis of many novel compounds. This included the synthesis of isonicotinyl hydrazine (isoniazid) from hydrazine compounds (Meyer & Mally, 1912). Experimentation continued throughout this period, and by the mid twentieth century, isoniazid was found to be an antitubercular agent (Lopez-Munoz, Alamo, Juckel, & Assion, 2007; Ramachandriah, Subramanyam, Bar, Baker, & Yeragani, 2011). In 1952, Zeller discovered that iproniazid, another hydrazine derivative, inhibited the monoamine oxidase (MAO) enzyme, which influences endogenous and exogenous amines. Examples of these include: serotonin, catecholamines, tyramine, betaphenylethylamine, benzylamine (Ramachandriah et al, 2011). In 1952, Selikoff and Robidzek observed that iproniazid had greater psychostimulatory effects, described as “mood elevating”, than isoniazid in patients with and without tuberculosis. Lurie, a private psychiatrist, coined the term “antidepressant” for the psychostimulatory effects (Ramachandriah et al, 2011). Cure Science: Breakthroughs/Obstacles Monoamine oxidase inhibitors (MAOI) such as isocarboxazid, tranylcypramine, phenelzine, mebanazine, nialamide, pheniprazine, and etryptamine, were first used in 1959. Later on, there were several reports of these drugs being associated with hepatotoxicity, or chemical-driven liver damage and death, due to hypertensive crises and intracranial hemorrhages (bleeding within the skull). Currently, MAOIs are not the first choice antidepressants given these potentially lethal side effects (Ramachandriah et al., 2011). Tricyclic antidepressants are derived from antihistaminic compounds, which were the predecessors of phenothiazines. In 1957, imipramine, which had a similar side chain as chlorpromazine, was given to patients with depressive psychosis who experienced a remarkable improvement within a few weeks. A one-year study with imipramine, which SGU Cures Index 240 was derived from antihistaminic compounds, showed that the drug was very effective in treating depressive psychosis (Ramachandriah et al., 2011). The finding was published in a Swiss journal in 1957, and it was marketed as "Tofranil" that same year. The first tetracyclic antidepressant, maprotiline, was developed by Wilhelm and Schmidt in 1967 (Ramachandriah et al., 2011). Loomer, Saunders, & Kline (1957) found a correlation between the effects of the antitubercular agents and their inhibitory action on MAO, and used iproniazid for the first time on a group of patients with depression. They recorded significant improvement in 70% of the patients. Later studies showed that its MAO-inhibiting property increased serotonin levels in the brain similar to the effects seen with 5hydroxytrytophan, a precursor of serotonin, which crosses the blood-brain barrier (Ramachandriah et al., 2011). The comparable effects of both MAO inhibitors and tricyclics led to the hypothesis that, despite different mechanisms, the final mode of action was common: increased availability of free serotonin and catecholamines in the brain (Pletscher, 1991). Arvid Carlsson was the first one to develop the antidepressant compound, zimeldine in the late 1970s, which was the first selective serotonin re-uptake inhibitor (SSRI). Zimeldine produced a serious neurological side effect, Guillian-Barre syndrome, in a few patients and thus was withdrawn from the market (Fagius, Osterman, Siden, & Wiholm, 1985). Five new SSRI antidepressants were designed by different pharmaceutical companies shortly thereafter (Butler & Meegan, 2008), and included, fluoxetine, fluvoxamine, paroxetine, sertraline, and citalopram. The ever increasing knowledge of pathophysiological mechanisms of depression has led to the synthesis of other drugs, which affect both serotonin and norepinephrine reuptake, such as venlafaxine, duloxetine (Ramachandriah et al, 2011). Cure Science/Future Obstacles and Targets The discovery of MAO inhibitors led to a new era in the development of psychotropics. This was the era of rational drug development, in which the drug molecules are designed to act on a particular brain region, neuronal receptors, enzymes, or reuptake pumps. This approach is more likely to avoid the undesirable side effects of serendipitously discovered drugs, which often have actions on multiple sites such as cholinergic, alpha-adrenergic, histaminic, and fast sodium ion channels. SGU Cures Index 241 Selective Serotonin Reuptake Inhibitors (SSRIs) act on serotonergic neurons, thus inherently causing side effects related to serotonin function. These include sexual dysfunction, nausea, motor incoordination, tremors, akathisia (unpleasant inner restlessness), and serotonin syndrome, a potentially life-threatening drug reaction. New targets continue to be investigated for potential antidepressant drugs. These include the amino acids GABA and glutamate, neuroactive steroids, corticotrophin releasing factor (CRF), substance P, cytokines, neurotrophic factors, and melatonin receptors. Future developments will likely involve the synthesis of more specific or targeted drugs and/or drugs that have been manufactured or chosen based on the genetic and biological make-up of each patient. Number of Patients Being Treated Currently Eleven percent of Americans aged 12 years and over took antidepressant medication during the period 2005-2008 (Pratt et al, 2011).From 1988-1994 through 2005-2008, the rate of antidepressant use in the US among all ages increased nearly 400% (National Center for Health Statistics, 2011).There were about 250 million residents in the USA in 2005, aged 12 and up (US Census Bureau). Number of Patients Requiring Treatment At least one study showed that primary care physicians missed the diagnosis of major depressive disorder in 66% of patients with the illness (Schwenk, 1994). Impact of Treatment on Years of Potential Life Lost (YPLL) Ironically, a link has been shown between selective serotonin reuptake inhibitors (SSRIs) and increased suicide rates, from 2 in 1,000 to 4 in 1,000 in children and adolescents (Lenzer, 2006), although it is not clear whether this increase is caused by the medication or is associated with the depression itself. For example, an alleviation of severe depression might provide someone with the energy and motivation to attempt suicide, while still depressed. The increased risk for suicide and suicidal behavior among adults under 25 approaches that seen in children and adolescents (Stone, Laughren, Jones, Levenson, Holland, Hughes, et al., 2009), and other studies have shown increased risk of suicidal behaviors across age groups (Jick, Kaye, & Jick, 2004). Subsequent follow-up studies have supported the hypothesis that antidepressant drugs reduce suicide risk (Beasley, Ball, Nilsson, Polzer, Tauscher-Wisniewski, Plewes, & Acharya, 2007; Bridge, Iyengar, Salary, Barbe, Birmaher, Pincus, et al., 2007; Gibbons, Brown, Hur, Davis, & SGU Cures Index 242 Mann, 2012), making it difficult to draw firm conclusions about suicide risk associated with antidepressant drugs at this time (Olfson & Shaffer, 2007). Impact of Treatment on Disability-adjusted Life Years (DALYs/QALYs) Ramsberg, Asseburg, & Henriksson (2012) describe cost of medications in Euros, the impact of different medications on QALYs, and cost-benefit analysis in areas such as drug cost, societal cost, healthcare cost vs. QALY gained, for antidepressants. The authors conclude that, "Employing a large body of randomized head-to-head evidence, escitalopram has the highest probability of remission of the investigated antidepressants and is the most effective and cost effective pharmacological treatment strategy for moderate to severe depression in a primary care setting, when evaluated over a one year time-horizon. The difference in effect is modest but even small differences in remission rates may be important when assessing costs and costeffectiveness of antidepressants." (need a page number here for direct quote). The cost of Escitalopram was 14,755 Euros in 2009, while the impact on QALY was +0.6978. Average Annual Total Cost of Antidepressant Retail prices for commonly prescribed antidepressants range from about $20 a month to more than $400 a month (Consumer Reports Health Best Buy Drugs, 2011). Medicaid spent $2.26 billion on antidepressant drugs in 2004, and $1.99 billion in 2005 (Chen, Kelton, Jing, Guo, Li, & Patel, 2008). Eleven percent of Americans aged 12 years and over took antidepressant medication during the period 2005-2008 (Pratt et al, 2011). There were 250 million Americans age 12+ in 2005. Eleven percent of 250 million = 27.5 million x $20 per month (minimal cost) = $550 million per month x 12 = $6.6 billion per year (best-case scenario drug cost only). Eleven percent of 250 million = 27.5 million x $200 per month (median cost) = $5.5 billion per month x 12 = $66 billion per year (median scenario drug cost only). Average Lifetime Cost of Antidepressant The average age of onset is 30 years, and average life expectancy in the USA is 79. The minimum drug cost of $20 per month x 12 months x 49 years = $11,760. Median annual drug cost is $200 per month x 12 months x 49 years = $117,600. SGU Cures Index 243 Economic Impact – Value of Life Added Self Inflicted Injuries: Given that 669,246 years of potential life were lost as a result of self-inflicted injuries in 2001 in the United States (WHO Department of Measurement and Health Information – Global Burden of Disease Study - Years of Life Lost, 2001), and 50 to 87 percent of suicides and attempted suicides are carried out by someone diagnosed with a major depressive episode (Rihmer, 2007), the potential economic impact of addressing depression, and consequently, suicide, is significant. If 50% of self-inflicted injuries in the USA are directly linked with depression, 334,623 years of potential life could be saved, giving an annual VMRR of $34.87 billion. Economic Impact – Value of Reduction in DALYs/Increase in QALYs While Ramsberg, Asseburg, & Henriksson (2012) have shown than the SSRI antidepressant Escitalopram has an impact on QALYs of +0.6978, we take the more conservative moderate to severe depression health state preference score of .60, and subtracting that from 1, which is perfect health, we assume a QALY associated with an effective treatment for depression of 1 - .60 = .40 (Pyne, Fortney, Tripathi, Feeny, Ubel, & Brazier, 2009). A .40 QALY increase x 14.8 million Americans aged 18+ diagnosed with clinical depression in a given year (Kessler et al, 2005; US Census Bureau, 2005) gives a VMRR of $616.8 billion. Other Cost Savings Associated with Antidepressants In sharp contrast to the 1950s through the 1970s, when most patients with depression were treated by a psychiatrist, the effectiveness, ease of use, and positive patient tolerability with SSRIs has made treatment in primary care the norm rather than the exception. Today, primary care physicians are among the most frequent prescribers of newer-generation antidepressant medications in the United States, and some depressed patients never receive treatment from a psychiatrist, which saves money on follow-up/specialist appointments. Bipolar Cure: Mood Stabilizers Cure Category Achieved, Functional Cure Identification/Description SGU Cures Index 244 Bipolar disorder, also known as bipolar affective disorder, manic-depressive disorder, or manic depression, is a mental illness characterized by episodes of a frenzied mood known as mania, alternating with episodes of depression. Mania can occur with different levels of severity. At milder levels, known as hypomania, individuals appear energetic, excitable, and may in fact be highly productive. As mania becomes more severe, individuals begin to behave erratically and impulsively, often making poor decisions due to unrealistic ideas about the future, and may have great difficulty with sleep. At the most severe level, individuals can experience very distorted beliefs about the world known as psychosis. Individuals who experience manic episodes also commonly experience depressive episodes; some experience a mixed state in which features of both mania and depression are present at the same time. Manic and depressive episodes last from a few days to several months. Because a bipolar diagnosis requires a manic or hypomanic episode, many patients are initially diagnosed and treated as having major depression (Muzina, Kemp, & McIntyre, 2007). Cure History Bipolar disorder likely follows a diathesis-stress model, in which a genetic predisposition is "triggered" by environmental factors such as stressors, leading to symptoms (Lazarus, 1993). In support of this view, twin study concordance rates are consistently identified at 40 % in monozygotic twins who have 100% gene overlap, compared to 0 to 10% in dizygotic twins who have 50% gene overlap, for Bipolar I Disorder (Kieseppa, Partonen, Haukka, Kaprio, & Lönnqvist, 2004). The overall heritability of the bipolar spectrum has been put at 0.71 (Edvardsen, Torgersen, Røysamb, Lygren, Skre, Onstad, & Øien, 2008). The relatively low concordance between dizygotic twins brought up together, suggests that shared family environmental effects are limited, although the ability to detect them has been limited by small sample sizes (Edvardsen et al., 2008). There is fairly consistent evidence from prospective studies that recent life events and interpersonal relationships contribute to the likelihood of onsets and recurrences of bipolar mood episodes (Alloy, Abramson, Urosevic, Walshaw, Nusslock, & Neeren, 2005). There have been repeated findings that between a third and a half of adults diagnosed with bipolar disorder report traumatic/abusive experiences in childhood, SGU Cures Index 245 which is associated on average with earlier onset, a worse course, and more cooccurring disorders such as PTSD (Leverich & Post, 2006). The total number of reported stressful events in childhood is higher in those with an adult diagnosis of bipolar spectrum disorder compared to those without, particularly events stemming from a harsh environment rather than from the child's own behavior (Miklowitz & Chang, 2008). Cure Science: Breakthroughs/Obstacles In the 1850s, the prognosis for those with bipolar disorder was considered to be “desperate, terrible and incurable” (Angst & Sellaro, 2000, pp. 445). Bipolar disorders are most often treated with a mood stabilizer, which is a psychiatric medication used to treat disorders characterized by intense and sustained mood shifts. These drugs are also used to treat borderline personality disorder and schizoaffective disorder. From the 1950s on, lithium was the only agent thought to be prophylactic against further episodes of manic-depressive illness (Healy, 2006). The term “mood stabilizer” became popular after 1995 when Abbott Laboratories obtained a license to use the anticonvulsant sodium valproate (Depakote) for treating acute mania, and by 2001, more than a hundred article titles a year featured the term (Healy, 2006). Reviews make it clear that the academic psychiatric community still has not come to a consensus on what the term “mood stabilizer” means (Bowden, 1998; Ghaemi, 2001; Sachs, 1996). However, this lack of consensus did not prevent the prescription of mood stabilizers (Ghaemi, Lenox, & Baldessarini, 2001; Ghaemi, Sachs, Chiou, Pandurangi, & Goodwin, 1999). The first group of drugs to colonize this new mood stabilizer niche was anticonvulsants. Further, antipsychotics have been used to treat bipolar disorders for several decades, especially in attempting to alleviate the symptoms of acute manic states. Against a background of epidemiological studies indicating that the prevalence of bipolar disorders might be greater than previously thought (National Advisory Mental Health Council, 1993), Lilly, Janssen, and Astra-Zeneca have marketed several antipsychotics as potential treatments for bipolar disorder (Healy, 2006). Cure Science/Future Obstacles and Targets Bipolar disorder has always been highly recurrent and considered to have a poor prognosis (Angst & Sellaro, 2000). Bipolar patients who have been hospitalized spend about 20% of their lifetime from the onset of their disorder experiencing episodes. Fifty percent of bipolar episodes last between two and seven months, with a median of three SGU Cures Index 246 months.The intervals between the first few episodes tend to shorten, then later the episodes return at an irregular rhythm of about 0.4 episodes per year with high interindividual variability (Angst & Sellaro, 2000). Important findings from a naturalistic study at the National Institute of Mental Health (NIMH) Collaborative Program on the Psychobiology of Depression, Clinical Studies (Keller, Lavori, Coryell, Endicott, & Mueller, 1993), showed a high rate of recurrence for pure mania (48% by one year and 81% by five years) and even higher rates for the mixed cycling group (57% by one year and 91% by five years). Over seven years the rate of recurrence was 81%. The length of sustained recovery was associated with a lower risk for recurrence over the subsequent four years, but over a period of 10 years this predictive power decreased considerably (Coryell, Endicott, Maser, Mueller, Lavori, & Keller, 1995 et al. 1995). The authors showed that even under sustained lithium prophylaxis recurrences were present in more than 70% of cases within five years of recovery. This finding is consistent with the outpatient study of Gitlin, Swendsen, Heller, and Hammen (1995), in which 73% of 82 bipolar patients had relapses/recurrences over an average of 4.3 years despite maintenance pharmacotherapy (two thirds of patients who relapsed experienced multiple relapses). Among the 26 patients who suffered no relapse, 46% continued to show significant symptoms of mania or depression. The lifetime outcome of bipolar disorder in a Zurich follow-up study demonstrates a poor prognosis despite modern treatments (Angst & Preisig, 1995a; 1995b). Up to a median age of 68 years, only 16% of patients had recovered, and 52% still suffered from recurrent episodes and the remaining patients had become chronically ill or had committed suicide. These data underline the poor outcome into old age and the need for intensive treatment. In summary, the prognosis for recurrence of depressive or manic episodes, even with existing mood stabilizers, is not very good. One study compared patterns of service utilization by bipolar patients in North-West Wales between the 1990s and the 1890s (Harris, Chandran, & Chakraborty, 2005). The results of the study showed that in the pre-lithium era, admissions for bipolar disorders occurred at a rate of 4 every 10 years. In the 1990s, they occurred at a rate of 6.3 every 10 years. Additionally, while there were 16 bipolar patients per million residents per day in hospital in the 1890s, in the 1990s there were 24 per million residents in acute service beds and more in non-acute beds. These data are incompatible with simple claims that mood-stabilizing drugs, which were widely available in the 1990s, ‘work’. An alternative is that these agents have treatment effects, and further research is needed to match treatments to patients in order to optimize outcomes. Number of Patients Being Treated Currently SGU Cures Index 247 While the number of Bipolar patients being actively treated is difficult to determine, Bipolar disorder is known to affect approximately 5.7 million American adults, or about 2.6 percent of the US population age 18 and older in a given year (Kessler et al, 2005; US Census Bureau, 2005). The median age of onset for bipolar disorders is 25 years (Kessler et al., 2005). Estimates for the prevalence of bipolar disorders have risen to 5% or more when the definition of bipolar disorders includes Bipolar I, Bipolar II, Cyclothymia, and Bipolar Not Otherwise Specified (NOS) (Angst, 1998). Patients with Bipolar I were symptomatically ill 47.3% of weeks throughout a mean of 12.8 years of follow-up. Depressive symptoms (31.9% of total follow-up weeks) predominated over manic/hypomanic symptoms (8.9% of weeks) or cycling/mixed symptoms (5.9% of weeks). Subsyndromal, minor depressive, and hypomanic symptoms combined were nearly 3 times more frequent than syndromal-level major depressive and manic symptoms (29.9% vs. 11.2% of weeks, respectively). Patients with BP-I changed symptom status an average of six times per year and polarity more than three times per year (Judd, Akiskal, Schettler, Endicott, Maser, Solomon, et al., 2002). Number of Patients Requiring Treatment Systematic application of DSM-IV criteria identified previously undiagnosed bipolar disorder in 40% of a referred population of patients with mood disorders (Ghaemi, Sachs, Chiou, Pandurangi, & Goodwin, 1999). Impact of Treatment on Years of Potential Life Lost (YPLL) The best available evidence shows that non-medicated patients with bipolar disorder do not have a higher risk of suicide (Healy, 2006). Impact of Treatment on Disability-adjusted Life Years (DALYs/QALYs) Bipolar disorder is associated with significant impairment in work, family and social life, beyond the acute phases of the illness (Sanchez-Moreno, Martinez-Aran, TabarésSeisdedos, Torrent, Vieta, & Ayuso-Mateos, 2009). Wyatt and Henter (1995) estimated the cost of bipolar disorder in 1991 in the USA to be $45 billion. SGU Cures Index 248 Chisholm, van Ommeren, Atuso-Mateos, & Saxena (2005) used data from the Global Burden of Disease study (Ayuso-Mateos, 2001) to determine that the composite disability weight of bipolar disorder from 0 (perfect health) to 1 (death), is .445. This is based on manic episode disability of .72, depressed episode disability of .76, and interim state disability of .14. This is also based on previous findings that bipolar patients spend about 50% of their time in a manic or depressed state before receiving treatment (Baldessarini & Tondo, 2000) and that people with the disorder experienced depression to mania in a 3:1 ratio (Judd et al., 2002). They also concluded that the following treatment regimens reduced the disability weight as such, after accounting for treatment response rates, efficacy, percent gain, and percent adherence (Chisholm, van Ommeren, Ayuso-Mateos, & Saxena, 2005): Lithium Alone .445 ---> .368 Lithium + Psychosocial Care .445 ---> .360 Valproic Acid Alone .445 ---> .365 Valproic Acid + Psychosocial Care .445 ---> .357 Bipolar patients who have been hospitalized spend about 20% of their lifetime from the onset of their disorder experiencing episodes. Fifty percent of bipolar episodes last between 2 and 7 months, with a median of 3 months. The intervals between the first few episodes tend to shorten, and later the episodes return at an irregular rhythm of about 0.4 episodes per year with high inter-individual variability (Angst & Sellaro, 2000). On the basis of an episode length of 3 months (median), bipolar patients spent about 2 months/year in episodes (Angst & Sellaro, 2000). In an attempt to estimate the cost-effectiveness of interventions for reducing the global burden of bipolar disorder, Chisholm, van Ommeren, Atuso-Mateos, & Saxena (2005) modeled hospital- and community-based delivery of two generic mood stabilizers: lithium and valproic acid, alone and in combination with psychosocial treatment. A population model was employed to estimate the impact of different strategies, relative to no intervention. Total costs in international dollars I$, and effectiveness disabilityadjusted life years (DALYs) averted, were combined to form cost-effectiveness ratios. Community-based treatment with lithium and psychosocial care was most cost-effective with a cost per DALY averted: $2,165 - $6,475 (International $) in developing subregions and $5,487 - $21,123 (International $) in developed sub-regions. Average Total Annual Cost of Mood Stabilizer Treatment Monthly cost of bipolar medications (averaged across three US Geographic regions) (rxpricequotes.com): SGU Cures Index 249 Aripiprazole (Abilify) $687 - 970 Carbamazepine (Tegetrol) $15 - 225 Divalproex (Depakote) $18 - 71 Quetiapine (Seroquel) $186 - 513 Risperidone (Risperdal) $19 - 36 Lithium Carbonate $13 - 25 Ziprasidone (Geodon) $259 - 408 Lamotrigine (Lamictal) $12 - 22 Asenapine (Saphris) $338 – 683 Using the high-end estimate for Risperidone ($36) x 5.7 million Americans diagnosed with Bipolar Disorder in any given year (2005) = $205.2 million per month x 12 months = $2.46 billion per year in medication cost. Average Lifetime Cost of Mood Stabilizer Treatment Average age of onset is 30 years, and average life expectancy in the USA is 79. Highend estimate for Lithium Carbonate is $25 per month x 12 months x 49 years = $14,700. Economic Impact – Value of Reduction in DALYs/Increase in QALYs Lithium Alone .445 ---> .368 Lithium + Psychosocial Care .445 ---> .360 Valproic Acid Alone .445 ---> .365 Valproic Acid + Psychosocial Care .445 ---> .357 As per Chisholm et al. (2005), taking a .077 disability index change for Lithium alone (.445 ---> .368) x 5.7 million Americans age 18+ diagnosed with bipolar disorder in a given year (Kessler et al, 2005; US Census Bureau, 2005) gives a VMRR of $45.7 billion. Economic impact – Value of Life Added $ 0 - Relatively few years of life lost as a result of bipolar disorder (and thus, very little gained in this regard from mood stabilizers). Citations Alarcon, F. J., Isaacson, J. H., & Franco-Bronson, K. (1998). Diagnosing and treating depression in primary care patients: Looking beyond physical complaints. Cleveland Clinic Journal of Medicine, 65, 251–260. SGU Cures Index 250 Alexander, F. & Selesnick, S. T. (1966). The history of psychiatry. Harper and Row: New York, NY). Alloy, L. B., Abramson, L. Y., Urosevic, S., Walshaw, P. D., Nusslock, R., & Neeren, A. M. (2005). The psychosocial context of bipolar disorder: Environmental, cognitive, and developmental risk factors. Clinical Psychology Review, 25 (8), 1043– 1075. Angst, J. (1998). The emerging epidemiology of hypomania and bipolar II disorder. Journal of Affective Disorders, 50, 163–173. Angst, J., & Preisig, M. (1995a). Course of a clinical cohort of unipolar, bipolar and schizoaffective patients. Results of a prospective study from 1959 to 1985. Schweizer Archiv fur Neurologie und Psychiatrie, 146, 5–16; Angst J., & Preisig, M. (1995b). Outcome of a clinical cohort of unipolar, bipolar and schizoaffective patients. Results of a prospective study from 1959 to 1985. Schweizer Archiv fur Neurologie und Psychiatrie, 146, 17–23). Angst, J., & Sellaro, R. (2000). Historical perspectives and natural history of bipolar d isorder. Biological Psychiatry, 48, 445-457. Ayuso-Mateos, J. L. (2001). Global burden of Bipolar Disorder in the year 2000: Version 1 estimates. World Health Organization. Retrieved on August 20, 2013 from: http:// www.who.int/evidence/bod Baldessarini, R. J. & Tondo, L. (2000). Does lithium treatment still work? Evidence of stable responses over three decades. Archives of General Psychiatry, 57,187-190. Beasley, C. M., Ball, S. G., Nilsson, M. E., Polzer, J., Tauscher-Wisniewski, S., Plewes, J., & Acharya, N. (2007). Fluoxetine and adult suicidality revisited. Journal of Clinical Psychopharmacology, 27 (6), 682–686. Berton, O., & Nestler, E. J. (2006). New approaches to antidepressant drug discovery: Beyond monoamines. Nature Reviews Neuroscience, 7, 137-151. Bowden, C. L. (1998). New concepts in mood stabilization: Evidence for the effectiveness of Valproate and Lamotrigine. Neuropsychopharmacology, 19, 194–199. SGU Cures Index 251 Bridge, J. A., Iyengar, S., Salary, C. B., Barbe, R. P., Birmaher, B., Pincus, H .A., Ren, L., & Brent, D. A. (2007). Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. Journal of the American medical Association, 297 (15), 1683–96. Butler, S. G., & Meegan, M. J. (2008). Recent developments in the design of antidepressant therapies: Targeting the serotonin transporter. Current Medicinal Chemistry, 15, 1737–1761. Chen, Y., Kelton, C. M. L., Jing, Y., Guo, J. J., Li, X., & Patel, N. C. (2008). Utilization, price, and spending trends for antidepressants in the US Medicaid program. Research in Social and Administrative Pharmacy, 4 (3), 244-257. Chisholm, D., van Ommeren, M., Ayuso-Mateos, J-L., & Saxena, S. (2005). Costeffectiveness of clinical interventions for reducing the global burden of bipolar disorder. British Journal of Psychiatry, 187, 559-567. Coryell, W., Endicott, J., Maser, J. D., Mueller, T., Lavori, P., & Keller, M. (1995). The likelihood of recurrence in bipolar affective disorder: The importance of episode recency. Journal of Affective Disorders, 33, 201–206. Davison, K. (2006). Historical aspects of mood disorders. Psychiatry, 5 (4), 115-118. Edvardsen, J., Torgersen, S., Røysamb, E., Lygren, S., Skre, I., Onstad, S., & Øien, P. A. (2008). Heritability of bipolar spectrum disorders. Unity or heterogeneity? Journal of Affective Disorders, 106 (3), 229–240. Fagius, J., Osterman, P. O., Siden, A., & Wiholm, B. E. (1985). Guillain-Barre syndrome following zimeldine treatment. Journal of Neurology, Neurosurgery, and Psychiatry, 48, 65–69. Falret, J. (1854). Memoire sur la folie circulaire. Bulletin de la Academie Imperiale de Medicin, 19, 382-400. Ghaemi S. N. (2001). On defining “mood stabilizer.” Bipolar Disorders, 3, 154–158. Ghaemi, S. N., Lenox, M. S., & Baldessarini, R. J. (2001). Effectiveness and safety of SGU Cures Index 252 long-term antidepressant treatment in bipolar disorder. Journal of Clinical Psychiatry, 62, 565–569). Ghaemi, S. N., Sachs, G. S., Chiou, A., Pandurangi, A. K., & Goodwin, F. K. (1999). Is bipolar disorder still under diagnosed? Are antidepressants over utilized? Journal of Affective Disorders, 52, 135–144). Gibbons, R. D., Brown, C. H., Hur, K., Davis, J. M., & Mann, J. J. (2012). Suicidal thoughts and behavior with antidepressant treatment: Reanalysis of the randomized placebo-controlled studies of Fluoxetine and Venlafaxine. Archives of General Psychiatry, 69 (6), 580–587. Gitlin, M. J., Swendsen, J., Heller, T. L. &, Hammen, C. (1995). Relapse and impairment in bipolar disorder. American Journal of Psychiatry, 152, 1635–1640. Goodwin, F. K.; Jamison, K. R. (2007). Manic–depressive illness: bipolar disorders and recurrent depression. Oxford University Press: Oxford, UK. Harris, M., Chandran, S., & Chakraborty, N (2005). The impact of mood stabilizers on bipolar disorder: the 1890s and 1990s compared History of Psychiatry, 16(4), 423-434. Healy, D. (2006). The Latest Mania: Selling Bipolar Disorder. PLoS Medicine, 3 (4), e185. Ingram, R. E. & Luxton, D. D. (2005). Vulnerability-Stress Models. In B.L. Hankin & J. R. Z. Abela (Eds.), Development of Psychopathology: A vulnerability stress perspective (pp. 32-46). Thousand Oaks, CA: Sage Publications Inc. Jaspers, K. (1986). An autobiographical account. In E. Ehrlich, L. Ehrlich, and G. Pepper (Eds.), Karl Jaspers: Basic Philosophical Writings (pp. 4–8). Humanities Press: Atlantic Highlands, NJ. Jick, H., Kaye, J. A., & Jick, S. S. (2004). Antidepressants and the risk of suicidal behaviors. Journal of the American Medical Association, 292 (3), 338–343. Judd, L. L., Akiskal, H. S., Schettler, P. J., Endicott, J., Maser, J., Solomon, D. A., Leon, A. C., Rice, J. A., & Keller, M. B. (2002). The Long-term natural history of the weekly symptomatic status of Bipolar I Disorder. Archives of General Psychiatry, 59(6), 530-537). SGU Cures Index 253 Keller, M. B., Lavori, P. W., Coryell, W., Endicott, J., & Mueller, T. I. (1993). Bipolar I: A five-year prospective follow-up. Journal of Nervous and Mental Disorders, 181, 238–245. Kessler, R. C., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K. R., Rush, A. J., Walters, E. E., & Wang, P. S. (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). Journal of the American Medical Association, 289 (23), 3095-4105. Kessler, R. C., Berglund, P. A., Demler, O., Jin, R., & Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Archives of General Psychiatry, 62 (6), 593-602. Kieseppa, T., Partonen, T., Haukka, J., Kaprio, J., & Lönnqvist, J. (2004). High Concordance of Bipolar I Disorder in a Nationwide Sample of Twins. American Journal of Psychiatry, 161 (10), 1814–1821. Lazarus, R. S. (1993). From psychological stress to the emotions: A history of changing outlooks. Annual Review of Psychology, 44,1-21. Lenzer, J. (2006). Antidepressants double suicidality in children, says FDA. British Medical Journal, 332, (7542), 626. Leverich, G. S., & Post, R. M. (2006). Course of bipolar illness after history of childhood trauma. The Lancet, 367 (9516), 1040–1042. Lieberman, J. A. (2003). History of the use of antidepressants in primary care. Primary Care Companion to the Journal of Clinical Psychiatry, 5 (7), 6-10. Lief, A. (1948). The commonsense psychiatry of Dr. Adolf Meyer. McGraw-Hill: New York, NY. Loomer, H. P., Saunders, J. C., & Kline, N. S. (1957). A clinical and pharmacodynamic evaluation of iproniazid as a psychic energizer. Psychiatric Research Report of the American Psychiatric Association, 8, 129-141. Lopez-Munoz, F., Alamo, C., Juckel, G., & Assion, H. J. (2007). Half a century of SGU Cures Index 254 antidepressants: On clinical introduction of monoamine oxidase inhibitors, tricyclics and tetracyclics. Part 1: Monoamine oxidase inhibitors. Journal of Clinical Pharmacology, 27, 555–559. Marnaeros, A. (2006). Mood disorders: Epidemiology and natural history. Psychiatry, 5 (4), 119-122. Meyer, H., & Mally, J. (1912). On hydrazine derivatives of pyridine carbonic acids. Monatshefte Chemie verwandte Teile anderer Wissenschaften, 33, 393–414). Miklowitz, D. J., & Chang, K. D. (2008). Prevention of bipolar disorder in at-risk children: Theoretical assumptions and empirical foundations. Development and Psychopathology, 20 (3), 881–897. Muzina, D. J., Kemp, D. E., & McIntyre, R. S. (2007). Differentiating bipolar disorders from major depressive disorders: treatment implications. Annals of Clinical Psychiatry, 19 (4), 305–312] National Advisory Mental Health Council. (1993). Health care reforms for Americans with severe mental illnesses. American Journal of Psychiatry, 150, 1447–1465. National Center for Health Statistics. Health, United States, 2010: With special feature on death and dying. Table 95. Hyattsville, MD. 2011. National Institutes of Health Research (2014). Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). Retrieved March 31, 2014, from: http://report.nih.gov/categorical_spending.aspx Oatley, K., Keltner, D. & Jenkins, J. M. (2006b). Emotions and mental health in childhood. Understanding emotions (2nd ed.)(pp. 321-351). Oxford, UK: Blackwell Publishing). Olfson, M., & Shaffer, D. (2007). SSRI prescriptions and the rate of suicide. American Journal of Psychiatry, 164 (12), 1907. Pichot, P. (2004). Circular insanity, 150 years on. Bulletin l’Academie Nationale de Medicine, 188 (2), 275–284.Pletscher, A. (1991). The Discovery of antidepressants: A winding path. Experientia, 47, 4–8. SGU Cures Index 255 Pratt, L. A., Brody, D. L., Gu, Q. (2011). US Department of Health and Human Services, Centers for Disease Control, NCHS Data Brief; 2011. Antidepressant use in persons aged 12 and over: United States, 2005–2008. Pyne, J. M., Fortney, J. C., Tripathi, S., Feeny, D., Ubel, P., & Brazier, J. (2009). How bad is depression? Preference score estimates from depressed patients and the general population. Health Services Research, 44 (4), 1406-1423. Ramachandriah, C. T., Subramanyam, N., Bar, K. J., Baker, G., & Yeragani, V. K. (2011). Antidepressants: From MAOIs to SSRIs and more. Indian Journal of Psychiatry, 53 (2), 180-182. Ramsberg, J., Asseburg, C., & Henriksson, M. (2012) Effectiveness and Cost Effectiveness of Antidepressants in Primary Care: A Multiple Treatment Comparison Meta-Analysis and Cost-Effectiveness Model. PLoS ONE 7 (8), e42003. Rihmer, Z. (2007). Suicide risk in mood disorders. Current Opinion in Psychiatry, 20, 1722. Sachs, G. (1996). Bipolar mood disorder: Practical strategies for acute and maintenance phase treatment. Journal of Clinical Psychopharmacology, 16, 32s–47s. Sanchez-Moreno, J., Martinez-Aran, A., Tabarés-Seisdedos, R., Torrent, C., Vieta, E., & Ayuso-Mateos, J. L. (2009). Functioning and disability in bipolar disorder: An extensive review. Journal of Psychotherapy & Psychosomatics, 78, 285–297. Schwenk, T. L. (1994). Depression: overcoming barriers to diagnosis. Consultant, 34, 1553–1559. Sheline, Y. I. (2003). Neuroimaging studies of mood disorder effects on the brain. Biological Psychiatry, 54, 338-352. Stone, M., Laughren, T., Jones, M. L., Levenson, M., Holland, P. C., Hughes, A. Hammad, T. A., Temple, R. et al. (2009). Risk of suicidality in clinical trials of antidepressants in adults: Analysis of proprietary data submitted to US Food and Drug Administration. British Medical Journal, 339, b2880. SGU Cures Index 256 US Census Bureau Population Estimates by Demographic Characteristics. Population Division, US Census Bureau Release Date: June 9, 2005. Retrieved on August 20, 2013 from: http://www.census/gov/popest/national/asrh/ World Health Organization (2009). Mortality and Burden of Disease Estimates for Member States in 2004: Age-std DALY rates for United States of America. Retrieved on June 4, 2014 from: http://apps.who.int/gho/data/node.main.1009?lang=en. World Health Organization (n.d.). Global Burden of Disease (GBD) 2001 Estimates by Subregion: Years of Life Lost (YLL). Retrieved on June 4, 2014 from: http://www.who.int/healthinfo/global_burden_disease/estimates_regional_2001/ en/. Wyatt, R. J. & Henter, I. (1995). An economic evaluation of manic-depressive illness 1991. Social Psychiatry and Psychiatric SGU Cures Index 257 MUSCULAR DYSTROPHY Jennifer Pak Disease Category Chronic Disease Identification, Description, and Diagnostic Criteria Muscular dystrophy is a group of diseases characterized mainly by progressive muscle wasting, of which more than 30 different types have been identified. Muscle wasting and weakness is a common characteristic of all types of muscular dystrophy, and is caused by a genetic mutation in a muscle protein. Individuals with the disease can pass the gene onto their offspring. In addition, parents who do not have the disease may have a child who spontaneously presents with the muscular dystrophy. Early signs of muscular dystrophy are different for each subtype (National Human Genome Research Institute, 2013). According to the National Institute of Child Health and Human Development (NICHD, 2012), there are nine primary subtypes of muscular dystrophy: (1) Duchenne (DMD) (2) Becker (3) Myotonic (4) Congenital (5) Emery-Dreifuss (6) Facioscapulohumeral (7) Limb-girdle (8) Distal (9) Oculopharyngeal The most common forms of muscular dystrophy are Duchenne (DMD) and Becker (BMD). These two types affect approximately 1 in every 5,600 to 7,700 males aged 5 to 24 in the US. Women are less likely to be affected by DMD because it is inherited in an X-linked pattern. Boys inherit one X chromosome from mom, and one Y chromosome from dad. Therefore, if that one X chromosome carries the DMD-causing mutation, then the boy will be affected. Girls on the other hand are less severely affected because they inherit one X chromosome from mom and another X chromosome from dad. Therefore, SGU Cures Index 258 even if one of the X chromosomes carries the DMD-causing mutation, the child will not have manifestations because one gene is still functional and able to produce the dystrophin protein. This report will focus on DMD, which constitutes 50% of all cases of muscular dystrophy. Due to the inheritance pattern, parents who do not have the phenotypic, or physical, manifestations of the disease can still pass the gene onto their children. In addition to cases linked to genetic inheritance, one-third of cases are new mutations. Women who are carriers of the defective gene may also present with some of the symptoms, although they tend to be less severe than for males. DMD is caused by the absence of a protein called dystrophin, which supports muscle fibers. Loss of dystrophin results in weak muscles, causing severe symptoms that progressively becomes worse. Difficulties walking due to weakened hip and thigh muscles are among the earliest signs of DMD. In addition, DMD patients often present with enlarged calf muscles (pseudohypertrophy), waddling gait, weakened heart function, difficulty breathing, and restricted activity of voluntary skeletal muscles. By early adolescence, patients with DMD typically are unable to walk and by late teens or early twenties, most die from weakness of the heart, respiratory complications, or infection. However, medicine has made profound advancements to allow these individuals to survive until their 30s and some even into their 40s (Centers for Disease Control and Prevention, 2009). Diagnostically, there are a variety of tests that can determine if an individual has DMD. Children with DMD will often have characteristically high levels of creatine kinase, an enzyme released into the bloodstream upon muscle deterioration. If this enzyme is elevated, then further testing is needed in order to rule out diagnoses of other conditions that may cause muscle wasting. Genetic testing is frequently utilized to detect missing or repeated mutations in the dystrophin gene. If through genetic testing, the lack of dystrophin gene is confirmed, then it can lead to a diagnosis of muscular dystrophy. Other tests that can be performed to diagnose muscular dystrophy include muscle biopsy, neurological tests, heart testing, exercise assessments, and imaging tests (NICHD, 2012). Disease Etiology (Cause) Many genes are involved in creating protein related to muscle function and structure. Each type of muscular dystrophy affects a certain gene that results in loss of normal SGU Cures Index 259 muscle function and/or structure. DMD is specifically caused by an absence of the protein dystrophin. This protein normally keeps muscles strong and acts as a support for muscle fibers. In the absence of this gene, the muscle’s integrity and structure is lost, causing the muscle weakness and associated symptoms. DMD is inherited in an Xlinked recessive pattern or by a spontaneous mutation in the gene and normally affects more males than females. In other words, males will inherit an X chromosome from mom and a Y chromosome from dad. Therefore, if the inherited X chromosome carries the DMD-causing mutation, then the boy will have DMD. On the other hand, females will inherit a X chromosome from mom and a second X chromosome from dad. Therefore, even though one X chromosome may have the DMD-causing mutation, the “normal” X chromosome will still produce the dystrophin protein, and the child will have none or mild symptoms of DMD. According to Ropper and Samuels (2009), approximately 30% of DMD represents spontaneous mutations. Females who inherit the gene from their fathers are carriers and may therefore manifest more subtle symptoms of DMD, compared to males with DMD. However, in either X-linked recessive inheritance or spontaneous mutation, the protein that is absent is dystrophin, which causes the muscle atrophy and weakness (National Institute of Neurological Disorders and Stroke, 2011) Current Prevalence/Incidence DMD is estimated to affect approximately 1 in 3,500 (2.9 per 10,000) male births (CDC, 2013). According to the Centers for Disease Control and Prevention, it is not known exactly how many people of all ages in the United States currently have Duchenne or Becker Muscular Dystrophy. In addition, the Muscular Dystrophy Association (MDA) in the US states that there is no known number for people with muscular dystrophy because it is not reportable. There are no requirements or healthcare laws in place that require patients or family to report someone with DMD (Personal communication with MDA, April 21, 2014). However, an estimated 1 of every 5,600 to 7,700 males 5 to 24 years of age had Duchenne or Becker Muscular Dystrophy (CDC, 2007). Using this information, we can approximate a range of people who may possibly have DMD. In 2012, there were 41, 667, 811 males aged 5-24 in the US (US Census Bureau, 2014). Divide the population by the incidence to get the range (41,667,811/7700 = 5,411 and 41,667,811/5,600 = 7,440). Thus, we estimate that there were between 5, 411 and 7, 400 males aged 5-24 with DMD or BMD in the US in 2012 (Author’s calculation using data from US Census Bureau, 2014). Estimated Un-Diagnosed/At-Risk SGU Cures Index 260 DMD is detected and diagnosed quickly due to the severe progression of the disease starting at an early age. Therefore, it is highly unlikely that a patient with DMD would go undiagnosed for long. Patients are at higher risk for the disease if the gene is present in their family history. Individuals who are carriers for the disease gene are more likely to have a male child with Duchenne Muscular Dystrophy. Females are more likely to only be carriers of DMD, present with less severe symptoms and go undetected as carriers of DMD. Typically, genetic counseling is advised if there is a history of DMD in the family and currently can be detected with 95% accuracy through genetic testing (National Center for Biotechnology Information, 2013). Disease Impact—Years of Potential Life Lost (YPLL) According to the Organization for Economic Co-operation and Development, the current life expectancy for males in the United States is 76 years. The life expectancy of patients with DMD is 25 years (Kaneshiro, Seckler, & Jasmin, 2012). Therefore, the years of potential life lost is 51 years (reference age – age at death = 76 years – 25 years = 51 years). Disease Impact—Disability Adjusted Life Years (DALY) According to the Muscular Dystrophy Association (MDA), patients with muscular dystrophy experience very high DALY rates because DALYs take into account quality of life due to disability and premature death rate, which are both high for these patients. For example, wheelchair use typically occurs prior to age 20, and life expectancy is currently about 25 years (Kaneshiro, Seckler, & Jasmin, 2012). There were an estimated 5,411 to 7,400 males aged 5-24 with DMD or BMD in the US in 2012, and that “for a person with MD (muscular dystrophy), more than two disability adjusted life years (DALYs) are lost each year" (Deloitte Access Economics, 2013), between 10,822 and 14,800 DALYs were lost to DMD/BMD in the US in 2012. Note that the DALY statistic used here is from the Australian Muscular Dystrophy Association (MDA). The US MDA does not know of a published DALY value for males in the US and has not conducted research in this area as of yet (Author’s personal communication with MDA, April 21, 2014). History of Disease and Breakthroughs Dr. Gaetano Conte and Dr. L. Gioja, two Neapolitan physicians, were the first to report an account of muscular dystrophy in 1836. In their account, they described two brothers SGU Cures Index 261 who began to have progressive muscle weakness at 10 years of age. During that time, it was thought that the brothers had tuberculosis. However, it is now known that the two brothers most likely had BMD, which is a milder form of muscular dystrophy (Do, Mehlman, Talavera, Thompson, Patel, 2012). Meryon reported four brothers who also had similar muscular weakness in 1852. He suspected that the underlying cause was a sarcolemma defect that only impacted males, but was transmitted through females. In 1868, a French neurologist named Guillaume Duchenne described 13 cases of patients with the disease and called it “paralysie musculaire pseudo-hypertrophique” or muscular pseudo-hypertrophic paralysis. Duchenne was widely known for his use of faradism (stimulation of muscles or nerves with an electric current) and eventually the most common form of muscular dystrophy was named after him—Duchenne Muscular Dystrophy (DMD). The exact cause of DMD was described with the advancement of molecular biotechnology techniques, which today is known to be an absence of the protein dystrophin (Do, Mehlman, Talavera, Thompson, Patel, 2012). Current Cure Status There is currently no known cure for DMD (Kaneshiro, Seckler, & Jasmin, 2012). However, there are methods that have been used to improve the quality of life for the affected individual. Treatment often involves a combination of physical and drug therapy with the goal of slowing disease progression. In addition, surgery may become an option to ease complications caused by muscular dystrophy. The most common drug therapy used for DMD patients involve corticosteroids. Steroid drugs can slow the rate at which muscle atrophies and can be started immediately upon diagnosis. Corticosteroids can help children maintain independent walking for several years. However, like any drug, there are side effects associated with corticosteroids. The most common side effects are facial changes, bone fragility, weight gain, and stunted growth. There are many other drugs used as part of therapy for DMD patients. Another type of therapy commonly used is assisted ventilation. DMD is commonly associated with respiratory muscle weakness, causing complications with breathing especially during the night. During sleep, muscles are usually more relaxed and in a state of “rest-or-digest”. As a result, muscles become weaker than they would during SGU Cures Index 262 the day and can lead to hypoventilation, or low oxygen levels. Assisted ventilation can be used day and/or night to help patients with DMD overcome respiratory difficulties. Patients with DMD may use one of the treatments listed above in addition to physical therapy. Activity is encouraged for patients with DMD, as bedrest can cause a faster rate of muscle atrophy and make the disease prognosis worse. Physical therapy is often used to promote movement, prevent deformities, and to encourage strong muscles. During a session of therapy, patients usually perform passive stretching, moderate exercise, and postural corrections. Corrective surgery is also an option for patients with DMD. Although surgery does not cure the disease, it eases the symptoms associated with DMD. Tendon or musclerelease surgery is sometimes performed and involves the lengthening of a tendon or muscle to improve range of movement (National Institute of Neurological Disorders and Stroke, 2011). Future Cure Obstacles Current treatments for DMD address the symptoms rather than the cause of the disease. The genetic basis of DMD makes it difficult to find a “definitive cure” for the disease at this time. Currently, extensive research is being done in gene replacement therapy and genetic modification therapy. However, there are a number of obstacles associated with such therapies. These include the identification of viable targets for therapy and finding a way to deliver the gene replacement while avoiding a potential immune response. Identifying viable targets for gene therapy is difficult with DMD, as the dystrophin gene is one of the largest in the human genome (National Center for Biotechnology Information, 2014). Therefore, finding an effective target that would produce results is difficult with such a large gene. In addition, finding an effective delivery process for the gene replacement represents a significant hurdle. Molecular biologists commonly use viral vectors to deliver genetic material into an organism. The vectors can be created from viruses due to their ability to infect cells and are modified so that the viral vector is not toxic to the cells. For DMD, viral vectors that accurately target skeletal and vascular muscles may not necessarily be as effective for cardiac muscle, given variation in how cells are targeted and respond to viral vectors. Research to overcome these obstacles is currently underway with government funded projects (National Institute of Neurological Disorders and Stroke, 2011). Research Development and Treatment Costs SGU Cures Index 263 NIH Spending on Duchenne/Becker Muscular Dystrophy (NIH RCDC, 2014): 2010: $38 million 2011: $32 million 2012: $34 million 2013: $33 million 2014 (estimated): $34 million 2015 (estimated): $34 million NIH Spending on Muscular Dystrophy (NIH RCDC, 2014): 2010: $86 million 2011: $75 million 2012: $75 million 2013: $76 million 2014 (estimated): $78 million 2015 (estimated): $78 million Duchenne Muscular Dystrophy Cure: Corticosteroids Cure Category Achieved, Functional Identification/Description At present, there is no known cure for DMD. However, there are treatments that may improve the quality of life for an individual with DMD (Kaneshiro, Seckler, & Jasmin, 2012). One such treatment is corticosteroids, which have been shown to slow the progression of the disease. Patients who were prescribed corticosteroids showed increases muscle strength and function (MDA, 2012). Physical therapy and surgery are also other preventative treatments that may help ease symptoms associated with DMD. Currently research is being performed in the fields of gene and stem cell therapy to target the molecular cause of DMD. However, these areas of research have high regulations and many technical challenges, which may serve as an obstacle for future advances (www.mda.org). Number of Patients Being Treated Currently SGU Cures Index 264 According to the CDC, DMD and BMD affect approximately 1 in every 3,500 boys, equating to approximately 400 to 600 live male births each year. Symptoms manifest at an average age of 4.9 years (CDC, 2009). We estimate that there were between 5,411 – 7, 400 males aged 5-24 with DMD or BMD in the U.S in 2012. It is likely that most, if not all, of these patients are receiving varying levels of treatment and cures. Number of Patients Requiring Treatment Although disease progression varies in DMD patients, most patients will be recommended a therapeutic treatment to help ease symptoms associated with DMD, improve their quality of life, and slow down disease progression. Therefore, most if not all patients require treatment (MDA, 2012). Impact of treatment on years of potential life lost (YPLL) Major advances in cardiac and respiratory care have recently increased life expectancy of individuals with DMD. In the past, most patients were not expected to live beyond their teens, and life expectancy is currently about 25 years (Kaneshiro, Seckler, & Jasmin, 2012). However, more patients are now surviving until their early 30s and there are even cases of patients living up to their 40s and 50s. A study by Passamano and colleagues (2012) showed a decade on decade improvement in survival when therapeutic treatment, specifically mechanical ventilatory support, was utilized. The study grouped patients based on the year of their birth. For patients born in the 1960s, survival rate was 23.3% at age 20. For patients born in the 1970s, survival rate at the age of 20 increased to 54% and for those born in the 1980s, survival rate was 59.8%. In addition, the study observed that the overall average age for respiratory death was 17.7 years for patients who did not use mechanical ventilatory support. However, for patients on mechanical ventilatory support, the average mean age for respiratory death increased to 27.9 years (Passamano, Taglia, Palladino, et al, 2012). The current average age of life expectancy for DMD patients is about 25 years, which is an improvement over the previous life expectancy. This improvement is a result of advances in research and treatments, including corticosteroids, physical therapy, assisted ventilation, etc. Further research may cause the YPLL to decrease even more in the future (MDA, 2012). Impact of Treatment on Disability-adjusted Life Years (DALYs/QALYs) A randomized study of DMD patients treated with corticosteroid demonstrated that the drug improves short-term (about six months to two years) muscle strength and function. Considering that the average age of wheel-chair use is 12, treatment with SGU Cures Index 265 corticosteroids may delay wheelchair use for up to two years (Manzur, Kuntzer, Pike, & Swan, 2008). No studies were found that examine the impact of improved therapies on DALY values among DMD/BMD patients. However, there is evidence that these advancements are increasing years of life lived, and are likely improving quality of life lived as well. It would be interesting to conduct such an historical analysis in the future Average Annual Total Cost of Corticosteroids A month supply of prednisone, a common corticosteroid prescribed for DMD patients, ranges from $5 to $10 per month for a dose of 20mg (www.goodrx.com). This means that each individual with DMD will use up to $120 per year (minimal cost), assuming that the patient takes a 20mg tablet once a day. Average Lifetime Cost of Corticosteroids Average age of onset is around 5 years for DMD patients and average life expectancy is currently 25 years. Minimum drug cost of $10 per month x 12 months x 20 years = $2,400. Average Overall Cost to Care for DMD Patient The Muscular Dystrophy Association recently funded a study published in 2014 that estimated the annual medical and non-medical cost to care for a patient with DMD. The result of the study showed that per-patient annual cost totaled to $50,953 per year. Nationally, the combined total is between $362-$488 million per year. Furthermore, most medical costs came from outpatient care, while non-medical costs were attributed to adapt housing and the expense of paid caregiving (Larkindale, Yang, Hogan et al, 2014). Economic Impact – Value of Life Added Advances in technology and therapies have increased the number of DMD patients who live beyond the age of 20 from 23.3% to 59.5% (Passamano, Taglia, Palladino, et al, 2012). The overall average age for respiratory death was 17.7 years for patients who did not use mechanical ventilatory support, but 27.9 years for patients on mechanical ventilatory support – an increase of 10.2 years of life (Passamano, Taglia, Palladino, et al, 2012). Multiplying that increase in life expectancy by the lowest estimate of the number of males aged 5-24 in the US with DMD/BMD (10.2 x $104,193 x 5,411) gives a VMRR of $5.75 billion associated with mechanical ventilatory support. SGU Cures Index 266 According to the Organization for Economic Co-operation and Development, the current life expectancy for males in the United States is 76 years. The life expectancy of patients with DMD is 25 years (Kaneshiro, Seckler, & Jasmin, 2012). Therefore, the years of potential life lost for each patient is 51 years (76 years – 25 years). Further, DMD is estimated to affect approximately 1 in 3,500 (2.9 per 10,000) male births (CDC, 2013). Given 1,976,420 male births in the US in 2012 (3,952,841 divided by half) (CDC National Vital Statistics, 2014), 565 babies were born with DMD that year. Multiplying 565 by 51 years of potential life lost to DMD gives an annual VMRR of $3 billion. Presuming an equivalent birth rate going forward, the ongoing economic impact of DMD/BMD will be approximately $3 billion each and every year indefinitely, in addition to the $28.75 billion in VMRR among those who are already living with DMD/BMD (given a conservative estimate of 5,411 existing patients). Duchenne Muscular Dystrophy Cure: Ataluren (Translarna) Cure Category Potential, functional Identification/Description Ataluren is a drug developed by PTC Therapeutics used “for the treatment of patients with genetic disorders that arise from a type of genetic mutation known as nonsense mutation”, which affects about 13 percent of patients with DMD. Ataluren is currently in Phase 3 of clinical trials for FDA approval and will recruit 220 patients to study the efficacy of the drug for 48 weeks. The drug targets the cellular machinery, allowing it to overcome the nonsense mutation and potentially produce a functioning dystrophin protein (PTC Therapeutics, 2014). Average Overall Cost for DMD Patient Patients who enroll in the study will have all costs of examination, screening, lab tests, and the cost of the drug covered by PTC Therapeutics. The drug is currently not available for sale anywhere in the world and therefore, no cost has been published by PTC Therapeutics (PTC Therapeutics, 2014). Research Development and Treatment Costs SGU Cures Index 267 The Muscular Dystrophy Association provided $1 million in funding for the study of Ataluren in patients with DMD (MDA, 2012). Beyond that, costs to develop the drug are covered by the private firm developing the drug (PTC Therapeutics, 2014). Citations Centers for Disease Control and Prevention. (2009). Prevalence of Duchenne/Becker muscular dystrophy among males aged 5-24 years—four states, 2007. MMWR Morbidity and Mortality Weekly Report, 58, 1119-1122. Retrieved March 5, 2014, from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5840a1.htm Centers for Disease Control and Prevention (2014). Muscular Dystrophy Data & Statistics. Retrieved June 10, 2014 from: http://www.cdc.gov/ncbddd/musculardystrophy/data.html. Deloitte Access Economics (2013). Economic Study of Muscular Dystrophy: Executive Summary. Retrieved on June 10, 2014 from: http://www.mda.org.au/Media/Publicity/Executive_Summary_Economic_study_of _muscular_dystrophy.pdf. Do, T. T., Mehlman, C., Talavera, F., Thompson, G., & Patel, D. (2012, September 12). Muscular Dystrophy (D. Grogan, Ed.). Retrieved March 23, 2014, from Medscape website: http://emedicine.medscape.com/article/1259041-overview Estimates of Funding for Various Research, Condition, and Disease Categories. (2014, March 7). Retrieved April 4, 2014, from NIH Research Portfolio Online Reporting Tools website: http://report.nih.gov/categorical_spending.aspx Kaneshiro, N., Seckler, B., & Jasmin, L. (2012, February 1). Duchenne muscular dystrophy [Fact sheet]. Retrieved March 23, 2014, from Medline Plus website: http://www.nlm.nih.gov/medlineplus/ency/article/000705.htm Larkindale, J., Yang, W., Hogan, P. F., Simon, C. J., Zhang, Y., Jain, A., Habeeb-Louks, E. M., Kennedy, A. and Cwik, V. A. (2014), Cost of illness for neuromuscular diseases in the United States. Muscle Nerve, 49: 431–438. doi: 10.1002/mus.23942 Manzur, A. Y., Kuntzer T., Pike, M., & Swan, A. V. (2008). Glucocorticoid corticosteroids SGU Cures Index 268 for Duchenne muscular dystrophy. Cochrane Database of Systematic Reviews, 1, Art. No.: CD003725. DOI: 10.1002/14651858.CD003725.pub3. Muscular Dystrophy Association – USA (n.d.). Medical Management. Retrieved April 1, 2014 from: http://mda.org/disease/myotonic-muscular-dystrophy/medicalmanagement. Myotonic Dystrophy Foundation. (n.d.). Types of DM. Retrieved March 10, 2014 from: http://www.myotonic.org/node/181. National Center for Biotechnology Information (n.d.). DMD Dystrophin Homo sapiens (Human) Fact Sheet. Retrieved on June 10, 2014 from: http://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=1756 National Human Genome Research Institute (2013). Learning about Duchenne Muscular Dystrophy. Retrieved March 6, 2014, from: http://www.genome.gov/19518854. National Institute of Child Health and Human Development (2012). What are the types of Muscular Dystrophy? Retrieved June 10, 2014 from: http://www.nichd.nih.gov/health/topics/musculardys/conditioninfo/pages/types. aspx. National Institute of Neurological Disorders and Stroke (NINDS). (2011). Muscular dystrophy: Hope through research. Retrieved March 23, 2014, from: http://www.ninds.nih.gov/disorders/md/detail_md.htm NIH Muscular Dystrophy Interagency Coordinating Committee. (2004). Muscular dystrophy research and education plan for the National Institutes of Health. Retrieved March 9, 2014, from: http://www.ninds.nih.gov/find_people/groups/mdcc/MD_Plan_submitted.pdf Organization for Economic Cooperation and Development (n.d.). United States Better Life Index. Downloaded on June 10, 2014 from: http://www.oecdbetterlifeindex.org/countries/united-states/. Passamano, L., Taglia, A., Palladino, A., Viggiano, E., D’Ambrosio, P., Scutifero, M., Cecio, M. R., Torre, V., De Luca, F., Picillo, E., Paciello, O., Piluso, G., Nigro, G., & Politano, L. (2012). Improvement of survival in Duchenne Muscular Dystrophy: Retrospective analysis of 835 patients. Acta Myologica, 31 (2), 121–125. SGU Cures Index 269 PTC Therapeutics. (2014). Therapeutic Areas: Ataluren for Genetic Disorders. Retrieved May 27, 2014 from PTC Therapeutics website: http://www.ptcbio.com/ataluren/ Ropper A.H., Samuels M.A. (Eds). (2009). The Muscular Dystrophies. Adams and Victor's Principles of Neurology, 9e. Chapter 50. US Census Bureau Population Estimates by Demographic and Housing Estimates. Retrieved on April 18, 2014 from: http:// http://factfinder2.census.gov SGU Cures Index 270 SICKLE CELL DISEASE Hilda Kyotakoze, Madlena Nalbandian, Tuula Jalonen Disease Category Genetic, Chronic Disease Identification, Description, and Diagnostic Criteria Sickle Cell Disease (SCD) is an inherited condition that results in the alteration of the shape of red blood cells into one resembling a farm tool, specifically, the sickle; hence, these altered cells are referred to as sickled cells. The change in shape of these red blood cells is in response to a change in the internal vascular environment to one with low oxygen levels, dehydration, extremes of temperature, and a number of other factors. Sickled red blood cells can block small blood vessels causing loss of blood supply to tissues, which can result in tissue damage and pain. In addition, the sickled red blood cells have a shorter half-life, and are broken down earlier than normal red blood cells, rendering affected individuals anemic. People with SCD present with a range of clinical symptoms, including life-threatening infections and organ failure, which contribute to slowed child development and increased morbidity and mortality. In the United States (US), the diagnosis of SCD is made by thin-layer isoelectric focusing (IEF), or high performance liquid chromatography (HPLC). Both these tests take advantage of the fact that the defective and normal hemoglobin molecules have different chemical properties creating characteristic patterns of movement. Either of these tests is recommended as part of routine newborn screening to allow early detection and management of the disease before the onset of symptoms (US Preventive Services Task Force, 2007). Prenatal diagnosis is also possible with the use of fetal blood sampling, amniocentesis and chorionic villous sampling, in which fetal cells and/or proteins are acquired in an invasive manner. A much newer and less invasive method of prenatal diagnosis is by isolation and testing of fetal DNA from maternal blood. On average, the disease manifests at about five months of life, when fetal hemoglobin is replaced by adult haemoglobin, which contains the defective protein. Usually the first manifestation involves swelling of the hands and feet of the baby, a condition known as dactylitis. Another manifestation at around this age is the abnormal function of the SGU Cures Index 271 spleen, which can result in acute splenic sequestration (a life-threatening condition in which blood elements, such as red blood cells and platelets, that flow into the spleen are retained within the organ, thus depleting the rest of the body of these vital blood components), decreased immunity to encapsulated microorganisms such as Streptococcus pneumoniae, and septicemia (Serjaent, 2013). In fact, the Cooperative Study of Sickle Cell Disease (CSSCD), the largest study on sickle cell disease involving more than 4,000 patients from 23 centers across the US, showed that the peak of mortality in patients with SCD under 20 years occurred at 1 to 3 years of age and was mainly due to pneumococcal infections (Leikin, Gallagher, Kinney, Sloanne, Klug, & Rida, 1989). Today, a major reason for the recommendation of newborn screening for SCD and early management of the disease is the high chance of life-threatening pneumococcal infections (U. S. Preventive Services Task Force, 2007). During childhood, a complex and potentially life-threatening condition called acute chest syndrome can arise as a major complication of SCD (Quinn, Rogers, & Buchanan, 2004; Serjaent, 2013). Infection and sequelae following decreased blood supply to lung tissue are contributors to this condition that is associated with decreasing lung function and increased mortality. Survival of this condition is associated with fibrosis of lung tissue and development of pulmonary hypertension later in life, which can impact heart function and contribute to heart failure. Strokes can occur in both childhood, between one and nine years (with a peak between two to five years), as well as later in life. The chance of experiencing a first stroke by 20 years of age is 11%, and by 45 years of age, is 24% (Ohene-Frempong et al., 1998; Verduzco & Nathan, 2009). After a first stroke, there is an increased risk of recurrence, making primary prevention of a first stroke a priority. The occurrence of this event is devastating, as it causes severe disability and impairs intellectual development in a young patient – affecting both child and family for years (Wang, 2007; Fullerton, Adams, Zhao & Johnston, 2004). Aplastic crisis is also sometimes seen in SCD. This is a decreased production of blood elements by the bone marrow. This decreased production is usually the result of a viral infection that would be almost harmless in someone without SCD. In adolescence and early adulthood, bone pain crises increase in incidence and are the most commonly reported symptom of SCD. During these crises, patients complain of debilitating pain affecting their ability to function normally. The Pain in Sickle Cell Epidemiology Study (PiSCES) reported SCD pain on 54.5% of the 31,017 days surveyed, also 29.3% of respondents had pain on more than 95% of the days surveyed SGU Cures Index 272 (Smith et al., 2005). These pain crises are associated with exposure to cold environments, wind, infection, dehydration, and stress, but still remain poorly understood. Additionally, increased frequency of pain episodes is associated with higher mortality in those over 20 years of age (Platt et al., 1991). While acute bone pain crises have been estimated to account for 5-35% of hospital admissions in SCD patients, it is important to remember that hospital admissions do not correctly estimate the prevalence of pain, as most patients do not seek treatment in hospitals. As such, the number of SCD pain cases seen in hospitals is likely the tip of the iceberg (Brookoff & Polomano, 1992; Smith & Scherer, 2010). Finally, SCD patients may also experience cell death of the femoral head, chronic leg ulcers, decreasing kidney function, and priapism (maintenance of an erection unrelated to sexual desire), which can cause infertility and tends to occur during early adulthood. In sum, SCD is a chronic condition that affects a wide range of tissues and organs, significantly impacting the quality of life of the affected individual and making it a potentially expensive disease to manage. Disease Etiology (Causes) In general, SCD results from a mutation in the gene for one of the components of hemoglobin, the protein contained in red blood cells that transports oxygen in the blood. This mutation causes the production of a defective protein, which changes shape in response to particular conditions such as low oxygen levels in the blood. The changed conformation promotes polymerization of hemoglobin molecules with each other and the eventual change in the overall shape of the red blood cell to a sickle shape. More specifically, adult hemoglobin (HbA) is composed of four subunits: two α subunits and two β subunits. Fetal hemoglobin (HbF) is also composed of four subunits but instead of two β subunits there are two γ subunits. In SCD, a mutation in the gene encoding for the β subunit results in abnormal adult hemoglobin molecules, which react to low oxygen levels and other conditions by aggregating with each other. This aggregation changes the configuration of a red blood cell into a sickled shape. SCD is therefore an inherited condition whose disease symptoms begin to manifest when fetal hemoglobin is replaced with adult hemoglobin at about five months of age. One must inherit two defective β globin genes, a state referred to as homozygous recessive or alternatively HbSS, in order to manifest the symptoms of SCD. The inheritance of only one defective gene, a state referred to as heterozygous or alternatively HbAS, results in SGU Cures Index 273 sickle cell trait where symptoms only manifest under extreme conditions. Individuals with sickle cell trait are said to be carriers of the condition and a child born from two carriers has a 25% chance of having SCD. The SCD mutation can be combined with other hemoglobin disorders resulting in variable disease severity. There can be combination with a hemoglobin C gene giving HbSC (this means that of the two β globin genes inherited from either parent, one gene has the sickle cell defect and the other gene has the haemoglobin C defect) which is milder than HbSS. Combination with the different types of β Thalassemia further expands the range in SCD severity: HbSβ0 has a severity similar to that of HbSS, and HbSβ+ is a milder disease. Much more rare variations of SCD include HbSE, HbSD, and HbSO. These variations may be challenging to differentiate from HbSS and may require definitive molecular diagnosis at specially equipped laboratories. Current Prevalence The National Newborn Screening Information System (NNSIS) was established to collect blood for measuring the prevalence of over 50 different infectious, genetic and metabolic diseases in newborns. NNSIS has been known globally as an effective method in early detection, in order to decrease mortality and morbidity (National Newborn Screening & Global Resource Center (NNSGRC), 2013). Every state is required to report its findings, in order to compile information about the incidence of SCD in the US, but not all states do (Hassell, 2010). Thus, the exact prevalence of SCD in the US is currently unknown. However, the Centers for Disease Control (CDC) is also working with the National Institutes of Health (NIH) to complete the Registry and Surveillance System for Hemoglobinopathies (RuSH) project (Centers for Disease Control, 2014). This project will also help determine the number of individuals living with SCD. RuSH has completed a pilot study to determine the prevalence of SCD in California, Georgia, Michigan, North Carolina, and New York. In California, there were 5,100 people living with SCD during the 2004-2008 assessment period. Approximately 89% of these SCD patients were African-American, 8% were Hispanic-American, and 5% were other. Also, 14% were younger than 6 years of age, 25% were 6-17 years of age, 22% were 30-50 years of age, and 11% were 51 years of age or older (Centers for Disease Control, 2014). In Georgia, there were 7,299 people living with SCD during the 2004-2008 assessment period. In Michigan, there were 1,392 people living with SCD during the 2004-2008 assessment period, Of these, 18% were younger than 5 years of age, 48% were between 5-14 years of age, and 34% were between 15-21 years of age. In North Carolina, there were 5,578 people living SGU Cures Index 274 with SCD during the 2004-2008 assessment period. Of these, 39% were younger than 18 years of age, 29% were between 18-35 years of age, and 32% were 35 years or older. In New York, 8,374 people were living with SCD during the 2004-2008 assessment period. Of these, 40% were younger than 21 years of age, 46% were between 21-50 years of age, and 14% were between 51 years of age or older. Overall, it has been estimated that SCD currently affects 90,000 to 100,000 Americans. Also, it is estimated that 1 in 12 African Americans have SCD trait (Centers for Disease Control, 2014). Current Incidence According to the Registry and Surveillance System for Hemoglobinopathies (RuSH) project, 486 babies were born with SCD in California during the 2004-2008 assessment period. This equates to 1 in every 5,644 live births. If the rates were relatively consistent across the five years of the assessment period, approximately 97 babies (486 / 5) are born with SCD in California each year. In Georgia, between 2004-2008, 1 in every 295 African American newborns had SCD. Also, it was estimated that 97% of newborns with SCD were African-American, while about 2% were Hispanic-American. In Michigan, 251 babies were born with SCD during the 2004-2008 assessment period, and 96% of them were African-American. If the rates were relatively consistent across the five years of the assessment period, approximately 50 babies (251 / 5) are born with SCD in Michigan each year. In Michigan , Kleyn, VanOchten, Grigorescu, and Young (2009) report that in 2008 specifically, 47 babies were born with SCD, equalling 1 in 2,539 screened infants. In North Carolina, 92 babies were born with SCD during the 2004-2008 assessment period. From these newborns, 95% were African-American, 1% were white, 0% were Asian-American, 1% were American Indian, 2% were Hispanic-American, 3% were other races. Also, SCD occurred in 1 in every 1,435 births, 1 in every 360 African-American births, and 1 in every 10,800 Hispanic-American births in North Carolina. If the rates were relatively consistent across the five years of the assessment period, approximately 18 babies (92/5) are born with SCD in North Carolina each year. In New York, in 2008, 197 babies were born with SCD. From these newborns, 89% were African-American, 8% were white, and 3% were other race. Also, 1 in every 1,259 births overall had SCD, while 1 in every 260 African-American, 1 in every 10,209 white births, and 1 in every 2,714 Hispanic-American births has a SCD. Overall, the incidence rate of SCD in the US has been estimated at 1 in every 2,500 births (McGann, Santos, Oliveira, Bernardino, Ware & Gross, 2013). Given 3,952,841 births in the US in 2012 (Centers for Disease Control, 2014a) and taking the overall SGU Cures Index 275 estimate of one SCD case in every 2,500 births gives 1,581 babies born with SCD in that year. Further, 1 in every 500 African American births and 1 in every 36,000 Hispanic American births have SCD (CDC, 2014). According to the National Newborn Screening Information System report for 2006 (NNSGRC, 2013), the apparent national incidence rate for Sickle-cell anemia is 0.253, S/C-disease is 0.140 and S-β-thalassemia is 0.031 per 1000 births. Estimated Undiagnosed/At-Risk People with SCD experience a number of symptoms ranging from swelling of the feet, life-threatening infections, acute chest syndrome, stroke, to the very common acute painful crises, all of which can significantly lower quality of life and contribute to endorgan damage (Leikin, Gallagher, Kinney, Sloanne, Klug, & Rida, 1989; Quinn, Rogers, & Buchanan, 2004; Serjaent, 2013; Smith et al., 2005). The increasing risk of severe symptoms as they age means that most, if not all children will be diagnosed and receive treatment as they get older. In addition, SCD is routinely screened for at birth to ensure these children get early treatment to avoid complications. This decreases the chances that an individual with SCD will not be diagnosed. As such, we estimate that the number of undiagnosed SCD cases is negligible to nonexistent in the US. Disease Impact – Years of Potential Life Lost (YPLL) In 2006, the CDC reported that the mean number of years lived with SCD was 39. From the 483 reported deaths, 9% were 20 years of age or younger, 28% were 20 to 34 years of age, 28% were 35 to 44 years of age, and 35% were over 45 years of age (Hassell, 2010). In 1994, the Cooperative Study of SCD (CSSCD) noted that the median survival age for Sickle-cell anemia for men was 42 years of age and for women it was 48 years of age (Hassell, 2010). Improvements in medical care, education, and the use of newborn screenings and prophylactic penicillin, have increased the age of survival in the last 30 years. About 50% of the SCD children didn’t reach adulthood previously. Today , 93.9% of sickle-cell anemia patients survive until adulthood (i.e., 18 years of age or older.) (Quinn, Rogers, McCavit & Buchanan, 2010). Additionally, there has been a relative mortality rate decrease of 68% from 1983 to 2002 for those aged 0-3 years, 39% for those aged 4-9 years, and 24% for those aged 10-14 years (Yanni, Grosse, Yang & Olney, 2009). Although the mortality rate decreased by 42% in children SGU Cures Index 276 between 0-3 years of age from 1995 to 2002, there was no significant decrease in survival rates for older children. Sickle-cell disease in the US has a much better prognosis than in developing countries, although even under the most sophisticated conditions, median survival is at least 20 years shorter than the general population (Serjeant, 2013). Given a current US life expectancy of 78.7 years (Centers for Disease Control, 2014b), SCD patients can expect to live until 58 or 59 under ideal health conditions, which is in line with the median survival age of 42 (men) and 48 (women) in 1994 (Hassell, 2010). Disease Impact – Disability Adjusted Life Years (DALYs) Years lived with disability (YLDs) across different diseases in the US have been calculated and compared from1990 to 2010 (Murray et al., 2013). The total YLD for SCD in 1990 in the US was 372,600, while in 2010, it was 472,000. Hence, there was a median change of 28.4% for YLD, and an age-standardized YLD rate change of 10.2% for SCD over the course of 20 years. The Disability Adjusted Life Years (DALYs) across different diseases in the US was calculated for all ages and per 100,000 people in 1990 and 2010 (Murray et al., 2013). While the US DALY figure associated with SCD is currently not available, the global DALY impact as a result of SCD has been calculated. In 1990, that value was 4.33 million (with a 95% confidence interval of 3.29 - 5.57 million), increasing to 5.64 million in 2010 (with a 95% confidence interval of 4.24 - 7.25 million). In 1990, the global DALYs associated with SCD was 82 (62 - 105) per 100,000 people, maintaining that level [82 (62 - 105) per 100,000 people] in 2010. The Disability Adjusted Life Years (DALYs) for the United Kingdom (UK) for Sickle-cell disorders was reported in 1990 and 2010 for both sexes combined and all ages (Murray et al., 2013). In 1990, the DALYs associated with SCD were 64,000 (44,000 – 90,000 range), while in 2010 they were 67,000 (46,000 – 95,000 range). Economic Impact – Years of Potential Life Lost (YPLL) The median survival age from the CSSCD was 42 years of age for males and 48 years of age for females (Platt et al., 1994). Given the current US life expectancy for males (76.4) and females (81.2) (CDC, 2014), the median years of potential life lost (YPLL) to SCD in the US are (76.4 - 42) 34.4 for males and (81.2 – 48) 33.2 for females. This is associated with a VMRR of $3.46 million for each female and $3.58 million for each male SGU Cures Index 277 diagnosed with SCD in the US. If 1,581 babies were born with SCD in 2012, split evenly between males and females, the total VMRR-based annual economic impact is $2.74 billion for females (790.5 female babies x $3.46 million) and $2.83 billion for males (790.5 male babies x $3.58 million), or $5.57 billion in total. The CDC and the Cooperative Study of SCD (CSSCD) report different survival age values; specifically, the 1994 values are higher than those in 2004, even though there were advancements in health care during that time (Hassell, 2010). Hassell reported that one of the underlying reasons for the differences in the values is that the CSSCD collected data from specific sickle-cell facilities; hence, the author suggests that there needs to be a more accurate and sophisticated approach utilized when reporting these values. We use the most conservative numbers from 1994 when reporting the years of potential life lost and their economic impacts. Economic Impact – Disability Adjusted Life Years (DALYs) The DALYs associated with SCD are not currently available for the US We provide SCDassociated DALYs for the United Kingdom as a comparison exercise. There were 67,000 DALYs lost to SCD in the UK in the year 2010, which equates to a US-based VMRR of $6.98 billion. This value is similar to the overall economic impact of potential lost life to SCD in the US as outlined above ($5.57 billion), and represents the potential value to be gained from further cure advancements for SCD. History of the Disease and Breakthroughs In 1910, the first case of SCD was reported in Walter Clement Noel, a Grenadian studying dentistry in Chicago. He approached Dr. James B. Herrick with complaints of pain and symptoms of anemia. Investigation by Dr. Herrick’s resident Dr. Ernest Irons revealed red blood cells with “a shape of a sickle” (Herrick, 1910; Winter, 2010). This year marked the official discovery of SCD (named so in 1922 by Dr. Verne Mason). However, it is worth noting the disease existed for centuries before this and was known by many names across Africa. Sickle cell disease has been shown to have a particularly high incidence among people of African origin, including African Americans, occurring in approximately 1 in 500 Black or African American births. This high incidence can be partly attributed to the fact that the SCD trait (the state in which one has one normal β globin gene and one defective β globin gene) offers an advantage against falciparum Malaria, which is endemic to Africa and some parts of the Mediterranean, Asia and the Middle East. SGU Cures Index 278 In 1927, Hahn and Gillespie found that sickling seemed to occur in the presence of low oxygen and tended to be seen in people related to each other (Hahn & Gillespie, 1927). It was not until 1949 that the genetics and inheritance of SCD was demonstrated independently by Col. E. A. Beet and Dr. James V. Neel (Neel, 1949; Winter, 2010). In the same year, Dr. Linus Pauling and colleagues demonstrated that SCD was characterized by a defect in the hemoglobin molecule making it a molecular disease (Pauling, Itano, Singer, & Wells, 1949). The molecular nature and behavior of this abnormal hemoglobin was later presented in detail by Dr. Vernon Ingram (Ingram, 1958). Knowledge of the genetic and molecular nature of SCD made it possible to develop screening tools and implement genetic counselling for prospective at-risk parents (Neal-Cooper & Scott, 1988). Of even more importance, prenatal and newborn screening using gel electrophoresis and similar techniques (i.e. isoelectric focusing and HPLC) has been developed to allow for early identification and management, thereby reducing the incidence of a variety of complications. In 1948, Watson and colleagues suggested that the presence of fetal hemoglobin and its replacement with adult hemoglobin was contributory to the manifestation of SCD at an age later than birth (Watson, Stahman, & Bilello, 1948). This is further supported by the association of fetal hemoglobin with milder forms of SCD (Frenette & Atweh, 2007) – high concentrations of defective adult hemoglobin in a red blood cell is associated with higher chances of aggregation and change in shape of the overall red blood cell; however, when fetal hemoglobin is being produced, there is a relatively lower concentration of defective adult hemoglobin in the red blood cell and thus lower chances of aggregation. Thus, increasing the expression of fetal hemoglobin can be a potential treatment for SCD, and is actually the mechanism of action of hydroxyurea, an increasingly used drug. Current Cure Status Current widely available and used treatments focus on addressing the symptoms of SCD and reducing the chance of complicating illnesses (i.e., pneumonia) rather than the underlying (genetic) cause. While these treatments have increased life expectancy for individuals with SCD, creating a functional cure that allows many to live into adulthood, an ideal cure for SCD will involve addressing the root cause - the red blood cells containing defective hemoglobin. SGU Cures Index 279 In 1960, Sir John Dacie described SCD as a disease of childhood with high mortality and relatively few patients reaching adult life, even with a high standard of medical care (cited in Platt et al., 1994). In 1973, Diggs estimated a median survival of 14.3 years, with 20% of deaths occurring in the first 2 years of life, one-third before five years, and half between 5 and 30 years. In the 1980s, median age of death in patients with SCD was reported to be 42 years for males and 48 years for females. This represented a decrease of roughly 25-30 years in life expectancy as compared with that of the black American population in general (Platt et al., 1994). Thus, average life expectancy of SCD patients has increased over time. Since the discovery of the condition, numerous studies have been conducted; and one of the most significant endeavours has been the Cooperative Study of Sickle Cell Disease (CSSCD). The CSSCD was commissioned in 1978 by the National Heart, Lung, and Blood Institute (NHLBI) to characterize prospectively the clinical course of SCD in a cohort of more than 4,000 patients from 23 centers across the US (Gaston & Rosse, 1982; Frenette & Atweh, 2007). This study revealed a number of complications and triggered the search for possible solutions, as outlined below. 1) In 1989, results from the CSSCD showed that 85% of patients with HbSS lived to 20 years of age and more (Leikin et al., 1989). This improvement from earlier estimates of 50% living past 20 years was attributed to parental education and counselling. Earlier studies had revealed acute splenic sequestration as a major cause of death and perhaps teaching parents how to feel for an enlarged spleen may have promoted early detection and management of the disease, and thus, better outcomes. This was evidenced by the presence of only one death from acute splenic sequestration in the particular study by CSSCD. This same study revealed a significant mortality between 1 and 3 years of age due to infection, particularly pneumococcal sepsis; whereas mortality in patients older than 10 years, but less than 20 years, was mainly due to stroke and traumatic events. A more recent longitudinal study by Quinn, Rogers, McCavit, and Buchanan (2010) has shown childhood deaths in patients with SCD to be a much less frequent event with survival at 18 years being 93.9%; additionally, acute chest syndrome and multi-organ failure syndrome have surpassed sepsis as a leading cause of death. It is noted that the burden of mortality is shifting to adulthood, especially the period of transition between pediatric and adult care. 2) The finding of significant mortality due to pneumococcal infections in children with SCD despite widespread use of pneumococcal vaccine, led to the Prophylactic Penicillin Study (PROPS). The PROPS trial (ClinicalTrials.gov Identifier: NCT00000585) resulted in a significant reduction in pneumococcal SGU Cures Index 280 sepsis in patients under 3 years old, which ultimately led to legislation by Congress for newborn screening for SCD and a recommendation for early management with prophylactic penicillin at 4 months to 5 years (Leikin et al., 1989; Gaston et al., 1986). Today, following detection of SCD via newborn screening, it is recommended that affected children begin prophylactic penicillin by 2 months of age, and receive pneumococcal immunizations (U. S. Preventive Services Task Force, 2007). Interestingly, the introduction of the 7-valent conjugated pneumococcal vaccine in 2000 coincided with a 42% decrease in SCD-related deaths in patients younger than 4 years in the period from 1999 to 2002 (Yanni, Grosse, Yang, & Olney, 2009). However, of increasing concern is the emergence of resistant strains of the organism (Cober & Phelps, 2010). 3) The incidence of stroke in the CSSCD prompted the Stroke Prevention Trial in Sickle Cell Anemia (STOP I; NCT00000592). This trial revealed that identification of at risk children with abnormal Transcranial Doppler Ultrasonography (TDU) and management with prophylactic chronic transfusion resulted in a 92% relative decrease in the rate of first stroke (Adams et al., 1998; Ohene-Frempong et al., 1998). TDU detects blood-flow velocity, which is inversely correlated with arterial diameter, and high flow velocity is associated with increased risk for stroke. These findings were further supported by a study in California that revealed a five-fold decline in incidence of stroke that coincided with the introduction of the intervention (Fullerton, et al., 2004). This California study also highlighted the socioeconomic impact of a stroke and thus the welcome of its reduction - the median length of stay for stroke-related admissions (6 days) was reported to be twice that for non-stroke SCD admissions (3 days), which translates into more work days lost for parents a trend which was also observed for cost of stay. However, it should be noted that TDU has a high sensitivity of 94-100% but a low specificity of 51% and therefore correctly predicts risk for stroke 36% of the timethus, raising a controversy over the use of transfusions for primary prevention based on TDU results (Adams et al., 1997; Verduzco et al., 2009) – especially considering the side effects of transfusion (i.e. iron overload, sensitization to foreign blood groups, and increased risk of infection). 4) Other complications and current methods of SCD management include the following: ● Patients with SCD are encouraged to keep hydrated and avoid extremes of temperature to reduce the probability of sickling and subsequent manifestations. ● Analgesia and transfusions are used in the management of pain crises. SGU Cures Index 281 ● Zinc supplements are recommended given that patients are often zinc deficient. This supplementation decreases incidence of infection and promotes healthy growth (Prasad 2002). ● One milligram of folic acid daily is given as the loss of red blood cells leads to increased need for this micronutrient. Vitamin B12 and Vitamin B6 may also be needed for the same reason (van der Dijs et al., 2002). ● Gallstones tend to manifest in patients with SCD necessitating removal of the gallbladder in a procedure called a cholecystectomy. ● Splenectomy (removal of the spleen) and regular transfusions are used in the management of acute splenic sequestration. ● Leg ulcers need to undergo proper wound care and dressing. They can become chronic and may require the use of other approaches such as skin grafting (Minniti, Eckman, Sebastiani, Steinberg, & Ballas, 2010). ● Transfusion in the management of an aplastic crisis. ● Acute chest syndrome can be managed using antibiotics, transfusion therapy, and supportive care. ● The more chronic manifestations such as pulmonary hypertension, congestive heart failure, and renal abnormalities are managed together with respective specialists. There are a range of manifestations of SCD and consequently a range of treatments depending on what the patient presents with. Being a disease with so many chances for complications, symptomatic treatment is bound to be demanding and expensive. In 2004, total hospitalization costs for SCD were $488 million (Agency for Healthcare Research and Quality, 2006). Research into the expression of hemoglobin genes during the late 1970s and 1980s contributed to the development of therapeutic interventions that promoted elevated levels of fetal hemoglobin (Frenette & Atweh, 2007). 5-Azacytidine was the first agent to show promising results, however, concerns about its potential carcinogenicity brought this to a halt. The controversy on the mechanism of action of 5-Azacytidine encouraged the study of another chemotherapeutic agent, hydroxyurea, as a possible treatment. Treatment with hydroxyurea is associated with lower incidence of pain crises and acute chest syndrome, and decreased need for transfusions; Further to this, improved survival is observed (Charache et al, 1995). However, a recent phase 3 Stroke with Transfusions Changing to Hydroxyurea trial (NCT00122980) has revealed that transfusions coupled with iron chelation remain a better alternative when compared to hydroxyurea coupled with phlebotomy (Ware & Helms, 2012). Ongoing concerns regarding the safety with 5Azacytidine and hydroxyurea promote the search for better agents. Another agent SGU Cures Index 282 called butyrate has shown ability to induce elevated fetal hemoglobin when given four days every four weeks to patients with SCD; however, administering large amounts of this drug via central venous catheters lowers its feasibility (Atweh et al., 1999). More recently, decitabine, a 5-azacytidine analog with a slightly different mode of action has shown promising results: 100% of patients responded including previous nonrespondents to hydroxyurea (DeSimone et al., 2002; Frenette & Atweh, 2007; Koshy et al., 2000). This agent still requires more rigorous testing to assess safety and efficacy. It should be noted that the agents mentioned are not cures but rather disease-modifying treatments - and of these, the only approved one is hydroxyurea, as it has been shown to be efficacious and has a low side-effect profile. Currently, research is focusing on the genetic risk factors for particular complications of sickle cell disease. 99.5% of human DNA is the same for all individuals, leaving 0.5% accounting for the diverse uniqueness of each individual – single nucleotide polymorphisms (SNPs) are just some of the areas of our DNA that contribute to this differentiation. The genome-wide association study (GWAS) is focused on collecting a profile of SNPs and other DNA variants and observe patterns associated with various diseases such as hypertension and diabetes; this is also being done to observe for patterns that contribute to increased risk for particular complications of SCD such as stroke, pain crises, and acute chest syndrome. This will hopefully allow for “personalized medicine” in the future, whereby a physician is able to tailor the treatment of a patient with SCD to target their risk for particular complications (Frenette & Atweh, 2007). To date, the only definitive cure for SCD being pursued is hematopoietic stem cell transplantation (HSCT), explained in greater detail in the next section. This procedure is motivated by the fact that red blood cells containing defective hemoglobin come from the bone marrow; therefore, replacing the cells in the bone marrow results in red blood cells containing normal hemoglobin. Clinical trials in children have shown more than 90% long-term survival following bone marrow transplantation. Future Cure Obstacles Hydroxyurea was licensed for use in patients with SCD in 1998, however, more than ten years later it is still being underutilized, leading to questions about the clinical effectiveness of the drug (Brandow & Panepinto, 2010; Lanzkron, Haywood Jr, Hassell & Rand, 2008). Various surveys have revealed the reasons for underutilization include: unwillingness to use a drug that is also used in cancer patients, unwillingness due to perception of the drug as experimental, and even lack of familiarity at the physicianSGU Cures Index 283 level (Haywood Jr et al., 2011; Brandow & Panepinto, 2010). Efforts must be made to uncover, and more importantly address, reasons why many physicians and patients are not using this drug. Hematopoietic stem cell transplant, the only definitive cure, is a procedure that has a risk of mortality, graft rejection, and Graft versus Host disease (GVHD – a condition in which donor immune cells attack host tissue) and so, candidates must meet criteria that have been set to minimize risk and maximize benefit. But even in suitable candidates, a matched sibling donor is available to less that 14% of SCD patients (Shenoy, 2013). Current efforts are geared towards improving safety and increasing availability (in terms of both supply and expertise) of this cure. In an effort to reach these goals, various approaches are being analyzed that will be discussed in the next section. Finally gene therapy is another potential cure for SCD but is still within its early stages of development. Current Treatment Costs Currently, the cost of hospital admissions is about $1,000 per day, and $350 every time an individual receives a blood transfusion (Shenoy, 2011). According to Jamison et al. (2006), a program in Toronto that provides SCD individuals with symptomatic care estimated that the annual costs for SCD outpatients, excluding transfusions, is $6,090 per year for chelated patients and $553.31 for non-chelated patients.2 The study also reported that in 1996, hospitalizations due to SCD would cost on average about $6,300, which resulted in a total spending of $575 million per year. According to Kauf, Coates, Huazhi, Mody-Patel, and Hartzema (2009), the health care costs for sickle-cell patients usually rises with age, and average cost from the Medicaid program was $1,389 per patient per year. According to Shenoy (2013), Medicaid and private insurers reported that children with SCD cost them 6-11 times more than children without the disease, which equates to about $9,000 to $13,000 higher per year. Research Development and Treatment Costs When a transfusion is performed there is introduction of red blood cells into the patient’s bloodstream. These red blood cells contain iron in the hemoglobin that they carry, which can create a state of iron excess in the patient’s body when added to the iron already present in the patient. Excess iron can be toxic and thus is controlled in a process known as chelation, wherein chelating agents are administered to remove heavy metals, including iron, from the body. 2 SGU Cures Index 284 The NIH has created a system called Research, Condition, and Disease Categorization (RCDC) to best estimate the amount of annual support and funding that is given to various diseases, such as SCD (National Institutes of Health Research, 2014). 2010 (non-ARRA): $73 million 2010 (ARRA): $12 million 2011: $65 million 2012: $65 million 2013: $70 million 2014 (estimated): $72 million 2015 (estimated): $72 million From 1972-2001, NHLBI has invested over $923 million in sickle-cell research (NIH 2014). Sickle Cell Disease Cure: Hematopoietic Stem Cell Transplant (HSCT) Cure Category Partially Achieved, Definitive Cure Identification/Description In hematopoietic stem cell transplantation (HSCT), the hematopoietic stem cells (cells that multiply to give blood elements) are obtained from the bone marrow of a donor that is either normal or has the sickle cell trait. Usually, this donor needs to have an HLA (Human Leukocyte Antigen) match with the recipient, which reduces the possibility of rejection of the cells by the recipient and also donor cells attacking recipient tissue. To provide the best chances for a match, the donor is often a SCD patient's brother or sister. The donor undergoes a procedure whereby the hematopoietic stem cells are obtained from the bone marrow. Meanwhile, the recipient undergoes a procedure to destroy his or her own hematopoietic stem cells. The donor cells are then introduced into the recipient via a vein, and they will travel to and repopulate the recipient’s bone marrow. Thus, following this one procedure, the recipient now has hematopoietic stem cells that will produce red blood cells with relatively normal hemoglobin and cure the SCD for life. Recovery can take one to two months during which the recipient is observed for signs of graft rejection, infection, and other complications. In the few cases of graft rejection, a second attempt tends to be successful. Cure History SGU Cures Index 285 The first bone marrow transplantation in a patient with SCD was performed in 1982 to treat a relapsed acute leukemia. Following the intervention, it was found that the patient’s SCD was cured as well (Johnson et al., 1984). Large clinical trials have been carried out in Europe and yielded promising results with overall survival over 90%. In one trial with 42 patients in Belgium and France, 36 had sustained engraftment (Vermylen & Cornu, 1994;Vermylen et al., 1998). The first relatively large trial carried out in the US with 22 patients aged 16 years and under who received marrow from matched sibling donors showed a 90% survival rate (20 patients survived) and sixteen of the patients showed stable engraftment and signs of being cured after a median follow-up time of 23.9 months (Walters et al., 1996a). A multicenter collaborative investigation of HLA identical sibling marrow transplantation in children with severe SCD was carried out between 1991 and 2000 by the same investigators - a total of 57 children from 27 transplant centers in the US, South America, and Europe were included (Walters et al., 1997; Walters et al., 2000; Walters et al., 2010). In the latest update published in 2010, follow-up revealed 55 patients survived and 50 survived free of the underlying disease. Furthermore, the majority of patients showed no further strokes and stable lung function (Walters et al., 2010). Overall, HSCT has been demonstrated to be curative for SCD – about 85% of patients show no more signs of the disease – but there is mortality ranging from 5-20% and risk for graft rejection, GVHD, interstitial pneumonitis, gonadal toxicity, sterility and other complications (Walters et al., 1997; Walters et al., 2000; Walters et al., 2010; Shenoy, 2013; Vermylen & Cornu, 1994; Vermylen et al., 1998). The procedure has been shown to be more successful in children than adults and research is ongoing to increase the safety and efficacy of the procedure. Cure Science: Breakthroughs/Obstacles A major challenge in the development of HSCT as an accessible cure for SCD is making it widely available to both adults and children. About 14-21% of eligible candidates have matched sibling donors (Walters et al., 1996b; Hsieh, Fitzhugh, & Tisdale, 2011). This dependency on sibling donors creates a barrier for many patients. Potential solutions for this low match rate include the use of non-sibling matched donors, nonsibling mismatched donors, cord blood transplantation (CBT - use of umbilical cord blood cells theoretically would allow for a greater degree of matching and therefore potential to expand the donor pool), and haploidentical donors (half-matched donors, usually a mother or father). These alternatives to matched sibling donors however carry higher risk for bad outcomes and research into increasing their efficacy and safety is under way. There is an ongoing clinical trial funded by the NHLBI into the safety and SGU Cures Index 286 efficacy of bone marrow transplants in children with SCD from unrelated donors (NCT00745420). Identifying which SCD patients should receive the procedure and at what age remain challenges given the variable course of the illness. Ideally, a young child with relatively healthy organs whose disease is most likely to be severe, is the perfect candidate. Children tend to experience less risk for complications from the procedure than do adults, and more benefit is gained before end-organ damage. However, it is difficult to predict whether a particular child will have a severe disease course, which would justify the high risks associated with undergoing the procedure. Movement has been made towards creating a system on which to base the decision of whether to carry out HSCT (Shenoy, 2013). Criteria used by Walter and colleagues (1996a) as indications for transplantation included a child less than 16 years of age with a history of stroke, or recurrent acute chest syndrome, or recurrent pain crises. NIH indications for HSCT in patients over 16 years of age include any of the following: irreversible end-organ damage, stroke or clinically significant central nervous system event, sickle-related renal insufficiency, reversible sickle complication not ameliorated by hydroxyurea, acute chest syndrome while on hydroxyurea, two or more pain crises requiring hospitalizations, among other indications (Hsieh, Fitzhugh, & Tisdale, 2011). Originally, HSCT in the treatment of SCD was done with myeloablative therapy (clearing out SCD patient marrow before replacing it with donor marrow), similar to the procedure used in malignancies. However, this approach appears to be toxic in adult patients with SCD and more promising results are observed when HSCT is carried out without myeloablative therapy – referred to as nonmyeloablative HSCT). This is an area of active research whose previous results have been disappointing (Hsieh, Fitzhugh, & Tisdale, 2011; Shenoy, 2013). During a recent clinical trial on nonmyeloablative HSCT in 30 patients aged 16-65, 87% (26 patients) experienced reversal of their SCD (Hsieh et al., 2014). From these participants, 14 completely stopped taking immunosuppressive medications, while all experienced normal hemoglobin and less hospitalization. This study represents a positive move towards making HSCT available and safer for adults with SCD. Currently, adults over 35 years of age often become refractory to hydroxyurea and hypersensitive to transfusion therapy and are thus left with few options for therapy. Cure Science: Future Obstacles and Targets Future cure obstacles and targets include making HSCT widely available as an option to patients without matched sibling donors, making HSCT a safer option for adults SGU Cures Index 287 (Shenoy, 2013), and reducing overall incidence of complications and mortality in both children and adults. One very recent and potentially promising treatment that may replace HSCT involves the use of induced pluripotent stem cells (iPSCs), which are derived from adult skin fibroblast cells (Harley, 2013). These cells can differentiate into most cell types, making the replacement of a β S allele with a normal β allele possible through stem cell transplantation into the SCD patient (Townes, 2008). However, this therapy is in very early stages of testing, and there are a number of obstacles that need to be overcome, such as the reprogramming method itself and preventing the stem cells from forming benign tumors (i.e., teratomas) (Teoh & Cheong, 2012). Number of Patients being Treated Currently In procedures after 2000, it has been reported that there was a 95.3% SCD-free survival rate among those who underwent HSCT (Bernaudin, 2007). The median follow-up time for these patients was 6 years, ranging from 2-18 years. The procedure is still under investigation and most of the individuals who have been treated are those that have been enrolled in clinical trials. The Health Resources and Services Administration (HRSA) reports that there were 382 transplants due to sickle cell anemia between 2008-2012 worldwide (HRSA, n.d.). In 2006, 1,336 hematopoietic stem cell transplantations were performed on hemoglobinopathies patients (Gratwohl et al., 2010). Number of Patients Requiring Treatment According to the NIH (2014), SCD affects over 90,000 Americans. However, HSCT treatment is only recommended for children given its toxic effects in adults. Globally, there is an annual global birth rate of 300,000 per year with sickle-cell anemia, the primary type of SCD treated with HSCT (Bolaños-Meade, 2012). Impact of the Treatment/cure on Years of Potential Life Lost (YPLL) Stem cell transplant is the only cure currently available for SCD, however it currently has a 5-20% chance of death from the procedure itself and from complications, such as infections, GVHD, etc. (Walters et al., 1997; Walters et al., 2000; Walters et al., 2010; Vermylen & Cornu, 1994; Vermylen et al., 1998). If successful, it can prevent episodes of pain, and further central nervous system and lung effects of SCD, which can extend years of potential life. Overall, it has been estimated that SCD currently affects 90,000 to 100,000 Americans (Centers for Disease Control, 2014). Approximately 40% of SCD patients are under age SGU Cures Index 288 17 in the state of California (CDC, 2014). Thus, we estimate there are 36,000 SCD patients under the age of 17 in the US, and this group defines the universe of individuals for whom transplantation is a potential cure. Currently approved treatment regimens (i.e., stem cell transplantation) for those with SCD under 17 years of age (36,000 Americans) are suitable for only 6% of cases based on the severity of SCD (i.e., experience of stroke, three or more pain crises annually, chest syndrome). This is risk-reward trade-off associated with the relatively high rates of death from the procedure (5-20%). Combining the 80% rate of those who survive the procedure (Walters et al., 1997; Walters et al., 2000; Walters et al., 2010; Vermylen & Cornu, 1994; Vermylen et al., 1998) with the 14% of suitable cases who are able to find a matching family donor for myeloablative HSCT (Hsieh et al., 2014; Walters et al., 1996b), we can estimate the number of SCD patients under age 17 in America who can potentially be cured with myeloablative HSCT at 242, and then use this result to determine the impact on years of potential life lost per patient as well America overall. According to Quinn et al. (2010), the average age of death was 9.22 among 23 severe SCD cases (i.e., chest syndrome, multi-organ failure syndrome, mycardial infarction, Pneumococcal sepsis, stroke and neurological events). Given that males with SCD live until 42, on average, and females live until 48, on average (i.e., 45 years combined) (Hassell, 2010) minus average age of death of 9.22, 36 years of life can be potentially saved for each of these severe SCD child patients. Multiplying by the 242 patients eligible for myeloablative HSCT gives 8,712 years of potential life saved by this treatment in the US More recent/cutting-edge treatment using nonmyeloablative HSCT in patients aged 1665 has demonstrated 87% effectiveness in reversing SCD (Hsieh et al., 2014). Unfortunately, this therapy has been found to have higher incidence of graft rejection in children, and is only available to adults over age 16. However, since 93.9% of sickle-cell anemia patients currently survive until adulthood (18+ years) (Quinn, Rogers, McCavit & Buchanan, 2010), the majority of patients will survive long enough to undergo this therapy. If 87% of the 94% of SCD patients who survive to adulthood can be cured from this therapy, 73,602 Americans with SCD could be cured [(90,000 current patients x 94% adulthood survival rate x 87% effectiveness rate]. Given the current American life expectancy of 78.7 years (Centers for Disease Control, 2014b), YPLL If the average SCD patient (male and female) current lives to age 45, 33.7 years of potential life can be saved per patent, multiplied by 73,602 American patients = 2.48 million years of potential life saved in the US SGU Cures Index 289 It is important to note that nonmyeloablative HSCT is a novel and rapidly emerging area of research, particularly among adult patients with severe disease. While the numbers we present above are based on all adult SCD patients in the US, this treatment may only apply to those experiencing severe disease, thereby reducing the numbers significantly. Cost of Treatment/Cure per Patient (Annualized and/or Lifetime) The cost of HSCT can range from $80,499 to $137,564 depending on the type of donor and conditioning that is utilized. Specifically, it costs approximately $83,583 for HCT from a relative, while umbilical cord HCT is approximately $137,564 (Preussler, Denzen, & Majhail, 2012). According to Jamison et al. (2006), the treatment is more costeffective than lifelong procedures for addressing complications of SCD. Economic Impact of the Treatment/Cure: Years of Potential Life Saved Given its restriction to the most severe SCD cases among children under 17 years of age (as a result of potentially lethal side effects and complication), and its low donor match rate of 14% of suitable cases, myeloablative HSCT has the potential to save relatively few (8,712) years of life in the US, with an associated VMRR of $907.7 million. While not yet realized, nonmyeloablative HSCT has the potential to treat adults (i.e., over 17 years of age) with severe SCD. While we have estimated the possible impact at 73,602 years of potential life saved among Americans currently diagnosed with SCD, this number presumes that all adults with SCD can undergo the treatment – which is not likely to be the case, as this treatment is in the very earliest stages of development. 73,602 years is associated with a VMRR of $7.669 billion. Total Research and Development Cost to Discover the Cure The NIH stem cell research funding for 2013 was $1.343 billion. This funding includes embryonic and non-embryonic human and non-human stem cell research (NIH, 2014). It is difficult to determine how much of the $1.343 billion in NIH stem cell research funding is directed specifically toward SCD. According to Gratwohl et al. (2010), approximately 348 stem cell transplantations for hemoglobinopathies patients were performed globally in 2006 compared to 11,928 stem cell transplantations. SGU Cures Index 290 Citations Adams, R. J., McKie, V. C., Carl, E. M., Nichols, F. T., Perry, R., Brock, K., McKie, K., Figueroa, R., Litaker, M., Weiner, S., & Brambilla, D. (1997). Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Annals of Neurology, 42(5), 699–704. Adams, R. J., McKie, V. C., Hsu, L. Files, B., Vichinsky, E., Pegelow, C., Abboud, M., Gallagher, D., Kutlar, A., Nichols, F. T., Bonds, D. R., & Brambilla, D. (1998). Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. The New England Journal of Medicine, 339(1), 5–11. Agency for Healthcare Research and Quality (2006). Healthcare Cost and Utilization. Statistical Brief #21: Sickle Cell Disease Patients in US Hospitals, 2004. Retrieved on 29th July 2014 from: http://www.hcupus.ahrq.gov/reports/statbriefs/sb21.pdf Atweh, G. F., Sutton, M., Nassif, I., Boosalis, V., Dover, G. J., Wallenstein, S., Wright, E., McMahon, L., Stamatoyannopoulos, G., Faller, D. V., & Perrine, S. P. (1999). Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood, 93(6), 1790-1797. Bernaudin, F., Socie, G., Kuentz, M., Chevret, S., Duval, M., Bertrand, Y., Vannier, J., Yakouben, K., Thuret, I., Bordigoni, P., Fischer, A., Lutz, P., Stephan, J., Dhedin, N., Plouvier, E., Margueritte, G., Bories, D., Verlhac, S., Esperou, H., Coic, L., Vernant, J., & Gluckman, E. (2007). Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood, 110(7), 2749-2756. Bolaños-Meade, J., Fuchs, E. J., Luznik, L., Lanzkron, S. M., Gamper, C. J., Jones, R. J., & Brodsky, R. A. (2012). HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood, 120(22), 4285-4291. Brandow, A. M., & Panepinto, J. A. (2010). Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert review of hematology, 3(3), 255-260. Brookoff, D., & Polomano, R. (1992). Treating sickle cell pain like cancer pain. Annals of SGU Cures Index 291 Internal Medicine, 116(5), 364–368. doi:10.7326/0003-4819-116-5-364. Charache, S., Terrin, M. L., Moore, R. D., Dover, G. J., Barton, F. B., Eckert, S. V., McMahon, R. P., Bonds, D. R., & the investigators of the Multicenter Study of Hydroxyurea in sickle cell anemia. (1995). The New England Journal of Medicine, 332(20), 1317–1322. doi: 10.1056/NEJM199505183322001 Centers for Disease Control (2014a). Births and Natality. Retrieved on September 3, 2014 from: http://www.cdc.gov/nchs/fastats/births.htm. Centers for Disease Control (2014b). Life Expectancy. Retrieved on May 23, 2014 from: http://www.cdc.gov/nchs/fastats/life-expectancy.htm. Cober, M. P., & Phelps, S. J. (2010). Penicillin prophylaxis in children with sickle cell disease. The Journal of Pediatric Pharmacology and Therapeutics, 15(3), 152-159 DeSimone, J., Koshy, M., Dorn, L., Lavelle, D., Bressler, L., Molokie R., & Talischy, N. (2002). Maintenance of elevated fetal hemoglobin levels by decitabine during interval treatment of sickle cell anemia. Blood, 99(11), 3905-3908. Fullerton, J. H., Adams, R. J., Zhao, S., & Johnston, C. (2004). Declining stroke rates in Californian children with sickle cell disease. Blood, 104(2), 336–339. doi:10.1182/blood-2004-02-0636 Gaston, M. H., & Rosse, W. F. (1982). The cooperative study of sickle cell disease: review of study design and objectives. The American journal of pediatric hematology/oncology, 4(2), 197–201. Gaston, M. H., Verter, J. I., Woods, G., Pegelow, C., Kelleher, J., Presbury, G., Zarkowsky, H., Vichinsky, E., Iyer, R., Lobel, J. S., & For the Prophylactic Penicillin Study Group. (1986). Prophylaxis with oral penicillin in children with sickle cell anemia: a randomized trial. The New England Journal of Medicine, 314, 1593– 1599. Gratwohl, A., Baldomero, H., Aljurf, M., Pasquini, M. C., Bouzas, L. F., Yoshimi, A., Szer, J., Lipton, J., Schwendener, A., Gratwohl, M., Frauendorfer, K., Niederwieser, D., Horowitz, M., & Kodera, Y. (2010). Hematopoietic stem cell transplantation: a global perspective. Journal of the American Medical Association, 303(16), 16171624. SGU Cures Index 292 Hahn, E. V., & Gillespie, E. B. (1927). Sickle cell anemia. Archives of Internal Medicine, 39, 233–254. Harley, M. N. (2013). Current treatments and prospective therapies to manage sickle cell disease. Lab Medicine, 44, e92-e96. Hassell, K. L. (2010). Population estimates of sickle cell disease in the US. American journal of preventive medicine, 38(4), S512-S521. doi: 10.1016/j.amepre.2009.12.022 Haywood, C., Beach, M. C., Bediako, S., Carroll, C. P., Lattimer, L., Jarrett, D., & Lanzkron, S. (2011). Examining the characteristics and beliefs of hydroxyurea users and nonusers among adults with sickle cell disease. American journal of hematology, 86(1), 85-87. Health Resources and Services Administration (HRSA) (n.d.). Number of transplants reported for sickle cell anemia from 2008-2012. Retrieved on 10th August, 2014 from: http://bloodcell.transplant.hrsa.gov/RESEARCH/Transplant_Data/US_Tx_Data/D ata_by_Disease/national.aspx Herrick, J. B. (1910). Peculiar elongated and sickle shaped red blood corpuscules in a case of severe anemia. Archives of Internal Medicine, 6, 517–521. Hsieh, M. M., Fitzhugh, C. D., & Tisdale, J. F. (2011). Allogeneic hematopoietic stem cell transplantation for sickle cell disease: the time is now. Blood, 118(5), 11971207. Hsieh, M. M., Fitzhugh, C. D., Weitzel, R. P., Link, M. E., Coles, W. A., Zhao, X., Rodgers, G. P., Powell, J. D., & Tisdale, J. F. (2014). Nonmyeloablative HLAmatched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. Journal of the American Medical Association, 312(1), 4856. Ingram, V. M. (1958). Abnormal human haemoglobins. I. The comparison of normal human and sickle-cell haemoglobins by fingerprinting. Biochimica et Biophysica Acta, 28, 539–545. SGU Cures Index 293 Jamison, D. T., Breman, J. G., Measham, A. R., Alleyne, G., Claeson, M., Evans, D. B., Prabhat, J., Mills, A., & Musgrove, P. (Eds.). (2006). Disease control priorities in developing countries. World Bank Publications. Johnson, F. L., Look, A. T., Gockerman, J., Ruggiero, M. R., Dalla-Pozza, L., & Billings, F. T. (1984). Bone-marrow transplantation in a patient with sickle-cell anemia. The New England Journal of Medicine, 311, 780-783. doi: 10.1056/NEJM198409203111207 Kauf, T. L., Coates, T. D., Huazhi, L., Mody-Patel, N.,& Hartzema, A. G. (2009). The cost of health care for children and adults with sickle cell disease. American Journal of Hematology, 84(6), 323-327. doi:10.1002/ajh.21408 Kleyn M., VanOchten K., Grigorescu V., Young W. (2009). Sickle Cell Disease in Michigan. Michigan Newborn Screening Follow-Up Brief 1(1). Retrieved on 30th August, 2014 from: http://www.michigan.gov/documents/mdch/SCBRIEF_MK_final_300653_7.pdf Koshy, M., Dorn, L., Bressler, L., Molokie, R., Lavelle, D., Talischy, N., Hoffman, & DeSimone, J. (2000). 2-deoxy 5-azacytidine and fetal hemoglobin induction in sickle cell anemia. Blood, 96(7), 23. Lanzkron, S., Haywood Jr, C., Hassell, K. L., & Rand, C. (2008). Provider barriers to hydroxyurea use in adults with sickle cell disease: a survey of the Sickle Cell Disease Adult Provider Network. Journal of the National Medical Association, 100(8), 968-973. Leikin S. L., Gallagher, D., Kinney, T. R., Sloane, D., Klug, P., & Rida, W. (1989). Mortality in children and adolescents with sickle cell disease. Pediatrics, 84(3), 500–508. Minniti, C. P., Eckman, J., Sebastiani, P., Steinberg, M. H., & Ballas, S. K. (2010). Leg ulcers in sickle cell disease. American Journal of Hematology, 85(10), 831-833. Murray, C. J., Vos, T., Lozano, R., Naghavi, M., Flaxman, A. D., Michaud, C., et al. 2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380(9859), 2197-2223. doi:10.1016/S0140-6736(12)61689-4 SGU Cures Index 294 National Institutes of Health. Estimates of Funding for Various Research, Conditions, and Disease Categories (RCDC). (2014). Retrieved on 30th July 2014 from: http://www.report.nih.gov/categorical_spending.aspx National Heart, Lung, and Blood Institute. (2014). Explore Blood and Marrow Stem Cell Transplant. Retrieved on 17th August 2014 from: http://www.nhlbi.nih.gov/health/health-topics/topics/bmsct/ National Newborn Screening & Global Resource Center (NNSGRC). (2013). Retrieved on 25th July 2014 from: http://genes-r-us.uthscsa.edu/newborn_reports Neal-Cooper, F., & Scott, R. B. (1988). Genetic counseling in sickle cell anemia: experiences with couples at risk. Public Health Reports, 103(2), 174–178. Neel, J. V. (1949). The inheritance of sickle cell anemia. Science, 110, 64–66. Ohene-Frempong, K., Weiner, S. J., Sleeper, L. A., Miller, S. T., Embury, S., Moohr, J. W., Wethers, D. L., Pegelow, C. H., & the Cooperative Study of Sickle Cell Disease. (1998). Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood, 91(1), 288–294. Pauling, L., Itano, H. A., Singer, S. J., & Wells, I. C. (1949). Sickle cell anemia, a molecular disease. Science, 110, 543–548. Platt, O. S., Brambilla, D. J., Rosse, W. F., Milner, P. F., Castro, O., Steinberg, M. H., & Klug, P.P. (1994). Mortality in sickle cell disease: Life expectancy and risk factors for early death. The New England Journal of Medicine, 330(23), 1639–1644. Platt, O. S., Thorington, B. D., Brambilla, D. J., Milner, P. F., Rosse, W. F., Vichinsky, E., & Kinney, T. R. (1991). Pain in sickle cell disease: Rates and risk factors. The New England Journal of Medicine 325(1), 11–16. Prasad, A. S. (2002). Zinc deficiency in patients with sickle cell disease. The American Journal of Clinical Nutrition, 72(2), 181-182. Preussler, J. M., Denzen, E. M., & Majhail, N. S. (2012). Costs and cost-effectiveness of hematopoietic cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation, 18(11), 1620. SGU Cures Index 295 Shenoy, S. (2013). Hematopoietic stem-cell transplantation for sickle cell disease: current evidence and opinions. Therapeutic Advances in Hematology, 4(5), 335344. doi: 10.1177/2040620713483063 Quinn, C. T., Rogers, Z. R., & Buchanan, G. R. (2004). Survival of children with sickle cell disease. Blood, 103(11), 4023–4027. doi:10.1182/blood-2003-11-3758 Quinn, C.T., Rogers, Z. R., McCavit, T. L., & Buchanan, G. R. (2010). Improved survival of children and adolescents with sickle cell disease. Blood, 115(17), 3447-3452. doi:10.1182/blood-2009-07-233700 Serjeant, G. R. (2013). The Natural History of Sickle Cell Disease. Cold Spring Harbor Perspectives in Medicine, 3(10), a011783. Smith, W. R., Bovbjerg, V. E., Penberthy, L. T., McClish, D. K., Levenson, J. L., Roberts, J. D., Gil, K., Roseff, S. D., & Aisiku, I. P. (2005). Understanding pain and improving management of sickle cell disease: the PiSCES study. Journal of the National Medical association, 97(2), 183. Smith, W. R., & Scherer, M. (2010). Sickle-cell pain: Advances in epidemiology and etiology. Hematology, 2010, 409 – 415. doi:10.1182/asheducation-2010.1.409 Teoh, H. K., & Cheong, S. K. (2012). Induced pluripotent stem cells in research and therapy. Malaysian Journal of Pathology, 34, 1-13. Townes, T. (2008). Gene replacement therapy for sickle cell disease and other blood disorders. American Society of Hematology Education Program, 193-196. US Burden of Disease Collaborators. (2013). The state of US health, 1990-2010: burden of diseases, injuries, and risk. The Journal of the American Medical Association, 310(6), 591-608. doi: 10.1001/jama.2013.13805 US Preventive Services Task Force. (2007). Retrieved on 8th July 2014 from: http://www.uspreventiveservicestaskforce.org/uspstf07/sicklecell/sicklers.pdf Van der Dijs, F. P., Fokkema, M. R., Dijck-Brouwer, D. A., Niessink, B., van der Wal, T. I., SGU Cures Index 296 Scnhog, J. J., Dutis, A. J., Muskiet, F. D., & Muskiet, F. A. (2002). Optimization of folic acid, vitamin B(12), and vitamin B(6) supplements in pediatric patients with sickle cell disease. American journal of hematology, 69(4), 239-246. Verduzco, L. A., & Nathan, D. G. (2009). Sickle cell disease and stroke. Blood, 114, 5117–5125. doi:10.1182/blood-2009-05-220921 Vermylen, C., & Cornu, G. (1994). Bone marrow transplantation for sickle cell disease. The European experience. The American Journal of Pediatrics Hematology/ Oncology, 16(1), 18-21. Vermylen, C., Cornu, G., Ferster, A., Brichard, B., Ninane, J., Ferrant, A., Zenebergh, A., Maes, P., Dhooge, C., Benoit, Y., Beguin, Y., Dresse, M. F., & Sariban, E. (1998). Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplantation, 22(1), 1-6. Walters, M. C., Patience, M., Leisenring, W., Eckman, J. R., Scott, J. P., Mentzer, W. C., Davies, S. C., Ohene-Frempong, K., Bernaudin, F., Matthews, D. C., Storb, R., & Sullivan, K. M. (1996a). Bone marrow transplantation for sickle cell disease. The New England Journal of Medicine, 335(6), 369-376. Walters, M. C., Patience, M., Leisenring, W., Eckman, J. R., Buchanan, G. R., Rogers, Z. R., Olivieri, N. E., Vichinsky, E., Davies, S. C., Mentzer, W. C., Powars, D., Scott, J. P., Bernaudin, F., Ohene-Frempong, K., Darbyshire, P. J., Wayne, A., Roberts, I. A., Dinndorf, P., Brandalise, S., Sanders, J. E., Matthews, D. C., Appelbaum, F. R., Storb, R., & Sullivan, K. M. (1996b). Barriers to bone marrow transplantation for sickle cell anemia. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation, 2(2), 100-104. Walters, M. C., Patience, M., Leisenring, W., Rogers, Z. R., Dinndorf, P., Davies, S. C., Roberts, I. A., Yeager, A., Kurtzberg, J., Bunin, N., Scott, J. P., Wall, D. A., Wayne, A. S., Wiley, J., Darbyshire, P. J., Mentzer, W. C., Smith, F. O., & Sullivan, K. M. (1997). Collaborative multicenter investigation of marrow transplantation for sickle cell disease: current results and future directions. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation, 3(6), 310-315. Walters, M. C., Storb, R., Patience, M., Leisenring, W., Taylor, T., Sanders, J. E., SGU Cures Index 297 Buchanan, G. E., Rogers, Z. R., Dinndorf, P., Davies, S. C., Roberts, I. A., Dickerhoff, R., Yeager, A. M., Hsu, L., Kurtzberg, J., Ohene-Frempong, K., Bunin, N., Bernaudin, F., Wong, W. Y., Scott, J. P., Margolis, D., Vichinsky, E., Wall, D. A., Wayne, A. S., Pegelow, C., Redding-Lallinger, R., Wiley, J., Klemperer, M., Mentzer, W. C., Smith, F. O., & Sullivan, K. M. (2000). Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Blood, 95(6), 1918-1924. Walters, M. C., Hardy, K., Edwards, S., Adamkiewicz, T., Barkovich, J., Bernaudin, F., Buchanan, G. R., Bunin, N., Dickerhoff, R., Giller, R., Haut, P. R., Horan, J., Hsu, L. L., Kamani, N., Levine, J. E., Margolis, D., Ohene-Frempong, K., Patience, M., Redding-Lallinger, R., Roberts, I. A. G., Rogers, Z. R., Sanders, J. E., Scott, J. P., Sullivan, K. M., & Multicenter Study of Bone Marrow Transplantation for Sickle Cell Disease. (2010). Pulmonary, gonadal, and central nervous system status after bone marrow transplantation for sickle cell disease. Biology of Blood and Marrow Transplantation, 16(2), 263-272. Wang, C. W. (2007). Central nervous system complications of sickle cell disease in children: An overview. Child Neuropsychology, 13(2), 103–119. doi:10.1080/09297040600788136 Ware, E. R., & Helms, R. W. (2012). Stroke with transfusions changing to hydroxyurea (SWiTCH). Blood, 119(17), 3925–3932. Watson, J., Stahman, A. W. & Bilello, F. P. (1948). The significance of the paucity of sickle cells in newborn Negro infants. The American Journal of the Medical Sciences, 215, 419–423. Winter, W.P. (2010). A brief history of sickle cell disease. Retrieved on 7th July 2014 from: http://sicklecell.howard.edu/ABriefHistoryofSickleCellDisease.htm Yanni, E., Grosse, S. D., Yang, Q., & Olney, R. S. (2009). Trends in pediatric sickle cell disease-related mortality in the United States, 1983-2002. The Journal of Pediatrics, 154(4), 541–545. doi: 10.1016/j.jpeds.2008.09.052 SGU Cures Index 298 C. Neoplasm (Cancers) SGU Cures Index 299 CHILD CANCERS Kayleigh Kangas Disease Category Neoplasm (Cancer) Disease Identification, Description, and Diagnostic Criteria While cancer affects both adults and children under age eighteen alike, child cancer is different from its adult counterpart in a few key ways. Firstly, different types of cancer usually affect children than are generally seen in adults. Secondly, children tend to respond better to treatment, although the adverse effects of treatment tend to result in negative long-term health impacts (American Cancer Society, 2014f). Finally, child cancers are associated with different risk factors than those associated with the development of cancer in adults (American Cancer Society, 2014f). Specifically, very few childhood cancers are caused by preventable factors such as avoiding tobacco smoke, excessive sun exposure, and a high fat diet (Ward, Desantis, Robbins, Kohler, & Jemal, 2014). The average age of onset varies between cancer types. Further, different types of cancer tend to be associated with specific ages in childhood development. The major types of childhood cancers include (Ward et al., 2014): • Acute lymphoblastic leukemia • Central nervous system tumors • Neuroblastoma • Non-Hodgkin’s lymphoma • Wilms tumor • Acute myeloid leukemia • Ewing sarcoma • Osteosarcoma • Hodgkin lymphoma • Rhabdomyosarcoma • Retinoblastoma • Thyroid carcinoma • Testicular germ cell tumors • Ovarian germ cell tumors • Melanoma SGU Cures Index 300 Ward, Desantis, Robbins, Kohler, and Jemal (2014) reported that acute lymphoblastic leukemia, central nervous system tumors, neuroblastoma, Non-Hodgkin’s lymphoma, Wilms tumor and acute myeloid leukemia account for 70% of all new cases of cancer in children age birth to fourteen. Since these six types constitute the largest impact, they are the main focus of the present analysis. Acute lymphoblastic leukemia (ALL) occurs within the bone marrow, or soft tissue inside bone, where underdeveloped lymphocytes, a type of white blood cell, are overproduced in the bodies of ALL patients (National Cancer Insitute, 2014). While weight loss is characteristic of ALL as in many other cancers, additional symptoms of ALL can include bone pain, joint pain, easy bleeding, tiredness, weakness, loss of appetite, paleness, pain or feeling of “fullness” below the ribs, petechiae (red spots on the skin), swollen glands, and night sweats. However, bruising and fever are the most commonly identified symptoms in children with ALL (Chen, Zieve, Blackman, Slon, & Wang, 2014; National Cancer Insitute, 2014). The reduction of healthy white blood cells that results from the increasing number of underdeveloped lymphocytes may lead to anemia, bleeding, and decreased immune function, which can increase the risk of infection (Chen et al., 2014). Ward et al. (2014), projected that ALL accounts for 26% of all cancer cases in young children, age birth to fourteen, making it the most prevalent of child cancers. Any mass of abnormal, cancerous cells in the brain or spinal cord is classified generally as a central nervous system (CNS) tumor, and this type of cancer will account for 21% of new cases in children in 2014, making it the cancer with the second highest impact in young children, birth to fourteen (Ward et al., 2014). Astrocytoma, brain stem glioma, ependymoma, germ cell tumor, and medulloblastoma are the most common childhood CNS cancers (American Society of Clinical Oncology, 2013). Symptoms related to CNS tumors may arise slowly and subtly, or they may appear suddenly (Chen, Zieve, Eltz, Slon, & Wang, 2012). Brain tumors usually cause symptoms when the tumor grows and places outward pressure on the skull. Symptoms can include headache, nausea, vomiting, crossed eyes, blurred vision, lack of balance, changes in behavior, seizures, drowsiness, and coma. Symptoms vary further based on their location in the central nervous system, and can range from numbness to bowel problems (American Cancer Society, 2014d). Neuroblastoma will account for 7% of all new cases of cancer in young children in 2014, and it is the most commonly diagnosed cancer in infants under one year of age (Ward et al., 2014). In 50% of cases, Neuroblastoma forms in the adrenal gland, which is located immediately above the kidneys. However, it can develop anywhere in the SGU Cures Index 301 sympathetic nervous system, which is a subsystem of the peripheral nervous system that includes nerves located beyond the brain and spinal cord (Ward et al., 2014). Symptoms experienced by patients with neuroblastoma include flushed or pale skin, excess sweating, and rapid pulse (Chen, Zieve, & Black, 2013). Approximately 6% of all new cases of cancer in children age 14 and under in 2014 will consist of non-Hodgkin lymphoma (NHL) (Ward et al., 2014). The tissue, fluid, and vessels of the lymphatic system extend through much of the body, so NHL can develop in almost any part of the body (National Cancer Institute, 2014b). In children with NHL, malignant cells gather in parts of the lymphatic system, which most commonly results in enlarged lymph nodes and breathing problems (National Cancer Institute, 2014b). Breathing problems can include wheezing, coughing, high-pitched sounds, or generalized difficulties breathing. Other symptoms can include unusual swelling of the head, neck, upper body, or arms, trouble swallowing, painless lump or swelling of the lymph nodes or testicles, fever unrelated to other illness, weight loss for an otherwise unknown reason, and night sweats(National Cancer Institute, 2014b). In children and adolescents, the most common types of NHL include Burkitt lymphoma, diffuse large Bcell lymphoma, lymphoblastic lymphoma, and anaplastic large cell lymphoma (Ward et al., 2014). Wilms tumor (WT) is the next most common type of childhood cancer, accounting for 5% of all new cases in 2014 in young children, age fourteen and under, However, it typically occurs in children under five years old (Ward et al., 2014). WT is a type of malignancy occurring in the kidney. An abdominal mass usually first indicates the presence of WT and prompts the initiation of testing, however other symptoms include high blood pressure, abdominal pain, and fever unrelated to other illness (Kaneshiro & Zieve, 2012). Acute myeloid leukemia (AML) will also account for 5% of new cases of cancer in children in 2014, with most cases appearing in children under one year of age (Ward et al., 2014). Much like ALL, AML develops in white blood cells in the bone marrow, however, it includes malignancies of myeloid stem cells, a different subset of white blood cells (National Cancer Institute, 2014a). Symptoms of AML include bruising, easy bleeding, fatigue and fever, while children may also experience night sweats, bone or joint pain, shortness of breath, skin rash, a painless lump, or weakness (National Cancer Institute, 2014a). A variety of tests and procedures are used in the diagnosis of cancer, following a physical exam and the assessment of symptoms. The specific method used is SGU Cures Index 302 determined by each individual case, but commonly used tests include tissue, fluid, or blood sampling and imaging. When symptoms indicate the possible presence of ALL, blood tests and bone marrow biopsy may be used. Bone marrow tissue may be assessed by cytogenetic analysis, in which changes to the chromosomes in lymphocytes may indicate the presence of ALL, or immunophenotyping, in which malignancies can be detected if ALL is present (National Cancer Insitute, 2014). When symptoms indicate the possible presence of a malignant mass in the nervous system or kidney, tests of blood, urine, cerebral spinal fluid, or tissue samples may follow. Additionally, imaging techniques, such as MRI, CT scan, PET scan, x-ray, and ultrasound are used to identify many cases (American Cancer Society, 2014c). Disease Etiology (Cause) The precise cause(s) of cancer in children are unknown, however several factors are associated with childhood cancers and thus contribute to increased risk. Any agent, whether it be an internal factor, an external or environmental agent, a familial predisposition, or a genetic factor, which has a statistically significant association with the occurrence of a given disease is said to be a risk factor of that disease (Linet, Wacholder, & Zahm, 2003). Clinically relevant risk factors include excessive exposure to radiation, certain chemicals and chemical mixtures, such as second-hand smoke, genetic disorders, such as Down Syndrome, and congenital immunodeficiency diseases (Linet, Wacholder, & Zahm, 2003; Ward et al., 2014). Risk factors vary with different types of childhood cancers, and they tend to be grouped into three evidence-based categories: (1) known; (2) suggestive but not conclusive; and (3), limited or inconsistent. Ward et al. (2014) describe some at-risk populations for each type of childhood cancer based on associated risk factors. For example, ALL is more common in boys than in girls, and it is also more common in white and hispanic children than in black children. Having Down syndrome is associated with a 10 to 20-fold increased risk of developing ALL. Children with particular genetic conditions are at a higher risk of developing CNS tumors. Children who have received radiation also make up a population at higher risk of developing a CNS tumor. Current epidemiological understanding of acute lymphoblastic leukemia (ALL) relates several known risk factors to its occurrence. These include sex, age, race, socioeconomic status, ionizing radiation (both in utero and postnatal), and a number of genetic conditions: Down syndrome, neurofibromatosis, Shwachman syndrome, Bloom syndrome, ataxia telangiectasia, Langerhans cell histiocytosis, and Klinefelter syndrome (Ries, Smith, Gurney, Linet, Tamra, Young, & Bunin, 1999; Ward et al., 2014). Evidence suggests that birth weight, maternal history of fetal loss, maternal age, and birth order SGU Cures Index 303 may also be risk factors (Ries et al., 1999; Ward et al., 2014). Limited or inconclusive evidence has shown that maternal smoking before or during pregnancy, parental occupational exposure, postnatal infection, maternal diet and postnatal chloramphenicol use could be possible risk factors for ALL (Ries et al., 1999; Ward et al., 2014). There are few known risk factors related to the development of CNS malignancies in children, including sex, ionizing radiation to the head, and genetic conditions such as neurofibromatosis, tuberous sclerosis, nevoid basal cell carcinoma syndrome (Gorlin syndrome), Turcot syndrome, and Li-Fraumeni syndrome (Ries et al., 1999; Ward et al., 2014). Research indicates that maternal diet during pregnancy, parent or sibling diagnosis of brain tumor, and family history of cancer, especially leukemia, lymphoma, or bone cancer, may also be risk factors. Inconsistent findings have also linked exposure to electromagnetic fields, parental occupational hazards, pesticides, patient head injury history, family history of epilepsy, and family history of intellectual disability as possible risk factors (Ries et al., 1999). Inconsistent evidence suggests that maternal medication during pregnancy, sex hormones, birth weight, congenital anomalies, previous spontaneous abortion (fetal death), use of alcohol or tobacco during pregnancy, and parental occupational exposure may be possible risk factors for neuroblastoma (Ries et al., 1999). Only one known factor has been associated with non-Hodgkin lymphoma (NHL) in children: immunodeficiency, which can be caused by a number of reasons, including HIV infection (Ries et al., 1999; Ward et al., 2014). Substantial evidence suggests that Epstein-Barr virus may be a risk factor in some populations (Ries et al., 1999). Inconsistent evidence indicates that radiation may also be a risk factor of pediatric NHL (Ries et al., 1999). The risk factors for Wilms tumor (WT) are more evident. They include gender, race, congenital anomalies and genetic conditions (Ries et al., 1999; Ward et al., 2014). Congenital anomalies and genetic conditions linked with WT include aniridia, genitourinary anomalies, WAGR syndrome, Beckwith-Wiedermann syndrome, Perlman syndrome, Denys-Drash syndrome, and Simpson-Golabi-Behmel syndrome (Ries et al., 1999; Ward et al., 2014). Evidence suggests paternal occupation as another potential risk factor. Limited, or inconsistent, evidence suggests that high birth weight, parental exposure to persticides, ionizing radiation in utero, maternal consumption of coffee and tea during pregnancy, maternal medication during pregnancy, maternal use of hair dye SGU Cures Index 304 during pregnancy, and maternal occupation may also be associated risk factors of WT (Ries et al., 1999). Like ALL, many risk factors have been identified for development of acute myeloid leukemia. These include race, chemotherapeutic agents, ionizing radiation in utero, and genetic conditions, such as Down syndrome, neurofibromatosis, Shwachman syndrome, Bloom syndrome, familial monosomy 7, Kostmann granulocytopenia, and Franconi anemia (Ries et al., 1999; Ward et al., 2014). Suggestive evidence indicates that alcohol consumption during pregnancy, parental and child exposure to pesticides, and parental exposure to benzene are also risk factors (Ries et al., 1999). More inconsistent or limited data suggests that recreational drug use during pregnancy, radon, and postnatal patient use of chloramphenicol may also be risk factors (Ries et al., 1999). Current Prevalence/Incidence US Prevalence: In 2010, there were 113,782 children with cancer in the United States, age zero to nineteen (Ward et al., 2014). US Incidence: In 1999, it was estimated that each year, 12,400 children under age 20 are diagnosed with cancer, and another 2,300 children under the age 20 die of cancer (Ries et al., 1999). In 2010, the cancer incidence rate for children age 0-14 was 159.8 per 1 million children (National Cancer Institute, 2014d). For children 0-19, the incidence rate of childhood cancer in 2010 was 173.4 per 1 million children (National Cancer Institute, 2014d). In 2010, there were about 61,227,000 children under 15 in the US, so the incidence rate that year was approximately 9,784 children in that age group (Howden & Meyer, 2011). Estimated Undiagnosed Childhood cancers are generally characterized differently than their adult equivalents. One important difference is that the most common of child cancers, leukemia and CNS tumors, are not staged according to the formal staging system used to describe adult cancers (i.e., stage I, II, III, IV). Because of this, more relevant factors related to prognosis include: (1) the child’s age at diagnosis, (2) cancer type and location, and (3) details related to the cancer type – for example, the white blood cell count at diagnosis of leukemia or grade of CNS tumor (American Cancer Society, 2014e; Children’s Cancer Research Fund, 2014). The grade of a tumor estimates how quickly the tumor may grow based on the appearance of the malignant cells. Due to the fact that the overwhelming SGU Cures Index 305 majority of children with cancer in the US are eventually diagnosed, childhood cancer is a condition where undiagnosed cases are few. Disease Impact – Years of Potential Life Lost (YPLL) In 2008, 97,000 years of life were lost to childhood cancer (National Cancer Institute, 2012). More recent data shows that an average of 10.4 years are lost in the individual life expectancy of those who reach five years of remission after diagnosis with a childhood cancer (Yeh, Nekhlyudov, Goldie, Mertens, & Diller, 2010). Approximately one out of four of these early deaths are estimated to be a result of cancer recurrence or second cancers related to late effects (Yeh et al., 2010). With an incidence of approximately 9,784 cases in 2010, the years of potential life lost are estimated to be at 101,754 years (Howden & Meyer, 2011; National Cancer Institute, 2014d). Disease Impact – Disability Adjusted Life Years (DALY) In 2004, 60,057 DALYs were lost to cancer in children aged 0-14 in the US (World Health Organization, 2009). History The first written description of cancer was produced in ancient Egypt, although Hippocrates, of ancient Greece, is most often credited as the first to name it as such around 400 BC, using the term carcinoma to describe a tumor. Like many other illnesses at the time, cancer was attributed to an imbalance in the body’s humors due to an excess of black bile. This theory was generally held for over a thousand years until it was replaced by the lymph theory, which stated that cancer was caused by the “fermenting” of lymphatic fluid in the body. Throughout most of the 17th and 18th centuries, it was believed by many that cancer was contagious. During the 1700s, epidemiological studies began exploring possible causes of cancer, including lifestyle, occupational exposure to carcinogens, and even tobacco. The 1800s saw two momentous steps forward. First, the invention of the microscope finally initiated the true birth of oncology. The introduction of the modern microscope also allowed tissue samples to be examined for malignancies—a method still sometimes used to diagnose cancers today. The second advance occurred when Johannes Muller, a German scientist, first demonstrated that cancer was made up, not of lymph or other fluid, but of cells. Soon after, Rudolph Virchow discovered that all cells are derivatives of other existing cells, yet another momentous discovery for the realm of oncology. In the early 1900s, Peyton Rous was the first to discover the existence of a carcinogenic virus. The SGU Cures Index 306 1970s brought CAT scanning, allowing medical professionals the opportunity to use imaging to guide diagnosis and treatment, and in 1986, researchers discovered genes associated with increased risk of developing cancer in childhood. (American Cancer Society, 2012; American Society of Clinical Oncology, 2014a, 2014b) Current Cure Status Child cancers are treated in a number of ways. The most common treatments for the cancer types discussed here include chemotherapy, bone marrow transplantation, surgery, radiation therapy, or some combination of treatments, such as chemotherapy and surgery (Ward et al., 2014). Future Cure Obstacles All types of current treatment can result in harmful, painful, or otherwise negative longterm side effects for patients, known as late effects. The heart, lungs, brain, spinal cord, bones, and joints can all be negatively affected by chemotherapy, for example. More specifically, neurocognitive deficits may follow the treatment of ALL; especially high risk is associated with chemotherapy and radiation applied to the central nervous system (Ward et al., 2014). Psychological and cognitive functioning (i.e., mood, thinking, and memory) can also suffer long-term negative effects following cancer treatment. Perhaps the most troubling, another possible late effect of treatment is increased risk of second cancers (National Cancer Institute, 2014c). Development of new treatments should seek to reduce or eliminate such late effects. Research Development and Treatment Costs NIH Spending on Childhood Leukemia Research: 2010: $55 million 2011: $59 million 2012: $77 million 2013: $67 million 2014: $68 million (estimated) NIH Spending on [General] Pediatric Research: 2010: $3.286 billion 2011: $3.277 billion 2012: $3.612 billion 2013: $3.266 billion SGU Cures Index 307 2014: $3.339 billion (estimated) NIH Spending on Cancer Research (All types): 2010: $5.823 billion 2011: $5.448 billion 2012: $5.621 billion 2013: $5.274 billion 2014: $5.418 billion (estimated) (National Institutes of Health, 2014) Child Cancer Cures: Surgery, Radiation, & Chemotherapy Cure Category Achieved, Functional Cure Identification/Description Treatment of childhood cancer varies from patient to patient. Because different cancer types affect different types of tissue, it follows that treatment varies by cancer type, among other factors. The main types of cures used to treat childhood cancer are (1) surgery / bone marrow transplant, (2) radiation, and (3) chemotherapy. It is difficult to describe prevalence, costs, and impact of each type separately, since many patients receive more than one form of treatment. These cures are each described below: 1) Surgery Curative surgery can be performed to remove cancerous tissues with the intention to preserve as much healthy tissue as possible. This type of treatment tends to be delivered only when the cancerous tissue is contained to a particular location. Surgical techniques vary depending on many circumstances, including, for example, location in the body and size or grade of the tumor. Neuroblastoma and WT tend to be treated with surgery, in combination with chemotherapy, radiation therapy, or both (Ward et al., 2014). Other types of childhood cancers such as ependymoma (a malignancy within the spinal cord), retinoblastoma (a malignancy within the eye), Ewing sarcoma (a malignant bone tumor), rhabdomyosarcoma (malignant cells which are precursors to skeletal muscles), and ovarian and testicular germ cell tumors are treated with surgery as well (Ward et al., 2014). SGU Cures Index 308 Bone marrow transplantation (BMT) is a procedure in which bone marrow tissue is harvested from a donor and then delivered by a method similar to intravenous line. BMT tends to be used following radiation therapy or chemotherapy, in order to replace tissue damaged by treatment with new, healthy tissue. This type of treatment is used most typically for children with ALL (Ward et al., 2014). 2) Radiation There are three types of radiation used to kill cancerous cells: X-ray, gamma ray, and charged particles. These radiation types can be delivered externally by photon beams (such as with x-ray or gamma ray), internally, by brachytherapy (radioactive material placed locally to the malignancy) or by systemic radiation (distribution of radioactive substances, such as one bound to an antibody, through the blood) (National Cancer Institute, 2010). The DNA of cancerous cells is damaged by radiation, causing these cells to die. Healthy cells, however, are also affected by radiation damage. In fact, radiation has been identified as a risk factor for later cancers such as leukemia (Ward et al., 2014). Still, it has been used to functionally cure many cancer types, especially Hodgkin’s Lymphoma which can sometimes be treated by radiation alone (Ward et al., 2014). 3) Chemotherapy Chemotherapy is a categorical descriptor for many different drugs used to treat cancer by either stopping the growth of a malignant mass or by killing existing cancerous cells. There are several different methods of delivering these drugs, including intravenous, oral, needle injection, topical cream, or injection locally to the cancer site, as in intrathecal chemotherapy (for some CNS tumors) (Mayo Clinic Staff, 2014). Depending on the method of delivery, treatments can be given as an outpatient procedure in a hospital or doctor’s office or at home, as with oral delivery in the form of a pill. ALL is often treated with several courses of chemotherapy (Ward et al., 2014). Lower doses of chemotherapy are also sometimes combined with radiation therapy in the treatment of HL, neuroblastoma, and some CNS tumors, and it tends to be used as the main treatment for patients with NHL (Ward et al., 2014). Combined treatments tend to be favorable because high-dose treatment using chemotherapy has been linked to infertility, second cancers, and other complications (Ward et al., 2014). Cure History 1) Surgery SGU Cures Index 309 Surgery was used as a treatment for cancer as early as ancient Rome, however in its earliest phases, surgery was not effective enough to cure cancer without relapse. It was even recognized by some that surgery could be more harmful than no treatment at all. While the introduction of anesthesia (1846) was momentous in all surgeries, the first truly pivotal discussion that propelled surgery for cancer treatment into the future was that of metastasis. English surgeon Stephan Paget hypothesized that cancer could spread throughout the body by way of the bloodstream, but could only live and grow in certain organs. Not only did this understanding help guide surgery approaches and techniques then, it continues to guide cancer treatment today. Throughout the 20th century, techniques were honed to increase the amount of normal tissue spared. (American Cancer Society, 2012; American Society of Clinical Oncology, 2014e) 2) Radiation Therapy The 1900s ushered in this relatively new form of cancer treatment, as compared to curative surgery. Radiation was almost immediately used as a treatment for cancer, just three years after it was discovered. From its inception through the 1950s, radiation was found capable of effectively curing HL in many patients. In the first half of the 20th century, radiation had been delivered only by use of photon beams. In the 1970s, brachytherapy, or the use of radioactive “seeds,” reemerged—a technique that had been employed more commonly before the use of external beams became widely used. (American Cancer Society, 2012; American Society of Clinical Oncology, 2014d) 3) Chemotherapy The use of drugs to treat cancer stemmed from the investigation of mustard gas during World War II. While studying its toxicity, it was discovered that a related compound— nitrogen mustard, which kills malignant cells by altering their DNA—could be used to treat lymphoma. In 1947 Sidney Farber achieved the first partial remission of ALL at a children’s hospital in Boston with a drug called aminopterin. The first drug approved by the FDA for treatment of cancer was nitrogen mustard in 1949. The severity of side effects was initially so great it necessitated hospitalization for some patients. The search began for new drugs to target the many forms of cancer—new drugs that elicited fewer harsh effects, a search that continues today. (American Cancer Society, 2012; American Society of Clinical Oncology, 2014c) Cure Science: Breakthroughs/Obstacles SGU Cures Index 310 1) Surgery In the 1990s, the emergence of laparoscopic surgery allowed patients to receive less invasive surgery, minimizing pain and recovery time. Newer technology still, including laser and robotic surgeries, continues to improve the preservation of healthy tissue and allow less invasive, more delicate procedures. (American Cancer Society, 2012; American Society of Clinical Oncology, 2014e). 2) Radiation Radiation therapy was used by two main delivery methods from its inception around the turn of the century. It was not until the 1980s, however that a revolutionary new method of radiation therapy was introduced to patients: stereotactic radiosurgery, or “Gamma knife.” With this approach, doctors can leave healthy tissue mostly untouched by using a very precise beam of radiation waves to damage and stop malignant cells from replicating. Another decade saw yet another new adoption of radiation therapy: the integration of imaging and radiation therapy to allow three-dimensionally focused treatment, further increasing the protection of surrounding, healthy tissue. By the end of the 1990s, intensity-modulated radiation therapy (IMRT) had been introduced, which even further decreases damage to surrounding, healthy tissue. (American Cancer Society, 2012; American Society of Clinical Oncology, 2014d) 3) Chemotherapy Not long after its emergence in the 1940s, chemotherapy was found to show increased remission in children with leukemia when delivered as “combination chemotherapy,” a method in which the patient receives multiple drugs for treatment. This breakthrough allows for the specific targeting of each case and minimization of side effects. In 1961, vinblastine and vincristine, also called “microtubule drugs,” were approved by the FDA for the treatment of leukemia, lymphoma, and other cancers. These drugs cause remission by halting division of cancerous cells. In the 1960s and 1970s, specific combination chemotherapy regimens were found to effectively cure as many as 70% of patients with HL. In 1967, researchers found that chemotherapy, used in combination with radiation therapy and intrathecal therapy (a CNS targeted chemotherapy), could cause long-term remission in children with ALL. Throughout the 1970s and 80s, researchers discovered new drugs for the treatment of adult cancers and childhood osteosarcoma. The next breakthrough occurred in the late 1980s, when cure rates for ALL significantly increased with the use of improved combination chemotherapy regimens. In 1998, a combination chemotherapy regimen was approved for safe use in SGU Cures Index 311 children with HL, reducing side effects, while maintaining positive outcomes of treatment. In 2007, an international trial found that reducing harsh chemotherapy treatment of neuroblastoma minimizes late effects without affecting survival outcome. Additional breakthroughs include new ways to reduce the harmful side effects of chemotherapy in children such as the use of stem cell transplantation following chemotherapy in order to restore and repair the immune system and production of blood in patients, both of which may be impaired by chemotherapy. (American Cancer Society, 2012; American Society of Clinical Oncology, 2014c) Cure Science: Future Obstacles and Targets 1) Surgery Late effects (i.e., long-term effects) associated with surgery often result from treatment of CNS tumors. Because all surgical treatment runs the risk of damaging healthy tissue, the functioning of the brain can be disrupted by a surgical procedure. Depending on the area of the brain affected, late effects can include headaches, seizures, impaired coordination and balance, hydrocephalus, nerve damage, impaired motor control, or even stroke (National Cancer Institute, 2014c). Cognitive and behavioral function can also be affected by surgery of the brain. These effects can range from change in behavior or memory to decreased academic achievement (American Cancer Society, 2014b). Future targets should include further minimizing damage to surrounding tissue in order to best preserve physical and cognitive functioning. 2) Radiation Therapy Future obstacles of radiation therapy will also include the reduction of late effects. Perhaps the most troubling late effect of radiation is the incidence of a second cancer (Ward et al., 2014). High doses of radiation therapy have been directly linked with development of CNS tumors (Ward et al., 2014). Reduced academic achievement, associated with decreased cognitive functioning, is a risk of radiation therapy in the treatment of children with ALL or CNS tumors (Robison et al., 2005). Heart-related health may suffer as a result of radiation therapy delivered to the chest (National Cancer Institute, 2014c; Ward et al., 2014). The risk of developing breast cancer has been estimated as high as 10% following treatment of HL with radiation therapy (Ward et al., 2014). Stroke has also been identified as a strongly-associated risk of radiation therapy, especially for children treated for HL (Bowers et al., 2005). In addition to reducing late effects, future treatment should seek to increase likelihood of survival. It is difficult to determine the unique effectiveness of individual radiation treatments, since many SGU Cures Index 312 patients are treated with combination therapy, however overall 5-year survival of childhood cancer is only about 80% (Ward et al., 2014). 3) Chemotherapy Chemotherapy, too, has been linked to cognitive deficits, as well as second malignancies, cardiac toxicity, and even infertility (Ward et al., 2014). In addition to physical health-related late effects, survivors of childhood leukemia who were treated with chemotherapy were found to be as much as 1.7 times more likely to develop depressive symptoms in young adulthood than their siblings ( Zebrack, Zeltzer, Whitton, Mertens, Odom, Berkow, & Robison, 2002). Intrathecal chemotherapy has been linked to CNS-related symptoms similar to those resulting from brain surgery, described above (National Cancer Institute, 2014c). Because chemotherapy late effects tend to be serious, reducing these harsh outcomes should be central in the development of new chemotherapy treatments. Number of Patients Treated Currently The number of children being treated currently is tied to prevalence, 113,782 children diagnosed each year (Ward et al., 2014). Not all children, however, receive optimal care due to the financial burden of treatment (American Cancer Society, 2014a). Number of Patients Requiring Treatment Virtually all children with cancer in the US are eventually diagnosed, so there is almost no contribution to the number of patients requiring, but not receiving, treatment. The financial burden of treatment can result in a later diagnosis, thereby eliminating some treatment options that might be optimal with early diagnosis. An estimated 10% of children are uninsured, but there is no definitive information on how many children who are diagnosed with cancer do not receive treatment as a result of the financial burden (American Cancer Society, 2014a). Instead, families who lack insurance coverage or the financial resources to pay for treatment out of pocket tend to find alternative means of acquiring funds for treatment. For example, through non-profit organizations, fundraisers, loans or gifts from friends or family, savings or retirement funds, credit cards, or by declaring bankruptcy (American Childhood Cancer Organization, 2013). There is a relative dearth of information regarding the number of children with cancer who do not receive the best practice of care in the US, making this a potentially interesting area for further examination. SGU Cures Index 313 Average Annual Total Cost of Treatment The total cost associated with cancer care was an estimated $124.57 billion in 2010 (Mariotto, Yabroff, Shao, Feuer, & Brown, 2010). Since approximately 1% of cases are under 20 years of age, the average annual total cost of child cancer treatment can be estimated at $1.25 billion (National Cancer Institute, 2014e). Average Lifetime Cost of Treatment Per Patient Because treatment varies so greatly from patient to patient, there is a wide range in lifetime cost of treatment per patient. Landrigan, Schechter, Lipton, Fahs, and Schwartz (2002) estimate that, including physician services, inpatient services, outpatient services, and laboratory services, the total mean cost of treating childhood cancer is $509,000 per case, in 1998 dollars (Landrigan et al., 2002). Economic impact – Value of Life Added 61 years of life added by the treatment(s) x 9,784 patients in any given year gives a total VMRR of $62.18 billion per year (Howden & Meyer, 2011; National Cancer Institute, 2011, 2014d; Yeh et al., 2010). Economic Impact—Value of Reduction in DALYs/Increase in QALYs Because if childhood cancer is left untreated it is ultimately terminal, treatment offers added life years but has little relevance to DALYs. Citations American Cancer Society. (2012). The History of Cancer. Retrieved March 24, 2014, from: http://www.cancer.org/cancer/cancerbasics/thehistoryofcancer/index?sitearea American Cancer Society. (2014a). Cancer Facts & Figures. Atlanta. Retrieved March 28, 2014, from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/inde x American Cancer Society. (2014b). Children Diagnosed With Cancer: Late Effects of Cancer Treatment. Retrieved May 18, 2014, from: SGU Cures Index 314 http://www.cancer.org/treatment/childrenandcancer/whenyourchildhascancer/chi ldren-diagnosed-with-cancer-late-effects-of-cancer-treatment American Cancer Society. (2014c). How are brain and spinal cord tumors in adults diagnosed? Retrieved April 03, 2014, from: http://www.cancer.org/cancer/braincnstumorsinadults/detailedguide/brain-andspinal-cord-tumors-in-adults-diagnosed American Cancer Society. (2014d). How are brain and spinal cord tumors in children diagnosed? Retrieved March 22, 2014, from: http://www.cancer.org/cancer/braincnstumorsinchildren/detailedguide/brainand-spinal-cord-tumors-in-children-diagnosis American Cancer Society. (2014e). How are brain and spinal cord tumors in children staged? Retrieved March 29, 2014, from: http://www.cancer.org/cancer/braincnstumorsinchildren/detailedguide/brainand-spinal-cord-tumors-in-children-staging American Cancer Society. (2014f). What are the differences between cancers in adults and children? Retrieved April 16, 2014, from: http://www.cancer.org/cancer/cancerinchildren/detailedguide/cancer-in-childrendifferences-adults-children American Childhood Cancer Organization. (2013). Financial Impact of Childhood Cancer. Retrieved April 28, 2014, from: https://www.acco.org/AwarenessAdvocacy/Awareness/FinancialImpact.aspx American Society of Clinical Oncology. (2013). Central Nervous System-Childhood. Retrieved March 21, 2014, from: http://www.cancer.net/cancer-types/centralnervous-system-childhood American Society of Clinical Oncology. (2014a). Major Cancer Milestones. CancerProgress.net. Retrieved March 25, 2014, from: http://www.cancerprogress.net/timeline/major-milestones-against-cancer American Society of Clinical Oncology. (2014b). Progress Against Pediatric Cancer. CancerProgress.net. Retrieved April 19, 2014, from: http://cancerprogress.net/timeline/pediatric-cancer SGU Cures Index 315 American Society of Clinical Oncology. (2014c). Progress in Cancer Chemotherapy. CancerProgress.net. Retrieved March 29, 2014, from: http://www.cancerprogress.net/timeline/chemotherapy American Society of Clinical Oncology. (2014d). Progress in Cancer Radiation. CancerProgress.net. Retrieved April 16, 2014, from: http://cancerprogress.net/timeline/radiation-therapy American Society of Clinical Oncology. (2014e). Progress in Cancer Surgery. CancerProgress.net. Retrieved April 27, 2014, from: http://cancerprogress.net/timeline/surgery Bowers, D. C., McNeil, D. E., Liu, Y., Yasui, Y., Stovall, M., Gurney, J. G., et al. (2005). Stroke as a late treatment effect of Hodgkin’s Disease: a report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 23(27), 6508–15. doi:10.1200/JCO.2005.15.107 Chen, Y., Zieve, D., & Black, B. (2013). Neuroblastoma. US National Library of Medicine. Retrieved March 24, 2014, from: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002381/ Chen, Y., Zieve, D., Blackman, B., Slon, S., & Wang, N. (2014). Acute Lymphocytic Leukemia (ALL). MedlinePlus Medical Encyclopedia. Retrieved March 14, 2014, from: http://www.nlm.nih.gov/medlineplus/ency/article/000541.htm Chen, Y., Zieve, D., Eltz, D., Slon, S., & Wang, N. (2012). Brain Tumor-Children. MedlinePlus Medical Encyclopedia. Retrieved March 22, 2014, from: http://www.nlm.nih.gov/medlineplus/ency/article/000768.htm Children’s Cancer Research Fund. (2014). Acute Lymphoblastic Leukemia (ALL). Retrieved March 28, 2014, from: http://www.childrenscancer.org/main/acute_lymphoblastic_leukemia_all/ Howden, L. M., & Meyer, J. A. (2011). Age and Sex Composition: 2010, 2010 Census Briefs. Washington, DC. Retrieved from: http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf SGU Cures Index 316 Kaneshiro, N., & Zieve, D. (2012). Wilms Tumor. MedlinePlus Medical Encyclopedia. Retrieved March 26, 2014, from: http://www.nlm.nih.gov/medlineplus/ency/article/001575.htm Linet, M. S., Wacholder, S., & Zahm, S. H. (2003). Interpreting Epidemiologic Research: Lessons From Studies of Childhood Cancer. Pediatrics, 112(1), 218–232. Retrieved from: http://pediatrics.aappublications.org/content/112/Supplement_1/218.long Mariotto, A. B., Yabroff, K. R., Shao, Y., Feuer, E. J., & Brown, M. L. (2010). Projections of the cost of cancer care in the United States: 2010-2020. Journal of the National Cancer Institute, 103(2), 117–28. doi:10.1093/jnci/djq495 Mayo Clinic Staff. (2014). Tests and Procedures: Chemotherapy. Retrieved May 08, 2014, from: http://www.mayoclinic.org/testsprocedures/chemotherapy/basics/definition/PRC-20023578?p=1 National Cancer Insitute. (2014). Childhood Acute Lymphoblastic Leukemia Treatment (PDQ®). Retrieved April 19, 2014, from: http://www.cancer.gov/cancertopics/pdq/treatment/childALL/Patient/page1 National Cancer Institute. (2010). Radiation Therapy for Cancer. Retrieved April 14, 2014, from: http://www.cancer.gov/cancertopics/factsheet/Therapy/radiation National Cancer Institute. (2011). SEER Cancer Statistics Review 1975-2008. Retrieved April 18, 2014, from: http://seer.cancer.gov/archive/csr/1975_2008/results_merged/topic_year_lost.pd f National Cancer Institute. (2012). Cancer Trends Progress Report-2011/2012 Update. Progress Report. Retrieved March 30, 2014, from: http://progressreport.cancer.gov/doc_detail.asp?pid=1&did=2009&chid=96&coi d=930 National Cancer Institute. (2014a). Childhood Acute Myeloid Leukemia/Other Myeloid Malignancies Treatment (PDQ®). Retrieved April 20, 2014, from: http://www.cancer.gov/cancertopics/pdq/treatment/childAML/Patient SGU Cures Index 317 National Cancer Institute. (2014b). Childhood Non-Hodgkin Lymphoma Treatment (PDQ®). Retrieved April 18, 2014, from: http://www.cancer.gov/cancertopics/pdq/treatment/child-non-hodgkins/Patient National Cancer Institute. (2014c). Late Effects of Treatment for Childhood Cancer (PDQ®). Retrieved April 11, 2014, from: http://www.cancer.gov/cancertopics/pdq/treatment/lateeffects/Patient National Cancer Institute. (2014d). SEER Cancer Statistics Review 1975-2010. Retrieved April 04, 2014, from: http://seer.cancer.gov/archive/csr/1975_2010/ National Cancer Institute. (2014e). SEER Stat Fact Sheets: All Cancer Sites. Retrieved March 27, 2014, from: http://seer.cancer.gov/statfacts/html/all.html National Institutes of Health. (2014). Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). Research Portfolio Online Reporting Tools (RePORT). Retrieved April 14, 2014, from: http://report.nih.gov/categorical_spending.aspx Ries, L. A. G., Smith, M. A., Gurney, J. G., Linet, M., Tamra, T., Young Jr., J. L., & Bunin, G. R. (1999). Cancer Incidence and Survival among Children and Adolescents : United States SEER Program 1975-1995. Retrieved from: http://seer.cancer.gov/archive/publications/childhood/index.html Robison, L. L., Ph, D., Green, D. M., Hudson, M., Meadows, A. T., Mertens, A. C., … Zeltzer, L. K. (2005). Long-Term Outcomes of Adult Survivors of Childhood: Results from the Childhood Cancer Survivor Study. Cancer, 104(11), 2557–2564. doi:10.1002/cncr.21249 Ward, E., Desantis, C., Robbins, A., Kohler, B., & Jemal, A. (2014). Childhood and Adolescent Cancer Statistics , 2014. CA: A Cancer Journal for Clinicians, 64(2), 83–103. doi:10.3322/caac.21219. World Health Organization. (2009). Mortality and Burden of Disease Estimates for WHO Member States in 2004 (p. DALY 0–14 2004). Retrieved from: http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/ Yeh, J. M., Nekhlyudov, L., Goldie, S. J., Mertens, A. C., & Diller, L. (2010). A ModelBased Estimate of Cumulative Excess Mortality in Survivors of Childhood Cancer. SGU Cures Index 318 Annals of Internal Medicine, 152(7), 1–16. doi:10.1059/0003-4819-152-7201004060-00005.A Zebrack, B. J., Zeltzer, L. K., Whitton, J., Mertens, A. C., Odom, L., Berkow, R., & Robison, L. L. (2002). Psychological Outcomes in Long-Term Survivors of Childhood Leukemia, Hodgkin’s Disease, and Non-Hodgkin's Lymphoma: A Report From the Childhood Cancer Survivor Study. Pediatrics, 110, 42–52. Retrieved from pediatrics.aappublications.org SGU Cures Index 319 INTRAEPITHELIAL NEOPLASIA (ANUS, CERVIX, OROPHARYNX) Krysta Fritzky, Colleen Angus-Yamada Disease Category Chronic Disease Identification, Description, and Diagnostic Criteria Viruses cause a large proportion of cervical, anal, and oropharyngeal cancers. Cancer (i.e., malignant neoplasm) involves a group of diseases characterized by unregulated cell growth. These cells can invade nearby tissue or spread to other parts of the body through the bloodstream or lymphatic system. Unregulated cell growth occurs as a result of deoxyribonucleic acid (DNA) damage. When DNA is damaged, the cell normally repairs the damage or the cell dies. However, in cancer cells the damaged DNA is not repaired, and the cell doesn’t die. Instead, it continues to replicate the DNA in new abnormal cells. These cells replicate out of control and form a tumor. Most DNA damage results from mistakes during cell replication or environmental factors. One of the first identifications of a virus-cancer link was made by Francis Peyton Rous in 1911 (Moore & Chang, 2010). His experiments had shown that cancer could be transmitted through cell-free tumor extracts, and was therefore caused by a small transmissible agent such as a virus (Moore & Chang, 2010). Worldwide in 2002, an estimated 561,200 new cancer cases (5.2% of all new cancers) were attributable to Human Papilloma Virus (HPV), making it one of the most important infectious causes of cancer (Parkin, 2006). A 2012 study showed increased incidence rates for oropharynx and anus HPVassociated cancers. This recent increase makes cervical, oropharynx, and anal cancers the most common HPV attributed cancers (Jemal et al., 2013). In this report, cancers of the cervix, oropharynx, and anus attributed to HPV will be discussed. Disease Etiology (Causes) Human Papilloma Virus is one of the most common sexually transmitted infections in the world and the most common in the United States (Muñoz et al., 2003). HPV is a DNA virus with over 120 types, 82 of which are carcinogenic, and 15 of which (types 16, SGU Cures Index 320 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82) are considered “high risk” carcinogenic (Muñoz et al., 2003). "High-risk" sexually transmitted HPVs may lead to the development of carcinomas. The majorities of the types do not cause any symptoms and subside undiagnosed, as the host’s immune system is able to clear the virus effectively. However, some types can cause warts, while others lead to cancers of the cervix, vagina, vulva, penis, oropharynx, and anus. Most infections occur in young women and cease to exist within two years (Muñoz et al., 2003). HPV is a DNA virus spread generally through direct contact, commonly via sexual activity. The virus enters the body through small tears or cuts on the outer layer of the skin (Mayo Clinic, 2014). The infection will cause cell abnormalities that may go away on their own, or could potentially progress to cancer. Some types of sexually transmitted human papillomaviruses (HPVs) can cause genital warts. The types that can cause cancer are called “high-risk” or “oncogenic” HPVs (National Cancer Institute, 2012). HPVs infect epithelial cells, the cells that cover the inner and outer surfaces of the body. When an HPV enters the cell it alters the DNA, leading to changes in production of RNA and proteins. These new proteins interfere with normal cell function, progressing to uncontrolled cell replication. These “rogue” cells can be eliminated by the immune system. However, if they are not, formation of a tumor may begin. Smoking, a weak immune system, long-term oral contraceptive use, and poor oral hygiene will increase the risk of developing cancer following an HPV infection (National Cancer Institute, 2012). Some of the HPV "early" genes, E6 and E7, act as oncogenes, keeping damaged cells from apoptosis (cell death) and therefore, building up mutations and promoting tumor growth (Yim & Park, 2005). The E6 protein is believed to promote cell proliferation by degrading the p53 protein, which normally prevents cell growth and stimulates apoptosis in the presence of DNA damage. P53 is a tumor suppressor gene that arrests the cell cycle when there is DNA damage (Yim & Park, 2005). Intraepithelial neoplasia is a precursor to cancer. It is a possibly cancerous abnormal growth of squamous cells in sites including, but not limited to, the anus and the cervix. Intraepithelial neoplasia is not cancer, and is usually curable or eliminated by the host's immune system without intervention. However, a small percentage of cases progress to become cervical cancer. There are multiple theories as to why some HPV infections lead to cervical cancer while the majority of infections do not. Lack of treatment/screening, smoking, a weak immune system, having children, and long-term oral contraception all increase the risk of developing cervical cancer (American Cancer Society, 2013). One theory is that hormonal changes could make the cervix more vulnerable to the effects of HPV (Brake & Lambert, 2005). Cervical intraepithelial neoplasia (CIN) is the abnormal SGU Cures Index 321 growth of squamous cells on the surface of the cervix (Sadler et al., 2004). CIN can lead to cervical cancer, which is the second most common cancer in women worldwide. In the United States, approximately 4,000 deaths a year are attributed to cervical cancer (Centers for Disease Control and Prevention, 2014a). Nearly all cases of cervical cancer are linked to a previous infection of an oncogenic type of HPV (Muñoz et al., 2003; Yim & Park, 2005). Currently, 63% of oropharyngeal cancers are caused by HPV (Centers for Disease Control and Prevention, 2012a). Oropharyngeal cancer attributed to HPV has significantly increased over the past 20 years and has been estimated that by 2020, HPV will cause more oropharyngeal cancers than cervical cancers in the United States (Chaturvedi et al., 2011). Cigarette smoking was significantly associated with acquisition of oral HPV in healthy men. Although the exact biological mechanism for this link has not yet been established, tobacco exposure induces pro-inflammatory and immunosuppressive effects, which might increase the likelihood of HPV infection and persistence, particularly at the oral cavity where tobacco carcinogens have direct contact with the oral epithelium (Kreimer et al., 2013). Disease Prevalence/Incidence HPV is the most common sexually transmitted infection in the United States. The Centers for Disease Control and Prevention (CDC) estimates there are approximately 6 million new cases of HPV each year. HPV is responsible for more than 90% of anal and cervical cancers (Centers for Disease Control and Prevention, 2013a). Of cervical cancer cases, 75% are attributed directly to HPV strains 16/18 (Gardasil, 2013). It is estimated that for every 1 million women infected, 10% (100,000) will develop precancerous changes in their cervical tissue and about 8% of these will develop early cancer (Yim & Park, 2005). Using these figures, 8,000 out of 1 million or .8% of those infected will develop early cervical cancer. 91% of all cervical cancer cases are attributable to HPV infection in the USA (Centers for Disease Control and Prevention, 2013a). The CDC estimates 10,300 cervical cancer cases per year attributed to HPV. In 2010, 11,818 women were diagnosed with cervical cancer, with 3,939 deaths (Centers for Disease Control and Prevention, 2013a). This is equivalent to 7.2 per 100,000 persons overall. 63% of oropharyngeal cancer cases in the United States are attributable to HPV (Centers for Disease Control and Prevention, 2012a). The CDC analyzed NPCR and SGU Cures Index 322 SEER data on cancers diagnosed during 2004-2007 in 50 states and the District of Colombia, and found that oropharyngeal cancer was the second most common (cervical as the most common) with an average of 11,726 cases annually (2,370 among females and 9,356 among males) (Centers for Disease Control and Prevention, 2012a). In 2010, there were approximately 275,193 people living with oral cavity and pharynx cancer in the United States (National Cancer Institute, 2014f). In 2014, it is estimated that there will be 42,440 new cases of oropharyngeal cancer with an estimated mortality rate of 8,390 (National Cancer Institute, 2014f). Death rates for oral cancer are higher among males, with the number of deaths being 2.5 per 100,000 men and women per year based on 2007-2011 deaths (National Cancer Institute, 2014g). 91% of anal cancer rates in the United States are attributable to HPV (Centers for Disease Control and Prevention, 2013a). In 2003, there were 4,000 new cases of anal cancer, 82.8% (or 3,312 cases) of which were attributable to HPV (Hu & Goldie, 2008). Medscape records show that in 2012 an estimated 2,900 women and 1,600 men (4,500 cases total) were diagnosed with HPV attributed anal cancer. The National Cancer Institute estimates that 249,496 women are currently living with cervical cancer and 275,193 people are currently living with oral cavity and pharynx cancer in the United States (National Cancer Institute, 2014f). There are approximately 7,060 new cases of anal cancer and 880 deaths per year (National Cancer Institute, 2014f). The average person lives with the disease for five years. Therefore, there are approximately ([7,060 x 5]-[880x5]) 30,900 people currently living with anal cancer (National Cancer Institute, 2014f). Between cervical, oropharyngeal, and anal cancer, there are about 60,780 new cases per year (National Cancer Institute, 2014g). Estimated Undiagnosed/At-risk More than half of all sexually active persons become infected with HPV at some point in their lives (Centers for Disease Control and Prevention, 2014b). Since most HPV infections cause no symptoms, there is a strong possibility that the infection will resolve itself undiagnosed. One study found that during 2003–2004, 26.8% of women aged 14 to 59 were infected with at least one type of HPV. This was higher than previous estimates and 15.2% were infected with one or more of the high-risk types that can cause cancer (Dunne, Unger, & Sternberg, 2007; Tanner, 2008). SGU Cures Index 323 Disease Impact – Years of Potential Life Lost (YPLL) Cervical Cancer: World Health Organization (WHO) statistics show YPLL to cervical cancer of 69,530 in 2001 in the United States (World Health Organization, 2001). Oropharyngeal Cancer: Word Health Organization statistics show YPLL of 83,795 for oropharyngeal cancer in 2001 in the United States (World Health Organization, 2001). Anal Cancer: Life expectancy in the US is 78.7 (Centers for Disease Control and Prevention, 2014), while for a patient with anal cancer life expectancy is 64 (National Cancer Institute, 2014b) resulting in YLL of 14.7. Multiplying this by the 880 anal cancer deaths in the US in 2013 (National Cancer Institute, 2014b) gives 12,936 total years of potential life lost to anal cancer in 2013. Disease Impact – Disability Adjusted Life Years (DALY) Cervical Cancer: The World Health Organization (2009) estimates that in 2004, 34 DALYS per 100,000 persons were lost due to cervical cancer in the US With a female population of 150.4 million that year, 51,133 DALYs were lost in 2004. A 2012 studied estimated 74 DALYs lost per 100,000 people in 2008 (Soerjomataram et al., 2012). Given a total population of approximately 158.6 million women in the USA at the time, 117,364 DALYs were lost to Cervical Cancer in 2008. Oropharyngeal Cancer: 23 DALYs per 100,000 people were lost for mouth and oropharyngeal cancers in the US in 2009 (World Health Organization, 2009). With a population of 293.7 million in 2004, this equates to 67,540 DALYs lost that year. Anal Cancer: The DALY for anal cancer was calculated by adding YLL to YLD. In 2013, the standard life expectancy in years was 78.7, while for a patient with anal cancer life expectancy was 64 (National Cancer Institute, 2014) resulting in YLL of 14.7. In 2013, there was an average duration from diagnosis to death of 4 years and an assumed disability weight of 0.51 (colorectal cancer disability weight) (Essink-Bot, Pereira, Packer, Schwarzinger, & Burstrom, 2002). Thus, YLD (4 x 0.51) = 2.04. YLL (14.7) + YLD (2.04) = 16.74 DALYs lost to anal cancer per 100,000 people in the US in 2013. With a population of 316.1 million in the US in 2013, that equates to 52,915 DALYs lost that year to anal cancer. Economic Impact – YPLL SGU Cures Index 324 69,530 years of potential life were lost to cervical cancer, 83,795 to oropharyngeal cancer, and 12,936 to anal cancer in one year (World Health Organization, 2001). The total lives lost (166,261) are associated with an annual VMRR of $17.323 billion. This represents the potential value to be gained from further cure advancements for these three cancers. Economic Impact – DALYs/QALYs 51,133 DALYs were lost to cervical cancer in 2004 (World Heath Organization, 2009), while another study estimated 117,364 DALYs lost to cervical cancer in 2008 (Soerjomataram et al., 2012). 67,540 DALYs were lost to oropharyngeal cancer in 2004 (World Heath Organization, 2009). 52,915 DALYs were lost to anal cancer in 2013 (see calculation in the Disease Impact – Disability Adjusted Life Years section above). The total DALYs (171,588) are associated with an annual VMRR of $17.878 billion. This represents the potential value to be gained from further cure advancements for these three cancers. History of the Disease and Breakthroughs Hippocrates believed cervical cancer to be incurable in 400 BCE (Gasparini & Panatto, 2009). In 1925, Hinselmann invented the colposcope, a magnifying instrument used to detect abnormalities in the cervix. Papanicolaou then developed the Papanicolaou technique (Pap test), a liquid based cytology used to detect abnormal cells in 1928 (Gasparini & Panatto, 2009). Pap tests have significantly reduced the incidence and deaths from cervical cancer (Arbyn et al., 2010). In 1951, the first successful in-vitro cell line, HeLa, was derived from a biopsy of cervical cancer of Henrietta Lacks. The mutant HeLa cell line contains extra DNA in its genome that originated from HPV (Picken & Yang, 1987). In the 1980s, zur Hausen published findings of HPV DNA in cervical cancer and warts (Gasparini & Panatto, 2009). In 1983 and 1984 zur Hausen and colleagues identified HPV16 and HPV18 in cervical cancer (The Shy Virus, 2008). Today, common treatments for cancer include surgery, radiation therapy, and chemotherapy. Surgery, removal of the cancer/tumor and some of the healthy tissue around it, could be followed up with radiation therapy, a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing, and/or chemotherapy, a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. Current Cure Status SGU Cures Index 325 Pap tests have significantly reduced the incidence and deaths from cervical cancer and Pap smear screening every three to five years can reduce cervical cancer incidence by up to 80% (Arbyn et al., 2010). In 2006, the FDA approved the first HPV vaccines: Gardasil and Cervarix. HPV types 16 and 18 are attributed to 70% of cervical cancer cases, and about 85 percent of all anal cancer cases caused by HPV-16. (National Cancer Institute, 2012) This recombinant vaccine contains a protein of the virus, which triggers the body’s immune response to protect itself against the infection. Immunization of those who are not infected will reduced the chance that they will become infected to nearly zero. Over time, HPV vaccines should cause a significant decrease in the incidences and fatalities of cervical cancer. Gardasil is also effective against HPV types 6 and 11, which can cause benign abnormalities of the cervical cells, anogenital warts, or papillomatosis (a disease of the respiratory tract) (Hariri, Dunne, Saraiya, Unger, & Markowitz, 2011). A patient with earlier stages of cervical cancer may be treated by hysterectomy (total or partial removal of the uterus) and, in more severe cases; the lymph nodes are removed as well. Patients treated with surgery who have high-risk features found through examination are given radiation therapy with or without chemotherapy in order to reduce the risk of relapse (National Cancer Institute, 2014). For patients with advanced oropharyngeal cancer, the combination of radiotherapy and chemotherapy appears to be as efficient as surgery (National Cancer Institute, 2014g). Although surgery is a common treatment for all stages of oropharyngeal cancer, some patients may be given chemotherapy or radiation therapy after surgery, to kill any cancer cells that are left. There are two new types of treatment, radiosensitizers and hyperthermia therapy, which are currently in clinical trials. Radiosensitizers are drugs that make tumor cells more sensitive to radiation therapy. The hope is that combining radiation therapy with radiosensitizers will kill more tumor cells while decreasing damage on healthy tissues. Hyperthermia therapy is a treatment in which body tissue is exposed to increased temperature to damage and kill cancer cells or to make cancer cells more sensitive to the effects of radiation and certain anticancer drugs (National Cancer Institute, 2014). Anal Pap smears, similar to those used in cervical cancer screening, have been studied for early detection of anal cancer in high-risk individuals (Chiao, Giordano, Palefsky, Tyring, & El Serag, 2006). Men with abnormal results receive further evaluation with high-resolution anoscopy. Previously, anal cancer was treated with surgery, and in early SGU Cures Index 326 stage disease, is often curative. The difficulty with this surgery has been the necessity of removing the anal sphincter, causing fecal incontinence, and therefore, requiring permanent colostomies. Chemotherapy and radiation treatment are often the preferred treatment today as they reduce the necessity of a debilitating surgery (National Cancer Institute, 2014). This approach improves quality of life after treatment. Because the vaccine is still relatively new, it is difficult to determine its overall effectiveness on the reduction of HPV cases over extended periods of time, and consequently, HPV-related cancer. The duration of protection is currently unknown, but is over 8.4 years for the bivalent vaccine and over 5 years for the quadrivalent vaccine (Poljak, 2012). Cervical, anal, and oropharyngeal cancer trends should show a significant decrease in the years to come as a result of the vaccine. Future Cure Obstacles There are 118 types of HPV not included in the vaccine, 15 of which are considered “high risk” carcinogenic. Although HPV types 16 and 18 contribute the majority (70%) of the cancer cases, the other 30% of cases are due to HPV types that do not have a vaccination. Gardasil and Cervarix only claim to protect against cervical cancer and genital warts, however HPV type 16 has been found in oral and anal cancer cases. For this reason, men should be taking the vaccine to prevent these other forms of cancer as well (Centers for Disease Control and Prevention, 2012). Treatment costs HPV is one of the most common and most expensive infections of the female genital tract (Ault, 2006). HPV-associated health care costs include routine Pap tests, treatment of genital warts, follow-up of cytological abnormalities, and management of malignancies. An observational study of the Kaiser Permanente health plan found that the annual cost of screening and treatment of HPV-related cervical neoplasia was $26.42 per woman (Insinga, Glass, & Rush, 2004). Given a total US adult (age 16+) female population of 127,751,000 in the US in 2012, this equates to a total cost of $3.375 billion. Another study conducted by Insinga, Dashach and Myers (2003) investigated the incidence and costs associated with genital warts. Each episode of genital warts required an average of 3.1 doctor visits, with an average total cost of $436 for all visits. Incidences ranged from 1.7 cases per 1,000 patient-years overall to a peak of 6.2 cases of genital warts per 1,000 patient-years, in women aged 20 to 24 years, at a cost of $1,692 per 1,000 patient-years. Further reported incidence was 5 cases per SGU Cures Index 327 1,000 patient-years in men aged 25 to 29 at a cost of $1,717 per 1,000 patient-years (Insinga et al., 2003). Cervical Cancer: Approximately $1.6 billion is spent annually on cervical cancer treatments in the US (National Cancer Institute, 2013). If 75%, a conservative percentage, of these cases is attributed to HPV, approximately $1.2 billion is spent annually treating HPV related cervical cancer. Mariotto, Yabroff, Shao, Feuer, and Brown (2011) estimated that it cost $1.55 billion to treat cervical cancer cases in 2010. Approximately 63,000 cases are treated per year, which translates to about $25,000 per case. Oropharynx: The Lifetime cost per case for oropharyngeal cancer is $46,800 (Hu & Goldie, 2008). The total lifetime cost for all new cases of oropharyngeal cancer in 2003 was $41.6 million (Hu & Goldie, 2008). From 2004 to 2007, there was a reported annual average of 25,110 cases of HPV-related cancers (Chesson, Ekwueme, Saraiya, Watson, Lowy, & Markowitz, 2012). It has been estimated that the total lifetime cost for all new cases of oropharyngeal cancer will rise to $306 million by the year 2010 (Chesson et al., 2012). Anal: Lifetime cost per case is $27,660 (Hu & Goldie, 2008). The total lifetime cost of all HPV attributed anal cancer cases in 2003 was $92 million (Hu & Goldie, 2008). Research and Development Costs Merck dedicated more than 20 years to Gardasil, and a 1998 report estimated that companies will spend approximately $250 million on the initial research and development of a vaccine (McGee, 2007). The National Institutes of Health (NIH) has allocated the following funds to cervical cancer and cancer overall, which includes the cervical cancer subcategory, over the past several years. The NIH does not include subcategories for spending on oropharyngeal and anal cancer (National Institutes of Health Research, 2014): Cervical Cancer: 2010: $101 million 2011: $119 million 2012: $112 million 2013: $98 million 2014: $101 million (estimated) SGU Cures Index 328 2015: $101 million (estimated) Cancer: 2010: $6,626 million 2011: $5,448 million 2012: $5,621 million 2013: $5,274 million 2014: $5,418 million (estimated) 2015: $5,418 million (estimated) Cervical Cancer Cure: Pap Test Cure Category Achieved, Functional Cure Identification/Description Cervicovaginal cytology, more commonly known as the Papanicolaou smear, or Pap test, is used worldwide as a clinical tool for the early detection of cancer (O’Meara, 2002). The test is performed by opening the vaginal canal with a speculum and collecting a sample of cells from the outer opening of the cervix of the uterus and the endocervix with an endocervical brush (O'Meara, 2002). The sample is then put onto a slide and examined under a microscope to look for abnormalities. The main purpose of the test is to detect abnormal cells that could develop into cancer if left untreated. Unusual findings are often followed up by more sensitive diagnostic procedures, and if necessary, intervention to prevent the progression to cervical cancer (O’Meara, 2002). According to the revised screening guidelines published by the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists (ACOG), and the United States Preventive Services Task Force (USPSTF), women should begin screening approximately 3 years after first sexual activity or by age 21, whichever comes first (Solomon, Breen & McNeel, 2007). In addition, the guidelines recommend that women ages 30 to 65 should have HPV and Pap co-testing every 5 years or a pap test alone every 3 years. Women with certain risk factors may need to have more frequent screening or continue to screen beyond the age of 65 (Solomon et al., 2007). The Pap test has proven to be a model for successful cancer prevention and is largely responsible for the 70% decrease in cervical cancer mortality in the US over the last 50 SGU Cures Index 329 years (Solomon et al., 2007; Diamantis, Magiorkinis, & Androutsos, 2010). Cure History The Pap test was first described by Aurel Babes in a presentation to the Romanian Society of Gynecology in Bucharest in 1927, and was later published in La Presse Médicale in 1928 (Tambouret, 2013). Babes examined cells from 20 women with biopsy-proven cervical cancer. The cells that were diagnostic of carcinoma were identified in 18 out of the 20 samples. Following this observation, Babes emphasized and illustrated diagnostic features of preinvasive and early invasive cancer (Tambouret, 2013). Babes, however, did not further publish on the topic, which is why cervicovaginal cytology does not bear his name (Tambouret, 2013). The Papanicolaou test (pap test) was named after George Papanicolaou. In 1917, he began his study of the reproductive cycle through the examination of vaginal smears. Consequently, Papanicolaou came to the discovery that cancer cells could be recognized cytologically (Tambouret, 2013). In 1928, one year after Babes described the Pap test to the Romanian Society of Gynecology, Papanicolaou independently described the technique at a conference in Battle Creek, Michigan. Papanicolaou’s method, however, was not put to clinical use until 1939, when Papanicolaou collaborated with Herbert Traut, a gynecologic pathologist at Cornell Medical College (Tambouret, 2013). They published the results of their studies in 1941. Once published, the method was rapidly adopted by gynecologic practices around North America (Tambouret, 2013). The Papanicolaou test received instrumental support from Massachusetts General Hospital (MGH) and in 1942, MGH set up the Gynecologic Cytology Laboratory. The American Cancer Society (ACS) sponsored the first National Cytology Conference in 1948 to promote the use of the Papanicolaou test to reduce the incidence of cervical cancer (Tambouret, 2013). Cure Science: Breakthroughs/Obstacles Despite the success of the traditional Pap test, false-negative rates occur at least 20% of the time (O’Meara, 2002). Causes of false negative smears include failure to sample the cervical lesions, small number of abnormal cells, abnormal cells of a very small size, or both, the presence of blood, mucus, or inflammation, and air-drying artifacts (Eltabbakh & Eltabbakh, 1999). SGU Cures Index 330 In response to this problem, the use of liquid-based technologies such as ThinPrep (Cytyc Corp., Boxborough, MA) and AutoCyte (TriPath Imaging, Burlington, NC) have gained popularity largely due to evidence that suggests a reduction in the incident of inadequate smears (O’Meara, 2002). Montz, Farber, Bristow, and Cornelison (2001) found that the false-negative rate dropped from 49% for the conventional Pap test to 27% for the thin layer technology. In these new methods, the cervix is sampled in the same way as the traditional Pap test, but the sampling device is placed in a liquid medium for transport to the laboratory, where the cells are collected by extraction across a filter (ThinPrep) or through layering onto a density reagent (AutoCyte Prep) and plated evenly on a slide for review (O’Meara, 2002). This method has been shown to reduce the number of slides compromised by obscuring blood, mucus, and inflammation, thereby improving specimen adequacy and diagnostic yield (Eltabbakh & Eltabbakh, 1999). In addition, the remaining sample in a liquid medium allows for further testing of the sample for HPV without having to bring the patient back for another examination (O’Meara, 2002). Cure Science: Future Obstacles The Pap test has limitations imposed by the patient, the specimen, the cytotechnologist, and the pathologist (Shingleton, Patrick, Johnston, & Smith, 1995). The Pap test is not diagnostic (Shingleton et al., 1995); it is a cervical cancer screening tool in which abnormal results are followed up by more sensitive diagnostic procedures, such as a colposcopy, before a formal diagnosis is made (O’Meara, 2002; World Health Organization, 2013). A colposcopy is a follow-up test that uses a special microscope to examine the cervix and vagina (World Health Organization, 2013). In addition, false-positive and false-negative results do occur (Shingleton et al., 1995). The false-positive rate for Pap tests, however, is fairly low, occurring only 2.05% of the time (Nanda et al., 2000). In addition, Pap tests are most commonly underused by women who have no source or no regular source of health care, women without health insurance, and women who have immigrated to the United States within the past 10 years (Scarinci et al., 2010). Additional research on subpopulations, such as those defined by geography, socioeconomic status, and insurance availability may help identify groups toward which targeted interventions should be focused (Hiatt, Klabunde, Breen, Swan, & BallardBarbash, 2002). SGU Cures Index 331 Number of Patients being Treated Currently The percent of US women 18 years of age and over who have had a Pap test within the past 3 years is 73.2% (National Center for Health Statistics, 2013). Number of Patients Requiring Treatment Given that 73.5% of women 18 years of age and over have had a Pap test in the past 3 years, 26.5% of adult women are in need of screening. The majority of these women are in specialized groups (i.e., no source or no regular source of health care, women without health insurance, and women who have immigrated to the United States within the past 10 years (Scarinci et al., 2010). More than 60% of cervical cancer cases occur in small regions of underserved, under-screened populations of women (Scarinci et al., 2010). Annual rates in these populations are 1.5 - 4 times higher than the national agestandardized rate of 8.4 per 100,000 and approach the rates of cervical cancer observed in much lower resource settings (Scarinci et al., 2010). Impact of Cure on Years of Potential Life Lost (YPLL) Cervical screening has been a successful model for cancer prevention, contributing significantly to the 70% decrease in cervical cancer mortality in the US over the last 50 years (Solomon et al., 2007). The scientific evidence for the efficacy of the Pap test in reducing the incidence of invasive disease and mortality comes from nonexperimental, observational and case-control studies (Shingleton et al., 1995). Some of the most telling observational studies are the evaluations of cervical cancer mortality rates in five Nordic countries before and after the introduction of screening programs. The study compared the mortality rates before and after the introduction of cytologic screening between 1963-1967 and 1978-1982, and mortality reductions between eight to 73% were observed (Shingleton et al., 1995). In 2003, HPV-associated cancers accounted for 181,026 YPLL, which represented 2.4% of the estimated 7.5 million YPLL attributable to all malignant cancers in the United States (Ekwueme, Chesson, Zhang, & Balamurugan, 2008). The average number of YPLL was 21.8 per HPV-associated cancer death (Ekwueme et al., 2008). The median age of cervical cancer diagnosis is 49 years of age (National Cancer Institute, 2014). The number of US deaths was 2.3 per 100,000 women per year based on 2007-2011 data, with the median age of death at 57 years of age (National Cancer Institute, 2014). In 2014, it is estimated that there will be 12,360 new cases of cervical SGU Cures Index 332 cancer and an estimated 4,020 people will die of the disease (National Cancer Institute, 2014). Impact of Cure on Disability-adjusted Life Years (DALY) A systematic analysis of disability-adjusted life-years (DALY) in 12 world regions was conducted in 2008 to assess the global burden of cancer. For women in North America, cervical cancer accounted for 74 DALYs per 100,000 in the population, compared to 293 DALYs per 100,000 worldwide (Soerjomataram et al., 2012). Cost of the Cure The Pap test costs can range from as inexpensive as $20 to 30 dollars per test (Bettigole, 2013), to as high as $81 per test ($101 in 2012 dollars), including a 10% rescreen rate (Sanders & Taira, 2003). The Pap test is covered by private insurance, Medicare, Medicaid and the ACA (Life Course Indicator, 2014). From 1993 to 2003, there was a steady increase in the number of Pap tests, with an estimated 65.6 million Pap tests performed in the US in 2003. In the most recent surveys from 1998 and 2000, approximately two thirds of women born after 1930 report having been screened in the last year, and approximately 85% within the previous 3 years (Solomon et al., 2007). The overall annual direct medical cost burden of preventing and treating HPVassociated disease was estimated to be $8.0 billion (2010 US dollars). Of this total cost, about $6.6 billion (82.3%) was for routine cervical cancer screening and follow-up, $1.0 billion (12.0%) was for cancer (including $0.4 billion for cervical cancer and $0.3 billion for oropharyngeal cancer), $0.3 billion (3.6%) was for genital warts, and $0.2 billion (2.1%) was for recurrent respiratory papillomatosis (RRP)(Chesson et al., 2012). Economic Impact – Value of Life Added Some of the most telling observational studies regarding the impact of Pap Tests on cervical cancer mortality rates come from five Nordic countries before and after the introduction of screening programs (1963-1967 and 1978-1982). Mortality reductions of 8% to 73% were observed (Shingleton et al., 1995), and Pap smear screening every three to five years can reduce cervical cancer incidence by up to 80% (Arbyn et al., 2010). The percent of US women 18 years of age and over who have had a Pap test within the past 3 years is only 73.2% (National Center for Health Statistics, 2013). In SGU Cures Index 333 2010, 3,939 women died in the US from cervical cancer (Centers for Disease Control and Prevention, 2013) and the median age at death from cervical cancer is 57 years (National Cancer Institute, 2014). If the other 26.8% of women aged 18+ in the US received a Pap test every 3 years, the maximum cost, at $101 per test in 2012 dollars (Sanders & Taira, 2003) would be (121.44 million x 26.8% = 32.546 million x $101) $3.287 billion, or $1.096 billion annually. Taking the current US female life expectancy of 81.2 years for females and subtracting the median age of death from cervical cancer of 57 gives 24.2 years of potential life lost. In line with this, Ekwueme and colleagues (2008) report 21.8 as the average number of YPLL to HPV-associated cancer death. If Pap Tests reduced cervical cancer mortality in the US by just 8% among the 32.546 million women 18+ who do not currently receive screening, we would expect that (3,939 x 8%), 315 fewer women would die from cervical cancer in the US each year. Given that each of those women would have died at 57 years of each, on average, (315 women x 24.2 YPLL), 7,623 years of potential life would be saved annually, giving a VMRR of $794.26 million as a result of further implementation of the Pap Test. If Pap Tests reduced cervical cancer mortality in the US by the upper limit in the Nordic research (73% - Shingleton et al., 1995), we would expect that (3,939 x 73%), 2,875 fewer women would die from cervical cancer in the US each year, resulting in 69,575 years of potential life saved annually, with a VMRR of $7.25 billion. To account for false negative Pap tests and other potential causes of cervical cancer, we estimate a maximum reduction in cervical cancer deaths of 73% despite 100% Pap smear screening of females 18+ in the US. In reality, the reduction in annual cervical cancer deaths is likely to be somewhere between 8% and 73%, which is why we have provided a range of impact in this analysis. HPV-related Cancer Cure: Vaccination Cure Category Achieved, Definitive Cure Identification/Description The HPV vaccines are prepared from empty protein shells called virus-like particles (VLP) produced by recombinant technology (Cutts et al., 2007). The vaccines do not contain any live biological products or DNA, making them non-infectious (Cutts et al., 2007). The quadrivalent HPV vaccine types 6,11,16,18 (GARDASILTM, manufactured by Merck and Co., Inc., Whitehouse Station, NJ) was licensed for use among females aged 9–26 years for the prevention of HPV-type–related cervical cancer, cervical cancer SGU Cures Index 334 precursors, vaginal and vulvar cancer precursors, and anogenital warts in 2006 (Centers for Disease Control and Prevention, 2012). The vaccine is administered intramuscularly as three separate 0.5-mL doses (Centers for Disease Control and Prevention, 2012). The second dose is administered 2 months after the first dose and the third dose 6 months after the first dose. The vaccine is available as a sterile suspension for injection in a single-dose vial or a prefilled syringe (Centers for Disease Control and Prevention, 2012). In the US, public health authorities have recommend that girls and women 11–26 years of age be vaccinated with the HPV vaccine to prevent cervical cancer, precancerous and low-grade lesions, and genital warts caused by HPV types 6, 11, 16, or 18 (Elbasha, Dasbach, & Insinga, 2007). It is recommended that girls should receive the 3 doses of the vaccine by ages 11-12 (Elbasha et al., 2007). The vaccination series can be started as young as 9 years of age (Centers for Disease Control and Prevention, 2007). The vaccine series is also recommended for females aged 13-26 years who have not been previously vaccinated or have not completed the full series. Ideally, the vaccine should be administered before potential exposure to HPV through sexual contact, however females who might have already been exposed to HPV should still be vaccinated. The HPV vaccine is also licensed for use among males aged 11-26 (Centers for Disease Control and Prevention, 2011). The Bivalent HPV (HPV2) vaccine (Cervarix, manufactured by GlaxoSmithKline) was approved by the Food and Drug Administration in October 2009. The vaccine is approved for females 10 through 25 years of age. The HPV2 vaccine is not approved for males (Centers for Disease Control and Prevention, 2012). Both HPV vaccines are highly immunogenic - more than 99% of recipients develop an antibody response to HPV types included in the respective vaccines 1 month after completing the three-dose series. Worldwide, approximately 70% of cervical cancers are caused by HPV types 16 and 18. The vaccine (when all three doses have been administered) has a high efficiency for the prevention of vaccine HPV type HPV 6-, 11-, 16-, and 18-, related persistent infection, vaccine type-related CIN (Cervical intraepithelial neoplasia), CIN 2/3, and external genital lesions (genital warts, VAIN). The vaccine will not, however, eliminate the need for cervical cancer screening, since not all HPV types that cause cervical cancer are included in the vaccine (Centers for Disease Control and Prevention, 2012). Cure History SGU Cures Index 335 The development of the VLP/L1 vaccine was a collaborative process with many contributors (McNeil, 2006). Research was initiated in the mid-1980s and investigators at Georgetown University, the University of Rochester, the University of Queensland in Australia, and the US National Cancer Institute (NCI) all contributed to the development of the vaccine. In 2006, the US Food and Drug Administration (FDA) approved the first preventative, quadrivalent HPV vaccine, known by the trade name Gardasil. In 2007, GlaxoSmithKline filed for approval in the US for a similar, Bivalent vaccine known as Cervarix. In 2009, the vaccine was approved for use in the US (McNeil, 2006). Cure Science: Breakthroughs/Obstacles There are five key discoveries that underlie both the public relations and patent claims of the HPV vaccine. In 1991, Jian Zhou, Ian Frazer, and colleagues at Queensland University in Brisbane found that the expression of the human papillomavirus L1 and L2 proteins together resulted in the formation of small virus-like particles (VLPs) (McNeil, 2006). VLPs are multiprotein structures that mimic the organization and conformation of authentic native viruses but lack the viral genome, yielding safer and cheaper vaccine candidates (Roldão, Mellado, Castilho, & Alves, 2010). In 1992, Shin-Je Chim, Bennet Jenson, and Richard Schlegal, from the Georgetown University in Washington, D.C., reported research on the HPV L1 expression in mammalian cells that were recognized by monoclonal antibodies. In the same year, Reinhard Kirnbauer, Doug Lowy, and John Schiller at NCI and colleagues discovered L1 from bovine papillomavirus type 1 self-assembled into morphologically correct VLPs that induced high levels of neutralizing antibodies in immunized animals (McNeil, 2006). In 1993, Robert Rose reported from the University of Rochester that L1 from HPV 11 self-assembled into VLPs, and were later shown to induce neutralizing antibodies. In the same year, Kirnbaueer, Lowy, and Schiller at NCI and colleagues, found L1 from HPV 16, taken from lesions that had not progressed to cancer, self-assembled more efficiently than the HPV 16 L1 that researchers everywhere at the time had been using. The old strain was shown to be a mutant, possibly because it had been isolated from a cancer (McNeil, 2006). In 2002, a landmark clinical trial was conducted by Koutsky and colleagues, which demonstrated that vaccination with HPV 16 L1 VLPs provided 100% protection from the natural acquisition of persistent HPV 16 infection over an average of 17.4 months SGU Cures Index 336 (Koutsky, Ault, Wheeler, Brown, Barr, & Alvarez, 2002; Monie, Hung, Roden, & Wu, 2008). The high degree of efficacy of the L1 VLP vaccines for protection against HPVassociated infection and disease has also been demonstrated in other clinical studies (Harper et al., 2004; Harper, Franco, & Wheeler, 2006; Monie et al., 2008). Cure Science: Future Obstacles The most important unresolved issues related to the HPV vaccines are the duration of protection, whether booster vaccinations are necessary, and if so, when. The duration of protection is currently unknown but is over 8.4 years for the bivalent vaccine and over 5 years for the quadrivalent vaccine (Poljak, 2012). Another important issue is the clinical significance of cross-protection. Cross protection refers to the efficacy of the vaccine against HPV types not specifically targeted by inclusion of the corresponding virus-like particles (VLPs) in the vaccine. Cross-protection against non-vaccine types is an important concern since non-vaccine HPV types are associated with 30% of cervical cancers worldwide (Lowy & Schiller, 2012). Although none of the phrase III trials were specifically designed to evaluate cross-protection, both vaccines have been evaluated post hoc for protection against infection and cervical disease associated with HPV types phylogenetically related to HPV-16 and HPV18 (Poljak, 2012). While some cross-protection has been demonstrated for both vaccines, there is an unknown duration of clinical efficacy (Cutts et al., 2007). Research on this issue is ongoing (Poljak, 2012). Both vaccines have demonstrated efficacy of over 90% against persistent infection due to genotypes 16 or 18 in women who received the 3 doses of the HPV vaccine (Cutts et al., 2007). In addition, the vaccines (when all 3 doses have been administered) have a high efficiency for the prevention of vaccine HPV type HPV 6-, 11-, related persistent infection, vaccine type-related cervical intraepithelial neoplasia (CIN), grade II CIN (2/3) and external genital lesions such as, genital warts, vaginal intraepithelial neoplasia (VAIN)(Centers for Disease Control and Prevention, 2012). Preliminary research has begun to look at the cross-protection effects of the vaccines. Both vaccines have shown some evidence of cross-protection against HPV 31 and HPV 45 (Cutts et al., 2007). In an extended follow-up of the phase II trails of the bivalent vaccine, a significant reduction was found in the incident infection with HPV type 45 (1 case in 528 vaccinated women and 17 cases in 518 controls (with a vaccine efficiency of 94.2%) and type 31 (14 versus 30 cases (vaccine efficiency of 54.5%) (Harper, Franco, & Wheeler, 2006). SGU Cures Index 337 The vaccine does not eliminate the need for cervical cancer screening, since only HPV types that cause 70% of cervical cancers are included in the vaccine (Lowy & Schiller, 2012; Poljak, 2012). The primary means of cervical cancer protection is the HPV vaccination, followed by the secondary method of cervical screening. Women who have an ambiguous or abnormal Pap test could be infected with any of more than 40 highrisk or low-risk genital HPV types. Women younger than 27 years with a previously abnormal Pap test may be vaccinated. However, women should be advised that current data does not indicate the vaccine will have any therapeutic effect on existing HPV infection or cervical lesions (Centers for Disease Control and Prevention, 2012). Number of Patients being Treated Currently Prevalence of HPV has decreased significantly since the vaccine was made available; falling from 11.5% to 5.1% among girls aged 14 to 19 between 2003-2006 and 20072010. In 2007-2010, approximately one-third of this group had received at least onedose of the vaccine (Markowitz et al., 2013). In 2013, just over one-third (37.6%) of US adolescent girls aged 13-17 received all 3 doses of the HPV vaccine (Elam-Evans et al., 2014). Given that there were about 10 million girls between 13-17 years of age in the US in 2012 (US Census Bureau, 2013), approximately 3.76 million received all 3 doses of the vaccine in 2013. The vaccination rates for adolescents in 2013 are only slightly higher than in 2012 (Elam-Evans et al., 2014). Among females, one vaccine dose (out of the full three vaccine doses), two and three set vaccine dose coverage was higher among Hispanic compared with Caucasian adolescents. African American females had lower HPV 3-dose series completed compared with Caucasian adolescent females. In addition, vaccination coverage did not vary by poverty level for HPV 3-dose series completion (for males or females). However, those living below the poverty level had higher dose coverage for both females and males compared with their counterparts living at or above the poverty level (Elam-Evans et al., 2014). Vaccination rates among boys and young men are much lower than for girls (14% in 2013), but have been increasing since the recommendation was issued in 2011. The vaccination rate for males ages 13 to 17 was 7% in 2012 and 1.3% in 2011 (Elam-Evans et al., 2014). Among males, one vaccine dose (out of the full three vaccine doses), two and three set vaccine dose coverage was higher among African American and Hispanic SGU Cures Index 338 adolescents compared with Caucasian adolescents. There were no statistically significant racial/ethnic differences among males for HPV 3-dose series completion (Elam-Evans et al., 2014). Male vaccination provides not only direct protection against HPV-related diseases (genital warts, anal, penile, oropharyngeal cancers) but also has a significant indirect effect by providing immunity benefits for their female (or male) partners (Poljak, 2012). Number of Patients Requiring Treatment Given that just 37.6% of US adolescent girls aged 13-17 received all 3 doses of the vaccine in 2013, 62.4%, or approximately 6.24 million girls, still required between 1-3 doses of the vaccine that year. Vaccine uptake has been slow due to several factors. Reasons cited by parents for not vaccinating or planning to vaccinate their adolescent children include: lack of knowledge about the vaccine, belief the vaccine is not necessary, concerns about the safety of the vaccine, side effects, it was not recommended to them, and their adolescent is not sexually active (Zimet, Rosberger, Fisher, Perez, & Stupiansky, 2013). Through a combination of initiatives such as increased public awareness, campaigns by professional and advocacy organizations, peer to peer education for physicians, and general health initiatives, HPV vaccination rates can be significantly increased. These initiatives were implemented in four states between 2012 and 2013 – IL, MS, NM, and SC- and led to significant increases in vaccination rates as a result (Centers for Disease Control and Prevention, 2014). Impact of Cure on Years of Potential Life Lost (YPLL) According to the Centers for Disease Control and Prevention (2013), 50,000 girls will develop cervical cancer over their lifetime that would have been prevented had the US reached the 80% vaccination rate goal by now (currently, only 37.6% have received the vaccine). For every year the US delays in achieving this goal, another 4,400 girls will develop cervical cancer in their lifetimes (Centers for Disease Control and Prevention, 2013). The number of new cases of cervical cancer was 7.8 per 100,000 women per year, while the number of deaths was 2.3 per 100,000 women per year. These rates are ageadjusted and based on 2007-2011 cases and deaths (National Cancer Institute, 2014). The 5-year survival rate for cervical cancer is 67.9%. The median age at diagnosis is 49 SGU Cures Index 339 years. Lastly, the percent of cervical cancer deaths is highest among women aged 4554, with a median age of death at 57 years (National Cancer Institute, 2014). Impact of Cure on Disability-adjusted Life Years (DALYs/QALYs) One study assumed 100% vaccine coverage, 90% vaccine efficacy against HPV16/18, and a cost of $377 per vaccination. An estimated 58% reduction was achieved in the lifetime risk for cervical cancer for the vaccinated cohort at a cost of $24,300 (2002 dollars) per QALY compared with no vaccination (Goldie et al., 2004). The cost per QALY gained by routine vaccination of females age 12 years in the published studies ranged from $3,000 to $24,300 (Elbasha, 2007; Garnett, 2006; Goldie et al., 2004; Kulasingam, 2003; Sanders, 2003; Taira, 2004). Under baseline assumptions, the cost-effectiveness ratio for extending a temporary catch-up program for girls to 18 years of age was $97,300 per QALY; the cost of extending vaccination of girls and women to the age of 21 years was $120,400 per QALY, and the cost for extension to the age of 26 years was $152,700 per QALY. The results were sensitive to the duration of vaccine-induced immunity; if immunity waned after 10 years, the cost of vaccination of preadolescent girls exceeded $140,000 per QALY, and catch-up strategies were less cost-effective than screening alone (Kim & Goldie, 2008). Cost of the Cure As of July 2012, the retail price of either vaccine is about $130 per dose ($390 for full series) (Centers for Disease Control and Prevention, 2012). Updated incidence and cost estimates from the literature found that the overall annual direct medical cost burden of preventing and treating HPV-associated disease was estimated to be $8.0 billion (2010 US dollars). Of this total cost, about $6.6 billion (82.3%) was for routine cervical cancer screening and follow-up (Chesson et al., 2012). The approximate cost to administer the vaccine to the 37.6% of girls who received all three doses in 2013 was $1.466 billion. The approximate cost to administer 3 doses of the vaccine to all 10 million girls aged 13-17 in 2013 would have been $3.9 billion. In the fiscal year of 2009 and 2010, the NCI supported $14.3 million in cervical cancer research using funding from the American Recovery and Reinvestment Act. In the fiscal year of 2013, NCI invested $63.4 million in cervical cancer research (National Cancer Institute, 2014). SGU Cures Index 340 Economic Impact – Value of Life Added According to the Centers for Disease Control and Prevention (2013), 50,000 girls will develop cervical cancer over their lifetime that would have been prevented had the US reached the 80% vaccination rate goal by now. In 2010, 11,818 women were diagnosed with cervical cancer, with 3,939 deaths – a 33.3% rate of all diagnoses (Centers for Disease Control and Prevention, 2013a). The median age at death from cervical cancer is 57 years (National Cancer Institute, 2014). Taking the current US female life expectancy of 81.2 years for females (Centers for Disease Control and Prevention, 2014c) and subtracting the median age of death from cervical cancer of 57 gives 24.2 years of potential life lost. In line with this, Ekwueme and colleagues (2008) report 21.8 as the average number of YPLL to HPV-associated cancer death. If vaccination could prevent 50,000 girls from developing cervical cancer, and 33.3% of those girls (16,500) would die at a median age of 57 years, (16,500 x 24.2 YPLL) 399,300 years of potential life would be saved by attaining an 80% vaccination rate. Those 399,300 years of potential life have a total VMRR of $41.604 billion. Citations American Cancer Society (2013). What are the risk factors for cervical cancer? Retrieved March 19, 2014 from: http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-riskfactors. American Cancer Society (2014). Oral Cavity and Oropharyngeal Cancer. Retrieved March 19, 2014 from: http://www.cancer.org/acs/groups/cid/documents/webcontent/003128-pdf.pdf. Arbyn M., Anttila, A., Jordan, J., Ronco, G., Schenck, U., Segnan, N., et al. (2010). European guidelines for quality assurance in cervical cancer screening, second edition—summary document. Annals of Oncology, 21 (3), 448–458. Ault, K. A. (2006). Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infectious Diseases in Obstetrics and Gynecology, 2006 Suppl, 40470-6. doi:10.1155/IDOG/2006/40470 Brake, T., & Lambert, P. F. (2005). Estrogen contributes to the onset, persistence, and SGU Cures Index 341 malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proceedings of the National Academy of Sciences USA, 102 (7), 2490-2495. Centers for Disease Control and Prevention. (2007). Quadrivalent Human Papillomavirus vaccine, recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report, 56, RR-2. Centers for Disease Control and Prevention. (2008). Chronic Disease Prevention: preventing chronic diseases: investing wisely in health. Screening to prevent cancer deaths. US department of health and human services. Retrieved October 1, 2014 from: http://www.cdc.gov/nccdphp/publications/factsheets/prevention/pdf/cancer.pdf Centers for Disease Control and Prevention. (2010). Pap tests and Cervical Cancer. Retrieved October 1, 2014 from: http://www.cdc.gov/nchs/fastats/pap-tests.htm Centers for Disease Control and Prevention. (2011a). Gynecological cancers. Retrieved March 19, 2014 from: http://www.cdc.gov/cancer/cervical/statistics/. Centers for Disease Control and Prevention. (2011b). Morbidity and Mortality Weekly Report. Quadrivalent Human Papillomavirus Vaccine, Recommendations of the Advisory Committee on Immunization Practices (ACIP). March 23, 2007/ Vol. 56/ RR-2. Centers for Disease Control and Prevention (2012a). HPV-Associated Oropharyngeal Cancer Rates by Race and Ethnicity. Retrieved March 5, 2014 from: http://www.cdc.gov/cancer/hpv/statistics/headneck.htm. Centers for Disease Control and Prevention. (2012b). HPV Vaccine Information For Young Women - Fact Sheet. Retrieved October 10, 2014 from: http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm Centers for Disease Control and Prevention. (2012c). Human Papillomavirus–Associated Cancers — United States, 2004–2008. Morbidity and Mortality Weekly Report, 61(15), 258-261. Centers for Disease Control and Prevention. (2012d). Vaccines and Immunizations. Human Papillomavirus. Epidemiology and Prevention of Vaccine-Preventable SGU Cures Index 342 Diseases. The Pink Book: Course Textbook - 12th Edition Second Printing (May 2012). Retrieved from http://www.cdc.gov/vaccines/pubs/pinkbook/hpv.html Centers for Disease Control and Prevention. (2012e). Vaginal and Vulvar Cancer. Retrieved October 22, 2014 from: http://www.cdc.gov/cancer/vagvulv/pdf/vagvulv_facts.pdf Centers for Disease Control and Prevention (2013). HPV-Associated Cancer Statistics. Retrieved March 5, 2014 from: http://www.cdc.gov/cancer/hpv/statistics/. Centers for Disease Control and Prevention. (2013b). Press Briefing Transcript: New study shows HPV vaccine helping lower HPV infection rates in teen girls. Retrieved October 9, 2014 from: http://www.cdc.gov/media/releases/2013/p0619-hpv-vaccinations.html Centers for Disease Control and Prevention (2014a). Cervical Cancer Statistics, Gynecological Cancers. Retrieved March 19, 2014 from: http://www.cdc.gov/cancer/cervical/statistics/. Centers for Disease Control and Prevention (2014b). Genital HPV Infection – Fact sheet. Retrieved January 15, 2014 from http://www.cdc.gov/std/hpv/stdfact-hpv.htm. Centers for Disease Control and Prevention (2014c). Life Expectancy. Retrieved on May 23, 2014 from: http://www.cdc.gov/nchs/fastats/life-expectancy.htm. Centers for Disease Control and Prevention. (2014d). Press Briefing Transcript: CDC Telebriefing on National Immunization Survey- Teen results and HPV vaccination coverage among adolescents. Retrieved October 9, 2014 from: http://www.cdc.gov/media/releases/2014/t0724-hpv-vaccination.html Chaturvedi, A. K., Engels, E. A., Pfeiffer, R. M., Hernandez, B. Y., Xiao, W., Kim, E., et al. (2011). Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of Clinical Oncology, 29 (32), 4294–4301. Chesson, H., Ekwueme, D., Saraiya, M., Watson, M., Lowy, D., Markowitz, L. (2012). Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine, 30(42), 6016-6019. doi: 10.1016/j.vaccine.2012.07.056 SGU Cures Index 343 Chiao, E. Y., Giordano, T.P., Palefsky, J.M., Tyring, S., & El Serag, H. (2006). Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clinical Infectious Diseases, 43(2), 223–33. Cutts, F. T., Harper, D. M., Markowitz, L., Franceschi, S., Goldie, S., Castellsague, X., et al. (2007). Human papillomavirus and HPV vaccines: A review. Bulletin of the World Health Organization, 85(9), 719-726. doi:10.2471/BLT.06.038414 Diamantis, A., Magiorkinis, E., & Androutsos, G. (2010). What's in a name? evidence that papanicolaou, not babes, deserves credit for the pap test. Diagnostic Cytopathology, 38(7), 473-476. doi:10.1002/dc.21226 Dunne, E. F., Unger, E. R., & Sternberg, M. (2007). Prevalence of HPV infection among females in the United States. Journal of the American Medical Association, 297(8), 813-819. “Figure 1. Prevalence of Low-Risk and High Risk HPV Types Among Females Aged 14 to 59 Years, NHANES 2003-2004”. Ekwueme, D. U., Chesson, H. W., Zhang, K. B., & Balamurugan, A. (2008). Years of potential life lost and productivity costs because of cancer mortality and for specific cancer sites where human papillomavirus may be a risk factor for carcinogenesis-united states, 2003. Cancer, 113(10 Suppl), 2936-2945. doi:10.1002/cncr.23761 Elam-Evans, L. D., Yankey, D., Jeyarajah, J., Singleton, J. A., Curtis, R. C., MacNeil, J., et al., Centers for Disease Control and Prevention (CDC). (2014). National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years--united states, 2013. MMWR. Morbidity and Mortality Weekly Report, 63(29), 625. Elbasha, E., Dasbach, E. J., & Insinga, R. P. (2007). Model for assessing human papillomavirus vaccination. Emerging Infectious Diseases, 13, 29-41. Eltabbakh, G. H., & Eltabbakh, G. D. (1999). Papanicolaou smear: Can we make a good test better? technical and interpretive challenges for the practitioner. Journal of Women's Health & Gender-Based Medicine, 8(4), 469-476. doi:10.1089/jwh.1.1999.8.469 SGU Cures Index 344 Essink-Bot, M-L., Pereira, J., Packer, C., Schwarzinger, M., & Burstrom, K. (2002) Crossnational comparability of burden of disease estimates: the European Disability Weights Project. Bulletin of the World Heath Organization, 80, 644-652. Garnett, G. P., Kim, J. J., French, K., & Goldie, S. J. (2006) Modeling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine, 24, S178-S186. Gasparini, R., & Panatto, D. (2009). Cervical cancer: from Hippocrates through RigoniStern to zur Hausen. Vaccine, 27, A4-A5. Gardasil. (2013). What is HPV? Retrieved January 15, 2014 from: http://www.gardasil.com/. Goldie, S. J., Kohli, M., Grima, D., Weinstein, M. C., Wright, T, C., Bosch, F. X., et al. (2004). Projected clinical benefits and cost effectiveness of a human papillomavirus 16/18 vaccine. Journal of the National Cancer Institute, 96, 604615. Hariri, S., Dunne, E., Saraiya, M., Unger, E., & Markowitz, L. (2011). Chapter 5: Human papillomavirus. VPD Surveillance Manual, 5th Edition. Retrieved June 9, 2014 from http://www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.pdf. Harper, D. M., De Carvalho, N. S., Roteli-Martins, C. M., Teixeira, J., Blatter, M. M., Korn, A. P., et al. (2004). Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. The Lancet, 364(9447), 1757-1765. doi:10.1016/S0140-6736(04)17398-4 Harper, D. M., Franco, E. L., & Wheeler, C. M. (2006). Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet (North American Edition) [H.W. Wilson - GS], 367, 1247. Hiatt, R. A., Klabunde, C., Breen, N., Swan, J., & Ballard-Barbash, R. (2002). Cancer screening practices from national health interview surveys: Past, present, and future. Journal of the National Cancer Institute, 94(24), 1837-1846. doi:10.1093/jnci/94.24.1837 SGU Cures Index 345 HPV – the Shy Virus. (2008) Soundprint.org radio program. Retrieved November 25, from: http://soundprint.org/radio/display_show/ID/774/name/HPV++the+Shy+Virus Hu, D., & Goldie, S. J. (2008). The economic burden of noncervical human papillomavirus in the United States. American Journal of Obstetrics and Gynecology, 198 (5), 500.e1-500.e7. Insinga, R. P., Dasbach, E. J., & Myers, E. R. (2003). The health and economic burden of genital warts in a set of private health plans in the United States. Clinical Infectious Diseases, 36 (11), 1397–1403. Insinga, R. P., Glass, A. G., & Rush, B. B. (2004). The health care costs of cervical human papillomavirus–related disease. American Journal of Obstetrics and Gynecology, 191(1), 114-120. doi:10.1016/j.ajog.2004.01.042 Jemal, A., Simard, E. P., Dorell, C., Noone, A., Markowitz, L., Kohler, B., et al. (2013). Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. Journal of the National Cancer Institute, 1-27. doi: 10.1093/jnci/djs491 Kim, J. J., & Goldie, S. J. (2008). Health and economic implications of HPV vaccination in the united states. The New England Journal of Medicine, 359(8), 821-832. doi:10.1056/NEJMsa0707052 Koutsky, L. A., Ault, K. A., Wheeler, C. M., Brown, D. R., Barr, E., Alvarez, F. B., et al. (2002). A controlled trial of a human papillomavirus type 16 vaccine. The New England Journal of Medicine, 347(21), 1645-1651. doi:10.1056/NEJMoa020586 Kreimer, A. R., Campbell, C. M. P., Lin, H.-Y., Fulp, W., Papenfuss, M., Abrahamsen, M., et al. (2013). Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. The Lancet, 382 (9895), 7-13. Kulasingam, S. L., & Myers, E. R. (2003). Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. Journal of the American Medical Association, 290, 780-789. SGU Cures Index 346 Life Course Indicator. (2014). Cervical Cancer Screening (LC-34). Retrieved October 3, 2014 from: http://www.amchp.org/programsandtopics/dataassessment/LifeCourseIndicatorDocuments/LC34_Cervical%20Cancer%20Screening_Final_9-15-2014.pdf Lowy, D. R., & Schiller, J. T. (2012). Reducing HPV-associated cancer globally. Cancer Prevention Research, 5(1), 18-23. doi:10.1158/1940-6207.CAPR-11-0542 Mariotto, A. B., Yabroff, K. R., Shao, Y. V., Feuer, E. J., & Brown, M. L., (2011). Projections of the cost of cancer care in the United States: 2010-2020. Journal of the National Cancer Institute, 103(2), 117-128. Markowitz, L. E., Hariri, S., Lin, C., Dunne, E. F., Steinau, M., McQuillan, G., et al. (2013). Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the united states, national health and nutrition examination surveys, 2003-2010. The Journal of Infectious Diseases, 208(3), 385. Mayo Clinic. (2014). HPV Infection. Mayo Clinic. Retrieved March 12, 2014 from http://www.mayoclinic.org/diseases-conditions/hpvinfection/basics/definition/con-20030343. Mcgee, G. (2007). How much should Gardasil cost? The Scientist. Retrieved December 15, 2013 from: http://www.thescientist.com/?articles.view/articleNo/25267/title/How-Much-Should-GardasilCost-/. McNeil, C. (2006). Who invented the VLP cervical cancer vaccines? Journal of the National Cancer Institute, 98(7), 433-433. doi:10.1093/jnci/djj144 Monie, A., Hung, C., Roden, R., & Wu, T. (2008). Cervarix: A vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics: Targets & Therapy, 2(1), 97113. Montz, F. J., Farber, F. L., Bristow, R. E., & Cornelison, T. E. R. R. I. (2001). Impact of increasing papanicolaou test sensitivity and compliance: A modeled cost and outcomes analysis. Obstetrics & Gynecology, 97(5), 781-788. doi:10.1016/S00297844(01)01322-9 SGU Cures Index 347 Moore, P. S., & Chang, Y. (2010). Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nature Reviews Cancer, 10, 879-889. Muñoz, N., Bosch, F. X., de Sanjosé, S., Herrero, R., Castellsagé, X., Shah, K. V., et al. (2003). Epidemiologic classification of human papillomavrius types associated with cervical cancer. New England Journal of Medicine, 348, 518-527. Nanda, K., McCrory, D. C., Myers, E. R., Bastian, L. A., Hasselblad, V., Hickey, J. D., et al. (2000). Accuracy of the papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: A systematic review. Annals of Internal Medicine, 132(10), 810. National Center for Health Statistics. (2013). Health, United States, 2013: With Special Feature on Prescription Drugs. Retrieved November 3, 2014 from: http://www.cdc.gov/nchs/data/hus/hus13.pdf#084 National Cancer Institute (2012). HPV and Cancer. Retrieved February 24, 2014 from: http://www.cancer.gov/cancertopics/factsheet/Risk/HPV. National Cancer Institute (2014). A Snapshot of Cervical Cancer. Retrieved March 14, 2014 from: http://www.cancer.gov/researchandfunding/snapshots/cervical. National Cancer Institute (2014b). Anal Cancer. Surveillance, Epidemiology, and End Results Program. Retrieved February 24, 2014 from: http://seer.cancer.gov/statfacts/html/anus.html. National Cancer Institute. (2014c). Anal Cancer Treatment (PDQ). Retrieved March 12, 2014 from: http://www.cancer.gov/cancertopics/pdq/treatment/anal/Patient/page4#Keypoin t17 National Cancer Institute (2014d). Cancer Snapshots: Disease Focused and Other Snapshots. Retrieved November 10, 2014 from: http://www.cancer.gov/researchandfunding/snapshots/cervical National Cancer Institute (2014e). Cervix Uteri Cancer. Surveillance, Epidemiology, and End Results Program. Retrieved November 3, 2014 from: http://seer.cancer.gov/statfacts/html/cervix.html SGU Cures Index 348 National Cancer Institute (2014f). Oral Cavity and Pharynx Cancer. Surveillance, Epidemiology, and End Results Program. Retrieved February 24, 2014 from: http://seer.cancer.gov/statfacts/html/oralcav.html. National Cancer Institute (2014g). Oropharyngeal Cancer Treatment (PDQ). Retrieved February 21, 2014 from: http://www.cancer.gov/cancertopics/pdq/treatment/oropharyngeal/Patient/page 1/AllPages/Print. National Institutes of Health Research (2014). Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). Retrieved March 31, 2014, from: http://report.nih.gov/categorical_spending.aspx. O'Meara, A. T. (2002). Present standards for cervical cancer screening. Current Opinion in Oncology, 14(5), 505-511. doi:10.1097/00001622-200209000-00006 Parkin, D. M. (2006). The global health burden of infection-associated cancers in the year 2002. International Journal of Cancer, 118, 3030-3044. Picken, R. N., & Yang, H. L. (1987). The integration of HPV-18 into HeLa cells has involved duplication of the viral genome as well as human DNA flanking sequences. Nucleic Acids Research, 15 (23), 10068. Poljak, M. (2012). Prophylactic human papillomavirus vaccination and primary prevention of cervical cancer: Issues and challenges. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases, 18 Suppl 5, 64-69. doi:10.1111/j.14690691.2012.03946.x Roldão, A., Mellado, M. C. M., Castilho, L. R., Carrondo, M. J., & Alves, P. M. (2010). Virus-like particles in vaccine development. Expert Review of Vaccines, 9(10), 1149-1176. doi:10.1586/erv.10.115 Sadler, L., Saftlas, A., Wang, W., Exeter, M., Whittaker, J., & McCowan, L. (2004). Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA: The Journal of the American Medical Association, 291(17), 2100-2106. doi:10.1001/jama.291.17.2100 Sanders, G. D., & Taira, A. V. (2003). Cost-effectiveness of a potential vaccine for SGU Cures Index 349 human papillomavirus. Emerging Infectious Diseases, 9, 37-48. Scarinci, I. C., Castle, P. E., Garcia, F. A. R., Kobetz, E., Partridge, E. E., Brandt, H. M., et al. (2010). Cervical cancer prevention: New tools and old barriers. Cancer, 116(11), 2531-NA. doi:10.1002/cncr.25065 Shingleton, H. M., Patrick, R. L., Johnston, W. W., & Smith, R. A. (1995). The current status of the papanicolaou smear. CA: A Cancer Journal for Clinicians, 45(5), 305320. doi:10.3322/canjclin.45.5.305 Soerjomataram, I., Lortet-Tieulent, J., Parkin, D. M., Ferlay, J., Mathers, C., Forman, D., et al. (2012). Global burden of cancer in 2008: A systematic analysis of disabilityadjusted life-years in 12 world regions. Lancet, 380(9856), 1840. doi:10.1016/S0140-6736(12)60919-2 Solomon, D., Breen, N., & McNeel, T. (2007). Cervical cancer screening rates in the united states and the potential impact of implementation of screening guidelines. CA: A Cancer Journal for Clinicians, 57(2), 105-111. doi:10.3322/canjclin.57.2.105 Soundprint (2008). HPV – The Shy Virus. Downloaded October 22, 2014 from http://soundprint.org/radio/display_show/ID/774/name/HPV+-+the+Shy+Virus Taira, A. V., Neukermans, C. P., & Sanders, G. D. (2004). Evaluating human papillomavirus vaccination programs. Emerging Infectious Diseases, 10, 1915-23. Tambouret, R. H. (2013). The evolution of the papanicolaou smear. Clinical Obstetrics and Gynecology, 56(1), 3-9. doi:10.1097/GRF.0b013e318282b982 Tanner, L. (2008). One in four girls in study has an std. Wisconsin State Journal, pp. A.1. US Census Bureau (2013). Current Population Survey, Annual Social and Economic Supplement, 2012. Downloaded October 27, 2014 from: https://www.census.gov/hhes/www/poverty/publications/pubs-cps.html Vilos, G. A. (1998). The history of the papanicolaou smear and the odyssey of george and andromache papanicolaou. Obstetrics & Gynecology, 91(3), 479-483. doi:10.1016/S0029-7844(97)00695-9 SGU Cures Index 350 World Health Organization. (2001). Department of Measurement and Health Information: Global Burden of Disease Study. Retrieved on January 23, 2014 from: http://www.who.int/healthinfo/global_burden_disease/en/. World Health Organization. (2001). Global Burden of Disease Study. Department of Measurement and Health Information. Retrieved on January 23, 2013 from: http://www.who.int/healthinfo/global_burden_disease/en/. World Health Organization. (2013). Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. Retrieved November 3, 2014 from: http://apps.who.int/iris/bitstream/10665/94830/1/9789241548694_eng.pdf Xu, F., Salandy, S. W., Jones, C. K., Crawford, C. A., Town, M., Balluz, L. S., et al. (2013). Surveillance for certain health behaviors among states and selected local areas united states, 2010. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C.: 2002), 62(1), 1. Yim, E-K. & Park, J-S. (2005). The role of HPV E6 and E7 oncoproteins in HPVassociated cervical carcinogenesis. Official Journal of Korean Cancer Association, 37 (6), 319-324. Zimet, G. D., Rosberger, Z., Fisher, W. A., Perez, S., & Stupiansky, N. W. (2013). Beliefs, behaviors and HPV vaccine: Correcting the myths and the misinformation. Preventive Medicine, 57(5), 414-418. doi:10.1016/j.ypmed.2013.05.013 SGU Cures Index 351 PANCREATIC CANCER Julia C. Brunet Disease Category Neoplasm (Cancer) Disease Identification, Description, and Diagnostic Criteria Pancreatic cancer is also called exocrine cancer, where cancer cells are found in pancreatic tissue. The pancreas is located in the abdomen, surrounded by the stomach and the liver. Pancreatic cancer can consist of a benign or malignant growth. In a benign growth, the cyst or tumor can be removed and it will not regrow; the tumor or cyst does not metastasize to other tissues or organs surrounding the pancreas, does not spread to different areas in the body, and is not life threating. Malignant growths may be removed but may regrow or metastasize into surrounding tissues and/or organs, may spread throughout different areas of the body, and are life threatening. In these cases, pancreatic cancer spreads as the tumor continues to grow and invade neighboring organs. The cancer can spread via different mechanisms. “Shedding” occurs when the tumor sheds off cancer cells and these cells eventually find their way into the abdomen and throughout different areas of the body, eventually leading to new tumors in other areas of the body. Pancreatic cancer can also spread when some cancer cells “break off” from the initial tumor and spread through the blood vessels to the liver and lungs or to lymph nodes and lymph vessels. This spreading of the cancer cells throughout the body can lead to more cancerous growths in different areas of the body (National Cancer Institute, 2010a). According to the National Cancer Institute (2010a), the stages of pancreatic cancer are: Stage I: The tumor is localized in the pancreas only. Stage II: The tumor has metastasized to surrounding tissues and possibly to nearby lymph nodes. The cancer has not reached neighboring blood vessels. Stage III: The tumor has metastasized to neighboring blood vessels. Stage IV: The cancer is no longer localized to the pancreas and has metastasized to surrounding organ(s). SGU Cures Index 352 The chances of surviving decline as the stage of diagnosis increases. According to the American Cancer Society (2013a), five-year survival rates from initial diagnosis are: Stage I: 12-14% Stage II: 5-7% Stage III: 3% Stage IV: 1% The overall rate of patients surviving for five years following diagnosis is only 6%. For localized non-metastasized pancreatic cancer, the five-year survival rate is 24.1%. But only 8.7% of pancreatic cancer patients are diagnosed at an early stage of the cancer. (Howlader, Noone, Krapcho, Garshell, Neyman, Altekruse, et al., 2013). Pancreatic cancer is so deadly because most patients are diagnosed at an advanced stage. The average (median) survival time after diagnosis of pancreatic cancer at stage IV is 4.6 months; at stage III is 10.6 months; at stage II is 12.7 to 15.4 months; and at stage I is 20.6 to 24.1 months (American Cancer Society, 2013a). The lack of distinct and characteristic symptoms means that patients often do not suspect anything is wrong and clinicians do not pursue pancreatic cancer as a possible diagnosis. It is only in the later stages of the disease where symptoms are persistent enough to be recognized and physicians may consider pancreatic cancer a possible diagnosis (Pancreatic Cancer Action, 2012). According to the American Cancer Society (2013a), any one or combination of the following tests/procedures is used for diagnosing pancreatic cancer: 1. History and physical examination: It is important to know if the individual has a family history of pancreatic cancer, which increases the individual’s risk of developing the cancer. Physical examination is performed, allowing the physician to find any tumors in the lymph nodes and/or liver. The eyes are also examined for any yellow coloration that may indicate jaundice. 2. Diagnostic tests such as the following: • CT scan (computerized tomography) • MRI (magnetic resonance imaging) • PET scan (positron emission tomography) • Ultrasound • ERCP (endoscope retrograde cholanglopancreotography) • Angiography • Blood tests SGU Cures Index 353 • Biopsy, which is conducted once initial examinations show abnormalities within the examined tissue Despite the many high technology options for diagnosing pancreatic cancer, the lack of distinct and characteristic symptoms, wherein patients often do not suspect that anything is wrong, means that the tests are often not conducted, as pancreatic cancer is not suspected. Pancreatic cancer risk increases with increasing age. Over seventy percent of patients diagnosed with pancreatic cancer are diagnosed at age 65 and older (O'Neill, Atoria, O'Reilly, LaFemina, Henamn, & Elkin, 2012). Men are minimally at higher risk than women. Pancreatic cancer rates are highest in individuals from 75 to 84 years of age with the median age of 71. Pancreatic cancer deaths are also highest at this same age range, with the median age of death at 73 years of age (Howlader, et al., 2013). Disease Etiology (Causes) Ductal adenocarcinoma (PDa) is caused by genetic factors and consists of numerous biological factors, including the cancer cell, tumor (Hidalgo, 2012) stoma, and its stem cell. The consecutive mutations and their accumulations are what lead to the development of pancreatic cancer. PDa contains a cancer stem cell compartment that is resistant to chemotherapy and radiation therapy and is responsible for its metastasis and resistance. Completely established pancreatic cancer nearly always conveys one or more of four genetic defects (Hidalgo, 2012). Smoking tobacco, a family history of pancreatic cancer, and having diabetes (Hsu & Saif, 2011) are the most direct and important risk factors for developing pancreatic cancer. Long-term pancreatitis, or the inflammation of the pancreas, and obesity are also significant risk factors for pancreatic cancer (National Cancer Institute, 2010a). Smokers are twice as likely to develop pancreatic cancer versus nonsmokers and over weight individuals have a 20% greater chance of developing pancreatic cancer compared to individuals of normal weight, as measured by BMI (American Cancer Society, 2014). A study conducted by Klein and colleagues (2013) looked at an array of risk factors and found current smokers were at the highest elevated risk to develop pancreatic cancer followed by heavy alcohol users, those with a BMI higher than 30, those diagnosed with diabetes for over 3 years, and a family history of pancreatic cancer. This same study showed a 30-44% increased risk of developing pancreatic cancer among those with a family history of pancreatitis. (Klein, Lindstrom, Mendelsohn, Steplowski, Arslan, Buenode-Mesquita, et al., 2013). This risk rises with people who smoke and have a family SGU Cures Index 354 history of pancreatitis in comparison to those who are non-smokers but have a family history of pancreatitis. Current Prevalence/Incidence/Mortality Rate US Prevalence: Pancreatic cancer currently affects approximately 41,609 people; 20,293 males and 21,316 females (Howlader et al., 2013). US Incidence: The estimated new cases for 2012 were 43,920 (American Cancer Society, 2012a). The estimated new cases for 2013 were 45,220 (Howlader et al., 2013). The age-adjusted incidence rate for pancreatic cancer from 1975 to 2010 was 11.78 per 100,000 for both sexes and all races. The incidence rate had generally stayed the same from the period of 1975 to 2011 with a few noted decreases in incidence rates in the years 1978, 1993, 1995, and 1999 (i.e., less than 11.1 per 100,000 people). From 2004 to 2011, the incidence rate was about 12.0 per 100,000, fluctuating in the range of 12.0-13.1 per 100,000 people (Howlader et al., 2013). US Age-Adjusted Mortality Rate: The estimated numbers of deaths for 2013 were 38,460. Pancreatic cancer showed an age-adjusted mortality rate of 10.70 per 100,000 Americans (Howlader et al., 2013). Estimated Undiagnosed/At-Risk The lifetime risk of an individual developing pancreatic cancer is 1 in every 67 individuals, with equivalent ratios between men and women (American Cancer Society, 2013a). The risk of developing pancreatic cancer increases with age. Nearly all patients are older than age 45 and 7 out of 10 individuals diagnosed with pancreatic cancer are over age 65 (American Cancer Society, 2013a). Disease Impact – Years of Potential Life Lost (YPLL) According to the United Nations and the World Health Organization, the YPLL to pancreatic cancer in the United States in 2001 was 114,952 for males of all ages, 100,694 for females of all ages, and 215,646 total for both males and females of all ages (World Health organization, n.d.). For both males and females, the YPLL value spiked up at ages 45-59 and then steadily decreased in progressing years (World Health Organization, 2009). Further, Carter and Nguyen (2012) calculated that 498,400 years of potential life were lost to pancreatic cancer in the US in 2007. For this report, SGU Cures Index 355 we used the more conservative number of 215,646 YPLL in our economic impact calculations, presented below. Disease Impact – Disability adjusted Life Years (DALYs) According to the United Nations and the World Health Organization, the AgeStandardized DALY value associated with pancreatic cancer was 63 per 100,000 US citizens in 2004 (World Health Organization, 2009). Given the population of the US in 2004, this rate equals 184,470 total DALYs lost to pancreatic cancer in that year (World Health Organization, 2009). Further, Carter and Nguyen (2012) provide an overall DALY impact of pancreatic cancer at 237,700 in the United States. For this report, we have taken a more conservative approach, using the lower DALY value of 184,470 in our calculations of economic impact presented below. History of Disease and Breakthroughs In the early 19th century, two pathologists named Giovanni Morgagni and Mathew Baillie paved the way for the discovery of pancreatic cancer. Morgagni indexed his case studies with autopsy findings and Baillie created the first depicted pathology textbook. Both of their works described pancreatic cancer in great detail (National Cancer Institute, 1987) Morgagni was the first to discuss cancer of the pancreas, as he found tumors in or around the pancreas while he performed his autopsies (Wagener, 2009). While progress has been made in methods to diagnose pancreatic cancer, treatment effectiveness has progressed more slowly. In the 20th century, X-ray and radium were used to combat cancer. Chemotherapy came later (National Cancer Institute, 1987). Treatments Depending on the stage at which the individual is diagnosed with pancreatic cancer, one or more of the following treatments may be used at the discretion of the physician or team treating the patient: -‐ Surgery -‐ Alternative tumor removal techniques -‐ Radiation Therapy -‐ Chemotherapy -‐ Additional drugs (American Cancer Society, 2013b) The two types of surgeries that may or may not be performed are “potentially curative surgery” and “palliative surgery”. Potentially curative surgery occurs when tests indicate SGU Cures Index 356 that all cancer has been removed during surgery and the cancer did not reach a ubiquitous location where some cancer cells remain. Palliative surgery occurs when surgery is performed in order to avoid further complications and/or to relieve the patient from pain. Palliative surgery does not remove all of the cancer, as the cells are too distributed to be completely removed (American Cancer Society, 2013a). The timing of diagnosis is critical. If diagnosed too late, treatment options are limited and surgery is no longer a viable option. There are various chemotherapy drugs used for pancreatic cancer. In some cases, depending on the physician and the patient, chemotherapy may be administered with radiation. Chemotherapy and radiation therapy can be combined to enhance treatment impact, but is often accompanied by increased side effects. The most commonly used chemotherapy drug for pancreatic cancer is gemcitabine. Gemcitabine is an “antitumor” agent that has proven effective against leukemia and other tumorous cancers. This medication has shown a positive benefit both clinically and in tumor development of over 5% in previous studies (Burris, Moore, Andersen, Green, Rothenberg, Modiano, et al., 1997). The FDA approved this drug in 1996 and it was used to treat cancer patients soon after its approval. By 2005, 95% of stage IV pancreatic cancer patients were being treated with gemcitabine. Currently, gemcitabine is the sole medication approved by the FDA to treat advanced stage pancreatic cancer. Various other drugs may be used in combination to gemcitabine. Folfirinox showed a higher survival rate but higher toxicity levels than gemcitabine in a study by Coroy and colleagues (2011). Because of its high toxicity levels, this drug is a viable option for patients who have metastatic pancreatic cancer and show a good reaction to chemotherapy drugs. Folfirinox showed no progression of the pancreatic cancer, with 52% survival at 6 months, 12.1% at 12 months, and 3.3% at 18 months. By comparison, patients under treatment with gemcitabine showed survival rates with no progression of the pancreatic cancer of 17.2% at 6 months, 3.5% at 12 months, and 0% at 18 months (Conroy, Desseigne, Ychou, Bouche, Guimbaude, Becouarn, et al., 2011). Current Cure Status There is presently no definitive cure for pancreatic cancer. Further, there are no blood tests or other procedures to identify or screen for this cancer until the individual has already developed more significant symptoms and therefore is at a more advanced stage of the disease (American Cancer Society, 2013a). As of now, only the removal of the pancreatic tumor gives the chance of curing pancreatic cancer, but not all patients are eligible (Ramirez & Vickers, 2004). Research targeting new drugs, clinical trials, and ways of diagnosing the disease at an earlier stage continues to illuminate the path to SGU Cures Index 357 finding new ways to prevent and/or cure this disease. The National Cancer Institute (NCI) has increased its focus on pancreatic cancer research over the past decade, resulting in a number of breakthroughs such as biomarker identifiers that may help with detecting, diagnosing and monitoring the disease (National Cancer Institute, 2012b). Nonetheless, these advances have yielded very limited improvement in survival rates. Future Cure Obstacles Many experts agree that the best way to increase pancreatic survival rates is through early detection, as demonstrated by the median 4.6 month survival rate at stage IV diagnosis versus the median 20.6 to 24.1 month survival rate at stage I. Finding a method to screen those at risk for pancreatic cancer is highly needed (Ramirez & Vickers, 2004). Despite this, early detection is limited in curing the disease, as demonstrated by the median 20.6 to 24.1 month survival rate at stage I. Beyond earlier detection and diagnosis, challenges in finding new treatments for pancreatic cancer are substantial. They include finding a balance of safety and efficacy, finding the right patients in the right stages of pancreatic cancer to incorporate into appropriate clinical trials, allowing genetic testing without compromising patient privacy and patient privacy laws set by the government and local institutions, and arranging support amongst laboratories and corresponding clinical trials. Arranging this support would in turn promote the review and funding of clinical trials and research conducted (Rothenberg, Carbone, & Johnson, 2003). Research Development and Treatment Costs The following are the recent “total relevant funding” figures provided for cancer of the pancreas, as reported by the National Cancer Institute (2012a): 2012: $105.4 million 2011: $99.5 million 2010: $97.1 million 2009: $89.7 million 2008: $87.2 million Pancreatic Cancer Cures: Chemotherapy/Radiation/Surgery Cure Category SGU Cures Index 358 Achieved, Functional (though extremely limited) Cure Identification/Description If diagnosed early enough, surgery followed by radiation/chemotherapy. If diagnosed later, surgery is not performed, radiation/chemotherapy are the treatments of choice. Cure History In the seventeenth century, surgeon John Hunter stated that it is up to the surgeons’ discretion to remove a “movable” tumor, and if the cancerous tumor were removed the cancer may be cured if it had not metastasized to nearby tissue. In the eighteenth century, the development of anesthesia allowed for the expansion of surgeries, thus increasing the number of cancer surgeries. In the nineteenth century, with the aid of the modern microscope, pathologists such as Rudolf Virchow examined tissues removed during surgery. He correlated Morgagni’s work with modern microscopes and helped identify if the cancer was eradicated during the surgery (American Cancer Society, 2012b). Allen Oldfather Whipple was a Persian (i.e., Iranian) born American surgeon who first developed a two-step surgery in 1935 to remove the pancreas and a portion of the duodenum. Thus, early diagnosis has the potential to extend lives by making surgery a valid option. Currently, less than 20% of pancreatic cancer is identified at an early enough stage to undergo surgical removal. Regardless of what treatment the patient has undergone, an estimated 75% of patients who undergo surgery will die from recurrent pancreatic cancer within three to four years of treatment and surgery (The Harvard Medical School Family Health Guide, 2007). While the Whipple two-step surgery is still performed today on the few pancreatic cancer patients whom have not reached an advanced stage of the disease (Wagener, 2009), it is still very much a functional, rather than definitive cure, as demonstrated by the limited median survival rate (20.6 to 24.1 months) at stage I diagnosis. It is at this stage of diagnosis that patients are most likely to undergo surgery, as the cancer is least likely to have spread to other tissues in the body. Cure Science: Breakthroughs/Obstacles Breakthroughs in pancreatic cancer cures are limited. As demonstrated by the limited median survival rate, even among those diagnosed with stage I pancreatic cancer, it remains a devastating disease with poor prognosis. The most notable breakthroughs SGU Cures Index 359 include technological diagnostic techniques and surgical techniques to remove the pancreas, and radiation / chemotherapy drugs to destroy cancerous cells. These are functional, rather than definitive cures. Cure Science/Future Obstacles and Targets Screening tests for genes that may increase an individual’s risk for pancreatic cancer are being developed. These tests would allow for constant screening and early detection of any cancer. This genetic testing is highly recommended for those individuals who have close family members with pancreatic cancer, or familial history of pancreatic cancer (American Cancer Society, 2013a). Genetic testing further provides an excellent example of how to find new ways to prevent pancreatic cancer and more effectively target future treatments. Genetic testing has opened many new possibilities in detecting pancreatic cancer at a much earlier stage. The International Cancer Genome Consortium has been trying to extract the genomes of 750 pancreatic cancer specimens, which will in turn produce more information on how to possibly conduct personalized treatments (Kanji & Gallinger, 2013). Research is being conducted to find new ways of understanding how the disease progresses, how to sequence possible genes linked to this cancer using mathematical strategies, investigating biomarkers that can increase the effectiveness of current pancreatic cancer treatments such as chemotherapy, investigating new therapeutic means for more precise and therefore targeted therapy, exploring further targeted treatment for self destruction of cancerous cells without harming healthy cells, and finding new ways of involving immunotherapy to current or new pancreatic cancer treatments (American Cancer Society, 2013a). Dakhel and colleagues (2014) have examined the genetic and biological processes associated with pancreatic cancer to block the metastasis-promoting protein S100P. Treatment based on blocking the activity of S100P extra-cellularly in combination with chemotherapy and target-directed drugs could be promising as a new treatment option for pancreatic cancer. Finally, researchers are collaborating to produce a “genetic knowledge bank of cancers”, which physicians can refer to when deciding which medication would be most efficacious for each individual patient based on his or her genetic profile (Phillips, 2012). Number of Patients being Treated Currently With an estimated pancreatic cancer treatment prevalence in the United States in 2010 of .0111%, this prevalence leads to an estimate of 41,609 individuals with pancreatic cancer (National Cancer Institute, 2010b). SGU Cures Index 360 Number of Patients Requiring Treatment US Age-Adjusted Prevalence: Pancreatic cancer affects approximately 41,609 people (20,293 males and 21,316 females) of all races. (Howlader, et al., 2013). While everyone diagnosed with pancreatic cancer requires treatment, there are no estimates of how many would be treated if everyone was diagnosed as soon as the cancer process began. Impact of Treatment on Years of Potential Life Lost (YPLL) A treatment, and the impact of that treatment, varies depending on the stage of pancreatic cancer experienced by each patient. It is critically important to be able to detect pancreatic cancer at an early stage. This is because those who are diagnosed at Stage I survive for about 20.6 to 24.1 months. By contrast, patients who are diagnosed at Stage IV survive for an average (median) of 4.6 months (American Cancer Society, 2013a). Because of the lethality of pancreatic cancer, we observe a YPLL value of 215,646 for both males and females (World Health Organization, 2009). Impact of Treatment on QALYs Tam and colleagues (2013) showed that Folfirinox increases the life expectancy of a pancreatic cancer patient by one year, during which they report a QALY value of 0.703. Both of these values are higher than patients treated with gemcitabine, which was associated with a life expectancy of 8 months and a QALY value of 0.487 (Tam, Ko, Cheung, & Chan, 2013). Only 8.7% of pancreatic cancer patients are diagnosed at an early stage of the cancer. From this information we can infer that 91.3% of patients are diagnosed at an advanced stage of pancreatic cancer (Howlader, et al., 2013). Average (median) time for survival after diagnosis of pancreatic cancer at stage IV is 4.6 months, at stage III is 10.6 months, at stage II is 12.7 to 15.4 months, and at stage I is 20.6 to 24.1 months. (American Cancer Society, 2013a). Pancreatic cancer is so deadly because most patients are diagnosed at an advanced stage, which only has a 5-year survival rate from 1 to 3 % (American Cancer Society, 2013a). Therefore, the impact of pancreatic cancer is best measured using YPLL rather than quality of life for the reason that survival of pancreatic cancer at an advanced stage is very short in time. SGU Cures Index 361 Average Costs to Treat The overall cost of treating the average pancreatic cancer patient who is enrolled in Medicare is (O'Neill et al., 2012): 1) $134,700 for resectable loco regional disease 2) $65,300 for unresectable loco regional disease 3) $49,000 for distant disease The overall average cost to treat all forms of pancreatic cancer is $61,700 USD. This is a "minimal cost" scenario, as it is based on Medicare figures. The overall costs may be higher for those with private health insurance. As such, the overall treatment costs included in our calculations are likely conservative. Average total medical costs are $65,500 for patients 65 years or older, which includes the majority of diagnosed pancreatic cancer patients. Average total costs are higher for those patients who have localized pancreatic cancer ($65,300) versus patients who have distant or metastasized pancreatic cancer ($49,000). Hospitalizations and cancer-related procedures together account for the majority of health care costs. From 2000 to 2007, average annual Medicare costs for all stages of pancreatic cancer were $14,700 for cancer related procedures, $8,700 for chemotherapy/radiotherapy, $21,900 for inpatient care, $4,500 for hospice care, and $15,700 for other expenses (O’Neill et al., 2012). Medical costs are lower for patients at an advanced stage of pancreatic cancer because those patients are mostly treated with only chemotherapy/radiation therapy, and often die after a shorter period of time. Also, patients who have operable or resectable pancreatic cancer at a stage in which it has not metastasized will undergo surgery to remove the tumor and therefore have a much higher possibility of recovery / longerterm survival. These patients may later be hospitalized again and undergo further procedures while hospitalized. The cost of procedures for patients with nonmetastasized resectable pancreatic cancer amounted to $51,000 in comparison to procedures for patients with non-metastasized unresectable pancreatic cancer, which amounted to $12,600, and procedures for patients with metastasized unresectable pancreatic cancer, which amounted at $7,000. Economic Impact - YPLLs SGU Cures Index 362 The economic impact of a potential cure for pancreatic cancer is significant. Given an estimated 215,646 years of potential life lost to pancreatic cancer in the US in 2001 (World Health Organization, n.d.), the associated annual VMRR is $22.469 billion. Economic Impact - DALYs/QALYs Given an estimated 184,470 DALYs lost to pancreatic cancer in the US in 2004 (World Health organization, 2009), the associated annual VMRR is $19.220 billion. Thus, a definitive cure for pancreatic cancer could have an estimated worth of at least $19.220 billion annually, based solely on VMRR values. Citations American Cancer Society. (2012a). Cancer Facts and Figures 2012. Retrieved January 22, 2014, from: www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/doc ument/acspc-031941.pdf American Cancer Society. (2012b). The History of Cancer. Retrieved February 13, 2014, from: www.cancer.org/cancer/cancerbasics/thehistoryofcancer/index American Cancer Society. (2013a). Pancreatic Cancer Overview. Retrieved Febuary 3, 2014: http://www.cancer.org/cancer/pancreaticcancer/overviewguide/pancreaticcancer-overview-key-statistics American Cancer Society. (2013b). Understanding Radiation Therapy: A Guide for Patients and Families. Retrieved March 5, 2014, from: http://www.cancer.org/treatment/treatmentandsideeffects/treatmettypes/radiati on/understandingradiationtherapyforpatientsandfamilies/understandingradiation-therapy-toc American Cancer Society. (2014). What are the risk factors for pancreatic cancer? Retrieved July 28, 2014, from: www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-riskfactors Bradley, C., Yabroff, K., Dahman, B., & Feuer, E. M. (2008). Productivity Costs of Cancer SGU Cures Index 363 Mortality in the United States: 2000-2020. Journal of the National Cancer Institute, 100 (24), 1763-1770. Burris, H.A. 3rd, Moore, M.J., Andersen, J., Green, M., Rothenberg, M., Modiano, M., Cripps, M.C., Portenoy, R.K., Storniolo, A.M., Tarassoff, P., Nelson, R., Dorr, F.A., Stephens, C.D., & Von Hoff, D.D. (1997). Improvements in survival and clinical benefit with gemcitabine as firstline therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinal Oncology, 15(6), 2403-2413. Cancer Treatment Centers of America. (2012). Survival Results: Pancreatic Cancer. Retrieved on February 1, 2014, from: www.cancercenter.com/pancreaticcancer/statistics/ Carpenter, W., Beskow, L., Blocker, E., Forlenza, M., Kim, A., Pevzner, E., Rose, J.M., Tran, A.N., Webber, K.H., Knight, K., & O’Malley, M.S. (2008). Towards a More Comprehensive Understanding of Cancer Burden in North Carolina: Priorities for Intervention. North Carolina Medical Journal, 69 (4), 275282. Carter, A. R., & Nguyen, C. N. (2012). A comparison of cancer burden and research spending reveals discrepencies in the distribution of research funding. BMC Public Health, 12(526), 1471-2458. Conroy, T., Desseigne, F., Ychou, M., Bouche, O., Guimbaude, R., Becouarn, Y., Adenis, A., Raoul, J.L., Gourgou-Bourgade, S., de la Fouchardière, C., Bennouna, J., Bachet, J.B., Khemissa-Akouz, F., Péré-Vergé, D., Delbaldo, C., Assenat, E., Chauffert, B., Michel, P., Montoto-Grillot, C., Ducreux, M., Groupe Tumeurs Digestives of Unicancer, & PRODIGE Intergroup. (2011). FOLFIRINOX verusus gemcitabine for matastatic pancreatic cancer. The New England Journal of Medicine, 364(19), 1817-1825. Dakhel, S., Padilla, L., Adan, J., Masa, M., Martinez, J., Roque, L., Coll, T., Hervas, R., Calvis, C., Messeguer, R., Mitjans, F., & Hernández, J.L. (2014). S100P antibody-mediated therapy as a new promising strategy for the treatment of pancreatic cancer. Oncogenesis, 3(3), 1-10. Hidalgo, M. (2012). New insights into pancreatic cancer biology. Oxford Journals, 23(10), 135-138. SGU Cures Index 364 Howlader, N., Noone, A.M., Krapcho, M., Garshell, J., Neyman, N., Altekruse, S. F., Kosary, C. L., Yu, M., Ruhl, J., Tatalovich, Z., Mariotto, A., Lewis, D.R., Chen, H.S., Feuer, E.J., & Cronin, K.A. (2013). SEER Cancer Statistics Review, 1975-2010. Retrieved January 20, 2014, from: http://seer.cancer.gov/csr/1975_2010/ Hsu, C., & Saif, M. (2011). Diabetes and pancreatic cancer. Highlights from the "2011 ASCO Annual Meeting". Journal Of The Pancreas, 12(4), 330-333. Kanji, Z., & Gallinger, S. (2013). Diagnosis and management of pancreatic cancer. Canadian Medical Association Journal, 185(14), 1219-1226. Klein, A. (2013). Identifying people at high risk of developing pancreatic cancer. Nature Reviews Cancer, 13(1), 66-74. Klein, A., Lindstrom, S., Mendelsohn, J., Steplowski, E., Arslan, A., Bueno-de-Mesquita, B., Fuchs, C.S., Gallinger, S., Gross, M., Helzlsouer, K., Holly, E.A., Jacobs, E.J., Lacroix, A., Li, D., Mandelson, M.T., Olson, S.H., Petersen, G.M., Risch, H.A., Stolzenberg-Solomon, R.Z., Zheng, W., Amundadottir, L., Albanes, D., Allen, N.E., Bamlet, W.R., Boutron-Ruault, M.C., Buring, J.E., Bracci, P.M., Canzian, F., Clipp. S., Cotterchio, M., Duell, E.J., Elena, J., Gaziano, J.M., Giovannucci, E.L., Goggins, M., Hallmans, G., Hassan, M., Hutchinson, A., Hunter, D.J., Kooperberg, C., Kurtz, R.C., Liu, S., Overvad, K., Palli, D., Patel, A.V., Rabe, K.G., Shu, X.O., Slimani, N., Tobias, G.S., Trichopoulos, D., Van Den Eeden, S.K., Vineis, P., Virtamo, J., Wactawski-Wende, J., Wolpin, B.M., Yu, H., Yu, K., Zeleniuch-Jacquotte, A., Chanock, S.J., Hoover, R.N., Hartge, P., & Kraft, P. (2013). An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. Plos ONE, 8(9), 1-10. National Cancer Institute. (1987). Closing in on cancer: Solving a 5000-year-old mystery. United States: US Department of Health and Human Services; Public Health Service; National Institutes of Health. National Cancer Institute. (2010a). What You Need To Know About Cancer Of the Pancreas. Retrieved January 16, 2014, from: www.cancer.gov National Cancer Institute. (2010b). Cancer of the pancreas, United States prevalence estimates. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. National institute of Health. SGU Cures Index 365 National Cancer Institute. (2012a). NCI Funded Research Portfolio. Retrieved Feburary 3, 2014, from: http://fundedresearch.cancer.gov/nciportfolio/search/resultmanager National Cancer Institute. (2012b). Pancreatic cancer: Summary of NCI's FY2010 and FY 2011 portfolio and selected research advances. National Institutes of Health, US Department of Health and Human Services. National Cancer Institute. (2013). Drugs Approved for Pancreatic Cancer. Retrieved March 8, 2014, from: www.cancer.gov/cancertopics/druginfo/pancreaticcancer Oberstein, P., Hershman, D., Khanna, L., Chabot, J., Insel, B., & and Neugut, A. (2013). Uptake and patterns of use of gencitabine for metastatic pancreatic cancer: a population-based study. Cancer Investigations, 31(5), 316-322. O'Neill, C. B., Atoria, C. L., O'Reilly, E. M., LaFemina, J., Henamn, M. C., & Elkin, E. B. (2012). Costs and trends in pancreatic cancer treatment. Cancer, 118(20), 51325136. Pancreatic Cancer Action. (2012). Symptoms of Pancreatic Cancer. Retrieved July 28, 2014, from: pancreaticcanceraction.org/pancreatic-cancer/symptoms/ Phillips, N. (2012). Australian breakthrough on lethal pancreatic cancer: 'Treat each patiently differently'. Sydney, Australia. The Sydney Morning Herald National. http://www.smh.com.au/national/health/australian-breakthrough-on-lethalpancreatic-cancer-treat-each-patient-differently-20121024-286d1.html Ramirez, P., & Vickers, S. (2004). Current status of gene therapy for pancreatic cancer. Current Surgery, 61(1), 84-92. Rothenberg, M., Carbone, D., & Johnson, D. (2003). Improving the evaluation of new cancer treatments: Challenges and opportunities. Nature Reviews Cancer, 3(4), 303-309. SEER Cancer Statistics Factsheets: Pancreas Cancer. (2013). Retrieved March 13, 2014, from: http://seer.cancer.gov/statfacts/html/pancreas.html Tam, V., Ko, Y. M., Cheung, M. K., & Chan, K. (2013). Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Current Oncology, 20(2), e90-106. SGU Cures Index 366 Harvard Medical School Family Health Guide. (2007). Pancreatic cancer: An update on a 'stealth' cancer. Retrieved July 28, 2014, from: www.health.harvard.edu/fhg/updates/Pancreatic-cancer-An-update-on-a-stealthcancer.shtml Wagener, D. (2009). The History of Oncology. Houten, GA, The Netherlands: Bohn Stafleu van Loghum. World Health Organization (2009). Mortality and Burden of Disease Estimates for Member States in 2004: Age-std DALY rates for United States of America. Retrieved on June 4, 2014 from: http://apps.who.int/gho/data/node.main.1009?lang=en. World Health Organization (n.d.). Global Burden of Disease (GBD) 2001 Estimates by Subregion: Years of Life Lost (YLL). Retrieved on June 4, 2014 from: http://www.who.int/healthinfo/global_burden_disease/estimates_regional_2001/ en/. SGU Cures Index 367 LIMITATIONS OF THE REPORT There are a number of significant limitations inherent in this report that need to be taken into consideration when reviewing the outcomes. First, we have not collected any new data for this report, but have instead relied on existing data. To accomplish this, the authors leveraged worldwide web-based search engines and sources. Information was drawn from peer-reviewed academic journals, government agency and policy reports, white papers, and nongovernmental societies (e.g., Alzheimer’s Society). Thus, the quality of the conclusions in our report is only as good as these data sources. To address this limitation, we have relied on a number of steps: 1. We drew information from verifiable sources as much as possible: Peerreviewed academic journals, official government websites, official government reports, international body reports (e.g., World Health Organization, United Nations), and nongovernmental societies (e.g., Alzheimer’s Society). 2. When drawing information from anything but the above top-tier sources, we attempted to verify the quality/validity of the information by cross-referencing it against data that addressed a similar topic, but drawn from different source. We also compared the data for logical validity against data drawn from a toptier source (e.g., 3,500 patients taking corticosteroids for Duchenne’s Muscular Dystrophy is consistent with estimates of 5,411 – 7,400 Duchenne’s or Becker Muscular Dystrophy patients in the US). 3. We provide full referencing for all of the sources of data utilized in the report, allowing for external, third party analysis and verification. Estimating economic impact values for each disease is a multi-step process that requires a number of inferences and assumptions. We have described the calculation of general values (i.e., Value of a Statistical Life-Year) in the “Methods and Terminology” section. Wherever we have used a unique value in the body of the report, we provide a breakdown of the calculations used to determine that value. Finally, in all of our calculations, we have used the more conservative input values to minimize overestimations and arrive at the most conservative economic impact figures. Thus, if anything, the impacts included in this report are likely on the conservative side. SGU Cures Index 368 We have only been able to review a limited number of diseases for this project. Ideally, we would like to have reviewed and compared at least 20 diseases, a number of which were included in our initial outline but never made it into this final report: Anxiety Disorders, Colon Cancer, Microbiotic Infections, Skin Cancer, Spina Bifada. Given limited time and resources, we attempted to include a mix of different disease categories, and also selected diseases based on health burden in the US Malaria was included given its global health burden and the importance of addressing this disease for the US Military. Now that the outline, methodology and groundwork have been laid for the report, perhaps other diseases can be added in future. We discuss behavioral health and prevention only briefly in this report. We understand the importance of behavior in “definitive cures” (i.e., preventing disease before it is able to take hold). While we only touch upon behavioral medicine, its inclusion as a cure that must be investigated is paramount. While it can be argued that cures for most chronic diseases (e.g., cardiovascular disease, lung cancer, diabetes) are known (i.e., exercise more, eat more whole foods and less processed foods, limit alcohol intake, stop smoking), most Americans do not follow these behavioral patterns sufficiently, and incidence rate for these chronic diseases continues to increase. In short, while the cure knowledge may exist in the form of everyday health behaviors, cure implementation remains an ongoing problem. Thus, further research is urgently needed to understand the process of behavioral cure implementation. This fits with a “cure strategy”. We cannot know the potential impact of research on future cures. The future is, by definition, unknown. For example, more than $8 billion has already been spent searching for an HIV vaccine (International AIDS Vaccine Initiative), which has not been found. The best we can do is examine the process of discovering cures, and the impact of historical cures as models of future cures potential. While past performance is not a guarantee of future outcomes, an historical analysis is the best we’ve got in devising current policy. SGU Cures Index 369 APPENDIX A: ST. GEORGE’S UNIVERSITY Since it’s founding as an independent School of Medicine in 1976, St. George’s University (SGU) has evolved into a top center of international education, drawing students and faculty from 140 countries to the island of Grenada. Over the past nearly 40 years, continual reinvestment into the school has resulted in a $250 million technologically advanced campus with all of the facilities of a world-class institution. The University’s 15,000+ graduates include physicians, veterinarians, scientists, and public health and business professionals across the world. The University offers advanced, premedical, and pre-veterinary degrees in its Schools of Medicine and Veterinary Medicine, and independent and dual graduate degrees in the sciences, public health, and business. Undergraduate degree programs are also available through its School of Arts and Sciences. The University programs are accredited and approved by many governing authorities. SGU is affiliated with educational institutions worldwide, including the United States, the United Kingdom, Canada, Australia and Ireland. Over 7,000 students from 90 countries are currently enrolled at the University, providing a thriving and active campus atmosphere. SGU faculty consists of on-island full-time members as well as part-time and visiting professors, which travel to Grenada for several weeks each semester to teach and engage in various on-campus activities. SGU Cures Index 370 APPENDIX B: WINDWARD ISLANDS RESEARCH AND EDUCATION FOUNDATION The Windward Islands Research and Education Foundation (WINDREF) promotes health, well-being, and sustainable environmental development through multi-disciplinary research and education programs. WINDREF strives for program excellence by promoting collaborative relationships between regional scientists and leading international scholars, and by adhering to the highest ethical and academic standards in the design and conduct of research. Founded in 1994, WINDREF is located on the St. George’s University (SGU) 42-acre campus in Grenada and collaborates closely with faculty from SGU‘s schools of Medicine, Public Health and Preventive Medicine, Nursing, Veterinary Medicine, Graduate Studies, and Arts and Sciences. WINDREF also collaborates with the Grenada Government Ministries in its research and community outreach work: Agriculture, Forestry, & Fisheries; Education & Human Resources; Health & Social Security; and Social Development. WINDREF is non-profit, holding 501(c)3 status in the United States (US), charitable trust status in the United Kingdom (UK), and non-profit status in Grenada. A USA Board of Directors, a UK Board of Trustees, and a Scientific Advisory Board oversee the activities SGU Cures Index 371 of the Institute. Over two decades, its work has been supported by funders such as the Bill & Melinda Gates Foundation, Centers for Disease Control and Prevention, International Development Research Centre, Global Environment Facility, UN Development Program, National Geographic, National Institutes of Health, PanAmerican Health Organization, Wellcome Trust, World Health Organization, World Bank, and many others. WINDREF has forged partnerships with academics, researchers, governments, and nongovernmental organizations throughout the Caribbean Region. Through SGU faculty and internationally-affiliated research fellows, WINDREF has access to consulting expertise in a number of areas: Anthropology, Behavioral Health, Bioethics, Climate Change Adaptation and Mitigation, Ecology, Epidemiology, Marine and Terrestrial Biology, Medicine, Infectious and Parasitic Diseases, International Business and Macroeconomics, Public Health (including occupational and environmental health), Research Methodology, and Sociology. More information about WINDREF can be found on the Institute’s website: http://etalk.sgu.edu/windref/index.html. SGU Cures Index 372