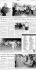Involvement of CaV3.1 T-type calcium channels in
Transcription
Involvement of CaV3.1 T-type calcium channels in
Am J Physiol Cell Physiol 298: C1414–C1423, 2010. First published March 24, 2010; doi:10.1152/ajpcell.00488.2009. Involvement of CaV3.1 T-type calcium channels in cell proliferation in mouse preadipocytes Atsushi Oguri,1 Tomofumi Tanaka,1 Haruko Iida,2 Kentarou Meguro,2 Haruhito Takano,2 Hitoshi Oonuma,3 Satoshi Nishimura,1,4,5 Toshihiro Morita,2 Tatsuya Yamasoba,6 Ryozo Nagai,1 and Toshiaki Nakajima2 1 Department of Cardiovascular Medicine, 2Department of Ischemic Circulatory Physiology, 3Department of Respiratory Medicine, and 4Translational Systems Biology and Medicine Initiative, University of Tokyo, 5PRESTO, Japan Science and Technology Agency, and 6Department of Otolaryngology, University of Tokyo, Tokyo, Japan Submitted 3 November 2009; accepted in final form 18 March 2010 3T3-L1; adipose; ␣1G; cell cycle a continuing toll on public health and is an established risk factor for diabetes, hypertension, hyperlipidemia, and cardiovascular disease (2, 6, 25, 33). It is evident that not only hypertrophy of adipocytes and increase of fat deposit but also proliferation of preadipocytes and differentiation from preadipocytes to adipocytes have important roles in forming obesity. In particular, proliferation of preadipocytes (hyperplasia) is a key process in body fat mass development in obesity since it conditions the number of potential new adipocytes in adipose tissues. Hypertrophy of adipocytes often precedes an OBESITY EXACTS Address for reprint requests and other correspondence: T. Nakajima, Dept. of Ischemic Circulatory Physiology, Univ. of Tokyo, 7-3-1 Hongo, Bunkyoku, Tokyo, Japan 113-8655 (e-mail: [email protected]). C1414 increase in adipocyte cell number (17), but the development of hyperplasia is associated with the severe form of obesity and associated diseases (19). Preadipocytes also contribute to the obesity-associated increase in circulating IL-6 (15). In light of these observations, drugs for the control of preadipocyte proliferation may represent a novel approach to the treatment of obesity and associated diseases. Ionic channels, e.g., K⫹ channels, play vital roles in regulating intracellular Ca2⫹ concentration ([Ca2⫹]i) and subsequently cellular processes such as proliferation and differentiation in various cells. Especially in proliferative cells such as cultured vascular smooth muscle cells and cancer cells, ion channel activities largely participate in cell proliferation (26, 29, 31, 34, 40). Preadipocytes are also a type of highly proliferating cell, and several studies have shown that K⫹ channels regulate cell proliferation (20, 30). Hu et al. (20) showed that transient outward current (Ito) encoded by voltagedependent K⫹ channel (KV 4.2) and large-conductance Ca2⫹activated K⫹ current (KCa1.1) participate in regulating proliferation of human preadipocytes. On the other hand, voltagegated Ca2⫹ channels (CaV) are ubiquitously expressed in various cell types, and they also play roles in regulation of cellular functions including proliferation (26, 29). So far, at least 10 genes that encode ␣-subunit of CaV have been described (3). The channels are classified in three broad families distinguished by the cell membrane potential at which they are active: Cav1 encoding high-voltage-activated (HVA) L-type CaV; Cav2 encoding HVA P/Q-type, N-type, and R-type CaV; and Cav3 encoding low-voltage-activated (LVA) T-type CaV. In adipocytes, the existence of L-type CaV has been shown by experimental and clinical studies. Gaur et al. (9, 10) reported that growth hormone regulates the distribution of L-type CaV in rat adipocyte membrane and then basal levels of [Ca2⫹]i. Nitrendipine, an L-type Ca2⫹ channel blocker, enhances insulin sensitivity in obese and glucose-intolerant subjects (4). In addition, LVA Ca2⫹ channel was first described in 3T3 fibroblasts, with the use of patch-clamp techniques (5, 32). Diaz-Velasquez et al. (8) showed the presence of ␣1G-subtype T-type CaV in 3T3F442A preadipocytes, a subclone of 3T3 Swiss mouse embryo fibroblasts (11, 12), by using RT-PCR analysis and immunohistochemical studies. Recent studies have reported that mice lacking Cav3.1 T-type CaV or mice treated with TTA-A2, a selective T-type CaV blocker, are resistant to high-fat diet-induced weight gain and fat increase and exhibit improved body composition (39). Additionally, TTA-A2 reduces body weight and fat mass of obese rodents 0363-6143/10 Copyright © 2010 the American Physiological Society http://www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 Oguri A, Tanaka T, Iida H, Meguro K, Takano H, Oonuma H, Nishimura S, Morita T, Yamasoba T, Nagai R, Nakajima T. Involvement of CaV3.1 T-type calcium channels in cell proliferation in mouse preadipocytes. Am J Physiol Cell Physiol 298: C1414–C1423, 2010. First published March 24, 2010; doi:10.1152/ajpcell.00488.2009.— Voltage-gated Ca2⫹ channels (CaV) are ubiquitously expressed in various cell types and play vital roles in regulation of cellular functions including proliferation. However, the molecular identities and function of CaV remained unexplored in preadipocytes. Therefore, whole cell voltage-clamp technique, conventional/ quantitative real-time RT-PCR, Western blot, small interfering RNA (siRNA) experiments, and immunohistochemical analysis were applied in mouse primary cultured preadipocytes as well as mouse 3T3-L1 preadipocytes. The effects of CaV blockers on cell proliferation and cell cycle were also investigated. Whole cell recordings of 3T3-L1 preadipocytes showed low-threshold CaV, which could be inhibited by mibefradil, Ni2⫹ (IC50 of 200 M), and NNC55-0396. Dominant expression of ␣1G mRNA was detected among CaV transcripts (␣1A–␣1I), supported by expression of CaV3.1 protein encoded by ␣1G gene, with immunohistochemical studies and Western blot analysis. siRNA targeted for ␣1G markedly inhibited CaV. Dominant expression of ␣1G mRNA and expression of CaV3.1 protein were also observed in mouse primary cultured preadipocytes. Expression level of ␣1G mRNA and CaV3.1 protein significantly decreased in differentiated adipocytes. Mibefradil, NNC55-0396, a selective T-type CaV blocker, but not diltiazem, inhibited cell proliferation in response to serum. NNC55-0396 and siRNA targeted for ␣1G also prevented cell cycle entry/progression. The present study demonstrates that the CaV3.1 T-type Ca2⫹ channel encoded by ␣1G subtype is the dominant CaV in mouse preadipocytes and may play a role in regulating preadipocyte proliferation, a key step in adipose tissue development. C1415 T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES Table 1. PCR primers used for amplification of voltage-gated Ca2⫹ channel genes Channel (gene symbol) Current Sequence (5=–3=) Sense CCGAGAACAGCCTTATCGT Antisense CCACTGACCACTATGAAGTC Sense ACAACCAGCGTAATGTCACC Antisense CACTGACAACGATGAAGTC Sense AGACGTGCTGTACTGGATGC Antisense CACTTCTGTGAGCCAGTGAG Sense AGTGATTTCATGCCTCAAGG Antisense GGTTCTGATGTGTACCATTC Sense GAAGGCAGCAGTGAACAAGC Antisense AGTCCAGGATGTTCCAGAGG Sense AAGCTGAGAACAACCCAGAAC Antisense CAAAGCGGGAAAGAATAGAC Sense GATGCGGGTGCTGAAGCTAG Antisense TGACAGGCAGCTGAATACAG Sense AGCAACATCGTGTTCACCAG Antisense GAAGAGCATTAGCAGCATGC Sense TGCATCCTGCACCACCAACT Antisense AACACGGAAGGCCATGCCAG Sense GGAAGAUCGUAGAUAGCAA Antisense UUGCUAUCUACGAUCUUCC CaV2.1(␣1A) P/Q-type 544 CaV2.2(␣1B) N-type 512 CaV1.2(␣1C) L-type 568 CaV1.3(␣1D) L-type 362 CaV2.3(␣1E) R-type 604 CaV1.4(␣1F) L-type 927 CaV3.1(␣1G) T-type 455 CaV3.2(␣1H) T-type 294 GAPDH 259 siRNA CaV3.1(␣1G) Negative control Allstars Negative Control (Qiagen, catalog no. 1027280) siRNA, small interfering RNA. (39). Thus CaV appear to provide a novel therapeutic target for the treatment of obesity. However, the molecular identities and physiological roles of CaV expressed in preadipocytes remained unexplored. Therefore, to clarify the characteristics of expression of CaV in preadipocytes, whole cell voltage-clamp technique, conventional/ quantitative real-time RT-PCR, Western blot, small interfering RNA (siRNA) experiments, and immunohistochemical analysis were applied to mouse 3T3-L1 preadipocytes, a subclone of 3T3 Swiss mouse embryo fibroblasts (11, 12), mouse primary cultured preadipocytes, and their differentiated adipocytes. Here we provide direct evidence that the CaV3.1 T-type Ca2⫹ channel encoded by ␣1G subtype is the dominant CaV in mouse preadipocytes and plays a role in the regulation of their proliferation. Fig. 1. Voltage-gated Ca2⫹ currents (ICa) expressed in 3T3-L1 preadipocytes. A: cells were held at ⫺100 mV, and command voltage pulses to ⫹0 mV (400 ms in duration) were applied. The bath solution contained 10 mM Ca2⫹ in Aa and Ab. In Ac, 10 mM Ca2⫹ was replaced by 10 mM Ba2⫹. Effects of TTX (10 M) and mibefradil (30 M) and nicardipine (1 M) are illustrated in Aa and Ab, respectively. Note that the transient inward current was blocked by mibefradil but not TTX and nicardipine. B: current voltage (I-V) relations measured at the peak of the transient inward current and the effects of holding potential (Vh). The current traces at various command voltage pulses were elicited from a Vh of ⫺40 mV (Ba) or ⫺100 mV (Bb). C: I-V relations measured at the peak. The transient inward current was elicited at a Vh of ⫺100 mV, but not at ⫺40 mV. D: steady-state activation (□) and inactivation (Œ) curves for ICa. Data obtained from 4 cells were fitted by Boltzmann equation. AJP-Cell Physiol • VOL 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 Size, bp C1416 T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES MATERIALS AND METHODS Fig. 3. A: expression of ␣-subunits of CaV genes detected by RT-PCR in 3T3-L1 preadipocytes and adult mouse brain. M, marker; 1A, ␣1A, CaV2.1; 1B, ␣1B, CaV2.2; 1C, ␣1C, CaV1.2; 1D, ␣1D, CaV1.3; 1E, ␣1E, CaV2.3; 1F, ␣1F, CaV1.4; 1G, ␣1G, CaV3.1; 1H, ␣1H, CaV3.2; GAPDH, internal control. B and C: real-time quantitative RT-PCR of CaV genes in 3T3-L1 and mouse primary cultured preadipocytes. Expression levels of CaV channel genes in 3T3-L1 preadipocytes (B) and mouse primary cultured preadipocytes (C) were normalized to 18S ribosomal RNA levels. Note that ␣1G appears to mainly encode for CaV in both 3T3-L1 preadipocytes and primary mouse preadipocytes. Fig. 2. Effects of various types of Ca2⫹ channel blockers on ICa expressed in 3T3-L1 preadipocytes. Cells were held at ⫺100 mV, and command voltage pulses to ⫹0 mV (400 ms in duration) were applied at 0.1 Hz. A–D: concentrationdependent inhibition of ICa by mibefradil (A and B) and Ni2⫹ (C and D). Original current traces are shown in A and C, and the inhibitory effect of these agents on the current amplitude measured at the peak is plotted against various concentrations of mibefradil (B) and Ni2⫹ (D). Data are shown as means ⫾ SE (n ⫽ 5) and fit by a Michaelis-Menten simple bimolecular model: % inhibition ⫽ 100/{1 ⫹ (IC50 [mibefradil or Ni2⫹]⫺1)}, where IC50 is 50% inhibitory concentration for drugs. The data were best fit with an IC50 value of 3 M for mibefradil and 200 M for Ni2⫹. E: effects of NNC55-0396 (10 M) on ICa. F: % inhibition of ICa by various Ca2⫹ channel blockers: mibefradil (Mib, 10 M), NNC55-0396 (NNC, 10 M), SNX-482(SNX, 100 nM), -agatoxin IVA (-agatoxin, 100 nM), -conotoxin GVIA (-conotoxin, 500 nM), and nicardipine (10 M). Note that both mibefradil and NNC55-0396 significantly inhibited ICa. Data are shown as means ⫾ SE (n ⫽ 5). AJP-Cell Physiol • VOL Isolation and culture of preadipocytes and adipocytes from mouse white adipose tissue. Mouse primary cultured preadipocytes were obtained from Primary Cell (Hokkaido, Japan). The isolation method was similar to that in previous reports (1, 18). Briefly, isolated epididymal white adipose tissue obtained from ICR mice at 2– 4 days after birth was rinsed in collagenase solution. The mixture was then allowed to digest with gentle shaking. The resulting cell suspension was allowed to settle in order to separate into a supernatant containing adipocytes and an inferior layer composed mainly of preadipocytes. The primary cultured preadipocytes were used for later experiments. The preadipocytes were then seeded in a plate (2.4 ⫻ 104 cells/plate) in DMEM supplemented with 10% fetal calf serum containing penicillin (10 U/ml), streptomycin (10 g/ml), (⫹)-biotin (33 M), pantothenic acid (17 M), ascorbic acid (100 M), octanoic acid (1 M), and 3,5,3=-triiodothyronine (T3; 50 nM). The medium was replaced with differentiating medium consisting of the above medium with dexamethasone (2.5 M) and insulin (10 g/ml). Under these conditions, adipocyte differentiation occurred a few days later (7), and differentiated adipocytes were maintained in the differentiation medium without dexamethasone. Full differentiation developed at 8 –10 days after induction of differentiation. Solutions and drugs. The composition of control Tyrode solution was as follows (in mM): 136.5 NaCl, 5.4 KCl, 1.8 CaCl2, 0.53 MgCl2, 5.5 glucose, and 5.5 HEPES-NaOH buffer (pH 7.4). To block K⫹ currents, the patch pipette contained (in mM) 140 CsCl, 10 EGTA, 2 MgCl2, 3 Na2ATP, 0.1 guanosine-5=-triphosphate (GTP, sodium salt; Sigma), and 5 HEPES-CsOH buffer (pH 7.2). To increase the current amplitude of voltage-gated Ca2⫹ current (ICa), 10 mM Ca2⫹ or 10 mM Ba2⫹ was applied to the bath solution. 4-Aminopyridine (4-AP, 4 mM), a KV blocker, and tetraethylammonium (2 mM), a Ca2⫹activated K⫹ current blocker, were added to the control Tyrode solution. Various types of CaV blockers (mibefradil, NNC55-0396, 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 The Animal Care and Use Committee of the University of Tokyo approved the present study. Cell culture of 3T3-L1 preadipocytes and adipocyte differentiation method. 3T3-L1 preadipocytes were obtained from the Japanese Collection of Research Bioresources (JCRB) Bank (Osaka, Japan) as previously described (35). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) containing 100 g/ml penicillin and 100 g/ml streptomycin. Cells were plated and grown until 2 days after confluence and then induced to differentiate into adipocytes by change of the culture medium to DMEM containing 0.5 mM methylisobutylxanthine, 0.25 M dexamethasone, and 10 g/ml insulin for 48 h (36). With this protocol, ⬎95% of the cells were differentiated into the adipocyte phenotype 3– 4 days after initiating differentiation. The differentiated 3T3-L1 adipocytes were switched to fresh DMEM containing 10% FBS. T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES Ca2⫹ equilibrium potential. ECa was obtained from the I-V curve, where the I-V curve crossed over the zero line. RNA extraction, reverse transcriptase-polymerase chain reaction, and real-time quantitative reverse transcriptase-polymerase chain reaction. Total cellular RNA was extracted from preadipocytes and differentiated adipocytes with the RNeasy mini kit (Qiagen, Cambridge, MA). For reverse transcriptase-polymerase chain reaction (RT-PCR), complementary DNA (cDNA) was synthesized from 1 g of total RNA with reverse transcriptase and random primers (Toyobo, Osaka, Japan). The reaction mixture was then subjected to PCR amplification with specific forward and reverse oligonucleotide primers for 35 cycles consisting of heat denaturation, annealing, and extension. PCR products were size-fractionated on 2% agarose gels and visualized under UV light. Primers were chosen on the basis of the sequence of mouse ␣-subunit genes of CaV (␣1A–␣1H) as shown in Table 1. Table 1 also shows the CaV protein created by each gene. Fig. 4. CaV3.1 T-type CaV expression in 3T3-L1 preadipocytes, mouse primary cultured preadipocytes, and differentiated adipocytes. Aa: microscopic image of 3T3-L1 preadipocytes. Ab: staining (green) for CaV3.1 in 3T3-L1 preadipocyte. Double staining of nuclei with Hoechst 33258 to visualize nuclei and anti-CaV3.1 is illustrated. Ac: negative controls without the antibody (anti-␣1G) in 3T3-L1 preadipocytes. Cells were counterstained with Hoechst 33258 to visualize nuclei. Ad: transfection of small interfering RNA (siRNA) for ␣1G mRNA. The transfected siRNA conjugated with rhodamine is visualized under fluorescent microscopy. Ae: Oil Red O staining of differentiated 3T3-L1 adipocytes. Ba: staining (green) for CaV3.1 in mouse primary cultured preadipocyte. Bb: differential interference contrast (DIC) image of mouse differentiated adipocytes. The differentiated adipocytes have lipid deposits. Af and Bc: staining (green) for CaV3.1 in differentiated 3T3-L1 adipocytes (Af) and mouse differentiated adipocytes (Bc). Scale bars, 50 m. AJP-Cell Physiol • VOL 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 SNX-482, -agatoxin IVA, -conotoxin GVIA, diltiazem, and nicardipine) were obtained from Sigma. All experiments were performed at room temperature (20 –25°C). Recording technique and data analysis. Membrane currents were recorded with tight-seal whole cell clamp techniques and a patchclamp amplifier (EPC-7, List Electronics, Darmstadt, Germany) as previously described (14, 38). Steady-state inactivation was estimated with a double-pulse protocol. Conditioning voltage pulses (500 ms in duration) of various membrane potentials were applied from a holding potential of ⫺100 mV. At 10 ms after the end of each conditioning pulse, a test pulse of ⫹0 mV was applied. The ratio of ICa amplitude with and without conditioning pulses was plotted against each conditioning voltage. From current-voltage (I-V) data, the steady-state activation curve was derived by using the following equation: gCa ⫽ ICa/[(Vm ⫺ ECa)], where ICa is the peak current amplitude at each membrane potential (Vm), gCa is the chord conductance, and ECa is the C1417 C1418 T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES AJP-Cell Physiol • VOL and color development was allowed to proceed for 3 h at 37°C and 5% CO2. Each plate was then read at an absorbance of 490 nm. Flow cytometry. 3T3-L1 preadipocytes were plated at ⬃5,000 cells/cm2 onto 10-cm plates and allowed to sit for 2 nights in FBS-free medium, and then cells were perfused into 5% FBS-containing medium in the presence or absence of CaV blockers. In siRNA experiments, cells treated with ␣1G siRNA and nonsilencing (negative control) siRNAs were plated at ⬃5,000 cells/cm2 onto 10-cm plates and allowed to sit for 24 h in FBS-free medium, and then cells were perfused into 5% FBS-containing medium. Cell cycle data were determined after 24-h treatment by flow cytometry in propidium iodide-stained mouse 3T3-L1 preadipocytes. Data analysis. All values are expressed as means ⫾ SE. Differences between multiple groups were compared by ANOVA. Twogroup analysis was performed with a Student’s t-test. Differences were considered significant if P ⬍ 0.05. RESULTS T-type CaV in 3T3-L1 preadipocytes. Figure 1, A–C, show ICa expressed in 3T3-L1 preadipocytes where the bath solution contained 10 mM Ca2⫹. The cells were held at ⫺100 mV, and command voltage pulses (400 ms in duration) were applied to ⫹0 mV. A fast transient inward current was elicited during the depolarizing pulse. TTX (10 M, Fig. 1Aa) or replacement of extracellular Na⫹ with N-methyl-D-glucamine⫹ (data not shown), an impermeant cation, did not affect the transient inward current at all. However, mibefradil (30 M, Fig. 1Aa), a potent inhibitor of T- and L-type currents (24), but not Fig. 5. Comparison of CaV3.1 protein expression between preadipocytes and differentiated adipocytes. Western blot analysis shows the marked difference of CaV3.1 protein expression between 3T3-L1 preadipocytes and differentiated adipocytes (Aa) and between mouse primary cultured preadipocytes and differentiated adipocytes (Ba). In Ab and Bb, the relative abundance of CaV3.1 protein between preadipocytes and differentiated adipocytes is compared. The expression level of CaV3.1 protein was compared with that of -actin, and the expression level in 3T3-L1 and mouse primary cultured preadipocytes is considered as 100%. Note that the expression level of CaV3.1 protein in differentiated adipocytes significantly decreased compared with 3T3-L1 preadipocytes and mouse primary cultured preadipocytes. **P ⬍ 0.01 vs. preadipocytes. 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 Total RNA of mouse adult brain was used for positive control. Real-time quantitative RT-PCR was performed with the use of realtime TaqMan technology and a sequence detector (ABI PRISM 7000, Applied Biosystems, Foster City, CA) (22). Gene-specific primers and TaqMan probes were used to analyze transcript abundance. The 18S ribosomal RNA level was analyzed as an internal control and used to normalize the values for transcript abundance of CaV family genes. The probes used in this study were purchased as Assay-on-Demand from Applied Biosystems: assay ID Mm00432190_ml for ␣1A , Mm00432226_ml for ␣1B, Mm00437917_ml for ␣1C, Mm00494444_ml for ␣1E, Mm01299131_ml for ␣1G, Mm00445369_ml for ␣1H, Mm01299033_ml for ␣1I, Mm01295675_ml for adipose protein 2 (aP2), Mm00494477_ml for Pref-1, and 4310893E for 18S rRNA endogenous control. We performed six independent experiments. Oil Red O staining. Differentiated 3T3-L1 adipocytes were rinsed in PBS before being fixed with 10% formaldehyde. Approximately 15 min after the fixation, Oil Red O stain was used to stain for lipid accumulation (20 min). After being rinsed with distilled water, photomicrographs were taken with a digital camera to document staining. Immunocytochemistry. Immunocytochemical analyses were performed on preadipocytes and differentiated adipocytes of 3T3-L1 and mouse primary cultured preadipocytes with anti-CaV3.1 (Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit polyclonal antibody against channel isoforms diluted to 1:100. The cells were cultured on Lab-Tek Chamber Slide Glass (Nalge Nunc International, Naperville, IL), fixed with 2% paraformaldehyde in PBS for 30 min, and then blocked for 10 min with 2% horse serum in PBS. The cells were incubated overnight with the primary antibodies diluted with 0.1% Triton X-100 and 0.01% NaN3 in PBS. For negative controls, cells were treated without antibody. Alexa Fluor 488-labeled donkey anti-rabbit IgG antibody diluted 1:1,000 (Molecular Probes, A21206) was used to visualize channel expression. The cells were also stained with Hoechst 33258 (Sigma Aldrich) to visualize nuclei. A confocal laser scanning microscope (Olympus FluoView FV300, Olympus, Tokyo, Japan) was used for observations. Transfection of synthetic small interfering RNA. ␣1G siRNA and nonsilencing (negative control) siRNAs as described in Table 1 were purchased from Qiagen (Cambridge, MA). They were transfected into 3T3-L1 preadipocytes to a final concentration of 5 nM with Lipofectamine 2000 (5 l/ml culture; Invitrogen) according to the instructions of the manufacturer. Transfected cells were incubated for 48 h in an atmosphere of 5% CO2 and 95% air at 37°C before each experiment. Analysis of mRNA and protein by real-time RT-PCR and Western blot was then performed. Rhodamine-conjugated siRNA was used to confirm the transfection of siRNA with Nikon ECLIPSE TE2000-u. The density of ICa and the effects of siRNA on cell cycle were also investigated in the transfected cells. Western blotting. Proteins were separated on a 7.5% polyacrylamide gel for 70 min at 200 V and then transferred to Amersham Hybond-P (GE Healthcare UK, Little Chalfont, UK) for 75 min at 72 mA. The membrane was blocked with 3% skim milk in PBS (0.01 M phosphate buffer, 0.138 M NaCl, 0.0027 M KCl, pH 7.4) with 0.1% Tween 20 (PBS-T) for 1 h. It was probed with anti-CaV3.1 polyclonal antibody diluted to 1:200 (Santa Cruz Biotechnology) and anti--actin monoclonal antibody (1:10,000; Sigma) overnight at 4°C, washed three times in PBS-T for 15 min each, and subsequently incubated with anti-rabbit IgG linked to peroxidase (Millipore) diluted to 1:1,000 with blocking buffer for 1 h at room temperature. After three additional washes, bound antibodies were detected with Amersham ECL-plus (GE Healthcare UK) and analyzed with an LAS-3000 mini image analyzer (Fuji-Film, Tokyo, Japan). Proliferation assay. Cell proliferation was assessed with the Cell Titer 96 Aqueous kit (Promega, Madison, WI). The preadipocytes were plated in 96-well plates (Becton Dickinson Labware, Franklin Lakes, NJ) at a density of 8 ⫻ 104 cells/well with experimental media with or without Ca2⫹ channel blockers. These plates were incubated for 24 h, after which the tetrazolium salt and dye solution were added T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES Fig. 6. Effects of treatment with siRNA targeted for ␣1G on ICa expressed in 3T3-L1 preadipocytes. A: effects of siRNA targeted for ␣1G on expression level of ␣1G mRNA. 3T3-L1 preadipocytes were treated with siRNA targeted for ␣1G or control siRNA. Ba: Western blot analysis showing the effects of siRNA on CaV3.1 protein expression. In Bb, the relative abundance of CaV3.1 protein in cells transfected with siRNA is compared with cells treated with control siRNA. The expression level of CaV3.1 protein was compared with that of -actin. The expression level in cells transfected with control siRNA is considered as 100%. Note that siRNA targeted for ␣1G decreased CaV3.1 protein expression in 3T3-L1 preadipocytes. C: current density of ICa in cells transfected with noncoding control and ␣1G siRNA. Note that the current density of ICa significantly decreased in siRNA-treated cells. **P ⬍ 0.01 vs. control siRNA. AJP-Cell Physiol • VOL Fig. 7. A: comparison of the expression level of ␣1G, adipose protein 2 (aP2), and Pref-1 mRNA between 3T3-L1 preadipocytes and differentiated adipocytes. B: comparison of the expression level of ␣1G, aP2, and Pref-1 mRNA between mouse primary cultured preadipocytes and differentiated adipocytes. Data are means ⫾ SE from 6 independent samples in A and B. The expression level of aP2 mRNA notably increased in 3T3-L1 adipocytes and mouse adipocytes, while the expression level of ␣1G and Pref-1 mRNA significantly decreased during the differentiation. **P ⬍ 0.01 vs. preadipocytes. obtained from the conductance and also fitted by Boltzmann equation. The values of Vh and k were ⫺30.0 ⫾ 0.3 and ⫺7.3 ⫾ 0.3 (n ⫽ 5), respectively. The overlap of the steadystate activation and inactivation curves at potentials more positive than ⫺50 mV determines the window currents. Figure 2, A and B, shows the concentration-dependent effects of mibefradil on ICa. Mibefradil inhibited ICa with an IC50 value of 3 M (Fig. 2B), and mibefradil at concentrations ⬎10 M nearly completely abolished ICa. Next, we investigated the Ni2⫹ sensitivity of ICa (Fig. 2, C and D). Ni2⫹ concentrationdependently inhibited ICa, with an IC50 value of 200 M, and 1 mM Ni2⫹ almost completely abolished it. Inhibitory effects were also observed with NNC55-0396 (10 M; Fig. 2E), a selective inhibitor of T-type CaV (21, 37). Furthermore, to determine which types of CaV are involved in ICa expressed in 3T3-L1 preadipocytes, we examined responses to a test stimulus applied in the absence or presence of various agents. The Ca2⫹ channel blockers used here were as follows: mibefradil (10 M), NNC55-0396 (10 M), SNX-482 (a R-type Ca2⫹ channel blocker; 100 nM), -agatoxin IVA (a P-type Ca2⫹ channel blocker; 100 nM), -conotoxin GVIA (a N-type Ca2⫹ channel blocker; 500 nM), and nicardipine (10 M). The percent inhibition of various Ca2⫹ channel blockers is illustrated in Fig. 2F. Mibefradil and NNC55-0396 inhibited ICa by 91 ⫾ 3% and 67.7 ⫾ 4.6%, respectively, but nicardipine inhibited it only by 3 ⫾ 1%. SNX-482, -agatoxin IVA, and -conotoxin GVIA were ineffective. Dominant expression of CaV3.1 encoded by ␣1G mRNA in 3T3-L1 preadipocytes. We investigated the expression of ␣-subunit genes of CaV family members (␣1A–␣1H mRNA). 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 nicardipine (1 M, Fig. 1Ab), a potent L-type Ca2⫹ channel blocker, completely abolished it. With replacement with 10 mM Ba2⫹ instead of Ca2⫹, the amplitude of the inward current slightly decreased (Fig. 1Ac). In addition, 1 M BAY K 8644 did not enhance it (data not shown), suggesting that highthreshold L-type CaV or voltage-gated Na⫹ channels do not contribute to form the inward current expressed in 3T3-L1 preadipocytes but non-L-type CaV do form it. Typical current data recorded at each membrane potential and I-V relations measured at the peak of ICa are shown in Fig. 1, B and C. Cells were held at ⫺40 mV (Fig. 1Ba) or ⫺100 mV (Fig. 1Bb) in the same cell. When the cell was held at ⫺100 mV, the transient inward currents were elicited at potentials more positive than ⫺50 mV. The peak amplitude of ICa was observed at approximately ⫺20 mV and reached zero at potentials of approximately ⫹50 to ⫹60 mV. On the other hand, ICa was not elicited during the depolarizing pulses at a holding potential of ⫺40 mV. Figure 1D shows the steadystate inactivation curve for ICa. The inactivation data were fitted to the following equation (Boltzmann equation) by using least-squares methods: I(V)/Imax ⫽ 1/{1 ⫹ exp[(V ⫺ Vh)k⫺1]}, where V is the membrane potential in millivolts, Vh is the membrane potential at half-maximum, and k is the slope factor. The values of Vh and k were ⫺51.7 ⫾ 0.9 mV and 8.4 ⫾ 0.8 (n ⫽ 5), respectively. The steady-state activation curves were C1419 C1420 T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES (Fig. 6C; P ⬍ 0.01), compared with nonsilencing (negative control) siRNA. CaV in mouse primary cultured preadipocytes. Expression of ␣-subunit genes of CaV family members (␣1A–␣1I) was also investigated by real-time quantitative RT-PCR (Fig. 3C) in mouse primary cultured preadipocytes, and ␣1G appeared to encode for ␣-subunit gene of CaV in a similar way to that in mouse 3T3-L1 preadipocytes. Expression of CaV protein was confirmed by immunostaining (Fig. 4Ba) and Western blot analysis using anti-CaV3.1 (Fig. 5, Ba and Bb), which was similar to 3T3-L1 preadipocytes. The cells were also counterstained with Hoechst 33258 to visualize nuclei, and the double staining of nucleus and CaV3.1 is shown in Fig. 4Ba. Comparison of ␣1G mRNA expression between preadipocytes and differentiated adipocytes. We examined the effects of differentiation on ␣1G mRNA expression in both 3T-L1 preadipocytes and mouse primary cultured preadipocytes. After differentiation, lipid accumulation stained by Oil Red O stain could be observed in 3T3-L1 cells (Fig. 4Ae). Similarly, lipid accumulation was observed in differentiated mouse adipocytes (Fig. 4Bb). Figure 7 compares the expression levels of ␣1G and aP2 mRNA, a differentiated marker gene of adipocyte, in both cell types. The expression level of aP2 mRNA was notably increased in 3T3-L1 adipocytes and mouse adipocytes, while the expression level of ␣1G mRNA was significantly decreased during the differentiation. In addition, the mRNA expression level of Pref-1 (also known as DLK-1), a marker of preadipocytes, was significantly high in both preadipocytes (Fig. 7). Similarly, Western blot analyses showed that the protein expression level of CaV3.1 in adipocytes was significantly decreased compared with 3T3-L1 preadipocytes (P ⬍ 0.01, n ⫽ 4; Fig. 5, Aa and Ab) and mouse preadipocytes (P ⬍ 0.01, n ⫽ Fig. 8. Effects of Ca2⫹ channel blockers on cell proliferation and cell cycle in 3T3-L1 preadipocytes. Aa and Ab: effects of diltiazem, nicardipine, and mibefradil (10 M; Aa), and NNC55-0396 (1–10 M; Ab) on cell proliferation. Note that cell proliferation was significantly inhibited by mibefradil and NNC550396, but not diltiazem and nicardipine. Data were obtained from 5–7 experiments. *P ⬍ 0.05 vs. control. B and C: effects of NNC550396 and diltiazem on cell cycle analyzed by flow cytometry. Serum-deprived cells were again treated with 5% FBS culture medium in the absence or presence of these drugs for 24 h and analyzed by flow cytometry. Representative data obtained from 3 experiments are shown in the absence and presence of NNC55– 039 (10 M; B) and diltiazem (10 M; C). D: summary data showing % of cells in G0/G1, S, and G2/M. Groups: 5% FBS (control), 5% FBS with NNC55-0396 10 M, 5% FBS with 10 M diltiazem. Treatment with NNC55-0396 showed a much smaller proportion of cells entering S and G2/M and a much higher proportion arrested in G0/G1, while diltiazem did not mimic the effects of NNC55-0396. **P ⬍ 0.01. AJP-Cell Physiol • VOL 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 By RT-PCR analysis, the transcripts of ␣1A, ␣1B, ␣1C, ␣1E, ␣1G, and ␣1H were detected (Fig. 3A) and the transcripts of ␣1D and ␣1F were not detected compared with control mouse brain. The amplitude of cDNA fragments was of predicted molecular size, identical to cDNA fragments amplified from reversely transcripted mRNA. Expression of CaV family members was also investigated by real-time quantitative RT-PCR (Fig. 3B). Transcript levels were normalized to 18S ribosomal housekeeping gene. Thus ␣1G appeared to dominantly encode for ␣-subunit gene of CaV in 3T3-L1 preadipocytes. Expression of CaV3.1 protein was confirmed by immunostaining (Fig. 4Ab) and Western blots using anti-CaV3.1 (Fig. 5, Aa and Ab) in 3T3-L1 preadipocytes. The cells were also counterstained with Hoechst 33258 to visualize nuclei, and the double staining of nucleus and CaV3.1 is shown in Fig. 4Ab. No expression was detected in negative controls without the antibody (Fig. 4Ac). We next investigated the effects of siRNA for ␣1G. Transfection of siRNA conjugated with rhodamine was confirmed by fluorescent microscopy (Fig. 4Ad). After siRNA treatment the inhibitory effect on ␣1G mRNA expression was analyzed by real-time RT-PCR. The expression level of ␣1G mRNA in 3T3-L1 preadipocytes transfected with siRNA was significantly decreased compared with nonsilencing (negative control) siRNA (Fig. 6A; P ⬍ 0.01, n ⫽ 6). Western blot analysis showed that the protein expression level of CaV3.1 in 3T3-L1 preadipocytes transfected with siRNA was also decreased compared with nonsilencing (negative control) siRNA (P ⬍ 0.01, n ⫽ 3; Fig. 6, Ba and Bb). Next, ICa was recorded with tight-seal whole cell clamp techniques in the transfected cells (20 cells, siRNA for ␣1G and negative control, respectively). The current density of ICa adjusted by cell capacitance was significantly reduced in cells transfected with siRNA for ␣1G T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES Fig. 9. Effects of siRNA targeted for ␣1G mRNA on cell proliferation and cell cycle in 3T3-L1 preadipocytes. A: effects of siRNA targeted for ␣1G on cell proliferation. Note that cell proliferation was significantly inhibited by siRNA. Data were obtained from 5–7 experiments. *P ⬍ 0.05 vs. control siRNA. B: effects of siRNA targeted for ␣1G mRNA on cell cycle analyzed by flow cytometry in 3T3-L1 preadipocytes. In B, siRNA-loaded cells were serum deprived for 24 h, and cells were again treated with 5% FBS-containing culture medium for 24 h and analyzed by flow cytometry. Representative data obtained from 3 experiments are shown for cells treated with siRNA targeted for ␣1G and control siRNA. C: summary data showing % of cells in G0/G1, S, and G2/M. *P ⬍ 0.05, **P ⬍ 0.01 vs. control siRNA. AJP-Cell Physiol • VOL ative control) siRNA-treated cells (Fig. 9, Bb and C), cells treated with siRNA targeted for ␣1G mRNA showed a much smaller proportion of cells entering G2/M and a much higher proportion arrested in G0/G1 in the presence of FBS (Fig. 9, Ba and C). On the other hand, siRNA did not affect the cell cycle in the absence of FBS (data not shown). Thus it is likely that siRNA targeted for ␣1G mRNA inhibited cell cycle entry/ progression, but not because of toxic effects. DISCUSSION The major findings of the present study are as follows. 1) T-type Ca2⫹ channel encoded by ␣1G subtype, blocked by mibefradil, Ni2⫹, NNC55-0396, and siRNA for ␣1G, was the dominant CaV in mouse 3T3-L1 preadipocytes. 2) RT-PCR, Western blot, and immunohistochemical analysis also showed the dominant expression of CaV3.1 T-type CaV in mouse primary cultured preadipocytes as well as 3T3-L1 preadipocytes. 3) The expression level of CaV3.1 encoded by ␣1G was significantly downregulated in differentiated adipocytes by decreasing the transcription level. 4) Mibefradil, NNC55-0396, but not diltiazem, and siRNA for ␣1G inhibited cell proliferation in response to serum. NNC55-0396 and siRNA for ␣1G inhibited cell cycle entry/progression. These results demonstrate for the first time that the T-type Ca2⫹ channel encoded by ␣1G subtype is the dominant CaV in mouse preadipocytes and plays a role in the regulation of their proliferation. CaV are ubiquitously expressed in various cell types and play vital roles in regulation of cellular functions such as proliferation. The existence of CaV has also been described in preadipocytes and adipocytes (4, 5, 10, 32), but the molecular identities and function of CaV remained quite unexplored. Recently, Diaz-Velasquez et al. (8) showed the presence of ␣1G-subtype T-type CaV in 3T3-F442A preadipocytes, a subclone of 3T3 Swiss mouse embryo fibroblasts (11, 12), by using RT-PCR analysis and immunohistochemical studies. The present study showed the presence of CaV in mouse 3T3-L1 preadipocytes by using patch-clamp experiments. Mibefradil, a T- and L-type Ca2⫹ channel blocker (24), inhibited it with an IC50 value of 3 M, and NNC55-0396, a selective T-type Ca2⫹ channel blocker (21, 37), also inhibited it. Also, the other Ca2⫹ channel blockers, SNX-482, -agatoxin IVA, -conotoxin GVIA, and nicardipine, were less or only a little effective to inhibit CaV, compared with mibefradil or NNC55-0396. Ni2⫹ inhibited it with an IC50 value of 200 M. In a heterologous expression system, the ␣1H subtype has been reported to exhibit a greater sensitivity to inhibition by NiCl2 (IC50 ⬃10 M) than either the ␣1G or ␣1I subtype (IC50 ⬃200 –300 M) (23, 27). Thus, from the sensitivity to Ni2⫹ and the other Ca2⫹ channel blockers, it is likely that T-type Ca2⫹ channel encoded by ␣1G subtype was the dominant CaV in 3T3-L1 preadipocytes. These results were supported by RT-PCR analysis, immunohistochemical studies, and Western blot analysis. The dominant expression of ␣1G mRNA was detected by conventional/quantitative real-time RT-PCR among ␣-subunits of CaV transcripts (␣1A–␣1I), and the expression of CaV3.1 protein encoded by ␣1G gene was detected by immunohistochemical studies and Western blot analysis. In addition, siRNA targeted for ␣1G markedly inhibited CaV expressed in 3T3-L1 preadipocytes. 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 4; Fig. 5, Ba and Bb). Immunohistochemical staining using anti-CaV3.1 showed the expression of ␣1G in both differentiated 3T3-L1 adipocytes (Fig. 4Af) and mouse differentiated adipocytes (Fig. 4Bc), but compared with preadipocytes, weak staining or no staining was observed in both differentiated adipocytes. T-type CaV blockers and siRNA for ␣1G mRNA inhibit cell proliferation in 3T3-L1 preadipocytes. Figure 8 shows the effects of various Ca2⫹ channel blockers on cell proliferation and cell cycle in 3T3-L1 preadipocytes. Mibefradil (10 M; Fig. 8Aa) and NNC55-0396 (1–10 M; Fig. 8Ab) significantly inhibited cell proliferation in response to FBS, while diltiazem and nicardipine (10 M; Fig. 8Aa) had little effect. In addition, we investigated the effects of NNC55-0396 (Fig. 8B) and diltiazem (Fig. 8C) on cell cycle. Cells were treated in the absence or presence of these drugs for 24 h and analyzed by flow cytometry. Treatment with NNC55-0396 (10 M; Fig. 8, B and D) showed a smaller proportion of cells entering S and G2/M and a higher proportion arrested in G0/G1, while diltiazem [10 M (Fig. 8, C and D) and 30 M (data not shown)] did not mimic the effects of NNC55-0396. The effects of siRNA targeted for ␣1G mRNA on cell proliferation and cell cycle in the response to serum were also investigated. As shown in Fig. 9A, siRNA targeted for ␣1G mRNA significantly inhibited cell proliferation in response to FBS compared with control siRNA. In addition, compared with nonsilencing (neg- C1421 C1422 T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES AJP-Cell Physiol • VOL thereby promoting a steady-state calcium entry. Consistent with a role for CaV3.1 in controlling the cell cycle, similar effects on proliferation have been reported in cultured human pulmonary arterial cells and also in nonexcitable cells such as tumor cells (28, 34). Thus it is very likely that T-type Ca2⫹ channels play a role in regulating [Ca2⫹]i, and subsequently preadipocyte proliferation. Recent in vivo studies have shown that mice lacking Cav3.1 T-type CaV or mice treated with TTA-A2, a selective T-type CaV blockade, are resistant to high-fat diet-induced weight gain and fat increase and exhibit improved body composition (39). These effects likely result from better alignment of diurnal feeding patterns with daily changes in circadian physiology, although the involvement of T-type CaV on adipose tissues has not been investigated. The present study showed that the T-type Ca2⫹ channel plays a role in the regulation of cell proliferation in mouse preadipocytes. Thus T-type Ca2⫹ channel blockers appear to be interesting new drugs for obesity. In conclusion, the present study demonstrates that the T-type Ca2⫹ channel encoded by ␣1G subtype is the dominant CaV in mouse preadipocytes and plays a role in the regulation of their proliferation, a key step in adipose tissue development. ACKNOWLEDGMENTS We thank Hiroyuki Imuta and Takaaki Hasegawa for help with the experiments. GRANTS This study was supported by Grants-in-Aid for Scientific Research of Japan to T. Nakajima. DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the author(s). REFERENCES 1. Ailhaud G, Grimaldi P, Négrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr 12: 207–233, 1992. 2. Bays HE. “Sick fat,” metabolic disease, atherosclerosis. Am J Med 122: S26 –S37, 2009. 3. Bootman MD, Lipp P, Berridge MJ. The organization and functions of local Ca2⫹ signals. J Cell Sci 114: 2213–2222, 2001. 4. Byyny RL, LoVerde M, Lloyd S, Mitchell W, Draznin B. Cytosolic calcium and insulin resistance in elderly patients with essential hypertension. Am J Hypertens 5: 459 –464, 1992. 5. Chen CF, Corbley MJ, Roberts TM, Hess P. Voltage-sensitive calcium channels in normal and transformed 3T3 fibroblasts. Science 239: 1024 – 1026, 1988. 6. Cho E, Manson JE, Stampfer MJ, Solomon CG, Colditz GA, Speizer FE, Willett WC, Hu FB. A prospective study of obesity and risk of coronary heart disease among diabetic women. Diabetes Care 25: 1142– 1148, 2002. 7. Deslex S, Negrel R, Ailhaud G. Development of a chemically defined serum-free medium for differentiation of rat adipose precursor cells. Exp Cell Res 168: 15–30, 1987. 8. Diaz-Velasquez CE, Castro-Muñozledo F, Kuri-Harcuch W. Staurosporine rapidly commits 3T3-F442A cells to the formation of adipocytes by activation of GSK-3beta and mobilization of calcium. J Cell Biochem 105: 147–157, 2008. 9. Gaur S, Morton ME, Frick GP, Goodman HM. Growth hormone regulates the distribution of L-type calcium channels in rat adipocyte membranes. Am J Physiol Cell Physiol 275: C505–C514, 1998. 10. Gaur S, Yamaguchi H, Goodman HM. Growth hormone regulates cytosolic free calcium in rat fat cells by maintaining L-type calcium channels. Am J Physiol Cell Physiol 270: C1478 –C1484, 1996. 11. Green H, Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell 7: 105–113, 1976. 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 The present study also provides the first evidence showing the expression of CaV transcripts in mouse primary cultured preadipocytes as well as 3T3-L1 preadipocytes. The dominant expression of ␣1G mRNA was detected by conventional/quantitative real-time RT-PCR among CaV transcripts (␣1A–␣1I), and the expression of CaV3.1 protein encoded by ␣1G gene was detected by immunohistochemical studies and Western blot analysis in both mouse preadipocytes. In addition, the protein level of Cav3.1 encoded by ␣1G mRNA was significantly downregulated in differentiated adipocytes by decreasing the transcription level. Thus T-type Ca2⫹ channel encoded by ␣1G subtype does not appear to function in mature white adipocytes, but alternatively it seems to play a vital role in the function of mouse preadipocytes. However, in contrast with the data of immunohistochemical studies and Western blot analysis, we have not been able to detect the obvious T-type CaV by patch-clamp experiments. Probably T-type CaV in mouse primary cultured preadipocytes may be very small for detection. Alternatively, it may be quickly decreased or run down under the culture conditions. Therefore, further studies using freshly isolated preadipocytes are needed to clarify the existence of T-type CaV in mouse preadipocytes. Diaz-Velasquez et al. (8) reported that T-type CaV may be involved in adipocyte differentiation, where mibefradil and Ni2⫹ partially inhibited staurosporine-induced preadipocyte conversion to adipocytes. They proposed that Ca2⫹ mobilization by at least T-type CaV is involved in adipocyte differentiation, where GSK3 activation depends on transient increase in [Ca2⫹]i (13, 16). Proliferation of preadipocytes is also an important key step in adipose tissue development since it conditions the number of potential new adipocytes in the adipose tissue. Electrophysiological studies have shown the expression of K⫹ channels in isolated cultured brown fat cells from neonatal rats and human preadipocytes, which may control membrane potential and then cellular proliferation and differentiation of preadipocytes. Hu et al. (20) reported that K⫹ channel blockers inhibited human preadipocyte proliferation by accumulating cells at G0/G1 and reducing S-phase population. In the present study, we showed that mibefradil inhibited 3T3-L1 proliferation in response to serum, while diltiazem and nicardipine were less effective. We also investigated the effects of NNC55-0396, a selective T-type Ca2⫹ channel blocker (21, 37), on cell proliferation and cell cycle. NNC55-0396 inhibited T-type CaV expressed in 3T3-L1 preadipocytes and significantly suppressed cell proliferation, by inhibiting cell cycle entry with accumulating cells at G0/G1 and reducing S- and G2/M-phase population. Similarly, siRNA targeted for ␣1G inhibited cell proliferation and reduced G0/G1-phase population in response to serum. However, siRNA also appears to inhibit progression of S phase into G2/M phase. The reason for the difference between NNC55-0396 and siRNA remains unclear, but the additional, nonspecific action of NNC55-0396 or the toxic effects of siRNA cannot be ruled out in this study. Further studies are needed to clarify the involvement of T-type CaV in the cell cycle. However, the present study showed that T-type CaV encoded by ␣1G subtype appears to play a role in the regulation of cell cycle entry/progression, and subsequently cell proliferation, in mouse preadipocytes. T-type Ca2⫹ channels may play a vital role in proliferation when activated by transient membrane depolarization or when they are open at resting membrane potentials as judged from window current, T-TYPE CALCIUM CHANNELS IN MOUSE PREADIPOCYTES AJP-Cell Physiol • VOL 26. Lory P, Bidaud I, Chemin J. T-type calcium channels in differentiation and proliferation. Cell Calcium 40: 135–146, 2006. 27. Monteil A, Chemin J, Bourinet E, Mennessier G, Lory P, Nargeot J. Molecular and functional properties of the human alpha1G subunit that forms T-type calcium channels. J Biol Chem 275: 6090 –6100, 2000. 28. Panner A, Cribbs LL, Zainelli GM, Origitano TC, Singh S, Wurster RD. Variation of T-type calcium channel protein expression affects cell division of cultured tumor cells. Cell Calcium 37: 105–119, 2005. 29. Panner A, Wurster RD. T-type calcium channels and tumor proliferation. Cell Calcium 40: 253–259, 2006. 30. Pappone PA, Ortiz-Miranda SI. Blockers of voltage-gated K channels inhibit proliferation of cultured brown fat cells. Am J Physiol Cell Physiol 264: C1014 –C1019, 1993. 31. Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 19: 285–292, 2004. 32. Peres A, Sturani E, Zippel R. Voltage-dependent calcium current in adherent mouse 3T3 fibroblasts. Exp Cell Res 180: 585–590, 1989. 33. Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA 280: 1843–1848, 1998. 34. Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res 96: 864 –872, 2005. 35. Shi H, Halvorsen YD, Ellis PN, Wilkison WO, Zemel MB. Role of intracellular calcium in human adipocyte differentiation. Physiol Genomics 3: 75–82, 2000. 36. Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem 255: 4745–4750, 1980. 37. Taylor JT, Huang L, Pottle JE, Liu K, Yang Y, Zeng X, Keyser BM, Agrawal KC, Hansen JB, Li M. Selective blockade of T-type Ca2⫹ channels suppresses human breast cancer cell proliferation. Cancer Lett 267: 116 –124, 2008. 38. Terasawa K, Nakajima T, Iida H, Iwasawa K, Oonuma H, Jo T, Morita T, Nakamura F, Fujimori Y, Toyo-oka T, Nagai R. Nonselective cation currents regulate membrane potential of rabbit coronary arterial cell: modulation by lysophosphatidylcholine. Circulation 106: 3111– 3119, 2002. 39. Uebele VN, Gotter AL, Nuss CE, Kraus RL, Doran SM, Garson SL, Reiss DR, Li Y, Barrow JC, Reger TS, Yang ZQ, Ballard JE, Tang C, Metzger JM, Wang SP, Koblan KS, Renger JJ. Antagonism of T-type calcium channels inhibits high-fat diet-induced weight gain in mice. J Clin Invest 119: 1659 –1667, 2009. 40. Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol 154: 91–107, 1996. 298 • JUNE 2010 • www.ajpcell.org Downloaded from http://ajpcell.physiology.org/ by 10.220.33.2 on October 25, 2016 12. Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol 101: 169 –171, 1979. 13. Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol 65: 391–426, 2001. 14. Hamill P, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981. 15. Harkins JM, Moustaid-Moussa N, Chung YJ, Penner KM, Pestka JJ, North CM, Claycombe KJ. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr 134: 2673–2677, 2004. 16. Hartigan JA, Johnson GV. Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem 274: 21395–21401, 1999. 17. Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev 2: 239 –254, 2001. 18. Hausman DB, Park HJ, Hausman GJ. Isolation and culture of preadipocytes from rodent white adipose tissue. Methods Mol Biol 456: 201– 219, 2008. 19. Hirsch J, Fried SK, Edens NK, Leibel RL. The fat cell. Med Clin North Am 73: 83–96, 1989. 20. Hu H, He ML, Tao R, Sun HY, Hu R, Zang WJ, Yuan BX, Lau CP, Tse HF, Li GR. Characterization of ion channels in human preadipocytes. J Cell Physiol 218: 427–435, 2009. 21. Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS, Li M. NNC 55-0396 [(1S,2S)-2-(2-(N-[(3benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther 309: 193–199, 2004. 22. Jo T, Nagata T, Iida H, Imuta H, Iwasawa K, Ma J, Hara K, Omata M, Nagai R, Takizawa H, Nagase T, Nakajima T. Voltage-gated sodium channel expressed in cultured human smooth muscle cells: involvement of SCN9A. FEBS Lett 567: 339 –343, 2004. 23. Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J 77: 3034 –3042, 1999. 24. Leuranguer V, Mangoni ME, Nargeot J, Richard S. Inhibition of T-type and L-type calcium channels by mibefradil: physiologic and pharmacologic bases of cardiovascular effects. J Cardiovasc Pharmacol 37: 649 –661, 2001. 25. Li TY, Rana JS, Manson JE, Willett WC, Stampfer MJ, Colditz GA, Rexrode KM, Hu FB. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation 113: 499 –506, 2006. C1423