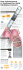BAL-CATH* Systems.
Transcription
BAL-CATH* Systems.
KIMBERLY-CLARK* BAL-CATH* Systems. Ventilator Associated Pneumonia (VAP) diagnosis The KIMBERLY-CLARK* BAL-CATH* System is a non-bronchoscopic bronchoalveolar lavage catheter (mini-BAL) that facilitates the diagnosis of VAP. BAL-CATH* is a 12 FR/16FR telescoping catheter that is positioned in the distal airway to obtain an alveolar lavage sample. CDC recommends targeting specific pathogens to prevent antimicrobial resistance in hospitalized adults. Semi-quantitative cultures of tracheal aspirates cannot be used as reliably as quantitative cultures to define the presence of pneumonia and the need for antibiotic therapy. Studies suggest that the use of quantitative cultures increase specificity of the diagnosis of hospital acquired pneumonia (HAP). If bronchoscopic sampling is not immediately available, non-bronchoscopic sampling can reliably obtain lower respiratory tract secretions for quantitative cultures, which can be used to guide antibiotic therapy decisions. The Kimberly-Clark BAL-CATH*catheter allows safe BAL, without loss of diagnostic yield, when visualization is not required in mechanically ventilated patients. BAL-CATH* allows maintenance of PEEP when used with the supplied ventilator circuit adapter. BAL-CATH* has shown to have similar accuracy to Bronchoscopic BAL and Bronchoscopic PSB. BAL samples can be obtained within a short period of time by using the BAL-CATH* system. Trained clinicians can safely and accurately perform mini-bronchoaveolar lavage with the BAL-CATH*catheter. The atraumatic radiopaque directional tip allows selective right or left lung sampling. Ventilator adapter with patented PEEP seal is included with kit. KIMBERLY-CLARK*BAL-CATH* Systems Is just one of the clinical solutions that you can depend on to meet the demands of your fast-paced world. Whether your needs involve preventing healthcare-associated infections, surgical and digestive solutions or pain management, with Kimberly-Clark you’ll always have one less worry. KIMBERLY-CLARK* BAL-CATH* System. KIMBERLY-CLARK* BAL-CATH* System Stock # Outer Catheter Inner Catheter Description 141 16Fr 12Fr Non-Bronchoscopic Bronchoalveolar Lavage Catheter Access Port Elbow Packaging 5/Case 1 1/2" 3-Way Stop Cock Ventilator adapter elbow included in kit Suction Tube Adapter Numeric Depth Markings Supplemental Oxygen Port Soft Atraumatic Radiopaque Tip Directional 16 Fr Outer Catheter Protected 12 Fr Inner Catheter t ity i +K * + Par ne er l y -Clar k mb rs i n Qual Commitment to Excellence If, for any reason, our products do not meet your expectations, please let us know your comments or suggestions for improvement. Your input will result in a concerted effort on our part to meet your requirements. Our goal is to provide quality products that completely meet your needs time after time. For more information, please call 1-800-KCHELPS in the United States, or visit our web site at www.kchealthcare.com. *Registered Trademark or Trademark of Kimberly-Clark Worldwide, Inc. or its affiliates. ©2006 KCWW. All rights reserved. KLM-256 H8346/Z0417
Similar documents
Peripheral I.V. Catheter Market Analysis and Demand Forecast to 2022
 The global peripheral I.V. catheter market was valued at $3,702.2 million in 2015, and it is expected to grow at a CAGR of 6.0% during the period 2016 - 2022. The global market is increasing, due to growing geriatric population and increasing incidence of chronic diseases. In addition, the growing demand for injectable drugs in comparison to the oral medications, increasing healthcare expenditure and technological advancements in peripheral I.V. catheter is encouraging the growth of the global peripheral I.V. catheter market.
The global peripheral I.V. catheter market was valued at $3,702.2 million in 2015, and it is expected to grow at a CAGR of 6.0% during the period 2016 - 2022. The global market is increasing, due to growing geriatric population and increasing incidence of chronic diseases. In addition, the growing demand for injectable drugs in comparison to the oral medications, increasing healthcare expenditure and technological advancements in peripheral I.V. catheter is encouraging the growth of the global peripheral I.V. catheter market.