Ecological Analysis in a Polluted Area of Algeciras Bay (Southern
Transcription
Ecological Analysis in a Polluted Area of Algeciras Bay (Southern
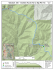
Pergamon PII: S0025-326X(97)00046-5 Marine Pollution Bulletin, Vol. 34, No. 10, pp. 780-793, 1997 © 1997 Elsevier Science Ltd All rights reserved. Printed in Great Britain 0025-326X/97 $17.00 + 0.00 Ecological Analysis in a Polluted Area of Algeciras Bay (Southern Spain): External 'Versus' Internal Outfalls and Environmental Implications F. J. ESTACIO*, E. M. GARCIA-ADIEGO*, D. A. FAt, J. C. GARCIA-G6MEZ*, J. L. DAZA*, F. HORTAS* and J. L. GtMEZ-ARIZA:~ *Laboratorio de Biologla Marina, Departamento de Fisiologla y Biologia Animal, Facultad de Biologla, Universidad de Sevilla. Apdo. 1095, E-41080 Sevilla, Espada tDepartment of Oceanography, University of Southampton, Highfield, Southampton S017 1BJ, U.K. ~Departamento de Quimica y Ciencias de los Materiales, Universidad de Huelva, 2189 La Rdbida, Huelva, Espada The effects of organic effluents both inside and outside the Saladillo Harbour (Algeciras, southern Spain) are investigated. Although the external outfall has a greater rate of discharge, the low levels of hydrodynamism inside the harbour create an area of relatively stagnant water, with markedly different environmental conditions. A clear gradient of decreasing pollution levels was observed from the interior to the exterior of the harbour. This was reflected both in the physieochemical water and sediment parameters and in their respective microbiological parameters. Macrobenthic species also mirrored the pollution gradients as did the applied analysis. The effects of the external outfall are seen to dissipate and clear much closer to the external pollution source than in the internal, enclosed harbour area. © 1997 Elsevier Science Ltd Keywords: macrobenthos; sediment; pollution; outfalls; southern Spain. The Saladillo Harbour in the Bay of Algeciras (southern Spain) has suffered a series of transformations due to the growth of the port of Algeciras. These have created conditions of reduced water movement and renewal in its interior. This situation is worsened by the existence of two raw sewage disposal points (one internal, one external) that originate from the City of Algeciras and the Saladillo River that transport a great quantity of organic waste. The internal outfall has a flow capacity of 3251 x 106 1 d a y - l and the external a flow capacity of 22580x106 1 day - l . The latter also has a subsidiary outflow into the harbour that is only used when outfall levels are higher than the norm. There are also two present small ship repair yards and mooring facilities for small fishing boats on its northern side. Via physical, 780 chemical and microbiological analysis of the waters and sediments undertaken in this study the presence of organic contamination in the Harbour and in the immediate vicinity of the exterior outfall has become evident. An extensive literature has described the close relationship between the macroinfauna in the sediment and the effect of contaminants on the medium (Reish, 1959, 1971, 1986; Pearson, 1975; Pearson and Rosenberg, 1976, 1978; Dauvin, 1982; Gray and Pearson, 1982; Bilyard, 1987; Warwick et al., 1990; Krtncke et al., 1992; Warwick, 1993; Simboura et al., 1995). Physical, chemical and microbiological analysis of the surface water and sediment characteristics were undertaken together with the application of univariate and multivariate techniques to the biotic data in order to measure changes caused to the community by pollution (Field, 1971; Gray, 1981; Ibafiez and Dauvin, 1988; Gray et al., 1988; Warwick et al., 1990). Through their relationship with physical and chemical variables in the sediments, it has been possible to show the existence of a gradient from the interior of the Harbour as the zone most affected by effluents to the less perturbed exterior (Gray and Pearson, 1982; Reish, 1986; Ltpez-Jamar and Cal, 1990; Krtncke et al., 1992). It has also been possible to compare the differing effects of the effluents both in the interior and the exterior of the Harbour due to different hydrodynamic conditions. M a t e r i a l s and M e t h o d s The study was conducted in June of 1993 in the Saladillo Harbour in the western side of the Bay of Algeciras (Fig. l(a and b)). Half of the Harbour bottom is formed by a rocky substratum and the remainder by sediments. A breakwater parallel to the coastline acts as a physical barrier between the interior and exterior of Volume M/Number 10/October 1997 A EXTERNAL j~.. OUTFALL~ ~/~ SALADILLO B Fig. 1 (a) Locationof SaladilloHarbour. (b) Locationof ettluentsand sampling areas. the Harbour. The sea bottom on the exterior is mainly composed of rocks. On the exterior side towards the northern end of the breakwater there exists a further urban effluent outfall which also originates from the City of Algeciras. Due to the morphology of the Harbour and its location it is well protected from the actions of the winds and waves. Only easterly (due to refraction by the Rock of Gibraltar) and south-easterly winds with a frequency equal to or greater than 3 5 0 affect the interior of the Harbour to any degree. Data supplied by Autoridad Portuaria de la Bahia de Algeciras (1993) on the range of values obtained for the coefficient of agitation (H/Ho where H is the mean height of the upper third of maximal wave sizes, and Ho is the wave height over indefinite depths, calculated using the MIKE21 matematical model) were averaged to obtain mean values for each station and show how even in conditions of predominant easterly and south-easterly wind, the area contained within stations 2, 3, 6 and 7 show lower values of agitation and consequently hydrodinamism. Figure 2 shows the distribution of the mean values obtained for all the stations. These values were also ineluded as abiotie parameters in the statistical analysis. The study zone, both inside and outside the Harbour, was divided into 200x200 m square areas. This was selected as the area for study as it is the same as the area formed by the ship repair yard at the northern end of the Harbour (Station 7, see Fig. 1(b)). Only 9 of the 30 quadrats sampled in the study were selected for the study of the benthic communities (Fig. 1(b)) and for the physical and chemical analysis of the sediments (depth between 2 and 9 m) due to the presence of rocky 781 Marine Pollution Bulletin :: ~:i ::: s 8~:::8:X:~:z, : : : : : :::.:".¢;;;: . >,-:,:,;. ii! i ;i!i!~ Fig. 2 Graphical representation of the mean valuescalculated for the agitation coeltieient (H/I-lo)for all the stations (Data s o u r c e : Autoridad Portuaria de la Bahiade Algeciras,1993). outcrops in the others. These were selected based on their varying distances from the pollution foci in both the interior and exterior of the Harbour. At the centre of all the quadrats water samples were taken for physical, chemical and microbiological analysis. At the same point in each of the nine selected quadrats, samples were taken for community data analysis using a van Veen grab (0.05 m 2) as the sampling tool. Five replicates were found to be sufficient to estimate the total diversity. The samples were sieved using a 0.5 mm mesh and specimens found were preserved in 4% formaldehyde, stained with bengal rose and were determined to species level almost in their totality. Water samples were taken in all 30 stations in the study area for physical and chemical analysis. For these, salinity, density, mean temperature, chlorophyll a, b and c, dissolved oxygen (DO), biological oxygen demand (BOD), total hydrocarbons, total fats, turbidity, nitrites, nitrates, ammonia and phosphates were measured. For the sediment, percentage of organic material, total nitrogen, phosphate, fats, hydrocarbons, granulometry and percentage water content were measured. Measurements of dissolved oxygen (ppm) were done in situ using an oxygen electrode. BOD (ppm) was ascertained after incubating the samples over 5 days at 20°C. Ammonia (ppm) was measured using a selective electrode. Nitrite (ppb), nitrate (ppm), chlorophyll (ppm) and phosphates (ppb) were measured using UV visible spectrophotometry. Fats and hydrocarbons (ppm) were measured by extraction and F T - I R spectrophotometry. Total nitrogen (ppm) in the sediment was assessed via Kjeldahl digestion and further determination with an ammonia selective electrode. Organic material (%) was analysed by ashing to 500°C (mean value for 3 replicates per sample) of subsamples of sediment previously dried at 100°C during 24h. Granulometry was determined by Buchanan and Kain's method (Buchanan and Kain, 1984) for 782 sediments with a percentage of silt less than 5 and with Bouyoucos method (Bouyoucos, 1934) for samples with a percentage higher than 5. In turn, and in the same way as for the earlier analysis, water samples were taken at the centre of the 30 quadrats and also at the outfall points for microbiological analysis. These samples were conserved in sterile containers and kept in cork and gelatine packs until their arrival at the laboratory where they were frozen. With these the quantity of total aerobes (gram positive and gram negative bacteria with growth in aerobic conditions) were obtained, as were total coliforms (bacilli gram negative or facultative anaerobes oxidases that ferment lactose with production of acids and gas at 307°C in a maximum time of 48 h) and faecal coliforms (total coliforms that ferment lactose with production of acid and gas at 35°C in a maximum time of 24 h). These last two are indicators of levels of recent faecal pollution such as stated in Directive 75/440/EEC for bathing waters. Results in all 3 cases were expressed as number of bacteria per 100 ml of sample and these were contrasted to the guide values (values beyond which remedial action is suggested) and imperative values (values beyond which remedial action is obligatory) that are prescribed by the normative for total and faecal coliforms. As only one sampling was undertaken, results obtained using the biotic and abiotic water parameter information will have a limited value as it was not possible to establish whether these readings were temporally representative. Nevertheless, it was considered that due to the conditions of reduced water movement found (particularly in the interior), their inclusion could still generate relevant results. The sediment parameters were also sampled on only one occasion but as these have a greater temporal stability they were considered as representative of overall conditions. To correctly examine the results obtained for the communities of the infaunal macrobenthos a number of diverse techniques and models were employed. Univariate analyses provided the total number of species, total abundance, total biomass, Shannon-Wiener and Pielou's equitativity index (Shannon and Weaver, 1963; Pielou, 1966) for each of the stations (calculated on the datasets generated by aggregating all five samples). The differences between all of these population parameters for all samples at each of the stations with the exception of the biomass were tested with one-tailed ANOVAs using Tuokey's test for statistical significance after the data was tested for normality using the KolmogorovSmirnov test and Bartlett's test for homogeneity of variances. Using the Plymouth Routines In Multivariate Ecological Analysis (PRIMER) package, the data were analysed using the Bray-Curtis index of similarity (Bray and Curtis, 1957) and a dendrogram created using the UPGMA method (Romesburg, 1984) with which Volume 34/Number 10/October 1997 the existence of groupings between stations based on their similarities was obtained. The data for this were previously transformed using a root-root transformation, given the high dominance levels of some species (Clarke and Warwick, 1994). Graphical representation of cumulative percentage of abundances and the cumulative percentage of biomass can be compared as ABC (abundance biomass comparison) curves, proposed by Warwick (1986) and based on the K-dominance curves of Lambshead et al. (1983). This technique has been employed in some studies to detect community perturbation (Warwick et al., 1987; Gray et al., 1988; Ibafiez and Dauvin, 1988; Austen et al., 1989; Ritz et al., 1989; Simboura et al., 1995). In order to quantify the interpretations deriving from this model the SEP index proposed by McManus and Pauly (1990) was used. This index is based on the relationship between the calculated Shannon diversity values (H') of the abundances and biomasses. An alternative method that attempts to elucidate the main factors affecting distributions was proposed by Clarke and Ainsworth (1993) and involves the comparison of the (rank) similarity matrices which underlie the resulting PCA ordinations of all possible permutations of the environmental data and correlating these with the similarity matrix obtained for an MDS of species abundances. The subset of environmental factors that best explains the observed patterns is then obtained by choosing the combination that gives the highest correlations using Spearman's test for non-parametric ranges. This was achieved using the BIOENV extension to the P R I M E R package. Results Physicochemical parameters and microbiological variables Table 1 shows the main results obtained. It will be seen that density does not show significant differences between sites and neither does the mean water temperature. In contrast, turbidity is higher (low Secchi disk values) and salinity lower in the internal stations most closely located to the effluents (1, 2 and 3), stabilizing as one approaches the harbour entrance. Decreased values for pH were also obtained at these sites. With respect to the chlorophyll values, it is interesting to note the high values obtained at stations 9 and 15. Low levels of DO were also found in the interior of the harbour, particularly so at station 3 and were also low close to the external outfall. Stations 4, 5, 6 and 7 showed intermediate DO values. Again, BOD is very high in stations close to effluents (including the exterior effluent), falling towards the harbour mouth, and this result is also found for levels of ammonia. Perhaps surprisingly, fats and hydrocarbon levels in the water are low; moreover, levels of nitrates, nitrites and phosphates can be considered normal, although these are higher at stations 1, 2, 3 and 21, especially the nitrites and phosphates. In general, 'exterior' values show an improvement in environmental conditions as one moves away from the harbour's sphere of influence. With regard to the sediments (Table 2), stations 2 and 3 show an increased level of organic material, higher even than that obtained for station 1. This may be due to the greater content of fine fractions in the first two. Nevertheless stations 6 and 7 stand out with even higher values of fine fractions than the aforementioned stations. To these must be added the highest levels of total nitrogen found and elevated hydrocarbon readings. Particularly high levels of hydrocarbons in station 3 should also be noted. In the same manner, phosphate levels are also high in stations 1, 2 and 3 and diminish in the same way as other sediment parameters towards the external stations. Granulometry is predominantly fine in the internal stations, again becoming more coarse as one moves out of the harbour. Values for all sediment quality parameters obtained for station 13 (next to external outfall) are similar in all respects to those obtained for the internal stations (1, 2 and 3), although probably due to a greater turnover of water, these conditions improve more rapidly as one moves away from this pollution source. Following the 'Guide' and 'Imperative' values proposed by Directive 75/440/EEC for bathing waters, Fig. 3 shows the levels of the different microbiological parameters found at each station. A simple scaled rank is used to show whether a particular sample proves higher (or not) than that required by law. High values are found close to the internal effluent points with a progressive decrease as one moves away towards the exterior where values can be considered as normal. Normal values are reached very close to the external station. Dendrograms based on the euclidean distance have been created from previously log (x+ 1) transformed and standardized water physicochemical data (abiotic) and the Bray-Curtis index of similarity from root-root transformed microbiological data. The resulting groupings are shown in Figs 4 and 5 in order to show similarities between methods. Some differences can be seen but a clear internal/external gradient is evident, approaching normal conditions in the stations farthest away from the harbour. The strong dilution of the exterior effluent is also clearly visible especially in a northerly direction. Macrobenthos The species found in the interior stations are characteristic of disturbed environments due to a high content of organic material. Highly dominant in this area is the polychaete Capitella capitata which drops in abundance towards the harbour mouth (98.5% to 16.8%). Accompanying polychaete species Cirratulus cirratus, Cirriformia tentaculata, Capitomastus minimus and Notomastus latericius have also been associated with high levels of organic enrichment (Theede et al., 783 Marine Pollution Bulletin ~z .~ e~ zg oo6ooo6o~o~ooooooooooooooooo~o 2, O' 0 ~'~ _= e~ 784 Volume M/Number 10/Oetober 1997 TABLE 2 Values obtained for sediments variables. Site 1 2 3 6 7 9 11 13 17 Depth (m) Hydrocarbons (ppm) Fats (ppm) Phosphate (P,ppm) Nitrogen (N,ppm) Organic matter (%) Sand (%) Water content (%) 2 3 3 4 4 4 7 8 9 332 863 4415 891 1179 106 20 25 13 38 97 229 82 0 11 31 38 8 933 800 1537 719 789 484 356 450 388 463 863 1028 1574 2011 223 120 79 120 2.9 6.8 6.3 8.1 13.2 3.7 2.9 2 3.3 74 33 40 25 20 98 99 99 98 32.58 40.12 56.38 54.65 61.04 30.77 26.87 23.99 27.68 Tailnledfurms I./100 NO O • >!0.000 10.000..500 • <500 Fueal ulfuu'iu (/!001 0 >2.000 • 2.ram '~ <100 N Fig. 3 Total values for total and faecal coliforms in the samples taken: the space internal of the spheres shown is directly related to the 'guide' and 'imperative' values for water quality (Directive 75/ 440/EEC). 1969; Pearson and Rosenberg, 1978). The only bivalves present in the interior stations, Abra alba and Mysella bidentata appear in the stations closest to the open sea. In Station 9 and for the exterior stations other bivalve species such as Digitaria digitaria, Gouldia minima, Dosinia lupinus and Clausinella fasciata indicate the presence of substratum composed of high levels of coarse material (Glemarec, 1969) in the form of bioclasts derived f r o m the proximal rocky substrata. Even though the proportion of polychaete abundance remains high, (72.72%), the proportion of mollusc abundance (3.07%) and crustaceans (22.84%) especially gammarids, increases in the external stations (Table 3). Univariate analysis Species richness, total n u m b e r of individuals, total biomass, diversity and equitability in each station consistently show a gradual increase f r o m the interior to the exterior of the h a r b o u r (Fig. 6). The results of a one-way test of variances between samples in each station using Tuckeys Test are also shown in Table 4. With the exception of stations 1 and 2 for diversity and 13 and 17 for number of species we can generalise that the stations closest to the interior effluents are not significantly different but are different with respect to stations 6 and 7 which are themselves very similar to each other. In turn, these show clear differences to the external stations which again show high levels of similarity between themselves. I f we compare the values obtained with these parameters at station 13, close to the external effluent to those obtained for stations 1, 2 and 3 we will find significant differences exist (Table 5). A oneway analysis of variance on each of the parameters 785 Marine Pollution Bulletin 4 EUCLIDEAN DISTANCES 3.53 2 . 5 2 1.5 1 0.5 0 , i ,.-i 1,-4 ~ , 1 ul, • Fig. 4 Cluster analysis of physicochemical water variables: the different groups are shown via differential shading in the above diagram. 1 2 3 4 9 II 7 ~17 'i 18 t'i BRAY-CURTIS SIMILARITY Fig. 5 Cluster analysis of the observed microbiologicalvariables: the different groups are shown via differential shading in the above diagram. indicates significant differences between the stations (Table 6). Similarity analysis Species abundance data was root-root transformed and a similarity matrix was calculated using the Bray786 Curtis index. The resulting dendrogram was obtained using the U P G M A method (Fig. 7). This cluster analysis clearly shows three groups which in turn integrate most internal stations, the intermediate stations close to the harbour m o u t h and the external stations including station 9. Volume 34/Number 10/October 1997 ~ B Diversity I 2.5 - • External outfall [ ] Evenness - 2 "Internal outfall 1.5 0 1 2 3 6 7 9 Station 1.6 11 13 0.5 ~o 17 3000 Ex~ 2500 ~. 1 Biomass 2000 !°fl • n] 0.6 0.4 nternal outfall 1 1500 500 I I II 2 3 6 7 11 9 13 17" Station External outfaU 80 60 20 0 _ Internal ouffall 1 2 3 6 7 9 11 1 13 17 Station Fig. 6 Graphical representation of the total species, number o f individuals, biomass (grs-station 17 is not included-), diversity (Shannon-Wiener) and evenness (Pielou) for each of the stations (calculated on the datasets generated by aggregating all five samples). TABLE 3 TABLE 4 Percentages for each taxonomic group found at the internal and external stations. One-way ANOVA (F-ratio values) o f values obtained for inter-station univariate analysis along the internal-external gradient (1 and 8 degrees of freedom). MoUusca Crustacea Polychaeta Echinodermata Sipunculida Ascidiacea Int. Ext. 1.22 0.52 98.25 0.00 0.00 0.00 3.07 22.84 72.72 0.07 1.27 0.02 Sites H' J' Total ind. Total spp. 1-2 2-3 3-6 6--7 7-9 9-11 11-13 13-17 12.62'* n.s. 32.58** 10.49" n.s. n.s. n.s. n.s. 7.23* n.s. 6.42* n.s. 32.75** 6.95* n.s. n.s. n.s. n.s. n.s. n.s. 46.53*** n.s. n.s. n.s. n.s. n.s. 26.45** n.s. 339.97*** n.s. n.s. 7.51" *p < 0.05; **p < 0.0 !; ***p < 0.001; n.s., not significant. 787 Marine Pollution Bulletin TABLE 5 One-way ANOVA of values obtained from the various univariate analysis of the stations closest to the internal effluents(1, 2 and 3) and closest to the external effluent (13). d.f. Sites 1-13 Total spp. Total ind. H' J' Total spp. Total ind. H' J' Total spp. Total ind. H' J' Sites 2-13 Sites 3-13 F 1, 8 1, 8 1, 8 1, 8 1, 8 1, 8 1, 8 1, 8 p 504.131 31.392 95.04 31.549 657.509 2.988 141.057 19.976 786.178 23.198 273.769 73.053 1, 8 1, 8 1, 8 1, 8 <0.0001 < 0.0005 < 0.0001 <0.0005 < 0.0001 n.s. < 0.0001 <0.01 < 0.0001 <0.01 < 0.0001 < 0.001 TABLE 6 One-way ANOVA of values obtained from the various univariate analysis applied. d.f. Tot. sp. Tot. ind H' 8 8 8 J' 8 M.S. F 1255.95 221035.89 4.7122 79.101'*** 9.544**** 79.657**** 0.1412 10 I / 7°tl 80+1 100-~ 17 11 13" P q External stations 7 ] 6 v 3 I 2 * Internal stations Fig. 7 Dendrogram of similarities of root-root transformed abundances using the Bray-Curtis index (Asterisks * indicate the stations closest to the internal and external effluents). Model for detecting pollution effects on communities Abundance biomass curves (ABC curves) show a gradient from the interior to the exterior (Fig. 8). Stations 1, 2, 3 and 13 are shown to be perturbed. Stations 6, 7 and 9 moderately perturbed and station 11 shows no perturbation. Values obtained for the SEP index (Fig. 9) again highlight a similar scenario. As the sampling was undertaken in June, there is the possibilty that juvenile settlement may affect the ABC curves (Warwick and Clarke, 1993). Although the observed gradient between stations would remain unchanged (as 788 Relationships between biotic and abiotic variables In order to achieve this the BIOENV programme was used which contrasts the station similarity matrix obtained for species abundance data via cluster analysis to the resulting matrix o f Euclidean distances obtained following PCA ordination of all the possible combinations of selected environmental variables. This has allowed us to extract those variables that show greatest correlations using a Spearman's test. Firstly, an analysis was undertaken which incorporated only the sediment variables. The subset of variables that gave the highest correlation were percentage of sand, phosphates and depth (0.88) (Table 7a). A further analysis incorporating the physicochemical water parameters give even higher correlation values (0.969 and 0.967) for two environmental subsets of percentage of sand, phosphates, depth and total nitrogen as sediment variables and DO, pH, nitrites and agitation as water parameters (Table 7b). 8.068**** ****/7<.0001. 00 l r settlement could be considered a constant) there may be difficulties when comparing results to established ABC distributions. A temporal extension would be required to establish to what extent, if any, this is affecting the results. Discussion Following the results obtained a clear gradient of environmental perturbation can be shown to exist from the internal stations to the outside of the Saladillo harbour, primarily correlated to DO, pH, nitrites, agitation, total nitrogen, phosphates, percentage of sand and water depth (which may be related to differing levels of sedimentation between the interior and exterior of the harbour). All these are indicative of high levels of organic matter stemming from the aforementioned effluents and a reduced level of hydrodynamism within the harbour itself. On the one hand, high levels of bacterial contamination, particularly faecal coliforms in the internal zones, indicate high levels of organic enrichment. These results are supported by those obtained from the chemical variables, either directly or indirectly related to the aforementioned microbiological parameters, such as low values of DO (Theede et al., 1969; Tenore, 1972; Driscoll, 1975; Thiel, 1978; Pearson and Rosenberg, 1978), high BOD (Frankenberg and Westerfield, 1968; L6pez-Jamar, 1978; Pearson and Rosenberg, 1978), high turbidity close to the effluents (Rhoads and Young, 1970) and also variables that show high correlations with the sampled bacterial flora and high values of reduced forms of nitrogen (Theede et al., 1969; Tenore, 1972). Also correlated with these variables are high levels of hydrocarbons (Reish, 1971) and fats in sediments together with increased values o f organic material in sediments (Tenore, 1972; Driscoll, 1975; Pearson, 1975; Reish, 1980; Tenore et al., 1984; L6pezJamar and Cal, 1990). On the other hand, the sheltered location of the harbour reduces both exposure to wind Volume 34/Number 10/October 1997 Station 2 Station 1 ,~ P == --= 100 90 8O 7o 60 50 40 3O 20 10 0 ~ "~ ~ = -~ E u ..j..,..../" ""~" ..... = II _= E o 100 90 80 70 ~ >• 60 50 "~ -E O 40 30 20 10 0 1 Species rank Species rank Station 3 soI, 100 ~ eel ¢¢ Station 6 ;, = 90 ~L~, . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E 0 "0 : =o 80 ~ - 70 60 .g E lOO 90 = - 70 60 w .~ so ¢ so 40 30 20 lO 0 t 1 ~ ~ E t~ 40 30 20 10 0 , > ~ ~ = O o 100. = i I i I t [ I g0 > ~ 40 = U 30 20 10 0 I 9 100, 80 70 60 50 -o i Station o 90. f "o I Species rank Station 7 80. 70" 60. 50' 40" 30" 20. 10. 0 ...m"' 10 Species rank o ,.," ...." ....." • : • • -:::: 10 Species rank Station = .~ N >e - e. m = S = o 11 80 70 60 = = == S t a t i o n 13 • O " -= E O .~ -= E ,1 lO Species rank i Species rank 100 90 50 40 30 20 10 0 : 10 ~ 100 90 80 70 60 50 4o 30 20 10 0 ....-"" 1 10 Species rank I t~ "~- -- ABUNDANCE BIOMASS I Fig. 8 Warwick's ABC model applied to each of the stations sampled (station 17 was omitted due to some biomass values not being available for some species). 789 M a r i n e P o l l u t i o n Bulletin 3.5 SEP= H' biomass / H'abundance 3 2.5 W 1.5 1 0 1 2 3 6 7 STATION 9 11 13 Fig. 9 Graphical representation of the SEP values for each station. and waves and may have created conditions of reduced water renewal within the harbour, favouring sedimentation processes (L6pez-Jamar, 1978; Parker, 1982; Sola and Ibafiez, 1986). This would have encouraged the formation of substrata with high levels of fine particles many of which emanate from the effluents. A different granulometry is found towards the harbour exit as greater exposure to wave and wind action increases the degree of water movement (Carballo et al., 1995; Conradi and Cervera, 1995), together with a greater proportion of rocky substrata. Levels of the measured variables soon return to normal in the exterior stations, even those close to the exterior effluent particularly towards the North. Concerning the substratum, dominance of opportunistic species such as C. capitata in the interior of the harbour are indicative of environmental perturbations. The decrease in its abundance towards the exterior stations reflects the effect of the effluents on the communities. The proximity of organic effluents, the results obtained from the different environmental variables measured in the surrounding sediments, and the presence of this species within them coincides with the zone described by Bellan (1967a,b) as a 'polluted' site. Numerous studies highlight that reproductive strategy based on lecitrophic and planktotrophic larvae (McCall, 1977) short life cycles (Grassle and Grassle, 1974) coupled with various annual cycles of reproduction (Warren, 1976), higher resistance to low oxygen levels (Glemarec, 1969; Theede et aL, 1969; Driscoll, 1975) and high resistance to hydrocarbon levels (Dauvin, 1982), a detrivorous habit (Fauchald and Jumars, 1979) and conditions of low interspecific competition (Warwick, 1986; L6pez-Jamar and Mejuto, 1988) go towards explaining the high level of dominance of this species. Stations 6 and 7 show differences when compared to the more internal stations. The first bivalves appear in these stations even though there are high levels of organic materials present which create unfavourable conditions (Tenore et aL, 1968). Crustaceans are poorly represented due to their being a taxonomic group which in general shows a high sensitivity to environmental 790 pollution (Pearson and Rosenberg, 1978; Parker, 1982). Based on the ABC curves and on the values obtained for the SEP index, these stations exhibit intermediate levels of environmental perturbation when compared to the more polluted internal and effectively normalized external stations. Similar results were obtained for the physical and chemical variables together with the microbiological parameters measured at these sites, although some of the sediment variables such as organic material give readings that would classify these sites as polluted. Station 9 is located at the mouth of the harbour and its environmental qualities are consequently improved as shown by the values of the measured physical, chemical and microbiological parameters. This is probably due to increased levels of hydrodynamism. Moreover, given the increased organic food resource it receives from the interior of the harbour it may also undergo 'biostimulation' for some species (Pearson and Rosenberg, 1978). The presence in this station of species such as Corbula gibba and Myrthea spinifera, characteristic of muddy habitats (note that percentage of sand= 98%) although not dominant, may be indicative of increased levels of organic enrichment (Gray et al., 1988). This station would be categorized as 'subnormal' by Bellan and Bourcier (1984). Population parameters show moderate values, similar to those encountered for the other external stations, and these similarities are confirmed by the multivariate analyses performed. The ABC curves also classify this station as 'moderately polluted'. Station 11 appears to be generally regarded by most methods of analysis as having the best environmental characteristics of all the stations in the study. Even though it would initially appear that station 13, which is located next to the external outfall, should be very similar to its apparent homologues in the interior of the harbour, this turns out not to be the case. Environmental parameters all show an improvement relative to the internal stations, although a slight deterioration is present when compared to the external stations. A less optimistic result is obtained from the ABC curves, and the SEP index wich highlight the presence of environmental perturbation at this location. Although subject to various climatic and physical factors such as wave action, wind, currents and rain, the various physical, chemical and microbiological variables of the water all appear to show a clear gradient of conditions which can be divided into three zones: the first would include the innermost (internal) stations, the second intermediate zone covering the area surrounding and including the mouth of the harbour, and the final zone encompassing the external stations. Sediment quality characteristics also break up the stations into three broad groups, although the degree of apparent environmental remediation is decreased; the intermediate sites are still classed as being moderately polluted Volume 34/Number 10/October 1997 TABLE7 (Best combinationsof variables selectedby the BIOENVprogramme.) (a) For sediment parameters only; k Best combinations of variables (p=) 1 PO43" % SAND HYDROC. WAT.CONT NITROGEN (.775) (.541) (.782) (.517) (.752) FATS .202 ORG.MAT. DEPTH (.332) (.667) 2 PO4Z',%SAND DEPTH.,PO43" HYDROC,PO43" HYDROC,DEPTH HYDROC,NITROGEN DEPTH',NITROGEN (.843) (.830) 3 4 (.816) (.807) PO4~',NITROGEN,DEPTH HIDROC.,NITROG.,PO4=" (.869) (.843) PO43",NITROGENO,HYDROC.,DEPTH PO43",HYDROC.,% SAND,DEPTH (.872) 5 6 7 (.806) (790) PO~,HYDROC,DEPTH (.840) PO~,NITROGENO,MAT.ORG.,DEPTH (.858) HYDROC.,PO4Z',NITROGENO,% SAND,DEPTH (.846) (.850) HIDROC,,PO4~',NITROGENO,ORG.MAT.,DEPTH (.841) HYDROC.,PO43",NITROGEN,%SAND,WAT.CONT.,DEPTH (.833) HYDROC, FATS,PO+=', NITROGEN,% SAND, DEPTH (.827) HYDROC.,FATS,PO4a-, NITROGEN,%SAND,WAT.CONT., DEPTH (.814) 8 HYDROC.,FATS,PO43",NITROG.,ORGMAT.,%SAND,WAT.CONT.,DEPTH (.739) (b) For all environmental variables measured. k 1 2 Bestcombinations of variables (p,) NO=" (.832) DO (.808) DO, NITROG. (.945) PO4Z'(s) pH SAND FATS NITROG. DEPTH ORG.MAT. AGIT. (.775) (.708) (.541) (.202) (.752) (.667) (.332) (.306) NO2", NITROG. (.939) NOz',PO4Z'(s) (.901) pH, NITROG. NO2",Sand NO=',AGIT. DO, NO2" (.898) (.883) NO=', DO,NITROG. NO2-,PO43"(s),NITROG. DO,NITROG,DEPTH NO=-,PO4='(s),S A N D (957) (.951) (.939) (.937) (.877) (.851) NO=-,DEPTH,AGIT. (.935) 4 pH,NITROG.,po43-(s),DEPTH pH,NITROG.,DEPTH,AGIT, pH, PO4~(s),SAND, DEPTH (.958*) (.954) (.951) 5 pH, DO,NITROG.,DEPTH,AGIT. DO,NO=',NITROG.,DEPTH,AGIT. pH,DO,NITROG.,SAND,DEPTH (.960) (.960) (.956) 8 DO, NO2,,PO4Z'(s),NITROG.,DEPTH,AGIT. pH,DO,PO4a'(s),NITROG.,DEPTH,AGIT. (.987) (.966) 8 pH,DO,NO=', PO43"(w),NITROG.,PO43"(s),DEPTH,AGIT. (.959) 9 pH,DO,NO=', pO+3"(w),NITROG.,pO,3"(s),SAND, DEPTH,AGIT. (.953) 10 pH,DO,NO=',PO43"(w),pO43"(s),NITROG.,ORG.MAT.,SAND,DEPHT,AGIT. (927) and the external stations only show a generally moderate environmental improvement. Taking these together, a generalized internal-external gradient of conditions is evident determined mainly by physical factors such as substratum type, hydrodynamism, depth and by chemical factors related to the organic content of the surrounding water such as DO and nitrites and the sediments, for example total nitrogen and phosphate, these being the overriding chemical factors in the sediment. Overall, the results of all the analysis help to distinguish between the effects of the effluents within 791 Marine Pollution Bulletin an almost enclosed system such as the Saladillo Harbour and their effects in open coastal waters. Environmental implications Poorly treated urban effluents that discharge into areas with reduced hydrodynamism affect much larger areas, both with regard to water quality and sediment parameters (biotic and abiotic) than outfalls that discharge in hydrodynamically energetic zones, where their effects are much more rapidly dispersed. Needless to say, this type of situation should be avoided wherever possible. Although the ideal solution would be a total treatment of the effluent before discharge, a transitional solution may be the channelling of these effluents to areas of higher water movement (making maximal use of the breakwaters and other structures that accentuate these effects) and/or constructing open-water channels under the breakwater that would allow a greater degree of water movement and renewal within the harbour itself. It must be borne in mind, however, that these remedial actions are only suggestions. It may even be the case that (although spatially more constrained) a greater impact on the existing biota in the surrounding area may occur due to the increased external outfall load. In order to establish what the possible outcome(s) could be, specific studies would be required for each proposed option. We would like to thank D. E. Morfin and D. F. del Castillo for having undertaken the microbiological analyses, and Dr M. Conradi for her help in the identification of gammarid species. We would also like to thank Mr A. Menez for his help regarding EEC legislation, and to the Autoridad Portuaria de la Bahia de Algeciras by have provided the agitation data and by their contribution, together with CEPSA (Compafiia Espafiola de Petrrleos, S.A.), Fundacibn Sevillana de Electricidad, Excmo. Ayuntamiento de los Barrios y Mancomunidad de Municipios del Campo de Gibraltar, in the financial support of this work. Austen, M. C., Warwick, R. M. and Rosado, M. C. (1989) Meiobenthic and macrobenthic community structure along a putative pollution gradient in southern Portugal. Marine Pollution Bulletin 20, 398-405. Autoridad Portuaria de la Bahia de Algeciras (1993) Proyecto de D~trsena de embarcaciones menores en el Saladillo. Autoridad Portuaria de la Bahia de Algeciras, Algeciras (unpublished). Bellan, G. (1967a) Pollution et peuplements benthiques de substrat meuble dans la rrgion de Marseille. I-Le secteur de Cortiou. Revue Internationale d'Oceanographie Medicale 6/7, 53-87. Bellan, G. (1967b) Pollution et peuplements benthiques de substrat meuble dans la rrgion de Marseille. II-L'ensemble portuaire marsellais. Revue lnternationale d'Oceanographie Medicale 8, 51-95. Bellan, G. and Bourcier, M. (1984) Bilan 6cologique du d&ournement permanent d'un petit fleuve crtier dans l'rmissaire d'eaux usres d'une grande ville. Marine Environmental Research 12, 11-83. Bilyard, G. R. (1987) The value of benthic infauna in marine pollution monitoring studies. Marine Pollution Bulletin 18, 581-585. Bouyoucos, G. J. (1934) The hydrometer method for making mechanical analysis of soils. Soil Science 38, 335--343. Bray, J. R. and Curtis, J. T. (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecological Monographs 27, 325-349. Buchannan, J. D. and Kain, J. M. (1984) Measurement of the physical and chemical environment. In Methods for the Study of Marine 792 Benthos, eds N. L. Holme and A. D. Mclntyre, pp. 30-50. Blackwell Scientific Publications, Oxford. Carbalto, J. L., Naranjo, S. and Garcia-Gbmez, J. C. (1995) Use of marine spongies as stress indicators in marine ecosystem at Algeciras Bay (southern Iberian Peninsula). Marine Ecology Progress Series 135, 109-122. Clarke, K. R. and Ainsworth, M. (1993) A method of linking multivariate community structure to environmental variables. Marine Ecology Progress Series 92, 205-219. Clarke, K. R. and Warwick, R. M. (1994) Change in Marine Communities: an Approach to Statistical Analysis and Interpretation, pp. 144. Natural Environment Research Council, UK. Conradi, M. and Cervera, J. L. (1995) Variability in trophic dominance of Amphipod associated with the hidrozoa Bugula neritina (Linneo, 1758) in Algeciras Bay (Southern Iberia Peninsula). Polish Archives of Hydrobiology 42(4), 483-494. Dauvin, J. C. (1982) Impact of the Amoco Cadiz oil spill on the muddy fine sand Abra alba and Melinna palmata community from the Bay of Morlaix. Estuarine and Coastal Shelf Science 14, 517531. Driscoll, E. G. (1975) Sediment-animal-water interaction, Buzzards Bay, Massachusetts. Journal of Marine Research 33(3), 275-302. Fauchald, K. and Jumars, P. A. (1979) The diet of worms: a study of polycbaete feeding guilds. Oceanography and Marine Biology Annual Review 17, 193-284. Field, J. G. (1971) A numerical analysis of changes in the soft-bottom fauna along a transect across False Bay, South Africa. Journal of Experimental Marine Biology and Ecology 7, 215-253. Frankenberg, D. and Westerfield, C. W. Jr. (1968) Oxygen demand and oxygen depletion capacity of sediments from Wassaw Saound, Georgia. Bulletin of the Georgia Academy of Science 26, 160-172. Glemarec, M. (1969) Les peuplements benthiques du plateau continental Nord Gascogne. PhD Thesis, University of Paris. Grassle, J. F. and Grassle, J. P. (1974) Opportunistic life histories and genetic systems in marine benthic polychaetes. Journal of Marine Research 32, 253-284. Gray, J. S. (198 i) Detecting pollution induced changes in communities using the log-normal distribution of individuals among species. Marine Pollution Bulletin 12, 173-176. Gray, J. S., Aschan, M., Carr, M. R., Clarke, K. R., Green, R. H., Pearson, T. H., Rosenberg, R. and Warwick, R. M. (1988) Analysis of community attributes of the benthic macrofauna of Frierljord/ Langesundljord and in a mesocosm experiment. Marine Ecology Progress Series 46, 151-165. Gray, J. S. and Pearson, T. H. (1982) Objective selection of sensitive species indicative of pollution-induced change in benthic communities. I-Comparative methodology. Marine Ecology Progress Series 9, 111-119. Ibafiez, F. and Dauvin, J. C. (1988) Long-term changes (1977-1987) in a muddy fine sand Abra alba-Melinna palmata community from the Western English Channel: multivariate time-series analysis. Marine Ecology Progress Series 49, 65-81. Krfncke, I., Duineveld, G. C. A., Raak, S., Rachor, E. and Daan, R. (1992) Effects of a former discharge of drill cutting on the macrofauna community. Marine Ecology Progress Series 91, 277287. Lambshead, P. J. D., Platt, H. M. and Shaw, K. M. (1983) The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. Journal of Natural History 17, 859-874. Lrpez-Jamar, E. (1978) Primeros datos sobre la biomasa y la composicirn del bentos infaunal de la Ria de Pontevedra, en relacirn con el contenido en materia org~mica del sedimento. Boletin de lnstituto EspaJqol d'Oceanograph6 240(4), 57-65. Lrpez-Jamar, E. and Cal, R. M. (1990) El sistema bentrnico de la zona submareal de la ria de Vlgo. Macroinfauna y microbiologia del sedimento. Boletin de Instituto Espa~qol d'Oceanograph6 6(2), 49-60. Lrpez-Jamar, E. and Mejuto, J. (1988) Infaunal benthic recolonization after dredging operations in La Corufia Bay, NW Spain. Cahiers de Biologie Marine 29, 37-49. McCall, P. L. (1977) Community patterns and adaptative strategies of the infaunal benthos of Long Island Sound. Journal of Marine Research 35(2), 221-265. McManus, J. W. and Pauly, D. (1990) Measuring ecological stress: variations on a theme by RM Warwick. Marine Biology 106, 305-308. Volume 34/Number 10/October 1997 Parker, J. G. (1982) Effects of pollutants upon the benthic ecology of Belfast Lough. Analytical Proceedings, 429-432. Pearson, T. H. (1975) The benthic ecology of Loeb Linnbe and Loch Eil, a sea-Itch system on the west coast of Scotland. IV. Changes in the benthic fauna attributable to organic enrichment. Journal of Experimental Marine Biology and Ecology 20, 1-41. Pearson, T. H. and Rosenberg, R. (1976) A comparative study of the effects on the marine environment of wastes from cellulose industries in Scotland and Sweden. Ambio 5, 77-79. Pearson, T. H. and Rosenberg, R. (1978) Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology Annual Review 16, 229-311. Pielou, E. C. (1966) The measurement of diversity in different types of biological collections. Journal of Theoretical Biology 13, 131-144. Reish, D. J. (1959) An ecological study of pollution in Los AngelesLong Beach harbors, California. Allan Hancock Foundation Publication Occasional 22, 119. Reish, D. J. (1971) Effect of pollution abatement in Los Angeles Harbours. Marine Pollution Bulletin 2, 71-74. Reish, D. J. (1980) Effect of domestic wastes on the benthic marine communities of southern California. Helgoldnder Meeresunterschun. gen 38, 377-383. Reish, D. J. (1986) Benthic invertebrates as indicators of marine pollution: 35 years of study. Proceed IEEE Oceans '86 Conference, pp. 885-888. Washington. Rhoads, D. C. and Young, D. K. (1970) The influence of depositfeeding organisms on sediment stability and community trophic structure. Journal of Marine Research 28, 150-178. Ritz, D. A., Lewis, M. E. and Shen, M. (1989) Response to organic enrichment of infaunal macrobenthic communities under salmonid seacages. Marine Biology 103, 211-214. Romesburg, C. H. (1984) Cluster Analysis for Researchers. Wadsworth, Belmont. Shannon, C. E. and Weaver, W. (1963) The Matematical Theory of Communication, pp. 117. University of Illinois Press, Urbana, Illinois. Simboura, N., Zenetos, A., Panayotidis, P. and Makra, A. (1995) Changes in benthic community structure along an environmental pollution gradient. Marine Pollution Bulletin 30(7), 470-474. Sola, J. C. and Ibafiez, M. (1986) Estudio de la fauna de antlidos poliquetos de los fondos blandos del estuario del Bidasoa. Larralde Investigaci6n y Espacio 9, 165-181. Tenore, K. R. (1972) Maerobenthos of the Pamlico River Estuary, North Carolina. Ecological Monographs 42, 51-69. Tenore, K. R., Horton, D. B. and Duke, T. W. (1968) Effects of bottom substrate on the brackish water bivalve Rangia caneata. Chesapeake Science 9, 238-248. Tenore, K. R., Cal, R. M., Hanson, R. B., L6pez-Jamar, E., Santiago, G. and Tietgen, J. H. (1984) Coastal upwelling off the Rias Bajas, Galicia, Nortwest Spain. II Benthic studies. Rhunion Conseil Internationale Exploration Met 183, 91-100. Theede, H., Ponat, A., Hiroki, K. and Sehlieper, C. (1969) Studies on the resistance of marine bottom invertebrates to oxygen-deficiency and hydrogen sulphide. Marine Biology 2(4), 325-337. Thiei, H. (1978) Benthos in upwelling regions. In Upwelling Ecosystems, eds R. Boje and M. Tomczak, pp. 124-138. Springer, Berlin. Warren, L. M. (1976) A population study of the polychaete Capitella capitata at Plymouth. Marine Biology 180, 209-216. Warwick, R. M. (1986) A new method for detecting pollution effects on marine macrobenthic communities. Marine Biology 92, 557-562. Warwick, R. M. (1993) Environmental impact studies on marine communities: pragmatical considerations. Australian Journal of Ecology 18, 63--80. Warwick, R. M. and Clarke, K. R. (1993) Comparing the severity of disturbance: a meta-analysis of marine macrobenthic community data. Marine Ecology Progress Series 92, 221-231. Warwick, R. M., Pearson, T. H. and Ruswahyuni (1987) Detection of pollution effects on marine macrobenthos: further evaluation of the species abundanceflaiomass method. Marine Biology 95, 193-200. Warwick, R. M., Platt, H. M., Clarke, K. R., Agard, J. and Gobin, J. (1990) Analysis of macrobenthic and meiobenthic community structure in relation to pollution and disturbance in Hamilton Harbour, Bermuda. Journal of Experimental Marine Biology and Ecology 138, 119-142. 793