Bacterin Biologics Catalog
Transcription
Bacterin Biologics Catalog
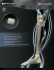
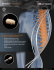
Bacterin Biologics 2015 Product Guide Contents Welcome to Bacterin Biologics 3 Product Codes Reference 4 BacteRinse Process 7 OsteoSponge 8 OsteoSponge SC 11 OsteoSelect DBM Putty 13 OsteoWrap | OrbitalWrap HD 15 OsteoSTX 16 3Demin 17 BacFast 18 Precision-Milled & Traditional Allografts 19 hMatrix ADM & hMatrix PR ADM 20 Sports Medicine 21 Need More Info? 22 Circle of Life 23 2 bacterin.com Bacterin is ISO 13485:2003 certified, registered with the FDA, accredited by the AATB, and has state licensures where required. All current licenses and certifications can be found at bacterin.com Welcome to Bacterin Biologics Bacterin upholds the highest levels of safety, efficacy, and quality with every product. About Bacterin Bacterin is a biotechnology organization focused on biologic-based applications for orthopedic and reconstructive surgical procedures. We take pride in our role as stewards of the gift of donation and the dimensional healing that occurs with both, patients and donor families. We thank you for the role you play in the Circle of Life and invite you to learn more about the Value Bacterin has to offer. Advanced Processing Technologies Bacterin has developed a patented demineralization technology that is engineered to preserve the native growth factors inherent bone. These growth factors are essential for the tissue regeneration process in the patients’ body. Bacterin has additional patent-pending methods focused on demineralization of tissue, designed to extend more desirable handling characteristics for the surgeon and clinical benefits for the patient. Our BacteRinse Process is also utilized ensuring all allografts are processed aseptically using progressive methods, and finalized with low-dose gamma irradiation. Proven Clinical Efficacy Clinical efficacy is of considerable importance to Bacterin and the continuum of care we strive to provide on behalf of our donors for the patients. Our products have been featured in various publications yielding results as high as 97.5% fusion rates. We take pride in knowing the science in our allografts provides the best possible outcomes for patients. Safety Patient safety is of the utmost importance. There have been many scientific advances in irradiation technologies that have minimized the effects on allograft integrity. 100% of Bacterin allografts and biologic devices are sterilized via low-dose gamma irradiation in the final packaging, with a sterility assurance level of 10 -6 (SAL 10 -6). This is commonly referred to as device level sterility and is accomplished using FDA approved, validated methods. bacterin.com 3 Bacterin Biologics Product Codes Call Point Legend FA Foot and Ankle H Hip UE Upper Extremity S Spine K Knee PR Plastic and Reconstructive C Cranio-Maxillofacial W Wound OsteoSponge Filler OsteoSponge Block FA FA H S UE C K H S UE 109405 OsteoSponge Filler Fine 0.5cc 1–4 mm Chips 109608 OsteoSponge Block 8 mm 109210 OsteoSponge Filler Fine 1.0cc 1–4 mm Chips 109609 OsteoSponge Block 8 mm — 10 pack 109225 OsteoSponge Filler Fine 2.5cc 1–4 mm Chips 109610 OsteoSponge Block 10 mm 109250 OsteoSponge Filler Fine 5.0cc 1–4 mm Chips 109612 OsteoSponge Block 12 mm 109310 OsteoSponge Filler Fine 10.0cc 1–4 mm Chips 109614 OsteoSponge Block 14 mm 109315 OsteoSponge Filler Fine 15.0cc 1–4 mm Chips 109330 OsteoSponge Filler Fine 30.0cc 1–4 mm Chips OsteoSponge SC 109410 OsteoSponge Filler 1.0cc 4–10 mm Chips FA 109425 OsteoSponge Filler 2.5cc 4–10 mm Chips 109550 OsteoSponge Filler 5.0cc 4–10 mm Chips 109816 OsteoSponge SC 6 mm 109510 OsteoSponge Filler 10.0cc 4–10 mm Chips 109818 OsteoSponge SC 8 mm 109515 OsteoSponge Filler 15.0cc 4–10 mm Chips 109830 OsteoSponge SC 10 mm 109530 OsteoSponge Filler 30.0cc 4–10 mm Chips 109832 OsteoSponge SC 12 mm 159550 OsteoSponge Filler 5.0cc — Syringe 159510 OsteoSponge Filler 10.0cc — Syringe OsteoSponge Disc 159515 OsteoSponge Filler 15.0cc — Syringe FA K C PR OsteoSponge Strip 109501 OsteoSponge Disc, 10 mm FA 109502 OsteoSponge Disc, 12 mm 109503 OsteoSponge Disc, 14 mm H S UE C 109618 OsteoSponge Strip 12 × 8 × 8 mm 109621 OsteoSponge Strip 50 × 5 × 5 mm OsteoWrap | OrbitalWrap HD 109622 OsteoSponge Strip 50 × 7 × 5 mm FA 109631 OsteoSponge Strip 20 × 14 × 5 mm 109632 OsteoSponge Strip 20 × 14 × 7 mm 109701 OsteoWrap 10 × 10 mm 109633 OsteoSponge Strip 26 × 19 × 7 mm 109702 OsteoWrap 15 × 10 mm 109634 OsteoSponge Strip 30 × 10 × 7 mm 109703 OsteoWrap 15 × 15 mm 109635 OsteoSponge Strip 50 × 10 × 7 mm 109711 OsteoWrap 20 × 20 mm 109636 OsteoSponge Strip 50 × 20 × 7 mm 109712 OsteoWrap 30 × 30 mm 109637 OsteoSponge Strip 26 × 19 × 7 mm — 2 pack 109713 OsteoWrap 50 × 25 mm 109638 OsteoSponge Strip 26 × 19 × 7 mm — 4 pack 109714 OsteoWrap 70 × 40 mm 109640 OsteoSponge Strip 40 × 15 × 5 mm 109715 OsteoWrap 60 × 50 mm 109722 OrbitalWrap HD - 20 × 20 × 2 mm 109733 OrbitalWrap HD - 30 × 30 × 2 mm 4 bacterin.com H S UE C PR 3Demin Cortical Fibers FA K H S OsteoSTX UE S 109762 3Demin Cortical Fibers 2.5 cc 109705 OsteoSTX 50 mm – 10 per pack 109765 3Demin Cortical Fibers 5.0 cc 109710 OsteoSTX 100 mm – 10 per pack 109760 3Demin Cortical Fibers 10.0 cc 109720 OsteoSTX 200 mm – 10 per pack 3Demin Boats Traditional Allografts FA FA K H S UE H S UE 109785 3Demin Boat 50 × 25 mm — 2 pack 101006 Ilium Tricortical Block 6 mm 109781 3Demin Boat 100 × 25 mm — 2 pack 101007 Ilium Tricortical Block 7 mm 101008 Ilium Tricortical Block 8 mm 3Demin Strips 101009 Ilium Tricortical Block 9 mm FA 101010 Ilium Tricortical Block 10 mm 101011 Ilium Tricortical Block 11 mm K H S UE 109775 3Demin Strip 50 × 10 mm — 2 pack 101012 Ilium Tricortical Block 12–14 mm 109771 3Demin Strip 100 × 10 mm — 2 pack 101015 Ilium Tricortical Block 15 mm 109772 3Demin Strip 200 × 10 mm — 2 pack 101016 Ilium Tricortical Block 16–21 mm 101022 Ilium Tricortical Block 22–25 mm 101040 Ilium Tricortical Strip 40 mm 101045 Ilium Tricortical Strip 45 mm Precision-Milled Allografts S 101050 Ilium Tricortical Strip 50 mm 100511 BacFast HD Facet Dowel, 5 mm 101055 Ilium Tricortical Strip 55 mm 100512 BacFast HD Facet Dowel, 6 mm 101060 Ilium Tricortical Strip 60 mm 1563305 Cervical Spacer, Lordotic, 5 mm 103015 Cancellous Crushed 0.1–4 mm 5cc 1563306 Cervical Spacer, Lordotic, 6 mm 103115 Cancellous Crushed 0.1–4 mm 15cc 1563307 Cervical Spacer, Lordotic, 7 mm 103130 Cancellous Crushed 0.1–4 mm 30cc 1563308 Cervical Spacer, Lordotic, 8 mm 103045 Cancellous Crushed 4–10 mm 5cc 1563309 Cervical Spacer, Lordotic, 9 mm 103415 Cancellous Crushed 4–10 mm 15cc 1563310 Cervical Spacer, Lordotic, 10 mm 103430 Cancellous Crushed 4–10 mm 30cc 104205 Unicortical Block 5 mm 104206 Unicortical Block 6 mm 104207 Unicortical Block 7 mm 104208 Unicortical Block 8 mm 104209 Unicortical Block 9 mm 104210 Unicortical Block 10 mm 106040 Fibula Segment 40–100 mm 106101 Fibula Segment 101–150 mm 105300 Femoral Cortical Strut — 200 mm Split OsteoSelect DBM Putty FA H S UE 309005 OsteoSelect DBM Putty 0.5cc 309010 OsteoSelect DBM Putty 1.0cc 309025 OsteoSelect DBM Putty 2.5cc 309050 OsteoSelect DBM Putty 5.0cc 309100 OsteoSelect DBM Putty 10.0cc 359025 OsteoSelect DBM Putty 2.5cc — Syringe 359050 OsteoSelect DBM Putty 5.0cc — Syringe 359100 OsteoSelect DBM Putty 10.0cc — Syringe bacterin.com 5 hMatrix ADM Sports Medicine FA FA W K 208030 hMatrix Dermis 2 × 1 cm, Thin 208000 Achilles Tendon w/Bone Block 208031 hMatrix Dermis 4 × 2 cm, Thin 208002 Anterior Tibialis Tendon 208032 hMatrix Dermis 5 × 4 cm, Thin 208003 Posterior Tibialis Tendon 208033 hMatrix Dermis 7 × 5 cm, Thin 208008 Gracilis Tendon 208034 hMatrix Dermis 10 × 5 cm, Thin 208009 Hemi-Patella Tendon 208052 hMatrix Dermis 2 × 1 cm, Medium 208011 Semitendinosus Tendon 208035 hMatrix Dermis 4 × 2 cm, Medium 208012 Whole Patella Tendon w/Quad 208036 hMatrix Dermis 5 × 4 cm, Medium 208013 Whole Patella Tendon w/o Quad 208037 hMatrix Dermis 7 × 5 cm, Medium 208014 Hemi-Patella Tendon w/Quad 208038 hMatrix Dermis 10 × 5 cm, Medium 208015 Peroneus 208043 hMatrix Dermis 2 × 1 cm, Thick 268012 SportMatrix ADM 12 × 1 cm, Medium 208039 hMatrix Dermis 4 × 2 cm, Thick 208040 hMatrix Dermis 5 × 4 cm, Thick 208041 hMatrix Dermis 7 × 5 cm, Thick 208042 hMatrix Dermis 10 × 5 cm, Thick 208231 hMatrix Dermis 4 × 2 cm, Thin, Meshed 208232 hMatrix Dermis 5 × 4 cm, Thin, Meshed 208233 hMatrix Dermis 7 × 5 cm, Thin, Meshed 208234 hMatrix Dermis 10 × 5 cm, Thin, Meshed hMatrix PR ADM PR 218166 hMatrix PR - Dermis 16 × 6 cm, Thin 228168 hMatrix PR - Dermis 16 ×8 cm, Medium 228160 hMatrix PR - Dermis 16 × 10 cm, Medium 228208 hMatrix PR - Dermis 20 × 8 cm, Medium 228320 hMatrix PR - Dermis 20 × 16 cm, Medium Pericardium and Fascia Lata FA W 108004 hMatrix Fascia Lata 30 × 30mm 108005 hMatrix Fascia Lata 50 × 40mm 108006 hMatrix Fascia Lata 70 × 50mm 108007 hMatrix Fascia Lata 100 × 50mm 108021 Pericardium 30 × 30mm 108010 Pericardium 40 × 40mm 108018 Pericardium 50 × 40mm 108019 Pericardium 70 × 50mm 108024 Pericardium 100 × 20mm 108020 Pericardium 100 × 50mm 6 bacterin.com The BacteRinse Proprietary Process Aseptic Recovery of Donor Tissue Tissue recovery takes place in prequalified settings Direct tissue sampling at time of recovery Donor Screening Exceeds FDA and AATB Standards Extensive microbiological testing on all recovered donor tissues Serological testing using the latest NAT (Nucleic Acid Testing) techniques Aseptic Processing to Prevent Introduction of Microbial Bioburden Utilization of ISO 5 cleanrooms Processing staff wears full isolation PPE v We process each graft in ISO 5 (Class 100) cleanrooms using the highest level of manual craftsmanship Meticulous Cleansing of Tissue Lysing of cellular components Removal of bone marrow and blood elements to consistently meet our product specifications for the surgical needs of medical providers. Bacterin has engineered its processing methods to effectively clean and decontaminate allograft tissue while minimizing Validated, Rigorous Disinfection of Tissue Proven log reduction against a panel of representative microbes Effective removal of processing reagents exposure of the extracellular matrices to potentially damaging treatment steps. Validated Sterilization with an SAL of 10-6 This preserves the osteoinductive Low dose, low temp potential of our DBM allografts ASNI/AAMI/ISO VD MAX or Method 2B and maintains the biomechanical integrity of our soft tissue grafts. bacterin.com 7 OsteoSponge Features JJ 100% human demineralized cancellous bone JJ Osteoconductive, interconnected porosity JJ Osteoinductive potential JJ Elastic, compressible handling characteristics JJ Radiolucent to allow for accurate follow-up JJ Sterility assurance level (SAL) 10 -6 JJ 5 year shelf life, room-temperature storage OsteoSponge can be cut to perfectly fit any cage OsteoSponge Benefits OsteoSponge is a novel form of demineralized bone matrix (DBM) made from 100% bone. Derived from trabecular bone, OsteoSponge provides a natural scaffold for cellular ingrowth and exposes bone-growth-inducing proteins to the healing environment. The malleable properties of OsteoSponge enable it to fill and conform to irregular bony defects. Due to its shape memory characteristics, OsteoSponge will expand to completely fill a void after graft placement. The unique mechanical and biological properties make OsteoSponge an ideal bone graft for use with all non-weight bearing applications where fusion is desired. The compressible characteristics of OsteoSponge OsteoSponge Strip OsteoSponge Block 8 bacterin.com “OsteoSponge absolutely works. I haven’t worked with a product that is as reliable and easy to work with as OsteoSponge.” Dr. Kevin Stevenson, MD The Science OsteoSponge is processed with a validated method which includes a patented demineralization step engineered to preserve growth factors native to cancellous bone. The efficacy and saftey of this processing method has been clinically proven. When utilized in TLIF procedures in the lumbar spine, OsteoSponge has demonstrated fusion rates equivalent to historical controls without any reported complications.1 OsteoSponge has been successfully utilized in foot and ankle fusion procedures. Both retrospective2 and prospective3 data indicate that OsteoSponge can produce Figure 1 fusion rates equivalent to or higher than historic controls. Reported pain and function outcomes were statistically improved over historical controls. Growth factors found in bone TGF-(Beta) 1-5 PDGF AA,AB,BB BMP 1-12 IGF 1,2 FGF 1 Girasole, G. et al,. (2013) Transforaminal lumbar interbody fusion rates in patients using a novel titanium implant and demineralized cancellous allograft bone sponge. International Journal of Spine Surgery. 7: e95-e100. 2 Brigido, S.A. et al., (2013) A Retrospective Analysis Evaluating Allogenic Cancellous Bone Sponge for Foot and Ankle Arthrodesis. The Journal of Foot and Ankle Surgery. 52: 28-31. Figure 2 3 Protzman, N.M. et al., (2014) Biologic Augementation of Foot and Ankle Arthrodesis With an Allogenic Cancellous Sponge. Orthopedics. March 37(3): e230-e236. Figures 1 and 2 (above) Neovascularization, osteoid formation, and new bone growth has been consistently observed in subcutaneous and intramuscular implants of OsteoSponge. These results suggest that in addition to providing an excellent natural scaffold for cellular ingrowth To see full, published articles regarding the clinical efficacy of Bacterin biologics, please visit bacterin.com. and proliferation, OsteoSponge also contains growth factors. bacterin.com 9 OsteoSponge Competitive Comparison Few allografts on the market achieve similar clinical efficacy as OsteoSponge, without compromising patient safety and ease of use Osteoinductive Response in Animal Model Contains Bioavailable BMPs Sterile (SAL) 10 -6 Trabecular Bone Structure Interconnected Porosity Osteoconductive Easily Loaded With BMA Room Temperature Storage Cervical Spine Indication OsteoSponge (Bacterin) Infuse ® (Medtronic) OsteoCel Plus (NuVasive) ® Trinity Elite™ (MTF/Orthofix) ® Chronos (DePuy) Vitoss ® (Stryker) Actifuse ® (Baxter) Crushed Cancellous PRP (Autologous) Bacterin was the first tissue bank to market an allograft specifically designed for its compressible handling characteristics, osteoconductivity, and osteoinductive potential. If you aren’t using OsteoSponge, you aren’t using the original ideal bone graft. 10 bacterin.com Posterior Spinal Fusion Indication OsteoSponge SC Features JJ Slightly contoured for ease of placement JJ Osteoconductive, interconnected porosity JJ Osteoinductive potential JJ Elastic, compressible handling characteristics JJ Radiolucent to allow for accurate follow-up JJ Sterility assurance level (SAL) 10 -6 JJ 5 year shelf life, room-temperature storage OsteoSponge SC Benefits OsteoSpnge SC OsteoSponge SC is sterile, radiolucent, has a 5-year shelf life at room temperature, and is characterized by malleable handling properties, making it an excellent alternative to autologous bone or synthetic materials to effectively, safely, and efficiently repair subchondral defects in the knee or other articulating joints. Published clinical data confirm the results of a large animal study demonstrating restoration of the subchondral bone and a healthy joint environment. OsteoSponge SC Surgical Instrumentation Kit Available Upon Request Side View Shows the Tapered Shape of OsteoSpnge SC bacterin.com 11 OsteoSponge Block OsteoSponge Strip 109608 OsteoSponge Block 8mm 109618 OsteoSponge Strip 12 × 8 × 8mm 109609 OsteoSponge Block 8mm – 10 pack 109621 OsteoSponge Strip 50 × 5 × 5mm 109610 OsteoSponge Block 10mm 109622 OsteoSponge Strip 50 × 7 × 5mm 109612 OsteoSponge Block 12mm 109631 OsteoSponge Strip 20 × 14 × 5mm 109614 OsteoSponge Block 14mm 109632 OsteoSponge Strip 20 × 14 × 7mm 109633 OsteoSponge Strip 26 × 19 × 7mm 109634 OsteoSponge Strip 30 × 10 × 7mm OsteoSponge Filler 109405 OsteoSponge Filler Fine 0.5cc 1–4 mm Chips 109635 OsteoSponge Strip 50 × 10 × 7mm 109210 OsteoSponge Filler Fine 1.0cc 1–4 mm Chips 109636 OsteoSponge Strip 50 × 20 × 7mm 109225 OsteoSponge Filler Fine 2.5cc 1–4 mm Chips 109637 OsteoSponge Strip 26 × 19 × 7mm - 2 pack 109250 OsteoSponge Filler Fine 5.0cc 1–4 mm Chips 109638 OsteoSponge Strip 26 × 19 × 7mm - 4 pack 109310 OsteoSponge Filler Fine 10.0cc 1–4 mm Chips 109640 OsteoSponge Strip 40 × 15 × 5mm 109315 OsteoSponge Filler Fine 15.0cc 1–4 mm Chips 109330 OsteoSponge Filler Fine 30.0cc 1–4 mm Chips 109410 OsteoSponge Filler 1.0cc 4–10 mm Chips 109425 OsteoSponge Filler 2.5cc 4–10 mm Chips 109550 OsteoSponge Filler 5.0cc 4–10 mm Chips 109510 OsteoSponge Filler 10.0cc 4–10 mm Chips 109515 OsteoSponge Filler 15.0cc 4–10 mm Chips 109530 OsteoSponge Filler 30.0cc 4–10 mm Chips 159550 OsteoSponge Filler 5.0cc — Syringe 109816 OsteoSponge SC 6 mm Defect 159510 OsteoSponge Filler 10.0cc — Syringe 109818 OsteoSponge SC 8 mm Defect 159515 OsteoSponge Filler 15.0cc — Syringe 109830 OsteoSponge SC 10 mm Defect 109832 OsteoSponge SC 12 mm Defect OsteoSponge Disc 109501 OsteoSponge Disc, 10 mm 109502 OsteoSponge Disc, 12 mm 109503 OsteoSponge Disc, 14 mm OsteoSponge SC See the unique handling characteristics of OsteoSponge, and other Bacterin biologics at: bacterin.com/videos 12 bacterin.com OsteoSelect DBM Putty Features JJ Osteoinductive JJ Osteoconductive JJ Sterility assurance level (SAL) 10 -6 JJ Exceptional handling characteristics JJ Sterile, room-temperature storage JJ Will not dissipate upon direct irrigation JJ Contains 74% demineralized bone matrix (DBM) by dry weight OsteoSelect DBM Putty OsteoSelect DBM Putty Benefits OsteoSelect DBM Putty is engineered with the surgeon in mind. With exceptional handling characteristics, OsteoSelect DBM Putty can be easily molded into any shape and compressed into bone voids. Taking the design a step further, Bacterin has validated a low-dose, low-temperature gamma sterilization process to provide maximum osteoinductive potential while still imparting device-level sterility. Every lot of OsteoSelect DBM Putty is tested for osteoinductivity after gamma sterilization in an animal model. Indications OsteoSelect DBM Putty is indicated for use as a bone void filler and bone graft substitute for voids or gaps that are not intrinsic to the stability of the bony structure. OsteoSelect DBM Putty is indicated for treatment of surgically created osseous defects or osseous defects from traumatic injury to the bone. OsteoSelect DBM Putty can be used as follows: Extremities Posterolateral Spine Pelvis OsteoSelect DBM Putty OsteoSelect DBM Putty in a syringe 309005 OsteoSelect DBM Putty 0.5 cc 309010 OsteoSelect DBM Putty 1.0 cc 309025 OsteoSelect DBM Putty 2.5 cc 309050 OsteoSelect DBM Putty 5.0 cc 309100 OsteoSelect DBM Putty 10.0 cc 359025 OsteoSelect DBM Putty 2.5 cc – syringe 359050 OsteoSelect DBM Putty 5.0 cc – syringe 359100 OsteoSelect DBM Putty 10.0 cc – syringe bacterin.com 13 OsteoSelect DBM Putty Competitive Comparison OsteoSelect DBM Putty is a highly competitive product in the demineralized bone matrix space, providing many benefits to the surgeon and the patient. Osteoconductive 100% lot testing for osteoinductive potential animal model (in vivo) Sterile R (SAL) 10 -6 Osteoinductive potential tested after sterilzation Room Temp Storage OsteoSelect DBM Putty (Bacterin) Progenix DBX ® ® (Medtronic) (DePuy Synthes) Grafton (Medtronic) N/A N/A Accell (Integra) Dynagraft II (Integra) The Science OsteoSelect DBM Putty utilizes a proprietary process engineered to preserve the growth factors found in cortical bone and provide the highest degree of safety (SAL 10 -6) This validated proprietary method consistently demonstrates its efficacy in vivo as every lot of OsteoSelect DBM Putty is proven to induce bone formation in a validated pre-clinical model after terminal sterilization. OsteoSelect DBM Putty has been shown to be equivalent to autograft when used as a bone graft substitute in a pre-clinical, un-instrumented posterior spinal fusion model.1 OsteoSelect DBM Putty has also been shown to be superior to a commercially available synthetic bone void filler.2 1 Kiely, P.D. et al., (2014) Evaluation of a new formulation of demineralized bone matrix putty in a rabbit posterolateral spinal fusion model. The Spine Journal. September 14(9): 2155-2163. 2 Schallenberger et al., (2014) Comparison of the Osteogenic Potential of OsteoSelect Demineralized Bone Matrix Putty to NovaBone Calcium-Phosphosilicate Synthetic Putty in a Cranial Defect Model. The Journal of Craniofacial Surgery. March 25(2): 657-661. 14 bacterin.com OsteoWrap & OrbitalWrap HD Features JJ JJ Demineralized cortical bone sheet Radiolucent to allow for accurate follow-up assessment of bone formation JJ Osteoconductive & osteoinductive potential JJ Flexible handling characteristics JJ Sterility assurance level (SAL) 10 -6 JJ 5 year shelf life, Room-temperature storage OsteoWrap OsteoWrap Benefits OsteoWrap is 100% human cortical bone demineralized through a proprietary process that makes the graft flexible while maintaining allograft integrity. In addition to its unique handling characteristics, OsteoWrap can be easily sized with a scalpel or scissors, and it may be sutured in place. The versatility of the OsteoWrap allograft makes it an ideal scaffold for use in spine, neuro, cranio-maxillofacial, reconstructive, and general orthopedic surgical procedures, to provide additional structural support as an overlay graft. OrbitalWrap HD Benefits OrbitalWrap HD is a unique and cost-competitive biologic solution that alleviates the need for permanent implants. This low-profile graft can be easily contoured over a defect in the patient’s orbital floor. If desired, OrbitalWrap HD can be used in conjunction with traditional implants. Designed to have similar attributes to OsteoWrap, OrbitalWrap HD is slightly less flexible. OrbitalWrap HD OsteoWrap | OrbitalWrap HD 109701 OsteoWrap 10 × 10 mm 109702 OsteoWrap 15 × 10 mm 109703 OsteoWrap 15 × 15 mm 109711 OsteoWrap 20 × 20 mm 109712 OsteoWrap 30 × 30 mm 109713 OsteoWrap 50 × 25 mm 109714 OsteoWrap 70 × 40 mm 109715 OsteoWrap 60 × 50 mm 109722 OrbitalWrap HD - 20 × 20 × 2 mm 109733 OrbitalWrap HD - 30 × 30 × 2 mm bacterin.com 15 OsteoSTX Features JJ Osteoconductive JJ Osteoinductive potential JJ Sterility assurance level (SAL) 10 -6 JJ Flexible handling characteristics JJ Sterile, room-temperature storage JJ Easily cut to size, available in multiple lengths JJ Conveniently distributed in packs of 10 OsteoSTX Benefits OsteoSTX are demineralized cortical sticks processed from donated human bone. Our proprietary demineralization technology gives the osteoconductive scaffold flexible handling characteristics and osteoinductive properties. When combined with BMA, OsteoSTX provide all of the necessary elements for bone regeneration. OsteoSTX are designed for posterolateral spine surgery applications including single- and multi-level fusions, as well as deformity procedures. OsteoSTX 109705 OsteoSTX 50 mm – 10 per pack 109710 OsteoSTX 100 mm – 10 per pack 109720 OsteoSTX 200 mm – 10 per pack OsteoSTX can be easily cut to size 16 bacterin.com 3Demin Features JJ Osteoconductive JJ Sterile, room-temperature storage JJ Osteoinductive potential JJ Will not dissipate upon direct irrigation JJ Sterility assurance level (SAL) 10 -6 JJ 100% human demineralized cortical bone JJ Exceptional handling characteristics 3Demin Benefits Bacterin’s 3Demin Technology utilizes demineralized cortical bone fibers that are entangled and shaped into sizes engineered to compliment specific surgical applications. This unique process creates an interconnected graft material that contains BMPs and other growth factors necessary for the promotion of new bone formation. 3Demin allografts are flexible upon hydration and each allograft has a sterility 3Demin Boat assurance level of 10 -6 via low-dose gamma sterilization. 3Demin allografts are also available as loose cortical fibers in three volume options. 3Demin Strip 3Demin Cortical Fibers 3Demin Cortical Fibers 109762 3Demin Cortical Fibers 2.5 cc 109765 3Demin Cortical Fibers 5.0 cc 109760 3Demin Cortical Fibers 10.0 cc 3Demin Boats 109785 3Demin Boat 50 × 25 mm — 2 pack 109781 3Demin Boat 100 × 25 mm — 2 pack 3Demin Strips 109775 3Demin Strip 50 × 10 mm — 2 pack 109771 3Demin Strip 100 × 10 mm — 2 pack 109772 3Demin Strip 200 × 10 mm — 2 pack bacterin.com 17 Precision-Milled Allografts BacFast HD BacFast HD BacFast HD Surface via a Scanning Electron Microscope (SEM) at a magnification of 22x BacFast HD is a facet stabilization dowel designed with a focus on osteoconductivity and preventing graft migration, making it an ideal graft to be used as a complement to common spinal fusion procedures as it preserves the posterior spine. Utilizing Bacterin’s proprietary demineralization technology, BacFast HD is hyper-demineralized to expose the growth factors and BMPs inherent to cortical bone. With the benefits of HD technology and increased collagen surface area, BacFast HD also provides the graft with osteoinductive properties without compromising the structural integrity of the graft. Cervical Interbody Spacers Precision-Milled Allografts offer the support your patient needs. We have a cervical footprint that is 12 mm × 14 mm with varying heights and 7 degree lordosis. Cervical spacers feature ridged contact surfaces to help reduce the incidence of graft migration. The central lumen is ideal for press-fit placement of OsteoSponge blocks for great fusion results. Precision-Milled Cervical Spacer BacFast HD on Inserter Instrumentation Surgical Kits Precision-Milled Allografts available upon request 18 bacterin.com 100511 BacFast HD Facet Dowel, 5 mm 100512 BacFast HD Facet Dowel, 6 mm 1563305 Cervical Spacer, Lordotic, 5 mm 1563306 Cervical Spacer, Lordotic, 6 mm 1563307 Cervical Spacer, Lordotic, 7 mm 1563308 Cervical Spacer, Lordotic, 8 mm 1563309 Cervical Spacer, Lordotic, 9 mm 1563310 Cervical Spacer, Lordotic, 10 mm Traditional Allografts Traditional Allografts In addition to Bacterin’s proprietary allograft portfolio, select traditional allografts are available. These matrices are used for multi-disciplinary applications including: Spine, Neuro, Trauma, Foot and Ankle, Sports Medicine, Cranio-Maxillofacial, and Plastic/Reconstructive Procedures. Unicortical Block Traditional Allografts Tricortical Block Femoral Cortical Strut, Split 101006 Ilium Tricortical Block 6 mm 101007 Ilium Tricortical Block 7 mm 101008 Ilium Tricortical Block 8 mm 101009 Ilium Tricortical Block 9 mm 101010 Ilium Tricortical Block 10 mm 101011 Ilium Tricortical Block 11 mm 101012 Ilium Tricortical Block 12–14 mm 101015 Ilium Tricortical Block 15 mm 101016 Ilium Tricortical Block 16–21 mm 101022 Ilium Tricortical Block 22–25 mm 101040 Ilium Tricortical Strip 40 mm 101045 Ilium Tricortical Strip 45 mm 101050 Ilium Tricortical Strip 50 mm 101055 Ilium Tricortical Strip 55 mm 101060 Ilium Tricortical Strip 60 mm 103015 Cancellous Crushed 0.1–4 mm 5cc 103115 Cancellous Crushed 0.1–4 mm 15cc 103130 Cancellous Crushed 0.1–4 mm 30cc 103045 Cancellous Crushed 4–10 mm 5cc 103415 Cancellous Crushed 4–10 mm 15cc 103430 Cancellous Crushed 4–10 mm 30cc 104205 Unicortical Block 5 mm 104206 Unicortical Block 6 mm 104207 Unicortical Block 7 mm 104208 Unicortical Block 8 mm 104209 Unicortical Block 9 mm 104210 Unicortical Block 10 mm 106040 Fibula Segment 40–100 mm 106101 Fibula Segment 101–150 mm 105300 Femoral Cortical Strut — 200 mm Split bacterin.com 19 hMatrix ADM Features JJ Sterility assurance level (SAL) 10 -6 JJ Superior strength * JJ Flexible matrix for precise placement JJ Decreased inflammatory response * JJ hMatrix ADM and hMatrix PR ADM are distributed as a frozen product JJ 5 year shelf life (with proper storage conditions) hMatrix Q Code Q4134 hMatrix PR ADM hMatrix ADM hMatrix ADM and hMatrix PR ADM Benefits hMatrix Acellular Dermis (ADM) and hMatrix PR ADM are allografts derived from donated human skin. The dermis is processed using a proprietary method to remove the cells minimizing the chance of an immunologic response. The natural collagen and elastin matrix is ideal for neovascularization and cellular proliferation and has the potential to expedite the healing process. Because it is processed from human donors, the risk of inflammation is significantly reduced relative to products of animal origin. Suture Retention Strength of hMatrix ADM and hMatrix PR ADM * Test results demonstrate an average suture retention strength of 310 Newtons (n = 12) for hMatrix ADM and hMatrix PR ADM compared to 229 Newtons (n = 10) for a leading competitive product. Overall, hMatrix ADM and hMatrix PR ADM were 35% stronger than the leading competitor (P = 0.002) with more consistent suture retention strength. †† * 20 Barber FA, Herbert MA, Coons DA (2006) Tendon Augmentation Grafts: Biomechanical Failure Loads and Failure Patterns. Arthroscopy: The Journal of Arthroscopic and Related Surgery. Vol 22, No 5 (May): 534-538. Data on File at Bacterin. bacterin.com 350 300 250 200 150 100 50 0 hMatrix ADM & hMatrix PR ADM Competitor † hMatrix ADM hMatrix PR ADM 208030 hMatrix Dermis 2 × 1 cm, Thin 218166 hMatrix PR - Dermis 16 × 6 cm, Thin 208031 hMatrix Dermis 4 × 2 cm, Thin 228168 hMatrix PR - Dermis 16 ×8 cm, Medium 208032 hMatrix Dermis 5 × 4 cm, Thin 228160 hMatrix PR - Dermis 16 × 10 cm, Medium 208033 hMatrix Dermis 7 × 5 cm, Thin 228208 hMatrix PR - Dermis 20 × 8 cm, Medium 208034 hMatrix Dermis 10 × 5 cm, Thin 228320 hMatrix PR - Dermis 20 × 16 cm, Medium 208052 hMatrix Dermis 2 × 1 cm, Medium 208035 hMatrix Dermis 4 × 2 cm, Medium Pericardium and Fascia Lata 208036 hMatrix Dermis 5 × 4 cm, Medium 108004 hMatrix Fascia Lata 30 × 30 mm 208037 hMatrix Dermis 7 × 5 cm, Medium 108005 hMatrix Fascia Lata 50 × 40 mm 208038 hMatrix Dermis 10 × 5 cm, Medium 108006 hMatrix Fascia Lata 70 × 50 mm 208043 hMatrix Dermis 2 × 1 cm, Thick 108007 hMatrix Fascia Lata 100 × 50 mm 208039 hMatrix Dermis 4 × 2 cm, Thick 108021 Pericardium 30 × 30 mm 208040 hMatrix Dermis 5 × 4 cm, Thick 108010 Pericardium 40 × 40 mm 208041 hMatrix Dermis 7 × 5 cm, Thick 108018 Pericardium 50 × 40 mm 208042 hMatrix Dermis 10 × 5 cm, Thick 108019 Pericardium 70 × 50 mm 208231 hMatrix Dermis 4 × 2 cm, Thin, Meshed 108024 Pericardium 100 × 20 mm 208232 hMatrix Dermis 5 × 4 cm, Thin, Meshed 108020 Pericardium 100 × 50 mm 208233 hMatrix Dermis 7 × 5 cm, Thin, Meshed 208234 hMatrix Dermis 10 × 5 cm, Thin, Meshed Sports Medicine Sports Medicine allografts are an excellent replacement option for deficient or torn cruciate ligament reconstructions and revisions. Benefits Achilles Tendon of Bacterin’s Sports Medicine allografts include consistent, superior quality and precise measurements. As with all of Bacterin’s allografts, safety and efficacy are an absolute priority. All tendons are distributed sterile via low-dose gamma irradiation. Hemi-Patella Tendon Sports Medicine 208000 Achilles Tendon w/Bone Block 208002 Anterior Tibialis Tendon 208003 Posterior Tibialis Tendon 208008 Gracilis Tendon 208009 Hemi-Patella Tendon 208011 Semitendinosus Tendon 208012 Whole Patella Tendon w/ Quad 208013 Whole Patella Tendon w/o Quad 208014 Hemi-Patella Tendon w/Quad 208015 Peroneus 268012 SportMatrix 12 × 1 cm bacterin.com 21 GPO Affiliations As part of our commitment to provide you with the most cost savings, we have partnered with Group Purchasing Organizations nationwide. Please contact our Customer Service Department at [email protected] or 1.888.886.9354 for more information. Bacterin is Just a Click Away If you are a Bacterin Distributor or Materials Manager and would like access to our Online Customer Service Portal, please contact our Customer Service Department for an application. Our portal will provide you with easy access for submitting online orders, a training calendar, and available downloads such as published papers, whitepapers, technique guides, instructions for use, and educational videos. We look forward to adding you as part of our online community and making access to information available at the click of a button. Bacterin Reimbursement Hotline Bacterin’s Reimbursement Hotline is dedicated to providing answers to all your reimbursement questions. It also serves as a resource for obtaining accurate billing information and reimbursement support for Bacterin products. hMatrix Q Code Q4134 1.844.252.4429 MONDAY–FRIDAY. 9AM–4PM CENTRAL. 22 bacterin.com Distributors Like what you see and want to become a distributor for Bacterin? Or if you have a recommendation for a Distributor that you enjoy working with, please contact us at bacterin. com/contact/distributors. Returns For information regarding returns, please contact our Customer Service Department at [email protected] or 1.888.886.9354. Not all products qualify. Honoring the Gift The Gift of Donation with Family Consent At Bacterin, we are honored to be stewards of the gift of donation. Recovery and Transfer of Tissue Patients Quality of Life is Greatly Enhanced Our respect for representing the wishes of the donor drives every decision we make. Bacterin serves a role throughout the entire process, from overseeing donor recovery to manufacturing, ensuring the highest possible graft quality and consistency, and Surgical Implantation of Tissue Bacterin Sterilely Processes and Packages Tissue distribution to our customers. In turn, this attention to detail in the recovery process, donor screening, and processing of our allografts reflects our respect not Hospital Accepts Tissue Tissue is Shipped via a Validated Carrier only for the donor, but extends to the recipient as well. Bacterin values the role you play in the Circle of Life. If you would like more information please visit: http://bacterin.com/DonateLife Donate Life America Bacterin is a proud supporter of Donate Life America and the Donate Life Rose Parade Float. Each year Bacterin, along with our recovery partners, sponsors hundreds of dedication roses, and in recent years memorial ‘floragraph’ portraits of organ, eye, and tissue donors honored and displayed on the Donate Life Rose Parade Float. bacterin.com 23 600 Cruiser Lane Belgrade, MT 59714 P: 1.888.886.9354 F: 1.406.388.3380 [email protected] www.bacterin.com 14008B 04/2015 © 2012 – 2015 Bacterin International, Inc. All Rights Reserved. BacFast , hMatrix , OrbitalWrap , OsteoLock , OsteoSelect , OsteoSponge , OsteoWrap , and BacteRinse are registered trademarks of Bacterin International, Inc. ® ® ® ® ® ® 3Demin™, SportMatrix™, and OsteoSTX™ are trademarks of Bacterin International, Inc. Records on file at Bacterin International, Inc. ® ®