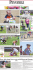Eggs-troidinary Eggs
Transcription
Eggs-troidinary Eggs
Eggs-troidinary Eggs-periment! Collect: • 2 eggs • 2 large jars or coffee mugs (clear works best for observing changes) • Corn syrup • Vinegar • Water • Food coloring Remember: Wash your hands after handling raw eggs. Day 1 & 2 1. Place both eggs in the bottom of one jar. Pour vinegar into the jar until both eggs are covered completely. 2. Wait two days before taking the eggs out of the vinegar. As you wait, observe any changes to the surface of the eggs. Day 3 3. Carefully take the eggs out of the jar without breaking the eggs. What happened to the shell? Do they both look and feel the same? 4. Rinse the jar and eggs to remove any remaining eggshell pieces. 5. Put one shell-less egg at the bottom of each jar. Cover one egg with corn syrup and the other egg with colored water. 6. Place them both in the refrigerator and wait one day before removing the eggs. As you wait, make a hypothesis of what you think might happen to each egg. Day 4 7. Carefully remove each egg from the jars. Do they look the same? What changed? What happened? Calcium makes eggshells strong in order to protect the inside of the egg. When the eggs are added to an acid like vinegar, the acid dissolves the calcium of the shell. Luckily, the egg has another defense. Underneath the shell are two thin membranes that protect the egg from harmful bacteria. These membranes are not made of calcium, so they don’t dissolve in the acidic vinegar. This leaves you with a shell-less, rubbery egg. 601 Light Street Baltimore, MD 21230 • www.marylandsciencecenter.org The two inner membranes of the egg are selectively permeable, which means that they will allow small molecules like water to pass through, but not larger molecules like sugar. Water molecules always move from areas with more water molecules, like tap water, to areas with fewer water molecules, like corn syrup. When the shell-less egg sits in water, the water molecules pass through the membrane into the egg which has a lower concentration of water molecules. If you use colored water, this changes the color of the egg and fills it with lots of water, keeping it firm. Corn-syrup is made up of a small percentage of water molecules and a larger percentage of sugar molecules. Sugar molecules are too big to pass through the membrane. Since the higher concentration of water inside of the egg can still pass through the membrane into the corn syrup, the egg loses its water molecules into the corn syrup which causes the egg to shrivel up. 601 Light Street Baltimore, MD 21230 • www.marylandsciencecenter.org