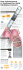Prevention and Management of Catheter- Associated UTIs
Transcription
Prevention and Management of Catheter- Associated UTIs
PRINTER-FRIENDLY VERSION AT IDSE.NET ll A Prevention and Management of CatheterAssociated UTIs s ite d. ib te oh no pr e is is rw on si he is ot m er ss le tp un ou up ith ro w G rt ng pa hi in is bl or Pu le ho on ah in w cM n M tio 10 uc 20 od © pr ht Re rig ed. py rv se re ht Co rig DIANE K. NEWMAN, RNC, MSN, CRNP, FAAN Co-Director Penn Center for Continence and Pelvic Health University of Pennsylvania Health System Division of Urology Philadelphia, Pennsylvania T he urinary tract is the most common 1 site of nosocomial infections and urinary tract infections (UTIs) account for approximately 40% of all hospital-acquired infections (HAIs) in the United States.2 More than 80% of HAIs are associated with an indwelling urinary catheter (IUC).3 Referred to as catheter- associated UTIs (CA-UTI), these infections can cause substantial morbidity in both men safety issue. Urinary catheters often are placed unnecessarily, remain in place without physician awareness, and are not removed as soon as possible when no longer needed.4 I N D E P E N D E N T LY D E V E L O P E D B Y M C M A H O N P U B L I S H I N G An IUC (often referred to as a “Foley”) is a commonly used medical device that allows for continuous urine drainage in patients with bladder dysfunction. IUCs are used in both men and women in all care settings. They are used in the acute care setting more than any other medical device. At least 15% to 25% of patients may have a urinary catheter inserted sometime during their hospital stay, with most used on a shortterm basis (<30 days). The prevalence of CAUTIs is greater in high-acuity patient units, as is evident by the high rate of IUC use in critical care and intensive care units. Inappropriate IUC use has been equated to a “one-point restraint,” and as a form of restraint, catheters are associated with functional impairment, discomfort, nosocomial infection, pressure ulcers, and death.5 Patients have reported that IUCs are uncomfortable, painful, and restrict activities of daily living.6 IUCs have been associated with a greater risk for death in hospitalized older patients—4 times as great during hospitalization and 2 times as great within 90 days after discharge.7 Extended IUC use in older patients sustaining hip fracture who are discharged to skilled nursing facilities with a catheter in place also has been associated with poor outcomes d. and women and are a preventable patient Prevalence of CA-UTI INFECTIOUS DISEASE SPECIAL EDITION • 2010 13 ll A as IUC placement puts these these individuals at higher risk for rehospitalization for CA-UTI and sepsis.8 IUCs are associated with many adverse events; the most frequent being CA-UTIs. Approximately 560,000 cases of CA-UTIs are reported yearly to the Centers for Disease Control and Prevention (CDC). Although frequently asymptomatic, between 20% and 30% of individuals with catheter-associated bacteriuria will develop symptoms of CA-UTI, secondary to long-term catheter use (>30 days). IUCs have specific indications that recently have been defined by the Healthcare Infection Control Practices Advisory Committee (HICPAC) and are listed in Table 1.9,10 Table 2 cites examples of inappropriate IUC use, as provided by HICPAC.9,10 Longer duration of IUC should be avoided as it provides access for bacteria and ultimately may result in a CA-UTI. Finally, another reason to avoid use of an IUC is cost. The cost of treating a single episode of a CA-UTI can range from $980 to $2,900, depending on the presence of associated bacteremia.11 this policy, CMS hopes to provide hospitals with the incentive to prevent hospital-acquired conditions by not reimbursing for their occurrence and treatment. Hospitals are now at risk for financial losses (nonpayment for additional costs) if CA-UTIs occur. So, in addition to improving patient care and safety, hospitals now have a financial incentive to minimize the use of IUCs. Causes of CA-UTIs s ite d. ib te oh no pr e is is rw on si he is ot m er ss le tp un ou up ith ro w G rt ng pa hi in is bl or Pu le ho on ah in w cM n M tio 10 uc 20 od © pr ht Re rig ed. py rv se re ht Co rig A UTI is an inflammatory response to colonization of the urinary tract from a bacterial or fungal pathogen.15 A CA-UTI is classified as a complicated UTI if the presence of a foreign body in the urinary tract not only predisposes the patient to UTI, but also alters the body’s ability to eradicate bacteria from the lower urinary tract. It is the most severe and common catheter-associated complication because it can lead to urosepsis and septicemia and most often is associated with long-term catheter use. CA-UTIs may occur at least twice a year in patients with long-term IUC and usually require hospitalization. CA-UTI is more likely to occur in women because of decreased estrogen hormone in the genitalia and the shorter length of the female urethra; as well as the urethra’s close proximity to the anus providing bacteria a shorter distance to travel.11 The bacteria causing CA-UTI gain access to the urinary tract either extraluminally or intraluminally.16 Approximately 66% of CA-UTIs are attributed to bacteria gaining access via the catheter–urethral lumen interface, and the remaining 34% are attributed to intraluminal migration associated with manipulation of the catheter and urinary drainage system.17 Extraluminal contamination may occur during insertion from contamination of the catheter from any source. Extraluminal contamination also is thought to occur when microorganisms ascend Nonpayment for CA-UTIs Changes by the Centers for Medicare & Medicaid Services (CMS) have reshaped reimbursement for inpatient prospective payment for acute care hospitals. CMS holds acute care hospitals accountable for failing to avert preventable harm resulting from medical care and will withhold additional payments to hospitals for “serious preventable events.”12-14 As part of the Hospital-Acquired Conditions Initiative (known as the “nopay rule”), CMS has placed a high priority on reducing CA-UTIs because they are viewed as unacceptable harm resulting from medical care. CMS can deny payment for 8 costly and sometimes deadly preventable hospitalacquired conditions, one of which is CA-UTIs. Through Table 1. Recommended Indications for Use of an IUC Acute urinary retention or bladder outlet obstruction Need for accurate measurements of urinary output in critically ill patients Perioperative use for selected surgical procedures: urologic surgery or other surgery on contiguous structures of the genitourinary tract Anticipated prolonged duration of surgery (if inserted, remove in postanesthesia care unit) Large-volume infusions or diuretics during surgery Need for intraoperative monitoring of urinary output Prolonged immobilization (eg, potentially unstable thoracic or lumbar spine, multiple traumatic injuries such as pelvic fractures) To improve comfort for end-of-life care IUC, indwelling urinary catheter Adapted from references 9 and 10. 14 I N D E P E N D E N T LY D E V E L O P E D B Y M C M A H O N P U B L I S H I N G d. To assist in healing of open sacral or perineal wounds in incontinent patients Table 2. Examples of Inappropriate Uses of an IUC As a substitute for nursing care of the patient with incontinence As a means of obtaining urine for culture or other diagnostic tests when the patient can voluntarily void ll A For prolonged postoperative duration without appropriate indications (eg, structural repair of urethra or contiguous structures, prolonged effect of epidural anesthesia) Bacteriuria and Infecting Organisms s ite d. ib te oh no pr e is is rw on si he is ot m er ss le tp un ou up ith ro w G rt ng pa hi in is bl or Pu le ho on ah in w cM n M tio 10 uc 20 od © pr ht Re rig ed. py rv se re ht Co rig Approximately 50% of hospitalized patients catheterized longer than 7 to 10 days develop bacteria or fungus in the urine (called bacteriuria). A large number and a variety of types of organisms are present in the periurethral area, in the distal part of the urethra, and they may be introduced into the bladder at the time of catheter insertion. Other factors that increase the risk for bacteriuria include the presence of residual urine in the bladder resulting from inadequate bladder drainage (urine stasis promotes bacterial growth), ischemic damage to the bladder mucosa through overdistention, mechanical irritation from the presence of a catheter, and biofilm formation on the catheter intraluminal surface. Most patients with bacteriuria are asymptomatic and although they should not be treated, many receive inappropriate antimicrobials. A 2009 article by Cope et al, reviewed all urine culture results of predominately male patients at a Veterans Affairs medical center during a 3-month period and noted that of the 164 episodes of catheter-associated asymptomatic bacteriuria, 68% (n=111) were managed appropriately (no treatment), whereas 32% (n=53) were treated with antibiotics (inappropriate treatment).21 However, between 20% and 30% of individuals with catheter-associated bacteriuria will develop symptoms of a CA-UTI. Most CA-UTIs involve multiple organisms and resistant bacteria from catheter-associated biofilms. These include Enterobacteriaceae, other than Escherichia coli (eg, Klebsiella, Enterobacter, Proteus, and Citrobacter), Pseudomonas aeruginosa, enterococci and staphylococci, and Candida. The infecting organism depends on hospital unit. For example, the predominant microorganisms causing CA-UTI in the intensive care unit are enteric gram-negative bacilli, enterococci, Candida species, and P. aeruginosa.22 Candiduria is especially common in individuals with prolonged IUC use who are receiving broad-spectrum systemic antimicrobial agents.23 However, because of increased antibiotic use, there has been an increase in antibioticresistant microorganisms, particularly P. aeruginosa and Candida albicans, 2 organisms frequently involved in device-associated nosocomial infections.24 A growing problem in hospitals is infection with vancomycinresistant Enterococcus (VRE) and methicillin-resistant Staphylococcus aureus (MRSA). IUC, indwelling urinary catheter Adapted from references 9 and 10. from the perineum along the surface of the catheter and occurs most often in women. Fecal strains contaminate the perineum and urethral meatus, are harbored in the labia, and subsequently ascend to the bladder along the external surface, thereby causing bacteriuria, catheter biofilm formation, and encrustation.18 Intraluminal contamination occurs by ascent of bacteria from a contaminated catheter, drainage tube, or urine drainage bag.19 Microorganisms can migrate up the catheter into the bladder within 1 to 3 days.20 There are 3 main catheter-associated entry points for bacteria: 1) the urethral meatus, with introduction of bacteria occurring on insertion of the catheter; 2) the junction of the catheter-bag connection, especially when a break in the closed catheter system occurs; and 3) the drainage port of the collection bag (Figure). All of these mechanisms involved in the pathogenesis of colonization and infection of the urinary tract combine to make CA-UTI very difficult to prevent in patients with an IUC in place for longer than 2 weeks. Catheter Drainage Tubing Junction Urethral MeatusCatheter Junction Catheter-Associated Biofilms d. Catheters are a good medium for bacterial growth because once they gain access to the urinary tract, bacteria produce various adhesions, including hairlike fimbriae that allow them to firmly attach to the catheter wall. These attached bacteria upregulate their expression of certain genes, resulting in altered phenotypes that ultimately lead to biofilms (living layers of organisms).19,25-28 Urine contains protein that adheres to and primes the catheter surface. Microorganisms bind to this protein layer and thus attach to the surface. Such Drainage Bag Outlet Tube Figure. IUC bacteria entry. IUC, indwelling urinary catheter I N D E P E N D E N T LY D E V E L O P E D B Y M C M A H O N P U B L I S H I N G 15 ll A bacteria are different from free-living planktonic bacteria (bacteria that float in urine). Initially, IUC biofilms may be composed of single organisms, but can lead to multiorganism biofilms because the presence of the biofilm inhibits antimicrobial activity.29 Biofilm provides a sustained reservoir for microorganisms that, after detachment, can infect the patient. These biofilms cause further problems if the bacteria (eg, P. mirabilis) produce the enzyme urease.24 The urine then becomes alkaline, causing the production of ammonium ions, followed by crystallization of calcium and magnesium phosphate within the urine. These crystals then are incorporated into the biofilm, resulting in s ite d. ib te oh no pr e is is rw on si he is ot m er ss le tp un ou up ith ro w G rt ng pa hi in is bl or Pu le ho on ah in w cM n M tio 10 uc 20 od © pr ht Re rig ed. py rv se re ht Co rig encrustation and blockage of the catheter over a period of time. Several features of biofilms have important implications for the development of antimicrobial resistance in organisms growing within the biofilm. The complex structures of the biofilm promote bacterial proliferation and protect bacteria from destruction by cleaners, antiseptics, antibiotics, and the host’s immune system. Bacteria within a biofilm exhibit a greater ability to communicate and exchange genetic information than do free-floating (planktonic) bacteria. This communication is hypothesized to promote antibiotic resistance and spread of the biofilm to other surfaces of the catheter and urinary epithelium. Table 3. Best Practices for Management of IUCs and Prevention of CA-UTIs Conduct daily assessment of the need for IUC, implement quality-improvement programs to reduce the risk for CA-UTIs and educate physician and nursing staff on indications for IUC Proper technique (aseptic) and equipment during catheter insertion Minimize urethral trauma during catheter insertion by using generous amounts of sterile lubricant and holding the penis in a near vertical position when catheterizing a male patient Wash hands before and after handling of the catheter site or apparatus as up to 15% of UTIs occur in clusters as a result of cross-infection. Always wear gloves when handling any part of the catheter system Ensure an unobstructed urine flow by preventing any kinks or loops from occurring in the catheter and tubing, which might restrict flow of urine Secure the catheter by anchoring it to the upper thigh in women or to the upper thigh or lower abdomen in men, to prevent excessive tension on the catheter, which can lead to urethral trauma and tears Avoid rigorous, frequent cleansing of the catheter entry site (urethral meatus or suprapubic) and do not use antiseptics for routine cleansing; wash the catheter entry site daily with soap and water or after bowel contamination Empty the drainage bag at least every 4 to 6 hours or when urine in the drainage bag reaches 400 mL to avoid migration of bacteria up the lumen of the catheter system. Empty bag prior to transporting patient Separate graduated containers for each patient and each patient drain. With multiple drainage devices for one patient, keep drainage devices on opposite sides of the bed and keep drainage devices in semiprivate rooms on opposite sides of the room Consider changing the catheter before obtaining a specimen for culture as cultures obtained through the old catheter may be inaccurate Routine urine should not be performed in the absence of infection because all chronically catheterized individuals have bacteria and the organisms change frequently (about 1-2 times per month). Urine cultures should only be obtained if the patient demonstrates clinical symptoms of a UTI. Consider changing the entire catheter and system if infection or obstruction occurs or before treating a CA-UTIs with antibiotics Encourage adequate fluid intake (approximately 30 mL/kg per day with a 1,500 mL/day minimum or as indicated based on the patient’s medical condition) d. Do not clamp the catheter or drainage tube Do not give the asymptomatic patient antibiotics and antimicrobials as a UTI prevention strategy Do not perform bladder or catheter irrigation unless medically necessary (eg, tissue/blood clots obstructing drainage). If catheter patency is questioned or occlusion is suspected, scan the bladder to assess urine volume CA-UTIs, catheter-associated urinary tract infections; IUC, indwelling urinary catheter; UTI, urinary tract infection Adapted from references 9, 14, 34-36. 16 I N D E P E N D E N T LY D E V E L O P E D B Y M C M A H O N P U B L I S H I N G ll A A 2010 article by Stickler and Feneley noted that P. mirabilis was the main cause of crystalline biofilms that encrust and block IUCs, and that elimination of this organism by antibiotics as soon as it appears could reduce associated complications.30 However, as the presence of the biofilm inhibits antimicrobial activity, organisms within the biofilm cannot be eradicated by antimicrobial therapy alone.29 The urinary biofilm provides a protective environment for the microorganisms, which allows evasion of the activity of antimicrobial agents. The biofilm also allows for microbial attachment to catheter surfaces in a manner that does not allow for removal with gentle rinsing, such as irrigation.20,31 Biofilms can begin to develop within the first 24 hours after catheter insertion and have reportedly grown so thick in some circumstances as to block a catheter lumen.26 The presence of urinary catheter biofilms has important implications for antimicrobial resistance, diagnosis of UTIs, and prevention and treatment of CA-UTIs. In those patients who need an IUC for long-term bladder management, prevention of associated complications relies on adherence to catheter care practices that are outlined in Table 3.9,14,34-36 unexplained fever may be the only symptoms of CA-UTI in elderly patients. Similarly, diagnosing catheter-related infection in patients with spinal cord injury may be especially challenging from the standpoint of history and physical examination because of frequent lack of localizing symptoms.32 Often, the only symptoms of CA-UTI in these patients are fever, diaphoresis, abdominal discomfort, or increased muscle spasticity.33 A New Focus on Strategies for Prevention Of CA-UTIs s ite d. ib te oh no pr e is is rw on si he is ot m er ss le tp un ou up ith ro w G rt ng pa hi in is bl or Pu le ho on ah in w cM n M tio 10 uc 20 od © pr ht Re rig ed. py rv se re ht Co rig The 2009 HICPAC Guideline for Prevention of Catheter-Associated Urinary Tract Infections has estimated that between 17% and 69% of hospital-acquired CA-UTIs may be prevented by implementation of an evidencebased prevention program.9 Evidence suggests that certain interventions can reduce the incidence of CA-UTI in patients managed by short-term IUC. These include staff education about catheter management, combined with regular monitoring of CA-UTI incidence, a hospitalwide program to ensure catheterization only when indicated, and prompt removal of the IUC. These practices are outlined in Table 3 and the strategies discussed here should be adopted to prevent CA-UTIs.9,14,34-36 Signs and Symptoms of a CA-UTI In patients with long-term IUC, symptoms are caused by an inflammatory response of the epithelium of the urinary tract to invasion and colonization by bacteria, but usually are nonspecific. Clinical manifestations of UTI (pain, urinary urgency, dysuria, fever, and leukocytosis) are uncommon even when bacteria or yeast is present, and are no more prevalent with positive urine culture results than with negative results. Confusion or REDUCE DEVICE DAYS A 2008 national survey reported by Saint, Kowalski, Kaufman, Hofer, Kauffman, Olmsted et al, noted that many hospitals were not paying attention to IUC use.37 Less than 50% of hospitals responding to the survey monitored whether patients had an IUC in place, and less than 25% knew the duration of catheterization (referred to as “Foley” days). The longer the IUC remains in place, Table 4. Bladder Bundlea Practices and Measures Measures Nurse-initiated IUC discontinuation protocol Prevalence rate of IUC utilization (number of IUC-days/ total number of patient-days during a period of time) Institute IUC reminder and removal prompts Review indications for insertion (Table 1) Determine urine volume in the bladder using portable ultrasound bladder monitoring (eg, BladderScan®) Prevalence rate of unnecessary IUC utilization (number of unnecessary urinary catheter-days/total number of patient-days during a period of time) Ensure catheter insertion care and maintenance Rate of discontinuation of unnecessary IUCs (number of unnecessary catheters discontinued/number of IUC evaluated for which no indication was found) d. Practices Consider alternatives to IUC IUC, indwelling urinary catheter a Bundle is a set of evidence-based practices that are generally mean to be implemented together. Adapted from reference 47. I N D E P E N D E N T LY D E V E L O P E D B Y M C M A H O N P U B L I S H I N G 17 ll A the greater the risk for development of a CA-UTI, so the cornerstone of a prevention program is to remove the IUC as soon as possible. The most important strategy for prevention of CA-UTIs is to avoid insertion of a catheter; however, if a catheter must be used, duration of catheterization should be limited to the shortest time possible. Given that the rate of infection is closely related to the duration of catheterization, the high frequency of inappropriate catheterization, and the finding that physicians often are unaware of catheter presence, it is apparent that an automatic urinary catheter “stop order” or other reminder would be appropriate. Such an innovative, systemwide administrative intervention, similar to an antibiotic stop order, ideally would remind physicians and nurses that a patient has an IUC in place, which in turn might help reduce inappropriate catheterization.38,39 deter the reinsertion of a catheter. Bladder monitoring should include the use of noninvasive techniques such as portable ultrasound bladder volume technology (eg, BladderScan) to detect postvoid residual (PVR) urine amounts as high PVR or unnecessary catheterizations can lead to a UTI. Catheters should not be used as a substitute for nursing care in the incontinent patient. They should not be inserted to obtain urine specimen for culture or other diagnostic tests in a patient who voids, nor should they be used postoperatively without appropriate indications. Approximately 15% of CA-UTIs have been linked to poor aseptic techniques when cleansing the urinary meatus area and inserting and maintaining the catheters. However, studies have shown that vigorous twice-daily meatal cleansing does not seem to reduce rates of urinary infections. Infection control-based IUC practices may enhance patient safety and decrease catheter-related costs, therefore, knowledge of and education on proper hand hygiene practices is appropriate. Hand washing is the best way to prevent spread of most nosocomial infections. Rates of hand washing among health care providers usually range from 20% to 50% per patient encounter, although some studies have reported hand-washing rates as high as 81%.48 Viable pathogens (eg, Acinetobacter spp., Clostridium difficile, Klebsiella spp., MRSA, Pseudomonas spp., VRE, and yeasts including Candida) often are found on the hands of health care providers. Alcohol-based handwashing solutions generally are considered to be more effective and are believed to have higher compliance rates than soap and water.49 s ite d. ib te oh no pr e is is rw on si he is ot m er ss le tp un ou up ith ro w G rt ng pa hi in is bl or Pu le ho on ah in w cM n M tio 10 uc 20 od © pr ht Re rig ed. py rv se re ht Co rig STAFF HYGIENE DEVELOP APPROPRIATE INFRASTRUCTURE AND SURVEILLANCE GUIDELINES FOR CATHETER USE Hospitals should provide and implement written guidelines for catheter use, insertion, and maintenance. It is necessary to develop a protocol that describes steps to take following catheter removal in order to 18 I N D E P E N D E N T LY D E V E L O P E D B Y M C M A H O N P U B L I S H I N G CATHETER CARE PRACTICES The recommended practices for IUC care are detailed Table 3 and include the following categories: aseptic catheter-insertion procedures; care of the drainage bag; maintenance of catheter patency; perineal care; catheter irrigation; fluid and hydration; hand washing and glove use; and patient, physician, and nursing staff education. In order to prevent CA-UTIs, a “closed” system should be used and the catheter should be removed as soon as possible. A systematic review suggested that use of sealed (eg, taped or presealed) drainage systems contribute to preventing bacteriuria.50 The basic components of a closed system include the catheter, a preconnected collecting tube with an attached sampling port, and a vented bag with a port for drainage. In general, the use of oral antibiotics and urinary acidifying agents, antimicrobial bladder irrigations, antimicrobial drainage bag solutions, and topical meatal antiseptics have been studied. It has been shown that bacteriuria and UTI can be suppressed temporarily, but resistant in • • • • • • • • d. The next strategy that hospitals should employ to prevent CA-UTIs is an appropriate infrastructure that includes some type of surveillance. Strong surveillance programs can control CA-UTIs and implementing appropriate “best-practice” protocols for catheter management can decrease prevalence of hospital-acquired UTIs. Multiple references in the literature deal with hospitalwide surveillance programs that have been successful in reducing hospital-acquired UTIs.35,39-45 Hospitals need to understand resources as well as current practices and problems so a plan can be developed for monitoring IUC use and preventing CA-UTIs. A 2008 article by Fakih, et al reported on the effect of nurse-led multidisciplinary rounds on 10 medicalsurgical units looking at the unnecessary use of IUCs.46 The group reviewed patient records to determine appropriate indication for IUC use. If indication for IUC placement was unfounded, the patient’s nurse was asked to contact the physician to request discontinuation. In this study, more than two-thirds of IUC placements did not have a clear indication. These authors found this simple monitoring intervention enabled a 10% reduction in unnecessary catheter placement. In 2010, Meddings et al conducted a systematic review and meta-analysis of the effectiveness of reminder systems to reduce CA-UTI and found that IUC reminders and stop orders decreased the rate of CA-UTIs by 50%.38 In 2009, Saint and colleagues implemented a “bladder bundle” that focused on preventing CA-UTIs by optimizing the use of IUCs with emphasis on daily assessment and removal as soon as possible. Saint’s bladder bundle practices and measures are described in Table 4.47 flora eventually appear. All staff caring for patients with IUCs in place should be taught methods that may prevent cross-contamination of bacteria. AVOID REPEAT CATHETERIZATIONS ll A Bladder monitoring should not be performed with repeat catheterizations, rather noninvasive technology (eg, portable bladder volume ultrasound) to avoid unnecessary catheterizations and to prevent UTIs is recommended. Portable bladder volume ultrasound has been used for more than 2 decades to measure urinary retention and has been advocated by some to reduce the need for catheterization.51 Portable bladder volume ultrasound accurately measures urine volume. Additionally, these “scanners” have been found to reduce the number of intermittent catheterizations and to perhaps even decrease the risk for UTIs.52 of their patients have indwelling urinary catheters? Am J Med. 2000;109(6):476-480. 5. Saint S, Lipsky BA, Goold SD. Indwelling urinary catheters: A one-point restraint? Ann Intern Med. 2002;137(2):125-127. 6. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. 7. Holroyd-Leduc JM, Sen S, Bertenthal D, et al. The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients. J Am Geriatric Soc. 2007;55(2):227-233. 8. Wald H, Epstein A, Kramer A. Extended use of indwelling urinary catheters in postoperative hip fracture patients. Med Care. 2005;43(10):1009-1017. s ite d. ib te oh no pr e is is rw on si he is ot m er ss le tp un ou up ith ro w G rt ng pa hi in is bl or Pu le ho on ah in w cM n M tio 10 uc 20 od © pr ht Re rig ed. py rv se re ht Co rig 9. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA; Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. Alternative Methods of Bladder Management There are several alternatives to management of patients with bladder dysfunction that cause fewer complications than use of IUC. These include urinals, external catheters in men, absorbent products for urinary incontinence, and intermittent catheterization for urinary retention.16,53 Urinals and absorbent products contain urine leakage in patients with incontinence. External devices are secured to the skin with adhesive or straps and are connected to a tube and collecting bag. They primarily are used in men with urinary incontinence. Intermittent catheterization is the insertion of a catheter into the bladder to allow for drainage. The catheter is removed after drainage. This type of catheterization is used in patients with urinary retention as it minimizes episodes of overdistention of bladder and frequent, regular bladder emptying prevents UTI. There is no limit to the number of times a patient can be intermittently catheterized safely. Conclusions IUCs are a significant source of hospital infections. The best prevention is to avoid insertion of an IUC at all costs, but if needed, the duration of catheterization should be limited. Nursing and physician staff can be instrumental in preventing a CA-UTI with the implementation of hospital-wide surveillance programs and appropriate catheter care protocols developed from evidence-based practices. Health care providers need to be aware of the current guidelines on the use of IUCs to ensure patient safety and best practices. 1. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160-166. 2. Kunin CM. Nosocomial urinary tract infections and the indwelling catheter: what is new and what is true? Chest. 2001;120(1):10-12. 3. Anderson DJ, Kirkland KB, Kaye KS, et al. Underresourced hospital infection control and prevention programs: penny wise, pound foolish? Infect Control Hosp Epidemiol. 2007;28(7):767-773. 4. Saint S, Wiese J, Amory JK, et al. Are physicians aware of which 11. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1): 68-75. 12. Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Federal Regist. 2007;72(162):47129-48175. http://www.cms.gov/ AcuteInpatientPPS/. Accessed August 10, 2010. 13. Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2009 rates. Federal Regist. 2008;73(161):48473-48491. http://www.cms.gov/ AcuteInpatientPPS/. Accessed August 10, 2010. 14. Wald H, Kramer AM. Nonpayment for harms resulting from medical care catheter-associated urinary tract infections. JAMA. 2007; 298(23):2782-2784. 15. Schaeffer AI, Schaeffer EM. Infections of the urinary tract. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, eds. CampbellWalsh Urology. 9th ed. Philadelphia, PA: Saunders; 2007:223-303. 16. Newman DK, Wein AJ. Managing and Treating Urinary Incontinence, 2nd Ed. Baltimore, MD: Health Professions Press;2009:365-483. 17. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents. 2001;17(4):299-303. 18. Mathur S, SullerMT, Stickler DJ, Feneley RC. Genotyping of urinary and fecal Proteus mirabilis isolates from individuals with long-term urinary catheters. Eur J Clin Microbiol Infect Dis. 2005;24(9): 643-644. 19. Pratt RJ, Pellowe CM, Wilson JA, et al. Epic2: national evidencebased guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2007;65(suppl 1):S1-S64. 20. Donlan RM Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167-193. 21. Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin Infect Dis. 2009;48(9):1182-1188. 22. Shuman EK, Chenoweth CE. Recognition and prevention of healthcare-associated urinary tract infections in the intensive care unit. Crit Care Med. 2010;38(suppl 8):S373-S379. d. References 10. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. 23. Goetz LL, Howard M, Cipher D, Revankar SG. Occurrence of candiduria in a population of chronically catheterized patients with spinal cord injury. Spinal Cord. 2010;48(1):51-54. 24. Trautner BW, Darouiche RO. Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control. 2004;32(3):177-183. 25. Morris NS, Stickler DJ, McLean RJ. The development of bacterial biofilms on indwelling urethral catheters. World J Urol. 1999;17(6):345-350. I N D E P E N D E N T LY D E V E L O P E D B Y M C M A H O N P U B L I S H I N G 19 26. Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infec Dis North Am. 2003;17(2):411-432. healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. 27. Saye D.E. Recurring and antimicrobial-resistant infections: considering the potential role of biofilms in clinical practice. Ostomy Wound Manage. 2007;53(4):46-48, 50, 52. 42. Rothfeld AF, Stickley A. A program to limit urinary catheter use at an acute care hospital. Am J Infect Control. 2010;38(7):568-571. 28. Talsma SS. Biofilms on medical devices. Home Healthcare Nurse. 2007;25(9):589-594. 43. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. 29. Falagas ME, Kapaskelis AM, Kouranos VD, Kakisi OK, Athanassa Z, Karageorgopoulos DE. Outcome of antimicrobial therapy in documented biofilm-associated infections: a review of the available clinical evidence. Drugs. 2009;69(10):1351-1361. 44. Topal J, Conklin S, Camp K, Morris V, Balcezak T, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual 2005;20(3):121-126. ll A 30. Stickler DJ, Feneley RC. The encrustation and blockage of longterm indwelling bladder. Spinal Cord. Apr 6, 2010 [Epub ahead of print]. 46. Fakih MG, Dueweke C, Meisner S, et al. Effect of nurse-led multidisciplinary rounds on reducing the unnecessary use of urinary catheterization in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(9):815-819. ite d. ib te oh no pr e is is rw on si he is ot m er ss le tp un ou up ith ro w G rt ng pa hi in is bl or Pu le ho on ah in w cM n M tio 10 uc 20 od © pr ht Re rig ed. py rv se re ht Co rig 31. Donlan RM.Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33(8):1387-1392. 45. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. s 32. Tambyah PA. Catheter-associated urinary tract infections: diagnosis and prophylaxis. Int J Antimicrob Agents. 2004;24 (suppl 1):S44-S48. 33. Biering-Sørensen F, Bagi P, Høiby N. Urinary tract infections in patients with spinal cord lesions: treatment and prevention. Drugs. 2001;61(9):1275-1287. 34. Gray ML. Securing the indwelling catheter. Am J Nurs. 2008; 108(12):44-50. 35. Lo E, Nicolle L, Classen D, et al. Strategies to prevent catheterassociated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S41-S50. 36. Newman D. The indwelling urinary catheter: Principles for best practice. J Wound, Ostomy, and Continence Nursing. 2007;24(6): 655-661. 37. Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospitalacquired urinary tract infection in the United States: a national study. Clin Infect Dis. 2008;46(2):243-50. 38. Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560. 39. Loeb M, Hunt D, O’Halloran K, Carusone SC, Dafoe N, Walter SD. Stop orders to reduce inappropriate urinary catheterization in hospitalized patients: a randomized controlled trial. J Gen Intern Med. 2008;23(6):816-820. 40. Wenger JE. Cultivating quality: reducing rates of catheter-associated urinary tract infection. Am J Nurs. 2010;110(8):40-45. 47. Saint S, Olmsted RN, Fakih MG, et al. Translating health careassociated urinary tract infection prevention research into practice via the bladder bundle. Jt Comm J Qual Patient Saf. 2009;35(9):449-455. 48. Boyce JM, Pittet D. Guideline for hand hygiene in healthcare settings: recommendations of hand hygiene in healthcare settings: recommendations of the Healthcare Infection Control Practices Advisory Commission and the KIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30(8):1-46. 49. Hilburn J, Hammond BS, Fendler EK, Groziak PA. Use of alcohol hand sanitizer as an infection control strategy in an acute care facility. Am J Infect Control. 2003;31(2):109-116. 50. Dunn S, Kowanko I, Paterson J, Pretty L. Systematic review of the effectiveness of urinary continence products. J Wound Ostomy Continence Nurs. 2002;29(3):129-142. 51. Sparks A, Boyer D, Gambrel A, et al. The clinical benefits of the bladder scanner: a research synthesis. J Nurs Care Qual. 2004;19(3):188-192. 52. Moore DA, Edwards K. Using a portable bladder scan to reduce the incidence of nosocomial urinary tract infections. MEDSURG Nursing. 1997;6(1):39-43. 53. Newman DK. Internal and external urinary catheters: a primer for clinical practice. Ostomy Wound Manage. 2008;54(12):18-35. 41. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce d. 20 I N D E P E N D E N T LY D E V E L O P E D B Y M C M A H O N P U B L I S H I N G