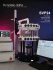integrated dual-layer system
Transcription
integrated dual-layer system
INTEGRATED DUAL-LAYER SYSTEM For Optimal Performance and Ease of Use · Easily Trackable with Partial Retrievability · Excellent Visualization · Optimal Stability and Wall Apposition Used with Used with Introducing the FRED™ System with inte 16-wire outer stent / 48-wire inner stent Braided self-expanding nickel titanium, paired-stent design. Enables simultaneous, dynamic push/ pull dual-stent deployment with single operator ease of use. Easy stent opening and superb vessel apposition, with partial resheathability. Four (4) atraumatic flared ends provide superb anchoring, vessel apposition and expansive conformability, to assist device placement stability and accuracy. Radiopaque distal wire tip, proximal and distal markers and interweaved working length helical strands. Provides full-stent length fluoroscopic visualization for good stent placement accuracy. Image courtesy of Drs. Civan Islak/Naci Kocer Carrahpaspa Medical University Hospital Istanbul, Turkey Caution: The FRED™ System is an investigational device limited by U.S. law to investigational use. egrated dual-layer stent for flow re-direction FRED™ System: Flow Re-direction Endoluminal Device An Integrated Dual-Layer System for Optimal Performance and Ease of Use The FRED System is a unique integrated dual-layer design that can be simultaneously deployed or partially retrieved by a single operator. Its self-expanding closed cell design improves ease of use while radiopaque end markers and helical strands assist device placement accuracy. • 3.5, 4.0, 4.5, 5.0 and 5.5mm fully expanded stent diameters in various lengths to target 3.0mm to 5.5mm parent vessels • Aneurysm neck coverage is provided by the FRED System’s proven 48-strand flow diversion inner stent layer plus the supporting 16-strand outer stent layer • Simple push-pull deployment method enables physicians the ability to better control and adjust the metal surface coverage across the aneurysm neck Integrated dual-layer coverage. 16-wire outer stent with 48-wire inner stent for enhanced radial force. Inner stent provides high percentage metal surface area for flow re-direction. • A 48-wire inner stent layer 22% to 44% metal surface area plus a 16-wire outer stent layer combine for a significant reduction of blood flow into the aneurysm while providing a stable scaffold with excellent vessel apposition. FRED™ System Flow Re-Direction Endoluminal Device One unit per box Product Code Fully Open Implant Diameter * (mm) Total Length / Working Length (mm) Fully Open Total Length / Working Length (mm) 3.0mm OD 3.5mm OD FRED3505 3.5 16 / 10 13 / 7 FRED3525 3.5 30 / 24 22 / 16 3.5mm OD 4.0mm OD FRED4015 4.0 24 / 18 18 / 12 FRED4025 4.0 FRED4515 4.5 FRED4525 4.5 31 / 26 23 / 17 4.0mm OD 4.5mm OD 26 / 19 20 / 13 34 / 27 25 / 18 4.5mm OD 5.0mm OD FRED5015 5.0 26 / 20 21 /14 FRED5025 5.0 35 / 28 26 / 20 5.0mm OD 5.5mm OD FRED5515 5.5 28 / 22 22 / 15 FRED5535 5.5 46 / 38 32 / 25 Notes: Compatible Microcatheter All Models: Headway® 27 (inner diameter = 0.027 inch or 0.69mm) * Fully Expanded (Max) OD with no constraints MicroVention, Inc. Worldwide Headquarters microvention.com 1311 Valencia Avenue Tustin, CA 92780 USA PH+1.714.247.8000 PH 1.800.990.8368 MicroVention UK Limited MicroVention Europe, S.A.R.L. MicroVention Deutschland GmbH Suite 3, The Barracks Building 10 Cliffords Fort, North Shields Tyne and Wear, NE30 1JE UK PH +44 (0) 191 258 6777 F +44 (0) 191 258 5999 30 bis, rue du Vieil Abreuvoir 78100 Saint-Germain-en-Laye France PH +33 (1) 39 21 77 46 F +33 (1) 39 21 16 01 Berliner Allee 61D-40212 Dusseldorf Germany PH +49 211 210 798-0 F +49 211 210 798-29 MICROVENTION and Headway are registered trademarks of MicroVention, Inc. FRED is a trademark of MicroVention, Inc. • Scientific and clinical data related to this document are on file at MicroVention, Inc. Refer to Instructions for Use for additional information. © 2013 MicroVention, Inc. MM281(I) 1/13
Similar documents
Stent Grafts Market Analysis, Market Size, Competitive Trends: Radiant Insights, Inc
 This market is expected to post a steady market growth rate of more than 6% by 2019 owing to the growing incidence of aortic aneurysm among the aging population.
Read More @ http://www.radiantinsights.com/research/global-stent-grafts-market-2015-2019
This market is expected to post a steady market growth rate of more than 6% by 2019 owing to the growing incidence of aortic aneurysm among the aging population.
Read More @ http://www.radiantinsights.com/research/global-stent-grafts-market-2015-2019