61-2014 Peterson FLS Fung. Res. Ann. Rep.
Transcription
61-2014 Peterson FLS Fung. Res. Ann. Rep.
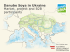
MISSISSIPPI SOYBEAN PROMOTION BOARD PROJECT NO. 61-2014 (YEAR 2) 2014 Annual Report Title: Investigations into Strobilurin Fungicide Resistance of Soybean Pathogens in Mississippi Investigator: Maria Tomaso-Peterson – [email protected] SUMMARY Thirty of 531 C. sojina isolates that were collected in Mississippi soybean fields were identified as sensitive to azoxystrobin based on the presence of glycine at position 143. The other 501 isolates expressed alanine at position 143, thus conferring resistance to azoxystrobin, as well as all QoI fungicides. The sensitive isolates originated from soybean fields in twenty Mississippi counties, all of which also had at least one field where a resistant isolate was present. The resistant C. sojina isolates are distributed among 73 soybean-producing counties in Mississippi. In 2014, a resistant isolate was collected from at least one field in every sampled county, and over 94% of these isolates were identified as QoI-resistant. Field applications of the QoI fungicides will not be effective for controlling FLS if the disease is incited by resistant C. sojina isolates. Plants inoculated with QoI-resistant isolates showed FLS symptoms, but severity and lesion count were significantly less when compared to the control that did not receive a QoI treatment. No disease was observed in a treatment where a triazole fungicide was applied regardless of QoI sensitivity. BACKGROUND AND OBJECTIVES Foliar soybean diseases can result in significant yield loss; therefore, cultural and chemical control practices are necessary. The strobilurin or quinone outside inhibitor (QoI) class of fungicides are primarily applied for late-season disease management as well as providing a minor yield benefit when applied at the appropriate growth stage (R3/R4). The two most commonly used fungicides are azoxystrobin (Quadris) and pyraclostrobin (Headline). The QoI site-specific mode of action puts this particular fungicide class at a high risk for fungicide resistance. This research project was initiated in April of 2013 to focus on screening C. sojina isolates that were collected from Mississippi soybean fields throughout the 2013 and 2014 growing seasons. Mr. Jeff Standish, graduate research assistant, began 1 April WWW.MSSOY.ORG Apr. 2015 1 2013 and has completed his responsibilities within this project to achieve a Master of Science degree in Plant Pathology under the supervision of Dr. Tomaso-Peterson. Objectives 1. Monitor MS soybean production fields and sentinel plots for QoI resistance within the aerial web blight, Cercospora blight, frogeye leaf spot, and target spot pathogen populations. 2. Identify the mechanism(s) of resistance and potential proportion within the prevailing fungal community to better understand the dynamics leading to QoI resistance. 3. Determine potential fitness costs associated with QoI resistance within the soybean pathogen populations present. PROGRESS A comprehensive sampling of 73 counties throughout Mississippi began in June 2014 and ended in September 2014. The survey generated 531 Cercospora sojina isolates from soybean fields symptomatic for frogeye leaf spot (FLS) (Fig. 1). Due to high occurrence of FLS in 2014, this project continued to focus on the FLS pathogen, C. sojina. In the lab, C. sojina was isolated from foliar lesions and maintained under long-term storage for fungicide resistance studies. Field coordinates, planting history, fungicide use (if available), water management practices, and soybean cultivar were recorded for each FLS sample. Azoxystrobin and other QoI fungicides inhibit the respiration process within fungi. The specific target site of this fungicide class is the cytochrome b gene (cyt B), which regulates electron transport and the production of ATP. QoI fungicides block ATP production, which reduces the fungus’ ability to maintain growth and development. The molecular mechanism of fungicide resistance to QoIs is well-documented. A specific amino acid that binds with the fungicide molecule is substituted with an amino acid that lacks the affinity to bind. This is referred to as G143A substitution, where glycine (binding) is replaced with alanine (non-binding). If the alanine is present at position 143 of cyt B, the fungus will not be sensitive to the fungicide, causing the product to be ineffective for disease control. Genomic DNA was extracted from all 531 C. sojina isolates and a PCR-RFLP method was utilized to identify the nucleotide point mutation responsible for the G143A substitution which confers resistance to the QoI fungicides (Fig. 2). We sequenced the cyt B gene of all isolates collected in 2014. Thirty C. sojina isolates were identified as sensitive to azoxystrobin based on the presence of glycine at position 143. The other 501 isolates expressed alanine at position 143, thus conferring resistance to azoxystrobin, as well as all QoI fungicides. The sensitive isolates originated from soybean fields in twenty Mississippi counties, all of which also had at least one field where a resistant isolate was present. The resistant C. sojina isolates are distributed among 73 soybean-producing counties in Mississippi (Fig. 3). In 2014, a resistant isolate was collected from at least one field in every sampled county, and over 94% of WWW.MSSOY.ORG Apr. 2015 2 these isolates were identified as QoI-resistant. Field applications of these QoI fungicides will not be effective for controlling FLS if the disease is incited by resistant C. sojina isolates. A greenhouse study was initiated in May and concluded in October. The study was designed to evaluate the virulence of six C. sojina isolates (3 sensitive; 3 resistant) on soybeans treated with QoI and triazole fungicides. Armor DK 4744 soybean seeds were planted into 3-in. clay pots, 2 plants per pot. When the plants reached V2 growth stage, three fungicide treatments— Headline (QoI), Quadris (QoI), and Topguard (triazole) were applied to three soybean replicates per treatment per C. sojina isolate. A water control was also included. Fungicides were applied in water equivalent to 4.0 gal/A at 40 psi using a CO2-pressurized sprayer with two XR teejet 8002-VS nozzles. Twenty-four hours post-fungicide applications, soybean plants were inoculated with a conidial suspension consisting of each C. sojina isolate and placed in a humid chamber for 4 days, after which they were transferred to a greenhouse bench under a shade cloth. Soybeans were assessed for FLS symptoms 21-days postinoculation. Results of the greenhouse studies indicate QoI and triazole fungicides inhibited infection caused by QoI-sensitive C. sojina isolates (Tables 1 and 2). Plants inoculated with QoI-resistant isolates showed FLS symptoms, but severity and lesion count were significantly less when compared to the control that did not receive a QoI treatment. No disease was observed in the triazole treatment regardless of QoI sensitivity. The reduction in FLS among QoI-resistant C. sojina isolates may be attributed to control failure that is observed in the field. IMPACTS/BENEFITS TO MISSISSIPPI SOYBEAN PRODUCERS This research project has and will continue to have a positive impact on Mississippi soybean production. The 2014 sampling of FLS has provided us with the most comprehensive disease distribution in Mississippi to date. Documented distribution of FLS may provide informed decision-making in terms of selecting a resistant soybean variety to reduce FLS outbreaks as well as monitoring the spread of the pathogen, C. sojina. This information will also raise awareness toward effective FLS disease management measures if fungicide resistance is an issue in specific production fields. Resultant information generated from this research will assist growers in choosing fungicides that are efficacious for FLS control. In soybean fields where practical resistance (fungal population is resistant to a class of fungicides) occurs, QoI fungicides will not be effective. Frogeye leaf spot-resistant soybean varieties should be considered as a tool in managing fungicide resistance along with crop rotation, applying fungicides at the label rate, and alternating fungicide classes for FLS control. WWW.MSSOY.ORG Apr. 2015 3 END PRODUCTS Oral presentations Investigation into the mechanism of resistance to azoxystrobin in Cercospora sojina, the causal agent of frogeye leaf spot. J. Standish, M. Tomaso-Peterson, T. W. Allen, S. Sabanadzovic, N. Aboughanem-Sabanadzovic. Annual Meeting of the American Phytopathological Society. Minneapolis, MN. 10 August 2014. Distribution of QoI resistant frogeye leaf spot pathogen throughout the Mississippi soybean production system. J. Standish*, M. Tomaso-Peterson, T. W. Allen, W. F. Moore, S. Sabanadzovic, N. Aboughanem-Sabanadzovic. Annual Meeting of the Mississippi Association of Plant Pathologists and Nematologists. Starkville, MS. 21 October 2014. *Second place in graduate student competition. Determining fitness cost in QoI resistant isolates of the frogeye leaf spot pathogen. Brochard, N., Tomaso-Peterson, M., Allen, T.W. Annual Meeting of the Mississippi Association of Plant Pathologists and Nematologists. Starkville, MS. 21 October 2014. An investigation into QoI resistance in isolates of Cercospora sojina throughout Mississippi soybean production fields. J. Standish*, M. Tomaso-Peterson, T. W. Allen, W. F. Moore, S. Sabanadzovic, N. Aboughanem-Sabanadzovic. Annual Meeting of the Southern Division-American Phytopathological Society. Atlanta, GA. 2 February 2015. * Second place in graduate student competition. Quinone outside inhibitor resistance in Cercospora sojina throughout Mississippi soybean. J. Standish*, M. Tomaso-Peterson, T. W. Allen, W. F. Moore, S. Sabanadzovic, N. Aboughanem-Sabanadzovic. 3rd Annual Future of Agriculture Graduate Student Competition. Starkville, MS. 5 February 2015. *Third place in graduate student competition (Section Three). Investigating fungicide sensitivities beyond the QoIs in Cercospora sojina from Mississippi. J. Standish*, M. Tomaso-Peterson, T. W. Allen, S. Sabanadzovic, N. Aboughanem-Sabanadzovic. 42nd Annual Meeting of the Southern Soybean Disease Workers. Pensacola, FL. 12 March 2015. *First place in graduate student competition. Poster presentations Distribution of Azoxystrobin resistant Cercospora sojina throughout soybean production fields in Mississippi. N. Brochard, J. Standish, M. Tomaso-Peterson, T. W. Allen. Annual Meeting of the American Phytopathological Society. Minneapolis, MN. 9 13 August 2014. WWW.MSSOY.ORG Apr. 2015 4 Referreed manuscripts J. Standish, M. Tomaso-Peterson, T. W. Allen, S. Sabanadzovic, N. AboughanemSabanadzovic. 2015. Occurrence of QoI fungicide resistance in Cercospora sojina from Mississippi soybean. Plant Disease (accepted). Thesis A comprehensive study into quinone outside inhibitor resistance in Cercospora sojina from Mississippi Soybean. Jeffrey Russell Standish. Mississippi State University. WWW.MSSOY.ORG Apr. 2015 5 Figure 1. Distribution of Cercospora sojina isolates collected from frogeye leaf spot epidemics during the 2013 and 2014 growing season. Green-shaded counties, specific locations, and sentinel plots indicated. (Courtesy T.W. Allen) WWW.MSSOY.ORG Apr. 2015 6 Figure 2. PCR-RFLP method: Electrophoresis gel, showing QoI-resistant (R) and QoI-sensitive (S) isolates, allows for easy detection of the G143A substitution. WWW.MSSOY.ORG Apr. 2015 7 Figure 3. Distribution of QoI- or strobilurin-resistant Cercospora sojina, causal organism of frogeye leaf spot. Red-shaded areas represent counties where at least one isolate was found to carry the G143A amino acid substitution conferring resistance to the QoI fungicides. (Courtesy T.W. Allen) WWW.MSSOY.ORG Apr. 2015 8 Table 1. Frogeye leaf spot severity on soybean resulting from infection by QoI-sensitive and QoI-resistant isolates of Cercospora sojina following an application of foliar fungicide in a greenhouse. Severity (%) x y Treatment and rate/ha 13-11-R 13-31-S 13-34-R 13-36-S 13-85-S 13-104-R Untreated Control 14.5 az 3.9 5.4 a 0.8 0.6 1.1 a Headline (0.877 L/ha) 4.2 b 0.3 2.3 b 0.0 0.3 0.3 b Quadris (1.133 L/ha) 3.1 b 1.4 1.1 b 0.6 0.0 0.1 b Topguard (1.023 L/ha) 0.0 b 0.0 0.0 b 0.0 0.0 0.0 b x Fungicides were applied at the highest label rate at the V2 growth stage. y R = Resistant isolates; S = Sensitive isolates. z Means (n=9) within columns followed by the same letter are not significantly different according to Fisher’s protected least significant difference test (P = 0.05). Table 2. Frogeye leaf spot lesion count on soybean resulting from infection by QoI-sensitive and QoI-resistant isolates of Cercospora sojina following an application of foliar fungicide in a greenhouse. Lesion countw Treatment and rate/hax 13-11-Ry 13-31-S 13-34-R 13-36-S 13-85-S 13-104-R Untreated Control 37 az 1 6 a 0 1 8 a Headline (876.9 ml/ha) 7 b 0 0 b 0 0 3 b Quadris (1132.7 ml/ha) 3 bc 0 1 b 0 0 1 b Topguard (1023.0 ml/ha) 0 c 0 0 b 0 0 0 b w Frogeye leaf spot rated as number of lesions per pot (two soybean plants/pot). x Fungicides were applied at the highest label rate at the V2 growth stage. y R = Resistant isolates; S = Sensitive isolates. z Means (n=9) within columns followed by the same letter are not significantly different according to Fisher’s protected least significant difference test (P = 0.05). WWW.MSSOY.ORG Apr. 2015 9