The Origin of Adakites in the Garibaldi Volcanic
Transcription
The Origin of Adakites in the Garibaldi Volcanic
The Origin of Adakites in the Garibaldi Volcanic Complex,
southwestern British Columbia, Canada
A Thesis
Submitted to the Faculty of Graduate Studies and Research
In Partial Fulfillment of the Requirements
For the Degree of
Master of Science
In
Geology
University of Regina
By
Julie Anne Fillmore
Regina, Saskatchewan
November 2014
Copyright 2014: J.A. Fillmore
UNIVERSITY OF REGINA
FACULTY OF GRADUATE STUDIES AND RESEARCH
SUPERVISORY AND EXAMINING COMMITTEE
Julie Anne Fillmore, candidate for the degree of Master of Science in Geology, has
presented a thesis titled, The Origin of Adakites in the Garibaldi Volcanic Complex,
Southwestern British Columbia, Canada, in an oral examination held on August 22,
2014. The following committee members have found the thesis acceptable in form and
content, and that the candidate demonstrated satisfactory knowledge of the subject
material.
External Examiner:
Dr. Martin Beech, Campion College
Supervisor:
Dr. Ian M. Coulson, Department of Geology
Committee Member:
Dr. Tsilavo Raharimahefa, Department of Geology
Chair of Defense:
Dr. Josef Buttigieg, Department of Biology
ii
Abstract
The Garibaldi Volcanic Complex (GVC) is located in southwestern British
Columbia, Canada. It comprises two volcanic fields: the Garibaldi Lake Volcanic
Field (GLVF) in the north and the Mount Garibaldi Volcanic Field (MGVF) in the
south. Petrographical and geochemical studies on volcanic rocks collected from
the GVC have determined that they exhibit adakitic characteristics; these
intermediate rocks range from basaltic andesite to dacite represented mainly by
lava flows, domes and minor pyroclastic material. All the lavas exhibit evidence
of magma mixing, which include sieve textured crystals, dehydration reaction
textures, differently sized phenocryst populations, xenocrysts and xenoliths. The
geochemistry of the GVC magmas exhibit several adakitic indicators which
include high Sr/Y (> 40), Mg# (~ 51), Al2O3 (> 15 wt. %), low K2O/Na2O (~ 0.3),
low Yb (< 1.9 ppm) and fractionated rare earth element (REE) compositions
(La/Yb(N)~ 10), which have not been identified in previous studies. Adakites are
the product of subducted slab partial melts within the garnet stability field,
subdivided into low silica adakites (LSA; < 60 wt. % SiO2) and high silica
adakites (HSA; > 60 wt. % SiO2) reflecting differing source regions, where HSA
are primary slab melts and LSA are partial melts of mantle wedge peridotite that
has been previously modified by slab-derived magmas. Identification of adakitic
rocks in tectonic regimes unrelated to subduction have led to the argument that
the distinctive high Sr/Y and La/Yb ratios are not unique and cannot be used as
an indicator of slab partial melting. Basalts in adakite suites are often enriched
iii
in high field strength elements (HFSE; Nb in particular) and are classified as
niobium enriched basalts (NEB); NEB is argued to originate from mantle wedge
peridotite that has been previously metasomatised by slab partial melts. Trace
element modelling illustrates that the GVC adakites can be generated by partial
melting of subducted ocean crust. Incompatible and compatible element ratios
and relative crustal thickness beneath the GVC preclude AFC processes or high
pressure partial melting or crystallisation of basalt as the source of the adakite
signature in the GVC rocks and suggests that the GVC adakites likely result from
slab partial melts.
iv
Acknowledgements
I would like to acknowledge everyone who provided funding and support for this
project. I thank my supervisor, Dr. Ian Coulson, who provided funding through
his Natural Sciences and Engineering Research Council (Discovery Grant) and
facilitated microprobe analysis of selected samples, as well as much support and
consultation. The University of Regina provided financial support through the
Faculty of Graduate Studies and Research scholarships, the Teaching
Assistantship program and through the Department of Geology Teaching
Assistantship program. I would especially like to thank Dr. Michael Clynne,
former editor in chief of the Bulletin of Volcanology for extremely helpful
comments and discussion on my paper published last year in the Bulletin and
strongly influenced this study. Dr. William Minarik and Glenna Keating, who
provided whole rock geochemical analysis of the samples studied at McGill
University. Finally, I would like to thank my family and all my friends for their
moral support and putting up with my frustrations.
Most of all, I have to thank my wonderful and loving husband, without whom this
project could not have been possible.
v
Table of Contents
Abstract
ii
Acknowledgements
iv
Table of Contents
v
List of Figures
viii
List of Appendices
x
List of Acronyms
xii
1. Introduction
1
1.1 Previous Work
2
1.2 Adakites in the GVC
6
2. Regional Geology
7
2.1 Eruptive History of the GVC
9
2.2 History and definition of adakites
12
2.3 Adakite – tonalite-trondhjemite-granodiorite (TTG)
association
2.4 Non-slab melt models for adakite genesis
3. Petrography
3.1 GLVF
15
18
20
20
3.1.1 Cheakamus Valley Basalts (CVB)
20
3.1.2 Helm Creek Basaltic Andesite (HCBA)
23
3.1.3 Desolation Valley Basaltic Andesite (DVBA)
27
3.1.4 Barrier andesite
29
3.1.5 Black Tusk
30
3.2 MGVF
3.2.1 Ring Creek andesite
33
33
vi
3.2.1.1 Proximal Ring Creek andesite
33
3.2.1.2 Distal Ring Creek andesite
35
3.2.2 Columnar Peak dacite
36
3.2.3 Paul Ridge andesite
37
4. Geochemistry
39
4.1 Analytical techniques
40
4.1.1 Whole Rock Geochemistry
40
4.1.2 Mineral Geochemistry
42
4.2 Results
43
4.2.1 Basalts and Basaltic Andesites
43
4.2.2 Adakites
48
5. Discussion
54
5.1 Adakite Geochemistry of the GVC
56
5.1.1 La and Cr
56
5.1.2 LSA versus HSA
57
5.1.3 Magma Mixing in the GVC
58
5.1.4 Interaction with Mantle Peridotite
70
5.1.5 Isotope Geochemistry
71
5.2 Possible models for Adakite Genesis
73
5.2.1 Partial melting of basaltic lower crust
73
5.2.2 High pressure fractionation/AFC of basaltic magma
77
5.2.3 Partial melting of subducted ocean crust
80
5.2.3.1 Association with niobium-enriched basalts (NEB)
84
5.2.3.2 Ni content in olivine
93
5.2.3.3 Regional tectonic regime for southwestern British
Columbia
96
vii
5.3 Petrogenetic model for Adakite Genesis beneath the GVC
98
6. Conclusions
104
References
107
Appendix A – Whole Rock Geochemistry
127
Appendix B – Normative Mineralogy
130
Appendix C – Mineral Geochemistry
132
Appendix D – Trace element data for modelling of slab partial melts
142
viii
List of Figures
Fig. 1: Location Map of the GVC
3
Fig. 2: Geology of the GVC (modified from Green 1977) with sample
locations
4
Fig. 3: Field Photographs
10
Fig. 4: Thin section photomicrographs of basalts and basaltic andesites
21
Fig. 5a, b: Mineral composition of mafic minerals (CVB and HCBA)
24
Fig. 5c, d: Mineral composition of mafic minerals (DVBA and PR)
25
Fig. 6a-f: Thin section photomicrographs of adakites (BF, BT, URC)
31
Fig. 6g-l: Thin section photomicrographs of adakites (LRC, CP, PR)
32
Fig. 7: Plot of total alkalis vs. silica
45
Fig. 8: Major element Harker diagrams
46
Fig. 9: Trace element Harker diagrams
47
Fig. 10: Primitive mantle normalised multi-element diagrams
49
Fig. 11: Chondrite normalised multi-element diagrams
50
Fig. 12: Adakite-normal arc rock differentiation plots (Sr/Y vs. Y, La/Yb
vs. Yb)
53
Fig. 13: K/Rb vs. SiO2/MgO and Sr-K/Rb-[(SiO2/MgO)*100] plots
55
Fig. 14: LSA-HSA differentiation plots
59
Fig. 15: SEM photomicrographs of the GVC adakites
62
Fig. 16: Incompatible-compatible element ratio plots for the GVC adakites 68
Fig. 17: Adakitic indices plots for GVC and lower crustal melts
76
Fig. 18: Modelled slab partial melts compared to observed GVC adakite
compositions
Fig. 19: Binary mixing plots for the CVB and HCBA
82
88
ix
Fig. 20: Simple three component mixing model for the CVB
90
Fig. 21: Incompatible-compatible element ratio plots for the CVB and
HCBA
91
Fig. 22: NiO vs. Fo content in GVC olivines
95
Fig. 23: Interpreted petrogenetic model for adakite genesis in the GVC
99
x
List of Appendices
Appendix A – Whole Rock Geochemistry
127
Table A1: Major and minor element composition of investigated
samples from the GVC
128
Table A2: Trace and rare earth element composition of
investigated samples from the GVC
129
Appendix B – Normative Mineralogy
Table B1: Normative mineralogy of representative samples
from the GVC
Appendix C – Mineral Geochemistry
130
131
132
Table C1: Electron microprobe compositions of olivine from the
GVC
133
Table C2: Electron microprobe compositions of clinopyroxene
from the GVC
136
Table C3: Electron microprobe compositions of orthopyroxene
from the GVC
137
Table C4: Electron microprobe compositions of plagioclase from
the GVC
138
Table C5: Electron microprobe compositions of oxides from the
GVC
140
Appendix D – Trace element modelling of slab partial melts
142
Table D1: Partition coefficients for elements used in slab partial
melt model
143
Table D2: Modelled compositions of slab melts at various
melt fractions (F); residue=70% Cpx, 10% Gt, 20% Hbl
143
Table D3: Modelled compositions of slab melts at various melt
fractions (F); residue=70% Cpx, 20% Gt, 9.5% Hbl, 0.5% Rut
143
Table D4: Modelled compositions of slab melts at various melt
fractions (F); residue=70 % Cpx, 25 % Gt, 4.5 % Hbl, 0.5% Rut
143
Table D5: Estimated starting composition of Juan de Fuca (JdF)
MORB
143
xi
Fig. D1: Failed slab partial melt models for various residual
compositions
144
xii
List of Acronyms
ADR
Andesite-dacite-rhyolite
AFC
Assimilation-fractional crystallisation
BF
Barrier flow
BT
Black Tusk
Cay
Mt. Cayley
CP
Columnar Peak dacite
CVB
Cheakamus Valley Basalt
DVBA
Desolation Valley Basaltic Andesite
GLVF
Garibaldi Lake Volcanic Field
GVB
Garibaldi Volcanic Belt
GVC
Garibaldi Volcanic Complex
HCBA
Helm Creek Basaltic Andesite
HFSE
High field strength element(s)
HREE
Heavy rare earth element(s)
HSA
High silica adakite
LC
Lower crust
LILE
Large ion lithophile element(s)
LOI
Loss on ignition
LRC
Lower Ring Creek andesite
LREE
Light rare earth element(s)
LSA
Low silica adakite
MGVF
Mt. Garibaldi Volcanic Field
MREE
Middle rare earth element(s)
NEB
Niobium-enriched basalt
(N)MORB
(Normal) mid-ocean ridge basalt
xiii
OIB
Ocean island basalt
PR
Paul Ridge andesite
REE
rare earth element(s)
RC
Ring Creek andesite
TTG
tonalite-trondhjemite-granodiorite
URC
Upper Ring Creek andesite
1
1.
Introduction
Partial melting of hydrated mantle wedge is a favoured mechanism in the
generation of arc-type magmas, and previous studies of magmatism within the
Garibaldi Volcanic Complex (GVC) have supported this model (Green 1977,
1981, 1990, 2006; Green and Harry 1999, Harry and Green 1999). The
distinctive chemistry of the intermediate to felsic rocks of the GVC, however,
suggests that mantle wedge partial melts are not the only possible source of
magmatism in the GVC. The GVC rocks exhibit geochemical characteristics that
identify them as adakites, with high Sr/Y (> 40) and low Yb (< 1.9 ppm). This
signature is attributed to partial melting of subducted basaltic ocean crust, at a
depth where garnet is stable but plagioclase is not (Defant and Drummond
1990), and generates felsic to intermediate melts. Adakite magmas are unique in
that they are defined almost solely on geochemistry (Richards and Kerrich
2007); the mineralogy of adakitic rocks and tectonic environments in which these
have been identified can be quite variable. However, in several environments
where adakites are found in association with basaltic rocks, basalt (and basaltic
andesite) rocks are commonly found to be unusually enriched in high field
strength elements (HFSE) and Nb in particular (Sajona et al. 1996). These mafic
rocks have been termed niobium enriched basalt (NEB) and are argued to
originate from mantle wedge peridotite altered by the interaction with ascending
slab partial melts. This may suggest that a petrogenetic link exists between
adakite and NEB however, further studies of this relationship have found that
2
they are not mutually exclusive and there are suites where adakite is present but
NEB are not (e.g. Chile and Argentina; Stern and Kilian 1996) and vice versa. As
a result, the definition of ‘adakite’ and whether or not it truly represents a distinct
magmatic process has been the subject of much discussion.
1.1
Previous Work
The GVC was first studied by Burwash (1914a), who described the relative
timing between volcanism therein and the effects of glaciation occurring in the
area that includes Mt. Garibaldi and Black Tusk. Mathews (1951, 1952), followed
up in this work and expanded upon the Quaternary geology and glacial geology.
More detailed studies were completed later by Mathews (1957, 1958), where the
rock types of the GVC and Mt. Cayley were characterised in terms of rock
petrography and major element chemistry, in addition to a description of the
Quaternary glacial geology and stratigraphy of both the Mt. Garibaldi Volcanic
Field (MGVF) and The Table (Figs. 1 & 2). Work in the GVC ceased until 1970’s
when a Ph.D. study by Fiesinger further investigated the petrography of rocks
collected from the GVC, Mt. Cayley and the Quesnel Highlands, as well as their
major element chemistry (Fiesinger 1975). A B.Sc. study by Sivertz (1976) was
the first to characterise the rocks of Opal Cone and the Ring Creek flow as
dacite through a combination of petrographic study and whole rock major and
limited trace element chemistry (Fig. 2). Sivertz (1976) proposed that the source
of the Ring Creek rocks was hydrous partial melting of quartz eclogite. The
subsequent Ph.D studies by Green (1977), however, suggested that the basalt
3
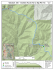
Figure 1: Location map of the GVC in southwestern British Columbia, with plate boundaries highlighted and relative plate
motions of the Juan de Fuca and Explorer plates indicated. Map modified after Hickson et al. (1999) and Madsen et al.
(2006).
4
Figure 2: Geology of the GLVF and the MGVF in the GVC. Sample locations are shown. Map modified after Green
(1977) and Green et al. (1988). Note: stratigraphy of the GLVF and MGVF is younging upwards independent of each
other.
5
and andesite rocks of the Garibaldi Lake Volcanic Field (GLVF) were the result
of multistage fractionation and partial melting of mantle wedge peridotite, based
on petrography, whole rock geochemistry, microprobe and isotopic data, and
geothermometry. The GLVF has been studied extensively, primarily by Green
(1981, 1990, 2006; Green et al. 1988), where the volcanology and eruptive
sequence of each centre has been described in depth. Despite these many
investigations, to date, there are no whole rock REE compositional data for the
MGVF and only one published study of a few samples from the GLVF (Green
and Henderson 1984)1. Recent studies of the GVC have focused primarily on the
basaltic lavas and how their chemistry (particularly the LILE, HFSE and REE) is
affected by the thermal structure of the Juan de Fuca Plate (Green and Harry
1999, Harry and Green 1999, Green and Sinha 2005, Green 2006). These
investigations have found that the subduction component (i.e., LILE, LREE
contents) decreases from the volcanoes of Glacier Peak in southern
Washington, northwards to north of Mt. Meager in central British Columbia and
that this is due to the decrease in age of the Juan de Fuca Plate further north,
and the resulting earlier onset of dehydration reactions within the slab (Green
and Sinha 2005, Green 2006). As older studies (e.g., Green 1977, Sivertz 1976)
have already described the mineralogy and major element chemistry of the
intermediate and felsic rocks of the GVC, very little new research has been done
on them. This is likely the reason that adakitic rocks have not been recognised in
1
Current research at the University of British Columbia at the time of completion of this thesis is revisiting
previously collected samples of the intermediate rocks in the GVC for geochemical and isotopic analysis.
6
the GVC until the study by Fillmore and Coulson (2013) and also by extension,
that the basalts in the GVC have not been recognised as NEB. There have been
some new isotopic studies on the basalts of the Cascades range (which includes
the Cheakamus Valley Basalts and the Helm Creek Basalt; Martindale et al.
2014) as well as on select evolved rocks from the GVC and Mt. Cayley
(Martindale et al. 2014). The NEB characteristics of the GVC basalts were also
identified in these works (although not explicitly identified as such), but this new
isotope data has not been evaluated in the context of the slab melt model
presented in this thesis and are not discussed further.
1.2
Adakites in the GVC
Geochemical and petrological attributes of andesite and dacite rocks that
comprise the GVC combined with associated field relationships (i.e., NEB)
strongly suggest that they are the result of slab melting, under the definition put
forth by Defant and Drummond (1990). Interactions between these melts and the
overlying mantle wedge are also evident in their trace element compositions, a
refinement made to the slab melt model by Martin et al. (2005). This study of the
GVC provides a unique opportunity to evaluate the following: (1) that partial
melting of subducted oceanic crust (i.e., subducted slab) is a process that is
more common in modern environments than previously thought; (2) magmas
produced by such a process can in fact migrate through the mantle wedge and
crust to be erupted at surface with the slab melt signature intact, and (3) the
presence of NEB and adakite in the same suite of rocks can suggest a
7
petrogenetic link and that these support a slab melt origin for adakitic volcanism
in the GVC.
The objectives of this study are: (1) to provide the first complete geochemical
characterisation of the intermediate and felsic rocks of the MGVF and the GLVF,
(2) be the first work to characterise the intermediate and felsic rocks in the GVC
as adakite, and basalt and basaltic andesite as NEB, (3) evaluate the various
models of adakite genesis in the context of the GVC, (4) introduce a potential
link between NEB and LSA as parent and daughter magmas, and (5) suggest
that the source for NEB in the GVC is mantle peridotite metasomatised by partial
melts of subducted oceanic lithosphere, which in turn, suggests a petrogenetic
link between the NEB and adakite rocks of the GVC.
2.
Regional Geology
The Garibaldi Volcanic Belt (GVB) extends from the Canada-U.S.A. border
northwards into British Columbia for approximately 140 km (Fig. 1; Sherrod and
Smith 1990). The GVC lies within the southern portion of this belt between the
towns of Whistler and Squamish, and comprises two fields; the GLVF in the
north and the MGVF in the south (Fig. 2). The volcanic rocks from the GLVF and
the MGVF range in composition from basalt to dacite and have been previously
interpreted to be the result of hydrous melting of the mantle wedge above the
Juan de Fuca Plate, which is subducting beneath the North American Plate, and
subsequent fractionation at various depths during ascent (Green 1977, 1981,
8
1990; Green and Harry 1999, Green and Sinha 2005, Green et al. 1988).
Basaltic volcanism in the GVC is thought to be related to the decreased volatile
content of the Juan de Fuca Plate and results in lower degrees of melting under
higher pressures and temperatures (Green 2006). The decreased slab flux is
attributed to both a northward decrease in plate movement and plate age
(Riddihough 1981, 1984; Green 1990, Wilson 2002). The younger, more buoyant
Explorer Plate separated from the Juan de Fuca Plate at 4 Ma (Wilson 2002,
Audet et al. 2008), and this deviation may relate to an increase in Quaternary
volcanism in the GVC.
Subduction of the Explorer Plate beneath North America is slower than that of
the Juan de Fuca Plate and it has been suggested that the Explorer Plate is
undergoing capture by the North American Plate (Audet et al. 2008). The
difference in subduction rates has caused a region of extension and slab
thinning along the Nootka Fault zone (Fig. 1), a transform fault that fractured in
response to an interval of ridge propagation and reorientation (Riddihough 1984,
Wilson 1988, Madsen et al. 2006) and created the Explorer and Juan de Fuca
plates. Independent movement of the Explorer Plate northward relative to the
east-northeasterly movement of the Juan de Fuca Plate suggests that the
separation encompasses both the subducted portions of the plates in addition to
the non-subducted oceanic parts (Madsen et al. 2006). This segmentation
coupled with the relative subduction vectors of the plates has resulted in a
change in mantle flow. Recent studies (Madsen et al. 2006, Audet et al. 2008)
9
have determined and modelled upwelling of mantle material along this boundary
and suggest the resulting structure has the potential to create a slab window.
Several workers (e.g., Breitsprecher et al. 2003, Thorkelson and Breitsprecher
2005, Ickert et al. 2009) have found a link between the formation of slab
windows or slab tears and adakitic volcanism, whereby asthenospheric
upwelling provides the heat flux necessary to melt the edges of slabs and in
doing so, facilitate the generation of adakite magmas.
2.1
Eruptive History of the GVC
The GLVF and the MGVF sit unconformably on the Coast Mountain Crystalline
Complex, which is a series of metamorphosed quartz diorite and granodiorite
plutons of Cretaceous age (Rusmore and Woodsworth 1991). Timing and
eruptive products from both fields have been summarised by previous authors
(Green 1977, 1981, 1990; Green et al. 1988) and are briefly outlined below.
Quaternary volcanic centres in the GLVF include Black Tusk, Cinder Cone,
Clinker Peak, Mt. Price and The Table, as well as the Cheakamus Valley Basalts
which were erupted from an unknown centre (Fig. 2). The oldest activity in the
GLVF was at Black Tusk and Mt. Price with episodic volcanism beginning at 1.3
Ma (Fig. 3a). The rocks of Black Tusk are hornblende andesite and
orthopyroxene andesite flows. The oldest rocks of Mt. Price are a series of
hornblende andesite and andesite flows followed by the formation of the
hornblende-biotite andesite satellite cone along Garibaldi Lake. Volcanism
ended in the Mt. Price area with the eruption of the Barrier and Culliton Creek
10
Figure 3: Field photographs. (a) Black Tusk; person for scale (red circle), (b) Barrier andesite (yellow) filling valley (red);
inset=porphyritic texture of the Barrier andesite, (c) the Cinder Cone (red), looking east, (d) The Table, looking south, (e)
columnar jointing in the CVB, near Daisy Lake; inset=vesicular texture in the CVB, (f) columnar jointing in the Columnar
Peak dacite, (g) Atwell Peak (back left) and Columnar Peak (front), (h) Opal Cone (red) and the Ring Creek flow (yellow).
11
andesite flows from Clinker Peak at 100 ka (Fig. 3b). Activity at Cinder Cone
began post 100 ka (Fig. 3c) with the formation of a tuff ring and the eruption of
the basaltic andesite of Desolation Valley, followed by the Helm Creek
composite flow. The Table formed approximately at 100 ka, when hornblende
andesite magma erupted beneath the Cordilleran Ice Sheet and melted its way
upwards to form a steep, flat topped tuya (Fig. 3d; Mathews 1951, Green 1981).
The olivine-bearing Cheakamus Valley Basalts were erupted from an unknown
vent post 100 ka and eruptions continued episodically to approximately 34 ka
(Fig. 3e; Green et al. 1988).
The MGVF is comprised of Mt. Garibaldi and its subsidiary vents, Dalton Dome
and Atwell Peak, Opal Cone and the Ring Creek andesite flow, Columnar Peak
and the andesite flows of Paul Ridge. Recent activity in this field began at
approximately 700 ka with the eruption of hornblende andesite flows atop pre 1.3
Ma hornblende-andesite and basaltic andesite along Paul Ridge. Increased
volcanism occurred between 260 ka and 220 ka with the eruption of hornblende
orthopyroxene dacite at Columnar Peak (Fig. 3f), followed by hornblende
orthopyroxene dacite flows and minor pyroclastic material from Mt. Garibaldi
(Green et al. 1988). The composite dacite cone of Dalton Dome formed later but
before the belt was overridden by glacial ice. Post 100 ka, dacitic pyroclastic
flows were erupted from Atwell Peak atop the glacial ice as well as additional
dacite flows from Dalton Dome. These flows and the west flank of Atwell Peak
collapsed following glacial retreat (Fig. 3g; Mathews 1952, 1958; Green 1990).
12
The most recent volcanism in the GVC was the eruption of the Ring Creek
andesite flow from Opal Cone between 10.7 ka and 9.3 ka (Fig. 3h; Brooks and
Friele 1992), which extends for some 17.5 km south around Paul Ridge and then
west towards Squamish River (Fig. 2).
2.2
History and definition of adakites
Adakites are a group of intermediate to felsic igneous rocks formed in
subduction zones involving relatively young, hot oceanic lithosphere (≤ 25 Ma).
Adakite is named after a suite of magnesian andesite rocks first described by
Kay (1978) from Adak Island in the Aleutian Arc. These rocks are believed to be
the result of partial melting of subducted ocean crust, creating typically sodic
‘slab melts’. Based on the work done by Kay (1978), Defant and Drummond
(1990) described the mineralogy and geochemistry of adakites more fully, as
well as field relationships and suggesting a petrogenetic model for adakite
genesis. Defant and Drummond (1990) argued that because adakites are most
commonly found in subduction zones that are young and hot, the subducted slab
still retains much of its heat and melts at shallower levels than older and colder
slabs, rather than dehydrate (or a combination of both). The partial melts remove
most or all of the plagioclase from the slab, resulting in the high Sr contents of
adakites as Sr strongly partitions into plagioclase (Rollinson 1993) and leaves
garnet as a residual phase, which strongly partitions Y and HREE. This process
generates the distinctive high Sr/Y and La/Yb ratios of adakite magmas. Defant
and Drummond (1990) developed the definition of adakite as volcanic or
13
intrusive arc rocks that contain ≥ 56 wt. % SiO2, ≥ 15 wt. % Al2O3, usually < 3 wt.
% MgO (but can be up to 6 wt. %), ≤ 18 ppm Y, ≤ 1.9 ppm Yb and Sr in excess
of 400 ppm. The mineralogy of adakites as described by Defant and Drummond
(1990) includes plagioclase and amphibole as the most common phases, with
clinopyroxene and orthopyroxene occurring frequently with some biotite and
opaques as well.
Following the work by Defant and Drummond (1990), adakites have been
identified and described at other “hot” subduction zones including: Panama
(Defant et al. 1991), Mt. St. Helens, U.S.A. (Dawes et al. 1994), southern Chile
and Argentina (Stern and Kilian 1996), and Ecuador (Samaniego et al. 2002),
suggesting that slab melting may be a more common process than once
thought. A study by Martin et al. (2005) expanded on the adakite definition by
analysing a geochemical database of > 340 samples (previously compiled by
Martin and Moyen 2003) and found that two groups were present, a low silica
adakite (LSA) and a high silica adakite (HSA). LSA contain < 60 wt. % SiO2,
higher MgO (4 - 9 wt. %), CaO + Na2O > 10 wt. %, Sr > 1000 ppm, generally
higher LREE and are Rb poor. HSA, by contrast, have SiO2 contents > 60 wt. %,
lower MgO (0.5 - 4 wt. %), CaO + Na2O < 11 wt. %, Sr < 1100 ppm and
generally lower HFSE and HREE than for LSA. Both groups display a low
K2O/Na2O ratio (~ 0.42), high Mg# (~ 51) and high Ni and Cr contents (24 and 36
ppm, respectively). Mineralogically, LSA differs from HSA in that LSA contains
pyroxene phenocrysts (Martin et al. 2005). Recognition of these distinct groups
14
led to the idea of different sources for magma genesis to explain the
geochemical characteristics of each; Martin et al. (2005) stated that HSA
magmas were direct partial melts of subducted basaltic crust that were
subsequently altered by interaction with mantle peridotite, as the melts migrated
upwards through the mantle wedge. This interaction increased the Mg#, Ni and
Cr contents, but left the adakitic signature (high Sr/Y, La/Yb, etc.) relatively
unchanged. Hence, the source of LSA magmas is not subducting crust, but the
overlying mantle wedge that has been metasomatised by the passage of
previous HSA melts (Martin et al. 2005). Partial melting of this altered mantle
wedge gives LSA their higher MgO, TiO2 and lower SiO2 contents than for HSA
magmas. Similarly, the higher Nb and Ta contents of LSA are transferred to the
mantle wedge by the HSA melts; melt is a more effective method of transferring
these elements out of the subducted slab than aqueous fluids (Tatsumi et al.
1986, Martin et al. 2005) and results in the higher amounts of these elements in
LSA versus HSA (on average).
Partial melting of basalt to generate adakite is supported by experimental work
(e.g., Rapp et al. 1999) and evidenced in natural rocks from subduction zones
(e.g., Schiano et al. 1995) and, as studies into magma genesis continued, an
ever widening group of different models were suggested. Consequently, a wide
range of magma compositions from different tectonic environments have been
classified as adakites. Models that explain the formation of adakite falls into two
main groups: genesis from slab partial melts and magmas generated by various
15
methods that can reproduce the distinctive adakite chemistry (which are not
necessarily subduction related). Further ambiguity arises when a suite of rocks
are classified as adakites (which may not follow the parameters outlined by
Defant and Drummond 1990) and are subsequently found not to be related to
slab melting. This leads to the conclusion that adakites are not slab melts and
has provided the means of pooling many different rock types under the one
name. Defant and Drummond (1990) first introduced the term ‘adakite’ and the
criterion by which they are defined. Hence the GVC adakites are evaluated
based upon this original definition and the adakite subdivisions of Martin et al.
(2005).
2.3
Adakite – tonalite-trondhjemite-granodiorite (TTG) association
Tonalite-trondhjemite-granodiorite (TTG) sequences comprise a large
component of Archaean greenstone belts and were first introduced by Jahn et al.
(1981). TTG are sodic plutonic rocks with several geochemical characteristics
that are very similar to adakites including high La/YbN (> 15), 20 < Sr/Y < 200,
high Ni and Cr (14 and 29 ppm respectively), low K2O/Na2O (0.3-0.6) and low Yb
(< 2 ppm; Martin et al. 2005, Moyen and Martin 2012). The high La/Yb and Sr/Y
ratios of TTG magmas were interpreted to originate from a source that was
plagioclase poor but garnet rich, and partial melting of hydrated metabasalt (with
residual garnet) became the generally accepted model for TTG petrogenesis
(Moyen and Martin 2012). The identification of the similar high La/Yb and Sr/Y
values in adakites led to studies comparing them with TTG rocks; the
16
observation that adakite magmas are generally rare and occur in predominantly
hotter subduction zones (as per the original definition by Defant and Drummond
1990) suggested that perhaps subduction was occurring in the Archaean and the
source of TTG magmas was partial melts of subducted basaltic crust (Moyen
and Martin 2012). The prevalence of TTG rocks compared to modern adakites is
attributed to the higher geothermal gradient in the Archaean, where slab melting
was likely easier and more common than in most present day subduction
environments which are cooler.
Martin et al. (2005) found a relationship between Archaean TTG’s and
sanukitoids and the HSA and LSA groups. TTG prior to approximately 3.3 Ga
are not adakitic and exhibit much higher SiO2 and lower Mg#, MgO and Sr
contents. Post 3.3 Ga TTG, however, are geochemically very similar to modern
HSA magmas with depleted HREE, higher Mg# (up to 65), Ni and Cr contents up
to 70 and 200 ppm respectively, and Sr up to 1200 ppm. LSA magmas have no
TTG analogue, but exhibit similar geochemical traits to sanukitoids, first
introduced by Shirey and Hanson (1984). Sanukitoids show high LILE and
fractionated REE with low Y and Yb concentrations, which are similar to TTG
and adakite but have lower silica (< 60 wt. %), higher Mg# (>> 62), MgO (> 6 wt.
%) and K2O (up to 4.5 wt. %), characteristic of modern LSA magmas (Martin et
al. 2005). The progression of non-adakite-like TTG in the Paleoarchaean to the
LSA-like sanukitoids in the Late Archaean was interpreted by Martin et al. (2005)
to reflect a petrogenetic shift that changed the source region. Pre 3.3 Ga, the
17
geothermal gradient was such that basalt melting occurred at a much shallower
depth and oceanic crust was much more buoyant, resulting in a very low
subduction angle that precluded the formation of a mantle wedge for the TTG
magmas to interact with. Post 3.3 Ga, the Earth (and ocean crust) had cooled
sufficiently to facilitate steeper subduction and basalt melting at greater depths;
this allowed for a mantle wedge to form and subsequent TTG melts interacted
with the wedge to generate the lower SiO2 and higher Mg# chemistry akin to
modern HSA. In the Neoarchaean, further cooling had reduced the efficiency of
slab melting and thereafter the majority of melts produced were consumed by
slab melt/peridotite reactions (Rapp et al. 1999, Martin et al. 2005), prior to
emplacement in the crust. Partial melting of this slab melt metasomatised mantle
peridotite resulted in magmas that had much lower silica and higher LILE, MgO,
Ni and Cr than Mesoarchaean TTG, very similar to LSA lavas (Martin et al.
2005).
The driving force behind comparing adakites and TTG is that the geochemical
similarities suggest magma genesis by a similar mechanism. The relative
abundance of TTG in the Archaean with the rare occurrence of true adakites (as
defined by Defant and Drummond 1990) that are only found in hot subduction
zones led to the interpretation that partial melting of subducted basaltic crust
was the method by which both magma types were formed. A review paper by
Moyen and Martin (2012) on TTG research noted some key points when
considering adakites as analogous to TTG, the most important of which is that
18
TTG are plutonic rocks and adakites are volcanic. As such, they likely should not
be considered equivalent because different processes can change the
geochemistry of volcanic rocks (e.g., differentiation/fractionation, magma mixing)
as compared to plutons (e.g., assimilation, H2O), despite having a similar source
region (Moyen and Martin 2012). In the literature, TTG have had similar
problems to adakites with the term being misused, where almost any somewhat
sodic Archaean pluton is identified as ‘TTG’, many of which exhibit few of the
original geochemical characteristics that define TTG (Jahn et al. 1981, Moyen
and Martin 2012). Hence, caution is needed when classifying a suite as either
‘TTG’ or ‘adakite’.
2.4
Non-slab melt models for adakite genesis
In tectonic regimes where there is no subduction or in subduction zones where
the slab is too cold to facilitate melting, the adakite signature needs to be
explained by a model that does not require slab partial melts. Several models
have been suggested to reproduce the adakite chemistry, the most common of
which include partial melting of mafic lower continental crust (Guo et al. 2006,
Huang and He 2010), fractional crystallisation of basaltic magma containing
garnet (Macpherson et al. 2006, Coldwell et al. 2011) and high pressure
assimilation, fractional crystallisation (AFC) processes of mantle-derived melts
and magma mixing (Castillo et al. 1999, Chiaradia et al. 2009). Potassic,
continental type adakites (K- and I-type adakites) have also been suggested,
originating from crustal thickening (e.g., Xiao et al. 2007). The commonality of all
19
of these models is that the source region is one where garnet is stable but
plagioclase is not. This implies that ‘adakite’ is not a petrogenetic term at all and
that rocks exhibiting an adakitic signature are simply the result of specific
temperature and pressure requirements being met in the source region, which
can occur in multiple tectonic environments.
In the study by Defant and Drummond (1990), basalt and basaltic andesite rocks
were rarely found in association with adakite. When basalt and adakite were
present in the same suite, the basalts were usually quite enriched in large ion
lithophile elements (LILE), approaching a shoshonitic composition. Further
studies into the nature of the mafic rocks associated with adakite showed that
contrary to the conclusions of Defant and Drummond (1990), the basalts were
enriched in high field strength elements (HFSE) rather than LILE. NEB were first
described by Sajona et al. (1993, 1994, 1996) and are defined as arc basalts
that contain 7 ppm < Nb << 20 ppm, as well as enrichments in other HFSE
including higher TiO2 contents (~ 1 - 2 wt. %) and low LILE/HFSE and
LREE/HFSE ratios (> 2 for both in normal arc rocks, Rollinson 1993). These
enrichments result in a much smaller, if not absent, Nb-Ta anomaly on primitive
mantle normalised multi-element plots; contrary to the steep negative anomaly
that is characteristic of normal subduction related magmas. NEB are commonly
found in adakitic suites and it was proposed by Sajona et al. (1993, 1996) that
the source of NEB was mantle wedge peridotite that had been previously
metasomatised by slab partial melts, suggesting a petrogenetic link between
20
adakite and NEB and reinforcing the slab melt model for adakite genesis.
However, other studies have shown that NEB and adakite are not always found
in association with each other and that one can be present without the other
(e.g., Southern Volcanic Zone (SVZ), South America; Stern and Kilian 1996).
This has led to some authors suggesting that it is only due to favourable tectonic
conditions that NEB and adakite erupt together and that there is no genetic link
between them (Macpherson et al. 2010), as there are other ways to generate
NEB independent of slab melt contribution to the mantle wedge (e.g. mixing of
OIB and MORB components in the mantle, Castillo et al. 2007).
3.
Petrography
3.1
GLVF
3.1.1 Cheakamus Valley Basalts (CVB)
Five samples were collected from the Cheakamus Dam phase of the
Cheakamus Valley Basalts (CVB) around the western edge of Daisy Lake (Fig.
2). The sampled flow is porphyritic, with 10 to 15 % total phenocryst content. The
most abundant phenocryst is plagioclase (~ 10 %), followed by approximately
equal amounts of olivine and augite (4 - 5 % each); phenocrysts of
orthopyroxene occur rarely (1 %). Plagioclase occurs as tabular to equant
crystals up to 3 mm in size. The plagioclase crystals are subhedral and
infrequently form glomeroporphyritic aggregates with augite (Fig. 4a). Several
textures are exhibited in plagioclase that includes sieve textured cores,
21
Figure 4: Thin section photomicrographs of the GLVF mafic rocks. (a) Ophitic texture in the CVB. (b) Plagioclase
oikocryst with included augite crystals in the CVB. (c) Zoned olivine phenocrysts in the CVB. (d) Olivine phenocrysts
altering to red iddingsite and flow banding in the HCBA. (e) Concentrically zoned augite phenocrysts with a sieve textured
core in the HCBA. (f) Zoned augite phenocrysts and rare pipe vesicles in the HCBA. (g) Strongly resorbed augite (upper
left corner) and orthopyroxene (lower left center) with primary, sub-ophitic orthopyroxene (center) in the DVBA. (h)
Twinned and zoned augite phenocrysts in the DVBA. All photomicrographs in cross-polarized light.
22
resorption of grain margins, rings of melt inclusions and rare poikilitic crystals
(Fig. 4b). Augite phenocrysts are generally smaller than plagioclase, with a
tabular habit of up to 2 mm in size. These grains are anhedral to subhedral,
occurring both as relatively unaltered phenocrysts and as strongly embayed and
resorbed crystals. Ophitic and subophitic are common textures between the
augite and plagioclase phenocrysts, with several smaller plagioclase laths
included within the augites (Fig. 4a). Olivine phenocrysts are of a similar size to
augite, approximately 1 - 2 mm. The crystals are anhedral to subhedral and
some exhibit poikilitic textures with plagioclase (Fig. 4b). Olivine also rarely
forms glomeroporphyritic aggregates with plagioclase and augite. Rare, weak
normal zoning is present in some olivine phenocrysts, that is best observed near
grain margins (Fig. 4c); some crystals also exhibit alteration to reddish orange
iddingsite along grain boundaries. Orthopyroxene phenocrysts are ~ 1 mm in
size and equant to prismatic in form. They include both anhedral to euhedral
varieties that occur as individual crystals as well as aggregates with plagioclase
and augite. Orthopyroxene locally exhibits ophitic and subophitic textures, and
some grains show evidence of resorption at their crystal margins (Fig. 4a,
bottom right). The groundmass to the CVB samples is weakly vesiculated (< 1
%), chiefly comprising microcrystalline plagioclase, augite and orthopyroxene,
forming an intergranular texture. Lesser amounts of olivine and opaque oxides
are also present. Where present, vesicles are spherical to slightly elongated, ~
0.5 to 1 mm in diameter.
23
Mineral compositions for olivine, clinopyroxene, plagioclase and oxide phases,
as determined by electron microprobe, are presented in Tables C1, C2, C4, and
C5 (Appendix C). Forsterite contents show moderate variation, ranging from Fo 78
to Fo68 (Table C1). Where present, zoning is weakly normal with only a 1-2 %
change in Fo (e.g., grain analysis 21-4, Table C1). Figure 5a illustrates typical
rim (open circles) and core (filled circles) compositions in olivine. Olivine
phenocrysts host appreciable Ni (up to 0.14 oxide wt. %). A single grain of
clinopyroxene was analysed; the phenocryst contains ~ 1 wt. % TiO2 (Table C2)
and is slightly more Mg-rich (En45 – Wo41). On the pyroxene quadrilateral, the
crystal (blue square) plots as a diopsidic augite (Fig. 5a). The determined
compositions of plagioclase phenocrysts are typically more felsic than would be
expected for such a primitive basalt (An63-65). Opaque oxides are chromian
magnetite, with Cr2O3 up to 23 wt. % (Table C5). These grains also contain
appreciable TiO2 (7.7 - 8.5 wt. % TiO2), Al2O3 (6.2 - 7.5 wt. %) and MgO (up to
5.5 wt. %).
3.1.2 Helm Creek Basaltic Andesite (HCBA)
Two samples (10JF013, 10JF024) were collected from the centre portion of the
HCBA flow (Fig. 2). This flow is porphyritic with ~ 10 to 15 % phenocrysts of
roughly equal amounts of olivine and augite. Olivine crystals are anhedral and
are up to 1 mm in size. Several, but not all, of the olivine phenocrysts are altered
with pervasive reddish orange iddingsite occurring along fractures and at grain
margins (Fig. 4d). Fine grained opaque phases also occur along the grain
24
Figure 5: Mineral compositions as determined by electron microprobe for (a) CVB and (b) HCBA. Aug=augite,
Di=diopside, Hd=hedenbergite. See text for explanation.
25
Figure 5 cont’d: Mineral compositions as determined by electron microprobe for (c) Desolation Valley and (d) Paul Ridge
andesite. Abbreviations as in Fig. 5 a & b. See text for explanation.
26
margins. Some olivine crystals also exhibit resorption and are embayed; opaque
inclusions are common. Rare, small glomeroporphyritic aggregates with augite
are present, up to 2.5 mm in size. Augite phenocrysts are slightly larger, ~ 2 mm
in size with rare larger phenocrysts up to 4 mm. The augite is euhedral to
subhedral and equant to prismatic in form. Simple twins are present in most
crystals as well as various patterns of zonation that include concentric zoning
(most common) and sector zoning. The larger augite crystals commonly exhibit a
distinct core mantled by a compositionally distinct, but uniform, rim and more
rarely have sieve textured cores (Fig. 4e). Augite also occurs as aggregates that
locally hosts inclusions of unaltered olivine. The groundmass to the Helm Creek
flow is trachytic, with several domains exhibiting different orientations. Strong
flow banding around the phenocrysts is also present. It is also weakly vesicular,
comprising mainly acicular plagioclase with lesser amounts of fine grained
augite, opaque oxide phases and brown glass. Vesicles (~ 1 %) are commonly
rounded to irregularly shaped, some 1 - 1.5 mm in diameter. Rare pipe vesicles
are also present, approximately 2 - 3 mm in length (Fig. 4f).
Mineral compositions for olivine, clinopyroxene, plagioclase and oxide phases as
determined by electron microprobe are presented in Tables C1, C2, C4 and C5
(Appendix C). The HCBA olivine phenocrysts are quite unique in that they are
near primitive (Fo86-89; Fig. 5b) despite being hosted in a basaltic andesite flow.
They show very little to no compositional zoning from core to rim and most fresh
crystals appear to be in equilibrium with the surrounding melt. Clinopyroxene is
27
again titaniferous, with up to 1.3 wt. % TiO2 (Table C2). Several crystals are
reversely zoned, with SiO2 decreasing from core to rim, but the En component
remains relatively unchanged or exhibits normal zoning. Clinopyroxene
phenocrysts are slightly more felsic than groundmass crystals (SiO2 of ~ 50 wt.
% versus ~ 48 wt. %; Table C2). Cr values span a wide range among the
analysed crystals, from below detection limits to as high as 0.9 wt. % Cr2O3. The
core compositions of the clinopyroxenes are augitic and trend towards diopside
(Fig. 5b). Groundmass clinopyroxene (filled diamonds) straddle the diopsideaugite boundary. Plagioclase compositions also exhibit a wide range, from An6
up to An56 (Table C4). BaO exhibits a general positive correlation with silica
(Table C4). Oxides are predominantly magnetite with a variable ulvöspinel
component (TiO2 up to 12 wt. %; Table C5). Cr contents are well below 0.5 wt.
%, with the exception of one chromian magnetite crystal that contains over 9 wt.
% Cr2O3 (24-6, Table C5). There is appreciable Al2O3 in the magnetite grains, as
well as MgO (up to 5.5 and up to 14.5 wt. % respectively, Table C5); however,
these do not always correlate with each other.
3.1.3 Desolation Valley basaltic andesite (DVBA)
One sample (10JF025) was collected from the flow terminus of the DVBA (Fig.
2). This flow is porphyritic, with ~ 20 % total phenocrysts, the most abundant of
which is plagioclase (~ 10 %), followed by olivine (~ 5 %) and roughly equal
amounts of orthopyroxene and augite (totaling ~ 5 %). Plagioclase crystals are
tabular to bladed, ~ 2 mm in size; it occurs both as discrete grains that display
28
oscillatory zoning, and as glomeroporphyritic aggregates with orthopyroxene and
augite. Some phenocrysts also exhibit resorption along grain margins. Olivine
occurs as anhedral grains of ~ 1 mm in diameter. The phenocrysts are strongly
resorbed and embayed, with some weak alteration to iddingsite along fractures.
Orthopyroxene forms equant to prismatic crystals of up to 2 mm. The
phenocrysts occur in two populations: strongly resorbed and embayed crystals,
and rare euhedral crystals. Euhedral orthopyroxene occur rarely as subophitic
grains with plagioclase (Fig. 4g) and on occasion, display weak oscillatory
zoning (Fig. 4f), whereas resorbed orthopyroxene commonly host melt
inclusions. Augite phenocrysts are typically smaller than plagioclase and
orthopyroxene, up to 1 mm in size. Augite may form equant subhedral to
anhedral crystals that are rarely sector zoned, and more commonly resorbed
and embayed along grain margins. Few of the augite crystals appear pristine;
those that are exhibit simple twins and a prismatic habit. The groundmass of the
DVBA has no evident fabric but weak flow banding is observed locally around
phenocrysts (Fig. 4g). The groundmass is microcrystalline with microlites of
bladed to acicular plagioclase and anhedral orthopyroxene with lesser amounts
of interstitial opaque oxides.
Mineral compositions for olivine, clinopyroxene, orthopyroxene, plagioclase and
oxide phases as determined by electron microprobe are presented in Tables C1C5 (Appendix C). The forsterite content of the olivine phenocrysts span a small
range, from Fo73 to Fo79; the rim and core compositions are slightly normally
29
zoned and overlap with core compositions (Table C1, Fig. 5c). SiO2 contents
decrease slightly from core to rim in the olivine grains, as does the NiO; the CaO
and MnO values increase towards the rim. Olivine lacks any significant TiO2 or
Cr2O3 and the phenocrysts are relatively inclusion free. Clinopyroxene shows
slight normal zoning as well, the rim composition is more Ca and Fe rich than the
core (Table C2) and when plotted on the pyroxene quadrilateral the core is
slightly richer in the augite component than the rim (Fig. 5c). TiO2 also increases
towards the rim; sector zoned crystals differ in their Ti contents. Cr2O3 decreases
significantly in the rim while MnO and Na2O both increase. Both phenocrystic
and groundmass orthopyroxene have been analysed; phenocrysts are more
mafic than the groundmass crystals with enstatite contents of En73, compared to
En68 in the groundmass (Table C3, Fig. 5c). The groundmass orthopyroxene is
also considerably richer in Al2O3 and Na2O, though CaO, MnO and NiO do not
differ significantly between phenocrysts and groundmass crystals. Plagioclase
compositions cover a relatively wide range of compositions, ranging from An65 to
An42 (Table C4). Oxide phases are mainly magnetite with a significant ulvöspinel
component, up to 9.7 wt. % TiO2 (Table C5). These are also chromian, ranging
from 0.95 - 2.3 wt. % Cr2O3. Al2O3 and MgO contents are similar for all
phenocrysts, between 2 - 3 wt. %.
3.1.4 Barrier andesite
Four samples (09JF004, 09JF005, 09JF006, 09JF012) were collected from the
Barrier andesite lava flow along the northern shore of Garibaldi Lake (Fig. 2).
30
This flow is porphyritic, with 10 to 15 % phenocrysts. Plagioclase is the most
abundant (~ 10 %), followed by approximately equal amounts of hornblende and
biotite (2 - 3 % each). Quartz phenocrysts occur rarely (< 1 %). Plagioclase
phenocrysts (up to 2 mm) form subhedral to anhedral equant to tabular crystals
that display complex zonation. Several grains contain concentric trails of melt
inclusions along their outer margins; sieve textures are also present. Hornblende
phenocrysts are smaller than plagioclase, ~ 1 mm in size and prismatic to bladed
in habit. The crystal edges are diffuse and commonly show alteration to fine
grained opaque minerals, possibly indicative of disequilibrium. Hornblende also
occurs as rare glomeroporphyritic aggregates (Fig. 6a). Biotite is the largest
phenocryst phase; up to 4 mm in size. The phenocrysts are equant, subhedral
and strongly pleochroic. The crystal margins are extensively altered and/or
heavily corroded; resulting in a rim opaque phases (Fig. 6b). Quartz
phenocrysts are anhedral, equant and ~ 1 mm in diameter. Where these occur,
quartz crystals are surrounded by a reaction rim of fine-grained, tabular, augite.
The groundmass of the Barrier andesite is comprised principally of brown glass
that comprises roughly equal amounts of crystallites and microlites of
plagioclase and rare hornblende. Rounded and irregularly shaped vesicles (~ 2
%) also occur in the groundmass. Flow banding is locally present around the
larger phenocrysts.
3.1.5 Black Tusk
31
Figure 6: Thin section photomicrographs of the GLVF and the MGVF adakite rocks. (a) Strongly altered biotite crystal
with a rim of oxide phases in the Barrier andesite, cross-polarized light. (b) Glomeroporphyritic hornblende in the Barrier
andesite, plane light. (c) Strongly resorbed orthopyroxene phenocryst in the Black Tusk andesite, cross-polarized light.
(d) Coarser grained crystal clot in the Black Tusk andesite, cross-polarized light. (e) Biotite xenocryst from the proximal
Ring Creek andesite, cross-polarized light. (f) Hornblende phenocrysts from the proximal Ring Creek andesite; note the
dark fine grained reaction rims along the crystal margins, plane light.
32
Figure 6 cont’d: Photomicrographs of the MGVF adakite rocks. (g) Mafic to intermediate xenolith comprised of
plagioclase and orthopyroxene in the distal Ring Creek andesite, plane light. (h) Quartz xenocryst with a reaction rim of
fine grained augite; weak flow banding is also present, cross-polarized light. (i) General texture observed (cross-polarized
light) and (j) concentrically zoned hornblende phenocrysts present within the Columnar Peak dacite, plane light. (k)
Plagioclase rich nature of the Paul Ridge andesite, with rounded olivine (lower right), cross-polarized light. (l) Olivine
(second order blue) being replaced by fibrous orthopyroxene (yellow) in the Paul Ridge andesite, cross-polarized light.
33
Two samples (10JF015, 10JF016) of orthopyroxene andesite were collected
from the west bluff of Black Tusk (Fig. 2). The andesite is essentially aphyric,
with rare (~ 1 %) phenocrysts of plagioclase and orthopyroxene. The plagioclase
phenocrysts are ~ 1 - 2 mm in size and are elongate to acicular in form. The
crystals are subhedral and fractured but are relatively clear. Orthopyroxene
phenocrysts (~ 1 - 2 mm) are equant, strongly fractured and in some examples,
exhibit resorption and disaggregation into the surrounding melt (Fig. 6c). Rare
sieve textured grains also occur as well as slightly coarser grained crystal clots
(Fig. 6d). The groundmass of the Black Tusk andesite comprises approximately
equal amounts of acicular, plagioclase microlites and brown glass. Strong flow
banding predominates in this unit.
3.2
MGVF
3.2.1 Ring Creek andesite
Four samples (09JF007, 09JF008, 10JF022, and 10JF023) were collected from
the Ring Creek andesite; two taken proximal to Opal Cone (09JF007, 09JF008)
and two taken approximately 2 km from the flow terminus (10JF022, 10JF023;
see Fig. 2). The mineralogy of the proximal Ring Creek andesite is different to
that of the distal portion of the flow (first noted by Sivertz 1976) and hence will be
described separately.
3.2.1.1
Proximal Ring Creek andesite
34
The andesite is porphyritic; main phenocrysts are plagioclase (15 %), followed
by hornblende (10 %) and augite (2 %). Quartz occurs in trace amounts (0.1 %).
Biotite (3 %) is present as an alteration product of hornblende and as rare large
xenocrysts (Fig. 6e). Plagioclase occurs in two size populations, the larger
phenocrysts are ~ 2 mm in size and the smaller less than 1 mm. The majority of
the plagioclase crystals are subhedral, equant to tabular; more rarely these form
glomeroporphyritic aggregates. Several features are exhibited in plagioclase that
includes sieve textures, resorption of grain margins and in some of the larger
crystals, seritization. However, an equal proportion of plagioclase phenocrysts
are inclusion free and ‘pristine’. Hornblende phenocrysts are second to
plagioclase in abundance and range in size from less than 0.5 mm up to 1 mm.
The majority of the crystals exhibit various disequilibrium textures including
fibrous cores of clinopyroxene and alteration to opaque oxides along the crystal
rims (Fig. 6f). Biotite occurs as subhedral xenocrysts up to 1 mm in size with rare
crystals of up to 3 mm. The edges of biotite crystals are diffuse and poorly
defined with rims showing alteration to a mass of fine grained opaque minerals.
The larger biotite xenocrysts are heavily embayed and have sieve textured
cores. With few exceptions these xenocrysts display extensive replacement by
opaque oxide phases; resorption of grain boundaries is also common. Augite
occurs as prismatic to equant crystals up to 3.5 mm in size. The margins of the
phenocrysts are resorbed and contain abundant inclusions of apatite and oxides.
The crystals are greenish brown and not distinctly pleochroic. Only a few quartz
35
crystals have been identified in this part of the flow. The phenocrysts are
anhedral and less than 0.5 mm in size and exhibit resorption along the grain
margins. The groundmass of the proximal Ring Creek andesite is approximately
equal parts crystallites and brown glass. Plagioclase, augite altered hornblende
and oxide phases comprise the majority of the crystallites. Local flow banding is
evident around the larger phenocrysts.
3.2.1.2
Distal Ring Creek andesite
The andesite is porphyritic, slightly less than the proximal portion with ~ 20 %
total phenocrysts. The mineralogy of the distal part of the flow differs from the
proximal portion in that the only phenocryst phases present are plagioclase and
augite. Xenoliths of mafic-intermediate cumulate inclusions that host
orthopyroxene occur rarely. The inclusions are heavily corroded and partially
melted (Fig. 6g). Plagioclase, again occurring in two size populations, is the
most abundant phenocryst (15 %), followed by augite (5 %). The larger
plagioclase crystals are up to 3.5 mm in size, the smaller approximately 1 mm;
all crystals are equant to tabular and subhedral, and display complex zonation
and various degrees of resorption. Sieve textured crystals are less common than
the plagioclase in the proximal portion of the flow but occur mainly in the larger
grains that also contain concentric trails of melt inclusions along their margins.
Augite phenocrysts are smaller than plagioclase, commonly less than 1 mm in
size. The crystals are equant and subhedral to euhedral; some grains display
simple twinning as well as glomeroporphyritic aggregates with plagioclase. Rare,
36
altered orthopyroxene crystals, likely derived from the mafic-intermediate
cumulate xenoliths, up to 2 mm in size are present but have been almost
completely altered to chlorite and opaque oxides. Rare quartz is also present
occurring as anhedral crystals that appear in disequilibrium with the surrounding
melt, in exhibiting reaction rims of fine-grained, radiating clusters of augite (Fig.
6h). This is in contrast to the proximal portion of the flow where the quartz
appears to be primary. The groundmass is predominantly crystallites of
plagioclase with lesser amounts of augite and brown glass. The groundmass
displays local, weakly developed flow foliation.
3.2.2 Columnar Peak dacite
Four samples from the orthopyroxene-hornblende dacite of Columnar Peak
(09JF009, 09JF010, 10JF019 and 10JF020) were examined as part of this study
(Fig. 2). This unit is described as a series of flows by Green (1977). The dacite is
porphyritic with 10 to 15 % phenocrysts (Fig. 6i). Plagioclase is the most
abundant phase (~ 7 %), followed by hornblende (~ 6 %) and orthopyroxene (< 1
%). Plagioclase occurs in two size populations; the larger crystals are up to 4.5
mm in size and the smaller crystals are ~ 1.5 mm. The phenocrysts are
subhedral to anhedral and are complexly zoned. Several disequilibrium textures
are observed in the large plagioclase phenocrysts including sieve textured cores,
resorption along crystal edges and several generations of concentric melt
inclusions. These textures are less common in the smaller plagioclase crystals.
Some of the larger grains exhibit fresh, clear overgrowths on the outer edges.
37
Hornblende is considerably smaller than plagioclase, up to 1 mm in size and is
subhedral to euhedral and equant to prismatic. The crystals are in disequilibrium
with the surrounding melt; the phenocrysts are commonly embayed with some
grains exhibiting sieve textured cores. All the crystals have resorbed margins
through disequilibria reaction rims comprised of opaque oxide phases. Weak
concentric zoning is also observed in some of the larger crystals (Fig. 6j).
Orthopyroxene crystals are quite small, less than 1 mm in size. The phenocrysts
are yellowish, equant to prismatic and mainly euhedral in form. The majority of
the orthopyroxene grains are relatively fresh, with occasional large melt
inclusions. Some crystals occur as aggregates or are perhaps fragments of a
single crystal separated by glass. The groundmass is comprised of roughly
equal amounts of brown glass and crystallites of plagioclase and opaque oxides.
Flow foliation is observed locally.
3.2.3 Paul Ridge andesite
Three samples of the Paul Ridge andesite (09JF011, 10JF017, 10JF018) were
collected in this study (Fig. 2). The andesite is described as a series of
hornblende andesite flows by Green (1977); these are porphyritic, with ~ 20 %
phenocrysts (Fig. 6k). Plagioclase phenocrysts are the most common (10 %),
followed by orthopyroxene and olivine (5 % each, marginally higher amounts in
09JF011) with trace amounts of xenocrystic quartz (< 0.5 %). The plagioclase
crystals are tabular and can also form aggregates of up to 3 mm in size. The
dominant crystal size is approximately 2 mm. Plagioclase is subhedral to
38
euhedral and is complexly zoned with reverse and oscillatory zoning. Some of
the phenocrysts exhibit uneven extinction, suggestive of strain deformation. The
crystals display varying degrees of sieve texture in the cores and resorption
along the edges. Some of the phenocrysts have clear cores with concentric rings
of melt inclusions near their margins. Orthopyroxene phenocrysts are markedly
less abundant than plagioclase, occurring mainly in the glassy groundmass with
some larger crystals. The relatively pristine orthopyroxene phenocrysts are up to
1 mm in size, green, broken and exhibits reverse zoning. Orthopyroxene also
commonly forms reaction rims on olivine and as an interstitial phase to crystal
aggregates. The majority of olivine crystals occur in a state of incipient
replacement by orthopyroxene (Fig. 6l); unaltered olivine is rare. These
replacement textures are slightly more common in 09JF011 than the other
samples. Larger olivine crystals occur occasionally in aggregates with
plagioclase; the olivine is anhedral and usually exhibits alteration to brown
iddingsite along crystal edges and fractures. Embayments are also common in
the crystals. A few quartz xenocrysts have been identified; the quartz is
anhedral, usually exhibiting weakly uneven extinction. The quartz xenocrysts
exhibit reaction rims and are surrounded by small, outwardly radiating augite
crystals. The groundmass is mainly brown glass with microlites of plagioclase
and opaques.
The compositions of the main mineral phases from two samples of the Paul
Ridge andesite (10JF017 and 10JF018) were determined by electron
39
microprobe and include olivine, orthopyroxene, plagioclase and oxides; these
values are listed in Tables C1, C2, C4 and C5 (Appendix C). Olivine
compositions between the samples are quite different; phenocrysts from
10JF018 are slightly normally zoned with more mafic olivine cores (~ Fo75) than
10JF017 (~ Fo65; Fig. 5d), but SiO2 contents of 10JF017 are lower than that of
10JF018 (Table C1). Orthopyroxene compositions between both samples are
generally similar with cores of En64-68, but 10JF018 exhibits strong reverse
zoning with rim compositions of up to En76 (Table C2, Fig. 5d). The Mg-Fe
contents of the olivine and orthopyroxene crystals in all samples probed span
very similar ranges (Fig. 5d). Reverse zoning is also observed for the TiO2 and
Cr2O3 contents in orthopyroxene; SiO2 remains relatively constant from core to
rim. Plagioclase also varies widely between samples; anorthite contents of
10JF018 are higher and span a much narrower range than the phenocrysts in
10JF017 (An66-72 versus An46-66 respectively, Table C4). With the exception of
one crystal (grain analysis 17-3, Table C4), SiO2 values of 10JF017 are only
slightly higher than 10JF018 (~ 51 wt. % versus ~ 50 wt. %). Oxide phases in
10JF018 are magnetite and ulvöspinel (Table C5), whereas 10JF017 contains
mainly magnetite. The magnetite grains in 10JF017 are more enriched in Cr than
10JF018 and have higher average MgO and Al2O3.
4.
Geochemistry
40
4.1
Analytical techniques
4.1.1 Whole Rock Geochemistry
Nine samples from the MGVF and nine from the GLVF have been analysed for
whole-rock major, trace and rare earth element (REE) concentrations. All
samples were crushed in a jaw crusher and then powdered to less than 74 µm in
a tungsten mill at the Dept. of Geology, University of Regina. All elements were
measured by the Trace Element Analysis Laboratories at McGill University,
Montréal, Canada. Data are presented in Tables A1 and A2 (Appendix A). Major
elements and some trace elements, which include Ba, Ce, Cr, Cu, Ni, V, Sc and
Zn were determined on a Philips PW 2440 X-ray fluorescence spectrometer
instrument, with samples prepared as fused glass discs. LOI was determined by
incrementally heating 4 g of rock powder in air to 400°C for 60 minutes, 800°C
for another 60 minutes, then to 1000°C for 180 minutes. The product was
weighed after cooling in a desiccator to calculate LOI. Other trace elements (Ga,
Nb, Rb, Sr, Th, U, Y, and Zr) were analysed on pressed powder pellets. Two
internal standards of known composition were sent with our samples to test the
accuracy and reproducibility of the results. The accuracy for silica is within 0.5
%. For other major elements it is within 1 % of the element analysed. For trace
elements as well, the accuracy is within 1 %, as determined from replicate
analyses of internal standards. The limiting factor for accuracy is the degree of
scatter of analyses from which the consensus values are determined
(Govindaraju et al. 1994, p. 256). Instrument precision is within 0.12 % relative.
41
This is the percent relative variation obtained when the same sample is analysed
repeatedly for the same element. Overall precision for glass discs and pressed
pellets is within 0.5 % relative. This is determined by repeatedly analysing two
glass discs or pressed pellets prepared from two different aliquots removed from
the same sample powder vial during the same day and used to prepare a fused
disc or pressed pellet by an experienced operator using routine procedures.
REE were measured by inductively coupled plasma-mass spectrometry (ICPMS) using a borate fusion decomposition method (after Panteeva et al. 2003).
The analyses of the REE were performed on solutions using a
PerkinElmer/SciEx Elan 6100 DRCplus ICP-MS. Samples (0.4 g, corrected for
LOI) were fused using a lithium metaborate mixture and then dissolved into nitric
acid and diluted. Standards and calibration solutions were prepared from fusion
blanks. Oxide corrections on the middle and heavy REE were made offline using
oxide production rates determined daily from single REE standard solutions.
Rock-sample detection limits (based upon three times the background standard
deviation) are 10 ppb for La through Pr, and 5 ppb for Nd through Lu. A set of
three internal laboratory reference materials were fused and run with each batch
of samples to evaluate long term precision. Precision was additionally evaluated
through repeat measurements of samples, including repeat fusions and dilution;
it is better than 3 % RSD in all cases. Accuracy was evaluated using a series of
six standard reference materials (SRMs) that span the sample concentration
range, prepared using the same procedure as the samples. Our determinations
42
agree with the accepted values for these SRMs with discrepancies of less than 5
%. Ferrous (FeO) iron was determined using an ammonium metavanadate
titration.
4.1.2 Mineral Geochemistry
The major mineral phases in two samples from the MGVF and four samples
from the GLVF were analysed utilising a Cameca SX-50 electron microprobe
housed in the Dept. of Earth, Atmospheric and Ocean Sciences, University of
British Columbia. Approximately 3 to 5 individual crystals were analysed of each
mineral phase in each thin section. Probed minerals included olivine,
clinopyroxene (where present), orthopyroxene, plagioclase and opaque oxides
(Tables C1-C5, Appendix C). Where zonation was present, core and rim
compositions were determined; complexly zoned crystals of olivine and
pyroxene were probed multiple times from core to rim or rim to rim.
Compositions of suitable microphenocrysts in the crystalline groundmass were
also determined, where they occurred. The operating conditions were:
accelerating voltage 20 kV; beam current 20 nA for all minerals; normally, the
beam was kept focused, giving a spot diameter in the region of 1 μm for most
minerals and counting time of 30 seconds over each spot. A rastered beam
covering ~ 12 μm × 12.5 μm was used for the analysis of plagioclase which may
be susceptible to decay under the electron beam. Larger scale rastering was not
acceptable due to spectrometer defocussing. Sodium was analysed for first
followed by other major elements to minimise artefacts due to decay. Mineral
43
standards were probed at regular intervals, approximately every 10 analyses.
Diopside standards were used for the olivine and pyroxene analyses, anorthite
for the plagioclase analyses and magnesium spinel for the opaque mineral
analyses. Additional compositional data for the investigated mineral phases
were determined by scanning electron microscopy-energy dispersivespectrometry (SEM-EDS) analysis, utilising a Jeol JSM-6360 SEM and Noran
system 7 EDS system, housed at the Electron Microbeam Facility, University of
Regina. Small scale textures and mineral-melt interactions were identified and
photographed for samples throughout the GVC, and X-ray element dispersal
maps were generated for crystals of interest. Mineral and glass compositions
were determined but due to the semi-qualitative nature of the EDS system, this
data is not discussed in the text. Backscattered scanning electron (BSE) images
of element distributions and zoning are included (see Section 5.1.3).
4.2
Results
4.2.1 Basalts and Basaltic Andesites
Three samples of the CVB and one sample from the HCBA were analysed as
part of this study. The geochemistry of the GLVF is well documented in several
other studies (Green 1977, 1981, 1990, 2006; Green and Henderson 1984) and
select data from these studies have been included for comparison in this work.
The CVB have determined compositions transitional between the alkaline and
sub-alkaline fields and exhibit a narrow range of total alkali contents (Na 2O +
44
K2O (wt. %) = 3.91 - 4.81; Fig. 7). The HCBA samples are predominantly
alkaline in character with a wide range of alkali and silica contents (4.14 - 7.13
wt. % Na2O + K2O) that reflect two distinct geochemical trends. The most mafic
samples plot along the alkaline-sub-alkaline boundary, closer to normal arc
basalts while the remainder fall well inside the alkaline field and range from
trachybasalt-hawaiite to basaltic trachyandesite-mugearite (Fig. 7). The CVB are
characterised by decreasing MgO, Fe2O3, CaO, TiO2, Ni and Cr with increasing
SiO2; whereas K2O, Na2O, P2O5 and V increase with SiO2 (Figs. 8, 9). Al2O3
does not vary significantly with increasing silica, as does Sr. The two distinct
trends identified in the HCBA data are more evident in the Harker diagrams
shown in Fig. 8. The mafic samples generally have the same characteristics
observed in the CVB samples; however, the more evolved samples exhibit
different geochemical trends. For example, Fe2O3 contents remain relatively
constant with increasing silica, whereas MgO, CaO, TiO2, K2O, Na2O and Cr all
decrease. P2O5 shows a strong negative correlation with increasing SiO2, while
Al2O3, Ni and Rb positively correlate with SiO2. V contents in the HCBA are
scattered and show no correlation with SiO2. Despite a normal decrease in MgO
contents for both the CVB and HCBA, the Mg#s increase with SiO2 in both
groups (Figs. 8, 9).
Primitive mantle normalised spider diagrams show that the HCBA samples have
stronger LILE and LREE enrichment than the CVB, as well as a prominent
positive Sr anomaly and Nb-Ta and Ti depletions. The CVB exhibit only a small
45
Figure 7: Total alkalis vs. SiO2 diagram with IUGS rock designations for the GVC rocks (after Le Bas and Streckeisen
1991). Dashed line represents the alkaline-subalkaline boundary from Macdonald (1968). Key: PR=Paul Ridge andesite,
RC=Ring Creek andesite, CP=Columnar Peak dacite, BT=Black Tusk andesite, BF=Barrier andesite, Table=Table
andesite, Cay=Mt. Cayley, CVB=Cheakamus Valley Basalts, HCBA=Helm Creek Basaltic Andesite. Open
symbols(Hist)=literature data, Mt. Cayley data from Kelman et al. (2001).
46
Figure 8: Harker diagrams illustrating variations in major element chemistry with increasing SiO 2 (wt. % oxide). All data
have been recalculated to 100 % on an anhydrous basis. Symbols as in Fig. 7.
47
Figure 9: Harker diagrams illustrating variations in trace element chemistry and Mg# with increasing silica for the GVC
rocks. All trace elements are in ppm, silica is reported in wt. % oxide. Symbols as in Fig. 7.
48
Nb-Ta depletion compared to the HCBA, despite both groups having very similar
Nb concentrations (~ 8.1 - 8.5 ppm). The CVB are also enriched in Sr, less so
than the HCBA, and have no Ti depletion (Fig. 10a, b). The Nb concentrations in
both the CVB and the HCBA classify them as Nb-enriched basalts (NEB), first
described by Sajona et al. (1993, 1994, 1996) as basalts and basaltic andesites
with Nb values of 7 ppm < Nb << 20 ppm. The CVB and HCBA both exhibit
additional geochemical characteristics of NEB, including higher TiO2 (~ 1 - 2 wt.
%) and little to no Zr depletion. NEB also have low LILE/HFSE and LREE/HFSE
ratios; Rb/NbPM and La/NbPM are > 2 in typical arc basalts. The CVB have
Rb/NbPM and La/NbPM well below that of arc basalts (0.85 and 1.1 respectively);
the HCBA has low Rb/NbPM (1.7) but higher La/NbPM than most NEB (~ 3). The
MREE and HREE contents of both the CVB and HCBA are very similar,
including a small Y depletion in both groups. These characteristics are also
evident in chondrite-normalised spider diagrams where HCBA rocks exhibit
stronger enrichment in LREE than for the CVB samples, but similar MREE and
HREE contents and behaviour. This results in a fractionated and slightly
concave upward pattern for the HCBA versus a less enriched, more linear trend
for CVB (Fig. 11a, b).
4.2.2 Adakites
Nine MGVF samples were analysed; 3 samples from Paul Ridge, 2 from
Columnar Peak dacite and 4 samples of Ring Creek (2 each from the proximal
and distal portions of this andesite flow). An additional 4 samples from the
49
Figure 10: Primitive mantle normalised spider diagrams for GVC samples analysed in this study (a & c) and for previously
published data (b & d). Data in (b) derive from Green (2006), data in (d) derive from Green and Henderson (1984).
Primitive mantle normalising values are from Sun and McDonough (1989). Symbols as in Fig. 7.
50
Figure 11: Chondrite normalised REE spider diagrams for GVC samples analysed in this study (a & c) and for previously
published data (b & d). Data in (b) and (d) derive from sources listed in Fig. 10. Symbols as in Fig. 7. Chondrite
normalising values from Sun and McDonough (1989).
51
Barrier andesite in the GLVF and 1 sample from Black Tusk were analysed (see
Tables A1 & A2, Appendix A). Data from Mt. Cayley (Kelman et al. 2001),
located north of the GVC, also exhibits adakitic characteristics and has been
included for comparison. The investigated MGVF and GLVF rocks are subalkaline in character ranging in composition from basaltic andesite to dacite, with
the bulk of samples falling in the andesite field (Fig. 7). All the GVC samples
define similar trends as individual centres in several major and trace element
variation diagrams (Figs. 8, 9). The volcanic products from each centre are
characterised by decreasing Fe2O3, CaO, MgO, TiO2, and V with increasing
SiO2. Al2O3 decreases only slightly as SiO2 increases, with alumina
concentrations spanning a range of about 1.5 wt. %. Ni, Cr, and Na2O do not
vary significantly with increasing SiO2, forming relatively flat trends. K2O and Rb
contents increase with SiO2, while Sr values increase to approximately 60 wt. %
SiO2 and then decrease sharply. The Mg#s of all the GVC rocks exhibit a narrow
range (46 to 52), except for the Paul Ridge andesite, where one sample has Mg#
of 62. Primitive mantle normalised multi-element spider diagrams show that all
centres have LILE enrichment and Nb-Ti negative anomalies, typical of
subduction related rocks (Fig. 10c, d). The Paul Ridge andesite rocks exhibit the
strongest depletion in Th and other incompatible elements and the highest
MREE-HREE abundances of all the GVC rocks. One Paul Ridge sample
(09JF011; see Tables A1 & A2, Appendix A) has higher MgO, TiO2, Ni and Cr
values than the others. Chondrite normalised REE spider diagrams (Fig. 11c, d)
52
for the GVC volcanic rocks display enrichment of LREE over HREE and lack any
significant Eu anomalies. The andesite rocks of Black Tusk display the lowest
LREE/HREE fractionation of all the GVC volcanic rocks with La/Yb N averaging ~
5.9. The Ring Creek andesite samples exhibit the highest fractionation with
La/YbN ranging from 8.5 to 9.6. All of the GVC rocks have similar MREE/HREE
fractionation with ratios of 1.5 to 1.9. On a Sr/Y versus Y diagram, all of the GVC
rocks plot within the adakite field, but fall outside of this field on the La/Yb versus
Yb diagram, having values typical of normal arc-rocks (Fig. 12a, b). The HCBA
has a silica content that is too low to be considered adakitic (Table A1, Appendix
A) but still exhibits several other adakitic indicators (see section 5.2.3.1) and has
been included in the adakite plots. The accepted minimum value for La/Yb as an
adakitic indicator ranges from as low as 8 (Drummond and Defant 1990) up to
20 and greater (Castillo et al. 1999, Richards and Kerrich 2007). The La/Yb
values for the GVC have a range of ~ 9 (Paul Ridge andesite) to 13.4 (Ring
Creek andesite), plotting in the lower end of the dashed adakite field. When
plotted against SiO2, both Sr/Y and La/Yb appears to increase for Paul Ridge
andesite rocks in contrast to the other GVC centres, for which Sr/Y decreases
with SiO2, but La/Yb increases (Fig. 12c, d). Other adakitic indices (Sr, Na2O,
Al2O3) do not show definitive trends with fractionation indices (SiO2, Ni, Cr),
suggesting that the chemistry is not controlled exclusively by fractionation
processes (Chiaradia 2009). The GVC samples may also be divided into the
low-silica and high-silica (LSA and HSA) groups on the basis of geochemical
53
Figure 12: Plots of adakitic indices (a & b) for the GVC rocks and variations in these indices with increasing silica (c & d).
Adakite and normal arc-rock fields derive from Castillo (2006) and Richards and Kerrich (2007). Trace elements are in
ppm, silica in wt. % oxide. Symbols as in Fig. 7. ADR=andesite-dacite-rhyolite
54
characteristics outlined for adakitic rocks by Martin et al. (2005). On a K/Rb
versus SiO2/MgO diagram, the Paul Ridge and Black Tusk andesite rocks exhibit
high K/Rb relative to SiO2/MgO and form a sub-vertical trend (Fig. 13a) indicative
of LSA; historical data for Table andesite samples also follow this trend. The
Ring Creek andesite and the Columnar Peak dacite have lower K/Rb values and
plot sub-horizontally. The Barrier andesite samples appear transitional, plotting
at the intersection between the LSA and HSA fields. This may relate to the Rb
contents of Barrier andesite samples, which are slightly elevated. The LSA and
HSA groupings are still evident, though not as well defined, in the Sr-K/Rb(SiO2/MgO)*100 ternary diagram (Fig. 13b). Here, only the Columnar Peak
dacite and the Ring Creek andesite are distinctly HSA and the Table andesite
rocks are clearly LSA.
5.
Discussion
Compositions determined for the GVC rocks analysed as part of this study and
those of previously published data conform to virtually all of the adakitic
geochemical traits put forward by Defant and Drummond (1990) and Martin et al.
(2005). The GVC rocks analysed in this study are characterised by high Sr/Y (46
- 98), Al2O3 (16.9 - 18.8 wt. %), Mg# (~ 51), low Y (≤ 17.1 ppm), and low Yb (≤
1.9; Tables A1 & A2, Appendix A). Some of the GVC adakites can be further
divided into HSA and LSA groups. For example, while the Ring Creek andesite
and Columnar Peak dacite units more closely represent HSA, with > 60 wt. %
SiO2, lower MgO (1.8 - 2.5 wt. %) and < 1100 ppm Sr (750 - 1078), the Paul
55
Figure 13: Discriminant diagrams for the LSA and HSA groups. (a) K/Rb vs. SiO2/MgO plot illustrating the distinction
between LSA and HSA groups within the investigated GVC rocks. LSA plots higher in K/Rb while HSA defines a subhorizontal trend. (b) Sr-K/Rb-{(SiO2/MgO)*100} ternary diagram distinguishing between LSA and HSA for the GVC
adakites. LSA and HSA fields modified from Martin et al. (2005). Symbols as in Fig. 7.
56
Ridge andesite generally resembles LSA, with < 60 wt. % SiO2, higher MgO (3.4
- 4.9 wt. %) and low Rb (8.4 - 13.1 ppm). Mineralogically, the Paul Ridge
andesite fits with LSA, but the Ring Creek andesite and the Columnar Peak
dacite differ from the HSA definition in that they both contain pyroxene
phenocrysts, which is a characteristic of LSA. The Black Tusk andesite samples
(and the Barrier andesite to a lesser degree) straddle the boundary between
LSA and HSA, with SiO2 contents ~ 60 wt. %. The Table andesite is the only unit
that exhibits all the geochemical traits characteristic of LSA (Fig. 13a, b), as
outlined by Martin et al. (2005). However, there are some variations in major and
trace element contents which illustrate a more typical arc-like magma
composition for the GVC as a whole and these are discussed below.
5.1
Adakitic Geochemistry of the GVC
5.1.1 La and Cr
The two key geochemical indicators for the identification of adakitic magmas are
Sr/Y ≥ 40 and La/Yb ≥ 20 (Defant and Drummond 1990). Determined Sr/Y ratios
for the GVC rocks investigated herein cover a wide range (38 - 109; Fig. 12,
Table A2; Appendix A), but the majority of values fall between 75 and 90. Some
of the previously published data (Black Tusk and the Barrier flow, in particular)
straddle the boundary between adakite and normal arc-rocks, which appears to
be related to elevated Y contents reported in these earlier published data. All of
the GVC rocks have La/Yb ratios that lie in the field of normal arc-magmas and
57
not in the adakite field (Fig. 12). This is a function of the low La in the GVC
relative to Yb content, which are typical of adakites (< 1.9 ppm). La
concentrations in the GVC range from 10.7 to 18.3 ppm, lower than the average
for typical intermediate rocks (~ 20 - 30; GERM, earthref.org). However, La
values for adakite from other localities are in fact quite variable, from ~ 30 ppm
(Cook Island, Stern and Kilian 1996) to as low as 10 - 12 ppm (Pinchincha
volcano and Tonga Trench; Bourdon et al. 2002, Falloon et al. 2008,
respectively). Similarly, the Cr concentrations from all the centres in the GVC are
found to be lower than that proposed by Martin et al. (2005) (~ 36 ppm), though
Cr contents of ≥ 30 ppm may be considered adakitic (Martin 1999, Richards and
Kerrich 2007), and these values are not significantly different from that of the
average Cr content for the GVC adakites (~ 27 - 32 ppm).
5.1.2 LSA versus HSA
The majority of the GVC adakite units exhibit geochemical traits of both the LSA
and HSA groups (except for The Table andesite). In general these units may be
subdivided based upon their SiO2 (and to a lesser extent, MgO) contents. This
may be the result of overlap of the LSA and HSA groups in the criterion outlined
by Martin et al. (2005) (e.g. CaO + Na2O < 11 wt. %, Sr < 1100 ppm for HSA,
CaO + Na2O > 10 wt. % and Sr > 1000 ppm for LSA). However, the trace
element data for the GVC also do not distinguish HSA from LSA. On a primitive
mantle normalised spider diagram (Fig. 10a, b), LSA differs from HSA in lower
Rb and higher Nb values, with a stronger positive Sr anomaly and no Ti anomaly
58
(Martin et al. 2005). The rocks with a stronger LSA character (Paul Ridge and
Black Tusk andesite rocks) have lower Rb, but also lower Nb and similar, if not
lower, Sr to HSA and a negative Ti anomaly. Similarly, the Ring Creek andesite
and the Columnar Peak dacite, which are predominantly HSA, have higher Nb
and Sr (on average) and this is a characteristic typical of LSA. The Barrier
andesite samples have Rb contents that fall between those of the Paul Ridge
and Columnar Peak rocks, but also the highest Nb contents of all the rocks of
the GVC (up to 8 ppm). Martin et al. (2005) used a series of binary plots to help
distinguish LSA from HSA (e.g., MgO vs. SiO2, Nb vs. SiO2 and Sr vs. CaO +
Na2O; Fig. 14). Differentiation between the two groups based upon these
element pairings is not, however, possible for the GVC adakite rocks as the
current data lack the extreme values of the adakite dataset used by Martin et al.
(2005) (e.g., Sr up to 3000 ppm, Nb > 20 ppm, Rb up to 150 ppm, etc.).
The tendency for the GVC rocks to display geochemical traits of both the LSA
and HSA groups (except for the Table andesite) in addition to the other
variations in major and trace element chemistry may suggest one of two things:
(1) the current dataset is insufficient to permit an adequate assessment of
adakite affinity for the investigated GVC rocks, or (2) that the GVC rocks have
been modified by other processes, that could have altered their adakitic
signature.
5.1.3 Magma Mixing in the GVC
59
Figure 14: (a) MgO vs. SiO2, (b) Nb vs. SiO2, and (c) CaO+Na2O discriminant diagrams illustrating the variability of HSA
and LSA compositions in the GVC dataset. LSA and HSA fields are modified from Martin et al. (2005). Major elements in
wt. % oxide, Nb and Sr are in ppm. Symbols as in Fig. 7.
60
All the GVC adakite magmas show petrographic evidence of high-level magma
mixing (Fig. 6) with the Paul Ridge andesite exhibiting the strongest. The
geochemical variation that suggest a more typical arc-type composition are most
likely the result of mixing between a pre-existing HSA magma and an intruding
non-adakitic magma (or magmas) of broadly similar composition. Mixing of HSA
magmas with normal, intermediate arc type magmas can decrease the adakitic
character of the GVC lavas relative to the average adakite. In the case of the
Paul Ridge andesite, multiple periods of mixing are observed across the
samples studied. For one sample (09JF011), the intruding magma is suggested
to be of a more basaltic composition. The occurrence of olivine and
orthopyroxene with quartz, and clear disequilibrium textures observed between
these phases and that of their surrounding groundmass (see Fig. 6) support the
interaction of compositionally distinct magmas in this sample, as has been
documented at other volcanoes in the GVB (cf. Mt. Meager, Hickson et al. 1999).
The other Paul Ridge samples also exhibit disequilibrium textures, but not so
markedly. There are no quartz xenocrysts observed in either 10JF017 or
10JF018, and while olivine crystals undergoing replacement by fibrous
clinopyroxene are observed, these are somewhat less abundant than in
09JF011; large, relatively unaltered olivine is also present. The geochemistry of
09JF011 is also distinct from the other samples; 09JF011 has a much higher
content of SiO2, MgO, Ni and Cr, but lower in Fe2O3 than determined for two
other Paul Ridge andesite samples. Similarly, 09JF011 has a stronger adakitic
61
affinity, with higher Mg#, Sr/Y and La/Yb values (Tables A1 & A2; Appendix A).
This suggests that perhaps this sample did not undergo the same degree of
mixing prior to eruption, than for the other Paul Ridge andesite samples, which
may have been more extensively mixed. Green (1977) noted that some minor
pyroclastic materials were present at Paul Ridge. It is possible that the 09JF011
sample taken from the northern part of Paul Ridge may represent a pyroclastic
component that has a stronger basaltic character. Microprobe analyses of
mineral phases from the Paul Ridge andesite (Tables C1-C5, Appendix C) and
SEM imaging supports these mixing relationships and that multiple flows of
distinct composition are present within the Paul Ridge centre. For example, the
compositions of phenocrystic olivine cores from sample 10JF018 are more
primitive than those determined for olivine found in sample 10JF017 (Fo 75 and
Fo65, respectively). Similarly, orthopyroxene phenocrysts present 10JF018
display strong reverse zoning towards their crystal margins, with rim
compositions exhibiting a ~ 10 % increase in the enstatite component as
compared to core compositions (Table C3; Fig. 15a, b). Plagioclase phenocrysts
also record similar trends, although their compositions are less pronounced
(e.g., plagioclase found in sample 10JF017 is less calcic than that from sample
10JF018; An61-66 versus An66-72). However, despite the more primitive
compositions identified in olivine, orthopyroxene and plagioclase phenocrysts,
the whole rock composition of sample 10JF018 is surprisingly more felsic than
that of sample 10JF017 (SiO2 is higher and Fe2O3 and TiO2 lower than for
62
Figure 15: Back scattered electron images (BSEI) photomicrographs of the GVC adakites illustrating magma mixing
processes. (a) reverse zoning in orthopyroxene from the Paul Ridge andesite (red line), (b) X-ray element map of
orthopyroxene in (a) showing the distribution of Mg in the crystal; note the more intense yellow colour representing an
increase in Mg towards the crystal rim which then falls again as the outer rim is approached, highlighting the effects of
fractionation on top of the magma mixing as the crystal grew. (c) plagioclase crystal from the Ring Creek andesite
illustrating several generations of partial melting and crystallisation; lighter grey shades indicate higher Ca content, darker
grey represents higher Na. (d) strongly resorbed original orthopyroxene (Px) crystal (light grey core) surrounded by new
orthopyroxene (darker mantle) crystallising from new magma pulse in the Black Tusk andesite; light grey=higher Fe,
dark grey=higher Mg. (e) quartz xenocryst (Qtz) surrounded by a reaction rim of augite from the Barrier andesite. (f)
hornblende phenocrysts (Hbl) from Columnar Peak with dehydration reaction rims (resulting from the crystal being
removed from its stability field) that is surrounded by a rim of fresh hornblende crystallising from the surrounding melt.
Plag=plagioclase, Gl=glass, TiMt=titanomagnetite.
63
10JF017; Table A1). The identification of the distinct chemical differences
between the Paul Ridge andesite samples may suggest a significant change in
either the source region and/or magmatic processes on ascent. The 09JF011
sample maintains a strong mafic character (high MgO, Ni, Cr and Mg#) despite
having the highest SiO2 content of all the Paul Ridge samples (Tables A1 & A2,
Appendix A) as well as incomplete mixing between very compositionally distinct
magmas, whereas mixing in 10JF017 and 10JF018 appears to be somewhat
more cryptic. A possible interpretation could be that 09JF011 originated as an
HSA magma and underwent more extensive interaction with mantle peridotite,
while 10JF017 and 10JF018 represent true LSA magmas. The potential
pyroclastic nature of 09JF011 suggests that it may be older than the other
andesite samples, which are likely to be flows, and this relative timing coupled
with the geochemistry and petrography indicates a possible transition from an
HSA source region to LSA source region. It must be noted that while different
sources could explain the geochemical differences between the Paul Ridge
andesite samples, all of these still exhibit traits of both the LSA and HSA groups.
For the Ring Creek andesite samples, there is no evidence of interaction with a
basaltic intruding magma and mixing relationships are less clear, suggesting that
the mixing components were perhaps of broadly similar composition or that they
were more effectively mixed. The mineralogy of the Ring Creek andesite varies
from an augite-hornblende-biotite-bearing assemblage in the proximal portion of
the flow to augite only-bearing in the distal portion. The proximal andesite also
64
appears to contain primary phenocrystic quartz, whereas quartz crystals in the
distal Ring Creek andesite show disequilibrium with the surrounding melt and
hence, is difficult to explain through a simple mixing process. However, Sivertz
(1976) in mapping the Ring Creek andesite and Opal Cone also noted this
difference in mineralogy and concluded that the hydrous mineral assemblage in
the proximal Ring Creek andesite was identical to the mineralogy of Opal Cone
itself. It is possible, therefore, that the proximal Ring Creek andesite entrained
material from Opal Cone during eruption and inherited then hornblende-biotitequartz mineralogy.
Mixing can result from convective overturn initiated by intrusion of hotter magma
in to sub-volcanic magma chamber from depth (Hickson et al. 1999), but there is
no evidence of mixing between compositionally distinct magmas in the Ring
Creek andesite. Magma chamber overturn between magmas of a similar
composition can be caused if the intruding magma has a high upward
momentum (Turner and Campbell 1986), or was significantly hotter. This is
evidenced in Figure 15c, which shows several generations of compositional
zoning with a distinct partial melting zone in a plagioclase phenocryst by a hotter
intruding melt. The Ring Creek andesite flow is unusually extensive for an
intermediate to felsic composition, having flowed for ~ 17 to 18 km from its vent
(Sivertz 1976; Green 1977; Brooks and Friele 1992). It represents predominantly
non-explosive eruptive volcanism with no evidence to support a coeval
pyroclastic component associated with this flow. Sivertz (1976) described lapilli
65
and block fragments of dacitic composition, but this material is only found within
Opal Cone. The large extent of the Ring Creek andesite flow and the lack of any
significant pyroclastic material might suggest that intrusion of a large volume of
relatively fast moving magma (the proximal andesite) of similar composition into
pre-existing, cooling magma (the distal andesite) within the magma chamber,
created turbulence that facilitated entrainment of wall-rock material from Opal
Cone as the eruption proceeded. The intermediate cumulate xenoliths observed
in the distal part of this andesite flow (but not found in the proximal andesite in
this study) were likely incorporated from the roof or walls of the magma
chamber, during rapid eruption. It is not known to what extent the hornblendebiotite-quartz mineralogy is present in the Ring Creek andesite further south and
access to the central portion of the flow is very limited (Sivertz 1976), making it
difficult to resolve further.
In the Black Tusk centre, mixing relationships in the andesite samples are not as
clear as for other centres within the GVC. The lava is essentially aphyric and the
few phenocrysts present are strongly resorbed. This suggests that an intruding
magma was likely significantly hotter than the pre-existing magma, resulting in
strong thermal disequilibrium that caused near total resorption of earlier formed
phenocrysts, thus obscuring any potential melt-crystal relationships. Additionally,
the intruding magma appears to be a very similar composition to the resident
magma. This may indicate that the volume of the intruding magma was large or
rapidly intruded, in order to superheat the system and allow for the
66
disaggregation of all the phenocrysts. SEM imaging of orthopyroxene
phenocrysts show rounded, strongly resorbed Mg-rich cores surrounded by a
reversely zoned, euhedral mantle (Fig. 15d), illustrating the progressive intrusion
of a much hotter magma.
Mixing in the Barrier andesite was likely between that of a dacitic pre-existing
magma and an andesitic intruding magma. This is evidenced by reaction rims of
augite on quartz phenocrysts (Fig. 15e) and strongly embayed biotite crystals.
Hornblende appears to be in equilibrium with the intruding andesitic lava; there
are few disequilibrium features in the phenocrysts except for rims of opaques
along the grain margins and these may be the result of hornblende being
removed from its stability field during ascent.
At Columnar Peak, both the pre-existing and intruding magmas appear to be
compositionally alike. There are disequilibrium features in the plagioclase and
hornblende phenocrysts, but the intruding magma generally contains the same
mineralogy, with the exception of minor orthopyroxene (< 1 %). These features
indicate that despite the similar composition, the relative temperatures of both
magmas were significantly different such that the larger, pre-existing
phenocrysts were strongly resorbed and embayed relative to the phenocrysts
from the intruding magma. Figure 15f shows fresh regrowth rims on pre-existing,
embayed hornblende crystals, further evidence of interaction between magmas
of similar composition.
67
Petrographic evidence for magma mixing is also supported in identified
geochemical traits and trends for the investigated GVC rocks. Schiano et al.
(2010) used trace element modelling, on a database of 700 rocks from the
Ecuadorian Andes, to illustrate that mixing was the major control on their
evolution. By plotting ratios of compatible and incompatible elements, fractional
crystallisation, partial melting and mixing processes can be distinguished from
each other (Allègre and Minster 1978, Schiano et al. 2010). By plotting an
incompatible element versus the ratio of that incompatible element and a
compatible element (e.g., Rb versus Rb/V), mixing and fractional crystallisation
will form a curved trend, whereas partial melting results in a more linear trend
(Fig. 16a). In the case of GVC centres, the Black Tusk and Paul Ridge andesites
show evidence of clear mixing or fractional crystallisation processes, while the
Ring Creek and Barrier andesites only show a slight curved trend, perhaps
reflecting the degree/efficiency of mixing (i.e., the Paul Ridge and Black Tusk
andesites show arguably stronger evidence of magma mixing while the Ring
Creek and Barrier andesites appear to be more effectively mixed). The limited
dataset from the Columnar Peak dacite precludes any interpretation; however,
when combined with other data for the GVC as a whole, this also illustrates a
curved array. To isolate the effects of mixing from fractional crystallisation, a
companion diagram is needed, where the incompatible/compatible element ratio
is plotted against 1/compatible element (e.g., 1/V versus Rb/V, Fig. 16b). On this
companion plot, mixing creates a linear trend, whereas partial melting and
68
Figure 16: Incompatible/compatible element ratio plots for (a) Rb vs. Rb/V and (b) 1/V vs. Rb/V distinguishing mixing from
both partial melting and fractional crystallisation (FC). Insets are modified from Schiano et al. (2010). In (a), mixing and/or
fractional crystallisation is represented by a curved trend. In (b), mixing is isolated and is illustrated by a straight line; this
supports the dominance of magma mixing in controlling the chemistry of the GVC adakites. Symbols as in Fig. 7.
69
fractional crystallisation will both appear as curves; all of the GVC samples plot
as linear trends. Figure 16b further illustrates that a mixing relationship reflects
only magma mixing and not mixing of sources. Partial melting of a
heterogeneous source would significantly modify the incompatible/compatible
element ratio, whereas ratios of incompatible elements alone would not cause a
change (Langmuir et al. 1978, Schiano et al. 2010). These plots and data
suggest that the mixing relations observed in the GVC occurred in the magma
chamber (or reservoirs) beneath each centre, after segregation from their solid
source and argues against significant fractional crystallisation processes
dominating the chemistry of the GVC adakites, as has been suggested for other
adakite magmas (e.g. Castillo et al. 1999).
The mixing relationships in the GVC rocks are significant for adakite genesis for
several reasons: (1) despite mixing with arguably non-adakitic magmas (in some
cases extensive mixing), the majority of the adakitic characteristics are
preserved in the GVC rocks (only Cr and La are affected); coherent, linear
trends of major and trace elements with increasing SiO2 (Figs. 8, 9) also argue
that both magmas are similar in composition, (2) these mixing processes may
explain the fact that the GVC rocks exhibit geochemical characteristics of both
the HSA and LSA groups, and (3) with no evidence of interaction with mafic
magmas (except for the Paul Ridge andesite), the adakitic Ni, Cr and Mg# of the
GVC rocks likely reflect interaction with mantle peridotite during ascent through
the mantle wedge (Rapp et al. 1999, Martin et al. 2005).
70
5.1.4 Interaction with Mantle Peridotite
The Ni, Cr and Mg# values for the GVC adakite rocks are generally higher than
for normal andesite or dacite (~ 20, ~ 25 ppm and ~ 42, respectively) and, due to
the lack of evidence of a basaltic mixing component for the majority of the GVC
(with the exception of the Paul Ridge andesite), these values perhaps reflect the
interaction of HSA magmas with mantle peridotite during ascent from their
source region. Increasing the Mg#, Ni and Cr concentrations in slab melts
through the assimilation of peridotite (as described for HSA by Martin et al.
2005), however, is an unlikely process for the GVC. Typical mantle peridotite
contains ~ 3200 ppm Cr, ~ 2300 ppm Ni and ~ 42 wt. % MgO (Sigurdsson et al.
2000). To inherit the values observed in the investigated GVC adakitic rocks,
assimilation of peridotite would be strongly limited, as assimilation of > 1 %
peridotite would result in Ni and Cr values higher than is observed in the GVC.
Partial melting of peridotite is an unlikely method to modify ascending adakitic
magmas as peridotite partial melts would be basaltic (e.g. McKenzie and Bickle
1988), and mixing of these melts would be an inefficient way to increase the
mafic content of HSA magmas while maintaining higher SiO2. Furthermore, there
is no petrographic evidence for mixing with basaltic magmas observed in the
GVC (with the exception of one sample of the Paul Ridge andesite). A more
likely process would be zone refining, whereby the ascending HSA magma gains
Ni, Cr and MgO by diffusion. This process enriched the adakite magma in mafic
components at the expense of SiO2, but by and large preserves the incompatible
71
element ratios of the slab melt; the diffusion rates of incompatible elements (e.g.,
REE, Y) would be too slow to significantly modify the ascending magma (Wilson
1989) and obscure the slab melt signature.
5.1.5 Isotope Geochemistry
Limited 87Sr/86Sr and 87Rb/86Sr data from previous studies (Green 1977, 1990) of
the GVC rocks show a smaller range of 87Sr/86Sr for intermediate compositions
(0.7031 - 0.7035; Green 1990) than for 87Rb/86Sr (0.025 - 0.120; Green 1990),
which appears to relate to the increasingly felsic character of the rocks analysed,
i.e. the rhyodacite and rhyolites from Mt. Garibaldi contain the highest ratios of
87
Rb/86Sr. Green (1990) stated that the MGVF rocks contain a significant Rb-rich
crustal component over the GLVF, which exhibit lower 87Rb/86Sr, and attributed
this to be the result of AFC processes combined with contamination from crustal
xenoliths and mixing with anatectic melts during ascent. While mixing of melts is
present in the MGVF, there is little evidence for the incorporation of crustal
xenoliths in any rocks examined as part of this study. Rare xenoliths are present
in the Ring Creek andesite, but are mafic to intermediate in composition and
would not significantly modify Rb values; the mineral phases present in the
xenoliths are predominantly pyroxene and plagioclase, neither of which
preferentially concentrates Rb (KdRb << 1 for orthopyroxene, clinopyroxene and
plagioclase, Rollinson 1993). Lower Rb in the GLVF (4 - 18 ppm) was suggested
by Green (1990) to reflect a depletion of LILE in the source region and less
crustal interaction than the MGVF. For the MGVF rocks in this study, the Rb
72
concentrations are comparable to that determined by Green (1990) for the GLVF
(average 14.5 ppm). Stern and Kilian (1996) noted that the effects of crustal
interactions were present in the adakite rocks from the Austral Volcanic Zone
(AVZ) and these decreased southward in the belt as the angle of subduction
became more orthogonal. This resulted in negligible interaction of the Cook
Island adakites with crustal material and hence, their Sr isotopic compositions
more closely resemble MORB (and by extension, slab partial melt) values.
87
Sr/86Sr data from the Cook Island adakite samples approximates to 0.7028
(Stern and Kilian 1996), which is quite similar to the
87
Sr/86Sr ratios determined
for the GVC adakites (Green 1977, 1990) as well as average
87
Sr/86Sr values for
MORB (~ 0.703; GERM, eartheref.org) and 87Sr/86Sr values for Juan de Fuca
MORB (~ 0.7025 – 0.7028; Hegner and Tatsumoto 1989, Chadwick et al. 2005).
The Cook Island adakite rocks are arguably the best known representation of
potentially pristine slab melt, and Stern and Kilian (1996) based this on several
geochemical traits that suggested a basaltic source, in addition to isotopic data.
The Rb/Sr ratios of the andesite rocks from Cook Island are extremely low, lower
than typical MORB (and by extension, slab partial melt) values (~ 0.002). The
Rb/Sr concentrations in the GVC rocks (~ 0.01) are not as low as those found in
the Cook Island andesites, but are comparable to MORB (0.006 - 0.009; Wilson
1989). Therefore, the possibility exists that: (1) the isotopic and element ratios of
the GVC are inherited from a basaltic (MORB) source, and (2) they also argue
against any significant contamination by crustal material.
73
5.2
Possible models for Adakite Genesis
Adakites were originally defined as felsic partial melts of subducted basaltic
crust, leaving a residuum that was garnet rich but plagioclase poor and giving
rise to the distinctive high Sr/Y and La/Yb ratios (Defant and Drummond 1990).
As studies continued, high Sr/Y and La/Yb ratios in arc-magmas were found to
not necessarily be indicative of slab melts as different processes in subduction
zone environments were shown to produce these high values; the most common
of which are partial melting of mafic lower crust (e.g. Wang et al. 2005,
Macpherson et al. 2006) and high pressure AFC processes +/- magma mixing
(e.g. Castillo et al 1999, Chiaradia 2009). This has cast doubt on the use of high
Sr/Y and La/Yb as unique characteristics of slab partial melts. The following
section evaluates the most common processes to generate adakite compositions
in the context of the GVC to determine the most likely scenario for the origins of
the GVC adakites.
5.2.1 Partial melting of basaltic lower crust
It has been shown that adakitic magmas can be generated from partial melting
of thickened lower continental crust (Guo et al. 2006, Huang and He 2010) or remelting of underplated basaltic magma (Macpherson et al. 2006). A condition of
these models is that magma genesis, whole or in part, must occur in the garnet
stability field to generate the low HREE and Y concentrations in the resulting
adakite melts. Garnet is stable in the lower crust as garnet amphibolite or
74
eclogite facies rocks, generally at ≥ 40 km depth (Richards and Kerrich 2007).
LITHOPROBE seismic transect studies from British Columbia, in the vicinity of
the GVC, have shown that the approximate thickness of the crust is ~ 34 km
(Perry et al. 2002), too thin for garnet to be a stable phase at the base of the
crust. Although some studies have found that garnet can be stable at depths as
shallow at ~ 30 - 35 km (Garrido et al. 2006, Macpherson et al. 2006), this
stability is dependent on the magmas being water saturated (Rooney et al.
2011). The pressure at the base of the GVC crust is approximately 1 GPa,
hence for garnet to be stable at this depth, H2O contents must exceed 4 - 5 wt.
% (Alonso-Perez et al. 2009). In the experiment conducted by Alonzo-Perez et
al. (2009), their liquids were corundum-normative and the fractionation of large
amounts of hornblende (> 50 %) played an important role in generating an
adakitic signature. In the case of the GVC, all adakite magmas are water
undersaturated, which precludes garnet stability in the crust beneath the GVC.
The absence or sparsity of primary hydrous phases in the GVC adakite samples
supports this interpretation. All of the GVC rocks are also mainly quartznormative (Table B1, Appendix B) and there is little evidence of fractionation of
significant amounts of hornblende (see Section 5.2.2). Moreover, studies of the
thermal structure of the Juan de Fuca subduction system (Harry and Green
1999, Green and Harry 1999, Green 2006) have concluded that the system
becomes increasingly anhydrous from southern California northwards to
southwestern British Columbia, thereby reducing the amount of available H2O.
75
Gomez-Tuena et al. (2007) argue that partial melts of mafic lower crust would
result in felsic magmas that have Mg#s less than 50; all the GVC adakites have
average Mg#s greater than 50 (Table A1, Appendix A). Mafic lower crust partial
melts would also not exhibit the elevated Ni and Cr contents typical of adakite
(Martin et al. 2005, Richards and Kerrich 2007). Fig. 17 shows adakitic indicator
diagrams for the GVC rocks plotted with the average composition of lower crust
partial melts from a study by Borg and Clynne (1998) in the Mt. Lassen volcanic
field in the Southern Cascades. The inverted triangles represent the average
composition of low silica partial melts leaving a plagioclase rich residue (filled
triangle, LC 1) and the average composition of high silica partial melts leaving a
hornblende rich residue (open triangle, LC 2). The two partial melts are
interpreted to result from lower crustal anatexis initiated by underplating of
hydrous basalt (hornblende residuum) versus anhydrous basalt (plagioclase
residuum, Borg and Clynne 1998). The geochemical characteristics of the lower
crustal melts are very different to those of the GVC adakites; they are both more
felsic than the GVC rocks, with lower Mg# (43 and 46, Fig. 17a) as well as Ni, Cr,
Sr and Sr/Y values that are significantly lower than typical adakites (Fig. 17b-e).
The chemistry is mainly controlled by the modelled residual mineralogy of the
crustal partial melts, which is made up of primarily plagioclase (40 – 50 wt. %)
and clinopyroxene (20 – 25 wt. %) with varying amounts of hornblende (up to 3
wt. % for LC 1, 25 wt. % for LC 2), magnetite and orthopyroxene (9 – 15 wt. %;
Borg and Clynne 1998). The high residual plagioclase in both models lowers the
76
Figure 17: Adakite indicator plots comparing the GVC adakites to modelled lower crustal partial melts from Borg and
Clynne (1998). Both partial melts plot well away from the GVC rocks in all diagrams and argues against partial melting of
mafic lower crust as the source for the GVC adakites; see text for explanation. LC 1=low silica partial melt with
plagioclase-rich residue, LC 2=high silica partial melt with a hornblende-rich residue. Silica is in wt. % oxide, trace
elements in ppm. Mg# calculated as outlined in Table A1. Symbols as in Fig. 7.
77
Sr concentration to that well outside of the adakite field (Fig. 17d, e), while the
high residual hornblende in LC 2 yields similar Y and Yb to the GVC rocks but
lowers the SiO2, Mg#, Ni and Cr (Fig. 17). The source of the GVC adakite
magma is plagioclase-poor and high amounts of residual hornblende is unlikely
because high water contents are required (> 5 % H2O; Borg and Clynne 1998)
and this is inconsistent with the erupted GVC products. The crust beneath Mt.
Lassen is marginally thicker than the crust beneath the GVC (~ 38 km versus ~
34 km respectively, Guffanti et al. 1990, Perry et al. 2002) an the absence of
garnet as a residual phase in the crustal melt models likely also precludes garnet
stability at the base of the GVC crust. As the HREE depletion in the GVC rocks
is most likely due to garnet (as compared to the crustal melts of Mt. Lassen
where HREE depletion is controlled by hornblende), the GVC adakites must be
sourced from the slab where garnet is stable to fractionate the HREE. Thus,
partial melting of basaltic lower crust does not adequately explain the presence
of adakite within the GVC, nor their compositions.
5.2.2 High pressure fractionation/AFC of basaltic magma
Fractionation of basaltic magma to generate adakite is the most common model
for adakite genesis independent of slab partial melting (e.g., Castillo et al. 1999,
Chiaradia 2009, Chiaradia et al. 2009, Coldwell et al. 2011). Primitive magmas
derived from partial melting of the mantle wedge may ascend to the base of
continental lithosphere, where they may be halted because of differences in
density. The elevated lithostatic pressures at this juncture may be sufficient to
78
form garnet, imparting low HREE and Y in melts that may form in this region and
result in evolved magmas which differentiate into adakitic compositions
(Macpherson et al. 2006). Variations in this model also include multiple
fractionations of mantle-derived basaltic magma and mixing of magmas
originating in deep crustal hot zones (Castillo et al. 1999). A key factor in these
processes is the necessity for garnet stability in the evolving magmas, for which
there is no evidence beneath the GVC (see Section 5.2.1). Adakite melts that
are generated from high pressure fractionation show correlations with
differentiation indices (i.e. decreasing Fe2O3, MgO, Al2O3 with increasing SiO2)
as well as adakitic indices (positive correlations between Sr/Y, La/Yb, Ni, Cr with
increasing SiO2) and exhibit a wide range of SiO2 contents. In the GVC, some
normal differentiation trends are observed (MgO, CaO, Fe 2O3, etc., Fig. 8), but
several major and trace element contents from each centre have either inverse
correlations with silica or remain relatively constant as SiO2 increases (e.g. TiO2,
Na2O, Ni, Cr, Mg#; Figs. 8, 9). Additionally, the GVC adakites exhibit a relatively
small range of silica contents (~ 8 wt. %) compared to other adakite suites (~ 17
wt. %, Macpherson et al. 2006). Fractionation of hornblende has been argued as
a proxy for garnet in controlling HREE and Y concentrations in adakite melts
(Richards and Kerrich 2007, Dessimoz et al. 2012). As basaltic magma
crystallises, the HREE and Y are generally incompatible in the major mafic
silicate phases (olivine, orthopyroxene) and these elements increase with
increasing SiO2 up to ~ 56 wt. % where hornblende may crystallise. As the result
79
of hornblende fractionation, Y and HREE can inversely correlate with SiO2,
illustrating a crystallisation path from normal arc rocks into the adakite field
(Richards and Kerrich 2007, Moyen 2009).
The CVB are the only mafic rocks in the GVC and previous studies have found
that they do not represent the parent magma to the GVC adakites (Green 1977,
1990; Green and Henderson 1984). There is an absence of data between the
SiO2 contents of the CVB (48 - 51 wt. %; Table A1, Appendix A; Figs. 7, 8, 9)
and the GVC adakites (> 56 wt. %) and as such, a trend illustrating hornblende
fractionation (if for illustrative purposes only) cannot be effectively demonstrated.
However, Y and Yb contents of the CVB are relatively constant over the, albeit
narrow, range of SiO2 concentrations (i.e. shows no increase with increasing
SiO2, which would be expected if these were controlled by fractionation). Y and
HREE generally increase with SiO2 for the GVC adakites (Table A2, Appendix
A); hornblende crystallisation would decrease these values as the magma
evolves to more felsic compositions. While hornblende can preferentially
fractionate HREE, the MREE have higher compatibilities (Rollinson 1993). This
can be illustrated in chondrite normalised spider diagrams where the effect of
hornblende can be identified by a listric or spoon shaped profile; a negative
correlation of MREE/HREE (Dy/Yb) with SiO2 also indicates interaction of
hornblende (Rollinson 1993, Richards and Kerrich 2007). The majority of the
GVC adakites have a smooth negative pattern on chondrite normalised spider
diagrams (Fig. 11), suggesting that small amounts of hornblende may be
80
present, but does not rule out the effect of residual garnet in the source region
and the MREE/HREE ratios remain relatively constant with increasing silica (not
shown). The Columnar Peak dacite does show a slight listric pattern which may
result from small amounts of hornblende phenocrysts (see Section 5.1.3).
Significant hornblende crystallisation would also lower the Mg content of the
resulting melts and hence lower the Mg#, which is not observed in the GVC
adakite rocks. Lastly, experimental studies on high pressure fractionation of
basalts showed that the resulting melts would be corundum normative (e.g.
Müntener et al. 2001; see previous section). This is in contrast to the GVC
adakites, which are all predominantly quartz normative (Table B1, Appendix B).
5.2.3 Partial melting of subducted ocean crust
The failure of the previously discussed models to generate the distinctive,
adakitic chemistry of the GVC rocks indicates that a slab partial melt scenario is
a perhaps a more likely process for generating adakite within the GVC. The
majority of geochemical traits as outlined by Defant and Drummond (1990) and
Martin et al. (2005) are observed in the GVC rocks; the few that are not (La, Cr,
LSA versus HSA) can be explained away in magma mixing (see Section 5.1.3).
This slab partial melt hypothesis is further explored in the context of the GVC
using trace element batch melting models, to determine if the GVC adakites can
be generated from a MORB source. Model concentrations of trace elements Rb,
Ba, Th, Nb, La, Ce, Sr, Nd, Sm, Zr, Eu, Dy, Y, Yb and Lu were determined using
an a restite composition of predominantly clinopyroxene, garnet and hornblende
81
+/- rutile. Based on the estimated depth and pressure of the Juan de Fuca slab
beneath the GVC (~ 60 km, 1.5-1.6 GPa; Audet et al. 2008), the starting
composition is assumed to be amphibolite. Partial melting experiments on
amphibolite (Wolf and Wyllie 1993, Rapp 1995, Rapp and Watson 1995) indicate
that garnet pyroxenite or eclogite is a suitable residuum for the approximated
conditions beneath the GVC. Experiments conducted by Rapp et al. 1999 show
that a Ti-bearing phase is ubiquitous in the residue and hence, rutile is included
in the model. The modal proportions of each mineral phase were varied
(sometimes significantly, from ~ 10 % up to 75 %) to test the effects on the
modelled composition compared to that of the GVC adakite samples, at
incremental melt percentages of up to 25 % partial melt. Mineral/melt partition
coefficients for silicic melts at temperatures and pressures comparable to the
Juan de Fuca subduction system, sources and values used are listed in
Appendix D. The starting composition for N-MORB representing the subducted
slab is derived from Karsten et al. (1990) and Chadwick et al. (2005); enriched,
depleted and transitional basalt magmas have been erupted from the Juan de
Fuca Ridge system (Cousens et al. 1995) and a slightly enriched composition
(compared to average Pacific MORB) has been used in the modelling. A few
model compositions can approximate the GVC adakites (see Fig. D1, Appendix
D), but the model that best reproduces the GVC adakites is a 15 to 20 % partial
melt of N-MORB leaving a residuum of ~ 70 % clinopyroxene, ~ 20 % garnet, ~
9.5 % hornblende with 0.5 % rutile (Fig. 18a). A second model where garnet is
82
Figure 18: Results of best fitting simple batch melting models (15 % melt fraction) to the GVC adakites. The residues
modelled fit the range of the GVC adakites reasonably well; the model composition in (b) is preferred based on previously
published experimental data on partial melting of basalt. N-MORB normalising values are estimated from Karsten et al.
(1990), Cousins et al. (1995) and Chadwick et al. (2005). Cpx=clinopyroxene, Gt=garnet, Hbl=hornblende, Ru=rutile. See
text for explanation.
83
increased to 25 % and hornblende is decreased to 4.5 % is also a good fit,
slightly less so than the former (Fig. 18b). Changes in the modal percentage of
clinopyroxene does not significantly change the modelled composition relative to
the GVC rocks; however, small to moderate increases in the amount of residual
garnet (> 25 %) results in lower HREE and Y than is observed. The presence of
hornblende (+/- rutile) is needed to moderate the HFSE and MREE contents; a
clinopyroxene and garnet only residue results in Nb, Zr and Eu (as well as Ce)
values that are too high. A similar model approximating the GVC chemistry can
be obtained with a residue of 60 % clinopyroxene, 15 % garnet and 25 %
hornblende (Fig. 18c); Zr, Sm and Eu values are closer to the observed
concentrations in the GVC rocks, but also result in lower Sr and Nd and higher
Nb and an overall poorer fit to the GVC data. Ickert (2006) tested the adakite
slab melt model for the Princeton Group adakites in south-central British
Columbia and noted that partial melting of amphibolite could not generate the
sodic, tonalitic melts that are consistent with adakites. This is the case only if
sufficient hornblende remains in the residue; removal of most or all residual
hornblende will result in partial melts that are adakitic (Rapp 1995). Additionally,
Ickert (2006) used the most primitive andesites to model, whereas primitive slab
melts are dacitic in composition. Thus, the model with 25 % garnet and 4.5 %
hornblende is the most likely residuum beneath the GVC (Fig. 18b). The
modelled composition fits the observed data quite well; discrepancies (slightly
lower Sr and slightly higher HFSE) could be influenced by magma mixing
84
processes (see section 5.1.3) and/or minor differentiation. Alternatively, the
partition coefficients used in the batch melting model (Barth et al. 2002) could
cause the elevated Zr and Eu anomalies as the determined Kd’s for these
elements are generally lower than other studies (e.g. Klein et al. 1999, Green et
al. 2000). The overall success of the model in replicating the chemistry of the
evolved GVC rocks suggests the following: (1) the model emphasizes the
petrographical and geochemical observations that the magma mixing
components are broadly similar in composition and adakitic (or near-adakitic),
(2) the mixing relationships do not significantly affect the model chemistry, even
the more mobile LILE, and most importantly (3) the trace element chemistry of
the GVC adakites can be generated from partial melting of N-MORB and the
good fit of the model composition to the observed composition suggests that the
trace and REE values of the GVC adakites may represent true slab melt values.
In addition to the trace element modelling and geochemical support for a slab
partial melt model, field relationships and the tectonic environment in
southwestern British Columbia also suggest that the GVC adakites originated
from slab melts and are discussed below.
5.2.3.1
Association with niobium-enriched basalts (NEB)
NEB were first identified and defined by Sajona et al. (1993, 1996) from the
Zamboanga arc in the Philippines. These basalts are characterised by
enrichment in HFSE as compared to ‘normal’ subduction related magmas, which
85
generally exhibit strong depletion in HFSE. Further, NEB contain relatively high
TiO2 (1 - 2 wt. %) and low LILE/HSFE and LREE/HSFE ratios (Sajona et al.
1993, 1996). These enrichments result in positive or weakly negative Nb
anomalies on mantle normalised multi-element plots. NEB and adakite are often
(but not always) associated with each other and this has led to the suggestion
that there is a petrogenetic link between them (Sajona et al. 1996, AguillónRobles et al. 2001), though some studies argue against this hypothesis (Castillo
et al. 2007, Hastie et al. 2011). Sajona et al. (1996) propose that NEB are
generated by partial melting of the mantle wedge that had been previously
metasomatised by slab melts. Interaction with the mantle wedge resulted in the
slab melts being consumed generating a metasomatic mineral assemblage that
included primarily orthopyroxene and amphibole. This mineral assemblage
scavenges HFSE from subsequent slab partial melts (likely by a process of zone
refining) as they ascend through the altered wedge. As subduction continues,
the metasomatic assemblage is pulled further down into the mantle where it
undergoes partial melting and generates a HFSE-enriched NEB basaltic melt
(Sajona et al. 1993, 1996). Some NEB are erupted at surface (e.g., Mexico,
Philippines) as primitive basalts while some likely differentiate and/or fractionate
at depth and erupt as more evolved compositions (discussed below). Other
studies suggest that the HFSE enrichment originates from mixing between a
MORB source and an OIB source (Castillo et al. 2007, Macpherson et al. 2010),
or partial melting of lower crust comprised of accreted oceanic terrains and/or
86
entrainment of asthenospheric material by convection into the mantle wedge
(Borg et al. 1997).
The CVB exhibit several geochemical traits that identify them as NEB, including
higher Nb (~ 8.2 ppm), high TiO2 (~ 1.5 wt. %) and low LILE/HFSE and
LREE/HFSE ratios (e.g., Rb/NbMN = 0.85, La/NbMN = 1.1; both ratios greater than
2 in normal arc rocks). On a primitive mantle normalised multi-element plot, the
CVB samples display weak Nb anomalies and no Ti anomaly, as well as, lower
total LILE than is typical of arc rocks (e.g., ~ 6 ppm Rb versus ~ 20 ppm; Fig. 8).
Partial melting of accreted oceanic terrains to generate the CVB NEB is thought
unlikely; the basement rocks below the GVC are quartz diorite and diorite
intrusions of Cretaceous age (Souther 1970, Green 1977, Rusmore and
Woodsworth 1991), that generally host silica contents of greater than 60 wt. %
SiO2 (Mathews 1958). Partial melting of such a composition could not generate
primitive basalts. Similarly, asthenospheric entrainment into the mantle wedge is
also deemed an unlikely process; a large slab window is present to the north of
the GVC that extends from the Alaska-Yukon border to Mount Itcha and the
Anahim-Wells Gray volcanic field (Thorkelson et al. 2011). Asthenospheric
material would generally flow in a northward direction into the slab window rather
than east-west into the mantle wedge beneath the GVC. A slab window has also
been suggested to be present between the Juan de Fuca and Explorer plates,
along the Nootka Fault Zone (Audet et al. 2008, Thorkelson et al. 2011), and
asthenospheric upwelling through this window (or around the eastward edge of
87
the Juan de Fuca Plate) may be the source of the alkaline volcanism found east
of the GVC in south-central British Columbia (e.g., Princeton Group volcanics,
Ickert et al. 2009, Thorkelson et al. 2011). However, these studies have
suggested that the slab window between the Juan de Fuca and Explorer plates
is in an early stage of formation and that it has not propagated westward enough
to cause significant alkaline volcanism in the GVC.
Mixing of MORB and OIB components has been argued as the source of several
NEB occurrences (Macpherson et al. 2006, 2010; Castillo 2008, Castillo et al.
2007) and is the most common model for generating NEB independent of slabmelt contribution. In the case of the CVB, a simple mixing model of MORB and
OIB cannot successfully explain the geochemistry. Figure 19 shows a simple
binary mixing plot between OIB and MORB using various pairs of trace
elements. Figure 19a shows that mixing of 10 % OIB into MORB could
potentially generate the CVB, but the data does not fit well and even less so for
the HCBA. This could be the influence of fluid interactions, as Ba is not as
incompatible as Nb, but the data does not appear to support an OIB component.
Figure 19b uses Zr instead of Ba, which is generally thought to behave as an
immobile element in aqueous fluids. The CVB data correlates well with a 10 %
OIB component; the HCBA shows less scatter but still trends towards more
MORB-like values. Based on these element pairs, it appears that the CVB can
originate from N-MORB mixed with 10 % OIB. However, further testing of this
simple mixing model with additional trace elements (e.g., HREE) fails to support
88
Figure 19: Simple binary mixing models between MORB and OIB components for (a) Ba vs. Nb and (b) Zr vs. Nb. In (a),
both the CVB and HCBA rocks plot off the mixing trend. The high Ba for the HCBA may be the result of fluid influence. In
(b), the data plots much closer to the mixing line and suggests that a mix of 90 % MORB and 10 % OIB can generate the
CVB rocks (and to a lesser extent, the HCBA). MORB composition derives from Sun and McDonough (1989), OIB from
Borg et al. (1997). Symbols as in Fig. 7.
89
OIB-MORB mixing. Figure 20 is a primitive mantle normalised multi-element
diagram comparing the CVB chemistry to a simple mixing model of 10 % OIB
and 90 % MORB (the data for the HCBA are not included as they represent
more evolved compositions). Some of the elevated HFSE values (Zr, Ti) can be
generated from mixing approximately 10 % OIB and 90 % MORB, however, this
composition results in HREE that are much higher than that of the CVB, and
LILE concentrations that are significantly lower with a strong negative Pb
anomaly. The addition of a bulk sediment component (~ 10 %) to the OIB-MORB
mixture increases LILE closer to CVB values, but does not lower the HREE and
requires 15 % OIB. Additionally, this three component model magma would
result in the 87Sr/86Sr isotopic ratios to be much higher (~ 0.7044; Plank and
Langmuir 1998) than published data for the CVB (0.7032; Green 1977, 1990).
Lastly, similar plots of incompatible and compatible element ratios (as discussed
at the end of Section 5.1.3) illustrate that the main control on the chemistry of the
CVB is partial melting (Fig. 21) and not fractional crystallisation or magma
mixing. For the CVB to be generated by OIB and MORB mixing, this would
require that the solid source be a heterogeneous mixture of OIB and MORB
components that subsequently underwent partial melting (Schiano et al. 2010).
Determining if the source was efficiently mixed cannot be effectively performed
with these plots but as discussed above, a heterogeneous mixture cannot
reproduce the CVB data. Thus, for the CVB NEB, the most effective way to
90
Figure 20: Primitive mantle normalised mixing models of MORB, OIB and bulk sediment components as compared to the
CVB. Mixing between 10 % OIB and 90 % MORB replicates the HFSE of the CVB but poorly matches the majority of the
data. Addition of 15 % bulk sediment and increasing the OIB component to 15 % results in a marginally better fit but
overall fails to reproduce the CVB chemistry. N-MORB composition from Sun and McDonough (1989), symbols as in Fig.
7.
91
Figure 21: Incompatible/compatible element ratio plots for (a) Ba vs. Ba/Ni, (b) 1/Ni vs. Ba/Ni, (c) La vs. La/Cr and (d)
1/Cr vs. La/Cr distinguishing mixing from both partial melting and fractional crystallisation (FC) processes. The
geochemistry of the CVB is controlled mainly by fractional crystallisation, whereas the HCBA defines a distinct trend from
both the CVB and the primitive Helm Creek basalts. Inset as in Fig. 16, symbols as in Fig. 7. Trace elements expressed
in ppm.
92
increase the HFSE without significant modification to LILE is partial melting of
slab melt-metasomatised mantle wedge.
In the case of the Helm Creek flow, the processes influencing the geochemistry
are more complex. The HCBA was originally mapped by Green (1977) as a
composite flow; the lava exhibits a range of silica contents (49-55 wt. % SiO2).
Primitive alkali olivine basalt is also described and occurs within the Cinder Cone
(Green 1977, 1990, 2006). Figure 11 (a and c) is a chondrite normalised plot
showing the REE values for the CVB, the HCBA from this study and the REE
values from primitive and evolved basalts taken from Green and Henderson
(1984) and Green (2006). The more evolved Helm Creek rocks exhibit higher
HFSE and lower HREE than the more primitive basalt; these concentrations
should remain relatively unchanged through differentiation from basalt to basaltic
andesite and the observed values may suggest that the source region for the
more evolved Helm Creek flow is different than that of the alkali olivine basalts.
Figure 11c also illustrates that the HREE contents of the HCBA are very similar
to that of the CVB. It is possible that the HCBA may have had a NEB parent that
either did not erupt at surface or experienced differentiation and/or fractional
crystallisation prior to eruption. The differences in the primitive basalts and the
HCBA are further illustrated in Fig. 21 as both groups form diverging trends. In
Fig. 21a, the HCBA show a positive curved relationship while the primitive rocks
form a linear negative trend. The companion plot shows the inverse of this
relationship illustrating that mixing is not the main control on the evolved
93
compositions but may affect the chemistry of the primitive Helm Creek basalts.
Ultimately, this shows that the source regions of the primitive and evolved Helm
Creek rocks are likely different.
These observations outlined above are significant to the argument about
whether or not NEB and adakite are genetically related; the HCBA exhibits
several adakitic traits such as high Sr (> 1400 ppm), low Y and Yb (15.4 and 1.6
ppm respectively), high Ni, Cr and Mg# (113 and 135 ppm,~ 55; Tables A1 & A2,
Appendix A). The only condition that precludes the HCBA from being classified
as adakite is that the silica content is too low (< 56 wt. %). Martin et al. (2005)
likened LSA to NEB based on their elevated Nb contents (up to 10 ppm), and the
source region described by Martin et al. (2005) for LSA is identical to that
proposed by Sajona et al. (1996) for NEB. Both models require a process that
enriches the mantle wedge in Nb; the elevated values in NEB (> 7 ppm; adakite
average of 6 ppm) cannot be obtained by fluid flux from the dehydrating slab
because these fluids are not effectively able to concentrate Nb (Tatsumi et al.
1986, Martin et al. 2005). A slab partial-melt can concentrate and transfer Nb as
it will preferentially go into the melt phase due to its incompatibility. Hence,
based on this data, it seems likely that NEB could represent a potential parental
magma to LSA and/or NEB are more extensive partial melts of slab meltmetasomatised mantle wedge; both of which suggest that NEB and adakites are
related in the GVC.
5.2.3.2
Ni content in olivine
94
Several recent works (Sobolev et al. 2005, 2007; Straub et al. 2008) have
recognized anomalously high nickel concentrations in olivine phenocrysts that
are inconsistent with magma generation in equilibrium with a peridotite source,
first noted in the Hawaiian basalts. This was explained by magma generation
from a pyroxenite source; partial melts from recycled oceanic crust (i.e. slab
melts) at depth infiltrate and interact with mantle peridotite, which consumes
olivine and creates solid pyroxenite. The pyroxenite melts at lower pressures
and the resulting liquids are highly enriched in Ni, as pyroxene has a lower
partition coefficient than olivine for Ni (~ 1 versus >> 1 for olivine, Herzberg
2011). Olivine phenocrysts that crystallise from this modified source reflect this
elevated Ni (Sobolev et al. 2005, 2007). A pyroxenite source region can be
distinguished from peridotite on a NiO versus forsterite content diagram where
olivines from a pyroxenite source plot at a higher NiO value for a given Fo
(Sobolev et al. 2005). The significance of this in the context of the GVC is that
both LSA and NEB are essentially generated from a pyroxenite source (Sajona
et al. 1996, Martin et al. 2005). Figure 22 is a NiO versus Fo plot for olivines
from the GVC with fields outlining peridotite and pyroxenite sources (fields
modified from Sobolev et al. 2005 and Gao et al. 2008). The HCBA is clearly
distinct from the other flows; it has the most primitive olivines and forms a
smooth, sub-vertical negative trend into the pyroxenite field. The olivine rim
compositions (open symbols) are considerably poorer in Ni than the cores and
reflect disequilibrium conditions between the phenocrysts and the surrounding
95
Figure 22: NiO (wt. %) vs. Fo (mole %) diagram of olivines in the GVC. Fields are modified from Sobolev et al. (2005)
and Gao et al. (2008) and differentiate between a pyroxenite source and a peridotite source. The HCBA and DVBA both
steeply trend into the pyroxenite field, whereas the CVB and PR have exhibit shallower trends towards the overlap
between the pyroxenite and peridotite fields and suggest the involvement of slab melts in the source region for all the
lavas. Filled symbols=core compositions, open symbols=rim compositions. PR, CVB, DVBA and HCBA acronyms as in
Fig. 7.
96
melt. The olivines from the Desolation Valley flow are much lower in their
forsterite content but strongly trend into the pyroxenite field as well (Fig. 22). The
Paul Ridge andesite and the CVB have a similar range of forsterite contents, but
the CVB have slightly less Ni; both datasets plot mainly outside the peridotite
and pyroxenite fields. The Fo values are not primitive for either the CVB or the
Paul Ridge andesite and the potential source region cannot be adequately
determined but the data for both flows trend towards the area of overlap
between both fields (Fig. 22). Similarly, the Ni contents are not unusually high,
however studies on the Mexican Volcanic Belt (MVB; Straub et al. 2008, 2011)
found that lower Ni and lower Mg olivines originated from a mixed
pyroxenite/peridotite source. Ultimately, the data trends potentially provide
further support for a slab melt component in the generation of the GVC NEB. It
should be noted that further microprobe analyses of olivine may or may not
corroborate these interpretations.
5.2.3.3
Regional tectonic regime for southwestern British Columbia
Studies on the thermal structure of the Juan de Fuca Plate (Green and Harry
1999, Harry and Green 1999, Green and Sinha 2005, Green 2006) have shown
that the Plate (and magmas generated above it) becomes increasingly
anhydrous further northwards. The geochemistry of erupted magmas also
changes further northwards; magmatism at Mt. Cayley, approximately 20 km
north of the GVC, exhibits an even stronger adakitic character, which has also
not been previously recognized. Sr/Y ratios for the Mt. Cayley adakites are as
97
high as 215, well above that of the GVC (Kelman et al. 2001). Average Mg# (~
53), Cr (~ 59) and Sr (~ 1123) are also higher than the GVC adakite rocks, with
lower Y (≤ 15 ppm) and lower range of K2O/Na2O (0.21 - 0.42; Kelman et al.
2001). The Mt. Cayley adakite magmas are predominantly HSA, and no basaltic
volcanism is associated with the suite (Kelman et al. 2001). Figures 10, 13 and
14 compare the adakitic characteristics of Mt. Cayley to that of the GVC
adakites. Mt. Meager, which is approximately 60 km north of the GVC,
represents another change in magma composition from the GVC to that of an
alkaline affinity. The rocks of Mt. Meager include trachydacite, trachyandesite,
basalt and trachybasalt (Stasiuk et al. 1994). An apparent transition northward
from adakitic calc-alkaline in the GVC to strongly adakitic calc-alkaline at Mt.
Cayley to alkaline volcanism at Mt. Meager appears to be linked to the extension
and thinning of the slab between the Juan de Fuca and Explorer plates, along
the Nootka Fault Zone (Audet et al. 2008). Recent studies (Madsen et al. 2006,
Audet et al. 2008) have shown that the Explorer Plate is likely being captured by
the North American Plate and is no longer subducting, whereas the Juan de
Fuca Plate subducts at a relatively slow rate (~ 4 cm/yr; Wilson 2002). This has
caused an approximate 40 km of stretched slab between the Explorer and Juan
de Fuca plates (Audet et al. 2008). Asthenospheric upwelling into this stretched
section would increase the heat flow and could facilitate partial melting; this
could potentially generate the alkaline character of the Mt. Meager centre, as the
volcano is situated on the Nootka Fault Zone (Thorkelson et al. 2011). Further
98
southward, the heat decreases and the alkaline signature changes to a strong
adakitic character (Mt. Cayley rocks), as the slab melt chemistry dominates.
Beneath the GVC, the slab is very young (~ 16 Ma; Green 2006) and retains
sufficient heat to melt, though the mantle wedge is more hydrous than further
north, which results in both adakitic and some normal arc-type melt-generation.
These normal (but likely near-adakitic) arc magmas mix with the adakitic partial
melts of the slab in the magma reservoirs to generate the hybrid GVC rocks (see
section 5.1.3). This along arc variation in magma geochemistry suggests that
partial melting of the subducted slab is not only occurring beneath the GVC, but
in fact may become an increasingly dominant magma generating process further
northwards.
5.3
Petrogenetic model for adakite genesis beneath the GVC
The model proposed herein illustrates that the most likely method to explain the
geochemical and mineralogical variations in the rocks of the GVC is partial
melting of subducted ocean crust, followed by magma mixing in upper level
magma chambers, and subsequent melting of slab melt-metasomatised mantle
wedge; Figure 23 illustrates the interpreted sequence of volcanic events
generating the adakites and NEB of the GVC. Based on the stratigraphy and
relative timing of eruption of the rocks in the GVC, there appears to be multiple
periods of both slab melting and melting of altered mantle wedge. Partial melts
of the subducting Juan de Fuca Plate ascended through the mantle wedge and
were consumed through interactions with mantle mineralogy, forming
99
Figure 23 (1 of 3): Simplified petrogenetic model for adakite and NEB genesis in the GVC. Estimated depths in km,
acronyms as in Fig. 7. *veins and partial melts may extend further upwards than is depicted in this schematic. NOTE:
model only includes volcanic centers studied in this work and does not represent all eruptive episodes in the presented
time scale.
100
Figure 23 (2 of 3): Simplified petrogenetic model for adakite and NEB genesis in the GVC. Estimated depths in km,
acronyms as in Fig. 7. *veins and partial melts may extend further upwards than is depicted in this schematic. NOTE:
model only includes volcanic centers studied in this work and does not represent all eruptive episodes in the presented
time scale.
101
Figure 23 (3 of 3): Simplified petrogenetic model for adakite and NEB genesis in the GVC. Estimated depths in km,
acronyms as in Fig. 7. *veins and partial melts may extend further upwards than is depicted in this schematic. **slab
window is north of the study area and not shown in this figure. NOTE: model only includes volcanic centers studied in this
work and does not represent all eruptive episodes in the presented time scale.
102
orthopyroxene and hornblende (+/- phlogopite) at the expense of olivine and
clinopyroxene and creating a metasomatic assemblage (i.e. pyroxenitic source).
This assemblage created a veined, metasomatised mantle wedge; these veins
of metasomatic minerals created pathways that allowed subsequent slab melts
to ascend into the magma chambers beneath the GVC with their HSA chemistry
relatively intact. As the altered mantle wedge was subducted further via slab pull,
the metasomatic mineral assemblage present in the veins melted to generate
the LSA magmas. These magmas ascended and in doing so mixed with the preexisting HSA already present in the GVC magma chambers reservoirs, such as
those occurring beneath Black Tusk at 1.3 Ma - which shows petrographic
evidence for the superheated mixing (see Section 5.1.3) of pre-existing HSA
magma and the much hotter intruding LSA magma, giving rise to the mixed
geochemical signature between LSA and HSA for this centre (Fig. 23, 1.3 Ma,
GLVF). The Paul Ridge andesite in the MGVF erupted next at approximately
700 ka whereby the intrusion of a hotter LSA magma caused a pyroclastic
eruption, possibly resulting from a temperature-driven convective overturn in the
magma chamber, and producing the distinct geochemical and petrographical
variations in sample 09JF011 (Fig. 23, 1.3 Ma and 700 ka, MGVF) compared to
the subsequent eruptions at Paul Ridge. A hiatus in volcanism in the GVC (700
ka to 260 ka) allowed for a second cycle of slab melts to be consumed and
metasomatise the mantle wedge (Fig. 23, pg. 2, MGVF), which provided
pathways for the eruption of HSA at Columnar Peak, beginning at 260 ka. This
103
newly metasomatised wedge subducts and partially melts, creating the largely
LSA Table and Barrier andesites which were approximately coeval eruptions at ~
100 ka. The DVBA also erupted at 100 ka; geochemical data is sparse for this
flow but the few published analyses indicate that it exhibits some characteristics
of NEB (~ 9 ppm Nb, ~ 118 ppm Zr, 0.94 wt. % TiO2,; Green 1981) and is also
close to an adakitic composition (Sr > 750 ppm, ~ 23 ppm Y, 55.9 wt. % SiO2).
The HCBA, which erupted at 40 ka, also shows some geochemical traits of NEB
(8.5 ppm Nb, 126 ppm Zr, 0.95 wt. % TiO2; Tables A1 & A2, Appendix A) but
exhibits a far stronger adakite signature than is present in Desolation Valley flow
rocks (Sr > 1400 ppm, Sr/Y = 92, La/Yb > 15; Table A2, Appendix A). Alkali
olivine basalts occur within the Cinder Cone volcano as the last eruptive
products; this progressive change in chemistry from near adakiticstrongly
adakiticalkaline over time may suggest a change in the magma source region.
The DVBA likely reflects a source where the slab has insufficient garnet to
generate an adakite signature; the resulting slab melts had higher, non-adakitic
concentrations of HREE and Y, which were transferred to the mantle wedge as
the slab melts interacted with the mantle wedge and were consumed creating a
metasomatic assemblage. The slab melts, however, were able to transfer HFSE
from the slab and imparted some geochemical characteristics of NEB.
Stratigraphically, the Barrier and Black Tusk andesites (which are adakitic) are
approximately coeval with the Desolation Valley flow and hence it is possible
that the DVBA was an LSA magma that mixed with normal arc magmas prior to
104
eruption (Fig. 23, 100 ka, GLVF). This is supported by the observation that
Clinker Peak, Black Tusk and Cinder Cone are not temporally far apart and it is
unlikely that the residual slab composition has significantly changed between
centres. The adakite signature is stronger in the HCBA, likely because the slab
became richer in garnet and as subduction continued, the mantle wedge was
altered by subsequent slab melts that were strongly adakitic, resulting in the
higher Sr/Y and La/Yb ratios. The source of the alkali basalts is speculative, but
it may reflect the start of a westward progression of alkaline volcanism;
volcanism that exhibits an intraplate character has been identified in southcentral British Columbia and believed to have started around 47 Ma and
continued to as recent as a few hundred years ago (Ickert et al. 2009,
Thorkelson et al. 2011 and references therein). The episodic and relatively
voluminous outpouring of the mildly alkaline CVB from a centre further north
suggests an increase in heat into the system, possibly due to the upwelling of
asthenospheric material into the Nootka Fault Zone, which melted out a large
portion of the altered mantle wedge and imparting the NEB characteristics to the
CVB. This increased heat flow also caused significant melting of newly
subducted ocean crust and resulted in a third cycle of HSA generation with the
eruption of the extensive Ring Creek andesite flow at 10 ka (Fig. 23, pg. 3, 10
ka, MGVF).
6.
Conclusions
105
The identification of adakites introduces new mechanisms of magma genesis
beneath the GVC which have not been previously considered. Evaluation of the
main models for generating adakites coupled with simple batch melting
modelling have shown that the most likely scenario for the generation of the
GVC adakites is partial melting of the subducting Juan de Fuca slab. The mixed
nature of the GVC rocks precludes classification of most of the adakites as LSA
or HSA, but despite mixing with arguably non-adakitic magmas the slab melt
signature is still evident. The presence of NEB associated with the GVC adakite
strengthens the slab melt model, as trace element modelling of the CVB and
HCBA argues against mixing between MORB and OIB components to generate
the GVC NEB. Higher Ni contents in olivine phenocrysts may also support a slab
melt component to the GVC NEB; however a larger dataset is needed to further
evaluate this. The observation that the HCBA (as well as the Desolation Valley
flow to a degree) exhibits geochemical traits of both NEB and adakite suggests
that some LSA magmas could be generated from differentiation/fractionation of
primary NEB magmas, especially as the proposed source region for both NEB
and LSA is mantle peridotite that has been altered by slab melts (Sajona et al.
1996, Martin et al. 2005). It must be noted that while the data presented in this
study does support: (1) a slab melt model for generating adakites in the GVC, (2)
the generation of NEB from slab melt metasomatised mantle wedge, and (3)
LSA magmas could be generated from primary NEB magmas, more extensive
sampling and analysis is needed to more rigorously test these hypotheses and
106
the smaller dataset carries with it a degree of speculation as to magma origins.
Additionally, while future studies may challenge this interpretation for the
presence of adakite (and NEB) in the GVC, this work introduces new
perspectives and ideas that are still worthwhile to evaluate in future studies.
107
References
Alonso-Perez, R., Müntener, O., Ulmer, P. (2009). Igneous garnet and
amphibole fractionation in the roots of island arcs: experimental constraints on
andesitic liquids. Contributions to Mineralogy and Petrology, vol. 157, p. 541558.
Aguillón-Robles, A., Calmus, T., Benoit, M., Bellon, H., Maury, R. C., Cotten, J.,
Bourgois, J., Michaud, F. (2001). Late Miocene adakites and Nb-enriched
basalts from Vizcaino Peninsula, Mexico: Indicators of East Pacific Rise
subduction below southern Baja California? Geology, vol. 29, p. 531-534.
Allègre, C. J., Minster, J. F. (1978). Quantitative models of trace element
behaviour in magmatic processes. Earth and Planetary Science Letters, vol. 38,
p. 1-25.
Audet, P., Bostock, M. G., Mercier, J. P., Cassidy, J. F. (2008). Morphology of
the Explorer-Juan de Fuca slab edge in northern Cascadia: imaging plate
capture at a ridge-trench-transform triple junction. Geology, vol. 36, p. 895-898.
Barth, M. G., Foley, S. F., Horn, I. (2002). Partial melting in Archean subduction
zones: constraints from experimentally determined trace element partition
coefficients between eclogitic minerals and tonalitic melts under upper mantle
conditions. Precambrian Research, vol. 113, p. 323-340.
108
Borg, L. E., Clynne, M. A., Bullen, T. D. (1997). The variable role of slab-derived
fluids in the generation of a suite of primitive calc-alkaline lavas from the
southernmost Cascades, California. The Canadian Mineralogist, vol. 35, p. 425452.
Borg, L. E., Clynne, M. A. (1998). The petrogenesis of felsic calc-alkaline
magmas from the southernmost Cascades, California: Origin by partial melting
of basaltic lower crust. Journal of Petrology, vol. 39, p. 1197-1222.
Bourdon, E., Eissen, J. P., Gutscher, M. A., Monzier, M., Samaniego, P., Robin,
C., Bollinger, C., Cotton, J. (2002). Slab melting and slab metasomatism in the
Northern Andean Volcanic Zone: adakites and high-Mg andesites from
Pichincha volcano (Ecuador). Bulletin de la Société Géologique de France, vol.
173, p. 195-206.
Breitsprecher, K., Thorkelson, D. J., Groome, W. G., Dostal, J. (2003).
Geochemical confirmation of the Kula-Farallon slab window beneath the Pacific
Northwest in Eocene time. Geology, vol. 31, p. 351-354.
Brooks, G. R., Friele, P. A. (1992). Bracketing ages for the formation of the Ring
Creek lava flow, Mount Garibaldi volcanic field, southwestern British Columbia.
Canadian Journal of Earth Sciences, vol. 29, p. 2425-2428.
Burwash, E. M. (1914). Pleistocene Vulcanism of the Coast Range of British
Columbia. Journal of Geology, vol. 22, p. 260-267.
109
Castillo, P. R. (2006). An overview of adakite petrogenesis. Chinese Science
Bulletin, vol. 51, p. 257-268.
Castillo, P. R. (2008). Origin of the adakite-high-Nb basalt association and its
implications for postsubduction magmatism in Baja California, Mexico.
Geological Society of America Bulletin, vol. 120, p. 451-462.
Castillo, P. R., Janney, P. E., Solidum, R. U. (1999). Petrology and geochemistry
of Camiguin Island, southern Philippines: insights to the source of adakites and
other lavas in a complex arc setting. Contributions to Mineralogy and Petrology,
vol. 134, p. 33-51.
Castillo, P. R., Rigby, S. J., Solidum, R. U. (2007). Origin of high field strength
element enrichment in volcanic arcs: Geochemical evidence from the Sulu Arc,
southern Philippines. Lithos, vol. 97, p. 271-288.
Chadwick J., Perfit, M., Ridley, I., Jonasson, I., Kamenov, G., Chadwick, W.,
Embley, R., le Roux, P., Smith, M. (2005). Magmatic effects of the Cobb hot spot
on the Juan de Fuca Ridge. Journal of Geophysical Research, vol. 110, B03101.
Chiaradia, M. (2009). Adakite-like magmas from fractional crystallization and
melting – assimilation of mafic lower crust (Eocene Macuchi arc, western
Cordillera, Ecuador). Chemical Geology, vol. 265, p. 468-487.
110
Chiaradia, M., Müntener, O., Beate, B., Fontignie, D. (2009). Adakite-like
volcanism of Ecuador: lower crust magmatic evolution and recycling.
Contributions to Mineralogy and Petrology, vol. 158, p. 563-588.
Coldwell, B., Adam, J., Rushmer, T., Macpherson, C. G. (2011). Evolution of the
East Philippine Arc: experimental constraints on magmatic phase relations and
adakitic melt formation. Contributions to Mineralogy and Petrology, vol. 162, p.
835-848.
Cousens, B. L., Allan, J. F., Leybourne, M. I., Chase, R. L., Wagoner, N. V.
(1995). Mixing of magmas from enriched and depleted mantle sources in the
northeast Pacific: West Valley segment, Juan de Fuca Ridge. Contributions to
Mineralogy and Petrology, vol. 120, p. 337-357.
Dawes, R. L., Green, N. L., Defant, M. J., Drummond, M. S. (1994). Mount St.
Helens: Potential example of the partial melting of the subducted lithosphere in a
volcanic arc: Comments and Reply. Geology, vol. 22, p. 187-190.
Defant, M. J., Drummond, M. S. (1990). Derivation of some modern arc magmas
by melting of young subducted lithosphere. Nature, vol. 367, p. 662-665.
Defant, M. J., Richerson, P. M., De Boer, J. Z., Stewart, R. H., Maury, R. C.,
Bellon, H., Drummond, M. S., Feigenson, M. D., Jackson, T. E. (1991). Dacite
Genesis via both Slab Melting and Differentiation: Petrogenesis of La Yeguada
Volcanic Complex, Panama. Journal of Petrology, vol. 32, p. 1101-1142.
111
Dessimoz, M., Müntener, O., Ulmer, P. (2012). A case for hornblende dominated
fractionation of aarc magmas: the Chelan Complex (Washington Cascades).
Contributions to Mineralogy and Petrology, vol. 163, p. 567-589.
Drummond, M. S., Defant, M. J. (1990). A model for trondhjemite-tonalite-dacite
genesis and crustal growth via slab melting: Archean to modern comparisons.
Journal of Geophysical Research, vol. 95, p. 21503-21521.
Falloon, T. J., Danyushevsky, L. V., Crawford, A. J., Meffre, S., Woodhead, J.
D., Bloomer, S. H. (2008). Boninites and adakites from the Northern Termination
of the Tonga Trench: implications for adakite petrogenesis. Journal of Petrology,
vol. 49, p. 697-715.
Fiesinger, D. W. (1975). Petrology of the Quaternary volcanic centres in the
Quesnel Highlands and Garibaldi Provincial Park areas, British Columbia. PhD
Dissertation, University of Calgary, 132p.
Fillmore, J., Coulson, I. M. (2013). Petrological and geochemical constraints on
the origin of adakites in the Garibaldi Volcanic Complex, southwestern British
Columbia, Canada. Bulletin of Volcanology, vol. 75, p. 730-753.
Foley, S. F., Barth, M. G., Jenner, G. A. (2000). Rutile/melt partition coefficients
for trace elements and an assessment of the influence of rutile on the trace
element characteristics of subduction zone magmas. Geochimica and
Cosmochimica Acta, vol. 64, p. 933-938.
112
Gao, S., Rudnick, R. L., Xu, W., Yuan, H., Liu, Y., Walker, R. J., Puchtel, I. S.,
Liu, X., Huang, H., Wang, X., Yang, J. (2008). Recycling deep cratonic
lithosphere and generation of intraplate magmatism in the North China Craton.
Earth and Planetary Science Letters, vol. 270, p. 41-53.
Garrido, C. J., Bodinier, J. L., Burg, J. P., Zeilinger, G., Hussain, S., Dawood, H.,
Nawaz Chaudhry, M., Gervilla, F. (2006). Petrogenesis of mafic garnet granulite
in the lower crust of the Kohistan Paleo-arc Complex (Northern Pakistan):
implications for intra-crustal differentiation of island arcs and generation of
continental crust. Journal of Petrology, vol. 47, p. 1873-1914.
Geochemical Earth Reference Model (GERM) Reservoir Database (2013).
earthref.org/GERMRD/datamodel/ oceanic crust, N-MORB, primitive mantle,
dacite, andesite, basalts. Accessed: Feb 1-4, 2013.
Gómez-Tuena, A., Langmuir, C. H., Goldstein, S. L., Straub, S. M., OrtegaGutiérrez, F. (2007). Geochemical Evidence for Slab Melting in the TransMexican Volcanic Belt. Journal of Petrology, vol. 4, p. 537-562.
Govindaraju, K., Potts, P. J., Webb, P. C., Watson, J. S. (1994). Report on Whin
Sill dolerite WS-E from England and Pitscurrie microgabbro PM-S from Scotland:
assessment by one hundred and four international laboratories. Geostandards
Newsletter, vol. 18, p. 211-300.
113
Green, N.L. (1977). Multistage andesite genesis in the Garibaldi Lake area,
southwestern British Columbia. PhD Dissertation, University of British Columbia,
265p.
Green, N. L. (1981). Geology and petrology of Quaternary volcanic rocks,
Garibaldi Lake area, southwestern British Columbia. Geological Society of
America Bulletin, vol. 92, p. 697-702.
Green, N. L. (1990). Late Cenozoic volcanism in the Mount Garibaldi and
Garibaldi Lake volcanic fields, Garibaldi volcanic belt, southwestern British
Columbia. Geoscience Canada, vol. 17, p. 171-174.
Green, N. L. (2006). Influence of slab thermal structure on basalt source regions
and melting conditions: REE and HFSE constraints from the Garibaldi volcanic
belt, northern Cascadia subduction system. Lithos, vol. 87, p. 23-49.
Green, N. L., Henderson, P. (1984). Rare earth element concentrations in
Quaternary volcanic rocks of southwestern British Columbia. Canadian Journal
of Earth Sciences, vol. 21, p. 731-736.
Green, N. L., Armstrong, R. L., Harakal, J. E., Souther, J. G., Read, P. B. (1988).
Eruptive history and K-Ar geochronology of the late Cenozoic Garibaldi volcanic
belt, southwestern British Columbia. Geological Science of America Bulletin, vol.
100, p. 563-579.
114
Green, N. L., Harry, D. L. (1999). On the relationship between subducted slab
age and arc basalt petrogenesis, Cascadia subduction system, North America.
Earth and Planetary Science Letters, vol. 171, p. 367-381.
Green, T. H., Blundy, J. D., Adam, J., Yaxley, G. M. (2000). SIMS determination
of trace element partition coefficients between garnet, clinopyroxene and
hydrous basaltic liquids at 2-7.5 GPa and 1080-1200°C. Lithos, vol. 53, p. 165187.
Green, N. L., Sinha, A. K. (2005). Consequences of varied slab age and thermal
structure on enrichment processes in the sub-arc mantle of the northern
Cascadia subduction system. Journal of Volcanology and Geothermal Research,
vol. 140, 107-132.
Guffanti, M., Clynne, M. A., Smith, J. G., Muffler, L. J. P., Bullen, T. D. (1990).
Late Cenozoic volcanism, subduction and extension in the Lassen region of
California, southern Cascade range. Journal of Geophysical Research, vol. 95,
p. 19453-19464.
Guo, Z., Wilson, M., Liu, J. (2006). Post-collisional adakites in south Tibet:
Products of partial melting of subduction-modified lower crust. Lithos, vol. 96, p.
205-224.
Harry, D. L., Green, N. L. (1999). Slab dehydration and basalt petrogenesis in
subduction systems involving very young oceanic lithosphere. Chemical
Geology, vol. 160, p. 309-333.
115
Hastie, A. R., Mitchell, S. F., Kerr, A. C., Minifie, M. J., Millar, I. L. (2011).
Geochemistry of rare high-Nb basalt lavas: Are they derived from a mantle
wedge metasomatized by slab melts? Geochimica and Cosmochimica Acta, vol.
75, p. 5049-5072.
Hegner, E., Tatsumoto, M. (1989). Pb, Sr, and Nd Isotopes in Seamount Basalts
From the Juan de Fuca Ridge and Kodiak-Bowie Seamount Chain, Northeast
Pacific. Journal of Geophysical Research, vol. 94, p. 17839-17846.
Herzberg, C. (2011). Identification of source lithology in the Hawaiian and
Canary Islands: implications for origins. Journal of Petrology, vol. 52, p. 113-146.
Hickson, C. J., Russell, J. K., Stasiuk, M. V. (1999). Volcanology of the 2350
B.P. eruption of Mount Meager volcanic complex, British Columbia, Canada:
implications for hazards from eruptions in topographically complex terrain.
Bulletin of Volcanology, vol. 60, p. 489-507.
Huang, F., He, Y. (2010). Partial melting of the dry mafic continental crust:
implications for petrogenesis of C-type adakites. Chinese Science Bulletin, vol.
55, p. 2428-2439.
Ickert, R. B. (2006). Adakitic Volcanism in Southern BC during the early Eocene:
Isotopic and geochemical constraints from the Princeton Group. MSc thesis,
University of Alberta, 116p.
116
Ickert, R. B., Thorkelson, D. J., Marshall, D. D., Ullrich, T. D. (2009). Eocene
adakitic volcanism in southern British Columbia: remelting of arc basalt above a
slab window. Tectonophysics, vol. 464, p. 164-185.
Jahn, B., Glickson, A. Y., Peucat, J.-J., Hickman, A. H. (1981). REE
geochemistry and isotopic data of Archaean silicic volcanics and granitoids from
the Pilbara block, western Australia: implications for early crustal evolution.
Geochimica and Cosmochimica Acta, vol. 45, p. 1633-1652.
Karsten, J. L., Delaney, J. R., Rhodes, J. M., Liias, R. A. (1990). Spatial and
Temporal Evolution of Magmatic Systems Beneath the Endeavour Segment,
Juan de Fuca Ridge: Tectonic and Petrologic Constrains. Journal of Geophysical
Research, vol. 95, p. 19235-19256.
Kay, R. W. (1978). Aleutian magnesian andesites: melts from subducted Pacific
Ocean crust. Journal of Volcanology and Geothermal Research, vol. 4, p. 117132.
Kelman, M. C., Russell, J. K., Hickson, C. J. (2001). Preliminary petrography and
chemistry of the Mount Cayley volcanic field, British Columbia. Geological
Survey of Canada, Current Research 2001-A11, 12p.
Klein, M., Stosch, H.-G., Seck, H. A., Shimizu, N. (1999). Experimental
partitioning of high field strength and rare earth elements between clinopyroxene
and garnet in andesitic to tonalitic systems. Geochimica and Cosmochimica
Acta, vol. 64, p. 99-115.
117
Langmuir, C. H., Vocke, R. D. Jr., Hanson, G. N., Hart, S. R. (1978). A general
mixing equation with applications to Icelandic basalts. Earth and Planetary
Science Letters, vol. 37, p. 380-392.
Le Bas, M. J., Streckeisen, A. L. (1991). The IUGS systematics of igneous rocks.
Journal of the Geological Society, London, vol. 148, p. 825-833.
Macdonald, G. A. (1968). Composition and origin of Hawaiian lavas. In: Coats,
R. R., Hay, R. L., Anderson, C. A. (eds). Studies in volcanology: a memoir in
honor of Howel Williams, vol. 116, Geological Science of America Memoir, p.
477-522.
Macpherson, C. G., Dreher, S. T., Thirlwall, M. F. (2006). Adakites without slab
melting: high pressure differentiation of island arc magma, Mindanao, the
Philippines. Earth and Planetary Science Letters, vol. 243, p. 581-593.
Macpherson, C. G., Chiang, K. K., Hall, R., Nowell, G. M., Castillo, P. R.,
Thirlwall, M. F. (2010). Plio-Pleistocene intra-plate magmatism from the southern
Sulu Arc, Semporna peninsula, Sabah, Borneo: Implications for high-Nb basalt
in subduction zones. Journal of Volcanology and Geothermal Research, vol.
190, p. 25-38.
Madsen, J. K., Thorkelson, D. J., Friedman, R. M., Marshall, D. D. (2006).
Cenozoic to recent plate configurations in the Pacific basin: ridge subduction and
slab window magmatism in western North America. Geosphere, vol. 2, p. 11-34.
118
Martin, H (1999). The adakitic magmas: modern analogues of Archaean
granitoids. Lithos, vol. 46, p. 411-429.
Martin, H., Moyen, J. F. (2003). Secular changes in TTG composition:
comparison with modern adakite. EGS-AGU-EUG Joint Meeting, Nice, Apr
VGP7-1 FR2O-001.
Martin, H., Smithies, R. H., Rapp, R., Moyen, J. F., Champion, D. (2005). An
overview of adakite, tonalite-trondhjemite-granodiorite (TTG) and sanukitoid:
relationships and some implications for crustal evolution. Lithos, vol. 79, p. 1-24.
Martindale, M., Mullen, E., Weis, D. (2014). Geochemical constraints on the
origin of evolved magmas in the Northern Cascades. Goldschmidt 2014, June 913; Sacramento, California; p. 1604.
Mathews, W. H. (1951). The Table, a flat topped volcano in southern British
Columbia. American Journal of Science, vol. 249, p. 830-841.
Mathews, W. H. (1952). Mount Garibaldi, a supraglacial volcano in southwestern
British Columbia. American Journal of Science, vol. 250, p. 81-103.
Mathews, W. H. (1957). Petrology of Quaternary Volcanics of the Mount
Garibaldi Map-Area, Southwestern British Columbia. American Journal of
Science, vol. 255, p. 400-415.
119
Mathews, W. H. (1958). Geology of the Mount Garibaldi map-area, southwestern
British Columbia, Canada. Geological Science of America Bulletin, vol. 69, p.
161-198.
McKenzie, D., Bickle, M. J. (1988). The Volume and Composition of Melt
Generated by Extension of the Lithosphere. Journal of Petrology, vol. 29, p. 625679.
Moyen, J. F. (2009). High Sr/Y and La/Yb ratios: the meaning of the “adakitic
signature”. Lithos, vol. 112, p. 556-574.
Moyen, J. F., Martin, H. (2012). Forty years of TTG research. Lithos, vol. 148, p.
312-336.
Müntener, O., Kelemen, P. B., Grove, T. L. (2001). The role of H2O during
crystallization of primitive arc magmas under uppermost mantle conditions and
genesis of igneous pyroxenites: an experimental study. Contributions to
Mineralogy and Petrology, vol. 141, p. 643-685.
Panteeva, S. V., Gladkochoub, D. P., Donskaya, T. V., Markova, V. V.,
Sandimirova, G. P. (2003). Determination of 24 trace elements in felsic rocks by
inductively coupled plasma mass spectrometry after lithium metaborate fusion.
Spectrochimica Acta, part B, vol. 58, p. 341-350.
120
Perry, H. K. C., Eaton, D. W. S., Forte, A. M. (2002). LITH5.0: a revised crustal
model for Canada based on lithoprobe results. Geophysical Journal
International, vol. 150, p. 285-294.
Plank, T., Langmuir, C. H. (1998). The chemical composition of subducting
sediment and its consequences for the crust and mantle. Chemical Geology, vol.
145, p. 325-394.
Rapp, R. P. (1995). Amphibole-out phase boundary in partially melted
metabasalt, its control over liquid fraction and composition and source
permeability. Journal of Geophysical Research, vol. 100, no. B8, p. 1560115610.
Rapp, R. P., Watson, E. B. (1995). Dehydration Melting of Metabasalt at 8-32
kbar: Implications for Continental Growth and Crust-Mantle Recycling. Journal of
Petrology, vol. 36, p. 891-931.
Rapp, R. P., Shimizu, N., Norman, M. D., Applegate, G. S. (1999). Reaction
between slab-derived melts and peridotite in the mantle wedge: experimental
constraints at 3.8 GPa. Chemical Geology, vol. 160, p. 335-356.
Richards, J. P., Kerrich, R. (2007). Special paper: adakite-like rocks: their
diverse origins and questionable role in metallogenesis. Economic Geology, vol.
102, p.537-576.
121
Riddihough, R. P. (1981). One hundred million years of plate tectonics in
western Canada. Geoscience Canada, vol. 9, p. 28-34.
Riddihough, R. P. (1984). Recent movements of the Juan de Fuca Plate system.
Journal of Geophysical Research, vol. 89 (B8), p. 6980-6994.
Rollinson, H. R. (1993). Using Geochemical Data: Evaluation, Presentation,
Interpretation. Longman Scientific and Technical, London. 352p.
Rooney, T. O., Franceschi, P., Hall, C. M. (2011). Water-saturated magmas in
the Panama Canal region: a precursor to adakite-like magma generation?
Contributions to Mineralogy and Petrology, vol. 161, p. 373-388.
Rusmore, M. E., Woodsworth, G. J. (1991). Coast Plutonic Complex: A midCretaceous contractional orogeny. Geology, vol. 19, p. 941-944.
Sajona, F. G., Maury, R. C., Bellon, H., Cotten, J., Defant, M., Pubellier, M.
(1993). Initiation of subduction and the generation of slab melts in the western
and eastern Mindanao, Philippines. Geology, vol. 21, p. 1007-1010.
Sajona, F. G., Bellon, H., Maury, R. C., Pubellier, M., Cotten, J., Rangin, C.
(1994). Magmatic response to abrupt changes in tectonic setting: PlioceneQuaternary calc-alkaline lavas and Nb-enriched basalts of Leyte and Mindanao
(Philippines). Tectonophysics, vol. 237, p. 47-72.
Sajona, F. G., Maury, R. C., Bellon, H., Cotten, J., Defant, M. (1996). High Field
Strength Element Enrichment of Pliocene-Pleistocene Island Arc Basalts,
122
Zamboanga Peninsula, Western Mindanao (Philippines). Journal of Petrology,
vol. 37, p. 693-726.
Samaniego, P., Martin, H., Robin, C., Monzier, M. (2002). Transition from calcalkalic to adakitic magmatism at Cayambe volcano, Ecuador: Insights into slab
melts and mantle wedge interactions. Geology, vol. 30, p. 967-970.
Schiano, P., Clocchiatti, R., Shimizu, N., Maury, R. C., Jochum, K. P., Hofmann,
A. W. (1995). Hydrous silica-rich melts in the sub-arc mantle and their
relationship with erupted arc lavas. Nature, vol. 377, p. 595-600.
Schiano, P., Monzier, M., Eissen, J. P., Martin, H., Koga, K. T. (2010). Simple
mixing as the major control of the evolution of volcanic suites in the Ecuadorian
Andes. Contributions to Mineralogy and Petrology, vol. 160, p. 297-312.
Sherrod, D. R., Smith, J. G. (1990). Quaternary extrusion rates of the Cascade
Range, northwestern United States and southern British Columbia. Journal of
Geophysical Research, vol. 95, p. 19465-19474.
Shirey, S. B., Hanson, G. N. (1984). Mantle-derived Archaean monzodiorites
and trachyandesites. Nature, vol. 310, p. 222-224.
Sigurdsson, H, Houghton, B, McNutt, S. R., Rymer, H., Stix, J. (eds) (2000).
Encyclopedia of volcanoes. Academic Press, California, 1417p.
123
Sivertz, G. W. G. (1976). Geology, petrology and petrogenesis of Opal Cone and
the Ring Creek lava flow, southern Garibaldi, British Columbia. B.Sc. Thesis,
University of British Columbia, 79p.
Sobolev, A. V., Hofmann, A. W., Sobolev, S. V., Nikogosian, I. K. (2005). An
olivine-free mantle source of Hawaiian shield basalts. Nature, vol. 434, p. 590597.
Sobolev, A. V., Hofmann, A. W., Kuzmin, D. V., Yaxley, G. M., Arndt, N. T.,
Chung, S., Danyushevsky, L. V., Elliott, T., Frey, F. A., Garcia, M. O., Gurenko,
A. A., Kamenetsky, V. S., Kerr, A. C., Krivolutskaya, N. A., Matvienkov, V. V.,
Nikogosian, I. K., Rocholl, A., Sigurdsson, I. A., Sushchevskaya, N. M., Teklay,
M. (2007). The Amount of Recycled Crust in Sources of Mantle-Derived Melts.
Science, vol. 316, p. 412-417.
Souther, J. G. (1970). Volcanism and its relationship to recent crustal
movements in the Canadian Cordillera. Canadian Journal of Earth Sciences, vol.
7, p. 553-568.
Stasiuk, M. V., Russell, J. K., Hickson, C. J. (1994). Influence of magma
chemistry on eruption behaviour from the distribution and nature of the 2400
Y.B.P. eruption products of Mount Meager, British Columbia. Geological Survey
of Canada Open File 2843, 38p.
Straub, S. M., LaGatta, A. B., Martin-Del Pozzo, A. L., Langmuir, C. H. (2008).
Evidence from high-Ni olivines for a hybridized peridotite/pyroxenite source for
124
orogenic andesites from the central Mexican Volcanic Belt. Geochemistry,
Geophysics, Geosystems, vol. 9, 33p.
Straub, S. M., Gomez-Tuena, A., Stuart, F. M., Zellmer, G. F., Espinasa-Perena,
R., Cai, Y., Iizuka, Y. (2011). Formation of hybrid arc andesites beneath thick
continental crust. Earth and Planetary Science Letters, vol. 303, p. 337-347.
Stern, C. R., Kilian, R. (1996). Role of subducted slab, mantle wedge and
continental crust in the generation of adakites from the Austral Volcanic Zone.
Contributions to Mineralogy and Petrology, vol. 123, p. 263-281.
Sun, S. S., McDonough, W. I. (1989). Chemical and isotopic systematics of
oceanic basalts: implications for mantle composition and processes. In:
Saunders, A. D., Norry, M. J. (eds). Magmatism in the ocean basins. Special
Publication 42, Geological Society of London, p. 313-345.
Tatsumi, Y., Hamilton, D. L., Nesbitt, R. W. (1986). Chemical characteristics of
fluid phase from the subducted lithosphere: evidence from high-pressure
experiments and natural rocks. Journal of Volcanology and Geothermal
Research, vol. 29, p. 293-309.
Thorkelson, D. J., Breitspretcher, K. (2005). Partial melting of slab window
margins: genesis of adakitic and non-adakitic magmas. Lithos, vol. 79, p. 25-41.
125
Thorkelson, D. J., Madsen, J. K., Sluggett, C. L. (2011). Mantle flow through the
Northern Cordilleran slab window revealed by volcanic geochemistry. Geology,
vol. 39, p. 267-270.
Turner, J. S., Campbell, I. H. (1986). Convection and mixing in magma
chambers. Earth Science Reviews, vol. 23, p. 255-352.
Wang, Q., McDermott, F., Xu, J., Bellon, H., Zhu, Y. (2005). Cenozoic K-rich
adakitic volcanic rocks in the Hohxil area, northern Tibet: Lower-crustal melting
in an intracontinental setting. Geology, vol. 33, p. 465-468.
Wilson, D. S. (1988). Tectonic history of the Juan de Fuca ridge over the last 40
million years. Journal of Geophysical Research, vol. 93, p. 11863-11876.
Wilson, M. (1989). Igneous petrogenesis. HarperCollinsAcademic, London,
485p.
Wilson, D. S. (2002). The Juan de Fuca plate and slab: isochron structure and
Cenozoic plate motions. In: Kirby, S. H., Wang, K., Dunlop, S. G. (eds). The
Cascadia subduction zone and related subduction systems. US Geological
Survey Open File Report 02-328, p. 9-12.
Wolf, M. B., Wyllie, P. J (1993). Garnet Growth during Amphibolite Anatexis:
Implications of a Garnetiferous Restite. The Journal of Geology, vol. 101, p. 357373.
126
Xiao, L., Zhang, H. F., Clemens, J. D., Wang, Q. W., Kan, Z. Z., Wang, K. M., Ni,
P. Z., Liu, X. M. (2007). Late Triassic granitoids of the eastern margin of the
Tibetan Plateau: Geochronology, petrogenesis and implications for tectonic
evolution. Lithos, vol. 96, p. 436-452.
127
Appendix A: Whole Rock Geochemistry
CVB
BF
BF
BF
BT
RC
RC
RC
RC
CP
CP
Mt. Garibaldi Volcanic Field
PR
PR
PR
0.27
733.00
15.92
100.15
0.19
881.00
16.46
99.91
0.27
713.00
11.80
100.34
0.37
706.00
33.11
99.73
0.39
674.00
35.78
100.07
0.34
668.45
26.07
100.27
0.36
660.00
24.79
99.93
0.32
653.00
26.94
99.97
0.32
628.00
26.10
100.13
0.28
788.00
19.53
99.85
0.26
686.00
19.46
100.08
0.25
619.00
20.61
100.26
0.27
648.00
21.80
100.46
678.00
20.46
100.20
100.37
100.47
-
-
100.40
-
-
100.59
K/Rb
SiO2/MgO
CVB - Cheakamus Valley Basalt, HCBA - Helm Creek Basaltic Andesite; BF - Barrier flow (andesite); BT - Black Tusk (andesite); RC - Ring Creek flow (andesite); CP - Columnar Peak (dacite);
PR - Paul Ridge (basaltic andesite/andesite)
Mg# = molar Mg/(Mg+Fe) x 100, where Fe = Total Fe as FeO
Total iron reported as FeO
Total
51.13
45.92
62.59
50.11
49.91
50.74
51.34
50.81
49.57
50.97
51.98
50.11
50.54
Mg#
0.26
0.21
1.02
0.77
1.48
0.63
0.63
3.15
0.78
0.10
0.46
4.69
0.20
0.37
0.04
d/l
d/l
d/l
LOI
51.23
0.05
0.33
0.21
0.16
0.16
0.24
0.25
0.24
0.26
0.24
0.26
0.28
0.28
0.25
0.51
0.24
0.24
0.25
P2O5
-
0.28
0.89
1.14
1.64
1.71
1.49
1.53
1.37
1.39
1.25
1.22
1.19
1.26
1.19
1.39
0.48
0.49
0.47
K2O
59.88
1.16
4.61
4.19
4.46
4.44
4.39
4.30
4.29
4.41
4.47
4.61
4.69
4.72
4.62
4.69
3.46
3.47
3.46
Na2O
-
4.37
6.39
6.62
4.52
4.39
5.65
5.51
5.28
5.78
6.09
6.27
6.36
6.13
6.22
7.33
8.84
8.83
8.90
CaO
55.05
6.70
3.42
4.96
1.95
1.81
2.40
2.52
2.24
2.37
3.05
3.05
2.88
2.76
2.92
5.54
7.94
7.93
7.84
MgO
-
3.61
0.14
0.10
0.09
0.08
0.08
0.09
0.08
0.09
0.10
0.10
0.10
0.09
0.09
0.13
0.17
0.17
0.17
MnO
55.23
0.12
7.25
5.34
3.49
3.27
4.19
4.30
3.90
4.34
5.28
5.07
5.16
4.86
5.00
6.68
11.67
11.57
11.60
FeOT
-
6.21
18.51
16.90
17.19
17.11
17.52
17.18
17.97
17.71
18.37
18.41
18.80
18.72
18.52
16.87
15.64
15.66
15.74
Al2O3
54.88
0.78
18.61
0.96
0.65
0.41
0.39
0.55
0.58
0.57
0.57
0.63
0.64
0.67
0.65
0.61
0.95
1.50
1.52
K2O/Na2O
57.46
56.31
58.51
64.56
64.76
62.57
62.46
60.34
61.86
59.58
59.34
59.35
60.16
59.74
55.32
49.12
49.10
1.53
09JF004 09JF005 09JF006 09JF012 10JF016 09JF007 09JF008 10JF022 10JF023 09JF009 09JF010 09JF011 10JF017 10JF018
BF
49.22
10JF013
HCBA
Garibaldi Lake Volcanic Field
TiO2
09JF001 09JF002 09JF003
CVB
SiO2
Sample
CVB
Table A1: Major and minor element composition of investigated samples from the Garibaldi Volcanic Complex.
128
54.6
7.86
43.9
6.24
81.5
12.91
78.0
12.72
79.2
12.89
81.5
14.20
76.7
13.38
98.6
13.73
89.9
12.50
75.8
8.58
89.5
10.11
78.7
10.44
73.4
10.43
83.9
9.53
92.2
15.46
5.86
5.64
5.66
Sr/Y
La/Yb
Elemental concentrations expressed in ppm
d/l - below detection limit
CVB - Cheakamus Valley Basalt; HCBA - Helm Creek Basaltic Andesite; BF - Barrier flow; BT - Black Tusk; RC - Ring Creek flow; CP - Columnar Peak; PR - Paul Ridge
12.03
27.27
3.74
16.63
3.56
1.12
3.48
0.48
2.87
0.57
1.58
0.23
1.53
0.22
11.54
26.54
3.78
17.49
4.02
1.35
4.00
0.58
3.42
0.68
1.98
0.28
1.85
0.28
15.11
32.12
4.04
16.46
2.98
0.98
2.48
0.36
2.11
0.43
1.18
0.17
1.17
0.18
12.85
26.83
3.30
12.90
2.42
0.76
1.98
0.30
1.73
0.34
0.98
0.14
1.01
0.16
13.02
27.09
3.30
12.90
2.36
0.75
1.93
0.29
1.71
0.34
0.94
0.14
1.01
0.15
16.90
36.37
4.73
19.46
3.65
1.08
2.94
0.41
2.30
0.45
1.27
0.18
1.19
0.18
17.13
37.25
4.87
20.11
3.83
1.07
3.05
0.42
2.39
0.46
1.27
0.19
1.28
0.19
15.24
33.40
4.39
17.82
3.36
0.99
2.40
0.36
2.07
0.39
1.13
0.16
1.11
0.17
14.87
32.56
4.25
17.48
3.31
1.03
2.72
0.39
2.24
0.43
1.20
0.17
1.19
0.18
10.73
24.00
3.27
14.32
3.09
1.00
2.80
0.41
2.37
0.46
1.33
0.19
1.25
0.19
12.03
26.21
3.53
14.78
3.05
0.99
2.73
0.39
2.22
0.45
1.27
0.17
1.19
0.18
13.68
29.27
3.86
16.09
3.24
1.09
2.86
0.42
2.47
0.49
1.33
0.19
1.31
0.20
13.46
28.80
3.77
15.79
3.19
1.06
2.96
0.42
2.48
0.47
1.32
0.19
1.29
0.20
12.01
26.61
3.58
15.11
3.22
1.03
2.68
0.39
2.33
0.47
1.25
0.19
1.26
0.19
24.74
51.68
6.54
26.62
4.75
1.40
3.83
0.53
3.12
0.61
1.68
0.24
1.60
0.23
8.97
20.69
2.93
13.66
3.65
1.37
4.25
0.64
3.75
0.71
1.83
0.25
1.53
0.22
8.95
20.83
2.94
13.81
3.77
1.37
4.33
0.63
3.71
0.70
1.82
0.24
1.59
0.22
9.25
21.19
3.03
14.23
3.81
1.41
4.33
0.65
3.88
0.73
1.94
0.25
1.63
0.23
La
Ce
Pr
Nd
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Table A2: Trace and rare earth element composition of investigated samples from the Garibaldi Volcanic Complex.
Mt. Garibaldi Volcanic Field
Garibaldi Lake Volcanic Field
PR
PR
PR
CP
CP
RC
RC
RC
RC
BT
BF
BF
BF
BF
HCBA
CVB
CVB
CVB
Sample 09JF001 09JF002 09JF003 10JF013 09JF004 09JF005 09JF006 09JF012 10JF016 09JF007 09JF008 10JF022 10JF023 09JF009 09JF010 09JF011 10JF017 10JF018
8.0
15.0
14.0
d/l
10.0
11.0
10.0
d/l
11.0
11.0
d/l
d/l
11.0
d/l
14.0
24.0
16.0
21.0
Sc
40.0
53.0
110.0
63.0
63.0
81.0
84.0
86.0
85.0
97.6
99.0
99.0
96.0
97.0
140.0
184.0
184.0
187.0
V
30.9
17.8
131.9
22.9
20.6
16.8
18.0
32.4
32.2
20.8
30.8
28.8
28.9
31.7
135.2
211.0
215.1
212.3
Cr
48.0
30.0
103.0
30.0
42.0
26.0
30.0
30.0
28.0
56.0
39.0
38.0
34.0
43.0
113.0
171.0
171.0
170.0
Ni
100.0
66.0
71.0
51.0
22.0
50.0
59.0
49.0
41.0
75.0
39.0
41.0
37.0
41.0
67.0
102.0
96.0
102.0
Cu
40.0
53.0
45.0
17.0
14.0
25.0
26.0
23.0
30.0
28.0
35.0
30.0
34.0
33.0
59.0
82.0
81.0
87.0
Zn
19.1
20.0
17.0
16.3
15.4
18.7
18.4
18.0
17.6
18.9
17.9
17.9
17.8
17.1
20.0
18.3
18.0
18.3
Ga
13.1
8.4
13.3
19.5
21.4
18.7
19.4
18.1
18.5
13.2
14.9
16.0
16.2
14.6
13.2
6.4
6.1
6.6
Rb
868.7
838.7
957.9
784.8
752.0
1026.8
1012.2
1078.6
1068.4
909.8
1023.5
982.4
957.8
1000.3
1419.8
431.0
433.8
429.6
Sr
15.9
19.1
11.8
10.1
9.5
12.6
13.2
10.9
11.9
12.0
11.4
12.5
13.1
11.9
15.4
15.5
16.8
16.3
Y
101.1
91.4
104.2
100.5
91.5
111.7
119.7
115.7
117.9
91.5
92.5
101.9
101.8
90.2
125.8
93.6
93.8
95.2
Zr
3.7
3.8
4.0
4.2
4.3
4.2
4.5
4.7
4.4
3.3
5.6
7.4
7.3
5.3
8.5
8.2
8.1
8.3
Nb
471.6
466.1
390.7
558.8
580.7
549.4
561.9
469.5
478.0
436.3
441.5
450.6
477.0
441.1
588.3
141.8
142.5
140.5
Ba
7.3
6.6
3.5
4.7
d/l
8.2
7.3
3.0
4.6
6.5
4.4
3.3
2.8
2.2
6.7
d/l
d/l
d/l
Pb
1.40
0.80
2.67
2.63
2.69
2.10
2.10
1.88
1.89
1.34
1.38
1.54
1.61
1.40
1.65
0.74
0.76
0.76
Th
0.50
0.30
0.88
1.05
1.08
0.80
0.80
0.68
0.70
0.55
0.58
0.63
0.65
0.61
0.71
0.28
0.26
0.28
U
129
130
Appendix B: Normative Mineralogy
Values expressed as wt. %
Table B1: Normative Mineralogy of representative samples from the GVC
MGVF
GLVF
09JF001 09JF002 09JF003 10JF013 09JF004 09JF005 09JF006 09JF012 10JF016 09JF007 09JF008 10JF022 10JF023 09JF009 09JF010 09JF011 10JF017 10JF018
PR
PR
PR
CP
CP
RC
RC
RC
RC
BT
BF
BF
BF
BF
HCBA
CVB
CVB
CVB
7.46
6.67
8.59
19.07
19.39
16.31
16.29
15.56
15.11
9.54
9.70
8.48
9.99
10.00
2.15
Qtz
64.71
66.48
59.84
60.08
59.44
60.92
59.76
63.46
62.07
64.43
65.18
66.36
65.93
65.46
60.25
55.31
55.42
55.53
Pl
7.22
5.68
7.09
10.25
10.79
9.30
9.54
8.83
8.64
7.81
7.63
7.40
7.81
7.45
8.72
2.98
3.04
2.92
Or
0.07
0.20
0.19
Co
3.33
2.30
6.45
2.62
2.58
2.54
2.29
3.23
2.87
2.34
2.84
10.07
13.84
13.82
13.78
Di
11.29
11.59
13.13
7.12
7.37
5.57
6.96
7.13
6.88
12.30
9.26
10.81
9.45
9.75
9.66
4.03
4.60
5.30
Hy
18.29
16.90
16.20
Ol
1.48
1.84
1.25
0.80
0.76
1.04
1.12
1.10
1.08
1.20
1.20
1.27
1.23
1.16
1.80
2.85
2.91
2.92
Ilm
3.80
4.63
3.09
2.19
1.65
3.58
3.07
3.07
3.00
1.83
3.13
2.09
2.54
2.70
6.08
2.09
2.71
2.71
Mt
0.65
0.76
0.49
0.37
0.37
0.56
0.58
0.58
0.60
0.56
0.60
0.65
0.65
0.58
1.18
0.56
0.56
0.58
Ap
0.01
0.01
0.01
0.01
0.01
0.03
0.03
0.03
0.03
0.01
0.01
0.03
0.01
0.01
0.03
0.01
0.01
0.01
Zr
99.95
99.96
99.94
99.96
99.98
99.93
99.93
99.95
99.95
99.97
99.94
99.96
99.95
99.95
99.94
99.96
99.97
99.95
Total
131
132
Appendix C: Mineral compositions for the GVC rocks
88.88
10.46
0.984
0.032
0.179
0.010
1.792
0.004
Cr2O3
FeO
MgO
CaO
MnO
Na2O
NiO
Total
Fo
Fa
Si
Ti
Al
Cr
Fe+3
Fe+2
Mn
Mg
Ca
0.975
0.049
0.152
0.009
1.810
0.005
89.41
9.94
0.00
9.79
49.42
0.18
0.41
0.03
0.08
99.62
0.00
0.02
0.988
0.001
0.022
0.181
0.010
1.793
0.004
89.2
10.09
9.94
49.28
0.17
0.47
0.15
100.55
0.02
0.02
-
0.981
0.038
0.169
0.010
1.797
0.005
89.04
10.22
10.11
49.41
0.18
0.49
0.11
100.52
0.01
-
0.980
0.040
0.164
0.010
1.800
0.005
89.13
10.11
10.01
49.50
0.19
0.50
0.02
0.18
100.61
0.02
0.974
0.051
0.156
0.010
1.804
0.005
89.09
10.19
0.02
10.06
49.32
0.18
0.48
0.03
0.13
99.94
0.02
0.01
0.976
0.047
0.173
0.009
1.791
0.004
88.51
10.85
0.01
10.72
49.07
0.16
0.43
0.29
100.58
0.02
0.01
-
0.978
0.001
0.001
0.042
0.395
0.006
1.571
0.006
77.9
21.52
0.06
20.43
41.19
0.23
0.26
0.03
0.14
100.6
0.03
0.982
0.002
0.034
0.431
0.006
1.539
0.005
76.35
23.08
0.02
21.57
40.03
0.19
0.29
0.05
0.12
100.39
0.05
0.977
0.001
0.044
0.473
0.006
1.491
0.007
73.76
25.57
0.01
23.79
38.50
0.26
0.29
0.10
100.62
0.03
0.01
21-3
37.62
0.972
0.002
0.054
0.487
0.006
1.472
0.007
72.68
26.68
0.02
24.95
38.12
0.24
0.29
0.10
101.3
0.06
-
21-4 c
37.53
Endmember compositions in mole %; Fe+3 calculated from charge balance of analyzed FeO
Cation values expressed per 4 oxygens
c - core composition; r - rim composition; 10JF025 - Desolation Valley flow (basaltic andesite; whole rock chemistry not analysed)
10JF021 - CVB, 10JF024 - HCBA (whole rock geochemistry not analysed)
0.01
10.33
49.23
0.14
0.47
0.04
0.25
100.78
Al2O3
0.02
-
0.01
TiO2
21-2
38.06
Table C1: Electron microprobe compositions of olivine from the Garibaldi Volcanic Complex
GLVF
10JF013
10JF021
13-1 c
13-1 r
13-2 c
13-2 r
13-3 c
13-3 r
13-4
21-1
SiO2
40.30
39.70
40.49
40.21
40.15
39.70
39.88
38.23
0.974
0.001
0.051
0.530
0.009
1.425
0.010
70.39
28.71
26.37
36.27
0.34
0.39
0.00
0.07
100.43
0.03
0.01
21-4 r
36.94
0.974
0.001
0.001
0.001
0.049
0.516
0.008
1.441
0.009
71.2
27.94
0.06
25.80
36.88
0.32
0.37
0.01
0.07
100.73
0.02
0.03
21-5
37.16
0.991
0.001
0.001
0.016
0.607
0.009
1.365
0.011
67.99
31.03
0.01
27.58
33.89
0.37
0.39
0.04
0.04
99.05
0.02
0.04
21-6
36.68
0.985
0.029
0.208
0.008
1.765
0.004
87.61
11.76
0.00
11.52
48.13
0.16
0.41
0.02
0.26
100.58
0.01
-
10JF024
24-1 c
40.06
133
86.59
12.94
0.981
0.038
0.223
0.005
1.748
0.004
87.57
11.74
0.986
0.001
0.028
0.208
0.010
1.764
0.004
0.978
0.001
0.001
0.043
0.172
0.010
1.792
0.004
88.66
10.64
0.03
10.48
49.01
0.16
0.48
0.13
100.19
0.978
0.001
0.043
0.192
0.008
1.774
0.004
87.75
11.66
0.00
11.50
48.56
0.17
0.37
0.03
0.27
100.85
0.03
-
24-3 c
39.91
0.983
0.034
0.172
0.010
1.797
0.005
89.07
10.21
10.07
49.29
0.19
0.46
0.00
0.14
100.37
-
-
24-3 r
40.21
0.979
0.001
0.041
0.184
0.008
1.783
0.004
88.28
11.13
10.92
48.58
0.14
0.40
0.02
0.39
101.07
0.02
-
24-4
39.76
0.985
0.001
0.029
0.192
0.009
1.781
0.004
88.44
10.96
0.02
10.82
49.00
0.14
0.42
0.00
0.23
101.07
0.02
-
24-5 c
40.41
0.991
0.018
0.189
0.011
1.787
0.004
88.97
10.29
10.19
49.46
0.16
0.52
0.02
0.09
101.33
0.01
0.01
24-5 r
40.86
0.971
0.001
0.056
0.456
0.011
1.499
0.006
73.91
25.24
23.45
38.53
0.21
0.52
0.02
0.08
100.08
0.03
0.01
10JF025
25-1
37.22
0.980
0.001
0.039
0.352
0.009
1.614
0.003
79.91
19.47
18.53
42.67
0.12
0.44
0.28
100.69
0.02
0.01
25-2 c
38.62
0.970
0.001
0.059
0.399
0.010
1.556
0.005
76.67
22.61
0.04
21.28
40.48
0.16
0.47
0.01
0.12
100.19
0.01
0.01
25-2 r
37.61
0.977
0.001
0.001
0.044
0.388
0.009
1.578
0.003
78.06
21.36
0.04
20.22
41.45
0.10
0.42
0.23
100.75
0.02
0.01
25-3 c
38.27
Endmember compositions in mole %; Fe+3 calculated from charge balance of analyzed FeO
Cation values expressed per 4 oxygens
c - core composition; r - rim composition; 10JF025 - Desolation Valley flow (basaltic andesite; whole rock chemistry not analysed)
10JF021 - CVB, 10JF024 - HCBA (whole rock geochemistry not analysed)
0.01
12.62
47.36
0.15
0.25
0.36
100.35
11.51
48.17
0.15
0.48
0.03
0.11
100.6
0.03
0.01
-
0.00
-
24-2 r
39.86
24-2 c
39.60
0.02
24-1 r
40.13
Table C1 cont'd
0.967
0.001
0.001
0.064
0.409
0.010
1.543
0.005
75.94
23.3
0.02
21.98
40.21
0.19
0.48
0.01
0.13
100.63
0.03
0.03
25-3 r
37.55
0.980
0.001
0.039
0.358
0.008
1.611
0.003
79.78
19.69
0.02
18.68
42.46
0.12
0.35
0.02
0.27
100.44
0.02
0.00
25-4 c
38.50
0.972
0.001
0.055
0.395
0.009
1.564
0.004
77.12
22.2
20.92
40.76
0.16
0.43
0.03
0.12
100.22
0.02
0.01
25-4 r
37.77
0.973
0.001
0.054
0.354
0.008
1.608
0.002
79.33
20.14
19.01
42.01
0.09
0.38
0.01
0.28
99.68
0.02
-
25-5 c
37.88
134
0.04
22.68
39.57
0.17
0.53
0.06
0.05
100.96
75.06
24.14
0.975
0.001
0.049
0.439
0.012
1.519
0.005
0.02
21.69
40.67
0.20
0.40
0.10
101.11
76.44
22.87
0.972
0.001
0.055
0.409
0.009
1.550
0.005
0.978
0.001
0.043
0.445
0.013
1.514
0.006
74.91
24.16
22.44
39.03
0.22
0.58
0.05
0.12
100.05
0.03
0.00
25-6 r
37.59
0.968
0.062
0.573
0.013
1.376
0.006
67.73
31.29
0.02
28.53
34.64
0.22
0.59
0.03
0.10
100.51
0.01
0.01
MGVF
10JF017
17-2
36.35
0.984
0.001
0.001
0.031
0.650
0.016
1.313
0.006
65.17
33.79
0.02
30.15
32.62
0.19
0.68
0.00
0.05
100.18
0.02
0.01
17-4
36.44
0.975
0.001
0.001
0.048
0.604
0.016
1.350
0.005
66.71
32.25
0.02
29.18
33.86
0.17
0.71
0.02
0.08
100.52
0.02
0.01
17-7
36.46
0.971
0.001
0.001
0.001
0.054
0.505
0.010
1.455
0.003
71.89
27.61
0.05
25.67
37.50
0.10
0.45
0.09
101.28
0.03
0.03
17-8
37.34
0.974
0.001
0.001
0.050
0.600
0.012
1.358
0.004
67.07
32.11
0.02
29.08
34.07
0.16
0.53
0.01
0.12
100.46
0.02
0.04
17-9
36.42
0.983
0.001
0.034
0.453
0.009
1.518
0.003
75.28
24.14
22.56
39.47
0.10
0.41
0.08
100.74
0.03
-
10JF018
18-1 r
38.09
0.986
0.001
0.026
0.422
0.007
1.553
0.003
77.19
22.27
0.02
20.96
40.75
0.13
0.34
0.10
100.9
0.03
0.01
18-1 c
38.56
0.984
0.001
0.032
0.480
0.009
1.492
0.003
74.02
25.41
0.02
23.68
38.70
0.10
0.40
0.02
0.10
101.07
0.02
-
18-1 r
38.03
0.974
0.001
0.049
0.413
0.007
1.551
0.003
76.63
22.86
0.02
21.45
40.34
0.11
0.33
0.14
100.21
0.02
0.03
18-2 c
37.77
Endmember compositions in mole %; Fe+3 calculated from charge balance of analyzed FeO
Cation values expressed per 4 oxygens
c - core composition; r - rim composition; 10JF025 - Desolation Valley flow (basaltic andesite; whole rock chemistry not analysed)
10JF021 - CVB, 10JF024 - HCBA (whole rock geochemistry not analysed)
-
0.01
0.01
25-6 c
37.85
25-5 r
38.03
Table C1 cont'd
0.975
0.001
0.048
0.446
0.008
1.517
0.004
74.97
24.42
22.82
39.31
0.13
0.38
0.02
0.18
100.57
0.04
0.02
18-2 r
37.66
0.979
0.001
0.001
0.040
0.419
0.007
1.550
0.003
76.75
22.75
0.02
21.58
40.84
0.11
0.33
0.04
0.17
101.58
0.02
0.03
18-3 c
38.44
0.976
0.048
0.424
0.007
1.542
0.003
76.21
23.3
21.96
40.30
0.12
0.31
0.03
0.13
100.88
0.01
0.02
18-3 r
38.00
0.980
0.001
0.039
0.424
0.008
1.546
0.003
76.59
22.89
0.03
21.44
40.24
0.11
0.34
0.13
100.35
0.00
0.02
18-4
38.03
135
0.36
98.62
44.86
12.41
42.72
1.875
0.02
0.132
0.005
0.099
0.142
0.007
0.868
0.827
0.026
0.81
0.02
99.24
45.63
10.63
43.73
1.842
0.033
0.142
0.166
0.038
0.007
0.875
0.839
0.058
0.74
0.02
96.62
43.31
10.62
46.07
1.799
0.035
0.145
0.209
0.006
0.849
0.904
0.054
0.42
0.03
97.96
43.92
10.86
45.21
1.883
0.02
0.123
0.101
0.109
0.006
0.851
0.876
0.031
0.35
0.00
98.92
45.45
10.31
44.24
1.87
0.019
0.12
0.001
0.125
0.077
0.006
0.89
0.866
0.025
0.43
98.66
44.69
10.63
44.68
1.867
0.019
0.134
0.124
0.082
0.005
0.869
0.868
0.031
0.80
98.51
44.98
11.27
43.75
1.822
0.038
0.153
0.184
0.032
0.007
0.864
0.84
0.058
0.73
0.02
98.89
45.06
10.56
44.38
1.841
0.037
0.143
0.153
0.05
0.006
0.865
0.852
0.052
0.49
0.05
98.92
45.15
9.84
45.02
1.876
0.021
0.139
0.01
0.09
0.098
0.003
0.864
0.862
0.035
0.53
98.83
48.23
11.18
40.6
1.896
0.022
0.09
0.113
0.105
0.007
0.939
0.79
0.038
0.43
0.02
99.34
43.72
12.18
44.1
1.842
0.025
0.171
0.001
0.125
0.109
0.007
0.841
0.848
0.031
0.37
0.00
99
47.05
10.75
42.2
1.892
0.014
0.101
0.001
0.113
0.097
0.005
0.922
0.827
0.027
0.39
0.04
99.26
45.27
13.51
41.21
1.895
0.028
0.115
0.021
0.046
0.212
0.866
0.789
0.028
0.53
0.06
98.41
47.89
7.46
44.65
1.893
0.013
0.111
0.027
0.088
0.055
0.004
0.917
0.854
0.038
0.51
98.34
41.7
12.13
46.17
1.789
0.035
0.225
0.165
0.067
0.003
0.797
0.883
0.037
0.43
0.02
98.31
45.45
10.29
44.26
1.874
0.018
0.122
0.123
0.178
0.003
0.887
0.864
0.031
0.38
98.98
45.83
10.51
43.65
1.892
0.019
0.116
0.089
0.115
0.003
0.89
0.848
0.028
0.40
99.24
42.14
12.12
45.74
1.816
0.033
0.202
0.002
0.126
0.106
0.005
0.806
0.875
0.029
0.37
0.05
99.26
45.3
9.99
44.7
1.878
0.017
0.114
0.001
0.121
0.075
0.005
0.887
0.876
0.026
0.40
0.04
98.69
42.76
11.41
45.82
1.823
0.03
0.192
0.004
0.126
0.093
0.004
0.82
0.879
0.029
En
Fs
Wo
Si
Ti
Al
Cr
Fe3+
Fe2+
Mn
Mg
Ca
Na
0.17
7.69
15.59
20.66
0.22
6.81
16.74
20.89
0.17
8.28
15.57
19.72
0.15
4.61
16.59
21.51
0.12
Endmember compositions in mole %; Fe+3 calculated from charge balance of analyzed FeO
Cation values expressed per 6 oxygens
c - core composition; r - rim composition; gm - groundmass crystal composition
NiO
Total
FeO
MgO
CaO
MnO
Na2O
7.40
14.27
21.99
0.09
6.62
15.92
21.23
0.21
6.57
15.02
22.23
0.18
0.02
6.65
15.81
21.61
0.20
0.03
6.51
16.11
21.82
0.20
6.64
15.67
21.80
0.16
0.01
6.96
15.58
21.08
0.24
6.54
15.66
21.46
0.20
0.34
6.07
15.63
21.69
0.10
7.03
17.02
19.94
0.23
0.04
7.56
15.22
21.36
0.22
6.44
15.96
21.63
0.09
2.99
3.28
3.25
2.75
2.76
3.05
3.50
3.28
3.19
2.06
3.91
6.57
16.08
21.30
0.09
0.72
1.17
1.22
0.71
0.68
0.70
1.37
1.32
0.77
0.78
0.89
7.45
14.54
21.96
0.15
50.21
49.97
47.41
49.78
50.46
50.21
48.97
49.68
50.59
51.24
49.69
6.34
16.11
22.12
0.16
10JF025
25-1 c
24-9 gm
24-8 r
24-8 c
24-7 r
24-7 c
24-6 gm
24-5 gm
24-4
24-3
24-2
7.01
14.74
21.98
0.11
Table C2: Electron microprobe compositions of clinopyroxene from the Garibaldi Volcanic Complex
GLVF
10JF021 10JF024
10JF013
24-1
21-1
13-6
13-5 r
13-4 c
13-3 r
13-3 c
13-2
13-1
SiO2
51.17
50.78
51.07
47.75
50.30
50.94
48.86
50.85
48.84
TiO2
0.50
1.01
0.45
1.23
0.65
0.67
1.18
0.62
1.08
Al2O3
2.32
2.61
2.55
5.09
2.77
2.65
4.62
2.62
4.36
Cr2O3
0.02
0.70
0.91
0.00
0.01
0.08
0.02
0.12
136
52.45
0.32
2.15
0.02
100.28
52.94
0.15
0.65
19.04
25.26
1.08
0.81
0.04
99.98
52.12
0.27
2.49
0.07
15.69
27.31
1.33
0.35
0.07
0.02
99.72
52.91
0.24
0.73
19.09
24.76
1.24
0.77
0.00
0.02
99.78
52.86
0.26
2.02
0.06
15.50
27.26
1.46
0.40
0.02
99.84
52.43
0.07
0.49
21.85
23.96
0.67
0.70
0.02
0.03
100.21
52.65
0.07
0.57
21.61
23.46
0.82
0.69
0.00
0.00
99.88
1.894
0.009
0.091
0.104
0.412
0.012
1.417
0.059
0.001
1.938
0.004
0.028
0.087
0.496
0.025
1.379
0.042
1.878
0.007
0.106
0.002
0.127
0.346
0.011
1.467
0.051
0.005
1.945
0.007
0.032
0.065
0.522
0.024
1.357
0.049
1.904
0.007
0.086
0.002
0.091
0.376
0.012
1.464
0.056
0.002
1.935
0.002
0.021
0.106
0.568
0.022
1.318
0.027
0.001
1.952
0.002
0.025
0.067
0.603
0.022
1.297
0.033
1.871
0.006
0.140
0.006
0.101
0.316
0.010
1.503
0.046
0.002
1.893
0.006
0.112
0.001
0.091
0.378
0.011
1.460
0.047
0.001
1.891
0.007
0.096
0.001
0.11
0.339
0.011
1.497
0.049
0.001
1.952
0.003
0.022
0.068
0.597
0.023
1.301
0.034
-
1.933
0.005
0.036
0.001
0.086
0.572
0.021
1.296
0.049
-
1.923
0.005
0.055
0.003
0.091
0.557
0.021
1.285
0.054
0.006
2.003
0.011
0.139
0.458
0.016
1.223
0.099
0.052
1.920
0.010
0.039
0.001
0.100
0.361
0.016
1.473
0.079
0.001
Si
Ti
Al
Cr
Fe3+
Fe2+
Mn
Mg
Ca
Na
Endmember compositions in mole %; Fe+3 calculated from charge balance of analyzed FeO
Cation values expressed per 6 oxygens
c - core composition; r - rim composition; gm - groundmass crystal composition
71.13
25.92
2.95
68.79
29.09
2.12
73.67
23.75
2.58
68.1
29.46
2.44
73.67
23.5
2.83
64.85
33.52
1.63
64.85
33.52
1.63
76.45
21.19
2.37
73.87
23.74
2.39
75.07
22.5
2.44
65.08
33.22
1.7
64.71
32.82
2.47
64.67
32.63
2.7
68.75
25.74
5.52
73.15
22.93
3.92
0.00
17.10
26.32
1.52
0.40
18-6 r
18-6 c
18-5 r
18-5 c
18-4 r
18-4 c
18-3 c
En
Fs
Wo
Table C3: Electron microprobe compositions of orthopyroxene from the Garibaldi Volcanic Complex
MGVF
GLVF
10JF018
10JF017
10JF025
18-2 r
18-2 c
18-1 r
18-1 c
17-1 r
17-1 c
25-2 gm
25-1
SiO2
52.44
52.73
52.73
52.81
52.13
51.68
53.63
52.95
TiO2
0.22
0.20
0.24
0.09
0.18
0.19
0.40
0.36
Al2O3
3.33
2.65
2.26
0.51
0.83
1.25
3.15
0.92
Cr2O3
0.22
0.03
0.02
0.04
0.11
0.02
13.95
15.62
14.96
21.48
21.20
20.84
14.65
15.22
FeO
28.25
27.27
28.01
23.61
23.45
23.17
21.96
27.23
MgO
1.20
1.23
1.26
0.86
1.25
1.34
2.47
2.03
CaO
0.33
0.37
0.37
0.73
0.67
0.65
0.50
0.53
MnO
Na2O
0.02
0.02
0.01
0.08
0.71
0.01
0.09
0.06
0.03
0.00
0.03
0.04
NiO
100.06
100.18
99.91
100.1
99.75
99.3
97.5
99.31
Total
137
2.732
1.222
0.055
0.001
0.001
0.237
0.002
0.607
0.143
Endmember compositions in mole %
Cation values expressed per 8 oxygens
2.713
1.257
0.042
0.001
0.002
0.263
0.006
0.616
0.101
2.912
1.065
0.033
0.001
0.001
0.065
0.003
0.607
0.313
2.582
1.395
0.052
0.001
0.002
0.416
0.003
0.508
0.040
2.394
1.589
0.021
0.009
0.628
0.352
0.007
2.377
1.595
0.020
0.009
0.650
0.342
0.007
65.03
34.22
0.76
0.082
0.005
0.674
0.232
2.894
1.073
0.041
-
8.25
68.24
23.51
2.666
1.319
0.046
0.322
0.002
0.558
0.085
63.65
35.64
0.71
Si
Al
Fe2+
Mn
Mg
Ca
Ba
Na
K
43.16
52.65
4.2
31.93
58.53
9.53
6.59
61.6
31.81
33.37
57.84
8.8
An
Ab
Or
24
61.53
14.46
0.39
0.07
98.74
26.8
62.9
10.3
4.69
2.667
1.312
0.052
0.003
0.308
0.003
0.564
0.092
2.447
1.528
0.036
0.003
0.544
0.001
0.417
0.023
55.27
42.39
2.34
28.23
0.94
0.05
11.07
-
24.42
1.37
0.04
6.30
1.58
0.17
98.74
53.30
58.49
6.38
24-3
24-2
Table C4: Electron microprobe compositions of plagioclase from the Garibaldi Volcanic Complex
GLVF
10JF013
10JF021
10JF024
13-1
13-2
13-3
13-4
13-5
21-1
21-2
24-1
SiO2
58.86
59.97
60.79
64.34
56.94
52.41
52.18
64.31
Al2O3
24.70
23.57
23.07
19.97
26.10
29.52
29.70
20.22
FeO
1.22
1.12
1.46
0.86
1.38
0.55
0.52
1.08
MgO
0.01
0.03
0.01
0.01
0.03
0.13
0.13
0.00
CaO
6.64
5.42
4.92
1.34
8.56
12.83
13.31
1.69
MnO
0.01
0.01
0.02
0.02
0.04
0.00
0.00
Na2O
6.36
7.03
6.97
6.92
5.77
3.97
3.87
7.73
K 2O
1.47
1.75
2.49
5.43
0.70
0.12
0.13
4.05
BaO
0.14
0.32
0.14
0.18
0.18
0.02
0.01
0.27
Total
99.41
99.21
99.88
99.08
99.70
99.55
99.84
99.35
2.424
1.552
0.036
0.001
0.004
0.558
0.002
0.405
0.019
56.84
41.26
1.9
0.32
0.12
98.57
4.54
28.61
0.94
0.06
11.31
0.01
52.66
24-4
2.859
1.112
0.031
0.001
0.110
0.003
0.628
0.257
11.05
63.12
25.83
4.46
0.20
99.01
7.16
20.87
0.81
0.01
2.27
-
63.24
24-5
2.563
1.380
0.047
0.008
0.424
0.001
0.556
0.020
42.42
55.61
1.97
0.34
0.05
99.12
6.33
25.81
1.25
0.12
8.73
-
56.49
10JF025
25-1
25-2
2.550
1.390
0.057
0.008
0.447
0.527
0.022
44.85
52.92
2.23
0.38
0.02
98.61
5.94
25.79
1.49
0.11
9.11
-
55.76
138
52.25
28.93
0.79
0.12
12.07
-
4.47
0.14
98.76
59.38
39.8
0.82
2.398
1.565
0.030
0.008
0.593
0.397
0.008
51.53
29.76
0.70
0.12
13.01
-
3.98
0.11
0.03
99.25
63.95
35.4
0.64
2.360
1.606
0.027
0.008
0.638
0.353
0.007
2.347
1.627
0.030
0.005
0.651
0.332
0.008
65.65
33.52
0.82
0.14
99.58
3.74
30.19
0.79
0.07
13.29
-
51.34
25-5
2.385
1.605
0.014
0.003
0.619
0.002
0.362
0.010
62.46
36.55
0.98
0.17
0.11
99.91
4.11
29.96
0.38
0.04
12.71
-
52.44
MGVF
10JF017
17-1
Endmember compositions in mole %
Cation values expressed per 8 oxygens
25-4
25-3
Table C4 cont'd
2.376
1.593
0.027
0.004
0.612
0.001
0.379
0.007
61.31
37.99
0.7
0.12
0.06
99.28
4.28
29.56
0.71
0.07
12.50
-
51.98
17-2
2.542
1.450
0.012
0.461
0.002
0.514
0.019
46.33
51.72
1.96
0.34
0.12
100.32
5.91
27.42
0.31
0.01
9.58
-
56.64
17-3
2.383
1.588
0.028
0.001
0.004
0.617
0.371
0.007
61.96
37.28
0.76
0.13
0.02
99.86
4.21
29.63
0.73
0.05
12.66
0.02
52.41
17-4
2.348
1.624
0.030
0.001
0.004
0.635
0.353
0.005
63.9
35.58
0.53
0.09
99.74
4.00
30.24
0.78
0.06
13.00
0.03
51.54
17-5
2.345
1.618
0.027
0.004
0.640
0.001
0.359
0.005
63.74
35.74
0.52
0.09
0.06
99.36
4.05
30.03
0.69
0.06
13.07
0.00
51.29
17-6
2.328
1.642
0.028
0.005
0.664
0.329
0.004
66.58
33.01
0.41
0.07
99.38
3.71
30.42
0.72
0.07
13.54
0.01
50.84
17-7
2.290
1.677
0.024
0.005
0.721
0.276
0.005
71.93
27.57
0.49
0.08
0.00
100.33
3.13
31.28
0.63
0.08
14.79
-
50.33
10JF018
18-1
18-2
2.282
1.689
0.022
0.005
0.723
0.001
0.275
0.003
72.25
27.42
0.32
0.06
0.04
99.36
3.08
31.18
0.58
0.07
14.69
0.01
49.65
18-3
2.326
1.642
0.020
0.006
0.688
0.311
0.006
68.78
31.16
0.06
0.10
0.02
100.00
3.52
30.58
0.53
0.08
14.10
0.01
51.05
18-4
2.319
1.650
0.025
0.005
0.669
0.327
0.005
66.83
32.65
0.52
0.09
99.81
3.70
30.71
0.64
0.07
13.71
0.01
50.87
18-5
2.315
1.673
0.020
0.006
0.696
0.001
0.283
0.005
70.78
28.74
0.48
0.08
0.06
99.50
3.18
30.94
0.53
0.09
14.17
-
50.45
139
0.011
0.843
1.239
0.019
13.674
2.265
5.221
0.025
0.325
0.057
Fe2O3
FeO
MgO
CaO
MnO
NiO
Total
Si
Ti
Al
Cr
Fe3+
Fe2+
Mg
Ca
Mn
Ni
0.18
0.403
1.338
0.043
14.115
6.304
1.410
0.052
0.148
0.036
62.03
24.93
3.13
0.16
0.58
0.15
96.68
0.21
0.016
0.309
1.738
0.044
13.812
1.538
5.767
0.029
0.549
0.077
68.82
6.89
14.51
0.10
2.43
0.36
100.45
0.010
2.282
0.230
0.027
12.878
5.935
1.646
0.017
0.107
0.009
57.36
23.79
3.70
0.05
0.42
0.04
96.34
0.11
0.65
0.002
2.795
0.336
0.019
12.149
6.680
0.888
0.025
0.052
0.006
53.68
26.56
1.98
0.08
0.20
0.02
95.92
0.08
0.95
0.012
0.866
1.893
0.173
12.837
5.142
2.605
0.033
0.078
0.032
60.83
21.92
6.23
0.11
0.33
0.14
100.22
0.78
5.73
4.11
0.014
0.339
1.166
0.065
14.329
5.064
2.563
0.015
0.269
0.044
66.41
21.12
6.00
0.05
1.11
0.19
100.23
0.29
3.45
1.57
7.55
7.78
0.014
1.619
2.465
5.198
6.295
5.279
2.422
0.007
0.063
0.025
30.21
22.80
5.87
0.02
0.27
0.11
98.41
23.75
Endmember compositions in mole %; Fe+3 calculated from charge balance of analyzed FeO
Cation values expressed per 4 oxygens
18-2a & b - single crystal with exsolved ilmenite component
0.09
67.07
10.00
12.93
0.09
1.42
0.26
99.91
Cr2O3
5.53
12.36
0.017
1.808
2.081
4.877
6.760
5.909
1.739
0.018
0.090
0.015
31.98
25.16
4.15
0.06
0.38
0.07
98.67
21.96
6.29
8.56
3.76
10.17
3.88
Al2O3
1.54
4.14
TiO2
1.77
21-2
0.06
Table C5: Electron microprobe compositions of oxides from the Garibaldi Volcanic Complex
GLVF
10JF013
10JF021
13-1
13-2
13-3
13-4
13-5
13-6
13-7
21-1
SiO2
0.04
0.06
0.03
0.01
0.04
0.05
0.05
0.003
0.441
0.387
0.138
14.920
4.101
3.567
0.053
0.181
0.044
69.11
17.09
8.34
0.17
0.75
0.19
99.5
0.61
1.14
2.04
10JF024
24-1
0.01
0.003
0.836
0.606
0.085
14.261
4.887
2.723
0.087
0.156
0.041
65.85
20.31
6.35
0.28
0.64
0.18
99.64
0.37
1.79
3.86
24-2
0.01
0.016
1.482
0.475
0.077
13.576
4.514
2.987
0.033
0.253
0.027
62.96
18.83
6.99
0.11
1.04
0.12
98.72
0.34
1.41
6.87
24-3
0.06
0.020
2.544
0.351
0.020
12.425
6.612
0.968
0.036
0.052
0.012
54.67
26.18
2.15
0.11
0.20
0.05
95.69
0.08
0.99
11.20
24-4
0.07
0.015
1.660
0.739
0.022
13.146
4.558
2.921
0.021
0.267
0.024
60.68
18.93
6.81
0.07
1.09
0.10
97.68
0.10
2.18
7.67
24-5
0.05
140
0.026
2.089
0.718
0.283
12.355
6.375
1.203
0.021
0.112
0.025
56.89
26.41
2.80
0.07
0.46
0.11
99.8
0.037
1.965
0.781
0.239
12.478
6.713
0.879
0.011
0.127
0.020
57.45
27.81
2.04
0.04
0.52
0.08
100.46
1.05
2.30
9.05
25-2
0.13
0.015
2.103
0.757
0.217
12.378
6.463
1.136
0.006
0.126
0.005
57.10
26.83
2.65
0.02
0.51
0.02
100.07
0.95
2.23
9.71
25-3
0.05
0.018
1.896
0.853
0.536
12.219
6.341
1.307
0.105
0.008
56.60
26.43
3.06
0.43
0.04
100.28
2.36
2.52
8.78
25-4
0.06
0.013
1.809
1.484
0.199
12.039
6.517
1.131
0.005
0.092
0.027
55.78
27.17
2.65
0.02
0.38
0.12
99.82
0.88
4.39
8.39
MGVF
10JF017
17-1
0.05
0.019
1.851
1.545
0.220
11.898
6.518
1.119
0.006
0.090
0.033
55.06
27.14
2.61
0.02
0.37
0.15
99.51
0.97
4.56
8.57
17-2
0.07
17-3
0.06
0.017
2.248
1.061
0.382
11.725
6.429
1.172
0.006
0.093
0.017
54.61
26.94
2.76
0.02
0.39
0.08
100.17
1.69
3.16
10.48
Endmember compositions in mole %; Fe+3 calculated from charge balance of analyzed FeO
Cation values expressed per 4 oxygens
18-2a & b - single crystal with exsolved ilmenite component
0.015
1.449
0.652
0.021
13.497
4.340
3.204
0.026
0.210
0.038
0.014
0.439
0.784
2.098
12.552
4.684
3.008
0.027
0.120
0.105
1.24
0.09
62.90
18.20
7.54
0.09
0.87
0.17
98.59
9.21
1.94
2.31
57.86
19.43
7.00
0.09
0.49
0.45
98.91
2.11
6.76
2.02
9.62
24-7
0.05
10JF025
25-1
0.09
24-6
0.05
Table C5 cont'd
0.016
3.061
0.325
0.117
11.713
6.810
0.676
0.005
0.115
0.009
54.40
28.46
1.59
0.02
0.47
0.04
100.73
0.52
0.96
14.22
17-4
0.05
0.015
2.034
0.839
0.058
12.542
6.828
0.777
0.005
0.109
0.026
57.20
28.02
1.79
0.02
0.44
0.11
99.61
0.25
2.44
9.28
10JF018
18-1
0.05
0.104
2.021
0.289
0.040
13.014
7.207
0.385
0.021
0.103
0.019
58.29
29.05
0.87
0.07
0.41
0.08
99.17
0.17
0.83
9.05
18-2a
0.35
1.443
8.223
0.007
3.910
5.950
0.674
0.041
0.126
-
20.46
28.01
1.78
0.15
0.59
99.74
0.04
-
43.04
18-2b
5.68
1.299
8.312
3.986
5.674
0.982
0.023
0.112
0.008
20.91
26.78
2.60
0.08
0.52
0.04
99.69
-
-
43.62
18-3
5.13
0.032
2.617
0.264
0.033
12.391
7.112
0.448
0.033
0.065
0.012
56.22
29.03
1.03
0.10
0.26
0.05
99.59
0.14
0.76
11.88
18-4
0.11
141
142
Appendix D: Trace Element Modelling Data
Table D5: Estimated starting composition of Juan de Fuca (JdF) MORB
Rb
Ba
Th
Nb
La
Ce
Sr
Nd
Sm
Zr
Eu
Dy*
JdF NMORB
3
50
0.31
3
3.61
9.72
121
8.41
2.8
72
0.82
4.55
*value from Sun and McDonough (1989); composition estimated from Karsten et al. (1990), Cousens et al. (1995) and Chadwick et al. (2005).
melts at various melt fractions (F) for a residue of 70% Cpx, 25% Gt, 4.5 % Hbl, 0.5% Ru
Nb
La
Ce
Sr
Nd
Sm
Zr
Eu
Dy
5.064
31.466
71.641
1056.59
16.95
3.929
163.663
2.354
1.76
4.887
22.378
53.652
750.97
16.09
3.847
153.385
2.143
1.818
4.722
17.363
42.884
582.49
15.31
3.769
144.322
1.967
1.881
4.568
14.184
35.715
475.75
14.61
3.694
136.27
1.817
1.948
Dy
2.11
2.17
2.23
2.306
Dy
6.2
1.4
0.01
3.08
Table D4: Modelled compositions of slab partial
Rb
Ba
Th
F=5
52.26
910.63
4.524
F=10
28.033
477.79
2.637
F=15
19.154
323.85
1.861
F=20
14.546
244.94
1.438
Mineral acronyms as in Fig. 18
Eu
1.64
1.55
1.48
1.417
Eu
0.327
0.232
0.004
1.39
melts at various melt fractions (F) for a residue of 70% Cpx, 20% Gt, 9.5% Hbl, 0.5% Ru
Nb
La
Ce
Sr
Nd
Sm
Zr
Eu
Dy
4.192
22.766
44.96
849.79
11.46
3.293
135.36
1.834
1.807
4.106
17.796
37.756
645.24
11.24
3.263
129.37
1.722
1.867
4.024
14.607
32.541
520.06
11.03
3.233
123.88
1.623
1.93
3.944
12.387
28.592
435.56
10.84
3.204
118.85
1.534
1.998
Zr
0.614
0.25
3.79
1.4
Table D3: Modelled compositions of slab partial
Rb
Ba
Th
F=5
49.437
706.73
3.778
F=10
27.243
417.86
2.378
F=15
18.802
296.62
1.735
F=20
14.354
229.91
1.366
Sm
0.712
0.59
2.4
2.1
melts at various melt fractions (F) for a residue of 70% Cpx, 10% Gt, 20% Hbl
Nb
La
Ce
Sr
Nd
Sm
Zr
10.03
19.595
37.515
763.77
9.501
3.08
133.19
8.929
15.891
32.607
596.89
9.437
3.064
127.48
8.046
13.365
28.835
489.85
9.373
3.048
122.25
7.322
11.532
25.845
415.37
9.311
3.032
117.43
Nd
0.131
0.44
0.684
2.79
Table D2: Modelled compositions of slab partial
Rb
Ba
Th
F=5
47.897
659.11
3.527
F=10
26.793
401.61
2.281
F=15
18.598
288.78
1.686
F=20
14.242
225.45
1.337
Table D1: Partition coefficients for elements used in slab partial melt model
Rb
Ba
Th
Nb
La
Ce
Sr
Gt
0.004
0.005
0.001
0.002
0.021
0.053
0.0303
Cpx
0.007
0.0096
0.014
0.003
0.056
0.05
0.073
Rut
0.019
0.02
0.54
102
0.237
0.296
0.048
Hbl
0.04
0.1
0.15
1.3
0.5
0.899
0.3
Kd’s derive from Rollinson (1993), Foley et al. (2000), Barth et al. (2002), and GERM
Y
28
Y
8.89
9.221
9.578
9.964
Y
9.629
9.973
10.34
10.74
Y
12.3
12.68
13.07
13.5
Y
8.7
1.39
0.459
2.5
Yb
2.59
Yb
0.752
0.782
0.813
0.847
Yb
0.848
0.879
0.912
0.948
Yb
1.14
1.18
1.21
1.257
Yb
10
1.42
0.0159
1.65
Lu
0.44
Lu
0.106
0.11
0.115
0.12
Lu
0.119
0.124
0.129
0.135
Lu
0.155
0.161
0.167
0.173
Lu
11
2.1
0.016
1.75
143
144
Figure D1: Failed slab partial melt models of the GVC adakites. Mineral acronyms as in Fig. 18. Grey shaded area
represents the range of the GVC adakites, the black line shows the best fit model to the GVC rocks. Normalising values
for N-MORB are from Sun and McDonough (1989).
145
Figure D1 cont’d: Failed slab partial melt models of the GVC adakites. Mineral acronyms as in Fig. 18. Grey shaded area
represents the range of the GVC adakites, the black line shows the best fit model to the GVC rocks. Normalising values
for N-MORB are from Sun and McDonough (1989).
146