breeding season habitat use of philippine hawk owl ninox
Transcription
breeding season habitat use of philippine hawk owl ninox
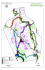
BREEDING SEASON HABITAT USE OF PHILIPPINE HAWK OWL NINOX PHILIPPENSIS SPILONOTA IN CEBU ISLAND, PHILIPPINES PHILIP GODFREY JAKOSALEM A Dissertation submitted to The School of BiologicalHABITAT Sciences of the University of East Anglia BREEDING SEASON USE OF PHILIPPINE in partial fulfillment of the requirements for the degree of Master of Science in Applied Ecology and Conservation HAWK OWL NINOX PHILIPPENSIS SPILONOTA IN September 2011 CEBU ISLAND, PHILIPPINES ACKNOWLEDGEMENTS I sincerely appreciate the advice, encouragement, support, guidance and direction provided by my supervisors Dr Jennifer Gill and Prof Nigel Collar. Their helpful comments greatly improved this paper. It’s been a great pleasure and honor to work with both of you. I thank my fellow course mates in Applied Ecology and Conservation for the friendship, hardship, support and advised, with special thanks extended to Alaa Mohamed Ali, Maxim Koshkin, Philip de Pous and Robert Hawkes for assisting me in some analysis, GIS and smart graph. This project was supported funded by the Chester Zoo Studentship 2011(Dr. Roger Wilkinson and Ms. Yvette Foulds), The World Owl Trust (Tony Warburton) Philippine Biodiversity Conservation Foundation, Birdlife International, Wetlands Trust and University of East Anglia this project will not be successful without their support. I’m also thankful to Dr Iain Barr for the additional transmitters and advice during the planning stage. This work will not be accomplished without the support and permission granted from the Department of Environment and Natural Resources, the municipalities of Alcoy, Dalaguete, Argao, Boljoon and the community of Tabunan and Nug-as. I thanks Protected Areas and Wildlife Department staff Reynaldo Yray for his assistance. My colleagues William L.R. Oliver, Lisa Marie, Paguntalan, Kail Zingapan, Orlyn Orlanes-Roxas, Edgar Lillio and Icarus, for their assistance, enthusiasm, and humor. I am grateful to the numerous flied assistant who assisted me in this research, Pedro Villarta, Fernando Anore, Ryan Anore, Teodoro Amaca and Tito Amaca for the skills and finesse. I’m also thankful to various volunteers Fe Anore, Romele Sandot, Sherry Paul Ramayla, Dexcem Pantinople, and Ara Patrice Kintanar for their assistance and dedication during the study. I thank my family for helping me and facilitating additional data needed for my study. Finally, I would like to thank Ford International Fellowship Program for the opportunity and unwavering encouragement and support on my fellowship. ABSTRACT 1. The largely deforested island of Cebu, Philippines, hosts a forest-dwelling hawkowl identified in the literature as Ninox philippensis spilonota but which is in fact a Cebu island endemic species or subspecies, soon to be named. 2. The five largest of 11 remaining forest patches on Cebu were surveyed between 28 March and 16 June 2011 involving 64 post-sunset 500-m long walked transects with three playback stations. Within the same period radio-telemetry studies were conducted on 10 owls but only for 3–5 days per owl as they removed the tailmounted transmitters. Habitat measurements were taken within a circular plot 10 m in radius at standard points along transects and at sites where birds were recorded. 3. A total of 52 owls were located across all five forests (at 16 sites two owls responded together to playback) but only the largest forest, Alcoy, contained enough transects for statistical analysis. Owls were detected in equal densities in forest interior, forest edge and forest-plantation mix, and on ridges and in gullies. Wind was the single variable measured that affected owl distribution, with the probability of owl occupancy decreasing with increasing wind speed. 4. Home ranges were calculated for 95% Minimum Convex Polygon, 95% kernel and 50% kernel, the 95% kernel yielding a mean 8.37 ha. Suitable forest studied covers roughly 1,670 ha, with six unstudied forests totalling 250 ha, so assuming a pair every 10 ha the global population of the Cebu Hawk-owl may be 192 pairs. If birds failed to cover all their home ranges while being radio-tracked, home ranges may be larger and the global population smaller. 5. Continuing habitat degradation triggers IUCN Red List category Endangered. Experiments with nest-box deployment are an obvious next step, provided this does not increase predator pressure on other rare species. TABLE OF CONTENTS Introduction……………………………………………………………………… 1 Decline of tropical forest……………………………………………………... 1 Decline of Philippine forest ………………………………………………….. 1 Fragmented forest of Cebu…………………………………………………… 2 Philippine Hawk Owl in Cebu……………………………………………….. 3 Methods………………………………………………………………………….. 4 Study species…………………………………………………………………. 4 Philippine Hawk Owl Ninox philippnensis spilonota……………………….. 4 Study sites……………………………………………………………………. 4 Alcoy Forest……………………………………………………………….. 4 Upper Beciril Forest and Nangka Forest………………………………….. 5 Argao Forest………………………………………………………………. 5 Dalaguete Forest…………………………………………………………... 5 Tabunan Forest……………………………………………………………. 6 Species capture, marking and movements…………………………………… 7 Owl detection transect……………………………………………………. 7 Habitat characteristic…………………...………………………………… 8 Radio-tracking of Philippine hawk-owls..……………………………….. 9 Dietary composition and nest site characteristics………………………... 10 Population estimates……………………………………………………… 11 Data analysis…………………………………………………………………. 11 Results…………………………………………………………………………… 12 Distribution of hawk-owls on Cebu Island………………………………….. 12 Factors influence the distribution of Philippine Hawk Owl…………………. 14 Home range characteristics...…………………………………………….. 15 Foraging habitat………………………………………………………….. 19 Roosting sites…………………………………………………………….. 19 Nest site characteristic……………………………………………………. 20 Dietary composition……………………………………………………… 21 Discussions………………………………………………………………………. 22 Conclusion……………………………………………………………………….. 25 Conservation implication……………………………………………………. 26 Reference………………………………………………………………………… 27 List of tables and figures List of tables Table 1. Name, unit and description of each of the habitat characteristics recorded in survey plots (314 m2) on hawk-owl detection transects in five forests on Cebu Island, Philippines. The surveys in which each variable was recorded (T = transect surveys, R = locations of radio-tracked individuals) are also shown……………………………………………………………………….. 9 Table 2. The total number of hawk owls and the estimated number of pairs recorded on transects through different habitats in five forest on Cebu Island..... 12 Table 3. Habitat characteristics of the five forests on Cebu Island on which hawk-owl surveys were carried out. The means (± SE) of each variable within circular plots (314 m2) on separate transects are shown, and the number of transects is given in parentheses………………………………………………… 13 Table 4. Differences in the habitat characteristics of the hawk-owl survey transects located on ridges (22 transects) and gulleys (19 transects) within Alcoy Forest, Cebu Island. The means of each variable within circular plots (314 m2) on separate transects are shown. (see Table 1 for details of habitat variables), and significant differences are highlighted in bold………………… 13 Table 5. . Estimates of home range size (from 95% minimum convex polygon (MCP), 95% kernel and 50% kernel home range analyses) of adult male and female and juvenile hawk owls radio-tracked within Alcoy Forest, Cebu Island. Names in bold indicate those with home ranges in the forest interior, and mean home range sizes for forest interior and forest edge are given………………….. 18 Table 6. Comparison between different radio fixes between different habitats during radio telemetry…………………………………………………………… 19 Table 7. Comparison between difference variable in forest and forest edge home range within the study sites……………………………………………….. 20 Table 8. Characteristics of the two hawk-owl nests that was located within Alcoy Forest, Cebu Island, one of which was active in 2011, and the one which had been used on 2005 but was inactive in 2011………………………………... Table 9. Variation of potential prey composition of Philippine Hawk Owl as 21 observed as reported and observed by Alcoy Forest Wardens. x= reported; x*=observed and – not reported…………………………………………………. 22 List of figures Figure 1. Map of Cebu showing the five study sites…………………………….. 6 Figure 2. The effect of wind-speed on the probability (dotted curve) of occupancy of hawk-owls on transects in Alcoy Forest, Cebu Island predicted from a logistic regression model. The wind speeds recorded on occupied (open bars) and unoccupied (filled bars) transects are shown, together with their frequencies……………………………………………………………………….. 14 Figure 3. Comparison of 95% minmum convex polygon (MPC) home range between forest and forest edge radio tagged owl………………………………... 17 Figure 4. Home ranges of five adult male (pale grey), four adult female (dark grey, within main forest) and one juvenile (dark grey out with main forest) radio-tagged hawk-owls in Alcoy Forest, Cebu Island. Inner lines indicate 50% and outer lines indicate 95% kernel estimates of home ranges………………….. 18 INTRODUCTION Decline of tropical forest Tropical forests are amongst the most threatened ecosystems in the world. Most tropical forests are found in Asia, Americas and Africa. Asia has the most tropical moist forest which totals 18%, while 58% and 25% are located in Americas and Africa, respectively. However, the rate of deforestation has been increasing since 1990s, with 0.8-0.9% per year in Asia and 0.4-0.5% in Americas and Africa. Among the Asian regions, Southeast Asia has suffered the highest rate of deforestation with annual net losses of forest of up to 0.9 million hectares per year (Laurance 2007; FAO 2011). Logging is one of the major causes of the decline of the forest in Southeast Asia, as well as conversion of forest into agriculture which has even more damaging impact, particularly in countries like Singapore and Philippines (Sodhi et al, 2004; Pulhin 2006). Decline of Philippine Forest The Philippines was once covered with tropical rainforest over 90% of the entire archipelago. A total of 27 million hectares of tropical forest covered the entire country during the start of the Spanish colonization in 1521. The country experienced widespread degradation and deforestation over 19th century (Pulhin et al, 2006). The major causes were increased growth of the human population and associated conversion of forest to agriculture during the Spanish colonization (Kummer 1992). In addition to agriculture, the Spanish regime established shipyards which required timber, particularly in the islands of Masbate, Cavite, Pangasinan, Albay, Mindoro, Marinduque and Cebu (Vitug 1993). After the Spanish colonization, America ruled the country and about 70% of the Philippines were forested. In this period, the Americans introduced logging and exported Philippine timber to the world market. After two decades in operation, about 163 saw mills operated throughout the country, which contributed to the decline from 70% to 57% of the total forest cover (Pulhin et al, 2006; ESSC 1999; de la Cruz 1941). The logging halted during the Japanese occupation; however exploitation and extraction of forest products continued and was concentrated in more accessible areas, particularly in the islands of Bohol and Cebu in central Philippines. 1 Fragmented forest of Cebu The island of Cebu is located in the centre of the Philippines. It is one of the largest islands in the Philippines; with a total land area of 5,088 sq. km. Cebu has been one of the progressive islands in the 19th country since 1900 (Rabor 1959), which has contributed to the destruction of natural forest and high demand of wood fuels and household used. The extent of forest exploitation meant that Cebu was viewed as the worst environmental disaster in the country (Bensel 2008), and resulted in the extinction of many species of plants and animals (McGregor 1904; Rabor 1952; Brooks et al, 1996). The remaining natural forest habitats amounts to less than 0.3% of the original area and these are broken into seven forest fragments. These forest fragments are widely distributed across the island (Gonzalez et al, 1999; Paguntalan & Jakosalem 2008). The destruction of the forest is one of the major causes of extinction of many species of wildlife in Cebu (Collar et al, 1999; Brooks et al, 1996; Rabor 1959). The island has been badly deforested since early 1900 (Brooks, et al, 1995; Collar 1998). The loss of forest has seriously affected the islands biodiversity. Cebu has three endemic large mammals. Two of these shared with Negros Panay Faunal Region the Visayan Spotted Deer Cervus alfredi and the Visayan Warty Pig Sus cebifrons were declared extinct in the island (Grubb & Groves 1983; Gonzalez et al, 1999). Recent finding of fossil remains in Cebu lead to the discovery a new species of buffalo, the Cebu Dwarf Buffalo Bubalus cebuensis (Croft et al, 2006) which once inhabited the forest of Cebu. There is also evidence of local extinction of birds in the island of Cebu; 39 of 79 forest resident bird species are feared extinct on the island (Dickinson et al, 1991; Brooks et. al. 1995). However recent studies conducted in Cebu lead to the rediscovery of supposedly extinct species, including the globally threatened Cebu Flowerpecker Dicaeum quadricolor and Streak-breasted Bulbul I. s. monticola (Dutson 1993; Magsalay 1993; Brooks et al, 1995). Some larger species of doves, pigeons and raptors were also rediscovered, including Pink-bellied Imperial-Pigeon D. poliocephala, Amethyst Brown Dove P. a. frontalis, Philippine Serpent-Eagle S. holospilus, and the Philippine Hawk Owl N. philippensis (Gonzalez et al, 1999; Paguntalan & Jakosalem 2008). 2 Philippine Hawk Owl in Cebu The Philippine Hawk Owl Ninox philippensis is an endemic hawk owl (or boobooks) in the Philippines. There are seven recognized subspecies which group in different categories based on morphology. However, the species taxonomy is under review. Some authors group these subspecies by location; the philippensis group comprised of N.p. philippensis found in the island of Luzon, Polillio, Catanduanes, Marinduque, Samar and Leyte, the N.p. centralis subspecies found in the islands of Siquijor, Panay, Guimaras and Negros and the subspecies N.p. proxima and N.p. ticaoensis located in the island of Masbate and Ticao, respectively. The second group is composed of the subspecies N.p. spilocephala are found in the islands of Mindanao and Basilan, and the subspecies N.p. reyi is found in the island of Sulu, Tawitawi and Bongao. The subspecies N.p. spilonota were found in the island of Tablas, Sibuyan, Camiguin Sur and Cebu (König et al, 1999). Collar & Rasmussen (1998) also suggested that this species should be grouped according to it plumage pattern namely the bar-bellied group from the islands of Mindoro, Tablas, Sibuyan, Cebu Camiguin Sur and Sulus and the streaked-breasted group composed of philippinesis and spilocephala. Several authors have suggested that there are two or more species and additional new subspecies in this group. König et al, (1999) suggested the subspecies spilocephala and spilonata were viewed as separate species. Another taxonomical review suggested that there are new species and new subspecies in this group like the subspecies spilonata may be restricted to the three islands of Tablas, Sibuyan and Camiguin Sur while the species in Cebu will be a full species (Rasmussen et al, in prep). The subspecies spilonota was first described by Bourns & Worcester (1894). The species is a forest dweller. It was recorded from lowland to montane forest (1600m ASL) and remnant forest patches (Kennedy et al, 2000; Warburton 2009). It is locally common all through its range. However, it was once feared extinct in Cebu where deforestation since 1900 has been severe. In surveys during 1950s and again in early 1990, the species was not recorded in Cebu and it was considered extinct (Rabor 1959; Brooks et al, 1995). An island-wide survey conducted in 1998 by Gonzalez et al. (1999) recorded the species in three different forest patches in Cebu; Tabunan, Tuburan and Alcoy forest. A juvenile N.p. spilocephala was caught by locals along the forest edge in Alcoy forest in 2003 and several were heard calling in Alcoy and Tabunan forest confirmed the existence of the species in the island (Paguntalan & Jakosalem 2008). Despite the rediscovery of this species there only few study conducted about its taxonomy and ecological requirements. Very little 3 information exists about the ecology of N.p spilonota on Cebu, particularly its breeding ecology, habitat associations, territory size, and population size. Such information is needed to identify appropriate forest conservation measures. This study focused on patterns of habitat use during the breeding season of N.p spilonota on Cebu Island, and aimed to address four major objectives: 1) quantify the characteristics of habitat in sites with and without owls; 2) estimate home range size if individual owls within the breeding season and the extent to which the species uses forest edge habitat and secondary forest; 3) summarize information on diet, particularly the contribution of prey from forest and farmland sources, and nest site characteristics. METHODS Study species Philippine Hawk Owl Ninox philippensis spilonota The Philippine Hawk Owl N. philippensis spilonota is a nocturnal avian predator that is widely distributed throughout the Philippine Islands, with the exception of Palawan. It is one of the smallest owls in the Philippines. The species is locally common throughout its range. It was listed as Least Concern by the IUCN but it is listed as a threatened species in the Philippine Republic Act 9147 of 2001 (otherwise known as ―Wildlife Resources Conservation and Protection Act‖). Study sites Alcoy Forest, Alcoy (9°52’N 123°30’E) Alcoy Forest is lowland secondary limestone forest with elevations ranging from the lowlands up to 960m ASL. The forest is comprised of native and endemic species of tree including Artocarpus sp., Syzygium sp., Ficus spp., Casuarina rumphiana, Melia dubia, Macaranga sp., Cinnamomum cebuense and Melastoma sp. (Paguntalan, 2009). The forest is managed and protected by four local organizations through a Community-Based Forest Management Agreement (CBFMA) with the Department of Environment and Natural Resources (DENR). It covers more than 1600 ha including plantations and scrubland (Fig. 1). The largest forest patch totals up to 800 ha surrounded by plantations composed of Mahogany Swietenia macrophylla, Gmelina arborea, Eucalyptus and Mountain Agoho Casuarina rumphiana with Rattan Calamus sp. (Paguntalan 2009). Vegetable farms are found at forest edges, in gullies and between exotic plantations. 4 Upper Beciril Forest (9°61’N 123°46’E) and Nangka Forest (9°68’N 123°43’E), Boljo-on Boljo-on forest is composed of five fragments (Paguntalan 2009), with the largest fragment located in Upper Beceril, Lower Beceril, Lunop and San Antonio range. This forest is confined to river gullies and is estimated at 160 ha. Elevation ranges from 50 to 400m ASL. The second largest fragment, estimated up to 130 ha, is located in Nangka and San Antonio forest block connecting to Alcoy forest, with elevation ranges from 600 to 900m ASL. Boljo-on is a secondary limestone forest composed of Ficus sp., Buchanania and Syzygium. Agroforestry and vegetable farms are found along ridges and forest edges. Argao Forest (9°54’N 123°32’E) Mt Lantoy Forest in the Municipality of Argao is a lowland secondary forest with a typical karst limestone formation with elevations up to 800m ASL (Fig. 1). There are some oldgrowth trees along the riverbanks and among the hills. Plantations of exotic trees like Gmelina, Mahogany, Teak Tectona grandis and Acacia Samanea saman surround the native vegetation. Fruit-bearing trees like Lanzones Lansium domisticum and Jackfruit Artocarpus have also been planted along riverbanks and on slopes. Farmlands and grazing areas dominate the landscape. Mt Lantoy is naturally bounded by the Argao River known as Argao River Watershed Forest Reserve (Paguntalan 2009). The watershed reserve covers some of the adjacent forest patches within the municipality. Dalaguete Forest, Dalaguete (9°82’N 123°49’E) Dalaguete Forest is made up of three separate forest fragments namely: Babayongan– Bulak–Malones forest (500–800 m), Obo–Sacsac–Mantalongon (300–500 m) forest and the Obong–Caliongan forest patch (60–200 m) (Paguntalan 2009). The natural limestone forest is dominated by Vitex parviflora, Buchanania, Ficus and Syzygium spp. These forest types are confined to very steep areas where farming is very difficult. The other dominant plant species in the area are Nauclea, Pittosporum pentandrum, Ficus pseudopalma, Dracaena, Sterculia philippensis, Leea manillensis, Leucosyke capitellata and Breynia. Large Ficus species are commonly located on riverbanks. Vines are abundant, creating an illusion of thick vegetation (Paguntalan 2009). Most of the forest patches are surrounded by vegetable farms and scrubland (Fig. 1). 5 Tabunan Forest, Tabunan Cebu City (10°26’N 123°49’E) The forest of Tabunan forms a thin, segmented strip of forest on steep limestone hillsides, with closed-canopy areas of only less than 10 ha. It is part of the Central Cebu Protected Landscape and managed by the Protected Area Management Board (PAMB). The elevation of the area ranges from 400 to 880 m ASL. Common species of trees include Ficus species, Homalanthus sp., Syzygium, Macaranga, Leea sp., Sterculia philippnensis, Dillenia sp., Leucosyke sp., Diospyrus philippensis and Mangifera sp. Farms abutting the forest perimeter are mostly planted with high-value crops, vegetables, ornamentals and cut flowers (Paguntalan 2009). A well-developed road from the Cebu Trans-central Highway leads to a high concentration of village houses about 100 metres from the forest edge (Fig. 1). Figure 1. Map of Cebu showing the five study sites. 6 Species capture, marking and movements Fieldwork was conducted at five study sites on Cebu between 28 March and 16 June 2011 (with environmental data gathering by assistants into July), and was divided up into three main components: nocturnal transects to determine owl presence or absence, specific habitat assessments of known owl foraging and roosting sites, and home-range assessments using radio-telemetry. In addition, incidental and anecdotal data were gathered on prey items and nest-sites. Owl detection transects The presence or absence of owls was assessed in a total of 64 transects in five different forests (41 in Alcoy, eight in Dalaguete, six in Boljo-on, five in Argao, four in Tabunan). Transects were 500 m in length, were at least 100 m apart, and followed established trails throughout the forests. Each transect was walked once soon after sunset with surveyors listening constantly for owl calls, and three calling stations were established on each transect (at 0, 250 and 500 m) at which playback of owl calls was carried out. Playback consisted of a two-minute broadcast of pre-recorded calls of the Cebu Ninox (using an mp3 player with portable speakers); including the species’ double call, several screeches and occasional chittering calls, followed by a two-minute listening period and finally a spotlight search of the survey site. In Alcoy Forest, each transect was resurveyed on the following night without playback, in order to confirm owl presence and absence (all owls were relocated and no new owls were identified on these second transects). Transect locations were stratified to ensure coverage of forest interior (at least 30 m from the forest edge; 27 transects), forest edge (within 30 m of the forest edge; 27 transects) and mixed plantation (forest with some planting of fruit trees and commercial exotic species; 10 transects) sites. All five forests are on steep-sided hills and trails tend be either in the gulley bottoms or the ridge-tops, thus transect locations were also stratified to cover ridges (36 transects) and gulleys (28 transects). For each transect, measures of forest structure were quantified either at the locations where owls had been recorded or, for transects without owls, at the mid-point of the transect. A circular plot of 10 m radius, centred either on the tree in which owls were recorded or on the nearest tree to the transect mid-point was established at each of these locations, and the variables described in Table 1 were all recorded. In addition to measures of mean tree height and density, we also recorded maximum tree height and the number of 7 tall (> 6 m) trees within each plot, as taller trees are more likely to provide suitable nesting cavities. In addition, Alcoy Forest is subject to strong winds in the north-west and southeast regions, particularly during the monsoon season. Discussions with local wardens suggested that wind speed may influence owl distribution, and thus each transect point was visited once with an anemometer in order to measure wind speed. Habitat characteristics This study identified four habitat categories: foraging habitat (n=14), roosting habitat (n=13), unoccupied habitat (n=11) and nesting habitat (n=2). The sites where hawk owls were located during the radio telemetry and on transect were documented for certain habitat characteristics. A 10m radius circular plot was created centred on the tree where each hawk owl was first located during radio telemetry (Martin et al, 1997). The plot was divided into four quadrants to facilitate the counting of trees and branches. Habitat features were recorded following Martin et al, (1997). Additional variables were measured during the line transects model. The site variable was added as a random factor to examine the differences between sites (Table 1). All the variables in the habitat models were included in the analysis since there was no colinearity between variables. In addition, because owls were considered likely to forage around the forest edges as well as inside forest, the habitat characteristics were recorded at any foraging locations and at randomly located points around the edge of the forest. These data allowed identification of the characteristics of locations used by the owls. The description of the foraging and roosting site characteristics were quantified in terms of canopy cover using densitometer, number and height of trees within the plot, and understorey cover. 8 Table 1: Name, unit and description of each of the habitat characteristics recorded in survey plots (314 m2) on hawk-owl detection transects in five forests on Cebu Island, Philippines. The surveys in which each variable was recorded (T = transect surveys, R = locations of radio-tracked individuals) are also shown. Variable Unit Survey Description m T, R m T, R No. tall trees number T, R Total no. trees number T, R m T, R km/hour T, R categorical number R R Canopy cover % R Mean dbh cm R Understory cover % R Visual estimate of the height of the tallest tree within each plot Mean height of all trees taller than 3 m… Number of trees taller than 6 m within each plot Total no of trees taller than 3 m within each plot Estimate of the distance of the plot from the nearest forest clearing or farm Wind speed measured with an anemometer at one location on each transects. Ridge-top or gulley Total number of branches suitable for owl perching (> 2 cm diameter) on trees taller than 6 m % canopy cover measured with a densiometer Diameter at breast height for all trees taller than 6 m % cover of understory vegetation (< 1 m height) Maximum tree height Mean tree height Distance to clearing Wind speed Transect location No. perching branches Radio-tracking of Philippine hawk-owls Between 11 April and 11 June 2011, ten hawk-owls were captured using mist-nets and playback and each was fitted with a tail-mounted Pip Ag393 transmitter (mass 2.3 g, supplied by Biotrack Ltd). Although these tags have a standard expected lifespan of 10 weeks at 50 pulses per minute (Kenward 2004), hawk-owls proved quite adept at removing the tags, and eight of the tags were found discarded within one week of deployment. Consequently, individual owls were tagged consecutively and tracked repeatedly (for 3-5 days) until the tag was removed. All captured owls were measured and checked for the presence or absence of a brood patch. Nine of the tagged owls were adults and four of these had brood patches. These four individuals were consistently smaller (wing length range = 9 175 – 181 mm, tail length range = 91 – 99 mm, mass range = 120 – 129 g) than the remaining five adults (wing length range = 184 - 190 mm, tail length range = 100-109 mm, mass range = 124 – 142 g), and we therefore assumed the individuals with brood patches to be female and those without to be male. The juvenile bird was within the size range of females (wing length = 175 mm, tail length = 96 mm, mass = 118 g) but the sex of this individual could not be determined with any certainty. Each radio-tagged owl was tracked every night from dusk (one hour after sunset) to dawn (two hours before sunrise), when they were deemed most likely to be active and foraging. In addition, daytime tracking was carried out to locate potential nest and roost sites, and to identify any diurnal activity. Locations of radio-tagged owls were assessed by triangulation (White and Garrott 1990) using three-element Yagi antennae and Sica 138 mHz receivers (Biotrack Ltd) and handheld GPS. The field team worked on foot along forest trails, roads, gulleys and ridgetops, handheld radios were used to facilitate synchronized tracking and to keep the team in contact. The actual direction of each located signal (azimuth) was determined using a compass, and triangulation of directional signals was obtained with an interval of five minutes (Mazur et al.1998, Frosman et al. 2005). The behavior of tagged individuals at each recorded location was classified as either foraging (signals of individuals moving or perching for short occasions only) or roosting (nonmoving signals for more than 30 minutes, mostly diurnal records). The habitat characteristics of the foraging and roosting locations of tagged individuals (together with the characteristics of the three locations from which roosting owls were disturbed during daytime fieldwork) were assessed. Circular plots of 10 m radius centred on the tree in which each owl was first located during radio telemetry were established, and divided into four quadrants to facilitate counting, and the variables listed in Table 1 were recorded. Dietary composition and nest site characteristics In each territory where the owls were located, a list of potential prey was recorded. Prey was classified according to likely origin (farmland vs. forest). Interviews with local forest wardens on diet were also conducted to add more information on the owls’ dietary requirements. Special efforts were made to find nests using the radio-tagged owls. Daytime tracking was conducted in order to identify whether these individuals were nesting. The location of the two nests found was marked and the characteristics of the habitat 10 around them were measured following Martin et al, (1997). Nest tree species, height and DBH, nest cavity height, nest hollow orientations, location, and names of dominant and emergent species of tree were collected. Information on status and threats to the nest tree was also noted. Population Estimates The actual and potential number of owl pairs on Cebu was estimated from a combination of home range size estimates for tagged owls and assessment of availability of foraging habitats. The forest of Alcoy and other remaining forests on the island have high degree of homogeneity, which help with extrapolation of the results to other habitats and locations around the island (Miller et al. 2004; Odum & Barrett 2005). Data analysis Differences in the measures of forest structure recorded on transect among the five forests were compared with a Kruskal-Wallis test. To explore the differences in habitat structure and wind speed on transects on which owls were and were not recorded, a logistic regression was constructed which included maximum tree height, number of tall trees, distance to the nearest clearing, location (ridge or gulley) and wind speed (Table 1). Only the transects in Alcoy Forest were included within this model because too few transects were available from the other sites (because of constraints imposed by forest area). The collinearity between predictor variables was checked prior to analysis, and location (ridge or gulley) was coded as a categorical predictor. In Alcoy Forest, differences between transects on ridges and gulleys in the measures of forest structure and wind speed were compared with t-tests. Estimates of the home range size used by individuals during the period of radiotracking were carried out using ArcView GIS version 3.2a with the Animal Movement Extension (Hooge & Eichenlaub 2000). We estimated home range using 95% minimum convex polygons (MCP) and kernels from 50% and 95% probability models, as the latter are less prone to the effects of outliers (Worton 1989, Mazur et al. 1998, Frosman et al. 2005). One location from each triangulation fix was included within the home range estimates. The sizes of owl home ranges that did and did not encompass forest edges, and the sizes of the home ranges of adult male and adult female owls were compared with Mann-Whitney tests. 11 RESULTS Surveys of the hawk owl were completed in five forest sites in Cebu Island; four in the southern part of the island and one in central Cebu, and owls were located in all five sites (owls detected on: Alcoy: 37/41 transects, Dalaguete Forest: 3/8 transects, Boljo-on Forest: 3/6 transects, Argao: 4/5 transects and Tabunan: 5/4 transects. Distribution of hawk-owls on Cebu Island Across the five forests, owls were detected in forest interior, forest edges and in areas of mixed forest and plantation, where some commercially used species are grown within the forest matrix (Table 2). In total, 52 owls were detected across these five forests, and at 16 locations there were two owls responding to playback together, suggesting that there were at least 16 pairs of owls within this total. Table 2. The total number of hawk owls and the estimated number of pairs recorded on transects through different habitats in five forest on Cebu Island (see text for details). Habitat Forest interior Forest edge Mixed plantation Total Alcoy No. No. owls pairs Dalaguete No. No. owls pairs Boljoon No. No. owls pairs Argao No. No. owls pairs Tabunan No. No. owls pairs 20 7 10 9 2 2 0 1 2 0 0 1 0 1 2 0 0 0 0 1 3 0 0 1 2 2 1 1 0 0 37 13 3 1 3 0 4 1 5 1 The five forests were similar in mean tree heights, total numbers of trees and numbers of tall tress recorded on the transect surveys, but the maximum tree heights were significantly taller in Alcoy and Tabunan Forests (Table 3). Transects in the five forests also did not differ significantly in their distances from clearings and farms, although the larger distances recorded in Alcoy forest plots reflect the the much larger area of continuous forest within Alcoy than within the other forest patches. 12 Table 3. Habitat characteristics of the five forests on Cebu Island on which hawk-owl surveys were carried out. The means (± SE) of each variable within circular plots (314 m2) on separate transects are shown, and the number of transects is given in parentheses (see Table 1 for details of habitat variables). Variables Max tree height Mean tree height No. of tall trees Total no. of trees Distance to clearing Alcoy (n=41) 10.24 ± 0.44 12.34 ± 0.68 27.00 ± 1.47 37.97 ± 1.88 136.8 ± 26.3 Dalaguete (n=8) 8.5 ± 0.26 14.92 ± 1.86 34.67 ± 4.65 47.05 ± 4.98 85.0 ± 21.38 Boljoon (n=6) 7.00 ± 0.86 14.7 ± 1.45 22.87 ± 1.11 41.03 ± 3.44 44.16 ± 26.4 Argao (n=5) 9.40 ± 1.02 12.36 ±1.73 26.36 ± 2.67 39.36 ± 4.64 47.0 ± 21.77 Tabunan (n=4) 12.50 ± 1.55 17.47 ± 3.79 42.22 ± 6.79 50.22 ± 8.82 105.0 ± 55.0 X2 p 11.27 0.02 5.57 0.23 9.20 0.06 5.81 0.21 2.79 0.59 Within Alcoy forest, owls were located on both ridges (10/22 transects) and gullies (12/19 transects). The mean tree height and total number of trees within survey plots was significantly higher on ridges than gulleys, and ridge plots were significantly further from clearings and farms. However, in general the structure of the forest in ridges and gulleys was very similar (Table 4). Table 4. Differences in the habitat characteristics of the hawk-owl survey transects located on ridges (22 transects) and gulleys (19 transects) within Alcoy Forest, Cebu Island. The means of each variable within circular plots (314 m2) on separate transects are shown. (see Table 1 for details of habitat variables), and significant differences are highlighted in bold. Variables Maximum tree height Mean tree height Number of tall trees Total number of trees Distance to clearing Wind speed Ridges Mean ± SE 9.77 ± 0.53 13.87 ± 0.97 28.81 ± 2.02 43.09 ± 2.38 190.9 ± 39.3 2.23 ± 0.45 Gullies Mean ± SE 10.78 ± 0.73 10.57 ± 3.55 24.89 ± 2.08 31.78 ± 2.43 74.21 ± 28.9 1.15 ± 0.4 T p 1.15 2.55 1.34 3.30 2.32 1.76 0.26 0.015 0.19 0.002 0.025 0.08 13 A logistic regression analysis of the factors influencing the presence and absence of owls on transects within Alcoy forest showed that only wind speed had a statistically significant influence on owl distribution (Wald = 11.71, df = 1, p < 0.001), with the probability of owl occupancy declining significantly with increasing wind speed (Figure 2). Wind speed correctly predicted 84.2% of the 19 transects without owls and 90.9% of the 22 transects with owls within the logistic regression model, with owls being very rarely recorded on transects on which wind speed readings exceeded 2 km/hour (Figure 2) . 1 0 Figure 2. The effect of wind-speed on the probability (dotted curve) of occupancy of hawkowls on transects in Alcoy Forest, Cebu Island predicted from a logistic regression model. The wind speeds recorded on occupied (open bars) and unoccupied (filled bars) transects are shown, together with their frequencies. 14 Home range characteristics A total of 11 owls were mist-netted and 10 owls were radio tracked. This study compared the home range between the 10 radio tracked hawk owls using ground-based radio telemetry. Each hawk owl was tracked for 5-6 days between April 11 and June 11, 2011 during the breeding season. Only hawk owls with more than 30 locations during triangulation were included in estimations of home range size (Table 5). The home range analysis indicates that estimates varied between 95% MCP, 95% kernel and 50% core kernel analyses (Table 5). The 95% kernel method estimates of home range size were larger than 95% MCP (Table 5, Fig. 2). The mean hawk owl home range size estimates during the breeding season were 7.24 ha (± 4.10 SD) 95% MCP, 8.31 ha (± 4.19 SD) 95% kernel and 2.67 ha (± 1.34 SD) 50% kernel core area (Fig. 3). For the 6 hawk owls that were located in the forest edge, mean home range size estimates were 6.0 5ha (± 4.61SD) 95% MCP, 7.33 ha (± 4.66 SD) 95% kernel and 2.67 ha (± 1.71) 50% kernel. By contrast, the home range size estimates for the four owls located within the forest were 9.01 ha (± 2.82 SD) 95% MCP, 9.28 ha (± 2.97 SD) 95% kernel and 2.68 ha (± 0.72 SD) 50% kernel (Appendix 1). The forest interior home ranges of hawk owls were therefore larger than those of owls in the forest edge (Table 5). Most of the location of the hawk owl’s location are clumped in distribution rather than spread throughout the territory (Appendix 2). The home range located in small forest patches has smaller home range size forest counterpart (Table 5). This three were located two to three kilometre away from the larger forest patch. This forest patch is composed of mixed native trees, exotic trees and agroforestry. The peak activity period during radio telemetry of hawk owls was from 1800-0100 hours, and activity slowed down between 0200 to 0400 hours. The species were recorded in different bearings within its home range in the early dusk. The most of the radio tagged hawk owls that were located between 0200 and 0400 were all motionless during this period. 15 Table 5. Estimates of home range size (from 95% minimum convex polygon (MCP), 95% kernel and 50% kernel home range analyses) of adult male and female and juvenile hawk owls radio-tracked within Alcoy Forest, Cebu Island. Names in bold indicate those with home ranges in the forest interior, and mean home range sizes for forest interior and forest edge are given Owl Radio 95% MCP Tracking Period Tracking Fixes (n) (ha) Days 95% Kernel (ha) 50% Kernel (ha) Adult males Ryan 8-11 May 2011 4 207 7.95 8.43 2.71 Godo 3-7 May 2011 5 140 4.54 5.67 3.23 Lisa 1-3 June 2011 3 54 1.13 1.59 .089 Doro 11-12 April 2011 3 31 5.29 9.45 5.09 Tito 22-25 May 2011 4 131 11.91 12.49 3.11 Mean ± SD 6.2 ± 4.0 7.52 ± 4.1 3.0 ± 1.5 Adult females Orlyn 11-14 May 2011 4 94 9.63 11.43 3.55 Elline 25-27 May 2011 3 53 10.63 12.87 3.25 Pedro 19-21 May 2011 3 64 12.62 12.98 2.44 Sidro 20-23May 2011 4 163 5.58 5.98 1.65 Mean ± SD 9.61 ± 3.0 10.81 ± 3.3 2.72 ± 0.8 Juvenile James 26-30April 2011 5 263 2.14 2.89 1.63 Overall mean ± 7.04 ± 4.0 8.37 ± 4.2 2.75 ± 1.2 SD Forest Interior 3.83 ± 1.0 107.66 5.89 ± 4.4 7.33 ± 4.3 2.80 ± 1.5 mean ± SD Forest Edge mean 138.5 ± 4.0 ± 0.8 9.01 ± 2.8 9.94 ± 2.7 0.72 ± 0.07 ± SD 64.9 16 Figure 3. Comparison of 95% minmum convex polygon (MPC) home range between forest and forest edge radio tagged owl 17 Figure 4. Home ranges of five adult male (pale grey), four adult female (dark grey, within main forest) and one juvenile (dark grey out with main forest) radio-tagged hawk-owls in Alcoy Forest, Cebu Island. Inner lines indicate 50% and outer lines indicate 95% kernel estimates of home ranges. 18 Foraging habitat There were six radio tagged owls observed foraging in different habitats with good. The species was mostly recorded foraging within the secondary forest 60% (59.5±38.7) and forest edge19% (19.0±16.1) (Table 6). However, they were also recorded in farmland (3%) with tall trees present. Two hawk owls were also tracked along the exotic plantation 6.0% adjacent to the secondary forest and scrubland along the forest edge. No territory overlap was observed during the study. The hawk owls were also recorded in mix plantation (12%) and small forest patches with large trees and dense branches. There was also a significant difference in term of canopy cover between forest and forest edge foraging habitat. The canopy was more open at mean 14.3 as compared to 11.1. However, the understorey within the forest edge was denser (mean 68.3%) than in the forest (59.0%). There was no significant difference between the number of trees, dbh, and number of branches. Table 6. Comparison between different radio fixes between different habitats during radio telemetry. Habitat use Percent Mean Std. Deviation Forest 60 59.5 38.7 Forest edge 19 19.0 16.1 Mix Forest 12 12.0 19.1 Plantation 6 6.0 8.0 Farm 3 3.5 4.1 Roosting sites All of the roosting (n=13) territory was positioned with in their home range. A total of 69% of the roosting sites were located within the forest and 31% were observed along the forest edge. Roosting sites were composed of thick foliage cover like vines, leaves and epiphytes. There is no difference between roosting sites in the forest and forest edge in term for foliage cover. However, the forest edge owl’s resident has shorter tree height with an average at 9.61 m while the forest resident owl was taller with a mean of 11.7 m (Table 7). The roosting tree was average dbh was 58.1 cm in the forest edge and 45 cm with the forest owls. Most of the roosting sites were characterized by high number of woody vines, and the presence of owl fecal on the ground. 19 Table 7. Comparison between difference variable in forest and forest edge home range within the study sites. Number of Understorey Canopy DBH tree Tree Height Forest Mean 35.5 55.0 11.1 45.0 11.7 Std Deviation 14.7 11.7 4.4 10.0 .9 29.8 57.6 14.8 58.1 9.6 9.2 20.1 13.2 22.3 3.2 Forest Edge Mean Std Deviation Nest site characteristics Nest finding efforts were concentrated on the 10 radio tagged owls supplemented by reports from forest wardens. Only 2 nests were recorded, one active and one in-active. The two nests were located in small mixed forest patches approximately 2-3 km from the main forest block. These patches were surrounded by vegetable farms and exotic tree plantations. The patch with the active nest was estimated to cover an area of 1.5 hectares that indicates the nest cover c.400 square metres (0.04 ha). The nest sites were located in the secondary forest a gully (active nest) and on a steep slope (inactive nest). The distance between the nests to forest edge was around 5 metres and 3 metres (Table 8). The nest trees were native species Melanolepis multiglandulosa and Vitex parviflora, the latter being a threatened species. The DBH of the two nesting tree was around 104 cm and estimated mean height of nest tree was 16.5 m. The mean height of the hollow from the ground was 2.28 m. The average size of the entrance to hollow was 17.78 x 11.43cm (Table 5). In the two nesting tree entrance was slightly smaller than the cavity with an average cavity depth 25.4 cm. The two nest sites were located in clumps of trees within the plot, with a mean canopy cover of 8.7 (using a densiometer). The mean height of the canopy was 6.23 metres. The active nest had denser understorey with 80% in the plot while the inactive nest had 10% understorey cover. The active nest site was dominated by Duabanga moluccana while the inactive nest site was dominated by Vitex parviflora. The active nest hollow was located 20 on the main truck. The hawk owl’s nest hollow was observed in mid height less than 3 m above the ground (Table 8). The entrances of the nest hollows were facing northeast and southwest. Table 8. Characteristics of the two hawk-owl nests that was located within Alcoy Forest, Cebu Island, one of which was active in 2011, and the one which had been used on 2005 but was inactive in 2011. Variables Nest site characteristics Tree species Tree status Height of nesting tree (m) DBH (cm) Nest hollow height (m) Nest hollow orientation Nest hollow entrance height (cm) Nest hollow entrance width (cm) Nest hollow depth (cm) Surrounding habitat characteristics Forest type Estimated forest patch size (ha) Canopy cover (%) Estimated canopy height (m) Dominant canopy species Tallest emergent tree species Understorey cover (%) Distance to clearing (m) Distance to main forest area (m) Topography Slope (%) Nest 1 (Active) Nest 2 (Inactive) Melanolepis multiglandulosa Alive 20 98 2.43 Northeast 20.32 12.7 33.2 Vitex parviflora Secondary plantation 1.4 9.67 7.04 Duabanga moluccnna Blume Melanolepis multiglandulosa 80 5 700 Gulley 75 Secondary forest 0.005 7.73 5.43 Vitex parviflora Dead 13 110 2.13 Southwest 15.24 10.16 17.78 Vitex parviflora 10 3 400 Near ridge 75 Dietary composition To quantify prey density in different habitat along forest and farmland, reports from the forest wardens and observations from the hawk owls nesting site were compiled. This study identified 15 potential prey items as reported by the forest wardens and four prey types were observed during the study (Table 9).The Philippine Hawk Owl N. p. spilonota diet is comprised of a range of different prey types, including small mammals, small birds, reptiles and insects (Table 9). They species appear to particularly prey on rats and small reptiles. This study observed the hawk owl preys on Cicada and Cricket. The hawk owl has also 21 been reported preying on amphibians along the forest edge or farmland particularly on Rana sp and Bufo sp. These amphibians are mostly observed in the forest edge and farmland or along creeks and water sources Table 9. Variation of potential prey composition of Philippine Hawk Owl as observed as reported and observed by Alcoy Forest Wardens. x= reported; x*=observed and – not reported. Prey Species Taxa Family/Order Common Name Forest Farmland Mammals Muridae Rats x x* Birds Nectariniidae Sunbird x x* Paridae Tits x x Dicaeidae Flowerpecker x x Muscicapidae Flycatcher x x Colubridae Snake x x Agamidae Gliding lizards x x Scincidae Common skinks x x Gekkonidae Gecko x x Ranidae Frog -- x Bufonidea Toad -- x Gryllidae Crickets x x* Lepidotera Moth x x Phasmatodea Stick insects x x Mantodea Mantis x x Cicadidae Cicada x x* Reptiles Amphibian Insects DISCUSSION The Philippine Hawk Owl breeding season habitat use is wide ranging. The study revealed that hawk owls can persist in different habitat. There are more hawk owls sighted within forest than at the forest edge. More hawk owls were recorded along gullies than on ridges, and most of larger stands of trees were located within the gullies. The three significant predictors that influence the presence and absence of hawk owls are the mean tree height, location between ridge and gully and wind—are all important in identifying suitable habitat 22 for the species. Hawk owls appear to prefer gully locations, with low levels of wind and tall trees. Wind significantly affects the probability of recording owl in all locations. In some instances hawk owls seemed to avoid windy areas within the forest or at forest edge. In particular, ridge top forests with apparently suitable tall trees may be too windy to be occupied by the species. The absence of owls in some areas may not necessarily indicate that the site was not suitable for the species. The study identified 11 sites where owl was recorded. The hawk owls avoid this areas since the site are windy especially during monsoon season. The understorey cover was 3.6% slightly less than occupied habitat. However, most of these sites are located along the ridge top forest. This implies that ridge forest has lower probability of holding owls. However, the presence of suitable nesting cavities within the home range may be important to the species. Tree cavities were observed in some of the radio-tagged owls’ territories. The number of cavities can limit the population of cavity-nesting species like owls (Newton 1994, 1998, Cockle et al, 2011). Consequently, lack of suitable nesting cavities could be one possible reason that the species declined to the point of being feared extinct in the island in 1950s (Rabor 1959; Brooks et al, 1995). The island had been deforested by early 1990 and only few old growth forests were found. It is therefore important to locate and protect tall trees with cavities for the owls and other species within the different forest patches. I recommend continuing long-term study on the species, particularly in respect of nest cavity availability and its effects on the population status of Cebu’s owls The home range of the Philippine Hawk Owl during the breeding season was extensive. Most of the larger home ranges were located within the forest rather than forest edge. Studies conducted on a similar species, Ninox connivens in Australia have shown that they have much larger territories of > 226 hectares (Taylor et al, 2004). This is typical of larger species of Ninox. The difference in home range size maybe due to several factors like size of the forest and human disturbance. The hawk owl home range tends to be smaller compared to the other species of the hawk owls. The species home range Some Philippine hawk owl home ranges were located within mixed mature exotic plantations with regenerating secondary forest along the forest edge. At the landscape level, the radio-tagged 23 hawk owl locations were mostly recorded within the forest than at the forest edge. However, the species also was also observed perching in the middle of the farmland and clearings perching in tall trees. However, all roosting sites were located within forested areas. The density of foliage seems to be a key characteristic of roosting sites. Dense foliage may provide protection from other predators, wind, rain, protection and disturbances from some species of birds particularly bulbuls. Radio telemetry within the forest is a real challenge because of radio signals bouncing off trees. It is important to look for a good position during homing to get a good radio signal. Another challenge was transmitter retention. The hawk owl can easily remove the transmitter or it will partially moult. The transmitters might be too big for the species and they have a strong enough beak to remove the tag. It is also important to consider other attachment methods like backpack for future study. It is better to look for other methods for the future studies in attaching radio transmitters with the owl. Furthermore, studies of the home range during the non-breeding season are needed, to check for differences between breeding and non-breeding territories. This could provide additional information on home range size, roosting and foraging habitat. This can provide more information on the conservation status of the species. This study is vital for the conservation of the potentially new endemic species of hawk owls in Cebu (Rasmussen et al, in prep.). Most of the remaining forests on Cebu are protected except Boljoon forest. Local legislation for the protection of wildlife was enacted particularly in Tabunan Forest, Dalaguete and Alcoy Forest where the species has been recorded. The home ranges located in small forest patches are more susceptible to habitat destruction. This study recommends protection of small forest patches potentially holding hawk owl territories. Planting native tree between forest patches will facilitate movement of individuals between forest sites. The hawk owls are generalist predators on a variety of vertebrates and invertebrates. Based on reports from the forest wardens and a few recorded observations, the owls exhibited considerable dietary breadth. However I was unable to conduct prey density and preference studies in territories. Hence I recommend more detailed study on prey abundance and preferences of the hawk owl within the different habitat use. It is also important to know whether dietary preferences exist. It is also important to know if there is 24 a significant relationship between the abundance of prey and the breeding success of the species. The results of the transect surveys support the concept that the hawk owl prefers forest over forest edge. The hawk owl also prefers gullies over ridge top forest. There were more proportionately records of owls along gullies than ridges. A total of 30 territories were recorded along the gully compared to 22 territories within the ridge (Fig. 11). Moreover, more territories of hawk owls were observed within the forest than forest edge. Among the radio-tagged owls nine had clearly defined territories, demonstrated by the fact that they responded to playback immediately. It is essential to understand its habitat requirements during the non-breeding season and compare the home range size. It is also important to explore other small forest patches in the island possible holding some population of hawk owls. This study recommends determining the differences in the ecological requirements of the other subspecies on the other islands. The use of radio telemetry is essential to know the different habitat requirements of the species before it is too late. Habitat restoration should be done in all the study sites using native species of tree and with thought given to the carrying capacity of current habitats. The results of this study can also provide appropriate information for conservation planning and management of the species and the improvement of its current status. CONCLUSION The Philippine Hawk Owl N.p. spilonota require breeding season home ranges of at least 8 to 12 hectares (using 95% maximum convex polygon and 95% kernel estimates). The species uses a variety of habitat categorized by different forest structures and types. In this study breeding hawk owls were located along the forest edge with a few large trees and suitable nest cavities. An estimate of breeding habitat used in forest edge almost the same as compared to the forest habitat. The hawk owl prefers forest over forest edge and plantation forest. There are more hawk owl observed with in the forest interior compared to forest edge. However the can survive in a different variety of habitat. There is more sighting of species territories within the forest. The territories within the forest are larger than expected home range. 25 The habitat characteristic provided more understanding of the species habitat requirements. Hence conservation of the species in a landscape-based approach is possible. This is important for maintaining the hawk owl population on the island. Small forest patches ranging from 1 to 2 hectares with good stands of tree are important to the species. Three of the radio-tagged owls were located in a small forest patch and this suggests that the species can survive in disturbed habitats. Most of their territories were surrounded by farmlands and exotic tree plantation. Thus the conservation of small forest patches needs to be promoted on the island. This study is an important step in conserving the last remaining forest in island. Several species of conservation interest were rediscovered bring back it population. The Philippine Hawk Owl was the first endemic raptor to be rediscovered in the island. Raptors are good indicators species for changes in the ecosystems (Rodriguez-Estralla et al. 1998). They are good indicators of habitat quality because of their sensitivity to human disturbance (Taylor, 1984). On the other hand the species was observed preying on small mammals in a vegetable farms near the forest edge. This could serve as natural pest control as most of the forest edge sites are owned by farmers producing vegetables for the town of Cebu. This study explored Philippine Hawk Owl breeding habitat use, home range, diet preference, and nest characteristics. The results indicate that habitat use varied in different scales. The species prefers forest over other habitat type. However, it manages to survive in mixed habitats and small forest patches. Proportionately more owls recorded in Alcoy forest than the rest of the sites. Conservation implication Taking this account together, they prefer larger forest patches like Alcoy forest. Most of the radio telemetry locations were concentrating with in the forest. Protecting the forest will save the species from extinction. Most of the forest sites are protected. However, the pressure from the booming economy means an increasing expansion of agricultural land in the island and the increasing human population creates more pressure on the last remaining forest in the island. There should be a good and realistic plan for the management and conservation of the species or perhaps the island’s biodiversity to ease the pressure on its habitat. The conservation programmes within the southern forest have been existing for 26 almost a decade particularly in the municipality of Alcoy, Dalaguete and Argao (Paguntalan 2009). The program is in partnership with community organization, non-government organizations, local government and the environment department. Tabunan forest within Central Cebu Protected Landscape manage by the local the department of environment and natural resources and protected areas management board should be retain and protected since it is the only important the last remaining old growth forest in the island. Yet there are still illegal activities within this forest sites. Strict implantation of laws is needed for the success of conservation and proper guidance to the community organization and local government unit. They should be guided in the right way in working for conservation. REFERENCE Bibby, C., M. Jones and S. Marsden (1998). Expedition Field Techniques: Bird Surveys. London: Expedition Advisory Center Royal Geographic Society pp 83,84,104. Bell, P. J. & Mooney, N. (2002). Distribution, habitat and abundance of Masked Owls (Tyto novaehollandiae) in Tasmania. Ecology and Conservation of Owls. (eds). Newton, I., Kavanagh, R., Olsen, J. & Taylor, I. Csiro Publishing Brooks, T.M., Magsalay, P., Dutson, G. and Allen, R. (1995). Forest loss, extinctions and last hope for birds on Cebu. Oriental Bird Club Bull.21:24-27. Bonaparte, Charles Lucien Jules Laurent (1855). Comptes Rendus Hebdomadaire des Séances de l'Académie des Sciences. Paris. 41, p. 655. Borlagdan, S.B. (1990). Social Forestry in Upland Cebu IN: Keepers of the Forest – Land Management Alternatives in Southeast Asia, M. Poffenberger (eds), Ateneo de Manila Press, p. 266-276. Bourns & Worcester (1894). Occasional Papers of the Minnesota Academy of Natural Science, 1, p. 8. Cockle, K.L, Martin, K.., Drever, M.C. (2010). Supply of tree-holes limits nest density of cavity-nesting birds in primary and logged subtropical Atlantic forest. Biological Conservation 143 (2851–2857 Cockle, K.L., Martin, K., & Wesotowski, T., (2011). Woodpecker, decay, and the future of cavity –nesting vertebrate communities worldwide. The Ecological Society of America Chokkalingan, U., A.P. Carandang, L.M. Pulhin, R.D. Lasco, R.J. Reras and T. 27 Toma. (2006). One century of forest rehabilitation in the Philippines: approaches, outcomes and lessons. SMK Grafika Desa Putera: Jakarta. Collar, N. J. & Rasmussen, P. C. (1998) Species limits in the Ninox philippensi complex. Ostrich 69: 398 (Proc. 22 Internatn. Orn. Congr.). Collar, N.J. (1998). Extinction by assumption; on the Romeo Error on Cebu. Oryx, 32 (4) Collar, N.J., N.A.D. Mallari and B.R. Tabaranza Jr. (1999).Threatened Birds of the Philippines. Bookmark: Philippines. Croft, D.A. L. R. Heaney, J. J. Flynn, and A. P. Bautista. (2006). Fossil Remains of A New, Diminutive Bubalus (Artiodactyla: Bovidae: Bovini) From Cebu Island, Philippines. Journal Of Mammalology 87:1037–1051 Dañguilan-Vitug, M. (1993). Power from the Forest the Politics of Logging by Philippine Center for Investigative Journalism Dickinson, E.C., R.S. Kennedy and K.C. Parkes (1991). The birds of the Philippines: An annotated checklist. Tring, U.K.: British Ornithologist’s Union (Checklist12). Dutson, G.C.L. (1993). Rediscovery of the Cebu Flowerpecker Dicaeum quadricolor confirmed. Oriental Bird Club Bull. 17:14. Environmental Science for Social Change. (1999). Decline of Philippine Forests. Makati, Philippines: ESSC Inc. and Bookmark Fabienne, H. (2000). Home Range and habitat use by the Long-Eared Owl in Northwestern Switzerland. J. Raptor Res. 34 (2) pp93-10. Forest Management Bureau. (2004). Philippine Forest Cover by Region and Province. Department of Environment and Natural Resources. Quezon City Frosman, E.D., Kaminske, T.J., Lewis, J.C., Maurice, K.J. and Sovern, S.G. (2005) Home Range and Habitat Use of Northern Spotted Owls on the Olympic Peninsula, Washington. J. Raptor Res.3 9 (4):365-377 Gerhardt. R., Gonzalez, N.B., Gerhardt. D.M., & Flattens, C.J. (1994). Breeding Biology and Ciccaba Home Range Of Two Owls. Willson Bull., 106(4) pp629-639 Githiru, M., Lens, L. Bennum, L.A. & Perrins, C. (2006). Experimental evidence of floaters in two isolated population of an Afrotropical Forest Birds. Ostrich; 77 (1&2): 28-35. Gonzales, J.C., Dans, A.T.L., Pedregosa, M.d.G., Chiu, C. and R. Villahermosa. (1999) Island-wide Survey of Forest and Fauna and Flora inventory of Selected Sites for Priority Conservation on Cebu. Fauna and Flora International Philippines 28 Gosler, A., (2004) Bird in Hand. In Bird Ecology and Conservation A Handbook of Techniques. Eds. Sutherland W.J., Newton I., Green R.E. Oxford University, New York Grubb, P. & Groves C.P. (1983). Note on the Taxonomy of Deer (Mammalia, Cervedae) of the Philippines. Zoo. Anz, Jena 210 ½, pp 199-144 Hardey, J., Crick, H., Wernham, C., Riley, H., Etheridge, B., and Thompson, D. (2006). Raptors: A Field Guide for Surveys and Monitoring. The Stationery Office: Edinburgh Harris, S., W. J. Cresswell, P. G. Forde, W. J. Trewhella, T. Woollard, and S. Wray. (1990). Home-range analysis using radio-tracking data - A review of problems and techniques particularly as applied to the study of mammals. Mammal Review 20:97-123. Heaney, L. R. and J. C. Regalado, Jr. (1998). Vanishing Treasures of the Philippine Rain Forest. The Field Museum, Chicago Hooge, P. N. and Eichenlaub, B., (2000). ―Animal movement extension to Arcview. ver. 2.0‖, Alaska Science Center - Biological Science Office, U.S. Geological Survey, Anchorage, AK, USA. Kavanagh, R.P. & Peake, (1993). Survey procedures of nocturnal forest bird: an evaluation of variability in census results due to temporal factors, weather and technique. (eds). Olsen P. Australian Raptor Studies 86-100 Melbourne; Australian Raptor Association, Royal Australian Ornithologist’s Union Kennedy, R. S., Gonzales, P. C., Dickinson, E. C., Miranda Jr., H. C. and Fisher, T. H. (2000). A Guide to the Birds of the Philippines. New York: Oxford University Press. Kenward, R.E. (2004). Radio tagging. In Bird Ecology and Conservation A Handbook of Techniques. Eds. Sutherland W.J., Newton I., Green R.E. Oxford University, New York König, C., Weick, F. & Becking, J.-H. (1999) Owls: a guide to the owls of the world. Robertsbridge, East Sussex, U.K.: Pica Press. Magsalay, P.M. (1993). Rediscovery of four Cebu endemic birds (Philippines) Asia Life Sci. 2: 141-148. Mallari, N. A. D., Tabaranza Jr., B. R. and Crosby, M. (2001) Key conservation sites in the Philippines: a Haribon Foundation and BirdLife International directory of Important Bird Areas. Makati City: Bookmark, Inc. 29 Martin, T. E., Paine, C., Conway, C. J., Hochachka, W. M., Allen, P. and Jenkins, W., (1997). BBIRD FIELD PROTOCOL: Breeding biology research & monitoring database, Biological Resources Division, Montana Cooperative Wildlife Research Unit, Missoula. Mazur, K.M., Frith, S.D., and James, P.C. (1998) Barred Owl Home Range and Habitat Selection in the Boreal Forest Of Central Saskatchewan. The Auk. 115 (3):746-754. McCrarym, .D. (1981). Effects of radio-tagging on the behaviour of RedShouldered Hawks. North Am. Bird Bander 6:138-141. McGregor, R.C. (1907). Notes on birds collected in Cebu. Philippine Journal of Science 2: p. 298-309 Miettemier, R.A., Myers, N., Thomsen, J.B., (1998). Biodiversity Hotspots and Major Tropical Wilderness Areas: Approaches to Setting Conservation Priorities. Conservation Biology, Pages 516–520 Volume 12, No. 3, June 1998 Miller, J.R., Turner, M.G., Smithwick, E.A.H., Dent, C.L., and Stanley E.H. (2004). Spatial extrapolation: the science of predicting ecological patterns and processes. BioScience Vol. 54 No. 4 pp 310-320 Millspaugh, J. J., and J. M. Marszluff, editors. (2001). Radio Tracking and Animal Populations. Academic Press, San Diego, California. Myers, N.,Mittermeier, R.A., Mittermeier, C.G., Da Fonseca, A.B. and Kent, J. (2000) Hotspot: earth’s biological richest and most endangered terrestrial ecoregions. Nature 403: 853-858 Newton, I., (1994). The role of nest sites in limiting the numbers of hole-nesting birds: a review. Biol. Conserv. 70, 265–276. Newton, I., (1998). Population Limitation in Birds. Academic Press, San Diego, California, USA. Odum, E.P. & Barrett, G.W., (2005). Fundamentals of Ecology. Fifth Edition. Thomson Brooks Oliver W.L.R. & Heaney L.R. (1997). Biodiversity and Conservation in the an Introduction to a Global Priority. eds Wildlife Conservation Society of the Philippines. Philippine Red Data Book. Makati City: Bookmark, Inc. Paguntalan, L.M.J. (2009). Cebu Local Forest Conservation Areas: Assessment and Action Plan. Cebu Biodiversity Conservation Foundation, Inc. and Department and Environment and Natural Resources-VII. Cebu, Philippines 30 Paguntalan, L.M.J. & Jakosalem, P.G.C. (2008). Significant records of birds in forests On Cebu island, central Philippines. FORKTAIL 24 (2008): 48–56 Pimm, S. L. (1991). The Balance of Nature? Ecological Issues in the Conservation of Species and Communities (Univ. of Chicago Press, Chicago). Pulhin. JM., Chokkalingam, U., Peras, R.J.J., Acosta R.T., Carangdang, A.P.C., Natividad, M.Q., Lasco R.D., & Razal, R.A. (2006). Chapter II Historical overview One century of forest rehabilitation in the Philippines Approaches, outcomes and lessons Eds. Chokkalingam, U., Carandang, A.P., Pulhin, J.M., Lasco, R.D., Peras, R.J.J., Toma, T. SMK Grafika Desa Putera: Jakarta Rabor, D.S. (1959). The impact of deforestation on birds of Cebu, Philippines, with new records for that island. Auk 76: 37-43. Republic Act No. 9147. (2001). ―Wildlife Resources Conservation and Protection Act‖ 11 th Congress of the Philippines Rodriguez-Estrella, R., Donazar, J.A., &Hiraldo, F. (1998). Raptors as indicators of environmental change in the scrub habitat of Baja California Sur, Mexico. Conservation Biology. Vol. 12, No. 4 Sergio, F., Marchesi, L. & Pedrini, P. (2003). Spatial refugia and the coexistence of a diurnal raptor with its intraguild owl predator. J. Anim. Ecol. 72: 232–245. Sodhi, N.S., Koh, L.P., Brook, B.W. & Ng P.K.L. (2004).Southeast Asian Biodiversity an impending Trends in Ecology and Evolution disaster Vol. 19 No 12 pp 654-660 Swihart, R., and N. Slade. (1997). On testing for independence of animal movements. Journal of Agricultural, Biological, and Environmental Statistics 2:48-63. Taylor, I.R., Kisten, I., & Peake P. (2002). Habitat, Breeding and Conservation of the Barking Owl Ninox connivens in Northeastern Victoria, Australia Taylor, R.J. (1984). Predation. Chapman and Hall, New York Warburton T. (2009). The Philippine Owl Conservation Programme: why is it needed? In: Jonhson D.H., Van Nieuwenhuyse D. & Duncan J.R. (eds) Proc. Fourth World Owl Conf. Oct-Nov 2007, Groningen, The Netherlands. Area 97(4): 429-438. Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home range studies. Ecology 70:164-168. 31