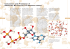Brain Research Phosphorylation
Transcription
Brain Research Phosphorylation
Brain Research 778 Ž1997. 107–119 Research report Phosphorylation modulates the activity of the ATP-sensitive Kq channel in the ventromedial hypothalamic nucleus V.H. Routh a a, ) , J.J. McArdle a , B.E. Levin b,c Departments of Pharmacology and Physiology, UniÕersity of Medicine and Dentistry of New Jersey (UMDNJ), 185 S. Orange AÕenue, Newark, NJ 07104, USA b Department of Neurosciences, UniÕersity of Medicine and Dentistry of New Jersey (UMDNJ), Newark, NJ 07104, USA c Neurology SerÕice (127C), Departments of Veterans Affairs Medical Center, E. Orange, NJ 07018, USA Accepted 12 August 1997 Abstract Regulation of the ATP-sensitive Kq ŽK-ATP. channel was examined in cell-attached and inside-out membrane patches of freshly isolated neurons from the ventromedial hypothalamic nucleus ŽVMN. of 7–14 day old male Sprague–Dawley rats. When inside-out patches were exposed to symmetrical Kq, the reversal potential was y2.85 " 1.65 mV, the single channel conductance 46 pS, and the total conductance varied as a multiple of this value. Glucose Ž10 mM. reversibly inhibited channel activity in cell-attached preparations by 81%. In the presence of 0.1 mM ADP, 10, 5, and 1 mM ATP reversibly inhibited VMN K-ATP channels in inside-out patches by 88, 83, and 60%, respectively. This inhibition was not dependent on phosphorylation since 5 mM AMPPNP, the non-hydrolyzable analog of ATP, reversibly inhibited channel activity by 67%. Relatively high concentrations of glibenclamide Ž100 mM. also reversibly inhibited VMN K-ATP channel activity in cell attached and inside-out patches by 67 and 79%, respectively. Finally, the non-specific kinase inhibitor H7 Ž200 mM. decreased channel activity by 53% while the non-specific phosphatase inhibitor microcystin Ž250 nM. increased channel activity by 218%. These data suggest that while the inhibitory effect of ATP is not phosphorylation dependent, phosphorylation state is an important regulator of the VMN K-ATP channel. q 1997 Elsevier Science B.V. Keywords: ATP-sensitive Kq channel; Ventromedial hypothalamic nucleus; Phosphorylation; Glibenclamide; AMPPNP; Metabolism; Glucose 1. Introduction The ventromedial hypothalamic nucleus ŽVMN. is a key site for monitoring glucose status and initiating a sympathoadrenal response. This control involves glucosensing neurons which increase their firing rate in the presence of glucose w25x. Furthermore, glucose infusions into the forebrain stimulate these neurons w7x and increase sympathetic activity w16x. Although the mechanism by which the VMN actually senses glucose is unknown, it is likely to be similar to that of the pancreatic b-cell where ATP inhibits a Kq channel ŽK-ATP.. When this channel closes, the cell depolarizes and causes voltage sensitive Ca2q channels to open. The resulting Ca2q influx ultimately causes insulin secretion w2x. This K-ATP channel is the target for the anti-diabetic sulfonylurea drugs w1x. ) Corresponding author. Fax: q1 Ž973. 972-4554; E-mail: [email protected] 0006-8993r97r$17.00 q 1997 Elsevier Science B.V. All rights reserved. PII S 0 0 0 6 - 8 9 9 3 Ž 9 7 . 0 1 0 4 3 - 3 K-ATP channels are found in many tissues, including brain w33x. Regulation of the activity of the K-ATP channel in the periphery is very complex. For example, ATP is known to exert a dual effect on the K-ATP channel; that is, low levels of ATP are necessary to maintain channel activity w34x, while higher levels are inhibitory w1x. Nucleoside diphosphates are also important regulators of K-ATP channel activity w34x. Furthermore, protein kinases and phosphatases have opposing effects on K-ATP channel activity in a variety of peripheral tissues w5,15,21,22x. Interestingly, the K-ATP channel described in the VMN differs from that in the periphery. For example, it has a reduced sensitivity to ATP w35x, a higher single channel conductance w3x, and an insensitivity to sulfonylureas in excised patches w4x. The present study was designed to further investigate the regulation of this VMN K-ATP channel. Our results are consistent with previous studies indicating that the VMN K-ATP channel is less sensitive to ATP than the peripheral K-ATP channel. However, we found that single channel conductance may be similar to 108 V.H. Routh et al.r Brain Research 778 (1997) 107–119 Punches were subjected to gentle trituration using firepolished Pasteur pipettes in order to isolate cells which were then incubated in glucose free extracellular buffer for 30 min. This lack of glucose maximizes the possibility of channel opening w3x. 2.2. Recording preparations Fig. 1. Current–voltage relations for the K-ATP channel in an inside-out patch of an isolated VMN neuron. The reversal potential for the VMN K-ATP channel in symmetrical Kq was y2.85"1.65 mV Ž ns9. and the single channel conductance was 46 pS Ž ns 7. at a holding potential of 20 mV. Single channel currents were studied in inside-out and cell-attached membrane patches using patch clamp techniques w8x. Patch pipettes were drawn from 1.5 mm thick walled borosilicate glass capillaries, coated with M-coat D ŽMeasurements Group Inc., Raleigh, NC., and heat polished ŽMF-83, Narishige, Japan.. The patch pipettes had resistances of 10–20 M V when filled with pipette solution. This solution contained ŽmM.: 140 KCl, 1.1 MgCl 2 , 2.6 CaCl 2 , and 10 HEPES ŽpH 7.4.. The seals were approximately 10 GV in resistance. The holding potential was 0 and 20 mV for cell-attached and inside-out patches, respectively. All experiments were carried out at room temperature Ž21–258C.. 2.3. Data collection and analysis that found in the periphery and that high concentrations of glibenclamide inhibit the activity of the VMN K-ATP channel. Additionally, we present a model suggesting how phosphorylation modulates the activity of the VMN K-ATP channel as well as its sensitivity to ATP. 2. Materials and methods 2.1. Cell isolation Brains from 8–18 day old Sprague–Dawley rats were dissected and placed in ice-cold oxygenated Ž95% O 2r5% CO 2 . isolation buffer. This buffer contained ŽmM.: 128 NaCl, 5 KCl, 1.2 NaH 2 PO4 , 2.4 CaCl 2 , 1.3 MgCl 2 , 26 NaHCO 3 , and 10 D-glucose ŽpH 7.4.. Brains were sliced into 400 mm sections on a vibratome. Slices were then enzymatically digested at room temperature for 20 min in pronase Ž1 mgr6 ml oxygenated isolation buffer; Calbiochem, San Diego, CA. followed by 20 min in thermolysine Ž1 mgr6 ml oxygenated isolation buffer; Calbiochem., and then allowed to recover for 30 min in enzyme free oxygenated isolation buffer. The VMN was punched with a 500 mm blunt needle and placed in a small culture dish in extracellular buffer. This buffer contained ŽmM.: 135 NaCl, 5 KCl, 1 CaCl 2 , 1 MgCl 2 , and 10 HEPES ŽpH 7.4.. Recordings were made with an Axopatch 200A amplifier ŽAxon Instruments, Foster City, CA.. Single channel data were stored on video tape using a pulse code modulator ŽMedical Systems Corp., Greenvale, NY. and subsequently analyzed via pClamp software ŽAxon Instruments.. Data were filtered at 2 kHz and digitized at 10 kHz. Due to the fluctuations in channel open probability Ž Po ., channel activity was quantified as the mean current Ž I . passed through the K-ATP channel for 60 s segments of data; I s NPori, where N is the number of channels and i is the single channel conductance. Following a specified experimental protocol the data were expressed as the percent of the peak response relative to the last recorded baseline Žcontrol. value and analyzed using a 1 sample t-test ŽJMP software, SAS Institute, Cary, NC.. Reversal potential for inside-out preparations was determined from current–voltage plots derived from voltage ramps that ranged from y50 to 50 mV. Both cell-attached and inside-out patches were allowed to stabilize for at least 5 min prior to data collection. Patches from cell-attached preparations were exposed to glucose or glibenclamide for approximately 4–8 min before recording data and were allowed to recover during washout for up to 10 min. Inside-out patches were exposed to the various treatments for 2–8 min and allowed to recover for a similar time. Fig. 2. The K-ATP channel in an inside-out preparation from an isolated VMN neuron has multiple subconductance states. This figure presents consecutive records taken from the same inside-out patch. Conductance states are designated as integers from 1–5 at the right of each record. A: The VMN K-ATP channel in control solution exhibits single channel conductances that are 2 to 5 times the magnitude of the 46 pS subconductance that is common to all recordings of the VMN K-ATP channel. B: Although higher amplitude conductances appear occasionally when the K-ATP channel is exposed to 10 mM ATPr0r1 mM ADP Župper record., it is the 46 pS subconductance that predominates under these conditions. V.H. Routh et al.r Brain Research 778 (1997) 107–119 109 110 V.H. Routh et al.r Brain Research 778 (1997) 107–119 2.4. Drugs For inside-out patch preparations the control solution contained 0.1 mM ATP and 0.1 mM ADP ŽKq salts, vanadium free; Sigma Chemical Co., St. Louis, MO. in the intracellular solution. This solution contained ŽmM.: 100 KCl, 40 KOH, 10 EDTA, and 10 HEPES ŽpH 7.2.. ATP, ADP, and AMPPNP Žadenyl-5X-yl imidodiphosphate; Sigma. were added to the intracellular solution within 1 h of use. Glibenclamide Ž10 mM stock; Smith Klein Beecham, King of Prussia, PA. was dissolved first in DMSO followed by an equal volume of ethanol. The stock solution was stored at 58C and used within 2 weeks. Microcystin Ž25 mM stock; Bethesda Research Lab. ŽBRL., Bethesda, MD. was dissolved in 10% DMSO. H7 Ž20 mM stock, BRL. was dissolved in water. Solutions were applied by gravity feed systems at a rate of approximately 1 ml miny1 . 3. Results 3.1. VMN K-ATP channel characterization The current–voltage relations of Fig. 1 demonstrate that the reversal potential for the K-ATP channel in inside-out patches from isolated VMN neurons in symmetrical Kq was y2.85 mV. The channel did not rectify in the Mg 2q free solution used in this study. Single channel conductance was 46 pS for single channel openings at a holding potential of 20 mV. This 46 pS conductance may represent a subconductance state since larger amplitude ATP sensitive conductances were observed in the same patches. However, while these larger conductances were multiples of 46 pS, they varied in total magnitude Žfrom 2–5 times the single channel conductance. from patch to patch, making it difficult to determine the total conductance of a single channel. This is illustrated in Fig. 2 which shows records of the K-ATP channel in a single inside out patch from an isolated VMN neuron that was exposed first to control solution ŽFig. 2A. and then to 10 mM ATPr0.1 mM ADP ŽFig. 2B.. There is a small current of slightly less than 1 pA shown in the lower record in Fig. 2B. According to Ohms law, a conductance of 46 pS produces a current of 0.92 pA at a holding potential of 20 mV. The records in Fig. 2A show discrete single channel conductances that range from 2–5 times this amplitude. When the channel is closed by ATP the lower amplitude conductances predominate ŽFig. 2B.. However, as seen in the upper record in Fig. 2B, higher amplitude conductances appear occasionally. Glucose Ž10 mM. reversibly inhibited the mean current passed through the VMN K-ATP channel by 81% in cell-attached preparations ŽTable 1.. The baseline Žcontrol. mean current and the time required for mean current to be affected by 10 mM glucose varied from patch to patch. Since this was also the case for all of the other treatments, the data in Table 1 were quantified as the peak response compared to the last recorded stable baseline Žcontrol. value. A decrease in mean current in response to 10 mM glucose was detectable within 3 to 5 min in most neurons and achieved a new stable level within 5–8 min. Recovery occurred between 5 and 10 min following washout of glucose. The records of Fig. 3A–C depict this effect for a single cell-attached preparation from an isolated VMN neuron. This figure demonstrates that the single channel current amplitude declined following exposure to glucose for all cell-attached preparations. This has been attributed to the decrease in the driving force for potassium between the cell and the patch pipette which is caused by the depolarisation resulting from the closure of a large number of K-ATP channels w3x. The VMN K-ATP channel was subject to rapid rundown following excision of an inside-out patch. Reducing Table 1 Summary of the effects of metabolic and pharmacologic agents on the mean current passed by the K-ATP channel in isolated VMN neurons Treatment Cell attached 10 mM Glucose 100 mM Glibenclamide Inside-out patch 10 mM ATPr0.1 mM ADP 5 mM ATPr0.1 mM ADP 1 mM ATPr0.1 mM ADP 5 mM AMPPNPr0.1 mM ADP 100 mM Glibenclamide 200 mM H7 250 nM Microcystin n Percent change from control p value Mean " S.E.M. Range 6 6 x81 " 5 x67 " 12 64–98 37–90 20 7 5 4 3 7 6 x88 " 3 x83 " 4 x60 " 18 67 " 12 x79 " 4 x53 " 8 ≠218 " 54 57–100 56–95 0–100 34–87 72–84 26–78 83–411 0.00003 0.0114 - 0.00001 0.00005 0.028 0.006 0.0021 0.0008 0.0099 The data were calculated as mean current for 60 s segments of data. Mean current was then expressed as the percent of the peak response relative to the last stable baseline Žcontrol. value. This percent change from control was analyzed using a 1 sample test. V.H. Routh et al.r Brain Research 778 (1997) 107–119 111 Fig. 3. The K-ATP channel in a cell-attached preparation from an isolated VMN neuron is closed by glucose and glibenclamide. This figure presents consecutive records from the same cell-attached patch preparation. A: the channel fluctuates and is primarily in the open state in the absence of glucose Žcontrol solution.; B: the channel is primarily in the closed state and the current being passed through the channel is very low following 5 min exposure to 10 mM glucose; C: channel activity and the mean current passed by the channel recover to control levels 7 min following washout of glucose; D: channel activity and mean current are reduced 4 min after 100 mM glibenclamide was added to the control solution; E: this response is reversed 5 min after washout of glibenclamide. 112 V.H. Routh et al.r Brain Research 778 (1997) 107–119 Fig. 4. The K-ATP channel in an inside-out patch from an isolated VMN neuron is closed by ATP. This figure presents consecutive records from the same inside-out patch preparation. A: the channel is primarily open in the control solution of 0.1 mM ATPr0.1 mM ADP; B: channel activity and mean current are reduced by approximately 50% after 5 min in 1 mM ATPr0.1 mM ADP; C: the response to ATP is reversed after 3 min in control solution; D: channel activity and the mean current passed by the channel are virtually abolished after 4 min in 10 mM ATPr0.1 mM ADP. V.H. Routh et al.r Brain Research 778 (1997) 107–119 the Mg 2q concentration to very low levels w13x and inclusion of 0.1 mM ATP with 0.1 mM ADP in the control internal solution ŽF.M. Ashcroft, personal communication. 113 prevented rundown. ATP in the presence of 0.1 mM ADP reversibly inhibited the VMN K-ATP channel ŽTable 1.; 1 mM, 5 mM, and 10 mM ATP decreased mean current by Fig. 5. The inhibitory effect of ATP on the K-ATP channel in an inside-out patch from an isolated VMN neuron is independent of phosphorylation. This figure presents consecutive records from the same inside-out patch preparation. A: the channel is primarily open in the control solution of 0.1 mM ATPr0.1 mM ADP; B: channel activity is reduced and mean current decreases to 40% of control values after 1 min in 5 mM AMPPNPr0.1 mM ADP; C: Channel activity and mean current recover completely after 2 min in control solution; D: channel activity and mean current are reduced to 15% of control values following 3 min exposure to 5 mM ATPr0.1 mM ADP; E: channel activity and mean current recover to 150% of control after a 1 min washout. 114 V.H. Routh et al.r Brain Research 778 (1997) 107–119 60%, 83%, and 88%, respectively. In most cases, the response to ATP appeared within the first minute and became stable by approximately 3 min. Occasionally, mean current continued to decrease until the ATP concentration was lowered Ž5–6 min. to control levels. Similarly, mean current increased within the first minute after return to control solution and became stable 1–4 min later. The records of Fig. 4 illustrate the effect of ATP on the K-ATP channel in a single inside-out patch from an isolated VMN neuron. In this case, 1 mM ATP reduced mean current by approximately 50% ŽFig. 4B. and 10 mM ATP completely abolished it ŽFig. 4D.. Finally, 5 mM AMPPNP, the non-hydrolyzable analog of ATP, inhibited channel activity by 67% ŽTable 1.. This effect followed the same time-course as ATP. Fig. 5 depicts this effect for a single inside out patch. Fig. 6. The K-ATP channel in an inside-out patch of an isolated VMN neuron is closed by glibenclamide. This figure presents consecutive records from the same inside-out patch preparation. A: the channel is open in control solution; B: channel activity and mean current are virtually abolished 3 min after 100 mM glibenclamide is added to the control solution; C: channel activity and mean current are greater than control values after 2 min wash in the control solution. V.H. Routh et al.r Brain Research 778 (1997) 107–119 Although these data indicate that half maximal inhibition should occur below 1 mM ATP, it is important to consider the variability in the response at that concentration ŽTable 1: range s 0–100%.. While the K-ATP channel in 1 out of the 5 inside-out patches was completely shut by 1 mM ATP, it immediately reopened at a level 50% of control and remained at that level during continued exposure to 1 mM ATP. Moreover, of the other 4 inside-out patches containing K-ATP channels, 1 was unaffected, 2 almost completely shut but also immediately re-opened to control values in the presence of 1 mM ATP, and mean current decreased by 44% in the last. Thus, 1 mM ATP in the presence of 0.1 mM ADP may actually inhibit the mean current passed through the VMN K-ATP channel slightly less than the 60% average indicated in Table 1. 3.2. Effects of sulfonylureas on cell-attached and inside-out patch preparations Within 1–5 min glibenclamide Ž100 mM. reversibly decreased the mean current of the VMN K-ATP channel by 67 and 79% in cell-attached and inside-out patches, respectively ŽTable 1.. After wash in the control solution for 1–5 min mean current recovered fully. This effect is depicted for the K-ATP channel in a single cell-attached 115 patch in Fig. 3C–E and for a single inside-out patch in Fig. 6A–C. Control experiments revealed that vehicle had no effect on channel activity. 3.3. Effects of phosphorylation on inside-out patch preparations The non-specific phosphatase inhibitor microcystin Ž250 nM. increased mean current by 218% ŽTable 1.. In all cases the response to microcystin appeared within the first minute and caused a new stable level of activity by 2 min. Recovery following washout was variable. The records in Fig. 7 show that the mean current passed through the K-ATP channel in a single inside-out patch was approximately doubled when phosphatase activity was inhibited by microcystin. In contrast, the non-specific kinase inhibitor H7 Ž200 mM. decreased mean current by 53% ŽTable 1.. In general, a response to H7 appeared within the first minute and a new stable level of activity was achieved within 2 min. Although mean current was increased within the first minute after washout of H7, full recovery to control levels generally required 4 to 10 min. However, some K-ATP channels responded more slowly as depicted in Fig. 8A–C for a single inside-out patch where 7 minutes were required for the full effect of H7 Ž8B.. In this case, recovery required 10 min of wash in control solution ŽFig. Fig. 7. The K-ATP channel in an inside-out patch from an isolated VMN neuron is opened by persistent phosphorylation due to inhibiting dephosphorylation. This figure presents consecutive records from the same inside-out patch preparation. A: channel activity and mean current are relatively high in control solution; B: channel activity and mean current have more than doubled 5 min after the non-specific phosphatase inhibitor, microcystin Ž250 nM., was added to the control solution. 116 V.H. Routh et al.r Brain Research 778 (1997) 107–119 Fig. 8. The K-ATP channel in an inside-out patch from an isolated VMN neuron is closed by inhibiting phosphorylation. This figure presents consecutive records from the same inside-out patch preparation. A: the channel is open in control solution; B: channel activity and mean current are reduced 7 min after the non-specific kinase inhibitor H7 Ž200 mM. was added to the control solution; C: channel activity and mean current have recovered 10 min after washout to control solution, although not to the levels of the initial control. 8C.. Control experiments revealed that vehicle had no effect on channel activity. 4. Discussion The reversal potential of approximately 0 mV in symmetrical Kq and the inhibitory response to glucose as well as ATP served to identify the K-ATP channel of VMN neurons. The fact that the VMN K-ATP channel did not inwardly rectify in our Mg 2q free control solution is not surprising since inward rectification is considered to be Mg 2q-dependent w34x. In agreement with earlier reports w3,35x, we found that the neuronal VMN K-ATP channel was much less sensitive to ATP ŽmM vs. mM. than the V.H. Routh et al.r Brain Research 778 (1997) 107–119 peripheral K-ATP channel and that the non-hydrolyzable ATP analog AMPPNP inhibited it. The single channel conductance of the VMN K-ATP channel which we studied was 46 pS. This is significantly lower than the 160 pS conductance reported previously for this tissue w3x, and in fact is much more similar to that found in peripheral tissues w1x. While there may be different subtypes of K-ATP channel in the VMN it is more likely that the 46 pS conductance is a minimal subconductance state, with much larger conductances arising as multiples of this value. Since the data from earlier studies w3x was filtered at a lower frequency Ž1 kHz. than ours Ž2 kHz., it would have been more difficult to observe this smaller conductance. Thus, it may be that a subconductance existed in these studies, but filtering prevented its detection. This would be compounded by any decrease in the signal to noise ratio. Also, it is possible that the recording conditions used by these investigators favored one specific multiple of the subconductance. Krouse et al. w14x described a similar phenomenon for a large anion selective channel in mouse alveolar cells which has six open states that are multiples of a smaller 60–70 pS conductance. They suggested that this is one channel with six ‘co-channels’ in parallel that share a common gating mechanism capable of opening or closing one or all of them simultaneously. As discussed below, co-channels may also mediate the conductance of the K-ATP channel of VMN neurons. While it has been reported that the VMN K-ATP channel in cell-attached preparations is insensitive to low doses Ž500 nM. of glibenclamide w4x, we found that high doses of glibenclamide Ž100 mM. reversibly inhibited channel activity by 67%. This suggests that sulfonylureas act at a low affinity receptor on VMN neurons. In fact, both high ŽSUR1. and low ŽSUR2. affinity sulfonylurea receptors have been cloned from rat brain w11,32x. Binding studies show that 15–20% of sulfonylurea receptors in the VMN 117 are low affinity and these are completely abolished when neuronal cell bodies are selectively destroyed with ibotenic acid w6x. This lesion has no effect on the remaining high affinity sites which are probably on GABA or glutamate containing nerve terminals. Interestingly, SUR2 sites are virtually non-existent in the VMN of a rodent model of dietary obesity w20x, suggesting that the VMN K-ATP channel may play a role in energy balance. Additionally, we found that 100 mM glibenclamide reversibly inhibited VMN K-ATP channel activity in inside-out patches, while others did not w4x. Since the inside-out patch contains only the excised membrane, the sulfonylurea binding site appears to be very closely associated with the channel protein, as suggested previously w32x. It is unclear why our data differ from those of previous studies. In order for the VMN K-ATP channel to serve as a neuronal glucosensor two conditions must be met. First, the channel must respond to a physiological ATPrADP ratio. Second, the concentration of ATP monitored by the K-ATP channel must vary with peripheral metabolism. In this study, a 10:1 ATP to ADP ratio inhibited the channel by approximately 50%. Although intraneuronal levels of ATP and ADP are not known in the VMN, this ratio is similar to that found in cortical neurons w26x. Thus, the VMN K-ATP channel responds to alterations in the ATP to ADP ratio found in the brain. The second condition is more difficult to evaluate, since changes in intracellular ATP are not easily measured. Moreover, cytosolic ATP levels are believed to be very highly buffered. However, it is conceivable that the local concentration of ATP around the channel, rather than the bulk cytosolic concentration, is the critical regulatory factor w10x. There is evidence to suggest that ATP may be compartmentalized within the cell w9x allowing intracellular ATP levels to regulate the VMN K-ATP channel w37x. This makes the channel a viable candidate as a neuronal glucosensing mechanism. Given the role of the VMN in autonomic activity and the Fig. 9. Model for ATP regulation of the VMN K-ATP channel. K AT P indicates the channel in a non-phosphorylated and non-conducting state. K ATP P results from the action of a cytoplasmic kinase ŽKNase-1.; the effect of KNase-1 vanishes when the patch is excised from the cell membrane. PHase-1 is a membrane bound and Mg 2q-dependent phosphatase which converts K AT P P to K ATP . KNase-2 is a membrane bound kinase which phosphorylates K ATP P to produce K AT P PP; PHase-2 is a phosphatase which reverses this process. Both K ATP P and K ATP PP can open to various subconductance states which are determined by co-channels activation. These states are indicated by the letter suffix appended to OPEN. There is a corresponding OPEN state for K AT P P and K AT P PP; i.e., OPENa – n and OPENaX – z . These OPEN states can be interconverted by either KNases 1 and 2 as well as PHases 1 and 2 or other Ž?. similar enzymes. Because of the extra phosphate group, opening of co-channels is energetically favored for K AT P PP as compared to K ATP P. At the same time, the affinity of open forms of K AT P PP for ATP is greater. Thus, our model predicts that while the mean current should be greater for K ATP PP, this current will also be more sensitive to block by ATP. This form of feedback would provide for fine control of K-ATP channel activity. 118 V.H. Routh et al.r Brain Research 778 (1997) 107–119 regulation of energy balance w7x, this channel could serve as a link with glucose metabolism. ATP exerts a dual effect on the K-ATP channel. In the periphery, although ATP inhibits channel activity w12x, its presence is also required for the maintenance of channel activity w34x. Our data and that of others w3x showing that the non-hydrolyzable analog of ATP, AMPPNP, mimics the inhibitory effect of ATP on the VMN K-ATP channel indicate that this inhibitory effect is not dependent on phosphorylation. In contrast, the facilitating effect of ATP is phosphorylation dependent w34x. In the periphery protein kinases increase K-ATP channel activity w21,36x, while protein phosphatases decrease channel activity w15,21x. This is consistent with our data showing that inhibiting phosphorylation decreased the activity of the VMN K-ATP channel while inhibiting dephosphorylation increased activity. This may also explain the non-stationarity of the VMN K-ATP channel w31x, as it does for other channels w28x. Since our data were obtained using excised patches, the regulatory kinases and phosphatases blocked in our studies appear to be closely associated with the channel protein andror the SUR2, both of which possess a number of possible phosphorylation sites w11,22x. Based on these phosphorylation data, we propose a simple model for the regulation of the VMN K-ATP channel ŽFig. 9. in which opposing actions of phosphatase and kinase enzymes set a ‘steady state’ level of activity. Phosphatase decreases phosphorylation and moves the channel toward an inactive closed state. Kinase phosphorylates the channel, placing it in a more active state. When one enzyme is blocked, activity of the opposing enzyme predominates. While our data do not allow determination of the type and number of kinases and phosphatases involved, they do suggest that at least two sets of phosphatases and kinases are involved. When the channel is excised into Mg 2q containing solution rundown occurs w34x. This suggests the presence of a cytosolic kinase ŽKNase-1. counteracting a membrane bound Mg 2q-dependent phosphatase ŽPHase-1. when the cell is intact. Excision of the patch strips the channel of the influence of the kinase and the phosphatase dominates, producing rundown ŽK ATP .. In contrast, when the channel is excised into Mg 2q free solution, rundown does not occur. This is presumably because the Mg 2q dependent PHase-1 is inactive. Thus, at excision, the channel exists in some phosphorylated state ŽK ATP P.. Since, at this point, blocking phosphorylation decreases while blocking dephosphorylation increases channel activity, it seems likely that at least one more set of Mg 2q-independent kinase and phosphatase enzymes exist; i.e., KNase-2 and PHase-2 in Fig. 9. Conceptually, our model is not unprecedented. For example, the activity of the K-ATP channel in kidney tubule cells is inhibited by both okadaic acid-sensitive and Mg 2q-dependent phosphatases and stimulated by protein kinase A w15x. In addition, K-ATP channels on ventricular myocytes are similarly inhibited by a type 2A phosphatase and stimu- lated by protein kinase C ŽPKC. w21x. Our model predicts that the K-ATP channel can open a varying number of co-channels from either of the phosphorylated states designated as K ATP P and K ATP PP. However, these two states are envisioned to differ in two ways. First, the probability that all co-channels are open increases with phosphorylation. Thus, the additional phosphate of K ATP PP provides the energy allowing transition to the fully open state ŽOPENz .. In contrast, the energy barrier to co-channel opening is much greater for K ATP P so that a smaller number of co-channels ŽOPENa – n . can be open simultaneously. We propose that even in their open states, K ATP P and K ATP PP are substrates for kinases and phosphatases which catalyze their interconversion; e.g., OPENa – n to OPENaX – z and back. The phosphatase and kinase mediating these conversions may be KNase and PHase 1 and 2 or others as indicated by the KNase-? and PHase-? in Fig. 9. Secondly, we suggest that while any of the open states can be reversibly blocked Ži.e., phosphorylation independent. by ATP, the affinity of the blocking site for ligand is conditioned by the level of phosphorylation. It is important to note that our data do not permit the determination of the exact number of phosphorylation states and, therefore, the state of the channel which first displays sensitivity to ATP inhibition. Rather, our model attempts to depict how phosphorylation pathways required for channel activity might interact with the blocking action of ATP. This model allows for precise modulation of the ability of the VMN to sense and respond to metabolic status. For example, many monoamine neuromodulators control the activity of protein kinases w24x. Thus, they could potentially regulate the activity of the K-ATP channel and its sensitivity to ATP via phosphorylation. Our suggestion that the VMN K-ATP channel may have a number of co-channels might provide a site at which monoamine induced phosphorylation could alter the number of open co-channels and thus modulate the mean current in the presence of the same level of ATP. Therefore, phosphorylation may modulate K-ATP channel activity by regulating the mean channel conductance. Our proposed interaction with monoamines could be responsible for the malfunction of glucosensing systems in obesity w16x and diabetes w27x. For example, multiple monoamine and neuropeptide modulators are altered in the VMN in rodent models of genetic w17,23,29,30x and dietary obesity w18x. These alterations in modulation may affect the steady state activity of the K-ATP channel via alterations in its phosphorylation state. This in turn could alter the response to the same metabolic cue leading to the altered ‘set-point’ seen in obesity. Acknowledgements The authors wish to thank Dr. F.M. Ascroft for advice concerning methods of measuring channel activity and V.H. Routh et al.r Brain Research 778 (1997) 107–119 preventing channel rundown, Dr. I.B. Levitan for advice concerning phosphorylation, and Dr. J.-H. Ye for assistance with cell isolation. This work was supported by grants from NIDDK ŽDK-3006. and the Research Service of the Dept. of Veterans Affairs to BEL, an Individual NRSA ŽNS10335. to VHR as well as a Grant-in-Aid from the American Heart Association to JJM. w17x w18x w20x w21x References w1x F.M. Ashcroft, S.J.H. Ashcroft, D.E. Harrison, Properties of single potassium channels modulated by glucose in rat pancreatic b-cells, J. Physiol. 400 Ž1988. 501–527. w2x F.M. Ashcroft, P. Proks, P.A. Smith, C. Ammala, K. Bokvist, P. Rorsman, Stimulus-secretion coupling in pancreatic b-cells, J. Cell. Biochem. 55s Ž1994. 54–65. w3x M.L.J. Ashford, P.R. Boden, J.M. Treherne, Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive Kq channels, Pflugers Arch. 415 Ž1990. 479–483. ¨ w4x M.L.J. Ashford, P.R. Boden, J.M. Treherne, Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurones by indirect inhibition of ATP-Kq channels, Br. J. Pharmacol. 101 Ž1990. 531–540. w5x J.R. De Weille, H. Schmid-Automarchi, M. Fosset, M. Lazdunski, Regulation of ATP sensitive Kq channels in insulinoma cells: activation by somatostatin and protein kinase C and the role of cAMP, Proc. Natl. Acad. Sci. USA 86 Ž1989. 2971–2975. w6x A.A. Dunn-Meynell, V.H. Routh, J.J. McArdle, B.E. Levin, Low affinity sulfonylurea binding sites reside on neuronal cell bodies in the brain, Brain Res. 745 Ž1997. 1–9. w7x A.A. Dunn-Meynell, E. Govek, B.E. Levin, Intracarotid glucose infusions selectively increase FOS-like immunoreactivity in paraventricular and ventromedial nucleus neurons, Brain Res. 748 Ž1997. 100–106. w8x O. Hamill, A. Marty, E. Neher, B. Sakmann, F.J. Sigworth, Improved patch-clamp techniques for high resolution recording from cells and cell-free membrane patches, Pflugers. Arch. 391 Ž1981. ¨ 85–100. w9x J.W. Han, R. Thielexczek, M. Varsanyi, L.M.G. Heilmeyer, Compartmentalized ATP synthesis in skeletal muscle triads, Biochemistry 31 Ž2. Ž1992. 377–384. w10x C.D. Hardin, L. Raeymaekers, R.J. Paul, Comparison of endogenous and exogenous sources of ATP in fueling Ca2q uptake in smooth muscle plasma membrane vesicle, J. Gen. Physiol. 99 Ž1992. 21–40. w11x N. Inagaki, T. Gonoi, J.P. Clement, C.Z. Wang, L. Aguilar-Bryan, J. Bryan, S. Seino, A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive Kq channels, Neuron 16 Ž1996. 1011–1017. w12x M. Kakei, A. Noma, T. Shibasaki, Properties of adenosine triphosphate regulated channels in guinea-pig ventricular cells, J. Physiol. 363 Ž1985. 441–462. w13x R.Z. Kozlowski, M.L.J. Ashford, ATP sensitive Kq channel rundown is Mg 2q dependent, Proc. R. Soc. Lond. B 240 Ž1990. 397–410. w14x M.E. Krouse, G.T. Schneider, P.W. Gage, A large anion-selective channel has seven conductance levels, Nature 319 Ž1986. 58–60. w15x M. Kubokawa, C.M. McNicholas, M.A. Higgins, W. Wang, G. Giebisch, Regulation of ATP-sensitive Kq channel by membranebound protein phosphatases in rat principal tubule cell, Am. J. Physiol. 269 Ž38. Ž1995. F355–F362. w16x B.E. Levin, Intra carotid glucose-induced norepinephrine response w22x w23x w24x w25x w26x w27x w28x w29x w30x w31x w32x w33x w34x w35x w36x w37x 119 and the development of diet-induced obesity, Int. J. Obes. 16 Ž1992. 451–457. B.E. Levin, B. Planas, V.H. Routh, J.S. Hamilton, J.S. Stern, B.A. Horwitz, Altered a 1 -adrenoreceptor binding in intact and adrenalectomized obese Zucker rats Ž far fa., Brain Res. 614 Ž1993. 146–154. B.E. Levin, Reduced norepinephrine turnover in organs and brains of obesity-prone rat, Int. J. Obes. 16 Ž1995. 451–457. B.E. Levin, K.L. Brown, A.A. Dunn-Meynell, Differential effects of diet and obesity on high and low affinity sulfonylurea binding sites in the rat brain, Brain Res. 739 Ž1996. 293–300. P.E. Light, B.G. Allen, M.P. Walsh, R.J. French, Regulation of adenosine triphosphate-sensitive potassium channels from rabbit ventricular myocytes by protein kinase C and type 2A protein phosphatase, Biochemistry 34 Ž1995. 7252–7257. P. Light, Regulation of ATP-sensitive potassium channels by phosphorylation, Biochim. Biophys. Acta 1286 Ž1996. 65–73. P.E. McKibbin, S.J. Cotton, S. McMillan, B. Holloway, R. Mayers, H.D. McCarthy, G. Williams, Altered neuropeptide Y concentrations in specific hypothalamic regions of obese Ž far fa. Zucker rats, Diabetes 40 Ž1991. 1423–1429. E.J. Nestler, P. Greengard, Protein phosphorylation and the regulation of neuronal function, in: G.J. Siegal ŽEd.., Basic Neurochemistry: Molecular, Cellular and Medicinal Aspects, 4th ed., Raven Press, NY, 1989. Y. Oomura, Glucose as a regulator of neuronal activity, in: A.J. Szabo ŽEd.., Advances in Metabolic Disorders, New York Academic Press, NY, 1983, pp. 31–65. H. Onodero, K. Iijima, K. Kogure, Mononucleotide metabolism in the rat brain after transient ischemia, J. Neurochem. 46 Ž1986. 1704–1710. C. Rattarsaran, S. Dagogo-Jack, J.J. Zachweija, P.E. Cryer, Hypoglycemia-induced autonomic failure in IDDM is specific for stimulus of hypoglycemia and is not attributable to prior autonomic activation, Diabetes 439 Ž1994. 809–818. P.H. Reinhart, I.B. Levitan, Kinase and phosphatase activities intimately associated with a reconstituted calcium-dependent potassium channel, J. Neurosci. 15 Ž6. Ž1995. 4572–4579. V.H. Routh, D.M. Murakami, J.S. Stern, C.A. Fuller, B.A. Horwitz, Neuronal activity in hypothalamic nuclei of obese and lean Zucker rats, Int. J. Obes. 14 Ž1990. 879–891. V.H. Routh, B.A. Horwitz, D.W. Geitzen, J.S. Stern, Hypothalamic monoaminergic activity in 11-week cold-exposed female lean Ž Far Fa. and obese Ž far fa. Zucker rats, Obes. Res. 2 Ž1994. 28–37. V.H. Routh, J.J. McArdle, B.E. Levin, Fluctuations in activity of the ATP-sensitive Kq channel in neurons isolated from the ventromedial hypothalamic nucleus, FASEB J. 10 Ž3. Ž1996. 1246. H. Sakura, C. Ammala, F.M. Gribble, F.M. Ashcroft, Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic b-cells, brain, heart and skeletal muscle, FEBS Lett. 377 Ž1995. 338–344. M. Takano, A. Noma, The ATP-sensitive Kq channel, Prog. Neurobiol. 41 Ž1993. 21–30. A. Terzic, I. Findlay, Y. Hosoya, Y. Kurachi, Dualistic behavior of ATP-sensitive Kq channels toward intracellular nucleoside diphosphates, Neuron 12 Ž1994. 1049–1058. J.M. Treherne, M.L.J. Ashford, Extracellular cations modulate the ATP sensitivity of ATP-Kq channels in rat ventromedial hypothalamic neurons, Proc. R. Soc. Lond. B 247 Ž1319. Ž1993. 121–124. W. Wang, G. Giebisch, Dual effects of adenosine triphosphate on the apical small conductance Kq channel of rat cortical collecting duct, J. Gen. Physiol. 98 Ž1991. 35–61. J.N. Weiss, S.T. Lamp, Cardiac ATP-sensitive Kq channels, J. Gen. Physiol. 94 Ž1989. 911–935.